User login
Risk factors for nonsuicidal self-injury: A review of the evidence
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.
NSSI risk factors for adolescents
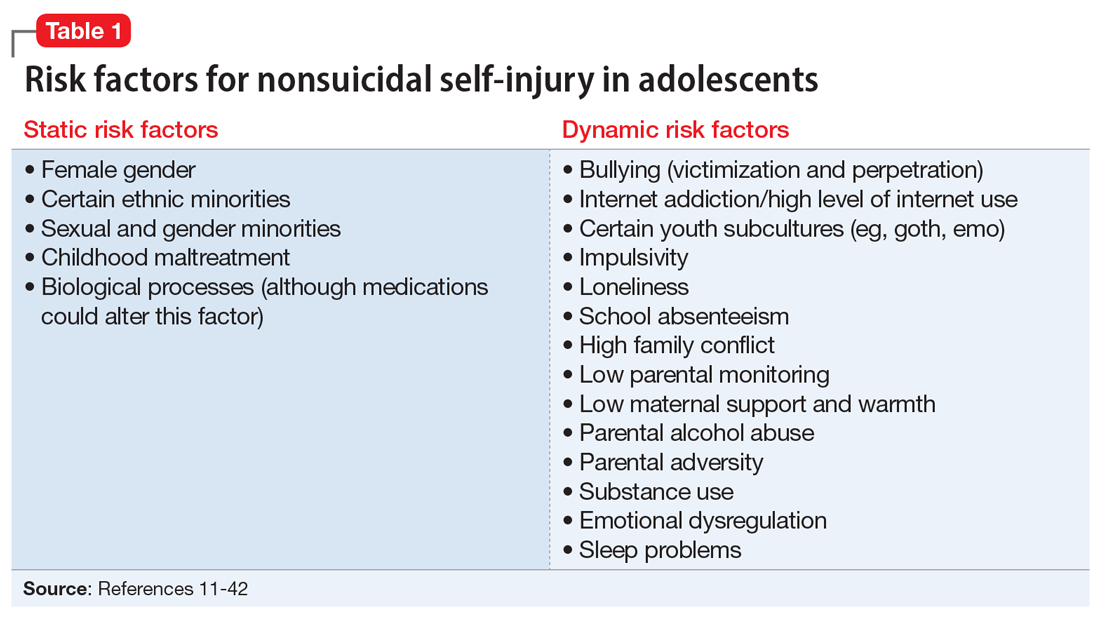
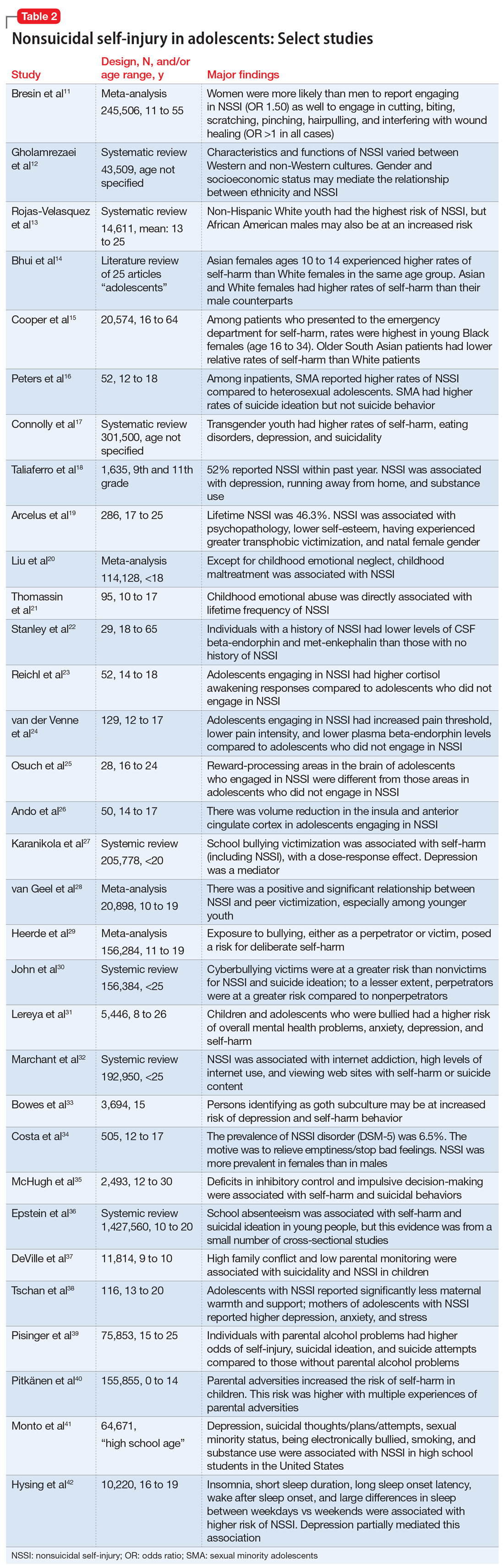
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.
Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
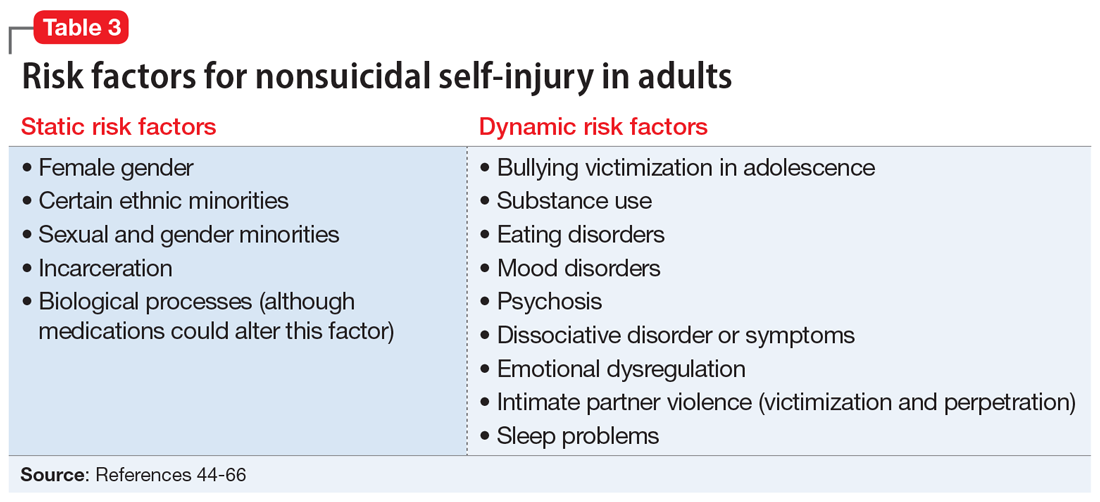
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.
Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11
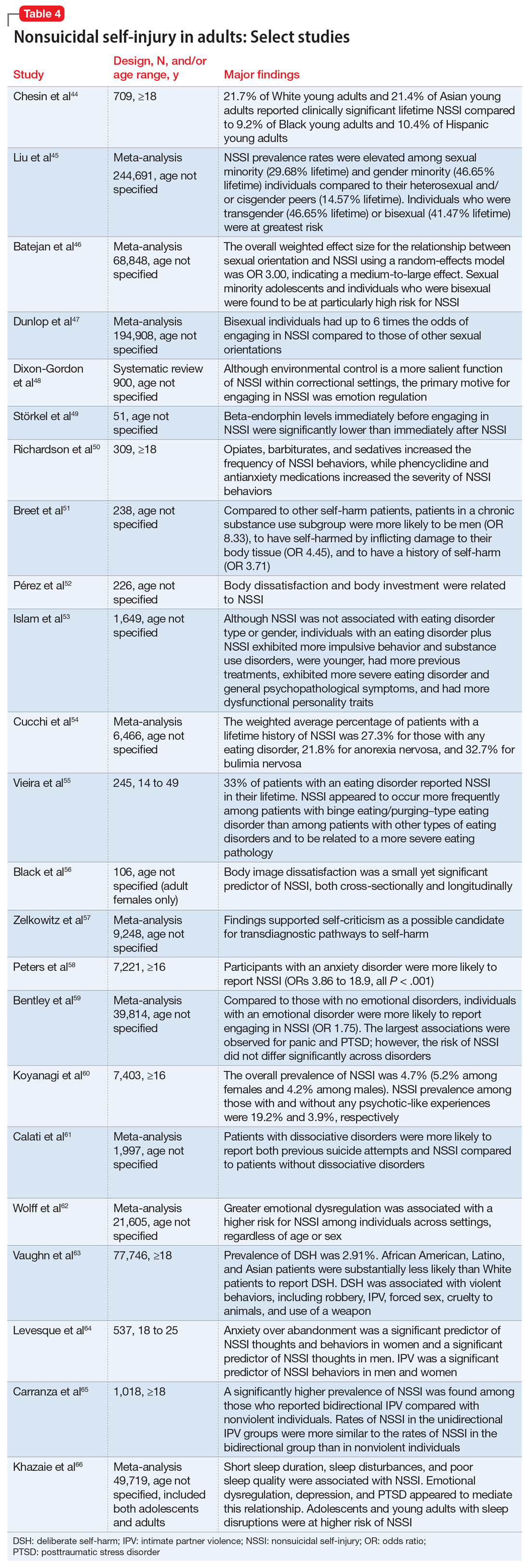
As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
Nonsuicidal self-injury (NSSI) is the direct and deliberate destruction of body tissue without intent to die.1 Common forms of NSSI include cutting, burning, scraping/scratching skin, biting, hitting, and interfering with wound healing.2 Functional theories suggest that NSSI temporarily alleviates overwhelming negative emotions and can produce feelings of relief, resulting in a reinforcing effect.3
NSSI has been shown to be a risk factor for future suicide attempts.4 A 2018 study found that NSSI is associated with an increased risk of subsequent suicidal ideation (odds ratio [OR] 2.8), suicide plan (OR 3.0), and suicide attempt (OR 5.5).5 NSSI is also associated with individuals who had suicidal ideation and formed a suicide plan, and individuals who had a suicide plan and attempted suicide (ORs 1.7 to 2.1).5 Another study found that 70% of adolescents who engage in NSSI have attempted suicide during their lifetime, and 55% have multiple attempts.6
Given the overlap between suicide attempts and NSSI, performing a thorough suicide risk assessment (which is beyond the scope of this article) is crucial. This article describes the static and dynamic risk factors for NSSI in adolescents and adults, which can help us perform a suicide risk assessment and allow us to formulate an appropriate treatment plan that includes safety-based interventions.

NSSI risk factors for adolescents
From developing sexual identity and undergoing puberty to achieving increased independence from their parents and developing a sense of autonomy, adolescents undergo many biological, psychological, and social changes before reaching adulthood.7 Data suggest that NSSI often begins in adolescence, with a typical onset at age 13 or 14.3 Community studies show that one-third to one-half of adolescents in the United States have engaged in NSSI.8,9 Previously, NSSI during adolescence was associated with 3 major diagnostic categories: eating disorders, developmental disabilities, and borderline personality disorder (BPD).10 However, recent data suggest that NSSI is also common outside of these categories. Here we describe static and dynamic risk factors for NSSI in adolescents (Table 111-42). Table 211-42 summarizes the studies of NSSI in adolescents that we reviewed.

Static risk factors
Female adolescents and adults engage in NSSI at higher rates than males. The difference is larger in clinical populations compared to the general population.11
A large portion of research about NSSI has been conducted in studies in which the majority of participants were White.12 Most studies report a higher prevalence of NSSI among non-Hispanic White youth,13 but some suggest other ethnic groups may also experience high rates of self-harm and NSSI.13-15 Several studies have demonstrated high rates of self-harm among South Asian adult females compared with White adult females, but this difference may be less pronounced in adolescents.14 One study in the United Kingdom found that White females age 10 to 14 had higher rates of self-harm compared to South Asian females,14 while another found that risk and rates of self-harm in young South Asian people varied by city and country of origin.15 Young Black females15 and young Black males13 also may be at an increased risk of self-harm. One review found that Black females were more likely to self-harm than Asian or White groups.15
Several studies suggest that sexual minority adolescents (SMA) (eg, lesbian, gay, bisexual, transgender, queer) are at greater risk for NSSI than heterosexual adolescents.16 SMA have been shown to engage in a significantly greater frequency of NSSI and more types of NSSI than heterosexual adolescents.16 Furthermore, on the Inventory of Statements about Self-Injury, SMA self-reported using NSSI for intrapersonal functions (eg, for affect regulation, antisuicide, self-punishment) significantly greater than their heterosexual peers; however, there were no significant differences between the 2 groups on interpersonal functions (eg, autonomy, interpersonal boundaries, peer bonding, sensation-seeking).16
Continue to: Transgender and gender nonconfirming...
Transgender and gender nonconfirming (GNC) youth are at a particularly high risk for NSSI; 30% to 45.5% of transgender adolescents report self-injury.17 Factors shown to distinguish transgender/GNC youth who engage in NSSI from those who do not include having a mental health problem, depression, running away from home, substance use, lower self-esteem/greater self-criticism, experiencing transphobia victimization, and having more interpersonal problems.18,19 Among transgender/GNC youth, those whose biological sex is female are more likely to report NSSI than those whose biological sex is male (ie, transgendered adolescent males are more likely to report NSSI than transgendered adolescent females).18,19
Most forms of childhood maltreatment have been associated with NSSI. In a recently published review, Liu et al20 found that childhood maltreatment (including sexual abuse, physical abuse, emotional abuse, and physical neglect) was associated with an increased risk for NSSI. However, conflicting evidence suggests that when confounders are removed, only childhood emotional abuse was directly associated with NSSI.21 Current evidence is modest for childhood emotional neglect as a risk factor for NSSI.20
Increasing research is investigating the biological processes that may be implicated in NSSI. Some studies suggest that endogenous opioids,22 monoamine neurotransmitters,22 and the hypothalamic-pituitary-adrenal (HPA) axis23 may play a role in NSSI. Compared to healthy controls, adolescents engaging in NSSI have been shown to have lower pain intensity (P = .036), higher pain thresholds (P = .040), and lower beta-endorphins (endogenous opioid hormones involved in mediating stress and pain) (P = .002).24 There may be alterations in the HPA axis among adolescents who engage in NSSI, more specifically stronger cortisol awakening responses.23 Both functional and standard MRI have been used to study the neurobiology of NSSI. One study demonstrated differences in functional connectivity between brain areas linked to neuroregulation of emotions in adolescents who engage in NSSI,25 while another found volume reduction in the insula of these adolescents, which suggests a possible neurobiological reason for impulsivity and the increased risk of suicidal behavior.26
Dynamic risk factors
Research has repeatedly shown bullying is a risk factor for NSSI.27 One study found that younger children who were victimized reported significantly more NSSI than older children.28 New data suggest that perpetrators of bullying are also at risk for deliberate self-harm behavior (SHB), which this study defined as a behavior that is intended to cause self-harm but without suicidal intent and having a nonfatal outcome.29 Victims of cyberbullying also are at a greater risk for self-harm, suicidal behaviors, and suicide attempt.30 To a lesser extent, cyberbullying perpetrators are at greater risk for suicidal behaviors and suicidal ideation.30 Bullying is a risk factor for NSSI not only in adolescence, but also in adulthood. Lereya et al31 found that victims of bullying in childhood and early adolescence were more likely to have mental health problems (including anxiety and depression) and more likely to engage in SHB—which this study defined as hurting oneself on purpose in any way—as adults.
The effects of internet use on adolescents’ mental health also has been investigated. A recent review that explored the relationship between all types of internet use (general use, internet addiction, social media, self-harm websites, forums, etc) and SHB/suicidal behavior found that young people with internet addiction, high levels of internet use, and a tendency to view websites with self-harm or suicidal content were at higher risk of engaging in SHB/suicidal behavior.32 This study did not use a specific definition for SHB or suicidal behavior.32
Continue to: Membership in certain youth...
Membership in certain youth subcultures (eg, emo or goth) has been evaluated as potential risk factors for depression and deliberate self-harm. Bowes et al33 found that for each unit increase in goth affiliation (not at all, not very much, somewhat, more than somewhat, very much), youth were 1.52 times more likely to engage in SHB; these researchers also reported a dose-response association between goth identification and future SHB. This study asked participants if they have ever tried to harm or hurt themselves in any manner, but did not distinguish between individuals who had harmed themselves with and without suicidal intent.33
Personality traits such as impulsiveness and loneliness have been linked to NSSI among adolescents.34,35 A recent study found that adolescents who met the proposed DSM-5 diagnostic criteria for NSSI scored higher on the Barratt Impulsiveness Scale, specifically in measures of:
- motor impulsiveness (ie, acting without thinking)
- attentional impulsiveness (ie, making decisions quickly)
- impulsiveness due to lack of planning (ie, failure to plan for the future).34
This study also found that adolescents who identified as being lonely based on scores on the Brazilian Loneliness Scale were at a higher risk for NSSI.34
A recent systematic review (32 studies) and meta-analysis (9 studies) found that school absenteeism was associated with a risk of self-harm (pooled aOR 1.37, P = .01) and suicidal ideation (pooled aOR 1.20, P = .03).36 This study suggested that school absenteeism, an important marker of social exclusion, was associated with both SHB and suicidal ideation in young people.36 It defined SHB as any act of self-injury or self-poisoning, regardless of intent.36
Finally, family-related factors have been associated with an increased risk of NSSI. One study of 11,814 children age 9 and 10 revealed that high family conflict (OR 1.09; 95% CI, 1.05 to 1.14) and low parental monitoring (OR 0.95; 95% CI, 0.93 to 0.98) were associated with NSSI.37 A smaller, community-based study found that adolescents with NSSI reported significantly less maternal support and warmth than nonclinical controls, but a cause-and-effect relationship has not yet been determined.38 Parental history alone may influence adolescents’ risk of NSSI. A study that included nearly 76,000 youth found that adolescents with perceived parental alcohol problems had higher odds of self-injury, suicidal ideation, and suicide attempts.39 Adolescents exposed to maternal or paternal adversities were also at a higher risk of self-harm (hazard ratio 1.5 to 5.4 among males, 1.7 to 3.9 among females).40
Continue to: NSSI risk factors for adults
NSSI risk factors for adults
Although data regarding the prevalence of NSSI in adults are lacking, available studies report a 12-month prevalence of 0.9%2 and a lifetime prevalence of 5.5% to 5.9%.43 There is a significant overlap in risk factors for NSSI in adolescent and adult populations, but there are also many important differences. The static and dynamic risk factors for NSSI in adults are described in Table 3.44-66 Table 444-66 summarizes the studies of NSSI in adults that we reviewed.

Static risk factors
Research findings regarding the prevalence of NSSI based on gender are varied. For years, it has been believed that women are more likely to engage in NSSI than men. Recent meta-analyses that have examined this relationship closely found that the gender difference is larger for clinical samples compared to community samples and more pronounced in younger individuals.11

As is the case with adolescents, there may be ethnic variations in rates of self-harm and NSSI among adults. A 2013 study by Chesin et al44 found that Asian and White young adults experience higher rates of NSSI than their Hispanic and Black counterparts. Evidence suggests that relative rates of self-harm for older South Asian adults are lower than in older White adults.15
Compared to heterosexual or cisgender individuals, members of sexual and gender minorities have a higher past-year and lifetime prevalence of NSSI.45 One study found that the weighted effect size between sexual orientation and NSSI had an OR of 3 (95% CI, 2.46 to 3.66), indicating a medium-to-large effect.46 Bisexual and transgender individuals appear to be at the highest risk for NSSI when compared to members of other sexual and gender minority groups.45 One review that included mostly cross-sectional studies found that individuals identifying as bisexual had up to 6 times the odds of engaging in NSSI when compared to those of other sexual orientations.47
Incarceration is a risk factor for NSSI. The rates of NSSI in criminal justice settings are higher (up to 61%) than in the general adult population (approximately 4%).48 Recent research found that NSSI serves similar functions in correctional and non-correctional settings, primarily to regulate emotions.48 However, there is also evidence of higher rates of NSSI being motivated by an attempt to influence the environment (ie, engaging in NSSI in order to be transferred to another prison unit) compared to NSSI in community settings.48
Continue to: Though less robust than data...
Though less robust than data published regarding adolescents, the role of biological processes in adults engaging in NSSI has also been studied. A 2021 study by Störkel et al49 found that levels of salivary beta-endorphins were significantly lower in adults immediately before engaging in NSSI compared to after NSSI. Furthermore, adults who engage in NSSI have lower levels of met-enkephalin (P < .01), an opioid growth factor, compared to adults who have never engaged in NSSI.22
Dynamic risk factors
Individuals who engage in NSSI often report substance use, but there is little data on whether substance use is an independent risk factor for NSSI. Although limited, recent evidence suggests illicit substance use in both adolescents41 and adults50 increases risk for NSSI. Richardson et al50 found that the use of barbiturates, opiates, and sedatives significantly increased the frequency of NSSI, whereas use of marijuana, phencyclidine, and medications used to treat anxiety significantly increased the severity of NSSI. A smaller study conducted in South Africa found that individuals who engage in substance use and NSSI were more likely to be male (P < .001).51
Eating disorders and NSSI are highly comorbid.52 The lifetime prevalence of NSSI among individuals with eating disorders ranges from 20.6%to 37.1%.52,53 Results are inconsistent regarding which eating disorders (if any) are greater risk factors for NSSI. One study found that the prevalence of NSSI in patients with bulimia nervosa was 32.7% (95% CI, 26.9% to 39.1%) vs 21.8% in patients with anorexia nervosa (95% CI, 18.5% to 25.6%).54 Another study found that individuals with binge eating/purging–type eating disorders reported engaging in NSSI more frequently than those with other types of eating disorders.55 Among patients with eating disorders who reported NSSI, risk factors included younger age of onset, more negative self-evaluation, more impulsive behavior, concomitant substance use, history of suicide attempts, childhood abuse, and peer aggression.53,55 Body image dissatisfaction and self-criticism, even in individuals not formally diagnosed with an eating disorder, are small but significant predictors of NSSI.56,57
Mood disorders have also been linked to NSSI.58,59 Anxiety disorders (including generalized anxiety disorder, social phobia, panic disorder, and agoraphobia) as well as anxiety-related disorders such as obsessive-compulsive disorder have been significantly associated with NSSI (P < .001), but this relationship decreased in strength when mood instability was removed as a confounder.58 Among patients with anxiety and anxiety-related disorders, panic disorder and posttraumatic stress disorder (PTSD) have shown the strongest association with NSSI, with pooled aORs of 2.67 and 2.06, respectively.59
Recent studies have examined the association of other mental health disorders and symptoms with NSSI, including psychosis60 and dissociative symptoms.61 One study found that paranoia, thought control, and auditory hallucinations were significantly associated with NSSI60; however, after controlling for concomitant BPD, only paranoia was significantly associated with NSSI.60 Individuals diagnosed with dissociative disorders were more likely than patients without such disorders to endorse NSSI and suicide attempts.61
Continue to: Emotional dysregulation...
Emotional dysregulation (EDR)—defined as difficulty understanding, recognizing, and managing one’s emotions—has been researched extensively in relation to NSSI.62 A recent review that included studies of both adolescents and adults reported a significant association between EDR and NSSI, with an OR of 2.40 (95% CI, 2.01 to 2.86).62 A larger effect size was observed between EDR and lifetime NSSI (OR 3.21; 95% CI, 2.63 to 3.91) compared to past-year NSSI (OR 2.32; 95% CI, 1.84 to 2.92).62 Patient age, sex, and sample type (clinical vs community) were not significant moderators of strength between the reported associations.62
Studies examining intimate partner violence (IPV) and NSSI have found that young adults who engage in IPV (both as victims and as perpetrators) are more likely to report NSSI.63-65 Researchers have proposed that anxiety over abandonment may explain this relationship.64 A recent study found that individuals with bidirectional IPV (ie, both victimization and perpetration) engaged in NSSI at a higher prevalence than those engaging in unidirectional IPV or no IPV.65 This suggests that relationship violence in general (rather than just being a victim of IPV) may be a risk factor for NSSI.65
Finally, studies suggest that adolescents and adults who have sleep problems (insomnia, short sleep duration, long sleep onset latency, waking after sleep onset, and poor quality sleep) are more likely to report self-harm or NSSI than those without sleep problems.42,66 In adults, this relationship is partially mediated by depressive symptoms, EDR, and PTSD.66 In adolescents, depressive symptoms are a mediator for this relationship.42
Bottom Line
Nonsuicidal self-injury (NSSI) is a significant health concern due to its association with suicide attempts. Although there are similarities in NSSI risk factors between adolescents and adults, there are also important differences. Understanding these differences is necessary to develop appropriate treatment plans.
Related Resources
- American Foundation for Suicide Prevention. https://afsp.org/
- Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: a systematic review. Front Psych. 2017;8:1946. doi:10.3389/ fpsyg.2017.01946
- Gold LH, Frierson RL, eds. Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020.
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
1. Nock MK. Self-injury. Annu Rev Clin Psychol. 2010;6:339-363.
2. Klonsky ED. Non-suicidal self-injury in United States adults: prevalence, sociodemographics, topography and functions. Psychol Med. 2011;41(9):1981-1986.
3. Klonsky ED. Nonsuicidal self-injury: what we know, and what we need to know. Can J Psychiatry. 2014;59(11):565-568.
4. Wilkinson P, Kelvin R, Roberts C, et al. Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT). Am J Psychiatry. 2011;168(5):495-501.
5. Kiekens G, Hasking P, Boyes M, et al. The associations between non-suicidal self-injury and first onset suicidal thoughts and behaviors. J Affect Disord. 2018;239:171-179.
6. Nock MK, Joiner TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
7. Christie D, Viner R. Adolescent development. BMJ. 2005;330(7486):301-304.
8. Yates TM, Tracy AJ, Luthar SS. Nonsuicidal self-injury among “privileged” youths: longitudinal and cross-sectional approaches to developmental process. J Consult Clin Psychol. 2008;76(1):52-62.
9. Lloyd-Richardson EE, Perrine N, Dierker L, et al. Characteristics and functions of non-suicidal self-injury in a community sample of adolescents. Psychol Med. 2007;37(8):1183-1192.
10. Peterson J, Freedenthal S, Sheldon C, et al. Nonsuicidal self injury in adolescents. Psychiatry(Edgmont). 2008;5(11):20-26.
11. Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: a meta-analysis. Clin Psychol Rev. 2015;38:55-64.
12. Gholamrezaei M, Stefano JD, Heath NL. Nonsuicidal self-injury across cultures and ethnic and racial minorities: a review. Int J Psychol. 2015;52(4):316-326.
13. Rojas-Velasquez DA, Pluhar EI, Burns PA, et al. Nonsuicidal self-injury among African American and Hispanic adolescents and young adults: a systematic review. Prev Sci. 2021;22:367-377.
14. Bhui K, McKenzie K, Rasul F. Rates, risk factors & methods of self harm among minority ethnic groups in the UK: a systematic review. BMC Public Health. 2007;7:336.
15. Cooper J, Murphy E, Webb R, et al. Ethnic differences in self-harm, rates, characteristics and service provision: three-city cohort study. Br J Psychiatry. 2010;197(3):212-218.
16. Peters JR, Mereish EH, Krek MA, et al. Sexual orientation differences in non-suicidal self-injury, suicidality, and psychosocial factors among an inpatient psychiatric sample of adolescents. Psychiatry Res. 2020;284:112664.
17. Connolly MD, Zervos MJ, Barone 2nd CJ, et al. The mental health of transgender youth: advances in understanding. J Adolesc Health. 2016;59(5):489-495.
18. Taliaferro LA, McMorris BJ, Rider GN, et al. Risk and protective factors for self-harm in a population-based sample of transgender youth. Archives Suicide Res. 2019;23(2):203-221.
19. Arcelus J, Claes L, Witcomb GL, et al. Risk factors for non-suicidal self-injury among trans youth. J Sex Med. 2016;13(3):402-412.
20. Liu RT, Scopelliti KM, Pittman SK, et al. Childhood maltreatment and non-suicidal self-injury: a systematic review and meta-analysis. Lancet Psychiatry. 2018;5(1):51-64.
21. Thomassin K, Shaffer A, Madden A, et al. Specificity of childhood maltreatment and emotion deficit in nonsuicidal self-injury in an inpatient sample of youth. Psychiatry Res. 2016;244:103-108.
22. Stanley B, Sher L, Wilson S, et al. Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord. 2010;124(1-2):134-140.
23. Reichl C, Heyer A, Brunner R, et al. Hypothalamic-pituitary-adrenal axis, childhood adversity and adolescent nonsuicidal self-injury. Psychoneuroendocrinology. 2016;74:203-211.
24. van der Venne P, Balint A, Drews E, et al. Pain sensitivity and plasma beta-endorphin in adolescent non-suicidal self-injury. J Affect Disord. 2021;278:199-209.
25. Osuch E, Ford K, Wrath A, et al. Functional MRI of pain application in youth who engaged in repetitive non-suicidal self-injury vs. psychiatric controls. Psychiatry Res. 2014;223(2):104-112.
26. Ando A, Reichl C, Scheu F, et al. Regional grey matter volume reduction in adolescents engaging in non-suicidal self-injury. Psychiatry Res Neuroimaging. 2018;280:48-55.
27. Karanikola MNK, Lyberg A, Holm A-L, et al. The association between deliberate self-harm and school bullying victimization and the mediating effect of depressive symptoms and self-stigma: a systematic review. BioMed Res Int. 2018;4745791. doi: 10.1155/2018/4745791
28. van Geel M, Goemans A, Vedder P. A meta-analysis on the relation between peer victimization and adolescent non-suicidal self-injury. Psychiatry Res. 2015;230(2):364-368.
29. Heerde JA, Hemphill SA. Are bullying perpetration and victimization associated with adolescent deliberate self-harm? A meta-analysis. Arch Suicide Res. 2019;23(3):353-381.
30. John A, Glendenning AC, Marchant A, et al. Self-harm, suicidal behaviours, and cyberbullying in children and young people: systematic review. J Med Internet Res. 2018;20(4):e129. doi: 10.2196/jmir.9044
31. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2(6):524-531.
32. Marchant A, Hawton K, Stewart A, et al. A systematic review of the relationship between internet use, self-harm and suicidal behaviour in young people: the good, the bad and the unknown. PLoS One. 2017;12(8):e0181722. doi: 10.1371/journal.pone.0181722
33. Bowes L, Carnegie R, Pearson R, et al. Risk of depression and self-harm in teenagers identifying with goth subculture: a longitudinal cohort study. Lancet Psychiatry. 2015;2(9):793-800.
34. Costa RPO, Peixoto ALRP, Lucas CCA, et al. Profile of non-suicidal self-injury in adolescents: interface with impulsiveness and loneliness. J Pediatr (Rio J). 2021;97(2):184-190.
35. McHugh CM, Lee RSC, Hermens DF, et al. Impulsivity in the self-harm and suicidal behavior of young people: a systematic review and meta-analysis. J Psychiatr Res. 2019;116:51-60.
36. Epstein S, Roberts E, Sedgwick R, et al. School absenteeism as a risk factor for self-harm and suicidal ideation in children and adolescents: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2020;29(9):1175-1194.
37. DeVille DC, Whalen D, Breslin FJ, et al. Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Netw Open. 2020;3(2):e1920956. doi: 10.1001/jamanetworkopen.2019.20956
38. Tschan T, Schmid M, In-Albon T. Parenting behavior in families of female adolescents with nonsuicidal self-injury in comparison to a clinical and a nonclinical control group. Child Adolesc Psychiatry Ment Health. 2015;9:17.
39. Pisinger V, Hawton K, Tolstrup JS. Self-injury and suicide behavior among young people with perceived parental alcohol problems in Denmark: a school-based survey. Eur Child Adolesc Psychiatry. 2018;27(2):201-208.
40. Pitkänen J, Remes H, Aaltonen M, et al. Experience of maternal and paternal adversities in childhood as determinants of self-harm in adolescence and young adulthood. J Epidemiol Community Health. 2019;73(11):1040-1046.
41. Monto MA, McRee N, Deryck FS. Nonsuicidal self-injury among a representative sample of US adolescents, 2015. Am J Public Health. 2018;108(8):1042-1048.
42. Hysing M, Sivertsen B, Stormark KM, et al. Sleep problems and self-harm in adolescence. Br J Psychiatry. 2015;207(4):306-312.
43. Swannell SV, Martin GE, Page A, et al. Prevalence of nonsuicidal self-injury in nonclinical samples: systematic review, meta-analysis and meta-regression. Suicide Life Threat Behav. 2014;44(3):273-303.
44. Chesin M, Moster A, Jeglic E. Non-suicidal self-injury among ethnically and racially diverse emerging adults: do factors unique to the minority experience matter? Current Psychology. 2013;32:318-328.
45. Liu RT, Sheehan AE, Walsh RFL, et al. Prevalence and correlates of non-suicidal self-injury among lesbian, gay, bisexual, and transgender individuals: a systematic review and meta-analysis. Clin Psychol Rev. 2019;74:101-783. doi:10.1016/j.cpr.2019.101783
46. Batejan KL, Jarvi SM, Swenson LP. Sexual orientation and non-suicidal self-injury: a meta-analytic review. Arch Suicide Res. 2015;19(2):131-150.
47. Dunlop BJ, Hartley S, Oladokun O, et al. Bisexuality and non-suicidal self-injury (NSSI): a narrative synthesis of associated variables and a meta-analysis of risk. J Affect Disord. 2020;276:1159-1172.
48. Dixon-Gordon K, Harrison N, Roesch R. Non-suicidal self-injury within offender populations: a systematic review. Int J Forensic Ment Health. 2012;11(1):33-50.
49. Störkel LM, Karabatsiakis A, Hepp K, et al. Salivary beta-endorphin in nonsuicidal self-injury: an ambulatory assessment study. Neuropsychopharmacology. 2021;46(7):1357-1363.
50. Richardson E, DePue MK, Therriault DJ, et al. The influence of substance use on engagement in non-suicidal self-injury (NSI) in adults. Subst Use Misuse. 2020;55(1):89-94.
51. Breet E, Bantjes J, Lewis I. Chronic substance use and self-harm in a primary health care setting. Afr J Prim Health Care Fam Med. 2018;10(1):e1-e9. doi: 10.4102/phcfm.v10i1.1544
52. Pérez S, Marco JH, Cañabate M. Non-suicidal self-injury in patients with eating disorders: prevalence, forms, functions, and body image correlates. Compr Psychiatry. 2018;84:32-38.
53. Islam MA, Steiger H, Jimenez-Murcia S, et al. Non-suicidal self-injury in different eating disorder types: relevance of personality traits and gender. Eur Eat Disord Rev. 2015;23(6):553-560.
54. Cucchi A, Ryan D, Konstantakopoulos G, et al. Lifetime prevalence of non-suicidal self-injury in patients with eating disorders: a systematic review and meta-analysis. Psychol Med. 2016;46(7):1345-1358.
55. Vieira AI, Machado BC, Machado PPP, et al. Putative risk factors for non-suicidal self-injury in eating disorders. Eur Eat Disord Rev. 2017;25(6):544-550.
56. Black EB, Garratt M, Beccaria G, et al. Body image as a predictor of nonsuicidal self-injury in women: a longitudinal study. Compr Psychiatry. 2019;88:83-89.
57. Zelkowitz RL, Cole DA. Self-criticism as a transdiagnostic process in nonsuicidal self-injury and disordered eating: systematic review and meta-analysis. Suicide Life Threat Behav. 2019;49(1):310-327.
58. Peters EM, Bowen R, Balbuena L. Mood instability contributes to impulsivity, non-suicidal self-injury, and binge eating/purging in people with anxiety disorders. Psychol Psychother. 2019;92(3):422-438.
59. Bentley KH, Cassiello-Robbins CF, Vittorio L, et al. The association between nonsuicidal self-injury and the emotional disorders: a meta-analytic review. Clin Psychol Rev. 2015;37:72-88.
60. Koyanagi A, Stickley A, Haro JM. Psychotic-like experiences and nonsuicidal self-injury in England: results from a national survey [corrected]. PLoS One. 2015;10(12):e0145533. doi: 10.1371/journal.pone.0145533
61. Calati R, Bensassi I, Courtet P. The link between dissociation and both suicide attempts and non-suicidal self-injury: meta-analyses. Psychiatry Res. 2017;251:103-114.
62. Wolff JC, Thompson E, Thomas SA, et al. Emotion dysregulation and non-suicidal self-injury: a systematic review and meta-analysis. Eur Psychiatry. 2019;59:25-36.
63. Vaughn MG, Salas-Wright CP, DeLisi M, et al. Deliberate self-harm and the nexus of violence, victimization, and mental health problems in the United States. Psychiatry Res. 2015;225(3):588-595.
64. Levesque C, Lafontaine M-F, Bureau J-F, et al. The influence of romantic attachment and intimate partner violence on nonsuicidal self-injury in young adults. J Youth Adolesc. 2010;39(5):474-483.
65. Carranza AB, Wallis CRD, Jonnson MR, et al. Nonsuicidal self-injury and intimate partner violence: directionality of violence and motives for self-injury. J Interpers Violence. 2020;886260520922372. doi: 10.1177/0886260520922372
66. Khazaie H, Zakiei A, McCall WV, et al. Relationship between sleep problems and self-injury: a systematic review. Behav Sleep Med. 2020;1-16. doi: 10.1080/15402002.2020.1822360
Bright light therapy for bipolar depression: A review of 6 studies
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
Depressive episodes are part of DSM-5 criteria for bipolar II disorder, and are also often experienced by patients with bipolar I disorder.1 Depressive episodes predominate the clinical course of bipolar disorder.2,3 Compared with manic and hypomanic episodes, bipolar depressive episodes have a stronger association with long-term morbidity, suicidal behavior, and impaired functioning.4,5 Approximately 20% to 60% of patients with bipolar disorder attempt suicide at least once in their lifetime, and 4% to 19% die by suicide. Compared with the general population, the risk of death by suicide is 10 to 30 times higher in patients with bipolar disorder.6
Treatment of bipolar depression is less investigated than treatment of unipolar depression or bipolar mania. The mainstays of treatment for bipolar depression include mood stabilizers (eg, lithium, valproic acid, or lamotrigine), second-generation antipsychotics (eg, risperidone, quetiapine, lurasidone, or olanzapine), adjunctive antidepressants (eg, selective serotonin reuptake inhibitors or bupropion), and combinations of the above. While significant progress has been made in the treatment of mania, achieving remission for patients with bipolar depression remains a challenge. Anti-manic medications reduce depressive symptoms in only one-third of patients.7 Antidepressant monotherapy can induce hypomania and rapid cycling.8 Electroconvulsive therapy has also been used for treatment-resistant bipolar depression, but is usually reserved as a last resort.9
Research to investigate novel therapeutics for bipolar depression is a high priority. Patients with bipolar disorder are susceptible to environmental cues that alter circadian rhythms and trigger relapse. Recent studies have suggested that bright light therapy (BLT), an accepted treatment for seasonal depression, also may be useful for treating nonseasonal depression.10 Patients with bipolar depression frequently have delayed sleep phase and atypical depressive features (hypersomnia, hyperphagia, and lethargy), which predict response to light therapy.11 In this article, we review 6 recent studies that evaluated the efficacy and safety of BLT for treating bipolar depression (Table12-17).
1. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
In this meta-analysis, Wang et al12 examined the role of BLT in treating bipolar depression. They also explored variables of BLT, including duration, timing, color, and color temperature, and how these factors may affect the severity of depressive symptoms.
Study design
- Two researchers conducted a systematic literature search on PubMed, Web of Science, Embase, Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL), as well as 4 Chinese databases from inception to March 2020. Search terms included “phototherapy,” “bright light therapy,” “bipolar disorder,” and “bipolar affective disorder.”
- Inclusion criteria called for randomized controlled trials (RCTs) or cohort studies that used a clearly defined diagnosis of bipolar depression. Five RCTs and 7 cohort studies with a total of 847 participants were included.
- The primary outcomes were depression severity, efficacy of duration/timing of BLT for depressive symptoms, and efficacy of different light color/color temperatures for depressive symptoms.
Outcomes
- As assessed by the Hamilton Depression Rating Scale (HAM-D); Inventory of Depressive Symptomatology, Clinician Rating; or the Structured Interview Guide for the HAM-D, depression severity significantly decreased (P < .05) with BLT intensity ≥5,000 lux when compared with placebo.
- Subgroup analyses suggested that BLT can improve depression severity with or without adjuvant therapy. Duration of <10 hours and >10 hours with morning light vs morning plus evening light therapy all produced a significant decrease in depressive symptoms (P < .05).
- White light therapy also significantly decreased depression severity (P < .05). Color temperatures >4,500K and <4,500K both significantly decreased depression severity (P < .05).
- BLT (at various durations, timings, colors, and color temperatures) can reduce depression severity.
- This analysis only included studies that showed short-term improvements in depressive symptoms, which brings into question the long-term utility of BLT.
2. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
Lam et al13 examined the role of BLT for patients with bipolar depression in a systematic review and meta-analysis.
Continue to: Study design
Study design
- Investigators conducted a systematic review of RCTs of BLT for patients with bipolar depression. Articles were obtained from Web of Science, Embase, MEDLINE, PsycInfo, and Clinicaltrials.gov using the search terms “light therapy,” “phototherapy,” “light treatment,” and “bipolar.”
- Inclusion criteria required patients diagnosed with bipolar disorder currently experiencing a depressive episode, a clinician-rated measure of depressive symptomatology, a specific light intervention, and a randomized trial design with a control.
- A total of 7 RCTs with 259 participants were reviewed. The primary outcome was improvement in depressive symptoms based on the 17-item HAM-D.
Outcomes
- BLT was associated with a significant improvement in clinician-rated depressive symptoms (P = .03).
- Data for clinical response obtained from 6 trials showed a significant difference favoring BLT vs control (P = .024). Data for remission obtained from 5 trials showed no significant difference between BLT and control (P = .09).
- Compared with control, BLT was not associated with an increased risk of affective switches (P= .67).
Conclusion
- This study suggests a small to moderate but significant effect of BLT in reducing depressive symptoms.
- Study limitations included inconsistent light parameters, short follow-up time, small sample sizes, and the possibility that control conditions had treatment effects (eg, dim light as control vs no light).
3. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
Hirakawa et al14 assessed the role of adjunctive BLT for treating bipolar depression. Previous meta-analyses focused on case-control studies that assessed the effects of BLT and sleep deprivation therapy on depressive symptoms, but this meta-analysis reviewed RCTs that did not include sleep deprivation therapy.
Continue to: Study design
Study design
- Two authors searched Embase, MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL, and Clinicaltrials.gov from inception to September 2019 using the terms “light therapy,” “phototherapy,” and “bipolar disorder.”
- Inclusion criteria called for RCTs, participants age ≥18, a diagnosis of bipolar disorder according to standard diagnostic criteria, evaluation by a standardized scale (HAM-D, Montgomery-Åsberg Depression Rating Scale [MADRS], Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement [SIGH-ADS]), and light therapy as the experimental group intervention.
- The main outcomes were response rate (defined as ≥50% reduction in depression severity based on a standardized scale) and remission rate (defined as a reduction to 7 points on HAM-D, reduction to 9 points on MADRS, and score <8 on SIGH-ADS).
- Four RCTs with a total of 190 participants with bipolar depression were evaluated.
Outcomes
- BLT had a significant effect on response rate (P = .002).
- There was no significant effect of BLT on remission rates (P = .34).
- No studies reported serious adverse effects. Minor effects included headache (14.9% for BLT vs 12.5% for control), irritability (4.26% for BLT vs 2.08% for control), and sleep disturbance (2.13% for BLT vs 2.08% for control). The manic switch rate was 1.1% in BLT vs 1.2% in control.
Conclusion
- BLT is effective in reducing depressive symptoms in bipolar disorder, but does not affect remission rates.
- This meta-analysis was based on a small number of RCTs, and light therapy parameters were inconsistent across the studies. Furthermore, most patients were also being treated with mood-stabilizing or antidepressant medications.
- It is unclear if BLT is effective as monotherapy, rather than as adjunctive therapy.
4. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
Triple chronotherapy is the combination of total sleep deprivation, sleep phase advance, and BLT. D’Agostino et al15 reviewed all available evidence on the efficacy of triple chronotherapy interventions in treating symptoms of major depressive disorder (MDD) and bipolar depression.
Study design
- Researchers conducted a systematic search on PubMed, Scopus, and Embase from inception to December 2019 using the terms “depression,” “sleep deprivation,” “chronotherapy,” and related words.
- The review included studies of all execution modalities, sequences of interventions, and types of control groups (eg, active control vs placebo). The population included participants of any age with MDD or bipolar depression.
- Two authors independently screened studies. Six articles published between 2009 and 2019 with a total of 190 patients were included.
Continue to: Outcomes
Outcomes
- All studies reported improvement in HAM-D scores at the end of treatment with triple chronotherapy, with response rates ranging from 50% to 84%.
- Most studies had a short follow-up period (up to 3 weeks). In these studies, response rates ranged from 58.3% to 61.5%. One study that had a 7-week follow-up also reported a statistically significant response rate in favor of triple chronotherapy.
- Remission rates, defined by different cut-offs depending on which version of the HAM-D was used, were evaluated in 5 studies. These rates ranged from 33.3% to 77%.
- Two studies that used the Columbia Suicide Severity Rating Scale to assess the effect of triple chronotherapy on suicide risk reported a significant improvement in scores.
Conclusion
- Triple chronotherapy may be an effective and safe adjunctive treatment for depression. Some studies suggest that it also may play a role in remission from depression and reducing suicide risk.
5. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
In a meta-analysis, Dallaspezia and Benedetti16 evaluated 11 studies to assess the role of BLT for treating depressive symptoms in patients with bipolar disorder.
Study design
- Researchers searched literature published on PubMed with the terms “mood disorder,” “depression,” and “light therapy.”
- Eleven studies with a total of 195 participants were included. Five studies were RCTs.
- The primary outcome was severity of depression based on scores on the HAM-D, Beck Depression Inventory, or SIGH-ADS. Secondary outcomes were light intensity (measured in lux) and duration of treatment.
Outcomes
- Analysis of all 11 studies revealed a positive effect of BLT on depressive symptoms (P < .001).
- Analysis of just the 5 RCTs found a significant effect of BLT on depressive symptoms (P < .001).
- The switch rate due to BLT was lower than rates for patients being treated with antidepressant monotherapy (15% to 40%) or placebo (4.2%).
- Duration of treatment influenced treatment outcomes (P = .05); a longer duration resulted in the highest clinical effect. However, regardless of duration, BLT showed higher antidepressant effects than placebo.
- Higher light intensity was also found to show greater efficacy.
Continue to: Conclusion
Conclusion
- BLT is an effective adjunctive treatment for bipolar depression.
- Higher light intensity and longer duration of BLT may result in greater antidepressant effects, although the optimum duration and intensity are unknown.
- A significant limitation of this study was that the studies reviewed had high heterogeneity, and only a few were RCTs.
6. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Takeshima et al17 conducted a systematic review and meta-analysis to evaluate the efficacy and safety of BLT for manic and depressive symptoms in patients with bipolar disorder. They also evaluated if BLT could prevent recurrent mood episodes in patients with bipolar disorder.
Study design
- Researchers searched for studies of BLT for bipolar disorder in MEDLINE, CENTRAL, Embase, PsychInfo, and Clincialtrials.gov using the terms “bipolar disorder,” “phototherapy,” and “randomized controlled trial.”
- Two groups of 2 authors independently screened titles and abstracts for the following inclusion criteria: RCTs, 80% of patients diagnosed clinically with bipolar disorder, any type of light therapy, and control groups that included sham treatment or no light. Three groups of 2 authors then evaluated the quality of the studies and risk of bias.
- Six studies with a total of 280 participants were included.
- Primary outcome measures included rates of remission from depressive or manic episodes, rates of relapse from euthymic states, and changes in score on depression or mania rating scales.
Outcomes
- No significant differences were found between BLT and placebo for rates of remission from depressive episodes (P = .42), rates of manic switching (P = .26), or depressive symptom scores (P = .30).
- Sensitivity analysis for 3 studies with low overall indirectness revealed that BLT did have a significant antidepressant effect (P = .006).
- The most commonly reported adverse effects of BLT were headache (4.7%) and sleep disturbance (1.4%).
Conclusion
- This meta-analysis suggests that BLT does not have a significant antidepressant effect. However, a sensitivity analysis of studies with low overall indirectness showed that BLT does have a significant antidepressant effect.
- This review was based on a small number of RCTs that had inconsistent placebos (dim light, negative ion, no light, etc.) and varying parameters of BLT (light intensity, exposure duration, color of light), which may have contributed to the inconsistent results.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Rihmer Z. S34.02 - Prediction and prevention of suicide in bipolar disorders. European Psychiatry. 2008;23(S2):S45-S45.
5. Simon GE, Bauer MS, Ludman EJ, et al. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237-1245.
6. Dome P, Rihmer Z, Gonda X. Suicide risk in bipolar disorder: a brief review. Medicina (Kaunas). 2019;55(8):403.
7. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
8. Post RM, Altshuler LL, Leverich GS, et al. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry. 2006;189:124-131.
9. Shah N, Grover S, Rao GP. Clinical practice guidelines for management of bipolar disorder. Indian J Psychiatry. 2017;59(Suppl 1):S51-S66.
10. Penders TM, Stanciu CN, Schoemann AM, et al. Bright light therapy as augmentation of pharmacotherapy for treatment of depression: a systematic review and meta-analysis. Prim Care Companion CNS Disord. 2016;18(5). doi: 10.4088/PCC.15r01906.
11. Terman M, Amira L, Terman JS, et al. Predictors of response and nonresponse to light treatment for winter depression. Am J Psychiatry. 1996;153(11):1423-1429.
12. Wang S, Zhang Z, Yao L, et al. Bright light therapy in treatment of patients with bipolar disorder: a systematic review and meta-analysis. PLoS ONE. 2020;15(5):e0232798. doi: 10.1371/journal.pone.0232798
13. Lam RW, Teng MY, Jung YE, et al. Light therapy for patients with bipolar depression: systematic review and meta-analysis of randomized controlled trials. Can J Psychiatry. 2020;65(5):290-300.
14. Hirakawa H, Terao T, Muronaga M, et al. Adjunctive bright light therapy for treating bipolar depression: a systematic review and meta-analysis of randomized controlled trials. Brain Behav. 2020;10(12):ee01876. doi.org/10.1002/brb3.1876
15. D’Agostino A, Ferrara P, Terzoni S, et al. Efficacy of triple chronotherapy in unipolar and bipolar depression: a systematic review of available evidence. J Affect Disord. 2020;276:297-304.
16. Dallaspezia S, Benedetti F. Antidepressant light therapy for bipolar patients: a meta-analyses. J Affect Disord. 2020;274:943-948.
17. Takeshima M, Utsumi T, Aoki Y, et al. Efficacy and safety of bright light therapy for manic and depressive symptoms in patients with bipolar disorder: a systematic review and meta-analysis. Psychiatry Clin Neurosci. 2020;74(4):247-256.
Pharmacologic management of autism spectrum disorder: A review of 7 studies
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.
1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and social interaction, including deficits in social reciprocity, nonverbal communicative behaviors used for social interaction, and skills in developing, maintaining, and understanding relationships.1 In addition, the diagnosis of ASD requires the presence of restricted, repetitive patterns of behavior, interests, or activities.
Initially, ASD was considered a rare condition. In recent years, the reported prevalence has increased substantially. The most recent estimated prevalence is 1 in 68 children at age 8, with a male-to-female ratio of 4 to 1.2
Behavioral interventions are considered to be the most effective treatment for the core symptoms of ASD. Pharmacologic interventions are used primarily to treat associated or comorbid symptoms rather than the core symptoms. Aggression, self-injurious behavior, and irritability are common targets of pharmacotherapy in patients with ASD. Studies have provided support for the use of antipsychotic agents to treat irritability and associated aggressive behaviors in patients with autism,3 but because these agents have significant adverse effects—including extrapyramidal side effects, somnolence, and weight gain—their use requires a careful risk/benefit assessment. Stimulants have also been shown to be effective in treating comorbid attention-deficit/hyperactivity symptoms. The use of selective serotonin reuptake inhibitors (SSRIs) to manage repetitive behaviors and anxiety is also common.
Here, we review 7 recent studies of the pharmacologic management of ASD (Table).4-10 These studies examined the role of SSRIs (sertraline, fluoxetine), an acetylcholinesterase inhibitor (donepezil), atypical antipsychotics (risperidone, aripiprazole, lurasidone), natural supplements (vitamin D, omega-3), a diuretic (bumetanide), and a glutamatergic modulator (riluzole) in the treatment of ASD symptoms.

1. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
Several studies have shown that SSRIs improve language development in children with Fragile X syndrome, based on the Mullen Scales of Early Learning (MSEL). A previously published trial involving children with Fragile X syndrome and comorbid ASD found that sertraline improved expressive language development. Potter et al4 examined the role of sertraline in children with ASD only.
Study Design
- In this randomized, double-blind, placebo-controlled trial, 58 children age 24 to 72 months with ASD received low-dose sertraline or placebo for 6 months.
- Of the 179 participants who were screened for eligibility, 58 were included in the study. Of these 58 participants, 32 received sertraline and 26 received placebo. The numbers of participants who discontinued from the sertraline and placebo arms were 8 and 5, respectively.
- Among those in the sertraline group, participants age <48 months received 2.5 mg/d, and those age ≥48 months received 5 mg/d.
Outcomes
- No significant differences were found on the primary outcome (MSEL expressive language raw score and age-equivalent combined score) or secondary outcomes (including Clinical Global Impressions–Improvement [CGI-I] scale at 3 months and end of treatment), as per intent-to-treat analyses.
- Sertraline was well tolerated. There was no difference in adverse effects between treatment groups and no serious adverse events.
Conclusion
- Although potentially useful for language development in patients with Fragile X syndrome with comorbid ASD, SSRIs such as sertraline have not proven efficacious for improving expressive language in patients with non-syndromic ASD.
- While 6-month treatment with low-dose sertraline in young children with ASD appears safe, the long-term effects are unknown.
Continue to: Gabis et al5 examined the safety...
2. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
Gabis et al5 examined the safety and efficacy of utilizing donepezil, an acetylcholinesterase inhibitor, plus a choline supplement to treat both core features and associated symptoms in children and adolescents with ASD.
Study design
- This 9-month randomized, double-blind trial included 60 children/adolescents with ASD who were randomly assigned to receive placebo or donepezil plus a choline supplement. Participants underwent a baseline evaluation (E1), 12 weeks of treatment and re-evaluation (E2), 6 months of washout, and a final evaluation (E3).
- The baseline and final evaluations assessed changes in language performance, adaptive functioning, sleep habits, autism severity, clinical impression, and intellectual abilities. The evaluation after 12 weeks of treatment (E2) included all of these measures except intellectual abilities.
Outcomes
- Patients treated with donepezil plus a choline supplement had significant improvement in receptive language skills between E1 and E3 (P = .003).
- Patients treated with donepezil plus a choline supplement had significant worsening in scores on the Autism Treatment Evaluation Checklist (ATEC) health/physical behavior subscale between E1 and E2 (P = .012) and between E1 and E3 (P = .021).
- Improvement in receptive language skills was significant only in patients age 5 to 10 years (P = .047), whereas worsening in ATEC health/physical behavior subscale score was significant only in patients age 10 to 16 years (P = .024).
- Patients treated with donepezil plus a choline supplement reported higher percentages of gastrointestinal disturbance when compared with placebo (P = .007), and patients in the adolescent subgroup had a significant increase in irritability (P = .035).
Conclusion
- Patients age 5 to 10 years treated with donepezil plus a choline supplement exhibited improved receptive language skills. This treatment was less effective in patients age >10 years, and this group also exhibited behavioral worsening.
- Gastrointestinal disturbances were the main adverse effect of treatment with donepezil plus a choline supplement.
Continue to: The persistence of excitatory...
3. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5):537-544.
The persistence of excitatory gamma-aminobutyric acid (GABA) signaling has been found in patients with ASD. Bumetanide is a sodium-potassium-chloride cotransporter 1 (NKCC1) antagonist that not only decreases intracellular chloride, but also aberrantly decreases GABA signaling. This potent loop diuretic is a proposed treatment for symptoms of ASD. James et al6 evaluated the safety and efficacy of bumetanide use in children with ASD.
Study design
- Researchers searched the PubMed and Ovid MEDLINE databases for the terms “autism” and “bumetanide” between 1946 and 2018. A total of 26 articles were screened by title, 7 were screened by full text, and 3 articles were included in the study. The remaining articles were excluded due to study design and use of non-human subjects.
- All 3 randomized controlled trials evaluated the effects of low-dose oral bumetanide (most common dose was 0.5 mg twice daily) in a total of 208 patients age 2 to 18 years.
- Measurement scales used in the 3 studies included the Childhood Autism Rating Scale (CARS), Clinical Global Impressions Scale (CGI), Autism Behavioral Checklist (ABC), Social Responsiveness Scale (SRS), and Autism Diagnostic Observation Schedule-Generic (ADOS-G).
Outcomes
- Bumetanide improved scores on multiple autism assessment scales, including CARS, but the degree of improvement was not consistent across the 3 trials.
- There was a statistically significant improvement in ASD symptoms as measured by CGI in all 3 trials, and statistically significant improvements on the ABC and SRS in 2 trials. No improvements were noted on the ADOS-G in any of the trials.
- No dose-effect correlation was identified, but hypokalemia and polyuria were more prevalent with higher doses of bumetanide.
Conclusion
- Low-dose oral bumetanide improved social communication, social interactions, and restricted interests in patients with moderate to severe ASD. However, the 3 trials used different evaluation methods and observed varying degrees of improvement, which makes it difficult to make recommendations for or against the use of bumetanide.
- Streamlined trials with a consensus on evaluation methodology are needed to draw conclusions about the efficacy and safety of bumetanide as a treatment for ASD.
Continue to: The use of SSRIs to target...
4. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
The use of SSRIs to target symptoms of ASD has been long studied because many children with ASD have elevated serotonin levels. Several SSRIs, including fluoxetine, are FDA-approved for the treatment of obsessive-compulsive disorder, anxiety, and depression. Currently, no SSRIs are FDA-approved for treating ASD. In a meta-analysis, Li et al7 evaluated the use of fluoxetine for ASD.
Study design
- Two independent researchers searched for studies of fluoxetine treatment for ASD in Embase, Google Scholar, Ovid SP, and PubMed, with disagreement resolved by consensus.
- The researchers extracted the study design, patient demographics, and outcomes (inter-rater reliability kappa = 0.93). The primary outcomes were response rate of patients treated with fluoxetine, and change from baseline in ABC, ATEC, CARS, CGI, and Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores after fluoxetine treatment.
Outcomes
- This meta-analysis included 13 studies in which fluoxetine was used to treat a total of 303 patients with ASD. The median treatment duration was 6 months, the average age of participants was 15.23 years, and most participants (72%) were male.
- The response rate of patients treated with fluoxetine was 75%, with significant mean changes from baseline in ABC score (Helvetica Neue LT Std−3.42), ATEC score (Helvetica Neue LT Std−2.04), CGI score (Helvetica Neue LT Std−0.93), and Y-BOCS score (Helvetica Neue LT Std−1.86).
- A significantly higher incidence of hyperactivity/restlessness/agitation was noted with fluoxetine.
Conclusion
- Although 75% of participants responded to fluoxetine, the limitations of this meta-analysis included low power, inadequate quality of the included studies, and high statistical heterogeneity. In addition, the analysis found a high incidence of hyperactivity/restlessness associated with fluoxetine.
- Future randomized controlled studies may provide further clarification on managing symptoms of ASD with SSRIs.
Continue to: Irritability is a common comorbid...
5. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
Irritability is a common comorbid symptom in children with ASD. Two second-generation antipsychotics (SGAs)—risperidone and aripiprazole—are FDA-approved for irritability associated with ASD. Fallah et al8 examined the efficacy of several SGAs for treating irritability.
Study design
- This review and meta-analysis included 8 studies identified from Medline, PsycINFO, and Embase from inception to March 2018. It included double-blind, randomized controlled trials that used the Aberrant Behavior Checklist Irritability (ABC-I) to measure irritability.
- The main outcome was change in degree of irritability.
- The 8 studies compared the efficacy of risperidone, aripiprazole, lurasidone, and placebo in a total of 878 patients.
Outcomes
- Risperidone reduced ABC-I scores more than aripiprazole, lurasidone, or placebo.
- Mean differences in ABC-I scores were Helvetica Neue LT Std−6.89 for risperidone, Helvetica Neue LT Std−6.62 for aripiprazole, and Helvetica Neue LT Std−1.61 for lurasidone.
Conclusion
- Risperidone and aripiprazole were efficacious and safe for children with ASD-associated irritability.
- Lurasidone may minimally improve irritability in children with ASD.
Continue to: Irritability and hyperactivity are common...
6. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
Irritability and hyperactivity are common comorbid symptoms in children with ASD and have been linked to lower quality of life, poor adaptive functioning, and lower responsiveness to treatments when compared to children without comorbid problem behaviors. Mazahery et al9 evaluated the efficacy of vitamin D, omega-3 long-chain polyunsaturated fatty acids (LCPUFA), or both on irritability and hyperactivity.
Study design
- In a 1-year, double-blind, placebo-controlled trial, 73 children age 2.5 to 8 years with ASD were randomly assigned to receive placebo; vitamin D, 2000 IU/d (VID); omega-3 LCPUFA, 722 mg/d (OM); or both in the aforementioned doses.
- The primary outcome was reduction in the Aberrant Behavior Checklist in the domains of irritability and hyperactivity. Caregivers also completed weekly surveys regarding adverse events, compliance, and utilization of behavioral therapies.
- Of 111 children enrolled, 73 completed the 12 months of treatment.
Outcomes
- Children who received OM and VID had a greater reduction in irritability than those who received placebo (P = .001 and P = .01, respectively).
- Children who received VID also had a reduction in irritability (P = .047).
- An explanatory analysis revealed that OM also reduced lethargy (based on the Aberrant Behavior Checklist) more significantly than placebo (P = .02 adjusted for covariates).
Conclusion
- Treatment with vitamin D, 2000 IU/d, reduced irritability and hyperactivity.
- Treatment with omega-3 LCPUFA, 722 mg/d, reduced hyperactivity and lethargy.
Continue to: Glutamatergic dysregulation has been...
7. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
Glutamatergic dysregulation has been identified as a potential cause of ASD. Riluzole, a glutamatergic modulator that is FDA-approved for treating amyotrophic lateral sclerosis, is a drug of interest for the treatment of ASD-related irritability due to this proposed mechanism. Wink et al10 evaluated riluzole for irritability in patients with ASD.
Study design
- This randomized, double-blind, placebo-controlled, crossover pilot study evaluated the tolerability and safety of adjunctive riluzole treatment for drug-refractory irritability in 8 patients with ASD.
- Participants were age 12 to 25 years, had a diagnosis of ASD confirmed by the autism diagnostic observation schedule 2, and an ABC-I subscale score ≥18. Participants receiving ≥2 psychotropic medications or glutamatergic/GABA-modulating medications were excluded.
- Participants received either 5 weeks of riluzole followed by 5 weeks of placebo, or vice versa; both groups then had a 2-week washout period.
- Riluzole was started at 50 mg/d, and then increased in 50 mg/d–increments to a maximum of 200 mg/d by Week 4.
- Primary outcome measures were change in score on the ABC-I and CGI-I.
Outcomes
- No significant treatment effects were identified.
- All participants tolerated riluzole, 200 mg/d, but increased dosages did not result in a higher overall treatment effect.
- There were no clinically significant adverse effects or laboratory abnormalities.
Conclusion
- Riluzole, 200 mg/d, was well tolerated but had no significant effect on irritability in adolescents with ASD.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.
1. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
2. Christensen DL, Baio J, Van Naarden Braun K, et al; Centers for Disease Control and Prevention. Prevalence and characteristics of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65(3):1-23.
3. Fung LK, Mahajan R, Nozzolillo A, et al. Pharmacologic treatment of severe irritability and problem behaviors in autism: a systematic review and meta-analysis. Pediatrics. 2016;137(suppl 2):S124-S135.
4. Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry. 2019;10:810.
5. Gabis LV, Ben-Hur R, Shefer S, et al. Improvement of language in children with autism with combined donepezil and choline treatment. J Mol Neurosci. 2019;69(2):224-234.
6. James BJ, Gales MA, Gales BJ. Bumetanide for autism spectrum disorder in children: a review of randomized controlled trials. Ann Pharmacother. 2019;53(5) 537-544.
7. Li C, Bai Y, Jin C, et al. Efficacy and safety of fluoxetine in autism spectrum disorder: a meta-analysis. Am J Ther. 2020;27(3):e312-e315.
8. Fallah MS, Shaikh MR, Neupane B, et al. Atypical antipsychotics for irritability in pediatric autism: a systematic review and network meta-analysis. J Child Adolesc Psychopharmacol. 2019;29(3):168-180.
9. Mazahery H, Conlon CA, Beck KL, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9-16.
10. Wink LK, Adams R, Horn PS, et al. A randomized placebo-controlled cross-over pilot study of riluzole for drug-refractory irritability in autism spectrum disorder. J Autism Dev Disord. 2018;48(9):3051-3060.