User login
Delirium in hospitalized patients: Risks and benefits of antipsychotics
Delirium is common in hospitalized patients and contributes to healthcare costs and poor patient outcomes, including death. Its diagnosis and management remain clinically challenging. Although consensus panel guidelines recommend antipsychotic medications to treat delirium when conservative measures fail, few head-to-head trials have been done to tell us which antipsychotic drug to select, and antipsychotic use poses risks in the elderly.
Here, we review the risks and benefits of using antipsychotic drugs to manage delirium and describe an approach to selecting and using 5 commonly used antipsychotics.
SCOPE OF THE PROBLEM
Delirium is common and serious, affecting 11% to 42% of patients hospitalized on general medical wards.1 The burden to the public and individual patient is extremely high. Delirium has been found to result in an additional $16,303 to $64,421 per delirious patient per year, with a subsequent total 1-year health-attributable cost between $38 billion and $152 billion in the United States.2 Furthermore, many patients who become delirious in the hospital lose their independence and are placed in long-term care facilities.3
Although delirium was originally thought to be a time-limited neurocognitive disorder, recent evidence shows that it persists much longer4 and that some patients never return to their previous level of function, suggesting that a single episode of delirium can significantly alter the course of an underlying dementia with the dramatic initiation of cognitive decline.3 Most alarmingly, delirium is associated with an increased rate of death.1
DSM-5 DEFINITION
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),5 delirium is a neurocognitive disorder characterized by the acute onset of disturbance in attention, awareness, and cognition that fluctuates in severity throughout the day and is the direct physiologic consequence of another medical condition. The cognitive impairment seen in delirium is typically global and can affect memory, orientation, language, visuospatial ability, and perception. Other prominent features include psychomotor disturbance, sleep-cycle derangement, and emotional lability.
The pathogenesis of delirium is not clearly delineated but may relate to cholinergic deficiency and dopaminergic excess.
THE FIRST STEPS: NONPHARMACOLOGIC MANAGEMENT
Inouye3 outlined a general 3-part approach to managing delirium:
Identify and address predisposing factors. All patients found to have an acute change in mental status should be evaluated for the underlying cause, with special attention to the most common causes, ie, infection, metabolic derangement, and substance intoxication and withdrawal. A thorough medication reconciliation should also be done to identify medications with psychoactive or anticholinergic effects.
Provide supportive care, eg, addressing volume and nutritional status, mobilizing the patient early, and giving prophylaxis against deep venous thrombosis.
Manage symptoms. Behavioral strategies should be instituted in every delirious patient and should include frequent reorientation, use of observers, encouragement of family involvement, avoidance of physical restraints and Foley catheters, use of vision and hearing aids, and normalizing the sleep-wake cycle.
ANTIPSYCHOTICS: ARE THEY SAFE AND EFFECTIVE?
The US Food and Drug Administration (FDA) has not approved any medications for delirium. However, multiple consensus statements, including those by the American Psychiatric Association,6 the Canadian Coalition for Seniors’ Mental Health,7 and the UK National Institute for Health and Care Excellence,8 advocate for psychopharmacologic management of delirium symptoms in the following situations:
- The patient is in significant distress from his or her symptoms
- The patient poses a safety risk to self or others
- The patient is impeding essential aspects of his or her medical care.
Guidelines from these organizations recommend antipsychotic medications as the first-line drugs for managing delirium symptoms not caused by substance withdrawal. Nevertheless, the use of antipsychotics in the management of delirium remains controversial. While a number of studies suggest these drugs are beneficial,9–11 others do not.12 These consensus panels advocate for the judicious use of antipsychotics, limited to the specific situations outlined above.
The use of antipsychotics in elderly and medically complex patients poses risks. One of the most significant safety concerns is increased risk of death due to adverse cardiac events caused by prolongation of the QT interval.
Antipsychotics, QT prolongation, and torsades de pointes
Most antipsychotics have the potential to prolong the time of ventricular depolarization and repolarization and the QT interval to some extent, which can lead to torsades de pointes.13 Other risk factors for prolonged QT interval and torsades de pointes include:
- Long QT syndrome (a genetic arrhythmia)
- Female sex
- Old age
- Electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia)
- Preexisting heart conditions such as bradycardia, left ventricular dysfunction, heart failure, mitral valve prolapse, and previous myocardial infarction
- Medical conditions that cause electrolyte derangements
- Medications, including antiarrhythmics, antibiotics (macrolides, quinolones), antifungals, antimalarials, antiemetics, some opioids (methadone), and most antipsychotics.
Haloperidol. Postmarketing analysis in 2007 found 73 cases of haloperidol-related torsades de pointes. However, many of these were confounded by other QT-prolonging medications and medical conditions.14
The QT-prolonging effect of haloperidol administered orally or intramuscularly is actually quite small. The equivalent oral dose of 15 mg of haloperidol (assuming 50% bioavailability) given orally or intramuscularly increases the corrected QT interval (QTc) by only 7 to 8 milliseconds. But intravenous haloperidol can cause much more significant QT prolongation: 8 of the 11 reported cases of fatal torsades de pointes occurred when haloperidol was given intravenously.14 Therefore, the FDA recommends cardiac monitoring for all patients receiving intravenous haloperidol.
Oral olanzapine, risperidone, and quetiapine prolong the QT interval approximately as much as oral haloperidol.
Aripiprazole has not been associated with significant QT prolongation.13
Atypical antipsychotics and stroke
The FDA has issued multiple warnings for prescribing antipsychotic medications in the elderly. In 2003, it warned prescribers of increased cerebrovascular adverse events, including stroke, in elderly patients with dementia who were treated with an atypical antipsychotic (risperidone, olanzapine, or aripiprazole) vs placebo.15
Atypical antipsychotics and risk of death
In 2005, the FDA issued a black-box warning about increased all-cause mortality risk in patients with dementia treated with atypical antipsychotics for behavioral disturbance (relative risk 1.6–1.7).16
This warning was likely based on a meta-analysis by Schneider et al17 of trials in which patients with dementia were randomized to receive either an atypical antipsychotic or placebo. The death rate was 3.5% in patients treated with an atypical antipsychotic vs 2.3% in patients treated with placebo, indicating a number needed to harm of 100. The most common causes of death were cardiovascular disease and pneumonia. However, the trials in this meta-analysis included only patients who were prescribed atypical antipsychotics for ongoing management of behavioral disturbances due to dementia in either the outpatient or nursing home setting. None of the trials looked at patients who were prescribed atypical antipsychotics for a limited time in a closely monitored inpatient setting.
Effectiveness of antipsychotics
While several studies since the FDA black-box warning have shown that antipsychotics are safe, the efficacy of these drugs in delirium management remains controversial.
In a 2016 meta-analysis, Kishi et al18 found that antipsychotics were superior to placebo in terms of response rate (defined as improvement of delirium severity rating scores), with a number needed to treat of 2.
In contrast, a meta-analysis by Neufeld et al12 found that antipsychotic use was not associated with a change in delirium duration, severity, or length of stay in the hospital or intensive care unit. However, the studies in this meta-analysis varied widely in age range, study design, drug comparison, and treatment strategy (with drugs given as both prophylaxis and treatment). Thus, the results are difficult to interpret.
Kishi et al18 found no difference in the incidence of death, extrapyramidal symptoms, akathisia, or QT prolongation between patients treated with antipsychotic drugs vs placebo.
In a prospective observational study, Hatta et al19 followed 2,453 inpatients who became delirious. Only 22 (0.9%) experienced adverse events attributable to antipsychotic use, the most common being aspiration pneumonia (0.7%), followed by cardiovascular events (0.2%). Notably, no patient died of antipsychotic-related events. In this study, the antipsychotic was stopped as soon as the delirium symptoms resolved, in most cases in 3 to 7 days.
Taken together, these studies indicate that despite the risk of QT prolongation with antipsychotic use and increased rates of morbidity with antipsychotic use in dementia, time-limited management of delirium with antipsychotics is effective9–11 and safe.
SELECTING AND USING ANTIPSYCHOTICS TO TREAT DELIRIUM
Identifying a single preferred agent is difficult, since we lack enough evidence from randomized controlled trials that directly compared the various antipsychotics used in delirium management.
Both typical and atypical antipsychotics are used in clinical practice to manage delirium. The typical antipsychotic most often used is haloperidol, while the most commonly used atypical antipsychotics for delirium include olanzapine, quetiapine, risperidone, and (more recently) aripiprazole.
The American Psychiatric Association guidelines6 suggest using haloperidol because it is the antipsychotic that has been most studied for delirium,20 and we have decades of experience with its use. Despite this, recent prospective studies have suggested that the atypical antipsychotics may be better because they have a faster onset of action and lower incidence of extrapyramidal symptoms.18,21
Because we lack enough head-to-head trials comparing the efficacy of the 5 most commonly used antipsychotics for the management of delirium, and because the prospective trials that do exist show equal efficacy across the antipsychotics studied,22 we suggest considering the unique pharmacologic properties of each drug within the patient’s clinical context when selecting which antipsychotic to use.
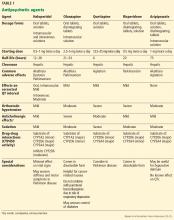
Table 123–25 summarizes some key characteristics of the 5 most commonly used antipsychotics.
Haloperidol
Haloperidol, a typical antipsychotic, is a potent antagonist of the dopamine D2 receptor.
Haloperidol has the advantage of having the strongest evidence base for use in delirium. In addition, it is available in oral, intravenous, and intramuscular dosage forms, and it has minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other medications.21
Intravenous haloperidol poses a significant risk of QT prolongation and so should be used judiciously in patients with preexisting cardiac conditions or other risk factors for QT prolongation as outlined above, and with careful cardiac monitoring. Parenteral haloperidol is approximately twice as potent as oral haloperidol.
Some evidence suggests a higher risk of acute dystonia and other extrapyramidal symptoms with haloperidol than with the atypical antipsychotics.21,26 In contrast, a 2013 prospective study showed that low doses of haloperidol (< 3.5 mg/day) did not result in a greater frequency of extrapyramidal symptoms.22 Nevertheless, if a patient has a history of extrapyramidal symptoms, haloperidol should likely be avoided in favor of an atypical antipsychotic.
Atypical antipsychotics
Olanzapine, quetiapine, and risperidone are atypical antipsychotics that, like haloperidol, antagonize the dopamine D2 receptor, but also have antagonist action at serotonin, histamine, and alpha-2 receptors. This multireceptor antagonism reduces the risk of extrapyramidal symptoms but increases the risk of orthostatic hypotension.
Quetiapine, in particular, imposes an unacceptably high risk of orthostatic hypotension and so is not recommended for use in delirium in the emergency department.27 Additionally, quetiapine is anticholinergic, raising concerns about constipation and urinary retention.
Although the association between fall risk and antipsychotic use remains controversial,28,29 a study found that olanzapine conferred a lower fall risk than quetiapine and risperidone.30
Of these drugs, only olanzapine is available in an intramuscular dosage form. Both risperidone and olanzapine are available in dissolvable tablets; however, they are not sublingually absorbed.
Randomized controlled trials have shown that olanzapine is effective in managing cancer-related nausea, and therefore it may be useful in managing delirium in oncology patients.31,32
Patients with Parkinson disease are exquisitely sensitive to the antidopaminergic effects of antipsychotics but are also vulnerable to delirium, so they present a unique treatment challenge. The agent of choice in patients with Parkinson disease is quetiapine, as multiple trials have shown it has no effect on the motor symptoms of Parkinson disease (reviewed by Desmarais et al in a systematic meta-analysis33).
Aripiprazole is increasingly used to manage delirium. Its mechanism of action differs from that of the other atypical antipsychotics, as it is a partial dopamine agonist. It is available in oral, orally dissolvable, and intramuscular forms. It appears to be slightly less effective than the other atypical antipsychotics,34 but it may be useful for hypoactive delirium as it is less sedating than the other agents.35 Because its effect on the QT interval is negligible, it may also be favored in patients who have a high baseline QTc or other predisposing factors for torsades de pointes.
BALANCING THE RISKS
Antipsychotic drugs have been shown to be effective and generally safe. Antipsychotics do prolong the QT interval. However, other than with intravenous administration of haloperidol, the absolute effect is minimal. Although large meta-analyses have shown a higher rate of all-cause mortality in elderly outpatients with dementia who are prescribed atypical antipsychotics, an increase in death rates has not been borne out by prospective studies focusing on hospitalized patients who receive low doses of antipsychotics for a limited time.
There are no head-to-head randomized controlled trials comparing the efficacy of all of the 5 most commonly used antipsychotics. Therefore, we suggest considering the unique psychopharmacologic properties of each agent within the patient’s clinical setting, specifically taking into account the risk of cardiac arrhythmia, risk of orthostasis and falls, history of extrapyramidal symptoms, other comorbidities such as Parkinson disease and cancer, and the desired route of administration.
At the time the patient is discharged, we recommend a careful medication reconciliation and discontinuation of the antipsychotic drug once delirium has resolved. Studies show that at least 26% of antipsychotics initiated in the hospital are continued after discharge.36,37
Current delirium consensus statements recommend limiting the use of antipsychotics to target patient distress, impediment of care, or safety, because of the putative risks of antipsychotic use in the elderly. However, a growing body of evidence shows that low-dose, time-limited antipsychotic use is safe and effective in the treatment of delirium. In fact, González et al found that delirium is an independent risk factor for death, and each 48-hour increase in delirium is associated with an increased mortality risk of 11%, suggesting that delay in treating delirium may actually increase the risk of death.38
Therefore, we must balance the risks of prescribing antipsychotics in medically vulnerable patients against the increasing burden of evidence supporting the serious risks of morbidity and mortality of delirium, as well as the costs. Much remains to be studied to optimize antipsychotic use in delirium.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006; 35:350–364.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Trzepacz P, Breitbart W, Franklin J, Levenson J, Martini DR, Wang P; American Psychiatric Association (APA). Practice guideline for the treatment of patients with delirium. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/delirium.pdf. Accessed July 13, 2017.
- Canadian Coalition for Seniors’ Mental Health. National guidelines for seniors’ mental health: the assessment and treatment of delirium. http://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_Delirium.pdf. Accessed July 13, 2017.
- National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. www.nice.org.uk/guidance/cg103. Accessed July 13, 2017.
- Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J Psychosom Res 2008; 65:273–282.
- Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009; 24:848–853.
- Devlin JW, Skrobik Y. Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harv Rev Psychiatry 2011; 19:59–67.
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64:705–714.
- Beach SR, Celano MC, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- US Food and Drug Administration (FDA). Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol decanoate and Haldol lactate). www.fda.gov/Drugs/DrugSafety/ucm085203.htm. Accessed July 13, 2017.
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval package for: Application Number: NDA 20-272/S-033, 20-588/S-021 & 21-444/S-004. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/020588_S021_RISPERDAL_TABLETS.pdf. Accessed July 13, 2017.
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed July 13, 2017.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943.
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87:767–774.
- Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry 2014; 29;253–262.
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996; 153:231–237.
- Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association For Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med 2012; 13:26–34.
- Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013; 13:240.
- American Psychiatric Association. Manual of Clinical Psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015.
- Conley RR, Kelly DL. Pharmacologic Treatment of Schizophrenia. 3rd ed. West Islip, NY: Professional Communications; 2007.
- American Psychiatric Association (APA). The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Psychiatric Care of the Medically Ill, 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011.
- Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 2015; 13:1079–1085.
- Currier GW, Trenton AJ, Walsh PG, van Wijngaarden E. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract 2006; 12:223–228.
- Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother 2012; 10:84–94.
- Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc 2013; 61: 715–722.
- Bozat-Emre S, Doupe M, Kozyrskyj AL, Grymonpre R, Mahmud SM. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int J Geriatr Psychiatry 2015; 30:842–850.
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011; 9:188–195.
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014; 722:180–186.
- Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol 2016; 29:227–236.
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry 2007; 68:1876–1885.
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003; 61:123–136.
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11:550–555.
- Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc 2016; 64:299–305.
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009; 50:234–238.
Delirium is common in hospitalized patients and contributes to healthcare costs and poor patient outcomes, including death. Its diagnosis and management remain clinically challenging. Although consensus panel guidelines recommend antipsychotic medications to treat delirium when conservative measures fail, few head-to-head trials have been done to tell us which antipsychotic drug to select, and antipsychotic use poses risks in the elderly.
Here, we review the risks and benefits of using antipsychotic drugs to manage delirium and describe an approach to selecting and using 5 commonly used antipsychotics.
SCOPE OF THE PROBLEM
Delirium is common and serious, affecting 11% to 42% of patients hospitalized on general medical wards.1 The burden to the public and individual patient is extremely high. Delirium has been found to result in an additional $16,303 to $64,421 per delirious patient per year, with a subsequent total 1-year health-attributable cost between $38 billion and $152 billion in the United States.2 Furthermore, many patients who become delirious in the hospital lose their independence and are placed in long-term care facilities.3
Although delirium was originally thought to be a time-limited neurocognitive disorder, recent evidence shows that it persists much longer4 and that some patients never return to their previous level of function, suggesting that a single episode of delirium can significantly alter the course of an underlying dementia with the dramatic initiation of cognitive decline.3 Most alarmingly, delirium is associated with an increased rate of death.1
DSM-5 DEFINITION
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),5 delirium is a neurocognitive disorder characterized by the acute onset of disturbance in attention, awareness, and cognition that fluctuates in severity throughout the day and is the direct physiologic consequence of another medical condition. The cognitive impairment seen in delirium is typically global and can affect memory, orientation, language, visuospatial ability, and perception. Other prominent features include psychomotor disturbance, sleep-cycle derangement, and emotional lability.
The pathogenesis of delirium is not clearly delineated but may relate to cholinergic deficiency and dopaminergic excess.
THE FIRST STEPS: NONPHARMACOLOGIC MANAGEMENT
Inouye3 outlined a general 3-part approach to managing delirium:
Identify and address predisposing factors. All patients found to have an acute change in mental status should be evaluated for the underlying cause, with special attention to the most common causes, ie, infection, metabolic derangement, and substance intoxication and withdrawal. A thorough medication reconciliation should also be done to identify medications with psychoactive or anticholinergic effects.
Provide supportive care, eg, addressing volume and nutritional status, mobilizing the patient early, and giving prophylaxis against deep venous thrombosis.
Manage symptoms. Behavioral strategies should be instituted in every delirious patient and should include frequent reorientation, use of observers, encouragement of family involvement, avoidance of physical restraints and Foley catheters, use of vision and hearing aids, and normalizing the sleep-wake cycle.
ANTIPSYCHOTICS: ARE THEY SAFE AND EFFECTIVE?
The US Food and Drug Administration (FDA) has not approved any medications for delirium. However, multiple consensus statements, including those by the American Psychiatric Association,6 the Canadian Coalition for Seniors’ Mental Health,7 and the UK National Institute for Health and Care Excellence,8 advocate for psychopharmacologic management of delirium symptoms in the following situations:
- The patient is in significant distress from his or her symptoms
- The patient poses a safety risk to self or others
- The patient is impeding essential aspects of his or her medical care.
Guidelines from these organizations recommend antipsychotic medications as the first-line drugs for managing delirium symptoms not caused by substance withdrawal. Nevertheless, the use of antipsychotics in the management of delirium remains controversial. While a number of studies suggest these drugs are beneficial,9–11 others do not.12 These consensus panels advocate for the judicious use of antipsychotics, limited to the specific situations outlined above.
The use of antipsychotics in elderly and medically complex patients poses risks. One of the most significant safety concerns is increased risk of death due to adverse cardiac events caused by prolongation of the QT interval.
Antipsychotics, QT prolongation, and torsades de pointes
Most antipsychotics have the potential to prolong the time of ventricular depolarization and repolarization and the QT interval to some extent, which can lead to torsades de pointes.13 Other risk factors for prolonged QT interval and torsades de pointes include:
- Long QT syndrome (a genetic arrhythmia)
- Female sex
- Old age
- Electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia)
- Preexisting heart conditions such as bradycardia, left ventricular dysfunction, heart failure, mitral valve prolapse, and previous myocardial infarction
- Medical conditions that cause electrolyte derangements
- Medications, including antiarrhythmics, antibiotics (macrolides, quinolones), antifungals, antimalarials, antiemetics, some opioids (methadone), and most antipsychotics.
Haloperidol. Postmarketing analysis in 2007 found 73 cases of haloperidol-related torsades de pointes. However, many of these were confounded by other QT-prolonging medications and medical conditions.14
The QT-prolonging effect of haloperidol administered orally or intramuscularly is actually quite small. The equivalent oral dose of 15 mg of haloperidol (assuming 50% bioavailability) given orally or intramuscularly increases the corrected QT interval (QTc) by only 7 to 8 milliseconds. But intravenous haloperidol can cause much more significant QT prolongation: 8 of the 11 reported cases of fatal torsades de pointes occurred when haloperidol was given intravenously.14 Therefore, the FDA recommends cardiac monitoring for all patients receiving intravenous haloperidol.
Oral olanzapine, risperidone, and quetiapine prolong the QT interval approximately as much as oral haloperidol.
Aripiprazole has not been associated with significant QT prolongation.13
Atypical antipsychotics and stroke
The FDA has issued multiple warnings for prescribing antipsychotic medications in the elderly. In 2003, it warned prescribers of increased cerebrovascular adverse events, including stroke, in elderly patients with dementia who were treated with an atypical antipsychotic (risperidone, olanzapine, or aripiprazole) vs placebo.15
Atypical antipsychotics and risk of death
In 2005, the FDA issued a black-box warning about increased all-cause mortality risk in patients with dementia treated with atypical antipsychotics for behavioral disturbance (relative risk 1.6–1.7).16
This warning was likely based on a meta-analysis by Schneider et al17 of trials in which patients with dementia were randomized to receive either an atypical antipsychotic or placebo. The death rate was 3.5% in patients treated with an atypical antipsychotic vs 2.3% in patients treated with placebo, indicating a number needed to harm of 100. The most common causes of death were cardiovascular disease and pneumonia. However, the trials in this meta-analysis included only patients who were prescribed atypical antipsychotics for ongoing management of behavioral disturbances due to dementia in either the outpatient or nursing home setting. None of the trials looked at patients who were prescribed atypical antipsychotics for a limited time in a closely monitored inpatient setting.
Effectiveness of antipsychotics
While several studies since the FDA black-box warning have shown that antipsychotics are safe, the efficacy of these drugs in delirium management remains controversial.
In a 2016 meta-analysis, Kishi et al18 found that antipsychotics were superior to placebo in terms of response rate (defined as improvement of delirium severity rating scores), with a number needed to treat of 2.
In contrast, a meta-analysis by Neufeld et al12 found that antipsychotic use was not associated with a change in delirium duration, severity, or length of stay in the hospital or intensive care unit. However, the studies in this meta-analysis varied widely in age range, study design, drug comparison, and treatment strategy (with drugs given as both prophylaxis and treatment). Thus, the results are difficult to interpret.
Kishi et al18 found no difference in the incidence of death, extrapyramidal symptoms, akathisia, or QT prolongation between patients treated with antipsychotic drugs vs placebo.
In a prospective observational study, Hatta et al19 followed 2,453 inpatients who became delirious. Only 22 (0.9%) experienced adverse events attributable to antipsychotic use, the most common being aspiration pneumonia (0.7%), followed by cardiovascular events (0.2%). Notably, no patient died of antipsychotic-related events. In this study, the antipsychotic was stopped as soon as the delirium symptoms resolved, in most cases in 3 to 7 days.
Taken together, these studies indicate that despite the risk of QT prolongation with antipsychotic use and increased rates of morbidity with antipsychotic use in dementia, time-limited management of delirium with antipsychotics is effective9–11 and safe.
SELECTING AND USING ANTIPSYCHOTICS TO TREAT DELIRIUM
Identifying a single preferred agent is difficult, since we lack enough evidence from randomized controlled trials that directly compared the various antipsychotics used in delirium management.
Both typical and atypical antipsychotics are used in clinical practice to manage delirium. The typical antipsychotic most often used is haloperidol, while the most commonly used atypical antipsychotics for delirium include olanzapine, quetiapine, risperidone, and (more recently) aripiprazole.
The American Psychiatric Association guidelines6 suggest using haloperidol because it is the antipsychotic that has been most studied for delirium,20 and we have decades of experience with its use. Despite this, recent prospective studies have suggested that the atypical antipsychotics may be better because they have a faster onset of action and lower incidence of extrapyramidal symptoms.18,21
Because we lack enough head-to-head trials comparing the efficacy of the 5 most commonly used antipsychotics for the management of delirium, and because the prospective trials that do exist show equal efficacy across the antipsychotics studied,22 we suggest considering the unique pharmacologic properties of each drug within the patient’s clinical context when selecting which antipsychotic to use.
Table 123–25 summarizes some key characteristics of the 5 most commonly used antipsychotics.
Haloperidol
Haloperidol, a typical antipsychotic, is a potent antagonist of the dopamine D2 receptor.
Haloperidol has the advantage of having the strongest evidence base for use in delirium. In addition, it is available in oral, intravenous, and intramuscular dosage forms, and it has minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other medications.21
Intravenous haloperidol poses a significant risk of QT prolongation and so should be used judiciously in patients with preexisting cardiac conditions or other risk factors for QT prolongation as outlined above, and with careful cardiac monitoring. Parenteral haloperidol is approximately twice as potent as oral haloperidol.
Some evidence suggests a higher risk of acute dystonia and other extrapyramidal symptoms with haloperidol than with the atypical antipsychotics.21,26 In contrast, a 2013 prospective study showed that low doses of haloperidol (< 3.5 mg/day) did not result in a greater frequency of extrapyramidal symptoms.22 Nevertheless, if a patient has a history of extrapyramidal symptoms, haloperidol should likely be avoided in favor of an atypical antipsychotic.
Atypical antipsychotics
Olanzapine, quetiapine, and risperidone are atypical antipsychotics that, like haloperidol, antagonize the dopamine D2 receptor, but also have antagonist action at serotonin, histamine, and alpha-2 receptors. This multireceptor antagonism reduces the risk of extrapyramidal symptoms but increases the risk of orthostatic hypotension.
Quetiapine, in particular, imposes an unacceptably high risk of orthostatic hypotension and so is not recommended for use in delirium in the emergency department.27 Additionally, quetiapine is anticholinergic, raising concerns about constipation and urinary retention.
Although the association between fall risk and antipsychotic use remains controversial,28,29 a study found that olanzapine conferred a lower fall risk than quetiapine and risperidone.30
Of these drugs, only olanzapine is available in an intramuscular dosage form. Both risperidone and olanzapine are available in dissolvable tablets; however, they are not sublingually absorbed.
Randomized controlled trials have shown that olanzapine is effective in managing cancer-related nausea, and therefore it may be useful in managing delirium in oncology patients.31,32
Patients with Parkinson disease are exquisitely sensitive to the antidopaminergic effects of antipsychotics but are also vulnerable to delirium, so they present a unique treatment challenge. The agent of choice in patients with Parkinson disease is quetiapine, as multiple trials have shown it has no effect on the motor symptoms of Parkinson disease (reviewed by Desmarais et al in a systematic meta-analysis33).
Aripiprazole is increasingly used to manage delirium. Its mechanism of action differs from that of the other atypical antipsychotics, as it is a partial dopamine agonist. It is available in oral, orally dissolvable, and intramuscular forms. It appears to be slightly less effective than the other atypical antipsychotics,34 but it may be useful for hypoactive delirium as it is less sedating than the other agents.35 Because its effect on the QT interval is negligible, it may also be favored in patients who have a high baseline QTc or other predisposing factors for torsades de pointes.
BALANCING THE RISKS
Antipsychotic drugs have been shown to be effective and generally safe. Antipsychotics do prolong the QT interval. However, other than with intravenous administration of haloperidol, the absolute effect is minimal. Although large meta-analyses have shown a higher rate of all-cause mortality in elderly outpatients with dementia who are prescribed atypical antipsychotics, an increase in death rates has not been borne out by prospective studies focusing on hospitalized patients who receive low doses of antipsychotics for a limited time.
There are no head-to-head randomized controlled trials comparing the efficacy of all of the 5 most commonly used antipsychotics. Therefore, we suggest considering the unique psychopharmacologic properties of each agent within the patient’s clinical setting, specifically taking into account the risk of cardiac arrhythmia, risk of orthostasis and falls, history of extrapyramidal symptoms, other comorbidities such as Parkinson disease and cancer, and the desired route of administration.
At the time the patient is discharged, we recommend a careful medication reconciliation and discontinuation of the antipsychotic drug once delirium has resolved. Studies show that at least 26% of antipsychotics initiated in the hospital are continued after discharge.36,37
Current delirium consensus statements recommend limiting the use of antipsychotics to target patient distress, impediment of care, or safety, because of the putative risks of antipsychotic use in the elderly. However, a growing body of evidence shows that low-dose, time-limited antipsychotic use is safe and effective in the treatment of delirium. In fact, González et al found that delirium is an independent risk factor for death, and each 48-hour increase in delirium is associated with an increased mortality risk of 11%, suggesting that delay in treating delirium may actually increase the risk of death.38
Therefore, we must balance the risks of prescribing antipsychotics in medically vulnerable patients against the increasing burden of evidence supporting the serious risks of morbidity and mortality of delirium, as well as the costs. Much remains to be studied to optimize antipsychotic use in delirium.
Delirium is common in hospitalized patients and contributes to healthcare costs and poor patient outcomes, including death. Its diagnosis and management remain clinically challenging. Although consensus panel guidelines recommend antipsychotic medications to treat delirium when conservative measures fail, few head-to-head trials have been done to tell us which antipsychotic drug to select, and antipsychotic use poses risks in the elderly.
Here, we review the risks and benefits of using antipsychotic drugs to manage delirium and describe an approach to selecting and using 5 commonly used antipsychotics.
SCOPE OF THE PROBLEM
Delirium is common and serious, affecting 11% to 42% of patients hospitalized on general medical wards.1 The burden to the public and individual patient is extremely high. Delirium has been found to result in an additional $16,303 to $64,421 per delirious patient per year, with a subsequent total 1-year health-attributable cost between $38 billion and $152 billion in the United States.2 Furthermore, many patients who become delirious in the hospital lose their independence and are placed in long-term care facilities.3
Although delirium was originally thought to be a time-limited neurocognitive disorder, recent evidence shows that it persists much longer4 and that some patients never return to their previous level of function, suggesting that a single episode of delirium can significantly alter the course of an underlying dementia with the dramatic initiation of cognitive decline.3 Most alarmingly, delirium is associated with an increased rate of death.1
DSM-5 DEFINITION
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5),5 delirium is a neurocognitive disorder characterized by the acute onset of disturbance in attention, awareness, and cognition that fluctuates in severity throughout the day and is the direct physiologic consequence of another medical condition. The cognitive impairment seen in delirium is typically global and can affect memory, orientation, language, visuospatial ability, and perception. Other prominent features include psychomotor disturbance, sleep-cycle derangement, and emotional lability.
The pathogenesis of delirium is not clearly delineated but may relate to cholinergic deficiency and dopaminergic excess.
THE FIRST STEPS: NONPHARMACOLOGIC MANAGEMENT
Inouye3 outlined a general 3-part approach to managing delirium:
Identify and address predisposing factors. All patients found to have an acute change in mental status should be evaluated for the underlying cause, with special attention to the most common causes, ie, infection, metabolic derangement, and substance intoxication and withdrawal. A thorough medication reconciliation should also be done to identify medications with psychoactive or anticholinergic effects.
Provide supportive care, eg, addressing volume and nutritional status, mobilizing the patient early, and giving prophylaxis against deep venous thrombosis.
Manage symptoms. Behavioral strategies should be instituted in every delirious patient and should include frequent reorientation, use of observers, encouragement of family involvement, avoidance of physical restraints and Foley catheters, use of vision and hearing aids, and normalizing the sleep-wake cycle.
ANTIPSYCHOTICS: ARE THEY SAFE AND EFFECTIVE?
The US Food and Drug Administration (FDA) has not approved any medications for delirium. However, multiple consensus statements, including those by the American Psychiatric Association,6 the Canadian Coalition for Seniors’ Mental Health,7 and the UK National Institute for Health and Care Excellence,8 advocate for psychopharmacologic management of delirium symptoms in the following situations:
- The patient is in significant distress from his or her symptoms
- The patient poses a safety risk to self or others
- The patient is impeding essential aspects of his or her medical care.
Guidelines from these organizations recommend antipsychotic medications as the first-line drugs for managing delirium symptoms not caused by substance withdrawal. Nevertheless, the use of antipsychotics in the management of delirium remains controversial. While a number of studies suggest these drugs are beneficial,9–11 others do not.12 These consensus panels advocate for the judicious use of antipsychotics, limited to the specific situations outlined above.
The use of antipsychotics in elderly and medically complex patients poses risks. One of the most significant safety concerns is increased risk of death due to adverse cardiac events caused by prolongation of the QT interval.
Antipsychotics, QT prolongation, and torsades de pointes
Most antipsychotics have the potential to prolong the time of ventricular depolarization and repolarization and the QT interval to some extent, which can lead to torsades de pointes.13 Other risk factors for prolonged QT interval and torsades de pointes include:
- Long QT syndrome (a genetic arrhythmia)
- Female sex
- Old age
- Electrolyte abnormalities (hypokalemia, hypocalcemia, hypomagnesemia)
- Preexisting heart conditions such as bradycardia, left ventricular dysfunction, heart failure, mitral valve prolapse, and previous myocardial infarction
- Medical conditions that cause electrolyte derangements
- Medications, including antiarrhythmics, antibiotics (macrolides, quinolones), antifungals, antimalarials, antiemetics, some opioids (methadone), and most antipsychotics.
Haloperidol. Postmarketing analysis in 2007 found 73 cases of haloperidol-related torsades de pointes. However, many of these were confounded by other QT-prolonging medications and medical conditions.14
The QT-prolonging effect of haloperidol administered orally or intramuscularly is actually quite small. The equivalent oral dose of 15 mg of haloperidol (assuming 50% bioavailability) given orally or intramuscularly increases the corrected QT interval (QTc) by only 7 to 8 milliseconds. But intravenous haloperidol can cause much more significant QT prolongation: 8 of the 11 reported cases of fatal torsades de pointes occurred when haloperidol was given intravenously.14 Therefore, the FDA recommends cardiac monitoring for all patients receiving intravenous haloperidol.
Oral olanzapine, risperidone, and quetiapine prolong the QT interval approximately as much as oral haloperidol.
Aripiprazole has not been associated with significant QT prolongation.13
Atypical antipsychotics and stroke
The FDA has issued multiple warnings for prescribing antipsychotic medications in the elderly. In 2003, it warned prescribers of increased cerebrovascular adverse events, including stroke, in elderly patients with dementia who were treated with an atypical antipsychotic (risperidone, olanzapine, or aripiprazole) vs placebo.15
Atypical antipsychotics and risk of death
In 2005, the FDA issued a black-box warning about increased all-cause mortality risk in patients with dementia treated with atypical antipsychotics for behavioral disturbance (relative risk 1.6–1.7).16
This warning was likely based on a meta-analysis by Schneider et al17 of trials in which patients with dementia were randomized to receive either an atypical antipsychotic or placebo. The death rate was 3.5% in patients treated with an atypical antipsychotic vs 2.3% in patients treated with placebo, indicating a number needed to harm of 100. The most common causes of death were cardiovascular disease and pneumonia. However, the trials in this meta-analysis included only patients who were prescribed atypical antipsychotics for ongoing management of behavioral disturbances due to dementia in either the outpatient or nursing home setting. None of the trials looked at patients who were prescribed atypical antipsychotics for a limited time in a closely monitored inpatient setting.
Effectiveness of antipsychotics
While several studies since the FDA black-box warning have shown that antipsychotics are safe, the efficacy of these drugs in delirium management remains controversial.
In a 2016 meta-analysis, Kishi et al18 found that antipsychotics were superior to placebo in terms of response rate (defined as improvement of delirium severity rating scores), with a number needed to treat of 2.
In contrast, a meta-analysis by Neufeld et al12 found that antipsychotic use was not associated with a change in delirium duration, severity, or length of stay in the hospital or intensive care unit. However, the studies in this meta-analysis varied widely in age range, study design, drug comparison, and treatment strategy (with drugs given as both prophylaxis and treatment). Thus, the results are difficult to interpret.
Kishi et al18 found no difference in the incidence of death, extrapyramidal symptoms, akathisia, or QT prolongation between patients treated with antipsychotic drugs vs placebo.
In a prospective observational study, Hatta et al19 followed 2,453 inpatients who became delirious. Only 22 (0.9%) experienced adverse events attributable to antipsychotic use, the most common being aspiration pneumonia (0.7%), followed by cardiovascular events (0.2%). Notably, no patient died of antipsychotic-related events. In this study, the antipsychotic was stopped as soon as the delirium symptoms resolved, in most cases in 3 to 7 days.
Taken together, these studies indicate that despite the risk of QT prolongation with antipsychotic use and increased rates of morbidity with antipsychotic use in dementia, time-limited management of delirium with antipsychotics is effective9–11 and safe.
SELECTING AND USING ANTIPSYCHOTICS TO TREAT DELIRIUM
Identifying a single preferred agent is difficult, since we lack enough evidence from randomized controlled trials that directly compared the various antipsychotics used in delirium management.
Both typical and atypical antipsychotics are used in clinical practice to manage delirium. The typical antipsychotic most often used is haloperidol, while the most commonly used atypical antipsychotics for delirium include olanzapine, quetiapine, risperidone, and (more recently) aripiprazole.
The American Psychiatric Association guidelines6 suggest using haloperidol because it is the antipsychotic that has been most studied for delirium,20 and we have decades of experience with its use. Despite this, recent prospective studies have suggested that the atypical antipsychotics may be better because they have a faster onset of action and lower incidence of extrapyramidal symptoms.18,21
Because we lack enough head-to-head trials comparing the efficacy of the 5 most commonly used antipsychotics for the management of delirium, and because the prospective trials that do exist show equal efficacy across the antipsychotics studied,22 we suggest considering the unique pharmacologic properties of each drug within the patient’s clinical context when selecting which antipsychotic to use.
Table 123–25 summarizes some key characteristics of the 5 most commonly used antipsychotics.
Haloperidol
Haloperidol, a typical antipsychotic, is a potent antagonist of the dopamine D2 receptor.
Haloperidol has the advantage of having the strongest evidence base for use in delirium. In addition, it is available in oral, intravenous, and intramuscular dosage forms, and it has minimal effects on vital signs, negligible anticholinergic activity, and minimal interactions with other medications.21
Intravenous haloperidol poses a significant risk of QT prolongation and so should be used judiciously in patients with preexisting cardiac conditions or other risk factors for QT prolongation as outlined above, and with careful cardiac monitoring. Parenteral haloperidol is approximately twice as potent as oral haloperidol.
Some evidence suggests a higher risk of acute dystonia and other extrapyramidal symptoms with haloperidol than with the atypical antipsychotics.21,26 In contrast, a 2013 prospective study showed that low doses of haloperidol (< 3.5 mg/day) did not result in a greater frequency of extrapyramidal symptoms.22 Nevertheless, if a patient has a history of extrapyramidal symptoms, haloperidol should likely be avoided in favor of an atypical antipsychotic.
Atypical antipsychotics
Olanzapine, quetiapine, and risperidone are atypical antipsychotics that, like haloperidol, antagonize the dopamine D2 receptor, but also have antagonist action at serotonin, histamine, and alpha-2 receptors. This multireceptor antagonism reduces the risk of extrapyramidal symptoms but increases the risk of orthostatic hypotension.
Quetiapine, in particular, imposes an unacceptably high risk of orthostatic hypotension and so is not recommended for use in delirium in the emergency department.27 Additionally, quetiapine is anticholinergic, raising concerns about constipation and urinary retention.
Although the association between fall risk and antipsychotic use remains controversial,28,29 a study found that olanzapine conferred a lower fall risk than quetiapine and risperidone.30
Of these drugs, only olanzapine is available in an intramuscular dosage form. Both risperidone and olanzapine are available in dissolvable tablets; however, they are not sublingually absorbed.
Randomized controlled trials have shown that olanzapine is effective in managing cancer-related nausea, and therefore it may be useful in managing delirium in oncology patients.31,32
Patients with Parkinson disease are exquisitely sensitive to the antidopaminergic effects of antipsychotics but are also vulnerable to delirium, so they present a unique treatment challenge. The agent of choice in patients with Parkinson disease is quetiapine, as multiple trials have shown it has no effect on the motor symptoms of Parkinson disease (reviewed by Desmarais et al in a systematic meta-analysis33).
Aripiprazole is increasingly used to manage delirium. Its mechanism of action differs from that of the other atypical antipsychotics, as it is a partial dopamine agonist. It is available in oral, orally dissolvable, and intramuscular forms. It appears to be slightly less effective than the other atypical antipsychotics,34 but it may be useful for hypoactive delirium as it is less sedating than the other agents.35 Because its effect on the QT interval is negligible, it may also be favored in patients who have a high baseline QTc or other predisposing factors for torsades de pointes.
BALANCING THE RISKS
Antipsychotic drugs have been shown to be effective and generally safe. Antipsychotics do prolong the QT interval. However, other than with intravenous administration of haloperidol, the absolute effect is minimal. Although large meta-analyses have shown a higher rate of all-cause mortality in elderly outpatients with dementia who are prescribed atypical antipsychotics, an increase in death rates has not been borne out by prospective studies focusing on hospitalized patients who receive low doses of antipsychotics for a limited time.
There are no head-to-head randomized controlled trials comparing the efficacy of all of the 5 most commonly used antipsychotics. Therefore, we suggest considering the unique psychopharmacologic properties of each agent within the patient’s clinical setting, specifically taking into account the risk of cardiac arrhythmia, risk of orthostasis and falls, history of extrapyramidal symptoms, other comorbidities such as Parkinson disease and cancer, and the desired route of administration.
At the time the patient is discharged, we recommend a careful medication reconciliation and discontinuation of the antipsychotic drug once delirium has resolved. Studies show that at least 26% of antipsychotics initiated in the hospital are continued after discharge.36,37
Current delirium consensus statements recommend limiting the use of antipsychotics to target patient distress, impediment of care, or safety, because of the putative risks of antipsychotic use in the elderly. However, a growing body of evidence shows that low-dose, time-limited antipsychotic use is safe and effective in the treatment of delirium. In fact, González et al found that delirium is an independent risk factor for death, and each 48-hour increase in delirium is associated with an increased mortality risk of 11%, suggesting that delay in treating delirium may actually increase the risk of death.38
Therefore, we must balance the risks of prescribing antipsychotics in medically vulnerable patients against the increasing burden of evidence supporting the serious risks of morbidity and mortality of delirium, as well as the costs. Much remains to be studied to optimize antipsychotic use in delirium.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006; 35:350–364.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Trzepacz P, Breitbart W, Franklin J, Levenson J, Martini DR, Wang P; American Psychiatric Association (APA). Practice guideline for the treatment of patients with delirium. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/delirium.pdf. Accessed July 13, 2017.
- Canadian Coalition for Seniors’ Mental Health. National guidelines for seniors’ mental health: the assessment and treatment of delirium. http://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_Delirium.pdf. Accessed July 13, 2017.
- National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. www.nice.org.uk/guidance/cg103. Accessed July 13, 2017.
- Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J Psychosom Res 2008; 65:273–282.
- Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009; 24:848–853.
- Devlin JW, Skrobik Y. Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harv Rev Psychiatry 2011; 19:59–67.
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64:705–714.
- Beach SR, Celano MC, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- US Food and Drug Administration (FDA). Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol decanoate and Haldol lactate). www.fda.gov/Drugs/DrugSafety/ucm085203.htm. Accessed July 13, 2017.
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval package for: Application Number: NDA 20-272/S-033, 20-588/S-021 & 21-444/S-004. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/020588_S021_RISPERDAL_TABLETS.pdf. Accessed July 13, 2017.
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed July 13, 2017.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943.
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87:767–774.
- Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry 2014; 29;253–262.
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996; 153:231–237.
- Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association For Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med 2012; 13:26–34.
- Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013; 13:240.
- American Psychiatric Association. Manual of Clinical Psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015.
- Conley RR, Kelly DL. Pharmacologic Treatment of Schizophrenia. 3rd ed. West Islip, NY: Professional Communications; 2007.
- American Psychiatric Association (APA). The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Psychiatric Care of the Medically Ill, 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011.
- Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 2015; 13:1079–1085.
- Currier GW, Trenton AJ, Walsh PG, van Wijngaarden E. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract 2006; 12:223–228.
- Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother 2012; 10:84–94.
- Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc 2013; 61: 715–722.
- Bozat-Emre S, Doupe M, Kozyrskyj AL, Grymonpre R, Mahmud SM. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int J Geriatr Psychiatry 2015; 30:842–850.
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011; 9:188–195.
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014; 722:180–186.
- Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol 2016; 29:227–236.
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry 2007; 68:1876–1885.
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003; 61:123–136.
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11:550–555.
- Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc 2016; 64:299–305.
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009; 50:234–238.
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006; 35:350–364.
- Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168:27–32.
- Inouye SK. Delirium in older persons. N Engl J Med 2006; 354:1157–1165.
- Levkoff SE, Evans DA, Liptzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
- Trzepacz P, Breitbart W, Franklin J, Levenson J, Martini DR, Wang P; American Psychiatric Association (APA). Practice guideline for the treatment of patients with delirium. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/delirium.pdf. Accessed July 13, 2017.
- Canadian Coalition for Seniors’ Mental Health. National guidelines for seniors’ mental health: the assessment and treatment of delirium. http://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_Delirium.pdf. Accessed July 13, 2017.
- National Institute for Health and Care Excellence (NICE). Delirium: prevention, diagnosis and management. www.nice.org.uk/guidance/cg103. Accessed July 13, 2017.
- Bourne RS, Tahir TA, Borthwick M, Sampson EL. Drug treatment of delirium: past, present and future. J Psychosom Res 2008; 65:273–282.
- Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults—a systematic evidence review. J Gen Intern Med 2009; 24:848–853.
- Devlin JW, Skrobik Y. Antipsychotics for the prevention and treatment of delirium in the intensive care unit: what is their role? Harv Rev Psychiatry 2011; 19:59–67.
- Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc 2016; 64:705–714.
- Beach SR, Celano MC, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54:1–13.
- US Food and Drug Administration (FDA). Information for healthcare professionals: haloperidol (marketed as Haldol, Haldol decanoate and Haldol lactate). www.fda.gov/Drugs/DrugSafety/ucm085203.htm. Accessed July 13, 2017.
- US Food and Drug Administration Center for Drug Evaluation and Research. Approval package for: Application Number: NDA 20-272/S-033, 20-588/S-021 & 21-444/S-004. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/020588_S021_RISPERDAL_TABLETS.pdf. Accessed July 13, 2017.
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Accessed July 13, 2017.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943.
- Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016; 87:767–774.
- Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry 2014; 29;253–262.
- Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry 1996; 153:231–237.
- Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association For Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med 2012; 13:26–34.
- Yoon HJ, Park KM, Choi WJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013; 13:240.
- American Psychiatric Association. Manual of Clinical Psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing; 2015.
- Conley RR, Kelly DL. Pharmacologic Treatment of Schizophrenia. 3rd ed. West Islip, NY: Professional Communications; 2007.
- American Psychiatric Association (APA). The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Psychiatric Care of the Medically Ill, 2nd ed. Arlington, VA: American Psychiatric Publishing; 2011.
- Boettger S, Jenewein J, Breitbart W. Haloperidol, risperidone, olanzapine and aripiprazole in the management of delirium: a comparison of efficacy, safety, and side effects. Palliat Support Care 2015; 13:1079–1085.
- Currier GW, Trenton AJ, Walsh PG, van Wijngaarden E. A pilot, open-label study of quetiapine for treatment of moderate psychotic agitation in the emergency setting. J Psychiatr Pract 2006; 12:223–228.
- Chatterjee S, Chen H, Johnson ML, Aparasu RR. Risk of falls and fractures in older adults using atypical antipsychotic agents: a propensity score-adjusted, retrospective cohort study. Am J Geriatr Pharmacother 2012; 10:84–94.
- Rigler SK, Shireman TI, Cook-Wiens GJ, et al. Fracture risk in nursing home residents initiating antipsychotic medications. J Am Geriatr Soc 2013; 61: 715–722.
- Bozat-Emre S, Doupe M, Kozyrskyj AL, Grymonpre R, Mahmud SM. Atypical antipsychotic drug use and falls among nursing home residents in Winnipeg, Canada. Int J Geriatr Psychiatry 2015; 30:842–850.
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011; 9:188–195.
- Navari RM. Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 2014; 722:180–186.
- Desmarais P, Massoud F, Filion J, Nguyen QD, Bajsarowicz P. Quetiapine for psychosis in Parkinson disease and neurodegenerative Parkinsonian disorders: a systematic review. J Geriatr Psychiatry Neurol 2016; 29:227–236.
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry 2007; 68:1876–1885.
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003; 61:123–136.
- Loh KP, Ramdass S, Garb JL, et al. Long-term outcomes of elders discharged on antipsychotics. J Hosp Med 2016; 11:550–555.
- Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic use in hospitalized adults: rates, indications, and predictors. J Am Geriatr Soc 2016; 64:299–305.
- González M, Martínez G, Calderón J, et al. Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics 2009; 50:234–238.
KEY POINTS
- Delirium is common in hospitalized patients and often leads to loss of independence and nursing-home placement.
- The first-line treatment is to identify and address predisposing factors, provide supportive care, and manage symptoms through behavioral strategies.
- Most antipsychotic medications can prolong the QT interval and thus pose a risk for torsades de pointes. The effect is greatest with intravenous haloperidol and least with aripiprazole.
- Lacking head-to-head trials of antipsychotics, we suggest selecting the drug based on its pharmacologic properties and the patient’s clinical context.