User login
Which test for CAD should be used in patients with left bundle branch block?
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
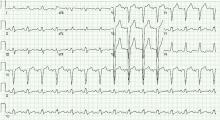
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
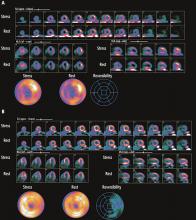
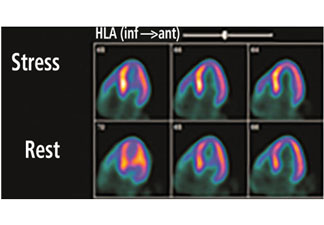
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
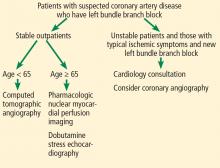
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
A 62-year-old woman with hypertension and type 2 diabetes mellitus has been experiencing shortness of breath on exertion and chest discomfort for 2 months. Her hypertension has been suboptimally controlled, and her most recent hemoglobin A1c measurement was 7.0%. She has never smoked and has no family history of premature coronary artery disease (CAD). She is otherwise well and walks for 30 minutes 3 times per week. A 12-lead electrocardiogram demonstrated normal sinus rhythm with left bundle branch block. Her physician suspects she has CAD. What testing does this patient need?
LIMITED DATA, GUIDELINES
For clinicians investigating suspected obstructive CAD in patients with left bundle branch block on resting electrocardiography, the data and guidelines are limited regarding the optimal noninvasive tests and how to interpret them.
Here, we present a practical review of the diagnostic utility of exercise stress electrocardiography, exercise stress echocardiography, dobutamine stress echocardiography, nuclear myocardial perfusion imaging, and computed tomographic (CT) angiography for assessing suspected obstructive CAD in patients with resting left bundle branch block.
WHAT IS LEFT BUNDLE BRANCH BLOCK?
In left bundle branch block, as the name implies, electrical conduction along the left bundle branch is blocked or delayed. Ventricular activation therefore begins in the right ventricle and the right side of the interventricular septum.1 Transseptal activation from the right ventricle to the left ventricle is slow, because it is transmyocardial.1 Left ventricular basal and posterolateral wall segments become activated last.1 Due to delay in the onset of left ventricular contraction, ventricular contraction is dyssynchronous. Classically, interventricular septal motion during systole has been described as paradoxical, with anterior septal motion.2–4
On electrocardiography, the QRS duration is widened (≥ 120 ms), with a distinctive morphology as shown in Figure 1. Left bundle branch block makes it difficult to accurately assess for dynamic ST-segment changes with exercise, rendering exercise stress electrocardiography a suboptimal test for obstructive CAD if left bundle branch block is present.
LEFT BUNDLE BRANCH BLOCK AND RISK OF DEATH
Although left bundle branch block can be an isolated finding, it can also be associated with underlying obstructive CAD5 or cardiomyopathy.6 When it occurs at rest, the risk of death from a cardiovascular event is 3 to 4 times higher.7 However, the exact incidence of significant obstructive CAD in asymptomatic patients with incidentally detected left bundle branch block is unknown.
Acute left bundle branch block accompanying acute myocardial infarction is associated with a high risk of death. Hindman et al,8 in a 1978 multicenter study, described 432 patients with acute myocardial infarction and left or right bundle branch block. In the 163 patients who had left bundle branch block, the in-hospital mortality rate was 24% and the 1-year mortality rate was 32%.
Freedman et al9 in 1987 reviewed 15,609 patients with chronic CAD who underwent coronary angiography, of whom 522 had left or right bundle branch block. During a follow-up of nearly 5 years, 2,386 patients died. The actuarial probability of death at 2 years in patients with left bundle branch block was more than 5 times that of patients without it (P < .0001).
During 18 years of observation in the Framingham study,10 55 participants developed left bundle branch block, at a mean age at onset of 62. Twenty-six (48%) of these participants developed clinically significant CAD or heart failure coincident with or subsequent to the onset of left bundle branch block. Fifty percent of the participants who developed left bundle branch block died of cardiovascular disease within 10 years of its onset.
EXERCISE STRESS ELECTROCARDIOGRAPHY
Exercise stress electrocardiography, although valuable for assessing functional capacity, cannot be used to diagnose obstructive CAD in patients with left bundle branch block.11
EXERCISE STRESS ECHOCARDIOGRAPHY
Exercise stress echocardiography is proven and widely used for assessing myocardial ischemia in patients with suspected obstructive CAD. But the data are limited on its diagnostic utility in patients with left bundle branch block. Until recently, recommendations for its use in this situation were based on only 1 small study.12
Peteiro et al12 in 2000 described 35 patients who underwent exercise stress echocardiography and coronary angiography. Detection of wall-motion abnormalities had high sensitivity (76%), specificity (83%), and diagnostic accuracy (80%).
Of note, 8 (23%) of the patients could not achieve at least 85% of the maximum predicted heart rate, and for them, the study was not diagnostic for ischemia. (Technically, the study is said to be nondiagnostic when the patient fails to achieve the target heart rate of at least 85% of the maximum predicted heart rate.)
Additionally, 18 of the 35 patients—over half—had a decrease in left ventricular ejection fraction in response to exercise. These 18 patients included 12 of the 17 patients with obstructive CAD and 6 of the 18 patients without obstructive CAD.12 It is unclear whether a significant proportion of these 18 patients would have been otherwise categorized as having a globally abnormal left ventricular contractile response to exercise according to contemporary (2007) reporting standards.13
Xu et al14,15 in 2016 examined the diagnostic utility of exercise stress echocardiography in assessing suspected obstructive CAD in 191 patients with resting left bundle branch block; 17 patients who failed to achieve a heart rate of at least 85% of the age-predicted maximum heart rate were excluded. Of the remaining 174 patients, 82 demonstrated a normal left ventricular contractile response to exercise and 92 had an abnormal response. In the abnormal group, 70 patients had a globally abnormal response, and 22 patients had a regional ischemic response. Of those who had a globally abnormal left ventricular contractile response who subsequently underwent angiography, only 30% were found to have obstructive CAD.
Although the sensitivity of exercise stress echocardiography was high (94%), its specificity and diagnostic accuracy were poor (specificity 21%, diagnostic accuracy 52%).14,15 These results suggest that for patients with resting left bundle branch block undergoing exercise stress echocardiography, obstructive CAD cannot be reliably diagnosed in those who develop a globally abnormal left ventricular contractile response. Therefore, an alternative imaging strategy should be considered.
DOBUTAMINE STRESS ECHOCARDIOGRAPHY
The evidence base for dobutamine stress echocardiography in patients with left bundle branch block is more robust than that for exercise stress echocardiography.
Geleijnse et al1 studied 64 patients with left bundle branch block undergoing dobutamine stress echocardiography who also underwent coronary angiography. Dobutamine stress echocardiography was moderately sensitive for detecting anterior and posterior myocardial wall ischemia (60% and 67%, respectively). Its specificity and diagnostic accuracy were high, at 94% and 98%, respectively.
Yanik et al16 studied 30 patients with left bundle branch block undergoing both dobutamine stress echocardiography and coronary angiography. The sensitivity of dobutamine stress echocardiography for identifying ischemia in the left anterior descending territory was 82%, the specificity was 95%, and the diagnostic accuracy was 90%. For identifying ischemia in the circumflex and right coronary artery territories, the sensitivity was 88%, specificity 96%, and accuracy 93%.
Mairesse et al17 studied 24 patients with left bundle branch block undergoing dobutamine stress echocardiography, myocardial perfusion tomography, and coronary angiography. Dobutamine stress echocardiography performed well in detecting ischemia in the left anterior descending territory, with a sensitivity of 83%, specificity 92%, and diagnostic accuracy 87%.
Of note, the available data come from very small studies published more than 15 years ago, and pharmacologic stress testing cannot provide the very important prognostic information derived from treadmill testing.
NUCLEAR MYOCARDIAL PERFUSION IMAGING
Exercise nuclear single-photon emission computed tomography (SPECT) myocardial perfusion imaging in patients with left bundle branch block is challenging, due to the development of septal perfusion defects at rest and during exercise in the absence of obstructive disease in the left anterior descending artery (Figure 2).18,19 Asynchronous contraction of the septum, with resulting compression of the septal arteries, decreased flow demands to the septal region, and attenuation artifacts are possible explanations for this phenomenon.20
Pharmacologic stress has been reported to improve the diagnostic accuracy of SPECT myocardial perfusion imaging.21
Biagini et al,21 in a meta-analysis of noninvasive techniques for diagnosing CAD in patients with left bundle branch block, found 1,785 patients from 39 studies who underwent nuclear myocardial perfusion imaging (48.8% with exercise, 41.9% with pharmacologic stress). Overall, sensitivity was high for both exercise and pharmacologic stress (92.9% and 88.5%). However, the reported specificity with exercise stress was significantly lower than with pharmacologic stress (23.3% vs 74.2%, P < .01).
Nuclear positron-emission tomography (PET) may further improve the diagnostic utility of nuclear myocardial perfusion imaging in patients with left bundle branch block. In a study of 440 patients with left bundle branch block undergoing myocardial perfusion imaging, 67 underwent PET and 373 underwent SPECT.22 Possible septal perfusion artifacts were significantly less common with PET than with SPECT (1.5% vs 19.3%, P < .001).
CT ANGIOGRAPHY
CT angiography has a high sensitivity and specificity for detecting significant obstructive CAD.23,24 Machines with 320 detector rows have been reported to have a sensitivity of 94% and specificity of 87% for detecting significant CAD and are not affected by resting left bundle branch block.25
Of note, coronary artery calcification increases in older patients, especially those age 65 and older,26 and this confers a higher likelihood of “bystander” CAD. Significant coronary artery calcification limits the diagnostic accuracy of multidetector cardiac CT. Additionally, the detection of bystander CAD leads to positive findings of uncertain clinical significance.
CURRENT GUIDELINES
Exercise stress echocardiography
American College of Cardiology Foundation/American Heart Association guidelines for diagnosis and management of patients with stable ischemic heart disease recommend exercise stress echocardiography for patients with an intermediate to high pretest probability of ischemic heart disease who have an uninterpretable electrocardiogram and at least moderate physical functioning or no disabling comorbidity (class 1 indication, level of evidence B).11
Current American Society of Echocardiography guidelines also support exercise stress echocardiography as an appropriate test for suspected obstructive CAD in patients with resting left bundle branch block.27 However, this recommendation is based on limited data.
Pharmacologic stress nuclear myocardial perfusion imaging
American Society of Nuclear Cardiology guidelines endorse pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators for evaluating suspected obstructive CAD in patients with resting left bundle branch block.28,29
THE POSSIBLE HARMS OF TESTING
Although current guidelines recommend it, recent data show that exercise stress echocardiography has poor specificity and diagnostic accuracy for significant obstructive CAD in patients with resting left bundle branch block. And performing this test in patients with left bundle branch block may result in further downstream investigations.
Based on limited data from a small number of studies published more than 15 years ago, dobutamine stress echocardiography has moderate sensitivity and specificity for significant CAD in patients with resting left bundle branch block. However, this test does not provide functional information about the patient’s exercise performance.
Pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators is an appropriate investigation strategy. However, radiation exposure is a limitation.30
CT angiography can assess for significant obstructive CAD in patients with resting left bundle branch block. However, its diagnostic accuracy can be affected by coronary calcification in older patients. Additionally, each scan is associated with a small amount of radiation exposure,31 and a small number of patients will have a true contrast allergy.32
CLINICAL BOTTOM LINE
For patients with typical ischemic symptoms and new left bundle branch block on electrocardiography, specialist cardiology consultation should be sought, with consideration given to proceeding directly to coronary angiography. For stable outpatients, we propose the following diagnostic approach (Figure 3).
Exercise stress echocardiography is recommended by current guidelines, but it cannot reliably detect significant obstructive CAD in patients with resting left bundle branch block—its specificity and diagnostic accuracy are poor.14,15 Alternative imaging strategies include CT angiography, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators, and dobutamine stress echocardiography.
For investigating suspected obstructive CAD in patients with resting left bundle branch block, we propose CT angiography as the first-line imaging test for patients under age 65 and pharmacologic stress nuclear myocardial perfusion imaging using coronary vasodilators or dobutamine stress echocardiography for those age 65 and older. For patients who cannot tolerate contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography may be used as alternatives.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
- Geleijnse ML, Vigna C, Kasprzak JD, et al. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J 2000; 21:1666–1673.
- Dillon JC, Chang S, Feigenbaum H. Echocardiographic manifestations of left bundle branch block. Circulation 1974; 49:876–880.
- Abbasi AS, Eber LM, Macalpin RN, Kattus AA. Paradoxical motion of interventricular septum in left bundle branch block. Circulation 1974; 49:423–427.
- McDonald IG. Echocardiographic demonstration of abnormal motion of the interventricular septum in left bundle branch block. Circulation 1973; 48:272–280.
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009; 2:251–259.
- Vaillant C, Martins RP, Donal E, et al. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol 2013; 61:1089–1095.
- Schneider JF, Thomas HE Jr, Sorlie P, Kreger BE, McNamara PM, Kannel WB. Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 1981; 47:931–940.
- Hindman MC, Wagner GS, JaRo M, et al. The clinical significance of bundle branch block complicating acute myocardial infarction. Circulation 1978; 58:689–699.
- Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 1987; 10:73–80.
- Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med 1979; 90:303–310.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. J Am Coll Cardiol 2012; 60:2564–2603.
- Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol 2000; 85:890–893, A9.
- Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr 2007; 20:1021–1041.
- Xu B, Dobson L, Mottram P, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? J Am Coll Cardiol 2016; 67:1570.
- Xu B, Dobson L, Mottram P, Nasis A, Cameron J, Moir S. Is exercise stress echocardiography useful in patients with suspected obstructive coronary artery disease who have resting left bundle branch block? Clin Cardiol 2018; in press.
- Yanik A, Yetkin E, Senen K, et al. Value of dobutamine stress echocardiography for diagnosis of coronary artery disease in patients with left bundle branch. Coron Artery Dis 2000; 11:545–548.
- Mairesse GH, Marwick TH, Arnese M, et al. Improved identification of coronary artery disease in patients with left bundle branch block by use of dobutamine stress echocardiography and comparison with myocardial perfusion tomography. Am J Cardiol 1995; 76:321–325.
- Vaduganathan P, He ZX, Raghavan C, Mahmarian JJ, Verani MS. Detection of left anterior descending coronary artery stenosis in patients with left bundle branch block: exercise, adenosine or dobutamine imaging? J Am Coll Cardiol 1996; 28:543–550.
- Jazmati B, Sadaniantz A, Emaus SP, Heller GV. Exercise thallium-201 imaging in complete left bundle branch block and the prevalence of septal perfusion defects. Am J Cardiol 1991; 67:46–49.
- Hasegawa S, Sakata Y, Ishikura F, et al. Mechanism for abnormal thallium-201 myocardial scintigraphy in patients with left bundle branch block in the absence of angiographic coronary artery disease. Ann Nucl Med 1999; 13:253–259.
- Biagini E, Shaw LJ, Poldermans D, et al. Accuracy of non-invasive techniques for diagnosis of coronary artery disease and prediction of cardiac events in patients with left bundle branch block: a meta-analysis. Eur J Nucl Med Mol Imaging 2006; 33:1442–1451.
- Cremer P, Brunken R, Menon V, Cerqueira M, Jaber W. Septal perfusion abnormalities are common in regadenoson SPECT myocardial perfusion imaging (MPI) but not PET MPI in patients with left bundle branch block (LBBB). J Am Coll Cardiol 2015; 65:A1148.
- Arbab-Zadeh A, Miller JM, Rochitte CE, et al. Diagnostic accuracy of computed tomography coronary angiography according to pre-test probability of coronary artery disease and severity of coronary arterial calcification. The CORE-64 (Coronary Artery Evaluation Using 64-Row Multidetector Computed Tomography Angiography) International Multicenter Study. J Am Coll Cardiol 2012; 59:379–387.
- Chow BJ, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009; 2:16–23.
- Nasis A, Leung MC, Antonis PR, et al. Diagnostic accuracy of noninvasive coronary angiography with 320-detector row computed tomography. Am J Cardiol 2010; 106:1429–1435.
- Whelton SP, Silverman MG, McEvoy JW, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. JACC Cardiovasc Imaging 2015; 8:1393–1400.
- Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. J Am Soc Echocardiogr 2011; 24:229–267.
- Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol 2016; 23:606–639.
- Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease. J Am Coll Cardiol 2014; 63:380–406.
- Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol 2010; 17:709–718.
- Halliburton SS, Abbara S, Chen MY, et al; Society of Cardiovascular Computed Tomography. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011; 5:198–224.
- Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol 2008; 191:409–415.
KEY POINTS
- Although current guidelines recommend exercise stress echocardiography, it cannot reliably detect significant obstructive CAD in patients who have left bundle branch block at rest.
- CT angiography is the first-line imaging test for these patients if they are under age 65. For those 65 and older, the first-line test is either pharmacologic stress nuclear myocardial perfusion imaging with coronary vasodilators or dobutamine stress echocardiography.
- For patients who cannot tolerate CT contrast due to renal impairment or who have a true contrast allergy, pharmacologic nuclear myocardial perfusion imaging using coronary vasodilators and dobutamine stress echocardiography can be alternatives.