User login
UPDATE ON CONTRACEPTION
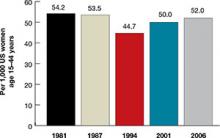
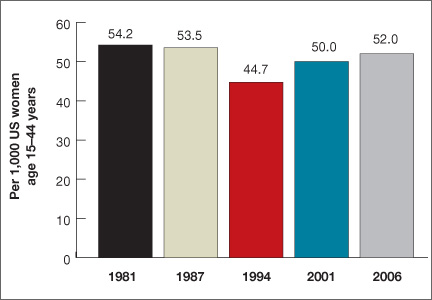
Women spend about 5 years of their reproductive lives trying to get pregnant and the other three decades trying to avoid it.1 Nearly half of all pregnancies are unintended, and 40% of these end in abortion.2 In the past 15 years, new contraceptive options have been developed to address this staggering statistic (FIGURE 1). Despite these innovations, the unintended pregnancy rate has increased continually since 1994 (FIGURE 2).23
What are we doing wrong? In this article, we will review how recent innovations are disseminated through the medical community in the context of three specific contraceptive technologies:
-
hysteroscopic sterilization (Essure)
-
ulipristal acetate emergency contraception (Ella)
-
the 13.5-mg levonorgestrel-releasing intrauterine system (Skyla).
In the process, we assess the available data on the intended and potential impacts of these technologies and describe how ObGyns can best translate these data when considering how to incorporate these new technologies into practice.
| FIGURE 2: Changes in the unintended pregnancy rate, 1981–2006 |
How contraceptive technologies spread in the medical community
Innovations spread through communication channels between individuals of a social network, who are then given time to adopt them. As opinion leaders of a social network become early adopters of a technology, dissemination of the innovation through the social network accelerates.4 This phenomenon is best described by the “diffusion of innovations theory” popularized in 1962 by sociologist Everett Rogers for agricultural applications; he also applied the model to public health.5 The variables he determined to be involved in the acceptance of an innovation are:
-
its relative advantage compared with existing technologies
-
compatibility with current practice
-
low complexity
-
high “trialability” (a potential adopter can easily attempt to use the innovation in his or her practice)
-
high “observability” (the results are easily observed and described to colleagues).
In contrast to new technology itself, medical evidence does not spread rapidly. Data generally spread far more slowly than new technology, typically taking longer than 10 years to influence medical practice.6,7 Opinion leaders can impair the dissemination of data by relying on anecdotal evidence to justify their recommendations.8 Negative findings that challenge these intuitive beliefs can take even longer to disseminate, allowing certain innovations to diffuse through the medical community faster than reports of any associated problems.9
Related article Let’s increase our use of IUDs and improve contraceptive effectiveness in this country Robert L. Barbieri, MD (Editorial, August 2012)
How hysteroscopic sterilization gained widespread adoption
Gariepy AM, Creinin MD, Schwarz EB, Smith KJ. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2):273–279.
Since its introduction into the market in 2002, more than 650,000 Essure hysteroscopic sterilization procedures have been performed worldwide.10 This procedure has diffused quickly through the medical community because of the characteristics we mentioned earlier, which ease acceptance in any network:
-
Relative advantage compared with existing technologies. Compared with existing laparoscopic sterilization methods, hysteroscopic sterilization was seen as a less invasive office procedure that could be performed more cost-effectively under local anesthesia, with very high efficacy, if successful.
-
Compatibility with current practice. Because many clinicians were providing in-office hysteroscopy, adding sterilization was a simple step.
-
Low complexity. Hysteroscopic sterilization builds on operative hysteroscopic skills with which gynecologists are familiar.
-
High trialability. The manufacturer’s representatives were willing to bring the instruments to any office for clinicians to try in their practice. The company worked with hysteroscopic equipment companies to create significant discounts for providers who would perform the procedure regularly.
-
High observability. Successful deployment of the devices, and the appearance of the confirmation test, were visualized and described easily as clinicians spoke to other clinicians, helping with dissemination.
Despite these features, however, new data suggest that hysteroscopic sterilization is less effective than laparoscopic sterilization. A successful Essure procedure requires:
-
visualization of both tubal ostia on hysteroscopy
-
successful deployment of the microinserts at the appropriate position
-
hysterosalpingography at least 3 months later (with use of an alternate form of contraception in the interim)
-
demonstrated tubal occlusion by the Essure devices (not by tubal spasm) on hysterosalpingogram.
Although 5-year data collected by the makers of Essure (and posted on their Web site) show a very high rate of efficacy and a failure rate of 0.17%, these data come from women who completed all of the required steps for successful sterilization and study follow-up.
How hysteroscopic sterilization compares with the laparoscopic approach
Gariepy and colleagues created an evidence-based clinical decision analysis to estimate the probability of successful sterilization after a hysteroscopic procedure in the operating room (OR) or office versus laparoscopic sterilization. A decision analysis, which includes the range of data available to assess different outcomes, is the best methodology to provide population-level information about likelihoods, including rare events (eg, pregnancy after sterilization), in the absence of a randomized trial.
A decision analysis assigns women to outcomes based on their intended method of sterilization, mimicking real-life situations created by the multiple steps required for successful completion of the procedure and confirmation of sterilization. When the probabilities of failing these steps are taken into account, 94% and 95% of women choosing hysteroscopic sterilization in the office and OR, respectively, would be successfully sterilized within 1 year, compared with a success rate of 99% in those who opt for laparoscopic sterilization. The estimates of hysteroscopic success include 6% of women who would attempt hysteroscopy but ultimately be sterilized via laparoscopy, and 5% of women who would decline further sterilization attempts after hysteroscopic sterilization fails.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE Hysteroscopic sterilization has its advantages, including a very high efficacy rate among women who meet all the criteria for successful occlusion. Among these criteria is confirmation, by hysterosalpingography, of occlusion 3 months after deployment of the microinserts.10 However, the efficacy of hysteroscopic sterilization is inferior at a population level; therefore, it should not be used indiscriminately. Rather, hysteroscopic sterilization may be a better option for women for whom laparoscopy itself carries a high risk, such as women with complicated diabetes or severe cardiopulmonary disease. While we await similar studies or further trials that evaluate population-based estimates of pregnancy rates, women considering sterilization should be counseled accordingly. |
How limits on access can prevent widespread use of effective contraception
American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol. 2012;120(5):1250–1253.
Ulipristal acetate as emergency contraception (EC) was introduced to the market in 2010. As was noted in this Update in 2011, ulipristal acetate is more effective than progestin-only emergency contraception and maintains this efficacy for a longer period of time.11 Despite these clear advantages, ulipristal acetate is unlikely to realize its full potential.
Data related to EC as a public health benefit have been largely disappointing. Increased access and availability have not yet reduced the unintended pregnancy rate in the United States. Although use of EC increased from 4.2% in 2002 to 11% in 2008,12 even women with a knowledge of EC do not always use it when needed.13,14
Use of ulipristal acetate, in particular, remains limited because it lacks one important requirement for rapid diffusion—access. Although it is clinically superior to the progestin-only method of EC, is compatible with current practice, and has both high trialability and high observability, access to the drug remains too complex for easy dissemination due to its prescription-only status. Because women can now obtain progestin-only EC over the counter, the use of ulipristal acetate is likely to remain low unless the access barrier to this effective oral EC regimen is reduced.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE When counseling women of reproductive age about contraception, offer them an advance prescription for ulipristal acetate and advise them of its greater efficacy, compared with progestin-only emergency contraception. |
Skyla versus other IUDs: What the data reveal
Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e1–e3.
The 13.5-mg levonorgestrel-releasing intrauterine system (Skyla) boasts a smaller frame and a narrower inserter than the two intrauterine devices (IUDs) already on the market (ParaGard and Mirena), a lower amount of levonorgestrel than the other levonorgestrel-releasing IUD (Mirena), and 3 years of continuous contraception. Both of the IUDs that predated Skyla are backed by data supporting their efficacy and safety in nulliparous women,15-18 but a number of clinicians and opinion leaders have stated that Skyla’s smaller frame and inserter make it an ideal IUD for the narrower cervical canal and smaller endometrial cavity of nulliparous women,19 including Gemzell-Danielsson and colleagues.
Skyla meets the prerequisites for rapid diffusion; it is highly compatible with current practice and easy to place and use. Of all these characteristics, the relative advantage granted by its size is most likely to promote its diffusion through the medical community.
Ease of placement versus Mirena
Clinical information about Skyla is currently available from two sources. The first is the product package insert, which includes selected data from the product’s Phase 3 study. This study included 1,432 participants, of whom 556 (38.8%) were nulliparous and 540 (37.7%) were treated in the United States.20
The second source is a published, peer-reviewed Phase 2 trial comparing Mirena with two smaller, lower-dose levonorgestrel-releasing devices, with the lowest-dose product corresponding to the marketed Skyla product.21 In the Phase 2 trial, all 738 women given Mirena or the smaller devices experienced successful placement, with 98.5% of placements achieved on the first attempt. Investigators rated placement for the smaller devices “easy” in 455 of 484 (94.0%) women, compared with 219 of 254 (86.2%) women given Mirena (P <.001). Most of the women given the smaller devices rated their pain with insertion as “mild pain” or “no pain,” compared with those given Mirena (72.3% vs 57.9%; P <.001). Adverse events were similar between users of the different products, except that significantly more women were classified as having an ovarian cyst among Mirena users than among users of the smaller, low-dose devices (22% vs 6%; P <.0001).
Little difference in "clinically relevant" effects
The claim that Skyla has an advantage over Mirena or ParaGard falls short on closer inspection. Although a clinician may prefer easy insertion and a patient with no pain, only very difficult or severely painful placements have clinical relevance.
Investigators rated only 4 of 254 (1.6%) Mirena insertions as “very difficult,” compared with 4 of 484 (0.8%) for the smaller devices (P=.46). Further, women found Mirena insertion to cause severe pain in only 17 of 254 (6.7%) insertions, compared with 21 of 484 (4.3%) placements of the smaller devices (P=.22). The smaller device and inserter, therefore, may have no clinical advantage.
Adverse events were similar
The data on adverse events are similarly misleading. Investigators in the Phase 2 study found that the lower-dose levonorgestrel-releasing IUDs had an 8.6% rate of ovarian cysts and the Mirena had a 22% rate (P <.0001). However, the Phase 2 study included a pelvic ultrasound examination at every visit, and ovarian cysts were included as an adverse event if the size was 3 cm or greater, regardless of symptoms.
Complaints of abdominal or low abdominal pain were as common among Mirena users as among users of the smaller devices, so this finding likely represents asymptomatic, clinically irrelevant cysts.
Most ovarian cysts found in users of the levonorgestrel-releasing intrauterine system are asymptomatic.22
Fewer Skyla users developed amenorrhea
Bleeding patterns differed between the products. Users of the smaller, low-dose device reported slightly more spotting and bleeding over the course of a month. In the Phase 2 trial, at the end of 3 years, only 12.7% of Skyla users achieved amenorrhea, compared with 23.6% of Mirena users. The amenorrhea rate for Mirena was very similar to the 20% rate reported in earlier studies,23,24 but the rate for Skyla was even lower (6%) in the larger Phase 3 study.
What about efficacy?
If there are no real advantages to be gained from the size of the device and inserter in terms of pain, and no real improvement in adverse effects or bleeding patterns, what about efficacy?
No direct comparisons are available, but if the devices are evaluated in terms of their first-year Pearl index rating from Phase 3 studies for approval in the United States, then among a cohort of 100,000 users, about 190 Mirena users would become pregnant in the first year, compared with 410 Skyla users.
All IUDs are considered highly effective contraceptives, but small relative differences can have a large impact on a population level if the methods are not used correctly or patients are not counseled appropriately. Although it is more effective than user-dependent contraceptives such as the pill, Skyla is the least effective of the highly effective methods available. If the device has any real benefits in comparison with the other IUDs, they must be better demonstrated with additional data.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
ObGyns have done much to increase the use of long-acting reversible contraceptives such as the IUD (Mirena, ParaGard), the etonogestrel implant (Implanon, Nexplanon), and the injectable contraceptive depot medroxyprogesterone acetate (Depo-Provera). We applaud this success and urge ObGyns to continue prescribing these options.
In addition, if we want to have a positive impact on the unintended pregnancy rate, we need to increase awareness of, access to, and use of the most effective contraceptive options in our community of providers and among our patients. We also need to eliminate barriers to use of the most effective methods—eg, discussing ulipristal acetate with our patients and providing advance prescriptions. We also need to be cautious about adopting some innovations, as the data for Skyla and Essure illustrate. They may be terrific options for very specific populations of women, but indiscriminate use may, paradoxically, increase the rate of unintended pregnancy.
Women spend about 5 years of their reproductive lives trying to get pregnant and the other three decades trying to avoid it.1 Nearly half of all pregnancies are unintended, and 40% of these end in abortion.2 In the past 15 years, new contraceptive options have been developed to address this staggering statistic (FIGURE 1). Despite these innovations, the unintended pregnancy rate has increased continually since 1994 (FIGURE 2).23
What are we doing wrong? In this article, we will review how recent innovations are disseminated through the medical community in the context of three specific contraceptive technologies:
-
hysteroscopic sterilization (Essure)
-
ulipristal acetate emergency contraception (Ella)
-
the 13.5-mg levonorgestrel-releasing intrauterine system (Skyla).
In the process, we assess the available data on the intended and potential impacts of these technologies and describe how ObGyns can best translate these data when considering how to incorporate these new technologies into practice.
| FIGURE 2: Changes in the unintended pregnancy rate, 1981–2006 |
How contraceptive technologies spread in the medical community
Innovations spread through communication channels between individuals of a social network, who are then given time to adopt them. As opinion leaders of a social network become early adopters of a technology, dissemination of the innovation through the social network accelerates.4 This phenomenon is best described by the “diffusion of innovations theory” popularized in 1962 by sociologist Everett Rogers for agricultural applications; he also applied the model to public health.5 The variables he determined to be involved in the acceptance of an innovation are:
-
its relative advantage compared with existing technologies
-
compatibility with current practice
-
low complexity
-
high “trialability” (a potential adopter can easily attempt to use the innovation in his or her practice)
-
high “observability” (the results are easily observed and described to colleagues).
In contrast to new technology itself, medical evidence does not spread rapidly. Data generally spread far more slowly than new technology, typically taking longer than 10 years to influence medical practice.6,7 Opinion leaders can impair the dissemination of data by relying on anecdotal evidence to justify their recommendations.8 Negative findings that challenge these intuitive beliefs can take even longer to disseminate, allowing certain innovations to diffuse through the medical community faster than reports of any associated problems.9
Related article Let’s increase our use of IUDs and improve contraceptive effectiveness in this country Robert L. Barbieri, MD (Editorial, August 2012)
How hysteroscopic sterilization gained widespread adoption
Gariepy AM, Creinin MD, Schwarz EB, Smith KJ. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2):273–279.
Since its introduction into the market in 2002, more than 650,000 Essure hysteroscopic sterilization procedures have been performed worldwide.10 This procedure has diffused quickly through the medical community because of the characteristics we mentioned earlier, which ease acceptance in any network:
-
Relative advantage compared with existing technologies. Compared with existing laparoscopic sterilization methods, hysteroscopic sterilization was seen as a less invasive office procedure that could be performed more cost-effectively under local anesthesia, with very high efficacy, if successful.
-
Compatibility with current practice. Because many clinicians were providing in-office hysteroscopy, adding sterilization was a simple step.
-
Low complexity. Hysteroscopic sterilization builds on operative hysteroscopic skills with which gynecologists are familiar.
-
High trialability. The manufacturer’s representatives were willing to bring the instruments to any office for clinicians to try in their practice. The company worked with hysteroscopic equipment companies to create significant discounts for providers who would perform the procedure regularly.
-
High observability. Successful deployment of the devices, and the appearance of the confirmation test, were visualized and described easily as clinicians spoke to other clinicians, helping with dissemination.
Despite these features, however, new data suggest that hysteroscopic sterilization is less effective than laparoscopic sterilization. A successful Essure procedure requires:
-
visualization of both tubal ostia on hysteroscopy
-
successful deployment of the microinserts at the appropriate position
-
hysterosalpingography at least 3 months later (with use of an alternate form of contraception in the interim)
-
demonstrated tubal occlusion by the Essure devices (not by tubal spasm) on hysterosalpingogram.
Although 5-year data collected by the makers of Essure (and posted on their Web site) show a very high rate of efficacy and a failure rate of 0.17%, these data come from women who completed all of the required steps for successful sterilization and study follow-up.
How hysteroscopic sterilization compares with the laparoscopic approach
Gariepy and colleagues created an evidence-based clinical decision analysis to estimate the probability of successful sterilization after a hysteroscopic procedure in the operating room (OR) or office versus laparoscopic sterilization. A decision analysis, which includes the range of data available to assess different outcomes, is the best methodology to provide population-level information about likelihoods, including rare events (eg, pregnancy after sterilization), in the absence of a randomized trial.
A decision analysis assigns women to outcomes based on their intended method of sterilization, mimicking real-life situations created by the multiple steps required for successful completion of the procedure and confirmation of sterilization. When the probabilities of failing these steps are taken into account, 94% and 95% of women choosing hysteroscopic sterilization in the office and OR, respectively, would be successfully sterilized within 1 year, compared with a success rate of 99% in those who opt for laparoscopic sterilization. The estimates of hysteroscopic success include 6% of women who would attempt hysteroscopy but ultimately be sterilized via laparoscopy, and 5% of women who would decline further sterilization attempts after hysteroscopic sterilization fails.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE Hysteroscopic sterilization has its advantages, including a very high efficacy rate among women who meet all the criteria for successful occlusion. Among these criteria is confirmation, by hysterosalpingography, of occlusion 3 months after deployment of the microinserts.10 However, the efficacy of hysteroscopic sterilization is inferior at a population level; therefore, it should not be used indiscriminately. Rather, hysteroscopic sterilization may be a better option for women for whom laparoscopy itself carries a high risk, such as women with complicated diabetes or severe cardiopulmonary disease. While we await similar studies or further trials that evaluate population-based estimates of pregnancy rates, women considering sterilization should be counseled accordingly. |
How limits on access can prevent widespread use of effective contraception
American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol. 2012;120(5):1250–1253.
Ulipristal acetate as emergency contraception (EC) was introduced to the market in 2010. As was noted in this Update in 2011, ulipristal acetate is more effective than progestin-only emergency contraception and maintains this efficacy for a longer period of time.11 Despite these clear advantages, ulipristal acetate is unlikely to realize its full potential.
Data related to EC as a public health benefit have been largely disappointing. Increased access and availability have not yet reduced the unintended pregnancy rate in the United States. Although use of EC increased from 4.2% in 2002 to 11% in 2008,12 even women with a knowledge of EC do not always use it when needed.13,14
Use of ulipristal acetate, in particular, remains limited because it lacks one important requirement for rapid diffusion—access. Although it is clinically superior to the progestin-only method of EC, is compatible with current practice, and has both high trialability and high observability, access to the drug remains too complex for easy dissemination due to its prescription-only status. Because women can now obtain progestin-only EC over the counter, the use of ulipristal acetate is likely to remain low unless the access barrier to this effective oral EC regimen is reduced.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE When counseling women of reproductive age about contraception, offer them an advance prescription for ulipristal acetate and advise them of its greater efficacy, compared with progestin-only emergency contraception. |
Skyla versus other IUDs: What the data reveal
Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e1–e3.
The 13.5-mg levonorgestrel-releasing intrauterine system (Skyla) boasts a smaller frame and a narrower inserter than the two intrauterine devices (IUDs) already on the market (ParaGard and Mirena), a lower amount of levonorgestrel than the other levonorgestrel-releasing IUD (Mirena), and 3 years of continuous contraception. Both of the IUDs that predated Skyla are backed by data supporting their efficacy and safety in nulliparous women,15-18 but a number of clinicians and opinion leaders have stated that Skyla’s smaller frame and inserter make it an ideal IUD for the narrower cervical canal and smaller endometrial cavity of nulliparous women,19 including Gemzell-Danielsson and colleagues.
Skyla meets the prerequisites for rapid diffusion; it is highly compatible with current practice and easy to place and use. Of all these characteristics, the relative advantage granted by its size is most likely to promote its diffusion through the medical community.
Ease of placement versus Mirena
Clinical information about Skyla is currently available from two sources. The first is the product package insert, which includes selected data from the product’s Phase 3 study. This study included 1,432 participants, of whom 556 (38.8%) were nulliparous and 540 (37.7%) were treated in the United States.20
The second source is a published, peer-reviewed Phase 2 trial comparing Mirena with two smaller, lower-dose levonorgestrel-releasing devices, with the lowest-dose product corresponding to the marketed Skyla product.21 In the Phase 2 trial, all 738 women given Mirena or the smaller devices experienced successful placement, with 98.5% of placements achieved on the first attempt. Investigators rated placement for the smaller devices “easy” in 455 of 484 (94.0%) women, compared with 219 of 254 (86.2%) women given Mirena (P <.001). Most of the women given the smaller devices rated their pain with insertion as “mild pain” or “no pain,” compared with those given Mirena (72.3% vs 57.9%; P <.001). Adverse events were similar between users of the different products, except that significantly more women were classified as having an ovarian cyst among Mirena users than among users of the smaller, low-dose devices (22% vs 6%; P <.0001).
Little difference in "clinically relevant" effects
The claim that Skyla has an advantage over Mirena or ParaGard falls short on closer inspection. Although a clinician may prefer easy insertion and a patient with no pain, only very difficult or severely painful placements have clinical relevance.
Investigators rated only 4 of 254 (1.6%) Mirena insertions as “very difficult,” compared with 4 of 484 (0.8%) for the smaller devices (P=.46). Further, women found Mirena insertion to cause severe pain in only 17 of 254 (6.7%) insertions, compared with 21 of 484 (4.3%) placements of the smaller devices (P=.22). The smaller device and inserter, therefore, may have no clinical advantage.
Adverse events were similar
The data on adverse events are similarly misleading. Investigators in the Phase 2 study found that the lower-dose levonorgestrel-releasing IUDs had an 8.6% rate of ovarian cysts and the Mirena had a 22% rate (P <.0001). However, the Phase 2 study included a pelvic ultrasound examination at every visit, and ovarian cysts were included as an adverse event if the size was 3 cm or greater, regardless of symptoms.
Complaints of abdominal or low abdominal pain were as common among Mirena users as among users of the smaller devices, so this finding likely represents asymptomatic, clinically irrelevant cysts.
Most ovarian cysts found in users of the levonorgestrel-releasing intrauterine system are asymptomatic.22
Fewer Skyla users developed amenorrhea
Bleeding patterns differed between the products. Users of the smaller, low-dose device reported slightly more spotting and bleeding over the course of a month. In the Phase 2 trial, at the end of 3 years, only 12.7% of Skyla users achieved amenorrhea, compared with 23.6% of Mirena users. The amenorrhea rate for Mirena was very similar to the 20% rate reported in earlier studies,23,24 but the rate for Skyla was even lower (6%) in the larger Phase 3 study.
What about efficacy?
If there are no real advantages to be gained from the size of the device and inserter in terms of pain, and no real improvement in adverse effects or bleeding patterns, what about efficacy?
No direct comparisons are available, but if the devices are evaluated in terms of their first-year Pearl index rating from Phase 3 studies for approval in the United States, then among a cohort of 100,000 users, about 190 Mirena users would become pregnant in the first year, compared with 410 Skyla users.
All IUDs are considered highly effective contraceptives, but small relative differences can have a large impact on a population level if the methods are not used correctly or patients are not counseled appropriately. Although it is more effective than user-dependent contraceptives such as the pill, Skyla is the least effective of the highly effective methods available. If the device has any real benefits in comparison with the other IUDs, they must be better demonstrated with additional data.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
ObGyns have done much to increase the use of long-acting reversible contraceptives such as the IUD (Mirena, ParaGard), the etonogestrel implant (Implanon, Nexplanon), and the injectable contraceptive depot medroxyprogesterone acetate (Depo-Provera). We applaud this success and urge ObGyns to continue prescribing these options.
In addition, if we want to have a positive impact on the unintended pregnancy rate, we need to increase awareness of, access to, and use of the most effective contraceptive options in our community of providers and among our patients. We also need to eliminate barriers to use of the most effective methods—eg, discussing ulipristal acetate with our patients and providing advance prescriptions. We also need to be cautious about adopting some innovations, as the data for Skyla and Essure illustrate. They may be terrific options for very specific populations of women, but indiscriminate use may, paradoxically, increase the rate of unintended pregnancy.
Women spend about 5 years of their reproductive lives trying to get pregnant and the other three decades trying to avoid it.1 Nearly half of all pregnancies are unintended, and 40% of these end in abortion.2 In the past 15 years, new contraceptive options have been developed to address this staggering statistic (FIGURE 1). Despite these innovations, the unintended pregnancy rate has increased continually since 1994 (FIGURE 2).23
What are we doing wrong? In this article, we will review how recent innovations are disseminated through the medical community in the context of three specific contraceptive technologies:
-
hysteroscopic sterilization (Essure)
-
ulipristal acetate emergency contraception (Ella)
-
the 13.5-mg levonorgestrel-releasing intrauterine system (Skyla).
In the process, we assess the available data on the intended and potential impacts of these technologies and describe how ObGyns can best translate these data when considering how to incorporate these new technologies into practice.
| FIGURE 2: Changes in the unintended pregnancy rate, 1981–2006 |
How contraceptive technologies spread in the medical community
Innovations spread through communication channels between individuals of a social network, who are then given time to adopt them. As opinion leaders of a social network become early adopters of a technology, dissemination of the innovation through the social network accelerates.4 This phenomenon is best described by the “diffusion of innovations theory” popularized in 1962 by sociologist Everett Rogers for agricultural applications; he also applied the model to public health.5 The variables he determined to be involved in the acceptance of an innovation are:
-
its relative advantage compared with existing technologies
-
compatibility with current practice
-
low complexity
-
high “trialability” (a potential adopter can easily attempt to use the innovation in his or her practice)
-
high “observability” (the results are easily observed and described to colleagues).
In contrast to new technology itself, medical evidence does not spread rapidly. Data generally spread far more slowly than new technology, typically taking longer than 10 years to influence medical practice.6,7 Opinion leaders can impair the dissemination of data by relying on anecdotal evidence to justify their recommendations.8 Negative findings that challenge these intuitive beliefs can take even longer to disseminate, allowing certain innovations to diffuse through the medical community faster than reports of any associated problems.9
Related article Let’s increase our use of IUDs and improve contraceptive effectiveness in this country Robert L. Barbieri, MD (Editorial, August 2012)
How hysteroscopic sterilization gained widespread adoption
Gariepy AM, Creinin MD, Schwarz EB, Smith KJ. Reliability of laparoscopic compared with hysteroscopic sterilization at 1 year: a decision analysis. Obstet Gynecol. 2011;118(2):273–279.
Since its introduction into the market in 2002, more than 650,000 Essure hysteroscopic sterilization procedures have been performed worldwide.10 This procedure has diffused quickly through the medical community because of the characteristics we mentioned earlier, which ease acceptance in any network:
-
Relative advantage compared with existing technologies. Compared with existing laparoscopic sterilization methods, hysteroscopic sterilization was seen as a less invasive office procedure that could be performed more cost-effectively under local anesthesia, with very high efficacy, if successful.
-
Compatibility with current practice. Because many clinicians were providing in-office hysteroscopy, adding sterilization was a simple step.
-
Low complexity. Hysteroscopic sterilization builds on operative hysteroscopic skills with which gynecologists are familiar.
-
High trialability. The manufacturer’s representatives were willing to bring the instruments to any office for clinicians to try in their practice. The company worked with hysteroscopic equipment companies to create significant discounts for providers who would perform the procedure regularly.
-
High observability. Successful deployment of the devices, and the appearance of the confirmation test, were visualized and described easily as clinicians spoke to other clinicians, helping with dissemination.
Despite these features, however, new data suggest that hysteroscopic sterilization is less effective than laparoscopic sterilization. A successful Essure procedure requires:
-
visualization of both tubal ostia on hysteroscopy
-
successful deployment of the microinserts at the appropriate position
-
hysterosalpingography at least 3 months later (with use of an alternate form of contraception in the interim)
-
demonstrated tubal occlusion by the Essure devices (not by tubal spasm) on hysterosalpingogram.
Although 5-year data collected by the makers of Essure (and posted on their Web site) show a very high rate of efficacy and a failure rate of 0.17%, these data come from women who completed all of the required steps for successful sterilization and study follow-up.
How hysteroscopic sterilization compares with the laparoscopic approach
Gariepy and colleagues created an evidence-based clinical decision analysis to estimate the probability of successful sterilization after a hysteroscopic procedure in the operating room (OR) or office versus laparoscopic sterilization. A decision analysis, which includes the range of data available to assess different outcomes, is the best methodology to provide population-level information about likelihoods, including rare events (eg, pregnancy after sterilization), in the absence of a randomized trial.
A decision analysis assigns women to outcomes based on their intended method of sterilization, mimicking real-life situations created by the multiple steps required for successful completion of the procedure and confirmation of sterilization. When the probabilities of failing these steps are taken into account, 94% and 95% of women choosing hysteroscopic sterilization in the office and OR, respectively, would be successfully sterilized within 1 year, compared with a success rate of 99% in those who opt for laparoscopic sterilization. The estimates of hysteroscopic success include 6% of women who would attempt hysteroscopy but ultimately be sterilized via laparoscopy, and 5% of women who would decline further sterilization attempts after hysteroscopic sterilization fails.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE Hysteroscopic sterilization has its advantages, including a very high efficacy rate among women who meet all the criteria for successful occlusion. Among these criteria is confirmation, by hysterosalpingography, of occlusion 3 months after deployment of the microinserts.10 However, the efficacy of hysteroscopic sterilization is inferior at a population level; therefore, it should not be used indiscriminately. Rather, hysteroscopic sterilization may be a better option for women for whom laparoscopy itself carries a high risk, such as women with complicated diabetes or severe cardiopulmonary disease. While we await similar studies or further trials that evaluate population-based estimates of pregnancy rates, women considering sterilization should be counseled accordingly. |
How limits on access can prevent widespread use of effective contraception
American College of Obstetricians and Gynecologists. Access to emergency contraception. Committee Opinion No. 542. Obstet Gynecol. 2012;120(5):1250–1253.
Ulipristal acetate as emergency contraception (EC) was introduced to the market in 2010. As was noted in this Update in 2011, ulipristal acetate is more effective than progestin-only emergency contraception and maintains this efficacy for a longer period of time.11 Despite these clear advantages, ulipristal acetate is unlikely to realize its full potential.
Data related to EC as a public health benefit have been largely disappointing. Increased access and availability have not yet reduced the unintended pregnancy rate in the United States. Although use of EC increased from 4.2% in 2002 to 11% in 2008,12 even women with a knowledge of EC do not always use it when needed.13,14
Use of ulipristal acetate, in particular, remains limited because it lacks one important requirement for rapid diffusion—access. Although it is clinically superior to the progestin-only method of EC, is compatible with current practice, and has both high trialability and high observability, access to the drug remains too complex for easy dissemination due to its prescription-only status. Because women can now obtain progestin-only EC over the counter, the use of ulipristal acetate is likely to remain low unless the access barrier to this effective oral EC regimen is reduced.
|
WHAT THIS EVIDENCE MEANS FOR PRACTICE When counseling women of reproductive age about contraception, offer them an advance prescription for ulipristal acetate and advise them of its greater efficacy, compared with progestin-only emergency contraception. |
Skyla versus other IUDs: What the data reveal
Gemzell-Danielsson K, Schellschmidt I, Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e1–e3.
The 13.5-mg levonorgestrel-releasing intrauterine system (Skyla) boasts a smaller frame and a narrower inserter than the two intrauterine devices (IUDs) already on the market (ParaGard and Mirena), a lower amount of levonorgestrel than the other levonorgestrel-releasing IUD (Mirena), and 3 years of continuous contraception. Both of the IUDs that predated Skyla are backed by data supporting their efficacy and safety in nulliparous women,15-18 but a number of clinicians and opinion leaders have stated that Skyla’s smaller frame and inserter make it an ideal IUD for the narrower cervical canal and smaller endometrial cavity of nulliparous women,19 including Gemzell-Danielsson and colleagues.
Skyla meets the prerequisites for rapid diffusion; it is highly compatible with current practice and easy to place and use. Of all these characteristics, the relative advantage granted by its size is most likely to promote its diffusion through the medical community.
Ease of placement versus Mirena
Clinical information about Skyla is currently available from two sources. The first is the product package insert, which includes selected data from the product’s Phase 3 study. This study included 1,432 participants, of whom 556 (38.8%) were nulliparous and 540 (37.7%) were treated in the United States.20
The second source is a published, peer-reviewed Phase 2 trial comparing Mirena with two smaller, lower-dose levonorgestrel-releasing devices, with the lowest-dose product corresponding to the marketed Skyla product.21 In the Phase 2 trial, all 738 women given Mirena or the smaller devices experienced successful placement, with 98.5% of placements achieved on the first attempt. Investigators rated placement for the smaller devices “easy” in 455 of 484 (94.0%) women, compared with 219 of 254 (86.2%) women given Mirena (P <.001). Most of the women given the smaller devices rated their pain with insertion as “mild pain” or “no pain,” compared with those given Mirena (72.3% vs 57.9%; P <.001). Adverse events were similar between users of the different products, except that significantly more women were classified as having an ovarian cyst among Mirena users than among users of the smaller, low-dose devices (22% vs 6%; P <.0001).
Little difference in "clinically relevant" effects
The claim that Skyla has an advantage over Mirena or ParaGard falls short on closer inspection. Although a clinician may prefer easy insertion and a patient with no pain, only very difficult or severely painful placements have clinical relevance.
Investigators rated only 4 of 254 (1.6%) Mirena insertions as “very difficult,” compared with 4 of 484 (0.8%) for the smaller devices (P=.46). Further, women found Mirena insertion to cause severe pain in only 17 of 254 (6.7%) insertions, compared with 21 of 484 (4.3%) placements of the smaller devices (P=.22). The smaller device and inserter, therefore, may have no clinical advantage.
Adverse events were similar
The data on adverse events are similarly misleading. Investigators in the Phase 2 study found that the lower-dose levonorgestrel-releasing IUDs had an 8.6% rate of ovarian cysts and the Mirena had a 22% rate (P <.0001). However, the Phase 2 study included a pelvic ultrasound examination at every visit, and ovarian cysts were included as an adverse event if the size was 3 cm or greater, regardless of symptoms.
Complaints of abdominal or low abdominal pain were as common among Mirena users as among users of the smaller devices, so this finding likely represents asymptomatic, clinically irrelevant cysts.
Most ovarian cysts found in users of the levonorgestrel-releasing intrauterine system are asymptomatic.22
Fewer Skyla users developed amenorrhea
Bleeding patterns differed between the products. Users of the smaller, low-dose device reported slightly more spotting and bleeding over the course of a month. In the Phase 2 trial, at the end of 3 years, only 12.7% of Skyla users achieved amenorrhea, compared with 23.6% of Mirena users. The amenorrhea rate for Mirena was very similar to the 20% rate reported in earlier studies,23,24 but the rate for Skyla was even lower (6%) in the larger Phase 3 study.
What about efficacy?
If there are no real advantages to be gained from the size of the device and inserter in terms of pain, and no real improvement in adverse effects or bleeding patterns, what about efficacy?
No direct comparisons are available, but if the devices are evaluated in terms of their first-year Pearl index rating from Phase 3 studies for approval in the United States, then among a cohort of 100,000 users, about 190 Mirena users would become pregnant in the first year, compared with 410 Skyla users.
All IUDs are considered highly effective contraceptives, but small relative differences can have a large impact on a population level if the methods are not used correctly or patients are not counseled appropriately. Although it is more effective than user-dependent contraceptives such as the pill, Skyla is the least effective of the highly effective methods available. If the device has any real benefits in comparison with the other IUDs, they must be better demonstrated with additional data.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
ObGyns have done much to increase the use of long-acting reversible contraceptives such as the IUD (Mirena, ParaGard), the etonogestrel implant (Implanon, Nexplanon), and the injectable contraceptive depot medroxyprogesterone acetate (Depo-Provera). We applaud this success and urge ObGyns to continue prescribing these options.
In addition, if we want to have a positive impact on the unintended pregnancy rate, we need to increase awareness of, access to, and use of the most effective contraceptive options in our community of providers and among our patients. We also need to eliminate barriers to use of the most effective methods—eg, discussing ulipristal acetate with our patients and providing advance prescriptions. We also need to be cautious about adopting some innovations, as the data for Skyla and Essure illustrate. They may be terrific options for very specific populations of women, but indiscriminate use may, paradoxically, increase the rate of unintended pregnancy.
UPDATE ON CONTRACEPTION
- An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
“Emergency contraception,” “the morning-after pill,” and “Plan B” are all phrases commonly used in most gynecologists’ offices. Regrettably, these phrases are not heard as frequently among patients. With half of all pregnancies unintended and 40% of these pregnancies ending in abortion, there is clearly an unmet need for both contraception and emergency contraception (EC). Although more women have turned to EC in recent years, this contraceptive approach remains highly underutilized in the US population. Despite some increase in usage, we have not yet realized a lower rate of unintended pregnancy or abortion.
Yuzpe and colleagues first published findings on the use of combined oral contraceptives (OCs) for postcoital contraception in 1974. Since then, researchers have been trying to manipulate various hormonal configurations in an attempt to best prevent pregnancy after unprotected intercourse. For years, we have quoted success rates as high as 85% when EC is initiated within 72 hours of unprotected intercourse1—but early studies may have overestimated the ability of EC to prevent unintended pregnancy. More recent investigations have shown that the magical “morning-after pill” and the physicians recommending it are long overdue for a wake-up call.
This installment of the Update on Contraception will review recent evidence on the efficacy of EC and make recommendations for practice, focusing on:
- the reasons EC has failed to reduce the rate of unintended pregnancy
- the efficacy of oral levonorgestrel (LNG) versus ulipristal acetate
- the impact of overweight and obesity on the efficacy of oral agents
- the overall superiority of the copper intrauterine device (IUD).
Half of all pregnancies are unintended, and 40% of unintended pregnancies end in abortion. These figures reflect an unmet need for both contraception and emergency contraception, which remains highly underutilized in the United States.
Access to EC is increasing, but women still lack basic information about it
Kavanaugh M, Schwarz EB. Counseling about and use of emergency contraception in the United States. Perspect Sex Reprod Health. 2008;40(2):81–86.
Kavanaugh M, Williams S, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578–2581.
In 1974, Yuzpe and colleagues first published findings on the use of combined estrogen-progestin OCs for postcoital contraception.2 At the same time, Kesseru and colleagues were evaluating progestin-only regimens for the same purpose.3
For many subsequent years, combinations of common OC pills containing ethinyl estradiol and LNG were used for EC, until 1998, when a progestin-only method containing two 0.75-mg LNG pills was approved by the Food and Drug Administration (FDA) and marketed in the United States under the brand name Plan B. That approval was based on a double-blind, randomized trial by the World Health Organization that demonstrated an almost threefold higher incidence of pregnancy with use of the Yuzpe regimen, compared with this LNG regimen.1
Access to the LNG-only method in the United States increased when the product was given behind-the-counter status in 2006, making it possible for women 18 years and older to obtain the medication without a prescription. In 2009, access was approved—also without a prescription—for 17-year-old women. The same year, the FDA approved Plan B One-Step, allowing women to take both 0.75-mg tablets together as a single tablet, theoretically improving treatment adherence.
Seeking a way to further increase use of EC, many investigators explored the potential benefits of advance provision. The idea was not new, as it had been proposed even for the Yuzpe method, and utilization increased significantly after 2006. Reviews of data from the National Survey of Family Growth (NSFG) showed an increase in EC use among women who had ever had sexual intercourse with a man from 4.2% of women surveyed in 2002 to 9.7% of women surveyed in 2006 to 2008, as reported by Kavanaugh and colleagues. Regrettably, this increase did not reduce the number of unintended pregnancies during the same time periods. Clearly, men and women fail to use EC every time they are at risk of unintended pregnancy.4
One of the biggest barriers to EC use is probably the lack of information patients receive from providers. Only 3% of respondents to the 2006–2008 NSFG indicated that they had received any counseling about EC in the past year, a number relatively unchanged from the 2002 survey. This finding suggests that the increase in EC use is likely due to the publicity surrounding the EC status change in 2006.
Greater availability and less restrictive access to EC has not reduced the rate of unintended pregnancy in the United States. However, improvements in the counseling of women may have an impact on the pregnancy rate. As the National Survey of Family Growth reveals, only about 3% of women receive any counseling about EC in a given year. For utilization of EC to increase, women need to be aware that it exists. Providers must begin to change their practices and discuss EC at all routine appointments before the public health benefit of a decrease in unintended pregnancies can ever be realized.
Ulipristal acetate makes its debut—and demonstrates superiority to LNG
Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555(9714)–562.
Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2pt1):257–263.
In 1998, the first-generation antiprogestin mifepristone was approved for use in France in medical abortion. As early as 1991, researchers were already investigating mifepristone as a method of EC, with great success.5,6 Overall, mifepristone was more effective and had fewer side effects than oral LNG, although the onset of menses was delayed with mifepristone.7 Mifepristone is available as EC in Russia and China, but its use in other countries is limited by social and political constraints.
Enter ulipristal acetate (UPA), a second-generation progesterone receptor modulator. Unlike its predecessor mifepristone, UPA (brand name, ella) is not approved for pregnancy termination, and no studies have been performed to evaluate the effects of UPA on an existing pregnancy. Because effects on pregnancy are unknown, the manufacturer states that exclusion of pregnancy is a requirement before UPA can be prescribed for EC.
The data on UPA as emergency contraception
UPA has been evaluated for EC in two large randomized trials.8,9 In the first study, UPA was administered in a 50-mg dose as long as 72 hours after unprotected intercourse. In the second study, conducted by Glasier and colleagues, a 30-mg micronized dose (bioequivalent to the 50-mg nonmicronized dose) was used as long as 120 hours after unprotected intercourse. Participants in both studies were randomized to UPA or oral LNG.
The first study showed UPA to be at least as effective as LNG in preventing pregnancy when taken within 72 hours after unprotected intercourse. The efficacy of UPA did not appear to decline even when it was taken 48 to 72 hours after unprotected intercourse, unlike the efficacy of LNG.
The second study similarly found UPA to be non-inferior to LNG. Although neither study was powered to demonstrate superiority, both did show that UPA seemed to prevent more pregnancies than LNG.
Glasier and colleagues then performed a meta-analysis of both studies, demonstrating that UPA almost halved the risk of pregnancy, compared with LNG, in women who received treatment within 120 hours after intercourse, with a reduction of almost two thirds when UPA was taken within 24 hours of unprotected intercourse.
UPA has FDA approval for use within 120 hours after unprotected intercourse and requires a prescription. Although the data leading to this approval are incredibly encouraging, fewer than 200 of more than 2,000 women in three studies performed with UPA took EC 96 to 120 hours after intercourse. With such a small number of women actually tested in this time range, physicians should use caution when counseling patients about the efficacy of UPA when it is taken more than 96 hours after unprotected intercourse.8-10
UPA is more expensive than LNG, which may be a barrier to use by some women. However, because the probability of becoming pregnant when taking UPA within 120 hours of unprotected intercourse is lower than with LNG, the cost differential between drugs is much smaller when total costs—including the cost of unintended pregnancy—are consid-ered.11
Although the LNG-only method is the only EC that is available without a prescription, UPA appears to be more effective, particularly when it is taken more than 72 hours after unprotected intercourse. However, providers should be aware that a relatively small number of women have been studied with UPA beyond 72 hours after unprotected intercourse.
Although LNG-only EC is available behind the counter, the superiority of UPA means that physicians should discuss EC with patients during routine appointments and consider advance provision. For patients, cost and access will be important issues when deciding whether to use LNG or UPA.
EC is more likely to fail in overweight and obese women
Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2, 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127.
Westhoff CT, Torgal AL, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use. Obstet Gynecol. 2010;116(2 pt 2):275–283.
As we observed, despite more widespread use of EC after the LNG-only method was made available without a prescription, we have not realized the public health benefit of a decreased rate of unintended pregnancy or abortion.4 Studies have shown that, despite taking EC, women who have further acts of intercourse in the same cycle of EC use are more likely to conceive.12,13
We now have clear information about another specific population in which EC is more likely to fail: overweight and obese women. Compared with women of normal weight (body mass index [BMI] <25), overweight women (BMI 25–30) had a risk of pregnancy 1.5 times greater, and obese women (BMI ≥30) had a risk of pregnancy more than three times greater.13
Pregnancy rate among obese women using LNG was the same as the background rate
Obese women who used LNG as EC had a pregnancy rate of 5.8%, which is approximately equivalent to the overall pregnancy rate expected in the absence of EC. Overweight women in the LNG group had a relative risk of pregnancy that was double that of normal or underweight women, whereas overweight women taking UPA had the same risk as normal or underweight women taking the same medication.
When researchers compared pregnancy rates by weight instead of BMI, differences persisted between the two treatment options, with a limit of efficacy reached at a weight of 70 kg (154 lb) for LNG, compared with 88 kg (194 lb) for UPA.
OC hormone absorption is slower in obesity
Two recent studies—by Edelman and colleagues and Westhoff and coworkers—have demonstrated that OC hormone absorption is slower in obese women than it is in women of normal weight. With EC, immediate absorption is important; this delay could explain the lower efficacy in obese women. No studies have evaluated whether a higher or double dose of LNG would improve efficacy. Like women who experience repeated acts of unprotected intercourse, overweight and obese women are at high risk of EC failure and should be counseled about this risk.
As the incidence of obesity continues to increase exponentially in the United States, the efficacy of our commonly used methods of EC will continue to decline. At a minimum, overweight and obese women should be counseled to take UPA rather than LNG because of its increased efficacy in this population. We also need to inform overweight patients that their risk of pregnancy is higher than is commonly quoted.
Have we overlooked the best available emergency contraceptive?
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205–1210.
Turok D, Gurtcheff S, Handley E, et al. A pilot study of the Copper T380A IUD and levonorgestral for emergency contraception. Contraception. 2010;82(6):520–525.
The copper IUD has always been the most effective EC available. Not only does it prevent pregnancy when inserted as EC, but it continues to provide long-term, reversible contraception for 10 years or longer. Two large studies—one of them published within the past year—found efficacy rates of 96.9% and 100%, much higher than those associated with oral EC, with only two pregnancies occurring in more than 2,000 women.14,15
Although use of the IUD as EC was described as early as 1976, adoption of this method has been minimal in the United States.16 One reason may be the need for a clinician to insert the device, but many providers undoubtedly dismiss the IUD as an option for EC, believing that American women are unwilling to accept it. Some providers maintain the longstanding opinion that the IUD is an option only for parous women, although this notion has been cast aside by layers of medical evidence, as reviewed by current Centers for Disease Control and Prevention (CDC) medical eligibility criteria for contraception.17
All women should be counseled about the long-term benefits of the copper IUD, the most reliable method of EC. The copper IUD not only provides effective emergency contraception but also long-term contraception for 10 years or more. Therefore, we should offer the copper IUD as first-line treatment for women seeking EC (FIGURE). This method is likely to be much more acceptable to patients than previously assumed.
Women are more accepting of the IUD than we thought
Schwarz and colleagues surveyed 412 women in Pittsburgh family planning clinics who were seeking EC or pregnancy testing and found that 15% of these women would be interested in same-day insertion of an IUD.18 This number increased if the IUD was free among women who reported difficulty with access to contraception.
In an observational study, Turok and colleagues offered women who were seeking EC a choice between the copper IUD and oral LNG and followed them for 6 months. Both methods were offered free of charge. They had assumed that, for every 20 women choosing oral LNG, one would choose the copper IUD. What they found was quite different: For every 1.5 women who chose oral LNG, one chose the copper IUD. Even more impressive was the number of women still using highly effective contraception (IUD, implant, or sterilization) 6 months later—4.5% in the oral LNG group and 61.5% in the IUD group. By the end of the 6-month period, two pregnancies had occurred in the oral LNG group and none in the IUD group.
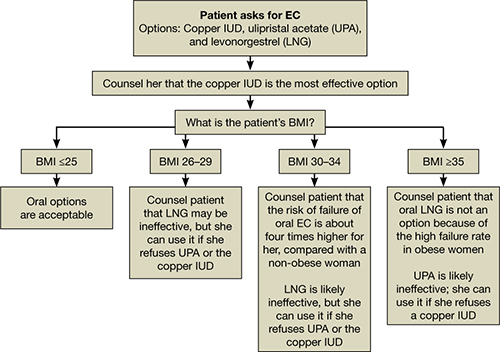
How to counsel a patient seeking emergency contraceptionWe want to hear from you! Tell us what you think.
1. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428-433.
2. Yuzpe A, Thurlow H, Ramzy I, Leyshon J. Post coital contraception–A pilot study. J Reprod Med. 1974;13(2):53-58.
3. Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10(4):411-424.
4. Polis CB, Schaffer K, Banchard K, et al. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev. 2007;(2):CD005497.-
5. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Postcoital contraception with mifepristone. Lancet. 1991;337(8754):1414-1415.
6. Webb AM. Alternative treatments in oral postcoital contraception: interim results. Adv Contracept. 1991;7(2–3):271-279.
7. Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.-
8. Creinin MD, Schlaff W, Archer D, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089-1097.
9. Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
10. Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 pt 1):257-263.
11. Thomas CM, Schmid R, Cameron S. Is it worth paying more for emergency hormonal contraception? The cost-effectiveness of ulipristal acetate versus levonorgestrel 1.5 mg. J Fam Plann Reprod Health Care. 2010;36(4):197-201.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepris-tone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803-1810.
13. Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
14. Zhou LY, Xiao BL. Emergency contraception with Multiload Cu-375 SL IUD: A multicenter clinical trial. Contraception. 2001;64(2):107-112.
15. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205-1210.
16. Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv Plan Parent. 1976;11(1):24-29.
17. Centers for Disease Control and Prevention. US medical eligibility criteria contraceptive use 2010. MMWR. 2010;59 (RR04):1-6.
18. Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113(4):833-839.
- An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
“Emergency contraception,” “the morning-after pill,” and “Plan B” are all phrases commonly used in most gynecologists’ offices. Regrettably, these phrases are not heard as frequently among patients. With half of all pregnancies unintended and 40% of these pregnancies ending in abortion, there is clearly an unmet need for both contraception and emergency contraception (EC). Although more women have turned to EC in recent years, this contraceptive approach remains highly underutilized in the US population. Despite some increase in usage, we have not yet realized a lower rate of unintended pregnancy or abortion.
Yuzpe and colleagues first published findings on the use of combined oral contraceptives (OCs) for postcoital contraception in 1974. Since then, researchers have been trying to manipulate various hormonal configurations in an attempt to best prevent pregnancy after unprotected intercourse. For years, we have quoted success rates as high as 85% when EC is initiated within 72 hours of unprotected intercourse1—but early studies may have overestimated the ability of EC to prevent unintended pregnancy. More recent investigations have shown that the magical “morning-after pill” and the physicians recommending it are long overdue for a wake-up call.
This installment of the Update on Contraception will review recent evidence on the efficacy of EC and make recommendations for practice, focusing on:
- the reasons EC has failed to reduce the rate of unintended pregnancy
- the efficacy of oral levonorgestrel (LNG) versus ulipristal acetate
- the impact of overweight and obesity on the efficacy of oral agents
- the overall superiority of the copper intrauterine device (IUD).

Half of all pregnancies are unintended, and 40% of unintended pregnancies end in abortion. These figures reflect an unmet need for both contraception and emergency contraception, which remains highly underutilized in the United States.
Access to EC is increasing, but women still lack basic information about it
Kavanaugh M, Schwarz EB. Counseling about and use of emergency contraception in the United States. Perspect Sex Reprod Health. 2008;40(2):81–86.
Kavanaugh M, Williams S, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578–2581.
In 1974, Yuzpe and colleagues first published findings on the use of combined estrogen-progestin OCs for postcoital contraception.2 At the same time, Kesseru and colleagues were evaluating progestin-only regimens for the same purpose.3
For many subsequent years, combinations of common OC pills containing ethinyl estradiol and LNG were used for EC, until 1998, when a progestin-only method containing two 0.75-mg LNG pills was approved by the Food and Drug Administration (FDA) and marketed in the United States under the brand name Plan B. That approval was based on a double-blind, randomized trial by the World Health Organization that demonstrated an almost threefold higher incidence of pregnancy with use of the Yuzpe regimen, compared with this LNG regimen.1
Access to the LNG-only method in the United States increased when the product was given behind-the-counter status in 2006, making it possible for women 18 years and older to obtain the medication without a prescription. In 2009, access was approved—also without a prescription—for 17-year-old women. The same year, the FDA approved Plan B One-Step, allowing women to take both 0.75-mg tablets together as a single tablet, theoretically improving treatment adherence.
Seeking a way to further increase use of EC, many investigators explored the potential benefits of advance provision. The idea was not new, as it had been proposed even for the Yuzpe method, and utilization increased significantly after 2006. Reviews of data from the National Survey of Family Growth (NSFG) showed an increase in EC use among women who had ever had sexual intercourse with a man from 4.2% of women surveyed in 2002 to 9.7% of women surveyed in 2006 to 2008, as reported by Kavanaugh and colleagues. Regrettably, this increase did not reduce the number of unintended pregnancies during the same time periods. Clearly, men and women fail to use EC every time they are at risk of unintended pregnancy.4
One of the biggest barriers to EC use is probably the lack of information patients receive from providers. Only 3% of respondents to the 2006–2008 NSFG indicated that they had received any counseling about EC in the past year, a number relatively unchanged from the 2002 survey. This finding suggests that the increase in EC use is likely due to the publicity surrounding the EC status change in 2006.
Greater availability and less restrictive access to EC has not reduced the rate of unintended pregnancy in the United States. However, improvements in the counseling of women may have an impact on the pregnancy rate. As the National Survey of Family Growth reveals, only about 3% of women receive any counseling about EC in a given year. For utilization of EC to increase, women need to be aware that it exists. Providers must begin to change their practices and discuss EC at all routine appointments before the public health benefit of a decrease in unintended pregnancies can ever be realized.
Ulipristal acetate makes its debut—and demonstrates superiority to LNG
Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555(9714)–562.
Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2pt1):257–263.
In 1998, the first-generation antiprogestin mifepristone was approved for use in France in medical abortion. As early as 1991, researchers were already investigating mifepristone as a method of EC, with great success.5,6 Overall, mifepristone was more effective and had fewer side effects than oral LNG, although the onset of menses was delayed with mifepristone.7 Mifepristone is available as EC in Russia and China, but its use in other countries is limited by social and political constraints.
Enter ulipristal acetate (UPA), a second-generation progesterone receptor modulator. Unlike its predecessor mifepristone, UPA (brand name, ella) is not approved for pregnancy termination, and no studies have been performed to evaluate the effects of UPA on an existing pregnancy. Because effects on pregnancy are unknown, the manufacturer states that exclusion of pregnancy is a requirement before UPA can be prescribed for EC.
The data on UPA as emergency contraception
UPA has been evaluated for EC in two large randomized trials.8,9 In the first study, UPA was administered in a 50-mg dose as long as 72 hours after unprotected intercourse. In the second study, conducted by Glasier and colleagues, a 30-mg micronized dose (bioequivalent to the 50-mg nonmicronized dose) was used as long as 120 hours after unprotected intercourse. Participants in both studies were randomized to UPA or oral LNG.
The first study showed UPA to be at least as effective as LNG in preventing pregnancy when taken within 72 hours after unprotected intercourse. The efficacy of UPA did not appear to decline even when it was taken 48 to 72 hours after unprotected intercourse, unlike the efficacy of LNG.
The second study similarly found UPA to be non-inferior to LNG. Although neither study was powered to demonstrate superiority, both did show that UPA seemed to prevent more pregnancies than LNG.
Glasier and colleagues then performed a meta-analysis of both studies, demonstrating that UPA almost halved the risk of pregnancy, compared with LNG, in women who received treatment within 120 hours after intercourse, with a reduction of almost two thirds when UPA was taken within 24 hours of unprotected intercourse.
UPA has FDA approval for use within 120 hours after unprotected intercourse and requires a prescription. Although the data leading to this approval are incredibly encouraging, fewer than 200 of more than 2,000 women in three studies performed with UPA took EC 96 to 120 hours after intercourse. With such a small number of women actually tested in this time range, physicians should use caution when counseling patients about the efficacy of UPA when it is taken more than 96 hours after unprotected intercourse.8-10
UPA is more expensive than LNG, which may be a barrier to use by some women. However, because the probability of becoming pregnant when taking UPA within 120 hours of unprotected intercourse is lower than with LNG, the cost differential between drugs is much smaller when total costs—including the cost of unintended pregnancy—are consid-ered.11
Although the LNG-only method is the only EC that is available without a prescription, UPA appears to be more effective, particularly when it is taken more than 72 hours after unprotected intercourse. However, providers should be aware that a relatively small number of women have been studied with UPA beyond 72 hours after unprotected intercourse.
Although LNG-only EC is available behind the counter, the superiority of UPA means that physicians should discuss EC with patients during routine appointments and consider advance provision. For patients, cost and access will be important issues when deciding whether to use LNG or UPA.
EC is more likely to fail in overweight and obese women
Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2, 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127.
Westhoff CT, Torgal AL, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use. Obstet Gynecol. 2010;116(2 pt 2):275–283.
As we observed, despite more widespread use of EC after the LNG-only method was made available without a prescription, we have not realized the public health benefit of a decreased rate of unintended pregnancy or abortion.4 Studies have shown that, despite taking EC, women who have further acts of intercourse in the same cycle of EC use are more likely to conceive.12,13
We now have clear information about another specific population in which EC is more likely to fail: overweight and obese women. Compared with women of normal weight (body mass index [BMI] <25), overweight women (BMI 25–30) had a risk of pregnancy 1.5 times greater, and obese women (BMI ≥30) had a risk of pregnancy more than three times greater.13
Pregnancy rate among obese women using LNG was the same as the background rate
Obese women who used LNG as EC had a pregnancy rate of 5.8%, which is approximately equivalent to the overall pregnancy rate expected in the absence of EC. Overweight women in the LNG group had a relative risk of pregnancy that was double that of normal or underweight women, whereas overweight women taking UPA had the same risk as normal or underweight women taking the same medication.
When researchers compared pregnancy rates by weight instead of BMI, differences persisted between the two treatment options, with a limit of efficacy reached at a weight of 70 kg (154 lb) for LNG, compared with 88 kg (194 lb) for UPA.
OC hormone absorption is slower in obesity
Two recent studies—by Edelman and colleagues and Westhoff and coworkers—have demonstrated that OC hormone absorption is slower in obese women than it is in women of normal weight. With EC, immediate absorption is important; this delay could explain the lower efficacy in obese women. No studies have evaluated whether a higher or double dose of LNG would improve efficacy. Like women who experience repeated acts of unprotected intercourse, overweight and obese women are at high risk of EC failure and should be counseled about this risk.
As the incidence of obesity continues to increase exponentially in the United States, the efficacy of our commonly used methods of EC will continue to decline. At a minimum, overweight and obese women should be counseled to take UPA rather than LNG because of its increased efficacy in this population. We also need to inform overweight patients that their risk of pregnancy is higher than is commonly quoted.
Have we overlooked the best available emergency contraceptive?
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205–1210.
Turok D, Gurtcheff S, Handley E, et al. A pilot study of the Copper T380A IUD and levonorgestral for emergency contraception. Contraception. 2010;82(6):520–525.
The copper IUD has always been the most effective EC available. Not only does it prevent pregnancy when inserted as EC, but it continues to provide long-term, reversible contraception for 10 years or longer. Two large studies—one of them published within the past year—found efficacy rates of 96.9% and 100%, much higher than those associated with oral EC, with only two pregnancies occurring in more than 2,000 women.14,15
Although use of the IUD as EC was described as early as 1976, adoption of this method has been minimal in the United States.16 One reason may be the need for a clinician to insert the device, but many providers undoubtedly dismiss the IUD as an option for EC, believing that American women are unwilling to accept it. Some providers maintain the longstanding opinion that the IUD is an option only for parous women, although this notion has been cast aside by layers of medical evidence, as reviewed by current Centers for Disease Control and Prevention (CDC) medical eligibility criteria for contraception.17
All women should be counseled about the long-term benefits of the copper IUD, the most reliable method of EC. The copper IUD not only provides effective emergency contraception but also long-term contraception for 10 years or more. Therefore, we should offer the copper IUD as first-line treatment for women seeking EC (FIGURE). This method is likely to be much more acceptable to patients than previously assumed.
Women are more accepting of the IUD than we thought
Schwarz and colleagues surveyed 412 women in Pittsburgh family planning clinics who were seeking EC or pregnancy testing and found that 15% of these women would be interested in same-day insertion of an IUD.18 This number increased if the IUD was free among women who reported difficulty with access to contraception.
In an observational study, Turok and colleagues offered women who were seeking EC a choice between the copper IUD and oral LNG and followed them for 6 months. Both methods were offered free of charge. They had assumed that, for every 20 women choosing oral LNG, one would choose the copper IUD. What they found was quite different: For every 1.5 women who chose oral LNG, one chose the copper IUD. Even more impressive was the number of women still using highly effective contraception (IUD, implant, or sterilization) 6 months later—4.5% in the oral LNG group and 61.5% in the IUD group. By the end of the 6-month period, two pregnancies had occurred in the oral LNG group and none in the IUD group.

How to counsel a patient seeking emergency contraceptionWe want to hear from you! Tell us what you think.
- An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
“Emergency contraception,” “the morning-after pill,” and “Plan B” are all phrases commonly used in most gynecologists’ offices. Regrettably, these phrases are not heard as frequently among patients. With half of all pregnancies unintended and 40% of these pregnancies ending in abortion, there is clearly an unmet need for both contraception and emergency contraception (EC). Although more women have turned to EC in recent years, this contraceptive approach remains highly underutilized in the US population. Despite some increase in usage, we have not yet realized a lower rate of unintended pregnancy or abortion.
Yuzpe and colleagues first published findings on the use of combined oral contraceptives (OCs) for postcoital contraception in 1974. Since then, researchers have been trying to manipulate various hormonal configurations in an attempt to best prevent pregnancy after unprotected intercourse. For years, we have quoted success rates as high as 85% when EC is initiated within 72 hours of unprotected intercourse1—but early studies may have overestimated the ability of EC to prevent unintended pregnancy. More recent investigations have shown that the magical “morning-after pill” and the physicians recommending it are long overdue for a wake-up call.
This installment of the Update on Contraception will review recent evidence on the efficacy of EC and make recommendations for practice, focusing on:
- the reasons EC has failed to reduce the rate of unintended pregnancy
- the efficacy of oral levonorgestrel (LNG) versus ulipristal acetate
- the impact of overweight and obesity on the efficacy of oral agents
- the overall superiority of the copper intrauterine device (IUD).

Half of all pregnancies are unintended, and 40% of unintended pregnancies end in abortion. These figures reflect an unmet need for both contraception and emergency contraception, which remains highly underutilized in the United States.
Access to EC is increasing, but women still lack basic information about it
Kavanaugh M, Schwarz EB. Counseling about and use of emergency contraception in the United States. Perspect Sex Reprod Health. 2008;40(2):81–86.
Kavanaugh M, Williams S, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578–2581.
In 1974, Yuzpe and colleagues first published findings on the use of combined estrogen-progestin OCs for postcoital contraception.2 At the same time, Kesseru and colleagues were evaluating progestin-only regimens for the same purpose.3
For many subsequent years, combinations of common OC pills containing ethinyl estradiol and LNG were used for EC, until 1998, when a progestin-only method containing two 0.75-mg LNG pills was approved by the Food and Drug Administration (FDA) and marketed in the United States under the brand name Plan B. That approval was based on a double-blind, randomized trial by the World Health Organization that demonstrated an almost threefold higher incidence of pregnancy with use of the Yuzpe regimen, compared with this LNG regimen.1
Access to the LNG-only method in the United States increased when the product was given behind-the-counter status in 2006, making it possible for women 18 years and older to obtain the medication without a prescription. In 2009, access was approved—also without a prescription—for 17-year-old women. The same year, the FDA approved Plan B One-Step, allowing women to take both 0.75-mg tablets together as a single tablet, theoretically improving treatment adherence.
Seeking a way to further increase use of EC, many investigators explored the potential benefits of advance provision. The idea was not new, as it had been proposed even for the Yuzpe method, and utilization increased significantly after 2006. Reviews of data from the National Survey of Family Growth (NSFG) showed an increase in EC use among women who had ever had sexual intercourse with a man from 4.2% of women surveyed in 2002 to 9.7% of women surveyed in 2006 to 2008, as reported by Kavanaugh and colleagues. Regrettably, this increase did not reduce the number of unintended pregnancies during the same time periods. Clearly, men and women fail to use EC every time they are at risk of unintended pregnancy.4
One of the biggest barriers to EC use is probably the lack of information patients receive from providers. Only 3% of respondents to the 2006–2008 NSFG indicated that they had received any counseling about EC in the past year, a number relatively unchanged from the 2002 survey. This finding suggests that the increase in EC use is likely due to the publicity surrounding the EC status change in 2006.
Greater availability and less restrictive access to EC has not reduced the rate of unintended pregnancy in the United States. However, improvements in the counseling of women may have an impact on the pregnancy rate. As the National Survey of Family Growth reveals, only about 3% of women receive any counseling about EC in a given year. For utilization of EC to increase, women need to be aware that it exists. Providers must begin to change their practices and discuss EC at all routine appointments before the public health benefit of a decrease in unintended pregnancies can ever be realized.
Ulipristal acetate makes its debut—and demonstrates superiority to LNG
Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555(9714)–562.
Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2pt1):257–263.
In 1998, the first-generation antiprogestin mifepristone was approved for use in France in medical abortion. As early as 1991, researchers were already investigating mifepristone as a method of EC, with great success.5,6 Overall, mifepristone was more effective and had fewer side effects than oral LNG, although the onset of menses was delayed with mifepristone.7 Mifepristone is available as EC in Russia and China, but its use in other countries is limited by social and political constraints.
Enter ulipristal acetate (UPA), a second-generation progesterone receptor modulator. Unlike its predecessor mifepristone, UPA (brand name, ella) is not approved for pregnancy termination, and no studies have been performed to evaluate the effects of UPA on an existing pregnancy. Because effects on pregnancy are unknown, the manufacturer states that exclusion of pregnancy is a requirement before UPA can be prescribed for EC.
The data on UPA as emergency contraception
UPA has been evaluated for EC in two large randomized trials.8,9 In the first study, UPA was administered in a 50-mg dose as long as 72 hours after unprotected intercourse. In the second study, conducted by Glasier and colleagues, a 30-mg micronized dose (bioequivalent to the 50-mg nonmicronized dose) was used as long as 120 hours after unprotected intercourse. Participants in both studies were randomized to UPA or oral LNG.
The first study showed UPA to be at least as effective as LNG in preventing pregnancy when taken within 72 hours after unprotected intercourse. The efficacy of UPA did not appear to decline even when it was taken 48 to 72 hours after unprotected intercourse, unlike the efficacy of LNG.
The second study similarly found UPA to be non-inferior to LNG. Although neither study was powered to demonstrate superiority, both did show that UPA seemed to prevent more pregnancies than LNG.
Glasier and colleagues then performed a meta-analysis of both studies, demonstrating that UPA almost halved the risk of pregnancy, compared with LNG, in women who received treatment within 120 hours after intercourse, with a reduction of almost two thirds when UPA was taken within 24 hours of unprotected intercourse.
UPA has FDA approval for use within 120 hours after unprotected intercourse and requires a prescription. Although the data leading to this approval are incredibly encouraging, fewer than 200 of more than 2,000 women in three studies performed with UPA took EC 96 to 120 hours after intercourse. With such a small number of women actually tested in this time range, physicians should use caution when counseling patients about the efficacy of UPA when it is taken more than 96 hours after unprotected intercourse.8-10
UPA is more expensive than LNG, which may be a barrier to use by some women. However, because the probability of becoming pregnant when taking UPA within 120 hours of unprotected intercourse is lower than with LNG, the cost differential between drugs is much smaller when total costs—including the cost of unintended pregnancy—are consid-ered.11
Although the LNG-only method is the only EC that is available without a prescription, UPA appears to be more effective, particularly when it is taken more than 72 hours after unprotected intercourse. However, providers should be aware that a relatively small number of women have been studied with UPA beyond 72 hours after unprotected intercourse.
Although LNG-only EC is available behind the counter, the superiority of UPA means that physicians should discuss EC with patients during routine appointments and consider advance provision. For patients, cost and access will be important issues when deciding whether to use LNG or UPA.
EC is more likely to fail in overweight and obese women
Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2, 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127.
Westhoff CT, Torgal AL, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use. Obstet Gynecol. 2010;116(2 pt 2):275–283.
As we observed, despite more widespread use of EC after the LNG-only method was made available without a prescription, we have not realized the public health benefit of a decreased rate of unintended pregnancy or abortion.4 Studies have shown that, despite taking EC, women who have further acts of intercourse in the same cycle of EC use are more likely to conceive.12,13
We now have clear information about another specific population in which EC is more likely to fail: overweight and obese women. Compared with women of normal weight (body mass index [BMI] <25), overweight women (BMI 25–30) had a risk of pregnancy 1.5 times greater, and obese women (BMI ≥30) had a risk of pregnancy more than three times greater.13
Pregnancy rate among obese women using LNG was the same as the background rate
Obese women who used LNG as EC had a pregnancy rate of 5.8%, which is approximately equivalent to the overall pregnancy rate expected in the absence of EC. Overweight women in the LNG group had a relative risk of pregnancy that was double that of normal or underweight women, whereas overweight women taking UPA had the same risk as normal or underweight women taking the same medication.
When researchers compared pregnancy rates by weight instead of BMI, differences persisted between the two treatment options, with a limit of efficacy reached at a weight of 70 kg (154 lb) for LNG, compared with 88 kg (194 lb) for UPA.
OC hormone absorption is slower in obesity
Two recent studies—by Edelman and colleagues and Westhoff and coworkers—have demonstrated that OC hormone absorption is slower in obese women than it is in women of normal weight. With EC, immediate absorption is important; this delay could explain the lower efficacy in obese women. No studies have evaluated whether a higher or double dose of LNG would improve efficacy. Like women who experience repeated acts of unprotected intercourse, overweight and obese women are at high risk of EC failure and should be counseled about this risk.
As the incidence of obesity continues to increase exponentially in the United States, the efficacy of our commonly used methods of EC will continue to decline. At a minimum, overweight and obese women should be counseled to take UPA rather than LNG because of its increased efficacy in this population. We also need to inform overweight patients that their risk of pregnancy is higher than is commonly quoted.
Have we overlooked the best available emergency contraceptive?
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205–1210.
Turok D, Gurtcheff S, Handley E, et al. A pilot study of the Copper T380A IUD and levonorgestral for emergency contraception. Contraception. 2010;82(6):520–525.
The copper IUD has always been the most effective EC available. Not only does it prevent pregnancy when inserted as EC, but it continues to provide long-term, reversible contraception for 10 years or longer. Two large studies—one of them published within the past year—found efficacy rates of 96.9% and 100%, much higher than those associated with oral EC, with only two pregnancies occurring in more than 2,000 women.14,15
Although use of the IUD as EC was described as early as 1976, adoption of this method has been minimal in the United States.16 One reason may be the need for a clinician to insert the device, but many providers undoubtedly dismiss the IUD as an option for EC, believing that American women are unwilling to accept it. Some providers maintain the longstanding opinion that the IUD is an option only for parous women, although this notion has been cast aside by layers of medical evidence, as reviewed by current Centers for Disease Control and Prevention (CDC) medical eligibility criteria for contraception.17
All women should be counseled about the long-term benefits of the copper IUD, the most reliable method of EC. The copper IUD not only provides effective emergency contraception but also long-term contraception for 10 years or more. Therefore, we should offer the copper IUD as first-line treatment for women seeking EC (FIGURE). This method is likely to be much more acceptable to patients than previously assumed.
Women are more accepting of the IUD than we thought
Schwarz and colleagues surveyed 412 women in Pittsburgh family planning clinics who were seeking EC or pregnancy testing and found that 15% of these women would be interested in same-day insertion of an IUD.18 This number increased if the IUD was free among women who reported difficulty with access to contraception.
In an observational study, Turok and colleagues offered women who were seeking EC a choice between the copper IUD and oral LNG and followed them for 6 months. Both methods were offered free of charge. They had assumed that, for every 20 women choosing oral LNG, one would choose the copper IUD. What they found was quite different: For every 1.5 women who chose oral LNG, one chose the copper IUD. Even more impressive was the number of women still using highly effective contraception (IUD, implant, or sterilization) 6 months later—4.5% in the oral LNG group and 61.5% in the IUD group. By the end of the 6-month period, two pregnancies had occurred in the oral LNG group and none in the IUD group.

How to counsel a patient seeking emergency contraceptionWe want to hear from you! Tell us what you think.
1. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428-433.
2. Yuzpe A, Thurlow H, Ramzy I, Leyshon J. Post coital contraception–A pilot study. J Reprod Med. 1974;13(2):53-58.
3. Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10(4):411-424.
4. Polis CB, Schaffer K, Banchard K, et al. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev. 2007;(2):CD005497.-
5. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Postcoital contraception with mifepristone. Lancet. 1991;337(8754):1414-1415.
6. Webb AM. Alternative treatments in oral postcoital contraception: interim results. Adv Contracept. 1991;7(2–3):271-279.
7. Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.-
8. Creinin MD, Schlaff W, Archer D, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089-1097.
9. Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
10. Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 pt 1):257-263.
11. Thomas CM, Schmid R, Cameron S. Is it worth paying more for emergency hormonal contraception? The cost-effectiveness of ulipristal acetate versus levonorgestrel 1.5 mg. J Fam Plann Reprod Health Care. 2010;36(4):197-201.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepris-tone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803-1810.
13. Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
14. Zhou LY, Xiao BL. Emergency contraception with Multiload Cu-375 SL IUD: A multicenter clinical trial. Contraception. 2001;64(2):107-112.
15. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205-1210.
16. Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv Plan Parent. 1976;11(1):24-29.
17. Centers for Disease Control and Prevention. US medical eligibility criteria contraceptive use 2010. MMWR. 2010;59 (RR04):1-6.
18. Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113(4):833-839.
1. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428-433.
2. Yuzpe A, Thurlow H, Ramzy I, Leyshon J. Post coital contraception–A pilot study. J Reprod Med. 1974;13(2):53-58.
3. Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10(4):411-424.
4. Polis CB, Schaffer K, Banchard K, et al. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev. 2007;(2):CD005497.-
5. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Postcoital contraception with mifepristone. Lancet. 1991;337(8754):1414-1415.
6. Webb AM. Alternative treatments in oral postcoital contraception: interim results. Adv Contracept. 1991;7(2–3):271-279.
7. Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.-
8. Creinin MD, Schlaff W, Archer D, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089-1097.
9. Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
10. Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 pt 1):257-263.
11. Thomas CM, Schmid R, Cameron S. Is it worth paying more for emergency hormonal contraception? The cost-effectiveness of ulipristal acetate versus levonorgestrel 1.5 mg. J Fam Plann Reprod Health Care. 2010;36(4):197-201.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepris-tone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803-1810.
13. Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
14. Zhou LY, Xiao BL. Emergency contraception with Multiload Cu-375 SL IUD: A multicenter clinical trial. Contraception. 2001;64(2):107-112.
15. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205-1210.
16. Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv Plan Parent. 1976;11(1):24-29.
17. Centers for Disease Control and Prevention. US medical eligibility criteria contraceptive use 2010. MMWR. 2010;59 (RR04):1-6.
18. Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113(4):833-839.
UPDATE: CONTRACEPTION
Dr. Gariepy reports no financial relationships relevant to this article. Dr. Creinin reports that he is a consultant to, and a speaker for, Schering-Plough.
Progestin-only contraception—a diverse group of oral (progestin-only pills, or so-called minipills), injectable (depot medroxyprogesterone acetate), intrauterine (the levonorgestrel intrauterine system), and implantable (etonogestrel implant) methods—may offer advantages over estrogen-containing contraception:
- the flexibility of distinctive methods of delivery
- the ability to initiate the method in postpartum breastfeeding women
- enhanced safety in women who should not be exposed to exogenous estrogens.
Unpredictable bleeding is a major disadvantage of progestin-only contraception, however, and can cause women to discontinue these methods—and discontinuation without an effective backup method creates a high risk of unplanned pregnancy. The significant variability in bleeding patterns among progestin-only contraceptive methods hinders our ability to counsel patients about them.
Furthermore, the lack of uniform definitions of bleeding patterns with hormonal contraception, including progestin-only methods, makes it difficult to counsel women accurately and compare bleeding patterns among methods.
Accurate prediction of the bleeding patterns associated with progestin-only contraception could lower the discontinuation rate. For example, studies have shown that pretreatment counseling about expected side effects increases approximately fourfold the acceptability and continuation of depot medroxyprogesterone acetate.1,2
In this Update, we review the data on bleeding patterns associated with progestin-only contraceptives, including the likelihood of 1) amenorrhea and 2) discontinuation due to changes in the bleeding pattern.
We also discuss what has been learned about the treatment of changes in bleeding patterns induced by progestin-only contraception.
Our goal? To summarize the findings in a comprehensive way that makes it easier for you to discuss expected bleeding patterns with your patients—so that women can choose the method of contraception that is the best fit for them.
Describing bleeding patterns is a challenging task
One of the difficulties of interpreting clinical data on bleeding patterns—with any type of contraception—is the lack of a universally accepted standard for collecting and reporting these data. The first suggestions for standardization were made in 1976, when Rodriguez and colleagues proposed using 90-day reference periods for analysis, as a way to minimize variability among individual menstrual cycles.3
Subsequently, the World Health Organization’s (WHO’s) Special Programme of Research, Development and Research Training in Human Reproduction developed recommendations for data collection, terminology, presentation, and data analysis when reporting vaginal bleeding during clinical trials of hormonal contraception. These recommendations became known as the WHO Belsey criteria (TABLE 1). They remain the standard.4
Under the WHO Belsey system:
- vaginal blood loss for which a woman uses sanitary protection is classified as bleeding
- vaginal blood loss that does not result in the use of sanitary protection is considered spotting.
This system also specifies indices for evaluating the bleeding pattern for each woman and reference period, including the number of bleeding-spotting days, number of bleeding-spotting episodes, lengths of bleeding-spotting episodes, and bleeding-spotting-free intervals. A bleeding-spotting episode is defined as one or more consecutive days during which blood loss (bleeding or spotting) has been recorded, each episode being bounded by bleeding-spotting-free days. The WHO Belsey criteria also identified subgroups that have “clinically important bleeding patterns” (TABLE 1).
But not all researchers use the WHO Belsey criteria. Many trials use, and report, their own system of analysis. Some researchers have chosen reference periods of other durations and study periods that range from 1 to 5 years. Some studies report bleeding patterns by number of days, and others report the percentage of women experiencing a given bleeding pattern during a reference period. The lack of uniformity results in data that are difficult to compare from one study to the next—and to explain to our patients.
It’s unclear whether any of our research definitions of clinically significant bleeding have ever been validated as clinically important to our patients. Multiple studies do show that changes in menstrual bleeding patterns are a significant cause of dissatisfaction with any given contraceptive method, but we don’t know if the number of days of bleeding-spotting or the predictability of this bleeding-spotting is the critical piece of information we should be relating to our patients.
In other words, do our beliefs about clinically important bleeding patterns reflect women’s beliefs?
TABLE 1
The WHO Belsey system of bleeding patterns
| Pattern | Definition |
|---|---|
| Amenorrhea | No bleeding |
| Prolonged bleeding | 1 or more bleeding-spotting episodes lasting longer than 14 days |
| Frequent bleeding | More than 5 bleeding-spotting episodes |
| Infrequent bleeding | 1 or 2 bleeding-spotting episodes |
| Irregular bleeding | 3 to 5 episodes with more than 3 bleeding-free intervals of 14 days or longer |
| Normal bleeding | None of the above are present |
| This system establishes criteria for defining clinically important bleeding patterns during a 90-day reference period. Adapted from: Belsey EM et al.4 | |
Implantable contraception
The etonogestrel (ENG) implant (Implanon) is the only implantable contraceptive available in the United States. This single-rod contraceptive can be used for as long as 3 years.
Contraceptive implants, including the levonorgestrel implants once sold in the United States and still available in some parts of the world, are highly effective. The Implanon prescribing information reports a first-year failure rate of 0.38 pregnancies for every 100 woman-years of use; Hatcher and co-workers reported a failure rate of 0.5 The difference is based on how the FDA defines pregnancy in contraceptive trials. In fact, the only pregnancies reported with the ENG implant happened after it was removed. Importantly, the studies evaluated by the FDA included only women not using any medications known to induce liver metabolism (the cytochrome P450 pathway) and who were between 80% and 130% of ideal body weight. The efficacy of the ENG implant for women who are taking medications that induce liver metabolism or who are greater than 130% of their ideal body weight is unknown.
The efficacy of the ENG implant is likely derived from suppression of ovulation and increased cervical mucus viscosity. Associated changes in the endometrium that occur with this low dosage of progestin are likely the primary cause of irregular and unpredictable bleeding.
Several studies have sought to describe the bleeding patterns experienced with the ENG implant.6-8 During the first 3 months, approximately 50% of all women using the ENG implant reported bleeding-spotting (TABLE 2) for 30 days, on average (TABLE 3). The number of days decreases to approximately 20 bleeding-spotting days for each 90-day reference period at 6 to 24 months, with wide variability. For example: From 3 to 6 months, women reported 22 days of bleeding-spotting (standard deviation, 20 days); from months 21 to 24, 20 days of bleeding-spotting (standard deviation, 14 days).7
After using the ENG implant for 2 years, therefore, most women can expect the number of bleeding-spotting days for every 90-day reference period to range between 6 and 34 days. These days of bleeding-spotting are often noncontinuous, however. On average, women reported three separate bleeding-spotting episodes for every 90-day reference period.7
Although individual bleeding patterns are unpredictable, women who had no bleeding, or infrequent bleeding, at the beginning of use of the ENG implant had only a “small chance” of bleeding frequently.6 The most common bleeding pattern observed throughout the study was infrequent bleeding, defined as fewer than three episodes of bleeding in a 90-day reference period (excluding amenorrhea).7
Amenorrhea may not persist. The amenorrhea rate at 6 months of use and beyond ranges from 10% to 20% (TABLE 4). Importantly, women who are amenorrheic in one 90-day reference period are not necessarily the ones who are amenorrheic in another reference period. So, unlike what is more commonly seen with other progestin-only methods, such as injectables, amenorrhea is not sustained for most women.
This unpredictable pattern affects continuation of the ENG implant (TABLE 5). Irregular bleeding is the most common reason women cite for discontinuation, accounting for 30% to 60% of all women who discontinue early.7,9
Overall, approximately 4% of ENG users discontinue the method at 1 year. Ten percent to 20% discontinue each year thereafter because of intolerance to bleeding changes.6-9
There are, however, differences in discontinuation rates across cultures. In an integrated analysis of 13 different trials that evaluated patterns of vaginal bleeding with the ENG implant where the rate of menstrual changes was similar, women from Europe and Canada were much more likely (23%) to discontinue the implant because of those changes than women from Southeast Asia and Chile were (2%).6 This finding may reflect differences in cultural beliefs or disparate access to other contraceptive options.10
TABLE 2
What percentage of women taking progestin-only contraception report bleeding-spotting?
| Months | ||||||
|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 18 | 24 |
| DMPA | ||||||
| Sangi-Haghpeykar (1996)33 | 46% | 43% | 40% | |||
| Cromer (1998)34 | 24% | 10% | ||||
| ENG implant | ||||||
| Croxatto (2000)9 | 40–50% | |||||
| LNG-IUS | ||||||
| Datey (1995)32 | 18% | 6% | 3% | 1% | 4% | |
| Hidalgo (2002)20 | 25% | 8% | 11% | |||
| Progestin-only pill | ||||||
| Sheth (1982)35 | 21–55%* | 6–42%* | ||||
| * Percentage reporting prolonged, frequent, or irregular bleeding. | ||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | ||||||
TABLE 3
How many days of bleeding-spotting do women have
when they use progestin-only contraception?
| Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 0–3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 | 19–21 | 22–24 | 36 |
| DMPA | |||||||||
| Belsey (1988)11 | 16* | 9 | 4 | 3 | |||||
| Hubacher (2009)31 | 21 | 18 | 14 | 10 | |||||
| ENG implant | |||||||||
| Affandi (1998)6 | 26 | 19 | 16 | 16 | 17 | 18 | 18 | 18 | |
| Zheng (1999)8 | 34 | 22 | 19–22 | ||||||
| Funk (2005)7 | 31 | 22 | 19 | 19 | 18 | 19 | 17 | 20 | |
| LNG-IUS | |||||||||
| Datey (1995)32 | |||||||||
| Total days of bleeding | 9 | 7 | 6 | 5 | 5 | 5 | |||
| Total days of spotting | 10 | 5 | 5 | 4 | 4 | 4 | |||
| Progestin-only pill | |||||||||
| Belsey (1988)11 | 15–18 | ||||||||
| *All values in the table represent an average number of days in a 90-day reference period. | |||||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | |||||||||
TABLE 4
What percentage of women taking progestin-only contraception report amenorrhea?
| Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 24 | ||||
| DMPA | |||||||||
| Belsey (1988)11 | 8% | 22% | 39% | 45% | |||||
| Sangi-Haghpeykar (1996)33 | 46% | 53% | 59% | ||||||
| Cromer (1998)34 | 34% | 60% | |||||||
| Polaneczky (1998)14 | 23% | 40% | 65% | 40% | |||||
| Canto (2001)1 | 35% | 70% | |||||||
| Jain (2004)13 (DMPA-SC) | 26% | 38% | 55% | ||||||
| Hubacher (2009)31 | 12% | 25% | 37% | 46% | |||||
| ENG implant | |||||||||
| Affandi (1998)6 | 2% | 19% | 25% | 23% | 21% | ||||
| Zheng (1999)8 | 2% | 19% | 10% | 15% | |||||
| Croxatto (2000)9 | 12-20% | ||||||||
| Funk (2005)7 | 2% | 14-20% | |||||||
| LNG-IUS | |||||||||
| Andersson (1994)21 | 17% | ||||||||
| Hidalgo (2002)20 | 44% | 50% | 50% | ||||||
| Progestin-only pill | |||||||||
| Belsey (1988)11 | 0% | 0% | 0% | 0% | |||||
| Sheth (1992)35 | 3-8% | 0-2% | |||||||
| Kovacs (1996)24 | 5-10% | ||||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | |||||||||
TABLE 5
What percentage discontinue progestin-only contraception
because of a change in bleeding pattern?
| Months | ||||||
|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 24 | 36 |
| DMPA | ||||||
| Potter (1997)36 | 43% | |||||
| Sangi-Haghpeykar (1996)33 | 34.1% | 58%* | 78%* | |||
| Davidson (1997)37 | 31% | 49%* | 58% | |||
| ENG implant | ||||||
| Croxatto (2000)9 | 19% | |||||
| Zheng (1999)8 | 4% | 6.1%* | 8.4%* | |||
| Affandi (1998)6 | 23% | |||||
| Funk (2005)7 | 13% | |||||
| LNG-IUS | ||||||
| Datey (1995)32 | 13.8% | |||||
| Luukkainen (1987)38 | 7.5% | |||||
| Andersson (1994)21 | 5.8%* | 8.3%* | 9.6%* | |||
| Progestin-only pill | ||||||
| Belsey (1988)39 | 10% | |||||
| Sheth (1982)35 | 25% | 34.5%* | ||||
| Graham (1992)25 | 18% | 25% | 35%* | |||
| *Percentages are cumulative across the months studied. | ||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | ||||||
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
The pattern of bleeding seen with the ENG implant is like the activity of the heart in atrial fibrillation: irregularly irregular. Still, most (80%) women continue to use it beyond 1 year. In fact, the discontinuation rate for the ENG implant is less than that of depot medroxyprogesterone acetate (DMPA) and progestin-only pills.
Most ENG implant users report no difficulty tolerating the associated unpredictable bleeding; it’s possible that they had unpredictable bleeding at baseline, or were drawn to the improvement in their dysmenorrhea.6
Importantly, unpredictable bleeding does not affect efficacy; the ENG implant remains one of the most effective long-acting reversible contraceptives. For women who can tolerate unpredictable bleeding, the ENG implant is a highly effective contraceptive option.
Injectable contraception
Approved by the FDA in 1992, DMPA (Depo-Provera) has good efficacy and long-acting protection. Disadvantages include unpredictable bleeding, weight gain, acne, depression, hair loss, and the controversial issue of decreased bone loss with prolonged use.
What are the expected changes in bleeding patterns with DMPA? Women often have unpredictable patterns, with infrequent but prolonged bleeding-spotting episodes.11 The overall incidence of irregular bleeding can be as high as 70% in the first year of use.12 Irregular bleeding decreases with continued use, to as low as 10% after the first year (TABLE 2).
Although the number of bleeding-spotting days decreases over time, women have reported as many as 10 days of irregular bleeding-spotting between 9 and 12 months of use (TABLE 3). The rates of irregular bleeding and amenorrhea are similar for the subcutaneous formulation of DMPA.13
DMPA is often used because of the high likelihood of amenorrhea. However, amenorrhea is not accomplished in most women in a short time. At 3 months of use, 10% to 45% of women report amenorrhea; after 1 year, the rate increases to 40% to 70% (TABLE 4). At 5 years, 80% of women report amenorrhea.12
A source of frustration. DMPA’s high discontinuation rate, compared with what is seen with other contraceptives, can be frustrating for patients and clinicians. Irregular bleeding is the most common reason for discontinuation. Approximately 35% of women who start DMPA discontinue it during the first 3 months of use because of irregular bleeding (TABLE 5). The cumulative discontinuation rate rises over time: At 1 year, 40% to 60% of women who started DMPA will have discontinued it because of changes in bleeding patterns (TABLE 5). Furthermore, 70% of women reporting DMPA discontinuation due to bleeding changes stopped the method after the first injection.14
Paul and colleagues conducted a telephone survey to determine the patterns of use and reasons for discontinuation among DMPA users.15 Of 252 DMPA users surveyed, 20% cited menstrual disturbances as the reason for discontinuation. These changes were equally distributed: amenorrhea, irregular bleeding, and heavy bleeding, all 6.8%.
Of approximately 7,000 women who participated in the 2002 National Survey for Family Growth, 600 had used DMPA in the past. Thirty-four percent pointed to a dislike of changes in menstrual periods as the reason for discontinuation.16
Not surprisingly, helping your patient develop realistic expectations about bleeding patterns with DMPA can decrease the discontinuation rate. Women who received repeated, structured information about DMPA were less likely to discontinue it because of menstrual disturbances (amenorrhea and irregular and heavy bleeding) than were women in a routine counseling group (OR, 0.20; 95% CI: 0.11, 0.37).17
Other investigators have reported similar findings, with a fourfold to sixfold lower likelihood of discontinuation because of bleeding changes among women who received detailed counseling about DMPA.1,2
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
DMPA is effective and convenient, but unpredictable bleeding in the first year of use is not uncommon. The irregularity is similar to that seen with the ENG implant in the first 6 months of use. Thereafter, DMPA users are more likely to achieve and maintain amenorrhea, compared to ENG implant users.
Intrauterine contraception
The main mechanism of contraceptive action in the levonorgestrel intrauterine system (LNG-IUS) (Mirena) is significant thickening of cervical mucus, resulting in a physical barrier to sperm penetration; ovulation inhibition may also contribute. In a study of women who had been using the LNG-IUS for 4 years, 88% (15/17 cycles) were still ovulatory according to progesterone levels, but only 47% (8/17 cycles) showed normal follicular growth and rupture by ultrasonography.18 The efficacy of the LNG-IUS is 99.8%.6
Advantages of the LNG-IUS include its high effectiveness; long-term action; increased rate of menstrual cycles that are shorter, lighter, and marked by less cramping as use continues; and a high likelihood of amenorrhea as duration of use lengthens.
As with other progestin-only contraceptives, the major disadvantage of the LNG-IUS is associated irregular bleeding that, as is the case with DMPA, appears to decrease with duration of use for most women.
What are the expected changes in bleeding patterns with LNG-IUS? Local effects of the LNG-IUS on the endometrial lining include stromal pseudodecidualization, glandular atrophy, and increased infiltration of leukocytes in the endometrium. These effects, combined with partial inhibition of ovulatory function, commonly result in irregular bleeding.
The number of days of bleeding-spotting is pronounced in the first 3 to 6 months after insertion. Approximately 18% of women reported bleeding-spotting in the first 3 months; 6% to 25%, at 6 months; and only 1% of women, approximately, at 12 months (TABLE 2).
In a survey of Finnish women who used the LNG-IUS, 45.2% reported irregular bleeding, and 18.1% reported spotting, at some point during use.19 Importantly, the prevalence of bleeding-spotting does decrease with duration of use. Nevertheless, as many as 10% of women still report irregular bleeding-spotting at 2 years (TABLE 2).
As with other progestin-only contraceptives, amenorrhea rates for the LNG-IUS vary (TABLE 4). In a Brazilian study of 256 women, 44% reported amenorrhea at 6 months; 50%, at 12 and 24 months.20 In a larger study of 1,821 Finnish women, however, only 17% of women reported amenorrhea at 12 months.21 A survey study of approximately 16,000 Finnish women who used the LNG-IUS found that 75% reported that they “totally or occasionally missed menses” at any time during as long as 5 years of use.19
The discontinuation rate for the LNG-IUS is lower than for the ENG implant or DMPA. Still, changes in bleeding patterns are the most common reason for discontinuation. At 1 year of use, approximately 10% of women discontinue the LNG-IUS because of changes in the bleeding pattern (TABLE 5).
In the most comprehensive study of early removal of the LNG-IUS, the total discontinuation rate—for all reasons—increased to 13% at 2 years, 19% at 3 years, 25% at 4 years, and 35% at 5 years.19 Women who reported excessive bleeding were almost three times more likely to discontinue the LNG-IUS early than women who did not report such a problem (RR, 2.77; 95% CI: 2.5, 3.07). Women who experience spotting are almost twice as likely to discontinue early (RR, 1.89; 95% CI: 1.75, 2.05). Others have reported the cumulative discontinuation rate to be as low as 14.4% at 5 years (when measuring discontinuation because of changes in menstrual bleeding) and as high as 35% at 5 years (when considering the total discontinuation rate for all reasons).21
Amenorrhea lowers the discontinuation rate. In one analysis, women who reported that they “totally or occasionally missed periods” were half as likely to discontinue the LNG-IUS as those who didn’t make such a report (RR, 0.46; 95% CI: 0.43, 0.50).19
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
Irregular bleeding is common with the LNG-IUS in the first 3 to 6 months of use, but overall discontinuation is relatively low—probably because of the high likelihood that bleeding patterns improve over time. Still, irregular bleeding remains the most common reason for discontinuation. Realistic expectations about bleeding patterns and the lower likelihood for amenorrhea, in comparison with DMPA, are important variables to discuss with women who are considering the LNG-IUS.
Progestin-only pills
Progestin-only pills (POPs) have a failure rate that ranges from 1.1 to 9.6 for every 100 users in the first year.22 A POP is used most often by women in whom estrogen is contraindicated, including those who are breastfeeding.23
Disadvantages. POPs require precise adherence and make irregular vaginal bleeding likely. Although 40% to 50% of women who take a POP have normal menstrual cycles, 40% have short, irregular cycles, and another 10% experience even more markedly irregular cycles—from spotting to amenorrhea.22
Studies that precede the WHO Belsey system showed that 1) as many as 70% of women who use a POP reported “breakthrough bleeding-spotting” in one or more cycles and 2) 6% to 16% have “breakthrough bleeding or inter-menstrual spotting” in all cycles (TABLES 2 AND 3).24,25
On average, 25% of women discontinue POPs because of changes in their menstrual cycle (TABLE 5).
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
The mechanism that results in irregular vaginal bleeding in women taking a POP is unclear; evidence suggests that incomplete suppression of ovulation and direct endometrial effects are possible. To the frustration of patients and clinicians, it isn’t possible to predict who will have irregular bleeding—i.e., there is no association between body weight, or age, and the risk of irregular bleeding. As with other progestin-only methods, irregular bleeding is the most common reason for discontinuing POPs.26 A Cochrane review of POPs is under way.27
Is unpredictable bleeding with progestin-only contraceptives treatable?
Bleeding and discontinuation rates associated with progestin-only contraceptives that are observed in clinical trials, especially rates used for FDA review and approval of a product, don’t always translate to real-life medicine. Typically, in such trials, no treatment for irregular or unacceptable bleeding patterns is permitted: If an effective treatment is available, overall acceptability and continuation of the contraceptive could, potentially, be boosted. This matter is most relevant with injectable, intrauterine, and implantable progestin-only methods.
Findings of one meta-analysis. A recent Cochrane review evaluated the literature until December 2006 on the treatment of vaginal bleeding irregularities induced by progestin-only contraceptives.28 Twenty-three randomized controlled trials, encompassing 2,674 subjects, were included. Seventy percent of the trials that were included were determined to reflect a low or moderate risk of bias.
Treatment with estrogen alone reduced the number of days of an ongoing bleeding episode among DMPA and levonorgestrel implant (Norplant) users. Treatment often led to individuals’ discontinuation in a study, however, because of gastrointestinal upset. Combined oral contraceptives can treat amenorrhea with success among DMPA users.
Antiprogestins such as mifepristone cause a reduction in bleeding among women using the levonorgestrel implant, but are not of benefit for ENG implant users.
Last, use of NSAIDs to treat irregular bleeding has shown variable efficacy. Additional small studies cited in the Cochrane review suggest that the following treatments were more effective than placebo for terminating an episode of bleeding among women using progestin-only contraception: the antiprogestin mifepristone for DMPA and POP users; mifepristone plus an estrogen for ENG implant users; and doxycycline for ENG implant users.28
Overall, some women benefit from attempts at treatment. The authors of the Cochrane review caution that their findings do not support the routine clinical use of any of the regimens included in the trials, particularly for obtaining a long-term effect.28
Newer trials, different findings? A more recent double-blind, randomized trial, in which the subjects were 100 Thai women, showed that irregular bleeding with DMPA ceased completely in 88% of those treated with tranexamic acid, 250 mg QID for 5 days, compared with 8% of women in whom bleeding ceased after treatment with placebo.29
Another recent randomized trial found that mifepristone, combined with ethinyl estradiol or doxycycline, was significantly more effective than placebo in ending an episode of bleeding in ENG implant users. No improvement was seen, however, in subsequent bleeding patterns, and improvement with treatment, compared with placebo, amounted to a decrease of only about 2 days.30
Noticeably missing from the literature are large trials that evaluate the use of combined hormonal contraceptives for bleeding irregularities in women using long-acting progestin-only contraceptives. True, some women use these methods because of a contraindication to estrogen-containing methods, but, in reality, most women who use these methods do so because of their high efficacy and ease of use.
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
For women who use the ENG implant or LNG-IUS and have no contraindication to estrogen-containing contraceptives, we often provide a short (1 or 2 months) course of a combined hormonal contraceptive when they find bleeding irregularities bothersome.
Because the serum progestin level provided with these methods is extremely low, adding a low-dose combined oral contraceptive, contraceptive patch, or contraceptive vaginal ring is not that different than using any of the combined hormonal contraceptives. A woman will not become pregnant if she forgets to take the pill or the ring falls out because she still has the progestin-only method in place. And if the short course of a combined hormonal contraceptive helps her continue the more effective method, then the overall goal of avoiding unintended pregnancy is better accomplished.
Large trials to evaluate the use of combined hormonal methods in such circumstances would, of course, be of great benefit.
Good counseling → informed choice → adherence and continuation
With all forms of progestin-only contraception, unpredictable bleeding occurs often and is the most common reason for method discontinuation.
Counseling that explicitly discusses the high likelihood of unpredictable menstrual bleeding allows women to prioritize this issue in their choice of a contraceptive.
Informed choice leads to a better continuation rate for progestin-only methods.
Seeking understanding. We lack full understanding of exactly what it is about changes in bleeding patterns that matter to women. Have definitions of bleeding and spotting that researchers utilize missed quality of life concerns that are more relevant to women? Are women concerned about how many days are spent avoiding sexual activity? Do religious restrictions figure prominently for some? How dissatisfied are they with days of cramping or bloating without bleeding? What do women want to know when they consider the bleeding patterns for their contraceptive options?
The answers to these questions likely vary from patient to patient—and that observation leads us back to grasping the art of contraceptive counseling: Our counseling needs to be concise, relatable, and honest.
1. Canto De Cetina TE, Canto P, Ordoñez Luna M. Effect of counseling to improve compliance in Mexican women receiving depot-medroxyprogesterone acetate. Contraception. 2001;63:143-146.
2. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception. 1996;53:357-361.
3. Rodriguez G, Faundes-Latham A, Atkinson LE. An approach to the analysis of menstrual patterns in the critical evaluation of contraceptives. Stud Fam Plann. 1976;7(2):42-51.
4. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253-260.
5. Hatcher RA, Trussell J, Nelson AL, Cates W, Jr, Stewart F, Kowal D. Contraceptive Technology. 19th ed. New York: Thomson Reuters; 2008.
6. Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception. 1998;58(6 Suppl):99S-107S.
7. Funk S, Miller MM, Mishell DR, Jr, et al. Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
8. Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
9. Croxatto HB. Clinical profile of Implanon: a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5 Suppl 2:21-28.
10. Power J, French R, Cowan F. Subdermal implantable contraceptives versus other forms of reversible contraceptives or other implants as effective methods of preventing pregnancy. Cochrane Database Syst Rev. 2007;(3):CD001326.-
11. Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
12. Haider S, Darney PD. Injectable contraception. Clin Obstet Gynecol. 2007;50:898-906.
13. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
14. Polaneczky M, Liblanc M. Long-term depot medroxyprogesterone acetate (Depo-Provera) use in inner-city adolescents. J Adolesc Health. 1998;23(2):81-88.
15. Paul C, Skegg DC, Williams S. Depot medroxyprogesterone acetate. Patterns of use and reasons for discontinuation. Contraception. 1997;56:209-214.
16. Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76:267-272.
17. Halpern V, Grimes DA, Lopez L, Gallo MF. Strategies to improve adherence and acceptability of hormonal methods for contraception. Cochrane Database Syst Rev. 2006;(1):CD004317.-
18. Barbosa I, Olsson SE, Odlind V, Goncalves T, Coutinho E. Ovarian function after seven years’ use of a levonorgestrel IUD. Adv Contracept. 1995;11(2):85-95.
19. Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG. 2000;107:335-339.
20. Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002;65:129-132.
21. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
22. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004.
23. Collins J, Crosignani PG. ESHRE Capri Workshop Group. Hormonal contraception without estrogens. Hum Reprod Update. 2003;9:373-386.
24. Kovacs G. Progestogen-only pills and bleeding disturbances. Hum Reprod. 1996;11 Suppl 2:20-23.
25. Graham S, Fraser IS. The progestogen-only minipill. Contraception. 1982;26:373-388.
26. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 Suppl 1):S1-S195.
27. Grimes DA, Lopez LM, O’Brien P, Raymond EG. Progestin-only pills for contraception (Protocol). Cochrane Database Syst Rev. 2009;(1):CD007541.-
28. Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gülmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst Rev. 2007;(4):CD003449.-
29. Senthong AJ, Taneepanichskul S. The effect of tranexamic acid for treatment irregular uterine bleeding secondary to DMPA use. J Med Assoc Thai. 2009;92:461-465.
30. Weisberg E, Hickey M, Palmer D, et al. A randomized controlled trial of treatment options for troublesome uterine bleeding in Implanon users. Hum Reprod. 2009;24:1852-1861.
31. Hubacher D, Lopez L, Steiner M, Dorflinger L. Menstrual pattern changes from levonorgestrel subdermal implants and DMPA: systematic review and evidence-based comparisons. Contraception. 2009. doi:10.1016/j.contraception.2009.02.008.
32. Datey S, Gaur LN, Saxena BN. Vaginal bleeding patterns of women using different contraceptive methods (implants, injectables, IUDs, oral pills)—an Indian experience. An ICMR Task Force Study. Indian Council of Medical Research. Contraception. 1995;51:155-165.
33. Sangi-Haghpeykar H, Poindexter AN, 3rd, Bateman L, Ditmore JR. Experiences of injectable contraceptive users in an urban setting. Obstet Gynecol. 1996;88:227-233.
34. Cromer BA, Berg-Kelly KS, Van Groningen JP, Seimer BS, Ruusuvaara L. Depot medroxyprogesterone acetate (Depo-Provera) and levonorgestrel (Norplant) use in adolescents among clinicians in Northern Europe and the United States. J Adolesc Health. 1998;23:74-80.
35. Sheth A, Jain U, Sharma S, et al. A randomized, double-blind study of two combined and two progestogen-only oral contraceptives. Contraception. 1982;25:243-252.
36. Potter LS, Dalberth BT, Cañamar R, Betz M. Depot medroxyprogesterone acetate pioneers. A retrospective study at a North Carolina Health Department. Contraception. 1997;56:305-312.
37. Davidson AR, Kalmuss D, Cushman LF, Romero D, Heartwell S, Rulin M. Injectable contraceptive discontinuation and subsequent unintended pregnancy among low-income women. Am J Public Health. 1997;87:1532-1534.
38. Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception. 1987;36:169-179.
39. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207-225.
Dr. Gariepy reports no financial relationships relevant to this article. Dr. Creinin reports that he is a consultant to, and a speaker for, Schering-Plough.
Progestin-only contraception—a diverse group of oral (progestin-only pills, or so-called minipills), injectable (depot medroxyprogesterone acetate), intrauterine (the levonorgestrel intrauterine system), and implantable (etonogestrel implant) methods—may offer advantages over estrogen-containing contraception:
- the flexibility of distinctive methods of delivery
- the ability to initiate the method in postpartum breastfeeding women
- enhanced safety in women who should not be exposed to exogenous estrogens.
Unpredictable bleeding is a major disadvantage of progestin-only contraception, however, and can cause women to discontinue these methods—and discontinuation without an effective backup method creates a high risk of unplanned pregnancy. The significant variability in bleeding patterns among progestin-only contraceptive methods hinders our ability to counsel patients about them.
Furthermore, the lack of uniform definitions of bleeding patterns with hormonal contraception, including progestin-only methods, makes it difficult to counsel women accurately and compare bleeding patterns among methods.
Accurate prediction of the bleeding patterns associated with progestin-only contraception could lower the discontinuation rate. For example, studies have shown that pretreatment counseling about expected side effects increases approximately fourfold the acceptability and continuation of depot medroxyprogesterone acetate.1,2
In this Update, we review the data on bleeding patterns associated with progestin-only contraceptives, including the likelihood of 1) amenorrhea and 2) discontinuation due to changes in the bleeding pattern.
We also discuss what has been learned about the treatment of changes in bleeding patterns induced by progestin-only contraception.
Our goal? To summarize the findings in a comprehensive way that makes it easier for you to discuss expected bleeding patterns with your patients—so that women can choose the method of contraception that is the best fit for them.
Describing bleeding patterns is a challenging task
One of the difficulties of interpreting clinical data on bleeding patterns—with any type of contraception—is the lack of a universally accepted standard for collecting and reporting these data. The first suggestions for standardization were made in 1976, when Rodriguez and colleagues proposed using 90-day reference periods for analysis, as a way to minimize variability among individual menstrual cycles.3
Subsequently, the World Health Organization’s (WHO’s) Special Programme of Research, Development and Research Training in Human Reproduction developed recommendations for data collection, terminology, presentation, and data analysis when reporting vaginal bleeding during clinical trials of hormonal contraception. These recommendations became known as the WHO Belsey criteria (TABLE 1). They remain the standard.4
Under the WHO Belsey system:
- vaginal blood loss for which a woman uses sanitary protection is classified as bleeding
- vaginal blood loss that does not result in the use of sanitary protection is considered spotting.
This system also specifies indices for evaluating the bleeding pattern for each woman and reference period, including the number of bleeding-spotting days, number of bleeding-spotting episodes, lengths of bleeding-spotting episodes, and bleeding-spotting-free intervals. A bleeding-spotting episode is defined as one or more consecutive days during which blood loss (bleeding or spotting) has been recorded, each episode being bounded by bleeding-spotting-free days. The WHO Belsey criteria also identified subgroups that have “clinically important bleeding patterns” (TABLE 1).
But not all researchers use the WHO Belsey criteria. Many trials use, and report, their own system of analysis. Some researchers have chosen reference periods of other durations and study periods that range from 1 to 5 years. Some studies report bleeding patterns by number of days, and others report the percentage of women experiencing a given bleeding pattern during a reference period. The lack of uniformity results in data that are difficult to compare from one study to the next—and to explain to our patients.
It’s unclear whether any of our research definitions of clinically significant bleeding have ever been validated as clinically important to our patients. Multiple studies do show that changes in menstrual bleeding patterns are a significant cause of dissatisfaction with any given contraceptive method, but we don’t know if the number of days of bleeding-spotting or the predictability of this bleeding-spotting is the critical piece of information we should be relating to our patients.
In other words, do our beliefs about clinically important bleeding patterns reflect women’s beliefs?
TABLE 1
The WHO Belsey system of bleeding patterns
| Pattern | Definition |
|---|---|
| Amenorrhea | No bleeding |
| Prolonged bleeding | 1 or more bleeding-spotting episodes lasting longer than 14 days |
| Frequent bleeding | More than 5 bleeding-spotting episodes |
| Infrequent bleeding | 1 or 2 bleeding-spotting episodes |
| Irregular bleeding | 3 to 5 episodes with more than 3 bleeding-free intervals of 14 days or longer |
| Normal bleeding | None of the above are present |
| This system establishes criteria for defining clinically important bleeding patterns during a 90-day reference period. Adapted from: Belsey EM et al.4 | |
Implantable contraception
The etonogestrel (ENG) implant (Implanon) is the only implantable contraceptive available in the United States. This single-rod contraceptive can be used for as long as 3 years.
Contraceptive implants, including the levonorgestrel implants once sold in the United States and still available in some parts of the world, are highly effective. The Implanon prescribing information reports a first-year failure rate of 0.38 pregnancies for every 100 woman-years of use; Hatcher and co-workers reported a failure rate of 0.5 The difference is based on how the FDA defines pregnancy in contraceptive trials. In fact, the only pregnancies reported with the ENG implant happened after it was removed. Importantly, the studies evaluated by the FDA included only women not using any medications known to induce liver metabolism (the cytochrome P450 pathway) and who were between 80% and 130% of ideal body weight. The efficacy of the ENG implant for women who are taking medications that induce liver metabolism or who are greater than 130% of their ideal body weight is unknown.
The efficacy of the ENG implant is likely derived from suppression of ovulation and increased cervical mucus viscosity. Associated changes in the endometrium that occur with this low dosage of progestin are likely the primary cause of irregular and unpredictable bleeding.
Several studies have sought to describe the bleeding patterns experienced with the ENG implant.6-8 During the first 3 months, approximately 50% of all women using the ENG implant reported bleeding-spotting (TABLE 2) for 30 days, on average (TABLE 3). The number of days decreases to approximately 20 bleeding-spotting days for each 90-day reference period at 6 to 24 months, with wide variability. For example: From 3 to 6 months, women reported 22 days of bleeding-spotting (standard deviation, 20 days); from months 21 to 24, 20 days of bleeding-spotting (standard deviation, 14 days).7
After using the ENG implant for 2 years, therefore, most women can expect the number of bleeding-spotting days for every 90-day reference period to range between 6 and 34 days. These days of bleeding-spotting are often noncontinuous, however. On average, women reported three separate bleeding-spotting episodes for every 90-day reference period.7
Although individual bleeding patterns are unpredictable, women who had no bleeding, or infrequent bleeding, at the beginning of use of the ENG implant had only a “small chance” of bleeding frequently.6 The most common bleeding pattern observed throughout the study was infrequent bleeding, defined as fewer than three episodes of bleeding in a 90-day reference period (excluding amenorrhea).7
Amenorrhea may not persist. The amenorrhea rate at 6 months of use and beyond ranges from 10% to 20% (TABLE 4). Importantly, women who are amenorrheic in one 90-day reference period are not necessarily the ones who are amenorrheic in another reference period. So, unlike what is more commonly seen with other progestin-only methods, such as injectables, amenorrhea is not sustained for most women.
This unpredictable pattern affects continuation of the ENG implant (TABLE 5). Irregular bleeding is the most common reason women cite for discontinuation, accounting for 30% to 60% of all women who discontinue early.7,9
Overall, approximately 4% of ENG users discontinue the method at 1 year. Ten percent to 20% discontinue each year thereafter because of intolerance to bleeding changes.6-9
There are, however, differences in discontinuation rates across cultures. In an integrated analysis of 13 different trials that evaluated patterns of vaginal bleeding with the ENG implant where the rate of menstrual changes was similar, women from Europe and Canada were much more likely (23%) to discontinue the implant because of those changes than women from Southeast Asia and Chile were (2%).6 This finding may reflect differences in cultural beliefs or disparate access to other contraceptive options.10
TABLE 2
What percentage of women taking progestin-only contraception report bleeding-spotting?
| Months | ||||||
|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 18 | 24 |
| DMPA | ||||||
| Sangi-Haghpeykar (1996)33 | 46% | 43% | 40% | |||
| Cromer (1998)34 | 24% | 10% | ||||
| ENG implant | ||||||
| Croxatto (2000)9 | 40–50% | |||||
| LNG-IUS | ||||||
| Datey (1995)32 | 18% | 6% | 3% | 1% | 4% | |
| Hidalgo (2002)20 | 25% | 8% | 11% | |||
| Progestin-only pill | ||||||
| Sheth (1982)35 | 21–55%* | 6–42%* | ||||
| * Percentage reporting prolonged, frequent, or irregular bleeding. | ||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | ||||||
TABLE 3
How many days of bleeding-spotting do women have
when they use progestin-only contraception?
| Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 0–3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 | 19–21 | 22–24 | 36 |
| DMPA | |||||||||
| Belsey (1988)11 | 16* | 9 | 4 | 3 | |||||
| Hubacher (2009)31 | 21 | 18 | 14 | 10 | |||||
| ENG implant | |||||||||
| Affandi (1998)6 | 26 | 19 | 16 | 16 | 17 | 18 | 18 | 18 | |
| Zheng (1999)8 | 34 | 22 | 19–22 | ||||||
| Funk (2005)7 | 31 | 22 | 19 | 19 | 18 | 19 | 17 | 20 | |
| LNG-IUS | |||||||||
| Datey (1995)32 | |||||||||
| Total days of bleeding | 9 | 7 | 6 | 5 | 5 | 5 | |||
| Total days of spotting | 10 | 5 | 5 | 4 | 4 | 4 | |||
| Progestin-only pill | |||||||||
| Belsey (1988)11 | 15–18 | ||||||||
| *All values in the table represent an average number of days in a 90-day reference period. | |||||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | |||||||||
TABLE 4
What percentage of women taking progestin-only contraception report amenorrhea?
| Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 24 | ||||
| DMPA | |||||||||
| Belsey (1988)11 | 8% | 22% | 39% | 45% | |||||
| Sangi-Haghpeykar (1996)33 | 46% | 53% | 59% | ||||||
| Cromer (1998)34 | 34% | 60% | |||||||
| Polaneczky (1998)14 | 23% | 40% | 65% | 40% | |||||
| Canto (2001)1 | 35% | 70% | |||||||
| Jain (2004)13 (DMPA-SC) | 26% | 38% | 55% | ||||||
| Hubacher (2009)31 | 12% | 25% | 37% | 46% | |||||
| ENG implant | |||||||||
| Affandi (1998)6 | 2% | 19% | 25% | 23% | 21% | ||||
| Zheng (1999)8 | 2% | 19% | 10% | 15% | |||||
| Croxatto (2000)9 | 12-20% | ||||||||
| Funk (2005)7 | 2% | 14-20% | |||||||
| LNG-IUS | |||||||||
| Andersson (1994)21 | 17% | ||||||||
| Hidalgo (2002)20 | 44% | 50% | 50% | ||||||
| Progestin-only pill | |||||||||
| Belsey (1988)11 | 0% | 0% | 0% | 0% | |||||
| Sheth (1992)35 | 3-8% | 0-2% | |||||||
| Kovacs (1996)24 | 5-10% | ||||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | |||||||||
TABLE 5
What percentage discontinue progestin-only contraception
because of a change in bleeding pattern?
| Months | ||||||
|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 24 | 36 |
| DMPA | ||||||
| Potter (1997)36 | 43% | |||||
| Sangi-Haghpeykar (1996)33 | 34.1% | 58%* | 78%* | |||
| Davidson (1997)37 | 31% | 49%* | 58% | |||
| ENG implant | ||||||
| Croxatto (2000)9 | 19% | |||||
| Zheng (1999)8 | 4% | 6.1%* | 8.4%* | |||
| Affandi (1998)6 | 23% | |||||
| Funk (2005)7 | 13% | |||||
| LNG-IUS | ||||||
| Datey (1995)32 | 13.8% | |||||
| Luukkainen (1987)38 | 7.5% | |||||
| Andersson (1994)21 | 5.8%* | 8.3%* | 9.6%* | |||
| Progestin-only pill | ||||||
| Belsey (1988)39 | 10% | |||||
| Sheth (1982)35 | 25% | 34.5%* | ||||
| Graham (1992)25 | 18% | 25% | 35%* | |||
| *Percentages are cumulative across the months studied. | ||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | ||||||
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
The pattern of bleeding seen with the ENG implant is like the activity of the heart in atrial fibrillation: irregularly irregular. Still, most (80%) women continue to use it beyond 1 year. In fact, the discontinuation rate for the ENG implant is less than that of depot medroxyprogesterone acetate (DMPA) and progestin-only pills.
Most ENG implant users report no difficulty tolerating the associated unpredictable bleeding; it’s possible that they had unpredictable bleeding at baseline, or were drawn to the improvement in their dysmenorrhea.6
Importantly, unpredictable bleeding does not affect efficacy; the ENG implant remains one of the most effective long-acting reversible contraceptives. For women who can tolerate unpredictable bleeding, the ENG implant is a highly effective contraceptive option.
Injectable contraception
Approved by the FDA in 1992, DMPA (Depo-Provera) has good efficacy and long-acting protection. Disadvantages include unpredictable bleeding, weight gain, acne, depression, hair loss, and the controversial issue of decreased bone loss with prolonged use.
What are the expected changes in bleeding patterns with DMPA? Women often have unpredictable patterns, with infrequent but prolonged bleeding-spotting episodes.11 The overall incidence of irregular bleeding can be as high as 70% in the first year of use.12 Irregular bleeding decreases with continued use, to as low as 10% after the first year (TABLE 2).
Although the number of bleeding-spotting days decreases over time, women have reported as many as 10 days of irregular bleeding-spotting between 9 and 12 months of use (TABLE 3). The rates of irregular bleeding and amenorrhea are similar for the subcutaneous formulation of DMPA.13
DMPA is often used because of the high likelihood of amenorrhea. However, amenorrhea is not accomplished in most women in a short time. At 3 months of use, 10% to 45% of women report amenorrhea; after 1 year, the rate increases to 40% to 70% (TABLE 4). At 5 years, 80% of women report amenorrhea.12
A source of frustration. DMPA’s high discontinuation rate, compared with what is seen with other contraceptives, can be frustrating for patients and clinicians. Irregular bleeding is the most common reason for discontinuation. Approximately 35% of women who start DMPA discontinue it during the first 3 months of use because of irregular bleeding (TABLE 5). The cumulative discontinuation rate rises over time: At 1 year, 40% to 60% of women who started DMPA will have discontinued it because of changes in bleeding patterns (TABLE 5). Furthermore, 70% of women reporting DMPA discontinuation due to bleeding changes stopped the method after the first injection.14
Paul and colleagues conducted a telephone survey to determine the patterns of use and reasons for discontinuation among DMPA users.15 Of 252 DMPA users surveyed, 20% cited menstrual disturbances as the reason for discontinuation. These changes were equally distributed: amenorrhea, irregular bleeding, and heavy bleeding, all 6.8%.
Of approximately 7,000 women who participated in the 2002 National Survey for Family Growth, 600 had used DMPA in the past. Thirty-four percent pointed to a dislike of changes in menstrual periods as the reason for discontinuation.16
Not surprisingly, helping your patient develop realistic expectations about bleeding patterns with DMPA can decrease the discontinuation rate. Women who received repeated, structured information about DMPA were less likely to discontinue it because of menstrual disturbances (amenorrhea and irregular and heavy bleeding) than were women in a routine counseling group (OR, 0.20; 95% CI: 0.11, 0.37).17
Other investigators have reported similar findings, with a fourfold to sixfold lower likelihood of discontinuation because of bleeding changes among women who received detailed counseling about DMPA.1,2
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
DMPA is effective and convenient, but unpredictable bleeding in the first year of use is not uncommon. The irregularity is similar to that seen with the ENG implant in the first 6 months of use. Thereafter, DMPA users are more likely to achieve and maintain amenorrhea, compared to ENG implant users.
Intrauterine contraception
The main mechanism of contraceptive action in the levonorgestrel intrauterine system (LNG-IUS) (Mirena) is significant thickening of cervical mucus, resulting in a physical barrier to sperm penetration; ovulation inhibition may also contribute. In a study of women who had been using the LNG-IUS for 4 years, 88% (15/17 cycles) were still ovulatory according to progesterone levels, but only 47% (8/17 cycles) showed normal follicular growth and rupture by ultrasonography.18 The efficacy of the LNG-IUS is 99.8%.6
Advantages of the LNG-IUS include its high effectiveness; long-term action; increased rate of menstrual cycles that are shorter, lighter, and marked by less cramping as use continues; and a high likelihood of amenorrhea as duration of use lengthens.
As with other progestin-only contraceptives, the major disadvantage of the LNG-IUS is associated irregular bleeding that, as is the case with DMPA, appears to decrease with duration of use for most women.
What are the expected changes in bleeding patterns with LNG-IUS? Local effects of the LNG-IUS on the endometrial lining include stromal pseudodecidualization, glandular atrophy, and increased infiltration of leukocytes in the endometrium. These effects, combined with partial inhibition of ovulatory function, commonly result in irregular bleeding.
The number of days of bleeding-spotting is pronounced in the first 3 to 6 months after insertion. Approximately 18% of women reported bleeding-spotting in the first 3 months; 6% to 25%, at 6 months; and only 1% of women, approximately, at 12 months (TABLE 2).
In a survey of Finnish women who used the LNG-IUS, 45.2% reported irregular bleeding, and 18.1% reported spotting, at some point during use.19 Importantly, the prevalence of bleeding-spotting does decrease with duration of use. Nevertheless, as many as 10% of women still report irregular bleeding-spotting at 2 years (TABLE 2).
As with other progestin-only contraceptives, amenorrhea rates for the LNG-IUS vary (TABLE 4). In a Brazilian study of 256 women, 44% reported amenorrhea at 6 months; 50%, at 12 and 24 months.20 In a larger study of 1,821 Finnish women, however, only 17% of women reported amenorrhea at 12 months.21 A survey study of approximately 16,000 Finnish women who used the LNG-IUS found that 75% reported that they “totally or occasionally missed menses” at any time during as long as 5 years of use.19
The discontinuation rate for the LNG-IUS is lower than for the ENG implant or DMPA. Still, changes in bleeding patterns are the most common reason for discontinuation. At 1 year of use, approximately 10% of women discontinue the LNG-IUS because of changes in the bleeding pattern (TABLE 5).
In the most comprehensive study of early removal of the LNG-IUS, the total discontinuation rate—for all reasons—increased to 13% at 2 years, 19% at 3 years, 25% at 4 years, and 35% at 5 years.19 Women who reported excessive bleeding were almost three times more likely to discontinue the LNG-IUS early than women who did not report such a problem (RR, 2.77; 95% CI: 2.5, 3.07). Women who experience spotting are almost twice as likely to discontinue early (RR, 1.89; 95% CI: 1.75, 2.05). Others have reported the cumulative discontinuation rate to be as low as 14.4% at 5 years (when measuring discontinuation because of changes in menstrual bleeding) and as high as 35% at 5 years (when considering the total discontinuation rate for all reasons).21
Amenorrhea lowers the discontinuation rate. In one analysis, women who reported that they “totally or occasionally missed periods” were half as likely to discontinue the LNG-IUS as those who didn’t make such a report (RR, 0.46; 95% CI: 0.43, 0.50).19
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
Irregular bleeding is common with the LNG-IUS in the first 3 to 6 months of use, but overall discontinuation is relatively low—probably because of the high likelihood that bleeding patterns improve over time. Still, irregular bleeding remains the most common reason for discontinuation. Realistic expectations about bleeding patterns and the lower likelihood for amenorrhea, in comparison with DMPA, are important variables to discuss with women who are considering the LNG-IUS.
Progestin-only pills
Progestin-only pills (POPs) have a failure rate that ranges from 1.1 to 9.6 for every 100 users in the first year.22 A POP is used most often by women in whom estrogen is contraindicated, including those who are breastfeeding.23
Disadvantages. POPs require precise adherence and make irregular vaginal bleeding likely. Although 40% to 50% of women who take a POP have normal menstrual cycles, 40% have short, irregular cycles, and another 10% experience even more markedly irregular cycles—from spotting to amenorrhea.22
Studies that precede the WHO Belsey system showed that 1) as many as 70% of women who use a POP reported “breakthrough bleeding-spotting” in one or more cycles and 2) 6% to 16% have “breakthrough bleeding or inter-menstrual spotting” in all cycles (TABLES 2 AND 3).24,25
On average, 25% of women discontinue POPs because of changes in their menstrual cycle (TABLE 5).
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
The mechanism that results in irregular vaginal bleeding in women taking a POP is unclear; evidence suggests that incomplete suppression of ovulation and direct endometrial effects are possible. To the frustration of patients and clinicians, it isn’t possible to predict who will have irregular bleeding—i.e., there is no association between body weight, or age, and the risk of irregular bleeding. As with other progestin-only methods, irregular bleeding is the most common reason for discontinuing POPs.26 A Cochrane review of POPs is under way.27
Is unpredictable bleeding with progestin-only contraceptives treatable?
Bleeding and discontinuation rates associated with progestin-only contraceptives that are observed in clinical trials, especially rates used for FDA review and approval of a product, don’t always translate to real-life medicine. Typically, in such trials, no treatment for irregular or unacceptable bleeding patterns is permitted: If an effective treatment is available, overall acceptability and continuation of the contraceptive could, potentially, be boosted. This matter is most relevant with injectable, intrauterine, and implantable progestin-only methods.
Findings of one meta-analysis. A recent Cochrane review evaluated the literature until December 2006 on the treatment of vaginal bleeding irregularities induced by progestin-only contraceptives.28 Twenty-three randomized controlled trials, encompassing 2,674 subjects, were included. Seventy percent of the trials that were included were determined to reflect a low or moderate risk of bias.
Treatment with estrogen alone reduced the number of days of an ongoing bleeding episode among DMPA and levonorgestrel implant (Norplant) users. Treatment often led to individuals’ discontinuation in a study, however, because of gastrointestinal upset. Combined oral contraceptives can treat amenorrhea with success among DMPA users.
Antiprogestins such as mifepristone cause a reduction in bleeding among women using the levonorgestrel implant, but are not of benefit for ENG implant users.
Last, use of NSAIDs to treat irregular bleeding has shown variable efficacy. Additional small studies cited in the Cochrane review suggest that the following treatments were more effective than placebo for terminating an episode of bleeding among women using progestin-only contraception: the antiprogestin mifepristone for DMPA and POP users; mifepristone plus an estrogen for ENG implant users; and doxycycline for ENG implant users.28
Overall, some women benefit from attempts at treatment. The authors of the Cochrane review caution that their findings do not support the routine clinical use of any of the regimens included in the trials, particularly for obtaining a long-term effect.28
Newer trials, different findings? A more recent double-blind, randomized trial, in which the subjects were 100 Thai women, showed that irregular bleeding with DMPA ceased completely in 88% of those treated with tranexamic acid, 250 mg QID for 5 days, compared with 8% of women in whom bleeding ceased after treatment with placebo.29
Another recent randomized trial found that mifepristone, combined with ethinyl estradiol or doxycycline, was significantly more effective than placebo in ending an episode of bleeding in ENG implant users. No improvement was seen, however, in subsequent bleeding patterns, and improvement with treatment, compared with placebo, amounted to a decrease of only about 2 days.30
Noticeably missing from the literature are large trials that evaluate the use of combined hormonal contraceptives for bleeding irregularities in women using long-acting progestin-only contraceptives. True, some women use these methods because of a contraindication to estrogen-containing methods, but, in reality, most women who use these methods do so because of their high efficacy and ease of use.
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
For women who use the ENG implant or LNG-IUS and have no contraindication to estrogen-containing contraceptives, we often provide a short (1 or 2 months) course of a combined hormonal contraceptive when they find bleeding irregularities bothersome.
Because the serum progestin level provided with these methods is extremely low, adding a low-dose combined oral contraceptive, contraceptive patch, or contraceptive vaginal ring is not that different than using any of the combined hormonal contraceptives. A woman will not become pregnant if she forgets to take the pill or the ring falls out because she still has the progestin-only method in place. And if the short course of a combined hormonal contraceptive helps her continue the more effective method, then the overall goal of avoiding unintended pregnancy is better accomplished.
Large trials to evaluate the use of combined hormonal methods in such circumstances would, of course, be of great benefit.
Good counseling → informed choice → adherence and continuation
With all forms of progestin-only contraception, unpredictable bleeding occurs often and is the most common reason for method discontinuation.
Counseling that explicitly discusses the high likelihood of unpredictable menstrual bleeding allows women to prioritize this issue in their choice of a contraceptive.
Informed choice leads to a better continuation rate for progestin-only methods.
Seeking understanding. We lack full understanding of exactly what it is about changes in bleeding patterns that matter to women. Have definitions of bleeding and spotting that researchers utilize missed quality of life concerns that are more relevant to women? Are women concerned about how many days are spent avoiding sexual activity? Do religious restrictions figure prominently for some? How dissatisfied are they with days of cramping or bloating without bleeding? What do women want to know when they consider the bleeding patterns for their contraceptive options?
The answers to these questions likely vary from patient to patient—and that observation leads us back to grasping the art of contraceptive counseling: Our counseling needs to be concise, relatable, and honest.
Dr. Gariepy reports no financial relationships relevant to this article. Dr. Creinin reports that he is a consultant to, and a speaker for, Schering-Plough.
Progestin-only contraception—a diverse group of oral (progestin-only pills, or so-called minipills), injectable (depot medroxyprogesterone acetate), intrauterine (the levonorgestrel intrauterine system), and implantable (etonogestrel implant) methods—may offer advantages over estrogen-containing contraception:
- the flexibility of distinctive methods of delivery
- the ability to initiate the method in postpartum breastfeeding women
- enhanced safety in women who should not be exposed to exogenous estrogens.
Unpredictable bleeding is a major disadvantage of progestin-only contraception, however, and can cause women to discontinue these methods—and discontinuation without an effective backup method creates a high risk of unplanned pregnancy. The significant variability in bleeding patterns among progestin-only contraceptive methods hinders our ability to counsel patients about them.
Furthermore, the lack of uniform definitions of bleeding patterns with hormonal contraception, including progestin-only methods, makes it difficult to counsel women accurately and compare bleeding patterns among methods.
Accurate prediction of the bleeding patterns associated with progestin-only contraception could lower the discontinuation rate. For example, studies have shown that pretreatment counseling about expected side effects increases approximately fourfold the acceptability and continuation of depot medroxyprogesterone acetate.1,2
In this Update, we review the data on bleeding patterns associated with progestin-only contraceptives, including the likelihood of 1) amenorrhea and 2) discontinuation due to changes in the bleeding pattern.
We also discuss what has been learned about the treatment of changes in bleeding patterns induced by progestin-only contraception.
Our goal? To summarize the findings in a comprehensive way that makes it easier for you to discuss expected bleeding patterns with your patients—so that women can choose the method of contraception that is the best fit for them.
Describing bleeding patterns is a challenging task
One of the difficulties of interpreting clinical data on bleeding patterns—with any type of contraception—is the lack of a universally accepted standard for collecting and reporting these data. The first suggestions for standardization were made in 1976, when Rodriguez and colleagues proposed using 90-day reference periods for analysis, as a way to minimize variability among individual menstrual cycles.3
Subsequently, the World Health Organization’s (WHO’s) Special Programme of Research, Development and Research Training in Human Reproduction developed recommendations for data collection, terminology, presentation, and data analysis when reporting vaginal bleeding during clinical trials of hormonal contraception. These recommendations became known as the WHO Belsey criteria (TABLE 1). They remain the standard.4
Under the WHO Belsey system:
- vaginal blood loss for which a woman uses sanitary protection is classified as bleeding
- vaginal blood loss that does not result in the use of sanitary protection is considered spotting.
This system also specifies indices for evaluating the bleeding pattern for each woman and reference period, including the number of bleeding-spotting days, number of bleeding-spotting episodes, lengths of bleeding-spotting episodes, and bleeding-spotting-free intervals. A bleeding-spotting episode is defined as one or more consecutive days during which blood loss (bleeding or spotting) has been recorded, each episode being bounded by bleeding-spotting-free days. The WHO Belsey criteria also identified subgroups that have “clinically important bleeding patterns” (TABLE 1).
But not all researchers use the WHO Belsey criteria. Many trials use, and report, their own system of analysis. Some researchers have chosen reference periods of other durations and study periods that range from 1 to 5 years. Some studies report bleeding patterns by number of days, and others report the percentage of women experiencing a given bleeding pattern during a reference period. The lack of uniformity results in data that are difficult to compare from one study to the next—and to explain to our patients.
It’s unclear whether any of our research definitions of clinically significant bleeding have ever been validated as clinically important to our patients. Multiple studies do show that changes in menstrual bleeding patterns are a significant cause of dissatisfaction with any given contraceptive method, but we don’t know if the number of days of bleeding-spotting or the predictability of this bleeding-spotting is the critical piece of information we should be relating to our patients.
In other words, do our beliefs about clinically important bleeding patterns reflect women’s beliefs?
TABLE 1
The WHO Belsey system of bleeding patterns
| Pattern | Definition |
|---|---|
| Amenorrhea | No bleeding |
| Prolonged bleeding | 1 or more bleeding-spotting episodes lasting longer than 14 days |
| Frequent bleeding | More than 5 bleeding-spotting episodes |
| Infrequent bleeding | 1 or 2 bleeding-spotting episodes |
| Irregular bleeding | 3 to 5 episodes with more than 3 bleeding-free intervals of 14 days or longer |
| Normal bleeding | None of the above are present |
| This system establishes criteria for defining clinically important bleeding patterns during a 90-day reference period. Adapted from: Belsey EM et al.4 | |
Implantable contraception
The etonogestrel (ENG) implant (Implanon) is the only implantable contraceptive available in the United States. This single-rod contraceptive can be used for as long as 3 years.
Contraceptive implants, including the levonorgestrel implants once sold in the United States and still available in some parts of the world, are highly effective. The Implanon prescribing information reports a first-year failure rate of 0.38 pregnancies for every 100 woman-years of use; Hatcher and co-workers reported a failure rate of 0.5 The difference is based on how the FDA defines pregnancy in contraceptive trials. In fact, the only pregnancies reported with the ENG implant happened after it was removed. Importantly, the studies evaluated by the FDA included only women not using any medications known to induce liver metabolism (the cytochrome P450 pathway) and who were between 80% and 130% of ideal body weight. The efficacy of the ENG implant for women who are taking medications that induce liver metabolism or who are greater than 130% of their ideal body weight is unknown.
The efficacy of the ENG implant is likely derived from suppression of ovulation and increased cervical mucus viscosity. Associated changes in the endometrium that occur with this low dosage of progestin are likely the primary cause of irregular and unpredictable bleeding.
Several studies have sought to describe the bleeding patterns experienced with the ENG implant.6-8 During the first 3 months, approximately 50% of all women using the ENG implant reported bleeding-spotting (TABLE 2) for 30 days, on average (TABLE 3). The number of days decreases to approximately 20 bleeding-spotting days for each 90-day reference period at 6 to 24 months, with wide variability. For example: From 3 to 6 months, women reported 22 days of bleeding-spotting (standard deviation, 20 days); from months 21 to 24, 20 days of bleeding-spotting (standard deviation, 14 days).7
After using the ENG implant for 2 years, therefore, most women can expect the number of bleeding-spotting days for every 90-day reference period to range between 6 and 34 days. These days of bleeding-spotting are often noncontinuous, however. On average, women reported three separate bleeding-spotting episodes for every 90-day reference period.7
Although individual bleeding patterns are unpredictable, women who had no bleeding, or infrequent bleeding, at the beginning of use of the ENG implant had only a “small chance” of bleeding frequently.6 The most common bleeding pattern observed throughout the study was infrequent bleeding, defined as fewer than three episodes of bleeding in a 90-day reference period (excluding amenorrhea).7
Amenorrhea may not persist. The amenorrhea rate at 6 months of use and beyond ranges from 10% to 20% (TABLE 4). Importantly, women who are amenorrheic in one 90-day reference period are not necessarily the ones who are amenorrheic in another reference period. So, unlike what is more commonly seen with other progestin-only methods, such as injectables, amenorrhea is not sustained for most women.
This unpredictable pattern affects continuation of the ENG implant (TABLE 5). Irregular bleeding is the most common reason women cite for discontinuation, accounting for 30% to 60% of all women who discontinue early.7,9
Overall, approximately 4% of ENG users discontinue the method at 1 year. Ten percent to 20% discontinue each year thereafter because of intolerance to bleeding changes.6-9
There are, however, differences in discontinuation rates across cultures. In an integrated analysis of 13 different trials that evaluated patterns of vaginal bleeding with the ENG implant where the rate of menstrual changes was similar, women from Europe and Canada were much more likely (23%) to discontinue the implant because of those changes than women from Southeast Asia and Chile were (2%).6 This finding may reflect differences in cultural beliefs or disparate access to other contraceptive options.10
TABLE 2
What percentage of women taking progestin-only contraception report bleeding-spotting?
| Months | ||||||
|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 18 | 24 |
| DMPA | ||||||
| Sangi-Haghpeykar (1996)33 | 46% | 43% | 40% | |||
| Cromer (1998)34 | 24% | 10% | ||||
| ENG implant | ||||||
| Croxatto (2000)9 | 40–50% | |||||
| LNG-IUS | ||||||
| Datey (1995)32 | 18% | 6% | 3% | 1% | 4% | |
| Hidalgo (2002)20 | 25% | 8% | 11% | |||
| Progestin-only pill | ||||||
| Sheth (1982)35 | 21–55%* | 6–42%* | ||||
| * Percentage reporting prolonged, frequent, or irregular bleeding. | ||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | ||||||
TABLE 3
How many days of bleeding-spotting do women have
when they use progestin-only contraception?
| Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 0–3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 | 19–21 | 22–24 | 36 |
| DMPA | |||||||||
| Belsey (1988)11 | 16* | 9 | 4 | 3 | |||||
| Hubacher (2009)31 | 21 | 18 | 14 | 10 | |||||
| ENG implant | |||||||||
| Affandi (1998)6 | 26 | 19 | 16 | 16 | 17 | 18 | 18 | 18 | |
| Zheng (1999)8 | 34 | 22 | 19–22 | ||||||
| Funk (2005)7 | 31 | 22 | 19 | 19 | 18 | 19 | 17 | 20 | |
| LNG-IUS | |||||||||
| Datey (1995)32 | |||||||||
| Total days of bleeding | 9 | 7 | 6 | 5 | 5 | 5 | |||
| Total days of spotting | 10 | 5 | 5 | 4 | 4 | 4 | |||
| Progestin-only pill | |||||||||
| Belsey (1988)11 | 15–18 | ||||||||
| *All values in the table represent an average number of days in a 90-day reference period. | |||||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | |||||||||
TABLE 4
What percentage of women taking progestin-only contraception report amenorrhea?
| Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 24 | ||||
| DMPA | |||||||||
| Belsey (1988)11 | 8% | 22% | 39% | 45% | |||||
| Sangi-Haghpeykar (1996)33 | 46% | 53% | 59% | ||||||
| Cromer (1998)34 | 34% | 60% | |||||||
| Polaneczky (1998)14 | 23% | 40% | 65% | 40% | |||||
| Canto (2001)1 | 35% | 70% | |||||||
| Jain (2004)13 (DMPA-SC) | 26% | 38% | 55% | ||||||
| Hubacher (2009)31 | 12% | 25% | 37% | 46% | |||||
| ENG implant | |||||||||
| Affandi (1998)6 | 2% | 19% | 25% | 23% | 21% | ||||
| Zheng (1999)8 | 2% | 19% | 10% | 15% | |||||
| Croxatto (2000)9 | 12-20% | ||||||||
| Funk (2005)7 | 2% | 14-20% | |||||||
| LNG-IUS | |||||||||
| Andersson (1994)21 | 17% | ||||||||
| Hidalgo (2002)20 | 44% | 50% | 50% | ||||||
| Progestin-only pill | |||||||||
| Belsey (1988)11 | 0% | 0% | 0% | 0% | |||||
| Sheth (1992)35 | 3-8% | 0-2% | |||||||
| Kovacs (1996)24 | 5-10% | ||||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | |||||||||
TABLE 5
What percentage discontinue progestin-only contraception
because of a change in bleeding pattern?
| Months | ||||||
|---|---|---|---|---|---|---|
| Study | 3 | 6 | 9 | 12 | 24 | 36 |
| DMPA | ||||||
| Potter (1997)36 | 43% | |||||
| Sangi-Haghpeykar (1996)33 | 34.1% | 58%* | 78%* | |||
| Davidson (1997)37 | 31% | 49%* | 58% | |||
| ENG implant | ||||||
| Croxatto (2000)9 | 19% | |||||
| Zheng (1999)8 | 4% | 6.1%* | 8.4%* | |||
| Affandi (1998)6 | 23% | |||||
| Funk (2005)7 | 13% | |||||
| LNG-IUS | ||||||
| Datey (1995)32 | 13.8% | |||||
| Luukkainen (1987)38 | 7.5% | |||||
| Andersson (1994)21 | 5.8%* | 8.3%* | 9.6%* | |||
| Progestin-only pill | ||||||
| Belsey (1988)39 | 10% | |||||
| Sheth (1982)35 | 25% | 34.5%* | ||||
| Graham (1992)25 | 18% | 25% | 35%* | |||
| *Percentages are cumulative across the months studied. | ||||||
| Key: DMPA, depot medroxyprogesterone acetate; ENG implant, etonogestrel implant; LNG-IUS, levonorgestrel intrauterine system. | ||||||
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
The pattern of bleeding seen with the ENG implant is like the activity of the heart in atrial fibrillation: irregularly irregular. Still, most (80%) women continue to use it beyond 1 year. In fact, the discontinuation rate for the ENG implant is less than that of depot medroxyprogesterone acetate (DMPA) and progestin-only pills.
Most ENG implant users report no difficulty tolerating the associated unpredictable bleeding; it’s possible that they had unpredictable bleeding at baseline, or were drawn to the improvement in their dysmenorrhea.6
Importantly, unpredictable bleeding does not affect efficacy; the ENG implant remains one of the most effective long-acting reversible contraceptives. For women who can tolerate unpredictable bleeding, the ENG implant is a highly effective contraceptive option.
Injectable contraception
Approved by the FDA in 1992, DMPA (Depo-Provera) has good efficacy and long-acting protection. Disadvantages include unpredictable bleeding, weight gain, acne, depression, hair loss, and the controversial issue of decreased bone loss with prolonged use.
What are the expected changes in bleeding patterns with DMPA? Women often have unpredictable patterns, with infrequent but prolonged bleeding-spotting episodes.11 The overall incidence of irregular bleeding can be as high as 70% in the first year of use.12 Irregular bleeding decreases with continued use, to as low as 10% after the first year (TABLE 2).
Although the number of bleeding-spotting days decreases over time, women have reported as many as 10 days of irregular bleeding-spotting between 9 and 12 months of use (TABLE 3). The rates of irregular bleeding and amenorrhea are similar for the subcutaneous formulation of DMPA.13
DMPA is often used because of the high likelihood of amenorrhea. However, amenorrhea is not accomplished in most women in a short time. At 3 months of use, 10% to 45% of women report amenorrhea; after 1 year, the rate increases to 40% to 70% (TABLE 4). At 5 years, 80% of women report amenorrhea.12
A source of frustration. DMPA’s high discontinuation rate, compared with what is seen with other contraceptives, can be frustrating for patients and clinicians. Irregular bleeding is the most common reason for discontinuation. Approximately 35% of women who start DMPA discontinue it during the first 3 months of use because of irregular bleeding (TABLE 5). The cumulative discontinuation rate rises over time: At 1 year, 40% to 60% of women who started DMPA will have discontinued it because of changes in bleeding patterns (TABLE 5). Furthermore, 70% of women reporting DMPA discontinuation due to bleeding changes stopped the method after the first injection.14
Paul and colleagues conducted a telephone survey to determine the patterns of use and reasons for discontinuation among DMPA users.15 Of 252 DMPA users surveyed, 20% cited menstrual disturbances as the reason for discontinuation. These changes were equally distributed: amenorrhea, irregular bleeding, and heavy bleeding, all 6.8%.
Of approximately 7,000 women who participated in the 2002 National Survey for Family Growth, 600 had used DMPA in the past. Thirty-four percent pointed to a dislike of changes in menstrual periods as the reason for discontinuation.16
Not surprisingly, helping your patient develop realistic expectations about bleeding patterns with DMPA can decrease the discontinuation rate. Women who received repeated, structured information about DMPA were less likely to discontinue it because of menstrual disturbances (amenorrhea and irregular and heavy bleeding) than were women in a routine counseling group (OR, 0.20; 95% CI: 0.11, 0.37).17
Other investigators have reported similar findings, with a fourfold to sixfold lower likelihood of discontinuation because of bleeding changes among women who received detailed counseling about DMPA.1,2
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
DMPA is effective and convenient, but unpredictable bleeding in the first year of use is not uncommon. The irregularity is similar to that seen with the ENG implant in the first 6 months of use. Thereafter, DMPA users are more likely to achieve and maintain amenorrhea, compared to ENG implant users.
Intrauterine contraception
The main mechanism of contraceptive action in the levonorgestrel intrauterine system (LNG-IUS) (Mirena) is significant thickening of cervical mucus, resulting in a physical barrier to sperm penetration; ovulation inhibition may also contribute. In a study of women who had been using the LNG-IUS for 4 years, 88% (15/17 cycles) were still ovulatory according to progesterone levels, but only 47% (8/17 cycles) showed normal follicular growth and rupture by ultrasonography.18 The efficacy of the LNG-IUS is 99.8%.6
Advantages of the LNG-IUS include its high effectiveness; long-term action; increased rate of menstrual cycles that are shorter, lighter, and marked by less cramping as use continues; and a high likelihood of amenorrhea as duration of use lengthens.
As with other progestin-only contraceptives, the major disadvantage of the LNG-IUS is associated irregular bleeding that, as is the case with DMPA, appears to decrease with duration of use for most women.
What are the expected changes in bleeding patterns with LNG-IUS? Local effects of the LNG-IUS on the endometrial lining include stromal pseudodecidualization, glandular atrophy, and increased infiltration of leukocytes in the endometrium. These effects, combined with partial inhibition of ovulatory function, commonly result in irregular bleeding.
The number of days of bleeding-spotting is pronounced in the first 3 to 6 months after insertion. Approximately 18% of women reported bleeding-spotting in the first 3 months; 6% to 25%, at 6 months; and only 1% of women, approximately, at 12 months (TABLE 2).
In a survey of Finnish women who used the LNG-IUS, 45.2% reported irregular bleeding, and 18.1% reported spotting, at some point during use.19 Importantly, the prevalence of bleeding-spotting does decrease with duration of use. Nevertheless, as many as 10% of women still report irregular bleeding-spotting at 2 years (TABLE 2).
As with other progestin-only contraceptives, amenorrhea rates for the LNG-IUS vary (TABLE 4). In a Brazilian study of 256 women, 44% reported amenorrhea at 6 months; 50%, at 12 and 24 months.20 In a larger study of 1,821 Finnish women, however, only 17% of women reported amenorrhea at 12 months.21 A survey study of approximately 16,000 Finnish women who used the LNG-IUS found that 75% reported that they “totally or occasionally missed menses” at any time during as long as 5 years of use.19
The discontinuation rate for the LNG-IUS is lower than for the ENG implant or DMPA. Still, changes in bleeding patterns are the most common reason for discontinuation. At 1 year of use, approximately 10% of women discontinue the LNG-IUS because of changes in the bleeding pattern (TABLE 5).
In the most comprehensive study of early removal of the LNG-IUS, the total discontinuation rate—for all reasons—increased to 13% at 2 years, 19% at 3 years, 25% at 4 years, and 35% at 5 years.19 Women who reported excessive bleeding were almost three times more likely to discontinue the LNG-IUS early than women who did not report such a problem (RR, 2.77; 95% CI: 2.5, 3.07). Women who experience spotting are almost twice as likely to discontinue early (RR, 1.89; 95% CI: 1.75, 2.05). Others have reported the cumulative discontinuation rate to be as low as 14.4% at 5 years (when measuring discontinuation because of changes in menstrual bleeding) and as high as 35% at 5 years (when considering the total discontinuation rate for all reasons).21
Amenorrhea lowers the discontinuation rate. In one analysis, women who reported that they “totally or occasionally missed periods” were half as likely to discontinue the LNG-IUS as those who didn’t make such a report (RR, 0.46; 95% CI: 0.43, 0.50).19
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
Irregular bleeding is common with the LNG-IUS in the first 3 to 6 months of use, but overall discontinuation is relatively low—probably because of the high likelihood that bleeding patterns improve over time. Still, irregular bleeding remains the most common reason for discontinuation. Realistic expectations about bleeding patterns and the lower likelihood for amenorrhea, in comparison with DMPA, are important variables to discuss with women who are considering the LNG-IUS.
Progestin-only pills
Progestin-only pills (POPs) have a failure rate that ranges from 1.1 to 9.6 for every 100 users in the first year.22 A POP is used most often by women in whom estrogen is contraindicated, including those who are breastfeeding.23
Disadvantages. POPs require precise adherence and make irregular vaginal bleeding likely. Although 40% to 50% of women who take a POP have normal menstrual cycles, 40% have short, irregular cycles, and another 10% experience even more markedly irregular cycles—from spotting to amenorrhea.22
Studies that precede the WHO Belsey system showed that 1) as many as 70% of women who use a POP reported “breakthrough bleeding-spotting” in one or more cycles and 2) 6% to 16% have “breakthrough bleeding or inter-menstrual spotting” in all cycles (TABLES 2 AND 3).24,25
On average, 25% of women discontinue POPs because of changes in their menstrual cycle (TABLE 5).
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
The mechanism that results in irregular vaginal bleeding in women taking a POP is unclear; evidence suggests that incomplete suppression of ovulation and direct endometrial effects are possible. To the frustration of patients and clinicians, it isn’t possible to predict who will have irregular bleeding—i.e., there is no association between body weight, or age, and the risk of irregular bleeding. As with other progestin-only methods, irregular bleeding is the most common reason for discontinuing POPs.26 A Cochrane review of POPs is under way.27
Is unpredictable bleeding with progestin-only contraceptives treatable?
Bleeding and discontinuation rates associated with progestin-only contraceptives that are observed in clinical trials, especially rates used for FDA review and approval of a product, don’t always translate to real-life medicine. Typically, in such trials, no treatment for irregular or unacceptable bleeding patterns is permitted: If an effective treatment is available, overall acceptability and continuation of the contraceptive could, potentially, be boosted. This matter is most relevant with injectable, intrauterine, and implantable progestin-only methods.
Findings of one meta-analysis. A recent Cochrane review evaluated the literature until December 2006 on the treatment of vaginal bleeding irregularities induced by progestin-only contraceptives.28 Twenty-three randomized controlled trials, encompassing 2,674 subjects, were included. Seventy percent of the trials that were included were determined to reflect a low or moderate risk of bias.
Treatment with estrogen alone reduced the number of days of an ongoing bleeding episode among DMPA and levonorgestrel implant (Norplant) users. Treatment often led to individuals’ discontinuation in a study, however, because of gastrointestinal upset. Combined oral contraceptives can treat amenorrhea with success among DMPA users.
Antiprogestins such as mifepristone cause a reduction in bleeding among women using the levonorgestrel implant, but are not of benefit for ENG implant users.
Last, use of NSAIDs to treat irregular bleeding has shown variable efficacy. Additional small studies cited in the Cochrane review suggest that the following treatments were more effective than placebo for terminating an episode of bleeding among women using progestin-only contraception: the antiprogestin mifepristone for DMPA and POP users; mifepristone plus an estrogen for ENG implant users; and doxycycline for ENG implant users.28
Overall, some women benefit from attempts at treatment. The authors of the Cochrane review caution that their findings do not support the routine clinical use of any of the regimens included in the trials, particularly for obtaining a long-term effect.28
Newer trials, different findings? A more recent double-blind, randomized trial, in which the subjects were 100 Thai women, showed that irregular bleeding with DMPA ceased completely in 88% of those treated with tranexamic acid, 250 mg QID for 5 days, compared with 8% of women in whom bleeding ceased after treatment with placebo.29
Another recent randomized trial found that mifepristone, combined with ethinyl estradiol or doxycycline, was significantly more effective than placebo in ending an episode of bleeding in ENG implant users. No improvement was seen, however, in subsequent bleeding patterns, and improvement with treatment, compared with placebo, amounted to a decrease of only about 2 days.30
Noticeably missing from the literature are large trials that evaluate the use of combined hormonal contraceptives for bleeding irregularities in women using long-acting progestin-only contraceptives. True, some women use these methods because of a contraindication to estrogen-containing methods, but, in reality, most women who use these methods do so because of their high efficacy and ease of use.
PERSPECTIVE AND GUIDANCE FOR YOUR PRACTICE
For women who use the ENG implant or LNG-IUS and have no contraindication to estrogen-containing contraceptives, we often provide a short (1 or 2 months) course of a combined hormonal contraceptive when they find bleeding irregularities bothersome.
Because the serum progestin level provided with these methods is extremely low, adding a low-dose combined oral contraceptive, contraceptive patch, or contraceptive vaginal ring is not that different than using any of the combined hormonal contraceptives. A woman will not become pregnant if she forgets to take the pill or the ring falls out because she still has the progestin-only method in place. And if the short course of a combined hormonal contraceptive helps her continue the more effective method, then the overall goal of avoiding unintended pregnancy is better accomplished.
Large trials to evaluate the use of combined hormonal methods in such circumstances would, of course, be of great benefit.
Good counseling → informed choice → adherence and continuation
With all forms of progestin-only contraception, unpredictable bleeding occurs often and is the most common reason for method discontinuation.
Counseling that explicitly discusses the high likelihood of unpredictable menstrual bleeding allows women to prioritize this issue in their choice of a contraceptive.
Informed choice leads to a better continuation rate for progestin-only methods.
Seeking understanding. We lack full understanding of exactly what it is about changes in bleeding patterns that matter to women. Have definitions of bleeding and spotting that researchers utilize missed quality of life concerns that are more relevant to women? Are women concerned about how many days are spent avoiding sexual activity? Do religious restrictions figure prominently for some? How dissatisfied are they with days of cramping or bloating without bleeding? What do women want to know when they consider the bleeding patterns for their contraceptive options?
The answers to these questions likely vary from patient to patient—and that observation leads us back to grasping the art of contraceptive counseling: Our counseling needs to be concise, relatable, and honest.
1. Canto De Cetina TE, Canto P, Ordoñez Luna M. Effect of counseling to improve compliance in Mexican women receiving depot-medroxyprogesterone acetate. Contraception. 2001;63:143-146.
2. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception. 1996;53:357-361.
3. Rodriguez G, Faundes-Latham A, Atkinson LE. An approach to the analysis of menstrual patterns in the critical evaluation of contraceptives. Stud Fam Plann. 1976;7(2):42-51.
4. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253-260.
5. Hatcher RA, Trussell J, Nelson AL, Cates W, Jr, Stewart F, Kowal D. Contraceptive Technology. 19th ed. New York: Thomson Reuters; 2008.
6. Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception. 1998;58(6 Suppl):99S-107S.
7. Funk S, Miller MM, Mishell DR, Jr, et al. Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
8. Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
9. Croxatto HB. Clinical profile of Implanon: a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5 Suppl 2:21-28.
10. Power J, French R, Cowan F. Subdermal implantable contraceptives versus other forms of reversible contraceptives or other implants as effective methods of preventing pregnancy. Cochrane Database Syst Rev. 2007;(3):CD001326.-
11. Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
12. Haider S, Darney PD. Injectable contraception. Clin Obstet Gynecol. 2007;50:898-906.
13. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
14. Polaneczky M, Liblanc M. Long-term depot medroxyprogesterone acetate (Depo-Provera) use in inner-city adolescents. J Adolesc Health. 1998;23(2):81-88.
15. Paul C, Skegg DC, Williams S. Depot medroxyprogesterone acetate. Patterns of use and reasons for discontinuation. Contraception. 1997;56:209-214.
16. Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76:267-272.
17. Halpern V, Grimes DA, Lopez L, Gallo MF. Strategies to improve adherence and acceptability of hormonal methods for contraception. Cochrane Database Syst Rev. 2006;(1):CD004317.-
18. Barbosa I, Olsson SE, Odlind V, Goncalves T, Coutinho E. Ovarian function after seven years’ use of a levonorgestrel IUD. Adv Contracept. 1995;11(2):85-95.
19. Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG. 2000;107:335-339.
20. Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002;65:129-132.
21. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
22. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004.
23. Collins J, Crosignani PG. ESHRE Capri Workshop Group. Hormonal contraception without estrogens. Hum Reprod Update. 2003;9:373-386.
24. Kovacs G. Progestogen-only pills and bleeding disturbances. Hum Reprod. 1996;11 Suppl 2:20-23.
25. Graham S, Fraser IS. The progestogen-only minipill. Contraception. 1982;26:373-388.
26. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 Suppl 1):S1-S195.
27. Grimes DA, Lopez LM, O’Brien P, Raymond EG. Progestin-only pills for contraception (Protocol). Cochrane Database Syst Rev. 2009;(1):CD007541.-
28. Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gülmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst Rev. 2007;(4):CD003449.-
29. Senthong AJ, Taneepanichskul S. The effect of tranexamic acid for treatment irregular uterine bleeding secondary to DMPA use. J Med Assoc Thai. 2009;92:461-465.
30. Weisberg E, Hickey M, Palmer D, et al. A randomized controlled trial of treatment options for troublesome uterine bleeding in Implanon users. Hum Reprod. 2009;24:1852-1861.
31. Hubacher D, Lopez L, Steiner M, Dorflinger L. Menstrual pattern changes from levonorgestrel subdermal implants and DMPA: systematic review and evidence-based comparisons. Contraception. 2009. doi:10.1016/j.contraception.2009.02.008.
32. Datey S, Gaur LN, Saxena BN. Vaginal bleeding patterns of women using different contraceptive methods (implants, injectables, IUDs, oral pills)—an Indian experience. An ICMR Task Force Study. Indian Council of Medical Research. Contraception. 1995;51:155-165.
33. Sangi-Haghpeykar H, Poindexter AN, 3rd, Bateman L, Ditmore JR. Experiences of injectable contraceptive users in an urban setting. Obstet Gynecol. 1996;88:227-233.
34. Cromer BA, Berg-Kelly KS, Van Groningen JP, Seimer BS, Ruusuvaara L. Depot medroxyprogesterone acetate (Depo-Provera) and levonorgestrel (Norplant) use in adolescents among clinicians in Northern Europe and the United States. J Adolesc Health. 1998;23:74-80.
35. Sheth A, Jain U, Sharma S, et al. A randomized, double-blind study of two combined and two progestogen-only oral contraceptives. Contraception. 1982;25:243-252.
36. Potter LS, Dalberth BT, Cañamar R, Betz M. Depot medroxyprogesterone acetate pioneers. A retrospective study at a North Carolina Health Department. Contraception. 1997;56:305-312.
37. Davidson AR, Kalmuss D, Cushman LF, Romero D, Heartwell S, Rulin M. Injectable contraceptive discontinuation and subsequent unintended pregnancy among low-income women. Am J Public Health. 1997;87:1532-1534.
38. Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception. 1987;36:169-179.
39. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207-225.
1. Canto De Cetina TE, Canto P, Ordoñez Luna M. Effect of counseling to improve compliance in Mexican women receiving depot-medroxyprogesterone acetate. Contraception. 2001;63:143-146.
2. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception. 1996;53:357-361.
3. Rodriguez G, Faundes-Latham A, Atkinson LE. An approach to the analysis of menstrual patterns in the critical evaluation of contraceptives. Stud Fam Plann. 1976;7(2):42-51.
4. Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction. Contraception. 1986;34:253-260.
5. Hatcher RA, Trussell J, Nelson AL, Cates W, Jr, Stewart F, Kowal D. Contraceptive Technology. 19th ed. New York: Thomson Reuters; 2008.
6. Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception. 1998;58(6 Suppl):99S-107S.
7. Funk S, Miller MM, Mishell DR, Jr, et al. Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
8. Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
9. Croxatto HB. Clinical profile of Implanon: a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5 Suppl 2:21-28.
10. Power J, French R, Cowan F. Subdermal implantable contraceptives versus other forms of reversible contraceptives or other implants as effective methods of preventing pregnancy. Cochrane Database Syst Rev. 2007;(3):CD001326.-
11. Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
12. Haider S, Darney PD. Injectable contraception. Clin Obstet Gynecol. 2007;50:898-906.
13. Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
14. Polaneczky M, Liblanc M. Long-term depot medroxyprogesterone acetate (Depo-Provera) use in inner-city adolescents. J Adolesc Health. 1998;23(2):81-88.
15. Paul C, Skegg DC, Williams S. Depot medroxyprogesterone acetate. Patterns of use and reasons for discontinuation. Contraception. 1997;56:209-214.
16. Moreau C, Cleland K, Trussell J. Contraceptive discontinuation attributed to method dissatisfaction in the United States. Contraception. 2007;76:267-272.
17. Halpern V, Grimes DA, Lopez L, Gallo MF. Strategies to improve adherence and acceptability of hormonal methods for contraception. Cochrane Database Syst Rev. 2006;(1):CD004317.-
18. Barbosa I, Olsson SE, Odlind V, Goncalves T, Coutinho E. Ovarian function after seven years’ use of a levonorgestrel IUD. Adv Contracept. 1995;11(2):85-95.
19. Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG. 2000;107:335-339.
20. Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002;65:129-132.
21. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
22. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004.
23. Collins J, Crosignani PG. ESHRE Capri Workshop Group. Hormonal contraception without estrogens. Hum Reprod Update. 2003;9:373-386.
24. Kovacs G. Progestogen-only pills and bleeding disturbances. Hum Reprod. 1996;11 Suppl 2:20-23.
25. Graham S, Fraser IS. The progestogen-only minipill. Contraception. 1982;26:373-388.
26. McCann MF, Potter LS. Progestin-only oral contraception: a comprehensive review. Contraception. 1994;50(6 Suppl 1):S1-S195.
27. Grimes DA, Lopez LM, O’Brien P, Raymond EG. Progestin-only pills for contraception (Protocol). Cochrane Database Syst Rev. 2009;(1):CD007541.-
28. Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gülmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives. Cochrane Database Syst Rev. 2007;(4):CD003449.-
29. Senthong AJ, Taneepanichskul S. The effect of tranexamic acid for treatment irregular uterine bleeding secondary to DMPA use. J Med Assoc Thai. 2009;92:461-465.
30. Weisberg E, Hickey M, Palmer D, et al. A randomized controlled trial of treatment options for troublesome uterine bleeding in Implanon users. Hum Reprod. 2009;24:1852-1861.
31. Hubacher D, Lopez L, Steiner M, Dorflinger L. Menstrual pattern changes from levonorgestrel subdermal implants and DMPA: systematic review and evidence-based comparisons. Contraception. 2009. doi:10.1016/j.contraception.2009.02.008.
32. Datey S, Gaur LN, Saxena BN. Vaginal bleeding patterns of women using different contraceptive methods (implants, injectables, IUDs, oral pills)—an Indian experience. An ICMR Task Force Study. Indian Council of Medical Research. Contraception. 1995;51:155-165.
33. Sangi-Haghpeykar H, Poindexter AN, 3rd, Bateman L, Ditmore JR. Experiences of injectable contraceptive users in an urban setting. Obstet Gynecol. 1996;88:227-233.
34. Cromer BA, Berg-Kelly KS, Van Groningen JP, Seimer BS, Ruusuvaara L. Depot medroxyprogesterone acetate (Depo-Provera) and levonorgestrel (Norplant) use in adolescents among clinicians in Northern Europe and the United States. J Adolesc Health. 1998;23:74-80.
35. Sheth A, Jain U, Sharma S, et al. A randomized, double-blind study of two combined and two progestogen-only oral contraceptives. Contraception. 1982;25:243-252.
36. Potter LS, Dalberth BT, Cañamar R, Betz M. Depot medroxyprogesterone acetate pioneers. A retrospective study at a North Carolina Health Department. Contraception. 1997;56:305-312.
37. Davidson AR, Kalmuss D, Cushman LF, Romero D, Heartwell S, Rulin M. Injectable contraceptive discontinuation and subsequent unintended pregnancy among low-income women. Am J Public Health. 1997;87:1532-1534.
38. Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception. 1987;36:169-179.
39. Belsey EM. The association between vaginal bleeding patterns and reasons for discontinuation of contraceptive use. Contraception. 1988;38:207-225.
UPDATE: contraception
The authors report no financial relationships relevant to this article.
We’ve heard that troubling statistic: Approximately 50% of pregnancies in the United States are unintended. But did you know that one half of those unintended pregnancies occur in women who were using some form of birth control at the time of conception?1 Such pregnancies are due to discontinuation of the method, incorrect use, or method failure.2 The focus of this article is contraceptive counseling, with special attention to:
- which methods of combination hormonal contraception women prefer
- the controversy surrounding the contraceptive patch in regard to thromboembolic disease
- long-acting reversible contraception (LARC), such as the intrauterine device (IUD) and the contraceptive implant, with an emphasis on how LARC is of benefit to both the patient and society.
The ultimate goal of good contraceptive counseling? To help women choose the easiest and most effective method with the fewest side effects.
In head-to-head comparison, women preferred the ring to the patch
Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch. Obstet Gynecol. 2008;111:267–277.
The ethinyl estradiol/etonogestril vaginal ring (NuvaRing) and the ethinyl estradiol/norelgestromin patch (OrthoEvra)—both approved by the Food and Drug Administration (FDA) in 2001—are the only nonoral forms of combined hormonal contraception on the market. These methods are said to increase patient compliance and, potentially, efficacy, because they are nondaily forms of contraception.
Until recently, these methods had been compared only with the combination oral contraceptive (OC), but a recent trial compared them directly to each other. At the conclusion of the study, 71% of ring users and 26.5% of patch users planned to continue using the assigned method (P<.001).
This information should aid clinicians in counseling women about which combination hormonal method to choose.
Participants started out using the OC
The multicenter, randomized, controlled clinical trial comparing the patch and ring included 479 women who were using, and happy with, the combination OC. After rating their satisfaction with the OC, women were randomized to the patch or ring and given 3 months’ worth of product. Follow-up involved only two telephone calls and one visit at the end of the third cycle, because this degree of monitoring was thought to mimic clinical practice.
The percentages of women who completed three cycles of their assigned product were 94.6% and 88.2% in the ring and patch groups, respectively (P=.03). The most common reasons for early discontinuation in the ring group were discomfort and adverse effects. In the patch group, the most common reasons were adverse effects, skin irritation, and adherence problems.
Even after adjusting for age, education, and whether an OC was actively being used at the time the study began, patch users were twice as likely to discontinue the patch at the end of three cycles and seven times more likely to state that they did not want to continue the patch.
Adverse effects were greater than with the pill
Women switching from pill to patch were significantly more likely to report breast pain, nausea, skin rash, longer menstrual bleeding, and menstrual pain than women who switched from the pill to the ring (P<.001).
Women who switched from the pill to the ring were more likely to experience vaginal discharge (P=.003) and a larger amount of vaginal discharge than patch users (P<.001).
These findings are similar to those of previous studies that compared the patch with the pill, noting that breast discomfort, application-site reaction, and dysmenorrhea were more common in patch than pill users.3 Earlier studies also found the ring to be associated with complaints of vaginal discharge.4,5
Findings may not be generalizable
The most important finding from this direct comparison is the difference in patient satisfaction between groups. Visual analog scales showed that women using the ring were happier with the ring than with the pill, whereas women using the patch were happier with the pill than with the patch (P<.001). Questionnaires revealed that women were more satisfied with the ring than they were with the patch, and were more likely to recommend the ring than the patch to a friend (P<.001).
Based on continuation rates, patient satisfaction, and adverse-effect profiles, women in this study clearly preferred the ring to the pill, and the pill to the patch. When using this information to counsel patients, however, it is important to recall that this population was specific. The women had been using an OC, with which they were happy. This study cannot necessarily be generalized to women who are just initiating combination hormonal contraception, but it can be helpful in counseling a patient who may want to switch from an OC to a method that involves nondaily dosing.
Does the contraceptive patch raise the risk of thromboembolism?
Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2006;73:223–228.
Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2007;76:4–7.
Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive users. Obstet Gynecol. 2007;109(2 Pt 1):339–346.
Both the media and regulatory agencies have raised concerns about whether the contraceptive patch heightens the risk of thromboembolism and is less effective in women above a certain body weight.
The controversy surrounding thromboembolic disease stems from a pharmacokinetics study by van den Heuvel and colleagues that compared serum ethinyl estradiol levels in users of the patch, vaginal ring, and a combination OC containing 30 μg of ethinyl estradiol and 150 μg of levonorgestrel.6 Women randomized to the patch had serum ethinyl estradiol levels 1.6 times higher than women randomized to an OC, and 3.4 times higher than women randomized to the ring.
These findings led the FDA to update package labeling of the patch to warn health-care providers and patients that the patch exposes women to 60% more estrogen and may increase the risk of thromboembolic events. Oddly, the FDA did not require any labeling change to combination OCs to indicate that they contain up to twice as much estrogen as the contraceptive ring.
A set of studies finds no elevated risk
Although the study by van den Heuvel and associates raised the possibility of increased blood clots in patch users, no association between the two had been corroborated at the time it was published.6 Since then, three epidemiological studies have explored the potential link between thromboembolic events and use of the patch.
In the first of these studies, Jick and colleagues used the PharMetrics database to extract data on users of the patch and norg-estimate-containing OCs. This database contains drug prescription information, patient demographic data, and ICD-9 billing codes submitted by managed care health plans. A nested case-control study design was used to compare patch and pill users and control for confounding variables.
The base population was women 15 to 44 years old who were new users of the patch or a norgestimate-containing OC. A thromboembolic event was diagnosed if the patient’s record included a diagnosis code for pulmonary embolus, deep vein thrombosis, or an emergency room visit or diagnostic testing indicating venous thromboembolism (VTE). These diagnosis codes, combined with the prescription of long-term anticoagulation therapy, strengthened the identification of cases. As many as four controls were selected for each case.
The 215,769 women included in this study contributed 147,323 woman-years of exposure to the drugs. There were 31 and 37 cases of VTE identified in the patch and pill groups, respectively, with an incidence of 52.8 for every 100,000 woman-years in the patch group and 41.8 for every 100,000 woman-years in the pill group and an unadjusted, matched odds ratio of VTE in patch versus pill users of 0.9. When the data were adjusted for duration of drug exposure, the odds ratio did not change.
A follow-up study by Jick and associates, published in 2007, had the same study design and included 17 additional months of data. Another 56 cases of VTE were diagnosed. The odds ratio for patch users, compared with pill users, was 1.1. When data from the two studies were combined, 73 and 51 total cases of VTE had occurred in the pill and patch groups, respectively. The overall odds ratio was 1.0.
A third study finds significantly heightened risk
Cole and associates studied insurance claims data from UnitedHealthcare, a database containing medical and prescription claim information as well as patient demographics. Because researchers used pharmacy dispensing records, they were able to include women 15 to 44 years old who had received at least one prescription for the contraceptive patch or a norgestimate-containing OC with 35 μg of ethinyl estradiol.
Unlike the studies by Jick and colleagues, the study by Cole and associates considered all women eligible, even if they had used OCs in the past. Cases of VTE, stroke, and acute myocardial infarction (AMI) were abstracted from this group, identified from insurance claim information, and confirmed by chart review. Review of medical records is an important strength of this study; no such review was done in the studies by Jick and colleagues. Four controls were matched to each case, by age and duration of contraceptive use.
(This study was commissioned in conjunction with both the FDA and Johnson & Johnson, makers of the contraceptive patch, but researchers had full control over the data and results and were not required to consult with Johnson & Johnson when reporting findings.)
There were 49,048 woman-years of exposure to the patch and 202,344 woman-years of exposure to the pill, with an incidence of VTE of 40.8 and 18.3 for every 100,000 woman-years in patch and pill users, respectively. The incidence of AMI was 6.1 and 3.5 for every 100,000 woman-years in patch and pill users, respectively. No ischemic strokes were noted in patch users.
The adjusted incidence ratio for VTE in patch users compared with pill users was 2.2, and for AMI it was 1.8. Following publication of this study, the FDA issued a statement in January of this year that women using the patch face an increased risk of VTE, compared with women using the pill. Package labeling was changed to reflect this heightened risk.
Reasons for different findings
The studies by Jick and colleagues and Cole and associates present very different findings. The studies by Jick and colleagues give the impression that there is no increased risk of VTE in patch users compared with pill users, but the studies have significant flaws. First, Jick and colleagues do not confirm the diagnosis of VTE in the medical record. This is particularly problematic because the reported number of pulmonary emboli (PE) is very high, compared with the number of deep vein thromboses. The 2006 study found 42 cases of PE and only 26 cases of deep vein thrombosis. Because the latter is more common than PE, this could indicate that deep vein thrombosis was underdiagnosed.
Another shortcoming is that Jick and colleagues included only nonfatal thromboembolic events, which may mean that they missed many cases of fatal VTE because they were not looking for this information. The inclusion of new initiators only also may have skewed the data. This would mean that former users of an OC may have been included in the patch group but were ineligible for inclusion in the pill group. This may bias the data toward experienced hormonal contraceptive users in the patch group, thereby falsely lowering the VTE rate.
The study by Cole and associates also has limitations. It included long-term users of hormonal contraceptives in both the patch and the OC groups, which may bias the data toward lower rates of VTE, AMI, and stroke for the same reasons cited above. One would assume that this bias was corrected, because prior use was allowed in both groups, making the bias equally distributed, but there is no way to confirm this with any degree of certainty.
All three studies have some flaws in common
All three studies used prescription information to determine exposure, but there is no guarantee that the women who filled the prescriptions actually used the agents. Patients given drug samples by their clinicians were overlooked because these samples are not tracked through pharmacy data.
Because the data were collected from insurance claims information of privately insured patients, it is impossible to generalize these findings to the general population. We cannot use the findings to determine whether the same results would be seen in uninsured women or women insured through nonprivate programs such as Medicaid or the Veterans Administration.
So what’s the bottom line?
Health-care providers should be cautious about citing these studies as “evidence” when advising patients about the risk of VTE while using the patch. The twofold increased risk of VTE observed in patch users and the almost twofold increased risk of AMI observed by Cole and associates cannot be completely ignored, however, particularly because this study was better designed than those by Jick and colleagues.
It is more important to remember that the incidence of VTE in patch users is extremely low. If a patient has been using the patch, is happy with the method, and has had no adverse effects, there is no reason, based on these findings, to discontinue it. When counseling new initiators, the best that can be done is to explain the potential risks and side effects associated with the method and allow the patient to make an informed choice using the information that is available.
If the increased risk of VTE is accurate, it would still be equal to or lower than the risk during pregnancy. A recent review found the overall incidence of VTE in pregnancy or the postpartum period to be 200 for every 100,000 woman-years.7
In a pooled analysis of the two studies of the contraceptive patch by Jick and colleagues and the one study by Cole and associates, the overall and method failure rates through 13 cycles were 0.8% and 0.6%, respectively, representing 15 pregnancies.1
Subject weights were divided into deciles to determine the number of pregnancies per decile. Interestingly, that number does not appear to be evenly distributed. In deciles 1 through 9, which represent women who weigh up to 80 kg, the number of pregnancies was eight, whereas seven pregnancies occurred in the 10th decile, which represents women weighing more than 80 kg. Because the number of pregnancies in decile 10 is essentially equivalent to all of the other deciles combined, women who weigh more than 80 kg (176 lb) appear to be at increased risk of pregnancy. Five of the seven pregnancies in decile 10 occurred in women weighing more than 90 kg (198 lb).
No studies have directly explored the reasons for this relationship or looked at body mass index or body surface area in relation to efficacy of the patch. Further research is clearly needed.
How to counsel overweight women
It is imperative that patients who weigh more than 198 lb be informed that the pregnancy rate is higher than the rate quoted for the patch. It may even be reasonable to counsel women in that 10thdecile—who weigh more than 176 lb—about alternative forms of hormonal contraception that would be more effective for them than the patch.
Reference
1. Ziemen M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77(2 Suppl 2):S13-S18.
Why don’t American women choose long-acting reversible contraception?
Do American women not want to use long-acting reversible contraception (LARC), or are we, as providers, failing to properly educate them about its benefits?
The ParaGard copper IUD, the Mirena levonorgestrel intrauterine system (LNGIUS), and the Implanon etonorgestrel contraceptive implant are all highly effective, convenient, long-duration, and reversible (FIGURE). Despite substantial evidence indicating that these methods are well tolerated and highly effective, only about 2% of American women are choosing them to prevent pregnancy.1 This rate lags far behind other countries in IUD utilization. In contrast, more than 50% of contraceptive users in China and Egypt are using intrauterine contraception.8
FIGURE
Copper IUD is effective for 12 years or longer
The copper IUD is FDA-approved for 10 years of use, although studies continue to support its continued efficacy for 12 years or longer.9 The 1-year perfect-use failure rate is 0.6%, and the typical use failure rate is 0.5% to 0.8%.10 The total failure rate over 12 years is 2.2%.9
Benefits. The copper IUD does not increase the risk of intrauterine infection and is safe to place in nulliparous patients.11 It is an excellent choice for women who clearly prefer to have monthly menses and for women who have personal or medical contraindications to hormonal birth control. Women using this method of birth control can expect excellent efficacy, rapid reversibility, and minimal side effects.
Adverse effects. The most common adverse events in copper IUD users are heavier menses and dysmenorrhea. Approximately 4.5% of women discontinue the copper IUD in the first year of use because of these particular side effects.12
LNG-IUS: Highly effective, with important noncontraceptive benefits
This method of birth control is comparable to the copper IUD in terms of efficacy and tolerability. It is FDA-approved for 5 years of use, with a cumulative 5-year failure rate of 0.7 for every 100 women.13 One small study demonstrated that this method is potentially effective up to 7 years, with a 1.1% pregnancy rate.11 With perfect use, the first-year pregnancy rate is 0.1% to 0.2%.14
Benefits. The progestin component provides noncontraceptive benefits, including a reduction in menstrual bleeding and dysmenorrhea,15 treatment of endometrial hyperplasia16 and endometrial cancer,17 endometrial protection in women using tamoxifen,18 treatment of endometriosis,19 and protection from pelvic inflammatory disease.20
Adverse effects. The primary disadvantage of this device is a change in bleeding pattern in some women, who may experience irregular spotting, primarily in the first 3 to 6 months.21 About 20% of users will become amenorrheic by 12 months of use, a feature that is highly desirable for many, but troubling to some.
Implant is essentially 100% effective
The newest LARC device is the etonorgestrel implant, which was approved by the FDA in July 2006. The single-rod implant is typically placed in the subcuticular tissue of the non-dominant arm, although placement in the dominant arm is fine if the patient prefers.
Benefits. In a 3-year study involving 635 subjects, no pregnancies were reported.22 The reported Pearl index of 0.38 pregnancies for every 100,000 woman-years of use relates to pregnancies that occurred shortly after discontinuation rather than during actual use. These studies included only women below 130% of their ideal body weight who were not using liver enzyme-inducing medications. The pregnancy rate in women who use such medications, or weigh above 130% of their ideal body weight, is unknown. Postmarketing surveillance has reported some pregnancies, as would be expected. The device is easily inserted and easily removed as long as 3 years later.
Adverse effects. The primary adverse effect of this implant is bleeding disturbances; discontinuation was usually due to this side effect.22 The cumulative discontinuation rate was 10% at 6 months, 20% at 12 months, 31% at 2 years, and 32.2% at 3 years.22
Training required. FDA approval included a stipulation that practitioners complete company-sponsored training (www.implanonusa.com) to insert and remove the device.
Overall benefits include minimal side effects, low cost
All LARC methods provide excellent protection against pregnancy (equal to or better than sterilization), have minimal side effects, and are rapidly reversible. They are also appropriate for women in whom combination hormonal contraception is contraindicated, such as smokers older than 35 years and women who have had VTE.
A final and important advantage: These methods are more cost-effective than other contraceptive methods, including combination OCs. They may require a higher initial investment, but the LNG-IUS and copper IUD are the least costly methods of contraception over 5 years of use.23
As providers continue to educate themselves and help women gain a better understanding of which methods are truly highly effective, they will likely begin to recommend LARC more often. Use of these devices has the potential to significantly decrease the high rate of unintended pregnancy.
Authors’ note: The figure at right depicts how the efficacy and convenience of contraceptive options rise (and side effects fall) along a continuum. LARC methods are “high up the ladder”—an observation that serves as food for thought as we counsel patients about what methods of birth control are best for them.
1. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24-29, 46.
2. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
3. Sibai BM, Odlind V, Meador ML, Shangold GA, Fisher AC, Creasy GW. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra/Evra). Fertil Steril. 2002;77(2 Suppl 2):S19-S26.
4. Arhendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 microg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451-457.
5. Oddson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive:a 1-year randomized trial. Contraception. 2005;71:176-182.
6. van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: The vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
7. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ, 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum:a 30-year population-based study. Ann Intern Med. 2005;143:697-706.
8. d’Arcangues C. Worldwide use of intrauterine devices for contraception. Contraception. 2007;75(6 Suppl):S2-S7.
9. Long-term reversible contraception. Twelve years of experience with TCu380A and TCu220C. Contraception. 1997;56:341-352.
10. Sivin I, Schmidt F. Effectiveness of IUDs: a review. Contraception. 1987;36:55-84.
11. Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
12. Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra Infante F, Guzmán-Rodríguez R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345:561-567.
13. Grimes DA. Intrauterine devices (IUDs). In:Hatcher RA, ed. Contraceptive Technology. 18th ed. New York: Ardent Media, Inc.;2004:495-530.
14. Sivin I, Stern J. Healthduring prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR). Fertil Steril. 1994;61:70-77.
15. Kadir RA, Chi C. Levonorgestrel intrauterine system: bleeding disorders and anticoagulant therapy. Contraception. 2007;75(6 Suppl):S123-S129.
16. Wildemeersch D, Dhont M. Treatment of non-atypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system. Am J Obstet Gynecol. 2003;188:1297-1298.
17. Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. 2005;97:924-927.
18. Gardner FJ, Konje JC, Abrams KR, et al. Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system:a randomised controlled trial. Lancet. 2000;356:1711-1717.
19. Lockhat FB, Emembolu JO, Konje JC. The efficacy, side-effects and continuation rates of women with symptomatic endometriosis undergoing treatment with an intra-uterine administered progestogen (levonorgestrel):a 3 year follow-up. Hum Reprod. 2005;20:789-793.
20. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’comparative experience of levonorgestrel-and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
21. Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system:a nationwide study of 17,360 users. BJOG. 2000;107:335-339.
22. Croxatto HB. Clinical profile of Implanon:a single-rod etonorgestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5(Suppl 2):21-28.
23. Chiou CF, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception. 2003;68:3-10.
The authors report no financial relationships relevant to this article.
We’ve heard that troubling statistic: Approximately 50% of pregnancies in the United States are unintended. But did you know that one half of those unintended pregnancies occur in women who were using some form of birth control at the time of conception?1 Such pregnancies are due to discontinuation of the method, incorrect use, or method failure.2 The focus of this article is contraceptive counseling, with special attention to:
- which methods of combination hormonal contraception women prefer
- the controversy surrounding the contraceptive patch in regard to thromboembolic disease
- long-acting reversible contraception (LARC), such as the intrauterine device (IUD) and the contraceptive implant, with an emphasis on how LARC is of benefit to both the patient and society.
The ultimate goal of good contraceptive counseling? To help women choose the easiest and most effective method with the fewest side effects.
In head-to-head comparison, women preferred the ring to the patch
Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch. Obstet Gynecol. 2008;111:267–277.
The ethinyl estradiol/etonogestril vaginal ring (NuvaRing) and the ethinyl estradiol/norelgestromin patch (OrthoEvra)—both approved by the Food and Drug Administration (FDA) in 2001—are the only nonoral forms of combined hormonal contraception on the market. These methods are said to increase patient compliance and, potentially, efficacy, because they are nondaily forms of contraception.
Until recently, these methods had been compared only with the combination oral contraceptive (OC), but a recent trial compared them directly to each other. At the conclusion of the study, 71% of ring users and 26.5% of patch users planned to continue using the assigned method (P<.001).
This information should aid clinicians in counseling women about which combination hormonal method to choose.
Participants started out using the OC
The multicenter, randomized, controlled clinical trial comparing the patch and ring included 479 women who were using, and happy with, the combination OC. After rating their satisfaction with the OC, women were randomized to the patch or ring and given 3 months’ worth of product. Follow-up involved only two telephone calls and one visit at the end of the third cycle, because this degree of monitoring was thought to mimic clinical practice.
The percentages of women who completed three cycles of their assigned product were 94.6% and 88.2% in the ring and patch groups, respectively (P=.03). The most common reasons for early discontinuation in the ring group were discomfort and adverse effects. In the patch group, the most common reasons were adverse effects, skin irritation, and adherence problems.
Even after adjusting for age, education, and whether an OC was actively being used at the time the study began, patch users were twice as likely to discontinue the patch at the end of three cycles and seven times more likely to state that they did not want to continue the patch.
Adverse effects were greater than with the pill
Women switching from pill to patch were significantly more likely to report breast pain, nausea, skin rash, longer menstrual bleeding, and menstrual pain than women who switched from the pill to the ring (P<.001).
Women who switched from the pill to the ring were more likely to experience vaginal discharge (P=.003) and a larger amount of vaginal discharge than patch users (P<.001).
These findings are similar to those of previous studies that compared the patch with the pill, noting that breast discomfort, application-site reaction, and dysmenorrhea were more common in patch than pill users.3 Earlier studies also found the ring to be associated with complaints of vaginal discharge.4,5
Findings may not be generalizable
The most important finding from this direct comparison is the difference in patient satisfaction between groups. Visual analog scales showed that women using the ring were happier with the ring than with the pill, whereas women using the patch were happier with the pill than with the patch (P<.001). Questionnaires revealed that women were more satisfied with the ring than they were with the patch, and were more likely to recommend the ring than the patch to a friend (P<.001).
Based on continuation rates, patient satisfaction, and adverse-effect profiles, women in this study clearly preferred the ring to the pill, and the pill to the patch. When using this information to counsel patients, however, it is important to recall that this population was specific. The women had been using an OC, with which they were happy. This study cannot necessarily be generalized to women who are just initiating combination hormonal contraception, but it can be helpful in counseling a patient who may want to switch from an OC to a method that involves nondaily dosing.
Does the contraceptive patch raise the risk of thromboembolism?
Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2006;73:223–228.
Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2007;76:4–7.
Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive users. Obstet Gynecol. 2007;109(2 Pt 1):339–346.
Both the media and regulatory agencies have raised concerns about whether the contraceptive patch heightens the risk of thromboembolism and is less effective in women above a certain body weight.
The controversy surrounding thromboembolic disease stems from a pharmacokinetics study by van den Heuvel and colleagues that compared serum ethinyl estradiol levels in users of the patch, vaginal ring, and a combination OC containing 30 μg of ethinyl estradiol and 150 μg of levonorgestrel.6 Women randomized to the patch had serum ethinyl estradiol levels 1.6 times higher than women randomized to an OC, and 3.4 times higher than women randomized to the ring.
These findings led the FDA to update package labeling of the patch to warn health-care providers and patients that the patch exposes women to 60% more estrogen and may increase the risk of thromboembolic events. Oddly, the FDA did not require any labeling change to combination OCs to indicate that they contain up to twice as much estrogen as the contraceptive ring.
A set of studies finds no elevated risk
Although the study by van den Heuvel and associates raised the possibility of increased blood clots in patch users, no association between the two had been corroborated at the time it was published.6 Since then, three epidemiological studies have explored the potential link between thromboembolic events and use of the patch.
In the first of these studies, Jick and colleagues used the PharMetrics database to extract data on users of the patch and norg-estimate-containing OCs. This database contains drug prescription information, patient demographic data, and ICD-9 billing codes submitted by managed care health plans. A nested case-control study design was used to compare patch and pill users and control for confounding variables.
The base population was women 15 to 44 years old who were new users of the patch or a norgestimate-containing OC. A thromboembolic event was diagnosed if the patient’s record included a diagnosis code for pulmonary embolus, deep vein thrombosis, or an emergency room visit or diagnostic testing indicating venous thromboembolism (VTE). These diagnosis codes, combined with the prescription of long-term anticoagulation therapy, strengthened the identification of cases. As many as four controls were selected for each case.
The 215,769 women included in this study contributed 147,323 woman-years of exposure to the drugs. There were 31 and 37 cases of VTE identified in the patch and pill groups, respectively, with an incidence of 52.8 for every 100,000 woman-years in the patch group and 41.8 for every 100,000 woman-years in the pill group and an unadjusted, matched odds ratio of VTE in patch versus pill users of 0.9. When the data were adjusted for duration of drug exposure, the odds ratio did not change.
A follow-up study by Jick and associates, published in 2007, had the same study design and included 17 additional months of data. Another 56 cases of VTE were diagnosed. The odds ratio for patch users, compared with pill users, was 1.1. When data from the two studies were combined, 73 and 51 total cases of VTE had occurred in the pill and patch groups, respectively. The overall odds ratio was 1.0.
A third study finds significantly heightened risk
Cole and associates studied insurance claims data from UnitedHealthcare, a database containing medical and prescription claim information as well as patient demographics. Because researchers used pharmacy dispensing records, they were able to include women 15 to 44 years old who had received at least one prescription for the contraceptive patch or a norgestimate-containing OC with 35 μg of ethinyl estradiol.
Unlike the studies by Jick and colleagues, the study by Cole and associates considered all women eligible, even if they had used OCs in the past. Cases of VTE, stroke, and acute myocardial infarction (AMI) were abstracted from this group, identified from insurance claim information, and confirmed by chart review. Review of medical records is an important strength of this study; no such review was done in the studies by Jick and colleagues. Four controls were matched to each case, by age and duration of contraceptive use.
(This study was commissioned in conjunction with both the FDA and Johnson & Johnson, makers of the contraceptive patch, but researchers had full control over the data and results and were not required to consult with Johnson & Johnson when reporting findings.)
There were 49,048 woman-years of exposure to the patch and 202,344 woman-years of exposure to the pill, with an incidence of VTE of 40.8 and 18.3 for every 100,000 woman-years in patch and pill users, respectively. The incidence of AMI was 6.1 and 3.5 for every 100,000 woman-years in patch and pill users, respectively. No ischemic strokes were noted in patch users.
The adjusted incidence ratio for VTE in patch users compared with pill users was 2.2, and for AMI it was 1.8. Following publication of this study, the FDA issued a statement in January of this year that women using the patch face an increased risk of VTE, compared with women using the pill. Package labeling was changed to reflect this heightened risk.
Reasons for different findings
The studies by Jick and colleagues and Cole and associates present very different findings. The studies by Jick and colleagues give the impression that there is no increased risk of VTE in patch users compared with pill users, but the studies have significant flaws. First, Jick and colleagues do not confirm the diagnosis of VTE in the medical record. This is particularly problematic because the reported number of pulmonary emboli (PE) is very high, compared with the number of deep vein thromboses. The 2006 study found 42 cases of PE and only 26 cases of deep vein thrombosis. Because the latter is more common than PE, this could indicate that deep vein thrombosis was underdiagnosed.
Another shortcoming is that Jick and colleagues included only nonfatal thromboembolic events, which may mean that they missed many cases of fatal VTE because they were not looking for this information. The inclusion of new initiators only also may have skewed the data. This would mean that former users of an OC may have been included in the patch group but were ineligible for inclusion in the pill group. This may bias the data toward experienced hormonal contraceptive users in the patch group, thereby falsely lowering the VTE rate.
The study by Cole and associates also has limitations. It included long-term users of hormonal contraceptives in both the patch and the OC groups, which may bias the data toward lower rates of VTE, AMI, and stroke for the same reasons cited above. One would assume that this bias was corrected, because prior use was allowed in both groups, making the bias equally distributed, but there is no way to confirm this with any degree of certainty.
All three studies have some flaws in common
All three studies used prescription information to determine exposure, but there is no guarantee that the women who filled the prescriptions actually used the agents. Patients given drug samples by their clinicians were overlooked because these samples are not tracked through pharmacy data.
Because the data were collected from insurance claims information of privately insured patients, it is impossible to generalize these findings to the general population. We cannot use the findings to determine whether the same results would be seen in uninsured women or women insured through nonprivate programs such as Medicaid or the Veterans Administration.
So what’s the bottom line?
Health-care providers should be cautious about citing these studies as “evidence” when advising patients about the risk of VTE while using the patch. The twofold increased risk of VTE observed in patch users and the almost twofold increased risk of AMI observed by Cole and associates cannot be completely ignored, however, particularly because this study was better designed than those by Jick and colleagues.
It is more important to remember that the incidence of VTE in patch users is extremely low. If a patient has been using the patch, is happy with the method, and has had no adverse effects, there is no reason, based on these findings, to discontinue it. When counseling new initiators, the best that can be done is to explain the potential risks and side effects associated with the method and allow the patient to make an informed choice using the information that is available.
If the increased risk of VTE is accurate, it would still be equal to or lower than the risk during pregnancy. A recent review found the overall incidence of VTE in pregnancy or the postpartum period to be 200 for every 100,000 woman-years.7
In a pooled analysis of the two studies of the contraceptive patch by Jick and colleagues and the one study by Cole and associates, the overall and method failure rates through 13 cycles were 0.8% and 0.6%, respectively, representing 15 pregnancies.1
Subject weights were divided into deciles to determine the number of pregnancies per decile. Interestingly, that number does not appear to be evenly distributed. In deciles 1 through 9, which represent women who weigh up to 80 kg, the number of pregnancies was eight, whereas seven pregnancies occurred in the 10th decile, which represents women weighing more than 80 kg. Because the number of pregnancies in decile 10 is essentially equivalent to all of the other deciles combined, women who weigh more than 80 kg (176 lb) appear to be at increased risk of pregnancy. Five of the seven pregnancies in decile 10 occurred in women weighing more than 90 kg (198 lb).
No studies have directly explored the reasons for this relationship or looked at body mass index or body surface area in relation to efficacy of the patch. Further research is clearly needed.
How to counsel overweight women
It is imperative that patients who weigh more than 198 lb be informed that the pregnancy rate is higher than the rate quoted for the patch. It may even be reasonable to counsel women in that 10thdecile—who weigh more than 176 lb—about alternative forms of hormonal contraception that would be more effective for them than the patch.
Reference
1. Ziemen M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77(2 Suppl 2):S13-S18.
Why don’t American women choose long-acting reversible contraception?
Do American women not want to use long-acting reversible contraception (LARC), or are we, as providers, failing to properly educate them about its benefits?
The ParaGard copper IUD, the Mirena levonorgestrel intrauterine system (LNGIUS), and the Implanon etonorgestrel contraceptive implant are all highly effective, convenient, long-duration, and reversible (FIGURE). Despite substantial evidence indicating that these methods are well tolerated and highly effective, only about 2% of American women are choosing them to prevent pregnancy.1 This rate lags far behind other countries in IUD utilization. In contrast, more than 50% of contraceptive users in China and Egypt are using intrauterine contraception.8
FIGURE
Copper IUD is effective for 12 years or longer
The copper IUD is FDA-approved for 10 years of use, although studies continue to support its continued efficacy for 12 years or longer.9 The 1-year perfect-use failure rate is 0.6%, and the typical use failure rate is 0.5% to 0.8%.10 The total failure rate over 12 years is 2.2%.9
Benefits. The copper IUD does not increase the risk of intrauterine infection and is safe to place in nulliparous patients.11 It is an excellent choice for women who clearly prefer to have monthly menses and for women who have personal or medical contraindications to hormonal birth control. Women using this method of birth control can expect excellent efficacy, rapid reversibility, and minimal side effects.
Adverse effects. The most common adverse events in copper IUD users are heavier menses and dysmenorrhea. Approximately 4.5% of women discontinue the copper IUD in the first year of use because of these particular side effects.12
LNG-IUS: Highly effective, with important noncontraceptive benefits
This method of birth control is comparable to the copper IUD in terms of efficacy and tolerability. It is FDA-approved for 5 years of use, with a cumulative 5-year failure rate of 0.7 for every 100 women.13 One small study demonstrated that this method is potentially effective up to 7 years, with a 1.1% pregnancy rate.11 With perfect use, the first-year pregnancy rate is 0.1% to 0.2%.14
Benefits. The progestin component provides noncontraceptive benefits, including a reduction in menstrual bleeding and dysmenorrhea,15 treatment of endometrial hyperplasia16 and endometrial cancer,17 endometrial protection in women using tamoxifen,18 treatment of endometriosis,19 and protection from pelvic inflammatory disease.20
Adverse effects. The primary disadvantage of this device is a change in bleeding pattern in some women, who may experience irregular spotting, primarily in the first 3 to 6 months.21 About 20% of users will become amenorrheic by 12 months of use, a feature that is highly desirable for many, but troubling to some.
Implant is essentially 100% effective
The newest LARC device is the etonorgestrel implant, which was approved by the FDA in July 2006. The single-rod implant is typically placed in the subcuticular tissue of the non-dominant arm, although placement in the dominant arm is fine if the patient prefers.
Benefits. In a 3-year study involving 635 subjects, no pregnancies were reported.22 The reported Pearl index of 0.38 pregnancies for every 100,000 woman-years of use relates to pregnancies that occurred shortly after discontinuation rather than during actual use. These studies included only women below 130% of their ideal body weight who were not using liver enzyme-inducing medications. The pregnancy rate in women who use such medications, or weigh above 130% of their ideal body weight, is unknown. Postmarketing surveillance has reported some pregnancies, as would be expected. The device is easily inserted and easily removed as long as 3 years later.
Adverse effects. The primary adverse effect of this implant is bleeding disturbances; discontinuation was usually due to this side effect.22 The cumulative discontinuation rate was 10% at 6 months, 20% at 12 months, 31% at 2 years, and 32.2% at 3 years.22
Training required. FDA approval included a stipulation that practitioners complete company-sponsored training (www.implanonusa.com) to insert and remove the device.
Overall benefits include minimal side effects, low cost
All LARC methods provide excellent protection against pregnancy (equal to or better than sterilization), have minimal side effects, and are rapidly reversible. They are also appropriate for women in whom combination hormonal contraception is contraindicated, such as smokers older than 35 years and women who have had VTE.
A final and important advantage: These methods are more cost-effective than other contraceptive methods, including combination OCs. They may require a higher initial investment, but the LNG-IUS and copper IUD are the least costly methods of contraception over 5 years of use.23
As providers continue to educate themselves and help women gain a better understanding of which methods are truly highly effective, they will likely begin to recommend LARC more often. Use of these devices has the potential to significantly decrease the high rate of unintended pregnancy.
Authors’ note: The figure at right depicts how the efficacy and convenience of contraceptive options rise (and side effects fall) along a continuum. LARC methods are “high up the ladder”—an observation that serves as food for thought as we counsel patients about what methods of birth control are best for them.
The authors report no financial relationships relevant to this article.
We’ve heard that troubling statistic: Approximately 50% of pregnancies in the United States are unintended. But did you know that one half of those unintended pregnancies occur in women who were using some form of birth control at the time of conception?1 Such pregnancies are due to discontinuation of the method, incorrect use, or method failure.2 The focus of this article is contraceptive counseling, with special attention to:
- which methods of combination hormonal contraception women prefer
- the controversy surrounding the contraceptive patch in regard to thromboembolic disease
- long-acting reversible contraception (LARC), such as the intrauterine device (IUD) and the contraceptive implant, with an emphasis on how LARC is of benefit to both the patient and society.
The ultimate goal of good contraceptive counseling? To help women choose the easiest and most effective method with the fewest side effects.
In head-to-head comparison, women preferred the ring to the patch
Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch. Obstet Gynecol. 2008;111:267–277.
The ethinyl estradiol/etonogestril vaginal ring (NuvaRing) and the ethinyl estradiol/norelgestromin patch (OrthoEvra)—both approved by the Food and Drug Administration (FDA) in 2001—are the only nonoral forms of combined hormonal contraception on the market. These methods are said to increase patient compliance and, potentially, efficacy, because they are nondaily forms of contraception.
Until recently, these methods had been compared only with the combination oral contraceptive (OC), but a recent trial compared them directly to each other. At the conclusion of the study, 71% of ring users and 26.5% of patch users planned to continue using the assigned method (P<.001).
This information should aid clinicians in counseling women about which combination hormonal method to choose.
Participants started out using the OC
The multicenter, randomized, controlled clinical trial comparing the patch and ring included 479 women who were using, and happy with, the combination OC. After rating their satisfaction with the OC, women were randomized to the patch or ring and given 3 months’ worth of product. Follow-up involved only two telephone calls and one visit at the end of the third cycle, because this degree of monitoring was thought to mimic clinical practice.
The percentages of women who completed three cycles of their assigned product were 94.6% and 88.2% in the ring and patch groups, respectively (P=.03). The most common reasons for early discontinuation in the ring group were discomfort and adverse effects. In the patch group, the most common reasons were adverse effects, skin irritation, and adherence problems.
Even after adjusting for age, education, and whether an OC was actively being used at the time the study began, patch users were twice as likely to discontinue the patch at the end of three cycles and seven times more likely to state that they did not want to continue the patch.
Adverse effects were greater than with the pill
Women switching from pill to patch were significantly more likely to report breast pain, nausea, skin rash, longer menstrual bleeding, and menstrual pain than women who switched from the pill to the ring (P<.001).
Women who switched from the pill to the ring were more likely to experience vaginal discharge (P=.003) and a larger amount of vaginal discharge than patch users (P<.001).
These findings are similar to those of previous studies that compared the patch with the pill, noting that breast discomfort, application-site reaction, and dysmenorrhea were more common in patch than pill users.3 Earlier studies also found the ring to be associated with complaints of vaginal discharge.4,5
Findings may not be generalizable
The most important finding from this direct comparison is the difference in patient satisfaction between groups. Visual analog scales showed that women using the ring were happier with the ring than with the pill, whereas women using the patch were happier with the pill than with the patch (P<.001). Questionnaires revealed that women were more satisfied with the ring than they were with the patch, and were more likely to recommend the ring than the patch to a friend (P<.001).
Based on continuation rates, patient satisfaction, and adverse-effect profiles, women in this study clearly preferred the ring to the pill, and the pill to the patch. When using this information to counsel patients, however, it is important to recall that this population was specific. The women had been using an OC, with which they were happy. This study cannot necessarily be generalized to women who are just initiating combination hormonal contraception, but it can be helpful in counseling a patient who may want to switch from an OC to a method that involves nondaily dosing.
Does the contraceptive patch raise the risk of thromboembolism?
Jick SS, Kaye JA, Russman S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2006;73:223–228.
Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2007;76:4–7.
Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive users. Obstet Gynecol. 2007;109(2 Pt 1):339–346.
Both the media and regulatory agencies have raised concerns about whether the contraceptive patch heightens the risk of thromboembolism and is less effective in women above a certain body weight.
The controversy surrounding thromboembolic disease stems from a pharmacokinetics study by van den Heuvel and colleagues that compared serum ethinyl estradiol levels in users of the patch, vaginal ring, and a combination OC containing 30 μg of ethinyl estradiol and 150 μg of levonorgestrel.6 Women randomized to the patch had serum ethinyl estradiol levels 1.6 times higher than women randomized to an OC, and 3.4 times higher than women randomized to the ring.
These findings led the FDA to update package labeling of the patch to warn health-care providers and patients that the patch exposes women to 60% more estrogen and may increase the risk of thromboembolic events. Oddly, the FDA did not require any labeling change to combination OCs to indicate that they contain up to twice as much estrogen as the contraceptive ring.
A set of studies finds no elevated risk
Although the study by van den Heuvel and associates raised the possibility of increased blood clots in patch users, no association between the two had been corroborated at the time it was published.6 Since then, three epidemiological studies have explored the potential link between thromboembolic events and use of the patch.
In the first of these studies, Jick and colleagues used the PharMetrics database to extract data on users of the patch and norg-estimate-containing OCs. This database contains drug prescription information, patient demographic data, and ICD-9 billing codes submitted by managed care health plans. A nested case-control study design was used to compare patch and pill users and control for confounding variables.
The base population was women 15 to 44 years old who were new users of the patch or a norgestimate-containing OC. A thromboembolic event was diagnosed if the patient’s record included a diagnosis code for pulmonary embolus, deep vein thrombosis, or an emergency room visit or diagnostic testing indicating venous thromboembolism (VTE). These diagnosis codes, combined with the prescription of long-term anticoagulation therapy, strengthened the identification of cases. As many as four controls were selected for each case.
The 215,769 women included in this study contributed 147,323 woman-years of exposure to the drugs. There were 31 and 37 cases of VTE identified in the patch and pill groups, respectively, with an incidence of 52.8 for every 100,000 woman-years in the patch group and 41.8 for every 100,000 woman-years in the pill group and an unadjusted, matched odds ratio of VTE in patch versus pill users of 0.9. When the data were adjusted for duration of drug exposure, the odds ratio did not change.
A follow-up study by Jick and associates, published in 2007, had the same study design and included 17 additional months of data. Another 56 cases of VTE were diagnosed. The odds ratio for patch users, compared with pill users, was 1.1. When data from the two studies were combined, 73 and 51 total cases of VTE had occurred in the pill and patch groups, respectively. The overall odds ratio was 1.0.
A third study finds significantly heightened risk
Cole and associates studied insurance claims data from UnitedHealthcare, a database containing medical and prescription claim information as well as patient demographics. Because researchers used pharmacy dispensing records, they were able to include women 15 to 44 years old who had received at least one prescription for the contraceptive patch or a norgestimate-containing OC with 35 μg of ethinyl estradiol.
Unlike the studies by Jick and colleagues, the study by Cole and associates considered all women eligible, even if they had used OCs in the past. Cases of VTE, stroke, and acute myocardial infarction (AMI) were abstracted from this group, identified from insurance claim information, and confirmed by chart review. Review of medical records is an important strength of this study; no such review was done in the studies by Jick and colleagues. Four controls were matched to each case, by age and duration of contraceptive use.
(This study was commissioned in conjunction with both the FDA and Johnson & Johnson, makers of the contraceptive patch, but researchers had full control over the data and results and were not required to consult with Johnson & Johnson when reporting findings.)
There were 49,048 woman-years of exposure to the patch and 202,344 woman-years of exposure to the pill, with an incidence of VTE of 40.8 and 18.3 for every 100,000 woman-years in patch and pill users, respectively. The incidence of AMI was 6.1 and 3.5 for every 100,000 woman-years in patch and pill users, respectively. No ischemic strokes were noted in patch users.
The adjusted incidence ratio for VTE in patch users compared with pill users was 2.2, and for AMI it was 1.8. Following publication of this study, the FDA issued a statement in January of this year that women using the patch face an increased risk of VTE, compared with women using the pill. Package labeling was changed to reflect this heightened risk.
Reasons for different findings
The studies by Jick and colleagues and Cole and associates present very different findings. The studies by Jick and colleagues give the impression that there is no increased risk of VTE in patch users compared with pill users, but the studies have significant flaws. First, Jick and colleagues do not confirm the diagnosis of VTE in the medical record. This is particularly problematic because the reported number of pulmonary emboli (PE) is very high, compared with the number of deep vein thromboses. The 2006 study found 42 cases of PE and only 26 cases of deep vein thrombosis. Because the latter is more common than PE, this could indicate that deep vein thrombosis was underdiagnosed.
Another shortcoming is that Jick and colleagues included only nonfatal thromboembolic events, which may mean that they missed many cases of fatal VTE because they were not looking for this information. The inclusion of new initiators only also may have skewed the data. This would mean that former users of an OC may have been included in the patch group but were ineligible for inclusion in the pill group. This may bias the data toward experienced hormonal contraceptive users in the patch group, thereby falsely lowering the VTE rate.
The study by Cole and associates also has limitations. It included long-term users of hormonal contraceptives in both the patch and the OC groups, which may bias the data toward lower rates of VTE, AMI, and stroke for the same reasons cited above. One would assume that this bias was corrected, because prior use was allowed in both groups, making the bias equally distributed, but there is no way to confirm this with any degree of certainty.
All three studies have some flaws in common
All three studies used prescription information to determine exposure, but there is no guarantee that the women who filled the prescriptions actually used the agents. Patients given drug samples by their clinicians were overlooked because these samples are not tracked through pharmacy data.
Because the data were collected from insurance claims information of privately insured patients, it is impossible to generalize these findings to the general population. We cannot use the findings to determine whether the same results would be seen in uninsured women or women insured through nonprivate programs such as Medicaid or the Veterans Administration.
So what’s the bottom line?
Health-care providers should be cautious about citing these studies as “evidence” when advising patients about the risk of VTE while using the patch. The twofold increased risk of VTE observed in patch users and the almost twofold increased risk of AMI observed by Cole and associates cannot be completely ignored, however, particularly because this study was better designed than those by Jick and colleagues.
It is more important to remember that the incidence of VTE in patch users is extremely low. If a patient has been using the patch, is happy with the method, and has had no adverse effects, there is no reason, based on these findings, to discontinue it. When counseling new initiators, the best that can be done is to explain the potential risks and side effects associated with the method and allow the patient to make an informed choice using the information that is available.
If the increased risk of VTE is accurate, it would still be equal to or lower than the risk during pregnancy. A recent review found the overall incidence of VTE in pregnancy or the postpartum period to be 200 for every 100,000 woman-years.7
In a pooled analysis of the two studies of the contraceptive patch by Jick and colleagues and the one study by Cole and associates, the overall and method failure rates through 13 cycles were 0.8% and 0.6%, respectively, representing 15 pregnancies.1
Subject weights were divided into deciles to determine the number of pregnancies per decile. Interestingly, that number does not appear to be evenly distributed. In deciles 1 through 9, which represent women who weigh up to 80 kg, the number of pregnancies was eight, whereas seven pregnancies occurred in the 10th decile, which represents women weighing more than 80 kg. Because the number of pregnancies in decile 10 is essentially equivalent to all of the other deciles combined, women who weigh more than 80 kg (176 lb) appear to be at increased risk of pregnancy. Five of the seven pregnancies in decile 10 occurred in women weighing more than 90 kg (198 lb).
No studies have directly explored the reasons for this relationship or looked at body mass index or body surface area in relation to efficacy of the patch. Further research is clearly needed.
How to counsel overweight women
It is imperative that patients who weigh more than 198 lb be informed that the pregnancy rate is higher than the rate quoted for the patch. It may even be reasonable to counsel women in that 10thdecile—who weigh more than 176 lb—about alternative forms of hormonal contraception that would be more effective for them than the patch.
Reference
1. Ziemen M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77(2 Suppl 2):S13-S18.
Why don’t American women choose long-acting reversible contraception?
Do American women not want to use long-acting reversible contraception (LARC), or are we, as providers, failing to properly educate them about its benefits?
The ParaGard copper IUD, the Mirena levonorgestrel intrauterine system (LNGIUS), and the Implanon etonorgestrel contraceptive implant are all highly effective, convenient, long-duration, and reversible (FIGURE). Despite substantial evidence indicating that these methods are well tolerated and highly effective, only about 2% of American women are choosing them to prevent pregnancy.1 This rate lags far behind other countries in IUD utilization. In contrast, more than 50% of contraceptive users in China and Egypt are using intrauterine contraception.8
FIGURE
Copper IUD is effective for 12 years or longer
The copper IUD is FDA-approved for 10 years of use, although studies continue to support its continued efficacy for 12 years or longer.9 The 1-year perfect-use failure rate is 0.6%, and the typical use failure rate is 0.5% to 0.8%.10 The total failure rate over 12 years is 2.2%.9
Benefits. The copper IUD does not increase the risk of intrauterine infection and is safe to place in nulliparous patients.11 It is an excellent choice for women who clearly prefer to have monthly menses and for women who have personal or medical contraindications to hormonal birth control. Women using this method of birth control can expect excellent efficacy, rapid reversibility, and minimal side effects.
Adverse effects. The most common adverse events in copper IUD users are heavier menses and dysmenorrhea. Approximately 4.5% of women discontinue the copper IUD in the first year of use because of these particular side effects.12
LNG-IUS: Highly effective, with important noncontraceptive benefits
This method of birth control is comparable to the copper IUD in terms of efficacy and tolerability. It is FDA-approved for 5 years of use, with a cumulative 5-year failure rate of 0.7 for every 100 women.13 One small study demonstrated that this method is potentially effective up to 7 years, with a 1.1% pregnancy rate.11 With perfect use, the first-year pregnancy rate is 0.1% to 0.2%.14
Benefits. The progestin component provides noncontraceptive benefits, including a reduction in menstrual bleeding and dysmenorrhea,15 treatment of endometrial hyperplasia16 and endometrial cancer,17 endometrial protection in women using tamoxifen,18 treatment of endometriosis,19 and protection from pelvic inflammatory disease.20
Adverse effects. The primary disadvantage of this device is a change in bleeding pattern in some women, who may experience irregular spotting, primarily in the first 3 to 6 months.21 About 20% of users will become amenorrheic by 12 months of use, a feature that is highly desirable for many, but troubling to some.
Implant is essentially 100% effective
The newest LARC device is the etonorgestrel implant, which was approved by the FDA in July 2006. The single-rod implant is typically placed in the subcuticular tissue of the non-dominant arm, although placement in the dominant arm is fine if the patient prefers.
Benefits. In a 3-year study involving 635 subjects, no pregnancies were reported.22 The reported Pearl index of 0.38 pregnancies for every 100,000 woman-years of use relates to pregnancies that occurred shortly after discontinuation rather than during actual use. These studies included only women below 130% of their ideal body weight who were not using liver enzyme-inducing medications. The pregnancy rate in women who use such medications, or weigh above 130% of their ideal body weight, is unknown. Postmarketing surveillance has reported some pregnancies, as would be expected. The device is easily inserted and easily removed as long as 3 years later.
Adverse effects. The primary adverse effect of this implant is bleeding disturbances; discontinuation was usually due to this side effect.22 The cumulative discontinuation rate was 10% at 6 months, 20% at 12 months, 31% at 2 years, and 32.2% at 3 years.22
Training required. FDA approval included a stipulation that practitioners complete company-sponsored training (www.implanonusa.com) to insert and remove the device.
Overall benefits include minimal side effects, low cost
All LARC methods provide excellent protection against pregnancy (equal to or better than sterilization), have minimal side effects, and are rapidly reversible. They are also appropriate for women in whom combination hormonal contraception is contraindicated, such as smokers older than 35 years and women who have had VTE.
A final and important advantage: These methods are more cost-effective than other contraceptive methods, including combination OCs. They may require a higher initial investment, but the LNG-IUS and copper IUD are the least costly methods of contraception over 5 years of use.23
As providers continue to educate themselves and help women gain a better understanding of which methods are truly highly effective, they will likely begin to recommend LARC more often. Use of these devices has the potential to significantly decrease the high rate of unintended pregnancy.
Authors’ note: The figure at right depicts how the efficacy and convenience of contraceptive options rise (and side effects fall) along a continuum. LARC methods are “high up the ladder”—an observation that serves as food for thought as we counsel patients about what methods of birth control are best for them.
1. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24-29, 46.
2. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
3. Sibai BM, Odlind V, Meador ML, Shangold GA, Fisher AC, Creasy GW. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra/Evra). Fertil Steril. 2002;77(2 Suppl 2):S19-S26.
4. Arhendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 microg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451-457.
5. Oddson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive:a 1-year randomized trial. Contraception. 2005;71:176-182.
6. van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: The vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
7. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ, 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum:a 30-year population-based study. Ann Intern Med. 2005;143:697-706.
8. d’Arcangues C. Worldwide use of intrauterine devices for contraception. Contraception. 2007;75(6 Suppl):S2-S7.
9. Long-term reversible contraception. Twelve years of experience with TCu380A and TCu220C. Contraception. 1997;56:341-352.
10. Sivin I, Schmidt F. Effectiveness of IUDs: a review. Contraception. 1987;36:55-84.
11. Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
12. Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra Infante F, Guzmán-Rodríguez R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345:561-567.
13. Grimes DA. Intrauterine devices (IUDs). In:Hatcher RA, ed. Contraceptive Technology. 18th ed. New York: Ardent Media, Inc.;2004:495-530.
14. Sivin I, Stern J. Healthduring prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR). Fertil Steril. 1994;61:70-77.
15. Kadir RA, Chi C. Levonorgestrel intrauterine system: bleeding disorders and anticoagulant therapy. Contraception. 2007;75(6 Suppl):S123-S129.
16. Wildemeersch D, Dhont M. Treatment of non-atypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system. Am J Obstet Gynecol. 2003;188:1297-1298.
17. Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. 2005;97:924-927.
18. Gardner FJ, Konje JC, Abrams KR, et al. Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system:a randomised controlled trial. Lancet. 2000;356:1711-1717.
19. Lockhat FB, Emembolu JO, Konje JC. The efficacy, side-effects and continuation rates of women with symptomatic endometriosis undergoing treatment with an intra-uterine administered progestogen (levonorgestrel):a 3 year follow-up. Hum Reprod. 2005;20:789-793.
20. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’comparative experience of levonorgestrel-and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
21. Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system:a nationwide study of 17,360 users. BJOG. 2000;107:335-339.
22. Croxatto HB. Clinical profile of Implanon:a single-rod etonorgestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5(Suppl 2):21-28.
23. Chiou CF, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception. 2003;68:3-10.
1. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30:24-29, 46.
2. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. J Reprod Med. 1995;40:355-360.
3. Sibai BM, Odlind V, Meador ML, Shangold GA, Fisher AC, Creasy GW. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra/Evra). Fertil Steril. 2002;77(2 Suppl 2):S19-S26.
4. Arhendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 microg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451-457.
5. Oddson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive:a 1-year randomized trial. Contraception. 2005;71:176-182.
6. van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: The vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
7. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ, 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum:a 30-year population-based study. Ann Intern Med. 2005;143:697-706.
8. d’Arcangues C. Worldwide use of intrauterine devices for contraception. Contraception. 2007;75(6 Suppl):S2-S7.
9. Long-term reversible contraception. Twelve years of experience with TCu380A and TCu220C. Contraception. 1997;56:341-352.
10. Sivin I, Schmidt F. Effectiveness of IUDs: a review. Contraception. 1987;36:55-84.
11. Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
12. Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra Infante F, Guzmán-Rodríguez R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345:561-567.
13. Grimes DA. Intrauterine devices (IUDs). In:Hatcher RA, ed. Contraceptive Technology. 18th ed. New York: Ardent Media, Inc.;2004:495-530.
14. Sivin I, Stern J. Healthduring prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR). Fertil Steril. 1994;61:70-77.
15. Kadir RA, Chi C. Levonorgestrel intrauterine system: bleeding disorders and anticoagulant therapy. Contraception. 2007;75(6 Suppl):S123-S129.
16. Wildemeersch D, Dhont M. Treatment of non-atypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system. Am J Obstet Gynecol. 2003;188:1297-1298.
17. Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. 2005;97:924-927.
18. Gardner FJ, Konje JC, Abrams KR, et al. Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system:a randomised controlled trial. Lancet. 2000;356:1711-1717.
19. Lockhat FB, Emembolu JO, Konje JC. The efficacy, side-effects and continuation rates of women with symptomatic endometriosis undergoing treatment with an intra-uterine administered progestogen (levonorgestrel):a 3 year follow-up. Hum Reprod. 2005;20:789-793.
20. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’comparative experience of levonorgestrel-and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
21. Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system:a nationwide study of 17,360 users. BJOG. 2000;107:335-339.
22. Croxatto HB. Clinical profile of Implanon:a single-rod etonorgestrel contraceptive implant. Eur J Contracept Reprod Health Care. 2000;5(Suppl 2):21-28.
23. Chiou CF, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception. 2003;68:3-10.