User login
Point-of-care ultrasound: Coming soon to primary care?
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
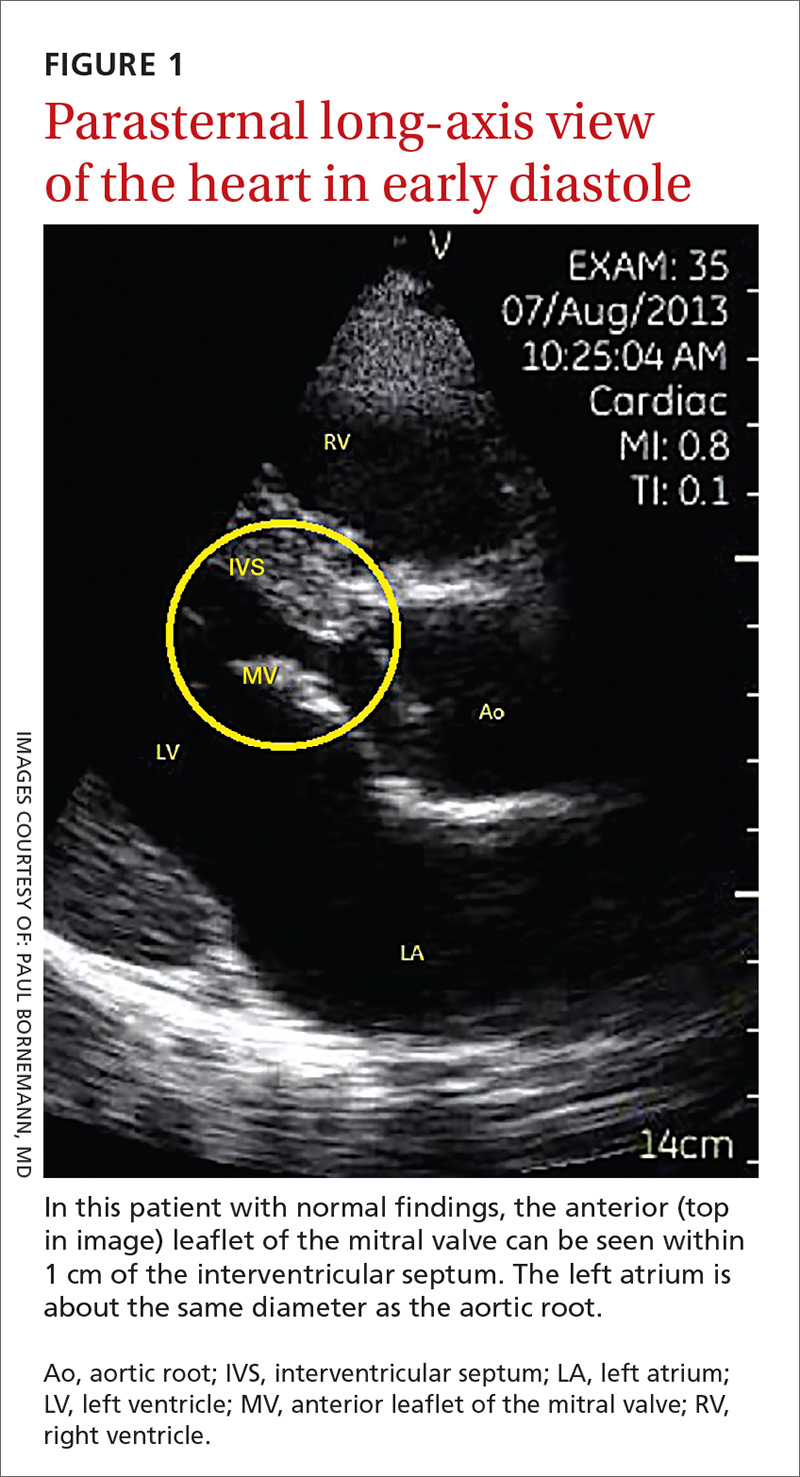
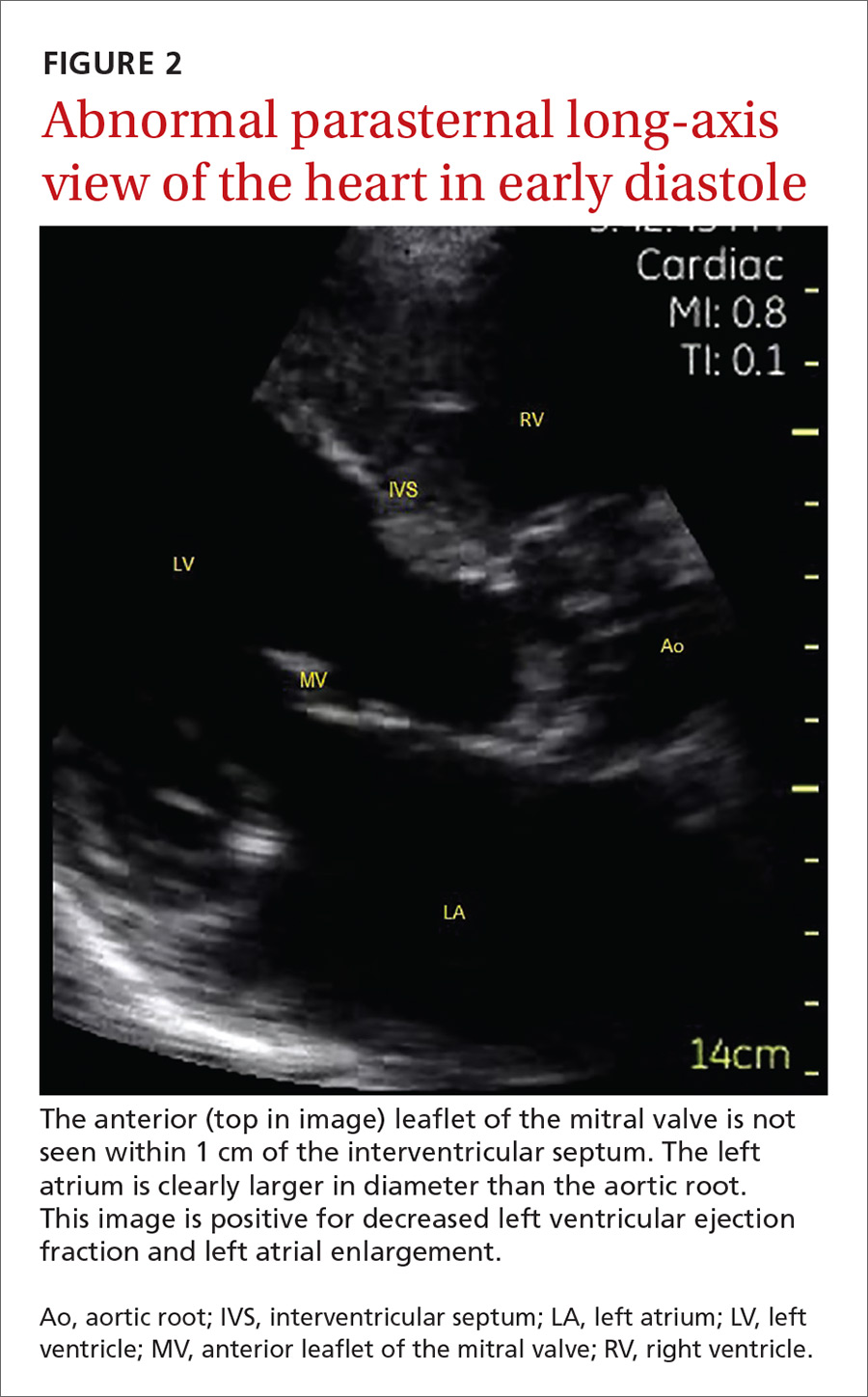
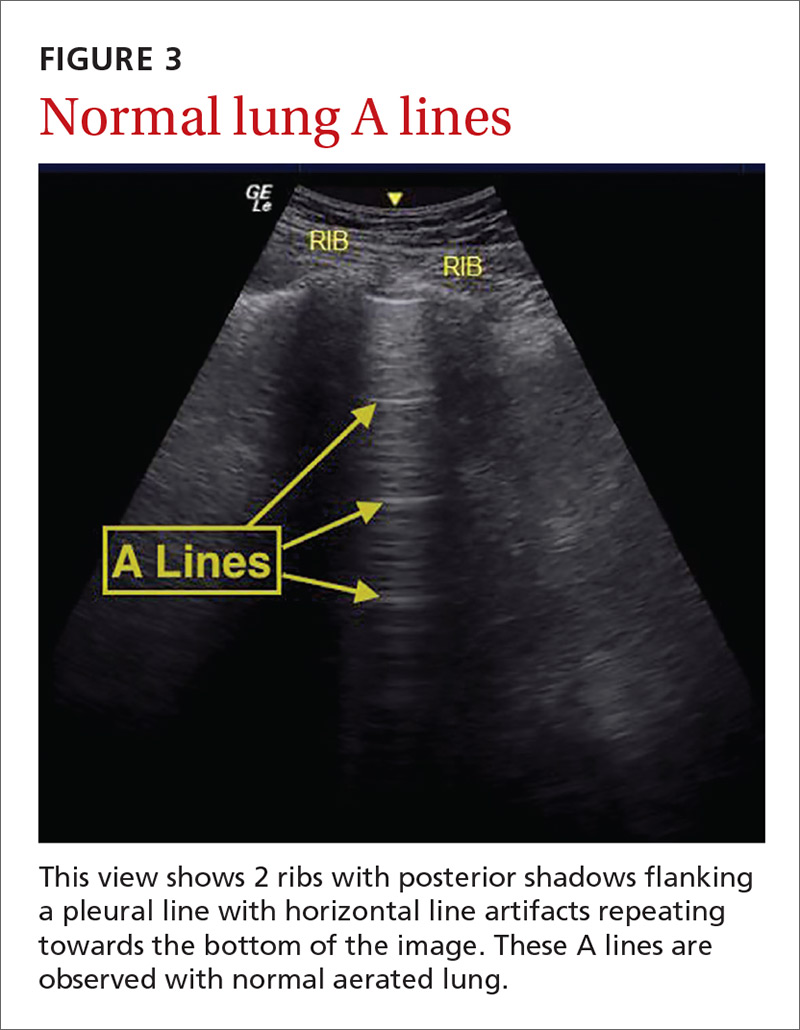
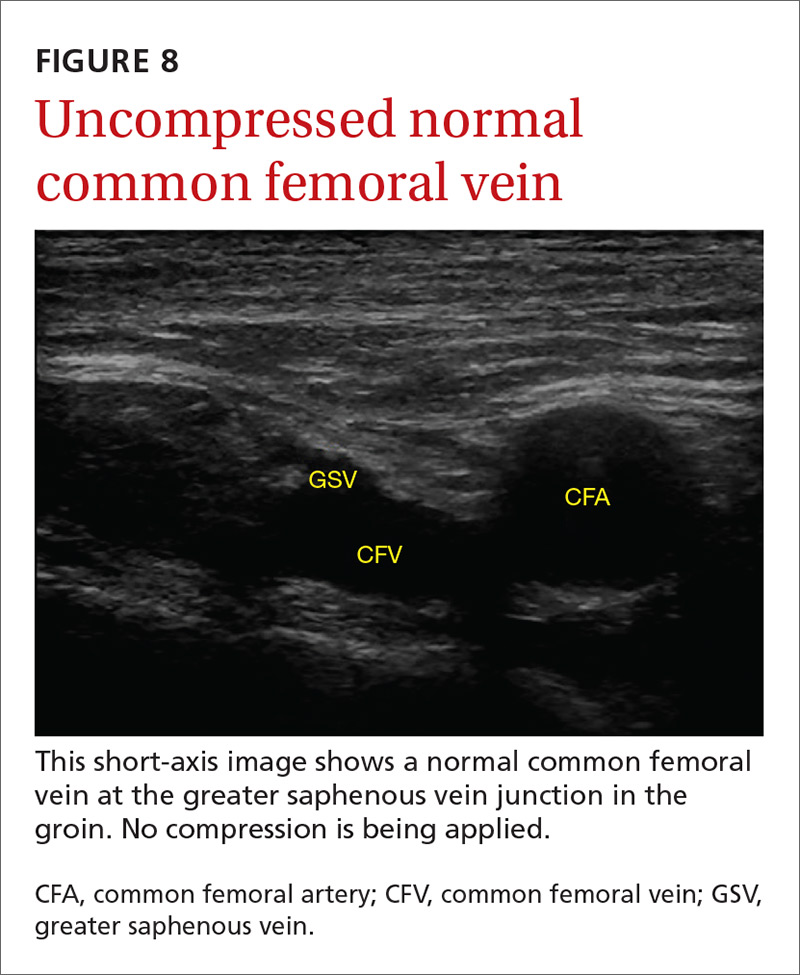
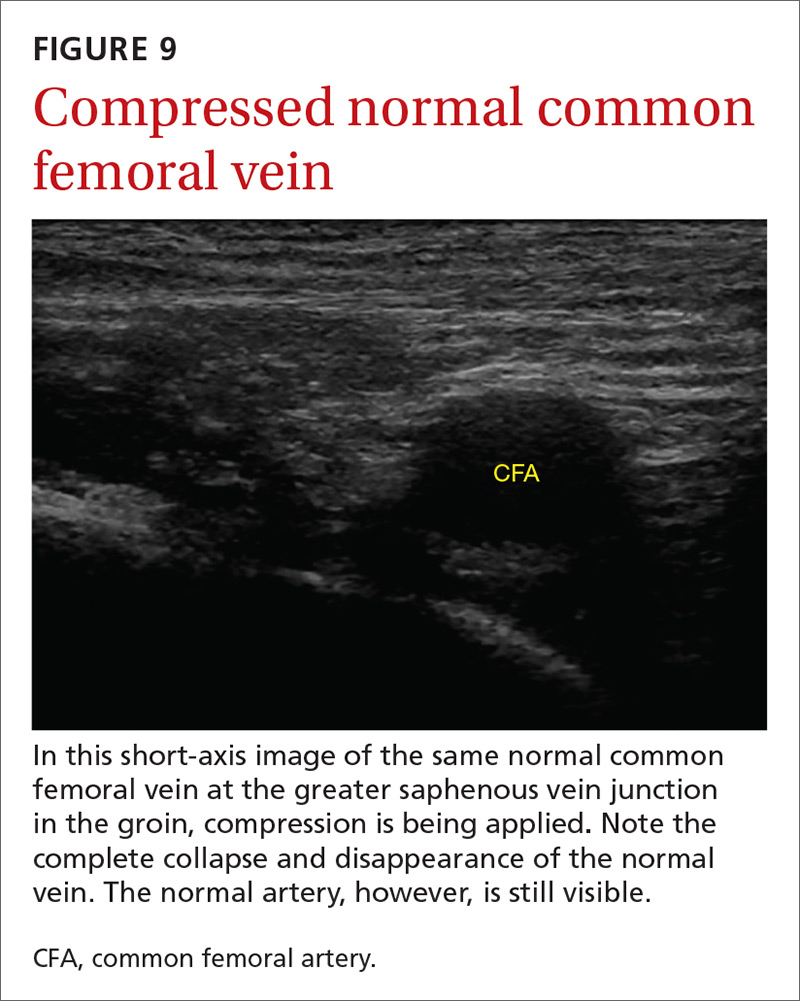
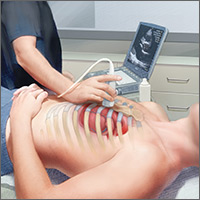
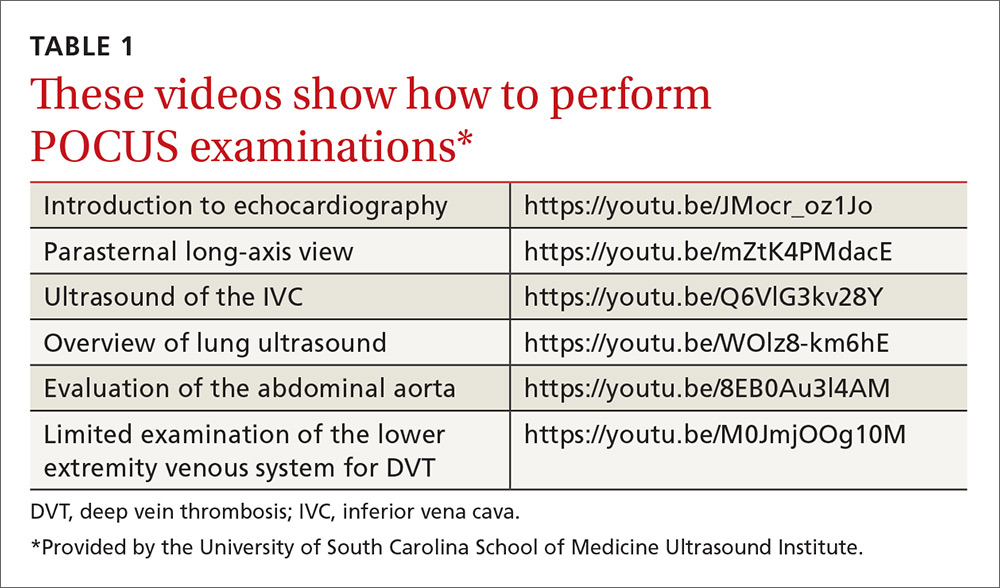
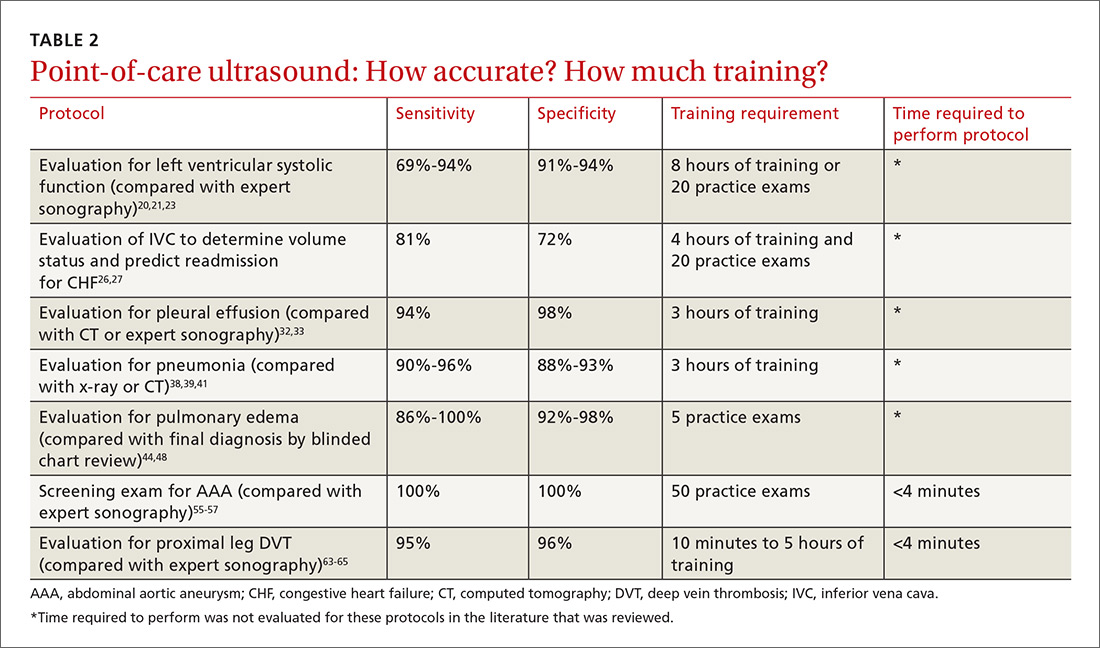
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).
Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
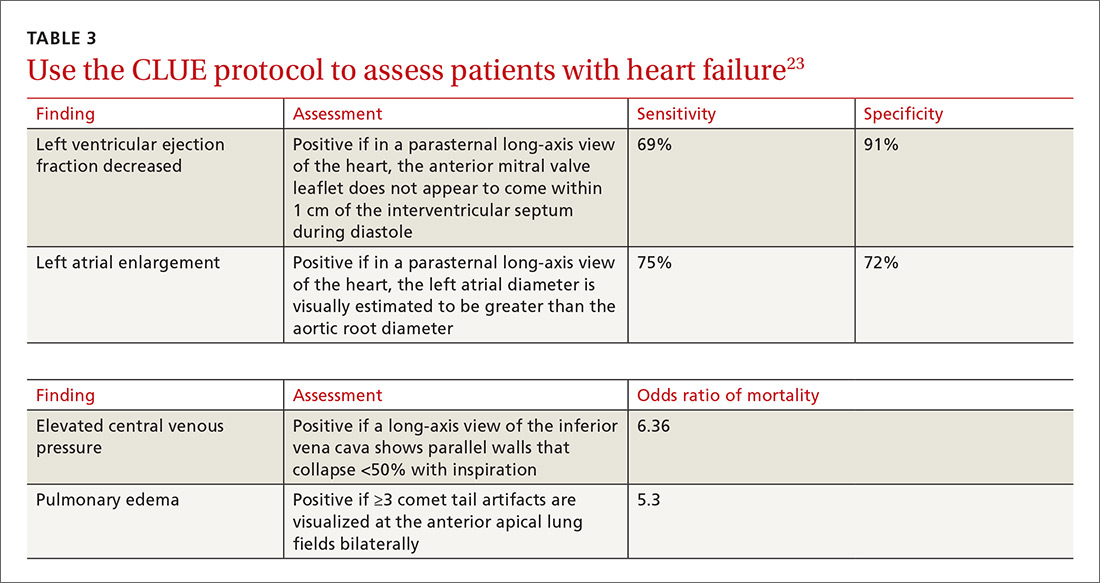
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
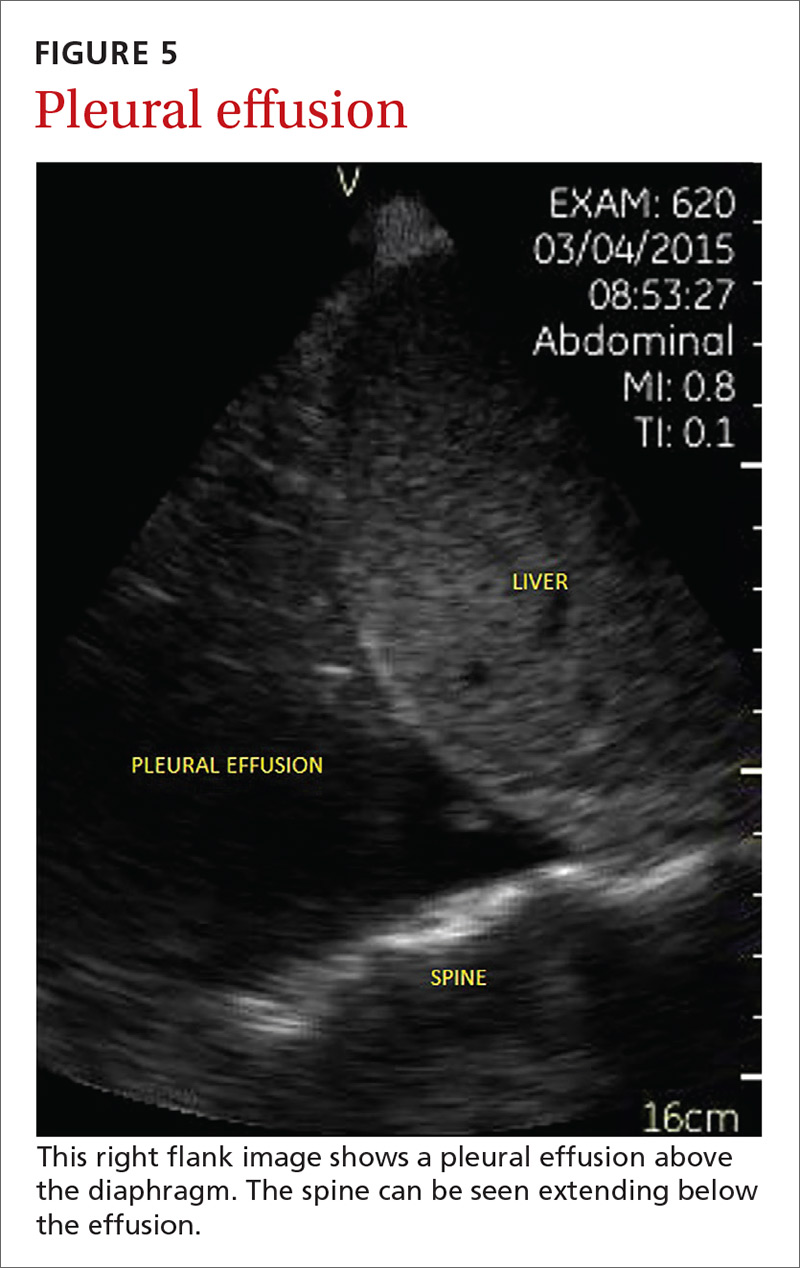
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
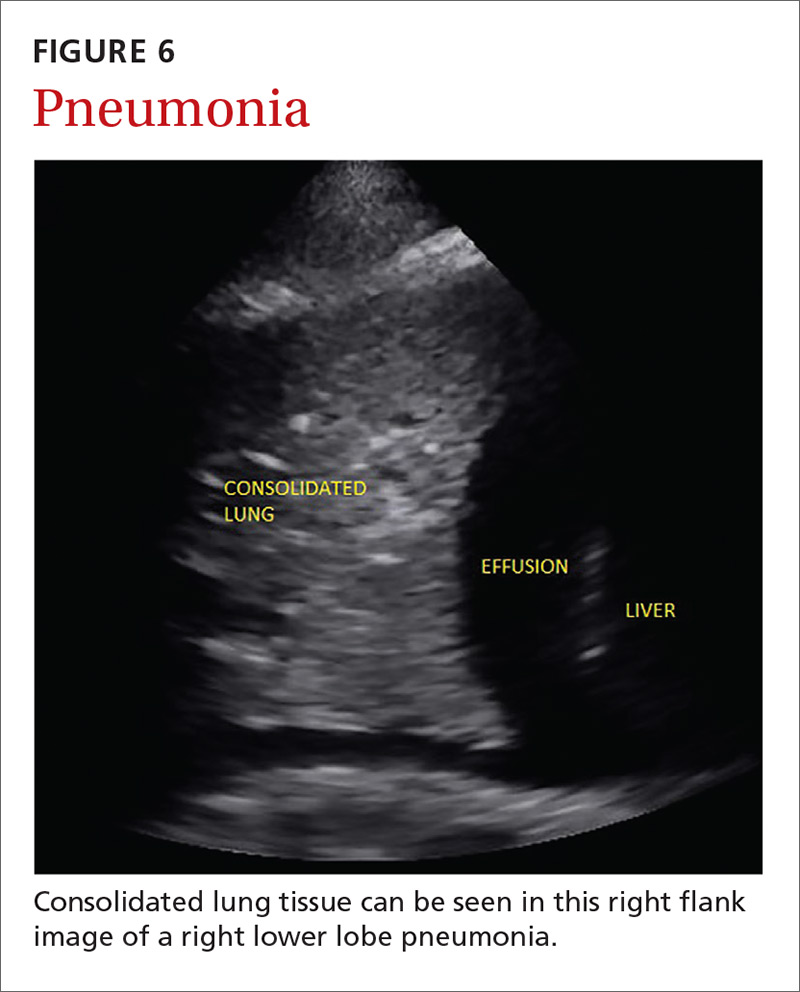
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
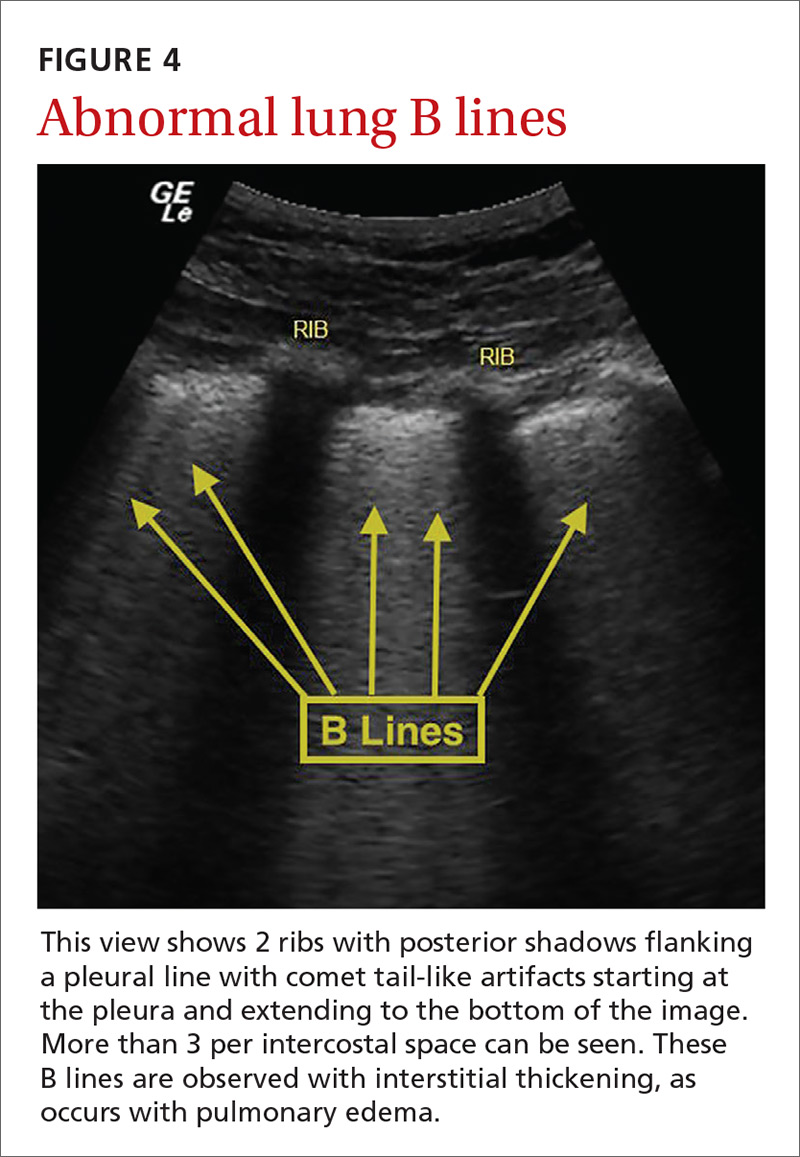
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
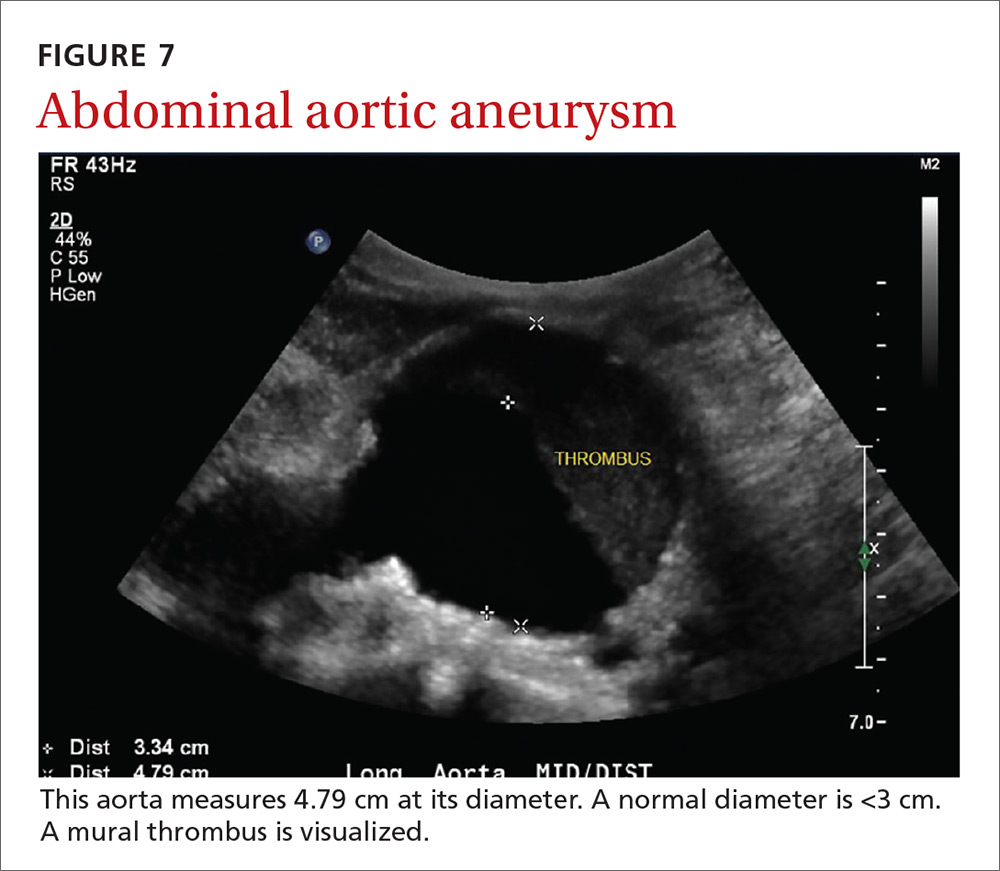
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
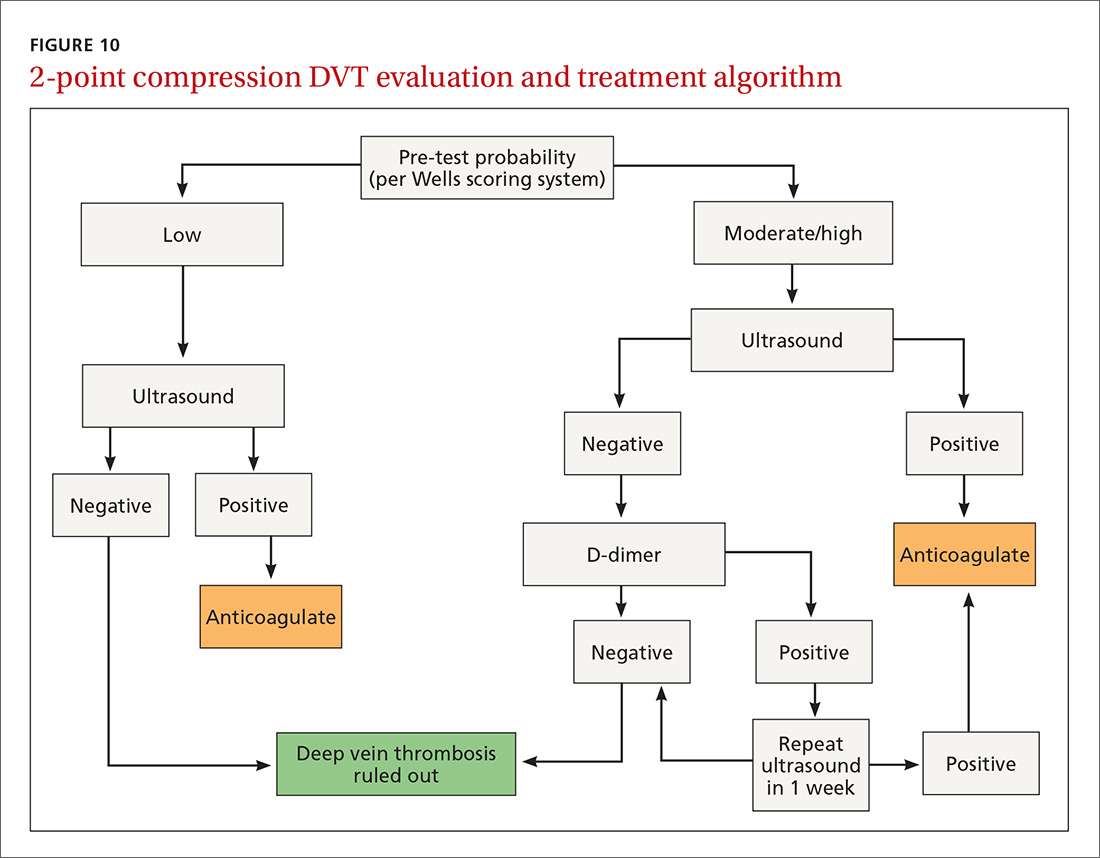
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).


Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).


Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
Smoking Cessation What Should You Recommend?
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
Smoking cessation: What should you recommend?
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
› Prescribe varenicline, bupropion, or nicotine replacement as first-line single pharmacotherapy for smoking cessation. A
› Provide counseling along with medication, as the combination has proven to be more effective than either option alone. A
› Refer patients to their state Quit Line—a toll-free tobacco cessation coaching service that has been shown to be an effective form of counseling. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
In its 2014 report, “The Health Consequences of Smoking—50 Years of Progress,”1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risks of such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 years cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family physicians with an evidence-based update.
The guideline: Treating tobacco use and dependence
Prescribing a first-line medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (See TABLE W1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (TABLE 1).4
Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratio [OR]=1.7 [1.4-2.0], 1.3 [1.1-1.6], and 1.2 [1.1-1.4], respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR=2.5 [1.9-3.4] when 2 different clinical disciplines were involved and 2.4 [2.1-2.9] for 3 or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see TABLE W2.)
For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at motivational interviewing.org/.
In counseling patients considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (TABLE 2)4,6,7 or methods used for prior quit attempts and reasons for relapse, if appropriate; and patient preference.
Evidence update: What's best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR]=1.60; 95% CI, 1.53-1.68).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form like a lozenge with a long-acting patch was found to be more effective than either one alone (RR=1.34; 95% CI, 1.18-1.51).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR=1.18; 95% CI 0.98-1.41). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts, but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a first-line agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a first-line pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR=2.27; 95% CI, 2.02-2.55).13 It also showed varenicline to be more effective than bupropion SR (RR=1.52; 95% CI, 1.22-1.88), but about as effective as NRT (RR=1.13; 95% CI, 0.94-1.35).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR=2.7; 95% CI, 2.1-3.5).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the US Food and Drug Administration (FDA) required that black box warnings be added to the labels of both varenicline and bupropion SR based on post-marketing safety reports showing the risk of neuropsychiatric symptoms, including suicidality.15 In 2011, a large case control study by the FDA Adverse Event Reporting System also showed an increased risk of suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group vs 19% for those in the placebo group (OR=6.2; 95% CI, 2.2-19.2). Although no increased risk of adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: a meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and 2 subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline's safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a first-line treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR=1.62; 95% CI, 1.49-1.76). No increased risk of serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline, (RR=0.68; 95% CI, 0.56-0.83). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30; 95% CI, 0.93-1.82) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a first-line agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a first-line agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation vs placebo (RR=1.63; 95% CI, 1.22-2.18),7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The 6-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (OR, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks vs 47% for varenicline alone (number needed to treat [NNT]=6; 95% CI, 4-11).24
In heavy smokers—defined as those who smoke ≥20 cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT= 9; 95% CI, 4.6-71.6), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT=6).26 Consider using these combinations in patients who have high nicotine dependency levels or have been unable to quit using a single first-line agent.
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 “Vaping,” as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of 2 RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at 6 months in users of e-cigarettes containing nicotine compared with placebo e-cigarettes (9% vs 4%; RR=2.29; 95% CI, 1.05-4.96). Additionally, e-cigarette use was associated with >50% decrease in cigarette smoking vs placebo (36% vs 27%; RR=1.31; 95% CI, 1.02-1.68) or patch (61% vs 44%; RR=1.41; 95% CI, 1.20-1.67).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at 8-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, light-headedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of 4 trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation, as well. A 2013 Cochrane review of 8 trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR=1.00; 95% CI, 0.66-1.51) or as an adjunct to NRT compared with NRT alone (RR=0.95; 95% CI, 0.70-1.30).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the United States.36
A 2012 Cochrane review identified 2 recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates vs placebo (RR=3.98; 95% CI, 2.01-7.87).13
CORRESPONDENCE
Paul Bornemann, MD, 3209 Colonial Drive, Columbia, SC 29203; paul.bornemann@uscmed.sc.edu.
ACKNOWLEDGEMENT
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. 2014. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed October 21, 2015.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service Report. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: Counseling and interventions. April 2009. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed October 21, 2015.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175:504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. US Food and Drug Administration. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148–2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: A randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla. man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. Available at: http:// www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed December 2, 2015.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. Available at: http://www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed August 3, 2015.