User login
In reply: The PARADIGM-HF trial
In Reply: We thank Dr. Blankfield for raising these two important points. Although the findings of the PARADIGM-HF study are compelling, the design and results of this trial have incited many questions.
To address his first point, about the differential dosages of the two drugs, we agree, and we did mention in our review that one concern about the results of PARADIGM-HF is the unequal dosages of valsartan and enalapril in the two different arms. We mentioned that this dosage of enalapril was chosen based on its survival benefit in previous trials. However, this still raises the question of whether the benefit seen in the sacubitril-valsartan group was due to greater inhibition of the renin-angiotensin-aldosterone system rather than to the new drug.
To address his second point, the decrease in blood pressure in the sacubitril-valsartan arm was significant, and the patients taking this drug were more likely to have symptomatic hypotension, which may contribute to patient intolerance and difficulty initiating treatment with this drug. Dr. Blankfield brings up an interesting point regarding reduction of blood pressure driving the decrease of events in the sacubitril-valsartan group. In the original trial results section, the authors mentioned that when the difference in blood pressure between the two groups was examined as a time-dependent covariate, it was not a significant predictor of the benefit of sacubitril-valsartan.1
Furthermore, although higher blood pressure is associated with worse cardiovascular outcomes in the general population, higher blood pressure has been shown to be protective in heart failure patients.2 Several studies have shown that the relationship between blood pressure and the mortality rate in patients with heart failure is paradoxical and complex.2–4 Lee et al3 found that this relationship was U-shaped, with increased mortality risk in those with high and low blood pressures (< 120 mm Hg). Ather et al4 also showed that the relationship was U-shaped in patients with a mild to moderate reduction in left ventricular ejection fraction, but linear in those with severely reduced ejection fraction. This study also found that a decrease in systolic blood pressure below 110 mm Hg was associated with increased mortality risk.
The findings of PARADIGM-HF have sparked much conversation and implementation of practice change in the treatment of heart failure patients, and we await additional data on the use and limitations of sacubitril-valsartan in this group of patients.
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Raphael CE, Whinnett ZI, Davies JE, et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart 2009; 95:56–62.
- Lee DS, Ghosh N, Floras JS, et al. Association of blood pressure at hospital discharge with mortality in patients diagnosed with heart failure. Circ Heart Fail 2009; 2:616-623.
- Ather S, Chan W, Chillar A, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J 2011; 161:567–573.
In Reply: We thank Dr. Blankfield for raising these two important points. Although the findings of the PARADIGM-HF study are compelling, the design and results of this trial have incited many questions.
To address his first point, about the differential dosages of the two drugs, we agree, and we did mention in our review that one concern about the results of PARADIGM-HF is the unequal dosages of valsartan and enalapril in the two different arms. We mentioned that this dosage of enalapril was chosen based on its survival benefit in previous trials. However, this still raises the question of whether the benefit seen in the sacubitril-valsartan group was due to greater inhibition of the renin-angiotensin-aldosterone system rather than to the new drug.
To address his second point, the decrease in blood pressure in the sacubitril-valsartan arm was significant, and the patients taking this drug were more likely to have symptomatic hypotension, which may contribute to patient intolerance and difficulty initiating treatment with this drug. Dr. Blankfield brings up an interesting point regarding reduction of blood pressure driving the decrease of events in the sacubitril-valsartan group. In the original trial results section, the authors mentioned that when the difference in blood pressure between the two groups was examined as a time-dependent covariate, it was not a significant predictor of the benefit of sacubitril-valsartan.1
Furthermore, although higher blood pressure is associated with worse cardiovascular outcomes in the general population, higher blood pressure has been shown to be protective in heart failure patients.2 Several studies have shown that the relationship between blood pressure and the mortality rate in patients with heart failure is paradoxical and complex.2–4 Lee et al3 found that this relationship was U-shaped, with increased mortality risk in those with high and low blood pressures (< 120 mm Hg). Ather et al4 also showed that the relationship was U-shaped in patients with a mild to moderate reduction in left ventricular ejection fraction, but linear in those with severely reduced ejection fraction. This study also found that a decrease in systolic blood pressure below 110 mm Hg was associated with increased mortality risk.
The findings of PARADIGM-HF have sparked much conversation and implementation of practice change in the treatment of heart failure patients, and we await additional data on the use and limitations of sacubitril-valsartan in this group of patients.
In Reply: We thank Dr. Blankfield for raising these two important points. Although the findings of the PARADIGM-HF study are compelling, the design and results of this trial have incited many questions.
To address his first point, about the differential dosages of the two drugs, we agree, and we did mention in our review that one concern about the results of PARADIGM-HF is the unequal dosages of valsartan and enalapril in the two different arms. We mentioned that this dosage of enalapril was chosen based on its survival benefit in previous trials. However, this still raises the question of whether the benefit seen in the sacubitril-valsartan group was due to greater inhibition of the renin-angiotensin-aldosterone system rather than to the new drug.
To address his second point, the decrease in blood pressure in the sacubitril-valsartan arm was significant, and the patients taking this drug were more likely to have symptomatic hypotension, which may contribute to patient intolerance and difficulty initiating treatment with this drug. Dr. Blankfield brings up an interesting point regarding reduction of blood pressure driving the decrease of events in the sacubitril-valsartan group. In the original trial results section, the authors mentioned that when the difference in blood pressure between the two groups was examined as a time-dependent covariate, it was not a significant predictor of the benefit of sacubitril-valsartan.1
Furthermore, although higher blood pressure is associated with worse cardiovascular outcomes in the general population, higher blood pressure has been shown to be protective in heart failure patients.2 Several studies have shown that the relationship between blood pressure and the mortality rate in patients with heart failure is paradoxical and complex.2–4 Lee et al3 found that this relationship was U-shaped, with increased mortality risk in those with high and low blood pressures (< 120 mm Hg). Ather et al4 also showed that the relationship was U-shaped in patients with a mild to moderate reduction in left ventricular ejection fraction, but linear in those with severely reduced ejection fraction. This study also found that a decrease in systolic blood pressure below 110 mm Hg was associated with increased mortality risk.
The findings of PARADIGM-HF have sparked much conversation and implementation of practice change in the treatment of heart failure patients, and we await additional data on the use and limitations of sacubitril-valsartan in this group of patients.
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Raphael CE, Whinnett ZI, Davies JE, et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart 2009; 95:56–62.
- Lee DS, Ghosh N, Floras JS, et al. Association of blood pressure at hospital discharge with mortality in patients diagnosed with heart failure. Circ Heart Fail 2009; 2:616-623.
- Ather S, Chan W, Chillar A, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J 2011; 161:567–573.
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Raphael CE, Whinnett ZI, Davies JE, et al. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart 2009; 95:56–62.
- Lee DS, Ghosh N, Floras JS, et al. Association of blood pressure at hospital discharge with mortality in patients diagnosed with heart failure. Circ Heart Fail 2009; 2:616-623.
- Ather S, Chan W, Chillar A, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J 2011; 161:567–573.
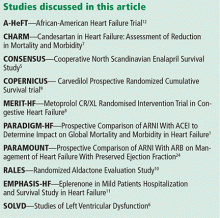
A new class of drugs for systolic heart failure: The PARADIGM-HF study
In a large phase trial, a combination drug that contains the angiotensin II receptor blocker (ARB) valsartan and the neprilysin inhibitor sacubitril was found to be superior to the angiotensin-converting enzyme (ACE) inhibitor enalapril in terms of important end points, including death and hospitalization for heart failure, in patients with heart failure with reduced ejection fraction.1
Recently approved by the US Food and Drug Administration, this combination drug, marketed under the brand name Entresto, represents a new drug class, angiotensin receptor-neprilysin inhibitors, or ARNIs.
This article is an overview of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial1 and the implications it may have on the care of patients with chronic heart failure.
NEED FOR NEW HEART FAILURE DRUGS
Heart failure is a major public health problem, and the care of patients with heart failure is challenging.
Almost 6 million US adults have heart failure, and the prevalence is projected to increase in the next few decades as the population continues to age.2 Furthermore, the total healthcare cost for heart failure patients was almost $31 billion in 2012 and is projected to rise to $70 billion by 2030.2
The care of patients with severely decompensated heart failure has changed dramatically in the last few decades with advances in heart transplantation and mechanical support devices. But day-to-day management of patients with chronic mildly to moderately symptomatic heart failure continues to pose a clinical challenge.
The drugs currently available for these patients include beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, digoxin, diuretics, and vasodilators. But even with these drugs, the death and readmission rates of patients with heart failure with reduced ejection fraction remain high. More than 50% of patients with heart failure die within 5 years of diagnosis,3 and 25% of patients hospitalized with heart failure are readmitted within 30 days of discharge.2 Furthermore, death rates are higher in those patients who have a history of heart failure hospitalization.4
Although heart failure with preserved ejection fraction encompasses an important group of heart failure patients with high morbidity, the focus of this article will be on patients with heart failure with reduced ejection fraction.
Available drugs to date
The cornerstone drugs that lower the odds of death in patients with heart failure with reduced ejection fraction are ACE inhibitors, ARBs, beta-blockers, and mineralocorticoid antagonists.
ACE inhibitors were the first class of drugs shown to reduce the death rate in patients with heart failure with reduced ejection fraction. The landmark CONSENSUS trial,5 published in 1987, found that the death rate in patients who received enalapril was 27% lower than in those receiving placebo, an effect driven entirely by a reduction in progressive heart failure. Similarly, the SOLVD trial,6 published in 1991, showed a 26% reduction in heart failure hospitalization and a 16% lower rate of death with enalapril compared with placebo, an effect driven predominantly by a decrease in the progression of heart failure.
ARBs have also been shown to decrease the rate of death, although not by as much as ACE inhibitors. In the CHARM trial,7 compared with placebo, candesartan significantly decreased the risk of death from any cause, of death from cardiovascular causes, and of hospitalization related to heart failure.7
Beta-blockers. The MERIT-HF trial,8 published in 1999, was stopped early because fewer patients were dying in the group receiving metoprolol succinate than in the group receiving placebo (relative risk 0.66). Similarly, in 2001, the COPERNICUS trial9 reported a 34% reduction in deaths in patients receiving carvedilol in addition to an ACE inhibitor compared with those receiving an ACE inhibitor alone.
Mineralocorticoid receptor antagonists were found to be beneficial when added to standard therapy for chronic symptomatic heart failure in the RALES10 and EMPHASIS-HF11 trials.
Vasodilators (specifically, the combination of isosorbide dinitrate and hydralazine) were found to have benefit in terms of mortality when added to standard therapy in African American patients in the A-HeEFT trial.12
WHY INHIBIT BOTH ANGIOTENSIN AND NEPRILYSIN?
The renin-angiotensin-aldosterone system is a major focus in treating heart failure, as overactivity of this system plays a key role in the pathophysiology of this disease. Therefore, essential drugs for heart failure patients include those that inhibit overactivity of this system such as ACE inhibitors, ARBs, and aldosterone antagonists.
The natriuretic peptide system is another important pathway that can be targeted in patients with heart failure. Natriuretic peptides are key molecules that counteract heart failure, as they contribute to diuresis and vasodilation and protect against vascular remodeling.13 An increased understanding of the importance of this system in slowing the progression of heart failure has motivated evaluation of drugs such as nesiritide in patients with symptomatic heart failure. However, these drugs can cause hypotension and have limited bioavailability.14
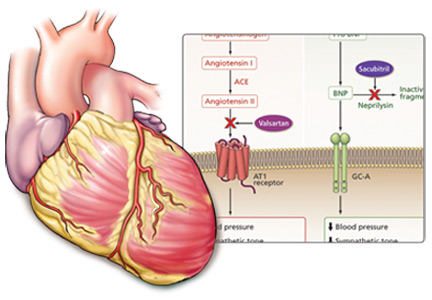
Neprilysin is an endopeptidase—an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides.14 Drugs that inhibit neprilysin increase the levels of these peptides and thus counteract neurohormonal stimuli that lead to cardiac remodeling, sodium retention, and vasoconstriction.15
However, neprilysin also hydrolyzes angiotensin I to angiotensin (1–7), an inhibitor of angiotensin II. Thus, inhibition of neprilysin alone could lead to increased activity of angiotensin II and so have an overall neutral effect. To be beneficial, neprilysin inhibition needs to be combined with renin-angiotensin system inhibition. Furthermore, the benefit of renin-angiotensin-aldosterone system blockade may be amplified by up-regulation of the endogenous natriuretic peptide system.15
Omapatrilat, the most studied combination neprilysin inhibitor and ACE inhibitor, improved cardiac function and decreased cardiac mass in animal experiments.15 In addition, this drug showed promise in terms of blood pressure, heart failure readmissions, death, and preservation of renal function when compared with ACE inhibitors in patients with heart failure.15–17 But in clinical trials this drug posed a greater risk of hypotension, dizziness, and, its major shortcoming, an unacceptably high incidence of angioedema compared with ACE inhibitors.15,16,18 This higher risk of angioedema is thought to be from inhibition of three enzymes that break down bradykinin: ACE, neprilysin, and aminopeptidase P.19
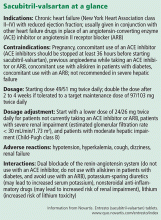
ARNIs contain an angiotensin receptor blocker rather than an ACE inhibitor, and thus in theory they may be associated with a lower risk of angioedema.19 Sacubitril-valsartan, the first drug of this class, contains its two constitutive drugs in a one-to-one molecular ratio (Figure 1).
PARADIGM-HF investigated the benefit of this drug in patients with systolic heart failure.1
STUDY DESIGN AND OBJECTIVES
PARADIGM-HF was a double-blind, randomized controlled trial comparing sacubitril-valsartan and enalapril in patients with chronic systolic heart failure. As such, it was the first trial in recent years to study a new drug in comparison with a well-established heart failure drug rather than as an add-on strategy.1
Inclusion criteria
To be included in the PARADIGM-HF trial, patients had to have:
- A left ventricular ejection fraction of 40% or less (later changed to ≤ 35%)
- New York Heart Association class II, III, or IV symptoms
- A B-type natriuretic peptide (BNP) level of at least 150 pg/mL or an N-terminal proBNP (NT-proBNP) level of at least 600 pg/mL; for patients hospitalized for heart failure within the previous 12 months, the cut points were lower (BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL).
End points
The primary end point was the composite of cardiovascular death or first hospitalization for heart failure. Other outcomes assessed were time to death from any cause, the change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to new-onset atrial fibrillation, and the time to decline in renal function (defined as end-stage renal disease or a decrease in estimated glomerular filtration rate of at least 50% from randomization). All end points were blindly adjudicated by a clinical end points committee.
Two run-in periods
The study enrolled 10,521 patients from 1,043 centers in 47 countries, who entered the initial run-in period consisting of 2 weeks of treatment with enalapril at the study dosage (10 mg twice daily) in order to ensure no unacceptable side effects. At this point, 1,102 patients exited the study, leaving 9,419 who entered the second run-in period.
The second run-in period consisted of 4 weeks of treatment with sacubitril-valsartan, initially at half the study regimen (100 mg twice daily) and eventually at the full study dosage (200 mg twice daily). During the second run-in period, 977 participants left the study, leaving a total of 8,442 patients who underwent randomization. Forty-three patients were then excluded (6 because of invalid randomization and 37 because of four sites that closed because of major violations of good clinical practice).
Of those randomized, 4,187 patients were assigned to the sacubitril-valsartan treatment group and 4,212 were assigned to the enalapril group. The investigators used an intention-to-treat analysis for this study.
Most patients had NYHA class II symptoms
The randomized patients had a mean age of 64 years, 75% were men, 66% were white, and 58% were from Europe (only 7% were from North America). The mean left ventricular ejection fraction was about 30%, and 60% of the study participants had an ischemic cause for their cardiomyopathy. Although one of the inclusion criteria was New York Heart Association class II, III, or IV symptoms, about 5% of the patients had class I symptoms. Seventy percent had class II symptoms, 24% had class III, and less than 1% had class IV symptoms.
At the time of randomization, 78% of the patients were taking an ACE inhibitor and 93% were taking a beta-blocker, but only a little more than half were taking a mineralocorticoid antagonist and only about 15% had an implantable cardioverter-defibrillator.
STUDY OUTCOMES
This study was designed to detect a 15% lower risk of cardiovascular death in the sacubitril-valsartan group. It was projected to continue for at least 34 months but was stopped early because of an overwhelming benefit of the new drug at a median follow-up of 27 months.
Major findings
The primary composite outcome (cardiovascular death or first hospitalization for heart failure)1 occurred in 21.8% of the patients in the sacubitril-valsartan group vs 26.5% of patients in the enalapril group (hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.73–0.87, P < .001). The number of patients who needed to be treated to prevent one occurrence of the primary composite outcome (100/absolute risk reduction) was only 21. The benefit was strong and consistent across both of the individual components of the composite outcome:
- Cardiovascular death 13.3% vs 16.5%, HR 0.80 (95% CI 0.71–0.89), P < .001
- First hospitalization for worsening heart failure 12.8% vs 15.6%, HR 0.79 (95% CI 0.71–0.89), P < .001.
Secondary outcomes. The sacubitril-valsartan group had a significantly lower rate of death from any cause (17.0% vs 19.8%, HR 0.84, 95% CI 0.76–0.93, P < .001) and a lower mean decrease in KCCQ clinical summary scores at 8 months (2.99 points vs 4.63 points, mean difference 1.64, 95% CI 0.63–2.65, P = .001). The KCCQ score measures subjective symptoms and physical limitations caused by heart failure; possible scores range from 0 to 100, with a higher score indicating better functional status. Notably, sacubitril-valsartan did not increase the KCCQ score in these patients; rather, sacubitril-valsartan recipients had a lower decrease in their scores than those in the enalapril group.
The incidence of new-onset atrial fibrillation was the same in both groups (3.1% and 3.1%).1 A decline in renal function (defined as end-stage renal disease, a decrease of 50% or more in estimated glomerular filtration rate from the value at randomization or a decrease in the estimated glomerular filtration rate of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2) occurred in 2.2% of the valsartan-sacubitril group and 2.6% of the enalapril group (P = .28).
The effects of the study drug on the primary composite outcome and on death from a cardiovascular cause were similar in all prespecified subgroups except for NYHA class: the reduction in the risk of the composite outcome was lower in sacubitril-valsartan recipients with NYHA I or II symptoms than in those with NYHA III or IV symptoms (P for interaction .03). However, there were no differences in the other prespecified subgroups, defined by age, sex, race, region, estimated glomerular filtration rate, diabetes, systolic blood pressure, ejection fraction, atrial fibrillation, NT-proBNP, hypertension, previous use of an ACE inhibitor, previous use of an aldosterone antagonist, previous heart failure hospitalization, and time since diagnosis of heart failure.
SAFETY: ANGIOEDEMA, HYPOTENSION, AND RENAL DYSFUNCTION
Angioedema
As noted above, the combination of neprilysin inhibitors and ACE inhibitors has been associated with an increased risk of angioedema. That was an important consideration before starting this study, which used a combination of a neprilysin inhibitor and an ARB in an attempt to avoid this serious side effect.
As it happened, there was no increased risk of significant angioedema with sacubitril-valsartan use compared with enalapril. Rates were similar to those in other studies, which showed a less than 1% risk of angioedema caused by ACE inhibitors.20,21 Only 19 patients (0.5%) in the sacubitril-valsartan group and 10 patients (0.2%) in the enalapril group experienced any angioedema. Of these, just three patients in the sacubitril-valsartan group and one patient in the enalapril group experienced angioedema that required hospitalization (P = .31). None of these patients had airway compromise due to angioedema.
Hypotension, cough, renal dysfunction, hyperkalemia
Other safety issues that were assessed included hypotension, worsening renal function, increase in potassium levels, and cough. Patients in the sacubitril-valsartan group were more likely to have symptomatic hypotension than patients in the enalapril group (14.0% vs 9.2%, P < .001); however, the authors noted that this was a rare cause of drug discontinuation.
Patients in the sacubitril-valsartan group were less likely to develop cough (11.3% vs 14.3%, P < .001), a serum creatinine level of 2.5 mg/dL or more (3.3% vs 4.5%, P = .007), or a serum potassium level of more than 6.0 mmol/L (11.3% vs 14.3%, P = .007).1
During the two run-in periods combined, 12% of the patients in the study withdrew because of adverse events, including cough, renal dysfunction, hyperkalemia, and symptomatic hypotension. During the enalapril run-in period, 591 patients (5.6%) withdrew due to adverse events, and 547 patients (5.8%) withdrew due to these events during the sacubitril-valsartan run-in period. After adjusting for the shorter time on enalapril, there was a higher rate of withdrawal because of adverse events from enalapril than from sacubitril-valsartan.
LOWER RISK OF CLINICAL PROGRESSION
In a separate paper,22 the PARADIGM-HF investigators reported that, among the survivors in the study, those who received sacubitril-valsartan fared better in terms of a number of markers of progression of heart failure, with lower rates of:
- Intensification of medical treatment for heart failure
- Emergency department visits for worsening heart failure
- Hospitalization for worsening heart failure
- Need for intensive care
- Need for intravenous inotropic agents
- Need for cardiac devices or heart transplants
- Worsening symptom scores
- Elevation of biomarkers of myocardial injury.
QUESTIONS AND CONCERNS
Although this study, which was funded by the manufacturer, showed consistent benefit for sacubitril-valsartan over enalapril, questions remain.
Are the findings generalizable?
Despite the study’s rigorous run-in period, 12% of patients dropped out because of adverse events, and thus the patients who completed the study may not be representative of the general population of heart failure patients. The authors included this double-level wash-out to ensure patient tolerance of both drugs. But in everyday practice, a significant number of patients may be unable to tolerate one of these drugs.
Moreover, after adjusting for the difference in the run-in periods, patients actually withdrew more often during the enalapril run-in period than during the sacubitril-valsartan run-in period. However, there may be overlap in tolerability in these two drugs, which both affect the renin-angiotensin-aldosterone system. Thus, the enalapril run-in period may have contributed to the lower tolerability of this drug compared with sacubitril-valsartan.
Were patients receiving the best possible therapy?
Another important point when considering how we treat heart failure patients in the United States is how few patients in this study were using cardiac implantable electronic devices. Only 15% of the patients in this study had an implantable cardioverter-defibrillator despite a mean left ventricular ejection fraction less than 30%. This likely reflects differences in practice internationally; however, based on American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines, these patients would have a class I indication for an implantable cardioverter-defibrillator for primary prevention of sudden cardiac death.23
Therefore, based on these recommendations, the patients in this study were not necessarily on optimal medical and device therapy and furthermore may not be representative of heart failure patients in the United States.
Was enalapril 10 mg twice a day a fair comparison?
Another concern about the results of this study relates to the dosages used in the two treatment groups. The sacubitril-valsartan formulation included full-dose valsartan, whereas the ACE inhibitor group received enalapril at less than a full dose.
Although the authors explained that the dose of enalapril chosen for the study was based on the one used in previous studies that showed survival benefit, this raises the question of whether the significant difference in outcomes in this trial was due to a greater inhibition of the renin-angiotensin-aldosterone system related to a higher dose of drug in the sacubitril-valsartan group.
What about black patients taking hydralazine-isosorbide?
Only about 5% of patients in the PARADIGM-HF trial were black. Based on the A-HeFT study results, black patients can be prescribed an ACE inhibitor as well as hydralazine and isosorbide dinitrate as tolerated to decrease the risk of death. Does sacubitril-valsartan offer benefit to these patients compared with a regimen of an ACE inhibitor, hydralazine, and isosorbide dinitrate?
Another concern is that the incidence of angioedema observed with ACE inhibitors and omapatrilat is higher in black patients.15,21 Thus, it would be prudent to investigate whether the risk of angioedema with sacubitril-valsartan would be higher if more black patients are studied.
IMPLICATIONS AND CONSIDERATIONS
In this study, sacubitril-valsartan showed impressive and consistent results, with an almost 20% decrease in the composite end point of heart failure hospitalization or cardiovascular death and a similar decrease in the composite outcomes with a very low number needed to treat (21 patients). It did not show a decrease in the incidence of new-onset atrial fibrillation; however, only 80 cases of atrial fibrillation were reported, so there may have been a lack of statistical power to detect a difference.
To avoid angioedema, wait at least 36 hours after stopping an ACE inhibitor. Sacubitril-valsartan was not associated with an increased risk of severe angioedema, and no patients experienced life-threatening angioedema. In the trial, the sacubitril-valsartan run-in period was started at least 24 hours after enalapril was stopped, and thus the authors recommended at least a 1-day washout period after discontinuing an ACE inhibitor to avoid angioedema in patients starting sacubitril-valsartan.
Hypotension is a concern. Although there was actually a decreased risk of renal dysfunction, hyperkalemia, and cough compared with enalapril, there was a significantly increased rate of symptomatic hypotension in the sacubitril-valsartan group, which raises the question of patient tolerance and physician comfort when prescribing and titrating this drug in clinical practice. This side effect will be an important consideration when attempting to titrate the drug to target doses.
Start treatment early. This trial largely consisted of patients with NYHA class II or III symptoms, with about 70% of patients with class II symptoms. Since this drug showed benefit in patients with mildly to moderately symptomatic heart failure, clinicians who are considering prescribing this drug should not wait until the patient is closer to end-stage disease. Patients with mildly symptomatic heart failure may be followed by a general cardiologist, internist, or both, and thus it is important to emphasize to the entire medical community the need to start this medication early on.
How much will it cost? Cost is a concern that could heavily weigh on the decision to prescribe this drug. Generic ACE inhibitors are relatively inexpensive, and it may difficult to switch from an affordable generic drug to a new drug that is likely to be much more expensive. Arguably, this drug may be cost-effective in the long run owing to a large decrease in heart failure readmissions. We await further analyses to evaluate this issue.
Will patients take a twice-a-day drug as prescribed? Most patients who are prescribed an ACE inhibitor take it just once a day, and switching from a daily to a twice-daily drug may present a challenge for some.
What about other outcomes? Based on this study, it is unclear what effect sacubitril-valsartan has on the incidence of fatal arrhythmias, sudden cardiac death, and pump failure. Furthermore, the effect on quality of life is still uncertain. Quality of life is an integral component in the evaluation of heart failure patients, and in this study the changes in KCCQ scores were not impressive. We hope to see further evaluations of this drug’s impact on quality of life of patients with heart failure. Furthermore, it would be interesting to study if this drug has any long-term effects on the need for advanced therapies such as left ventricular assist devices and orthotopic heart transplant.
What about patients with heart failure with preserved ejection fraction? This study included only patients with heart failure with reduced ejection fraction. However PARAMOUNT, a phase 2 study that evaluated the benefit of sacubitril-valsartan in patients with heart failure with preserved ejection fraction, has shown encouraging results.24 We look forward to further investigation of this agent in patients with heart failure with preserved ejection fraction.
Sacubitril-valsartan, the first ARNI to be studied in humans, has a dual action in that it enhances the activity of the natriuretic peptide system and inhibits that of the renin-angiotensin-aldosterone system. It is the first drug in over a decade to show mortality benefit in patients with chronic systolic heart failure when compared with an already well-established heart failure medication. It appears to decrease rates of mortality and heart failure hospitalization without increasing the risk of severe angioedema in patients with mild or moderate chronic systolic heart failure. Symptomatic hypotension and high cost may pose the largest barriers to the use of this new drug. And we have yet to see how the clinical community and patients with heart failure will respond to it.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Dunlay SM, Pereira NL, Kushwaha SS. Contemporary strategies in the diagnosis and management of heart failure. Mayo Clin Proc 2014; 89:662–676.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7:590–595.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Schreiner GF, Protter AA. B-type natriuretic peptide for the treatment of congestive heart failure. Curr Opin Pharmacol 2002; 2:142–147.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 2004; 102:223–241.
- Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106:920–926.
- Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000; 356:615–620.
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17:103–111.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Packer M, McMurray JJV, Desai AS, et al; on behalf of the PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131:54–61.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm 2008; 5:934–955.
- Solomon SD, Zile M, Pieske B, et al; Prospective comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380:1387–1395.
In a large phase trial, a combination drug that contains the angiotensin II receptor blocker (ARB) valsartan and the neprilysin inhibitor sacubitril was found to be superior to the angiotensin-converting enzyme (ACE) inhibitor enalapril in terms of important end points, including death and hospitalization for heart failure, in patients with heart failure with reduced ejection fraction.1
Recently approved by the US Food and Drug Administration, this combination drug, marketed under the brand name Entresto, represents a new drug class, angiotensin receptor-neprilysin inhibitors, or ARNIs.
This article is an overview of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial1 and the implications it may have on the care of patients with chronic heart failure.
NEED FOR NEW HEART FAILURE DRUGS
Heart failure is a major public health problem, and the care of patients with heart failure is challenging.
Almost 6 million US adults have heart failure, and the prevalence is projected to increase in the next few decades as the population continues to age.2 Furthermore, the total healthcare cost for heart failure patients was almost $31 billion in 2012 and is projected to rise to $70 billion by 2030.2
The care of patients with severely decompensated heart failure has changed dramatically in the last few decades with advances in heart transplantation and mechanical support devices. But day-to-day management of patients with chronic mildly to moderately symptomatic heart failure continues to pose a clinical challenge.
The drugs currently available for these patients include beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, digoxin, diuretics, and vasodilators. But even with these drugs, the death and readmission rates of patients with heart failure with reduced ejection fraction remain high. More than 50% of patients with heart failure die within 5 years of diagnosis,3 and 25% of patients hospitalized with heart failure are readmitted within 30 days of discharge.2 Furthermore, death rates are higher in those patients who have a history of heart failure hospitalization.4
Although heart failure with preserved ejection fraction encompasses an important group of heart failure patients with high morbidity, the focus of this article will be on patients with heart failure with reduced ejection fraction.
Available drugs to date
The cornerstone drugs that lower the odds of death in patients with heart failure with reduced ejection fraction are ACE inhibitors, ARBs, beta-blockers, and mineralocorticoid antagonists.
ACE inhibitors were the first class of drugs shown to reduce the death rate in patients with heart failure with reduced ejection fraction. The landmark CONSENSUS trial,5 published in 1987, found that the death rate in patients who received enalapril was 27% lower than in those receiving placebo, an effect driven entirely by a reduction in progressive heart failure. Similarly, the SOLVD trial,6 published in 1991, showed a 26% reduction in heart failure hospitalization and a 16% lower rate of death with enalapril compared with placebo, an effect driven predominantly by a decrease in the progression of heart failure.
ARBs have also been shown to decrease the rate of death, although not by as much as ACE inhibitors. In the CHARM trial,7 compared with placebo, candesartan significantly decreased the risk of death from any cause, of death from cardiovascular causes, and of hospitalization related to heart failure.7
Beta-blockers. The MERIT-HF trial,8 published in 1999, was stopped early because fewer patients were dying in the group receiving metoprolol succinate than in the group receiving placebo (relative risk 0.66). Similarly, in 2001, the COPERNICUS trial9 reported a 34% reduction in deaths in patients receiving carvedilol in addition to an ACE inhibitor compared with those receiving an ACE inhibitor alone.
Mineralocorticoid receptor antagonists were found to be beneficial when added to standard therapy for chronic symptomatic heart failure in the RALES10 and EMPHASIS-HF11 trials.
Vasodilators (specifically, the combination of isosorbide dinitrate and hydralazine) were found to have benefit in terms of mortality when added to standard therapy in African American patients in the A-HeEFT trial.12
WHY INHIBIT BOTH ANGIOTENSIN AND NEPRILYSIN?
The renin-angiotensin-aldosterone system is a major focus in treating heart failure, as overactivity of this system plays a key role in the pathophysiology of this disease. Therefore, essential drugs for heart failure patients include those that inhibit overactivity of this system such as ACE inhibitors, ARBs, and aldosterone antagonists.
The natriuretic peptide system is another important pathway that can be targeted in patients with heart failure. Natriuretic peptides are key molecules that counteract heart failure, as they contribute to diuresis and vasodilation and protect against vascular remodeling.13 An increased understanding of the importance of this system in slowing the progression of heart failure has motivated evaluation of drugs such as nesiritide in patients with symptomatic heart failure. However, these drugs can cause hypotension and have limited bioavailability.14
Neprilysin is an endopeptidase—an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides.14 Drugs that inhibit neprilysin increase the levels of these peptides and thus counteract neurohormonal stimuli that lead to cardiac remodeling, sodium retention, and vasoconstriction.15
However, neprilysin also hydrolyzes angiotensin I to angiotensin (1–7), an inhibitor of angiotensin II. Thus, inhibition of neprilysin alone could lead to increased activity of angiotensin II and so have an overall neutral effect. To be beneficial, neprilysin inhibition needs to be combined with renin-angiotensin system inhibition. Furthermore, the benefit of renin-angiotensin-aldosterone system blockade may be amplified by up-regulation of the endogenous natriuretic peptide system.15
Omapatrilat, the most studied combination neprilysin inhibitor and ACE inhibitor, improved cardiac function and decreased cardiac mass in animal experiments.15 In addition, this drug showed promise in terms of blood pressure, heart failure readmissions, death, and preservation of renal function when compared with ACE inhibitors in patients with heart failure.15–17 But in clinical trials this drug posed a greater risk of hypotension, dizziness, and, its major shortcoming, an unacceptably high incidence of angioedema compared with ACE inhibitors.15,16,18 This higher risk of angioedema is thought to be from inhibition of three enzymes that break down bradykinin: ACE, neprilysin, and aminopeptidase P.19
ARNIs contain an angiotensin receptor blocker rather than an ACE inhibitor, and thus in theory they may be associated with a lower risk of angioedema.19 Sacubitril-valsartan, the first drug of this class, contains its two constitutive drugs in a one-to-one molecular ratio (Figure 1).
PARADIGM-HF investigated the benefit of this drug in patients with systolic heart failure.1
STUDY DESIGN AND OBJECTIVES
PARADIGM-HF was a double-blind, randomized controlled trial comparing sacubitril-valsartan and enalapril in patients with chronic systolic heart failure. As such, it was the first trial in recent years to study a new drug in comparison with a well-established heart failure drug rather than as an add-on strategy.1
Inclusion criteria
To be included in the PARADIGM-HF trial, patients had to have:
- A left ventricular ejection fraction of 40% or less (later changed to ≤ 35%)
- New York Heart Association class II, III, or IV symptoms
- A B-type natriuretic peptide (BNP) level of at least 150 pg/mL or an N-terminal proBNP (NT-proBNP) level of at least 600 pg/mL; for patients hospitalized for heart failure within the previous 12 months, the cut points were lower (BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL).
End points
The primary end point was the composite of cardiovascular death or first hospitalization for heart failure. Other outcomes assessed were time to death from any cause, the change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to new-onset atrial fibrillation, and the time to decline in renal function (defined as end-stage renal disease or a decrease in estimated glomerular filtration rate of at least 50% from randomization). All end points were blindly adjudicated by a clinical end points committee.
Two run-in periods
The study enrolled 10,521 patients from 1,043 centers in 47 countries, who entered the initial run-in period consisting of 2 weeks of treatment with enalapril at the study dosage (10 mg twice daily) in order to ensure no unacceptable side effects. At this point, 1,102 patients exited the study, leaving 9,419 who entered the second run-in period.
The second run-in period consisted of 4 weeks of treatment with sacubitril-valsartan, initially at half the study regimen (100 mg twice daily) and eventually at the full study dosage (200 mg twice daily). During the second run-in period, 977 participants left the study, leaving a total of 8,442 patients who underwent randomization. Forty-three patients were then excluded (6 because of invalid randomization and 37 because of four sites that closed because of major violations of good clinical practice).
Of those randomized, 4,187 patients were assigned to the sacubitril-valsartan treatment group and 4,212 were assigned to the enalapril group. The investigators used an intention-to-treat analysis for this study.
Most patients had NYHA class II symptoms
The randomized patients had a mean age of 64 years, 75% were men, 66% were white, and 58% were from Europe (only 7% were from North America). The mean left ventricular ejection fraction was about 30%, and 60% of the study participants had an ischemic cause for their cardiomyopathy. Although one of the inclusion criteria was New York Heart Association class II, III, or IV symptoms, about 5% of the patients had class I symptoms. Seventy percent had class II symptoms, 24% had class III, and less than 1% had class IV symptoms.
At the time of randomization, 78% of the patients were taking an ACE inhibitor and 93% were taking a beta-blocker, but only a little more than half were taking a mineralocorticoid antagonist and only about 15% had an implantable cardioverter-defibrillator.
STUDY OUTCOMES
This study was designed to detect a 15% lower risk of cardiovascular death in the sacubitril-valsartan group. It was projected to continue for at least 34 months but was stopped early because of an overwhelming benefit of the new drug at a median follow-up of 27 months.
Major findings
The primary composite outcome (cardiovascular death or first hospitalization for heart failure)1 occurred in 21.8% of the patients in the sacubitril-valsartan group vs 26.5% of patients in the enalapril group (hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.73–0.87, P < .001). The number of patients who needed to be treated to prevent one occurrence of the primary composite outcome (100/absolute risk reduction) was only 21. The benefit was strong and consistent across both of the individual components of the composite outcome:
- Cardiovascular death 13.3% vs 16.5%, HR 0.80 (95% CI 0.71–0.89), P < .001
- First hospitalization for worsening heart failure 12.8% vs 15.6%, HR 0.79 (95% CI 0.71–0.89), P < .001.
Secondary outcomes. The sacubitril-valsartan group had a significantly lower rate of death from any cause (17.0% vs 19.8%, HR 0.84, 95% CI 0.76–0.93, P < .001) and a lower mean decrease in KCCQ clinical summary scores at 8 months (2.99 points vs 4.63 points, mean difference 1.64, 95% CI 0.63–2.65, P = .001). The KCCQ score measures subjective symptoms and physical limitations caused by heart failure; possible scores range from 0 to 100, with a higher score indicating better functional status. Notably, sacubitril-valsartan did not increase the KCCQ score in these patients; rather, sacubitril-valsartan recipients had a lower decrease in their scores than those in the enalapril group.
The incidence of new-onset atrial fibrillation was the same in both groups (3.1% and 3.1%).1 A decline in renal function (defined as end-stage renal disease, a decrease of 50% or more in estimated glomerular filtration rate from the value at randomization or a decrease in the estimated glomerular filtration rate of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2) occurred in 2.2% of the valsartan-sacubitril group and 2.6% of the enalapril group (P = .28).
The effects of the study drug on the primary composite outcome and on death from a cardiovascular cause were similar in all prespecified subgroups except for NYHA class: the reduction in the risk of the composite outcome was lower in sacubitril-valsartan recipients with NYHA I or II symptoms than in those with NYHA III or IV symptoms (P for interaction .03). However, there were no differences in the other prespecified subgroups, defined by age, sex, race, region, estimated glomerular filtration rate, diabetes, systolic blood pressure, ejection fraction, atrial fibrillation, NT-proBNP, hypertension, previous use of an ACE inhibitor, previous use of an aldosterone antagonist, previous heart failure hospitalization, and time since diagnosis of heart failure.
SAFETY: ANGIOEDEMA, HYPOTENSION, AND RENAL DYSFUNCTION
Angioedema
As noted above, the combination of neprilysin inhibitors and ACE inhibitors has been associated with an increased risk of angioedema. That was an important consideration before starting this study, which used a combination of a neprilysin inhibitor and an ARB in an attempt to avoid this serious side effect.
As it happened, there was no increased risk of significant angioedema with sacubitril-valsartan use compared with enalapril. Rates were similar to those in other studies, which showed a less than 1% risk of angioedema caused by ACE inhibitors.20,21 Only 19 patients (0.5%) in the sacubitril-valsartan group and 10 patients (0.2%) in the enalapril group experienced any angioedema. Of these, just three patients in the sacubitril-valsartan group and one patient in the enalapril group experienced angioedema that required hospitalization (P = .31). None of these patients had airway compromise due to angioedema.
Hypotension, cough, renal dysfunction, hyperkalemia
Other safety issues that were assessed included hypotension, worsening renal function, increase in potassium levels, and cough. Patients in the sacubitril-valsartan group were more likely to have symptomatic hypotension than patients in the enalapril group (14.0% vs 9.2%, P < .001); however, the authors noted that this was a rare cause of drug discontinuation.
Patients in the sacubitril-valsartan group were less likely to develop cough (11.3% vs 14.3%, P < .001), a serum creatinine level of 2.5 mg/dL or more (3.3% vs 4.5%, P = .007), or a serum potassium level of more than 6.0 mmol/L (11.3% vs 14.3%, P = .007).1
During the two run-in periods combined, 12% of the patients in the study withdrew because of adverse events, including cough, renal dysfunction, hyperkalemia, and symptomatic hypotension. During the enalapril run-in period, 591 patients (5.6%) withdrew due to adverse events, and 547 patients (5.8%) withdrew due to these events during the sacubitril-valsartan run-in period. After adjusting for the shorter time on enalapril, there was a higher rate of withdrawal because of adverse events from enalapril than from sacubitril-valsartan.
LOWER RISK OF CLINICAL PROGRESSION
In a separate paper,22 the PARADIGM-HF investigators reported that, among the survivors in the study, those who received sacubitril-valsartan fared better in terms of a number of markers of progression of heart failure, with lower rates of:
- Intensification of medical treatment for heart failure
- Emergency department visits for worsening heart failure
- Hospitalization for worsening heart failure
- Need for intensive care
- Need for intravenous inotropic agents
- Need for cardiac devices or heart transplants
- Worsening symptom scores
- Elevation of biomarkers of myocardial injury.
QUESTIONS AND CONCERNS
Although this study, which was funded by the manufacturer, showed consistent benefit for sacubitril-valsartan over enalapril, questions remain.
Are the findings generalizable?
Despite the study’s rigorous run-in period, 12% of patients dropped out because of adverse events, and thus the patients who completed the study may not be representative of the general population of heart failure patients. The authors included this double-level wash-out to ensure patient tolerance of both drugs. But in everyday practice, a significant number of patients may be unable to tolerate one of these drugs.
Moreover, after adjusting for the difference in the run-in periods, patients actually withdrew more often during the enalapril run-in period than during the sacubitril-valsartan run-in period. However, there may be overlap in tolerability in these two drugs, which both affect the renin-angiotensin-aldosterone system. Thus, the enalapril run-in period may have contributed to the lower tolerability of this drug compared with sacubitril-valsartan.
Were patients receiving the best possible therapy?
Another important point when considering how we treat heart failure patients in the United States is how few patients in this study were using cardiac implantable electronic devices. Only 15% of the patients in this study had an implantable cardioverter-defibrillator despite a mean left ventricular ejection fraction less than 30%. This likely reflects differences in practice internationally; however, based on American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines, these patients would have a class I indication for an implantable cardioverter-defibrillator for primary prevention of sudden cardiac death.23
Therefore, based on these recommendations, the patients in this study were not necessarily on optimal medical and device therapy and furthermore may not be representative of heart failure patients in the United States.
Was enalapril 10 mg twice a day a fair comparison?
Another concern about the results of this study relates to the dosages used in the two treatment groups. The sacubitril-valsartan formulation included full-dose valsartan, whereas the ACE inhibitor group received enalapril at less than a full dose.
Although the authors explained that the dose of enalapril chosen for the study was based on the one used in previous studies that showed survival benefit, this raises the question of whether the significant difference in outcomes in this trial was due to a greater inhibition of the renin-angiotensin-aldosterone system related to a higher dose of drug in the sacubitril-valsartan group.
What about black patients taking hydralazine-isosorbide?
Only about 5% of patients in the PARADIGM-HF trial were black. Based on the A-HeFT study results, black patients can be prescribed an ACE inhibitor as well as hydralazine and isosorbide dinitrate as tolerated to decrease the risk of death. Does sacubitril-valsartan offer benefit to these patients compared with a regimen of an ACE inhibitor, hydralazine, and isosorbide dinitrate?
Another concern is that the incidence of angioedema observed with ACE inhibitors and omapatrilat is higher in black patients.15,21 Thus, it would be prudent to investigate whether the risk of angioedema with sacubitril-valsartan would be higher if more black patients are studied.
IMPLICATIONS AND CONSIDERATIONS
In this study, sacubitril-valsartan showed impressive and consistent results, with an almost 20% decrease in the composite end point of heart failure hospitalization or cardiovascular death and a similar decrease in the composite outcomes with a very low number needed to treat (21 patients). It did not show a decrease in the incidence of new-onset atrial fibrillation; however, only 80 cases of atrial fibrillation were reported, so there may have been a lack of statistical power to detect a difference.
To avoid angioedema, wait at least 36 hours after stopping an ACE inhibitor. Sacubitril-valsartan was not associated with an increased risk of severe angioedema, and no patients experienced life-threatening angioedema. In the trial, the sacubitril-valsartan run-in period was started at least 24 hours after enalapril was stopped, and thus the authors recommended at least a 1-day washout period after discontinuing an ACE inhibitor to avoid angioedema in patients starting sacubitril-valsartan.
Hypotension is a concern. Although there was actually a decreased risk of renal dysfunction, hyperkalemia, and cough compared with enalapril, there was a significantly increased rate of symptomatic hypotension in the sacubitril-valsartan group, which raises the question of patient tolerance and physician comfort when prescribing and titrating this drug in clinical practice. This side effect will be an important consideration when attempting to titrate the drug to target doses.
Start treatment early. This trial largely consisted of patients with NYHA class II or III symptoms, with about 70% of patients with class II symptoms. Since this drug showed benefit in patients with mildly to moderately symptomatic heart failure, clinicians who are considering prescribing this drug should not wait until the patient is closer to end-stage disease. Patients with mildly symptomatic heart failure may be followed by a general cardiologist, internist, or both, and thus it is important to emphasize to the entire medical community the need to start this medication early on.
How much will it cost? Cost is a concern that could heavily weigh on the decision to prescribe this drug. Generic ACE inhibitors are relatively inexpensive, and it may difficult to switch from an affordable generic drug to a new drug that is likely to be much more expensive. Arguably, this drug may be cost-effective in the long run owing to a large decrease in heart failure readmissions. We await further analyses to evaluate this issue.
Will patients take a twice-a-day drug as prescribed? Most patients who are prescribed an ACE inhibitor take it just once a day, and switching from a daily to a twice-daily drug may present a challenge for some.
What about other outcomes? Based on this study, it is unclear what effect sacubitril-valsartan has on the incidence of fatal arrhythmias, sudden cardiac death, and pump failure. Furthermore, the effect on quality of life is still uncertain. Quality of life is an integral component in the evaluation of heart failure patients, and in this study the changes in KCCQ scores were not impressive. We hope to see further evaluations of this drug’s impact on quality of life of patients with heart failure. Furthermore, it would be interesting to study if this drug has any long-term effects on the need for advanced therapies such as left ventricular assist devices and orthotopic heart transplant.
What about patients with heart failure with preserved ejection fraction? This study included only patients with heart failure with reduced ejection fraction. However PARAMOUNT, a phase 2 study that evaluated the benefit of sacubitril-valsartan in patients with heart failure with preserved ejection fraction, has shown encouraging results.24 We look forward to further investigation of this agent in patients with heart failure with preserved ejection fraction.
Sacubitril-valsartan, the first ARNI to be studied in humans, has a dual action in that it enhances the activity of the natriuretic peptide system and inhibits that of the renin-angiotensin-aldosterone system. It is the first drug in over a decade to show mortality benefit in patients with chronic systolic heart failure when compared with an already well-established heart failure medication. It appears to decrease rates of mortality and heart failure hospitalization without increasing the risk of severe angioedema in patients with mild or moderate chronic systolic heart failure. Symptomatic hypotension and high cost may pose the largest barriers to the use of this new drug. And we have yet to see how the clinical community and patients with heart failure will respond to it.
In a large phase trial, a combination drug that contains the angiotensin II receptor blocker (ARB) valsartan and the neprilysin inhibitor sacubitril was found to be superior to the angiotensin-converting enzyme (ACE) inhibitor enalapril in terms of important end points, including death and hospitalization for heart failure, in patients with heart failure with reduced ejection fraction.1
Recently approved by the US Food and Drug Administration, this combination drug, marketed under the brand name Entresto, represents a new drug class, angiotensin receptor-neprilysin inhibitors, or ARNIs.
This article is an overview of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial1 and the implications it may have on the care of patients with chronic heart failure.
NEED FOR NEW HEART FAILURE DRUGS
Heart failure is a major public health problem, and the care of patients with heart failure is challenging.
Almost 6 million US adults have heart failure, and the prevalence is projected to increase in the next few decades as the population continues to age.2 Furthermore, the total healthcare cost for heart failure patients was almost $31 billion in 2012 and is projected to rise to $70 billion by 2030.2
The care of patients with severely decompensated heart failure has changed dramatically in the last few decades with advances in heart transplantation and mechanical support devices. But day-to-day management of patients with chronic mildly to moderately symptomatic heart failure continues to pose a clinical challenge.
The drugs currently available for these patients include beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, digoxin, diuretics, and vasodilators. But even with these drugs, the death and readmission rates of patients with heart failure with reduced ejection fraction remain high. More than 50% of patients with heart failure die within 5 years of diagnosis,3 and 25% of patients hospitalized with heart failure are readmitted within 30 days of discharge.2 Furthermore, death rates are higher in those patients who have a history of heart failure hospitalization.4
Although heart failure with preserved ejection fraction encompasses an important group of heart failure patients with high morbidity, the focus of this article will be on patients with heart failure with reduced ejection fraction.
Available drugs to date
The cornerstone drugs that lower the odds of death in patients with heart failure with reduced ejection fraction are ACE inhibitors, ARBs, beta-blockers, and mineralocorticoid antagonists.
ACE inhibitors were the first class of drugs shown to reduce the death rate in patients with heart failure with reduced ejection fraction. The landmark CONSENSUS trial,5 published in 1987, found that the death rate in patients who received enalapril was 27% lower than in those receiving placebo, an effect driven entirely by a reduction in progressive heart failure. Similarly, the SOLVD trial,6 published in 1991, showed a 26% reduction in heart failure hospitalization and a 16% lower rate of death with enalapril compared with placebo, an effect driven predominantly by a decrease in the progression of heart failure.
ARBs have also been shown to decrease the rate of death, although not by as much as ACE inhibitors. In the CHARM trial,7 compared with placebo, candesartan significantly decreased the risk of death from any cause, of death from cardiovascular causes, and of hospitalization related to heart failure.7
Beta-blockers. The MERIT-HF trial,8 published in 1999, was stopped early because fewer patients were dying in the group receiving metoprolol succinate than in the group receiving placebo (relative risk 0.66). Similarly, in 2001, the COPERNICUS trial9 reported a 34% reduction in deaths in patients receiving carvedilol in addition to an ACE inhibitor compared with those receiving an ACE inhibitor alone.
Mineralocorticoid receptor antagonists were found to be beneficial when added to standard therapy for chronic symptomatic heart failure in the RALES10 and EMPHASIS-HF11 trials.
Vasodilators (specifically, the combination of isosorbide dinitrate and hydralazine) were found to have benefit in terms of mortality when added to standard therapy in African American patients in the A-HeEFT trial.12
WHY INHIBIT BOTH ANGIOTENSIN AND NEPRILYSIN?
The renin-angiotensin-aldosterone system is a major focus in treating heart failure, as overactivity of this system plays a key role in the pathophysiology of this disease. Therefore, essential drugs for heart failure patients include those that inhibit overactivity of this system such as ACE inhibitors, ARBs, and aldosterone antagonists.
The natriuretic peptide system is another important pathway that can be targeted in patients with heart failure. Natriuretic peptides are key molecules that counteract heart failure, as they contribute to diuresis and vasodilation and protect against vascular remodeling.13 An increased understanding of the importance of this system in slowing the progression of heart failure has motivated evaluation of drugs such as nesiritide in patients with symptomatic heart failure. However, these drugs can cause hypotension and have limited bioavailability.14
Neprilysin is an endopeptidase—an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides.14 Drugs that inhibit neprilysin increase the levels of these peptides and thus counteract neurohormonal stimuli that lead to cardiac remodeling, sodium retention, and vasoconstriction.15
However, neprilysin also hydrolyzes angiotensin I to angiotensin (1–7), an inhibitor of angiotensin II. Thus, inhibition of neprilysin alone could lead to increased activity of angiotensin II and so have an overall neutral effect. To be beneficial, neprilysin inhibition needs to be combined with renin-angiotensin system inhibition. Furthermore, the benefit of renin-angiotensin-aldosterone system blockade may be amplified by up-regulation of the endogenous natriuretic peptide system.15
Omapatrilat, the most studied combination neprilysin inhibitor and ACE inhibitor, improved cardiac function and decreased cardiac mass in animal experiments.15 In addition, this drug showed promise in terms of blood pressure, heart failure readmissions, death, and preservation of renal function when compared with ACE inhibitors in patients with heart failure.15–17 But in clinical trials this drug posed a greater risk of hypotension, dizziness, and, its major shortcoming, an unacceptably high incidence of angioedema compared with ACE inhibitors.15,16,18 This higher risk of angioedema is thought to be from inhibition of three enzymes that break down bradykinin: ACE, neprilysin, and aminopeptidase P.19
ARNIs contain an angiotensin receptor blocker rather than an ACE inhibitor, and thus in theory they may be associated with a lower risk of angioedema.19 Sacubitril-valsartan, the first drug of this class, contains its two constitutive drugs in a one-to-one molecular ratio (Figure 1).
PARADIGM-HF investigated the benefit of this drug in patients with systolic heart failure.1
STUDY DESIGN AND OBJECTIVES
PARADIGM-HF was a double-blind, randomized controlled trial comparing sacubitril-valsartan and enalapril in patients with chronic systolic heart failure. As such, it was the first trial in recent years to study a new drug in comparison with a well-established heart failure drug rather than as an add-on strategy.1
Inclusion criteria
To be included in the PARADIGM-HF trial, patients had to have:
- A left ventricular ejection fraction of 40% or less (later changed to ≤ 35%)
- New York Heart Association class II, III, or IV symptoms
- A B-type natriuretic peptide (BNP) level of at least 150 pg/mL or an N-terminal proBNP (NT-proBNP) level of at least 600 pg/mL; for patients hospitalized for heart failure within the previous 12 months, the cut points were lower (BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL).
End points
The primary end point was the composite of cardiovascular death or first hospitalization for heart failure. Other outcomes assessed were time to death from any cause, the change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to new-onset atrial fibrillation, and the time to decline in renal function (defined as end-stage renal disease or a decrease in estimated glomerular filtration rate of at least 50% from randomization). All end points were blindly adjudicated by a clinical end points committee.
Two run-in periods
The study enrolled 10,521 patients from 1,043 centers in 47 countries, who entered the initial run-in period consisting of 2 weeks of treatment with enalapril at the study dosage (10 mg twice daily) in order to ensure no unacceptable side effects. At this point, 1,102 patients exited the study, leaving 9,419 who entered the second run-in period.
The second run-in period consisted of 4 weeks of treatment with sacubitril-valsartan, initially at half the study regimen (100 mg twice daily) and eventually at the full study dosage (200 mg twice daily). During the second run-in period, 977 participants left the study, leaving a total of 8,442 patients who underwent randomization. Forty-three patients were then excluded (6 because of invalid randomization and 37 because of four sites that closed because of major violations of good clinical practice).
Of those randomized, 4,187 patients were assigned to the sacubitril-valsartan treatment group and 4,212 were assigned to the enalapril group. The investigators used an intention-to-treat analysis for this study.
Most patients had NYHA class II symptoms
The randomized patients had a mean age of 64 years, 75% were men, 66% were white, and 58% were from Europe (only 7% were from North America). The mean left ventricular ejection fraction was about 30%, and 60% of the study participants had an ischemic cause for their cardiomyopathy. Although one of the inclusion criteria was New York Heart Association class II, III, or IV symptoms, about 5% of the patients had class I symptoms. Seventy percent had class II symptoms, 24% had class III, and less than 1% had class IV symptoms.
At the time of randomization, 78% of the patients were taking an ACE inhibitor and 93% were taking a beta-blocker, but only a little more than half were taking a mineralocorticoid antagonist and only about 15% had an implantable cardioverter-defibrillator.
STUDY OUTCOMES
This study was designed to detect a 15% lower risk of cardiovascular death in the sacubitril-valsartan group. It was projected to continue for at least 34 months but was stopped early because of an overwhelming benefit of the new drug at a median follow-up of 27 months.
Major findings
The primary composite outcome (cardiovascular death or first hospitalization for heart failure)1 occurred in 21.8% of the patients in the sacubitril-valsartan group vs 26.5% of patients in the enalapril group (hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.73–0.87, P < .001). The number of patients who needed to be treated to prevent one occurrence of the primary composite outcome (100/absolute risk reduction) was only 21. The benefit was strong and consistent across both of the individual components of the composite outcome:
- Cardiovascular death 13.3% vs 16.5%, HR 0.80 (95% CI 0.71–0.89), P < .001
- First hospitalization for worsening heart failure 12.8% vs 15.6%, HR 0.79 (95% CI 0.71–0.89), P < .001.
Secondary outcomes. The sacubitril-valsartan group had a significantly lower rate of death from any cause (17.0% vs 19.8%, HR 0.84, 95% CI 0.76–0.93, P < .001) and a lower mean decrease in KCCQ clinical summary scores at 8 months (2.99 points vs 4.63 points, mean difference 1.64, 95% CI 0.63–2.65, P = .001). The KCCQ score measures subjective symptoms and physical limitations caused by heart failure; possible scores range from 0 to 100, with a higher score indicating better functional status. Notably, sacubitril-valsartan did not increase the KCCQ score in these patients; rather, sacubitril-valsartan recipients had a lower decrease in their scores than those in the enalapril group.
The incidence of new-onset atrial fibrillation was the same in both groups (3.1% and 3.1%).1 A decline in renal function (defined as end-stage renal disease, a decrease of 50% or more in estimated glomerular filtration rate from the value at randomization or a decrease in the estimated glomerular filtration rate of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2) occurred in 2.2% of the valsartan-sacubitril group and 2.6% of the enalapril group (P = .28).
The effects of the study drug on the primary composite outcome and on death from a cardiovascular cause were similar in all prespecified subgroups except for NYHA class: the reduction in the risk of the composite outcome was lower in sacubitril-valsartan recipients with NYHA I or II symptoms than in those with NYHA III or IV symptoms (P for interaction .03). However, there were no differences in the other prespecified subgroups, defined by age, sex, race, region, estimated glomerular filtration rate, diabetes, systolic blood pressure, ejection fraction, atrial fibrillation, NT-proBNP, hypertension, previous use of an ACE inhibitor, previous use of an aldosterone antagonist, previous heart failure hospitalization, and time since diagnosis of heart failure.
SAFETY: ANGIOEDEMA, HYPOTENSION, AND RENAL DYSFUNCTION
Angioedema
As noted above, the combination of neprilysin inhibitors and ACE inhibitors has been associated with an increased risk of angioedema. That was an important consideration before starting this study, which used a combination of a neprilysin inhibitor and an ARB in an attempt to avoid this serious side effect.
As it happened, there was no increased risk of significant angioedema with sacubitril-valsartan use compared with enalapril. Rates were similar to those in other studies, which showed a less than 1% risk of angioedema caused by ACE inhibitors.20,21 Only 19 patients (0.5%) in the sacubitril-valsartan group and 10 patients (0.2%) in the enalapril group experienced any angioedema. Of these, just three patients in the sacubitril-valsartan group and one patient in the enalapril group experienced angioedema that required hospitalization (P = .31). None of these patients had airway compromise due to angioedema.
Hypotension, cough, renal dysfunction, hyperkalemia
Other safety issues that were assessed included hypotension, worsening renal function, increase in potassium levels, and cough. Patients in the sacubitril-valsartan group were more likely to have symptomatic hypotension than patients in the enalapril group (14.0% vs 9.2%, P < .001); however, the authors noted that this was a rare cause of drug discontinuation.
Patients in the sacubitril-valsartan group were less likely to develop cough (11.3% vs 14.3%, P < .001), a serum creatinine level of 2.5 mg/dL or more (3.3% vs 4.5%, P = .007), or a serum potassium level of more than 6.0 mmol/L (11.3% vs 14.3%, P = .007).1
During the two run-in periods combined, 12% of the patients in the study withdrew because of adverse events, including cough, renal dysfunction, hyperkalemia, and symptomatic hypotension. During the enalapril run-in period, 591 patients (5.6%) withdrew due to adverse events, and 547 patients (5.8%) withdrew due to these events during the sacubitril-valsartan run-in period. After adjusting for the shorter time on enalapril, there was a higher rate of withdrawal because of adverse events from enalapril than from sacubitril-valsartan.
LOWER RISK OF CLINICAL PROGRESSION
In a separate paper,22 the PARADIGM-HF investigators reported that, among the survivors in the study, those who received sacubitril-valsartan fared better in terms of a number of markers of progression of heart failure, with lower rates of:
- Intensification of medical treatment for heart failure
- Emergency department visits for worsening heart failure
- Hospitalization for worsening heart failure
- Need for intensive care
- Need for intravenous inotropic agents
- Need for cardiac devices or heart transplants
- Worsening symptom scores
- Elevation of biomarkers of myocardial injury.
QUESTIONS AND CONCERNS
Although this study, which was funded by the manufacturer, showed consistent benefit for sacubitril-valsartan over enalapril, questions remain.
Are the findings generalizable?
Despite the study’s rigorous run-in period, 12% of patients dropped out because of adverse events, and thus the patients who completed the study may not be representative of the general population of heart failure patients. The authors included this double-level wash-out to ensure patient tolerance of both drugs. But in everyday practice, a significant number of patients may be unable to tolerate one of these drugs.
Moreover, after adjusting for the difference in the run-in periods, patients actually withdrew more often during the enalapril run-in period than during the sacubitril-valsartan run-in period. However, there may be overlap in tolerability in these two drugs, which both affect the renin-angiotensin-aldosterone system. Thus, the enalapril run-in period may have contributed to the lower tolerability of this drug compared with sacubitril-valsartan.
Were patients receiving the best possible therapy?
Another important point when considering how we treat heart failure patients in the United States is how few patients in this study were using cardiac implantable electronic devices. Only 15% of the patients in this study had an implantable cardioverter-defibrillator despite a mean left ventricular ejection fraction less than 30%. This likely reflects differences in practice internationally; however, based on American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines, these patients would have a class I indication for an implantable cardioverter-defibrillator for primary prevention of sudden cardiac death.23
Therefore, based on these recommendations, the patients in this study were not necessarily on optimal medical and device therapy and furthermore may not be representative of heart failure patients in the United States.
Was enalapril 10 mg twice a day a fair comparison?
Another concern about the results of this study relates to the dosages used in the two treatment groups. The sacubitril-valsartan formulation included full-dose valsartan, whereas the ACE inhibitor group received enalapril at less than a full dose.
Although the authors explained that the dose of enalapril chosen for the study was based on the one used in previous studies that showed survival benefit, this raises the question of whether the significant difference in outcomes in this trial was due to a greater inhibition of the renin-angiotensin-aldosterone system related to a higher dose of drug in the sacubitril-valsartan group.
What about black patients taking hydralazine-isosorbide?
Only about 5% of patients in the PARADIGM-HF trial were black. Based on the A-HeFT study results, black patients can be prescribed an ACE inhibitor as well as hydralazine and isosorbide dinitrate as tolerated to decrease the risk of death. Does sacubitril-valsartan offer benefit to these patients compared with a regimen of an ACE inhibitor, hydralazine, and isosorbide dinitrate?
Another concern is that the incidence of angioedema observed with ACE inhibitors and omapatrilat is higher in black patients.15,21 Thus, it would be prudent to investigate whether the risk of angioedema with sacubitril-valsartan would be higher if more black patients are studied.
IMPLICATIONS AND CONSIDERATIONS
In this study, sacubitril-valsartan showed impressive and consistent results, with an almost 20% decrease in the composite end point of heart failure hospitalization or cardiovascular death and a similar decrease in the composite outcomes with a very low number needed to treat (21 patients). It did not show a decrease in the incidence of new-onset atrial fibrillation; however, only 80 cases of atrial fibrillation were reported, so there may have been a lack of statistical power to detect a difference.
To avoid angioedema, wait at least 36 hours after stopping an ACE inhibitor. Sacubitril-valsartan was not associated with an increased risk of severe angioedema, and no patients experienced life-threatening angioedema. In the trial, the sacubitril-valsartan run-in period was started at least 24 hours after enalapril was stopped, and thus the authors recommended at least a 1-day washout period after discontinuing an ACE inhibitor to avoid angioedema in patients starting sacubitril-valsartan.
Hypotension is a concern. Although there was actually a decreased risk of renal dysfunction, hyperkalemia, and cough compared with enalapril, there was a significantly increased rate of symptomatic hypotension in the sacubitril-valsartan group, which raises the question of patient tolerance and physician comfort when prescribing and titrating this drug in clinical practice. This side effect will be an important consideration when attempting to titrate the drug to target doses.
Start treatment early. This trial largely consisted of patients with NYHA class II or III symptoms, with about 70% of patients with class II symptoms. Since this drug showed benefit in patients with mildly to moderately symptomatic heart failure, clinicians who are considering prescribing this drug should not wait until the patient is closer to end-stage disease. Patients with mildly symptomatic heart failure may be followed by a general cardiologist, internist, or both, and thus it is important to emphasize to the entire medical community the need to start this medication early on.
How much will it cost? Cost is a concern that could heavily weigh on the decision to prescribe this drug. Generic ACE inhibitors are relatively inexpensive, and it may difficult to switch from an affordable generic drug to a new drug that is likely to be much more expensive. Arguably, this drug may be cost-effective in the long run owing to a large decrease in heart failure readmissions. We await further analyses to evaluate this issue.
Will patients take a twice-a-day drug as prescribed? Most patients who are prescribed an ACE inhibitor take it just once a day, and switching from a daily to a twice-daily drug may present a challenge for some.
What about other outcomes? Based on this study, it is unclear what effect sacubitril-valsartan has on the incidence of fatal arrhythmias, sudden cardiac death, and pump failure. Furthermore, the effect on quality of life is still uncertain. Quality of life is an integral component in the evaluation of heart failure patients, and in this study the changes in KCCQ scores were not impressive. We hope to see further evaluations of this drug’s impact on quality of life of patients with heart failure. Furthermore, it would be interesting to study if this drug has any long-term effects on the need for advanced therapies such as left ventricular assist devices and orthotopic heart transplant.
What about patients with heart failure with preserved ejection fraction? This study included only patients with heart failure with reduced ejection fraction. However PARAMOUNT, a phase 2 study that evaluated the benefit of sacubitril-valsartan in patients with heart failure with preserved ejection fraction, has shown encouraging results.24 We look forward to further investigation of this agent in patients with heart failure with preserved ejection fraction.
Sacubitril-valsartan, the first ARNI to be studied in humans, has a dual action in that it enhances the activity of the natriuretic peptide system and inhibits that of the renin-angiotensin-aldosterone system. It is the first drug in over a decade to show mortality benefit in patients with chronic systolic heart failure when compared with an already well-established heart failure medication. It appears to decrease rates of mortality and heart failure hospitalization without increasing the risk of severe angioedema in patients with mild or moderate chronic systolic heart failure. Symptomatic hypotension and high cost may pose the largest barriers to the use of this new drug. And we have yet to see how the clinical community and patients with heart failure will respond to it.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Dunlay SM, Pereira NL, Kushwaha SS. Contemporary strategies in the diagnosis and management of heart failure. Mayo Clin Proc 2014; 89:662–676.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7:590–595.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Schreiner GF, Protter AA. B-type natriuretic peptide for the treatment of congestive heart failure. Curr Opin Pharmacol 2002; 2:142–147.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 2004; 102:223–241.
- Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106:920–926.
- Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000; 356:615–620.
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17:103–111.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Packer M, McMurray JJV, Desai AS, et al; on behalf of the PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131:54–61.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm 2008; 5:934–955.
- Solomon SD, Zile M, Pieske B, et al; Prospective comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380:1387–1395.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Dunlay SM, Pereira NL, Kushwaha SS. Contemporary strategies in the diagnosis and management of heart failure. Mayo Clin Proc 2014; 89:662–676.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7:590–595.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Schreiner GF, Protter AA. B-type natriuretic peptide for the treatment of congestive heart failure. Curr Opin Pharmacol 2002; 2:142–147.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 2004; 102:223–241.
- Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106:920–926.
- Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000; 356:615–620.
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17:103–111.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Packer M, McMurray JJV, Desai AS, et al; on behalf of the PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131:54–61.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm 2008; 5:934–955.
- Solomon SD, Zile M, Pieske B, et al; Prospective comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380:1387–1395.
KEY POINTS
- Neprilysin is an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides. Inhibition of neprilysin raises the levels of these peptides, leading to less cardiac remodeling, less sodium retention, and less vasoconstriction.
- Neprilysin inhibition must be combined with inhibition of the renin-angiotensin-aldosterone system, optimally with an angiotensin II receptor blocker.
- PARADIGM-HF showed a 20% reduction in the primary outcome of death from cardiovascular causes or hospitalization for heart failure with sacubitril-valsartan 200 mg twice daily vs enalapril 10 mg twice daily at a median follow-up of 27 months.
- The ultimate role of combined neprilysin and angiotensin receptor inhibitors remains to be determined.