User login
Dual antiplatelet therapy for acute coronary syndromes: How long to continue?
Percutaneous coronary intervention for acute coronary syndromes has evolved, and so, hand in hand, has antiplatelet therapy. With the advent of clopidogrel and newer agents, several studies demonstrated the benefits of dual antiplatelet therapy in preventing major vascular ischemic complications. The findings culminated in a guideline recommendation for at least 12 months of dual antiplatelet therapy after placement of a drug-eluting stent, when feasible—a class I recommendation (treatment should be given), level of evidence B (limited populations evaluated).1,2 But extending dual antiplatelet therapy beyond 12 months had no strong favorable evidence until the recent Dual Antiplatelet Therapy (DAPT) study3 shed light on this topic.
Here, we review the evidence thus far on the optimal duration of dual antiplatelet therapy in the secondary prevention of coronary artery disease.
PLATELETS IN ACUTE CORONARY SYNDROMES AND STENT THROMBOSIS
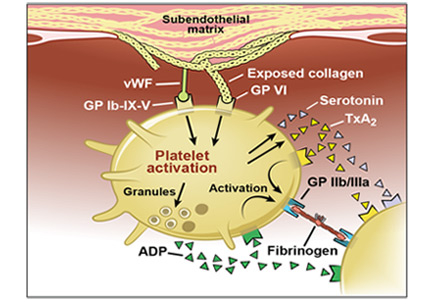
Acute coronary syndromes begin with fissuring or ulceration of a vulnerable atherosclerotic plaque, followed by thrombosis and occlusion, mediated by platelet adhesion, activation, and aggregation (Figure 1). Transient occlusion results in unstable angina or non-ST-elevation myocardial infarction, while total occlusion usually results in ST-elevation myocardial infarction.
Platelet aggregation is prominent among the mechanisms leading to stent thrombosis and vaso-occlusive ischemic complications after percutaneous coronary intervention. Thus, antiplatelet agents play a vital role in both primary and secondary prevention of cardiovascular events.4–6
Adhesion, activation, and aggregation
Adhesion. Disruption of the vascular endothelium as a result of vulnerable plaque fissuring or ulceration exposes subendothelial thrombogenic collagen and von Willebrand factor to blood. Collagen engages platelets through their glycoprotein (GP) Ia, IIa, and VI receptors, and von Willebrand factor binds platelets through the GP Ib-IX-V receptor.
Activation. Once platelets adhere to the subendothelium, they undergo a conformational change and become activated. Simultaneous release of various autocrine and paracrine mediators including adenosine diphosphate, serotonin, epinephrine, thromboxane, and various ligand-receptor interactions all contribute to the activation cascade. Adenosine diphosphate binds to the platelet receptor P2Y1, leading to an increase in intracellular calcium, and it binds to P2Y12, leading to a decrease in cyclic adenosine monophosphate, both of which cause GP IIb/IIIa receptor activation. Thromboxane A2 released by platelets by cyclo-oxygenase 1 binds to alpha or beta variant receptors and contributes to GP IIb/IIIa activation through elevation of intracellular calcium levels.
Aggregation and thrombosis. Exposure of tissue factor to plasma following plaque rupture activates the coagulation cascade via the extrinsic pathway, which generates thrombin, a powerful platelet activator that causes thrombus formation via fibrin. Thrombin binds to protease-activated receptors PAR-1 and PAR-4 on platelets, causing an increase in intracellular calcium and a decrease in cyclic adenosine monophosphate with subsequent GP IIb/IIIa activation. GP IIb/IIIa facilitates platelet aggregation by binding to fibrinogen and forming a stable platelet thrombus.
In the early stages of thrombus formation, platelets predominate (“white” thrombi); further organization with fibrin results in older “red” thrombi. The stages of thrombi vary in non-ST-elevation and ST-elevation myocardial infarction and are prognostic markers of death.4–8
PERCUTANEOUS INTERVENTION, RESTENOSIS, AND STENT THROMBOSIS
Percutaneous coronary intervention, the preferred means of revascularization for many patients, is performed emergently in patients with ST-elevation myocardial infarction, urgently in those with acute coronary syndromes without ST elevation, and electively in those with stable ischemic symptoms.
Percutaneous revascularization techniques have evolved from balloon angioplasty to bare-metal stents to drug-eluting stents, but each of these procedures has been associated with a periprocedural and postprocedural risk of thrombosis.
Balloon angioplasty was associated with vascular intimal injury, inciting elastic vascular recoil and smooth muscle cell proliferation leading to restenosis.
Bare-metal stents reduced the restenosis rate by eliminating vascular recoil, although restenosis still occurred within the stent because of neointimal proliferation of vascular smooth muscle cells. This was an important limitation, as both acute and subacute stent thrombosis were refractory to aggressive anticoagulation regimens that were associated with major bleeding complications and longer hospital length of stay. Stenting became mainstream practice only after the ISAR9 and STARS10 trials showed that dual antiplatelet therapy controlled stent thrombosis.
Drug-eluting stents coated with anti-proliferative and anti-inflammatory polymers markedly reduced in-stent restenosis rates by suppressing the initial vascular smooth-muscle proliferative response. However, they were still associated with late and very late stent thrombosis with incomplete endothelialization, even up to 40 months after implantation. Proposed mechanisms include incomplete stent apposition and inflammatory hypersensitivity reactions to the polymer coating. Incomplete stent apposition associated with low-velocity blood flow at the junction of the stent strut and vessel wall, together with delayed endothelialization, promotes platelet adhesion and aggregation, followed by thrombus formation.11
Second-generation drug-eluting stents have thinner struts and more biocompatible polymers and are thought to favor more complete re-endothelialization, reducing the rates of stent thrombosis.8,12,13
Predictors of early stent thrombosis
The Dutch Stent Thrombosis Registry and other studies looked at risk factors for stent thrombosis.14,15
Procedure-related factors included:
- Stent undersizing
- Residual uncovered dissections after angioplasty
- Longer stents
- Low flow after angioplasty (< 3 on the 0–3 Thrombolysis in Myocardial Infarction [TIMI] scale).
Lesion-related factors included:
- Intermediate coronary artery disease both proximal and distal to the culprit lesions
- Bifurcation lesions.
Patient-related factors included:
- Low left ventricular ejection fraction
- Diabetes mellitus
- Peripheral arterial disease Premature discontinuation of clopidogrel.
ANTIPLATELET AGENTS: MECHANISM OF ACTION
Various pathways play synergistic roles in platelet activation and aggregation and thrombus formation, and different antiplatelet agents inhibit these specific pathways, thus complementing each other and having additive effects (Figure 2, Table 1).5,16–21
Aspirin inhibits cyclo-oxygenase 1
Cyclo-oxygenase 1, found in platelets, endothelial cells, and other cells, catalyzes the conversion of arachidonic acid to thromboxane A2. Aspirin irreversibly inhibits cyclo-oxygenase 1 by acetylating its serine residue, preventing formation of thromboxane A2 and preventing platelet activation and aggregation.
P2Y12 ADP receptor antagonists
Clopidogrel and prasugrel are thienopyridine agents that irreversibly inhibit the P2Y12 receptor, thereby preventing binding of adenosine diphosphate and the subsequent platelet activation-aggregation cascade. They are both prodrugs and require conversion by cytochrome P450 enzymes to active metabolites. Prasugrel is 10 times more potent than clopidogrel due to more efficient formation of its active metabolite, and it achieves a comparable effect on platelet inhibition 30 minutes faster than the peak effect of clopidogrel at 6 hours. The overall peak inhibitory effect of prasugrel is twice that of clopidogrel.22
Ticagrelor, a cyclopentyl-triazolo-pyrimidine, directly and reversibly inhibits the P2Y12 ADP receptor. Unlike clopidogrel and prasugrel, it does not need to be converted to an active metabolite, and it noncompetitively inhibits P2Y12 at a site different from the adenosine diphosphate binding site.23 Like prasugrel, ticagrelor inhibits platelet function more rapidly and more completely than clopidogrel.
Cangrelor, an intravenously administered analogue of adenosine triphosphate, reversibly inhibits the P2Y12 receptor. It has undergone phase 3 trials but is not yet approved for clinical use.24
WHY DUAL ANTIPLATELET THERAPY?
Aspirin is good, clopidogrel is better
Aspirin has a well-validated role in both primary and secondary prevention of coronary and noncoronary atherosclerotic vascular disease.
The CAPRIE trial found clopidogrel monotherapy to be superior to aspirin monotherapy in patients with established atherosclerotic vascular disease.25
After stenting, short-term dual therapy is better than short-term warfarin
Thrombotic complications in the early postprocedural period were a major limitation of stenting, and existing anticoagulation regimens were ineffective in preventing them.26,27
The ISAR trial studied the benefit of combined antiplatelet vs anticoagulant therapy after stent placement. Patients randomized to receive combined aspirin plus ticlopidine (an early P2Y12 inhibitor) had significantly lower rates of primary cardiac, hemorrhagic, and vascular events at 30 days.9 Two other trials confirmed this finding.28,29
STARS10 also confirmed the benefit of aspirin and ticlopidine after stenting. Patients were randomly assigned to aspirin alone, aspirin plus warfarin, or aspirin plus ticlopidine after stent placement. The rate of stent thrombosis at 30 days was significantly lower in the dual antiplatelet group than in the other two groups. The dual antiplatelet group had a higher rate of bleeding than the aspirin-alone group, but the rate was similar to that of the aspirin-plus-warfarin group.
Long-term dual antiplatelet therapy is beneficial in several situations
ISAR and STARS were landmark trials that showed stent thrombosis could be reduced by dual antiplatelet therapy for a 30-day period. However, the long-term role of dual antiplatelet therapy was still unknown.
The CURE trial30–32 randomized patients presenting with acute coronary syndromes without ST elevation to receive clopidogrel plus aspirin or placebo plus aspirin for 3 to 12 months. The rate of the primary end point (cardiac death, nonfatal myocardial infarction, or stroke) was significantly lower in the clopidogrel-plus-aspirin group. A similar benefit of dual antiplatelet therapy was seen in the subgroup of patients who underwent percutaneous coronary intervention. Both pretreatment with clopidogrel plus aspirin for a median of 10 days prior to percutaneous intervention and continuing it for a mean of 9 months reduced major adverse cardiovascular events.
The CREDO trial20 found that the combination of clopidogrel and aspirin significantly reduced the incidence of death, myocardial infarction, or stroke at 1 year after percutaneous coronary intervention. A subgroup of patients in this trial who had a longer pretreatment interval with a loading clopidogrel dose showed a benefit at 28 days, which was not as evident with a shorter loading dose interval.
The CLARITY-TIMI 28 trial33,34 showed the advantage of adding clopidogrel to aspirin in patients receiving fibrinolytic therapy for ST-elevation myocardial infarction. Adding clopidogrel both improved the patency of the infarct-related artery and reduced ischemic complications. In patients who subsequently underwent percutaneous coronary intervention and stenting, clopidogrel pretreatment was associated with a significant decrease in ischemic complications before and after the procedure. There was no significant increase in bleeding complications in either group.
COMMIT/CCS 235 also showed the benefit of dual antiplatelet therapy in patients with ST-elevation myocardial infarction. Clopidogrel added to aspirin during the short-term in-hospital or postdischarge treatment period significantly reduced a composite end point of reinfarction, death, or stroke as well as death from any cause.
The CHARISMA trial36–38 aimed to determine if patients who were more stable (ie, no recent acute coronary syndrome event or percutaneous coronary intervention) would benefit. Overall, CHARISMA showed no benefit of adding clopidogrel to aspirin compared with aspirin alone in a broad population of patients with established vascular disease (secondary prevention) or risk factors for vascular disease (primary prevention).
But importantly, though no benefit was seen in the primary prevention group, the large subgroup of patients with established atherosclerotic vascular disease (12,153 of the 15,603 patients in the trial) did benefit from dual antiplatelet therapy.36,37 This subgroup showed an overall reduction in absolute risk of 1.5% (relative risk 0.88, P = .046) over a median follow-up of 27.6 months. This benefit was even more apparent in the 9,478 patients with prior myocardial infarction, stroke, or peripheral artery disease, for whom the relative risk reduction was 17.1% (P = .01) and the reduction in absolute risk 1.5%.38
These results are comparable to the 2% absolute risk reduction in the CURE trial for similar end points over 9 months. In both studies, there was no significant increase in the risk of major bleeding or intracranial bleeding in the clopidogrel-plus-aspirin groups, although minor bleeding was increased by dual antiplatelet therapy.
The rate of severe bleeding, which was the primary safety end point in CHARISMA, was not significantly different in the clopidogrel-plus-aspirin group compared with the placebo-plus-aspirin group (relative risk 1.25, 95% CI 0.97–1.61, P = .09).
Thus, although the CHARISMA findings were negative overall, the positive finding observed in the predominant subgroup of patients with established vascular disease can therefore be considered supportive of the results of the subsequent trials discussed below.
The PEGASUS-TIMI 54 trial39 studied the benefit of adding ticagrelor (60 or 90 mg) to low-dose aspirin in patients with stable coronary artery disease who had had a myocardial infarction 1 to 3 years earlier.
Confirming the results of the CHARISMA subgroup analysis, the incidence of the ischemic primary efficacy end point (a composite of cardiovascular death, myocardial infarction, and stroke) was significantly lower in both groups receiving ticagrelor plus aspirin compared with those receiving placebo plus aspirin. The Kaplan-Meier rate at 3 years for the ticagrelor 90 mg-plus-aspirin group was 7.85% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.85, 95% confidence interval [CI] 0.75–0.96, P = .008). The rate for the ticagrelor 60 mg-plus-aspirin group was 7.77% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.84, 95% CI 0.74–0.95, P = .004).
The rates of all TIMI major and minor bleeding, as well as bleeding requiring transfusion or discontinuation of the study drug, were significantly higher in both ticagrelor dosing groups than in the placebo group (P < .01 for both groups vs placebo). The rates of fatal bleeding and nonfatal intracranial hemorrhage were not significantly higher. Although there was an overall reduction in ischemic end points with the addition of ticagrelor, there was also a significantly higher incidence of bleeding in this group.
Comment. Thus, with or without percutaneous coronary intervention in acute coronary syndrome as well as in stable coronary artery disease, dual antiplatelet therapy was shown to improve outcomes and decrease ischemic complications compared with aspirin alone. It provided benefit in the setting of acute coronary syndrome (in the CURE trial) and percutaneous coronary intervention (in the CREDO trial) for up to 1 year.
Major questions remained to be addressed:
- Do the results of CREDO, which was performed before the current interventional era and the use of drug-eluting stents, reflect outcomes after current interventional practice?
- Could shorter periods of dual antiplatelet therapy be sufficient, especially with newer stents with less risk of late thrombosis?
- Does the benefit of dual antiplatelet therapy extend beyond the 1-year time period tested in those trials to date?
RECOMMENDATIONS FOR DOSING
The American College of Cardiology Foundation/American Heart Association guidelines for dosing of antiplatelet agents for non-ST-elevation myocardial infarction are summarized in Table 2, and those for ST-elevation myocardial infarction are summarized in Table 3.1,2
WOULD SHORTER THERAPY AFTER STENTING WORK AS WELL?
The American College of Cardiology Foundation/American Heart Association currently recommend dual antiplatelet therapy for at least 12 months after drug-eluting stent placement, with shorter courses appropriate for patients who develop excessive bleeding complications or who are at high risk of bleeding.
Four trials (Table 4) evaluated whether shorter durations of dual antiplatelet therapy would suffice: SECURITY,40 EXCELLENT,41 OPTIMIZE,42 and RESET.43 All of them showed that short-duration therapy was not inferior to standard-duration therapy.44 These studies were comparable in that:
- Patients were randomized at the time of percutaneous coronary intervention or within 24 hours of it.
- Most patients received a second-generation drug-eluting stent, with the following exceptions: in EXCELLENT,41 one-fourth of patients received a Cypher first-generation drug-eluting stent, and in RESET,43 approximately one-fourth of the patients received a sirolimus-eluting stent in the standard-duration group for short lesions. Those patients with longer lesions in the RESET standard-duration group received an everolimus drug-eluting stent.
- The second antiplatelet added to aspirin in all studies was clopidogrel, with the exception of the SECURITY trial, in which fewer than 2% of patients received ticagrelor or prasugrel.40
- All the trials except RESET excluded patients who had had a myocardial infarction within 72 hours, and thus most patients studied had a lower risk profile.
- All of the trials sought to study noninferiority of short- vs standard-duration dual antiplatelet therapy, defined as the occurrence of a primary end point at 1 year (a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, target vessel failure or revascularization, or bleeding).
Their low-risk patient populations and infrequent end points rendered these studies underpowered to make definitive conclusions about the relative efficacy of 6-months vs 12-months of dual antiplatelet therapy.
WOULD LONGER THERAPY BE BETTER?
The PRODIGY trial45 assessed durations of dual antiplatelet therapy both shorter and longer than the conventional 1 year, randomizing patients undergoing placement of a bare-metal stent, first-generation drug-eluting stent, or second-generation drug-eluting stent to receive aspirin and clopidogrel for either 6 months or 24 months. The study showed no significant difference in primary outcomes in the short- or long-duration groups.
Other trials that compared the standard 12 months of dual antiplatelet therapy with extended duration beyond 12 months were DAPT,3 ARCTIC-Interruption,46 and DES-LATE.47 The trials were comparable in that:
- All patients were randomized after completing 12 months of dual antiplatelet therapy following drug-eluting stent placement.
- All patients who were included had been free of major cardiac ischemic events or bleeding during the 12 months following stent placement.
- The primary aim of all three studies was to compare primary end points in groups receiving aspirin alone vs extended dual antiplatelet therapy. The primary end point was a composite of death due to a cardiovascular cause, nonfatal myocardial infarction, stroke, or stent thrombosis.
- The principal safety end point was bleeding.
Although the two earlier studies (ARCTIC-Interruption and DES-LATE) did not show any benefit of extended dual antiplatelet therapy compared with the standard 12-month duration, the recent DAPT study did.
The DAPT study
The DAPT study3 was an international, multicenter, placebo-controlled, double-blind randomized trial designed to examine the benefit of dual antiplatelet therapy beyond 1 year in a patient population large enough to provide definitive assessment of benefit and risk.
A total of 9,961 patients who received drug-eluting stents were randomized after 12 months of dual antiplatelet therapy to receive either a thienopyridine (clopidogrel or prasugrel) plus aspirin or placebo plus aspirin. They were followed for an additional 18 months. The coprimary efficacy end points were stent thrombosis and a composite of death, myocardial infarction, or stroke, while the primary safety end point was moderate or severe bleeding. The patients were also observed from months 30 to 33 on aspirin alone after stopping the thienopyridine.
Results. Longer therapy substantially reduced the risks of stent thrombosis (hazard ratio [HR] 0.29, 95% confidence interval [CI] 0.17–0.48) and the composite ischemic end point (HR 0.71, 95% CI 0.59–0.85). Follow-up during the 3-month thienopyridine discontinuation phase starting at 30 months revealed convergence of the ischemic event-rate curves in the two groups, which suggested that continuing dual antiplatelet therapy beyond 30 months might have been beneficial. Myocardial infarction unrelated to stent thrombosis accounted for 55% of the treatment benefit of dual antiplatelet therapy.
The risk of bleeding was higher in the thienopyridine group during the treatment period (2.5% vs 1.6%, P = .001). There was also a higher rate of noncardiovascular mortality in the thienopyridine group, although this difference may have been due to chance.3,48
Why were the results different?
All three trials included first- and second-generation drug-eluting stents, with different proportions in different trials. In ARCTIC-Interruption,46 43% of the patients in the continuation group had a first-generation stent, as did 64% of the patients in the dual antiplatelet group of DES-LATE.47 In the DAPT trial,3 38% of the patients in the longer-duration arm had a first-generation stent, and in 26% of cases it was a paclitaxel-eluting stent.
Only clopidogrel was used as the second antiplatelet agent in DES-LATE, whereas prasugrel was used in 10% of patients in ARCTIC-Interruption and 35% in DAPT.
Yet none of these differences seem to explain the differences in outcome among the studies. ARCTIC-Interruption and DES-LATE did not show any benefit of continued dual antiplatelet therapy beyond 12 months. DAPT showed benefit of extended therapy with prasugrel or with clopidogrel, and with first-generation or second-generation drug-eluting stents. The most likely explanation for the different results was that DAPT was the only trial sufficiently powered to definitively assess the end points, including stent thrombosis.
A balance between ischemic efficacy and bleeding risk is the major consideration with any antithrombotic and antiplatelet therapy. In the three largest trials we discussed (the vascular disease subgroups of CHARISMA,38 PEGASUS,39 and DAPT3), comparison of the prespecified efficacy and safety end points of each trial suggests that dual antiplatelet therapy has a net benefit, particularly given the irreversible nature of ischemic end points.
In CHARISMA,38 60 cardiovascular deaths, myocardial infarctions, or strokes were prevented per year per 10,000 patients treated, at the cost of 28 excess moderate bleeding events.
In PEGASUS,39 42 cardiovascular deaths, myocardial infarctions, or strokes were prevented, at the cost of 79 excess bleeding events requiring transfusion.
In DAPT (a selected population who had tolerated dual antiplatelet therapy for 1 year), 106 deaths, myocardial infarctions, or stroke events were prevented, at the cost of 47 excess moderate bleeding events.3
Indirect comparisons between trials are problematic, given different end point definitions, populations, and background therapies. But their results suggest that less-intensive inhibition with clopidogrel as the second antiplatelet long-term (as in CHARISMA) may provide the best balance of benefit vs risk.
BALANCING RISK AND BENEFIT
The evidence is unequivocal that dual antiplatelet therapy suppresses coronary ischemic complications resulting from thrombosis at sites of spontaneous plaque rupture following acute coronary syndromes or mechanical plaque disruption and foreign body implantation associated with percutaneous coronary intervention.
Three large-scale trials (DAPT,3 PEGASUS,39 and the secondary prevention subgroup of CHARISMA38) showed that the protective effect of dual antiplatelet therapy continues with prolonged therapy in patients who have experienced an acute coronary syndrome event or have received a drug-eluting stent. That benefit seems to be due to the action of these therapies on the culprit vessel (the one that caused the acute coronary syndrome or the site of stenting), as well as nonculprit arteries, emphasizing that dual antiplatelet therapy protects against atherosclerosis progression and future plaque rupture events.
For the durations studied in the longest trials thus far, 30 months (DAPT3) and 36 months (PEGASUS39), event curves continue to diverge, indicating that the advantage of dual antiplatelet therapy may persist for an indefinite period of time. Thus, indefinite therapy with dual antiplatelet agents can be supported, particularly in patients with advanced coronary artery disease or those who have had multiple coronary events.
We believe that the balance of evidence suggests that smaller studies that failed to show a benefit of longer-term therapy were underpowered to do so.
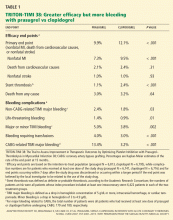
The ischemic protection is associated with the adverse effect of increased bleeding risk. Unfortunately, there has been little success in guiding dual antiplatelet therapy based on ischemic vs bleeding risk, in part because the same factors that predict risk of ischemic complications seem to predict increased susceptibility to bleeding. Nevertheless, indirect comparisons between studies suggest that for longer-term therapy clopidogrel may be superior to ticagrelor or prasugrel: the absolute excess bleeding risk with dual antiplatelet therapy vs aspirin in the CHARISMA secondary prevention subgroup was less than that in PEGASUS, with similar absolute reductions in ischemic events. So while the TRITON-TIMI 3822 and PLATO23 trials support the superiority of prasugrel or ticagrelor over clopidogrel for the first year after acute coronary syndrome, subsequent years of therapy may best be provided with clopidogrel.
Some patients may have identifiable factors that place them at very high risk of bleeding—need for surgical procedures, need for anticoagulation, or occurrence of bleeding complications or excessive “nuisance bleeding.” In those patients, the data suggest that dual antiplatelet therapy could be discontinued after 6 months, or perhaps even 3 months in the highest bleeding risk circumstances after second-generation drug-eluting stent placement.
WOEST49 was an open-label randomized controlled trial that studied the safety of antiplatelet regimens in patients on anticoagulation requiring percutaneous coronary interventions. Patients were randomized to double therapy with anticoagulant and clopidogrel vs triple therapy with additional aspirin following percutaneous coronary intervention. The primary end point was bleeding events within 1 year. Clopidogrel without aspirin was associated with significantly fewer bleeding events compared with triple therapy, with no increase in adverse ischemic events. The strategy tested in the WOEST trial seems reasonable in the specific group of patients who require ongoing anticoagulant therapy after drug-eluting stent placement, recognizing that the trial was somewhat underpowered to make definitive conclusions, particularly in patients at high risk for stent thrombosis.
Based on the results of PEGASUS and the CHARISMA subgroup with established ischemic burden, in which dual antiplatelet therapy was started after an interruption following the index coronary event, it is also reasonable to restart long-term dual antiplatelet therapy in patients who require interruption for short-term indications such as a surgical procedure.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426.
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–2166.
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010; 74:597–607.
- Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc 2013; 53:81–96.
- Showkathali R, Natarajan A. Antiplatelet and antithrombin strategies in acute coronary syndrome: state-of-the-art review. Curr Cardiol Rev 2012; 8:239–249.
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72:2087–2116.
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014; 7:1081–1092.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48:193–202.
- Nikam N, Steinberg TB, Steinberg DH. Advances in stent technologies and their effect on clinical efficacy and safety. Med Devices (Auckl) 2014; 7:165–178.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol 2014; 30:35–45.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015; 36:3320–3331.
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009; 53:1399–1409.
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol 2013; 112:737–745.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015; 12:30–47.
- Patrono C, Rocca B. The future of antiplatelet therapy in cardiovascular disease. Annu Rev Med 2010; 61:49–61.
- Park SJ, Kang SM, Park DW. Dual antiplatelet therapy after drug-eluting stents: defining the proper duration. Coron Artery Dis 2014; 25:83–89.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Nusca A, Patti G. Platelet function and inhibition in ischemic heart disease. Curr Cardiol Rep 2012; 14:457–467.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Genereux P, Stone GW, Harrington RA, et al; CHAMPION PHOENIX Investigators. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: Insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol 2014; 63:619–629.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Warren J, Baber U, Mehran R. Antiplatelet therapy after drug-eluting stent implantation. J Cardiol 2015; 65:98–104.
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998; 98:2126–2132.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358:527–533.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002; 13(suppl 1):17–21.
- Ferguson JJ. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy—CLARITY-TIMI 28. Future Cardiol 2005; 1:605–610.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791–1800.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64:2086–2097.
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125:505–513.
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510–2522.
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340–1348.
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014; 114:236–242.
- Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012; 125:2015–2026.
- Collet JP, Silvain J, Barthelemy O, et al; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384:1577–1585.
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014; 129:304–312.
- Kwok CS, Bulluck H, Ryding AD, Loke YK. Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. ScientificWorldJournal 2014; 2014:794078.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
Percutaneous coronary intervention for acute coronary syndromes has evolved, and so, hand in hand, has antiplatelet therapy. With the advent of clopidogrel and newer agents, several studies demonstrated the benefits of dual antiplatelet therapy in preventing major vascular ischemic complications. The findings culminated in a guideline recommendation for at least 12 months of dual antiplatelet therapy after placement of a drug-eluting stent, when feasible—a class I recommendation (treatment should be given), level of evidence B (limited populations evaluated).1,2 But extending dual antiplatelet therapy beyond 12 months had no strong favorable evidence until the recent Dual Antiplatelet Therapy (DAPT) study3 shed light on this topic.
Here, we review the evidence thus far on the optimal duration of dual antiplatelet therapy in the secondary prevention of coronary artery disease.
PLATELETS IN ACUTE CORONARY SYNDROMES AND STENT THROMBOSIS
Acute coronary syndromes begin with fissuring or ulceration of a vulnerable atherosclerotic plaque, followed by thrombosis and occlusion, mediated by platelet adhesion, activation, and aggregation (Figure 1). Transient occlusion results in unstable angina or non-ST-elevation myocardial infarction, while total occlusion usually results in ST-elevation myocardial infarction.
Platelet aggregation is prominent among the mechanisms leading to stent thrombosis and vaso-occlusive ischemic complications after percutaneous coronary intervention. Thus, antiplatelet agents play a vital role in both primary and secondary prevention of cardiovascular events.4–6
Adhesion, activation, and aggregation
Adhesion. Disruption of the vascular endothelium as a result of vulnerable plaque fissuring or ulceration exposes subendothelial thrombogenic collagen and von Willebrand factor to blood. Collagen engages platelets through their glycoprotein (GP) Ia, IIa, and VI receptors, and von Willebrand factor binds platelets through the GP Ib-IX-V receptor.
Activation. Once platelets adhere to the subendothelium, they undergo a conformational change and become activated. Simultaneous release of various autocrine and paracrine mediators including adenosine diphosphate, serotonin, epinephrine, thromboxane, and various ligand-receptor interactions all contribute to the activation cascade. Adenosine diphosphate binds to the platelet receptor P2Y1, leading to an increase in intracellular calcium, and it binds to P2Y12, leading to a decrease in cyclic adenosine monophosphate, both of which cause GP IIb/IIIa receptor activation. Thromboxane A2 released by platelets by cyclo-oxygenase 1 binds to alpha or beta variant receptors and contributes to GP IIb/IIIa activation through elevation of intracellular calcium levels.
Aggregation and thrombosis. Exposure of tissue factor to plasma following plaque rupture activates the coagulation cascade via the extrinsic pathway, which generates thrombin, a powerful platelet activator that causes thrombus formation via fibrin. Thrombin binds to protease-activated receptors PAR-1 and PAR-4 on platelets, causing an increase in intracellular calcium and a decrease in cyclic adenosine monophosphate with subsequent GP IIb/IIIa activation. GP IIb/IIIa facilitates platelet aggregation by binding to fibrinogen and forming a stable platelet thrombus.
In the early stages of thrombus formation, platelets predominate (“white” thrombi); further organization with fibrin results in older “red” thrombi. The stages of thrombi vary in non-ST-elevation and ST-elevation myocardial infarction and are prognostic markers of death.4–8
PERCUTANEOUS INTERVENTION, RESTENOSIS, AND STENT THROMBOSIS
Percutaneous coronary intervention, the preferred means of revascularization for many patients, is performed emergently in patients with ST-elevation myocardial infarction, urgently in those with acute coronary syndromes without ST elevation, and electively in those with stable ischemic symptoms.
Percutaneous revascularization techniques have evolved from balloon angioplasty to bare-metal stents to drug-eluting stents, but each of these procedures has been associated with a periprocedural and postprocedural risk of thrombosis.
Balloon angioplasty was associated with vascular intimal injury, inciting elastic vascular recoil and smooth muscle cell proliferation leading to restenosis.
Bare-metal stents reduced the restenosis rate by eliminating vascular recoil, although restenosis still occurred within the stent because of neointimal proliferation of vascular smooth muscle cells. This was an important limitation, as both acute and subacute stent thrombosis were refractory to aggressive anticoagulation regimens that were associated with major bleeding complications and longer hospital length of stay. Stenting became mainstream practice only after the ISAR9 and STARS10 trials showed that dual antiplatelet therapy controlled stent thrombosis.
Drug-eluting stents coated with anti-proliferative and anti-inflammatory polymers markedly reduced in-stent restenosis rates by suppressing the initial vascular smooth-muscle proliferative response. However, they were still associated with late and very late stent thrombosis with incomplete endothelialization, even up to 40 months after implantation. Proposed mechanisms include incomplete stent apposition and inflammatory hypersensitivity reactions to the polymer coating. Incomplete stent apposition associated with low-velocity blood flow at the junction of the stent strut and vessel wall, together with delayed endothelialization, promotes platelet adhesion and aggregation, followed by thrombus formation.11
Second-generation drug-eluting stents have thinner struts and more biocompatible polymers and are thought to favor more complete re-endothelialization, reducing the rates of stent thrombosis.8,12,13
Predictors of early stent thrombosis
The Dutch Stent Thrombosis Registry and other studies looked at risk factors for stent thrombosis.14,15
Procedure-related factors included:
- Stent undersizing
- Residual uncovered dissections after angioplasty
- Longer stents
- Low flow after angioplasty (< 3 on the 0–3 Thrombolysis in Myocardial Infarction [TIMI] scale).
Lesion-related factors included:
- Intermediate coronary artery disease both proximal and distal to the culprit lesions
- Bifurcation lesions.
Patient-related factors included:
- Low left ventricular ejection fraction
- Diabetes mellitus
- Peripheral arterial disease Premature discontinuation of clopidogrel.
ANTIPLATELET AGENTS: MECHANISM OF ACTION
Various pathways play synergistic roles in platelet activation and aggregation and thrombus formation, and different antiplatelet agents inhibit these specific pathways, thus complementing each other and having additive effects (Figure 2, Table 1).5,16–21
Aspirin inhibits cyclo-oxygenase 1
Cyclo-oxygenase 1, found in platelets, endothelial cells, and other cells, catalyzes the conversion of arachidonic acid to thromboxane A2. Aspirin irreversibly inhibits cyclo-oxygenase 1 by acetylating its serine residue, preventing formation of thromboxane A2 and preventing platelet activation and aggregation.
P2Y12 ADP receptor antagonists
Clopidogrel and prasugrel are thienopyridine agents that irreversibly inhibit the P2Y12 receptor, thereby preventing binding of adenosine diphosphate and the subsequent platelet activation-aggregation cascade. They are both prodrugs and require conversion by cytochrome P450 enzymes to active metabolites. Prasugrel is 10 times more potent than clopidogrel due to more efficient formation of its active metabolite, and it achieves a comparable effect on platelet inhibition 30 minutes faster than the peak effect of clopidogrel at 6 hours. The overall peak inhibitory effect of prasugrel is twice that of clopidogrel.22
Ticagrelor, a cyclopentyl-triazolo-pyrimidine, directly and reversibly inhibits the P2Y12 ADP receptor. Unlike clopidogrel and prasugrel, it does not need to be converted to an active metabolite, and it noncompetitively inhibits P2Y12 at a site different from the adenosine diphosphate binding site.23 Like prasugrel, ticagrelor inhibits platelet function more rapidly and more completely than clopidogrel.
Cangrelor, an intravenously administered analogue of adenosine triphosphate, reversibly inhibits the P2Y12 receptor. It has undergone phase 3 trials but is not yet approved for clinical use.24
WHY DUAL ANTIPLATELET THERAPY?
Aspirin is good, clopidogrel is better
Aspirin has a well-validated role in both primary and secondary prevention of coronary and noncoronary atherosclerotic vascular disease.
The CAPRIE trial found clopidogrel monotherapy to be superior to aspirin monotherapy in patients with established atherosclerotic vascular disease.25
After stenting, short-term dual therapy is better than short-term warfarin
Thrombotic complications in the early postprocedural period were a major limitation of stenting, and existing anticoagulation regimens were ineffective in preventing them.26,27
The ISAR trial studied the benefit of combined antiplatelet vs anticoagulant therapy after stent placement. Patients randomized to receive combined aspirin plus ticlopidine (an early P2Y12 inhibitor) had significantly lower rates of primary cardiac, hemorrhagic, and vascular events at 30 days.9 Two other trials confirmed this finding.28,29
STARS10 also confirmed the benefit of aspirin and ticlopidine after stenting. Patients were randomly assigned to aspirin alone, aspirin plus warfarin, or aspirin plus ticlopidine after stent placement. The rate of stent thrombosis at 30 days was significantly lower in the dual antiplatelet group than in the other two groups. The dual antiplatelet group had a higher rate of bleeding than the aspirin-alone group, but the rate was similar to that of the aspirin-plus-warfarin group.
Long-term dual antiplatelet therapy is beneficial in several situations
ISAR and STARS were landmark trials that showed stent thrombosis could be reduced by dual antiplatelet therapy for a 30-day period. However, the long-term role of dual antiplatelet therapy was still unknown.
The CURE trial30–32 randomized patients presenting with acute coronary syndromes without ST elevation to receive clopidogrel plus aspirin or placebo plus aspirin for 3 to 12 months. The rate of the primary end point (cardiac death, nonfatal myocardial infarction, or stroke) was significantly lower in the clopidogrel-plus-aspirin group. A similar benefit of dual antiplatelet therapy was seen in the subgroup of patients who underwent percutaneous coronary intervention. Both pretreatment with clopidogrel plus aspirin for a median of 10 days prior to percutaneous intervention and continuing it for a mean of 9 months reduced major adverse cardiovascular events.
The CREDO trial20 found that the combination of clopidogrel and aspirin significantly reduced the incidence of death, myocardial infarction, or stroke at 1 year after percutaneous coronary intervention. A subgroup of patients in this trial who had a longer pretreatment interval with a loading clopidogrel dose showed a benefit at 28 days, which was not as evident with a shorter loading dose interval.
The CLARITY-TIMI 28 trial33,34 showed the advantage of adding clopidogrel to aspirin in patients receiving fibrinolytic therapy for ST-elevation myocardial infarction. Adding clopidogrel both improved the patency of the infarct-related artery and reduced ischemic complications. In patients who subsequently underwent percutaneous coronary intervention and stenting, clopidogrel pretreatment was associated with a significant decrease in ischemic complications before and after the procedure. There was no significant increase in bleeding complications in either group.
COMMIT/CCS 235 also showed the benefit of dual antiplatelet therapy in patients with ST-elevation myocardial infarction. Clopidogrel added to aspirin during the short-term in-hospital or postdischarge treatment period significantly reduced a composite end point of reinfarction, death, or stroke as well as death from any cause.
The CHARISMA trial36–38 aimed to determine if patients who were more stable (ie, no recent acute coronary syndrome event or percutaneous coronary intervention) would benefit. Overall, CHARISMA showed no benefit of adding clopidogrel to aspirin compared with aspirin alone in a broad population of patients with established vascular disease (secondary prevention) or risk factors for vascular disease (primary prevention).
But importantly, though no benefit was seen in the primary prevention group, the large subgroup of patients with established atherosclerotic vascular disease (12,153 of the 15,603 patients in the trial) did benefit from dual antiplatelet therapy.36,37 This subgroup showed an overall reduction in absolute risk of 1.5% (relative risk 0.88, P = .046) over a median follow-up of 27.6 months. This benefit was even more apparent in the 9,478 patients with prior myocardial infarction, stroke, or peripheral artery disease, for whom the relative risk reduction was 17.1% (P = .01) and the reduction in absolute risk 1.5%.38
These results are comparable to the 2% absolute risk reduction in the CURE trial for similar end points over 9 months. In both studies, there was no significant increase in the risk of major bleeding or intracranial bleeding in the clopidogrel-plus-aspirin groups, although minor bleeding was increased by dual antiplatelet therapy.
The rate of severe bleeding, which was the primary safety end point in CHARISMA, was not significantly different in the clopidogrel-plus-aspirin group compared with the placebo-plus-aspirin group (relative risk 1.25, 95% CI 0.97–1.61, P = .09).
Thus, although the CHARISMA findings were negative overall, the positive finding observed in the predominant subgroup of patients with established vascular disease can therefore be considered supportive of the results of the subsequent trials discussed below.
The PEGASUS-TIMI 54 trial39 studied the benefit of adding ticagrelor (60 or 90 mg) to low-dose aspirin in patients with stable coronary artery disease who had had a myocardial infarction 1 to 3 years earlier.
Confirming the results of the CHARISMA subgroup analysis, the incidence of the ischemic primary efficacy end point (a composite of cardiovascular death, myocardial infarction, and stroke) was significantly lower in both groups receiving ticagrelor plus aspirin compared with those receiving placebo plus aspirin. The Kaplan-Meier rate at 3 years for the ticagrelor 90 mg-plus-aspirin group was 7.85% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.85, 95% confidence interval [CI] 0.75–0.96, P = .008). The rate for the ticagrelor 60 mg-plus-aspirin group was 7.77% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.84, 95% CI 0.74–0.95, P = .004).
The rates of all TIMI major and minor bleeding, as well as bleeding requiring transfusion or discontinuation of the study drug, were significantly higher in both ticagrelor dosing groups than in the placebo group (P < .01 for both groups vs placebo). The rates of fatal bleeding and nonfatal intracranial hemorrhage were not significantly higher. Although there was an overall reduction in ischemic end points with the addition of ticagrelor, there was also a significantly higher incidence of bleeding in this group.
Comment. Thus, with or without percutaneous coronary intervention in acute coronary syndrome as well as in stable coronary artery disease, dual antiplatelet therapy was shown to improve outcomes and decrease ischemic complications compared with aspirin alone. It provided benefit in the setting of acute coronary syndrome (in the CURE trial) and percutaneous coronary intervention (in the CREDO trial) for up to 1 year.
Major questions remained to be addressed:
- Do the results of CREDO, which was performed before the current interventional era and the use of drug-eluting stents, reflect outcomes after current interventional practice?
- Could shorter periods of dual antiplatelet therapy be sufficient, especially with newer stents with less risk of late thrombosis?
- Does the benefit of dual antiplatelet therapy extend beyond the 1-year time period tested in those trials to date?
RECOMMENDATIONS FOR DOSING
The American College of Cardiology Foundation/American Heart Association guidelines for dosing of antiplatelet agents for non-ST-elevation myocardial infarction are summarized in Table 2, and those for ST-elevation myocardial infarction are summarized in Table 3.1,2
WOULD SHORTER THERAPY AFTER STENTING WORK AS WELL?
The American College of Cardiology Foundation/American Heart Association currently recommend dual antiplatelet therapy for at least 12 months after drug-eluting stent placement, with shorter courses appropriate for patients who develop excessive bleeding complications or who are at high risk of bleeding.
Four trials (Table 4) evaluated whether shorter durations of dual antiplatelet therapy would suffice: SECURITY,40 EXCELLENT,41 OPTIMIZE,42 and RESET.43 All of them showed that short-duration therapy was not inferior to standard-duration therapy.44 These studies were comparable in that:
- Patients were randomized at the time of percutaneous coronary intervention or within 24 hours of it.
- Most patients received a second-generation drug-eluting stent, with the following exceptions: in EXCELLENT,41 one-fourth of patients received a Cypher first-generation drug-eluting stent, and in RESET,43 approximately one-fourth of the patients received a sirolimus-eluting stent in the standard-duration group for short lesions. Those patients with longer lesions in the RESET standard-duration group received an everolimus drug-eluting stent.
- The second antiplatelet added to aspirin in all studies was clopidogrel, with the exception of the SECURITY trial, in which fewer than 2% of patients received ticagrelor or prasugrel.40
- All the trials except RESET excluded patients who had had a myocardial infarction within 72 hours, and thus most patients studied had a lower risk profile.
- All of the trials sought to study noninferiority of short- vs standard-duration dual antiplatelet therapy, defined as the occurrence of a primary end point at 1 year (a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, target vessel failure or revascularization, or bleeding).
Their low-risk patient populations and infrequent end points rendered these studies underpowered to make definitive conclusions about the relative efficacy of 6-months vs 12-months of dual antiplatelet therapy.
WOULD LONGER THERAPY BE BETTER?
The PRODIGY trial45 assessed durations of dual antiplatelet therapy both shorter and longer than the conventional 1 year, randomizing patients undergoing placement of a bare-metal stent, first-generation drug-eluting stent, or second-generation drug-eluting stent to receive aspirin and clopidogrel for either 6 months or 24 months. The study showed no significant difference in primary outcomes in the short- or long-duration groups.
Other trials that compared the standard 12 months of dual antiplatelet therapy with extended duration beyond 12 months were DAPT,3 ARCTIC-Interruption,46 and DES-LATE.47 The trials were comparable in that:
- All patients were randomized after completing 12 months of dual antiplatelet therapy following drug-eluting stent placement.
- All patients who were included had been free of major cardiac ischemic events or bleeding during the 12 months following stent placement.
- The primary aim of all three studies was to compare primary end points in groups receiving aspirin alone vs extended dual antiplatelet therapy. The primary end point was a composite of death due to a cardiovascular cause, nonfatal myocardial infarction, stroke, or stent thrombosis.
- The principal safety end point was bleeding.
Although the two earlier studies (ARCTIC-Interruption and DES-LATE) did not show any benefit of extended dual antiplatelet therapy compared with the standard 12-month duration, the recent DAPT study did.
The DAPT study
The DAPT study3 was an international, multicenter, placebo-controlled, double-blind randomized trial designed to examine the benefit of dual antiplatelet therapy beyond 1 year in a patient population large enough to provide definitive assessment of benefit and risk.
A total of 9,961 patients who received drug-eluting stents were randomized after 12 months of dual antiplatelet therapy to receive either a thienopyridine (clopidogrel or prasugrel) plus aspirin or placebo plus aspirin. They were followed for an additional 18 months. The coprimary efficacy end points were stent thrombosis and a composite of death, myocardial infarction, or stroke, while the primary safety end point was moderate or severe bleeding. The patients were also observed from months 30 to 33 on aspirin alone after stopping the thienopyridine.
Results. Longer therapy substantially reduced the risks of stent thrombosis (hazard ratio [HR] 0.29, 95% confidence interval [CI] 0.17–0.48) and the composite ischemic end point (HR 0.71, 95% CI 0.59–0.85). Follow-up during the 3-month thienopyridine discontinuation phase starting at 30 months revealed convergence of the ischemic event-rate curves in the two groups, which suggested that continuing dual antiplatelet therapy beyond 30 months might have been beneficial. Myocardial infarction unrelated to stent thrombosis accounted for 55% of the treatment benefit of dual antiplatelet therapy.
The risk of bleeding was higher in the thienopyridine group during the treatment period (2.5% vs 1.6%, P = .001). There was also a higher rate of noncardiovascular mortality in the thienopyridine group, although this difference may have been due to chance.3,48
Why were the results different?
All three trials included first- and second-generation drug-eluting stents, with different proportions in different trials. In ARCTIC-Interruption,46 43% of the patients in the continuation group had a first-generation stent, as did 64% of the patients in the dual antiplatelet group of DES-LATE.47 In the DAPT trial,3 38% of the patients in the longer-duration arm had a first-generation stent, and in 26% of cases it was a paclitaxel-eluting stent.
Only clopidogrel was used as the second antiplatelet agent in DES-LATE, whereas prasugrel was used in 10% of patients in ARCTIC-Interruption and 35% in DAPT.
Yet none of these differences seem to explain the differences in outcome among the studies. ARCTIC-Interruption and DES-LATE did not show any benefit of continued dual antiplatelet therapy beyond 12 months. DAPT showed benefit of extended therapy with prasugrel or with clopidogrel, and with first-generation or second-generation drug-eluting stents. The most likely explanation for the different results was that DAPT was the only trial sufficiently powered to definitively assess the end points, including stent thrombosis.
A balance between ischemic efficacy and bleeding risk is the major consideration with any antithrombotic and antiplatelet therapy. In the three largest trials we discussed (the vascular disease subgroups of CHARISMA,38 PEGASUS,39 and DAPT3), comparison of the prespecified efficacy and safety end points of each trial suggests that dual antiplatelet therapy has a net benefit, particularly given the irreversible nature of ischemic end points.
In CHARISMA,38 60 cardiovascular deaths, myocardial infarctions, or strokes were prevented per year per 10,000 patients treated, at the cost of 28 excess moderate bleeding events.
In PEGASUS,39 42 cardiovascular deaths, myocardial infarctions, or strokes were prevented, at the cost of 79 excess bleeding events requiring transfusion.
In DAPT (a selected population who had tolerated dual antiplatelet therapy for 1 year), 106 deaths, myocardial infarctions, or stroke events were prevented, at the cost of 47 excess moderate bleeding events.3
Indirect comparisons between trials are problematic, given different end point definitions, populations, and background therapies. But their results suggest that less-intensive inhibition with clopidogrel as the second antiplatelet long-term (as in CHARISMA) may provide the best balance of benefit vs risk.
BALANCING RISK AND BENEFIT
The evidence is unequivocal that dual antiplatelet therapy suppresses coronary ischemic complications resulting from thrombosis at sites of spontaneous plaque rupture following acute coronary syndromes or mechanical plaque disruption and foreign body implantation associated with percutaneous coronary intervention.
Three large-scale trials (DAPT,3 PEGASUS,39 and the secondary prevention subgroup of CHARISMA38) showed that the protective effect of dual antiplatelet therapy continues with prolonged therapy in patients who have experienced an acute coronary syndrome event or have received a drug-eluting stent. That benefit seems to be due to the action of these therapies on the culprit vessel (the one that caused the acute coronary syndrome or the site of stenting), as well as nonculprit arteries, emphasizing that dual antiplatelet therapy protects against atherosclerosis progression and future plaque rupture events.
For the durations studied in the longest trials thus far, 30 months (DAPT3) and 36 months (PEGASUS39), event curves continue to diverge, indicating that the advantage of dual antiplatelet therapy may persist for an indefinite period of time. Thus, indefinite therapy with dual antiplatelet agents can be supported, particularly in patients with advanced coronary artery disease or those who have had multiple coronary events.
We believe that the balance of evidence suggests that smaller studies that failed to show a benefit of longer-term therapy were underpowered to do so.
The ischemic protection is associated with the adverse effect of increased bleeding risk. Unfortunately, there has been little success in guiding dual antiplatelet therapy based on ischemic vs bleeding risk, in part because the same factors that predict risk of ischemic complications seem to predict increased susceptibility to bleeding. Nevertheless, indirect comparisons between studies suggest that for longer-term therapy clopidogrel may be superior to ticagrelor or prasugrel: the absolute excess bleeding risk with dual antiplatelet therapy vs aspirin in the CHARISMA secondary prevention subgroup was less than that in PEGASUS, with similar absolute reductions in ischemic events. So while the TRITON-TIMI 3822 and PLATO23 trials support the superiority of prasugrel or ticagrelor over clopidogrel for the first year after acute coronary syndrome, subsequent years of therapy may best be provided with clopidogrel.
Some patients may have identifiable factors that place them at very high risk of bleeding—need for surgical procedures, need for anticoagulation, or occurrence of bleeding complications or excessive “nuisance bleeding.” In those patients, the data suggest that dual antiplatelet therapy could be discontinued after 6 months, or perhaps even 3 months in the highest bleeding risk circumstances after second-generation drug-eluting stent placement.
WOEST49 was an open-label randomized controlled trial that studied the safety of antiplatelet regimens in patients on anticoagulation requiring percutaneous coronary interventions. Patients were randomized to double therapy with anticoagulant and clopidogrel vs triple therapy with additional aspirin following percutaneous coronary intervention. The primary end point was bleeding events within 1 year. Clopidogrel without aspirin was associated with significantly fewer bleeding events compared with triple therapy, with no increase in adverse ischemic events. The strategy tested in the WOEST trial seems reasonable in the specific group of patients who require ongoing anticoagulant therapy after drug-eluting stent placement, recognizing that the trial was somewhat underpowered to make definitive conclusions, particularly in patients at high risk for stent thrombosis.
Based on the results of PEGASUS and the CHARISMA subgroup with established ischemic burden, in which dual antiplatelet therapy was started after an interruption following the index coronary event, it is also reasonable to restart long-term dual antiplatelet therapy in patients who require interruption for short-term indications such as a surgical procedure.
Percutaneous coronary intervention for acute coronary syndromes has evolved, and so, hand in hand, has antiplatelet therapy. With the advent of clopidogrel and newer agents, several studies demonstrated the benefits of dual antiplatelet therapy in preventing major vascular ischemic complications. The findings culminated in a guideline recommendation for at least 12 months of dual antiplatelet therapy after placement of a drug-eluting stent, when feasible—a class I recommendation (treatment should be given), level of evidence B (limited populations evaluated).1,2 But extending dual antiplatelet therapy beyond 12 months had no strong favorable evidence until the recent Dual Antiplatelet Therapy (DAPT) study3 shed light on this topic.
Here, we review the evidence thus far on the optimal duration of dual antiplatelet therapy in the secondary prevention of coronary artery disease.
PLATELETS IN ACUTE CORONARY SYNDROMES AND STENT THROMBOSIS
Acute coronary syndromes begin with fissuring or ulceration of a vulnerable atherosclerotic plaque, followed by thrombosis and occlusion, mediated by platelet adhesion, activation, and aggregation (Figure 1). Transient occlusion results in unstable angina or non-ST-elevation myocardial infarction, while total occlusion usually results in ST-elevation myocardial infarction.
Platelet aggregation is prominent among the mechanisms leading to stent thrombosis and vaso-occlusive ischemic complications after percutaneous coronary intervention. Thus, antiplatelet agents play a vital role in both primary and secondary prevention of cardiovascular events.4–6
Adhesion, activation, and aggregation
Adhesion. Disruption of the vascular endothelium as a result of vulnerable plaque fissuring or ulceration exposes subendothelial thrombogenic collagen and von Willebrand factor to blood. Collagen engages platelets through their glycoprotein (GP) Ia, IIa, and VI receptors, and von Willebrand factor binds platelets through the GP Ib-IX-V receptor.
Activation. Once platelets adhere to the subendothelium, they undergo a conformational change and become activated. Simultaneous release of various autocrine and paracrine mediators including adenosine diphosphate, serotonin, epinephrine, thromboxane, and various ligand-receptor interactions all contribute to the activation cascade. Adenosine diphosphate binds to the platelet receptor P2Y1, leading to an increase in intracellular calcium, and it binds to P2Y12, leading to a decrease in cyclic adenosine monophosphate, both of which cause GP IIb/IIIa receptor activation. Thromboxane A2 released by platelets by cyclo-oxygenase 1 binds to alpha or beta variant receptors and contributes to GP IIb/IIIa activation through elevation of intracellular calcium levels.
Aggregation and thrombosis. Exposure of tissue factor to plasma following plaque rupture activates the coagulation cascade via the extrinsic pathway, which generates thrombin, a powerful platelet activator that causes thrombus formation via fibrin. Thrombin binds to protease-activated receptors PAR-1 and PAR-4 on platelets, causing an increase in intracellular calcium and a decrease in cyclic adenosine monophosphate with subsequent GP IIb/IIIa activation. GP IIb/IIIa facilitates platelet aggregation by binding to fibrinogen and forming a stable platelet thrombus.
In the early stages of thrombus formation, platelets predominate (“white” thrombi); further organization with fibrin results in older “red” thrombi. The stages of thrombi vary in non-ST-elevation and ST-elevation myocardial infarction and are prognostic markers of death.4–8
PERCUTANEOUS INTERVENTION, RESTENOSIS, AND STENT THROMBOSIS
Percutaneous coronary intervention, the preferred means of revascularization for many patients, is performed emergently in patients with ST-elevation myocardial infarction, urgently in those with acute coronary syndromes without ST elevation, and electively in those with stable ischemic symptoms.
Percutaneous revascularization techniques have evolved from balloon angioplasty to bare-metal stents to drug-eluting stents, but each of these procedures has been associated with a periprocedural and postprocedural risk of thrombosis.
Balloon angioplasty was associated with vascular intimal injury, inciting elastic vascular recoil and smooth muscle cell proliferation leading to restenosis.
Bare-metal stents reduced the restenosis rate by eliminating vascular recoil, although restenosis still occurred within the stent because of neointimal proliferation of vascular smooth muscle cells. This was an important limitation, as both acute and subacute stent thrombosis were refractory to aggressive anticoagulation regimens that were associated with major bleeding complications and longer hospital length of stay. Stenting became mainstream practice only after the ISAR9 and STARS10 trials showed that dual antiplatelet therapy controlled stent thrombosis.
Drug-eluting stents coated with anti-proliferative and anti-inflammatory polymers markedly reduced in-stent restenosis rates by suppressing the initial vascular smooth-muscle proliferative response. However, they were still associated with late and very late stent thrombosis with incomplete endothelialization, even up to 40 months after implantation. Proposed mechanisms include incomplete stent apposition and inflammatory hypersensitivity reactions to the polymer coating. Incomplete stent apposition associated with low-velocity blood flow at the junction of the stent strut and vessel wall, together with delayed endothelialization, promotes platelet adhesion and aggregation, followed by thrombus formation.11
Second-generation drug-eluting stents have thinner struts and more biocompatible polymers and are thought to favor more complete re-endothelialization, reducing the rates of stent thrombosis.8,12,13
Predictors of early stent thrombosis
The Dutch Stent Thrombosis Registry and other studies looked at risk factors for stent thrombosis.14,15
Procedure-related factors included:
- Stent undersizing
- Residual uncovered dissections after angioplasty
- Longer stents
- Low flow after angioplasty (< 3 on the 0–3 Thrombolysis in Myocardial Infarction [TIMI] scale).
Lesion-related factors included:
- Intermediate coronary artery disease both proximal and distal to the culprit lesions
- Bifurcation lesions.
Patient-related factors included:
- Low left ventricular ejection fraction
- Diabetes mellitus
- Peripheral arterial disease Premature discontinuation of clopidogrel.
ANTIPLATELET AGENTS: MECHANISM OF ACTION
Various pathways play synergistic roles in platelet activation and aggregation and thrombus formation, and different antiplatelet agents inhibit these specific pathways, thus complementing each other and having additive effects (Figure 2, Table 1).5,16–21
Aspirin inhibits cyclo-oxygenase 1
Cyclo-oxygenase 1, found in platelets, endothelial cells, and other cells, catalyzes the conversion of arachidonic acid to thromboxane A2. Aspirin irreversibly inhibits cyclo-oxygenase 1 by acetylating its serine residue, preventing formation of thromboxane A2 and preventing platelet activation and aggregation.
P2Y12 ADP receptor antagonists
Clopidogrel and prasugrel are thienopyridine agents that irreversibly inhibit the P2Y12 receptor, thereby preventing binding of adenosine diphosphate and the subsequent platelet activation-aggregation cascade. They are both prodrugs and require conversion by cytochrome P450 enzymes to active metabolites. Prasugrel is 10 times more potent than clopidogrel due to more efficient formation of its active metabolite, and it achieves a comparable effect on platelet inhibition 30 minutes faster than the peak effect of clopidogrel at 6 hours. The overall peak inhibitory effect of prasugrel is twice that of clopidogrel.22
Ticagrelor, a cyclopentyl-triazolo-pyrimidine, directly and reversibly inhibits the P2Y12 ADP receptor. Unlike clopidogrel and prasugrel, it does not need to be converted to an active metabolite, and it noncompetitively inhibits P2Y12 at a site different from the adenosine diphosphate binding site.23 Like prasugrel, ticagrelor inhibits platelet function more rapidly and more completely than clopidogrel.
Cangrelor, an intravenously administered analogue of adenosine triphosphate, reversibly inhibits the P2Y12 receptor. It has undergone phase 3 trials but is not yet approved for clinical use.24
WHY DUAL ANTIPLATELET THERAPY?
Aspirin is good, clopidogrel is better
Aspirin has a well-validated role in both primary and secondary prevention of coronary and noncoronary atherosclerotic vascular disease.
The CAPRIE trial found clopidogrel monotherapy to be superior to aspirin monotherapy in patients with established atherosclerotic vascular disease.25
After stenting, short-term dual therapy is better than short-term warfarin
Thrombotic complications in the early postprocedural period were a major limitation of stenting, and existing anticoagulation regimens were ineffective in preventing them.26,27
The ISAR trial studied the benefit of combined antiplatelet vs anticoagulant therapy after stent placement. Patients randomized to receive combined aspirin plus ticlopidine (an early P2Y12 inhibitor) had significantly lower rates of primary cardiac, hemorrhagic, and vascular events at 30 days.9 Two other trials confirmed this finding.28,29
STARS10 also confirmed the benefit of aspirin and ticlopidine after stenting. Patients were randomly assigned to aspirin alone, aspirin plus warfarin, or aspirin plus ticlopidine after stent placement. The rate of stent thrombosis at 30 days was significantly lower in the dual antiplatelet group than in the other two groups. The dual antiplatelet group had a higher rate of bleeding than the aspirin-alone group, but the rate was similar to that of the aspirin-plus-warfarin group.
Long-term dual antiplatelet therapy is beneficial in several situations
ISAR and STARS were landmark trials that showed stent thrombosis could be reduced by dual antiplatelet therapy for a 30-day period. However, the long-term role of dual antiplatelet therapy was still unknown.
The CURE trial30–32 randomized patients presenting with acute coronary syndromes without ST elevation to receive clopidogrel plus aspirin or placebo plus aspirin for 3 to 12 months. The rate of the primary end point (cardiac death, nonfatal myocardial infarction, or stroke) was significantly lower in the clopidogrel-plus-aspirin group. A similar benefit of dual antiplatelet therapy was seen in the subgroup of patients who underwent percutaneous coronary intervention. Both pretreatment with clopidogrel plus aspirin for a median of 10 days prior to percutaneous intervention and continuing it for a mean of 9 months reduced major adverse cardiovascular events.
The CREDO trial20 found that the combination of clopidogrel and aspirin significantly reduced the incidence of death, myocardial infarction, or stroke at 1 year after percutaneous coronary intervention. A subgroup of patients in this trial who had a longer pretreatment interval with a loading clopidogrel dose showed a benefit at 28 days, which was not as evident with a shorter loading dose interval.
The CLARITY-TIMI 28 trial33,34 showed the advantage of adding clopidogrel to aspirin in patients receiving fibrinolytic therapy for ST-elevation myocardial infarction. Adding clopidogrel both improved the patency of the infarct-related artery and reduced ischemic complications. In patients who subsequently underwent percutaneous coronary intervention and stenting, clopidogrel pretreatment was associated with a significant decrease in ischemic complications before and after the procedure. There was no significant increase in bleeding complications in either group.
COMMIT/CCS 235 also showed the benefit of dual antiplatelet therapy in patients with ST-elevation myocardial infarction. Clopidogrel added to aspirin during the short-term in-hospital or postdischarge treatment period significantly reduced a composite end point of reinfarction, death, or stroke as well as death from any cause.
The CHARISMA trial36–38 aimed to determine if patients who were more stable (ie, no recent acute coronary syndrome event or percutaneous coronary intervention) would benefit. Overall, CHARISMA showed no benefit of adding clopidogrel to aspirin compared with aspirin alone in a broad population of patients with established vascular disease (secondary prevention) or risk factors for vascular disease (primary prevention).
But importantly, though no benefit was seen in the primary prevention group, the large subgroup of patients with established atherosclerotic vascular disease (12,153 of the 15,603 patients in the trial) did benefit from dual antiplatelet therapy.36,37 This subgroup showed an overall reduction in absolute risk of 1.5% (relative risk 0.88, P = .046) over a median follow-up of 27.6 months. This benefit was even more apparent in the 9,478 patients with prior myocardial infarction, stroke, or peripheral artery disease, for whom the relative risk reduction was 17.1% (P = .01) and the reduction in absolute risk 1.5%.38
These results are comparable to the 2% absolute risk reduction in the CURE trial for similar end points over 9 months. In both studies, there was no significant increase in the risk of major bleeding or intracranial bleeding in the clopidogrel-plus-aspirin groups, although minor bleeding was increased by dual antiplatelet therapy.
The rate of severe bleeding, which was the primary safety end point in CHARISMA, was not significantly different in the clopidogrel-plus-aspirin group compared with the placebo-plus-aspirin group (relative risk 1.25, 95% CI 0.97–1.61, P = .09).
Thus, although the CHARISMA findings were negative overall, the positive finding observed in the predominant subgroup of patients with established vascular disease can therefore be considered supportive of the results of the subsequent trials discussed below.
The PEGASUS-TIMI 54 trial39 studied the benefit of adding ticagrelor (60 or 90 mg) to low-dose aspirin in patients with stable coronary artery disease who had had a myocardial infarction 1 to 3 years earlier.
Confirming the results of the CHARISMA subgroup analysis, the incidence of the ischemic primary efficacy end point (a composite of cardiovascular death, myocardial infarction, and stroke) was significantly lower in both groups receiving ticagrelor plus aspirin compared with those receiving placebo plus aspirin. The Kaplan-Meier rate at 3 years for the ticagrelor 90 mg-plus-aspirin group was 7.85% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.85, 95% confidence interval [CI] 0.75–0.96, P = .008). The rate for the ticagrelor 60 mg-plus-aspirin group was 7.77% vs 9.04% for the placebo-plus-aspirin group (hazard ratio 0.84, 95% CI 0.74–0.95, P = .004).
The rates of all TIMI major and minor bleeding, as well as bleeding requiring transfusion or discontinuation of the study drug, were significantly higher in both ticagrelor dosing groups than in the placebo group (P < .01 for both groups vs placebo). The rates of fatal bleeding and nonfatal intracranial hemorrhage were not significantly higher. Although there was an overall reduction in ischemic end points with the addition of ticagrelor, there was also a significantly higher incidence of bleeding in this group.
Comment. Thus, with or without percutaneous coronary intervention in acute coronary syndrome as well as in stable coronary artery disease, dual antiplatelet therapy was shown to improve outcomes and decrease ischemic complications compared with aspirin alone. It provided benefit in the setting of acute coronary syndrome (in the CURE trial) and percutaneous coronary intervention (in the CREDO trial) for up to 1 year.
Major questions remained to be addressed:
- Do the results of CREDO, which was performed before the current interventional era and the use of drug-eluting stents, reflect outcomes after current interventional practice?
- Could shorter periods of dual antiplatelet therapy be sufficient, especially with newer stents with less risk of late thrombosis?
- Does the benefit of dual antiplatelet therapy extend beyond the 1-year time period tested in those trials to date?
RECOMMENDATIONS FOR DOSING
The American College of Cardiology Foundation/American Heart Association guidelines for dosing of antiplatelet agents for non-ST-elevation myocardial infarction are summarized in Table 2, and those for ST-elevation myocardial infarction are summarized in Table 3.1,2
WOULD SHORTER THERAPY AFTER STENTING WORK AS WELL?
The American College of Cardiology Foundation/American Heart Association currently recommend dual antiplatelet therapy for at least 12 months after drug-eluting stent placement, with shorter courses appropriate for patients who develop excessive bleeding complications or who are at high risk of bleeding.
Four trials (Table 4) evaluated whether shorter durations of dual antiplatelet therapy would suffice: SECURITY,40 EXCELLENT,41 OPTIMIZE,42 and RESET.43 All of them showed that short-duration therapy was not inferior to standard-duration therapy.44 These studies were comparable in that:
- Patients were randomized at the time of percutaneous coronary intervention or within 24 hours of it.
- Most patients received a second-generation drug-eluting stent, with the following exceptions: in EXCELLENT,41 one-fourth of patients received a Cypher first-generation drug-eluting stent, and in RESET,43 approximately one-fourth of the patients received a sirolimus-eluting stent in the standard-duration group for short lesions. Those patients with longer lesions in the RESET standard-duration group received an everolimus drug-eluting stent.
- The second antiplatelet added to aspirin in all studies was clopidogrel, with the exception of the SECURITY trial, in which fewer than 2% of patients received ticagrelor or prasugrel.40
- All the trials except RESET excluded patients who had had a myocardial infarction within 72 hours, and thus most patients studied had a lower risk profile.
- All of the trials sought to study noninferiority of short- vs standard-duration dual antiplatelet therapy, defined as the occurrence of a primary end point at 1 year (a composite of cardiovascular death, myocardial infarction, stroke, stent thrombosis, target vessel failure or revascularization, or bleeding).
Their low-risk patient populations and infrequent end points rendered these studies underpowered to make definitive conclusions about the relative efficacy of 6-months vs 12-months of dual antiplatelet therapy.
WOULD LONGER THERAPY BE BETTER?
The PRODIGY trial45 assessed durations of dual antiplatelet therapy both shorter and longer than the conventional 1 year, randomizing patients undergoing placement of a bare-metal stent, first-generation drug-eluting stent, or second-generation drug-eluting stent to receive aspirin and clopidogrel for either 6 months or 24 months. The study showed no significant difference in primary outcomes in the short- or long-duration groups.
Other trials that compared the standard 12 months of dual antiplatelet therapy with extended duration beyond 12 months were DAPT,3 ARCTIC-Interruption,46 and DES-LATE.47 The trials were comparable in that:
- All patients were randomized after completing 12 months of dual antiplatelet therapy following drug-eluting stent placement.
- All patients who were included had been free of major cardiac ischemic events or bleeding during the 12 months following stent placement.
- The primary aim of all three studies was to compare primary end points in groups receiving aspirin alone vs extended dual antiplatelet therapy. The primary end point was a composite of death due to a cardiovascular cause, nonfatal myocardial infarction, stroke, or stent thrombosis.
- The principal safety end point was bleeding.
Although the two earlier studies (ARCTIC-Interruption and DES-LATE) did not show any benefit of extended dual antiplatelet therapy compared with the standard 12-month duration, the recent DAPT study did.
The DAPT study
The DAPT study3 was an international, multicenter, placebo-controlled, double-blind randomized trial designed to examine the benefit of dual antiplatelet therapy beyond 1 year in a patient population large enough to provide definitive assessment of benefit and risk.
A total of 9,961 patients who received drug-eluting stents were randomized after 12 months of dual antiplatelet therapy to receive either a thienopyridine (clopidogrel or prasugrel) plus aspirin or placebo plus aspirin. They were followed for an additional 18 months. The coprimary efficacy end points were stent thrombosis and a composite of death, myocardial infarction, or stroke, while the primary safety end point was moderate or severe bleeding. The patients were also observed from months 30 to 33 on aspirin alone after stopping the thienopyridine.
Results. Longer therapy substantially reduced the risks of stent thrombosis (hazard ratio [HR] 0.29, 95% confidence interval [CI] 0.17–0.48) and the composite ischemic end point (HR 0.71, 95% CI 0.59–0.85). Follow-up during the 3-month thienopyridine discontinuation phase starting at 30 months revealed convergence of the ischemic event-rate curves in the two groups, which suggested that continuing dual antiplatelet therapy beyond 30 months might have been beneficial. Myocardial infarction unrelated to stent thrombosis accounted for 55% of the treatment benefit of dual antiplatelet therapy.
The risk of bleeding was higher in the thienopyridine group during the treatment period (2.5% vs 1.6%, P = .001). There was also a higher rate of noncardiovascular mortality in the thienopyridine group, although this difference may have been due to chance.3,48
Why were the results different?
All three trials included first- and second-generation drug-eluting stents, with different proportions in different trials. In ARCTIC-Interruption,46 43% of the patients in the continuation group had a first-generation stent, as did 64% of the patients in the dual antiplatelet group of DES-LATE.47 In the DAPT trial,3 38% of the patients in the longer-duration arm had a first-generation stent, and in 26% of cases it was a paclitaxel-eluting stent.
Only clopidogrel was used as the second antiplatelet agent in DES-LATE, whereas prasugrel was used in 10% of patients in ARCTIC-Interruption and 35% in DAPT.
Yet none of these differences seem to explain the differences in outcome among the studies. ARCTIC-Interruption and DES-LATE did not show any benefit of continued dual antiplatelet therapy beyond 12 months. DAPT showed benefit of extended therapy with prasugrel or with clopidogrel, and with first-generation or second-generation drug-eluting stents. The most likely explanation for the different results was that DAPT was the only trial sufficiently powered to definitively assess the end points, including stent thrombosis.
A balance between ischemic efficacy and bleeding risk is the major consideration with any antithrombotic and antiplatelet therapy. In the three largest trials we discussed (the vascular disease subgroups of CHARISMA,38 PEGASUS,39 and DAPT3), comparison of the prespecified efficacy and safety end points of each trial suggests that dual antiplatelet therapy has a net benefit, particularly given the irreversible nature of ischemic end points.
In CHARISMA,38 60 cardiovascular deaths, myocardial infarctions, or strokes were prevented per year per 10,000 patients treated, at the cost of 28 excess moderate bleeding events.
In PEGASUS,39 42 cardiovascular deaths, myocardial infarctions, or strokes were prevented, at the cost of 79 excess bleeding events requiring transfusion.
In DAPT (a selected population who had tolerated dual antiplatelet therapy for 1 year), 106 deaths, myocardial infarctions, or stroke events were prevented, at the cost of 47 excess moderate bleeding events.3
Indirect comparisons between trials are problematic, given different end point definitions, populations, and background therapies. But their results suggest that less-intensive inhibition with clopidogrel as the second antiplatelet long-term (as in CHARISMA) may provide the best balance of benefit vs risk.
BALANCING RISK AND BENEFIT
The evidence is unequivocal that dual antiplatelet therapy suppresses coronary ischemic complications resulting from thrombosis at sites of spontaneous plaque rupture following acute coronary syndromes or mechanical plaque disruption and foreign body implantation associated with percutaneous coronary intervention.
Three large-scale trials (DAPT,3 PEGASUS,39 and the secondary prevention subgroup of CHARISMA38) showed that the protective effect of dual antiplatelet therapy continues with prolonged therapy in patients who have experienced an acute coronary syndrome event or have received a drug-eluting stent. That benefit seems to be due to the action of these therapies on the culprit vessel (the one that caused the acute coronary syndrome or the site of stenting), as well as nonculprit arteries, emphasizing that dual antiplatelet therapy protects against atherosclerosis progression and future plaque rupture events.
For the durations studied in the longest trials thus far, 30 months (DAPT3) and 36 months (PEGASUS39), event curves continue to diverge, indicating that the advantage of dual antiplatelet therapy may persist for an indefinite period of time. Thus, indefinite therapy with dual antiplatelet agents can be supported, particularly in patients with advanced coronary artery disease or those who have had multiple coronary events.
We believe that the balance of evidence suggests that smaller studies that failed to show a benefit of longer-term therapy were underpowered to do so.
The ischemic protection is associated with the adverse effect of increased bleeding risk. Unfortunately, there has been little success in guiding dual antiplatelet therapy based on ischemic vs bleeding risk, in part because the same factors that predict risk of ischemic complications seem to predict increased susceptibility to bleeding. Nevertheless, indirect comparisons between studies suggest that for longer-term therapy clopidogrel may be superior to ticagrelor or prasugrel: the absolute excess bleeding risk with dual antiplatelet therapy vs aspirin in the CHARISMA secondary prevention subgroup was less than that in PEGASUS, with similar absolute reductions in ischemic events. So while the TRITON-TIMI 3822 and PLATO23 trials support the superiority of prasugrel or ticagrelor over clopidogrel for the first year after acute coronary syndrome, subsequent years of therapy may best be provided with clopidogrel.
Some patients may have identifiable factors that place them at very high risk of bleeding—need for surgical procedures, need for anticoagulation, or occurrence of bleeding complications or excessive “nuisance bleeding.” In those patients, the data suggest that dual antiplatelet therapy could be discontinued after 6 months, or perhaps even 3 months in the highest bleeding risk circumstances after second-generation drug-eluting stent placement.
WOEST49 was an open-label randomized controlled trial that studied the safety of antiplatelet regimens in patients on anticoagulation requiring percutaneous coronary interventions. Patients were randomized to double therapy with anticoagulant and clopidogrel vs triple therapy with additional aspirin following percutaneous coronary intervention. The primary end point was bleeding events within 1 year. Clopidogrel without aspirin was associated with significantly fewer bleeding events compared with triple therapy, with no increase in adverse ischemic events. The strategy tested in the WOEST trial seems reasonable in the specific group of patients who require ongoing anticoagulant therapy after drug-eluting stent placement, recognizing that the trial was somewhat underpowered to make definitive conclusions, particularly in patients at high risk for stent thrombosis.
Based on the results of PEGASUS and the CHARISMA subgroup with established ischemic burden, in which dual antiplatelet therapy was started after an interruption following the index coronary event, it is also reasonable to restart long-term dual antiplatelet therapy in patients who require interruption for short-term indications such as a surgical procedure.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426.
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–2166.
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010; 74:597–607.
- Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc 2013; 53:81–96.
- Showkathali R, Natarajan A. Antiplatelet and antithrombin strategies in acute coronary syndrome: state-of-the-art review. Curr Cardiol Rev 2012; 8:239–249.
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72:2087–2116.
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014; 7:1081–1092.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48:193–202.
- Nikam N, Steinberg TB, Steinberg DH. Advances in stent technologies and their effect on clinical efficacy and safety. Med Devices (Auckl) 2014; 7:165–178.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol 2014; 30:35–45.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015; 36:3320–3331.
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009; 53:1399–1409.
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol 2013; 112:737–745.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015; 12:30–47.
- Patrono C, Rocca B. The future of antiplatelet therapy in cardiovascular disease. Annu Rev Med 2010; 61:49–61.
- Park SJ, Kang SM, Park DW. Dual antiplatelet therapy after drug-eluting stents: defining the proper duration. Coron Artery Dis 2014; 25:83–89.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Nusca A, Patti G. Platelet function and inhibition in ischemic heart disease. Curr Cardiol Rep 2012; 14:457–467.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Genereux P, Stone GW, Harrington RA, et al; CHAMPION PHOENIX Investigators. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: Insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol 2014; 63:619–629.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Warren J, Baber U, Mehran R. Antiplatelet therapy after drug-eluting stent implantation. J Cardiol 2015; 65:98–104.
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998; 98:2126–2132.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358:527–533.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002; 13(suppl 1):17–21.
- Ferguson JJ. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy—CLARITY-TIMI 28. Future Cardiol 2005; 1:605–610.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791–1800.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64:2086–2097.
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125:505–513.
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510–2522.
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340–1348.
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014; 114:236–242.
- Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012; 125:2015–2026.
- Collet JP, Silvain J, Barthelemy O, et al; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384:1577–1585.
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014; 129:304–312.
- Kwok CS, Bulluck H, Ryding AD, Loke YK. Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. ScientificWorldJournal 2014; 2014:794078.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
- American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61:e78–e140.
- Amsterdam EA, Wenger NK, Brindis RG, et al; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426.
- Mauri L, Kereiakes DJ, Yeh RW, et al; DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371:2155–2166.
- Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J 2010; 74:597–607.
- Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc 2013; 53:81–96.
- Showkathali R, Natarajan A. Antiplatelet and antithrombin strategies in acute coronary syndrome: state-of-the-art review. Curr Cardiol Rev 2012; 8:239–249.
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012; 72:2087–2116.
- Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014; 7:1081–1092.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med 1998; 339:1665–1671.
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006; 48:193–202.
- Nikam N, Steinberg TB, Steinberg DH. Advances in stent technologies and their effect on clinical efficacy and safety. Med Devices (Auckl) 2014; 7:165–178.
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O’Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol 2014; 30:35–45.
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015; 36:3320–3331.
- van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol 2009; 53:1399–1409.
- Berger JS. Aspirin, clopidogrel, and ticagrelor in acute coronary syndromes. Am J Cardiol 2013; 112:737–745.
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015; 12:30–47.
- Patrono C, Rocca B. The future of antiplatelet therapy in cardiovascular disease. Annu Rev Med 2010; 61:49–61.
- Park SJ, Kang SM, Park DW. Dual antiplatelet therapy after drug-eluting stents: defining the proper duration. Coron Artery Dis 2014; 25:83–89.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Nusca A, Patti G. Platelet function and inhibition in ischemic heart disease. Curr Cardiol Rep 2012; 14:457–467.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Genereux P, Stone GW, Harrington RA, et al; CHAMPION PHOENIX Investigators. Impact of intraprocedural stent thrombosis during percutaneous coronary intervention: Insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects Who Require Percutaneous Coronary Intervention). J Am Coll Cardiol 2014; 63:619–629.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Brilakis ES, Patel VG, Banerjee S. Medical management after coronary stent implantation: a review. JAMA 2013; 310:189–198.
- Warren J, Baber U, Mehran R. Antiplatelet therapy after drug-eluting stent implantation. J Cardiol 2015; 65:98–104.
- Urban P, Macaya C, Rupprecht HJ, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the Multicenter Aspirin and Ticlopidine Trial After Intracoronary Stenting (MATTIS). Circulation 1998; 98:2126–2132.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Mehta SR, Yusuf S, Peters RJ, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001; 358:527–533.
- Morais J. Insights from CURE: using clopidogrel on top of standard therapy. Cerebrovasc Dis 2002; 13(suppl 1):17–21.
- Ferguson JJ. Clopidogrel plus aspirin in patients with acute myocardial infarction treated with fibrinolytic therapy—CLARITY-TIMI 28. Future Cardiol 2005; 1:605–610.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al; CHARISMA Investigators. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Bonaca MP, Bhatt DL, Cohen M, et al; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372:1791–1800.
- Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014; 64:2086–2097.
- Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012; 125:505–513.
- Feres F, Costa RA, Abizaid A, et al; OPTIMIZE Trial Investigators. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013; 310:2510–2522.
- Kim BK, Hong MK, Shin DH, et al; RESET Investigators. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012; 60:1340–1348.
- El-Hayek G, Messerli F, Bangalore S, et al. Meta-analysis of randomized clinical trials comparing short-term versus long-term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 2014; 114:236–242.
- Valgimigli M, Campo G, Monti M, et al; Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012; 125:2015–2026.
- Collet JP, Silvain J, Barthelemy O, et al; ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384:1577–1585.
- Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014; 129:304–312.
- Kwok CS, Bulluck H, Ryding AD, Loke YK. Benefits and harms of extending the duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stents: a meta-analysis. ScientificWorldJournal 2014; 2014:794078.
- Dewilde WJ, Oirbans T, Verheugt FW, et al; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013; 381:1107–1115.
KEY POINTS
- The outcomes of patients with acute coronary syndrome events have been improving as percutaneous coronary intervention and its accompanying medical therapy have evolved.
- Newer, more potent antiplatelet agents are preferred over clopidogrel when possible.
- Two earlier studies showed no advantage of extended dual antiplatelet therapy over the standard 12-month duration, but the recent Dual Antiplatelet Therapy trial did.
- The protection against ischemia afforded by dual antiplatelet therapy comes at the price of increased risk of bleeding.
Should patients with stable ischemic heart disease undergo revascularization?
The answer is less clear for these patients than for patients with acute coronary syndromes. In the latter group, percutaneous or surgical revascularization reduces the rates of morbidity and mortality, whereas in patients with stable ischemic heart disease, benefits may be limited to the improvement of angina. Certain markers and criteria may help us in this decision, and trials are ongoing.
Of importance, all patients with coronary artery disease should receive guideline-directed medical therapy as tolerated, regardless of whether they undergo revascularization.
MEDICAL THERAPY FOR ALL
In all the relevant trials, patients with stable ischemic heart disease in both the revascularization groups and the unrevascularized groups received guideline-directed medical therapy. Current guidelines1 give class I recommendations (ie, treatment should be given) for:
- Lipid management
- Blood pressure management
- Physical activity
- Weight management
- Smoking cessation
- Antiplatelet therapy
- Beta-blockers for patients with normal left ventricular function after an acute coronary syndrome event, and for those with an ejection fraction of 40% or less
- Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients who have hypertension, diabetes mellitus, a left ventricular ejection fraction of 40% or less, or chronic kidney disease
- Annual influenza vaccination
- Anti-ischemic medications (beta-blockers, calcium channel blockers, nitrates) for relief of symptoms.
REVASCULARIZATION FOR SOME?
Results of the studies outlined below will help in deciding when to use guideline-directed medical therapy alone or medical therapy plus revascularization.
COURAGE trial: No added benefit in patients at low risk
The findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE), published in 2007, suggested that in select patients, percutaneous coronary intervention for stable coronary artery disease was no better than guideline-directed medical therapy alone for reducing the outcomes of death, myocardial infarction, or hospitalization for acute coronary syndrome.2
Of note, however, is that the 2,287 patients included in COURAGE were a low-risk subset of the more than 35,000 patients initially evaluated. The investigators reviewed the patients’ coronary angiograms before enrollment, and thus many patients with complex or high-risk anatomy were likely excluded based on an a priori assessment of angiographic images.
Also, coronary stent technology has substantially improved since COURAGE (which primarily used bare-metal stents and early drug-eluting stents), and this brings into question whether the results are applicable to current patients.
Moreover, in subsequent substudies from COURAGE, revascularization significantly improved symptoms of angina and quality-of-life scores compared with medical therapy alone.3,4
Also important is that more than one-third of the patients in the medical therapy group crossed over to revascularization during the study, most often for worsening symptoms of angina.
Regardless of its limitations, COURAGE played an important role in delineating the use of guideline-directed medical therapy alone in certain low-risk patients and sparked debate about when and if to revascularize other patients.
BARI 2D trial: CABG may benefit those with diabetes
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, published in 2009, aimed to find out if revascularization in patients with stable ischemic heart disease and diabetes was beneficial compared with medical therapy alone.5
While it was not designed to directly compare percutaneous coronary intervention vs coronary artery bypass grafting (CABG), it did find that medical therapy plus CABG might reduce the rate of adverse cardiovascular events in this population compared with medical therapy alone or medical therapy plus percutaneous intervention.
As with COURAGE, however, the patients in the medical therapy group in BARI 2D also had a high rate of crossover to revascularization, primarily driven by worsening anginal symptoms.
FREEDOM and the 2014 updated guideline
Based on the findings of BARI 2D and those of FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease),6 the American College of Cardiology and American Heart Association updated their recommendations in 2014.7 This focused update states that for patients with diabetes and multivessel coronary artery disease, if revascularization is likely to improve survival (for example, in three-vessel disease or complex two-vessel disease involving the proximal left anterior descending artery), then CABG should be performed if a left internal mammary artery graft can be anastomosed to the left anterior descending artery. Otherwise, percutaneous coronary intervention should be reserved for those patients with diabetes and high-risk or complex multivessel coronary artery disease who are not good surgical candidates.
FAME 2 trial: Fractional flow reserve as a guide
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial,8 published in 2012, evaluated whether clinical outcomes differ between patients who undergo percutaneous revascularization plus medical therapy and those who are treated with medical therapy alone, using fractional flow reserve as a means to determine which stenoses should be considered for intervention. Fractional flow reserve performed during invasive angiography determines the ratio of intracoronary pressure to aortic pressure using a wire advanced across a coronary obstruction.
FAME 2 found a markedly lower incidence of the primary composite end point of death, myocardial infarction, and urgent revascularization with randomization to percutaneous revascularization plus medical therapy compared with medical therapy only (4.3% vs 12.7%, P = .001) in patients with a fractional flow reserve less than 0.80 (considered a hemodynamically significant obstruction). The trial was stopped early because of the markedly different outcomes.
Of note, however, the reduction in adverse clinical outcomes was driven primarily by a reduction in urgent revascularizations in those treated with percutaneous coronary intervention in the revascularization arm. Regardless, using fractional flow reserve to guide whether obstructive coronary lesions should be treated with percutaneous coronary intervention has appropriately become a mainstay in interventional cardiology.
Stress testing
Noninvasive stress testing has played a role in helping to guide revascularization decisions in stable ischemic heart disease. In particular, revascularization in the setting of greater than 10% ischemia on perfusion imaging has been associated with a lower risk of cardiac death than in those who were revascularized with an ischemic burden less than 10%.9
A substudy of COURAGE found that percutaneous coronary intervention reduced ischemia to a greater degree than medical therapy alone on serial nuclear stress tests in patients with stable ischemic heart disease.10 In this substudy, when both groups were combined, the investigators also found that there were fewer adverse events in those who had an overall reduction of ischemia regardless of treatment strategy.
ISCHEMIA: Revascularize those with ischemia?
While COURAGE, BARI 2D, and FAME 2 suggested that early revascularization for low-risk patients with coronary artery disease does not confer a benefit over medical treatment alone with regard to hard clinical end points, it remains unclear whether an early revascularization strategy is advantageous in patients with stable ischemic heart disease who have at least a moderate amount of ischemia on noninvasive stress testing.
The ongoing ISCHEMIA (International Study of Comparative Effectiveness With Medical and Invasive Approaches) trial will help to answer that question. In this study, 8,000 patients with stable angina and at least moderate ischemia on noninvasive stress testing are being randomized before coronary angiography either to guideline-directed medical therapy plus revascularization (percutaneous or surgical) or to medical therapy alone.11 The ISCHEMIA study population reflects current practice more closely than the previous studies discussed above in its inclusion of fractional flow reserve and later-generation drug-eluting stents.
The results of ISCHEMIA will be an important piece of the puzzle to answer whether patients with stable ischemic heart disease benefit from revascularization in terms of cardiovascular mortality or myocardial infarction (the primary end point of the study).
Studies in additional subsets
It is important to recognize that there are additional subsets of patients with stable ischemic heart disease (those with multivessel disease, left main coronary disease, or low ejection fractions, for example) who have been studied to help determine when and how to perform revascularization. In addition, there are guidelines12 for both interventional cardiologists and cardiac surgeons that help delineate which patients should undergo revascularization. While a complete review is beyond the scope of this discussion, three trials are worth mentioning:
The Coronary Artery Surgery Study (CASS)13 revealed that revascularization in left main coronary artery disease is associated with lower mortality rates than medical therapy alone. This study, along with others, eventually led to recommendations for revascularization to be performed in all patients with significant left main coronary disease, regardless of symptoms or stress test findings.14,15
The Surgical Treatment for Ischemic Heart Failure (STICH) trial16 found that patients with a low ejection fraction (< 35%) and ischemic heart disease had no difference in all-cause mortality rates when treated with CABG plus medical therapy compared with medical therapy alone (although the study’s design has been heavily criticized).
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) study17 found that CABG was associated with fewer adverse events in three-vessel coronary artery disease or complex left main coronary artery disease compared with percutaneous coronary intervention. The study used early-generation paclitaxel drug-eluting stents that are no longer used in contemporary practice. This study established the SYNTAX score, which is often used to help make revascularization decisions. A low SYNTAX score of 0 to 22 (meaning less-severe coronary artery disease) was associated with equivalent outcomes for both percutaneous coronary intervention and CABG. Thus, even if there is multivessel disease or left main disease, if the SYNTAX score is low, then percutaneous coronary intervention is an acceptable method for revascularization with similar results as for CABG.
A TEAM APPROACH
Due to the complexity of stable ischemic heart disease and the subtleties of managing these patients, a multidisciplinary “heart team” approach may be the best way to navigate treating stable ischemic heart disease via revascularization or with medical therapy alone. The heart team approach could take advantage of the particular expertise that the primary care physician, cardiologist, interventional cardiologist, and cardiac surgeon provide.
The upcoming results of studies such as the ISCHEMIA trial will help to provide additional guidance for these teams in long-term management of patients with stable ischemic heart disease.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012; 126:e354–e471.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Blankenship J, Marshall JJ, Pinto DS, et al; Society for Cardiovascular Angiography and Interventions. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81:243–249.
- BARI 2D Study Group; Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Farkouh ME, Domanski M, Sleep LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:1929–1949.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998; 97:535–543.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Stone GW, Hochman JS, Williams DO, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol 2016; 67:81–99.
- Patel M, Dehmer G, Hirshfeld J, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol 2012; 59:857–881.
- Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990; 82:1629–1646.
- Hillis L, Smith P, Anderson J, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine G, Bates E, Blankenship J, et al. 2011 ACCF/AHA guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Velazquez EJ, Lee KL, Deja MA, et al, for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364:1607–1616.
- Serruys PW, Morice M-C, Kappetein AP, et al, for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
The answer is less clear for these patients than for patients with acute coronary syndromes. In the latter group, percutaneous or surgical revascularization reduces the rates of morbidity and mortality, whereas in patients with stable ischemic heart disease, benefits may be limited to the improvement of angina. Certain markers and criteria may help us in this decision, and trials are ongoing.
Of importance, all patients with coronary artery disease should receive guideline-directed medical therapy as tolerated, regardless of whether they undergo revascularization.
MEDICAL THERAPY FOR ALL
In all the relevant trials, patients with stable ischemic heart disease in both the revascularization groups and the unrevascularized groups received guideline-directed medical therapy. Current guidelines1 give class I recommendations (ie, treatment should be given) for:
- Lipid management
- Blood pressure management
- Physical activity
- Weight management
- Smoking cessation
- Antiplatelet therapy
- Beta-blockers for patients with normal left ventricular function after an acute coronary syndrome event, and for those with an ejection fraction of 40% or less
- Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients who have hypertension, diabetes mellitus, a left ventricular ejection fraction of 40% or less, or chronic kidney disease
- Annual influenza vaccination
- Anti-ischemic medications (beta-blockers, calcium channel blockers, nitrates) for relief of symptoms.
REVASCULARIZATION FOR SOME?
Results of the studies outlined below will help in deciding when to use guideline-directed medical therapy alone or medical therapy plus revascularization.
COURAGE trial: No added benefit in patients at low risk
The findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE), published in 2007, suggested that in select patients, percutaneous coronary intervention for stable coronary artery disease was no better than guideline-directed medical therapy alone for reducing the outcomes of death, myocardial infarction, or hospitalization for acute coronary syndrome.2
Of note, however, is that the 2,287 patients included in COURAGE were a low-risk subset of the more than 35,000 patients initially evaluated. The investigators reviewed the patients’ coronary angiograms before enrollment, and thus many patients with complex or high-risk anatomy were likely excluded based on an a priori assessment of angiographic images.
Also, coronary stent technology has substantially improved since COURAGE (which primarily used bare-metal stents and early drug-eluting stents), and this brings into question whether the results are applicable to current patients.
Moreover, in subsequent substudies from COURAGE, revascularization significantly improved symptoms of angina and quality-of-life scores compared with medical therapy alone.3,4
Also important is that more than one-third of the patients in the medical therapy group crossed over to revascularization during the study, most often for worsening symptoms of angina.
Regardless of its limitations, COURAGE played an important role in delineating the use of guideline-directed medical therapy alone in certain low-risk patients and sparked debate about when and if to revascularize other patients.
BARI 2D trial: CABG may benefit those with diabetes
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, published in 2009, aimed to find out if revascularization in patients with stable ischemic heart disease and diabetes was beneficial compared with medical therapy alone.5
While it was not designed to directly compare percutaneous coronary intervention vs coronary artery bypass grafting (CABG), it did find that medical therapy plus CABG might reduce the rate of adverse cardiovascular events in this population compared with medical therapy alone or medical therapy plus percutaneous intervention.
As with COURAGE, however, the patients in the medical therapy group in BARI 2D also had a high rate of crossover to revascularization, primarily driven by worsening anginal symptoms.
FREEDOM and the 2014 updated guideline
Based on the findings of BARI 2D and those of FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease),6 the American College of Cardiology and American Heart Association updated their recommendations in 2014.7 This focused update states that for patients with diabetes and multivessel coronary artery disease, if revascularization is likely to improve survival (for example, in three-vessel disease or complex two-vessel disease involving the proximal left anterior descending artery), then CABG should be performed if a left internal mammary artery graft can be anastomosed to the left anterior descending artery. Otherwise, percutaneous coronary intervention should be reserved for those patients with diabetes and high-risk or complex multivessel coronary artery disease who are not good surgical candidates.
FAME 2 trial: Fractional flow reserve as a guide
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial,8 published in 2012, evaluated whether clinical outcomes differ between patients who undergo percutaneous revascularization plus medical therapy and those who are treated with medical therapy alone, using fractional flow reserve as a means to determine which stenoses should be considered for intervention. Fractional flow reserve performed during invasive angiography determines the ratio of intracoronary pressure to aortic pressure using a wire advanced across a coronary obstruction.
FAME 2 found a markedly lower incidence of the primary composite end point of death, myocardial infarction, and urgent revascularization with randomization to percutaneous revascularization plus medical therapy compared with medical therapy only (4.3% vs 12.7%, P = .001) in patients with a fractional flow reserve less than 0.80 (considered a hemodynamically significant obstruction). The trial was stopped early because of the markedly different outcomes.
Of note, however, the reduction in adverse clinical outcomes was driven primarily by a reduction in urgent revascularizations in those treated with percutaneous coronary intervention in the revascularization arm. Regardless, using fractional flow reserve to guide whether obstructive coronary lesions should be treated with percutaneous coronary intervention has appropriately become a mainstay in interventional cardiology.
Stress testing
Noninvasive stress testing has played a role in helping to guide revascularization decisions in stable ischemic heart disease. In particular, revascularization in the setting of greater than 10% ischemia on perfusion imaging has been associated with a lower risk of cardiac death than in those who were revascularized with an ischemic burden less than 10%.9
A substudy of COURAGE found that percutaneous coronary intervention reduced ischemia to a greater degree than medical therapy alone on serial nuclear stress tests in patients with stable ischemic heart disease.10 In this substudy, when both groups were combined, the investigators also found that there were fewer adverse events in those who had an overall reduction of ischemia regardless of treatment strategy.
ISCHEMIA: Revascularize those with ischemia?
While COURAGE, BARI 2D, and FAME 2 suggested that early revascularization for low-risk patients with coronary artery disease does not confer a benefit over medical treatment alone with regard to hard clinical end points, it remains unclear whether an early revascularization strategy is advantageous in patients with stable ischemic heart disease who have at least a moderate amount of ischemia on noninvasive stress testing.
The ongoing ISCHEMIA (International Study of Comparative Effectiveness With Medical and Invasive Approaches) trial will help to answer that question. In this study, 8,000 patients with stable angina and at least moderate ischemia on noninvasive stress testing are being randomized before coronary angiography either to guideline-directed medical therapy plus revascularization (percutaneous or surgical) or to medical therapy alone.11 The ISCHEMIA study population reflects current practice more closely than the previous studies discussed above in its inclusion of fractional flow reserve and later-generation drug-eluting stents.
The results of ISCHEMIA will be an important piece of the puzzle to answer whether patients with stable ischemic heart disease benefit from revascularization in terms of cardiovascular mortality or myocardial infarction (the primary end point of the study).
Studies in additional subsets
It is important to recognize that there are additional subsets of patients with stable ischemic heart disease (those with multivessel disease, left main coronary disease, or low ejection fractions, for example) who have been studied to help determine when and how to perform revascularization. In addition, there are guidelines12 for both interventional cardiologists and cardiac surgeons that help delineate which patients should undergo revascularization. While a complete review is beyond the scope of this discussion, three trials are worth mentioning:
The Coronary Artery Surgery Study (CASS)13 revealed that revascularization in left main coronary artery disease is associated with lower mortality rates than medical therapy alone. This study, along with others, eventually led to recommendations for revascularization to be performed in all patients with significant left main coronary disease, regardless of symptoms or stress test findings.14,15
The Surgical Treatment for Ischemic Heart Failure (STICH) trial16 found that patients with a low ejection fraction (< 35%) and ischemic heart disease had no difference in all-cause mortality rates when treated with CABG plus medical therapy compared with medical therapy alone (although the study’s design has been heavily criticized).
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) study17 found that CABG was associated with fewer adverse events in three-vessel coronary artery disease or complex left main coronary artery disease compared with percutaneous coronary intervention. The study used early-generation paclitaxel drug-eluting stents that are no longer used in contemporary practice. This study established the SYNTAX score, which is often used to help make revascularization decisions. A low SYNTAX score of 0 to 22 (meaning less-severe coronary artery disease) was associated with equivalent outcomes for both percutaneous coronary intervention and CABG. Thus, even if there is multivessel disease or left main disease, if the SYNTAX score is low, then percutaneous coronary intervention is an acceptable method for revascularization with similar results as for CABG.
A TEAM APPROACH
Due to the complexity of stable ischemic heart disease and the subtleties of managing these patients, a multidisciplinary “heart team” approach may be the best way to navigate treating stable ischemic heart disease via revascularization or with medical therapy alone. The heart team approach could take advantage of the particular expertise that the primary care physician, cardiologist, interventional cardiologist, and cardiac surgeon provide.
The upcoming results of studies such as the ISCHEMIA trial will help to provide additional guidance for these teams in long-term management of patients with stable ischemic heart disease.
The answer is less clear for these patients than for patients with acute coronary syndromes. In the latter group, percutaneous or surgical revascularization reduces the rates of morbidity and mortality, whereas in patients with stable ischemic heart disease, benefits may be limited to the improvement of angina. Certain markers and criteria may help us in this decision, and trials are ongoing.
Of importance, all patients with coronary artery disease should receive guideline-directed medical therapy as tolerated, regardless of whether they undergo revascularization.
MEDICAL THERAPY FOR ALL
In all the relevant trials, patients with stable ischemic heart disease in both the revascularization groups and the unrevascularized groups received guideline-directed medical therapy. Current guidelines1 give class I recommendations (ie, treatment should be given) for:
- Lipid management
- Blood pressure management
- Physical activity
- Weight management
- Smoking cessation
- Antiplatelet therapy
- Beta-blockers for patients with normal left ventricular function after an acute coronary syndrome event, and for those with an ejection fraction of 40% or less
- Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for patients who have hypertension, diabetes mellitus, a left ventricular ejection fraction of 40% or less, or chronic kidney disease
- Annual influenza vaccination
- Anti-ischemic medications (beta-blockers, calcium channel blockers, nitrates) for relief of symptoms.
REVASCULARIZATION FOR SOME?
Results of the studies outlined below will help in deciding when to use guideline-directed medical therapy alone or medical therapy plus revascularization.
COURAGE trial: No added benefit in patients at low risk
The findings of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE), published in 2007, suggested that in select patients, percutaneous coronary intervention for stable coronary artery disease was no better than guideline-directed medical therapy alone for reducing the outcomes of death, myocardial infarction, or hospitalization for acute coronary syndrome.2
Of note, however, is that the 2,287 patients included in COURAGE were a low-risk subset of the more than 35,000 patients initially evaluated. The investigators reviewed the patients’ coronary angiograms before enrollment, and thus many patients with complex or high-risk anatomy were likely excluded based on an a priori assessment of angiographic images.
Also, coronary stent technology has substantially improved since COURAGE (which primarily used bare-metal stents and early drug-eluting stents), and this brings into question whether the results are applicable to current patients.
Moreover, in subsequent substudies from COURAGE, revascularization significantly improved symptoms of angina and quality-of-life scores compared with medical therapy alone.3,4
Also important is that more than one-third of the patients in the medical therapy group crossed over to revascularization during the study, most often for worsening symptoms of angina.
Regardless of its limitations, COURAGE played an important role in delineating the use of guideline-directed medical therapy alone in certain low-risk patients and sparked debate about when and if to revascularize other patients.
BARI 2D trial: CABG may benefit those with diabetes
The Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, published in 2009, aimed to find out if revascularization in patients with stable ischemic heart disease and diabetes was beneficial compared with medical therapy alone.5
While it was not designed to directly compare percutaneous coronary intervention vs coronary artery bypass grafting (CABG), it did find that medical therapy plus CABG might reduce the rate of adverse cardiovascular events in this population compared with medical therapy alone or medical therapy plus percutaneous intervention.
As with COURAGE, however, the patients in the medical therapy group in BARI 2D also had a high rate of crossover to revascularization, primarily driven by worsening anginal symptoms.
FREEDOM and the 2014 updated guideline
Based on the findings of BARI 2D and those of FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease),6 the American College of Cardiology and American Heart Association updated their recommendations in 2014.7 This focused update states that for patients with diabetes and multivessel coronary artery disease, if revascularization is likely to improve survival (for example, in three-vessel disease or complex two-vessel disease involving the proximal left anterior descending artery), then CABG should be performed if a left internal mammary artery graft can be anastomosed to the left anterior descending artery. Otherwise, percutaneous coronary intervention should be reserved for those patients with diabetes and high-risk or complex multivessel coronary artery disease who are not good surgical candidates.
FAME 2 trial: Fractional flow reserve as a guide
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial,8 published in 2012, evaluated whether clinical outcomes differ between patients who undergo percutaneous revascularization plus medical therapy and those who are treated with medical therapy alone, using fractional flow reserve as a means to determine which stenoses should be considered for intervention. Fractional flow reserve performed during invasive angiography determines the ratio of intracoronary pressure to aortic pressure using a wire advanced across a coronary obstruction.
FAME 2 found a markedly lower incidence of the primary composite end point of death, myocardial infarction, and urgent revascularization with randomization to percutaneous revascularization plus medical therapy compared with medical therapy only (4.3% vs 12.7%, P = .001) in patients with a fractional flow reserve less than 0.80 (considered a hemodynamically significant obstruction). The trial was stopped early because of the markedly different outcomes.
Of note, however, the reduction in adverse clinical outcomes was driven primarily by a reduction in urgent revascularizations in those treated with percutaneous coronary intervention in the revascularization arm. Regardless, using fractional flow reserve to guide whether obstructive coronary lesions should be treated with percutaneous coronary intervention has appropriately become a mainstay in interventional cardiology.
Stress testing
Noninvasive stress testing has played a role in helping to guide revascularization decisions in stable ischemic heart disease. In particular, revascularization in the setting of greater than 10% ischemia on perfusion imaging has been associated with a lower risk of cardiac death than in those who were revascularized with an ischemic burden less than 10%.9
A substudy of COURAGE found that percutaneous coronary intervention reduced ischemia to a greater degree than medical therapy alone on serial nuclear stress tests in patients with stable ischemic heart disease.10 In this substudy, when both groups were combined, the investigators also found that there were fewer adverse events in those who had an overall reduction of ischemia regardless of treatment strategy.
ISCHEMIA: Revascularize those with ischemia?
While COURAGE, BARI 2D, and FAME 2 suggested that early revascularization for low-risk patients with coronary artery disease does not confer a benefit over medical treatment alone with regard to hard clinical end points, it remains unclear whether an early revascularization strategy is advantageous in patients with stable ischemic heart disease who have at least a moderate amount of ischemia on noninvasive stress testing.
The ongoing ISCHEMIA (International Study of Comparative Effectiveness With Medical and Invasive Approaches) trial will help to answer that question. In this study, 8,000 patients with stable angina and at least moderate ischemia on noninvasive stress testing are being randomized before coronary angiography either to guideline-directed medical therapy plus revascularization (percutaneous or surgical) or to medical therapy alone.11 The ISCHEMIA study population reflects current practice more closely than the previous studies discussed above in its inclusion of fractional flow reserve and later-generation drug-eluting stents.
The results of ISCHEMIA will be an important piece of the puzzle to answer whether patients with stable ischemic heart disease benefit from revascularization in terms of cardiovascular mortality or myocardial infarction (the primary end point of the study).
Studies in additional subsets
It is important to recognize that there are additional subsets of patients with stable ischemic heart disease (those with multivessel disease, left main coronary disease, or low ejection fractions, for example) who have been studied to help determine when and how to perform revascularization. In addition, there are guidelines12 for both interventional cardiologists and cardiac surgeons that help delineate which patients should undergo revascularization. While a complete review is beyond the scope of this discussion, three trials are worth mentioning:
The Coronary Artery Surgery Study (CASS)13 revealed that revascularization in left main coronary artery disease is associated with lower mortality rates than medical therapy alone. This study, along with others, eventually led to recommendations for revascularization to be performed in all patients with significant left main coronary disease, regardless of symptoms or stress test findings.14,15
The Surgical Treatment for Ischemic Heart Failure (STICH) trial16 found that patients with a low ejection fraction (< 35%) and ischemic heart disease had no difference in all-cause mortality rates when treated with CABG plus medical therapy compared with medical therapy alone (although the study’s design has been heavily criticized).
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) study17 found that CABG was associated with fewer adverse events in three-vessel coronary artery disease or complex left main coronary artery disease compared with percutaneous coronary intervention. The study used early-generation paclitaxel drug-eluting stents that are no longer used in contemporary practice. This study established the SYNTAX score, which is often used to help make revascularization decisions. A low SYNTAX score of 0 to 22 (meaning less-severe coronary artery disease) was associated with equivalent outcomes for both percutaneous coronary intervention and CABG. Thus, even if there is multivessel disease or left main disease, if the SYNTAX score is low, then percutaneous coronary intervention is an acceptable method for revascularization with similar results as for CABG.
A TEAM APPROACH
Due to the complexity of stable ischemic heart disease and the subtleties of managing these patients, a multidisciplinary “heart team” approach may be the best way to navigate treating stable ischemic heart disease via revascularization or with medical therapy alone. The heart team approach could take advantage of the particular expertise that the primary care physician, cardiologist, interventional cardiologist, and cardiac surgeon provide.
The upcoming results of studies such as the ISCHEMIA trial will help to provide additional guidance for these teams in long-term management of patients with stable ischemic heart disease.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012; 126:e354–e471.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Blankenship J, Marshall JJ, Pinto DS, et al; Society for Cardiovascular Angiography and Interventions. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81:243–249.
- BARI 2D Study Group; Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Farkouh ME, Domanski M, Sleep LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:1929–1949.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998; 97:535–543.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Stone GW, Hochman JS, Williams DO, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol 2016; 67:81–99.
- Patel M, Dehmer G, Hirshfeld J, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol 2012; 59:857–881.
- Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990; 82:1629–1646.
- Hillis L, Smith P, Anderson J, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine G, Bates E, Blankenship J, et al. 2011 ACCF/AHA guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Velazquez EJ, Lee KL, Deja MA, et al, for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364:1607–1616.
- Serruys PW, Morice M-C, Kappetein AP, et al, for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2012; 126:e354–e471.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007; 356:1503–1516.
- Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008; 359:677–687.
- Blankenship J, Marshall JJ, Pinto DS, et al; Society for Cardiovascular Angiography and Interventions. Effect of percutaneous coronary intervention on quality of life: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013; 81:243–249.
- BARI 2D Study Group; Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009; 360:2503–2515.
- Farkouh ME, Domanski M, Sleep LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:1929–1949.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012; 367:991–1001.
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998; 97:535–543.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008; 117:1283–1291.
- Stone GW, Hochman JS, Williams DO, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol 2016; 67:81–99.
- Patel M, Dehmer G, Hirshfeld J, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol 2012; 59:857–881.
- Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990; 82:1629–1646.
- Hillis L, Smith P, Anderson J, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011; 58:e123–e210.
- Levine G, Bates E, Blankenship J, et al. 2011 ACCF/AHA guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011; 58:e44–e122.
- Velazquez EJ, Lee KL, Deja MA, et al, for the STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364:1607–1616.
- Serruys PW, Morice M-C, Kappetein AP, et al, for the SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
Managing acute coronary syndromes: Decades of progress
Most decisions for managing acute coronary syndromes can be based on ample data from large randomized trials with hard clinical end points, so there is little reason to provide care that is not evidence-based.
This article reviews some of the trials that provide guidance on diagnosing and managing acute coronary syndromes, including the timing of reperfusion and adjunctive therapies in different situations.
MOST ACUTE CORONARY SYNDROMES ARE NON-ST-ELEVATION CONDITIONS
Acute coronary syndromes range from unstable angina and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation MI (STEMI), reflecting a continuum of severity of coronary stenosis. The degree of coronary occlusion may ultimately determine whether a patient has unstable angina or MI with or without ST elevation.1
The substrate for all of these is vulnerable plaque. Angiographic studies have indicated that in many cases medium-size plaques (30%–40% stenosis) are more likely to rupture than larger, more obstructive ones. Moderate plaques may be vulnerable because they are less mature, with a large lipid core and a thin cap prone to rupture or erode, exposing the thrombogenic subendothelial components.2
Because the vulnerability of a coronary plaque may not correlate with the severity of stenosis before the plaque ruptures, stress tests and symptoms may not predict the risk of MI. The key role of thrombosis in the pathogenesis also highlights the importance of antithrombotic therapy in the acute phases of acute coronary syndromes, which can significantly reduce mortality and morbidity rates.
Perhaps because of the widespread use of aspirin and statins, most patients who currently present with an acute coronary syndrome have either unstable angina or NSTEMI: of about 1.57 million hospital admissions in 2004 for acute coronary syndromes, for example, only 330,000 (21%) were for STEMI.3
DIAGNOSING ACUTE CORONARY SYNDROME
Symptoms may not be classic
The classic symptoms of acute coronary syndromes are intense, oppressive chest pressure radiating to the left arm, but nearly any discomfort “between the nose and navel” (eg, including the jaw, arm, and epigastric and abdominal areas) may be an acute coronary syndrome. Associated symptoms may include chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or arms, and dyspnea.
Particularly in older, female, postoperative, or diabetic patients, the presentation may be atypical or “silent,” including nausea or vomiting; breathlessness; sweating; arrhythmias; or light-headedness. Especially in these groups, symptoms may be mild or subtle, and acute coronary syndrome may manifest only as “not feeling well.”
The differential diagnosis of acute coronary syndromes is broad. Most important to immediately consider are pulmonary embolism and aortic dissection, as they are life-threatening and are treated differently from acute coronary syndromes. Otherwise, it is best to err on the side of caution and treat for an acute coronary syndrome until it is proven otherwise.
Electrocardiography is critical
Electrocardiography (ECG) gives valuable information about the location, extent, and prognosis of infarction, and it is critically important for distinguishing STEMI from NSTEMI, with ST elevation classically diagnostic of complete coronary occlusion. Q waves can occur early and do not necessarily signify completed infarction, as traditionally thought. ST depression or T inversion indicates that total coronary occlusion is unlikely unless they are in a pattern of circumflex infarct associated with an enlarging R wave in lead V1. An ST elevation in RV4 indicates right ventricular infarction.
The appearance on ECG may evolve over time, so a patient with atypical symptoms and a nonspecific electrocardiogram should be observed for 24 hours or until more specific criteria develop.
Biomarkers in NSTEMI
In MI, cardiac troponin levels begin to rise about 3 hours after the onset of chest pain, and elevations can last for up to 14 days. Levels can also be mildly elevated chronically in patients with renal dysfunction, so positive biomarker tests in that population should be interpreted cautiously.
For STEMI, the opportunity to reperfuse is lost if one waits for cardiac biomarkers to become elevated. But for NSTEMI, they are highly sensitive and specific for identifying patients at high risk and determining who should be treated aggressively. Patients who are biomarker-negative have a better prognosis than patients with identical symptoms and electrocardiograms who are biomarker-positive.
MI is currently defined as a rise in any biomarker (usually troponin) above the 99th percentile for a reference population, with at least one of the following:
- Ischemic symptoms
- New ST/T changes or left bundle branch block
- Pathologic Q waves
- Loss of myocardium or abnormal wall motion seen by imaging
- Intracoronary thrombus.
REPERFUSION FOR ACUTE STEMI
Because acute coronary syndromes have a common pathophysiology, for the most part, lessons from clinical trials in one syndrome are relevant to the others. However, important differences exist regarding the need for immediate reperfusion in STEMI, since in most cases these patients have total rather than partial occlusion.
Fibrinolysis has limitations
The standard of management for STEMI is immediate reperfusion. The goal is to interrupt the wave front of myocardial necrosis, salvage threatened myocardium, and ultimately improve survival.
Five placebo-controlled trials showed a 30% reduction in the death rate in patients who received fibrinolytic therapy within 6 to 12 hours of presentation.4
Patients with ST elevation or with new bundle branch block benefit most from fibrinolytic therapy. Those with ST depression, T inversion, or nonspecific changes on ECG do not benefit; they probably do not have complete coronary occlusion, so the prothrombotic or platelet-activating effects of fibrinolytic therapy may make them worse.5 Further, fibrinolytic therapy poses the risk of intracranial hemorrhage, which, although rare (occurring in up to 1% of cases depending on the drug regimen), is a devastating complication.
In general, absolute contraindications to fibrinolysis include intracranial abnormalities, hemorrhage, and head trauma. An important relative contraindication is uncontrolled blood pressure (> 180/110 mm Hg at any point during hospitalization, including during the immediate presentation). Studies show that even if blood pressure can be controlled, the risk of intracranial hemorrhage is substantially higher, although the risk may not outweigh the benefit of reperfusion, particularly for large infarctions when percutaneous coronary intervention (PCI) is not available as an alternative to fibrinolysis.
Prompt PCI is preferable to fibrinolysis
If PCI is available on site, there is nearly no role for fibrinolytic therapy. PCI is better than fibrinolytic therapy in terms of the degree of reperfusion, reocclusion, MI recurrence, and mortality rate, and it poses little or no risk of intracranial hemorrhage.6
For either fibrinolytic therapy or percutaneous therapy, “time is muscle”: the longer the ischemic time, the higher the mortality rate (relative risk = 1.075 for every 30 minutes of delay, P = .041).7
At centers that do not have PCI on site, studies (mainly from Europe) have shown that it is better to transport the patient for PCI than to give immediate fibrinolytic therapy.7,8 But because the centers studied tended to have short transport times (usually 40 minutes or less), it is uncertain whether the results are applicable throughout the United States.
The delay between symptom onset and presentation is also relevant. Reperfusion within the first 1 to 2 hours after the onset of symptoms provides the greatest degree of myocardial salvage and of reduction in the risk of death; the extent of benefit thereafter is substantially less. As a result, patients who present very early after symptom onset have the most to lose if their reperfusion is delayed by even a few more hours, whereas patients who have already experienced several hours of pain are affected less by additional delay.9 Thus, patients presenting within the “golden” 1 or 2 hours after symptoms begin should be considered for fibrinolytic therapy if transfer for PCI cannot be done expeditiously. It is important for hospitals without PCI available on site to have a system in place for rapid transport of patients when needed.
Guidelines advise that patients with STEMI should undergo PCI rather than receive fibrinolytic therapy as long as PCI is available within 90 minutes of first medical contact. Otherwise, fibrinolysis should be started within 30 minutes.10 For patients who present several hours after symptom onset, PCI may still be preferable even if the transport time is somewhat longer.
PCI after fibrinolytic therapy
In prior decades, PCI immediately after fibrinolytic therapy was associated with an increased risk of bleeding complications and reinfarction. That has changed with improvements in equipment and antithrombotic therapy.
Two large trials conclusively found that routinely transferring high-risk patients for PCI immediately after receiving fibrinolytic therapy (combined half-dose reteplase [Retavase] and abciximab [ReoPro]11 or full-dose tenecteplase [TNKase]12) resulted in much lower rates of ischemic end points without an increase in bleeding complications compared with transferring patients only for rescue PCI after fibrinolytic therapy.
Routine transfer is now the standard of care for high-risk patients after fibrinolytic therapy and probably is best for all patients after an MI.
MANAGING NSTEMI AND UNSTABLE ANGINA
For patients with NSTEMI, immediate reperfusion is usually not required, although initial triage for “early invasive” vs “initial conservative” management must be done early in the hospital course. Randomized trials have evaluated these two approaches, with most studies in the contemporary era reporting improved outcomes with an early invasive approach.
The TACTICS trial,13 the most important of these, enrolled more than 2,200 patients with unstable angina or NSTEMI and randomized them to an early invasive strategy or a conservative strategy. Overall, results were better with the early invasive strategy.
The ICTUS trial.14 Although several studies showed that an early invasive approach was better, the most recent study using the most modern practices—the ICTUS trial—did not find that it reduced death rates. Most patients eventually underwent angiography and revascularization, but not early on. However, all studies showed that rates of recurrent unstable angina and hospitalization were reduced by an early invasive approach, so revascularization does have a role in stabilizing the patient. But in situations of aggressive medical management with antithrombotic and other therapies, an early conservative approach may be an appropriate alternative for many patients.15
The selection of an invasive vs a conservative approach should include a consideration of risk, which can be estimated using a number of criteria, including the Thrombolysis in Myocardial Infarction (TIMI) or the GRACE risk score. When risk was stratified using the TIMI risk score,16 in the TACTICS trial, the higher the risk score, the more likely patients were to benefit from early revascularization.
When an invasive approach is chosen, it does not appear necessary to take patients to catheterization immediately (within 2–24 hours) compared with later during the hospital course.
The TIMACS trial,17 with more than 3,000 patients, tested the benefits of very early vs later revascularization for patients with NSTEMI and unstable angina. Early intervention did not significantly improve outcomes for the primary composite end point of death, MI, and stroke in the overall population enrolled in the trial, but when the secondary end point of refractory ischemia was added in, early intervention was found to be beneficial overall. Moreover, when stratified by risk, high-risk patients significantly benefited from early intervention for the primary end point.
Guidelines for NSTEMI and unstable angina continue to prefer an early invasive strategy, particularly for high-risk patients, although a conservative strategy is considered acceptable if patients receive intensive evidence-based medical therapy and remain clinically stable.18
ANTITHROMBOTIC THERAPIES
Once a revascularization strategy has been chosen, adjunctive therapies should be considered. The most important are the antithrombotic therapies.
Many drugs target platelet activity. Most important are the thromboxane inhibitor aspirin, the adenosine diphosphate (ADP) receptor antagonists clopidogrel (Plavix), prasugrel (Effient), and ticagrelor (Brilinta), and the glycoprotein (GP) IIb/IIIa antagonists abciximab and eptifibatide (Integrilin). Others, such as thrombin receptor antagonists, are under investigation.19
Aspirin for secondary prevention
Evidence is unequivocal for the benefit of aspirin therapy in patients with established or suspected vascular disease.
The ISIS-2 trial20 compared 35-day mortality rates in 16,000 patients with STEMI who were given aspirin, streptokinase, combined streptokinase and aspirin, or placebo. Mortality rates were reduced by aspirin compared with placebo by an extent similar to that achieved with streptokinase, with a further reduction when aspirin and streptokinase were given together.
Therefore, patients with STEMI should be given aspirin daily indefinitely unless they have true aspirin allergy. The dose is 165 to 325 mg initially and 75 to 162 mg daily thereafter.
For NSTEMI and even for secondary prevention in less-acute situations, a number of smaller trials also provide clear evidence of benefit from aspirin therapy.
The CURRENT-OASIS 7 trial21 showed that low maintenance dosages of aspirin (75–100 mg per day) resulted in the same incidence of ischemic end points (cardiovascular death, MI, or stroke) as higher dosages. Although rates of major bleeding events did not differ, a higher rate of gastrointestinal bleeding was evident at just 30 days in patients taking the higher doses. This large trial clearly established that there is no advantage to daily aspirin doses of more than 100 mg.
DUAL ANTIPLATELET THERAPY IS STANDARD
Standard practice now is to use aspirin plus another antiplatelet agent that acts by inhibiting either the ADP receptor (for which there is the most evidence) or the GP IIb/IIIa receptor (which is becoming less used). Dual therapy should begin early in patients with acute coronary syndrome.
Clopidogrel: Well studied with aspirin
The most commonly used ADP antagonist is clopidogrel, a thienopyridine. Much evidence exists for its benefit.
The CURE trial22 randomized more than 12,000 patients with NSTEMI or unstable angina to aspirin plus either clopidogrel or placebo. The incidence of the combined end point of MI, stroke, and cardiovascular death was 20% lower in the clopidogrel group than in the placebo group over 12 months of follow-up. The benefit of clopidogrel began to occur within the first 24 hours after randomization, with a 33% relative risk reduction in the combined end point of cardiovascular death, MI, stroke, and severe ischemia, demonstrating the importance of starting this agent early in the hospital course.
COMMIT23 found a benefit in adding clopidogrel to aspirin in patients with acute STEMI. Although it was only a 30-day trial, significant risk reduction was found in the dual-therapy group for combined death, stroke, or reinfarction. The results of this brief trial were less definitive, but the pathophysiology was similar to non-ST-elevation acute coronary syndromes, so it is reasonable to extrapolate the long-term findings to this setting.
The CURRENT-OASIS 7 trial21 randomized more than 25,000 patients to either clopidogrel in a double dosage (600 mg load, 150 mg/day for 6 days, then 75 mg/day) or standard dosage (300 mg load, 75 mg/day thereafter). Although no overall benefit was found for the higher dosage, a subgroup of more than 17,000 patients who underwent PCI after randomization had a lower risk of developing stent thrombosis. On the other hand, higher doses of clopidogrel caused more major bleeding events.
Ticagrelor and prasugrel: New alternatives to clopidogrel
The principal limitation of clopidogrel is its metabolism. It is a prodrug, ie, it is not active as taken and must be converted to its active state by cytochrome P450 enzymes in the liver. Patients who bear certain polymorphisms in the genes for these enzymes or who are taking other medications that affect this enzymatic pathway may derive less platelet inhibition from the drug, leading to considerable patient-to-patient variability in the degree of antiplatelet effect.
Alternatives to clopidogrel have been developed that inhibit platelets more intensely, are activated more rapidly, and have less interpatient variability. Available now are ticagrelor and prasugrel.24 Like clopidogrel, prasugrel is absorbed as an inactive prodrug, but it is efficiently metabolized by esterases to an active form, and then by a simpler step within the liver to its fully active metabolite.25 Ticagrelor is active as absorbed.26
Pharmacodynamically, the two drugs perform almost identically and much faster than clopidogrel, with equilibrium platelet inhibition reached in less than 1 hour. The degree of platelet inhibition is also more—sometimes twice as much—with the new drugs compared with clopidogrel, and the effect is much more consistent between patients.
Both clopidogrel and prasugrel permanently inhibit the platelet ADP receptor, and 3 to 7 days are therefore required for their antiplatelet effects to completely wear off. In contrast, ticagrelor is a reversible inhibitor and its effects wear off more rapidly. Despite achieving a much higher level of platelet inhibition than clopidogrel, ticagrelor’s activity falls below that of clopidogrel’s by 48 hours of discontinuing the drugs.
Trial of prasugrel vs clopidogrel
The TRITON-TIMI 38 trial27 enrolled more than 13,000 patients with acute coronary syndromes, randomized to receive, either prasugrel or clopidogrel, in addition to aspirin. The patients were all undergoing PCI, so the findings do not apply to patients treated medically with an early conservative approach. The study drug was given only after the decision was made to perform PCI in patients with non-ST-elevation acute coronary syndrome (but given immediately for patients with STEMI, because nearly all those patients undergo PCI).
Prasugrel was clearly beneficial, with a significant 20% lower rate of the combined end point of cardiovascular death, MI, and stroke at 15 months. However, bleeding risk was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% confidence interval 1.02–1.68, P = .03). Looking at individual end points, the advantages of prasugrel were primarily in reducing rates of stent thrombosis and nonfatal MI. Death rates with the two drugs were equivalent, possibly because of the higher risk of bleeding with prasugrel. Bleeding in the prasugrel group was particularly increased in patients who underwent bypass surgery; more patients also needed transfusion.
Subgroup analysis showed that patients with a history of stroke or transient ischemic attack had higher rates of ischemic and bleeding events with prasugrel than with clopidogrel, leading to these being labeled as absolute contraindications to prasugrel. Patients over age 75 or who weighed less than 60 kg experienced excess bleeding risk that closely matched the reduction in ischemic event rates and thus did not have a net benefit with prasugrel.
Trial of ticagrelor vs clopidogrel
The PLATO trial28 included 18,000 patients, of whom 65% underwent revascularization and 35% were treated medically. The drug—clopidogrel or ticagrelor—was given in addition to aspirin at randomization (within 24 hours of symptom onset); this more closely follows clinical practice, in which dual antiplatelet therapy is started as soon as possible. This difference makes the PLATO study more relevant to practice for patients with non-ST-elevation acute coronary syndrome. Also, because they gave the drugs to all patients regardless of whether they were to undergo PCI, this study likely had a higher-risk population, which may be refected in the higher mortality rate at 30 days (5.9% in the clopidogrel group in the PLATO study vs 3.2% in the clopidogrel group in the TRITON study).
Another important difference between the trials testing prasugrel and ticagrelor is that patients who had already received a thienopyridine were excluded from the prasugrel trial but not from the ticagrelor trial. Nearly half the patients in the ticagrelor group were already taking clopidogrel. The clinical implication is that for patients who arrive from another facility and already have been given clopidogrel, it is safe to give ticagrelor. There is limited information about whether that is also true for prasugrel, although there is no known reason why the safety of adding prasugrel to clopidogrel should be different from that of ticagrelor.
The rate of ischemic events was 20% lower in the ticagrelor group than in the clopidogrel group, importantly including reductions in the incidence of death, MI, and stent thrombosis. There was no increase with ticagrelor compared with clopidogrel in bleeding associated with coronary artery bypass graft surgery, likely because of the more rapid washout of the ticagrelor effect, or in the need for blood transfusions. However, the rate of bleeding unrelated to coronary artery bypass was about 20% higher with ticagrelor.
In summary, more intense platelet inhibition reduces the risk of ischemic events, but, particularly for the irreversible inhibitor prasugrel, at the cost of a higher risk of bleeding. In general, the net benefit of these agents in preventing the irreversible complications of MI and (in the case of ticagrelor) death favor the use of the more intense ADP inhibitors in appropriate patients. Ticagrelor is indicated in patients with acute coronary syndromes undergoing invasive or conservative management; prasugrel is indicated in patients undergoing PCI, but contraindicated in patients with a previous stroke or transient ischemic event. Neither drug is indicated in patients undergoing elective PCI outside the setting of acute coronary syndromes, although these agents may be appropriate in patients with intolerance or allergy to clopidogrel.
Glycoprotein IIb/IIIa antagonists for select cases only
GP IIb/IIIa antagonists such as abciximab were previously used more commonly than they are today. Now, with routine pretreatment using thienopyridines, their role in acute coronary syndromes is less clear. They still play a role when routine dual antiplatelet therapy is not used, when prasugrel or ticagrelor is not used, and when heparin rather than an alternative antithrombin agent is used.
A meta-analysis29 of 3,755 patients showed a clear reduction in ischemic complications with abciximab as an adjunct to primary PCI for STEMI in patients treated with heparin.
Kastrati et al30 found that patients with non-ST-elevation acute coronary syndromes benefited from abciximab at the time of PCI with heparin, even though they had been routinely pretreated with clopidogrel. However, benefits were seen only in high-risk patients who had presented with elevated troponins.
On the other hand, the role of GP IIb/IIIa blockade for “upstream” medical management in patients with acute coronary syndromes has been eroded by several studies.
The ACUITY trial31 randomized more than 9,000 patients to receive either routine treatment with a GP IIb/IIIa inhibitor before angiography or deferred selective use in the catheterization laboratory only for patients undergoing PCI. No significant differences were found in rates of MI and death.
The Early ACS trial32 compared early routine eptifibatide vs delayed, provisional eptifibatide in 9,492 patients with acute coronary syndromes without ST elevation and who were assigned to an invasive strategy. The early-eptifibatide group received two boluses and an infusion of eptifibatide before angiography; the others received a placebo infusion, with provisional eptifibatide after angiography if the patient underwent PCI and was deemed at high risk. No significant difference in rates of death or MI were noted, and the early-eptifibatide group had significantly higher rates of bleeding and need for transfusion.
The FINESSE trial33 also discredited “facilitating” PCI by giving GP IIb/IIIa antagonists in patients with STEMI before arrival in the catheterization laboratory, with no benefit to giving abciximab ahead of time vs in the catheterization laboratory, and with an increased risk of bleeding complications.
These studies have helped narrow the use of GP IIb/IIIa inhibitors to the catheterization laboratory in conjunction with heparin anticoagulation (as compared with bivalirudin [Angiomax]; see below) and only in select or high-risk cases. These drugs are indicated in the medical phase of management only if patients cannot be stabilized by aspirin or ADP inhibition.
NEWER ANTITHROMBOTICS: ADVANTAGES UNCLEAR
The complex coagulation cascade has a number of components, but only a few are targeted by drugs that are approved and recommended: fondaparinux (Arixtra) and oral factor Xa inhibitors affect the prothrombinase complex (including factor X); bivalirudin and oral factor IIa inhibitors affect thrombin; and heparin and the low-molecular-weight heparins inhibit both targets.
Low-molecular-weight heparins
The SYNERGY trial34 randomized nearly 10,000 patients with non-ST-elevation acute coronary syndromes at high risk for ischemic cardiac complications managed with an invasive approach to either the low-molecular-weight heparin enoxaparin (Lovenox) or intravenous unfractionated heparin immediately after enrollment. Most patients underwent catheterization and revascularization. No clinical advantage was found for enoxaparin, and bleeding complications were increased.
The EXTRACT-TIMI 25 trial35 randomized more than 20,000 patients with STEMI who were about to undergo fibrinolysis to receive either enoxaparin throughout hospitalization (average of 8 days) or unfractionated heparin for at least 48 hours. The enoxaparin group had a lower rate of recurrent MI, but it was unclear if the difference was in part attributable to the longer therapy time. The enoxaparin group also had more bleeding.
Fondaparinux
The OASIS-5 trial36,37 compared enoxaparin and fondaparinux, an exclusive factor Xa inhibitor, in more than 20,000 patients with unstable angina or NSTEMI. Fondaparinux was associated with a lower risk of death and reinfarction as well as fewer bleeding events. However, the benefits were almost exclusively in patients treated medically. In those undergoing PCI within the first 8 days, no benefit was found, although there was still a significant reduction in major bleeding events. Catheter thrombosis was also increased in patients taking fondaparinux, but only in those who did not receive adequate unfractionated heparin treatment before PCI.
Bivalirudin superior at time of catheterization
The most significant advance in antithrombotic therapy for patients with acute coronary syndromes is bivalirudin. This drug has a clear role only in the catheterization laboratory, where patients can be switched to it from heparin, low-molecular-weight heparin, or fondaparinux.
Three trials38–40 evaluated the drug in a total of more than 20,000 patients receiving invasive management of coronary artery disease undergoing PCI for elective indications, NSTEMI, or STEMI.
Results were remarkably similar across the three trials. Patients who were treated with bivalirudin alone had the same rate of ischemic end points at 30 days as those receiving heparin plus a GP IIb/IIIa inhibitor, but bivalirudin was associated with a consistent and significant 40% to 50% lower bleeding risk. For the highest-risk patients, those with STEMI, the bivalirudin group also had a significantly lower risk of death at 1 year.41
OTHER DRUGS: EARLY TREATMENT NO LONGER ROUTINE
Most data for the use of therapies aside from antithrombotics are from studies of patients with STEMI, but findings can logically be extrapolated to those with non-ST-elevation acute coronary syndromes.
Beta-blockers: Cardiogenic shock a risk
For beta-blockers, many historical trials were done in stable coronary disease, but there are no large trials in the setting of NSTEMI or unstable angina, and only recently have there been large trials for STEMI. Before the availability of recent evidence, standard practice was to treat STEMI routinely with intravenous metoprolol (Lopressor) and then oral metoprolol.
When large studies were finally conducted, the results were sobering.
COMMIT.42 Nearly 46,000 patients with suspected acute MI were randomized to receive either metoprolol (up to 15 mg intravenously, then 200 mg by mouth daily until discharge or for up to 4 weeks in the hospital) or placebo. Surprisingly, although rates of reinfarction and ventricular fibrillation were lower with metoprolol, a higher risk of cardiogenic shock with early beta-blockade offset these benefits and the net mortality rate was not reduced. This study led to a reduction in the early use of beta-blockers in patients with STEMI.
The standard of care has now shifted from beta-blockers in everyone as early as possible after MI to being more cautious in patients with contraindications, including signs of heart failure or a low-output state, or even in those of advanced age or with borderline low blood pressure or a high heart rate. Patients who present late and therefore may have a larger infarct are also at higher risk.
Although the goal should be to ultimately discharge patients on beta-blocker therapy after an MI, there should be no rush to start one early.
Carvedilol now preferred after STEMI
The CAPRICORN trial43 randomized nearly 2,000 patients following MI with left ventricular dysfunction (an ejection fraction of 40% or below) to either placebo or the beta-blocker carvedilol (Coreg). Patients taking the drug had a clear reduction in rates of death and reinfarction, leading to this drug becoming the beta-blocker of choice in patients with ventricular dysfunction after STEMI.
Angiotensin-converting enzyme inhibitors: Early risk of cardiogenic shock
The use of angiotensin-converting enzyme (ACE) inhibitors after MI is also supported by several studies.44 Two very large studies, one of nearly 60,000 patients and one of nearly 20,000, showed a clear reduction in the mortality rate in those who received an ACE inhibitor. Most of the benefit was in patients with an ejection fraction of less than 40%. On the basis of these trials, ACE inhibitors are indicated for all patients for the first 30 days after MI and then indefinitely for those with left ventricular dysfunction. However, the trial in which an ACE inhibitor was given intravenously early on had to be stopped prematurely because of worse outcomes owing to cardiogenic shock.
These studies highlight again that for patients who are unstable in the first few days of an acute coronary syndrome, it is best to wait until their condition stabilizes and to start these therapies before hospital discharge.
Intensive statin therapy
In the last 20 years, unequivocal evidence has emerged to support the beneficial role of statins for secondary prevention in patients with established coronary artery disease. More-recent trials have also shown that intensive statin therapy (a high dose of a potent statin) improves outcomes better than lower doses.
The PROVE-IT TIMI 22 trial45 randomized patients after an acute coronary syndrome to receive either standard therapy (pravastatin [Pravachol] 40 mg) or intensive therapy (atorvastatin [Lipitor] 80 mg). The intensive-therapy group had a significantly lower rate of major cardiovascular events, and the difference persisted and grew over 30 months of follow-up.
A number of studies confirmed this and broadened the patient population to those with unstable or stable coronary disease. Regardless of the risk profile, the effects were consistent and showed that high-dose statins were better in preventing coronary death and MI.46
Guidelines are evolving toward recommendation of highest doses of statins independently of the target level of low-density lipoprotein cholesterol.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2004; 110:e82–e292. Erratum in: Circulation 2005; 111:2013–2014.
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000; 83:361–366.
- Rosamond W, Flegal K, Friday G, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115:e69–e171.
- Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992; 44:293–325.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343:311–322.
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109:1223–1225.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 2005; 293:979–986.
- Antman EM, Hand M, Armstron PW, et al; Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Cannon CP, Weintraub WS, Demopoulos LA, et al; TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344:1879–1887.
- Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55:858–864.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284:835–842.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Yousef O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8:547–559.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Yusuf S, Mehta SR, Zhao F, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003; 107:966–972.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Schömig A. Ticagrelor—is there need for a new player in the antiplatelet-therapy field? N Engl J Med 2009; 361:1108–1111.
- Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010; 122:394–403.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120:2577–2585.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Fiho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J 2004; 148:937–943.
- Kastrati A, Mehilli J, Neuman FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006; 295:1531–1538.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Giugliano RP, White JA, Bode C, et al; Early ACS Investigators. Early vs delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009; 360:2176–2190.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Fergusson JJ, Califf RM, Antman EM, et al; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004; 292:45–54.
- Antman EM, Morrow DA, McCabe CH; EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354:1477–1488.
- The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Stone GW, Witzenbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2007; 358:2218–2230.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Chen ZM, Pan HC, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Dargie JH. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med 1996; 335:1660–1667.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48:438–445.
Most decisions for managing acute coronary syndromes can be based on ample data from large randomized trials with hard clinical end points, so there is little reason to provide care that is not evidence-based.
This article reviews some of the trials that provide guidance on diagnosing and managing acute coronary syndromes, including the timing of reperfusion and adjunctive therapies in different situations.
MOST ACUTE CORONARY SYNDROMES ARE NON-ST-ELEVATION CONDITIONS
Acute coronary syndromes range from unstable angina and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation MI (STEMI), reflecting a continuum of severity of coronary stenosis. The degree of coronary occlusion may ultimately determine whether a patient has unstable angina or MI with or without ST elevation.1
The substrate for all of these is vulnerable plaque. Angiographic studies have indicated that in many cases medium-size plaques (30%–40% stenosis) are more likely to rupture than larger, more obstructive ones. Moderate plaques may be vulnerable because they are less mature, with a large lipid core and a thin cap prone to rupture or erode, exposing the thrombogenic subendothelial components.2
Because the vulnerability of a coronary plaque may not correlate with the severity of stenosis before the plaque ruptures, stress tests and symptoms may not predict the risk of MI. The key role of thrombosis in the pathogenesis also highlights the importance of antithrombotic therapy in the acute phases of acute coronary syndromes, which can significantly reduce mortality and morbidity rates.
Perhaps because of the widespread use of aspirin and statins, most patients who currently present with an acute coronary syndrome have either unstable angina or NSTEMI: of about 1.57 million hospital admissions in 2004 for acute coronary syndromes, for example, only 330,000 (21%) were for STEMI.3
DIAGNOSING ACUTE CORONARY SYNDROME
Symptoms may not be classic
The classic symptoms of acute coronary syndromes are intense, oppressive chest pressure radiating to the left arm, but nearly any discomfort “between the nose and navel” (eg, including the jaw, arm, and epigastric and abdominal areas) may be an acute coronary syndrome. Associated symptoms may include chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or arms, and dyspnea.
Particularly in older, female, postoperative, or diabetic patients, the presentation may be atypical or “silent,” including nausea or vomiting; breathlessness; sweating; arrhythmias; or light-headedness. Especially in these groups, symptoms may be mild or subtle, and acute coronary syndrome may manifest only as “not feeling well.”
The differential diagnosis of acute coronary syndromes is broad. Most important to immediately consider are pulmonary embolism and aortic dissection, as they are life-threatening and are treated differently from acute coronary syndromes. Otherwise, it is best to err on the side of caution and treat for an acute coronary syndrome until it is proven otherwise.
Electrocardiography is critical
Electrocardiography (ECG) gives valuable information about the location, extent, and prognosis of infarction, and it is critically important for distinguishing STEMI from NSTEMI, with ST elevation classically diagnostic of complete coronary occlusion. Q waves can occur early and do not necessarily signify completed infarction, as traditionally thought. ST depression or T inversion indicates that total coronary occlusion is unlikely unless they are in a pattern of circumflex infarct associated with an enlarging R wave in lead V1. An ST elevation in RV4 indicates right ventricular infarction.
The appearance on ECG may evolve over time, so a patient with atypical symptoms and a nonspecific electrocardiogram should be observed for 24 hours or until more specific criteria develop.
Biomarkers in NSTEMI
In MI, cardiac troponin levels begin to rise about 3 hours after the onset of chest pain, and elevations can last for up to 14 days. Levels can also be mildly elevated chronically in patients with renal dysfunction, so positive biomarker tests in that population should be interpreted cautiously.
For STEMI, the opportunity to reperfuse is lost if one waits for cardiac biomarkers to become elevated. But for NSTEMI, they are highly sensitive and specific for identifying patients at high risk and determining who should be treated aggressively. Patients who are biomarker-negative have a better prognosis than patients with identical symptoms and electrocardiograms who are biomarker-positive.
MI is currently defined as a rise in any biomarker (usually troponin) above the 99th percentile for a reference population, with at least one of the following:
- Ischemic symptoms
- New ST/T changes or left bundle branch block
- Pathologic Q waves
- Loss of myocardium or abnormal wall motion seen by imaging
- Intracoronary thrombus.
REPERFUSION FOR ACUTE STEMI
Because acute coronary syndromes have a common pathophysiology, for the most part, lessons from clinical trials in one syndrome are relevant to the others. However, important differences exist regarding the need for immediate reperfusion in STEMI, since in most cases these patients have total rather than partial occlusion.
Fibrinolysis has limitations
The standard of management for STEMI is immediate reperfusion. The goal is to interrupt the wave front of myocardial necrosis, salvage threatened myocardium, and ultimately improve survival.
Five placebo-controlled trials showed a 30% reduction in the death rate in patients who received fibrinolytic therapy within 6 to 12 hours of presentation.4
Patients with ST elevation or with new bundle branch block benefit most from fibrinolytic therapy. Those with ST depression, T inversion, or nonspecific changes on ECG do not benefit; they probably do not have complete coronary occlusion, so the prothrombotic or platelet-activating effects of fibrinolytic therapy may make them worse.5 Further, fibrinolytic therapy poses the risk of intracranial hemorrhage, which, although rare (occurring in up to 1% of cases depending on the drug regimen), is a devastating complication.
In general, absolute contraindications to fibrinolysis include intracranial abnormalities, hemorrhage, and head trauma. An important relative contraindication is uncontrolled blood pressure (> 180/110 mm Hg at any point during hospitalization, including during the immediate presentation). Studies show that even if blood pressure can be controlled, the risk of intracranial hemorrhage is substantially higher, although the risk may not outweigh the benefit of reperfusion, particularly for large infarctions when percutaneous coronary intervention (PCI) is not available as an alternative to fibrinolysis.
Prompt PCI is preferable to fibrinolysis
If PCI is available on site, there is nearly no role for fibrinolytic therapy. PCI is better than fibrinolytic therapy in terms of the degree of reperfusion, reocclusion, MI recurrence, and mortality rate, and it poses little or no risk of intracranial hemorrhage.6
For either fibrinolytic therapy or percutaneous therapy, “time is muscle”: the longer the ischemic time, the higher the mortality rate (relative risk = 1.075 for every 30 minutes of delay, P = .041).7
At centers that do not have PCI on site, studies (mainly from Europe) have shown that it is better to transport the patient for PCI than to give immediate fibrinolytic therapy.7,8 But because the centers studied tended to have short transport times (usually 40 minutes or less), it is uncertain whether the results are applicable throughout the United States.
The delay between symptom onset and presentation is also relevant. Reperfusion within the first 1 to 2 hours after the onset of symptoms provides the greatest degree of myocardial salvage and of reduction in the risk of death; the extent of benefit thereafter is substantially less. As a result, patients who present very early after symptom onset have the most to lose if their reperfusion is delayed by even a few more hours, whereas patients who have already experienced several hours of pain are affected less by additional delay.9 Thus, patients presenting within the “golden” 1 or 2 hours after symptoms begin should be considered for fibrinolytic therapy if transfer for PCI cannot be done expeditiously. It is important for hospitals without PCI available on site to have a system in place for rapid transport of patients when needed.
Guidelines advise that patients with STEMI should undergo PCI rather than receive fibrinolytic therapy as long as PCI is available within 90 minutes of first medical contact. Otherwise, fibrinolysis should be started within 30 minutes.10 For patients who present several hours after symptom onset, PCI may still be preferable even if the transport time is somewhat longer.
PCI after fibrinolytic therapy
In prior decades, PCI immediately after fibrinolytic therapy was associated with an increased risk of bleeding complications and reinfarction. That has changed with improvements in equipment and antithrombotic therapy.
Two large trials conclusively found that routinely transferring high-risk patients for PCI immediately after receiving fibrinolytic therapy (combined half-dose reteplase [Retavase] and abciximab [ReoPro]11 or full-dose tenecteplase [TNKase]12) resulted in much lower rates of ischemic end points without an increase in bleeding complications compared with transferring patients only for rescue PCI after fibrinolytic therapy.
Routine transfer is now the standard of care for high-risk patients after fibrinolytic therapy and probably is best for all patients after an MI.
MANAGING NSTEMI AND UNSTABLE ANGINA
For patients with NSTEMI, immediate reperfusion is usually not required, although initial triage for “early invasive” vs “initial conservative” management must be done early in the hospital course. Randomized trials have evaluated these two approaches, with most studies in the contemporary era reporting improved outcomes with an early invasive approach.
The TACTICS trial,13 the most important of these, enrolled more than 2,200 patients with unstable angina or NSTEMI and randomized them to an early invasive strategy or a conservative strategy. Overall, results were better with the early invasive strategy.
The ICTUS trial.14 Although several studies showed that an early invasive approach was better, the most recent study using the most modern practices—the ICTUS trial—did not find that it reduced death rates. Most patients eventually underwent angiography and revascularization, but not early on. However, all studies showed that rates of recurrent unstable angina and hospitalization were reduced by an early invasive approach, so revascularization does have a role in stabilizing the patient. But in situations of aggressive medical management with antithrombotic and other therapies, an early conservative approach may be an appropriate alternative for many patients.15
The selection of an invasive vs a conservative approach should include a consideration of risk, which can be estimated using a number of criteria, including the Thrombolysis in Myocardial Infarction (TIMI) or the GRACE risk score. When risk was stratified using the TIMI risk score,16 in the TACTICS trial, the higher the risk score, the more likely patients were to benefit from early revascularization.
When an invasive approach is chosen, it does not appear necessary to take patients to catheterization immediately (within 2–24 hours) compared with later during the hospital course.
The TIMACS trial,17 with more than 3,000 patients, tested the benefits of very early vs later revascularization for patients with NSTEMI and unstable angina. Early intervention did not significantly improve outcomes for the primary composite end point of death, MI, and stroke in the overall population enrolled in the trial, but when the secondary end point of refractory ischemia was added in, early intervention was found to be beneficial overall. Moreover, when stratified by risk, high-risk patients significantly benefited from early intervention for the primary end point.
Guidelines for NSTEMI and unstable angina continue to prefer an early invasive strategy, particularly for high-risk patients, although a conservative strategy is considered acceptable if patients receive intensive evidence-based medical therapy and remain clinically stable.18
ANTITHROMBOTIC THERAPIES
Once a revascularization strategy has been chosen, adjunctive therapies should be considered. The most important are the antithrombotic therapies.
Many drugs target platelet activity. Most important are the thromboxane inhibitor aspirin, the adenosine diphosphate (ADP) receptor antagonists clopidogrel (Plavix), prasugrel (Effient), and ticagrelor (Brilinta), and the glycoprotein (GP) IIb/IIIa antagonists abciximab and eptifibatide (Integrilin). Others, such as thrombin receptor antagonists, are under investigation.19
Aspirin for secondary prevention
Evidence is unequivocal for the benefit of aspirin therapy in patients with established or suspected vascular disease.
The ISIS-2 trial20 compared 35-day mortality rates in 16,000 patients with STEMI who were given aspirin, streptokinase, combined streptokinase and aspirin, or placebo. Mortality rates were reduced by aspirin compared with placebo by an extent similar to that achieved with streptokinase, with a further reduction when aspirin and streptokinase were given together.
Therefore, patients with STEMI should be given aspirin daily indefinitely unless they have true aspirin allergy. The dose is 165 to 325 mg initially and 75 to 162 mg daily thereafter.
For NSTEMI and even for secondary prevention in less-acute situations, a number of smaller trials also provide clear evidence of benefit from aspirin therapy.
The CURRENT-OASIS 7 trial21 showed that low maintenance dosages of aspirin (75–100 mg per day) resulted in the same incidence of ischemic end points (cardiovascular death, MI, or stroke) as higher dosages. Although rates of major bleeding events did not differ, a higher rate of gastrointestinal bleeding was evident at just 30 days in patients taking the higher doses. This large trial clearly established that there is no advantage to daily aspirin doses of more than 100 mg.
DUAL ANTIPLATELET THERAPY IS STANDARD
Standard practice now is to use aspirin plus another antiplatelet agent that acts by inhibiting either the ADP receptor (for which there is the most evidence) or the GP IIb/IIIa receptor (which is becoming less used). Dual therapy should begin early in patients with acute coronary syndrome.
Clopidogrel: Well studied with aspirin
The most commonly used ADP antagonist is clopidogrel, a thienopyridine. Much evidence exists for its benefit.
The CURE trial22 randomized more than 12,000 patients with NSTEMI or unstable angina to aspirin plus either clopidogrel or placebo. The incidence of the combined end point of MI, stroke, and cardiovascular death was 20% lower in the clopidogrel group than in the placebo group over 12 months of follow-up. The benefit of clopidogrel began to occur within the first 24 hours after randomization, with a 33% relative risk reduction in the combined end point of cardiovascular death, MI, stroke, and severe ischemia, demonstrating the importance of starting this agent early in the hospital course.
COMMIT23 found a benefit in adding clopidogrel to aspirin in patients with acute STEMI. Although it was only a 30-day trial, significant risk reduction was found in the dual-therapy group for combined death, stroke, or reinfarction. The results of this brief trial were less definitive, but the pathophysiology was similar to non-ST-elevation acute coronary syndromes, so it is reasonable to extrapolate the long-term findings to this setting.
The CURRENT-OASIS 7 trial21 randomized more than 25,000 patients to either clopidogrel in a double dosage (600 mg load, 150 mg/day for 6 days, then 75 mg/day) or standard dosage (300 mg load, 75 mg/day thereafter). Although no overall benefit was found for the higher dosage, a subgroup of more than 17,000 patients who underwent PCI after randomization had a lower risk of developing stent thrombosis. On the other hand, higher doses of clopidogrel caused more major bleeding events.
Ticagrelor and prasugrel: New alternatives to clopidogrel
The principal limitation of clopidogrel is its metabolism. It is a prodrug, ie, it is not active as taken and must be converted to its active state by cytochrome P450 enzymes in the liver. Patients who bear certain polymorphisms in the genes for these enzymes or who are taking other medications that affect this enzymatic pathway may derive less platelet inhibition from the drug, leading to considerable patient-to-patient variability in the degree of antiplatelet effect.
Alternatives to clopidogrel have been developed that inhibit platelets more intensely, are activated more rapidly, and have less interpatient variability. Available now are ticagrelor and prasugrel.24 Like clopidogrel, prasugrel is absorbed as an inactive prodrug, but it is efficiently metabolized by esterases to an active form, and then by a simpler step within the liver to its fully active metabolite.25 Ticagrelor is active as absorbed.26
Pharmacodynamically, the two drugs perform almost identically and much faster than clopidogrel, with equilibrium platelet inhibition reached in less than 1 hour. The degree of platelet inhibition is also more—sometimes twice as much—with the new drugs compared with clopidogrel, and the effect is much more consistent between patients.
Both clopidogrel and prasugrel permanently inhibit the platelet ADP receptor, and 3 to 7 days are therefore required for their antiplatelet effects to completely wear off. In contrast, ticagrelor is a reversible inhibitor and its effects wear off more rapidly. Despite achieving a much higher level of platelet inhibition than clopidogrel, ticagrelor’s activity falls below that of clopidogrel’s by 48 hours of discontinuing the drugs.
Trial of prasugrel vs clopidogrel
The TRITON-TIMI 38 trial27 enrolled more than 13,000 patients with acute coronary syndromes, randomized to receive, either prasugrel or clopidogrel, in addition to aspirin. The patients were all undergoing PCI, so the findings do not apply to patients treated medically with an early conservative approach. The study drug was given only after the decision was made to perform PCI in patients with non-ST-elevation acute coronary syndrome (but given immediately for patients with STEMI, because nearly all those patients undergo PCI).
Prasugrel was clearly beneficial, with a significant 20% lower rate of the combined end point of cardiovascular death, MI, and stroke at 15 months. However, bleeding risk was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% confidence interval 1.02–1.68, P = .03). Looking at individual end points, the advantages of prasugrel were primarily in reducing rates of stent thrombosis and nonfatal MI. Death rates with the two drugs were equivalent, possibly because of the higher risk of bleeding with prasugrel. Bleeding in the prasugrel group was particularly increased in patients who underwent bypass surgery; more patients also needed transfusion.
Subgroup analysis showed that patients with a history of stroke or transient ischemic attack had higher rates of ischemic and bleeding events with prasugrel than with clopidogrel, leading to these being labeled as absolute contraindications to prasugrel. Patients over age 75 or who weighed less than 60 kg experienced excess bleeding risk that closely matched the reduction in ischemic event rates and thus did not have a net benefit with prasugrel.
Trial of ticagrelor vs clopidogrel
The PLATO trial28 included 18,000 patients, of whom 65% underwent revascularization and 35% were treated medically. The drug—clopidogrel or ticagrelor—was given in addition to aspirin at randomization (within 24 hours of symptom onset); this more closely follows clinical practice, in which dual antiplatelet therapy is started as soon as possible. This difference makes the PLATO study more relevant to practice for patients with non-ST-elevation acute coronary syndrome. Also, because they gave the drugs to all patients regardless of whether they were to undergo PCI, this study likely had a higher-risk population, which may be refected in the higher mortality rate at 30 days (5.9% in the clopidogrel group in the PLATO study vs 3.2% in the clopidogrel group in the TRITON study).
Another important difference between the trials testing prasugrel and ticagrelor is that patients who had already received a thienopyridine were excluded from the prasugrel trial but not from the ticagrelor trial. Nearly half the patients in the ticagrelor group were already taking clopidogrel. The clinical implication is that for patients who arrive from another facility and already have been given clopidogrel, it is safe to give ticagrelor. There is limited information about whether that is also true for prasugrel, although there is no known reason why the safety of adding prasugrel to clopidogrel should be different from that of ticagrelor.
The rate of ischemic events was 20% lower in the ticagrelor group than in the clopidogrel group, importantly including reductions in the incidence of death, MI, and stent thrombosis. There was no increase with ticagrelor compared with clopidogrel in bleeding associated with coronary artery bypass graft surgery, likely because of the more rapid washout of the ticagrelor effect, or in the need for blood transfusions. However, the rate of bleeding unrelated to coronary artery bypass was about 20% higher with ticagrelor.
In summary, more intense platelet inhibition reduces the risk of ischemic events, but, particularly for the irreversible inhibitor prasugrel, at the cost of a higher risk of bleeding. In general, the net benefit of these agents in preventing the irreversible complications of MI and (in the case of ticagrelor) death favor the use of the more intense ADP inhibitors in appropriate patients. Ticagrelor is indicated in patients with acute coronary syndromes undergoing invasive or conservative management; prasugrel is indicated in patients undergoing PCI, but contraindicated in patients with a previous stroke or transient ischemic event. Neither drug is indicated in patients undergoing elective PCI outside the setting of acute coronary syndromes, although these agents may be appropriate in patients with intolerance or allergy to clopidogrel.
Glycoprotein IIb/IIIa antagonists for select cases only
GP IIb/IIIa antagonists such as abciximab were previously used more commonly than they are today. Now, with routine pretreatment using thienopyridines, their role in acute coronary syndromes is less clear. They still play a role when routine dual antiplatelet therapy is not used, when prasugrel or ticagrelor is not used, and when heparin rather than an alternative antithrombin agent is used.
A meta-analysis29 of 3,755 patients showed a clear reduction in ischemic complications with abciximab as an adjunct to primary PCI for STEMI in patients treated with heparin.
Kastrati et al30 found that patients with non-ST-elevation acute coronary syndromes benefited from abciximab at the time of PCI with heparin, even though they had been routinely pretreated with clopidogrel. However, benefits were seen only in high-risk patients who had presented with elevated troponins.
On the other hand, the role of GP IIb/IIIa blockade for “upstream” medical management in patients with acute coronary syndromes has been eroded by several studies.
The ACUITY trial31 randomized more than 9,000 patients to receive either routine treatment with a GP IIb/IIIa inhibitor before angiography or deferred selective use in the catheterization laboratory only for patients undergoing PCI. No significant differences were found in rates of MI and death.
The Early ACS trial32 compared early routine eptifibatide vs delayed, provisional eptifibatide in 9,492 patients with acute coronary syndromes without ST elevation and who were assigned to an invasive strategy. The early-eptifibatide group received two boluses and an infusion of eptifibatide before angiography; the others received a placebo infusion, with provisional eptifibatide after angiography if the patient underwent PCI and was deemed at high risk. No significant difference in rates of death or MI were noted, and the early-eptifibatide group had significantly higher rates of bleeding and need for transfusion.
The FINESSE trial33 also discredited “facilitating” PCI by giving GP IIb/IIIa antagonists in patients with STEMI before arrival in the catheterization laboratory, with no benefit to giving abciximab ahead of time vs in the catheterization laboratory, and with an increased risk of bleeding complications.
These studies have helped narrow the use of GP IIb/IIIa inhibitors to the catheterization laboratory in conjunction with heparin anticoagulation (as compared with bivalirudin [Angiomax]; see below) and only in select or high-risk cases. These drugs are indicated in the medical phase of management only if patients cannot be stabilized by aspirin or ADP inhibition.
NEWER ANTITHROMBOTICS: ADVANTAGES UNCLEAR
The complex coagulation cascade has a number of components, but only a few are targeted by drugs that are approved and recommended: fondaparinux (Arixtra) and oral factor Xa inhibitors affect the prothrombinase complex (including factor X); bivalirudin and oral factor IIa inhibitors affect thrombin; and heparin and the low-molecular-weight heparins inhibit both targets.
Low-molecular-weight heparins
The SYNERGY trial34 randomized nearly 10,000 patients with non-ST-elevation acute coronary syndromes at high risk for ischemic cardiac complications managed with an invasive approach to either the low-molecular-weight heparin enoxaparin (Lovenox) or intravenous unfractionated heparin immediately after enrollment. Most patients underwent catheterization and revascularization. No clinical advantage was found for enoxaparin, and bleeding complications were increased.
The EXTRACT-TIMI 25 trial35 randomized more than 20,000 patients with STEMI who were about to undergo fibrinolysis to receive either enoxaparin throughout hospitalization (average of 8 days) or unfractionated heparin for at least 48 hours. The enoxaparin group had a lower rate of recurrent MI, but it was unclear if the difference was in part attributable to the longer therapy time. The enoxaparin group also had more bleeding.
Fondaparinux
The OASIS-5 trial36,37 compared enoxaparin and fondaparinux, an exclusive factor Xa inhibitor, in more than 20,000 patients with unstable angina or NSTEMI. Fondaparinux was associated with a lower risk of death and reinfarction as well as fewer bleeding events. However, the benefits were almost exclusively in patients treated medically. In those undergoing PCI within the first 8 days, no benefit was found, although there was still a significant reduction in major bleeding events. Catheter thrombosis was also increased in patients taking fondaparinux, but only in those who did not receive adequate unfractionated heparin treatment before PCI.
Bivalirudin superior at time of catheterization
The most significant advance in antithrombotic therapy for patients with acute coronary syndromes is bivalirudin. This drug has a clear role only in the catheterization laboratory, where patients can be switched to it from heparin, low-molecular-weight heparin, or fondaparinux.
Three trials38–40 evaluated the drug in a total of more than 20,000 patients receiving invasive management of coronary artery disease undergoing PCI for elective indications, NSTEMI, or STEMI.
Results were remarkably similar across the three trials. Patients who were treated with bivalirudin alone had the same rate of ischemic end points at 30 days as those receiving heparin plus a GP IIb/IIIa inhibitor, but bivalirudin was associated with a consistent and significant 40% to 50% lower bleeding risk. For the highest-risk patients, those with STEMI, the bivalirudin group also had a significantly lower risk of death at 1 year.41
OTHER DRUGS: EARLY TREATMENT NO LONGER ROUTINE
Most data for the use of therapies aside from antithrombotics are from studies of patients with STEMI, but findings can logically be extrapolated to those with non-ST-elevation acute coronary syndromes.
Beta-blockers: Cardiogenic shock a risk
For beta-blockers, many historical trials were done in stable coronary disease, but there are no large trials in the setting of NSTEMI or unstable angina, and only recently have there been large trials for STEMI. Before the availability of recent evidence, standard practice was to treat STEMI routinely with intravenous metoprolol (Lopressor) and then oral metoprolol.
When large studies were finally conducted, the results were sobering.
COMMIT.42 Nearly 46,000 patients with suspected acute MI were randomized to receive either metoprolol (up to 15 mg intravenously, then 200 mg by mouth daily until discharge or for up to 4 weeks in the hospital) or placebo. Surprisingly, although rates of reinfarction and ventricular fibrillation were lower with metoprolol, a higher risk of cardiogenic shock with early beta-blockade offset these benefits and the net mortality rate was not reduced. This study led to a reduction in the early use of beta-blockers in patients with STEMI.
The standard of care has now shifted from beta-blockers in everyone as early as possible after MI to being more cautious in patients with contraindications, including signs of heart failure or a low-output state, or even in those of advanced age or with borderline low blood pressure or a high heart rate. Patients who present late and therefore may have a larger infarct are also at higher risk.
Although the goal should be to ultimately discharge patients on beta-blocker therapy after an MI, there should be no rush to start one early.
Carvedilol now preferred after STEMI
The CAPRICORN trial43 randomized nearly 2,000 patients following MI with left ventricular dysfunction (an ejection fraction of 40% or below) to either placebo or the beta-blocker carvedilol (Coreg). Patients taking the drug had a clear reduction in rates of death and reinfarction, leading to this drug becoming the beta-blocker of choice in patients with ventricular dysfunction after STEMI.
Angiotensin-converting enzyme inhibitors: Early risk of cardiogenic shock
The use of angiotensin-converting enzyme (ACE) inhibitors after MI is also supported by several studies.44 Two very large studies, one of nearly 60,000 patients and one of nearly 20,000, showed a clear reduction in the mortality rate in those who received an ACE inhibitor. Most of the benefit was in patients with an ejection fraction of less than 40%. On the basis of these trials, ACE inhibitors are indicated for all patients for the first 30 days after MI and then indefinitely for those with left ventricular dysfunction. However, the trial in which an ACE inhibitor was given intravenously early on had to be stopped prematurely because of worse outcomes owing to cardiogenic shock.
These studies highlight again that for patients who are unstable in the first few days of an acute coronary syndrome, it is best to wait until their condition stabilizes and to start these therapies before hospital discharge.
Intensive statin therapy
In the last 20 years, unequivocal evidence has emerged to support the beneficial role of statins for secondary prevention in patients with established coronary artery disease. More-recent trials have also shown that intensive statin therapy (a high dose of a potent statin) improves outcomes better than lower doses.
The PROVE-IT TIMI 22 trial45 randomized patients after an acute coronary syndrome to receive either standard therapy (pravastatin [Pravachol] 40 mg) or intensive therapy (atorvastatin [Lipitor] 80 mg). The intensive-therapy group had a significantly lower rate of major cardiovascular events, and the difference persisted and grew over 30 months of follow-up.
A number of studies confirmed this and broadened the patient population to those with unstable or stable coronary disease. Regardless of the risk profile, the effects were consistent and showed that high-dose statins were better in preventing coronary death and MI.46
Guidelines are evolving toward recommendation of highest doses of statins independently of the target level of low-density lipoprotein cholesterol.
Most decisions for managing acute coronary syndromes can be based on ample data from large randomized trials with hard clinical end points, so there is little reason to provide care that is not evidence-based.
This article reviews some of the trials that provide guidance on diagnosing and managing acute coronary syndromes, including the timing of reperfusion and adjunctive therapies in different situations.
MOST ACUTE CORONARY SYNDROMES ARE NON-ST-ELEVATION CONDITIONS
Acute coronary syndromes range from unstable angina and non-ST-elevation myocardial infarction (NSTEMI) to ST-elevation MI (STEMI), reflecting a continuum of severity of coronary stenosis. The degree of coronary occlusion may ultimately determine whether a patient has unstable angina or MI with or without ST elevation.1
The substrate for all of these is vulnerable plaque. Angiographic studies have indicated that in many cases medium-size plaques (30%–40% stenosis) are more likely to rupture than larger, more obstructive ones. Moderate plaques may be vulnerable because they are less mature, with a large lipid core and a thin cap prone to rupture or erode, exposing the thrombogenic subendothelial components.2
Because the vulnerability of a coronary plaque may not correlate with the severity of stenosis before the plaque ruptures, stress tests and symptoms may not predict the risk of MI. The key role of thrombosis in the pathogenesis also highlights the importance of antithrombotic therapy in the acute phases of acute coronary syndromes, which can significantly reduce mortality and morbidity rates.
Perhaps because of the widespread use of aspirin and statins, most patients who currently present with an acute coronary syndrome have either unstable angina or NSTEMI: of about 1.57 million hospital admissions in 2004 for acute coronary syndromes, for example, only 330,000 (21%) were for STEMI.3
DIAGNOSING ACUTE CORONARY SYNDROME
Symptoms may not be classic
The classic symptoms of acute coronary syndromes are intense, oppressive chest pressure radiating to the left arm, but nearly any discomfort “between the nose and navel” (eg, including the jaw, arm, and epigastric and abdominal areas) may be an acute coronary syndrome. Associated symptoms may include chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or arms, and dyspnea.
Particularly in older, female, postoperative, or diabetic patients, the presentation may be atypical or “silent,” including nausea or vomiting; breathlessness; sweating; arrhythmias; or light-headedness. Especially in these groups, symptoms may be mild or subtle, and acute coronary syndrome may manifest only as “not feeling well.”
The differential diagnosis of acute coronary syndromes is broad. Most important to immediately consider are pulmonary embolism and aortic dissection, as they are life-threatening and are treated differently from acute coronary syndromes. Otherwise, it is best to err on the side of caution and treat for an acute coronary syndrome until it is proven otherwise.
Electrocardiography is critical
Electrocardiography (ECG) gives valuable information about the location, extent, and prognosis of infarction, and it is critically important for distinguishing STEMI from NSTEMI, with ST elevation classically diagnostic of complete coronary occlusion. Q waves can occur early and do not necessarily signify completed infarction, as traditionally thought. ST depression or T inversion indicates that total coronary occlusion is unlikely unless they are in a pattern of circumflex infarct associated with an enlarging R wave in lead V1. An ST elevation in RV4 indicates right ventricular infarction.
The appearance on ECG may evolve over time, so a patient with atypical symptoms and a nonspecific electrocardiogram should be observed for 24 hours or until more specific criteria develop.
Biomarkers in NSTEMI
In MI, cardiac troponin levels begin to rise about 3 hours after the onset of chest pain, and elevations can last for up to 14 days. Levels can also be mildly elevated chronically in patients with renal dysfunction, so positive biomarker tests in that population should be interpreted cautiously.
For STEMI, the opportunity to reperfuse is lost if one waits for cardiac biomarkers to become elevated. But for NSTEMI, they are highly sensitive and specific for identifying patients at high risk and determining who should be treated aggressively. Patients who are biomarker-negative have a better prognosis than patients with identical symptoms and electrocardiograms who are biomarker-positive.
MI is currently defined as a rise in any biomarker (usually troponin) above the 99th percentile for a reference population, with at least one of the following:
- Ischemic symptoms
- New ST/T changes or left bundle branch block
- Pathologic Q waves
- Loss of myocardium or abnormal wall motion seen by imaging
- Intracoronary thrombus.
REPERFUSION FOR ACUTE STEMI
Because acute coronary syndromes have a common pathophysiology, for the most part, lessons from clinical trials in one syndrome are relevant to the others. However, important differences exist regarding the need for immediate reperfusion in STEMI, since in most cases these patients have total rather than partial occlusion.
Fibrinolysis has limitations
The standard of management for STEMI is immediate reperfusion. The goal is to interrupt the wave front of myocardial necrosis, salvage threatened myocardium, and ultimately improve survival.
Five placebo-controlled trials showed a 30% reduction in the death rate in patients who received fibrinolytic therapy within 6 to 12 hours of presentation.4
Patients with ST elevation or with new bundle branch block benefit most from fibrinolytic therapy. Those with ST depression, T inversion, or nonspecific changes on ECG do not benefit; they probably do not have complete coronary occlusion, so the prothrombotic or platelet-activating effects of fibrinolytic therapy may make them worse.5 Further, fibrinolytic therapy poses the risk of intracranial hemorrhage, which, although rare (occurring in up to 1% of cases depending on the drug regimen), is a devastating complication.
In general, absolute contraindications to fibrinolysis include intracranial abnormalities, hemorrhage, and head trauma. An important relative contraindication is uncontrolled blood pressure (> 180/110 mm Hg at any point during hospitalization, including during the immediate presentation). Studies show that even if blood pressure can be controlled, the risk of intracranial hemorrhage is substantially higher, although the risk may not outweigh the benefit of reperfusion, particularly for large infarctions when percutaneous coronary intervention (PCI) is not available as an alternative to fibrinolysis.
Prompt PCI is preferable to fibrinolysis
If PCI is available on site, there is nearly no role for fibrinolytic therapy. PCI is better than fibrinolytic therapy in terms of the degree of reperfusion, reocclusion, MI recurrence, and mortality rate, and it poses little or no risk of intracranial hemorrhage.6
For either fibrinolytic therapy or percutaneous therapy, “time is muscle”: the longer the ischemic time, the higher the mortality rate (relative risk = 1.075 for every 30 minutes of delay, P = .041).7
At centers that do not have PCI on site, studies (mainly from Europe) have shown that it is better to transport the patient for PCI than to give immediate fibrinolytic therapy.7,8 But because the centers studied tended to have short transport times (usually 40 minutes or less), it is uncertain whether the results are applicable throughout the United States.
The delay between symptom onset and presentation is also relevant. Reperfusion within the first 1 to 2 hours after the onset of symptoms provides the greatest degree of myocardial salvage and of reduction in the risk of death; the extent of benefit thereafter is substantially less. As a result, patients who present very early after symptom onset have the most to lose if their reperfusion is delayed by even a few more hours, whereas patients who have already experienced several hours of pain are affected less by additional delay.9 Thus, patients presenting within the “golden” 1 or 2 hours after symptoms begin should be considered for fibrinolytic therapy if transfer for PCI cannot be done expeditiously. It is important for hospitals without PCI available on site to have a system in place for rapid transport of patients when needed.
Guidelines advise that patients with STEMI should undergo PCI rather than receive fibrinolytic therapy as long as PCI is available within 90 minutes of first medical contact. Otherwise, fibrinolysis should be started within 30 minutes.10 For patients who present several hours after symptom onset, PCI may still be preferable even if the transport time is somewhat longer.
PCI after fibrinolytic therapy
In prior decades, PCI immediately after fibrinolytic therapy was associated with an increased risk of bleeding complications and reinfarction. That has changed with improvements in equipment and antithrombotic therapy.
Two large trials conclusively found that routinely transferring high-risk patients for PCI immediately after receiving fibrinolytic therapy (combined half-dose reteplase [Retavase] and abciximab [ReoPro]11 or full-dose tenecteplase [TNKase]12) resulted in much lower rates of ischemic end points without an increase in bleeding complications compared with transferring patients only for rescue PCI after fibrinolytic therapy.
Routine transfer is now the standard of care for high-risk patients after fibrinolytic therapy and probably is best for all patients after an MI.
MANAGING NSTEMI AND UNSTABLE ANGINA
For patients with NSTEMI, immediate reperfusion is usually not required, although initial triage for “early invasive” vs “initial conservative” management must be done early in the hospital course. Randomized trials have evaluated these two approaches, with most studies in the contemporary era reporting improved outcomes with an early invasive approach.
The TACTICS trial,13 the most important of these, enrolled more than 2,200 patients with unstable angina or NSTEMI and randomized them to an early invasive strategy or a conservative strategy. Overall, results were better with the early invasive strategy.
The ICTUS trial.14 Although several studies showed that an early invasive approach was better, the most recent study using the most modern practices—the ICTUS trial—did not find that it reduced death rates. Most patients eventually underwent angiography and revascularization, but not early on. However, all studies showed that rates of recurrent unstable angina and hospitalization were reduced by an early invasive approach, so revascularization does have a role in stabilizing the patient. But in situations of aggressive medical management with antithrombotic and other therapies, an early conservative approach may be an appropriate alternative for many patients.15
The selection of an invasive vs a conservative approach should include a consideration of risk, which can be estimated using a number of criteria, including the Thrombolysis in Myocardial Infarction (TIMI) or the GRACE risk score. When risk was stratified using the TIMI risk score,16 in the TACTICS trial, the higher the risk score, the more likely patients were to benefit from early revascularization.
When an invasive approach is chosen, it does not appear necessary to take patients to catheterization immediately (within 2–24 hours) compared with later during the hospital course.
The TIMACS trial,17 with more than 3,000 patients, tested the benefits of very early vs later revascularization for patients with NSTEMI and unstable angina. Early intervention did not significantly improve outcomes for the primary composite end point of death, MI, and stroke in the overall population enrolled in the trial, but when the secondary end point of refractory ischemia was added in, early intervention was found to be beneficial overall. Moreover, when stratified by risk, high-risk patients significantly benefited from early intervention for the primary end point.
Guidelines for NSTEMI and unstable angina continue to prefer an early invasive strategy, particularly for high-risk patients, although a conservative strategy is considered acceptable if patients receive intensive evidence-based medical therapy and remain clinically stable.18
ANTITHROMBOTIC THERAPIES
Once a revascularization strategy has been chosen, adjunctive therapies should be considered. The most important are the antithrombotic therapies.
Many drugs target platelet activity. Most important are the thromboxane inhibitor aspirin, the adenosine diphosphate (ADP) receptor antagonists clopidogrel (Plavix), prasugrel (Effient), and ticagrelor (Brilinta), and the glycoprotein (GP) IIb/IIIa antagonists abciximab and eptifibatide (Integrilin). Others, such as thrombin receptor antagonists, are under investigation.19
Aspirin for secondary prevention
Evidence is unequivocal for the benefit of aspirin therapy in patients with established or suspected vascular disease.
The ISIS-2 trial20 compared 35-day mortality rates in 16,000 patients with STEMI who were given aspirin, streptokinase, combined streptokinase and aspirin, or placebo. Mortality rates were reduced by aspirin compared with placebo by an extent similar to that achieved with streptokinase, with a further reduction when aspirin and streptokinase were given together.
Therefore, patients with STEMI should be given aspirin daily indefinitely unless they have true aspirin allergy. The dose is 165 to 325 mg initially and 75 to 162 mg daily thereafter.
For NSTEMI and even for secondary prevention in less-acute situations, a number of smaller trials also provide clear evidence of benefit from aspirin therapy.
The CURRENT-OASIS 7 trial21 showed that low maintenance dosages of aspirin (75–100 mg per day) resulted in the same incidence of ischemic end points (cardiovascular death, MI, or stroke) as higher dosages. Although rates of major bleeding events did not differ, a higher rate of gastrointestinal bleeding was evident at just 30 days in patients taking the higher doses. This large trial clearly established that there is no advantage to daily aspirin doses of more than 100 mg.
DUAL ANTIPLATELET THERAPY IS STANDARD
Standard practice now is to use aspirin plus another antiplatelet agent that acts by inhibiting either the ADP receptor (for which there is the most evidence) or the GP IIb/IIIa receptor (which is becoming less used). Dual therapy should begin early in patients with acute coronary syndrome.
Clopidogrel: Well studied with aspirin
The most commonly used ADP antagonist is clopidogrel, a thienopyridine. Much evidence exists for its benefit.
The CURE trial22 randomized more than 12,000 patients with NSTEMI or unstable angina to aspirin plus either clopidogrel or placebo. The incidence of the combined end point of MI, stroke, and cardiovascular death was 20% lower in the clopidogrel group than in the placebo group over 12 months of follow-up. The benefit of clopidogrel began to occur within the first 24 hours after randomization, with a 33% relative risk reduction in the combined end point of cardiovascular death, MI, stroke, and severe ischemia, demonstrating the importance of starting this agent early in the hospital course.
COMMIT23 found a benefit in adding clopidogrel to aspirin in patients with acute STEMI. Although it was only a 30-day trial, significant risk reduction was found in the dual-therapy group for combined death, stroke, or reinfarction. The results of this brief trial were less definitive, but the pathophysiology was similar to non-ST-elevation acute coronary syndromes, so it is reasonable to extrapolate the long-term findings to this setting.
The CURRENT-OASIS 7 trial21 randomized more than 25,000 patients to either clopidogrel in a double dosage (600 mg load, 150 mg/day for 6 days, then 75 mg/day) or standard dosage (300 mg load, 75 mg/day thereafter). Although no overall benefit was found for the higher dosage, a subgroup of more than 17,000 patients who underwent PCI after randomization had a lower risk of developing stent thrombosis. On the other hand, higher doses of clopidogrel caused more major bleeding events.
Ticagrelor and prasugrel: New alternatives to clopidogrel
The principal limitation of clopidogrel is its metabolism. It is a prodrug, ie, it is not active as taken and must be converted to its active state by cytochrome P450 enzymes in the liver. Patients who bear certain polymorphisms in the genes for these enzymes or who are taking other medications that affect this enzymatic pathway may derive less platelet inhibition from the drug, leading to considerable patient-to-patient variability in the degree of antiplatelet effect.
Alternatives to clopidogrel have been developed that inhibit platelets more intensely, are activated more rapidly, and have less interpatient variability. Available now are ticagrelor and prasugrel.24 Like clopidogrel, prasugrel is absorbed as an inactive prodrug, but it is efficiently metabolized by esterases to an active form, and then by a simpler step within the liver to its fully active metabolite.25 Ticagrelor is active as absorbed.26
Pharmacodynamically, the two drugs perform almost identically and much faster than clopidogrel, with equilibrium platelet inhibition reached in less than 1 hour. The degree of platelet inhibition is also more—sometimes twice as much—with the new drugs compared with clopidogrel, and the effect is much more consistent between patients.
Both clopidogrel and prasugrel permanently inhibit the platelet ADP receptor, and 3 to 7 days are therefore required for their antiplatelet effects to completely wear off. In contrast, ticagrelor is a reversible inhibitor and its effects wear off more rapidly. Despite achieving a much higher level of platelet inhibition than clopidogrel, ticagrelor’s activity falls below that of clopidogrel’s by 48 hours of discontinuing the drugs.
Trial of prasugrel vs clopidogrel
The TRITON-TIMI 38 trial27 enrolled more than 13,000 patients with acute coronary syndromes, randomized to receive, either prasugrel or clopidogrel, in addition to aspirin. The patients were all undergoing PCI, so the findings do not apply to patients treated medically with an early conservative approach. The study drug was given only after the decision was made to perform PCI in patients with non-ST-elevation acute coronary syndrome (but given immediately for patients with STEMI, because nearly all those patients undergo PCI).
Prasugrel was clearly beneficial, with a significant 20% lower rate of the combined end point of cardiovascular death, MI, and stroke at 15 months. However, bleeding risk was higher with prasugrel (2.4% vs 1.8%, hazard ratio 1.32, 95% confidence interval 1.02–1.68, P = .03). Looking at individual end points, the advantages of prasugrel were primarily in reducing rates of stent thrombosis and nonfatal MI. Death rates with the two drugs were equivalent, possibly because of the higher risk of bleeding with prasugrel. Bleeding in the prasugrel group was particularly increased in patients who underwent bypass surgery; more patients also needed transfusion.
Subgroup analysis showed that patients with a history of stroke or transient ischemic attack had higher rates of ischemic and bleeding events with prasugrel than with clopidogrel, leading to these being labeled as absolute contraindications to prasugrel. Patients over age 75 or who weighed less than 60 kg experienced excess bleeding risk that closely matched the reduction in ischemic event rates and thus did not have a net benefit with prasugrel.
Trial of ticagrelor vs clopidogrel
The PLATO trial28 included 18,000 patients, of whom 65% underwent revascularization and 35% were treated medically. The drug—clopidogrel or ticagrelor—was given in addition to aspirin at randomization (within 24 hours of symptom onset); this more closely follows clinical practice, in which dual antiplatelet therapy is started as soon as possible. This difference makes the PLATO study more relevant to practice for patients with non-ST-elevation acute coronary syndrome. Also, because they gave the drugs to all patients regardless of whether they were to undergo PCI, this study likely had a higher-risk population, which may be refected in the higher mortality rate at 30 days (5.9% in the clopidogrel group in the PLATO study vs 3.2% in the clopidogrel group in the TRITON study).
Another important difference between the trials testing prasugrel and ticagrelor is that patients who had already received a thienopyridine were excluded from the prasugrel trial but not from the ticagrelor trial. Nearly half the patients in the ticagrelor group were already taking clopidogrel. The clinical implication is that for patients who arrive from another facility and already have been given clopidogrel, it is safe to give ticagrelor. There is limited information about whether that is also true for prasugrel, although there is no known reason why the safety of adding prasugrel to clopidogrel should be different from that of ticagrelor.
The rate of ischemic events was 20% lower in the ticagrelor group than in the clopidogrel group, importantly including reductions in the incidence of death, MI, and stent thrombosis. There was no increase with ticagrelor compared with clopidogrel in bleeding associated with coronary artery bypass graft surgery, likely because of the more rapid washout of the ticagrelor effect, or in the need for blood transfusions. However, the rate of bleeding unrelated to coronary artery bypass was about 20% higher with ticagrelor.
In summary, more intense platelet inhibition reduces the risk of ischemic events, but, particularly for the irreversible inhibitor prasugrel, at the cost of a higher risk of bleeding. In general, the net benefit of these agents in preventing the irreversible complications of MI and (in the case of ticagrelor) death favor the use of the more intense ADP inhibitors in appropriate patients. Ticagrelor is indicated in patients with acute coronary syndromes undergoing invasive or conservative management; prasugrel is indicated in patients undergoing PCI, but contraindicated in patients with a previous stroke or transient ischemic event. Neither drug is indicated in patients undergoing elective PCI outside the setting of acute coronary syndromes, although these agents may be appropriate in patients with intolerance or allergy to clopidogrel.
Glycoprotein IIb/IIIa antagonists for select cases only
GP IIb/IIIa antagonists such as abciximab were previously used more commonly than they are today. Now, with routine pretreatment using thienopyridines, their role in acute coronary syndromes is less clear. They still play a role when routine dual antiplatelet therapy is not used, when prasugrel or ticagrelor is not used, and when heparin rather than an alternative antithrombin agent is used.
A meta-analysis29 of 3,755 patients showed a clear reduction in ischemic complications with abciximab as an adjunct to primary PCI for STEMI in patients treated with heparin.
Kastrati et al30 found that patients with non-ST-elevation acute coronary syndromes benefited from abciximab at the time of PCI with heparin, even though they had been routinely pretreated with clopidogrel. However, benefits were seen only in high-risk patients who had presented with elevated troponins.
On the other hand, the role of GP IIb/IIIa blockade for “upstream” medical management in patients with acute coronary syndromes has been eroded by several studies.
The ACUITY trial31 randomized more than 9,000 patients to receive either routine treatment with a GP IIb/IIIa inhibitor before angiography or deferred selective use in the catheterization laboratory only for patients undergoing PCI. No significant differences were found in rates of MI and death.
The Early ACS trial32 compared early routine eptifibatide vs delayed, provisional eptifibatide in 9,492 patients with acute coronary syndromes without ST elevation and who were assigned to an invasive strategy. The early-eptifibatide group received two boluses and an infusion of eptifibatide before angiography; the others received a placebo infusion, with provisional eptifibatide after angiography if the patient underwent PCI and was deemed at high risk. No significant difference in rates of death or MI were noted, and the early-eptifibatide group had significantly higher rates of bleeding and need for transfusion.
The FINESSE trial33 also discredited “facilitating” PCI by giving GP IIb/IIIa antagonists in patients with STEMI before arrival in the catheterization laboratory, with no benefit to giving abciximab ahead of time vs in the catheterization laboratory, and with an increased risk of bleeding complications.
These studies have helped narrow the use of GP IIb/IIIa inhibitors to the catheterization laboratory in conjunction with heparin anticoagulation (as compared with bivalirudin [Angiomax]; see below) and only in select or high-risk cases. These drugs are indicated in the medical phase of management only if patients cannot be stabilized by aspirin or ADP inhibition.
NEWER ANTITHROMBOTICS: ADVANTAGES UNCLEAR
The complex coagulation cascade has a number of components, but only a few are targeted by drugs that are approved and recommended: fondaparinux (Arixtra) and oral factor Xa inhibitors affect the prothrombinase complex (including factor X); bivalirudin and oral factor IIa inhibitors affect thrombin; and heparin and the low-molecular-weight heparins inhibit both targets.
Low-molecular-weight heparins
The SYNERGY trial34 randomized nearly 10,000 patients with non-ST-elevation acute coronary syndromes at high risk for ischemic cardiac complications managed with an invasive approach to either the low-molecular-weight heparin enoxaparin (Lovenox) or intravenous unfractionated heparin immediately after enrollment. Most patients underwent catheterization and revascularization. No clinical advantage was found for enoxaparin, and bleeding complications were increased.
The EXTRACT-TIMI 25 trial35 randomized more than 20,000 patients with STEMI who were about to undergo fibrinolysis to receive either enoxaparin throughout hospitalization (average of 8 days) or unfractionated heparin for at least 48 hours. The enoxaparin group had a lower rate of recurrent MI, but it was unclear if the difference was in part attributable to the longer therapy time. The enoxaparin group also had more bleeding.
Fondaparinux
The OASIS-5 trial36,37 compared enoxaparin and fondaparinux, an exclusive factor Xa inhibitor, in more than 20,000 patients with unstable angina or NSTEMI. Fondaparinux was associated with a lower risk of death and reinfarction as well as fewer bleeding events. However, the benefits were almost exclusively in patients treated medically. In those undergoing PCI within the first 8 days, no benefit was found, although there was still a significant reduction in major bleeding events. Catheter thrombosis was also increased in patients taking fondaparinux, but only in those who did not receive adequate unfractionated heparin treatment before PCI.
Bivalirudin superior at time of catheterization
The most significant advance in antithrombotic therapy for patients with acute coronary syndromes is bivalirudin. This drug has a clear role only in the catheterization laboratory, where patients can be switched to it from heparin, low-molecular-weight heparin, or fondaparinux.
Three trials38–40 evaluated the drug in a total of more than 20,000 patients receiving invasive management of coronary artery disease undergoing PCI for elective indications, NSTEMI, or STEMI.
Results were remarkably similar across the three trials. Patients who were treated with bivalirudin alone had the same rate of ischemic end points at 30 days as those receiving heparin plus a GP IIb/IIIa inhibitor, but bivalirudin was associated with a consistent and significant 40% to 50% lower bleeding risk. For the highest-risk patients, those with STEMI, the bivalirudin group also had a significantly lower risk of death at 1 year.41
OTHER DRUGS: EARLY TREATMENT NO LONGER ROUTINE
Most data for the use of therapies aside from antithrombotics are from studies of patients with STEMI, but findings can logically be extrapolated to those with non-ST-elevation acute coronary syndromes.
Beta-blockers: Cardiogenic shock a risk
For beta-blockers, many historical trials were done in stable coronary disease, but there are no large trials in the setting of NSTEMI or unstable angina, and only recently have there been large trials for STEMI. Before the availability of recent evidence, standard practice was to treat STEMI routinely with intravenous metoprolol (Lopressor) and then oral metoprolol.
When large studies were finally conducted, the results were sobering.
COMMIT.42 Nearly 46,000 patients with suspected acute MI were randomized to receive either metoprolol (up to 15 mg intravenously, then 200 mg by mouth daily until discharge or for up to 4 weeks in the hospital) or placebo. Surprisingly, although rates of reinfarction and ventricular fibrillation were lower with metoprolol, a higher risk of cardiogenic shock with early beta-blockade offset these benefits and the net mortality rate was not reduced. This study led to a reduction in the early use of beta-blockers in patients with STEMI.
The standard of care has now shifted from beta-blockers in everyone as early as possible after MI to being more cautious in patients with contraindications, including signs of heart failure or a low-output state, or even in those of advanced age or with borderline low blood pressure or a high heart rate. Patients who present late and therefore may have a larger infarct are also at higher risk.
Although the goal should be to ultimately discharge patients on beta-blocker therapy after an MI, there should be no rush to start one early.
Carvedilol now preferred after STEMI
The CAPRICORN trial43 randomized nearly 2,000 patients following MI with left ventricular dysfunction (an ejection fraction of 40% or below) to either placebo or the beta-blocker carvedilol (Coreg). Patients taking the drug had a clear reduction in rates of death and reinfarction, leading to this drug becoming the beta-blocker of choice in patients with ventricular dysfunction after STEMI.
Angiotensin-converting enzyme inhibitors: Early risk of cardiogenic shock
The use of angiotensin-converting enzyme (ACE) inhibitors after MI is also supported by several studies.44 Two very large studies, one of nearly 60,000 patients and one of nearly 20,000, showed a clear reduction in the mortality rate in those who received an ACE inhibitor. Most of the benefit was in patients with an ejection fraction of less than 40%. On the basis of these trials, ACE inhibitors are indicated for all patients for the first 30 days after MI and then indefinitely for those with left ventricular dysfunction. However, the trial in which an ACE inhibitor was given intravenously early on had to be stopped prematurely because of worse outcomes owing to cardiogenic shock.
These studies highlight again that for patients who are unstable in the first few days of an acute coronary syndrome, it is best to wait until their condition stabilizes and to start these therapies before hospital discharge.
Intensive statin therapy
In the last 20 years, unequivocal evidence has emerged to support the beneficial role of statins for secondary prevention in patients with established coronary artery disease. More-recent trials have also shown that intensive statin therapy (a high dose of a potent statin) improves outcomes better than lower doses.
The PROVE-IT TIMI 22 trial45 randomized patients after an acute coronary syndrome to receive either standard therapy (pravastatin [Pravachol] 40 mg) or intensive therapy (atorvastatin [Lipitor] 80 mg). The intensive-therapy group had a significantly lower rate of major cardiovascular events, and the difference persisted and grew over 30 months of follow-up.
A number of studies confirmed this and broadened the patient population to those with unstable or stable coronary disease. Regardless of the risk profile, the effects were consistent and showed that high-dose statins were better in preventing coronary death and MI.46
Guidelines are evolving toward recommendation of highest doses of statins independently of the target level of low-density lipoprotein cholesterol.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2004; 110:e82–e292. Erratum in: Circulation 2005; 111:2013–2014.
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000; 83:361–366.
- Rosamond W, Flegal K, Friday G, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115:e69–e171.
- Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992; 44:293–325.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343:311–322.
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109:1223–1225.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 2005; 293:979–986.
- Antman EM, Hand M, Armstron PW, et al; Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Cannon CP, Weintraub WS, Demopoulos LA, et al; TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344:1879–1887.
- Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55:858–864.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284:835–842.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Yousef O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8:547–559.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Yusuf S, Mehta SR, Zhao F, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003; 107:966–972.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Schömig A. Ticagrelor—is there need for a new player in the antiplatelet-therapy field? N Engl J Med 2009; 361:1108–1111.
- Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010; 122:394–403.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120:2577–2585.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Fiho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J 2004; 148:937–943.
- Kastrati A, Mehilli J, Neuman FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006; 295:1531–1538.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Giugliano RP, White JA, Bode C, et al; Early ACS Investigators. Early vs delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009; 360:2176–2190.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Fergusson JJ, Califf RM, Antman EM, et al; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004; 292:45–54.
- Antman EM, Morrow DA, McCabe CH; EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354:1477–1488.
- The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Stone GW, Witzenbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2007; 358:2218–2230.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Chen ZM, Pan HC, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Dargie JH. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med 1996; 335:1660–1667.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48:438–445.
- Antman EM, Anbe DT, Armstrong PW, et al; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Canadian Cardiovascular Society. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation 2004; 110:e82–e292. Erratum in: Circulation 2005; 111:2013–2014.
- Davies MJ. The pathophysiology of acute coronary syndromes. Heart 2000; 83:361–366.
- Rosamond W, Flegal K, Friday G, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007; 115:e69–e171.
- Granger CB, Califf RM, Topol EJ. Thrombolytic therapy for acute myocardial infarction. A review. Drugs 1992; 44:293–325.
- Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 1994; 343:311–322.
- Keely EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003; 361:13–20
- De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109:1223–1225.
- Dalby M, Bouzamondo A, Lechat P, Montalescot G. Transfer for primary angioplasty versus immediate thrombolysis in acute myocardial infarction: a meta-analysis. Circulation 2003; 108:1809–1814.
- Gersh BJ, Stone GW, White HD, Holmes DR Jr. Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 2005; 293:979–986.
- Antman EM, Hand M, Armstron PW, et al; Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008; 51:210–247.
- Di Mario C, Dudek D, Piscione F, et al; CARESS-in-AMI (Combined Abciximab Reteplase Stent Study in Acute Myocardial Infarction) Investigators. Immediate angioplasty versus standard therapy with rescue angioplasty after thrombolysis in the Combined Abciximab REteplase Stent Study in Acute Myocardial Infarction (CARESS-in-AMI): an open, prospective, randomised, multicentre trial. Lancet 2008; 371:559–568.
- Cantor WJ, Fitchett D, Borgundvaag B, et al; TRANSFER-AMI Trial Investigators. Routine early angioplasty after fibrinolysis for acute myocardial infarction. N Engl J Med 2009; 360:2705–2718.
- Cannon CP, Weintraub WS, Demopoulos LA, et al; TACTICS (Treat Angina With Aggrastat and Determine Cost of Therapy With an Invasive or Conservative Strategy)–Thrombolysis in Myocardial Infarction 18 Investigators. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 2001; 344:1879–1887.
- Damman P, Hirsch A, Windhausen F, Tijssen JG, de Winter RJ; ICTUS Investigators. 5-year clinical outcomes in the ICTUS (Invasive versus Conservative Treatment in Unstable coronary Syndromes) trial a randomized comparison of an early invasive versus selective invasive management in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010; 55:858–864.
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta-analysis of contemporary randomized clinical trials. J Am Coll Cardiol 2006; 48:1319–1325.
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000; 284:835–842.
- Mehta SR, Granger CB, Boden WE, et al; TIMACS Investigators. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med 2009; 360:2165–2175.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction. J Am Coll Cardiol 2007; 50:e1–e157.
- Yousef O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol 2011; 8:547–559.
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988; 2:349–360.
- CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010; 363:930–942.
- Yusuf S, Mehta SR, Zhao F, et al; Clopidogrel in Unstable angina to prevent Recurrent Events Trial Investigators. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003; 107:966–972.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Schömig A. Ticagrelor—is there need for a new player in the antiplatelet-therapy field? N Engl J Med 2009; 361:1108–1111.
- Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010; 122:394–403.
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009; 120:2577–2585.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wallentin L, Becker RC, Budaj A, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- de Queiroz Fernandes Araujo JO, Veloso HH, Braga De Paiva JM, Fiho MW, Vincenzo De Paola AA. Efficacy and safety of abciximab on acute myocardial infarction treated with percutaneous coronary interventions: a meta-analysis of randomized, controlled trials. Am Heart J 2004; 148:937–943.
- Kastrati A, Mehilli J, Neuman FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 2006; 295:1531–1538.
- Stone GW, Bertrand ME, Moses JW, et al; ACUITY Investigators. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY Timing trial. JAMA 2007; 297:591–602.
- Giugliano RP, White JA, Bode C, et al; Early ACS Investigators. Early vs delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009; 360:2176–2190.
- Ellis SG, Tendera M, de Belder MA, et al; FINESSE Investigators. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med 2008; 358:2205–2217.
- Fergusson JJ, Califf RM, Antman EM, et al; SYNERGY Trial Investigators. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA 2004; 292:45–54.
- Antman EM, Morrow DA, McCabe CH; EXTRACT-TIMI 25 Investigators. Enoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarction. N Engl J Med 2006; 354:1477–1488.
- The Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators. Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006; 354:1464–1476.
- Mehta SR, Granger CB, Eikelboom JW, et al. Efficacy and safety of fondaparinux versus enoxaparin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: results from the OASIS-5 trial. J Am Coll Cardiol 2007; 50:1742–1751.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003; 289:853–863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006; 355:2203–2216.
- Stone GW, Witzenbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2007; 358:2218–2230.
- Mehran R, Lansky AJ, Witzenbichler B, et al; HORIZONS-AMI Trial Investigators. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet 2009; 374:1149–1159.
- Chen ZM, Pan HC, Chen YP, et al; COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative Group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1622–1632.
- Dargie JH. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001; 357:1385–1390.
- Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction—evidence from clinical trials. N Engl J Med 1996; 335:1660–1667.
- Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol 2006; 48:438–445.
KEY POINTS
- For acute ST-elevation myocardial infarction, primary percutaneous coronary intervention is preferred over fibrinolytic therapy if it is available within 90 minutes of first medical contact.
- For non-ST-elevation acute coronary syndromes, either an early invasive or conservative strategy is recommended depending on patient risk and whether intensive medical therapy is available and appropriate.
- Daily aspirin therapy is indicated for all patients with acute coronary syndromes unless they have a true aspirin allergy.
- Adenosine diphosphate receptor inhibitors—clopidogrel, prasugrel, and ticagrelor—reduce ischemic events but increase bleeding risk and should be used only for patients with no history of stroke or transient ischemic attack.
A rare complication of infective endocarditis
An 85-year-old woman presented to the emergency department with a 2-hour history of dyspnea, dizziness, generalized weakness, nausea, and diaphoresis. Her medical history included hypertension, end-stage renal disease with hemodialysis, and atrial fibrillation.
She had an arteriovenous fistula for dialysis access in her right upper arm, with erythema around the site.
Her creatine kinase level was 1,434 U/L (normal range 30–220), creatine kinase MB 143.4 ng/mL (0.0–8.8 ng/mL), and troponin T 0.1 ng/mL (0.0–0.1 ng/mL). She had ST elevation in leads I and aVL. She was taken for emergency cardiac catheterization.
Three blood cultures were drawn on the day of cardiac catheterization. Two grew gram-positive organisms: one grew coagulase-negative Staphylococcus, and the other grew gram-positive bacilli (anaerobic, non-sporeforming). On the basis of these findings, intravenous vancomycin (Vancocin) was started. Seventy-two hours later, one of two blood cultures again grew coagulase-negative Staphylococcus. Five days after the start of antibiotic treatment, blood cultures were negative, and the patient received intravenous vancomycin for 4 weeks (from the time the blood cultures became negative) for native mitral valve endocarditis.
EMBOLISM AND ENDOCARDITIS: KEY FEATURES
An embolic event occurs in 22% to 50% of cases of infective endocarditis and can involve the lungs, bowel, other organs, or extremities.1 The incidence of embolization of the coronary arteries in patients with infective endocarditis is unknown, but in one case series2 it occurred in 8 (7.5%) of 107 cases. The most common site of coronary embolism is the LAD.3
Myocardial infarction is a rare complication of coronary artery embolization.2 It was reported in 17 (2.9%) of 586 consecutive patients with infective endocarditis.4 In patients with infectious endocarditis complicated by myocardial infarction, the death rate was nearly double that seen in patients with infective endocarditis without myocardial infarction (64% vs 33%).4
TREATMENT
The best treatment for this complication of infective endocarditis is not known, as it has not been well studied. The high death rate in these patients makes restoration of coronary perfusion essential.
Thrombolytics are usually avoided in patients with septic embolization because of concerns about concurrent intracerebral mycotic aneurysms and the risk of hemorrhage.
Percutaneous transluminal angioplasty carries a risk of distal mobilization of emboli, development of mycotic aneurysm at the balloon dilation site, or reocclusion due to a mobile embolus.5 Stent placement may improve vessel patency but carries a theoretic risk of infection in bacteremic patients. Percutaneous embolectomy has also been used either prior to or instead of stent placement.6 Surgical options include embolectomy in patients who may require surgery, and coronary artery bypass grafting for patients with chronic embolization.7
- Baddour LM, Wilson WR, Bayer AS, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434.
- Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at Columbia-Presbyterian Medical Center, 1968–1973. Medicine (Baltimore) 1978; 57:105–127.
- Glazier JJ. Interventional treatment of septic coronary embolism: sailing into uncharted and dangerous waters. J Interv Cardiol 2002; 15:305–307.
- Manzano MC, Vilacosta I, San Roman JA, et al. Acute cornary syndrome in infective endocarditis. Rev Esp Cardiol 2007; 60:24–31.
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect 2005; 51:e101–105.
- Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997; 20:885–888.
- Baek MJ, Kim HK, Yu CW, Na CY. Surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008; 33:116–118.
An 85-year-old woman presented to the emergency department with a 2-hour history of dyspnea, dizziness, generalized weakness, nausea, and diaphoresis. Her medical history included hypertension, end-stage renal disease with hemodialysis, and atrial fibrillation.
She had an arteriovenous fistula for dialysis access in her right upper arm, with erythema around the site.
Her creatine kinase level was 1,434 U/L (normal range 30–220), creatine kinase MB 143.4 ng/mL (0.0–8.8 ng/mL), and troponin T 0.1 ng/mL (0.0–0.1 ng/mL). She had ST elevation in leads I and aVL. She was taken for emergency cardiac catheterization.
Three blood cultures were drawn on the day of cardiac catheterization. Two grew gram-positive organisms: one grew coagulase-negative Staphylococcus, and the other grew gram-positive bacilli (anaerobic, non-sporeforming). On the basis of these findings, intravenous vancomycin (Vancocin) was started. Seventy-two hours later, one of two blood cultures again grew coagulase-negative Staphylococcus. Five days after the start of antibiotic treatment, blood cultures were negative, and the patient received intravenous vancomycin for 4 weeks (from the time the blood cultures became negative) for native mitral valve endocarditis.
EMBOLISM AND ENDOCARDITIS: KEY FEATURES
An embolic event occurs in 22% to 50% of cases of infective endocarditis and can involve the lungs, bowel, other organs, or extremities.1 The incidence of embolization of the coronary arteries in patients with infective endocarditis is unknown, but in one case series2 it occurred in 8 (7.5%) of 107 cases. The most common site of coronary embolism is the LAD.3
Myocardial infarction is a rare complication of coronary artery embolization.2 It was reported in 17 (2.9%) of 586 consecutive patients with infective endocarditis.4 In patients with infectious endocarditis complicated by myocardial infarction, the death rate was nearly double that seen in patients with infective endocarditis without myocardial infarction (64% vs 33%).4
TREATMENT
The best treatment for this complication of infective endocarditis is not known, as it has not been well studied. The high death rate in these patients makes restoration of coronary perfusion essential.
Thrombolytics are usually avoided in patients with septic embolization because of concerns about concurrent intracerebral mycotic aneurysms and the risk of hemorrhage.
Percutaneous transluminal angioplasty carries a risk of distal mobilization of emboli, development of mycotic aneurysm at the balloon dilation site, or reocclusion due to a mobile embolus.5 Stent placement may improve vessel patency but carries a theoretic risk of infection in bacteremic patients. Percutaneous embolectomy has also been used either prior to or instead of stent placement.6 Surgical options include embolectomy in patients who may require surgery, and coronary artery bypass grafting for patients with chronic embolization.7
An 85-year-old woman presented to the emergency department with a 2-hour history of dyspnea, dizziness, generalized weakness, nausea, and diaphoresis. Her medical history included hypertension, end-stage renal disease with hemodialysis, and atrial fibrillation.
She had an arteriovenous fistula for dialysis access in her right upper arm, with erythema around the site.
Her creatine kinase level was 1,434 U/L (normal range 30–220), creatine kinase MB 143.4 ng/mL (0.0–8.8 ng/mL), and troponin T 0.1 ng/mL (0.0–0.1 ng/mL). She had ST elevation in leads I and aVL. She was taken for emergency cardiac catheterization.
Three blood cultures were drawn on the day of cardiac catheterization. Two grew gram-positive organisms: one grew coagulase-negative Staphylococcus, and the other grew gram-positive bacilli (anaerobic, non-sporeforming). On the basis of these findings, intravenous vancomycin (Vancocin) was started. Seventy-two hours later, one of two blood cultures again grew coagulase-negative Staphylococcus. Five days after the start of antibiotic treatment, blood cultures were negative, and the patient received intravenous vancomycin for 4 weeks (from the time the blood cultures became negative) for native mitral valve endocarditis.
EMBOLISM AND ENDOCARDITIS: KEY FEATURES
An embolic event occurs in 22% to 50% of cases of infective endocarditis and can involve the lungs, bowel, other organs, or extremities.1 The incidence of embolization of the coronary arteries in patients with infective endocarditis is unknown, but in one case series2 it occurred in 8 (7.5%) of 107 cases. The most common site of coronary embolism is the LAD.3
Myocardial infarction is a rare complication of coronary artery embolization.2 It was reported in 17 (2.9%) of 586 consecutive patients with infective endocarditis.4 In patients with infectious endocarditis complicated by myocardial infarction, the death rate was nearly double that seen in patients with infective endocarditis without myocardial infarction (64% vs 33%).4
TREATMENT
The best treatment for this complication of infective endocarditis is not known, as it has not been well studied. The high death rate in these patients makes restoration of coronary perfusion essential.
Thrombolytics are usually avoided in patients with septic embolization because of concerns about concurrent intracerebral mycotic aneurysms and the risk of hemorrhage.
Percutaneous transluminal angioplasty carries a risk of distal mobilization of emboli, development of mycotic aneurysm at the balloon dilation site, or reocclusion due to a mobile embolus.5 Stent placement may improve vessel patency but carries a theoretic risk of infection in bacteremic patients. Percutaneous embolectomy has also been used either prior to or instead of stent placement.6 Surgical options include embolectomy in patients who may require surgery, and coronary artery bypass grafting for patients with chronic embolization.7
- Baddour LM, Wilson WR, Bayer AS, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434.
- Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at Columbia-Presbyterian Medical Center, 1968–1973. Medicine (Baltimore) 1978; 57:105–127.
- Glazier JJ. Interventional treatment of septic coronary embolism: sailing into uncharted and dangerous waters. J Interv Cardiol 2002; 15:305–307.
- Manzano MC, Vilacosta I, San Roman JA, et al. Acute cornary syndrome in infective endocarditis. Rev Esp Cardiol 2007; 60:24–31.
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect 2005; 51:e101–105.
- Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997; 20:885–888.
- Baek MJ, Kim HK, Yu CW, Na CY. Surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008; 33:116–118.
- Baddour LM, Wilson WR, Bayer AS, et al; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111:e394–e434.
- Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at Columbia-Presbyterian Medical Center, 1968–1973. Medicine (Baltimore) 1978; 57:105–127.
- Glazier JJ. Interventional treatment of septic coronary embolism: sailing into uncharted and dangerous waters. J Interv Cardiol 2002; 15:305–307.
- Manzano MC, Vilacosta I, San Roman JA, et al. Acute cornary syndrome in infective endocarditis. Rev Esp Cardiol 2007; 60:24–31.
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect 2005; 51:e101–105.
- Glazier JJ, McGinnity JG, Spears JR. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997; 20:885–888.
- Baek MJ, Kim HK, Yu CW, Na CY. Surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg 2008; 33:116–118.
Prasugrel for acute coronary syndromes: Faster, more potent, but higher bleeding risk
Prasugrel (Effient) is more potent and consistent in its effects than clopidogrel (Plavix), thus preventing more thrombotic events—but at a price of more bleeding. Therefore, the drugs must be appropriately selected for the individual patient.
Over the last 9 years, the thienopyridines—ticlopidine (Ticlid), clopidogrel, and now prasugrel—have become essential tools for treating acute coronary syndromes.
The usual underlying mechanism of acute coronary syndromes is thrombosis, caused by rupture of atherosclerotic plaque.1 Accordingly, antithrombotic agents—aspirin, heparin, lowmolecular-weight heparin, glycoprotein IIb/IIIa inhibitors, the direct thrombin inhibitor bivalirudin (Angiomax), and thienopyridines—have all been shown to reduce the risk of major adverse cardiac outcomes in this setting.
In this article, we review the pharmacology and evidence of effectiveness of the thienopyridine drugs, focusing on prasugrel, the latest thienopyridine to be approved by the US Food and Drug Administration (FDA).
THIENOPYRIDINES INHIBIT PLATELET ACTIVATION AND AGGREGATION
Thienopyridines are prodrugs that require conversion by hepatic cytochrome P450 enzymes. The active metabolites bind irreversibly to platelet P2Y12 receptors. Consequently, they permanently block signalling mediated by platelet adenosine diphosphate-P2Y12 receptors, thereby inhibiting glycoprotein IIb/IIIa receptor activation and platelet aggregation.
Aspirin, in contrast, inhibits platelets by blocking the thromboxane-mediated pathway. Therefore, the combination of aspirin plus a thienopyridine has an additive effect.2
The effect of thienopyridines on platelets is irreversible. Therefore, although the half-life of prasugrel’s active metabolite is 3.7 hours, its inhibitory effects last for 96 hours, essentially the time for half the body’s circulating platelets to be replaced.
TICLOPIDINE, THE FIRST THIENOPYRIDINE
Ticlopidine was the first thienopyridine to be approved by the FDA. Its initial studies in unstable angina were small, their designs did not call for patients to concurrently receive aspirin, and all they showed was that ticlopidine was about as beneficial as aspirin. Consequently, the studies had little impact on clinical practice.3
In a pivotal trial,4 patients who received coronary stents were randomized to afterward receive either the combination of ticlopidine plus aspirin or anticoagulation therapy with heparin, phenprocoumon (a coumarin derivative available in Europe), and aspirin. At 30 days, an ischemic complication (death, myocardial infarction [MI], repeat intervention) had occurred in 6.2% of the anticoagulation therapy group vs 1.6% of the ticlopidine group, a risk reduction of 75%. Rates of stent occlusion, MI, and revascularization were 80% to 85% lower in the ticlodipine group. This study paved the way for widespread use of thienopyridines.
Ticlopidine’s use was limited, however, by a 2.4% incidence of serious granulocytopenia and rare cases of thrombocytopenic purpura.
BENEFIT OF CLOPIDOGREL
Although prasugrel is the focus of this review, the trials of prasugrel all compared its efficacy with that of clopidogrel. Furthermore, many patients should still receive clopidogrel and not prasugrel, so it is important to be familiar with the evidence of clopidogrel’s benefit.
Once approved for clinical use, clopidogrel was substituted for ticlopidine in patients undergoing coronary stenting on the basis of studies showing it to be at least as effective as ticlopidine and more tolerable. A series of trials of clopidogrel were done in patients across a spectrum of risk groups, from those at high risk of coronary heart disease to those presenting with ST-elevation MI. The time of pretreatment in the studies ranged from 3 hours to 6 days before percutaneous coronary intervention, and the duration of treatment following intervention ranged from 30 days to 1 year.
Clopidogrel in non-ST-elevation acute coronary syndromes
The CURE trial2 (Clopidogrel in Unstable Angina to Prevent Recurrent Events), published in 2001, established clopidogrel as a therapy for unstable ischemic syndromes, whether treated medically or with revascularization. In that trial, 12,562 patients with acute coronary syndromes without ST elevation (ie, unstable angina or non-ST-elevation MI), as defined by electrocardiographic changes or positive cardiac markers, were randomized to receive clopidogrel (a 300-mg loading dose followed by 75-mg maintenance doses) or placebo for a mean duration of 9 months. All patients also received aspirin 75 mg to 325 mg daily.
The composite outcome of death from cardiovascular causes, nonfatal MI, or stroke occurred in 20% fewer patients treated with clopidogrel than with placebo (9.3% vs 11.4%). The benefit was similar in patients undergoing revascularization compared with those treated medically.
Although there were significantly more cases of major bleeding in the clopidogrel group than in the placebo group (3.7% vs 2.7%), the number of episodes of life-threatening bleeding or hemorrhagic strokes was the same.
PCI-CURE5 was a substudy of the CURE trial in patients who underwent a percutaneous coronary intervention. Patients were pretreated with clopidogrel or placebo for a mean of 6 days before the procedure. Afterward, they all received clopidogrel plus aspirin in an unblinded fashion for 2 to 4 weeks, and then the randomized study drug was resumed for a mean of 8 months.
Significantly fewer adverse events occurred in the clopidogrel group as tallied at the time of the intervention, 1 month later, and 8 months later.
Clopidogrel in ST-elevation acute MI
The CLARITY-TIMI 28 trial6 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) showed that adding clopidogrel (a 300-mg loading dose, then 75 mg daily) to aspirin benefitted patients with ST-elevation MI receiving fibrinolytic therapy. At 30 days, cardiovascular death, recurrent MI, or urgent revascularization had occurred in 11.6% of the clopidogrel group vs 14.1% of the placebo group, a statistically significant difference. The rates of major or minor bleeding were no higher in the clopidogrel group than in the placebo group, an especially remarkable finding in patients receiving thrombolytic therapy.
PCI-CLARITY.7 About half of the patients in the CLARITY trial ultimately underwent a percutaneous coronary intervention after fibrinolytic therapy, with results reported as the PCI-CLARITY substudy. Like those in PCI-CURE, these patients were randomized to receive pretreatment with either clopidogrel or placebo before the procedure, in this study for a median of 3 days. Both groups received clopidogrel afterward. At 30 days from randomization, the outcome of cardiovascular death, MI, or stroke had occurred in 7.5% of the clopidogrel group compared with 12.0% of the placebo group, which was statistically significant, without any significant excess in the rates of major or minor bleeding.
COMMIT8 (the Clopidogrel and Metoprolol in Myocardial Infarction Trial) also showed clopidogrel to be beneficial in patients with acute MI. This trial included more than 45,000 patients in China with acute MI, 93% of whom had ST-segment elevation. In contrast to CLARITY, in COMMIT barely more than half of the patients received fibrinolysis, fewer than 5% proceeded to percutaneous interventions, and no loading dose was given: patients in the clopidogrel group received 75 mg/day from the outset.
At 15 days, the incidence of death, reinfarction, or stroke was 9.2% with clopidogrel compared with 10.1% with placebo, a small but statistically significant difference. Again, the rate of major bleeding was not significantly higher, either overall or in patients over age 70.
Of note, patients over age 75 were excluded from CLARITY, and as mentioned, no loading dose was used in COMMIT. Thus, for patients receiving fibrinolysis who are over age 75, there is no evidence to support the safety of a loading dose, and clopidogrel should be started at 75 mg daily.
Clopidogrel in elective percutaneous coronary intervention
The CREDO trial9 (Clopidogrel for the Reduction of Events During Observation) was in patients referred for elective percutaneous coronary intervention. Three to 24 hours before the procedure, the patients received either a 300-mg loading dose of clopidogrel or placebo; afterward, all patients received clopidogrel 75 mg/day for 28 days. All patients also received aspirin.
A clopidogrel loading dose 3 to 24 hours before the intervention did not produce a statistically significant reduction in ischemic events, although a post hoc subgroup analysis suggested that patients who received the loading dose between 6 and 24 hours before did benefit, with a relative risk reduction of 38.6% in the composite end point (P = .051).
After 28 days, the patients who had received the clopidogrel loading dose were continued on clopidogrel, while those in the placebo group were switched back to placebo. At 1 year, the investigators found a significantly lower rate of the composite end point with the prolonged course of clopidogrel (8.5% vs 11.5%).
In summary, these studies found clopidogrel to be beneficial in a broad spectrum of coronary diseases. Subgroup analyses suggest that pretreatment before percutaneous coronary intervention provides additional benefit, particularly if clopidogrel is given at least 6 hours in advance (the time necessary for clopidogrel to cause substantial platelet inhibition).
SOME PATIENTS RESPOND LESS TO CLOPIDOGREL
The level of platelet inhibition induced by clopidogrel varies. In different studies, the frequency of clopidogrel “nonresponsiveness” ranged from 5% to 56% of patients, depending on which test and which cutoff values were used. The distribution of responses to clopidogrel is wide and fits a normal gaussian curve.10
A large fraction of the population carries a gene that may account for some of the interpatient variation in platelet inhibition with clopidogrel. Carriers of a reduced-function CYP2C19 allele—approximately 30% of people in one study—have significantly lower levels of the active metabolite of clopidogrel, less platelet inhibition from clopidogrel therapy, and a 53% higher rate of death from cardiovascular causes, MI, or stroke.11
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel, FDA-approved in July 2009 for the treatment of acute coronary syndromes, is given in an oral loading dose of 60 mg followed by an oral maintenance dose of 10 mg daily.
Pharmacology of prasugrel vs clopidogrel
As noted previously, the thienopyridines are prodrugs that require hepatic conversion to exert antiplatelet effects.
Metabolism. Prasugrel’s hepatic activation involves a single step, in contrast to the multiple-step process required for activation of clopidogrel. Clopidogrel is primarily hydrolyzed by intestinal and plasma esterases to an inactive terminal metabolite, with the residual unhydrolized drug undergoing a two-step metabolism that depends on cytochrome P450 enzymes. Prasugrel is also extensively hydrolyzed by these esterases, but the intermediate product is then metabolized in a single step to the active sulfhydryl compound, mainly by CYP3A4 and CYP2B6.
Thus, about 80% of an orally absorbed dose of prasugrel is converted to active drug, compared with only 10% to 20% of absorbed clopidogrel.
Time to peak effect. With clopidogrel, maximal inhibition of platelet aggregation occurs 3 to 5 days after starting therapy with 75 mg daily without a loading dose, but within 4 to 6 hours if a loading dose of 300 to 600 mg is given. In contrast, a prasugrel loading dose produces more than 80% of its platelet inhibitory effects by 30 minutes, and peak activity is observed within 4 hours.12 The platelet inhibition induced by prasugrel at 30 minutes after administration is comparable to the peak effect of clopidogrel at 6 hours.13
Dose-response. Prasugrel’s inhibition of platelet aggregation is dose-related.
Prasugrel is about 10 times more potent than clopidogrel and 100 times more potent than ticlopidine. Thus, treatment with 5 mg of prasugrel results in inhibition of platelet activity (distributed in a gaussian curve) very similar to that produced by 75 mg of clopidogrel. On the other hand, even a maintenance dose of 150 mg of clopidogrel inhibits platelet activity to a lesser degree than 10 mg of prasugrel (46% vs 61%),14 so clopidogrel appears to reach a plateau of platelet inhibition that prasugrel can overcome.
At the approved dose of prasugrel, inhibition of platelet aggregation is significantly greater and there are fewer “nonresponders” than with clopidogrel.
Interactions. Drugs that inhibit CYP3A4 do not inhibit the efficacy of prasugrel, but they can inhibit that of clopidogrel. Some commonly used drugs that have this effect are the statins (eg, atorvastain [Lipitor]) and the macrolide antibiotics (eg, erythromycin). Furthermore, whereas proton pump inhibitors have been shown to diminish the effect of clopidogrel by reducing the formation of its active metabolite, no such effect has been noted with prasugrel.
Prasugrel in phase 2 trials: Finding the optimal dosage
A phase 2 trial compared three prasugrel regimens (loading dose/daily maintenance dose of 40 mg/7.5 mg, 60 mg/10 mg, and 60 mg/15 mg) and standard clopidogrel therapy (300 mg/75 mg) in patients undergoing elective or urgent percutaneous coronary intervention.15 No significant difference in outcomes was seen in the groups receiving the three prasugrel regimens. However, more “minimal bleeding events” (defined by the criteria of the TIMI trial16) occurred with high-dose prasugrel than with lower-dose prasugrel or with clopidogrel, leading to use of the intermediate-dose prasugrel regimen (60-mg loading dose, 10-mg daily maintenance) for later trials.
Another phase 2 trial randomized 201 patients undergoing elective percutaneous coronary intervention to receive prasugrel 60 mg/10 mg or clopidogrel 600 mg/150 mg.14 In all patients, the loading dose was given about 1 hour before cardiac catheterization. As soon as 30 minutes after the loading dose, platelet inhibition was superior with prasugrel (31% vs 5% inhibition of platelet aggregation), and it remained significantly higher at 6 hours (75% vs 32%) and during the maintenance phase (61% vs 46%).
Phase 3 trial of prasugrel vs clopidogrel: TRITON-TIMI 38
Only one large phase 3 trial of prasugrel has been completed: TRITON-TIMI 38 (the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction),17 which enrolled adults with moderate-risk to high-risk acute coronary syndromes scheduled to undergo a percutaneous coronary intervention. In this trial, 10,074 patients were enrolled who had moderate-to high-risk unstable angina or non-ST-elevation MI, and 3,534 patients were enrolled who had ST-elevation MI.
Patients were randomized to receive prasugrel (a 60-mg loading dose, then 10 mg daily) or clopidogrel (a 300-mg loading dose, then 75 mg daily) and were treated for 6 to 15 months. All patients also received aspirin.
These benefits came at a price of more bleeding. Of those patients who did not undergo coronary artery bypass grafting, more experienced bleeding in the prasugrel group than in the clopidogrel group (2.4% vs 1.8%, P = .03), including a higher rate of life-threatening bleeding (1.4% vs 0.89%, P = .01) and fatal bleeding (0.4% vs 0.1%, P = .002). More patients discontinued prasugrel because of hemorrhage (2.5% vs 1.4%, P < .001). In patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in those who received prasugrel than in those who received clopidogrel (13.4% vs 3.2%, P < .001).
A higher rate of adverse events related to colon cancer was also noted in patients treated with prasugrel, although the authors suggest this may have resulted from the stronger antiplatelet effects of prasugrel bringing more tumors to medical attention due to bleeding.
Overall death rates did not differ significantly between the treatment groups.
In a post hoc analysis,18 prasugrel was superior to clopidogrel in preventing ischemic events both during the first 3 days following randomization (the “loading phase”) and for the remainder of the trial (the “maintenance phase”). Whereas bleeding risk was similar with the two drugs during the loading phase, prasugrel was subsequently associated with more bleeding during the maintenance phase.
Certain patient subgroups had no net benefit or even suffered harm from prasugrel compared with clopidogrel.17 Patients with previous stroke or transient ischemic attack had net harm from prasugrel (hazard ratio 1.54, P = .04) and showed a strong trend toward a greater rate of major bleeding (P = .06). Patients age 75 and older and those weighing less than 60 kg had no net benefit from prasugrel.
Cost of prasugrel
Prasugrel is currently priced at 18% more than clopidogrel, with average wholesale prices per pill of $6.65 for prasugrel 10 mg compared with $5.63 for clopidogrel 75 mg. (Prasugrel 10-mg pills cost $6.33 at drugstore.com or $7.60 at CVS; clopidogrel 75-mg pills cost $5.33 at drugstore.com or $6.43 at CVS.) The patent on clopidogrel expires in November 2011, after which the price differential is expected to become significantly greater.
TICAGRELOR, A REVERSIBLE ORAL AGENT
Ticagrelor, the first reversible oral P2Y12 receptor antagonist, is an alternative to thienopyridine therapy for acute coronary syndromes.
Ticagrelor is quickly absorbed, does not require metabolic activation, and has a rapid antiplatelet effect and offset of effect, which closely follow drug-exposure levels. In a large randomized controlled trial in patients with acute coronary syndromes with or without STsegment elevation, treatment with ticagrelor compared with clopidogrel resulted in a significant reduction in death from vascular causes, MI, or stroke (9.8% vs 11.7%).19
Given its reversible effect on platelet inhibition, ticagrelor may be preferred in patients whose coronary anatomy is unknown and for whom coronary artery bypass grafting is deemed probable. It is still undergoing trials and is not yet approved.
TAKE-HOME POINTS
Prasugrel is more potent, more rapid in onset, and more consistent in inhibiting platelet aggregation than clopidogrel. A large clinical trial17 found prasugrel to be superior to clopidogrel for patients with moderate-to high-risk acute coronary syndromes with high probability of undergoing a percutaneous coronary intervention.
Who should receive prasugrel, and how?
Prasugrel should be given after angiography to patients with non-ST-elevation acute coronary syndromes or at presentation to patients with ST-elevation MI. When used for planned percutaneous coronary intervention, prasugrel should be given at least 30 minutes before the intervention, as was done in phase 2 trials (although its routine use in this situation is not recommended—see below).
It is given in a one-time loading dose of 60 mg by mouth and then maintained with 10 mg by mouth once daily for at least 1 year. (At least 9 months of treatment with a thienopyridine is indicated for patients with acute coronary syndromes who are medically treated, and at least 1 year is indicated following urgent or elective percutaneous coronary intervention, including balloon angioplasty and placement of a bare-metal or drug-eluting stent.)
Who should not receive prasugrel?
For now, prasugrel should be avoided in favor of clopidogrel in patients at higher risk of bleeding. It is clearly contraindicated in patients with prior transient ischemic attack or stroke, for whom the risk of serious bleeding seems to be prohibitive. It should generally be avoided in patients age 75 and older, although it might be considered in those at particularly high risk of stent thrombosis, such as those with diabetes or prior MI. In patients weighing less than 60 kg, the package insert advises a reduced dose (5 mg), although clinical evidence for this practice is lacking.
As yet, we have no data assuring that prasugrel is safe to use in combination with fibrinolytic agents, so patients on thrombolytic therapy for acute MI should continue to receive clopidogrel starting immediately after lysis. Furthermore, in patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in the prasugrel group than in the clopidogrel group in the TRITON-TIMI 38 trial.17 No thienopyridine should be given to patients likely to proceed to coronary artery bypass grafting.
Only clopidogrel has evidence supporting its use as an alternative to aspirin for patients with atherosclerotic disease who cannot tolerate aspirin. Neither drug has evidence for use for primary prevention.
Other areas of uncertainty
Prior to angiography. Indications for prasugrel are currently limited by the narrow scope of the trial data. TRITON-TIMI 38,17 the only large trial completed to date, randomized patients to receive prasugrel only after their coronary anatomy was known, except for ST-elevation MI patients. It is unknown whether the benefits of prasugrel will outweigh the higher risk of bleeding in patients with acute coronary syndromes who do not proceed to percutaneous coronary interventions.
A clinical trial is currently under way comparing prasugrel with clopidogrel in 10,000 patients with acute coronary syndromes who will be medically managed without planned revascularization: A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS), ClinicalTrials.gov Identifier: NCT00699998. The trial has an estimated completion date of March 2011.
In cases of non-ST-elevation acute coronary syndrome, it is reasonable to wait to give a thienopyridine until after the coronary anatomy has been defined, if angiography will be completed soon after presentation. For example, a 1-hour delay before giving prasugrel still delivers antiplatelet therapy more quickly than giving clopidogrel on presentation. If longer delays are expected before angiography, however, the patient should be given a loading dose of clopidogrel “up front,” in accordance with guidelines published by the American College of Cardiology, American Heart Association, and European Society of Cardiology,20 which recommend starting a thienopyridine early during hospitalization based on trial data with clopidogrel.
Patients undergoing elective percutaneous coronary intervention are at lower risk of stent thrombosis and other ischemic complications, so it is possible that the benefits of prasugrel would not outweigh the risks in these patients. Thus, prasugrel cannot yet be recommended for routine elective percutaneous coronary intervention except in individual cases in which the interventionalist feels that the patient may be at higher risk of thrombosis.
- Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med 2000; 342:101–114.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Balsano F, Rizzon P, Violi F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 1990; 82:17–26.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Mehta SR, Yusuf S, Peters RJG, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Sabatine MS, Cannon CP, Gibson CM, et al; CLA RITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with STsegment elevation. N Engl J Med 2005; 352:1179–1189.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005: 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of a front-loaded regimen of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20:2316–2321.
- Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol 2007; 100:331–336.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLETIMI 44 Investigators. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Wiviott SD, Antman EM, Winters KJ, et al; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 Trial. Circulation 2005; 111:3366–3373.
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Ann Intern Med 1991; 115:256–265.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51:2028–2033.
- Wallentin L, Becker RC, Budaj A, Freij A, Thorsén M, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—summary article*1: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002; 40:1366–1374.
Prasugrel (Effient) is more potent and consistent in its effects than clopidogrel (Plavix), thus preventing more thrombotic events—but at a price of more bleeding. Therefore, the drugs must be appropriately selected for the individual patient.
Over the last 9 years, the thienopyridines—ticlopidine (Ticlid), clopidogrel, and now prasugrel—have become essential tools for treating acute coronary syndromes.
The usual underlying mechanism of acute coronary syndromes is thrombosis, caused by rupture of atherosclerotic plaque.1 Accordingly, antithrombotic agents—aspirin, heparin, lowmolecular-weight heparin, glycoprotein IIb/IIIa inhibitors, the direct thrombin inhibitor bivalirudin (Angiomax), and thienopyridines—have all been shown to reduce the risk of major adverse cardiac outcomes in this setting.
In this article, we review the pharmacology and evidence of effectiveness of the thienopyridine drugs, focusing on prasugrel, the latest thienopyridine to be approved by the US Food and Drug Administration (FDA).
THIENOPYRIDINES INHIBIT PLATELET ACTIVATION AND AGGREGATION
Thienopyridines are prodrugs that require conversion by hepatic cytochrome P450 enzymes. The active metabolites bind irreversibly to platelet P2Y12 receptors. Consequently, they permanently block signalling mediated by platelet adenosine diphosphate-P2Y12 receptors, thereby inhibiting glycoprotein IIb/IIIa receptor activation and platelet aggregation.
Aspirin, in contrast, inhibits platelets by blocking the thromboxane-mediated pathway. Therefore, the combination of aspirin plus a thienopyridine has an additive effect.2
The effect of thienopyridines on platelets is irreversible. Therefore, although the half-life of prasugrel’s active metabolite is 3.7 hours, its inhibitory effects last for 96 hours, essentially the time for half the body’s circulating platelets to be replaced.
TICLOPIDINE, THE FIRST THIENOPYRIDINE
Ticlopidine was the first thienopyridine to be approved by the FDA. Its initial studies in unstable angina were small, their designs did not call for patients to concurrently receive aspirin, and all they showed was that ticlopidine was about as beneficial as aspirin. Consequently, the studies had little impact on clinical practice.3
In a pivotal trial,4 patients who received coronary stents were randomized to afterward receive either the combination of ticlopidine plus aspirin or anticoagulation therapy with heparin, phenprocoumon (a coumarin derivative available in Europe), and aspirin. At 30 days, an ischemic complication (death, myocardial infarction [MI], repeat intervention) had occurred in 6.2% of the anticoagulation therapy group vs 1.6% of the ticlopidine group, a risk reduction of 75%. Rates of stent occlusion, MI, and revascularization were 80% to 85% lower in the ticlodipine group. This study paved the way for widespread use of thienopyridines.
Ticlopidine’s use was limited, however, by a 2.4% incidence of serious granulocytopenia and rare cases of thrombocytopenic purpura.
BENEFIT OF CLOPIDOGREL
Although prasugrel is the focus of this review, the trials of prasugrel all compared its efficacy with that of clopidogrel. Furthermore, many patients should still receive clopidogrel and not prasugrel, so it is important to be familiar with the evidence of clopidogrel’s benefit.
Once approved for clinical use, clopidogrel was substituted for ticlopidine in patients undergoing coronary stenting on the basis of studies showing it to be at least as effective as ticlopidine and more tolerable. A series of trials of clopidogrel were done in patients across a spectrum of risk groups, from those at high risk of coronary heart disease to those presenting with ST-elevation MI. The time of pretreatment in the studies ranged from 3 hours to 6 days before percutaneous coronary intervention, and the duration of treatment following intervention ranged from 30 days to 1 year.
Clopidogrel in non-ST-elevation acute coronary syndromes
The CURE trial2 (Clopidogrel in Unstable Angina to Prevent Recurrent Events), published in 2001, established clopidogrel as a therapy for unstable ischemic syndromes, whether treated medically or with revascularization. In that trial, 12,562 patients with acute coronary syndromes without ST elevation (ie, unstable angina or non-ST-elevation MI), as defined by electrocardiographic changes or positive cardiac markers, were randomized to receive clopidogrel (a 300-mg loading dose followed by 75-mg maintenance doses) or placebo for a mean duration of 9 months. All patients also received aspirin 75 mg to 325 mg daily.
The composite outcome of death from cardiovascular causes, nonfatal MI, or stroke occurred in 20% fewer patients treated with clopidogrel than with placebo (9.3% vs 11.4%). The benefit was similar in patients undergoing revascularization compared with those treated medically.
Although there were significantly more cases of major bleeding in the clopidogrel group than in the placebo group (3.7% vs 2.7%), the number of episodes of life-threatening bleeding or hemorrhagic strokes was the same.
PCI-CURE5 was a substudy of the CURE trial in patients who underwent a percutaneous coronary intervention. Patients were pretreated with clopidogrel or placebo for a mean of 6 days before the procedure. Afterward, they all received clopidogrel plus aspirin in an unblinded fashion for 2 to 4 weeks, and then the randomized study drug was resumed for a mean of 8 months.
Significantly fewer adverse events occurred in the clopidogrel group as tallied at the time of the intervention, 1 month later, and 8 months later.
Clopidogrel in ST-elevation acute MI
The CLARITY-TIMI 28 trial6 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) showed that adding clopidogrel (a 300-mg loading dose, then 75 mg daily) to aspirin benefitted patients with ST-elevation MI receiving fibrinolytic therapy. At 30 days, cardiovascular death, recurrent MI, or urgent revascularization had occurred in 11.6% of the clopidogrel group vs 14.1% of the placebo group, a statistically significant difference. The rates of major or minor bleeding were no higher in the clopidogrel group than in the placebo group, an especially remarkable finding in patients receiving thrombolytic therapy.
PCI-CLARITY.7 About half of the patients in the CLARITY trial ultimately underwent a percutaneous coronary intervention after fibrinolytic therapy, with results reported as the PCI-CLARITY substudy. Like those in PCI-CURE, these patients were randomized to receive pretreatment with either clopidogrel or placebo before the procedure, in this study for a median of 3 days. Both groups received clopidogrel afterward. At 30 days from randomization, the outcome of cardiovascular death, MI, or stroke had occurred in 7.5% of the clopidogrel group compared with 12.0% of the placebo group, which was statistically significant, without any significant excess in the rates of major or minor bleeding.
COMMIT8 (the Clopidogrel and Metoprolol in Myocardial Infarction Trial) also showed clopidogrel to be beneficial in patients with acute MI. This trial included more than 45,000 patients in China with acute MI, 93% of whom had ST-segment elevation. In contrast to CLARITY, in COMMIT barely more than half of the patients received fibrinolysis, fewer than 5% proceeded to percutaneous interventions, and no loading dose was given: patients in the clopidogrel group received 75 mg/day from the outset.
At 15 days, the incidence of death, reinfarction, or stroke was 9.2% with clopidogrel compared with 10.1% with placebo, a small but statistically significant difference. Again, the rate of major bleeding was not significantly higher, either overall or in patients over age 70.
Of note, patients over age 75 were excluded from CLARITY, and as mentioned, no loading dose was used in COMMIT. Thus, for patients receiving fibrinolysis who are over age 75, there is no evidence to support the safety of a loading dose, and clopidogrel should be started at 75 mg daily.
Clopidogrel in elective percutaneous coronary intervention
The CREDO trial9 (Clopidogrel for the Reduction of Events During Observation) was in patients referred for elective percutaneous coronary intervention. Three to 24 hours before the procedure, the patients received either a 300-mg loading dose of clopidogrel or placebo; afterward, all patients received clopidogrel 75 mg/day for 28 days. All patients also received aspirin.
A clopidogrel loading dose 3 to 24 hours before the intervention did not produce a statistically significant reduction in ischemic events, although a post hoc subgroup analysis suggested that patients who received the loading dose between 6 and 24 hours before did benefit, with a relative risk reduction of 38.6% in the composite end point (P = .051).
After 28 days, the patients who had received the clopidogrel loading dose were continued on clopidogrel, while those in the placebo group were switched back to placebo. At 1 year, the investigators found a significantly lower rate of the composite end point with the prolonged course of clopidogrel (8.5% vs 11.5%).
In summary, these studies found clopidogrel to be beneficial in a broad spectrum of coronary diseases. Subgroup analyses suggest that pretreatment before percutaneous coronary intervention provides additional benefit, particularly if clopidogrel is given at least 6 hours in advance (the time necessary for clopidogrel to cause substantial platelet inhibition).
SOME PATIENTS RESPOND LESS TO CLOPIDOGREL
The level of platelet inhibition induced by clopidogrel varies. In different studies, the frequency of clopidogrel “nonresponsiveness” ranged from 5% to 56% of patients, depending on which test and which cutoff values were used. The distribution of responses to clopidogrel is wide and fits a normal gaussian curve.10
A large fraction of the population carries a gene that may account for some of the interpatient variation in platelet inhibition with clopidogrel. Carriers of a reduced-function CYP2C19 allele—approximately 30% of people in one study—have significantly lower levels of the active metabolite of clopidogrel, less platelet inhibition from clopidogrel therapy, and a 53% higher rate of death from cardiovascular causes, MI, or stroke.11
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel, FDA-approved in July 2009 for the treatment of acute coronary syndromes, is given in an oral loading dose of 60 mg followed by an oral maintenance dose of 10 mg daily.
Pharmacology of prasugrel vs clopidogrel
As noted previously, the thienopyridines are prodrugs that require hepatic conversion to exert antiplatelet effects.
Metabolism. Prasugrel’s hepatic activation involves a single step, in contrast to the multiple-step process required for activation of clopidogrel. Clopidogrel is primarily hydrolyzed by intestinal and plasma esterases to an inactive terminal metabolite, with the residual unhydrolized drug undergoing a two-step metabolism that depends on cytochrome P450 enzymes. Prasugrel is also extensively hydrolyzed by these esterases, but the intermediate product is then metabolized in a single step to the active sulfhydryl compound, mainly by CYP3A4 and CYP2B6.
Thus, about 80% of an orally absorbed dose of prasugrel is converted to active drug, compared with only 10% to 20% of absorbed clopidogrel.
Time to peak effect. With clopidogrel, maximal inhibition of platelet aggregation occurs 3 to 5 days after starting therapy with 75 mg daily without a loading dose, but within 4 to 6 hours if a loading dose of 300 to 600 mg is given. In contrast, a prasugrel loading dose produces more than 80% of its platelet inhibitory effects by 30 minutes, and peak activity is observed within 4 hours.12 The platelet inhibition induced by prasugrel at 30 minutes after administration is comparable to the peak effect of clopidogrel at 6 hours.13
Dose-response. Prasugrel’s inhibition of platelet aggregation is dose-related.
Prasugrel is about 10 times more potent than clopidogrel and 100 times more potent than ticlopidine. Thus, treatment with 5 mg of prasugrel results in inhibition of platelet activity (distributed in a gaussian curve) very similar to that produced by 75 mg of clopidogrel. On the other hand, even a maintenance dose of 150 mg of clopidogrel inhibits platelet activity to a lesser degree than 10 mg of prasugrel (46% vs 61%),14 so clopidogrel appears to reach a plateau of platelet inhibition that prasugrel can overcome.
At the approved dose of prasugrel, inhibition of platelet aggregation is significantly greater and there are fewer “nonresponders” than with clopidogrel.
Interactions. Drugs that inhibit CYP3A4 do not inhibit the efficacy of prasugrel, but they can inhibit that of clopidogrel. Some commonly used drugs that have this effect are the statins (eg, atorvastain [Lipitor]) and the macrolide antibiotics (eg, erythromycin). Furthermore, whereas proton pump inhibitors have been shown to diminish the effect of clopidogrel by reducing the formation of its active metabolite, no such effect has been noted with prasugrel.
Prasugrel in phase 2 trials: Finding the optimal dosage
A phase 2 trial compared three prasugrel regimens (loading dose/daily maintenance dose of 40 mg/7.5 mg, 60 mg/10 mg, and 60 mg/15 mg) and standard clopidogrel therapy (300 mg/75 mg) in patients undergoing elective or urgent percutaneous coronary intervention.15 No significant difference in outcomes was seen in the groups receiving the three prasugrel regimens. However, more “minimal bleeding events” (defined by the criteria of the TIMI trial16) occurred with high-dose prasugrel than with lower-dose prasugrel or with clopidogrel, leading to use of the intermediate-dose prasugrel regimen (60-mg loading dose, 10-mg daily maintenance) for later trials.
Another phase 2 trial randomized 201 patients undergoing elective percutaneous coronary intervention to receive prasugrel 60 mg/10 mg or clopidogrel 600 mg/150 mg.14 In all patients, the loading dose was given about 1 hour before cardiac catheterization. As soon as 30 minutes after the loading dose, platelet inhibition was superior with prasugrel (31% vs 5% inhibition of platelet aggregation), and it remained significantly higher at 6 hours (75% vs 32%) and during the maintenance phase (61% vs 46%).
Phase 3 trial of prasugrel vs clopidogrel: TRITON-TIMI 38
Only one large phase 3 trial of prasugrel has been completed: TRITON-TIMI 38 (the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction),17 which enrolled adults with moderate-risk to high-risk acute coronary syndromes scheduled to undergo a percutaneous coronary intervention. In this trial, 10,074 patients were enrolled who had moderate-to high-risk unstable angina or non-ST-elevation MI, and 3,534 patients were enrolled who had ST-elevation MI.
Patients were randomized to receive prasugrel (a 60-mg loading dose, then 10 mg daily) or clopidogrel (a 300-mg loading dose, then 75 mg daily) and were treated for 6 to 15 months. All patients also received aspirin.
These benefits came at a price of more bleeding. Of those patients who did not undergo coronary artery bypass grafting, more experienced bleeding in the prasugrel group than in the clopidogrel group (2.4% vs 1.8%, P = .03), including a higher rate of life-threatening bleeding (1.4% vs 0.89%, P = .01) and fatal bleeding (0.4% vs 0.1%, P = .002). More patients discontinued prasugrel because of hemorrhage (2.5% vs 1.4%, P < .001). In patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in those who received prasugrel than in those who received clopidogrel (13.4% vs 3.2%, P < .001).
A higher rate of adverse events related to colon cancer was also noted in patients treated with prasugrel, although the authors suggest this may have resulted from the stronger antiplatelet effects of prasugrel bringing more tumors to medical attention due to bleeding.
Overall death rates did not differ significantly between the treatment groups.
In a post hoc analysis,18 prasugrel was superior to clopidogrel in preventing ischemic events both during the first 3 days following randomization (the “loading phase”) and for the remainder of the trial (the “maintenance phase”). Whereas bleeding risk was similar with the two drugs during the loading phase, prasugrel was subsequently associated with more bleeding during the maintenance phase.
Certain patient subgroups had no net benefit or even suffered harm from prasugrel compared with clopidogrel.17 Patients with previous stroke or transient ischemic attack had net harm from prasugrel (hazard ratio 1.54, P = .04) and showed a strong trend toward a greater rate of major bleeding (P = .06). Patients age 75 and older and those weighing less than 60 kg had no net benefit from prasugrel.
Cost of prasugrel
Prasugrel is currently priced at 18% more than clopidogrel, with average wholesale prices per pill of $6.65 for prasugrel 10 mg compared with $5.63 for clopidogrel 75 mg. (Prasugrel 10-mg pills cost $6.33 at drugstore.com or $7.60 at CVS; clopidogrel 75-mg pills cost $5.33 at drugstore.com or $6.43 at CVS.) The patent on clopidogrel expires in November 2011, after which the price differential is expected to become significantly greater.
TICAGRELOR, A REVERSIBLE ORAL AGENT
Ticagrelor, the first reversible oral P2Y12 receptor antagonist, is an alternative to thienopyridine therapy for acute coronary syndromes.
Ticagrelor is quickly absorbed, does not require metabolic activation, and has a rapid antiplatelet effect and offset of effect, which closely follow drug-exposure levels. In a large randomized controlled trial in patients with acute coronary syndromes with or without STsegment elevation, treatment with ticagrelor compared with clopidogrel resulted in a significant reduction in death from vascular causes, MI, or stroke (9.8% vs 11.7%).19
Given its reversible effect on platelet inhibition, ticagrelor may be preferred in patients whose coronary anatomy is unknown and for whom coronary artery bypass grafting is deemed probable. It is still undergoing trials and is not yet approved.
TAKE-HOME POINTS
Prasugrel is more potent, more rapid in onset, and more consistent in inhibiting platelet aggregation than clopidogrel. A large clinical trial17 found prasugrel to be superior to clopidogrel for patients with moderate-to high-risk acute coronary syndromes with high probability of undergoing a percutaneous coronary intervention.
Who should receive prasugrel, and how?
Prasugrel should be given after angiography to patients with non-ST-elevation acute coronary syndromes or at presentation to patients with ST-elevation MI. When used for planned percutaneous coronary intervention, prasugrel should be given at least 30 minutes before the intervention, as was done in phase 2 trials (although its routine use in this situation is not recommended—see below).
It is given in a one-time loading dose of 60 mg by mouth and then maintained with 10 mg by mouth once daily for at least 1 year. (At least 9 months of treatment with a thienopyridine is indicated for patients with acute coronary syndromes who are medically treated, and at least 1 year is indicated following urgent or elective percutaneous coronary intervention, including balloon angioplasty and placement of a bare-metal or drug-eluting stent.)
Who should not receive prasugrel?
For now, prasugrel should be avoided in favor of clopidogrel in patients at higher risk of bleeding. It is clearly contraindicated in patients with prior transient ischemic attack or stroke, for whom the risk of serious bleeding seems to be prohibitive. It should generally be avoided in patients age 75 and older, although it might be considered in those at particularly high risk of stent thrombosis, such as those with diabetes or prior MI. In patients weighing less than 60 kg, the package insert advises a reduced dose (5 mg), although clinical evidence for this practice is lacking.
As yet, we have no data assuring that prasugrel is safe to use in combination with fibrinolytic agents, so patients on thrombolytic therapy for acute MI should continue to receive clopidogrel starting immediately after lysis. Furthermore, in patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in the prasugrel group than in the clopidogrel group in the TRITON-TIMI 38 trial.17 No thienopyridine should be given to patients likely to proceed to coronary artery bypass grafting.
Only clopidogrel has evidence supporting its use as an alternative to aspirin for patients with atherosclerotic disease who cannot tolerate aspirin. Neither drug has evidence for use for primary prevention.
Other areas of uncertainty
Prior to angiography. Indications for prasugrel are currently limited by the narrow scope of the trial data. TRITON-TIMI 38,17 the only large trial completed to date, randomized patients to receive prasugrel only after their coronary anatomy was known, except for ST-elevation MI patients. It is unknown whether the benefits of prasugrel will outweigh the higher risk of bleeding in patients with acute coronary syndromes who do not proceed to percutaneous coronary interventions.
A clinical trial is currently under way comparing prasugrel with clopidogrel in 10,000 patients with acute coronary syndromes who will be medically managed without planned revascularization: A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS), ClinicalTrials.gov Identifier: NCT00699998. The trial has an estimated completion date of March 2011.
In cases of non-ST-elevation acute coronary syndrome, it is reasonable to wait to give a thienopyridine until after the coronary anatomy has been defined, if angiography will be completed soon after presentation. For example, a 1-hour delay before giving prasugrel still delivers antiplatelet therapy more quickly than giving clopidogrel on presentation. If longer delays are expected before angiography, however, the patient should be given a loading dose of clopidogrel “up front,” in accordance with guidelines published by the American College of Cardiology, American Heart Association, and European Society of Cardiology,20 which recommend starting a thienopyridine early during hospitalization based on trial data with clopidogrel.
Patients undergoing elective percutaneous coronary intervention are at lower risk of stent thrombosis and other ischemic complications, so it is possible that the benefits of prasugrel would not outweigh the risks in these patients. Thus, prasugrel cannot yet be recommended for routine elective percutaneous coronary intervention except in individual cases in which the interventionalist feels that the patient may be at higher risk of thrombosis.
Prasugrel (Effient) is more potent and consistent in its effects than clopidogrel (Plavix), thus preventing more thrombotic events—but at a price of more bleeding. Therefore, the drugs must be appropriately selected for the individual patient.
Over the last 9 years, the thienopyridines—ticlopidine (Ticlid), clopidogrel, and now prasugrel—have become essential tools for treating acute coronary syndromes.
The usual underlying mechanism of acute coronary syndromes is thrombosis, caused by rupture of atherosclerotic plaque.1 Accordingly, antithrombotic agents—aspirin, heparin, lowmolecular-weight heparin, glycoprotein IIb/IIIa inhibitors, the direct thrombin inhibitor bivalirudin (Angiomax), and thienopyridines—have all been shown to reduce the risk of major adverse cardiac outcomes in this setting.
In this article, we review the pharmacology and evidence of effectiveness of the thienopyridine drugs, focusing on prasugrel, the latest thienopyridine to be approved by the US Food and Drug Administration (FDA).
THIENOPYRIDINES INHIBIT PLATELET ACTIVATION AND AGGREGATION
Thienopyridines are prodrugs that require conversion by hepatic cytochrome P450 enzymes. The active metabolites bind irreversibly to platelet P2Y12 receptors. Consequently, they permanently block signalling mediated by platelet adenosine diphosphate-P2Y12 receptors, thereby inhibiting glycoprotein IIb/IIIa receptor activation and platelet aggregation.
Aspirin, in contrast, inhibits platelets by blocking the thromboxane-mediated pathway. Therefore, the combination of aspirin plus a thienopyridine has an additive effect.2
The effect of thienopyridines on platelets is irreversible. Therefore, although the half-life of prasugrel’s active metabolite is 3.7 hours, its inhibitory effects last for 96 hours, essentially the time for half the body’s circulating platelets to be replaced.
TICLOPIDINE, THE FIRST THIENOPYRIDINE
Ticlopidine was the first thienopyridine to be approved by the FDA. Its initial studies in unstable angina were small, their designs did not call for patients to concurrently receive aspirin, and all they showed was that ticlopidine was about as beneficial as aspirin. Consequently, the studies had little impact on clinical practice.3
In a pivotal trial,4 patients who received coronary stents were randomized to afterward receive either the combination of ticlopidine plus aspirin or anticoagulation therapy with heparin, phenprocoumon (a coumarin derivative available in Europe), and aspirin. At 30 days, an ischemic complication (death, myocardial infarction [MI], repeat intervention) had occurred in 6.2% of the anticoagulation therapy group vs 1.6% of the ticlopidine group, a risk reduction of 75%. Rates of stent occlusion, MI, and revascularization were 80% to 85% lower in the ticlodipine group. This study paved the way for widespread use of thienopyridines.
Ticlopidine’s use was limited, however, by a 2.4% incidence of serious granulocytopenia and rare cases of thrombocytopenic purpura.
BENEFIT OF CLOPIDOGREL
Although prasugrel is the focus of this review, the trials of prasugrel all compared its efficacy with that of clopidogrel. Furthermore, many patients should still receive clopidogrel and not prasugrel, so it is important to be familiar with the evidence of clopidogrel’s benefit.
Once approved for clinical use, clopidogrel was substituted for ticlopidine in patients undergoing coronary stenting on the basis of studies showing it to be at least as effective as ticlopidine and more tolerable. A series of trials of clopidogrel were done in patients across a spectrum of risk groups, from those at high risk of coronary heart disease to those presenting with ST-elevation MI. The time of pretreatment in the studies ranged from 3 hours to 6 days before percutaneous coronary intervention, and the duration of treatment following intervention ranged from 30 days to 1 year.
Clopidogrel in non-ST-elevation acute coronary syndromes
The CURE trial2 (Clopidogrel in Unstable Angina to Prevent Recurrent Events), published in 2001, established clopidogrel as a therapy for unstable ischemic syndromes, whether treated medically or with revascularization. In that trial, 12,562 patients with acute coronary syndromes without ST elevation (ie, unstable angina or non-ST-elevation MI), as defined by electrocardiographic changes or positive cardiac markers, were randomized to receive clopidogrel (a 300-mg loading dose followed by 75-mg maintenance doses) or placebo for a mean duration of 9 months. All patients also received aspirin 75 mg to 325 mg daily.
The composite outcome of death from cardiovascular causes, nonfatal MI, or stroke occurred in 20% fewer patients treated with clopidogrel than with placebo (9.3% vs 11.4%). The benefit was similar in patients undergoing revascularization compared with those treated medically.
Although there were significantly more cases of major bleeding in the clopidogrel group than in the placebo group (3.7% vs 2.7%), the number of episodes of life-threatening bleeding or hemorrhagic strokes was the same.
PCI-CURE5 was a substudy of the CURE trial in patients who underwent a percutaneous coronary intervention. Patients were pretreated with clopidogrel or placebo for a mean of 6 days before the procedure. Afterward, they all received clopidogrel plus aspirin in an unblinded fashion for 2 to 4 weeks, and then the randomized study drug was resumed for a mean of 8 months.
Significantly fewer adverse events occurred in the clopidogrel group as tallied at the time of the intervention, 1 month later, and 8 months later.
Clopidogrel in ST-elevation acute MI
The CLARITY-TIMI 28 trial6 (Clopidogrel as Adjunctive Reperfusion Therapy—Thrombolysis in Myocardial Infarction 28) showed that adding clopidogrel (a 300-mg loading dose, then 75 mg daily) to aspirin benefitted patients with ST-elevation MI receiving fibrinolytic therapy. At 30 days, cardiovascular death, recurrent MI, or urgent revascularization had occurred in 11.6% of the clopidogrel group vs 14.1% of the placebo group, a statistically significant difference. The rates of major or minor bleeding were no higher in the clopidogrel group than in the placebo group, an especially remarkable finding in patients receiving thrombolytic therapy.
PCI-CLARITY.7 About half of the patients in the CLARITY trial ultimately underwent a percutaneous coronary intervention after fibrinolytic therapy, with results reported as the PCI-CLARITY substudy. Like those in PCI-CURE, these patients were randomized to receive pretreatment with either clopidogrel or placebo before the procedure, in this study for a median of 3 days. Both groups received clopidogrel afterward. At 30 days from randomization, the outcome of cardiovascular death, MI, or stroke had occurred in 7.5% of the clopidogrel group compared with 12.0% of the placebo group, which was statistically significant, without any significant excess in the rates of major or minor bleeding.
COMMIT8 (the Clopidogrel and Metoprolol in Myocardial Infarction Trial) also showed clopidogrel to be beneficial in patients with acute MI. This trial included more than 45,000 patients in China with acute MI, 93% of whom had ST-segment elevation. In contrast to CLARITY, in COMMIT barely more than half of the patients received fibrinolysis, fewer than 5% proceeded to percutaneous interventions, and no loading dose was given: patients in the clopidogrel group received 75 mg/day from the outset.
At 15 days, the incidence of death, reinfarction, or stroke was 9.2% with clopidogrel compared with 10.1% with placebo, a small but statistically significant difference. Again, the rate of major bleeding was not significantly higher, either overall or in patients over age 70.
Of note, patients over age 75 were excluded from CLARITY, and as mentioned, no loading dose was used in COMMIT. Thus, for patients receiving fibrinolysis who are over age 75, there is no evidence to support the safety of a loading dose, and clopidogrel should be started at 75 mg daily.
Clopidogrel in elective percutaneous coronary intervention
The CREDO trial9 (Clopidogrel for the Reduction of Events During Observation) was in patients referred for elective percutaneous coronary intervention. Three to 24 hours before the procedure, the patients received either a 300-mg loading dose of clopidogrel or placebo; afterward, all patients received clopidogrel 75 mg/day for 28 days. All patients also received aspirin.
A clopidogrel loading dose 3 to 24 hours before the intervention did not produce a statistically significant reduction in ischemic events, although a post hoc subgroup analysis suggested that patients who received the loading dose between 6 and 24 hours before did benefit, with a relative risk reduction of 38.6% in the composite end point (P = .051).
After 28 days, the patients who had received the clopidogrel loading dose were continued on clopidogrel, while those in the placebo group were switched back to placebo. At 1 year, the investigators found a significantly lower rate of the composite end point with the prolonged course of clopidogrel (8.5% vs 11.5%).
In summary, these studies found clopidogrel to be beneficial in a broad spectrum of coronary diseases. Subgroup analyses suggest that pretreatment before percutaneous coronary intervention provides additional benefit, particularly if clopidogrel is given at least 6 hours in advance (the time necessary for clopidogrel to cause substantial platelet inhibition).
SOME PATIENTS RESPOND LESS TO CLOPIDOGREL
The level of platelet inhibition induced by clopidogrel varies. In different studies, the frequency of clopidogrel “nonresponsiveness” ranged from 5% to 56% of patients, depending on which test and which cutoff values were used. The distribution of responses to clopidogrel is wide and fits a normal gaussian curve.10
A large fraction of the population carries a gene that may account for some of the interpatient variation in platelet inhibition with clopidogrel. Carriers of a reduced-function CYP2C19 allele—approximately 30% of people in one study—have significantly lower levels of the active metabolite of clopidogrel, less platelet inhibition from clopidogrel therapy, and a 53% higher rate of death from cardiovascular causes, MI, or stroke.11
PRASUGREL, THE NEWEST THIENOPYRIDINE
Prasugrel, FDA-approved in July 2009 for the treatment of acute coronary syndromes, is given in an oral loading dose of 60 mg followed by an oral maintenance dose of 10 mg daily.
Pharmacology of prasugrel vs clopidogrel
As noted previously, the thienopyridines are prodrugs that require hepatic conversion to exert antiplatelet effects.
Metabolism. Prasugrel’s hepatic activation involves a single step, in contrast to the multiple-step process required for activation of clopidogrel. Clopidogrel is primarily hydrolyzed by intestinal and plasma esterases to an inactive terminal metabolite, with the residual unhydrolized drug undergoing a two-step metabolism that depends on cytochrome P450 enzymes. Prasugrel is also extensively hydrolyzed by these esterases, but the intermediate product is then metabolized in a single step to the active sulfhydryl compound, mainly by CYP3A4 and CYP2B6.
Thus, about 80% of an orally absorbed dose of prasugrel is converted to active drug, compared with only 10% to 20% of absorbed clopidogrel.
Time to peak effect. With clopidogrel, maximal inhibition of platelet aggregation occurs 3 to 5 days after starting therapy with 75 mg daily without a loading dose, but within 4 to 6 hours if a loading dose of 300 to 600 mg is given. In contrast, a prasugrel loading dose produces more than 80% of its platelet inhibitory effects by 30 minutes, and peak activity is observed within 4 hours.12 The platelet inhibition induced by prasugrel at 30 minutes after administration is comparable to the peak effect of clopidogrel at 6 hours.13
Dose-response. Prasugrel’s inhibition of platelet aggregation is dose-related.
Prasugrel is about 10 times more potent than clopidogrel and 100 times more potent than ticlopidine. Thus, treatment with 5 mg of prasugrel results in inhibition of platelet activity (distributed in a gaussian curve) very similar to that produced by 75 mg of clopidogrel. On the other hand, even a maintenance dose of 150 mg of clopidogrel inhibits platelet activity to a lesser degree than 10 mg of prasugrel (46% vs 61%),14 so clopidogrel appears to reach a plateau of platelet inhibition that prasugrel can overcome.
At the approved dose of prasugrel, inhibition of platelet aggregation is significantly greater and there are fewer “nonresponders” than with clopidogrel.
Interactions. Drugs that inhibit CYP3A4 do not inhibit the efficacy of prasugrel, but they can inhibit that of clopidogrel. Some commonly used drugs that have this effect are the statins (eg, atorvastain [Lipitor]) and the macrolide antibiotics (eg, erythromycin). Furthermore, whereas proton pump inhibitors have been shown to diminish the effect of clopidogrel by reducing the formation of its active metabolite, no such effect has been noted with prasugrel.
Prasugrel in phase 2 trials: Finding the optimal dosage
A phase 2 trial compared three prasugrel regimens (loading dose/daily maintenance dose of 40 mg/7.5 mg, 60 mg/10 mg, and 60 mg/15 mg) and standard clopidogrel therapy (300 mg/75 mg) in patients undergoing elective or urgent percutaneous coronary intervention.15 No significant difference in outcomes was seen in the groups receiving the three prasugrel regimens. However, more “minimal bleeding events” (defined by the criteria of the TIMI trial16) occurred with high-dose prasugrel than with lower-dose prasugrel or with clopidogrel, leading to use of the intermediate-dose prasugrel regimen (60-mg loading dose, 10-mg daily maintenance) for later trials.
Another phase 2 trial randomized 201 patients undergoing elective percutaneous coronary intervention to receive prasugrel 60 mg/10 mg or clopidogrel 600 mg/150 mg.14 In all patients, the loading dose was given about 1 hour before cardiac catheterization. As soon as 30 minutes after the loading dose, platelet inhibition was superior with prasugrel (31% vs 5% inhibition of platelet aggregation), and it remained significantly higher at 6 hours (75% vs 32%) and during the maintenance phase (61% vs 46%).
Phase 3 trial of prasugrel vs clopidogrel: TRITON-TIMI 38
Only one large phase 3 trial of prasugrel has been completed: TRITON-TIMI 38 (the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction),17 which enrolled adults with moderate-risk to high-risk acute coronary syndromes scheduled to undergo a percutaneous coronary intervention. In this trial, 10,074 patients were enrolled who had moderate-to high-risk unstable angina or non-ST-elevation MI, and 3,534 patients were enrolled who had ST-elevation MI.
Patients were randomized to receive prasugrel (a 60-mg loading dose, then 10 mg daily) or clopidogrel (a 300-mg loading dose, then 75 mg daily) and were treated for 6 to 15 months. All patients also received aspirin.
These benefits came at a price of more bleeding. Of those patients who did not undergo coronary artery bypass grafting, more experienced bleeding in the prasugrel group than in the clopidogrel group (2.4% vs 1.8%, P = .03), including a higher rate of life-threatening bleeding (1.4% vs 0.89%, P = .01) and fatal bleeding (0.4% vs 0.1%, P = .002). More patients discontinued prasugrel because of hemorrhage (2.5% vs 1.4%, P < .001). In patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in those who received prasugrel than in those who received clopidogrel (13.4% vs 3.2%, P < .001).
A higher rate of adverse events related to colon cancer was also noted in patients treated with prasugrel, although the authors suggest this may have resulted from the stronger antiplatelet effects of prasugrel bringing more tumors to medical attention due to bleeding.
Overall death rates did not differ significantly between the treatment groups.
In a post hoc analysis,18 prasugrel was superior to clopidogrel in preventing ischemic events both during the first 3 days following randomization (the “loading phase”) and for the remainder of the trial (the “maintenance phase”). Whereas bleeding risk was similar with the two drugs during the loading phase, prasugrel was subsequently associated with more bleeding during the maintenance phase.
Certain patient subgroups had no net benefit or even suffered harm from prasugrel compared with clopidogrel.17 Patients with previous stroke or transient ischemic attack had net harm from prasugrel (hazard ratio 1.54, P = .04) and showed a strong trend toward a greater rate of major bleeding (P = .06). Patients age 75 and older and those weighing less than 60 kg had no net benefit from prasugrel.
Cost of prasugrel
Prasugrel is currently priced at 18% more than clopidogrel, with average wholesale prices per pill of $6.65 for prasugrel 10 mg compared with $5.63 for clopidogrel 75 mg. (Prasugrel 10-mg pills cost $6.33 at drugstore.com or $7.60 at CVS; clopidogrel 75-mg pills cost $5.33 at drugstore.com or $6.43 at CVS.) The patent on clopidogrel expires in November 2011, after which the price differential is expected to become significantly greater.
TICAGRELOR, A REVERSIBLE ORAL AGENT
Ticagrelor, the first reversible oral P2Y12 receptor antagonist, is an alternative to thienopyridine therapy for acute coronary syndromes.
Ticagrelor is quickly absorbed, does not require metabolic activation, and has a rapid antiplatelet effect and offset of effect, which closely follow drug-exposure levels. In a large randomized controlled trial in patients with acute coronary syndromes with or without STsegment elevation, treatment with ticagrelor compared with clopidogrel resulted in a significant reduction in death from vascular causes, MI, or stroke (9.8% vs 11.7%).19
Given its reversible effect on platelet inhibition, ticagrelor may be preferred in patients whose coronary anatomy is unknown and for whom coronary artery bypass grafting is deemed probable. It is still undergoing trials and is not yet approved.
TAKE-HOME POINTS
Prasugrel is more potent, more rapid in onset, and more consistent in inhibiting platelet aggregation than clopidogrel. A large clinical trial17 found prasugrel to be superior to clopidogrel for patients with moderate-to high-risk acute coronary syndromes with high probability of undergoing a percutaneous coronary intervention.
Who should receive prasugrel, and how?
Prasugrel should be given after angiography to patients with non-ST-elevation acute coronary syndromes or at presentation to patients with ST-elevation MI. When used for planned percutaneous coronary intervention, prasugrel should be given at least 30 minutes before the intervention, as was done in phase 2 trials (although its routine use in this situation is not recommended—see below).
It is given in a one-time loading dose of 60 mg by mouth and then maintained with 10 mg by mouth once daily for at least 1 year. (At least 9 months of treatment with a thienopyridine is indicated for patients with acute coronary syndromes who are medically treated, and at least 1 year is indicated following urgent or elective percutaneous coronary intervention, including balloon angioplasty and placement of a bare-metal or drug-eluting stent.)
Who should not receive prasugrel?
For now, prasugrel should be avoided in favor of clopidogrel in patients at higher risk of bleeding. It is clearly contraindicated in patients with prior transient ischemic attack or stroke, for whom the risk of serious bleeding seems to be prohibitive. It should generally be avoided in patients age 75 and older, although it might be considered in those at particularly high risk of stent thrombosis, such as those with diabetes or prior MI. In patients weighing less than 60 kg, the package insert advises a reduced dose (5 mg), although clinical evidence for this practice is lacking.
As yet, we have no data assuring that prasugrel is safe to use in combination with fibrinolytic agents, so patients on thrombolytic therapy for acute MI should continue to receive clopidogrel starting immediately after lysis. Furthermore, in patients who proceeded to coronary artery bypass grafting, the rate of major bleeding was more than four times higher in the prasugrel group than in the clopidogrel group in the TRITON-TIMI 38 trial.17 No thienopyridine should be given to patients likely to proceed to coronary artery bypass grafting.
Only clopidogrel has evidence supporting its use as an alternative to aspirin for patients with atherosclerotic disease who cannot tolerate aspirin. Neither drug has evidence for use for primary prevention.
Other areas of uncertainty
Prior to angiography. Indications for prasugrel are currently limited by the narrow scope of the trial data. TRITON-TIMI 38,17 the only large trial completed to date, randomized patients to receive prasugrel only after their coronary anatomy was known, except for ST-elevation MI patients. It is unknown whether the benefits of prasugrel will outweigh the higher risk of bleeding in patients with acute coronary syndromes who do not proceed to percutaneous coronary interventions.
A clinical trial is currently under way comparing prasugrel with clopidogrel in 10,000 patients with acute coronary syndromes who will be medically managed without planned revascularization: A Comparison of Prasugrel and Clopidogrel in Acute Coronary Syndrome Subjects (TRILOGY ACS), ClinicalTrials.gov Identifier: NCT00699998. The trial has an estimated completion date of March 2011.
In cases of non-ST-elevation acute coronary syndrome, it is reasonable to wait to give a thienopyridine until after the coronary anatomy has been defined, if angiography will be completed soon after presentation. For example, a 1-hour delay before giving prasugrel still delivers antiplatelet therapy more quickly than giving clopidogrel on presentation. If longer delays are expected before angiography, however, the patient should be given a loading dose of clopidogrel “up front,” in accordance with guidelines published by the American College of Cardiology, American Heart Association, and European Society of Cardiology,20 which recommend starting a thienopyridine early during hospitalization based on trial data with clopidogrel.
Patients undergoing elective percutaneous coronary intervention are at lower risk of stent thrombosis and other ischemic complications, so it is possible that the benefits of prasugrel would not outweigh the risks in these patients. Thus, prasugrel cannot yet be recommended for routine elective percutaneous coronary intervention except in individual cases in which the interventionalist feels that the patient may be at higher risk of thrombosis.
- Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med 2000; 342:101–114.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Balsano F, Rizzon P, Violi F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 1990; 82:17–26.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Mehta SR, Yusuf S, Peters RJG, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Sabatine MS, Cannon CP, Gibson CM, et al; CLA RITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with STsegment elevation. N Engl J Med 2005; 352:1179–1189.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005: 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of a front-loaded regimen of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20:2316–2321.
- Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol 2007; 100:331–336.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLETIMI 44 Investigators. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Wiviott SD, Antman EM, Winters KJ, et al; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 Trial. Circulation 2005; 111:3366–3373.
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Ann Intern Med 1991; 115:256–265.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51:2028–2033.
- Wallentin L, Becker RC, Budaj A, Freij A, Thorsén M, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—summary article*1: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002; 40:1366–1374.
- Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med 2000; 342:101–114.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Balsano F, Rizzon P, Violi F, et al. Antiplatelet treatment with ticlopidine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlopidina nell'Angina Instabile Group. Circulation 1990; 82:17–26.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med 1996; 334:1084–1089.
- Mehta SR, Yusuf S, Peters RJG, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- Sabatine MS, Cannon CP, Gibson CM, et al; CLA RITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with STsegment elevation. N Engl J Med 2005; 352:1179–1189.
- Sabatine MS, Cannon CP, Gibson CM, et al; Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005: 294:1224–1232.
- Chen ZM, Jiang LX, Chen YP, et al; COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Steinhubl SR, Berger PB, Mann JT, et al; CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362.
- Helft G, Osende JI, Worthley SG, et al. Acute antithrombotic effect of a front-loaded regimen of clopidogrel in patients with atherosclerosis on aspirin. Arterioscler Thromb Vasc Biol 2000; 20:2316–2321.
- Weerakkody GJ, Jakubowski JA, Brandt JT, et al. Comparison of speed of onset of platelet inhibition after loading doses of clopidogrel versus prasugrel in healthy volunteers and correlation with responder status. Am J Cardiol 2007; 100:331–336.
- Wiviott SD, Trenk D, Frelinger AL, et al; PRINCIPLETIMI 44 Investigators. Prasugrel compared with high loading-and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Wiviott SD, Antman EM, Winters KJ, et al; JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 Trial. Circulation 2005; 111:3366–3373.
- Bovill EG, Terrin ML, Stump DC, et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) Phase II Trial. Ann Intern Med 1991; 115:256–265.
- Wiviott SD, Braunwald E, McCabe CH, et al; TRITONTIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008; 51:2028–2033.
- Wallentin L, Becker RC, Budaj A, Freij A, Thorsén M, et al; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–1057.
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction—summary article*1: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002; 40:1366–1374.
KEY POINTS
- The thienopyridines—ticlopidine (Ticlid), clopidogrel (Plavix), and now prasugrel—reduce the risk of death from and serious complications of acute coronary syndromes by inhibiting platelet aggregation.
- Compared with clopidogrel, prasugrel is more potent, faster in onset, and more consistent in inhibiting platelets.
- Prasugrel should be avoided in patients at higher risk of bleeding, including those with a history of stroke or transient ischemic attack, those age 75 or older, or those who weigh less than 60 kg.