User login
When the evidence suggests that placebo is best
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
In this issue of JFP, the Clinical Inquiry seeks to answer the question: What are effective injection treatments for lateral epicondylitis? Answering this question proved to be a daunting task for the authors. The difficulty lies in answering this question: effective compared to what?
The injections evaluated in their comprehensive review—corticosteroids, botulinum toxin, hyaluronic acid, platelet-rich plasma,
There are 2 choices for an ideal comparison group. One choice compares the active intervention to an adequate placebo, the other compares it to another treatment that has previously been proven effective. Ideally, the other treatment would be a “gold standard”—that is, the best treatment currently available. Unfortunately, for treatment of lateral epicondylitis, no gold standard has been established.
So, what is an “adequate placebo” for injection therapy? This is a very difficult question. The placebo should probably include putting a needle into the treatment site and injecting a nonactive substance, such as saline solution. This is the comparison group Vukelic et al chose for their review. But even saline could theoretically be therapeutic.
Another fair comparison for the treatment of lateral epicondylitis would be an injection near, but not at, the lateral epicondyle. Yet another comparison—dry needling without any medication to the lateral epicondyle vs dry needling of an adjacent location—would also be a fair comparison to help understand the effect of needling alone. Unfortunately, these comparisons have not been explored in randomized controlled trials. Although several studies have evaluated dry needling for lateral epicondylitis,2-4 none have used a fair comparison.
Some studies1 evaluating treatments for lateral epicondylitis used comparisons to agents that are ineffective or of uncertain effectiveness. Comparing 1 agent to another ineffective or potentially harmful agent obscures our knowledge. Evidence-based medicine must be built on a reliable foundation.
Vukelic and colleagues did an admirable job of selecting studies with an appropriate comparison group—that is, saline injection, the best comparator that has been studied. What they discovered is that no type of injection therapy has been proven to be better than a saline injection.
So, if your patient is not satisfied with conservative therapy for epicondylitis and wants an injection, salt water seems as good as anything.
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
1. Sims S, Miller K, Elfar J, et al. Non-surgical treatment of lateral epicondylitis: a systematic review of randomized controlled trials. Hand (NY). 2014;9:419-446. doi: 10.1007/s11552-014-9642-x
2. Uygur E, Aktas B, Ozkut A, et al. Dry needling in lateral epicondylitis: a prospective controlled study. Int Orthop. 2017; 41:2321-2325. doi: 10.1007/s00264-017-3604-1
3. Krey D, Borchers J, McCamey K. Tendon needling for treatment of tendinopathy: A systematic review. Phys Sportsmed. 2015;43:80-86. doi: 10.1080/00913847.2015.1004296
4. Jayaseelan DJ, Faller BT, Avery MH. The utilization and effects of filiform dry needling in the management of tendinopathy: a systematic review. Physiother Theory Pract. Published online April 27, 2021. doi: 10.1080/09593985.2021.1920076
Do A-fib patients continue to benefit from vitamin K antagonists with advancing age?
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
EVIDENCE SUMMARY
A meta-analysis of 12 randomized trials of stroke prevention in patients with atrial fibrillation (8932 patients, 63% male, mean age 72 years, 19.6% ≥ 80 years) examined outcomes of ischemic stroke, serious bleeding (systemic or intracranial hemorrhages requiring hospitalization, transfusion, or surgery) and cardiovascular events (ischemic stroke, myocardial infarction, systemic emboli, and vascular death).1 Patients were randomized to oral anticoagulants (3430 patients), antiplatelet therapy (3531 patients), or no therapy (1971 patients).
Warfarin target international normalized ratios (INRs) ranged from 1.5 to 4.2. Previous stoke or transient ischemic attack varied across studies but averaged 22% (patient baseline characteristics were evenly distributed among all arms of all 12 studies, suggesting appropriate randomizations). Fifteen percent of patients had diabetes, 50% had hypertension, and 20% had congestive heart failure. They were followed for a mean of 2 years.
Overall, patients experienced 623 ischemic strokes, 289 serious bleeds, and 1210 cardiovascular events. After adjusting for treatment and covariates, age was independently associated with higher risk for each outcome. For every decade increase in age, the hazard ratio (HR) for ischemic stroke was 1.45 (95% confidence interval [CI], 1.26-1.66); serious hemorrhage, 1.61 (95% CI, 1.47-1.77); and cardiovascular events, 1.43 (95% CI, 1.33-1.53).
Benefits of warfarin outweigh increased risk of hemorrhage
Treatment with vitamin K antagonists, compared with placebo, reduced ischemic strokes (HR = 0.36; 95% CI, 0.29-0.45) and cardiovascular events (HR = 0.59; 95% CI, 0.52-0.66) but increased the risk of serious hemorrhage (HR = 1.56; 95% CI, 1.03-2.37) in patients from 50 to 90 years of age. The benefits of decreased ischemic strokes and cardiovascular events consistently surpassed the increased risk of hemorrhage, however.
Across all age groups, the absolute risk reductions (ARRs) for ischemic stroke and cardiovascular events were 2% to 3% and 3% to 8%, respectively, whereas the absolute risk increase for serious hemorrhage was 0.5% to 1%. For those ages 70 to 75, for example, warfarin decreased the rate of ischemic stroke by 3% per year (number needed to treat [NNT] = 34; rates estimated from graphs) and the rate of cardiovascular events by 7% (NNT = 14) but increased the risk of serious hemorrhage by approximately 0.5% per year (number need to harm = 200).
Warfarin prevents major strokes more effectively than aspirin
A randomized open-label trial with blind assessment of endpoints, included in the meta-analysis, followed 973 patients older than 75 years (mean 81.5 years) with atrial fibrillation for 2 to 7 years.2 Researchers evaluated warfarin compared with aspirin for the outcomes of major stroke, arterial embolism, and intracranial hemorrhage. Major strokes comprised fatal or disabling strokes. Researchers excluded patients with minor strokes, rheumatic heart disease, a major nontraumatic hemorrhage within the previous 5 years, intracranial hemorrhage, peptic ulcer disease, esophageal varices, or a terminal illness.
Compared with aspirin, warfarin significantly reduced all primary events (ARR = 1.8% vs 3.8%; relative risk reduction [RRR] = 0.48; 95% CI, 0.28-0.80; NNT = 50). Warfarin decreased major strokes more than aspirin (21 vs 44 strokes; ARR = 1.8%; relative risk [RR] = 0.46; 95% CI, 0.26-0.79; NNT = 56) but didn’t alter the risk of hemorrhagic strokes (6 vs 5 absolute events, respectively; RRR = 1.15, 95% CI, 0.29-4.77) or other intracranial hemorrhages (2 vs 1 event, respectively; RR = 1.92; 95% CI, 0.10-113.3). Wide confidence intervals and the small number of hemorrhagic events suggest that the study wasn’t powered to detect a significant difference in hemorrhagic events.
Continue to: Large study finds net benefit for warfarin treatment
Large study finds net benefit for warfarin treatment
A retrospective cohort including all 182,678 Swedish Hospital Discharge Register patients with atrial fibrillation (260,000 patient-years) evaluated the net benefit of anticoagulation treatment decisions over an average of 1.5 years.3 The Swedish National Prescribed Drugs Registry, which includes all Swedish pharmacies, identified all patients who were prescribed warfarin during the study years of July 2005 through December 2008. The patients were divided into 2 groups, warfarin or no warfarin, and assigned risk scores using CHA2DS2-VASc and HAS-BLED.4,5
Researchers defined net benefit as the number of ischemic strokes avoided in patients taking warfarin, minus the number of excess intracranial bleeds. They assigned a weight of 1.5 to intracranial bleeds vs 1 for ischemic strokes to compensate for the generally more severe outcomes of intracranial bleeding.
Warfarin produced a net benefit at every CHA2DS2-VASc score greater than 0 (aggregate result of 3.9 fewer events per 100 patient-years; 95% CI, 3.8-4.1; NNT = 26). Kaplan-Meier composite plots of all-cause mortality, ischemic stroke, and intracranial bleeds showed a net benefit favoring warfarin use for all combinations of CHA2DS2-VASc greater than 0 (patients older than 65 years never have a CHA2DS2-VASc score of 0 because they’re assigned 1 point at ages 65 to 74 years and 2 points at 75 years and older) and HAS-BLED scores (all curves P < .00001).
Hazard ratios (HRs) of every combination of scores favored warfarin use (HRs ranged from 0.26-0.72; 95% CIs, less than 1 for all HRs; aggregate benefit at all risk scores: HR = 0.51; 95% CI, 0.50-0.52,). The risk of intracranial bleed, or any bleed, on warfarin at all risk strata was less than the corresponding risk of ischemic stroke (or thromboembolic event) without warfarin except among the lowest risk patients (CHA2DS2-VASc = 0). The difference between thromboses and hemorrhages increased as the CHA2DS2-VASc score increased. Of note, a smaller percentage of the highest risk patients were on warfarin.
EDITOR’S TAKEAWAY
We have solid evidence that, although the risks of systemic and intracranial bleeding from warfarin therapy in older patients with atrial fibrillation increase steadily with advancing age, so do the benefits in reduced ischemic stroke, myocardial infarction, thrombotic emboli, and overall cardiovascular death. Most important, the benefits continue to outweigh the risks by a factor of 2 to 4, even in the oldest age groups.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
1. van Walraven C, Hart R, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation. Stroke. 2009;40:1410-1416.
2. Mant J, Hobbs FD. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study): a randomised controlled trial. Lancet. 2007;370:493–503.
3. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298-2307.
4. Friberg L, Rosenqvist M, Lip G. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500-1510.
5. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093-1100.
EVIDENCE-BASED ANSWER:
Yes, patients with atrial fibrilla- tion who are between the ages of 50 and 90 years continue to benefit from vitamin K antagonist therapy (warfarin) (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs] and large cohorts). Regardless of age, warfarin produces a reduction in risk of thrombotic events that is 2- to 4-fold greater than the risk of hemorrhagic events.
What clinical clues differentiate migraine from sinus headaches?
Patients with sinus headaches have thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath (SOR: B, cross-sectional study).
The 5 symptoms that are most predictive of migraine are: pulsatile quality, duration of 4 to 72 hours, unilateral location, nausea or vomiting, and disabling intensity (SOR: B, retrospective cohort). As the number of these symptoms increases, so too, does the likelihood that the patient has a migraine (SOR: B, systematic review of retrospective cohort studies).
Most patients diagnosed with sinus headache actually have a migraine headache (SOR: B, 2 cross-sectional studies).
EVIDENCE SUMMARY
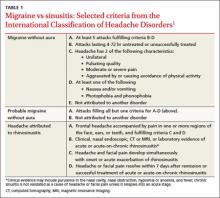
Clinical signs and symptoms define headache types. The International Headache Society (IHS)’s definition of migraine (which is considered the gold standard) includes many of the same symptoms associated with headaches attributed to rhinosinusitis (TABLE 1).1 In order for a headache to be attributed to rhinosinusitis, the patient must meet the definition of acute rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS). The definition includes purulent discharge, nasal obstruction, and facial pain-pressure-fullness.
Migraine headache symptoms include nasal discharge but not purulent discharge or nasal obstruction. The IHS doesn’t accept chronic sinusitis as a cause of headaches unless the patient meets the criteria for acute rhinosinusitis.1,2
When a “sinus” headache isn’t
A cross-sectional study enrolled 100 patients (78 female, 22 male) 18 to 81 years of age who responded to an advertisement seeking people with self-diagnosed “sinus” headaches.3 A neurologist used the 2004 IHS criteria to classify the correct headache type. In 86% of patients, the investigators reclassified the patients with a migraine or probable migraine. Only 3 patients retained the sinus headache diagnosis. All 3 had at least one of the following: thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath. The remaining 11 patients suffered from other headache subtypes.
The big 5 migraine symptoms
A prospective cohort study of 166 French railway employees evaluated the sensitivity and specificity of individual components of the IHS criteria. Patients enrolled had an average age of 39 years and a female-to-male ratio of 1:2. A neurologist diagnosed the headache type and placed patients into either a migraine or nonmigraine cohort. Researchers asked participants about IHS defined migraine symptoms and then compared the frequency of positive responses from migraineurs vs nonmigraineurs.
Five specific IHS criteria were found to be useful in identifying patients with migraine: duration between 4 and 72 hours (odds ratio [OR]=2.5; P=.02), unilateral location (OR=2.3; P=.03), pulsating quality (OR=2.4; P=.02), disturbance of daily activity (OR=2.5; P=.02), and nausea or vomiting (OR=2.8; P=.009). Any 4 of these features indicated a probability of migraine headache of ≥70%. The presence of photophobia and phonophobia (OR=0.5; P=.11) and aggravation by physical activity (OR=1.7; P=.14) didn’t improve diagnostic accuracy.4
A systematic review of retrospective cohort studies later analyzed the data.5 The authors derived the POUND mnemonic (TABLE 2)5 to aid clinicians in using the 5 clinical features to determine the likelihood of migraine headache. When any 4 of the 5 screening questions are positively, the likelihood ratio (LR) for migraine is 24 (95% confidence interval [CI], 1.5-388). Conversely, when 2 or fewer screening questions elicit positive responses, migraine is less likely (LR=0.41; 95% CI, 0.32-0.52).5
Most “sinus” headaches found to be migraine
A multicenter cross-sectional study evaluated 2991 male and female primary care patients, 18 to 65 years of age, who reported at least 6 self-described or physician-diagnosed sinus headaches within the preceding 6 months.6 At baseline, patients reported the following symptoms: pulsing or throbbing pain (89%), pain that worsened with physical activity (85%), sinus pressure (84%), sinus pain (82%), nasal congestion (63%), photophobia (79%), nausea (73%), and phonophobia (67%). Patients were excluded if they had a previous diagnosis of migraine, used a triptan, had a radiologic diagnosis of sinusitis, or had purulent drainage or fever.
Using the patients’ headache histories, reported symptoms, and the IHS criteria, researchers reclassified 88% of these “sinus headache” patients as having migraine type headaches.
Recommendations
IHS recommends using strict criteria for diagnosing migraines and headaches attributed to rhinosinusitis.1
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(1 suppl):S8-S160.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31.
3. Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213-224.
4. Michel P, Henry P, Letenneur L, et al. Diagnostic screen for assessment of the IHS criteria for migraine by general practitioners. Cephalagia.1993;13(12suppl):S54-S59.
5. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
6. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769-1772.
Patients with sinus headaches have thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath (SOR: B, cross-sectional study).
The 5 symptoms that are most predictive of migraine are: pulsatile quality, duration of 4 to 72 hours, unilateral location, nausea or vomiting, and disabling intensity (SOR: B, retrospective cohort). As the number of these symptoms increases, so too, does the likelihood that the patient has a migraine (SOR: B, systematic review of retrospective cohort studies).
Most patients diagnosed with sinus headache actually have a migraine headache (SOR: B, 2 cross-sectional studies).
EVIDENCE SUMMARY
Clinical signs and symptoms define headache types. The International Headache Society (IHS)’s definition of migraine (which is considered the gold standard) includes many of the same symptoms associated with headaches attributed to rhinosinusitis (TABLE 1).1 In order for a headache to be attributed to rhinosinusitis, the patient must meet the definition of acute rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS). The definition includes purulent discharge, nasal obstruction, and facial pain-pressure-fullness.
Migraine headache symptoms include nasal discharge but not purulent discharge or nasal obstruction. The IHS doesn’t accept chronic sinusitis as a cause of headaches unless the patient meets the criteria for acute rhinosinusitis.1,2
When a “sinus” headache isn’t
A cross-sectional study enrolled 100 patients (78 female, 22 male) 18 to 81 years of age who responded to an advertisement seeking people with self-diagnosed “sinus” headaches.3 A neurologist used the 2004 IHS criteria to classify the correct headache type. In 86% of patients, the investigators reclassified the patients with a migraine or probable migraine. Only 3 patients retained the sinus headache diagnosis. All 3 had at least one of the following: thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath. The remaining 11 patients suffered from other headache subtypes.
The big 5 migraine symptoms
A prospective cohort study of 166 French railway employees evaluated the sensitivity and specificity of individual components of the IHS criteria. Patients enrolled had an average age of 39 years and a female-to-male ratio of 1:2. A neurologist diagnosed the headache type and placed patients into either a migraine or nonmigraine cohort. Researchers asked participants about IHS defined migraine symptoms and then compared the frequency of positive responses from migraineurs vs nonmigraineurs.
Five specific IHS criteria were found to be useful in identifying patients with migraine: duration between 4 and 72 hours (odds ratio [OR]=2.5; P=.02), unilateral location (OR=2.3; P=.03), pulsating quality (OR=2.4; P=.02), disturbance of daily activity (OR=2.5; P=.02), and nausea or vomiting (OR=2.8; P=.009). Any 4 of these features indicated a probability of migraine headache of ≥70%. The presence of photophobia and phonophobia (OR=0.5; P=.11) and aggravation by physical activity (OR=1.7; P=.14) didn’t improve diagnostic accuracy.4
A systematic review of retrospective cohort studies later analyzed the data.5 The authors derived the POUND mnemonic (TABLE 2)5 to aid clinicians in using the 5 clinical features to determine the likelihood of migraine headache. When any 4 of the 5 screening questions are positively, the likelihood ratio (LR) for migraine is 24 (95% confidence interval [CI], 1.5-388). Conversely, when 2 or fewer screening questions elicit positive responses, migraine is less likely (LR=0.41; 95% CI, 0.32-0.52).5
Most “sinus” headaches found to be migraine
A multicenter cross-sectional study evaluated 2991 male and female primary care patients, 18 to 65 years of age, who reported at least 6 self-described or physician-diagnosed sinus headaches within the preceding 6 months.6 At baseline, patients reported the following symptoms: pulsing or throbbing pain (89%), pain that worsened with physical activity (85%), sinus pressure (84%), sinus pain (82%), nasal congestion (63%), photophobia (79%), nausea (73%), and phonophobia (67%). Patients were excluded if they had a previous diagnosis of migraine, used a triptan, had a radiologic diagnosis of sinusitis, or had purulent drainage or fever.
Using the patients’ headache histories, reported symptoms, and the IHS criteria, researchers reclassified 88% of these “sinus headache” patients as having migraine type headaches.
Recommendations
IHS recommends using strict criteria for diagnosing migraines and headaches attributed to rhinosinusitis.1
Patients with sinus headaches have thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath (SOR: B, cross-sectional study).
The 5 symptoms that are most predictive of migraine are: pulsatile quality, duration of 4 to 72 hours, unilateral location, nausea or vomiting, and disabling intensity (SOR: B, retrospective cohort). As the number of these symptoms increases, so too, does the likelihood that the patient has a migraine (SOR: B, systematic review of retrospective cohort studies).
Most patients diagnosed with sinus headache actually have a migraine headache (SOR: B, 2 cross-sectional studies).
EVIDENCE SUMMARY
Clinical signs and symptoms define headache types. The International Headache Society (IHS)’s definition of migraine (which is considered the gold standard) includes many of the same symptoms associated with headaches attributed to rhinosinusitis (TABLE 1).1 In order for a headache to be attributed to rhinosinusitis, the patient must meet the definition of acute rhinosinusitis as defined by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS). The definition includes purulent discharge, nasal obstruction, and facial pain-pressure-fullness.
Migraine headache symptoms include nasal discharge but not purulent discharge or nasal obstruction. The IHS doesn’t accept chronic sinusitis as a cause of headaches unless the patient meets the criteria for acute rhinosinusitis.1,2
When a “sinus” headache isn’t
A cross-sectional study enrolled 100 patients (78 female, 22 male) 18 to 81 years of age who responded to an advertisement seeking people with self-diagnosed “sinus” headaches.3 A neurologist used the 2004 IHS criteria to classify the correct headache type. In 86% of patients, the investigators reclassified the patients with a migraine or probable migraine. Only 3 patients retained the sinus headache diagnosis. All 3 had at least one of the following: thick nasal discharge, fever, chills, sweats, or abnormally malodorous breath. The remaining 11 patients suffered from other headache subtypes.
The big 5 migraine symptoms
A prospective cohort study of 166 French railway employees evaluated the sensitivity and specificity of individual components of the IHS criteria. Patients enrolled had an average age of 39 years and a female-to-male ratio of 1:2. A neurologist diagnosed the headache type and placed patients into either a migraine or nonmigraine cohort. Researchers asked participants about IHS defined migraine symptoms and then compared the frequency of positive responses from migraineurs vs nonmigraineurs.
Five specific IHS criteria were found to be useful in identifying patients with migraine: duration between 4 and 72 hours (odds ratio [OR]=2.5; P=.02), unilateral location (OR=2.3; P=.03), pulsating quality (OR=2.4; P=.02), disturbance of daily activity (OR=2.5; P=.02), and nausea or vomiting (OR=2.8; P=.009). Any 4 of these features indicated a probability of migraine headache of ≥70%. The presence of photophobia and phonophobia (OR=0.5; P=.11) and aggravation by physical activity (OR=1.7; P=.14) didn’t improve diagnostic accuracy.4
A systematic review of retrospective cohort studies later analyzed the data.5 The authors derived the POUND mnemonic (TABLE 2)5 to aid clinicians in using the 5 clinical features to determine the likelihood of migraine headache. When any 4 of the 5 screening questions are positively, the likelihood ratio (LR) for migraine is 24 (95% confidence interval [CI], 1.5-388). Conversely, when 2 or fewer screening questions elicit positive responses, migraine is less likely (LR=0.41; 95% CI, 0.32-0.52).5
Most “sinus” headaches found to be migraine
A multicenter cross-sectional study evaluated 2991 male and female primary care patients, 18 to 65 years of age, who reported at least 6 self-described or physician-diagnosed sinus headaches within the preceding 6 months.6 At baseline, patients reported the following symptoms: pulsing or throbbing pain (89%), pain that worsened with physical activity (85%), sinus pressure (84%), sinus pain (82%), nasal congestion (63%), photophobia (79%), nausea (73%), and phonophobia (67%). Patients were excluded if they had a previous diagnosis of migraine, used a triptan, had a radiologic diagnosis of sinusitis, or had purulent drainage or fever.
Using the patients’ headache histories, reported symptoms, and the IHS criteria, researchers reclassified 88% of these “sinus headache” patients as having migraine type headaches.
Recommendations
IHS recommends using strict criteria for diagnosing migraines and headaches attributed to rhinosinusitis.1
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(1 suppl):S8-S160.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31.
3. Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213-224.
4. Michel P, Henry P, Letenneur L, et al. Diagnostic screen for assessment of the IHS criteria for migraine by general practitioners. Cephalagia.1993;13(12suppl):S54-S59.
5. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
6. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769-1772.
1. Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(1 suppl):S8-S160.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31.
3. Eross E, Dodick D, Eross M. The sinus, allergy and migraine study (SAMS). Headache. 2007;47:213-224.
4. Michel P, Henry P, Letenneur L, et al. Diagnostic screen for assessment of the IHS criteria for migraine by general practitioners. Cephalagia.1993;13(12suppl):S54-S59.
5. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
6. Schreiber CP, Hutchinson S, Webster CJ, et al. Prevalence of migraine in patients with a history of self-reported or physician-diagnosed “sinus” headache. Arch Intern Med. 2004;164:1769-1772.
Evidence-based answers from the Family Physicians Inquiries Network
What treatment is best for hypertrophic scars and keloids?
NO ONE TREATMENT IS BEST (strength of recommendation [SOR]: C, meta-analysis of heterogenous studies); no good evidence exists comparing treatments with each other.
Triamcinolone injections, triamcinolone injections combined with excision, and cryotherapy all improve hypertrophic and keloid scars (SOR: C, case series studies).
Silicone gel products have weak evidence of efficacy (SOR: C, Cochrane review with no clear recommendation).
Evidence summary
The TABLE summarizes the evidence for the best-studied treatments.1-5 A systematic review of 396 studies, 36 of which were included in an accompanying meta-analysis, concluded that, overall, any treatment gave patients a 70% (95% confidence interval [CI], 49%-91%) chance of improvement.6 The mean improvement in scar appearance or symptoms was 60% for all the studies combined (no CI reported).
The review found no statistically significant difference between outcomes of 27 different treatments or combinations of treatments. The authors concluded that no optimal evidence-based therapy exists and recommended choosing treatment based on cost and adverse effect profile.6
TABLE
What the evidence tells us about these scar treatments
| Treatment | Study design | Number of scars treated | Inclusion/ exclusion criteria | Results | Comment |
|---|---|---|---|---|---|
| Triamcinolone injections1 | Case-control | 195 | None | >90% of scars showed moderate to marked improvement in 3 wk | Only study with control group; no controls showed improvement |
| Triamcinolone injections plus excision2 | Case series | 58 | None | 100% of patients were symptom-free in 5 wk | No recurrences in 91.9% of keloids and 95.2% of hypertrophic scars at a mean follow-up of 30.5 mo |
| Cryotherapy study 13 | Case series | 119 | Only fair-skinned patients | 61.3% of patients had good to excellent results; most patients needed ≥3 treatments. Hypertrophic scars responded better than keloids | Side effect of hypopigmentation limits use of this therapy in dark-skinned patients Lesions <2 y responded better than older scars (P<.5); no recurrences were noted |
| Cryotherapy study 24 | Case series | 65 | None | Complete flattening in 73% of scars; improvement in 17% | All lesions that responded showed hypopigmentation that persisted in mean 31-mo follow-up 6 lesions didn’t respond; all had been present >2 y |
| Silicone gel products5 | Cochrane review | NA | NA | Weak evidence of reduction in scar thickness and color | Poor-quality studies, highly susceptible to bias |
| NA, not applicable | |||||
Many studies have limitations
Studies often don’t distinguish between hypertrophic and keloid scars, although much evidence supports important differences in their natural histories and response to therapy.7 Hypertrophic scars may resolve spontaneously, can improve with surgical revision, and are less likely to recur.
Moreover, many studies looked only at initial response, although good initial response to therapy doesn’t translate into a low recurrence rate, particularly for keloid scars. Studies were also flawed by lack of controls, nonvalidated outcome measures, and small size.
No available evidence supports using over-the-counter products such as Mederma and other creams, gels, and oils, to treat scars.
Recommendations
The American Academy of Dermatology does not make any recommendations about hypertrophic or keloid scars.
The International Clinical Recommendations on Scar Management (written for the International Advisory Panel on Scar Management) recommend silicone gel sheeting and intralesional corticosteroids as first-line therapy, based on a systematic review of the clinical literature. For secondary management, the authors accepted localized pressure therapy, specific wavelength laser therapy, and surgical revision with adjuvant silicone gel therapy as standard practice based on expert opinion. They conclude that many standard practices and emerging therapies need to be studied in well-designed trials before being conclusively recommended.8
1. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1966;38:209-218.
2. Chowdri NA, Mattoo M, Mattoo A, et al. Keloids and hypertrophic scars: results with intralesional and serial postoperative corticosteroid injection therapy. Aust NZ J Surg. 1999;69:655-659.
3. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. A prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
4. Rusciani L, Rossi G, Bono R. Use of cryotherapy in the treatment of keloids. J Dermatol Surg Oncol. 1993;19:529-534.
5. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
6. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
7. English R, Shenefelt P. Keloids and hypertrophic scars. Dermatol Surg. 1999;25:631-638.
8. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
NO ONE TREATMENT IS BEST (strength of recommendation [SOR]: C, meta-analysis of heterogenous studies); no good evidence exists comparing treatments with each other.
Triamcinolone injections, triamcinolone injections combined with excision, and cryotherapy all improve hypertrophic and keloid scars (SOR: C, case series studies).
Silicone gel products have weak evidence of efficacy (SOR: C, Cochrane review with no clear recommendation).
Evidence summary
The TABLE summarizes the evidence for the best-studied treatments.1-5 A systematic review of 396 studies, 36 of which were included in an accompanying meta-analysis, concluded that, overall, any treatment gave patients a 70% (95% confidence interval [CI], 49%-91%) chance of improvement.6 The mean improvement in scar appearance or symptoms was 60% for all the studies combined (no CI reported).
The review found no statistically significant difference between outcomes of 27 different treatments or combinations of treatments. The authors concluded that no optimal evidence-based therapy exists and recommended choosing treatment based on cost and adverse effect profile.6
TABLE
What the evidence tells us about these scar treatments
| Treatment | Study design | Number of scars treated | Inclusion/ exclusion criteria | Results | Comment |
|---|---|---|---|---|---|
| Triamcinolone injections1 | Case-control | 195 | None | >90% of scars showed moderate to marked improvement in 3 wk | Only study with control group; no controls showed improvement |
| Triamcinolone injections plus excision2 | Case series | 58 | None | 100% of patients were symptom-free in 5 wk | No recurrences in 91.9% of keloids and 95.2% of hypertrophic scars at a mean follow-up of 30.5 mo |
| Cryotherapy study 13 | Case series | 119 | Only fair-skinned patients | 61.3% of patients had good to excellent results; most patients needed ≥3 treatments. Hypertrophic scars responded better than keloids | Side effect of hypopigmentation limits use of this therapy in dark-skinned patients Lesions <2 y responded better than older scars (P<.5); no recurrences were noted |
| Cryotherapy study 24 | Case series | 65 | None | Complete flattening in 73% of scars; improvement in 17% | All lesions that responded showed hypopigmentation that persisted in mean 31-mo follow-up 6 lesions didn’t respond; all had been present >2 y |
| Silicone gel products5 | Cochrane review | NA | NA | Weak evidence of reduction in scar thickness and color | Poor-quality studies, highly susceptible to bias |
| NA, not applicable | |||||
Many studies have limitations
Studies often don’t distinguish between hypertrophic and keloid scars, although much evidence supports important differences in their natural histories and response to therapy.7 Hypertrophic scars may resolve spontaneously, can improve with surgical revision, and are less likely to recur.
Moreover, many studies looked only at initial response, although good initial response to therapy doesn’t translate into a low recurrence rate, particularly for keloid scars. Studies were also flawed by lack of controls, nonvalidated outcome measures, and small size.
No available evidence supports using over-the-counter products such as Mederma and other creams, gels, and oils, to treat scars.
Recommendations
The American Academy of Dermatology does not make any recommendations about hypertrophic or keloid scars.
The International Clinical Recommendations on Scar Management (written for the International Advisory Panel on Scar Management) recommend silicone gel sheeting and intralesional corticosteroids as first-line therapy, based on a systematic review of the clinical literature. For secondary management, the authors accepted localized pressure therapy, specific wavelength laser therapy, and surgical revision with adjuvant silicone gel therapy as standard practice based on expert opinion. They conclude that many standard practices and emerging therapies need to be studied in well-designed trials before being conclusively recommended.8
NO ONE TREATMENT IS BEST (strength of recommendation [SOR]: C, meta-analysis of heterogenous studies); no good evidence exists comparing treatments with each other.
Triamcinolone injections, triamcinolone injections combined with excision, and cryotherapy all improve hypertrophic and keloid scars (SOR: C, case series studies).
Silicone gel products have weak evidence of efficacy (SOR: C, Cochrane review with no clear recommendation).
Evidence summary
The TABLE summarizes the evidence for the best-studied treatments.1-5 A systematic review of 396 studies, 36 of which were included in an accompanying meta-analysis, concluded that, overall, any treatment gave patients a 70% (95% confidence interval [CI], 49%-91%) chance of improvement.6 The mean improvement in scar appearance or symptoms was 60% for all the studies combined (no CI reported).
The review found no statistically significant difference between outcomes of 27 different treatments or combinations of treatments. The authors concluded that no optimal evidence-based therapy exists and recommended choosing treatment based on cost and adverse effect profile.6
TABLE
What the evidence tells us about these scar treatments
| Treatment | Study design | Number of scars treated | Inclusion/ exclusion criteria | Results | Comment |
|---|---|---|---|---|---|
| Triamcinolone injections1 | Case-control | 195 | None | >90% of scars showed moderate to marked improvement in 3 wk | Only study with control group; no controls showed improvement |
| Triamcinolone injections plus excision2 | Case series | 58 | None | 100% of patients were symptom-free in 5 wk | No recurrences in 91.9% of keloids and 95.2% of hypertrophic scars at a mean follow-up of 30.5 mo |
| Cryotherapy study 13 | Case series | 119 | Only fair-skinned patients | 61.3% of patients had good to excellent results; most patients needed ≥3 treatments. Hypertrophic scars responded better than keloids | Side effect of hypopigmentation limits use of this therapy in dark-skinned patients Lesions <2 y responded better than older scars (P<.5); no recurrences were noted |
| Cryotherapy study 24 | Case series | 65 | None | Complete flattening in 73% of scars; improvement in 17% | All lesions that responded showed hypopigmentation that persisted in mean 31-mo follow-up 6 lesions didn’t respond; all had been present >2 y |
| Silicone gel products5 | Cochrane review | NA | NA | Weak evidence of reduction in scar thickness and color | Poor-quality studies, highly susceptible to bias |
| NA, not applicable | |||||
Many studies have limitations
Studies often don’t distinguish between hypertrophic and keloid scars, although much evidence supports important differences in their natural histories and response to therapy.7 Hypertrophic scars may resolve spontaneously, can improve with surgical revision, and are less likely to recur.
Moreover, many studies looked only at initial response, although good initial response to therapy doesn’t translate into a low recurrence rate, particularly for keloid scars. Studies were also flawed by lack of controls, nonvalidated outcome measures, and small size.
No available evidence supports using over-the-counter products such as Mederma and other creams, gels, and oils, to treat scars.
Recommendations
The American Academy of Dermatology does not make any recommendations about hypertrophic or keloid scars.
The International Clinical Recommendations on Scar Management (written for the International Advisory Panel on Scar Management) recommend silicone gel sheeting and intralesional corticosteroids as first-line therapy, based on a systematic review of the clinical literature. For secondary management, the authors accepted localized pressure therapy, specific wavelength laser therapy, and surgical revision with adjuvant silicone gel therapy as standard practice based on expert opinion. They conclude that many standard practices and emerging therapies need to be studied in well-designed trials before being conclusively recommended.8
1. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1966;38:209-218.
2. Chowdri NA, Mattoo M, Mattoo A, et al. Keloids and hypertrophic scars: results with intralesional and serial postoperative corticosteroid injection therapy. Aust NZ J Surg. 1999;69:655-659.
3. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. A prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
4. Rusciani L, Rossi G, Bono R. Use of cryotherapy in the treatment of keloids. J Dermatol Surg Oncol. 1993;19:529-534.
5. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
6. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
7. English R, Shenefelt P. Keloids and hypertrophic scars. Dermatol Surg. 1999;25:631-638.
8. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
1. Ketchum LD, Smith J, Robinson DW, et al. The treatment of hypertrophic scar, keloid and scar contracture by triamcinolone acetonide. Plast Reconstr Surg. 1966;38:209-218.
2. Chowdri NA, Mattoo M, Mattoo A, et al. Keloids and hypertrophic scars: results with intralesional and serial postoperative corticosteroid injection therapy. Aust NZ J Surg. 1999;69:655-659.
3. Zouboulis CC, Blume U, Büttner P, et al. Outcomes of cryosurgery in keloids and hypertrophic scars. A prospective consecutive trial of case series. Arch Dermatol. 1993;129:1146-1151.
4. Rusciani L, Rossi G, Bono R. Use of cryotherapy in the treatment of keloids. J Dermatol Surg Oncol. 1993;19:529-534.
5. O’Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826.-
6. Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362-368.
7. English R, Shenefelt P. Keloids and hypertrophic scars. Dermatol Surg. 1999;25:631-638.
8. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560-571.
Evidence-based answers from the Family Physicians Inquiries Network
When should you consider implanted nerve stimulators for lower back pain?
CONSIDER IT FOR PATIENTS WITH FAILED BACK SURGERY SYNDROME. These patients can gain more pain relief from spinal cord stimulation (SCS) than from reoperation (strength of recommendation [SOR]: A, 2 randomized controlled trials [RCTs]). SCS can also treat chronic low back pain effectively (SOR: B, cohort studies). It’s indicated when conservative measures have failed (SOR: C, expert opinion).
The side effects and failure rates of SCS are well documented and should be considered before recommending the therapy to patients (SOR: A, systematic review of RCTs and cohort studies).
Evidence summary
SCS systems comprise transcutaneously inserted leads that deliver low-voltage electronic stimulation to the spinal cord or targeted peripheral nerves. The resulting dermatomal parasthesia can be preferable to chronic painful stimuli. The voltage generator is located externally or implanted internally.
SCS can be used to treat patients with chronic and intractable pain, such as the pain caused by failed back surgery syndrome. The syndrome, defined as persistent or recurrent pain after lumbosacral spine surgery, occurs in 10% to 40% of patients who have undergone lumbosacral spine surgery.1
A 2005 prospective RCT enrolled 50 patients with failed back surgery syndrome who were considering reoperation.1 Twenty-four were randomized to SCS and 26 to reoperation. Success was defined as >50% pain relief measured by a validated visual analog pain scale. The average length of follow-up was 3 years. An intention-to-treat analysis demonstrated that 9 of 24 (38%) SCS insertions were successful, compared with 3 of 26 (12%) reoperations (P=.04; number needed to treat=3.8).
Low back pain shows significant response to stimulation
A 2004 systematic review of SCS for all indications included 51 studies and 2973 patients.2 Sixteen of the studies, with a total of 616 patients, focused on low back pain, specifically chronic back pain and failed back surgery syndrome. Two of the 16 studies were prospective controlled trials, 8 were prospective trials without controls, and 6 were retrospective studies.
Both prospective, controlled trials (total of 62 patients) demonstrated statistically significant (P<.05) results with SCS. One measured subjective pain and the other used crossover to the other treatment arm (SCS vs surgery) as a marker for treatment failure.
Consider the side effects
SCS isn’t without side effects. Cameron’s systematic review of 51 SCS studies reported rates for a number of complications ( TABLE ).2 The most common complication was lead migration—displacement of the spinal electrodes that can cause pain to recur.
TABLE
Major complications of SCS
| Complication | Rate |
|---|---|
| Lead migration | 13.2% |
| Lead breakage | 9.1% |
| Infection | 3.4% |
| Hardware malfunction | 2.9% |
| Unwanted stimulation | 2.4% |
| Battery failure | 1.6% |
| Pain over implant | 0.9% |
| SCS, spinal cord stimulation. | |
| Adapted from: Cameron T, et al. J Neurosurg.2 | |
Recommendations
Evidence-based guidelines for interventional techniques to control chronic pain, published in the January 2007 edition of Pain Physician, classify indications for SCS as follows:3
Strong indication: complex regional pain syndrome (CRPS)
Moderate indication: failed back surgery syndrome, chronic low back pain, and chronic lower extremity pain.
The Society of British Neurological Surgeons lists the following conditions as “good indications” for SCS: failed back surgery syndrome, CRPS, neuropathic pain from peripheral nerve damage, pain secondary to peripheral vascular disease, refractory angina, and brachial plexopathy.4
1. North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98-106.
2. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100(suppl 3 Spine):254-267.
3. Boswell MV, Trescot AM, Datta S, et al. American Society of Interventional Pain Physicians Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
4. Society of British Neurological Surgeons/British Pain Society Spinal Cord Stimulation for the Management of Pain: Recommendations for Best Clinical Practice. London: British Pain Society; 2009. Available at: www.britishpainsociety.org/book_scs_main.pdf. Accessed October 3, 2009.
CONSIDER IT FOR PATIENTS WITH FAILED BACK SURGERY SYNDROME. These patients can gain more pain relief from spinal cord stimulation (SCS) than from reoperation (strength of recommendation [SOR]: A, 2 randomized controlled trials [RCTs]). SCS can also treat chronic low back pain effectively (SOR: B, cohort studies). It’s indicated when conservative measures have failed (SOR: C, expert opinion).
The side effects and failure rates of SCS are well documented and should be considered before recommending the therapy to patients (SOR: A, systematic review of RCTs and cohort studies).
Evidence summary
SCS systems comprise transcutaneously inserted leads that deliver low-voltage electronic stimulation to the spinal cord or targeted peripheral nerves. The resulting dermatomal parasthesia can be preferable to chronic painful stimuli. The voltage generator is located externally or implanted internally.
SCS can be used to treat patients with chronic and intractable pain, such as the pain caused by failed back surgery syndrome. The syndrome, defined as persistent or recurrent pain after lumbosacral spine surgery, occurs in 10% to 40% of patients who have undergone lumbosacral spine surgery.1
A 2005 prospective RCT enrolled 50 patients with failed back surgery syndrome who were considering reoperation.1 Twenty-four were randomized to SCS and 26 to reoperation. Success was defined as >50% pain relief measured by a validated visual analog pain scale. The average length of follow-up was 3 years. An intention-to-treat analysis demonstrated that 9 of 24 (38%) SCS insertions were successful, compared with 3 of 26 (12%) reoperations (P=.04; number needed to treat=3.8).
Low back pain shows significant response to stimulation
A 2004 systematic review of SCS for all indications included 51 studies and 2973 patients.2 Sixteen of the studies, with a total of 616 patients, focused on low back pain, specifically chronic back pain and failed back surgery syndrome. Two of the 16 studies were prospective controlled trials, 8 were prospective trials without controls, and 6 were retrospective studies.
Both prospective, controlled trials (total of 62 patients) demonstrated statistically significant (P<.05) results with SCS. One measured subjective pain and the other used crossover to the other treatment arm (SCS vs surgery) as a marker for treatment failure.
Consider the side effects
SCS isn’t without side effects. Cameron’s systematic review of 51 SCS studies reported rates for a number of complications ( TABLE ).2 The most common complication was lead migration—displacement of the spinal electrodes that can cause pain to recur.
TABLE
Major complications of SCS
| Complication | Rate |
|---|---|
| Lead migration | 13.2% |
| Lead breakage | 9.1% |
| Infection | 3.4% |
| Hardware malfunction | 2.9% |
| Unwanted stimulation | 2.4% |
| Battery failure | 1.6% |
| Pain over implant | 0.9% |
| SCS, spinal cord stimulation. | |
| Adapted from: Cameron T, et al. J Neurosurg.2 | |
Recommendations
Evidence-based guidelines for interventional techniques to control chronic pain, published in the January 2007 edition of Pain Physician, classify indications for SCS as follows:3
Strong indication: complex regional pain syndrome (CRPS)
Moderate indication: failed back surgery syndrome, chronic low back pain, and chronic lower extremity pain.
The Society of British Neurological Surgeons lists the following conditions as “good indications” for SCS: failed back surgery syndrome, CRPS, neuropathic pain from peripheral nerve damage, pain secondary to peripheral vascular disease, refractory angina, and brachial plexopathy.4
CONSIDER IT FOR PATIENTS WITH FAILED BACK SURGERY SYNDROME. These patients can gain more pain relief from spinal cord stimulation (SCS) than from reoperation (strength of recommendation [SOR]: A, 2 randomized controlled trials [RCTs]). SCS can also treat chronic low back pain effectively (SOR: B, cohort studies). It’s indicated when conservative measures have failed (SOR: C, expert opinion).
The side effects and failure rates of SCS are well documented and should be considered before recommending the therapy to patients (SOR: A, systematic review of RCTs and cohort studies).
Evidence summary
SCS systems comprise transcutaneously inserted leads that deliver low-voltage electronic stimulation to the spinal cord or targeted peripheral nerves. The resulting dermatomal parasthesia can be preferable to chronic painful stimuli. The voltage generator is located externally or implanted internally.
SCS can be used to treat patients with chronic and intractable pain, such as the pain caused by failed back surgery syndrome. The syndrome, defined as persistent or recurrent pain after lumbosacral spine surgery, occurs in 10% to 40% of patients who have undergone lumbosacral spine surgery.1
A 2005 prospective RCT enrolled 50 patients with failed back surgery syndrome who were considering reoperation.1 Twenty-four were randomized to SCS and 26 to reoperation. Success was defined as >50% pain relief measured by a validated visual analog pain scale. The average length of follow-up was 3 years. An intention-to-treat analysis demonstrated that 9 of 24 (38%) SCS insertions were successful, compared with 3 of 26 (12%) reoperations (P=.04; number needed to treat=3.8).
Low back pain shows significant response to stimulation
A 2004 systematic review of SCS for all indications included 51 studies and 2973 patients.2 Sixteen of the studies, with a total of 616 patients, focused on low back pain, specifically chronic back pain and failed back surgery syndrome. Two of the 16 studies were prospective controlled trials, 8 were prospective trials without controls, and 6 were retrospective studies.
Both prospective, controlled trials (total of 62 patients) demonstrated statistically significant (P<.05) results with SCS. One measured subjective pain and the other used crossover to the other treatment arm (SCS vs surgery) as a marker for treatment failure.
Consider the side effects
SCS isn’t without side effects. Cameron’s systematic review of 51 SCS studies reported rates for a number of complications ( TABLE ).2 The most common complication was lead migration—displacement of the spinal electrodes that can cause pain to recur.
TABLE
Major complications of SCS
| Complication | Rate |
|---|---|
| Lead migration | 13.2% |
| Lead breakage | 9.1% |
| Infection | 3.4% |
| Hardware malfunction | 2.9% |
| Unwanted stimulation | 2.4% |
| Battery failure | 1.6% |
| Pain over implant | 0.9% |
| SCS, spinal cord stimulation. | |
| Adapted from: Cameron T, et al. J Neurosurg.2 | |
Recommendations
Evidence-based guidelines for interventional techniques to control chronic pain, published in the January 2007 edition of Pain Physician, classify indications for SCS as follows:3
Strong indication: complex regional pain syndrome (CRPS)
Moderate indication: failed back surgery syndrome, chronic low back pain, and chronic lower extremity pain.
The Society of British Neurological Surgeons lists the following conditions as “good indications” for SCS: failed back surgery syndrome, CRPS, neuropathic pain from peripheral nerve damage, pain secondary to peripheral vascular disease, refractory angina, and brachial plexopathy.4
1. North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98-106.
2. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100(suppl 3 Spine):254-267.
3. Boswell MV, Trescot AM, Datta S, et al. American Society of Interventional Pain Physicians Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
4. Society of British Neurological Surgeons/British Pain Society Spinal Cord Stimulation for the Management of Pain: Recommendations for Best Clinical Practice. London: British Pain Society; 2009. Available at: www.britishpainsociety.org/book_scs_main.pdf. Accessed October 3, 2009.
1. North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56:98-106.
2. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100(suppl 3 Spine):254-267.
3. Boswell MV, Trescot AM, Datta S, et al. American Society of Interventional Pain Physicians Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
4. Society of British Neurological Surgeons/British Pain Society Spinal Cord Stimulation for the Management of Pain: Recommendations for Best Clinical Practice. London: British Pain Society; 2009. Available at: www.britishpainsociety.org/book_scs_main.pdf. Accessed October 3, 2009.
Evidence-based answers from the Family Physicians Inquiries Network