User login
Overview and Discussion of the 2017 VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care (FULL)
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
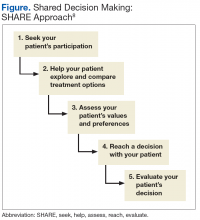
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
Diabetes mellitus (DM) is an epidemic in the U.S. More than 30 million people (9.4% of the total population) have DM; type 2 DM (T2SM) accounts for 95% of these cases.1 The estimated prevalence of DM among individuals aged > 65 years is about 3 times higher at 26%. The prevalence among veterans enrolled in the VA is higher than in the general population; about 25% of VA users have been diagnosed with DM.2 As a result, DM is the leading cause of blindness, end stage renal disease, amputations, and a significant contributor to myocardial infarction and stroke. Older adults with DM have an increased risk of mortality compared with individuals without DM.3 In 2012, DM was estimated to cost $176 billion in direct and indirect medical costs.4 These health and cost implications make effective management of DM a priority for health care providers (HCPs), policy makers, and patients nationwide.
The 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of T2DM in Primary Care provides the primary care team an evidence-based and individualized approach to holistic care of the patient with T2DM.5 Key recommendations were developed based on methods established by the VA/DoD Evidence-Based Practice Working Group (EBPWG) and are aligned with standards for trustworthy guidelines by using the Grading of Recommendations Assessment, Development and Evaluation system to assess the quality of the evidence base and assign a grade for the strength for each recommendation.6,7 The EBPWG included a multidisciplinary panel of practicing clinician stakeholders, including primary care physicians, endocrinologists, medical nutritionists, pharmacists, diabetes educators, and nurse practitioners. The CPG development process also included a patient focus group. Important themes from the focus group were shared with the EBPWG to help address the needs and perspectives of patients receiving treatment for DM in the VA and DoD.
In this article, the authors briefly review several of the most pertinent CPG updates for the busy clinician.
Shared Decision Making
Shared decision making (SDM) is a central component of the approach to patients with DM. Shared decision making involves the patient and care providers together making important decisions about the treatment plan and goals of care, using communication tools and exploring patient preferences.8
Using an empathetic and nonjudgmental approach facilitates discussions about a patient’s specific health care needs and goals for care. Shared decision making also can provide culturally appropriate treatment and care information to meet the needs of those with limited literacy or numeracy skills, or other learning barriers, such as physical, sensory, or learning disabilities. Family involvement is an important component of SDM when desired by the patient.9
The goals of successful SDM include a decrease in patient anxiety and an increase in trust in the health care team, ideally leading to improvement in adherence and patient outcomes.8,10-12 Improved patient-clinician communication conveys openness to discuss any future concerns. Furthermore, SDM does not need to take a significant amount of a clinician’s time to create an environment of consideration and goal formation. Training in communication skills may be helpful for those unfamiliar with SDM techniques. Patients are most likely to participate in the SDM process when they are comfortable speaking with clinicians and have some knowledge about their specific disease process.13
The clinical team can review all prior treatment attempts with the patient to understand the patient's perspective on these interventions. Lastly, patients are involved in prioritizing problems to be addressed and in setting specific goals. A 5-step SDM process prompted by the SHARE acronym can be used:
- Seek your patient’s participation;
- Help your patient explore and compare treatment options;
- Assess your patient’s values and preferences;
- Reach a decision with your patient; and
- Evaluate your patient’s decision (Figure).8
The VA/DoD CPG noted that there is high-quality evidence supporting SDM for improving patients’ knowledge, satisfaction, and engagement with their treatment plan.14-16 Specific methodologic approaches to SDM are not well defined for individual patient groups, which represents a significant research gap. Patients diagnosed with T2DM might respond differently to SDM depending on personal goals, life experiences, and coping strategies.14-16 Shared decision making should be used at every decision point in the treatment process, from the diagnosis of pre-diabetes to the patient with advanced complications. This includes— at a minimum—at initial diagnosis, when experiencing difficulties in management, and at times of transition or development of complications.16
A shared understanding is critical to the SDM process. Diabetes self-management education and diabetes self-management support provide a framework that involves a collaborative, ongoing, interactive process to help patients gain knowledge, modify behavior, and successfully manage the disease. The goal of DM education in SDM is to ensure that the patient has sufficient knowledge and skills to achieve the treatment goals they set with their health care team. Assessment of patient understanding in the clinic could include use of the “teach-back method.”17 Health coaching and motivational interviewing strategies also may help clinicians understand patients’ perceptions, values, and beliefs regarding their condition, treatment, and self-management options, particularly when patients seem to be reluctant to fully participate in decisions and care.
A challenge for HCPs is to help patients understand how they can successfully manage DM and partner with health care teams to express their goals and preferences to aid in individualized health care decisions. Using SDM tools and ensuring that clinicians can use patient-centered communication skills increase patients’ willingness to share in decision making and engage in the treatment plan.
Nutrition Recommendations
Nutrition therapy is a key component of any successful DM management plan. The EBPWG added 2 strong recommendations for DM nutrition strategies. The first recommendation is to follow a Mediterranean diet, if this resonates with the patient’s values and preferences (Table 1).
- High intake of vegetables, fruits, nuts, unrefined grains, and olive oil;
- Moderate intake of fish and poultry;
- Low or moderate intake of wine; and
- Low intake of red meat, processed meat, dairy, and sweets.
The Mediterranean diet effectively improves glycemic control, delays the time to first pharmacologic intervention, and reduces cardiovascular risk factors in individuals with diabetes.18 An additional benefits of this dietary pattern includes significant hemoglobin A1c (HbA1c) reduction.19,20 A Mediterranean diet also has been linked to improved cardiovascular outcomes and weight loss. In general, the evidence supporting a Mediterranean diet are robust, but securing and adapting to these types of foods can be challenging for some patients.
The second nutrition recommendation is to reduce the percentage of energy from carbohydrates to between 14% and 45% per day and/or eat foods with lower glycemic index. Patients who do not choose a Mediterranean diet can employ this dietary pattern. A systematic review compared dietary interventions, including lower carbohydrate and low-glycemic index diets, and showed both dietary interventions improved glycemic control.18 Unfortunately, many studies compare different intervention diets rather than comparing an intervention against a control diet. However, based on the available evidence, the Working Group endorses a Mediterranean diet and carbohydrate reduction and low glycemic index foods as dietary options in which the benefits seem to outweigh harms.
Target Hemoglobin A1c
The EBPWG reviewed several large, intensive glucose control trials to apply recent evidence to ongoing HbA1c treatment targets. The CPG strongly reaffirms that rather than assigning a single glycemic goal for all patients, clinicians should use SDM to develop an HbA1c target range that is risk-stratified (Table 2).
The ARR of complications must be balanced against the risk of therapy. Several major trials tested the hypothesis that intensive glycemic control (target HbA1c at least < 7%) improved cardiovascular outcomes in patients with T2DM.23-25 These trials did not demonstrate cardiovascular benefit from intensive control to reach HbA1c < 7%, and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study revealed possible cardiovascular harm.24
In addition, because these studies enrolled patients with established T2DM, they demonstrated less reduction in microvascular complications than was seen in newly diagnosed patients in UKPDS.22 Systematic reviews comparing intensive and conventional glucose control showed no significant differences in all-cause mortality or death from cardiovascular disease.26,27 Therefore, intensive control of T2DM has the greatest impact on microvascular complications and is most successful when initiated early in the disease process.
A target HbA1c range is recommended rather than a threshold value (eg, HbA1c < 8.0%) for several reasons. Most important the clinical trials that provide evidence for improved glycemic control used an HbA1c value recorded over time, not a single value measured at one point in time. Many factors influence HbA1c measurements other than just glycemic control.28 These include anemia, chronic kidney disease, race/ethnicity, and hemoglobinapathies.29-32 Patients can have clinically significant variation in HbA1c results between test samples, even when obtained from the same laboratory.33 For these reasons, the CPG continues to recommend use of fasting glucose ≥ 126 mg/dL to establish a DM diagnosis when the HbA1c is < 7.0%. This limits the likelihood that patients will be incorrectly diagnosed with DM, which can affect insurability, disability, or the trajectory of a military career. For patients with diagnosed T2DM, glycemic control over time remains important, but overreliance on a single HbA1c test could lead to overtreatment and potential adverse outcomes.
The EBPWG considered the target HbA1c and outcomes in UKPDS, ACCORD, Veterans Affairs Diabetes Trial (VADT), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) when considering HbA1c target ranges.23,25,34,35 Indeed, target HbA1c ranges, with both lower and upper bounds, were considered a better way to balance the potential risks and benefits of therapy. For example, a target HbA1c range of 6% to 7% might be appropriate in patients with a life expectancy more than 10 to 15 years with no significant microvascular disease and no other socioeconomic limitations to therapy. For patients with established microvascular disease or a life expectancy < 10 years, target ranges from 7% to 9% might be appropriate depending on patient-specific factors. A patient with advanced disease or limited life expectancy is less likely to derive benefit from intensive control, yet they would be exposed to the adverse effects from intensive therapy. For these patients, consider a less-intensive HbA1c target range. Although life expectancy can be difficult to estimate, this framework can be helpful to reach a target range using SDM with the patient.
An important issue in current DM management is potential overtreatment, which sits at the intersection of overuse of low value practices and medication safety. Up to 65% of older veterans with DM taking hypoglycemic agents might be overtreated based on the presence of DM complications, medical comorbidities, and decreased life expectancy that confer more risk than benefit from lower HbA1c levels.36 Harms from intensive glycemic control, such as increased risk of death from cardiovascular events and severe hypoglycemia must be considered.24 Patient-specific factors that could increase risk of hypoglycemia include the use of specific drugs (insulin and sulfonylureas), advanced age (> 75 years), cognitive impairment, chronic renal insufficiency, and food insufficiency.37-39
The CPG did not address specific pharmacologic treatment options because these can change rapidly as the literature evolves. Instead, the CPG refers clinicians to current criteria issued by the VA and DoD, which are updated frequently. In line with recent reviews, the CPG continues to recommend metformin as a first-line therapy for most patients with T2DM.40 An important consideration in the future will be the potential for cardiovascular risk reduction from specific medications or classes of medication independent of HbA1c reduction. As ongoing clinical trials are completed, SDM, ARR, and potential harm from therapy will remain important considerations.
Conclusion
The VA/DoD Diabetes Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care strongly recommend SDM in setting management and treatment goals, lifestyle changes that favor a Mediterranean or reduced carbohydrate diet, and targeting HbA1c levels to a range that balances benefits and harms for an individual patient.
This CPG represents a significant step foward in improving the treatment and management of patients with DM in the VA and DoD. This document represents a synthesis of the best available evidence regarding DM care as of March 2016. It is the authors' hope that such recommendations are implemented at the individual practice level. The CPG can help HCPs, but use of such recommendations should be placed in the context of clinical judgment, the patient’s values and preferences, and other available evidence as scientific knowledge and technology advance and treatment patterns evolve.
Application of these CPG recommendations will help VA and DoD clinicians deliver high-quality DM care in a personalized, proactive, and patient-driven manner, that inspires patients to achieve a state of health and well-being that is tailored to their unique characteristics and goals of care.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.
1. Centers for Disease Control and Prevention. 2017 National Diabetes Statistics Report. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics -report.pdf. Accessed 9/7/2017.
2. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10-B21.
3. Bethel MA, Sloan FA, Belsky D, Feinglos MN. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167(9):921-927.
4. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. U.S. Department of Veteran Affairs, U.S. Department of Defense.VA/DoD clinical practice guidelines: management of diabetes mellitus in primary care. https://www.healthquality.va.gov/guidelines/CD/diabetes/. Updated April 18, 2017. Accessed August 28, 2017.
6. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guidelines: CPG policy guidance: guidelines for guidelines. https://www.healthquality.va.gov/documents/cpgGuidelinesForGuidelinesFinalRevisions051214.docx . Updated February 8, 2017. Accessed August 28,2017.
7. Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719-725.
8. Agency for Healthcare Research and Quality. The SHARE approach. https://www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/index.html. Updated February 2017. Accessed August 28, 2017.
9. Kirkman MS, Briscoe VJ, Clark N, et al; Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60(12):2342-2356.
10. VA/DoD Evidence-based Practice. Shared Decision Making, A Guide for Busy Clinicians. https://www.qmo.amedd.army.mil/asthma/SDM-POCKETGuide.pdf. Accessed 3/17/2017.
11. Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med. 2011;24(3):229-239.
12. Robinson JH, Callister LC, Berry JA, Dearing KA. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract. 2008;20(12):600-607.
13. Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560-1568.
14. Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Serv Res. 2013;13:301.
15. Hsu WC, Lau KH, Huang R, et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18(2):59-67.
16. Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5(11):e009116.
17. Agency for Healthcare Research and Quality. Health literacy universal precautions tool kit, 2nd edition. Use the teach-back method: tool #5. http://www.ahrq .gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/healthlittoolkit2-tool5.html. Updated February 2015. Accessed August 28, 2017.
18. Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516.
19. Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200-1208.
20. Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222.
21. Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med. 2011;154(11):774-775.
22. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837-853.
23. Beulens JW, Patel A, Vingerling JR, et al; AdRem project team; ADVANCE management committee. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: a randomised controlled trial. Diabetologia. 2009;52(10):2027-2036.
24. ACCORD Study Group, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818-828.
25. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
26. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;(6):CD008143.
27. Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg. 2016;63(suppl 2):22S-28S.e1-2.
28. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. 2014;29(2):388-394.
29. English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58(7):1409-1421.
30. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761-1773.
31. Herman WH, Ma Y, Uwaifo G, et al; Diabetes Prevention Program Research Group. Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453-2457.
32. Little RR, Rohlfing CL, Hanson S, et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin Chem. 2008;54(8):1277-1282.
33. Sacks DB, Arnold M, Bakris GL, et al; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61-e99.
34. Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197-2206.
35. Zoungas S, Chalmers J, Neal B, et al; ADVANCE-ON Collaborative Group. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392-1406.
36. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395.
38. ORIGIN Trial Investigators. Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care. 2015;38(1):22-28.
39. Bruderer SG, Bodmer M, Jick SS, Bader G, Schlienger RG, Meier CR. Incidence of and risk factors for severe hypoglycaemia in treated type 2 diabetes mellitus patients in the UK—a nested case-control analysis. Diabetes Obes Metab. 2014;16(9):801-811.
40. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164(11):740-751.