User login
Quitting smoking: Still a challenge, but newer tools show promise
Tobacco is a dirty weed,
I like it.
It satisfies no normal need,
I like it.
It makes you thin, it makes you lean,
It takes the hair right off your bean.
It’s the worst darn stuff I’ve ever seen.
I like it.
Graham Lee Hemminger. The Penn State Froth, November 1915: 19. Courtesy of Paul J. Dzyak, Jr., Paterno Library, Pennsylvania State University, State College, PA.
All physicians recognize the harm in tobacco smoking and try to convince patients to quit for health reasons, but quitting is challenging and frustrating for both doctor and patient. Physicians can improve quitting outcomes by applying their knowledge of the physiologic basis of nicotine addiction and newer tools that are making a real difference in smoking cessation.
THE NO. 1 PREVENTABLE CAUSE OF DEATH
Tobacco use remains the single largest preventable cause of death and disease in the United States: 443,000 US adults die of smoking-related illnesses each year, or one every 8 seconds.1 Tobacco smoking is currently responsible for 18% of all deaths and 37% of all preventable deaths. One-third of all smokers die early, with men losing 13 years of life and women losing 15 years. (See The rise and partial fall of smoking for a historical overview.)
Smoking, the leading cause of lung cancer, is also implicated in cancers of the mouth, larynx, esophagus, stomach, kidney, bladder, and cervix and has been linked to leukemia. (Though nicotine is responsible for the addictive properties of tobacco, it does not cause cancer itself: other substances in tobacco smoke, many of them byproducts of combustion, are carcinogenic.)
Running a close second to cancer as a smoking-related cause of death is cardiovascular disease, including stroke, myocardial infarction, microvascular dementia, peripheral vascular disease, and aortic aneurysm. Pulmonary and respiratory diseases, including chronic obstructive pulmonary disease, pneumonia, and asthma, are the third most common fatal smoking-related ailments.
Other medical consequences include erectile dysfunction, infertility, pregnancy complications, and low birth weight. Smoking also causes adverse surgical outcomes, poor wound healing, hip fractures, low bone density, peptic ulcer disease, and cataracts.
Smoking is estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.2
On the positive side, quitting smoking has health benefits at any age, and smokers who quit before age 35 have death rates similar to those in people who have never smoked.1,3
WHY IS IT SO HARD TO QUIT?
Most smokers want to quit, and many try to—but few succeed. In the 2010 National Health Interview Surveys, 68.8% of adult smokers said they wanted to stop smoking, and 52.4% had tried to in the past year, but only 6.2% had succeeded.4 Many recovering alcoholics and drug addicts say that quitting tobacco was much harder than abstaining from other substances of choice.
Why is it so hard to quit?
Smoking is a classic addiction
Addictions are usually diagnosed by behavioral signs, and nicotine addiction has many of the clinical hallmarks, eg:
- Tolerance, with a trend toward increasing the potency of the dose and the frequency of smoking over time
- Mental preoccupation with smoking, as it often becomes woven into one’s daily schedule and is associated with almost everything the smoker does throughout the day. Having no cigarettes in the house can generate anxiety that is relieved only by obtaining more
- Squandering scarce financial resources on nicotine products, over time amounting to substantial sums, and since smoking rates are higher in poor people than in the affluent, these are people who can least afford it
- Withdrawal symptoms, characterized by jitteriness, irritability, headache, insomnia, anxiety, and increased appetite.
People continue to smoke despite adverse consequences such as falling asleep while smoking and setting fire to the bed or to the house, or losing digits to peripheral vascular disease. Being unable to quit and to stay off smoking is a hallmark of tobacco dependence. Relapses are often triggered by being near other smokers or seeing a billboard advertising cigarettes. Eventually, the nicotine addict comes to value and crave nicotine more than health or life itself.
Nicotine stimulates ‘reward’ centers in the brain
Nicotine is an alkaloid found in many plants (including potatoes) but in especially high concentrations in tobacco. In mammals, it is a stimulant, rapidly producing dependence and addiction.
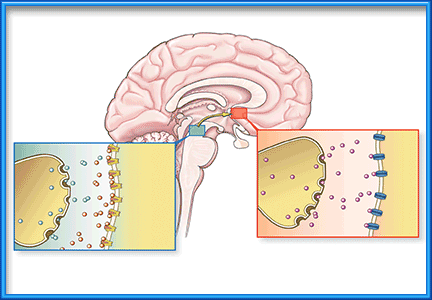
Inhaled by smoking, nicotine is absorbed across the large alveolar surface, avoids first-pass metabolism, and is transported rapidly to the brain (Figure 1). In fact, nicotine reaches the brain less than 20 seconds after inhalation, which is slightly faster even than when drugs are injected intravenously.5
Tobacco smoke contains approximately 4,800 compounds, many of which activate neurotransmitter systems such as dopamine, norepinephrine, acetylcholine, glutamate, serotonin, beta-endorphin, and gamma-aminobutyric acid. The most significant of these is the dopamine reward system known as the mesoaccumbens pathway. This system is activated within seconds of smoking and produces a sense of pleasure.
Nicotine binds to nicotinic acetylcholine receptors, primarily to alpha-4, beta-2 receptors in the ventral tegmental area of the midbrain. Once this binding occurs, a neurochemical message is conveyed to the nucleus accumbens via the release of dopamine in the mesoaccumbens pathway—the final common reward pathway triggered by all drugs of abuse. Since these structures and pathways of the brain are anatomically central, the addiction is driven by the basal ganglia and midbrain, the phylogenetically oldest parts of the brain. Nicotine therefore drives its addicts to continue smoking by producing strong neurochemical rewards and by causing strongly negative reactions when discontinued.
Genetically mediated susceptibility probably contributes to addiction. People whose neurochemical pathways are easily stimulated by this drug are probably at far greater risk of addiction. Paradoxically, people who are rapid metabolizers of nicotine are at greater risk than slow metabolizers.6 (Nicotine is metabolized by cytochrome P450 2A6 in the liver.)
Tolerance and withdrawal
Tolerance develops with long-term use, mediated by up-regulation (increased numbers) of alpha-4, beta-2 cholinergic receptors in the ventral tegmental area. Any reduction in nicotine level causes distress because receptors are unoccupied; with more receptors, nicotine intake must increase to keep physiologic balance and avoid withdrawal. Since the half-life of nicotine is only about 2 hours, the smoker must smoke almost constantly to satisfy receptors hungry for the stimulating drug. If drug levels drop, withdrawal occurs very quickly.
Eventually, smokers use nicotine less for pleasure and more as a way to avoid withdrawal. The cycle of pleasure, eventual tolerance, withdrawal, craving, and compulsion is biologically driven, like the drives of thirst, reproduction, and hunger. Nicotine hijacks species-sustaining reward mechanisms, leading to the malignant, compulsive disease of nicotine addiction.
Treatment doomed to fail?
Because nicotine addiction involves the midbrain, cessation strategies that rely on higher cerebral function are not likely to succeed. Counseling, common sense, and willpower simply cannot overcome the dopaminergic stimulating power or assuage the withdrawal sickness of nicotine dependence. Telling patients that smoking is bad for them misses the mark in most cases. Patients want to quit, but the drive to smoke is too powerful. Attempts to cut down rather than abstain from smoking also fail.
Nicotine is a formidable adversary for the patient and for the doctor or other health professional. Until recently, treatment was usually ineffective.
So, what does work against nicotine addiction?
PHARMACOTHERAPIES FOR SMOKING CESSATION
Nicotine replacement therapy
The oldest of the pharmacotherapies for nicotine addiction is nicotine replacement, in the form of patch, gum, lozenge, or nasal spray.
Advantages:
- Nicotine replacement therapy eliminates exposure to the other harmful compounds in tobacco, with few to none of the health risks associated with smoking.
- By delivering nicotine by a different route, nicotine replacement therapy breaks the association between smoking and feeling good. The addict is already dopamine-stimulated before putting a cigarette in the mouth, merely by association and suggestion. Using a different route of nicotine administration avoids that associative stimulation from the act of smoking, so that quitting becomes easier.
- The dose of nicotine is lower with replacement therapy than with smoking. The cigarette is the most efficient delivery mechanism for getting nicotine into the body. A smoked cigarette produces a rapid spike in plasma nicotine levels, far higher and faster than nicotine gum, nasal spray, or transdermal patch. Peak levels of plasma nicotine from nicotine replacement therapy are only 30% to 50% as high as those achieved by smoking.7–9
- It is inexpensive.
Disadvantages:
- Nicotine replacement therapy maintains the addiction to nicotine, with its neurophysiologic distortions.
- Some patients continue nicotine replacement therapy for years.
Use of nicotine gum can be a problem because of the need for frequent administration. The gum is chewed until the user feels a tingling or peppery taste in the mouth, after which the gum must be placed inside the cheek to allow for maximal absorption of the nicotine. Once the tingling has faded, the user is to chew another piece and repeat the cycle as long as craving is perceived. On the other hand, the nicotine patch is applied once daily. Both of these products are available over-the-counter.
Caution is indicated when starting nicotine replacement therapy in those with recent myocardial infarction, angina, or arrhythmia.
Effectiveness. Nicotine replacement therapy has been shown to be as effective as bupropion (see below) but not as effective as varenicline when used in single administration form (patch, gum, lozenge, or inhaler alone). The four single-administration forms of nicotine replacement therapy are all equally efficacious. Combinations of nicotine replacement formulations have been reported to be as effective as varenicline and superior to single formulations.10
How about electronic cigarettes? Electronic cigarettes, or e-cigarettes, supply nicotine in a noncombustion vapor and are advertised as an alternative to smoking. No claim is made for reducing smoking, so the products, including the liquids involved, are not regulated by the US Food and Drug Administration (FDA). Controversy exists as to whether they actually increase the number of smokers by introducing young people to “vaping” to get nicotine. Since nicotine is still inhaled, the addictive potential remains unabated. E-cigarettes are unregulated vehicles for supplying nicotine and may pose other health risks, and there is very limited evidence to support the efficacy of e-cigarettes as aids to smoking cessation. Since no controlled study has demonstrated successful cessation of smoking with e-cigarettes, they are best regarded for now as merely another way to introduce nicotine into the body.
Bupropion
Bupropion, an antidepressant also sold as Wellbutrin SR, was approved in 1997 for use in smoking cessation under the trade name Zyban. The manufacturer, Glaxo SmithKline, learned serendipitously that depressive patients taking bupropion were able to quit smoking. After some field trials, this “new” medication was born. It was the first nonnicotine drug for tobacco dependence to gain FDA approval.
Its mechanism of action in combating smoking is unknown but is thought to be related to mild inhibition of dopamine re-uptake in the midbrain.
The drug is approved for smokers over age 18 who are smoking at least nine cigarettes daily. It requires a prescription, and the typical dose is 150 mg twice daily for 8 to 12 weeks, up to 12 months. Smoking is allowed for the first 7 days of drug use.
Contraindications include a history of seizures, concurrent use of bupropion, bulimia, anorexia, detoxification from alcohol or sedatives, use of monoamine oxidase inhibitors, and allergy to bupropion. Warnings are noted for diseases of heart, liver, or kidney; for use with selective serotonin reuptake inhibitors or tricyclic antidepressants; for pregnancy; and for adolescents because of heightened suicide risk.
Side effects. Seizure risk has been estimated at 1 in 1,000 bupropion users at dosages of up to 300 mg daily and is 10 times greater at dosages of 450 to 600 mg/day.11
The most common side effect reported is insomnia, which occurs in about one-third of people who take the medication. Less common side effects include dry mouth, anxiety, and hypertension. Pretreatment screening should include a history of seizure, closed head trauma, brain surgery, stroke, and the eating disorders anorexia nervosa and bulimia. The FDA has required a boxed warning regarding the association of bupropion with psychiatric symptoms.12
Effectiveness. Compared with placebo, bupropion reduces withdrawal symptoms such as irritability, frustration, anger, restlessness, depression, craving, poor concentration, and urge to smoke. Bupropion SR, 150 or 300 mg per day, has been reported to lead to substantial abstinence rates when used with intensive telephone counseling. In a randomized trial,13 side effects were common, especially at the higher dose, but there were no serious adverse effects such as deaths or seizures.13
Buproprion has been found to be as efficacious in improving the odds of quitting as single forms of nicotine replacement therapy, but not as efficacious as nicotine replacement therapy forms used in combination. Bupropion does not appear to be as effective as varenicline.9 US Public Health Service guidelines since 2000 have included nicotine replacement therapy and sustained-release bupropion in combination.
Disadvantages. Bupropion is significantly more expensive than nicotine replacement therapy, but it is often covered by insurance when it is used for smoking cessation. Bupropion has many contraindications, produces drug-drug interactions, is often poorly tolerated, and has many side effects. Some deaths have been reported. Zyban is available by prescription only, an indicator of its relative risk, with the added drawback of higher cost to patients.
Varenicline
Varenicline (Chantix, Champix) was granted a priority review by the FDA in 2005, as it showed significantly better results than other current therapies. It was approved in 2006 and added as a first-line agent in the 2008 guidelines.12
Mechanism of action. A synthetic “designer” drug made for its specific purpose, the varenicline molecule is a modified version of cytisine, a naturally occurring alkaloid previously marketed as Tabex in Eastern Europe. Cytisine is a selective alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist. The high-affinity alpha-4, beta-2 nicotinic acetylcholine receptors exist in the mesolimbic dopaminergic system, the reward center of the brain.14
Varenicline has the same mechanism of action as cytisine but penetrates the central nervous system better. This mechanism of action allows varenicline to block the attachment of the nicotine molecule to this receptor, preventing nicotine’s dominant effect. Varenicline, however, is a partial agonist, so that when it attaches itself to the receptor, it causes a partial agonist effect, which is an opening of the receptor channel to sodium ions, causing partial stimulation of the cells in the ventral tegmental area, and ultimately causing a mild release of dopamine in the nucleus accumbens.15,16 Thus, varenicline effectively stimulates the receptor partially, while at the same time blocking the effects of nicotine.
Pharmacokinetics. After oral intake, the maximal plasma concentration of varenicline is reached in 3 to 4 hours. Food does not inhibit absorption. There is minimal hepatic metabolism, with 92% of the drug excreted unchanged in the urine. There are no known drug-drug interactions. The 24-hour half-life of varenicline allows for once-daily dosing.
Effectiveness. Several phase 2 and phase 3 studies compared varenicline with placebo and other drugs in terms of efficacy, dosing, and safety in 3,600 smokers. The initial phase 2 study, lasting 7 weeks, showed a 4-week abstinence rate of 48% with varenicline compared with 17% with placebo.17
Two phase 3 trials with 2,052 participants demonstrated that, at 12 weeks, abstinence rates were 44% with varenicline, 17% with bupropion, and 17% with placebo. At the end of 1 year, those groups again demonstrated significant differences in nicotine abstinence—22% in the varenicline group vs 15% with bupropion and 9% with placebo. Also, varenicline was superior to bupropion and placebo in reducing craving.18,19 For those who were nicotine-free after 12 weeks of treatment, continuing varenicline for another 12 weeks boosted nicotine abstinence rates from 36% to 44% at 1 year.20
Though varenicline produces a mild physiologic dependence, it is not addictive and does not produce tolerance to itself. There is no need to increase the dose over time. Three percent of patients have reported mild irritability on stopping varenicline.
In sum, varenicline has been shown to be more effective than bupropion and any of the four single formulations of nicotine replacement when they are used alone. It has not been shown to be more effective than combinations of nicotine replacement therapy.10
Safety considerations with varenicline. Psychiatric adverse events associated with varenicline have included severe depression, agitation, and suicidal behavior—including completed suicide. Motor vehicle accidents and erratic behaviors have led to a ban on varenicline use by airline pilots, truck drivers, and maritime workers. Skin rashes (including Stevens-Johnson syndrome), renal failure, and cataracts have also been reported. Safety has not been established with schizophrenia, bipolar disorder, or major depression. The physician should ask about prior psychiatric history, illnesses, and reactions before prescribing varenicline. Generally, it is prudent to avoid varenicline in patients with a significant psychiatric history.
Nausea and sleep disturbances such as vivid dreams and insomnia are the most frequently reported side effects.
Black box warnings with bupropion and varenicline. In July 2009, the FDA issued boxed warnings for bupropion SR and for varenicline for smoking cessation because of reports of neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, attempted suicide, and completed suicide.21 These can occur in people with or without a history of mental illness, and whether the patient has stopped smoking or not. Providers should inform patients, family members, and caregivers about the potential for these symptoms and what to do if symptoms develop—ie, stop the medication immediately and contact the health care provider.
Patients should also be told to use caution when driving, operating machinery, or performing hazardous activities until they know how the medication will affect them.21
When prescribing varenicline. Advise patients to set a “quit date” 7 days after starting varenicline—they can continue smoking for the first 7 days on the drug. The starter packet for varenicline comes as 0.5 mg daily for 3 days, then twice daily for 2 days; the dose increases to 1 mg twice daily thereafter. Smokers report that it is much easier to quit after 7 days on varenicline.
Maintenance packs are available for 1 month of daily dosing. Generally, one starter pack is prescribed, with a second prescription for continuing packs for 2 to 5 more months. Varenicline is best taken with a full glass of water. If the smoker abstains for the first 3 months of therapy, it is best to prescribe an additional 3 months of medication to improve long-term abstinence from nicotine. With nausea or renal disease, lower the dose. Avoid prescribing varenicline for the elderly, teens, and pregnant women.
Varenicline is available only by prescription, and no generic equivalent is available.
WHEN IT’S TIME TO QUIT
A useful prescribing plan is:
- For most people, begin with nicotine patches plus gum
- If nicotine replacement therapy fails, prescribe varenicline
- Prescribe bupropion for patients with depression or if varenicline fails.
According to the US Public Health Service guideline,12 in a meta-analysis comparing various tobacco cessation medications with placebo and nicotine patch, the combination of nicotine patch (> 14 weeks) plus gum was 3.6 times as effective as placebo and 1.9 times as effective as nicotine patch alone. Varenicline at 2 mg per day was 3.1 times as effective as placebo and 1.6 times as effective as nicotine patch alone. Therefore, the combination of nicotine patch and gum is an inexpensive yet effective way to begin a course of smoking cessation therapy.
Behavioral counseling
Timing is important to successful quitting. Patients generally know when it’s a good time to quit—and when it’s not. Avoid trying to get patients to quit when they are stressed, overly busy, fatigued, or anxious. Try to get the patient to set a time to quit that’s ideal, and then encourage the patient to stick to it. For example, scheduling the quit day on a celebration, anniversary, or birthday gives that date added significance and enhances motivation. Follow the patient frequently for 6 to 12 months with intense monitoring and encouragement, and to assess for any adverse effects of medication.
The 2008 update to the Public Health Service Clinical Practice Guidelines on treating tobacco use and dependence concludes that counseling and medication are each effective alone in increasing smoking cessation and are even more effective when used together.12 Even very brief, 3-minute discussions and encouragement have been shown to be helpful. The Public Health Service evidence-based clinical practice guideline on cessation states that brief advice by medical providers to quit smoking is an effective intervention.12
Doctors who show great interest in smoking cessation seem to be more effective in persuading patients to quit. They should take note of smoking rather than ignoring it. A modified version of the CAGE questionnaire to assess problem drinking is recommended as a tool to assess patients’ smoking behavior and initiate a discussion about it (Table 1).22 Emphasize the health and financial costs to the patient. Try to form a therapeutic alliance with the patient against smoking: “Let’s see what we can do about this problem.” Be positive and optimistic in offering help with counseling, support, and medications.
Caution smokers against switching to “light” tar and nicotine cigarettes, as controlled experiments have failed to show consistent reductions in the amounts of tar and nicotine these products deliver into the lungs. Smokers also appear to compensate or adapt their smoking habits to increase the yield from these products. There is insufficient evidence to support the supposed health benefits of such low-yield smoking products.23
Always refer the patient for counseling with the pharmaceutical company help line or with a supported quit line. Some manufacturers of smoking cessation medications offer counseling or web-based support for patients trying to quit. For example, patients who are prescribed varenicline are offered the GETQUIT Plan, a free program that includes online education, tracking of progress, and “check-ins with slip-up support.” These services are often underused yet represent a ready source of helpful support.
If relapses occur, encourage the patient to keep trying again and again, as it may take several attempts to succeed.
Quit lines
To help smokers and other tobacco users quit, all states now have a toll-free cessation quit line, a telephone service accessible through a national toll-free number (1-800-QUIT-NOW). Quit lines also can be a referral source for health care providers who might not have the time or staff to provide all of the steps in the recommended “five-A” cessation counseling model,12 ie:
- Ask about tobacco use
- Advise to quit
- Assess willingness to make a quit attempt
- Assist in quit attempt
- Arrange follow-up.
Quit lines have been shown to improve outcomes when compared with people trying to stop on their own.12 Quit line services have evolved from their modest beginnings as providers of information and counseling to a level at which in many states, evidence-based medications are provided through quit lines.13,24 Medication use, coupled with quit line counseling intervention, increases the likelihood of tobacco abstinence and is consistent with US Public Health Service guideline recommendations that all tobacco users should be offered at least one medication as part of their quit attempt.12
WOMEN SMOKERS HAVE UNIQUE HEALTH RISKS
Women have unique health risks arising from smoking: low-birth-weight babies, sudden infant death syndrome, cervical cancer, and an increasing rate of lung cancer. In general, women have poorer responses to nicotine replacement therapy, are more concerned about gaining weight after quitting, and demonstrate more mood lability after quitting. Women seem more energized by the taste, smell, and overall sensations involved in smoking.
Weight gain will occur when quitting smoking; this is hard to overcome. More exercise may help, and a trial of bupropion with nicotine replacement therapy may mitigate weight gain.
Women who are pregnant present a special challenge when it comes to weighing the benefit of medications against continued smoking. For pregnant women who want to quit smoking, the best treatment is counseling without nicotine replacement or other pharmacotherapy. There are inadequate data for the use of varenicline or bupropion in pregnancy. If medication is needed, start nicotine replacement therapy early in pregnancy, as its risk is the same as or less than the smoking risk to the fetus.
The US Public Health Service guideline provides a useful discussion and bibliography related to this topic.12 All of the FDA-approved medications for tobacco cessation carry an FDA pregnancy category designation of C or D—ie, not recommended for use by pregnant women. These designations are not absolute contraindications and do allow for use in life-threatening situations or when other treatment modalities have failed. Some clinicians and their patients may decide that the potential for fetal harm, including fetal death, with continued smoking is high enough to warrant use of medications.
A careful and thorough discussion of the risks and benefits is recommended between the patient and her physician regarding this issue.
A CALL TO ARMS
The statistics are incontrovertible but do not tell the whole story. The day-to-day practices of physicians bear witness to the suffering that compulsive smoking creates for the smoker. As in all addictions, those around the addict suffer as well, from secondary smoke but also from fear and anxiety about premature loss of their loved ones. Smoking causes suffering and early death, and it is vitally important that doctors—the front-line troops—take up the fight against it as America’s number-one preventable cause of health problems and death.
To be effective champions in the public health fight against smoking, doctors must develop an understanding of compulsive smoking as a biologically driven process of addiction. The smoker attempting to quit is literally in the fight of his or her life and needs emotional support, cognitive-behavioral tools, and state-of-the-art pharmacology to overcome the slow destruction caused by the “dirty weed.”
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. Fifty-years’ observations on male British doctors. BMJ 2004; 328:1519-1528.
- Centers for Disease Control and Prevention (CDC). Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011; 60:1513–1519.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192:29–60.
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction 2013; 108:406–412.
- Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 1988; 44:23–28.
- Schneider NG, Lunell E, Olmstead RE, Fagerström KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clin Pharmacokinet 1996; 31:65–80.
- Benowitz NL. Nicotine replacement therapy. What has been accomplished—can we do better? Drugs 1993; 45:157–170.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329.
- Committee on Safety in Medicines and the Medicines Control Agency. Zyban safety reminder. Current Problems in Pharmacovigilance 2001; 27:5.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. Am J Prev Med 2008; 35:158–176.
- Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003; 163:2337–2344.
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000; 2:19–37.
- Coe JW, Brooks PR, Wirtz MC, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett 2005; 15:4889–4897.
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1999; 1(suppl 2):S121–S125.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166:1561–1568.
- Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296:56–63.
- Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR; Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006; 296:64–71.
- US Food and Drug Administration (FDA). Public health advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Accessed October 8, 2014.
- Rustin TA. Assessing nicotine dependence. Am Fam Physician 2000; 62:579–592.
- Centers for Disease Control and Prevention (CDC). Smoking & tobacco use. Low-yield cigarettes. www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/low_yield_cigarettes/index.htm. Accessed October 8, 2014.
- Biazzo LL, Froshaug DB, Harwell TS, et al. Characteristics and abstinence outcomes among tobacco quitline enrollees using varenicline or nicotine replacement therapy. Nicotine Tob Res 2010; 12:567–573.
- US Department of Health and Human Services. The health consequences of smoking—nicotine addiction; a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 1988.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Current cigarette smoking among adults—United States, 2011. MMWR, 2012; 61(44):889–894.
Tobacco is a dirty weed,
I like it.
It satisfies no normal need,
I like it.
It makes you thin, it makes you lean,
It takes the hair right off your bean.
It’s the worst darn stuff I’ve ever seen.
I like it.
Graham Lee Hemminger. The Penn State Froth, November 1915: 19. Courtesy of Paul J. Dzyak, Jr., Paterno Library, Pennsylvania State University, State College, PA.
All physicians recognize the harm in tobacco smoking and try to convince patients to quit for health reasons, but quitting is challenging and frustrating for both doctor and patient. Physicians can improve quitting outcomes by applying their knowledge of the physiologic basis of nicotine addiction and newer tools that are making a real difference in smoking cessation.
THE NO. 1 PREVENTABLE CAUSE OF DEATH
Tobacco use remains the single largest preventable cause of death and disease in the United States: 443,000 US adults die of smoking-related illnesses each year, or one every 8 seconds.1 Tobacco smoking is currently responsible for 18% of all deaths and 37% of all preventable deaths. One-third of all smokers die early, with men losing 13 years of life and women losing 15 years. (See The rise and partial fall of smoking for a historical overview.)
Smoking, the leading cause of lung cancer, is also implicated in cancers of the mouth, larynx, esophagus, stomach, kidney, bladder, and cervix and has been linked to leukemia. (Though nicotine is responsible for the addictive properties of tobacco, it does not cause cancer itself: other substances in tobacco smoke, many of them byproducts of combustion, are carcinogenic.)
Running a close second to cancer as a smoking-related cause of death is cardiovascular disease, including stroke, myocardial infarction, microvascular dementia, peripheral vascular disease, and aortic aneurysm. Pulmonary and respiratory diseases, including chronic obstructive pulmonary disease, pneumonia, and asthma, are the third most common fatal smoking-related ailments.
Other medical consequences include erectile dysfunction, infertility, pregnancy complications, and low birth weight. Smoking also causes adverse surgical outcomes, poor wound healing, hip fractures, low bone density, peptic ulcer disease, and cataracts.
Smoking is estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.2
On the positive side, quitting smoking has health benefits at any age, and smokers who quit before age 35 have death rates similar to those in people who have never smoked.1,3
WHY IS IT SO HARD TO QUIT?
Most smokers want to quit, and many try to—but few succeed. In the 2010 National Health Interview Surveys, 68.8% of adult smokers said they wanted to stop smoking, and 52.4% had tried to in the past year, but only 6.2% had succeeded.4 Many recovering alcoholics and drug addicts say that quitting tobacco was much harder than abstaining from other substances of choice.
Why is it so hard to quit?
Smoking is a classic addiction
Addictions are usually diagnosed by behavioral signs, and nicotine addiction has many of the clinical hallmarks, eg:
- Tolerance, with a trend toward increasing the potency of the dose and the frequency of smoking over time
- Mental preoccupation with smoking, as it often becomes woven into one’s daily schedule and is associated with almost everything the smoker does throughout the day. Having no cigarettes in the house can generate anxiety that is relieved only by obtaining more
- Squandering scarce financial resources on nicotine products, over time amounting to substantial sums, and since smoking rates are higher in poor people than in the affluent, these are people who can least afford it
- Withdrawal symptoms, characterized by jitteriness, irritability, headache, insomnia, anxiety, and increased appetite.
People continue to smoke despite adverse consequences such as falling asleep while smoking and setting fire to the bed or to the house, or losing digits to peripheral vascular disease. Being unable to quit and to stay off smoking is a hallmark of tobacco dependence. Relapses are often triggered by being near other smokers or seeing a billboard advertising cigarettes. Eventually, the nicotine addict comes to value and crave nicotine more than health or life itself.
Nicotine stimulates ‘reward’ centers in the brain
Nicotine is an alkaloid found in many plants (including potatoes) but in especially high concentrations in tobacco. In mammals, it is a stimulant, rapidly producing dependence and addiction.
Inhaled by smoking, nicotine is absorbed across the large alveolar surface, avoids first-pass metabolism, and is transported rapidly to the brain (Figure 1). In fact, nicotine reaches the brain less than 20 seconds after inhalation, which is slightly faster even than when drugs are injected intravenously.5
Tobacco smoke contains approximately 4,800 compounds, many of which activate neurotransmitter systems such as dopamine, norepinephrine, acetylcholine, glutamate, serotonin, beta-endorphin, and gamma-aminobutyric acid. The most significant of these is the dopamine reward system known as the mesoaccumbens pathway. This system is activated within seconds of smoking and produces a sense of pleasure.
Nicotine binds to nicotinic acetylcholine receptors, primarily to alpha-4, beta-2 receptors in the ventral tegmental area of the midbrain. Once this binding occurs, a neurochemical message is conveyed to the nucleus accumbens via the release of dopamine in the mesoaccumbens pathway—the final common reward pathway triggered by all drugs of abuse. Since these structures and pathways of the brain are anatomically central, the addiction is driven by the basal ganglia and midbrain, the phylogenetically oldest parts of the brain. Nicotine therefore drives its addicts to continue smoking by producing strong neurochemical rewards and by causing strongly negative reactions when discontinued.
Genetically mediated susceptibility probably contributes to addiction. People whose neurochemical pathways are easily stimulated by this drug are probably at far greater risk of addiction. Paradoxically, people who are rapid metabolizers of nicotine are at greater risk than slow metabolizers.6 (Nicotine is metabolized by cytochrome P450 2A6 in the liver.)
Tolerance and withdrawal
Tolerance develops with long-term use, mediated by up-regulation (increased numbers) of alpha-4, beta-2 cholinergic receptors in the ventral tegmental area. Any reduction in nicotine level causes distress because receptors are unoccupied; with more receptors, nicotine intake must increase to keep physiologic balance and avoid withdrawal. Since the half-life of nicotine is only about 2 hours, the smoker must smoke almost constantly to satisfy receptors hungry for the stimulating drug. If drug levels drop, withdrawal occurs very quickly.
Eventually, smokers use nicotine less for pleasure and more as a way to avoid withdrawal. The cycle of pleasure, eventual tolerance, withdrawal, craving, and compulsion is biologically driven, like the drives of thirst, reproduction, and hunger. Nicotine hijacks species-sustaining reward mechanisms, leading to the malignant, compulsive disease of nicotine addiction.
Treatment doomed to fail?
Because nicotine addiction involves the midbrain, cessation strategies that rely on higher cerebral function are not likely to succeed. Counseling, common sense, and willpower simply cannot overcome the dopaminergic stimulating power or assuage the withdrawal sickness of nicotine dependence. Telling patients that smoking is bad for them misses the mark in most cases. Patients want to quit, but the drive to smoke is too powerful. Attempts to cut down rather than abstain from smoking also fail.
Nicotine is a formidable adversary for the patient and for the doctor or other health professional. Until recently, treatment was usually ineffective.
So, what does work against nicotine addiction?
PHARMACOTHERAPIES FOR SMOKING CESSATION
Nicotine replacement therapy
The oldest of the pharmacotherapies for nicotine addiction is nicotine replacement, in the form of patch, gum, lozenge, or nasal spray.
Advantages:
- Nicotine replacement therapy eliminates exposure to the other harmful compounds in tobacco, with few to none of the health risks associated with smoking.
- By delivering nicotine by a different route, nicotine replacement therapy breaks the association between smoking and feeling good. The addict is already dopamine-stimulated before putting a cigarette in the mouth, merely by association and suggestion. Using a different route of nicotine administration avoids that associative stimulation from the act of smoking, so that quitting becomes easier.
- The dose of nicotine is lower with replacement therapy than with smoking. The cigarette is the most efficient delivery mechanism for getting nicotine into the body. A smoked cigarette produces a rapid spike in plasma nicotine levels, far higher and faster than nicotine gum, nasal spray, or transdermal patch. Peak levels of plasma nicotine from nicotine replacement therapy are only 30% to 50% as high as those achieved by smoking.7–9
- It is inexpensive.
Disadvantages:
- Nicotine replacement therapy maintains the addiction to nicotine, with its neurophysiologic distortions.
- Some patients continue nicotine replacement therapy for years.
Use of nicotine gum can be a problem because of the need for frequent administration. The gum is chewed until the user feels a tingling or peppery taste in the mouth, after which the gum must be placed inside the cheek to allow for maximal absorption of the nicotine. Once the tingling has faded, the user is to chew another piece and repeat the cycle as long as craving is perceived. On the other hand, the nicotine patch is applied once daily. Both of these products are available over-the-counter.
Caution is indicated when starting nicotine replacement therapy in those with recent myocardial infarction, angina, or arrhythmia.
Effectiveness. Nicotine replacement therapy has been shown to be as effective as bupropion (see below) but not as effective as varenicline when used in single administration form (patch, gum, lozenge, or inhaler alone). The four single-administration forms of nicotine replacement therapy are all equally efficacious. Combinations of nicotine replacement formulations have been reported to be as effective as varenicline and superior to single formulations.10
How about electronic cigarettes? Electronic cigarettes, or e-cigarettes, supply nicotine in a noncombustion vapor and are advertised as an alternative to smoking. No claim is made for reducing smoking, so the products, including the liquids involved, are not regulated by the US Food and Drug Administration (FDA). Controversy exists as to whether they actually increase the number of smokers by introducing young people to “vaping” to get nicotine. Since nicotine is still inhaled, the addictive potential remains unabated. E-cigarettes are unregulated vehicles for supplying nicotine and may pose other health risks, and there is very limited evidence to support the efficacy of e-cigarettes as aids to smoking cessation. Since no controlled study has demonstrated successful cessation of smoking with e-cigarettes, they are best regarded for now as merely another way to introduce nicotine into the body.
Bupropion
Bupropion, an antidepressant also sold as Wellbutrin SR, was approved in 1997 for use in smoking cessation under the trade name Zyban. The manufacturer, Glaxo SmithKline, learned serendipitously that depressive patients taking bupropion were able to quit smoking. After some field trials, this “new” medication was born. It was the first nonnicotine drug for tobacco dependence to gain FDA approval.
Its mechanism of action in combating smoking is unknown but is thought to be related to mild inhibition of dopamine re-uptake in the midbrain.
The drug is approved for smokers over age 18 who are smoking at least nine cigarettes daily. It requires a prescription, and the typical dose is 150 mg twice daily for 8 to 12 weeks, up to 12 months. Smoking is allowed for the first 7 days of drug use.
Contraindications include a history of seizures, concurrent use of bupropion, bulimia, anorexia, detoxification from alcohol or sedatives, use of monoamine oxidase inhibitors, and allergy to bupropion. Warnings are noted for diseases of heart, liver, or kidney; for use with selective serotonin reuptake inhibitors or tricyclic antidepressants; for pregnancy; and for adolescents because of heightened suicide risk.
Side effects. Seizure risk has been estimated at 1 in 1,000 bupropion users at dosages of up to 300 mg daily and is 10 times greater at dosages of 450 to 600 mg/day.11
The most common side effect reported is insomnia, which occurs in about one-third of people who take the medication. Less common side effects include dry mouth, anxiety, and hypertension. Pretreatment screening should include a history of seizure, closed head trauma, brain surgery, stroke, and the eating disorders anorexia nervosa and bulimia. The FDA has required a boxed warning regarding the association of bupropion with psychiatric symptoms.12
Effectiveness. Compared with placebo, bupropion reduces withdrawal symptoms such as irritability, frustration, anger, restlessness, depression, craving, poor concentration, and urge to smoke. Bupropion SR, 150 or 300 mg per day, has been reported to lead to substantial abstinence rates when used with intensive telephone counseling. In a randomized trial,13 side effects were common, especially at the higher dose, but there were no serious adverse effects such as deaths or seizures.13
Buproprion has been found to be as efficacious in improving the odds of quitting as single forms of nicotine replacement therapy, but not as efficacious as nicotine replacement therapy forms used in combination. Bupropion does not appear to be as effective as varenicline.9 US Public Health Service guidelines since 2000 have included nicotine replacement therapy and sustained-release bupropion in combination.
Disadvantages. Bupropion is significantly more expensive than nicotine replacement therapy, but it is often covered by insurance when it is used for smoking cessation. Bupropion has many contraindications, produces drug-drug interactions, is often poorly tolerated, and has many side effects. Some deaths have been reported. Zyban is available by prescription only, an indicator of its relative risk, with the added drawback of higher cost to patients.
Varenicline
Varenicline (Chantix, Champix) was granted a priority review by the FDA in 2005, as it showed significantly better results than other current therapies. It was approved in 2006 and added as a first-line agent in the 2008 guidelines.12
Mechanism of action. A synthetic “designer” drug made for its specific purpose, the varenicline molecule is a modified version of cytisine, a naturally occurring alkaloid previously marketed as Tabex in Eastern Europe. Cytisine is a selective alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist. The high-affinity alpha-4, beta-2 nicotinic acetylcholine receptors exist in the mesolimbic dopaminergic system, the reward center of the brain.14
Varenicline has the same mechanism of action as cytisine but penetrates the central nervous system better. This mechanism of action allows varenicline to block the attachment of the nicotine molecule to this receptor, preventing nicotine’s dominant effect. Varenicline, however, is a partial agonist, so that when it attaches itself to the receptor, it causes a partial agonist effect, which is an opening of the receptor channel to sodium ions, causing partial stimulation of the cells in the ventral tegmental area, and ultimately causing a mild release of dopamine in the nucleus accumbens.15,16 Thus, varenicline effectively stimulates the receptor partially, while at the same time blocking the effects of nicotine.
Pharmacokinetics. After oral intake, the maximal plasma concentration of varenicline is reached in 3 to 4 hours. Food does not inhibit absorption. There is minimal hepatic metabolism, with 92% of the drug excreted unchanged in the urine. There are no known drug-drug interactions. The 24-hour half-life of varenicline allows for once-daily dosing.
Effectiveness. Several phase 2 and phase 3 studies compared varenicline with placebo and other drugs in terms of efficacy, dosing, and safety in 3,600 smokers. The initial phase 2 study, lasting 7 weeks, showed a 4-week abstinence rate of 48% with varenicline compared with 17% with placebo.17
Two phase 3 trials with 2,052 participants demonstrated that, at 12 weeks, abstinence rates were 44% with varenicline, 17% with bupropion, and 17% with placebo. At the end of 1 year, those groups again demonstrated significant differences in nicotine abstinence—22% in the varenicline group vs 15% with bupropion and 9% with placebo. Also, varenicline was superior to bupropion and placebo in reducing craving.18,19 For those who were nicotine-free after 12 weeks of treatment, continuing varenicline for another 12 weeks boosted nicotine abstinence rates from 36% to 44% at 1 year.20
Though varenicline produces a mild physiologic dependence, it is not addictive and does not produce tolerance to itself. There is no need to increase the dose over time. Three percent of patients have reported mild irritability on stopping varenicline.
In sum, varenicline has been shown to be more effective than bupropion and any of the four single formulations of nicotine replacement when they are used alone. It has not been shown to be more effective than combinations of nicotine replacement therapy.10
Safety considerations with varenicline. Psychiatric adverse events associated with varenicline have included severe depression, agitation, and suicidal behavior—including completed suicide. Motor vehicle accidents and erratic behaviors have led to a ban on varenicline use by airline pilots, truck drivers, and maritime workers. Skin rashes (including Stevens-Johnson syndrome), renal failure, and cataracts have also been reported. Safety has not been established with schizophrenia, bipolar disorder, or major depression. The physician should ask about prior psychiatric history, illnesses, and reactions before prescribing varenicline. Generally, it is prudent to avoid varenicline in patients with a significant psychiatric history.
Nausea and sleep disturbances such as vivid dreams and insomnia are the most frequently reported side effects.
Black box warnings with bupropion and varenicline. In July 2009, the FDA issued boxed warnings for bupropion SR and for varenicline for smoking cessation because of reports of neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, attempted suicide, and completed suicide.21 These can occur in people with or without a history of mental illness, and whether the patient has stopped smoking or not. Providers should inform patients, family members, and caregivers about the potential for these symptoms and what to do if symptoms develop—ie, stop the medication immediately and contact the health care provider.
Patients should also be told to use caution when driving, operating machinery, or performing hazardous activities until they know how the medication will affect them.21
When prescribing varenicline. Advise patients to set a “quit date” 7 days after starting varenicline—they can continue smoking for the first 7 days on the drug. The starter packet for varenicline comes as 0.5 mg daily for 3 days, then twice daily for 2 days; the dose increases to 1 mg twice daily thereafter. Smokers report that it is much easier to quit after 7 days on varenicline.
Maintenance packs are available for 1 month of daily dosing. Generally, one starter pack is prescribed, with a second prescription for continuing packs for 2 to 5 more months. Varenicline is best taken with a full glass of water. If the smoker abstains for the first 3 months of therapy, it is best to prescribe an additional 3 months of medication to improve long-term abstinence from nicotine. With nausea or renal disease, lower the dose. Avoid prescribing varenicline for the elderly, teens, and pregnant women.
Varenicline is available only by prescription, and no generic equivalent is available.
WHEN IT’S TIME TO QUIT
A useful prescribing plan is:
- For most people, begin with nicotine patches plus gum
- If nicotine replacement therapy fails, prescribe varenicline
- Prescribe bupropion for patients with depression or if varenicline fails.
According to the US Public Health Service guideline,12 in a meta-analysis comparing various tobacco cessation medications with placebo and nicotine patch, the combination of nicotine patch (> 14 weeks) plus gum was 3.6 times as effective as placebo and 1.9 times as effective as nicotine patch alone. Varenicline at 2 mg per day was 3.1 times as effective as placebo and 1.6 times as effective as nicotine patch alone. Therefore, the combination of nicotine patch and gum is an inexpensive yet effective way to begin a course of smoking cessation therapy.
Behavioral counseling
Timing is important to successful quitting. Patients generally know when it’s a good time to quit—and when it’s not. Avoid trying to get patients to quit when they are stressed, overly busy, fatigued, or anxious. Try to get the patient to set a time to quit that’s ideal, and then encourage the patient to stick to it. For example, scheduling the quit day on a celebration, anniversary, or birthday gives that date added significance and enhances motivation. Follow the patient frequently for 6 to 12 months with intense monitoring and encouragement, and to assess for any adverse effects of medication.
The 2008 update to the Public Health Service Clinical Practice Guidelines on treating tobacco use and dependence concludes that counseling and medication are each effective alone in increasing smoking cessation and are even more effective when used together.12 Even very brief, 3-minute discussions and encouragement have been shown to be helpful. The Public Health Service evidence-based clinical practice guideline on cessation states that brief advice by medical providers to quit smoking is an effective intervention.12
Doctors who show great interest in smoking cessation seem to be more effective in persuading patients to quit. They should take note of smoking rather than ignoring it. A modified version of the CAGE questionnaire to assess problem drinking is recommended as a tool to assess patients’ smoking behavior and initiate a discussion about it (Table 1).22 Emphasize the health and financial costs to the patient. Try to form a therapeutic alliance with the patient against smoking: “Let’s see what we can do about this problem.” Be positive and optimistic in offering help with counseling, support, and medications.
Caution smokers against switching to “light” tar and nicotine cigarettes, as controlled experiments have failed to show consistent reductions in the amounts of tar and nicotine these products deliver into the lungs. Smokers also appear to compensate or adapt their smoking habits to increase the yield from these products. There is insufficient evidence to support the supposed health benefits of such low-yield smoking products.23
Always refer the patient for counseling with the pharmaceutical company help line or with a supported quit line. Some manufacturers of smoking cessation medications offer counseling or web-based support for patients trying to quit. For example, patients who are prescribed varenicline are offered the GETQUIT Plan, a free program that includes online education, tracking of progress, and “check-ins with slip-up support.” These services are often underused yet represent a ready source of helpful support.
If relapses occur, encourage the patient to keep trying again and again, as it may take several attempts to succeed.
Quit lines
To help smokers and other tobacco users quit, all states now have a toll-free cessation quit line, a telephone service accessible through a national toll-free number (1-800-QUIT-NOW). Quit lines also can be a referral source for health care providers who might not have the time or staff to provide all of the steps in the recommended “five-A” cessation counseling model,12 ie:
- Ask about tobacco use
- Advise to quit
- Assess willingness to make a quit attempt
- Assist in quit attempt
- Arrange follow-up.
Quit lines have been shown to improve outcomes when compared with people trying to stop on their own.12 Quit line services have evolved from their modest beginnings as providers of information and counseling to a level at which in many states, evidence-based medications are provided through quit lines.13,24 Medication use, coupled with quit line counseling intervention, increases the likelihood of tobacco abstinence and is consistent with US Public Health Service guideline recommendations that all tobacco users should be offered at least one medication as part of their quit attempt.12
WOMEN SMOKERS HAVE UNIQUE HEALTH RISKS
Women have unique health risks arising from smoking: low-birth-weight babies, sudden infant death syndrome, cervical cancer, and an increasing rate of lung cancer. In general, women have poorer responses to nicotine replacement therapy, are more concerned about gaining weight after quitting, and demonstrate more mood lability after quitting. Women seem more energized by the taste, smell, and overall sensations involved in smoking.
Weight gain will occur when quitting smoking; this is hard to overcome. More exercise may help, and a trial of bupropion with nicotine replacement therapy may mitigate weight gain.
Women who are pregnant present a special challenge when it comes to weighing the benefit of medications against continued smoking. For pregnant women who want to quit smoking, the best treatment is counseling without nicotine replacement or other pharmacotherapy. There are inadequate data for the use of varenicline or bupropion in pregnancy. If medication is needed, start nicotine replacement therapy early in pregnancy, as its risk is the same as or less than the smoking risk to the fetus.
The US Public Health Service guideline provides a useful discussion and bibliography related to this topic.12 All of the FDA-approved medications for tobacco cessation carry an FDA pregnancy category designation of C or D—ie, not recommended for use by pregnant women. These designations are not absolute contraindications and do allow for use in life-threatening situations or when other treatment modalities have failed. Some clinicians and their patients may decide that the potential for fetal harm, including fetal death, with continued smoking is high enough to warrant use of medications.
A careful and thorough discussion of the risks and benefits is recommended between the patient and her physician regarding this issue.
A CALL TO ARMS
The statistics are incontrovertible but do not tell the whole story. The day-to-day practices of physicians bear witness to the suffering that compulsive smoking creates for the smoker. As in all addictions, those around the addict suffer as well, from secondary smoke but also from fear and anxiety about premature loss of their loved ones. Smoking causes suffering and early death, and it is vitally important that doctors—the front-line troops—take up the fight against it as America’s number-one preventable cause of health problems and death.
To be effective champions in the public health fight against smoking, doctors must develop an understanding of compulsive smoking as a biologically driven process of addiction. The smoker attempting to quit is literally in the fight of his or her life and needs emotional support, cognitive-behavioral tools, and state-of-the-art pharmacology to overcome the slow destruction caused by the “dirty weed.”
Tobacco is a dirty weed,
I like it.
It satisfies no normal need,
I like it.
It makes you thin, it makes you lean,
It takes the hair right off your bean.
It’s the worst darn stuff I’ve ever seen.
I like it.
Graham Lee Hemminger. The Penn State Froth, November 1915: 19. Courtesy of Paul J. Dzyak, Jr., Paterno Library, Pennsylvania State University, State College, PA.
All physicians recognize the harm in tobacco smoking and try to convince patients to quit for health reasons, but quitting is challenging and frustrating for both doctor and patient. Physicians can improve quitting outcomes by applying their knowledge of the physiologic basis of nicotine addiction and newer tools that are making a real difference in smoking cessation.
THE NO. 1 PREVENTABLE CAUSE OF DEATH
Tobacco use remains the single largest preventable cause of death and disease in the United States: 443,000 US adults die of smoking-related illnesses each year, or one every 8 seconds.1 Tobacco smoking is currently responsible for 18% of all deaths and 37% of all preventable deaths. One-third of all smokers die early, with men losing 13 years of life and women losing 15 years. (See The rise and partial fall of smoking for a historical overview.)
Smoking, the leading cause of lung cancer, is also implicated in cancers of the mouth, larynx, esophagus, stomach, kidney, bladder, and cervix and has been linked to leukemia. (Though nicotine is responsible for the addictive properties of tobacco, it does not cause cancer itself: other substances in tobacco smoke, many of them byproducts of combustion, are carcinogenic.)
Running a close second to cancer as a smoking-related cause of death is cardiovascular disease, including stroke, myocardial infarction, microvascular dementia, peripheral vascular disease, and aortic aneurysm. Pulmonary and respiratory diseases, including chronic obstructive pulmonary disease, pneumonia, and asthma, are the third most common fatal smoking-related ailments.
Other medical consequences include erectile dysfunction, infertility, pregnancy complications, and low birth weight. Smoking also causes adverse surgical outcomes, poor wound healing, hip fractures, low bone density, peptic ulcer disease, and cataracts.
Smoking is estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.2
On the positive side, quitting smoking has health benefits at any age, and smokers who quit before age 35 have death rates similar to those in people who have never smoked.1,3
WHY IS IT SO HARD TO QUIT?
Most smokers want to quit, and many try to—but few succeed. In the 2010 National Health Interview Surveys, 68.8% of adult smokers said they wanted to stop smoking, and 52.4% had tried to in the past year, but only 6.2% had succeeded.4 Many recovering alcoholics and drug addicts say that quitting tobacco was much harder than abstaining from other substances of choice.
Why is it so hard to quit?
Smoking is a classic addiction
Addictions are usually diagnosed by behavioral signs, and nicotine addiction has many of the clinical hallmarks, eg:
- Tolerance, with a trend toward increasing the potency of the dose and the frequency of smoking over time
- Mental preoccupation with smoking, as it often becomes woven into one’s daily schedule and is associated with almost everything the smoker does throughout the day. Having no cigarettes in the house can generate anxiety that is relieved only by obtaining more
- Squandering scarce financial resources on nicotine products, over time amounting to substantial sums, and since smoking rates are higher in poor people than in the affluent, these are people who can least afford it
- Withdrawal symptoms, characterized by jitteriness, irritability, headache, insomnia, anxiety, and increased appetite.
People continue to smoke despite adverse consequences such as falling asleep while smoking and setting fire to the bed or to the house, or losing digits to peripheral vascular disease. Being unable to quit and to stay off smoking is a hallmark of tobacco dependence. Relapses are often triggered by being near other smokers or seeing a billboard advertising cigarettes. Eventually, the nicotine addict comes to value and crave nicotine more than health or life itself.
Nicotine stimulates ‘reward’ centers in the brain
Nicotine is an alkaloid found in many plants (including potatoes) but in especially high concentrations in tobacco. In mammals, it is a stimulant, rapidly producing dependence and addiction.
Inhaled by smoking, nicotine is absorbed across the large alveolar surface, avoids first-pass metabolism, and is transported rapidly to the brain (Figure 1). In fact, nicotine reaches the brain less than 20 seconds after inhalation, which is slightly faster even than when drugs are injected intravenously.5
Tobacco smoke contains approximately 4,800 compounds, many of which activate neurotransmitter systems such as dopamine, norepinephrine, acetylcholine, glutamate, serotonin, beta-endorphin, and gamma-aminobutyric acid. The most significant of these is the dopamine reward system known as the mesoaccumbens pathway. This system is activated within seconds of smoking and produces a sense of pleasure.
Nicotine binds to nicotinic acetylcholine receptors, primarily to alpha-4, beta-2 receptors in the ventral tegmental area of the midbrain. Once this binding occurs, a neurochemical message is conveyed to the nucleus accumbens via the release of dopamine in the mesoaccumbens pathway—the final common reward pathway triggered by all drugs of abuse. Since these structures and pathways of the brain are anatomically central, the addiction is driven by the basal ganglia and midbrain, the phylogenetically oldest parts of the brain. Nicotine therefore drives its addicts to continue smoking by producing strong neurochemical rewards and by causing strongly negative reactions when discontinued.
Genetically mediated susceptibility probably contributes to addiction. People whose neurochemical pathways are easily stimulated by this drug are probably at far greater risk of addiction. Paradoxically, people who are rapid metabolizers of nicotine are at greater risk than slow metabolizers.6 (Nicotine is metabolized by cytochrome P450 2A6 in the liver.)
Tolerance and withdrawal
Tolerance develops with long-term use, mediated by up-regulation (increased numbers) of alpha-4, beta-2 cholinergic receptors in the ventral tegmental area. Any reduction in nicotine level causes distress because receptors are unoccupied; with more receptors, nicotine intake must increase to keep physiologic balance and avoid withdrawal. Since the half-life of nicotine is only about 2 hours, the smoker must smoke almost constantly to satisfy receptors hungry for the stimulating drug. If drug levels drop, withdrawal occurs very quickly.
Eventually, smokers use nicotine less for pleasure and more as a way to avoid withdrawal. The cycle of pleasure, eventual tolerance, withdrawal, craving, and compulsion is biologically driven, like the drives of thirst, reproduction, and hunger. Nicotine hijacks species-sustaining reward mechanisms, leading to the malignant, compulsive disease of nicotine addiction.
Treatment doomed to fail?
Because nicotine addiction involves the midbrain, cessation strategies that rely on higher cerebral function are not likely to succeed. Counseling, common sense, and willpower simply cannot overcome the dopaminergic stimulating power or assuage the withdrawal sickness of nicotine dependence. Telling patients that smoking is bad for them misses the mark in most cases. Patients want to quit, but the drive to smoke is too powerful. Attempts to cut down rather than abstain from smoking also fail.
Nicotine is a formidable adversary for the patient and for the doctor or other health professional. Until recently, treatment was usually ineffective.
So, what does work against nicotine addiction?
PHARMACOTHERAPIES FOR SMOKING CESSATION
Nicotine replacement therapy
The oldest of the pharmacotherapies for nicotine addiction is nicotine replacement, in the form of patch, gum, lozenge, or nasal spray.
Advantages:
- Nicotine replacement therapy eliminates exposure to the other harmful compounds in tobacco, with few to none of the health risks associated with smoking.
- By delivering nicotine by a different route, nicotine replacement therapy breaks the association between smoking and feeling good. The addict is already dopamine-stimulated before putting a cigarette in the mouth, merely by association and suggestion. Using a different route of nicotine administration avoids that associative stimulation from the act of smoking, so that quitting becomes easier.
- The dose of nicotine is lower with replacement therapy than with smoking. The cigarette is the most efficient delivery mechanism for getting nicotine into the body. A smoked cigarette produces a rapid spike in plasma nicotine levels, far higher and faster than nicotine gum, nasal spray, or transdermal patch. Peak levels of plasma nicotine from nicotine replacement therapy are only 30% to 50% as high as those achieved by smoking.7–9
- It is inexpensive.
Disadvantages:
- Nicotine replacement therapy maintains the addiction to nicotine, with its neurophysiologic distortions.
- Some patients continue nicotine replacement therapy for years.
Use of nicotine gum can be a problem because of the need for frequent administration. The gum is chewed until the user feels a tingling or peppery taste in the mouth, after which the gum must be placed inside the cheek to allow for maximal absorption of the nicotine. Once the tingling has faded, the user is to chew another piece and repeat the cycle as long as craving is perceived. On the other hand, the nicotine patch is applied once daily. Both of these products are available over-the-counter.
Caution is indicated when starting nicotine replacement therapy in those with recent myocardial infarction, angina, or arrhythmia.
Effectiveness. Nicotine replacement therapy has been shown to be as effective as bupropion (see below) but not as effective as varenicline when used in single administration form (patch, gum, lozenge, or inhaler alone). The four single-administration forms of nicotine replacement therapy are all equally efficacious. Combinations of nicotine replacement formulations have been reported to be as effective as varenicline and superior to single formulations.10
How about electronic cigarettes? Electronic cigarettes, or e-cigarettes, supply nicotine in a noncombustion vapor and are advertised as an alternative to smoking. No claim is made for reducing smoking, so the products, including the liquids involved, are not regulated by the US Food and Drug Administration (FDA). Controversy exists as to whether they actually increase the number of smokers by introducing young people to “vaping” to get nicotine. Since nicotine is still inhaled, the addictive potential remains unabated. E-cigarettes are unregulated vehicles for supplying nicotine and may pose other health risks, and there is very limited evidence to support the efficacy of e-cigarettes as aids to smoking cessation. Since no controlled study has demonstrated successful cessation of smoking with e-cigarettes, they are best regarded for now as merely another way to introduce nicotine into the body.
Bupropion
Bupropion, an antidepressant also sold as Wellbutrin SR, was approved in 1997 for use in smoking cessation under the trade name Zyban. The manufacturer, Glaxo SmithKline, learned serendipitously that depressive patients taking bupropion were able to quit smoking. After some field trials, this “new” medication was born. It was the first nonnicotine drug for tobacco dependence to gain FDA approval.
Its mechanism of action in combating smoking is unknown but is thought to be related to mild inhibition of dopamine re-uptake in the midbrain.
The drug is approved for smokers over age 18 who are smoking at least nine cigarettes daily. It requires a prescription, and the typical dose is 150 mg twice daily for 8 to 12 weeks, up to 12 months. Smoking is allowed for the first 7 days of drug use.
Contraindications include a history of seizures, concurrent use of bupropion, bulimia, anorexia, detoxification from alcohol or sedatives, use of monoamine oxidase inhibitors, and allergy to bupropion. Warnings are noted for diseases of heart, liver, or kidney; for use with selective serotonin reuptake inhibitors or tricyclic antidepressants; for pregnancy; and for adolescents because of heightened suicide risk.
Side effects. Seizure risk has been estimated at 1 in 1,000 bupropion users at dosages of up to 300 mg daily and is 10 times greater at dosages of 450 to 600 mg/day.11
The most common side effect reported is insomnia, which occurs in about one-third of people who take the medication. Less common side effects include dry mouth, anxiety, and hypertension. Pretreatment screening should include a history of seizure, closed head trauma, brain surgery, stroke, and the eating disorders anorexia nervosa and bulimia. The FDA has required a boxed warning regarding the association of bupropion with psychiatric symptoms.12
Effectiveness. Compared with placebo, bupropion reduces withdrawal symptoms such as irritability, frustration, anger, restlessness, depression, craving, poor concentration, and urge to smoke. Bupropion SR, 150 or 300 mg per day, has been reported to lead to substantial abstinence rates when used with intensive telephone counseling. In a randomized trial,13 side effects were common, especially at the higher dose, but there were no serious adverse effects such as deaths or seizures.13
Buproprion has been found to be as efficacious in improving the odds of quitting as single forms of nicotine replacement therapy, but not as efficacious as nicotine replacement therapy forms used in combination. Bupropion does not appear to be as effective as varenicline.9 US Public Health Service guidelines since 2000 have included nicotine replacement therapy and sustained-release bupropion in combination.
Disadvantages. Bupropion is significantly more expensive than nicotine replacement therapy, but it is often covered by insurance when it is used for smoking cessation. Bupropion has many contraindications, produces drug-drug interactions, is often poorly tolerated, and has many side effects. Some deaths have been reported. Zyban is available by prescription only, an indicator of its relative risk, with the added drawback of higher cost to patients.
Varenicline
Varenicline (Chantix, Champix) was granted a priority review by the FDA in 2005, as it showed significantly better results than other current therapies. It was approved in 2006 and added as a first-line agent in the 2008 guidelines.12
Mechanism of action. A synthetic “designer” drug made for its specific purpose, the varenicline molecule is a modified version of cytisine, a naturally occurring alkaloid previously marketed as Tabex in Eastern Europe. Cytisine is a selective alpha-4, beta-2 nicotinic acetylcholine receptor partial agonist. The high-affinity alpha-4, beta-2 nicotinic acetylcholine receptors exist in the mesolimbic dopaminergic system, the reward center of the brain.14
Varenicline has the same mechanism of action as cytisine but penetrates the central nervous system better. This mechanism of action allows varenicline to block the attachment of the nicotine molecule to this receptor, preventing nicotine’s dominant effect. Varenicline, however, is a partial agonist, so that when it attaches itself to the receptor, it causes a partial agonist effect, which is an opening of the receptor channel to sodium ions, causing partial stimulation of the cells in the ventral tegmental area, and ultimately causing a mild release of dopamine in the nucleus accumbens.15,16 Thus, varenicline effectively stimulates the receptor partially, while at the same time blocking the effects of nicotine.
Pharmacokinetics. After oral intake, the maximal plasma concentration of varenicline is reached in 3 to 4 hours. Food does not inhibit absorption. There is minimal hepatic metabolism, with 92% of the drug excreted unchanged in the urine. There are no known drug-drug interactions. The 24-hour half-life of varenicline allows for once-daily dosing.
Effectiveness. Several phase 2 and phase 3 studies compared varenicline with placebo and other drugs in terms of efficacy, dosing, and safety in 3,600 smokers. The initial phase 2 study, lasting 7 weeks, showed a 4-week abstinence rate of 48% with varenicline compared with 17% with placebo.17
Two phase 3 trials with 2,052 participants demonstrated that, at 12 weeks, abstinence rates were 44% with varenicline, 17% with bupropion, and 17% with placebo. At the end of 1 year, those groups again demonstrated significant differences in nicotine abstinence—22% in the varenicline group vs 15% with bupropion and 9% with placebo. Also, varenicline was superior to bupropion and placebo in reducing craving.18,19 For those who were nicotine-free after 12 weeks of treatment, continuing varenicline for another 12 weeks boosted nicotine abstinence rates from 36% to 44% at 1 year.20
Though varenicline produces a mild physiologic dependence, it is not addictive and does not produce tolerance to itself. There is no need to increase the dose over time. Three percent of patients have reported mild irritability on stopping varenicline.
In sum, varenicline has been shown to be more effective than bupropion and any of the four single formulations of nicotine replacement when they are used alone. It has not been shown to be more effective than combinations of nicotine replacement therapy.10
Safety considerations with varenicline. Psychiatric adverse events associated with varenicline have included severe depression, agitation, and suicidal behavior—including completed suicide. Motor vehicle accidents and erratic behaviors have led to a ban on varenicline use by airline pilots, truck drivers, and maritime workers. Skin rashes (including Stevens-Johnson syndrome), renal failure, and cataracts have also been reported. Safety has not been established with schizophrenia, bipolar disorder, or major depression. The physician should ask about prior psychiatric history, illnesses, and reactions before prescribing varenicline. Generally, it is prudent to avoid varenicline in patients with a significant psychiatric history.
Nausea and sleep disturbances such as vivid dreams and insomnia are the most frequently reported side effects.
Black box warnings with bupropion and varenicline. In July 2009, the FDA issued boxed warnings for bupropion SR and for varenicline for smoking cessation because of reports of neuropsychiatric symptoms, including changes in behavior, hostility, agitation, depressed mood, suicidal thoughts and behavior, attempted suicide, and completed suicide.21 These can occur in people with or without a history of mental illness, and whether the patient has stopped smoking or not. Providers should inform patients, family members, and caregivers about the potential for these symptoms and what to do if symptoms develop—ie, stop the medication immediately and contact the health care provider.
Patients should also be told to use caution when driving, operating machinery, or performing hazardous activities until they know how the medication will affect them.21
When prescribing varenicline. Advise patients to set a “quit date” 7 days after starting varenicline—they can continue smoking for the first 7 days on the drug. The starter packet for varenicline comes as 0.5 mg daily for 3 days, then twice daily for 2 days; the dose increases to 1 mg twice daily thereafter. Smokers report that it is much easier to quit after 7 days on varenicline.
Maintenance packs are available for 1 month of daily dosing. Generally, one starter pack is prescribed, with a second prescription for continuing packs for 2 to 5 more months. Varenicline is best taken with a full glass of water. If the smoker abstains for the first 3 months of therapy, it is best to prescribe an additional 3 months of medication to improve long-term abstinence from nicotine. With nausea or renal disease, lower the dose. Avoid prescribing varenicline for the elderly, teens, and pregnant women.
Varenicline is available only by prescription, and no generic equivalent is available.
WHEN IT’S TIME TO QUIT
A useful prescribing plan is:
- For most people, begin with nicotine patches plus gum
- If nicotine replacement therapy fails, prescribe varenicline
- Prescribe bupropion for patients with depression or if varenicline fails.
According to the US Public Health Service guideline,12 in a meta-analysis comparing various tobacco cessation medications with placebo and nicotine patch, the combination of nicotine patch (> 14 weeks) plus gum was 3.6 times as effective as placebo and 1.9 times as effective as nicotine patch alone. Varenicline at 2 mg per day was 3.1 times as effective as placebo and 1.6 times as effective as nicotine patch alone. Therefore, the combination of nicotine patch and gum is an inexpensive yet effective way to begin a course of smoking cessation therapy.
Behavioral counseling
Timing is important to successful quitting. Patients generally know when it’s a good time to quit—and when it’s not. Avoid trying to get patients to quit when they are stressed, overly busy, fatigued, or anxious. Try to get the patient to set a time to quit that’s ideal, and then encourage the patient to stick to it. For example, scheduling the quit day on a celebration, anniversary, or birthday gives that date added significance and enhances motivation. Follow the patient frequently for 6 to 12 months with intense monitoring and encouragement, and to assess for any adverse effects of medication.
The 2008 update to the Public Health Service Clinical Practice Guidelines on treating tobacco use and dependence concludes that counseling and medication are each effective alone in increasing smoking cessation and are even more effective when used together.12 Even very brief, 3-minute discussions and encouragement have been shown to be helpful. The Public Health Service evidence-based clinical practice guideline on cessation states that brief advice by medical providers to quit smoking is an effective intervention.12
Doctors who show great interest in smoking cessation seem to be more effective in persuading patients to quit. They should take note of smoking rather than ignoring it. A modified version of the CAGE questionnaire to assess problem drinking is recommended as a tool to assess patients’ smoking behavior and initiate a discussion about it (Table 1).22 Emphasize the health and financial costs to the patient. Try to form a therapeutic alliance with the patient against smoking: “Let’s see what we can do about this problem.” Be positive and optimistic in offering help with counseling, support, and medications.
Caution smokers against switching to “light” tar and nicotine cigarettes, as controlled experiments have failed to show consistent reductions in the amounts of tar and nicotine these products deliver into the lungs. Smokers also appear to compensate or adapt their smoking habits to increase the yield from these products. There is insufficient evidence to support the supposed health benefits of such low-yield smoking products.23
Always refer the patient for counseling with the pharmaceutical company help line or with a supported quit line. Some manufacturers of smoking cessation medications offer counseling or web-based support for patients trying to quit. For example, patients who are prescribed varenicline are offered the GETQUIT Plan, a free program that includes online education, tracking of progress, and “check-ins with slip-up support.” These services are often underused yet represent a ready source of helpful support.
If relapses occur, encourage the patient to keep trying again and again, as it may take several attempts to succeed.
Quit lines
To help smokers and other tobacco users quit, all states now have a toll-free cessation quit line, a telephone service accessible through a national toll-free number (1-800-QUIT-NOW). Quit lines also can be a referral source for health care providers who might not have the time or staff to provide all of the steps in the recommended “five-A” cessation counseling model,12 ie:
- Ask about tobacco use
- Advise to quit
- Assess willingness to make a quit attempt
- Assist in quit attempt
- Arrange follow-up.
Quit lines have been shown to improve outcomes when compared with people trying to stop on their own.12 Quit line services have evolved from their modest beginnings as providers of information and counseling to a level at which in many states, evidence-based medications are provided through quit lines.13,24 Medication use, coupled with quit line counseling intervention, increases the likelihood of tobacco abstinence and is consistent with US Public Health Service guideline recommendations that all tobacco users should be offered at least one medication as part of their quit attempt.12
WOMEN SMOKERS HAVE UNIQUE HEALTH RISKS
Women have unique health risks arising from smoking: low-birth-weight babies, sudden infant death syndrome, cervical cancer, and an increasing rate of lung cancer. In general, women have poorer responses to nicotine replacement therapy, are more concerned about gaining weight after quitting, and demonstrate more mood lability after quitting. Women seem more energized by the taste, smell, and overall sensations involved in smoking.
Weight gain will occur when quitting smoking; this is hard to overcome. More exercise may help, and a trial of bupropion with nicotine replacement therapy may mitigate weight gain.
Women who are pregnant present a special challenge when it comes to weighing the benefit of medications against continued smoking. For pregnant women who want to quit smoking, the best treatment is counseling without nicotine replacement or other pharmacotherapy. There are inadequate data for the use of varenicline or bupropion in pregnancy. If medication is needed, start nicotine replacement therapy early in pregnancy, as its risk is the same as or less than the smoking risk to the fetus.
The US Public Health Service guideline provides a useful discussion and bibliography related to this topic.12 All of the FDA-approved medications for tobacco cessation carry an FDA pregnancy category designation of C or D—ie, not recommended for use by pregnant women. These designations are not absolute contraindications and do allow for use in life-threatening situations or when other treatment modalities have failed. Some clinicians and their patients may decide that the potential for fetal harm, including fetal death, with continued smoking is high enough to warrant use of medications.
A careful and thorough discussion of the risks and benefits is recommended between the patient and her physician regarding this issue.
A CALL TO ARMS
The statistics are incontrovertible but do not tell the whole story. The day-to-day practices of physicians bear witness to the suffering that compulsive smoking creates for the smoker. As in all addictions, those around the addict suffer as well, from secondary smoke but also from fear and anxiety about premature loss of their loved ones. Smoking causes suffering and early death, and it is vitally important that doctors—the front-line troops—take up the fight against it as America’s number-one preventable cause of health problems and death.
To be effective champions in the public health fight against smoking, doctors must develop an understanding of compulsive smoking as a biologically driven process of addiction. The smoker attempting to quit is literally in the fight of his or her life and needs emotional support, cognitive-behavioral tools, and state-of-the-art pharmacology to overcome the slow destruction caused by the “dirty weed.”
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. Fifty-years’ observations on male British doctors. BMJ 2004; 328:1519-1528.
- Centers for Disease Control and Prevention (CDC). Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011; 60:1513–1519.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192:29–60.
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction 2013; 108:406–412.
- Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 1988; 44:23–28.
- Schneider NG, Lunell E, Olmstead RE, Fagerström KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clin Pharmacokinet 1996; 31:65–80.
- Benowitz NL. Nicotine replacement therapy. What has been accomplished—can we do better? Drugs 1993; 45:157–170.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329.
- Committee on Safety in Medicines and the Medicines Control Agency. Zyban safety reminder. Current Problems in Pharmacovigilance 2001; 27:5.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. Am J Prev Med 2008; 35:158–176.
- Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003; 163:2337–2344.
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000; 2:19–37.
- Coe JW, Brooks PR, Wirtz MC, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett 2005; 15:4889–4897.
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1999; 1(suppl 2):S121–S125.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166:1561–1568.
- Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296:56–63.
- Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR; Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006; 296:64–71.
- US Food and Drug Administration (FDA). Public health advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Accessed October 8, 2014.
- Rustin TA. Assessing nicotine dependence. Am Fam Physician 2000; 62:579–592.
- Centers for Disease Control and Prevention (CDC). Smoking & tobacco use. Low-yield cigarettes. www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/low_yield_cigarettes/index.htm. Accessed October 8, 2014.
- Biazzo LL, Froshaug DB, Harwell TS, et al. Characteristics and abstinence outcomes among tobacco quitline enrollees using varenicline or nicotine replacement therapy. Nicotine Tob Res 2010; 12:567–573.
- US Department of Health and Human Services. The health consequences of smoking—nicotine addiction; a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 1988.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Current cigarette smoking among adults—United States, 2011. MMWR, 2012; 61(44):889–894.
- US Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010.
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep 2008; 57:1226–1228.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. Fifty-years’ observations on male British doctors. BMJ 2004; 328:1519-1528.
- Centers for Disease Control and Prevention (CDC). Quitting smoking among adults—United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011; 60:1513–1519.
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009; 192:29–60.
- Rubinstein ML, Shiffman S, Moscicki AB, Rait MA, Sen S, Benowitz NL. Nicotine metabolism and addiction among adolescent smokers. Addiction 2013; 108:406–412.
- Benowitz NL, Porchet H, Sheiner L, Jacob P 3rd. Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther 1988; 44:23–28.
- Schneider NG, Lunell E, Olmstead RE, Fagerström KO. Clinical pharmacokinetics of nasal nicotine delivery. A review and comparison to other nicotine systems. Clin Pharmacokinet 1996; 31:65–80.
- Benowitz NL. Nicotine replacement therapy. What has been accomplished—can we do better? Drugs 1993; 45:157–170.
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329.
- Committee on Safety in Medicines and the Medicines Control Agency. Zyban safety reminder. Current Problems in Pharmacovigilance 2001; 27:5.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US public health service report. Am J Prev Med 2008; 35:158–176.
- Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med 2003; 163:2337–2344.
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res 2000; 2:19–37.
- Coe JW, Brooks PR, Wirtz MC, et al. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett 2005; 15:4889–4897.
- Picciotto MR, Zoli M, Changeux JP. Use of knock-out mice to determine the molecular basis for the actions of nicotine. Nicotine Tob Res 1999; 1(suppl 2):S121–S125.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med 2006; 166:1561–1568.
- Jorenby DE, Hays JT, Rigotti NA, et al; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006; 296:56–63.
- Gonzales D, Rennard SI, Nides M, et al; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296:47–55.
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR; Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA 2006; 296:64–71.
- US Food and Drug Administration (FDA). Public health advisory: FDA requires new boxed warnings for the smoking cessation drugs Chantix and Zyban. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm. Accessed October 8, 2014.
- Rustin TA. Assessing nicotine dependence. Am Fam Physician 2000; 62:579–592.
- Centers for Disease Control and Prevention (CDC). Smoking & tobacco use. Low-yield cigarettes. www.cdc.gov/tobacco/data_statistics/fact_sheets/tobacco_industry/low_yield_cigarettes/index.htm. Accessed October 8, 2014.
- Biazzo LL, Froshaug DB, Harwell TS, et al. Characteristics and abstinence outcomes among tobacco quitline enrollees using varenicline or nicotine replacement therapy. Nicotine Tob Res 2010; 12:567–573.
- US Department of Health and Human Services. The health consequences of smoking—nicotine addiction; a report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, 1988.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Current cigarette smoking among adults—United States, 2011. MMWR, 2012; 61(44):889–894.
KEY POINTS
- Nicotine dependence is a life-threatening, biochemically based disease, driven by changes in midbrain receptors and reward mechanisms.
- The state of the art in smoking cessation involves encouragement, persistence, and evidence-based pharmacotherapy.
- Physicians should be assertive in addressing nicotine dependence, approaching patients with encouragement to quit, consistent monitoring and support, telephone “quit lines,” and counseling, as well as persistence and optimism. The combination of proactive, engaged, brief counseling and pharmacotherapy will yield the best results.