User login
Metastatic angiosarcoma arising in a patient with long-standing treatment-refractory hemangioma
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
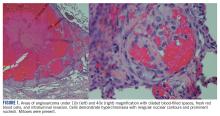
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
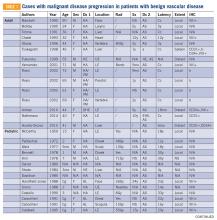
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.