User login
Sarcoidosis: An FP’s primer on an enigmatic disease
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
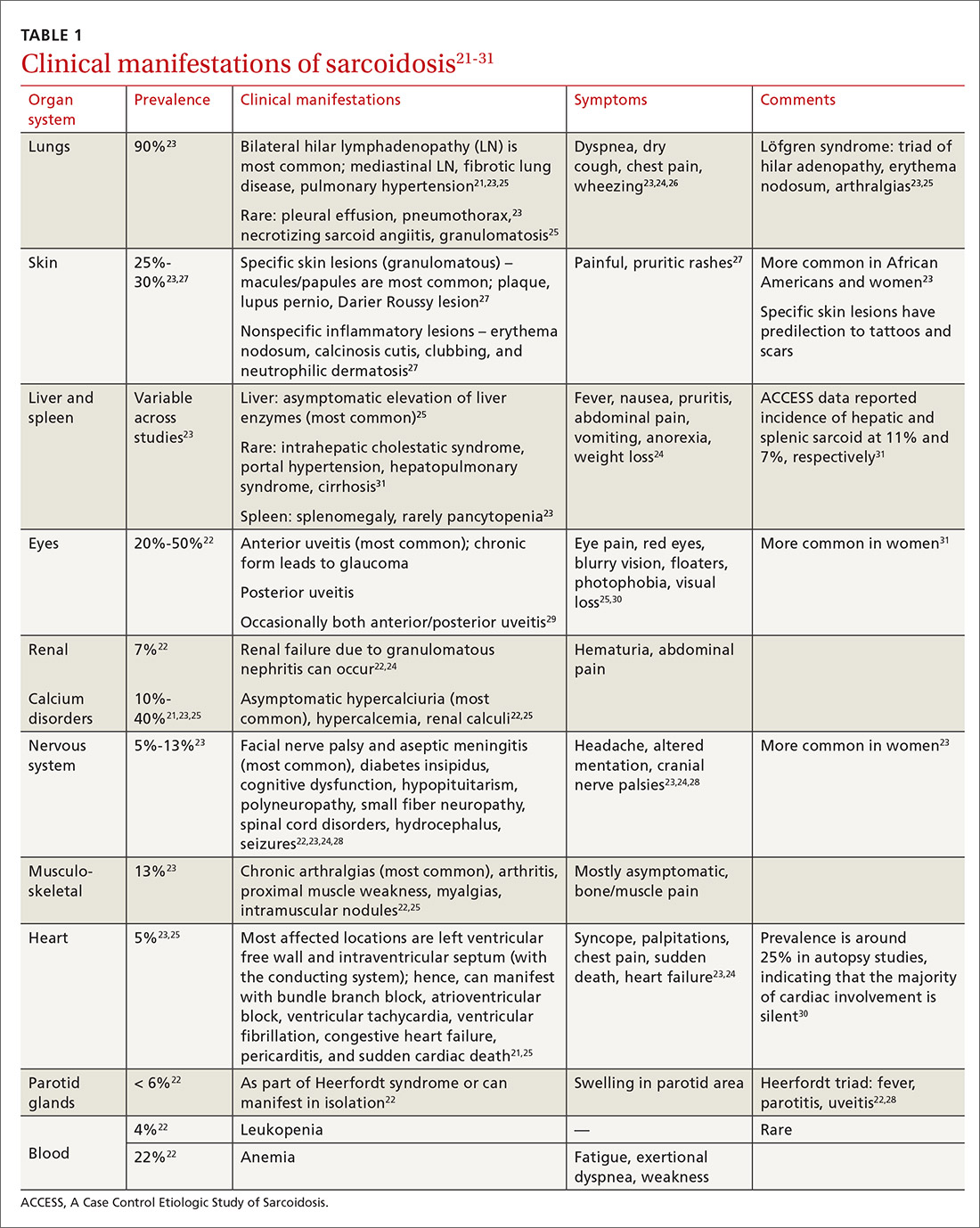
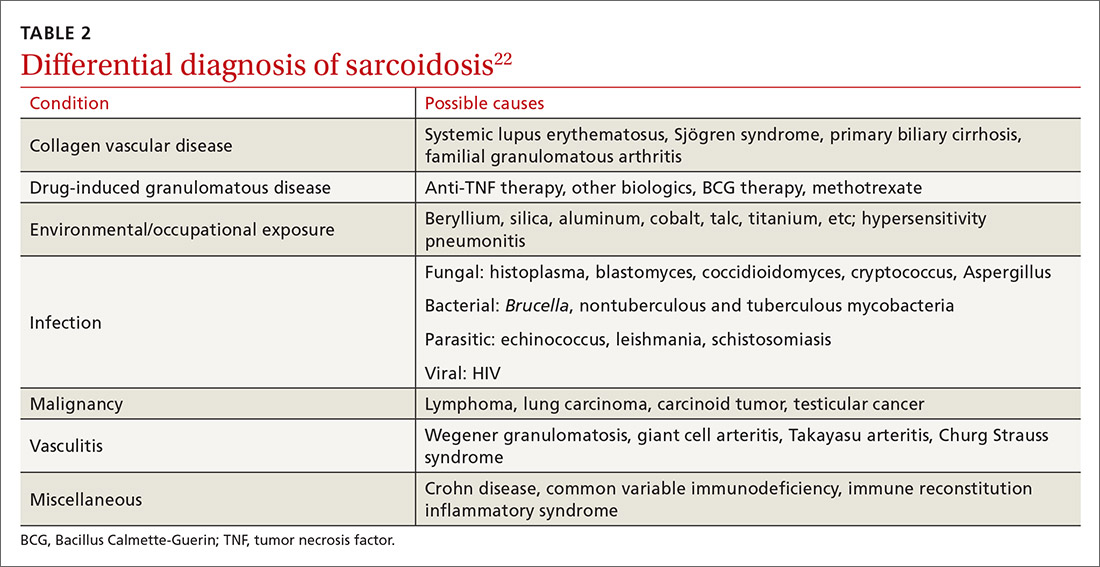
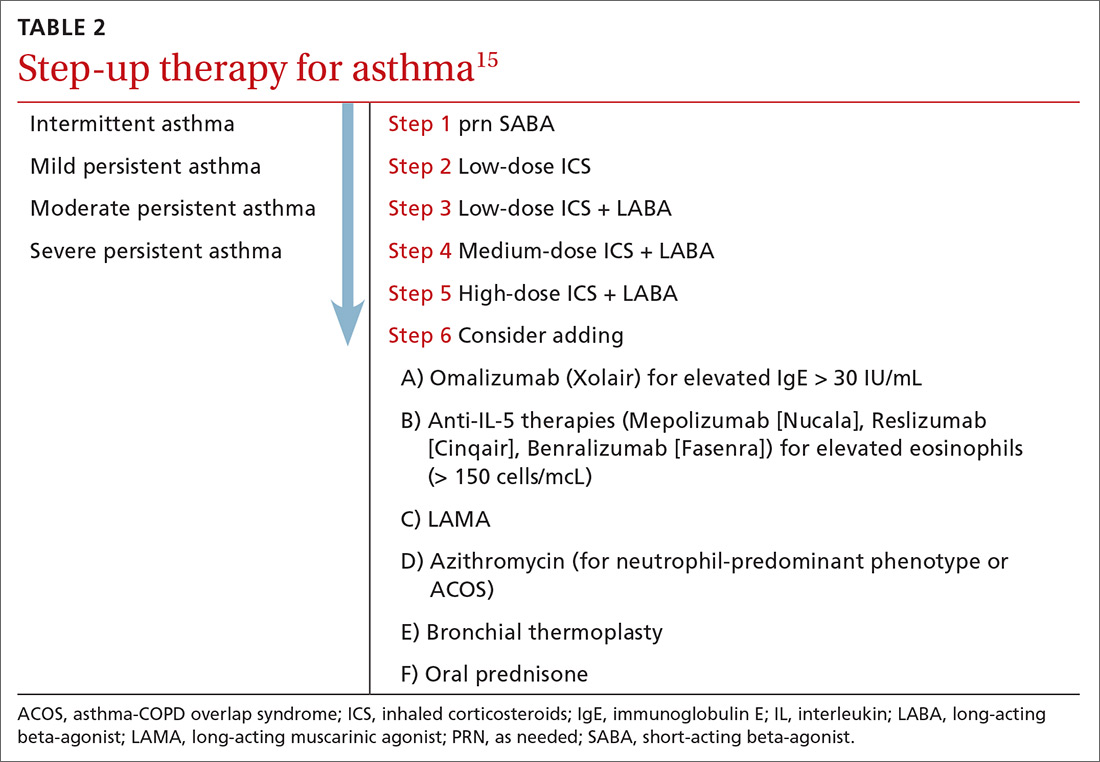
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
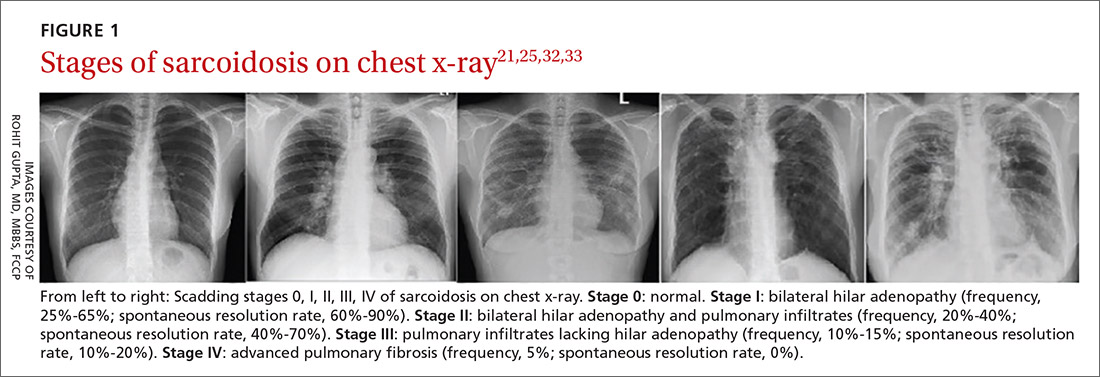
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
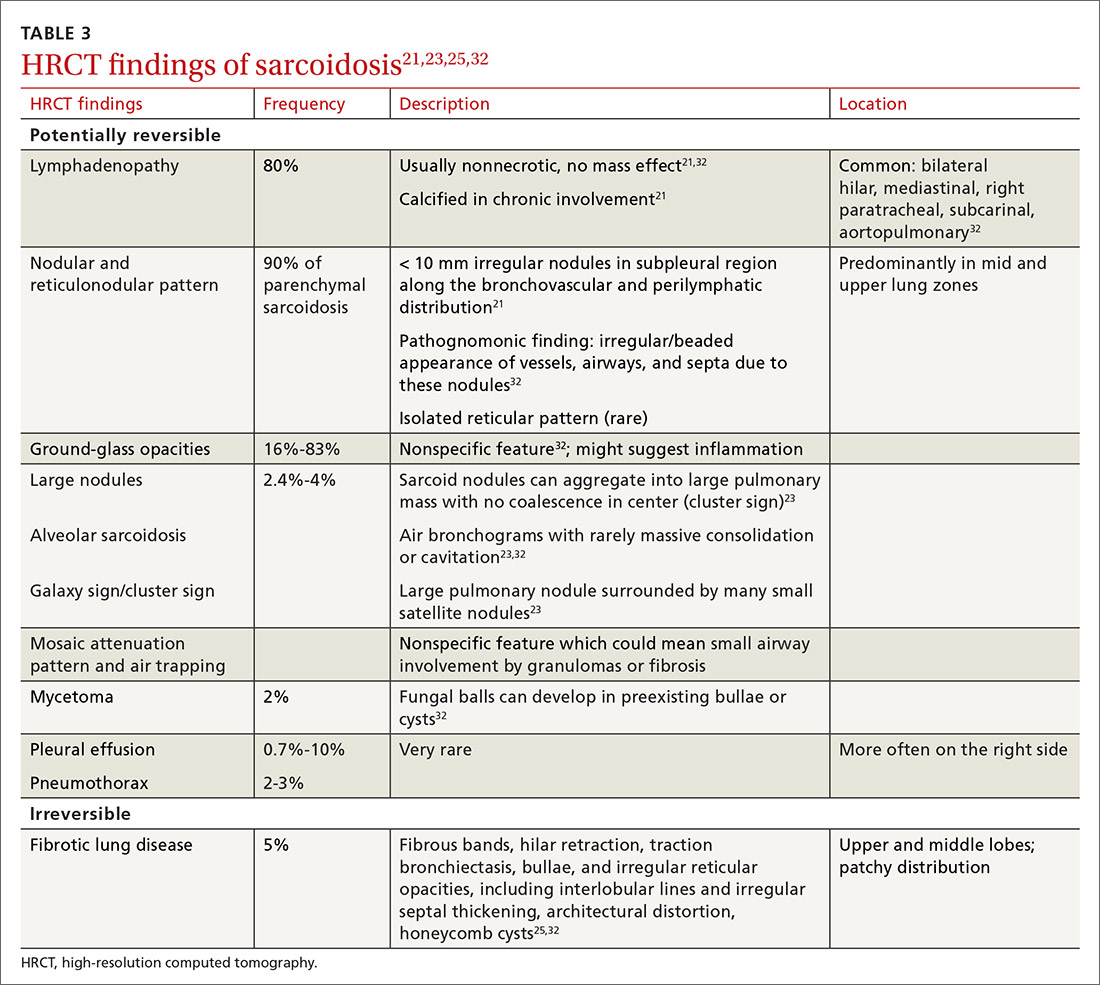
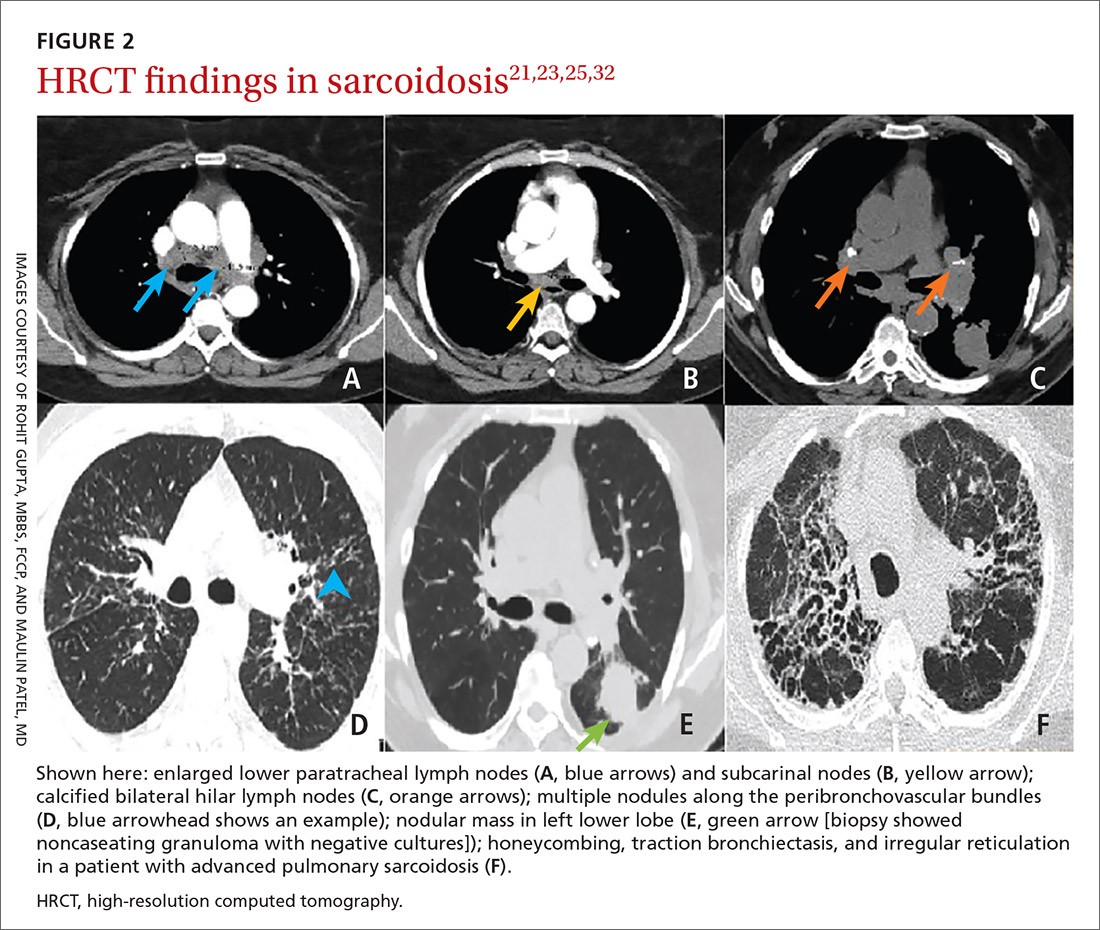
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
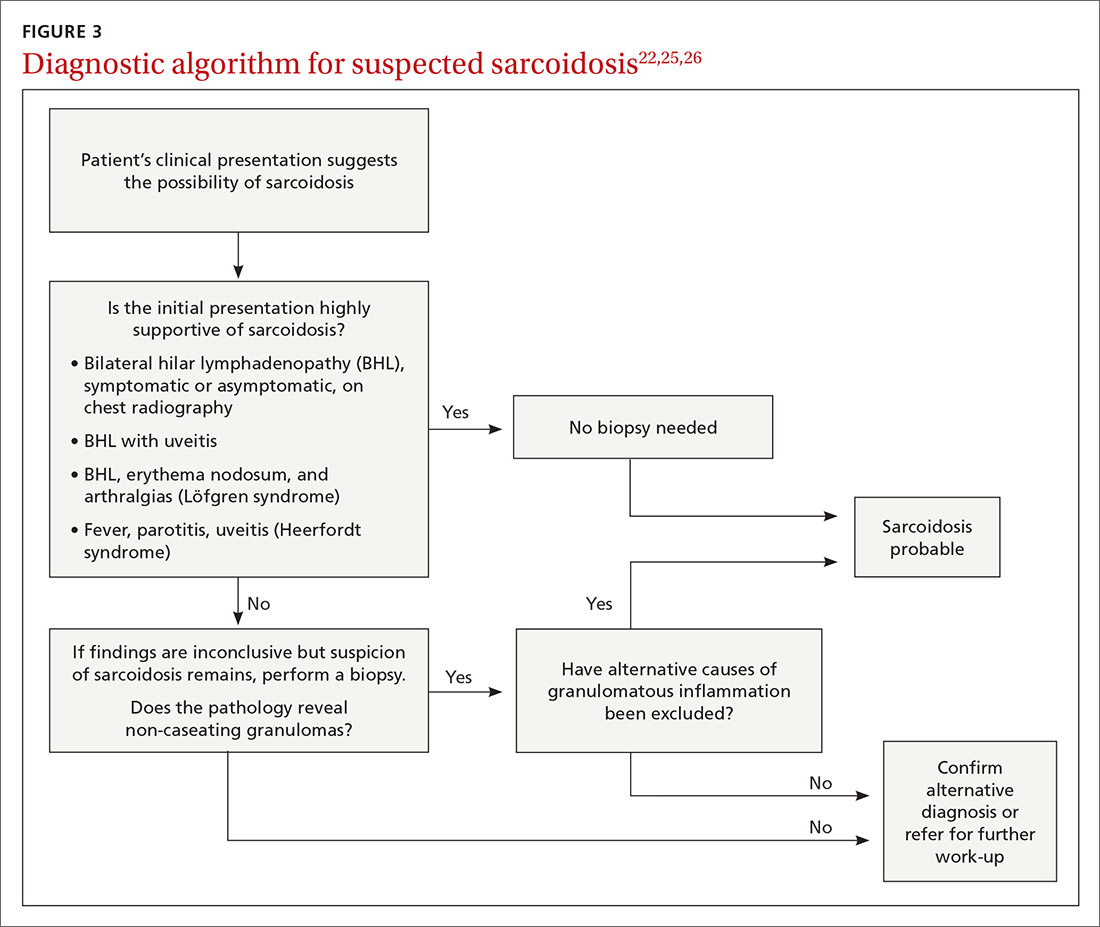
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
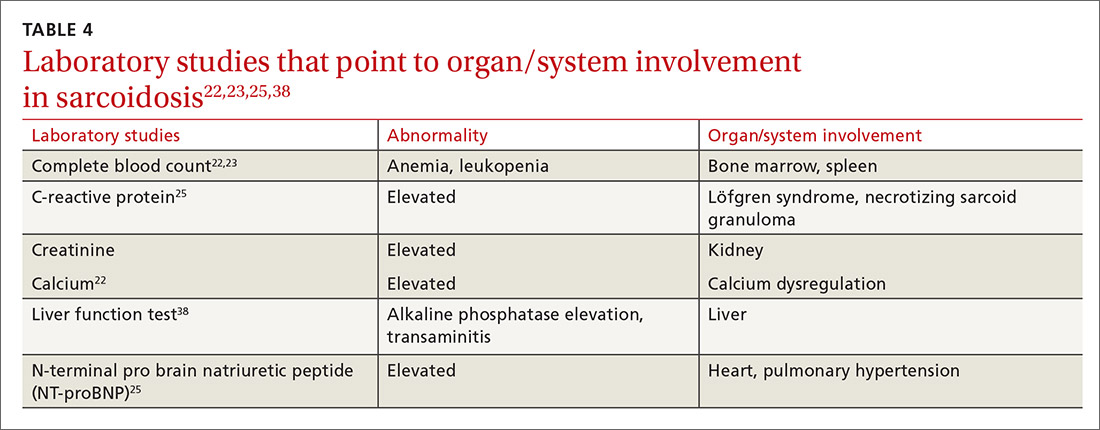
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
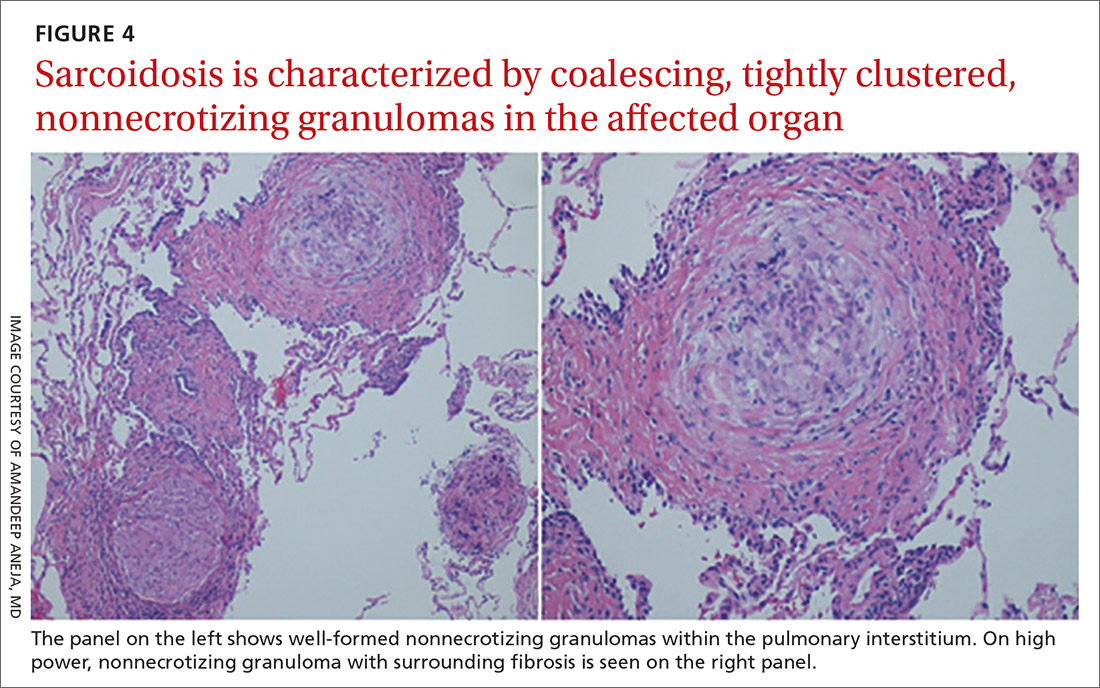
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
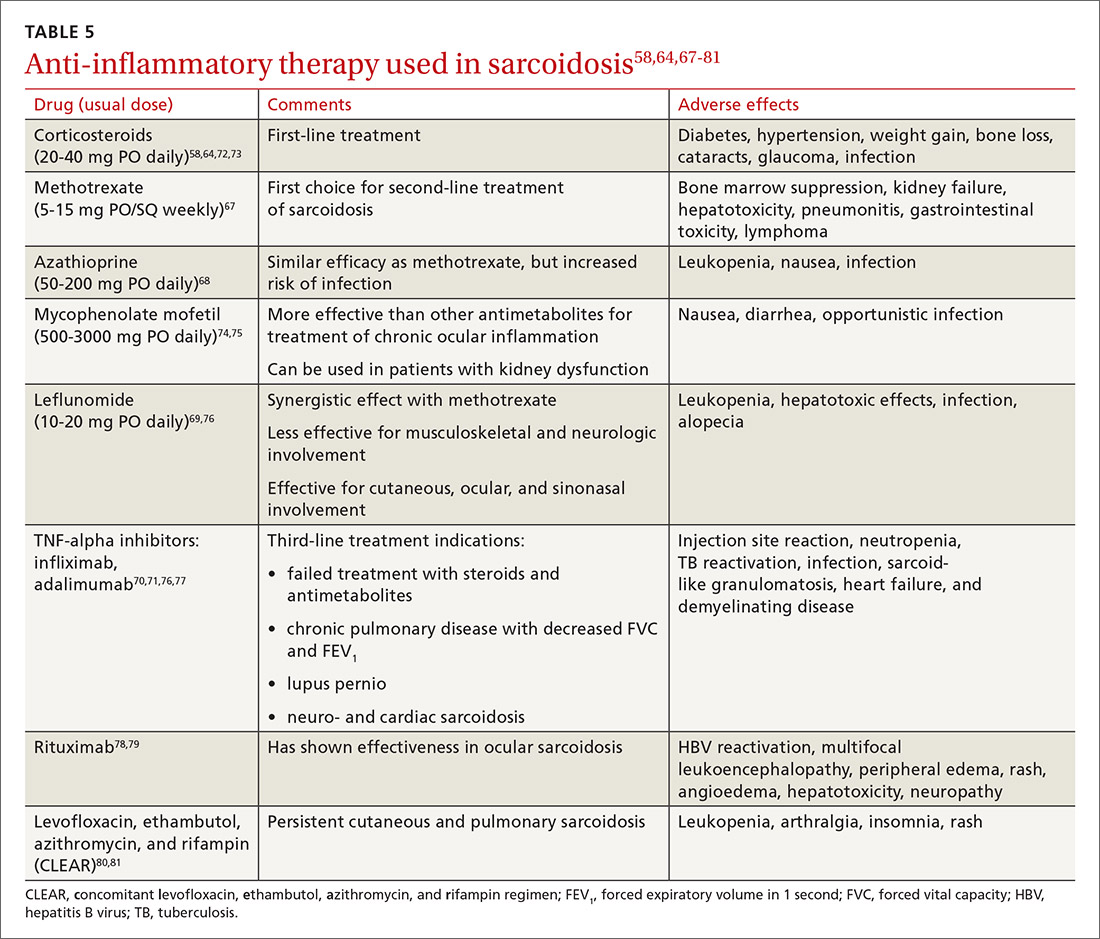
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; rohit.gupta@tuhs.temple.edu
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; rohit.gupta@tuhs.temple.edu
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; rohit.gupta@tuhs.temple.edu
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
PRACTICE RECOMMENDATIONS
› Consider biopsy to aid in diagnosing sarcoidosis; it may be avoided with a high clinical suspicion for sarcoidosis (eg, Löfgren syndrome, lupus pernio, or Heerfordt syndrome). C
› Rule out alternative diagnoses such as infection, malignancy, collagen vascular disease, and vasculitis. C
› Identify extra-pulmonary organ involvement, as clinically indicated, by screening with a baseline eye examination; complete blood count; creatinine, alkaline phosphatase, and calcium levels; electrocardiogram, and other organ-specific studies. C
› Make a patient-centered decision whether to begin antiinflammatory treatment based on symptomatology and risk of organ failure or death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Asthma: Newer Tx options mean more targeted therapy
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
PRACTICE RECOMMENDATIONS
› Consider inhaled corticosteroids (ICS) as your first choice for a long-term control agent to treat asthma; add a long-acting beta agonist (LABA) when needed. A
› Use long-acting muscarinic antagonists (LAMA) as add-on therapy for patients whose asthma is uncontrolled despite the use of low-dose ICS-LABA, or as an alternative to high-dose ICS-LABA. A
› Consider biological therapies for patients with asthma exacerbations that require steroids at least twice a year. B
› Use azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series