User login
Delirium in Hospitalized Patients
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
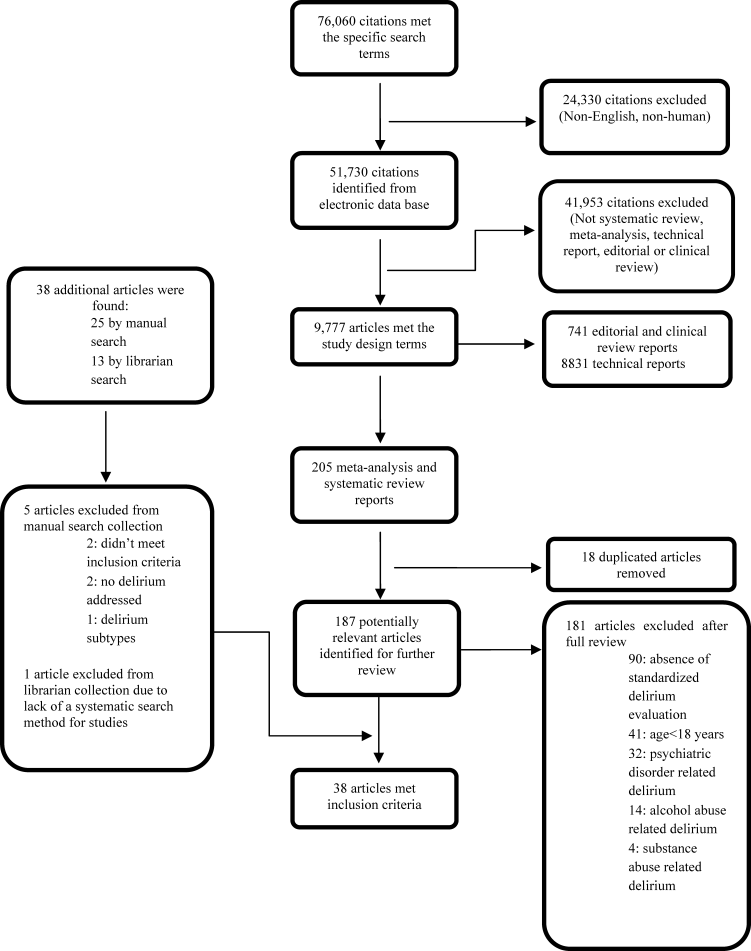
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.
| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
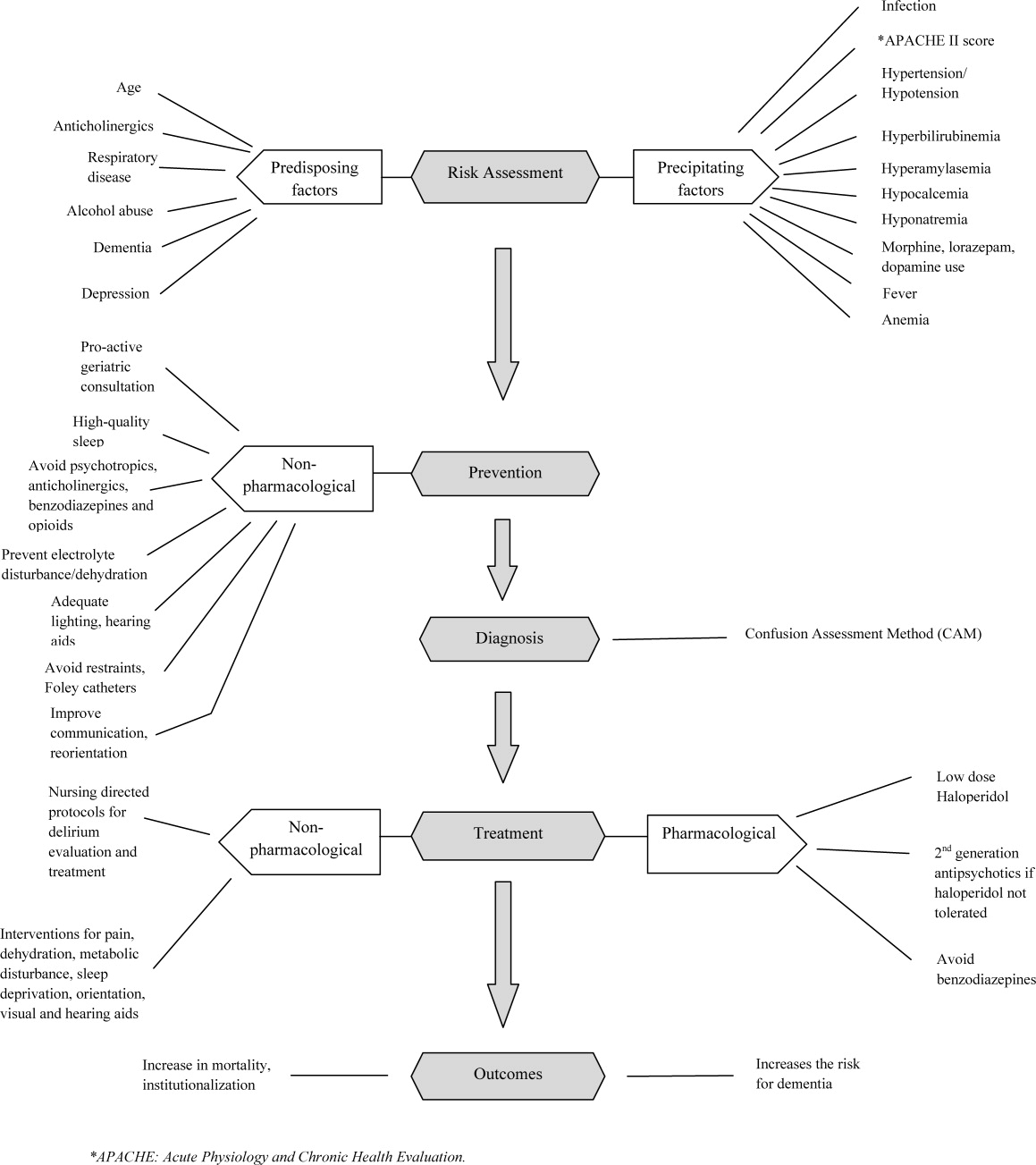
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.
FUTURE RESEARCH DIRECTIONS
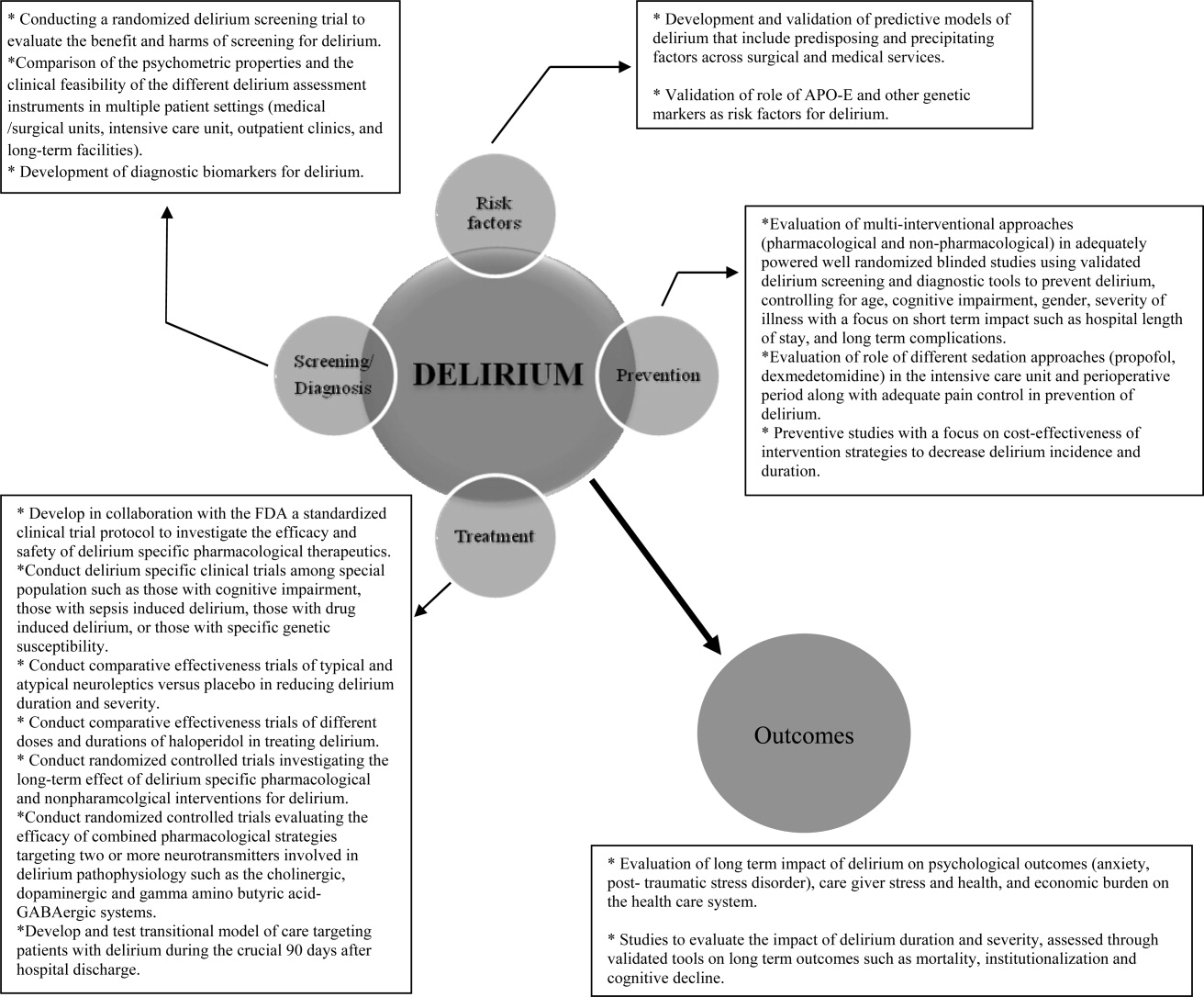
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.
To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.

| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.

FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.

To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
Delirium is a syndrome of disturbance of consciousness, with reduced ability to focus, sustain, or shift attention, that occurs over a short period of time and fluctuates over the course of the day.1 It encompasses a variety of cognitive, behavioral, and psychological symptoms including inattention, short‐term memory loss, sleep disturbances, agitated behaviors, delusions, and visual hallucinations.2 Delirium complicates the care of 70% to 80% of mechanically ventilated patients in intensive care units (ICUs).3 Of 13 million patients aged 65 and older hospitalized in 2002, 10% to 52% had delirium at some point during their admission.4, 5
Patients experiencing delirium have a higher probability of death during their hospital stay, adjusted for age, gender, race, and comorbidities.3, 6, 7 They are more vulnerable to hospital‐acquired complications leading to prolonged ICU and hospital stay, new institutionalization, and higher healthcare costs.3, 6, 7 Even with such a range of poor outcomes, the rates of delirium recognition are low,8 resulting in inadequate management.9 There has been considerable growth in the number of articles published on delirium in recent years. Therefore, it is of value to provide a state‐of‐the‐art summary of robust evidence in the field to healthcare personnel and delirium investigators.
We systematically reviewed the literature to identify published systematic evidence reviews (SERs), which evaluated the evidence on delirium risk factors, diagnosis, pathogenesis, prevention, treatment, and outcomes. We then summarized the data from the methodologically sound SERs to provide the reader with a clinically oriented summary of delirium literature for patient care. We also identify current gaps in delirium literature, and present future directions for delirium investigators to design studies that will enhance delirium care.
DATA SOURCES AND REVIEW METHODS
The domains of risk factors, diagnosis, pathophysiology, prevention, treatment, and outcomes were selected a priori to capture all relevant SERs regarding delirium based on the framework suggested by the American Delirium Society task force.10 To maximize article retrieval, a 3‐step search strategy was applied. First, we searched the electronic database utilizing OVID Medline, PubMed, the Cochrane Library, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using the following delirium‐specific search terms: delirium, confusion, agitation, mental status change, inattention, encephalopathy, organic mental disorders, and disorientation. We combined the above terms with the following study design terms: technical report, systematic evidence review, systematic review, meta‐analysis, editorial, and clinical reviews. We limited our search to human subjects. We excluded studies that: a) enrolled patients aged <18; b) enrolled patients with current or past Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I psychotic disorders; c) did not have standardized delirium evaluation; d) evaluated alcohol or substance abuse‐related delirium; e) did not use a systematic search method for identifying delirium‐related articles; and f) evaluated delirium sub‐types. We searched articles published from January 1966 through April 2011. Second, a manual search of references of the retrieved papers plus an Internet search using Google Scholar was conducted to find additional SERs. Titles and abstracts were screened by 2 reviewers (B.A.K., M.Z.). Authors of the included studies were contacted as necessary. Third, a library professional at the Indiana University School of Medicine independently performed a literature search, and those results were compared with our search to retrieve any missing SERs.
The methodological quality of each SER was independently assessed by 2 reviewers (B.A.K., M.Z.) using the United States Preventive Services Task Force (USPSTF) Critical Appraisal for SER.11 This scale assesses parameters that are critical to the scientific credibility of an SER and categorizes the SER as poor, fair, or good (Table 1). The 2 reviewers (B.A.K., M.Z.) used a data extraction form to record the following information from each SER: primary author, publication year, number and type of studies, number of participants and their mean age, study population, method for delirium diagnosis, risk factors, preventive and therapeutic interventions, and outcomes. Any disagreement between reviewers in SER selection, data extraction, or SER appraisal was resolved through discussion with a third reviewer (M.A.B.). The conflicting findings among SERs were resolved by consensus and by including the findings from a good SER over a fair SER.
| Criteria | Rating Definition |
|---|---|
| Recent, relevant review with comprehensive sources and search strategies | Good: If all the criteria are met |
| Explicit and relevant selection criteria | |
| Standard appraisal of included studies | |
| Valid conclusion | |
| Recent, relevant review that is not clearly biased but lacks comprehensive sources and search strategies | Fair: If this criterion is met |
| Outdated, irrelevant, or biased review | Poor: If one or more of the criteria are met |
| There is no systematic search for studies | |
| There are no explicit selection criteria | |
| There is no standard appraisal of studies |
RESULTS
Our search yielded 76,060 potential citations, out of which we identified 38 SERs meeting our inclusion criteria (Table 2). Figure 1 outlines our search strategy. Based on the USPSTF criteria, 22 SERs graded as good or fair provided the data to establish our review.

| Author (Year) | Studies (n)/ Participants (n) | Mean Age (Years) | Study Type | Service | Delirium/Cognition Assessment Scales | Review Objectives* | Rating |
|---|---|---|---|---|---|---|---|
| |||||||
| Van Rompaey et al15 (2008) | 6/7,114 | 61.2 | Prospective cohort, retrospective analysis | ICU (medical, surgical, coronary, mixed) | CAM‐ICU, psychiatric interview, ICU delirium screening checklist | 1/Risk factors | Fair |
| Bryson and Wyand13 (2006) | 18/3,473 | 71.93 | RCT | Surgery | MattisKovner Verbal Recall and Recognition, GDS, DST, DSM‐III, AMT, PRT, FOMTL, DCT, FPU, GEMS, WAIS‐R, Meta Memory Questionnaire, National Adult Reading Test | 1/Risk factors | Good |
| Fong et al14 (2006) | 9/1,078 | 63.1 | RCT, case control, prospective cohort, retrospective cohort | Surgery | CAM, DSM‐III, MMSE, SPMSQ, Digit Symbol Substitution Test, Trailmaking B Test | 1/Risk factors | Fair |
| Adamis et al53 (2009) | 6/882 | 54.59 | Case control | Medicine, ICU, surgery | CAM, DRS, DSM‐III‐R, DSM‐IV, ICD‐10 | 1/Risk factors | Poor |
| Balasundaram and Holmes12 (2007) | 4/364 | 66.8 | Prospective cohort | Surgery | CAM, DRS, HDS‐R, DSM‐IV | 1/Risk factors | Good |
| Dasgupta and Dumbrell49 (2006) | 25/5,175 | 72.5 | Prospective observational | Surgery | CAM, DSM‐III/IV | 1/Risk factors | Poor |
| Elie et al50 (1998) | 27/1,365 | 75.7 | Prospective | Medicine, surgery, psychiatry | CAM, NFRD, MMSE, MSQ, SPMSQ | 1/Risk factors | Poor |
| Van Munster et al52 (2009) | 5/1,099 | 77.86 | Cohort | Medicine, surgery | CAM, DRS | 1/Risk factors | Poor |
| Van der Mast and Roest51 (1996) | 57/6,129 | 48.2 | Prospective control, retrospective | Surgery | Psychiatric interview, chart review for signs of delirium, DSM‐III, MMSE | 1/Risk factors | Poor |
| Campbell et al16 (2009) | 27/8,492 | 71.35 | Longitudinal cohort, cross‐sectional, case control | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DSI, DSM‐III/III‐R/IV, SDC, MMSE, Verbal N‐Back Test, BCRS, WMS | 1/Risk factors | Fair |
| Soiza et al17 (2008) | 12/764 | 72.4 | Cohort, case control, case series | Medicine, ICU, psychiatry | CAM, DSM‐III/III‐R/IV | 1/Risk factors | Good |
| Michaud et al9 (2007) | 29/NA | 76.7 | RCT, cohort | Medicine, surgery | CAM, BOMC, DRS, MDAS, ICD‐10, DSM‐IV, MMSE | 1/Risk factors, 2/Diagnosis, 4/Prevention, 5/Treatment | Fair |
| Steis and Fick54 (2008) | 10/3,059 | 72.5 | Prospective clinical trials, retrospective, observational, case study | Medicine, surgery, ICU | DSM‐III/IV | 2/Diagnosis | Poor |
| Wei et al20 (2008) | 7/1,071 | 70.17 | Validation, adaptation, translation, application | ICU, ED, medicine, surgery | CAM, CAM‐ICU, DSM‐IV, NH‐CAM, DI | 2/Diagnosis | Good |
| Wong et al18 (2010) | 25/3,027 | 72.76 | Prospective clinical studies | Medicine, surgery | CAC, CAM, DOSS, DRS, DRS‐R‐98, Digit Span Test, GAR, MDAS, MMSE, Nu‐DESC, Vigilance A Test | 2/Diagnosis | Fair |
| Devlin et al55 (2007) | 12/2,106 | 61.8 | Validation studies | ICU | CAM, ICDSC, CTD, ROC, DSM‐III/IV, DDS, MMSE | 2/Diagnosis | Poor |
| Fick et al47 (2002) | 14/7,701 | 79.51 | Prospective cohort, retrospective cohort, cross‐sectional, clinical trials | Medicine, surgery, ED | CAM, DRS, DSM‐III/III‐R/IV, CERAD, NINCDS‐ADRDA, IQCODE, MMSE | 2/Diagnosis, 4/Prevention, 6/Prognosis | Fair |
| Siddiqi et al46 (2006) | 40/12,220 | 78.8 | Prospective cohort, cross‐sectional, case‐controlled trials | Medicine | CAM, DRS, MDAS, SPMSQ, DSM‐III/III‐R/IV, MSQ, MMSE,BPRS, IQCODE, GHQ BAS | 2/Diagnosis, 6/Prognosis | Fair |
| 28/4,915 | |||||||
| Hall et al21 (2011) | 5/315 | 71.13 | Prospective cohort | Medicine, surgery, psychogeriatric | DSM‐III/III‐R/IV, MMSE, DRS, CAM, IQCODE, GDS | 3/Pathophysiology | Good |
| Cole et al56 (1996) | 10/999 | 71.6 | Randomized and nonrandomized trials | Medicine, surgery | DSM‐III, SPMSQ | 4/Prevention | Poor |
| Siddiqi et al25 (2007) | 6/833 | 76.67 | RCT | Surgery | CAM, DRS‐R‐98, DSM‐III/IV, DSI, MDAS, AMT, MMSE, OBS | 4/Prevention | Good |
| Campbell et al27 (2009) | 13/1,305 | 65.8 | RCT | Medicine, surgery, ICU | MDAS, DRS‐R‐98 | 4/Prevention, 5/Treatment | Good |
| Weber et al41 (2004) | 13/1,650 | 73.99 | RCT, non‐RCT, clinical trials, meta‐analysis, case report | Medicine, surgery | CAM, MDAS, DSI, DRS, DSM‐III‐R/IV, MMSE | 4/Prevention, 5/Treatment | Fair |
| Milisen et al22 (2005) | 7/1,683 | 80.73 | RCT, controlled trials, beforeafter study | Medicine, surgery | CAM, DSM‐III, SPMSQ, MMSE | 4/Prevention, 5/Treatment | Good |
| Lonergan et al39 (2009) | 3/629 | 74.5 | RCT | Medicine, surgery | CAM, DRS, DRS‐R‐98, MDAS, CGI, DSM‐IV | 5/Treatment | Good |
| Jackson and Lipman40 (2004) | 1/30 | 39.2 | RCT | Medicine | DRS, DSM‐III‐R | 5/Treatment | Good |
| Lonergan et al42 (2009) | 1/106 | 54.5 | RCT | ICU | CAM‐ICU | 5/Treatment | Good |
| Bourne et al57 (2008) | 33/1,880 | 60.99 | RCT, prospective trials, comparative trials | Medicine, surgery | DRS | 4/Prevention, 5/Treatment | Poor |
| Bitsch et al58 (2004) | 12/1,823 | 79.02 | Prospective, descriptive | Surgery | CAM, MDAS, DSI, OBS, MMSE | 4/Prevention, 5/Treatment | Poor |
| Overshott et al43 (2008) | 1/80 | 67 | RCT | Surgery | CAM, DSI, DSM‐IV, MMSE | 5/Treatment | Good |
| Lacasse et al59 (2006) | 4/158 | 60.8 | RCT | Medicine, surgery | CAM, DRS‐R‐98, MDAS, DI, DSM‐III‐R/IV, MMSE | 5/Treatment | Poor |
| Peritogiannis et al60 (2009) | 23/538 | 62.84 | RCT, retrospective, open label | Medicine, surgery | DRS, DRS‐R‐98, DRS‐R‐98‐J, MDAS, DI, 10‐Point Visual Analog Scale | 5/Treatment | Poor |
| Seitz et al38 (2007) | 14/448 | 63.09 | Prospective | Medicine, surgery, ICU | DSM‐III/III‐R/IV/IV‐TR, CAM, DRS‐R‐98, MDAS, DI | 5/Treatment | Good |
| Britton and Russell37 (2001/2004) | 1/227 | 82.35 | RCT | Medicine | CAM, SPMSQ, DSM‐III‐R, MMSE | 5/Treatment | Good |
| Jackson et al6 (2004) | 9/1,885 | 77.68 | Prospective, descriptive | Medicine, surgery, ICU, psychiatry | CAM, CAM‐ICU, DRS, MMSE, DSM | 6/Prognosis | Poor |
| Cole et al44 (2009) | 18/1,322 | 81.3 | Prospective cohort | Medicine, surgery | CAM, DSM‐III/III‐R/IV, ICD‐10, OBS | 6/Prognosis | Good |
| Witlox et al45 (2010) | 42/5,777 | 79.96 | Observational | Medicine, surgery | DSM, patient interview | 6/Prognosis | Good |
| Cole and Primeau61 (1993) | 8/573 | 77.25 | Prospective trials | Medicine, surgery, psychiatry | DSM‐I/III | 6/Prognosis | Poor |
1: What Are the Risk Factors for Development of Delirium in Hospitalized Patients?
We found 6 SERs1217 that evaluated risk factors for the development of delirium. Three reviews included only surgical patients,1214 1 focused on the intensive care unit (ICU),15 and the remaining 2 had both medical and surgical patients.16, 17 Risk factors identified in an elective vascular surgery population were age >64, preoperative cognitive impairment, depression, intraoperative blood transfusions, and previous amputation.12 The risk of incident delirium conferred by general anesthesia compared to regional anesthesia in non‐cardiac surgery patients was not significantly different among both groups.13 One SER14 focused on the effects of different opioid analgesics on postoperative delirium, and whether route of administration of medicines (intravenous vs epidural) had any impact on delirium. Mepiridine was consistently associated with an increased risk of delirium in elderly surgical patients, but there were no significant differences in postoperative delirium rates among those receiving morphine, fentanyl, or hydromorphone. The rates of delirium did not differ significantly between intravenous and epidural routes of analgesic administration, except in one study where epidural route had more delirium cases, but in 85% of those cases, mepiridine was used as an epidural agent. Risk factors explored in an ICU setting found multiple predisposing and precipitating risk factors, with the surprising finding that age was not a strong predictor of delirium.15 An association between delirium and drugs with anticholinergic properties was found in 1 SER.16 There was no causal relationship between structural or functional neuroimaging findings and delirium development.17
2: What Is the Clinical Utility of Bedside Tools in Delirium Diagnosis?
The accuracy of bedside instruments in diagnosing delirium was assessed in an SER of 25 prospective studies.18 Among the 11 scales reviewed, the Confusion Assessment Method (CAM) had the most evidence supporting its use as a bedside tool (+likelihood ratio [LR], 9.6; 95% CI [confidence interval], 5.816.0; LR, 0.16; 95% CI, 0.090.29). The Folstein mini‐mental status examination (MMSE)19 (score <24) was the least useful test for identifying delirium (LR, 1.6; 95% CI, 1.22.0). Another SER evaluating the psychometric properties of CAM demonstrated a sensitivity of 94% (CI, 91%97%) and specificity of 89% (CI, 85%94%).20 CAM also showed prognostic value with worsening of delirium outcomes depending on the number of CAM items present.20
3: What Is the Underlying Pathophysiology of Delirium and Is There a Role of Measuring Biomarkers for Delirium?
We found only 1 SER which examined the associations between cerebrospinal fluid biomarkers and delirium.21 Delirium was associated with raised levels of serotonin metabolites, interleukin‐8, cortisol, lactate, and protein. Additionally, higher acetylcholinesterase predicted poor outcome after delirium, and higher dopamine metabolites were associated with psychotic features. Delirium was also associated with reduced levels of somatostatin, ‐endorphin, and neuron‐specific enolase.
4: Can Delirium Be Prevented?
Nonpharmacologic Interventions
An SER22 reviewing multicomponent interventions to prevent delirium identified 2 studies23, 24 showing statistically significant results. In the Yale Delirium Prevention Trial,23 the intervention was targeted toward minimizing 6 risk factors in elderly patients (70 years of age) admitted to a general medicine service, who did not have delirium at the time of admission, but were at risk for delirium development. The interventions included: orientation activities for the cognitively impaired, early mobilization, preventing sleep deprivation, minimizing the use of psychoactive drugs, use of eyeglasses and hearing aids, and treating volume depletion. The incidence of delirium was 9.9% with this intervention compared with 15% in the usual care group (OR [odds ratio], 0.60; 95% CI, 0.390.92).23 The other studied patients with hip fractures, randomized to either standard care versus the addition of a geriatrics consultation preoperatively or immediately after hip repair, providing recommendations based on a structured protocol.24 The incidence of delirium during hospitalization was 32% in the geriatrics consultation group versus 50% in the standard care group (OR, 0.48; 95% CI, 0.230.98; relative risk [RR], 0.64; 95% CI, 0.370.98), but there was no difference in duration of delirium.24
Pharmacologic Interventions
A Cochrane review found 6 randomized controlled trials for preventing delirium in hospitalized surgical patients.25 Low‐dose haloperidol prophylaxis was found to be effective in reducing the severity (mean difference in delirium rating scale score of 4.0 (95% CI, 2.05.8) and duration of delirium (RR, 6.44; 95% CI, 7.64 to 5.24), along with shortening the length of hospital stay (mean difference in hospital days, 5.5; 95% CI, 1.42.3) in hip surgery patients, but it did not prevent delirium occurrence.26 A review by Campbell et al evaluated 9 studies testing pharmacological interventions in preventing delirium in surgical patients.27 Use of a single‐dose risperidone after cardiac surgery decreased delirium incidence compared to placebo.28 Donepezil and citicoline showed no benefit in preventing delirium.2931 Early restoration of sleep cycles with the use of a benzodiazepine/opiate combination and pain control with gabapentin postoperatively reduced delirium incidence.32, 33 Interventions started on day of surgery and continued for up to 3 days postoperatively were found to be effective in reducing delirium incidence.27
5: How Should Delirium Be Treated?
Nonpharmacologic Interventions
The multicomponent intervention SER22 mentioned above evaluated the efficacy of interventions ranging from a geriatric psychiatric consultation and a nursing liaison to assess patients' daily pain management, to treating hypoxemia and other metabolic derangements along with a standardized screening tool for early detection of delirium. Delirious patients randomized to a geriatrician or a geriatric psychiatrist's consultation making treatment decisions, along with daily visits by a nursing liaison, resulted in improvement in short portable mental status questionnaire scores (SPMSQ) from 8.2 to 7.9, two weeks after admission, whereas the usual care group showed a deterioration in scores (8.4 to 9.1).34 Though by week 8, the difference between both groups disappeared. While the severity and recurrence rates of delirium were unchanged, the trial by Inouye et al23 evaluating 6 standardized intervention protocols showed a significant reduction in the total number of hospital days with delirium (105 vs 161 days, P = 0.02). Training of nurses to use a delirium screening instrument to identify delirium in hip fracture patients, along with prompt implementation of interventions based on a nursing guide for evaluation of causes of delirium, resulted in a shorter duration of delirium (median = 1 day vs 4 days, P = 0.03) and severity, compared to the usual care group.35 Daily assessment by a gerontological nurse resulted in greater improvement in functional status (21% vs 10%).36 No difference in patients' length of stay or mortality was demonstrated in any of the studies included in the review.22 A Cochrane review assessing efficacy of multidisciplinary interventions for reducing delirium in cognitively impaired patients did not identify any studies.37
Pharmacologic Interventions
We identified 7 SERs,27, 3843 addressing the efficacy and safety of various pharmacological interventions to treat delirium. Campbell et al suggested that blocking the dopaminergic system with neuroleptics, and reducing the exposure to lorazepam, might reduce delirium severity and duration among hospitalized elders, including those in the ICU.27 There was no advantage of using atypical neuroleptics over haloperidol. Low‐dose haloperidol use was associated with reduced delirium severity and duration in hip surgery patients.26 Seitz et al38 evaluated the efficacy and safety of antipsychotics (haloperidol, olanzapine, quetiapine, risperidone, mianserin, and lorazepam) in treating delirium symptoms. They evaluated prospective single‐agent and comparison trials. None of the studies included a placebo group. An improvement in delirium severity was observed in the majority of studies, but there was no advantage of one agent over the other in comparison trials. Most trials were underpowered to detect a clinically significant difference and are of short duration (<7 days) to adequately assess for delirium resolution.
A Cochrane review39 comparing the efficacy of haloperidol over risperidone and olanzapine for treating delirium showed similar findings as Campbell and colleagues' SER.27 The decrease in delirium severity scores was not significantly different using low‐dose haloperidol (<3.0 mg per day) compared with olanzapine and risperidone (OR, 0.63; 95% CI, 0.291.38; P = 0.25). High‐dose haloperidol (>4.5 mg per day) was associated with an increased incidence of extrapyramidal adverse effects. The role of drug therapy for delirium in terminally ill adult patients was evaluated in a Cochrane review40 and by Weber et al.41 They suggested the use of haloperidol or chlorpromazine in reducing delirium in acquired immune deficiency syndrome (AIDS) patients. Benzodiazepines were ineffective for treatment of non‐alcohol withdrawal delirium.42 In mechanically ventilated ICU patients, dexmedetomidine treatment increased number of delirium/coma‐free days compared with lorazepam (7 vs 3 days, P = 0.01).42 Cholinesterase inhibitor donepezil did not decrease duration of delirium compared to placebo in postoperative orthopedic patients.43
6: What Is the Impact of Delirium on Patient Outcomes?
We found 4 SERs.4447 Persistent delirium defined as delirium present on admission and at the time of discharge or beyond, and its impact on outcomes in older hospitalized patients, was evaluated in 1 SER. The combined proportions of patients with persistent delirium at discharge, 1, 3, and 6 months were 44.7%, 32.8%, 25.6%, and 21%, respectively.44 Evaluation of prognosis was complicated by small number of subjects and differences in length of follow up.
Delirium in elderly (>65 years) patients was associated with an increased risk of death45, 46 compared with controls, with a mortality rate of 38% in delirious patients compared to 27.5% in controls (hazard ratio[HR], 1.95; 95% CI, 1.512.52).45 This association persisted independent of preexisting dementia. Patients with delirium compared to controls were also at increased risk of institutionalization (33.4% vs 10.7%) (OR, 2.41; 95% CI, 1.773.29) and dementia (62.5% vs 8.1%) (OR, 12.52; 95% CI, 1.8684.21).45 In patients with dementia, delirium increased the risk of 30‐day rehospitalization and admission to long‐term care, compared to patients with dementia or delirium alone.47
DISCUSSION AND CLINICAL IMPLICATIONS
Our study identified age, cognitive impairment, depression, and mepiridine use for analgesia as risk factors for delirium in surgical patients. Drugs with anticholinergic properties were implicated in delirium development in both medical and surgical patients. The CAM has the best available data to be used as a diagnostic tool for delirium. Multicomponent interventions to prevent delirium occurrence are effective in a non‐cognitively impaired population, and low‐dose haloperidol prophylaxis decreases delirium duration and severity without affecting delirium incidence in hip surgery patients. There is no advantage of using atypical antipsychotics over haloperidol in treating delirium, and low‐dose haloperidol is as effective as a higher dose without unwarranted extrapyramidal side effects. Delirium carries a poor prognosis with an increased risk of death, institutionalization, and dementia.
Hospitals may benefit from implementing multicomponent strategies, focusing on at‐risk elderly medical and surgical patients, administered by a multidisciplinary team to reduce delirium incidence. For ICU physicians and administrators, development of sedation guidelines minimizing the use of benzodiazepines will decrease the risk of delirium development.
A structured approach in diagnosing delirium is required to maximize identification. Use of the CAM, based on best available data is recommended. However, the length of time in doing the CAM (more than 10 minutes with the requisite mental status examination) and insensitivity in nonexpert hands suggest a need for alternative screening tools. Haloperidol should be the preferred first‐line pharmacological therapy for delirium, with atypical antipsychotics reserved for patients with contraindications to haloperidol or those who are refractory to therapy with haloperidol. Figure 2 delineates a clinical model for delirium management derived from the findings in the Results section.

FUTURE RESEARCH DIRECTIONS
We identified multiple areas without clear guidelines that could provide opportunities for future research. A role for routine delirium screening can be clarified through a well‐designed delirium screening trial investigating the benefits of delirium screening, coupled with a multicomponent intervention versus usual care. Use of pharmacotherapy in delirium prevention needs to be explored further in a large randomized trial, with 3 arms to compare typical antipsychotics, atypical antipsychotics, and placebo in patients at risk for delirium with a primary outcome of delirium incidence. In regard to delirium treatment, a large randomized trial to compare haloperidol with atypical antipsychotics, with a placebo arm focusing not only on delirium duration and severity, but also on long‐term outcomes such as rehospitalizations, institutionalization, cognitive impairment, and mortality, is warranted. Figure 3 points out potential areas for researchers to investigate hypotheses generated by our review and thereby improve delirium care.

To our knowledge, our SER presents the first summary of SERs in delirium. Prior to this review, Michaud et al9 and National Institute for Health and Clinical Excellence48 published delirium guidelines, but in both of these guidelines, evidence was collected from a multitude of studies ranging in methodology from scientific review and meta‐analysis to observational studies, and the majority of recommendations were based on expert opinion. On the contrary, our review was limited to rigorously conducted SERs; hence, we utilized the highest level, critically appraised evidence to provide guidance to clinicians and researchers.
Limitations include a diverse group of studies with a heterogeneous population of patients, preventing pooling of results. We did not review each individual study included in the 38 SERs. We excluded non‐English language SERs, studies evaluating delirium subtypes, alcohol or substance abuse‐related delirium, or delirium associated with psychiatric disorders. As we only reviewed SERs, some notable studies not included in the SERs may have been missed.
CONCLUSION
Delirium among hospitalized patients is a common syndrome with a significant burden to the healthcare system and society. The field of delirium has seen considerable advances in diagnosis, prevention, and treatment over the last decade. Even with this advancement, there are still areas of uncertainty, such as: the benefits and costs of delirium screening; the benefits and harms of single or combined pharmacological agents for delirium prevention and treatment; the development of a set of reliable biomarkers for delirium diagnosis, prognosis, and response to therapy; the long‐term effect of delirium‐specific therapeutics on patients' cognitive, physical, and psychological functions; and the relationship between delirium and the development of Alzheimer's disease. As our understanding of delirium's impact on patients and healthcare improves, delirium should be identified as an indicator of poor long‐term prognosis, and should prompt immediate and effective evidence‐based management strategies, like any other critical illness.
Note Added in Proof
Disclosure: This study was supported by the National Institute on Aging (NIA), grant R01AG054205‐02; and the National Institute of Mental Health (NIMH), grant R24MH080827‐04.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.
- ,,,,,.Clarifying delirium: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113(12):941–948.
- ,,.Delirium; a subcortical phenomenon?J Neuropsychiatry Clin Neurosci.1989;1(3):283–290.
- ,,, et al.The impact of delirium on the survival of mechanically ventilated patients.Crit Care Med.2004;32(11):2254–2259.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004;342:1–29.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, Bernard M, Flaherty E, eds.Primary Care Geriatrics, a Case‐Based Approach.5th ed.Philadelphia, PA:Mosby/Elsevier;2007:210–218.
- ,,,,.The association between delirium and cognitive decline: a review of the empirical literature.Neuropsychol Rev.2004;14(2):87–98.
- ,,, et al.Costs associated with delirium in mechanically ventilated patients.Crit Care Med.2004;32(4):955–962.
- ,,,.Detection in delirium in the acute hospital.Age Ageing.2010;39(1):131–135.
- ,,, et al;for the Delirium Guidelines Development Group.Delirium: guidelines for general hospitals.J Psychosom Res.2007;62(3):371–383.
- ,,,,.Delirium: a strategic plan to bring an ancient disease into the 21st century.J Am Geriatr Soc.2011;59:S237–S240.
- ,,, et al;for the Methods Work Group.Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process.Am J Prev Med.2001;20:21–35.
- ,.Delirium in vascular surgery.Eur J Vasc Endovasc Surg.2007;34(2):131–134.
- ,.Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction.Can J Anaesth.2006;53(7):669–677.
- ,,.The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review.Anesth Analg.2006(4):1255–1266.
- ,,,,.Risk factors for intensive care delirium: a systematic review.Intensive Crit Care Nurs.2008;24(2):98–107.
- ,,, et al.The cognitive impact of anticholinergics: a clinical review.Clin Interv Aging.2009;4:225–233.
- ,,,,,.Neuroimaging studies of delirium: a systematic review.J Psychosom Res.2008;65(3):239–248.
- ,,,.Does this patient have delirium? Value of bedside instruments.JAMA.2010;304(7):779–786.
- ,,.“Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res.1975;12(3):189–198.
- ,,,.The confusion assessment method: a systematic review of current usage.J Am Geriatr Soc.2008;56(5):823–830.
- ,,.A systematic literature review of cerebrospinal fluid biomarkers in delirium.Dement Geriatr Cogn Disord.2011;32:9–93.
- ,,,.Multicomponent intervention strategies for managing delirium in hospitalized older people; systematic review.J Adv Nurs.2005;52(1):79–90.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340(9):669–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,.Interventions for preventing delirium in hospitalized patients.Cochrane Database Syst Rev.2007;2:CD005563. DOI: 10.1002/14651858.CD005563.
- ,,, et al.Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study.J Am Geriatr Soc.2005;53(10):1658–1666.
- ,,, et al.Pharmacological management of delirium in hospitalized adults: a systematic evidence review.J Gen Intern Med.2009;24:848–853.
- ,.Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery.Anaesth Intensive Care.2007;35(5):714–719.
- ,,,,.Donepezil in the prevention and treatment of post‐surgical delirium.Am J Geriatr Psychiatry.2005;13:1100–1106.
- ,,, et al.A randomized,doubleblind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement.Int J Geriatr Psychiatry.2007;22:343–349.
- ,,, et al.Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial [in Spanish].Rev Neurol.2001;33(8):716–719.
- ,,, et al.A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery.Surg Today.2002;32:310–314.
- ,,, et al.Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients.Neurology.2006;67(7):1251–1253.
- ,,, et al.Systematic intervention for elderly inpatients with delirium: a randomized clinical trial.Can Med Assoc J.1994;151:965–970.
- ,,, et al.A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients.J Am Geriatr Soc.2001;49:523–532.
- ,,,.Functional status outcomes of a nursing intervention in hospitalized elderly.Image J Nurs Sch.1992;24:201–220.
- ,.Multidisciplinary team interventions for delirium in patients with chronic cognitive impairment.Cochrane Database Syst Rev.2001;1:CD000395. Update in: Cochrane Database Syst Rev. year="2004"2004;2:CD000395.
- ,,.Antipsychotics in the treatment of delirium: a systematic review.J Clin Psychiatry.2007;68(1):11–21.
- ,,.Antipsychotics for delirium. The Cochrane Collaboration.The Cochrane Library.2009;1:1–117.
- ,.Drug therapy for delirium in terminally ill patients.Cochrane Database Syst Rev.2004;2:CD004770.
- ,,.Delirium: current trends in prevention and treatment.J Intern Med.2004;34(3):115–121.
- ,,,.Benzodiazepines for delirium.Cochrane Database Syst Rev.2009;1:CD006379. Update in: Cochrane Database Syst Rev.year="2009"2009;4:CD006379.
- ,,.Cholinesterase inhibitors for delirium.Cochrane Database Syst Rev.2008;1:CD005317.
- ,,,.Persistent delirium in older hospital patients: a systematic review of frequency and prognosis.Age Ageing.2009;38(1):19–26.
- ,,,,,.Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis.JAMA.2010;304(4):443–451.
- ,,.Occurrence and outcome of delirium in medical in‐patients: a systematic literature review.Age Ageing.2006;35(4):350–364.
- ,,.Delirium superimposed on dementia: a systematic review.J Am Geriatr Soc.2002;50(10):1723–1732.
- National Institute for Health and Clinical Excellence. NICE guidelines for delirium diagnosis, prevention and management. Available at: http://www.ice.ork.uk/guidelines. Accessed October 1,2011.
- ,.Preoperative risk assessment for delirium after noncardiac surgery: a systematic review.J Am Geriatr Soc.2006;54(10):1578–1589.
- ,,,.Delirium risk factors in elderly hospitalized patients.J Gen Intern Med.1998;13(3):204–212.
- ,.Delirium after cardiac surgery: a critical review.J Psychosom Res.1996;41(1):13–30.
- ,,,,.The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta‐analysis.Am J Geriatr Psychiatry.2009;17:856–862.
- ,,.The genetics of deliria.Int Rev Psychiatry.2009;21(1):20–29.
- ,.Are nurses recognizing delirium? A systematic review.J Gerontol Nurs.2008;34(9):40–48.
- ,,,.Delirium assessment in the critically ill.Intensive Care Med.2007;33(6):929–940.
- ,,.Effectiveness of interventions to prevent delirium in hospitalized patients: a systematic review.Can Med Assoc J.1996;155(9):1263–1268.
- ,,,.Drug treatment of delirium: past, present and future.J Psychosom Res.2008;65(3):273–282.
- ,,,.Pathogensis of and management strategies for postoperative delirium after hip fracture: a review.Acta Orthop Scand.2004;75(4):378–389.
- ,,.Systematic review of antipsychotics for the treatment of hospital‐associated delirium in medically or surgically ill patients.Ann Pharmacother.2006;40(11):1966–1973.
- ,,,,.Atypical antipsychotics in the treatment of delirium.Psychiatry Clin Neurosci.2009;63(5):623–631.
- ,.Prognosis of delirium in elderly hospital patients.Can Med Assoc J.1993;149(1):41–46.