User login
The COVID-19 Pandemic and Changes in Healthcare Utilization for Pediatric Respiratory and Nonrespiratory Illnesses in the United States
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 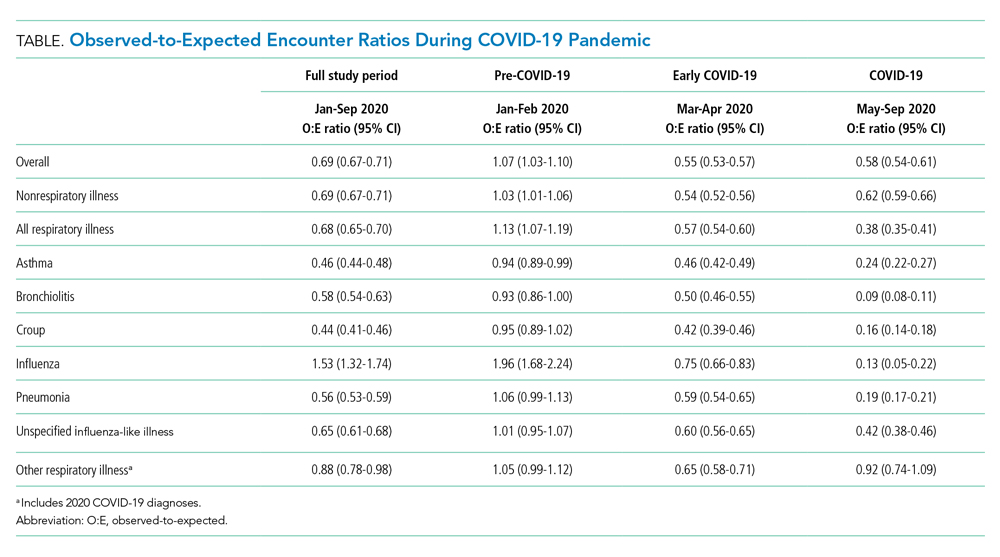

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
© 2021 Society of Hospital Medicine