User login
Redefining treatment success in type 2 diabetes mellitus: Comprehensive targeting of core defects
According to the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), glycosylated hemoglobin (HbA1c) in patients with diabetes should be maintained at 6.5% or less (AACE) or at less than 7.0% (ADA). Both organizations support an aggressive stepwise approach that includes medication and lifestyle modification, with strategies and clinical attention devoted to avoiding significant hypoglycemia.1,2 Yet, despite the introduction of new antidiabetes agents, most current management strategies are offset by limitations in achieving and maintaining glycemic targets needed to provide optimal care for patients with diabetes, more than 90% of whom have type 2 diabetes mellitus (T2DM).3,4
Nationally, glycemic control among patients with T2DM has improved but is still far from optimal. According to data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), glycemic control (HbA1c < 7.0%) rates were 35.8% for patients with T2DM.5 In a more recent report (NHANES 1999–2004), fewer than half (48.4%) of adult patients with diagnosed diabetes achieved HbA1c levels below 7.0%.5,6 Factors contributing to these data include earlier onset and earlier detection of T2DM.7
CHANGING TREATMENT TRENDS
Available treatments for patients with T2DM include secretagogues, such as sulfonylureas and “glinides” (repaglinide and nateglinide), metformin, thiazolidinediones (TZDs), and dipeptidyl peptidase–4 (DPP-4) inhibitors among oral medications, and insulin and glucagon-like peptide–1 (GLP-1) receptor agonists among parenterally administered agents. According to the latest published data on prescribing patterns for patients with T2DM, analyses of the National Disease and Therapeutic Index (1994–2007) and the National Prescription Audit (2001–2007), sulfonylurea use decreased from 67% of treatment visits in 1994 to 34% of visits in 2007.8 By 2007, metformin, used in 54% of treatment visits, and TZDs, used in 28%, were the most frequently administered antidiabetes agents. Insulin use declined from 38% of visits during which a treatment was administered in 1994 to 25% of visits in 2000, but had increased subsequently to 28% of visits in 2007.
SIGNIFICANCE OF CARDIOVASCULAR RISK
Clinical research has suggested that focusing solely on improving glycemic control may be insufficient to reduce overall morbidity and mortality associated with diabetes. Specifically, data from recent studies, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), emphasized that lowering HbA1c below 7% in a high-risk population of individuals with T2DM did not improve cardiovascular (CV) outcomes.9–11 The observations confirm that risk factors, including weight, blood pressure (BP), and lipid levels, are vitally important in reducing morbidity and mortality in this population. This perception is further underscored by the NHANES 1999–2004 data, which showed poor concurrent control of HbA1c, BP, and lipids; only 13.2% of patients with diagnosed diabetes achieved all three target goals simultaneously.6 Similarly, a nationwide survey in Norway showed that only 13% of patients with T2DM concurrently achieved goals for HbA1c, BP, and lipids.12
In the Danish Steno-2 Study, patients with T2DM and persistent microalbuminuria were treated with either intensive target-driven therapy using multiple drugs or conventional multifactorial treatment. Over a mean period of 13.3 years (7.8 years of treatment plus 5.5 years of follow-up), intensive multifactorial intervention to control multiple CV risk factors, including HbA1c, BP, and lipids, was associated with a lower risk of death from CV causes (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19 to 0.94; P = .04) and a lower risk of CV events (HR, 0.41; 95% CI, 0.25 to 0.67; P < .001) than was conventional therapy.13
This article clarifies the redefinition of treatment success in patients with T2DM based on targeting the underlying physiologic defects of the disease.
T2DM, OVERWEIGHT/OBESITY, AND CV DISEASE: CLOSELY LINKED
The incidence and prevalence of T2DM, overweight/obesity, and CV disease (CVD) are increasing worldwide. It is estimated that the worldwide prevalence of diabetes will increase from 171 million in 2000 to 366 million by 203014; T2DM increases the risk of morbidity and mortality from microvascular (eg, neuropathic, retinopathic, nephropathic) and macrovascular (eg, coronary, peripheral vascular disease) complications.15 According to a Michigan health maintenance organization study (N = 1,364), the median annual direct cost of medical care for Caucasian patients with T2DM who were diet controlled, had a body mass index (BMI) of 30 kg/m2 or higher, and had no vascular complications was estimated to be $1,700 for men and $2,100 for women.16 The actual cost of care for patients with T2DM may be much higher, since most patients present with multiple CV risk factors in addition to being overweight.
NHANES data show that approximately two-thirds of Americans are either overweight or obese17; overweight/obesity affects about 80% of adults diagnosed with T2DM.18 Overweight or obesity can increase the risk for developing T2DM by more than 90-fold and, in women, it can increase the risk for developing coronary heart disease (CHD) by sixfold.19 The close link between T2DM and CVD is underscored further with recent data from the Framingham Heart Study, which showed a high lifetime risk of CVD in patients with diabetes, heightened further by obesity. During the 30-year study period, the lifetime risk of CVD in normal-weight people with diabetes was 78.6% in men and 54.8% in women; the risk increased to 86.9% in obese men with diabetes and to 78.8% in obese women with diabetes.20 The NHANES data also showed that the prevalence of T2DM increased in the past decade and that patients are being diagnosed at a younger age, from a mean age of 52 years in 1988–1994 to 46 years in 1999–2000.7
BRIDGING THE GAP FROM PATHOPHYSIOLOGY TO UNMET NEEDS
The paradigm behind the pathophysiology of T2DM has shifted from its perception as a simple “dual-defect” disease (ie, deficiency in insulin secretion and peripheral tissue insulin resistance) to a multidimensional disorder.1,21 This new model includes overweight/obesity, insulin resistance, qualitative and quantitative defects in insulin secretion, and dysregulation in the secretion of other hormones, including the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the gastrointestinal incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide.21–23
CLINICAL GUIDELINES AND CV RISK FACTOR MANAGEMENT
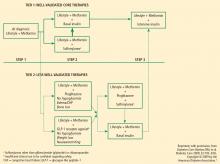
The best strategy for managing T2DM is a comprehensive approach that addresses the fundamental core defects plus associated factors that contribute to increased CV risk. Several specialty groups have suggested guidelines and algorithms for the management of T2DM and its comorbidities. These guidelines, including the ADA standards of medical care, the AACE standards in tandem with the American College of Endocrinology guidelines, and the recent joint statement from the ADA and the European Association for the Study of Diabetes (EASD), acknowledge that the core defects of T2DM and the associated CV risk factors (eg, weight gain, obesity, hypertension, dyslipidemia) are important in developing optimal treatment strategies.1–3 Medical nutrition guidelines advocate weight loss as a key initial step in managing T2DM and the comorbidities that lead to elevated CV risk.25,26 The National Institutes of Health and the US Department of Health and Human Services/US Department of Agriculture advocate regular physical activity, dietary assessment, and periodic comorbidity and weight assessment for all people, not just those with T2DM or CVD.26,27
Weight reduction
Evidence in support of effective lifestyle intervention was demonstrated in the Action for Health in Diabetes (Look AHEAD) study. After 1 year, patients with T2DM treated with intensive lifestyle intervention lost an average of 8.6% of their initial weight compared with 0.7% in patients treated only with diabetes support and education (P < 0.001). The intensive-intervention patients also had a significant drop in HbA1c (from 7.3% to 6.6%; P < 0.001) and were able to reduce their antidiabetes, antihypertensive, and lipid-lowering medications.28 More recent data from the Look AHEAD study reported that overweight patients with T2DM enrolled in a weight management program experienced significant weight loss, improved physical fitness, reduced physical symptoms, and overall improvement in health-related quality of life.29 Thus, weight reduction appears to be a key component in reducing CV risk and improving quality of life in most patients with T2DM.28–30
Hypertension
Hypertension is a major risk factor for microvascular complications and CVD, and may be associated with, or be the underlying result of, nephropathy.2 BP control is clearly important in reducing the morbidity and mortality associated with T2DM. The recommended BP goal in patients with T2DM is less than 130/80 mm Hg.1,2
Hyperlipidemia
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III [ATP III]), diabetes is considered a CHD risk equivalent because it confers a high risk of new CHD developing within 10 years.31 In addition to the NCEP–ATP III guidelines, the ADA and the AACE have set target levels for lipids in patients with diabetes, including T2DM.1,2,31 All three organizations have defined 100 mg/dL as the target level for low-density lipoprotein.
HbA1c and lifestyle intervention
EVOLUTION OF ANTIDIABETES THERAPIES
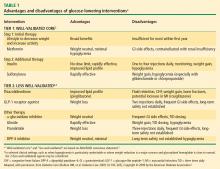
Traditional antidiabetes agents used in the treatment of patients with T2DM have focused mainly on insulin secretion and insulin resistance, with treatment success defined as achieving HbA1c goals with a reduced incidence of hypoglycemia.23 Secretagogues, such as sulfonylureas and glinides, stimulate the pancreas to release insulin. Insulin sensitizers, such as TZDs and metformin, enhance the action of insulin in muscle and fat1,3,23 and lower hepatic glucose production. The alpha-glucosidase inhibitors alter carbohydrate absorption from the gastrointestinal tract.1 The extent to which each agent achieves treatment success in terms of glucose lowering depends on several factors, including intrinsic attributes, duration of disease, and baseline glycemic control.3
GLP-1 receptor agonists
Exenatide effects. Although many agents are in development, to date exenatide is the only GLP-1 receptor agonist approved by the US Food and Drug Administration (FDA).8,33 Exenatide is an exendin-4 GLP-1 receptor agonist with multiple glucoregulatory effects, including enhanced glucose-dependent insulin secretion, reduced glucagon secretion and food intake, and slowed gastric emptying.22,34 Exenatide is detectable in the circulation for up to 10 hours following subcutaneous (SC) administration22 and has a greater potency in reducing plasma glucose than GLP-1 in preclinical studies.35,36
By virtue of its beneficial effects on glycemic control, weight, BP, and lipids, exenatide addresses some of the components of the metabolic syndrome.37–41 In pivotal 30-week studies, exenatide was associated with HbA1c reductions that ranged from –0.40% to –0.86% from baseline and decreases in body weight of approximately –1 kg to –3 kg from baseline, without severe hypoglycemia.37–39 The percentage of patients who reached the ADA goal of HbA1c less than 7.0% at 30 weeks ranged from 24% to 34%. The addition of exenatide to TZD therapy in a 16-week study was associated with mean reductions in HbA1c of –0.98%, fasting plasma glucose (FPG) concentration of –1.69 mmol/L (–30.42 mg/dL), and body weight of –1.51 kg.40
A posthoc analysis of an open-label extension study involving patients who completed the original 30-week placebo-controlled studies showed that 46% of patients who remained on exenatide achieved the ADA goal of HbA1c less than 7.0% at 3 years.41 Exenatide administered for up to 3.5 years was associated with sustained reductions in HbA1c of –1.0% (P < .0001) and body weight of –5.3 kg (P < .001). Pancreatic beta-cell function, assessed by homeostasis model assessment, improved, as did BP, triglyceride, high-density lipoprotein, low-density lipoprotein, and aspartate aminotransferase levels.41
Comparison with insulin analogues. Comparative studies have highlighted the contrasting effects of exenatide and insulin analogues (eg, insulin glargine and fixed-ratio insulin).42–45 In a 26-week trial comparing exenatide with insulin glargine in subjects with T2DM, both agents resulted in similar decreases in HbA1c. Exenatide was also associated with a –2.3-kg weight reduction, whereas insulin glargine was associated with a +1.8-kg weight gain.42 Although rates of symptomatic hypoglycemia were similar, there were fewer cases of nocturnal hypoglycemia with exenatide (0.9 event/patient-year vs 2.4 events/patient-year with insulin).
In a 32-week study comparing exenatide BID with titrated insulin glargine QD, the HbA1c reductions for exenatide and insulin glargine were comparable. However, body weight decreased –4.2 kg over two 16-week treatment periods with exenatide, but increased +3.3 kg over the same periods with the basal insulin analogue.43 The incidence of hypoglycemia was lower with exenatide than with insulin glargine (14.7% vs 25.2%), although the difference was not statistically significant.
In another study that compared exenatide with biphasic insulin aspart, patients who were treated with exenatide also lost weight while those who received the fast-acting insulin analogue gained weight (between-group difference, –5.4 kg). Patients treated with exenatide also demonstrated greater reductions in postprandial plasma glucose (PPG) excursions following their morning (P < .001), midday (P = .002), and evening meals (P < .001).44 Overall, hypoglycemia rates were similar at study end between exenatide and insulin aspart (4.7 events/patient-year vs 5.6 events/patient-year). In all of these studies, significant gastrointestinal adverse events (nausea and vomiting) occurred more frequently with exenatide, and more patients withdrew from exenatide than from insulin.
Formulations in development. Other advances in GLP-1 receptor agonist therapy include novel formulations under clinical development, such as exenatide once weekly36,46 and liraglutide, a human analogue GLP-1 receptor agonist formulated for once-daily administration.47,48 In a 52-week study in patients with T2DM, liraglutide significantly reduced HbA1c; the 1.2-mg SC QD dosage reduced HBA1c by –0.84% (P = .0014) and the 1.8-mg SC QD dosage by –1.14% (P < .0001). In comparison, glimepiride 8 mg orally QD achieved a –0.51% reduction. Liraglutide was also associated with greater reductions in weight, hypoglycemia, and systolic BP than glimepiride.47
A 26-week study compared liraglutide (0.6, 1.2, and 1.8 mg SC QD), placebo, and glimepiride 4 mg QD in combination with metformin 1 g BID. HbA1c was reduced significantly in all liraglutide groups compared with placebo (P < .0001). Mean HbA1c decreased –1.0% with liraglutide 1.2 mg and 1.8 mg and with glimepiride; it decreased –0.7% with liraglutide 0.6 mg; and it increased +0.1% with placebo. Body weight decreased –1.8 kg to –2.8 kg in all liraglutide groups but increased +1.0 kg in the glimepiride group (P < .0001). The incidence of minor hypoglycemia with liraglutide (~3%) was comparable to that observed with placebo but less than that with glimepiride (17%; P < .001).48
A once-weekly long-acting release (LAR) formulation of exenatide submitted to the FDA for approval may provide enhanced glycemic and weight control, potentially improving patient acceptance and adherence.36,46 In a 15-week study, exenatide once weekly produced significant reductions in HbA1c, FPG, PPG, and body weight. There were no withdrawals due to adverse events, and the formation of anti-exenatide antibodies was not predictive of therapeutic end point response or adverse safety outcome. Instances of hypoglycemia were mild and not dose related.36 In a 30-week study comparing exenatide LAR once weekly with exenatide BID, patients given exenatide LAR once weekly had significantly greater HbA1c reductions than did patients given exenatide BID (–1.9% vs –1.5%; P = .0023). Treatment adherence was 98% with both exenatide regimens, and no episodes of major hypoglycemia occurred with either formulation regardless of background sulfonylurea use. Favorable effects on BP and lipid profile were observed with both exenatide regimens.46
DPP-4 inhibitors
The DPP-4 inhibitors (commonly called gliptins) inhibit the proteolytic cleavage of circulating GLP-1 by binding to the DPP-4 enzyme, increasing the concentration of endogenous GLP-1 approximately two- to threefold.49–51 These concentrations result in more prompt and appropriate secretion of insulin and suppression of glucagon in response to a carbohydrate-containing snack or meal, with the change in glucagon correlating linearly with improved glucose tolerance.51
DPP-4 inhibitors, which are given orally, include sitagliptin and saxagliptin (approved in the United States) and vildagliptin (not approved in the United States but used in the European Union and Latin America).8,22,33,52 Sitagliptin can be used either as monotherapy or in combination with metformin or a TZD.8,49–55 Recently, a single-tablet formulation of sitagliptin plus metformin was granted regulatory approval.8
When used alone or in combination with metformin or pioglitazone, sitagliptin has been associated with significant reductions in HbA1c (of ~0.5% to 0.6% when used alone, ~0.7% with metformin, and ~0.9% with pioglitazone [P < .001 vs placebo]), with hypoglycemia occurring in 1.3% or less of the population.54 In an 18-week study in which patients with T2DM who were inadequately controlled with metformin monotherapy were randomized to receive add-on sitagliptin (100 mg QD), rosiglitazone (8 mg QD), or placebo, sitagliptin reduced HbA1c –0.73% (P < .001 vs placebo) and reduced body weight –0.4 kg, while rosiglitazone reduced HbA1c –0.79% and increased body weight +1.5 kg.55
To evaluate the effectiveness of sitagliptin and metformin as initial therapy, a 54-week study was completed in 885 patients with T2DM and inadequate glycemic control (HbA1c 7.5–11%) on diet and exercise.56 Patients were evaluated on monotherapy with either sitagliptin (100 mg QD) or metformin (1 g or 2 g QD), or on initial therapy with the two in combination (sitagliptin 100 mg + metformin 1 mg or 2 mg QD). At week 54, in the all-patients-treated analysis, mean changes in HbA1c from baseline were –1.8% with sitagliptin plus metformin 2 g QD, –1.4% with sitagliptin plus metformin 1 g QD, –1.3% with metformin 2 g QD monotherapy, –1.0% with metformin 1 g QD monotherapy, and –0.8% with sitagliptin 100 mg QD monotherapy.
All treatments improved measures of beta-cell function (eg, homeostasis model assessment [HOMA]-beta, proinsulin/insulin ratio). Mean body weight decreased from baseline in the combination and metformin monotherapy groups and was unchanged from baseline in the sitagliptin monotherapy group. The incidence of hypoglycemia was low (1%–3%) across treatment groups. The incidence of gastrointestinal adverse experiences was evaluated with the coadministration of sitagliptin and metformin and appeared similar to that observed with use of metformin as monotherapy.56 Thus, this study suggested that an initial combination of a DPP-IV inhibitor with metformin can improve glycemic control and markers of beta-cell function in patients with T2DM.
Incretin-based therapies compared
Studies in both healthy individuals and in patients with T2DM have shown that oral DPP-4 inhibitors such as sitagliptin increase endogenous GLP-1 concentrations by about twofold compared with placebo.22,50 The pharmacologic concentration of subcutaneously administered exenatide available for activating the GLP-1 receptor is significantly greater than the increased endogenous GLP-1 concentrations achieved with sitagliptin. In a recent clinical study comparing exenatide and sitagliptin in patients with T2DM, the mean 2-hour plasma concentration for exenatide was 64 pM compared with the mean 2-hour postprandial GLP-1 concentration of 15 pM for sitagliptin (baseline GLP-1 concentration was 7.2 pM).57 While both agents were shown to be effective, exenatide appeared to have had a greater effect than sitagliptin in increasing insulin secretion and reducing postprandial glucagon secretion, leading to significantly (P < 0.0001) greater reductions in PPG.57
Sitagliptin has been minimally associated with nausea, whereas patients who take exenatide need to be informed of the risk of usually mild to moderate, but sometimes severe, nausea and vomiting that tends to decrease over time.
For a detailed comparison of the effects of GLP-1 receptor agonists and DPP-4 inhibitors on HbA1c, weight, and hypoglycemia, see “Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors.”
CONCLUSION
Despite advances in diagnosis and treatment, T2DM, overweight/obesity, CVD, and their complications remain major public health burdens worldwide. The concepts that explain the pathophysiology of T2DM include the contribution of various factors beyond insulin secretion and insulin resistance, such as the role of incretin hormones in disease progression. A comprehensive approach to managing patients with T2DM requires targeting the fundamental defects of the disease and its comorbidities. Newer agents, including incretin-based therapies such as GLP-1 receptor agonists and DPP-4 inhibitors, address the fundamental defects of T2DM. The definition of treatment success in the management of T2DM will be redefined as more data become available on agents that exert beneficial effects not only on glycemia but on parameters that may influence overall CV health, such as weight, BP, and lipid profiles.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Koopman RJ, Mainous AG III, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3:60–63.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Jenssen TG, Tonstad S, Claudi T, Midthjell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26:2300–2304.
- National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control. Eur J Endocrinol 2008; 158:773–784.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care 2008; 31:1582–1584.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 2007; 81:636–649.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- US Department of Health and Human Services (HHS) and US Department of Agriculture. Dietary guidelines for Americans, 2005. US Department of HHS Web site. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Published January 2005. Accessed September 25, 2009.
- National Heart, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health Web site. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Updated: October 2000. Accessed September 28, 2009.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K; for the Look AHEAD Research Group. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169:163–171.
- Klein S, Sheard NF, Pi-Sunyer X, et al; for the American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117:77–88.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:2078–2084.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med 2009; 121:40–45.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther 2007; 29:2614–2634.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009; 25:569–583.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
According to the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), glycosylated hemoglobin (HbA1c) in patients with diabetes should be maintained at 6.5% or less (AACE) or at less than 7.0% (ADA). Both organizations support an aggressive stepwise approach that includes medication and lifestyle modification, with strategies and clinical attention devoted to avoiding significant hypoglycemia.1,2 Yet, despite the introduction of new antidiabetes agents, most current management strategies are offset by limitations in achieving and maintaining glycemic targets needed to provide optimal care for patients with diabetes, more than 90% of whom have type 2 diabetes mellitus (T2DM).3,4
Nationally, glycemic control among patients with T2DM has improved but is still far from optimal. According to data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), glycemic control (HbA1c < 7.0%) rates were 35.8% for patients with T2DM.5 In a more recent report (NHANES 1999–2004), fewer than half (48.4%) of adult patients with diagnosed diabetes achieved HbA1c levels below 7.0%.5,6 Factors contributing to these data include earlier onset and earlier detection of T2DM.7
CHANGING TREATMENT TRENDS
Available treatments for patients with T2DM include secretagogues, such as sulfonylureas and “glinides” (repaglinide and nateglinide), metformin, thiazolidinediones (TZDs), and dipeptidyl peptidase–4 (DPP-4) inhibitors among oral medications, and insulin and glucagon-like peptide–1 (GLP-1) receptor agonists among parenterally administered agents. According to the latest published data on prescribing patterns for patients with T2DM, analyses of the National Disease and Therapeutic Index (1994–2007) and the National Prescription Audit (2001–2007), sulfonylurea use decreased from 67% of treatment visits in 1994 to 34% of visits in 2007.8 By 2007, metformin, used in 54% of treatment visits, and TZDs, used in 28%, were the most frequently administered antidiabetes agents. Insulin use declined from 38% of visits during which a treatment was administered in 1994 to 25% of visits in 2000, but had increased subsequently to 28% of visits in 2007.
SIGNIFICANCE OF CARDIOVASCULAR RISK
Clinical research has suggested that focusing solely on improving glycemic control may be insufficient to reduce overall morbidity and mortality associated with diabetes. Specifically, data from recent studies, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), emphasized that lowering HbA1c below 7% in a high-risk population of individuals with T2DM did not improve cardiovascular (CV) outcomes.9–11 The observations confirm that risk factors, including weight, blood pressure (BP), and lipid levels, are vitally important in reducing morbidity and mortality in this population. This perception is further underscored by the NHANES 1999–2004 data, which showed poor concurrent control of HbA1c, BP, and lipids; only 13.2% of patients with diagnosed diabetes achieved all three target goals simultaneously.6 Similarly, a nationwide survey in Norway showed that only 13% of patients with T2DM concurrently achieved goals for HbA1c, BP, and lipids.12
In the Danish Steno-2 Study, patients with T2DM and persistent microalbuminuria were treated with either intensive target-driven therapy using multiple drugs or conventional multifactorial treatment. Over a mean period of 13.3 years (7.8 years of treatment plus 5.5 years of follow-up), intensive multifactorial intervention to control multiple CV risk factors, including HbA1c, BP, and lipids, was associated with a lower risk of death from CV causes (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19 to 0.94; P = .04) and a lower risk of CV events (HR, 0.41; 95% CI, 0.25 to 0.67; P < .001) than was conventional therapy.13
This article clarifies the redefinition of treatment success in patients with T2DM based on targeting the underlying physiologic defects of the disease.
T2DM, OVERWEIGHT/OBESITY, AND CV DISEASE: CLOSELY LINKED
The incidence and prevalence of T2DM, overweight/obesity, and CV disease (CVD) are increasing worldwide. It is estimated that the worldwide prevalence of diabetes will increase from 171 million in 2000 to 366 million by 203014; T2DM increases the risk of morbidity and mortality from microvascular (eg, neuropathic, retinopathic, nephropathic) and macrovascular (eg, coronary, peripheral vascular disease) complications.15 According to a Michigan health maintenance organization study (N = 1,364), the median annual direct cost of medical care for Caucasian patients with T2DM who were diet controlled, had a body mass index (BMI) of 30 kg/m2 or higher, and had no vascular complications was estimated to be $1,700 for men and $2,100 for women.16 The actual cost of care for patients with T2DM may be much higher, since most patients present with multiple CV risk factors in addition to being overweight.
NHANES data show that approximately two-thirds of Americans are either overweight or obese17; overweight/obesity affects about 80% of adults diagnosed with T2DM.18 Overweight or obesity can increase the risk for developing T2DM by more than 90-fold and, in women, it can increase the risk for developing coronary heart disease (CHD) by sixfold.19 The close link between T2DM and CVD is underscored further with recent data from the Framingham Heart Study, which showed a high lifetime risk of CVD in patients with diabetes, heightened further by obesity. During the 30-year study period, the lifetime risk of CVD in normal-weight people with diabetes was 78.6% in men and 54.8% in women; the risk increased to 86.9% in obese men with diabetes and to 78.8% in obese women with diabetes.20 The NHANES data also showed that the prevalence of T2DM increased in the past decade and that patients are being diagnosed at a younger age, from a mean age of 52 years in 1988–1994 to 46 years in 1999–2000.7
BRIDGING THE GAP FROM PATHOPHYSIOLOGY TO UNMET NEEDS
The paradigm behind the pathophysiology of T2DM has shifted from its perception as a simple “dual-defect” disease (ie, deficiency in insulin secretion and peripheral tissue insulin resistance) to a multidimensional disorder.1,21 This new model includes overweight/obesity, insulin resistance, qualitative and quantitative defects in insulin secretion, and dysregulation in the secretion of other hormones, including the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the gastrointestinal incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide.21–23
CLINICAL GUIDELINES AND CV RISK FACTOR MANAGEMENT
The best strategy for managing T2DM is a comprehensive approach that addresses the fundamental core defects plus associated factors that contribute to increased CV risk. Several specialty groups have suggested guidelines and algorithms for the management of T2DM and its comorbidities. These guidelines, including the ADA standards of medical care, the AACE standards in tandem with the American College of Endocrinology guidelines, and the recent joint statement from the ADA and the European Association for the Study of Diabetes (EASD), acknowledge that the core defects of T2DM and the associated CV risk factors (eg, weight gain, obesity, hypertension, dyslipidemia) are important in developing optimal treatment strategies.1–3 Medical nutrition guidelines advocate weight loss as a key initial step in managing T2DM and the comorbidities that lead to elevated CV risk.25,26 The National Institutes of Health and the US Department of Health and Human Services/US Department of Agriculture advocate regular physical activity, dietary assessment, and periodic comorbidity and weight assessment for all people, not just those with T2DM or CVD.26,27
Weight reduction
Evidence in support of effective lifestyle intervention was demonstrated in the Action for Health in Diabetes (Look AHEAD) study. After 1 year, patients with T2DM treated with intensive lifestyle intervention lost an average of 8.6% of their initial weight compared with 0.7% in patients treated only with diabetes support and education (P < 0.001). The intensive-intervention patients also had a significant drop in HbA1c (from 7.3% to 6.6%; P < 0.001) and were able to reduce their antidiabetes, antihypertensive, and lipid-lowering medications.28 More recent data from the Look AHEAD study reported that overweight patients with T2DM enrolled in a weight management program experienced significant weight loss, improved physical fitness, reduced physical symptoms, and overall improvement in health-related quality of life.29 Thus, weight reduction appears to be a key component in reducing CV risk and improving quality of life in most patients with T2DM.28–30
Hypertension
Hypertension is a major risk factor for microvascular complications and CVD, and may be associated with, or be the underlying result of, nephropathy.2 BP control is clearly important in reducing the morbidity and mortality associated with T2DM. The recommended BP goal in patients with T2DM is less than 130/80 mm Hg.1,2
Hyperlipidemia
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III [ATP III]), diabetes is considered a CHD risk equivalent because it confers a high risk of new CHD developing within 10 years.31 In addition to the NCEP–ATP III guidelines, the ADA and the AACE have set target levels for lipids in patients with diabetes, including T2DM.1,2,31 All three organizations have defined 100 mg/dL as the target level for low-density lipoprotein.
HbA1c and lifestyle intervention
EVOLUTION OF ANTIDIABETES THERAPIES
Traditional antidiabetes agents used in the treatment of patients with T2DM have focused mainly on insulin secretion and insulin resistance, with treatment success defined as achieving HbA1c goals with a reduced incidence of hypoglycemia.23 Secretagogues, such as sulfonylureas and glinides, stimulate the pancreas to release insulin. Insulin sensitizers, such as TZDs and metformin, enhance the action of insulin in muscle and fat1,3,23 and lower hepatic glucose production. The alpha-glucosidase inhibitors alter carbohydrate absorption from the gastrointestinal tract.1 The extent to which each agent achieves treatment success in terms of glucose lowering depends on several factors, including intrinsic attributes, duration of disease, and baseline glycemic control.3
GLP-1 receptor agonists
Exenatide effects. Although many agents are in development, to date exenatide is the only GLP-1 receptor agonist approved by the US Food and Drug Administration (FDA).8,33 Exenatide is an exendin-4 GLP-1 receptor agonist with multiple glucoregulatory effects, including enhanced glucose-dependent insulin secretion, reduced glucagon secretion and food intake, and slowed gastric emptying.22,34 Exenatide is detectable in the circulation for up to 10 hours following subcutaneous (SC) administration22 and has a greater potency in reducing plasma glucose than GLP-1 in preclinical studies.35,36
By virtue of its beneficial effects on glycemic control, weight, BP, and lipids, exenatide addresses some of the components of the metabolic syndrome.37–41 In pivotal 30-week studies, exenatide was associated with HbA1c reductions that ranged from –0.40% to –0.86% from baseline and decreases in body weight of approximately –1 kg to –3 kg from baseline, without severe hypoglycemia.37–39 The percentage of patients who reached the ADA goal of HbA1c less than 7.0% at 30 weeks ranged from 24% to 34%. The addition of exenatide to TZD therapy in a 16-week study was associated with mean reductions in HbA1c of –0.98%, fasting plasma glucose (FPG) concentration of –1.69 mmol/L (–30.42 mg/dL), and body weight of –1.51 kg.40
A posthoc analysis of an open-label extension study involving patients who completed the original 30-week placebo-controlled studies showed that 46% of patients who remained on exenatide achieved the ADA goal of HbA1c less than 7.0% at 3 years.41 Exenatide administered for up to 3.5 years was associated with sustained reductions in HbA1c of –1.0% (P < .0001) and body weight of –5.3 kg (P < .001). Pancreatic beta-cell function, assessed by homeostasis model assessment, improved, as did BP, triglyceride, high-density lipoprotein, low-density lipoprotein, and aspartate aminotransferase levels.41
Comparison with insulin analogues. Comparative studies have highlighted the contrasting effects of exenatide and insulin analogues (eg, insulin glargine and fixed-ratio insulin).42–45 In a 26-week trial comparing exenatide with insulin glargine in subjects with T2DM, both agents resulted in similar decreases in HbA1c. Exenatide was also associated with a –2.3-kg weight reduction, whereas insulin glargine was associated with a +1.8-kg weight gain.42 Although rates of symptomatic hypoglycemia were similar, there were fewer cases of nocturnal hypoglycemia with exenatide (0.9 event/patient-year vs 2.4 events/patient-year with insulin).
In a 32-week study comparing exenatide BID with titrated insulin glargine QD, the HbA1c reductions for exenatide and insulin glargine were comparable. However, body weight decreased –4.2 kg over two 16-week treatment periods with exenatide, but increased +3.3 kg over the same periods with the basal insulin analogue.43 The incidence of hypoglycemia was lower with exenatide than with insulin glargine (14.7% vs 25.2%), although the difference was not statistically significant.
In another study that compared exenatide with biphasic insulin aspart, patients who were treated with exenatide also lost weight while those who received the fast-acting insulin analogue gained weight (between-group difference, –5.4 kg). Patients treated with exenatide also demonstrated greater reductions in postprandial plasma glucose (PPG) excursions following their morning (P < .001), midday (P = .002), and evening meals (P < .001).44 Overall, hypoglycemia rates were similar at study end between exenatide and insulin aspart (4.7 events/patient-year vs 5.6 events/patient-year). In all of these studies, significant gastrointestinal adverse events (nausea and vomiting) occurred more frequently with exenatide, and more patients withdrew from exenatide than from insulin.
Formulations in development. Other advances in GLP-1 receptor agonist therapy include novel formulations under clinical development, such as exenatide once weekly36,46 and liraglutide, a human analogue GLP-1 receptor agonist formulated for once-daily administration.47,48 In a 52-week study in patients with T2DM, liraglutide significantly reduced HbA1c; the 1.2-mg SC QD dosage reduced HBA1c by –0.84% (P = .0014) and the 1.8-mg SC QD dosage by –1.14% (P < .0001). In comparison, glimepiride 8 mg orally QD achieved a –0.51% reduction. Liraglutide was also associated with greater reductions in weight, hypoglycemia, and systolic BP than glimepiride.47
A 26-week study compared liraglutide (0.6, 1.2, and 1.8 mg SC QD), placebo, and glimepiride 4 mg QD in combination with metformin 1 g BID. HbA1c was reduced significantly in all liraglutide groups compared with placebo (P < .0001). Mean HbA1c decreased –1.0% with liraglutide 1.2 mg and 1.8 mg and with glimepiride; it decreased –0.7% with liraglutide 0.6 mg; and it increased +0.1% with placebo. Body weight decreased –1.8 kg to –2.8 kg in all liraglutide groups but increased +1.0 kg in the glimepiride group (P < .0001). The incidence of minor hypoglycemia with liraglutide (~3%) was comparable to that observed with placebo but less than that with glimepiride (17%; P < .001).48
A once-weekly long-acting release (LAR) formulation of exenatide submitted to the FDA for approval may provide enhanced glycemic and weight control, potentially improving patient acceptance and adherence.36,46 In a 15-week study, exenatide once weekly produced significant reductions in HbA1c, FPG, PPG, and body weight. There were no withdrawals due to adverse events, and the formation of anti-exenatide antibodies was not predictive of therapeutic end point response or adverse safety outcome. Instances of hypoglycemia were mild and not dose related.36 In a 30-week study comparing exenatide LAR once weekly with exenatide BID, patients given exenatide LAR once weekly had significantly greater HbA1c reductions than did patients given exenatide BID (–1.9% vs –1.5%; P = .0023). Treatment adherence was 98% with both exenatide regimens, and no episodes of major hypoglycemia occurred with either formulation regardless of background sulfonylurea use. Favorable effects on BP and lipid profile were observed with both exenatide regimens.46
DPP-4 inhibitors
The DPP-4 inhibitors (commonly called gliptins) inhibit the proteolytic cleavage of circulating GLP-1 by binding to the DPP-4 enzyme, increasing the concentration of endogenous GLP-1 approximately two- to threefold.49–51 These concentrations result in more prompt and appropriate secretion of insulin and suppression of glucagon in response to a carbohydrate-containing snack or meal, with the change in glucagon correlating linearly with improved glucose tolerance.51
DPP-4 inhibitors, which are given orally, include sitagliptin and saxagliptin (approved in the United States) and vildagliptin (not approved in the United States but used in the European Union and Latin America).8,22,33,52 Sitagliptin can be used either as monotherapy or in combination with metformin or a TZD.8,49–55 Recently, a single-tablet formulation of sitagliptin plus metformin was granted regulatory approval.8
When used alone or in combination with metformin or pioglitazone, sitagliptin has been associated with significant reductions in HbA1c (of ~0.5% to 0.6% when used alone, ~0.7% with metformin, and ~0.9% with pioglitazone [P < .001 vs placebo]), with hypoglycemia occurring in 1.3% or less of the population.54 In an 18-week study in which patients with T2DM who were inadequately controlled with metformin monotherapy were randomized to receive add-on sitagliptin (100 mg QD), rosiglitazone (8 mg QD), or placebo, sitagliptin reduced HbA1c –0.73% (P < .001 vs placebo) and reduced body weight –0.4 kg, while rosiglitazone reduced HbA1c –0.79% and increased body weight +1.5 kg.55
To evaluate the effectiveness of sitagliptin and metformin as initial therapy, a 54-week study was completed in 885 patients with T2DM and inadequate glycemic control (HbA1c 7.5–11%) on diet and exercise.56 Patients were evaluated on monotherapy with either sitagliptin (100 mg QD) or metformin (1 g or 2 g QD), or on initial therapy with the two in combination (sitagliptin 100 mg + metformin 1 mg or 2 mg QD). At week 54, in the all-patients-treated analysis, mean changes in HbA1c from baseline were –1.8% with sitagliptin plus metformin 2 g QD, –1.4% with sitagliptin plus metformin 1 g QD, –1.3% with metformin 2 g QD monotherapy, –1.0% with metformin 1 g QD monotherapy, and –0.8% with sitagliptin 100 mg QD monotherapy.
All treatments improved measures of beta-cell function (eg, homeostasis model assessment [HOMA]-beta, proinsulin/insulin ratio). Mean body weight decreased from baseline in the combination and metformin monotherapy groups and was unchanged from baseline in the sitagliptin monotherapy group. The incidence of hypoglycemia was low (1%–3%) across treatment groups. The incidence of gastrointestinal adverse experiences was evaluated with the coadministration of sitagliptin and metformin and appeared similar to that observed with use of metformin as monotherapy.56 Thus, this study suggested that an initial combination of a DPP-IV inhibitor with metformin can improve glycemic control and markers of beta-cell function in patients with T2DM.
Incretin-based therapies compared
Studies in both healthy individuals and in patients with T2DM have shown that oral DPP-4 inhibitors such as sitagliptin increase endogenous GLP-1 concentrations by about twofold compared with placebo.22,50 The pharmacologic concentration of subcutaneously administered exenatide available for activating the GLP-1 receptor is significantly greater than the increased endogenous GLP-1 concentrations achieved with sitagliptin. In a recent clinical study comparing exenatide and sitagliptin in patients with T2DM, the mean 2-hour plasma concentration for exenatide was 64 pM compared with the mean 2-hour postprandial GLP-1 concentration of 15 pM for sitagliptin (baseline GLP-1 concentration was 7.2 pM).57 While both agents were shown to be effective, exenatide appeared to have had a greater effect than sitagliptin in increasing insulin secretion and reducing postprandial glucagon secretion, leading to significantly (P < 0.0001) greater reductions in PPG.57
Sitagliptin has been minimally associated with nausea, whereas patients who take exenatide need to be informed of the risk of usually mild to moderate, but sometimes severe, nausea and vomiting that tends to decrease over time.
For a detailed comparison of the effects of GLP-1 receptor agonists and DPP-4 inhibitors on HbA1c, weight, and hypoglycemia, see “Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors.”
CONCLUSION
Despite advances in diagnosis and treatment, T2DM, overweight/obesity, CVD, and their complications remain major public health burdens worldwide. The concepts that explain the pathophysiology of T2DM include the contribution of various factors beyond insulin secretion and insulin resistance, such as the role of incretin hormones in disease progression. A comprehensive approach to managing patients with T2DM requires targeting the fundamental defects of the disease and its comorbidities. Newer agents, including incretin-based therapies such as GLP-1 receptor agonists and DPP-4 inhibitors, address the fundamental defects of T2DM. The definition of treatment success in the management of T2DM will be redefined as more data become available on agents that exert beneficial effects not only on glycemia but on parameters that may influence overall CV health, such as weight, BP, and lipid profiles.
According to the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), glycosylated hemoglobin (HbA1c) in patients with diabetes should be maintained at 6.5% or less (AACE) or at less than 7.0% (ADA). Both organizations support an aggressive stepwise approach that includes medication and lifestyle modification, with strategies and clinical attention devoted to avoiding significant hypoglycemia.1,2 Yet, despite the introduction of new antidiabetes agents, most current management strategies are offset by limitations in achieving and maintaining glycemic targets needed to provide optimal care for patients with diabetes, more than 90% of whom have type 2 diabetes mellitus (T2DM).3,4
Nationally, glycemic control among patients with T2DM has improved but is still far from optimal. According to data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), glycemic control (HbA1c < 7.0%) rates were 35.8% for patients with T2DM.5 In a more recent report (NHANES 1999–2004), fewer than half (48.4%) of adult patients with diagnosed diabetes achieved HbA1c levels below 7.0%.5,6 Factors contributing to these data include earlier onset and earlier detection of T2DM.7
CHANGING TREATMENT TRENDS
Available treatments for patients with T2DM include secretagogues, such as sulfonylureas and “glinides” (repaglinide and nateglinide), metformin, thiazolidinediones (TZDs), and dipeptidyl peptidase–4 (DPP-4) inhibitors among oral medications, and insulin and glucagon-like peptide–1 (GLP-1) receptor agonists among parenterally administered agents. According to the latest published data on prescribing patterns for patients with T2DM, analyses of the National Disease and Therapeutic Index (1994–2007) and the National Prescription Audit (2001–2007), sulfonylurea use decreased from 67% of treatment visits in 1994 to 34% of visits in 2007.8 By 2007, metformin, used in 54% of treatment visits, and TZDs, used in 28%, were the most frequently administered antidiabetes agents. Insulin use declined from 38% of visits during which a treatment was administered in 1994 to 25% of visits in 2000, but had increased subsequently to 28% of visits in 2007.
SIGNIFICANCE OF CARDIOVASCULAR RISK
Clinical research has suggested that focusing solely on improving glycemic control may be insufficient to reduce overall morbidity and mortality associated with diabetes. Specifically, data from recent studies, including the Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), and the Veterans Affairs Diabetes Trial (VADT), emphasized that lowering HbA1c below 7% in a high-risk population of individuals with T2DM did not improve cardiovascular (CV) outcomes.9–11 The observations confirm that risk factors, including weight, blood pressure (BP), and lipid levels, are vitally important in reducing morbidity and mortality in this population. This perception is further underscored by the NHANES 1999–2004 data, which showed poor concurrent control of HbA1c, BP, and lipids; only 13.2% of patients with diagnosed diabetes achieved all three target goals simultaneously.6 Similarly, a nationwide survey in Norway showed that only 13% of patients with T2DM concurrently achieved goals for HbA1c, BP, and lipids.12
In the Danish Steno-2 Study, patients with T2DM and persistent microalbuminuria were treated with either intensive target-driven therapy using multiple drugs or conventional multifactorial treatment. Over a mean period of 13.3 years (7.8 years of treatment plus 5.5 years of follow-up), intensive multifactorial intervention to control multiple CV risk factors, including HbA1c, BP, and lipids, was associated with a lower risk of death from CV causes (hazard ratio [HR], 0.43; 95% confidence interval [CI], 0.19 to 0.94; P = .04) and a lower risk of CV events (HR, 0.41; 95% CI, 0.25 to 0.67; P < .001) than was conventional therapy.13
This article clarifies the redefinition of treatment success in patients with T2DM based on targeting the underlying physiologic defects of the disease.
T2DM, OVERWEIGHT/OBESITY, AND CV DISEASE: CLOSELY LINKED
The incidence and prevalence of T2DM, overweight/obesity, and CV disease (CVD) are increasing worldwide. It is estimated that the worldwide prevalence of diabetes will increase from 171 million in 2000 to 366 million by 203014; T2DM increases the risk of morbidity and mortality from microvascular (eg, neuropathic, retinopathic, nephropathic) and macrovascular (eg, coronary, peripheral vascular disease) complications.15 According to a Michigan health maintenance organization study (N = 1,364), the median annual direct cost of medical care for Caucasian patients with T2DM who were diet controlled, had a body mass index (BMI) of 30 kg/m2 or higher, and had no vascular complications was estimated to be $1,700 for men and $2,100 for women.16 The actual cost of care for patients with T2DM may be much higher, since most patients present with multiple CV risk factors in addition to being overweight.
NHANES data show that approximately two-thirds of Americans are either overweight or obese17; overweight/obesity affects about 80% of adults diagnosed with T2DM.18 Overweight or obesity can increase the risk for developing T2DM by more than 90-fold and, in women, it can increase the risk for developing coronary heart disease (CHD) by sixfold.19 The close link between T2DM and CVD is underscored further with recent data from the Framingham Heart Study, which showed a high lifetime risk of CVD in patients with diabetes, heightened further by obesity. During the 30-year study period, the lifetime risk of CVD in normal-weight people with diabetes was 78.6% in men and 54.8% in women; the risk increased to 86.9% in obese men with diabetes and to 78.8% in obese women with diabetes.20 The NHANES data also showed that the prevalence of T2DM increased in the past decade and that patients are being diagnosed at a younger age, from a mean age of 52 years in 1988–1994 to 46 years in 1999–2000.7
BRIDGING THE GAP FROM PATHOPHYSIOLOGY TO UNMET NEEDS
The paradigm behind the pathophysiology of T2DM has shifted from its perception as a simple “dual-defect” disease (ie, deficiency in insulin secretion and peripheral tissue insulin resistance) to a multidimensional disorder.1,21 This new model includes overweight/obesity, insulin resistance, qualitative and quantitative defects in insulin secretion, and dysregulation in the secretion of other hormones, including the beta-cell hormone amylin, the alpha-cell hormone glucagon, and the gastrointestinal incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide.21–23
CLINICAL GUIDELINES AND CV RISK FACTOR MANAGEMENT
The best strategy for managing T2DM is a comprehensive approach that addresses the fundamental core defects plus associated factors that contribute to increased CV risk. Several specialty groups have suggested guidelines and algorithms for the management of T2DM and its comorbidities. These guidelines, including the ADA standards of medical care, the AACE standards in tandem with the American College of Endocrinology guidelines, and the recent joint statement from the ADA and the European Association for the Study of Diabetes (EASD), acknowledge that the core defects of T2DM and the associated CV risk factors (eg, weight gain, obesity, hypertension, dyslipidemia) are important in developing optimal treatment strategies.1–3 Medical nutrition guidelines advocate weight loss as a key initial step in managing T2DM and the comorbidities that lead to elevated CV risk.25,26 The National Institutes of Health and the US Department of Health and Human Services/US Department of Agriculture advocate regular physical activity, dietary assessment, and periodic comorbidity and weight assessment for all people, not just those with T2DM or CVD.26,27
Weight reduction
Evidence in support of effective lifestyle intervention was demonstrated in the Action for Health in Diabetes (Look AHEAD) study. After 1 year, patients with T2DM treated with intensive lifestyle intervention lost an average of 8.6% of their initial weight compared with 0.7% in patients treated only with diabetes support and education (P < 0.001). The intensive-intervention patients also had a significant drop in HbA1c (from 7.3% to 6.6%; P < 0.001) and were able to reduce their antidiabetes, antihypertensive, and lipid-lowering medications.28 More recent data from the Look AHEAD study reported that overweight patients with T2DM enrolled in a weight management program experienced significant weight loss, improved physical fitness, reduced physical symptoms, and overall improvement in health-related quality of life.29 Thus, weight reduction appears to be a key component in reducing CV risk and improving quality of life in most patients with T2DM.28–30
Hypertension
Hypertension is a major risk factor for microvascular complications and CVD, and may be associated with, or be the underlying result of, nephropathy.2 BP control is clearly important in reducing the morbidity and mortality associated with T2DM. The recommended BP goal in patients with T2DM is less than 130/80 mm Hg.1,2
Hyperlipidemia
According to the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III [ATP III]), diabetes is considered a CHD risk equivalent because it confers a high risk of new CHD developing within 10 years.31 In addition to the NCEP–ATP III guidelines, the ADA and the AACE have set target levels for lipids in patients with diabetes, including T2DM.1,2,31 All three organizations have defined 100 mg/dL as the target level for low-density lipoprotein.
HbA1c and lifestyle intervention
EVOLUTION OF ANTIDIABETES THERAPIES
Traditional antidiabetes agents used in the treatment of patients with T2DM have focused mainly on insulin secretion and insulin resistance, with treatment success defined as achieving HbA1c goals with a reduced incidence of hypoglycemia.23 Secretagogues, such as sulfonylureas and glinides, stimulate the pancreas to release insulin. Insulin sensitizers, such as TZDs and metformin, enhance the action of insulin in muscle and fat1,3,23 and lower hepatic glucose production. The alpha-glucosidase inhibitors alter carbohydrate absorption from the gastrointestinal tract.1 The extent to which each agent achieves treatment success in terms of glucose lowering depends on several factors, including intrinsic attributes, duration of disease, and baseline glycemic control.3
GLP-1 receptor agonists
Exenatide effects. Although many agents are in development, to date exenatide is the only GLP-1 receptor agonist approved by the US Food and Drug Administration (FDA).8,33 Exenatide is an exendin-4 GLP-1 receptor agonist with multiple glucoregulatory effects, including enhanced glucose-dependent insulin secretion, reduced glucagon secretion and food intake, and slowed gastric emptying.22,34 Exenatide is detectable in the circulation for up to 10 hours following subcutaneous (SC) administration22 and has a greater potency in reducing plasma glucose than GLP-1 in preclinical studies.35,36
By virtue of its beneficial effects on glycemic control, weight, BP, and lipids, exenatide addresses some of the components of the metabolic syndrome.37–41 In pivotal 30-week studies, exenatide was associated with HbA1c reductions that ranged from –0.40% to –0.86% from baseline and decreases in body weight of approximately –1 kg to –3 kg from baseline, without severe hypoglycemia.37–39 The percentage of patients who reached the ADA goal of HbA1c less than 7.0% at 30 weeks ranged from 24% to 34%. The addition of exenatide to TZD therapy in a 16-week study was associated with mean reductions in HbA1c of –0.98%, fasting plasma glucose (FPG) concentration of –1.69 mmol/L (–30.42 mg/dL), and body weight of –1.51 kg.40
A posthoc analysis of an open-label extension study involving patients who completed the original 30-week placebo-controlled studies showed that 46% of patients who remained on exenatide achieved the ADA goal of HbA1c less than 7.0% at 3 years.41 Exenatide administered for up to 3.5 years was associated with sustained reductions in HbA1c of –1.0% (P < .0001) and body weight of –5.3 kg (P < .001). Pancreatic beta-cell function, assessed by homeostasis model assessment, improved, as did BP, triglyceride, high-density lipoprotein, low-density lipoprotein, and aspartate aminotransferase levels.41
Comparison with insulin analogues. Comparative studies have highlighted the contrasting effects of exenatide and insulin analogues (eg, insulin glargine and fixed-ratio insulin).42–45 In a 26-week trial comparing exenatide with insulin glargine in subjects with T2DM, both agents resulted in similar decreases in HbA1c. Exenatide was also associated with a –2.3-kg weight reduction, whereas insulin glargine was associated with a +1.8-kg weight gain.42 Although rates of symptomatic hypoglycemia were similar, there were fewer cases of nocturnal hypoglycemia with exenatide (0.9 event/patient-year vs 2.4 events/patient-year with insulin).
In a 32-week study comparing exenatide BID with titrated insulin glargine QD, the HbA1c reductions for exenatide and insulin glargine were comparable. However, body weight decreased –4.2 kg over two 16-week treatment periods with exenatide, but increased +3.3 kg over the same periods with the basal insulin analogue.43 The incidence of hypoglycemia was lower with exenatide than with insulin glargine (14.7% vs 25.2%), although the difference was not statistically significant.
In another study that compared exenatide with biphasic insulin aspart, patients who were treated with exenatide also lost weight while those who received the fast-acting insulin analogue gained weight (between-group difference, –5.4 kg). Patients treated with exenatide also demonstrated greater reductions in postprandial plasma glucose (PPG) excursions following their morning (P < .001), midday (P = .002), and evening meals (P < .001).44 Overall, hypoglycemia rates were similar at study end between exenatide and insulin aspart (4.7 events/patient-year vs 5.6 events/patient-year). In all of these studies, significant gastrointestinal adverse events (nausea and vomiting) occurred more frequently with exenatide, and more patients withdrew from exenatide than from insulin.
Formulations in development. Other advances in GLP-1 receptor agonist therapy include novel formulations under clinical development, such as exenatide once weekly36,46 and liraglutide, a human analogue GLP-1 receptor agonist formulated for once-daily administration.47,48 In a 52-week study in patients with T2DM, liraglutide significantly reduced HbA1c; the 1.2-mg SC QD dosage reduced HBA1c by –0.84% (P = .0014) and the 1.8-mg SC QD dosage by –1.14% (P < .0001). In comparison, glimepiride 8 mg orally QD achieved a –0.51% reduction. Liraglutide was also associated with greater reductions in weight, hypoglycemia, and systolic BP than glimepiride.47
A 26-week study compared liraglutide (0.6, 1.2, and 1.8 mg SC QD), placebo, and glimepiride 4 mg QD in combination with metformin 1 g BID. HbA1c was reduced significantly in all liraglutide groups compared with placebo (P < .0001). Mean HbA1c decreased –1.0% with liraglutide 1.2 mg and 1.8 mg and with glimepiride; it decreased –0.7% with liraglutide 0.6 mg; and it increased +0.1% with placebo. Body weight decreased –1.8 kg to –2.8 kg in all liraglutide groups but increased +1.0 kg in the glimepiride group (P < .0001). The incidence of minor hypoglycemia with liraglutide (~3%) was comparable to that observed with placebo but less than that with glimepiride (17%; P < .001).48
A once-weekly long-acting release (LAR) formulation of exenatide submitted to the FDA for approval may provide enhanced glycemic and weight control, potentially improving patient acceptance and adherence.36,46 In a 15-week study, exenatide once weekly produced significant reductions in HbA1c, FPG, PPG, and body weight. There were no withdrawals due to adverse events, and the formation of anti-exenatide antibodies was not predictive of therapeutic end point response or adverse safety outcome. Instances of hypoglycemia were mild and not dose related.36 In a 30-week study comparing exenatide LAR once weekly with exenatide BID, patients given exenatide LAR once weekly had significantly greater HbA1c reductions than did patients given exenatide BID (–1.9% vs –1.5%; P = .0023). Treatment adherence was 98% with both exenatide regimens, and no episodes of major hypoglycemia occurred with either formulation regardless of background sulfonylurea use. Favorable effects on BP and lipid profile were observed with both exenatide regimens.46
DPP-4 inhibitors
The DPP-4 inhibitors (commonly called gliptins) inhibit the proteolytic cleavage of circulating GLP-1 by binding to the DPP-4 enzyme, increasing the concentration of endogenous GLP-1 approximately two- to threefold.49–51 These concentrations result in more prompt and appropriate secretion of insulin and suppression of glucagon in response to a carbohydrate-containing snack or meal, with the change in glucagon correlating linearly with improved glucose tolerance.51
DPP-4 inhibitors, which are given orally, include sitagliptin and saxagliptin (approved in the United States) and vildagliptin (not approved in the United States but used in the European Union and Latin America).8,22,33,52 Sitagliptin can be used either as monotherapy or in combination with metformin or a TZD.8,49–55 Recently, a single-tablet formulation of sitagliptin plus metformin was granted regulatory approval.8
When used alone or in combination with metformin or pioglitazone, sitagliptin has been associated with significant reductions in HbA1c (of ~0.5% to 0.6% when used alone, ~0.7% with metformin, and ~0.9% with pioglitazone [P < .001 vs placebo]), with hypoglycemia occurring in 1.3% or less of the population.54 In an 18-week study in which patients with T2DM who were inadequately controlled with metformin monotherapy were randomized to receive add-on sitagliptin (100 mg QD), rosiglitazone (8 mg QD), or placebo, sitagliptin reduced HbA1c –0.73% (P < .001 vs placebo) and reduced body weight –0.4 kg, while rosiglitazone reduced HbA1c –0.79% and increased body weight +1.5 kg.55
To evaluate the effectiveness of sitagliptin and metformin as initial therapy, a 54-week study was completed in 885 patients with T2DM and inadequate glycemic control (HbA1c 7.5–11%) on diet and exercise.56 Patients were evaluated on monotherapy with either sitagliptin (100 mg QD) or metformin (1 g or 2 g QD), or on initial therapy with the two in combination (sitagliptin 100 mg + metformin 1 mg or 2 mg QD). At week 54, in the all-patients-treated analysis, mean changes in HbA1c from baseline were –1.8% with sitagliptin plus metformin 2 g QD, –1.4% with sitagliptin plus metformin 1 g QD, –1.3% with metformin 2 g QD monotherapy, –1.0% with metformin 1 g QD monotherapy, and –0.8% with sitagliptin 100 mg QD monotherapy.
All treatments improved measures of beta-cell function (eg, homeostasis model assessment [HOMA]-beta, proinsulin/insulin ratio). Mean body weight decreased from baseline in the combination and metformin monotherapy groups and was unchanged from baseline in the sitagliptin monotherapy group. The incidence of hypoglycemia was low (1%–3%) across treatment groups. The incidence of gastrointestinal adverse experiences was evaluated with the coadministration of sitagliptin and metformin and appeared similar to that observed with use of metformin as monotherapy.56 Thus, this study suggested that an initial combination of a DPP-IV inhibitor with metformin can improve glycemic control and markers of beta-cell function in patients with T2DM.
Incretin-based therapies compared
Studies in both healthy individuals and in patients with T2DM have shown that oral DPP-4 inhibitors such as sitagliptin increase endogenous GLP-1 concentrations by about twofold compared with placebo.22,50 The pharmacologic concentration of subcutaneously administered exenatide available for activating the GLP-1 receptor is significantly greater than the increased endogenous GLP-1 concentrations achieved with sitagliptin. In a recent clinical study comparing exenatide and sitagliptin in patients with T2DM, the mean 2-hour plasma concentration for exenatide was 64 pM compared with the mean 2-hour postprandial GLP-1 concentration of 15 pM for sitagliptin (baseline GLP-1 concentration was 7.2 pM).57 While both agents were shown to be effective, exenatide appeared to have had a greater effect than sitagliptin in increasing insulin secretion and reducing postprandial glucagon secretion, leading to significantly (P < 0.0001) greater reductions in PPG.57
Sitagliptin has been minimally associated with nausea, whereas patients who take exenatide need to be informed of the risk of usually mild to moderate, but sometimes severe, nausea and vomiting that tends to decrease over time.
For a detailed comparison of the effects of GLP-1 receptor agonists and DPP-4 inhibitors on HbA1c, weight, and hypoglycemia, see “Advances in therapy for type 2 diabetes: GLP–1 receptor agonists and DPP–4 inhibitors.”
CONCLUSION
Despite advances in diagnosis and treatment, T2DM, overweight/obesity, CVD, and their complications remain major public health burdens worldwide. The concepts that explain the pathophysiology of T2DM include the contribution of various factors beyond insulin secretion and insulin resistance, such as the role of incretin hormones in disease progression. A comprehensive approach to managing patients with T2DM requires targeting the fundamental defects of the disease and its comorbidities. Newer agents, including incretin-based therapies such as GLP-1 receptor agonists and DPP-4 inhibitors, address the fundamental defects of T2DM. The definition of treatment success in the management of T2DM will be redefined as more data become available on agents that exert beneficial effects not only on glycemia but on parameters that may influence overall CV health, such as weight, BP, and lipid profiles.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Koopman RJ, Mainous AG III, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3:60–63.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Jenssen TG, Tonstad S, Claudi T, Midthjell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26:2300–2304.
- National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control. Eur J Endocrinol 2008; 158:773–784.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care 2008; 31:1582–1584.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 2007; 81:636–649.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- US Department of Health and Human Services (HHS) and US Department of Agriculture. Dietary guidelines for Americans, 2005. US Department of HHS Web site. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Published January 2005. Accessed September 25, 2009.
- National Heart, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health Web site. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Updated: October 2000. Accessed September 28, 2009.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K; for the Look AHEAD Research Group. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169:163–171.
- Klein S, Sheard NF, Pi-Sunyer X, et al; for the American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117:77–88.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:2078–2084.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med 2009; 121:40–45.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther 2007; 29:2614–2634.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009; 25:569–583.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
- AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):S4–S68.
- American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009; 32(suppl 1):S13–S61.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007 fact sheet. National Institutes of Health Web site. http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Published 2008. Accessed September 16, 2009.
- Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care 2004; 27:17–20.
- Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the US National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008; 18:222–229.
- Koopman RJ, Mainous AG III, Diaz VA, Geesey ME. Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 2005; 3:60–63.
- Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008; 168:2088–2094.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360:129–139.
- Jenssen TG, Tonstad S, Claudi T, Midthjell K, Cooper J. The gap between guidelines and practice in the treatment of type 2 diabetes: a nationwide survey in Norway. Diabetes Res Clin Pract 2008; 80:314–320.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–591.
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053.
- Rosenstock J. Management of type 2 diabetes mellitus in the elderly: special considerations. Drugs Aging 2001; 18:31–44.
- Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26:2300–2304.
- National Center for Health Statistics. Prevalence of overweight and obesity among adults: United States 2003–2004. Centers for Disease Contral and Prevention Web site. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overwght_adult_03.htm. Published: April 2006. Accessed September 23, 2009.
- Van Gaal LF, Gutkin SW, Nauck MA. Exploiting the antidiabetic properties of incretins to treat type 2 diabetes mellitus: glucagon-like peptide 1 receptor agonists or insulin for patients with inadequate glycemic control. Eur J Endocrinol 2008; 158:773–784.
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331–339.
- Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care 2008; 31:1582–1584.
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58:773–795.
- Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev 2008; 4:101–109.
- Cefalu WT. Pharmacotherapy for the treatment of patients with type 2 diabetes mellitus: rationale and specific agents. Clin Pharmacol Ther 2007; 81:636–649.
- Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin 2008; 24:2189–2202.
- American Diabetes Association. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008; 31(suppl 1):S61−S78.
- US Department of Health and Human Services (HHS) and US Department of Agriculture. Dietary guidelines for Americans, 2005. US Department of HHS Web site. http://www.health.gov/DietaryGuidelines/dga2005/document/default.htm. Published January 2005. Accessed September 25, 2009.
- National Heart, Lung, and Blood Institute. The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. National Institutes of Health Web site. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Updated: October 2000. Accessed September 28, 2009.
- Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007; 30:1374–1383.
- Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K; for the Look AHEAD Research Group. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169:163–171.
- Klein S, Sheard NF, Pi-Sunyer X, et al; for the American Diabetes Association; North American Association for the Study of Obesity; American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27:2067–2073.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497.
- Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007; 298:194–206.
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117:77–88.
- Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 1999; 48:1026–1034.
- Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30:1487–1493.
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2628–2635.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28:1083–1091.
- Zinman B, Hoogwerf BJ, Durán García S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146:477–485.
- Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24:275–286.
- Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; for the GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143:559–569.
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther 2007; 29:2333–2348.
- Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50:259–267.
- Glass LC, Qu Y, Lenox S, et al. Effects of exenatide versus insulin analogues on weight change in subjects with type 2 diabetes: a pooled post-hoc analysis. Curr Med Res Opin 2008; 24:639–644.
- Drucker DJ, Buse JB, Taylor K, et al; for the DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372:1240–1250.
- Garber A, Henry R, Ratner R, et al; for the LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481.
- Nauck M, Frid A, Hermansen K, et al; for the LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009; 32:84–90.
- Ahrén B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004; 89:2078–2084.
- Herman GA, Stevens C, Van Dyck K, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther 2005; 78:675–688.
- Bohannon N. Overview of the gliptin class (dipeptidyl peptidase-4 inhibitors) in clinical practice. Postgrad Med 2009; 121:40–45.
- US Department of Health and Human Services. FDA approves new drug treatment for type 2 diabetes. US Food and Drug Administration Web site. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm174780.htm. Published July 31, 2009. Accessed September 18, 2009.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; for the Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther 2007; 29:2614–2634.
- Scott R, Loeys T, Davies MJ, Engel SS; for the Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008; 10:959–969.
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin 2009; 25:569–583.
- DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008; 24:2943–2952.
KEY POINTS
- The NHANES 1999–2004 data showed that only 13.2% of patients with diagnosed diabetes achieved concurrent weight, blood pressure, and lipid level goals.
- Among patients with T2DM, lifestyle intervention (control of weight, blood pressure, lipid levels) should be reinforced at every physician visit; glycosylated hemoglobin (HbA1c) should be monitored every 3 months until it is less than 7.0%, and then rechecked every 6 months.
- The effects of GLP-1 agonists on HbA1c are comparable to insulin analogues, but GLP-1 agonists are associated with weight reduction, while insulin is associated with weight gain.
- DPP-4 inhibitors have been associated with significant reductions in HbA1c when used alone or with metformin or pioglitazone.