User login
What is the differential diagnosis of chronic leg edema in primary care?
The differential diagnosis, in descending order, includes: elevated pulmonary artery pressure (often due to obstructive sleep apnea), congestive heart failure, idiopathic causes, venous insufficiency, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and proteinuria (>1 g daily) (strength of recommendation: B, based on a nonconsecutive diagnostic cohort study with good reference standards).
Test for DVT in those with unilateral leg edema
Marcia Lu, MD
Department of Family and Community Medicine, University of Nevada School of Medicine, Reno
Based on presented evidence, it is premature to make the initial diagnosis of venous insufficiency without further evaluation through cardiovascular testing in patients >45 years of age.
Though this Clinical Inquiry is very convincing, I recommend cautious interpretation of this data due to the relatively small sample size, type of study, and demographics of the study population. A final note: remember to exclude pregnancy in women of reproductive age, and consider tests to exclude deep venous thrombosis in patients presenting with unilateral leg edema.
Evidence summary
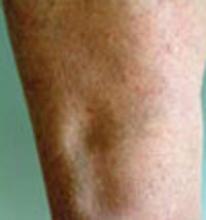
Chronic leg edema is defined as palpable swelling caused by an increase in interstitial fluid volume lasting at least 72 hours.1
We were able to find only 1 moderate-quality study regarding the diagnosis of bilateral lower extremity edema that included a thorough cardiovascular evaluation.
Single study in bilateral leg edema: What the FPs thought…
A nonconsecutive cohort study2 evaluated the causes of bilateral leg edema among 58 ambulatory adults (between 29 and 83 years of age) enrolled from an inner-city family medicine clinic in Cleveland. Edema was present for >3 months in 78% of patients, and 84% were obese. Patients were excluded if the edema was known to be due to nifedipine, intra-abdominal malignancy, hypothyroidism, or idiopathic cyclic edema.
Family physicians obtained a history and performed a physical exam on all patients and recorded a clinical diagnosis for the edema. Initial clinical impressions included: venous insufficiency (71%), congestive heart failure (18%), nephrotic syndrome (13%), uncertain (7%), and other causes (2% each). Other causes included lymphedema, pulmonary hypertension, cor pulmonale, hypoalbuminemia, NSAID or corticosteroid use, sleep apnea, and obesity. (Total percentages exceed 100% because some patients had multiple conditions.)
All patients were then evaluated with a serum albumin, a 24-hour urine protein collection, echocardiogram, and lower extremity duplex venous ultrasound (13 of 58 patients did not complete the echocardiogram and venous duplex ultrasound). Investigators developed a final diagnosis using the results of this evaluation in conjunction with the clinical information obtained by the physician.
…and what the testing revealed
Final diagnoses included pulmonary hypertension/borderline pulmonary hyper-tension (>30 mm Hg) (42%), congestive heart failure (29%), idiopathic edema (27%), venous insufficiency (22%), medication use (15%, primarily corticosteroids and NSAIDs), proteinuria >1 g/day (15%), and other causes (2% each). These other causes included transient renal disease, hypoalbuminemia, lymphedema, and stenosis of the inferior vena cava. (Total percentages again exceed 100% because some patients had multiple diagnoses.)
All 15 patients with cardiac conditions and 17 of 19 patients with pulmonary hypertension were over 45 years of age. Of the 19 patients with pulmonary hypertension, 6 had CHF, 4 had chronic obstructive pulmonary disease, 4 had sleep apnea diagnosed in subsequent testing, 1 had an atrial septal defect, and 4 appeared to have primary pulmonary hypertension.
Investigators recommend 3 steps
The investigators recommended 3 steps to evaluate chronic leg edema.
- Stop any potentially causative medicines, such as NSAIDs or calcium-channel blockers.
- Obtain an echocardiogram if the patient is 45 years of age or older.
- Obtain a sleep study if the echo-cardiogram reveals pulmonary hypertension without an apparent cause.
Recommendations from others
The authors of a recent narrative systematic review1 stated that “most patients with chronic leg edema can be assumed to have venous insufficiency, CHF, or cyclic edema, unless another cause is suspected after a history and physical examination.” They also stated that: “pulmonary hypertension and early CHF can both cause leg edema before they become clinically obvious” and reiterate that patients over 45 years of age with edema of unclear cause should have an echocardiogram to rule out pulmonary hypertension.
The differential diagnosis, in descending order, includes: elevated pulmonary artery pressure (often due to obstructive sleep apnea), congestive heart failure, idiopathic causes, venous insufficiency, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and proteinuria (>1 g daily) (strength of recommendation: B, based on a nonconsecutive diagnostic cohort study with good reference standards).
Test for DVT in those with unilateral leg edema
Marcia Lu, MD
Department of Family and Community Medicine, University of Nevada School of Medicine, Reno
Based on presented evidence, it is premature to make the initial diagnosis of venous insufficiency without further evaluation through cardiovascular testing in patients >45 years of age.
Though this Clinical Inquiry is very convincing, I recommend cautious interpretation of this data due to the relatively small sample size, type of study, and demographics of the study population. A final note: remember to exclude pregnancy in women of reproductive age, and consider tests to exclude deep venous thrombosis in patients presenting with unilateral leg edema.
Evidence summary
Chronic leg edema is defined as palpable swelling caused by an increase in interstitial fluid volume lasting at least 72 hours.1
We were able to find only 1 moderate-quality study regarding the diagnosis of bilateral lower extremity edema that included a thorough cardiovascular evaluation.
Single study in bilateral leg edema: What the FPs thought…
A nonconsecutive cohort study2 evaluated the causes of bilateral leg edema among 58 ambulatory adults (between 29 and 83 years of age) enrolled from an inner-city family medicine clinic in Cleveland. Edema was present for >3 months in 78% of patients, and 84% were obese. Patients were excluded if the edema was known to be due to nifedipine, intra-abdominal malignancy, hypothyroidism, or idiopathic cyclic edema.
Family physicians obtained a history and performed a physical exam on all patients and recorded a clinical diagnosis for the edema. Initial clinical impressions included: venous insufficiency (71%), congestive heart failure (18%), nephrotic syndrome (13%), uncertain (7%), and other causes (2% each). Other causes included lymphedema, pulmonary hypertension, cor pulmonale, hypoalbuminemia, NSAID or corticosteroid use, sleep apnea, and obesity. (Total percentages exceed 100% because some patients had multiple conditions.)
All patients were then evaluated with a serum albumin, a 24-hour urine protein collection, echocardiogram, and lower extremity duplex venous ultrasound (13 of 58 patients did not complete the echocardiogram and venous duplex ultrasound). Investigators developed a final diagnosis using the results of this evaluation in conjunction with the clinical information obtained by the physician.
…and what the testing revealed
Final diagnoses included pulmonary hypertension/borderline pulmonary hyper-tension (>30 mm Hg) (42%), congestive heart failure (29%), idiopathic edema (27%), venous insufficiency (22%), medication use (15%, primarily corticosteroids and NSAIDs), proteinuria >1 g/day (15%), and other causes (2% each). These other causes included transient renal disease, hypoalbuminemia, lymphedema, and stenosis of the inferior vena cava. (Total percentages again exceed 100% because some patients had multiple diagnoses.)
All 15 patients with cardiac conditions and 17 of 19 patients with pulmonary hypertension were over 45 years of age. Of the 19 patients with pulmonary hypertension, 6 had CHF, 4 had chronic obstructive pulmonary disease, 4 had sleep apnea diagnosed in subsequent testing, 1 had an atrial septal defect, and 4 appeared to have primary pulmonary hypertension.
Investigators recommend 3 steps
The investigators recommended 3 steps to evaluate chronic leg edema.
- Stop any potentially causative medicines, such as NSAIDs or calcium-channel blockers.
- Obtain an echocardiogram if the patient is 45 years of age or older.
- Obtain a sleep study if the echo-cardiogram reveals pulmonary hypertension without an apparent cause.
Recommendations from others
The authors of a recent narrative systematic review1 stated that “most patients with chronic leg edema can be assumed to have venous insufficiency, CHF, or cyclic edema, unless another cause is suspected after a history and physical examination.” They also stated that: “pulmonary hypertension and early CHF can both cause leg edema before they become clinically obvious” and reiterate that patients over 45 years of age with edema of unclear cause should have an echocardiogram to rule out pulmonary hypertension.
The differential diagnosis, in descending order, includes: elevated pulmonary artery pressure (often due to obstructive sleep apnea), congestive heart failure, idiopathic causes, venous insufficiency, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and proteinuria (>1 g daily) (strength of recommendation: B, based on a nonconsecutive diagnostic cohort study with good reference standards).
Test for DVT in those with unilateral leg edema
Marcia Lu, MD
Department of Family and Community Medicine, University of Nevada School of Medicine, Reno
Based on presented evidence, it is premature to make the initial diagnosis of venous insufficiency without further evaluation through cardiovascular testing in patients >45 years of age.
Though this Clinical Inquiry is very convincing, I recommend cautious interpretation of this data due to the relatively small sample size, type of study, and demographics of the study population. A final note: remember to exclude pregnancy in women of reproductive age, and consider tests to exclude deep venous thrombosis in patients presenting with unilateral leg edema.
Evidence summary
Chronic leg edema is defined as palpable swelling caused by an increase in interstitial fluid volume lasting at least 72 hours.1
We were able to find only 1 moderate-quality study regarding the diagnosis of bilateral lower extremity edema that included a thorough cardiovascular evaluation.
Single study in bilateral leg edema: What the FPs thought…
A nonconsecutive cohort study2 evaluated the causes of bilateral leg edema among 58 ambulatory adults (between 29 and 83 years of age) enrolled from an inner-city family medicine clinic in Cleveland. Edema was present for >3 months in 78% of patients, and 84% were obese. Patients were excluded if the edema was known to be due to nifedipine, intra-abdominal malignancy, hypothyroidism, or idiopathic cyclic edema.
Family physicians obtained a history and performed a physical exam on all patients and recorded a clinical diagnosis for the edema. Initial clinical impressions included: venous insufficiency (71%), congestive heart failure (18%), nephrotic syndrome (13%), uncertain (7%), and other causes (2% each). Other causes included lymphedema, pulmonary hypertension, cor pulmonale, hypoalbuminemia, NSAID or corticosteroid use, sleep apnea, and obesity. (Total percentages exceed 100% because some patients had multiple conditions.)
All patients were then evaluated with a serum albumin, a 24-hour urine protein collection, echocardiogram, and lower extremity duplex venous ultrasound (13 of 58 patients did not complete the echocardiogram and venous duplex ultrasound). Investigators developed a final diagnosis using the results of this evaluation in conjunction with the clinical information obtained by the physician.
…and what the testing revealed
Final diagnoses included pulmonary hypertension/borderline pulmonary hyper-tension (>30 mm Hg) (42%), congestive heart failure (29%), idiopathic edema (27%), venous insufficiency (22%), medication use (15%, primarily corticosteroids and NSAIDs), proteinuria >1 g/day (15%), and other causes (2% each). These other causes included transient renal disease, hypoalbuminemia, lymphedema, and stenosis of the inferior vena cava. (Total percentages again exceed 100% because some patients had multiple diagnoses.)
All 15 patients with cardiac conditions and 17 of 19 patients with pulmonary hypertension were over 45 years of age. Of the 19 patients with pulmonary hypertension, 6 had CHF, 4 had chronic obstructive pulmonary disease, 4 had sleep apnea diagnosed in subsequent testing, 1 had an atrial septal defect, and 4 appeared to have primary pulmonary hypertension.
Investigators recommend 3 steps
The investigators recommended 3 steps to evaluate chronic leg edema.
- Stop any potentially causative medicines, such as NSAIDs or calcium-channel blockers.
- Obtain an echocardiogram if the patient is 45 years of age or older.
- Obtain a sleep study if the echo-cardiogram reveals pulmonary hypertension without an apparent cause.
Recommendations from others
The authors of a recent narrative systematic review1 stated that “most patients with chronic leg edema can be assumed to have venous insufficiency, CHF, or cyclic edema, unless another cause is suspected after a history and physical examination.” They also stated that: “pulmonary hypertension and early CHF can both cause leg edema before they become clinically obvious” and reiterate that patients over 45 years of age with edema of unclear cause should have an echocardiogram to rule out pulmonary hypertension.
Evidence-based answers from the Family Physicians Inquiries Network
What are effective treatments for oppositional defiant behaviors in adolescents?
Psychological interventions for the family—such as parenting skills training and behavioral therapy for the child, the parents, or the whole family—reduce conflict behaviors in adolescents with oppositional defiant disorder (ODD) (strength of recommendation [SOR]: C, based on extrapolation from systematic reviews of younger children with ODD and adolescents with conduct disorder).
ODD most commonly does not occur as a solitary diagnosis. When ODD is associated with attention deficit/hyperactivity disorder (ADHD) or other medication-responsive comorbid conditions, medical treatment reduces overall symptoms (SOR: B, based on a meta-analysis of adolescent and younger children with both ODD and ADHD).
Model good parenting skills, educate parents about basic behavioral tools, provide referral as resources allow
Elizabeth A. Rulon, MD
Family Medicine Residency of Idaho, Boise
It can be challenging to distinguish oppositional defiant behaviors from variations of normal development as adolescents try to become “independent” from their parents. However, adolescents may engage in many dangerous risk-taking behaviors during this period, so timely diagnosis and interventions are important. Affected adolescents often have a difficult home life, with parents who may have very poor social support and coping skills. Typically, such parents must be convinced that the oppositional and defiant behaviors are a family problem requiring a family solution with no quick fix. Significant financial barriers to counseling and other resources are also common in many of these families. At a minimum, the family doctor can model good parenting skills in the exam room, educate parents about basic behavioral tools to use when interacting with their adolescents, and provide referral as resources allow.
Evidence summary
No studies specifically evaluate effective treatments for ODD (distinguished by chronic argumentativeness and refusal to comply with adult requests) for adolescent patients. However, there are treatment studies of younger children with ODD and studies of adolescents with the more disruptive behavior problem of conduct disorder (distinguished by a persistent pattern of violating other’s rights, aggression, and illegal acts).
A Clinical Inquiry summarized 8 well-done systematic reviews of ODD treatments of preadolescent children and found improved behavior with parenting interventions and behavioral therapy.1 Each of the systematic reviews assessed multiple randomized controlled trials (RCTs) using a variety of parenting and behavioral therapy interventions. The most rigorous systematic review (which included 16 RCTs), compared group-based parenting skills training with untreated wait-list controls and found decreased aggression, noncompliance, and temper tantrums by children aged 3 to 10 years (total number of subjects not given) by an average effect size of 0.6 to 2.9. (Effect size is the difference between the means of the experimental and control groups expressed in standard deviations. An effect size of 0.2 is considered small, 0.5 is medium, and 0.8 is moderate to large.) Behavioral therapy (cognitive-behavioral therapy, social problem-solving skills training, parent management training), comprising 12 to 25 sessions with either the child alone or with teachers or parents, decreased disruptive or aggressive behaviors by 20% to 30%.
A 2-year case-control study2 of 158 self-referred families with young adolescents (11 to 14 years old) without a formal ODD diagnosis but with reported problem behaviors (defined as smoking, negative engagement in family problem solving, and parental ratings of unpleasant events) found significant improvements (P<.01) with parent-only, teen-only, and parent-teen behavioral interventions for negative engagement behaviors (average of 30% reduction in scores), and with parent and teen interventions for unpleasant events (average of 9% reduction in scores). Interventions comprised 12 weekly 90-minute sessions, with the parent-only group targeting family management practices and communication skills, the teen-only group targeting adolescent self-regulation and pro-social behavior, and the parent-teen group following a structured curriculum.
A meta-analysis3 of 8 RCTs (with a total of 749 children) of various behavioral treatments for conduct disorder and juvenile delinquency among children aged 10 to 17 years found significant reductions in rearrest rates (relative risk [RR]=0.66; 95% confidence interval [CI], 0.44–0.98; number needed to treat [NNT] to prevent 1 rearrest=3.7) and time spent in institutions (mean difference, 51 days) with family and parenting interventions (comprising 1 to 6 months of individual and group parenting training, short and long-term family therapy, and individual and group adolescent interventions).
ODD comorbid with other psychiatric conditions
Approximately half to two-thirds of adolescents with ODD also have ADHD.4 A meta-analysis5 evaluated 28 studies of stimulant medication (methylphenidate, amphetamine, or pemoline) for children with comorbid ADHD and ODD. A total of 683 patients aged 8 to 18 years were included. Stimulants reduced aggression-related behaviors in these children by an effect size of 0.84 for overt aggression and 0.69 for covert aggression. Stimulants typically reduce aggressive behaviors by similar effect sizes when prescribed for children with ADD alone. The study groups did not separate children with ADHD and ODD from those with ADHD and conduct disorder; they also grouped adolescents together with younger children.
An RCT6 of different doses of atomoxetine (Strattera) treatment vs placebo for children ages 8 to 18 (mean age=11) with ADHD alone (N=178) and children with both ADHD and ODD (N=115) found significant effect sizes for atomoxetine in both groups. Two dosages of atomoxetine (1.2 and 1.8 mg/kg/d) produced equivalent effect sizes in the ADHD-only group (0.55 and 0.56); however, the higher dosage had a greater effect size (0.49 vs 0.69) in the group with ODD comorbid with ADHD.
A double-blind crossover RCT7 evaluated divalproex (Depakote) vs placebo for 20 children (aged 10 to 18 years) with explosive temper and mood lability who also met DSM-IV criteria for either ODD or conduct disorder. Patients with significant medical problems, such as bipolar disorder, major depression, or mental retardation, were excluded. Divalproex significantly (P=.003) reduced aggressive behaviors and anger-hostility items by approximately 33% as reported by child, parent, school, and clinician on 2 standardized scales.
Experts say antidepressant medications may be helpful in treating children with conduct disorder and comorbid major depression.8
Recommendations by others
An international consensus statement on ADHD and disruptive behavior disorders (comprising ODD, conduct disorder, and disruptive behavior not otherwise specified) says that psychopharmacologic treatment would not be appropriate for cases of ODD in the absence of psychiatric comorbidity, unless severe aggression or destructive behavior persisted despite attempts at psychosocial interventions of established efficacy.4
1. Farley SE, Adams JS, Lutton ME, Scoville C, Fulkerson RC, Webb AR. What are effective treatments for oppositional and defiant behaviors in preadolescents? J Fam Pract 2005;54:162-165.
2. Dishion TJ, Andrews DW. Preventing escalation of problem behaviors with high-risk young adolescents: immediate and 1-year outcomes. J Consult Clin Psychol 1995;63:538-548.
3. Woolfenden SR, Williams K, Peat JK. Family and parenting interventions for conduct disorder and delinquency: a meta-analysis of randomized controlled trials. Arch Dis Child 2002;86:251-256.
4. Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol 2004;14:11-28.
5. Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH, Jr. Psychopharmacology and aggression. I: a meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry 2002;41:253-261.
6. Newcorn JH, Spencer TJ, Biederman J, Milton DR, Michelson D. Atomoxetine treatment in children and adolescents with attention deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 2005;44:240-248.
7. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000;157:818-820.
8. Searight HR, Rottneck F, Abby SL. Conduct disorder: diagnosis and treatment in primary care. Am Fam Physician 2001;63:1579-1592.
Psychological interventions for the family—such as parenting skills training and behavioral therapy for the child, the parents, or the whole family—reduce conflict behaviors in adolescents with oppositional defiant disorder (ODD) (strength of recommendation [SOR]: C, based on extrapolation from systematic reviews of younger children with ODD and adolescents with conduct disorder).
ODD most commonly does not occur as a solitary diagnosis. When ODD is associated with attention deficit/hyperactivity disorder (ADHD) or other medication-responsive comorbid conditions, medical treatment reduces overall symptoms (SOR: B, based on a meta-analysis of adolescent and younger children with both ODD and ADHD).
Model good parenting skills, educate parents about basic behavioral tools, provide referral as resources allow
Elizabeth A. Rulon, MD
Family Medicine Residency of Idaho, Boise
It can be challenging to distinguish oppositional defiant behaviors from variations of normal development as adolescents try to become “independent” from their parents. However, adolescents may engage in many dangerous risk-taking behaviors during this period, so timely diagnosis and interventions are important. Affected adolescents often have a difficult home life, with parents who may have very poor social support and coping skills. Typically, such parents must be convinced that the oppositional and defiant behaviors are a family problem requiring a family solution with no quick fix. Significant financial barriers to counseling and other resources are also common in many of these families. At a minimum, the family doctor can model good parenting skills in the exam room, educate parents about basic behavioral tools to use when interacting with their adolescents, and provide referral as resources allow.
Evidence summary
No studies specifically evaluate effective treatments for ODD (distinguished by chronic argumentativeness and refusal to comply with adult requests) for adolescent patients. However, there are treatment studies of younger children with ODD and studies of adolescents with the more disruptive behavior problem of conduct disorder (distinguished by a persistent pattern of violating other’s rights, aggression, and illegal acts).
A Clinical Inquiry summarized 8 well-done systematic reviews of ODD treatments of preadolescent children and found improved behavior with parenting interventions and behavioral therapy.1 Each of the systematic reviews assessed multiple randomized controlled trials (RCTs) using a variety of parenting and behavioral therapy interventions. The most rigorous systematic review (which included 16 RCTs), compared group-based parenting skills training with untreated wait-list controls and found decreased aggression, noncompliance, and temper tantrums by children aged 3 to 10 years (total number of subjects not given) by an average effect size of 0.6 to 2.9. (Effect size is the difference between the means of the experimental and control groups expressed in standard deviations. An effect size of 0.2 is considered small, 0.5 is medium, and 0.8 is moderate to large.) Behavioral therapy (cognitive-behavioral therapy, social problem-solving skills training, parent management training), comprising 12 to 25 sessions with either the child alone or with teachers or parents, decreased disruptive or aggressive behaviors by 20% to 30%.
A 2-year case-control study2 of 158 self-referred families with young adolescents (11 to 14 years old) without a formal ODD diagnosis but with reported problem behaviors (defined as smoking, negative engagement in family problem solving, and parental ratings of unpleasant events) found significant improvements (P<.01) with parent-only, teen-only, and parent-teen behavioral interventions for negative engagement behaviors (average of 30% reduction in scores), and with parent and teen interventions for unpleasant events (average of 9% reduction in scores). Interventions comprised 12 weekly 90-minute sessions, with the parent-only group targeting family management practices and communication skills, the teen-only group targeting adolescent self-regulation and pro-social behavior, and the parent-teen group following a structured curriculum.
A meta-analysis3 of 8 RCTs (with a total of 749 children) of various behavioral treatments for conduct disorder and juvenile delinquency among children aged 10 to 17 years found significant reductions in rearrest rates (relative risk [RR]=0.66; 95% confidence interval [CI], 0.44–0.98; number needed to treat [NNT] to prevent 1 rearrest=3.7) and time spent in institutions (mean difference, 51 days) with family and parenting interventions (comprising 1 to 6 months of individual and group parenting training, short and long-term family therapy, and individual and group adolescent interventions).
ODD comorbid with other psychiatric conditions
Approximately half to two-thirds of adolescents with ODD also have ADHD.4 A meta-analysis5 evaluated 28 studies of stimulant medication (methylphenidate, amphetamine, or pemoline) for children with comorbid ADHD and ODD. A total of 683 patients aged 8 to 18 years were included. Stimulants reduced aggression-related behaviors in these children by an effect size of 0.84 for overt aggression and 0.69 for covert aggression. Stimulants typically reduce aggressive behaviors by similar effect sizes when prescribed for children with ADD alone. The study groups did not separate children with ADHD and ODD from those with ADHD and conduct disorder; they also grouped adolescents together with younger children.
An RCT6 of different doses of atomoxetine (Strattera) treatment vs placebo for children ages 8 to 18 (mean age=11) with ADHD alone (N=178) and children with both ADHD and ODD (N=115) found significant effect sizes for atomoxetine in both groups. Two dosages of atomoxetine (1.2 and 1.8 mg/kg/d) produced equivalent effect sizes in the ADHD-only group (0.55 and 0.56); however, the higher dosage had a greater effect size (0.49 vs 0.69) in the group with ODD comorbid with ADHD.
A double-blind crossover RCT7 evaluated divalproex (Depakote) vs placebo for 20 children (aged 10 to 18 years) with explosive temper and mood lability who also met DSM-IV criteria for either ODD or conduct disorder. Patients with significant medical problems, such as bipolar disorder, major depression, or mental retardation, were excluded. Divalproex significantly (P=.003) reduced aggressive behaviors and anger-hostility items by approximately 33% as reported by child, parent, school, and clinician on 2 standardized scales.
Experts say antidepressant medications may be helpful in treating children with conduct disorder and comorbid major depression.8
Recommendations by others
An international consensus statement on ADHD and disruptive behavior disorders (comprising ODD, conduct disorder, and disruptive behavior not otherwise specified) says that psychopharmacologic treatment would not be appropriate for cases of ODD in the absence of psychiatric comorbidity, unless severe aggression or destructive behavior persisted despite attempts at psychosocial interventions of established efficacy.4
Psychological interventions for the family—such as parenting skills training and behavioral therapy for the child, the parents, or the whole family—reduce conflict behaviors in adolescents with oppositional defiant disorder (ODD) (strength of recommendation [SOR]: C, based on extrapolation from systematic reviews of younger children with ODD and adolescents with conduct disorder).
ODD most commonly does not occur as a solitary diagnosis. When ODD is associated with attention deficit/hyperactivity disorder (ADHD) or other medication-responsive comorbid conditions, medical treatment reduces overall symptoms (SOR: B, based on a meta-analysis of adolescent and younger children with both ODD and ADHD).
Model good parenting skills, educate parents about basic behavioral tools, provide referral as resources allow
Elizabeth A. Rulon, MD
Family Medicine Residency of Idaho, Boise
It can be challenging to distinguish oppositional defiant behaviors from variations of normal development as adolescents try to become “independent” from their parents. However, adolescents may engage in many dangerous risk-taking behaviors during this period, so timely diagnosis and interventions are important. Affected adolescents often have a difficult home life, with parents who may have very poor social support and coping skills. Typically, such parents must be convinced that the oppositional and defiant behaviors are a family problem requiring a family solution with no quick fix. Significant financial barriers to counseling and other resources are also common in many of these families. At a minimum, the family doctor can model good parenting skills in the exam room, educate parents about basic behavioral tools to use when interacting with their adolescents, and provide referral as resources allow.
Evidence summary
No studies specifically evaluate effective treatments for ODD (distinguished by chronic argumentativeness and refusal to comply with adult requests) for adolescent patients. However, there are treatment studies of younger children with ODD and studies of adolescents with the more disruptive behavior problem of conduct disorder (distinguished by a persistent pattern of violating other’s rights, aggression, and illegal acts).
A Clinical Inquiry summarized 8 well-done systematic reviews of ODD treatments of preadolescent children and found improved behavior with parenting interventions and behavioral therapy.1 Each of the systematic reviews assessed multiple randomized controlled trials (RCTs) using a variety of parenting and behavioral therapy interventions. The most rigorous systematic review (which included 16 RCTs), compared group-based parenting skills training with untreated wait-list controls and found decreased aggression, noncompliance, and temper tantrums by children aged 3 to 10 years (total number of subjects not given) by an average effect size of 0.6 to 2.9. (Effect size is the difference between the means of the experimental and control groups expressed in standard deviations. An effect size of 0.2 is considered small, 0.5 is medium, and 0.8 is moderate to large.) Behavioral therapy (cognitive-behavioral therapy, social problem-solving skills training, parent management training), comprising 12 to 25 sessions with either the child alone or with teachers or parents, decreased disruptive or aggressive behaviors by 20% to 30%.
A 2-year case-control study2 of 158 self-referred families with young adolescents (11 to 14 years old) without a formal ODD diagnosis but with reported problem behaviors (defined as smoking, negative engagement in family problem solving, and parental ratings of unpleasant events) found significant improvements (P<.01) with parent-only, teen-only, and parent-teen behavioral interventions for negative engagement behaviors (average of 30% reduction in scores), and with parent and teen interventions for unpleasant events (average of 9% reduction in scores). Interventions comprised 12 weekly 90-minute sessions, with the parent-only group targeting family management practices and communication skills, the teen-only group targeting adolescent self-regulation and pro-social behavior, and the parent-teen group following a structured curriculum.
A meta-analysis3 of 8 RCTs (with a total of 749 children) of various behavioral treatments for conduct disorder and juvenile delinquency among children aged 10 to 17 years found significant reductions in rearrest rates (relative risk [RR]=0.66; 95% confidence interval [CI], 0.44–0.98; number needed to treat [NNT] to prevent 1 rearrest=3.7) and time spent in institutions (mean difference, 51 days) with family and parenting interventions (comprising 1 to 6 months of individual and group parenting training, short and long-term family therapy, and individual and group adolescent interventions).
ODD comorbid with other psychiatric conditions
Approximately half to two-thirds of adolescents with ODD also have ADHD.4 A meta-analysis5 evaluated 28 studies of stimulant medication (methylphenidate, amphetamine, or pemoline) for children with comorbid ADHD and ODD. A total of 683 patients aged 8 to 18 years were included. Stimulants reduced aggression-related behaviors in these children by an effect size of 0.84 for overt aggression and 0.69 for covert aggression. Stimulants typically reduce aggressive behaviors by similar effect sizes when prescribed for children with ADD alone. The study groups did not separate children with ADHD and ODD from those with ADHD and conduct disorder; they also grouped adolescents together with younger children.
An RCT6 of different doses of atomoxetine (Strattera) treatment vs placebo for children ages 8 to 18 (mean age=11) with ADHD alone (N=178) and children with both ADHD and ODD (N=115) found significant effect sizes for atomoxetine in both groups. Two dosages of atomoxetine (1.2 and 1.8 mg/kg/d) produced equivalent effect sizes in the ADHD-only group (0.55 and 0.56); however, the higher dosage had a greater effect size (0.49 vs 0.69) in the group with ODD comorbid with ADHD.
A double-blind crossover RCT7 evaluated divalproex (Depakote) vs placebo for 20 children (aged 10 to 18 years) with explosive temper and mood lability who also met DSM-IV criteria for either ODD or conduct disorder. Patients with significant medical problems, such as bipolar disorder, major depression, or mental retardation, were excluded. Divalproex significantly (P=.003) reduced aggressive behaviors and anger-hostility items by approximately 33% as reported by child, parent, school, and clinician on 2 standardized scales.
Experts say antidepressant medications may be helpful in treating children with conduct disorder and comorbid major depression.8
Recommendations by others
An international consensus statement on ADHD and disruptive behavior disorders (comprising ODD, conduct disorder, and disruptive behavior not otherwise specified) says that psychopharmacologic treatment would not be appropriate for cases of ODD in the absence of psychiatric comorbidity, unless severe aggression or destructive behavior persisted despite attempts at psychosocial interventions of established efficacy.4
1. Farley SE, Adams JS, Lutton ME, Scoville C, Fulkerson RC, Webb AR. What are effective treatments for oppositional and defiant behaviors in preadolescents? J Fam Pract 2005;54:162-165.
2. Dishion TJ, Andrews DW. Preventing escalation of problem behaviors with high-risk young adolescents: immediate and 1-year outcomes. J Consult Clin Psychol 1995;63:538-548.
3. Woolfenden SR, Williams K, Peat JK. Family and parenting interventions for conduct disorder and delinquency: a meta-analysis of randomized controlled trials. Arch Dis Child 2002;86:251-256.
4. Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol 2004;14:11-28.
5. Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH, Jr. Psychopharmacology and aggression. I: a meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry 2002;41:253-261.
6. Newcorn JH, Spencer TJ, Biederman J, Milton DR, Michelson D. Atomoxetine treatment in children and adolescents with attention deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 2005;44:240-248.
7. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000;157:818-820.
8. Searight HR, Rottneck F, Abby SL. Conduct disorder: diagnosis and treatment in primary care. Am Fam Physician 2001;63:1579-1592.
1. Farley SE, Adams JS, Lutton ME, Scoville C, Fulkerson RC, Webb AR. What are effective treatments for oppositional and defiant behaviors in preadolescents? J Fam Pract 2005;54:162-165.
2. Dishion TJ, Andrews DW. Preventing escalation of problem behaviors with high-risk young adolescents: immediate and 1-year outcomes. J Consult Clin Psychol 1995;63:538-548.
3. Woolfenden SR, Williams K, Peat JK. Family and parenting interventions for conduct disorder and delinquency: a meta-analysis of randomized controlled trials. Arch Dis Child 2002;86:251-256.
4. Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol 2004;14:11-28.
5. Connor DF, Glatt SJ, Lopez ID, Jackson D, Melloni RH, Jr. Psychopharmacology and aggression. I: a meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry 2002;41:253-261.
6. Newcorn JH, Spencer TJ, Biederman J, Milton DR, Michelson D. Atomoxetine treatment in children and adolescents with attention deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 2005;44:240-248.
7. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000;157:818-820.
8. Searight HR, Rottneck F, Abby SL. Conduct disorder: diagnosis and treatment in primary care. Am Fam Physician 2001;63:1579-1592.
Evidence-based answers from the Family Physicians Inquiries Network
Can patients with steatohepatitis take statins?
Patients with steatohepatitis who take HMG Co-A reductase inhibitors (statins) lower their elevated liver enzymes and show evidence of improvement in fatty liver on follow-up imaging (strength of recommendation [SOR]: C, based on very small, short-term prospective studies).
Statins do not further increase transaminase levels for patients with pre-existing transaminase elevations (SOR: B, based on 2 retrospective cohort studies). However, for patients with decompensated liver disease or advanced cirrhosis, balance the benefits of statins against the risks (SOR: C, based on expert opinion).
Remain cautious in prescribing statins for those with nonalcoholic steatohepatitis
Robert C. Oh, MD, MPH
Department of Family Medicine, Tripler Army Medical Center, Honolulu, Hawaii
It is encouraging to see that statins may not worsen nonalcoholic steatohepatitis (NASH) and can potentially improve the process. However, these conclusions are supported by small clinical trials, and clinicians should remain cautious in prescribing statins for patients with NASH.
Importantly, if liver enzyme elevations are revealed during baseline examinations, consider statins only if a systematic work-up is unrevealing and suggests only NASH.7-9 However, I generally avoid statins for those with more than mild to moderate elevations (greater than 100). Before starting statins, I inform patients of the small but potential risk of worsening hepatotoxicity and the importance of close follow-up. If the patient is agreeable, obtaining hepatic enzymes after each statin dose change and periodically after cholesterol goals are achieved is integral in the successful management of the NASH patient requiring statin therapy.
Evidence summary
A prospective study1 evaluated 5 patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) who took 20 mg of pravastatin daily for 6 months. Liver enzyme levels at baseline were no more than 3 times the upper limit of normal. All 5 patients had normalized liver enzymes at the end of the study.
A 6-month unblinded study2 found similar results among 44 adult patients with biopsy-confirmed NASH. Twenty-seven hyperlipidemic patients (aged 50±1.4 years) with an average alanine aminotransferase (ALT) of 81.8 U/L took 10 mg of atorvastatin daily. Seventeen normolipidemic patients (aged 43.7±1.8 years) with an average ALT of 76.0 U/L took ursodeoxycholic acid (UDCA) 13–15 mg/kg/d for the same duration; 59% of atorvastatin-treated patients normalized liver enzyme levels compared with 23% in the UDCA group. On computed tomography scanning, both groups showed improvement in liver densities, suggesting improvement of fatty liver.2
Another study3 included patients with biopsy-confirmed fatty liver and elevated ALT levels greater than 1.5 times the upper limit of normal. In this 24-week study, 23 predominantly hypertriglyceridemic patients took omega-3 fatty acids, 5 mL 3 times daily, 28 hypercholesterolemic patients took atorvastatin 20 mg daily, and 21 dyslipidemic patients with a body mass index >27.0 took orlistat 120 mg 3 times daily. ALT levels decreased in all 3 groups during the study. Ultrasonography showed normal liver echo pattern at the end of treatment for 35% of omega-3 patients, 61% of atorvastatin patients, and 86% of orlistat patients. No serious adverse events were observed.
Two retrospective studies of patients with baseline elevated transaminases who took statins showed no significant increase in transaminase levels during treatment compared with patients with elevated transaminases who did not take statins. One study4 reviewed electronic medical records for patients with preexisting elevated liver enzymes who initiated statin therapy (atorvastatin, simvastatin, or fluvastatin) and had follow-up labs drawn 6 months later (cohort 1, n=342). The comparison groups included patients with normal liver enzymes who initiated statins (cohort 2, n=1437) and patients with elevated baseline liver enzymes who did not take statins (cohort 3, n=2245). At follow-up, 4.7% of cohort 1 patients had mild-to-moderate elevations in liver enzymes, which did not differ significantly (P=.2) from those in cohort 3. Within cohort 2, 1.9% experienced mild-to-moderate elevations of transaminases (defined as less than 10 times the upper limit of normal).
Another retrospective cohort study5 of patients with preexisting elevated liver enzymes found comparable results with lovastatin. Among lovastatin patients (n=135), 6.6% had mild-to-moderate elevations in transaminases during therapy vs 11% of the cohort of patients with preexisting elevated liver enzymes who did not take statins. This difference was not statistically significant (P=.2).
Recommendations from others
The National Cholesterol Education Project6 states that “the incidence of clinically important transaminase elevations in the large statin trials is the same for statins as for placebo. Progression to liver failure is exceedingly rare, if it occurs.” They further state that the use of statins for persons with decompensated liver disease or advanced cirrhosis depends on clinical judgment, but that their use in NASH is considered safe.
The FDA states that statins are contraindicated in cholestasis and active liver disease, and that statins should be discontinued when liver enzymes increase to 3 times the upper limits of normal.
1. Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004;174:193-196.
2. Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 2003;17:713-718.
3. Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 2004;23:131-134.
4. Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patient with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287-1292.
5. Vuppalanchi R, Teal E, Chalasani N. Patient with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from Lovastatin than those with normal baseline liver enzymes. Am J Med Sci 2005;329:62-65.
6. National Heart, Lung and Blood Institute. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, Md: US Department of Health and Human Services, Public Health Service; 2001 May.
7. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician 2005;71:1105-1110.
8. American Gastroenterological Association. Medical position statement: evaluation of liver chemistry tests. Gastroenterology 2002;123:1364-1366.
9. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266-1271.
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Patients with steatohepatitis who take HMG Co-A reductase inhibitors (statins) lower their elevated liver enzymes and show evidence of improvement in fatty liver on follow-up imaging (strength of recommendation [SOR]: C, based on very small, short-term prospective studies).
Statins do not further increase transaminase levels for patients with pre-existing transaminase elevations (SOR: B, based on 2 retrospective cohort studies). However, for patients with decompensated liver disease or advanced cirrhosis, balance the benefits of statins against the risks (SOR: C, based on expert opinion).
Remain cautious in prescribing statins for those with nonalcoholic steatohepatitis
Robert C. Oh, MD, MPH
Department of Family Medicine, Tripler Army Medical Center, Honolulu, Hawaii
It is encouraging to see that statins may not worsen nonalcoholic steatohepatitis (NASH) and can potentially improve the process. However, these conclusions are supported by small clinical trials, and clinicians should remain cautious in prescribing statins for patients with NASH.
Importantly, if liver enzyme elevations are revealed during baseline examinations, consider statins only if a systematic work-up is unrevealing and suggests only NASH.7-9 However, I generally avoid statins for those with more than mild to moderate elevations (greater than 100). Before starting statins, I inform patients of the small but potential risk of worsening hepatotoxicity and the importance of close follow-up. If the patient is agreeable, obtaining hepatic enzymes after each statin dose change and periodically after cholesterol goals are achieved is integral in the successful management of the NASH patient requiring statin therapy.
Evidence summary
A prospective study1 evaluated 5 patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) who took 20 mg of pravastatin daily for 6 months. Liver enzyme levels at baseline were no more than 3 times the upper limit of normal. All 5 patients had normalized liver enzymes at the end of the study.
A 6-month unblinded study2 found similar results among 44 adult patients with biopsy-confirmed NASH. Twenty-seven hyperlipidemic patients (aged 50±1.4 years) with an average alanine aminotransferase (ALT) of 81.8 U/L took 10 mg of atorvastatin daily. Seventeen normolipidemic patients (aged 43.7±1.8 years) with an average ALT of 76.0 U/L took ursodeoxycholic acid (UDCA) 13–15 mg/kg/d for the same duration; 59% of atorvastatin-treated patients normalized liver enzyme levels compared with 23% in the UDCA group. On computed tomography scanning, both groups showed improvement in liver densities, suggesting improvement of fatty liver.2
Another study3 included patients with biopsy-confirmed fatty liver and elevated ALT levels greater than 1.5 times the upper limit of normal. In this 24-week study, 23 predominantly hypertriglyceridemic patients took omega-3 fatty acids, 5 mL 3 times daily, 28 hypercholesterolemic patients took atorvastatin 20 mg daily, and 21 dyslipidemic patients with a body mass index >27.0 took orlistat 120 mg 3 times daily. ALT levels decreased in all 3 groups during the study. Ultrasonography showed normal liver echo pattern at the end of treatment for 35% of omega-3 patients, 61% of atorvastatin patients, and 86% of orlistat patients. No serious adverse events were observed.
Two retrospective studies of patients with baseline elevated transaminases who took statins showed no significant increase in transaminase levels during treatment compared with patients with elevated transaminases who did not take statins. One study4 reviewed electronic medical records for patients with preexisting elevated liver enzymes who initiated statin therapy (atorvastatin, simvastatin, or fluvastatin) and had follow-up labs drawn 6 months later (cohort 1, n=342). The comparison groups included patients with normal liver enzymes who initiated statins (cohort 2, n=1437) and patients with elevated baseline liver enzymes who did not take statins (cohort 3, n=2245). At follow-up, 4.7% of cohort 1 patients had mild-to-moderate elevations in liver enzymes, which did not differ significantly (P=.2) from those in cohort 3. Within cohort 2, 1.9% experienced mild-to-moderate elevations of transaminases (defined as less than 10 times the upper limit of normal).
Another retrospective cohort study5 of patients with preexisting elevated liver enzymes found comparable results with lovastatin. Among lovastatin patients (n=135), 6.6% had mild-to-moderate elevations in transaminases during therapy vs 11% of the cohort of patients with preexisting elevated liver enzymes who did not take statins. This difference was not statistically significant (P=.2).
Recommendations from others
The National Cholesterol Education Project6 states that “the incidence of clinically important transaminase elevations in the large statin trials is the same for statins as for placebo. Progression to liver failure is exceedingly rare, if it occurs.” They further state that the use of statins for persons with decompensated liver disease or advanced cirrhosis depends on clinical judgment, but that their use in NASH is considered safe.
The FDA states that statins are contraindicated in cholestasis and active liver disease, and that statins should be discontinued when liver enzymes increase to 3 times the upper limits of normal.
Patients with steatohepatitis who take HMG Co-A reductase inhibitors (statins) lower their elevated liver enzymes and show evidence of improvement in fatty liver on follow-up imaging (strength of recommendation [SOR]: C, based on very small, short-term prospective studies).
Statins do not further increase transaminase levels for patients with pre-existing transaminase elevations (SOR: B, based on 2 retrospective cohort studies). However, for patients with decompensated liver disease or advanced cirrhosis, balance the benefits of statins against the risks (SOR: C, based on expert opinion).
Remain cautious in prescribing statins for those with nonalcoholic steatohepatitis
Robert C. Oh, MD, MPH
Department of Family Medicine, Tripler Army Medical Center, Honolulu, Hawaii
It is encouraging to see that statins may not worsen nonalcoholic steatohepatitis (NASH) and can potentially improve the process. However, these conclusions are supported by small clinical trials, and clinicians should remain cautious in prescribing statins for patients with NASH.
Importantly, if liver enzyme elevations are revealed during baseline examinations, consider statins only if a systematic work-up is unrevealing and suggests only NASH.7-9 However, I generally avoid statins for those with more than mild to moderate elevations (greater than 100). Before starting statins, I inform patients of the small but potential risk of worsening hepatotoxicity and the importance of close follow-up. If the patient is agreeable, obtaining hepatic enzymes after each statin dose change and periodically after cholesterol goals are achieved is integral in the successful management of the NASH patient requiring statin therapy.
Evidence summary
A prospective study1 evaluated 5 patients with biopsy-confirmed nonalcoholic steatohepatitis (NASH) who took 20 mg of pravastatin daily for 6 months. Liver enzyme levels at baseline were no more than 3 times the upper limit of normal. All 5 patients had normalized liver enzymes at the end of the study.
A 6-month unblinded study2 found similar results among 44 adult patients with biopsy-confirmed NASH. Twenty-seven hyperlipidemic patients (aged 50±1.4 years) with an average alanine aminotransferase (ALT) of 81.8 U/L took 10 mg of atorvastatin daily. Seventeen normolipidemic patients (aged 43.7±1.8 years) with an average ALT of 76.0 U/L took ursodeoxycholic acid (UDCA) 13–15 mg/kg/d for the same duration; 59% of atorvastatin-treated patients normalized liver enzyme levels compared with 23% in the UDCA group. On computed tomography scanning, both groups showed improvement in liver densities, suggesting improvement of fatty liver.2
Another study3 included patients with biopsy-confirmed fatty liver and elevated ALT levels greater than 1.5 times the upper limit of normal. In this 24-week study, 23 predominantly hypertriglyceridemic patients took omega-3 fatty acids, 5 mL 3 times daily, 28 hypercholesterolemic patients took atorvastatin 20 mg daily, and 21 dyslipidemic patients with a body mass index >27.0 took orlistat 120 mg 3 times daily. ALT levels decreased in all 3 groups during the study. Ultrasonography showed normal liver echo pattern at the end of treatment for 35% of omega-3 patients, 61% of atorvastatin patients, and 86% of orlistat patients. No serious adverse events were observed.
Two retrospective studies of patients with baseline elevated transaminases who took statins showed no significant increase in transaminase levels during treatment compared with patients with elevated transaminases who did not take statins. One study4 reviewed electronic medical records for patients with preexisting elevated liver enzymes who initiated statin therapy (atorvastatin, simvastatin, or fluvastatin) and had follow-up labs drawn 6 months later (cohort 1, n=342). The comparison groups included patients with normal liver enzymes who initiated statins (cohort 2, n=1437) and patients with elevated baseline liver enzymes who did not take statins (cohort 3, n=2245). At follow-up, 4.7% of cohort 1 patients had mild-to-moderate elevations in liver enzymes, which did not differ significantly (P=.2) from those in cohort 3. Within cohort 2, 1.9% experienced mild-to-moderate elevations of transaminases (defined as less than 10 times the upper limit of normal).
Another retrospective cohort study5 of patients with preexisting elevated liver enzymes found comparable results with lovastatin. Among lovastatin patients (n=135), 6.6% had mild-to-moderate elevations in transaminases during therapy vs 11% of the cohort of patients with preexisting elevated liver enzymes who did not take statins. This difference was not statistically significant (P=.2).
Recommendations from others
The National Cholesterol Education Project6 states that “the incidence of clinically important transaminase elevations in the large statin trials is the same for statins as for placebo. Progression to liver failure is exceedingly rare, if it occurs.” They further state that the use of statins for persons with decompensated liver disease or advanced cirrhosis depends on clinical judgment, but that their use in NASH is considered safe.
The FDA states that statins are contraindicated in cholestasis and active liver disease, and that statins should be discontinued when liver enzymes increase to 3 times the upper limits of normal.
1. Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004;174:193-196.
2. Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 2003;17:713-718.
3. Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 2004;23:131-134.
4. Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patient with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287-1292.
5. Vuppalanchi R, Teal E, Chalasani N. Patient with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from Lovastatin than those with normal baseline liver enzymes. Am J Med Sci 2005;329:62-65.
6. National Heart, Lung and Blood Institute. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, Md: US Department of Health and Human Services, Public Health Service; 2001 May.
7. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician 2005;71:1105-1110.
8. American Gastroenterological Association. Medical position statement: evaluation of liver chemistry tests. Gastroenterology 2002;123:1364-1366.
9. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266-1271.
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
1. Rallidis LS, Drakoulis CK, Parasi AS. Pravastatin in patients with nonalcoholic steatohepatitis: results of a pilot study. Atherosclerosis 2004;174:193-196.
2. Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol 2003;17:713-718.
3. Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 2004;23:131-134.
4. Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patient with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287-1292.
5. Vuppalanchi R, Teal E, Chalasani N. Patient with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from Lovastatin than those with normal baseline liver enzymes. Am J Med Sci 2005;329:62-65.
6. National Heart, Lung and Blood Institute. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, Md: US Department of Health and Human Services, Public Health Service; 2001 May.
7. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician 2005;71:1105-1110.
8. American Gastroenterological Association. Medical position statement: evaluation of liver chemistry tests. Gastroenterology 2002;123:1364-1366.
9. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000;342:1266-1271.
The views expressed in this article are those of the author(s) and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Evidence-based answers from the Family Physicians Inquiries Network
How can we best treat and monitor VTE during pregnancy?
Unfractionated heparin and low-molecular-weight heparin are equally effective for the treatment of acute venous thromboembolism (VTE) in pregnancy (strength of recommendation [SOR]: C; based on expert opinion and 1 low-power cohort study). Low-molecular-weight heparin may be associated with fewer bleeding events than unfractionated heparin (SOR: B; extrapolated from a randomized controlled trial of thromboprophylaxis in pregnancy).
Unfractionated heparin for treatment of VTE should be given by IV bolus followed by continuous infusion, maintaining the activated partial thromboplastin time (aPTT) in therapeutic range for at least 5 days, followed by subcutaneous heparin 2 or 3 times daily to maintain aPTT levels 1.5 to 2.5 times normal for at least 3 months (SOR: C, expert opinion). Low-molecular-weight heparin should be initially dosed based on weight as for nonpregnant patients, then adjusted to goal peak antifactor Xa levels of 0.5–1.2 IU/mL (SOR: C; expert opinion). The US Food and Drug Administration has labeled warfarin as category X, indicating that it is contraindicated during pregnancy due to fetal loss and probable teratogenicity.
Safety is most important when treating pregnant women
Linda French, MD, FAAFP
Michigan State University, East Lansing
We have enough evidence to conclude that unfractionated heparin and low-molecular-weight heparin are both effective treatments for acute VTE in pregnant women. Unfortunately, we don’t know whether 1 treatment is safer or more effective than the other. The safety issue is the most important consideration in treating pregnant women. A large number of patients would need to be studied to identify a small but significant difference between the 2. We as clinicians would want to know if 1 therapy had even a slightly increased risk of a catastrophic harm. Clinical experience is not enough to tell us that; we need more research.
Evidence summary
Pulmonary embolism remains one of the leading causes of maternal mortality in developed nations. For nonpregnant populations, low-molecular-weight heparin has equal efficacy as unfractionated heparin with a lower overall mortality.1,2
The only direct comparison of unfractionated with low-molecular-weight heparin for treatment of VTE in pregnancy was a prospective cohort study of 31 patients.3 For the initial week of treatment, the unfractionated heparin group received an IV bolus followed by infusion titrated to aPTT levels (goal 70–100s), while lowmolecular-weight heparin group received subcutaneous dalteparin 115 IU/kg twice daily adjusted to target antifactor Xa levels of 1 to 1.5 IU/mL 3 hours after injection. After 7 days, both groups received prophylactic doses of dalteparin throughout the remainder of pregnancy. There were no significant differences in outcome including bleeding or fetal effects. No cases of thrombocytopenia or pulmonary embolus were seen. There was 1 case of progressive thrombosis for a patient on low-molecular-weight heparin.
One randomized controlled trial compared unfractionated with low-molecular-weight heparin for VTE prophylaxis among 107 high-risk pregnant patients.4 The unfractionated heparin group received 7500 IU subcutaneously twice daily adjusted to aPTT levels, while the dalteparin group received weight-adjusted doses to target antifactor Xa levels >0.2 IU/mL at 3 hours. No thromboembolic complications occurred in either group (95% confidence interval, 0 to 2 in both groups). Minor bleeding complications were significantly more common with unfractionated heparin than with low-molecular-weight heparin. Two bleeds requiring transfusion and 2 lumbosacral compression fractures were also observed in the unfractionated heparin group, compared with none in the dalteparin group (difference not statistically significant).
Heparinoid metabolism appears to significantly alter in pregnancy. Several studies of low-molecular-weight heparin for the treatment of VTE in pregnancy used target antifactor Xa levels of 0.5 to 1.5 at 3 hours and found patients often need doses greater than those used for nonpregnant patients.3,5-7 The only study of unfractionated heparin for the treatment of VTE in pregnancy used aPTT levels extrapolated from nonpregnant patients, with a mean heparin dose of 25,430 IU/d, similar to mean doses for nonpregnant patients.3
There are no studies of repeat lower extremity ultrasounds for pregnant patients; however, 1 study of nonpregnant patients revealed proximal extension of deep venous thrombosis despite anticoagulation predicted increased risk of pulmonary embolism.8
Recommendations from others
Both the American College of Obstetrics and Gynecologists9 and the American College of Chest Physicians10 recommends treating acute VTE in pregnancy with either weight-adjusted-dose low-molecular-weight heparin (goal antifactor Xa levels, 0.5–1.2) throughout pregnancy or full-dose intravenous unfractionated heparin, followed by adjusted-dose unfractionated or low-molecular-weight heparin, for the remainder of the pregnancy and at least 6 weeks postpartum.
1 Dolovich LR, Ginsberg JS, Douketis JD, Holbrook AM, Cheah G. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med 2000;160:181-188.
2. Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med 1999;130:800-809.
3. Ulander VM, Stenqvist P, Kaaja R. Treatment of deep venous thrombosis with low-molecular-weight heparin during pregnancy. Thromb Res 2002;106:13-17.
4. Pettila V, Kaaja R, Leinonen P, Ekblad U, Kataja M, Ikkala E. Thromboprophylaxis with low molecular weight heparin (dalteparin) in pregnancy. Thromb Res 1999;96:275-282.
5. Jacobsen AF, Qvigstad E, Sandset PM. Low molecular weight heparin (dalteparin) for the treatment of venous thromboembolism in pregnancy. BJOG 2003;110:139-144.
6. Rodie VA, Thomson AJ, Stewart FM, Quinn AJ, Walker ID, Greer IA. Low molecular weight heparin for the treatment of venous thromboembolism in pregnancy: a case series. BJOG 2002;109:1020-1024.
7. Rowan JA, McLintock C, Taylor RS, North RA. Prophylactic and therapeutic enoxaparin during pregnancy: indications, outcomes and monitoring. Aust N Z J Obstet Gynaecol 2003;43:123-128.
8. Ascher E, Depippo PS, Hingorani A, Yorkovich W, Salles-Cunha S. Does repeat duplex ultrasound for lower extremity deep vein thrombosis influence patient management? Vasc Endovascular Surg 2004;38:525-531.
9. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Thromboembolism in pregnancy. Int J Gynaecol Obstet 2001;75:203-212.
10. Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:627S-644S.
Unfractionated heparin and low-molecular-weight heparin are equally effective for the treatment of acute venous thromboembolism (VTE) in pregnancy (strength of recommendation [SOR]: C; based on expert opinion and 1 low-power cohort study). Low-molecular-weight heparin may be associated with fewer bleeding events than unfractionated heparin (SOR: B; extrapolated from a randomized controlled trial of thromboprophylaxis in pregnancy).
Unfractionated heparin for treatment of VTE should be given by IV bolus followed by continuous infusion, maintaining the activated partial thromboplastin time (aPTT) in therapeutic range for at least 5 days, followed by subcutaneous heparin 2 or 3 times daily to maintain aPTT levels 1.5 to 2.5 times normal for at least 3 months (SOR: C, expert opinion). Low-molecular-weight heparin should be initially dosed based on weight as for nonpregnant patients, then adjusted to goal peak antifactor Xa levels of 0.5–1.2 IU/mL (SOR: C; expert opinion). The US Food and Drug Administration has labeled warfarin as category X, indicating that it is contraindicated during pregnancy due to fetal loss and probable teratogenicity.
Safety is most important when treating pregnant women
Linda French, MD, FAAFP
Michigan State University, East Lansing
We have enough evidence to conclude that unfractionated heparin and low-molecular-weight heparin are both effective treatments for acute VTE in pregnant women. Unfortunately, we don’t know whether 1 treatment is safer or more effective than the other. The safety issue is the most important consideration in treating pregnant women. A large number of patients would need to be studied to identify a small but significant difference between the 2. We as clinicians would want to know if 1 therapy had even a slightly increased risk of a catastrophic harm. Clinical experience is not enough to tell us that; we need more research.
Evidence summary
Pulmonary embolism remains one of the leading causes of maternal mortality in developed nations. For nonpregnant populations, low-molecular-weight heparin has equal efficacy as unfractionated heparin with a lower overall mortality.1,2
The only direct comparison of unfractionated with low-molecular-weight heparin for treatment of VTE in pregnancy was a prospective cohort study of 31 patients.3 For the initial week of treatment, the unfractionated heparin group received an IV bolus followed by infusion titrated to aPTT levels (goal 70–100s), while lowmolecular-weight heparin group received subcutaneous dalteparin 115 IU/kg twice daily adjusted to target antifactor Xa levels of 1 to 1.5 IU/mL 3 hours after injection. After 7 days, both groups received prophylactic doses of dalteparin throughout the remainder of pregnancy. There were no significant differences in outcome including bleeding or fetal effects. No cases of thrombocytopenia or pulmonary embolus were seen. There was 1 case of progressive thrombosis for a patient on low-molecular-weight heparin.
One randomized controlled trial compared unfractionated with low-molecular-weight heparin for VTE prophylaxis among 107 high-risk pregnant patients.4 The unfractionated heparin group received 7500 IU subcutaneously twice daily adjusted to aPTT levels, while the dalteparin group received weight-adjusted doses to target antifactor Xa levels >0.2 IU/mL at 3 hours. No thromboembolic complications occurred in either group (95% confidence interval, 0 to 2 in both groups). Minor bleeding complications were significantly more common with unfractionated heparin than with low-molecular-weight heparin. Two bleeds requiring transfusion and 2 lumbosacral compression fractures were also observed in the unfractionated heparin group, compared with none in the dalteparin group (difference not statistically significant).
Heparinoid metabolism appears to significantly alter in pregnancy. Several studies of low-molecular-weight heparin for the treatment of VTE in pregnancy used target antifactor Xa levels of 0.5 to 1.5 at 3 hours and found patients often need doses greater than those used for nonpregnant patients.3,5-7 The only study of unfractionated heparin for the treatment of VTE in pregnancy used aPTT levels extrapolated from nonpregnant patients, with a mean heparin dose of 25,430 IU/d, similar to mean doses for nonpregnant patients.3
There are no studies of repeat lower extremity ultrasounds for pregnant patients; however, 1 study of nonpregnant patients revealed proximal extension of deep venous thrombosis despite anticoagulation predicted increased risk of pulmonary embolism.8
Recommendations from others
Both the American College of Obstetrics and Gynecologists9 and the American College of Chest Physicians10 recommends treating acute VTE in pregnancy with either weight-adjusted-dose low-molecular-weight heparin (goal antifactor Xa levels, 0.5–1.2) throughout pregnancy or full-dose intravenous unfractionated heparin, followed by adjusted-dose unfractionated or low-molecular-weight heparin, for the remainder of the pregnancy and at least 6 weeks postpartum.
Unfractionated heparin and low-molecular-weight heparin are equally effective for the treatment of acute venous thromboembolism (VTE) in pregnancy (strength of recommendation [SOR]: C; based on expert opinion and 1 low-power cohort study). Low-molecular-weight heparin may be associated with fewer bleeding events than unfractionated heparin (SOR: B; extrapolated from a randomized controlled trial of thromboprophylaxis in pregnancy).
Unfractionated heparin for treatment of VTE should be given by IV bolus followed by continuous infusion, maintaining the activated partial thromboplastin time (aPTT) in therapeutic range for at least 5 days, followed by subcutaneous heparin 2 or 3 times daily to maintain aPTT levels 1.5 to 2.5 times normal for at least 3 months (SOR: C, expert opinion). Low-molecular-weight heparin should be initially dosed based on weight as for nonpregnant patients, then adjusted to goal peak antifactor Xa levels of 0.5–1.2 IU/mL (SOR: C; expert opinion). The US Food and Drug Administration has labeled warfarin as category X, indicating that it is contraindicated during pregnancy due to fetal loss and probable teratogenicity.
Safety is most important when treating pregnant women
Linda French, MD, FAAFP
Michigan State University, East Lansing
We have enough evidence to conclude that unfractionated heparin and low-molecular-weight heparin are both effective treatments for acute VTE in pregnant women. Unfortunately, we don’t know whether 1 treatment is safer or more effective than the other. The safety issue is the most important consideration in treating pregnant women. A large number of patients would need to be studied to identify a small but significant difference between the 2. We as clinicians would want to know if 1 therapy had even a slightly increased risk of a catastrophic harm. Clinical experience is not enough to tell us that; we need more research.
Evidence summary
Pulmonary embolism remains one of the leading causes of maternal mortality in developed nations. For nonpregnant populations, low-molecular-weight heparin has equal efficacy as unfractionated heparin with a lower overall mortality.1,2
The only direct comparison of unfractionated with low-molecular-weight heparin for treatment of VTE in pregnancy was a prospective cohort study of 31 patients.3 For the initial week of treatment, the unfractionated heparin group received an IV bolus followed by infusion titrated to aPTT levels (goal 70–100s), while lowmolecular-weight heparin group received subcutaneous dalteparin 115 IU/kg twice daily adjusted to target antifactor Xa levels of 1 to 1.5 IU/mL 3 hours after injection. After 7 days, both groups received prophylactic doses of dalteparin throughout the remainder of pregnancy. There were no significant differences in outcome including bleeding or fetal effects. No cases of thrombocytopenia or pulmonary embolus were seen. There was 1 case of progressive thrombosis for a patient on low-molecular-weight heparin.
One randomized controlled trial compared unfractionated with low-molecular-weight heparin for VTE prophylaxis among 107 high-risk pregnant patients.4 The unfractionated heparin group received 7500 IU subcutaneously twice daily adjusted to aPTT levels, while the dalteparin group received weight-adjusted doses to target antifactor Xa levels >0.2 IU/mL at 3 hours. No thromboembolic complications occurred in either group (95% confidence interval, 0 to 2 in both groups). Minor bleeding complications were significantly more common with unfractionated heparin than with low-molecular-weight heparin. Two bleeds requiring transfusion and 2 lumbosacral compression fractures were also observed in the unfractionated heparin group, compared with none in the dalteparin group (difference not statistically significant).
Heparinoid metabolism appears to significantly alter in pregnancy. Several studies of low-molecular-weight heparin for the treatment of VTE in pregnancy used target antifactor Xa levels of 0.5 to 1.5 at 3 hours and found patients often need doses greater than those used for nonpregnant patients.3,5-7 The only study of unfractionated heparin for the treatment of VTE in pregnancy used aPTT levels extrapolated from nonpregnant patients, with a mean heparin dose of 25,430 IU/d, similar to mean doses for nonpregnant patients.3
There are no studies of repeat lower extremity ultrasounds for pregnant patients; however, 1 study of nonpregnant patients revealed proximal extension of deep venous thrombosis despite anticoagulation predicted increased risk of pulmonary embolism.8
Recommendations from others
Both the American College of Obstetrics and Gynecologists9 and the American College of Chest Physicians10 recommends treating acute VTE in pregnancy with either weight-adjusted-dose low-molecular-weight heparin (goal antifactor Xa levels, 0.5–1.2) throughout pregnancy or full-dose intravenous unfractionated heparin, followed by adjusted-dose unfractionated or low-molecular-weight heparin, for the remainder of the pregnancy and at least 6 weeks postpartum.
1 Dolovich LR, Ginsberg JS, Douketis JD, Holbrook AM, Cheah G. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med 2000;160:181-188.
2. Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med 1999;130:800-809.
3. Ulander VM, Stenqvist P, Kaaja R. Treatment of deep venous thrombosis with low-molecular-weight heparin during pregnancy. Thromb Res 2002;106:13-17.
4. Pettila V, Kaaja R, Leinonen P, Ekblad U, Kataja M, Ikkala E. Thromboprophylaxis with low molecular weight heparin (dalteparin) in pregnancy. Thromb Res 1999;96:275-282.
5. Jacobsen AF, Qvigstad E, Sandset PM. Low molecular weight heparin (dalteparin) for the treatment of venous thromboembolism in pregnancy. BJOG 2003;110:139-144.
6. Rodie VA, Thomson AJ, Stewart FM, Quinn AJ, Walker ID, Greer IA. Low molecular weight heparin for the treatment of venous thromboembolism in pregnancy: a case series. BJOG 2002;109:1020-1024.
7. Rowan JA, McLintock C, Taylor RS, North RA. Prophylactic and therapeutic enoxaparin during pregnancy: indications, outcomes and monitoring. Aust N Z J Obstet Gynaecol 2003;43:123-128.
8. Ascher E, Depippo PS, Hingorani A, Yorkovich W, Salles-Cunha S. Does repeat duplex ultrasound for lower extremity deep vein thrombosis influence patient management? Vasc Endovascular Surg 2004;38:525-531.
9. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Thromboembolism in pregnancy. Int J Gynaecol Obstet 2001;75:203-212.
10. Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:627S-644S.
1 Dolovich LR, Ginsberg JS, Douketis JD, Holbrook AM, Cheah G. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med 2000;160:181-188.
2. Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med 1999;130:800-809.
3. Ulander VM, Stenqvist P, Kaaja R. Treatment of deep venous thrombosis with low-molecular-weight heparin during pregnancy. Thromb Res 2002;106:13-17.
4. Pettila V, Kaaja R, Leinonen P, Ekblad U, Kataja M, Ikkala E. Thromboprophylaxis with low molecular weight heparin (dalteparin) in pregnancy. Thromb Res 1999;96:275-282.
5. Jacobsen AF, Qvigstad E, Sandset PM. Low molecular weight heparin (dalteparin) for the treatment of venous thromboembolism in pregnancy. BJOG 2003;110:139-144.
6. Rodie VA, Thomson AJ, Stewart FM, Quinn AJ, Walker ID, Greer IA. Low molecular weight heparin for the treatment of venous thromboembolism in pregnancy: a case series. BJOG 2002;109:1020-1024.
7. Rowan JA, McLintock C, Taylor RS, North RA. Prophylactic and therapeutic enoxaparin during pregnancy: indications, outcomes and monitoring. Aust N Z J Obstet Gynaecol 2003;43:123-128.
8. Ascher E, Depippo PS, Hingorani A, Yorkovich W, Salles-Cunha S. Does repeat duplex ultrasound for lower extremity deep vein thrombosis influence patient management? Vasc Endovascular Surg 2004;38:525-531.
9. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin. Thromboembolism in pregnancy. Int J Gynaecol Obstet 2001;75:203-212.
10. Bates SM, Greer IA, Hirsh J, Ginsberg JS. Use of antithrombotic agents during pregnancy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:627S-644S.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best approach for patients with ASCUS detected on Pap smear?
DNA testing for human papillomavirus (HPV), especially if the sample can be obtained at the same time as the Papanicolaou (Pap) smear, can guide the management of women whose test result shows atypical squamous cells of undetermined significance (ASCUS). Those who test positive for high-risk types of HPV should be referred for colposcopy (strength of recommendation [SOR]: B), and those with a negative test result may resume regular Pap testing in 12 months (SOR: B). If HPV testing is unavailable, an alternative strategy is to repeat the Pap smear at 4- to 6-month intervals. After 2 negative Pap smears are obtained, usual screening may resume. But if either of the repeat Pap smears results in ASCUS or worse, the woman should be referred for colposcopy (SOR: B).
Evidence summary
Although only 5% to 10% of women with the result of ASCUS on a Pap smear have a high-grade squamous intraepithelial lesion (HSIL), estimates suggest that more than one third of these lesions are identified during follow-up to ASCUS Pap smears.1
The recent ASCUS-LSIL Triage Study (ALTS), a multicenter randomized trial, directly addressed the appropriate evaluation of ASCUS.2 The trial compared 3 management strategies for ASCUS Pap smears: reflex HPV-DNA testing (the initial Pap sample is tested for HPV only if the results are ASCUS), immediate referral for colposcopy, and repeat Pap smears. Reflex HPV testing had a sensitivity of 96% for detecting HSIL and a negative predictive value of 98%. The 44% of women with ASCUS who tested negative for high-risk HPV were able to avoid colposcopy. A single repeat Pap smear within 4 to 6 months, with referral for colposcopy if abnormal, had a sensitivity of 85% (sensitivity might be expected to improve with a second repeat test) and a similar colposcopy referral rate.2
A cost-effectiveness analysis that modeled data from the trial found that reflex HPV testing was most cost-effective.3 For women aged 29 years or older, HPV testing resulted in a much lower colposcopy referral rate, 31% vs 65% for younger women, without sacrificing sensitivity.4
Recommendations from others
Evidenced-based guidelines were developed at a consensus conference sponsored by the American Society for Colposcopy and Cervical Pathology in September 2001.5 Recommendations were also made for women with ASCUS in special circumstances. Pregnant women should be managed the same way as nonpregnant women; immunosuppressed women should be referred for colposcopy; and postmenopausal women, who are at a lower risk for HSIL, may try a 3- to 6-week course of intravaginal estrogen followed by repeat Pap smears 1 week after estrogen treatment and again 4 to 6 months later.
If either repeat test is reported as ASCUS or greater, the woman should be referred for colposcopy. Any woman with a Pap smear reported as ASCH (atypical squamous cells, cannot exclude HSIL) should be referred for colposcopy.5
The US Preventive Services Task Force recently concluded that evidence is insufficient to recommend for or against the routine use of HPV testing as a primary screening test for cervical cancer, but they did not address the management of abnormal Pap smears.6
Thin-prep Pap smears can make workup of ASCUS easier for physician and patient
John Hill, MD
University of Colorado Health Sciences Center, Denver
The management of ASCUS Pap smears has often confused primary care doctors. This is confounded by the fact that it is often a challenge to ensure that patients follow our recommendations. How could we blame them—after all, who wants to undergo 4 Pap smears instead of 1? The advent of thin-prep Pap smears, with reflex HPV testing on the same specimen, has simplified our lives. By obtaining routine thin-prep Pap smears and then reflex HPV testing for only high-risk HPV types, fewer Pap smears and colposcopic exams are needed, without reducing the detection of HSIL. Best of all, fewer women are overtreated or lost to follow-up.
1. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999;281:1605-1610.
2. Solomon D, Schiffman M, Tarone R; ALTS Study Group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-299.
3. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA 2002;287:2382-2390.
4. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003;127:946-949.
5. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson. EJ; ASCCP-Sponsored Consensus Conference. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-2129.
6. US Preventive Services Task Force. Screening for cervical cancer: recommendations and rationale. AHRQ Publication No. 03-515A. January 2003. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahcpr.gov/clinic/uspstf/uspscerv.htm. Accessed on January 27, 2004.
DNA testing for human papillomavirus (HPV), especially if the sample can be obtained at the same time as the Papanicolaou (Pap) smear, can guide the management of women whose test result shows atypical squamous cells of undetermined significance (ASCUS). Those who test positive for high-risk types of HPV should be referred for colposcopy (strength of recommendation [SOR]: B), and those with a negative test result may resume regular Pap testing in 12 months (SOR: B). If HPV testing is unavailable, an alternative strategy is to repeat the Pap smear at 4- to 6-month intervals. After 2 negative Pap smears are obtained, usual screening may resume. But if either of the repeat Pap smears results in ASCUS or worse, the woman should be referred for colposcopy (SOR: B).
Evidence summary
Although only 5% to 10% of women with the result of ASCUS on a Pap smear have a high-grade squamous intraepithelial lesion (HSIL), estimates suggest that more than one third of these lesions are identified during follow-up to ASCUS Pap smears.1
The recent ASCUS-LSIL Triage Study (ALTS), a multicenter randomized trial, directly addressed the appropriate evaluation of ASCUS.2 The trial compared 3 management strategies for ASCUS Pap smears: reflex HPV-DNA testing (the initial Pap sample is tested for HPV only if the results are ASCUS), immediate referral for colposcopy, and repeat Pap smears. Reflex HPV testing had a sensitivity of 96% for detecting HSIL and a negative predictive value of 98%. The 44% of women with ASCUS who tested negative for high-risk HPV were able to avoid colposcopy. A single repeat Pap smear within 4 to 6 months, with referral for colposcopy if abnormal, had a sensitivity of 85% (sensitivity might be expected to improve with a second repeat test) and a similar colposcopy referral rate.2
A cost-effectiveness analysis that modeled data from the trial found that reflex HPV testing was most cost-effective.3 For women aged 29 years or older, HPV testing resulted in a much lower colposcopy referral rate, 31% vs 65% for younger women, without sacrificing sensitivity.4
Recommendations from others
Evidenced-based guidelines were developed at a consensus conference sponsored by the American Society for Colposcopy and Cervical Pathology in September 2001.5 Recommendations were also made for women with ASCUS in special circumstances. Pregnant women should be managed the same way as nonpregnant women; immunosuppressed women should be referred for colposcopy; and postmenopausal women, who are at a lower risk for HSIL, may try a 3- to 6-week course of intravaginal estrogen followed by repeat Pap smears 1 week after estrogen treatment and again 4 to 6 months later.
If either repeat test is reported as ASCUS or greater, the woman should be referred for colposcopy. Any woman with a Pap smear reported as ASCH (atypical squamous cells, cannot exclude HSIL) should be referred for colposcopy.5
The US Preventive Services Task Force recently concluded that evidence is insufficient to recommend for or against the routine use of HPV testing as a primary screening test for cervical cancer, but they did not address the management of abnormal Pap smears.6
Thin-prep Pap smears can make workup of ASCUS easier for physician and patient
John Hill, MD
University of Colorado Health Sciences Center, Denver
The management of ASCUS Pap smears has often confused primary care doctors. This is confounded by the fact that it is often a challenge to ensure that patients follow our recommendations. How could we blame them—after all, who wants to undergo 4 Pap smears instead of 1? The advent of thin-prep Pap smears, with reflex HPV testing on the same specimen, has simplified our lives. By obtaining routine thin-prep Pap smears and then reflex HPV testing for only high-risk HPV types, fewer Pap smears and colposcopic exams are needed, without reducing the detection of HSIL. Best of all, fewer women are overtreated or lost to follow-up.
DNA testing for human papillomavirus (HPV), especially if the sample can be obtained at the same time as the Papanicolaou (Pap) smear, can guide the management of women whose test result shows atypical squamous cells of undetermined significance (ASCUS). Those who test positive for high-risk types of HPV should be referred for colposcopy (strength of recommendation [SOR]: B), and those with a negative test result may resume regular Pap testing in 12 months (SOR: B). If HPV testing is unavailable, an alternative strategy is to repeat the Pap smear at 4- to 6-month intervals. After 2 negative Pap smears are obtained, usual screening may resume. But if either of the repeat Pap smears results in ASCUS or worse, the woman should be referred for colposcopy (SOR: B).
Evidence summary
Although only 5% to 10% of women with the result of ASCUS on a Pap smear have a high-grade squamous intraepithelial lesion (HSIL), estimates suggest that more than one third of these lesions are identified during follow-up to ASCUS Pap smears.1
The recent ASCUS-LSIL Triage Study (ALTS), a multicenter randomized trial, directly addressed the appropriate evaluation of ASCUS.2 The trial compared 3 management strategies for ASCUS Pap smears: reflex HPV-DNA testing (the initial Pap sample is tested for HPV only if the results are ASCUS), immediate referral for colposcopy, and repeat Pap smears. Reflex HPV testing had a sensitivity of 96% for detecting HSIL and a negative predictive value of 98%. The 44% of women with ASCUS who tested negative for high-risk HPV were able to avoid colposcopy. A single repeat Pap smear within 4 to 6 months, with referral for colposcopy if abnormal, had a sensitivity of 85% (sensitivity might be expected to improve with a second repeat test) and a similar colposcopy referral rate.2
A cost-effectiveness analysis that modeled data from the trial found that reflex HPV testing was most cost-effective.3 For women aged 29 years or older, HPV testing resulted in a much lower colposcopy referral rate, 31% vs 65% for younger women, without sacrificing sensitivity.4
Recommendations from others
Evidenced-based guidelines were developed at a consensus conference sponsored by the American Society for Colposcopy and Cervical Pathology in September 2001.5 Recommendations were also made for women with ASCUS in special circumstances. Pregnant women should be managed the same way as nonpregnant women; immunosuppressed women should be referred for colposcopy; and postmenopausal women, who are at a lower risk for HSIL, may try a 3- to 6-week course of intravaginal estrogen followed by repeat Pap smears 1 week after estrogen treatment and again 4 to 6 months later.
If either repeat test is reported as ASCUS or greater, the woman should be referred for colposcopy. Any woman with a Pap smear reported as ASCH (atypical squamous cells, cannot exclude HSIL) should be referred for colposcopy.5
The US Preventive Services Task Force recently concluded that evidence is insufficient to recommend for or against the routine use of HPV testing as a primary screening test for cervical cancer, but they did not address the management of abnormal Pap smears.6
Thin-prep Pap smears can make workup of ASCUS easier for physician and patient
John Hill, MD
University of Colorado Health Sciences Center, Denver
The management of ASCUS Pap smears has often confused primary care doctors. This is confounded by the fact that it is often a challenge to ensure that patients follow our recommendations. How could we blame them—after all, who wants to undergo 4 Pap smears instead of 1? The advent of thin-prep Pap smears, with reflex HPV testing on the same specimen, has simplified our lives. By obtaining routine thin-prep Pap smears and then reflex HPV testing for only high-risk HPV types, fewer Pap smears and colposcopic exams are needed, without reducing the detection of HSIL. Best of all, fewer women are overtreated or lost to follow-up.
1. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999;281:1605-1610.
2. Solomon D, Schiffman M, Tarone R; ALTS Study Group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-299.
3. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA 2002;287:2382-2390.
4. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003;127:946-949.
5. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson. EJ; ASCCP-Sponsored Consensus Conference. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-2129.
6. US Preventive Services Task Force. Screening for cervical cancer: recommendations and rationale. AHRQ Publication No. 03-515A. January 2003. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahcpr.gov/clinic/uspstf/uspscerv.htm. Accessed on January 27, 2004.
1. Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999;281:1605-1610.
2. Solomon D, Schiffman M, Tarone R; ALTS Study Group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst 2001;93:293-299.
3. Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA 2002;287:2382-2390.
4. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003;127:946-949.
5. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB. Wilkinson. EJ; ASCCP-Sponsored Consensus Conference. 2001 Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-2129.
6. US Preventive Services Task Force. Screening for cervical cancer: recommendations and rationale. AHRQ Publication No. 03-515A. January 2003. Rockville, Md: Agency for Healthcare Research and Quality. Available at: www.ahcpr.gov/clinic/uspstf/uspscerv.htm. Accessed on January 27, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Which postmenopausal women should be offered combined HRT?
Recent studies have demonstrated a small but significant risk of adverse effects from combined hormone replacement therapy (HRT), including cardiovascular disease, thromboembolic disease, and breast cancer. Time-limited HRT will control intolerable menopausal symptoms and prevent risk of fractures in newly menopausal women. However, HRT achieves its maximum efficacy in 35 years, and the risk of adverse outcomes increases as time progresses. Women considering HRT, particularly those at higher risk for vascular disease and breast cancer, should be informed of the potential risks.
There is inadequate evidence to determine the extent of these risks in women who have had a hysterectomy and are taking unopposed estrogen (strength of recommendation: A, based on large randomized controlled trials).
Evidence summary
The Women’s Health Initiative (WHI),1 the largest randomized trial of HRT, showed that long-term use of HRT poses more risks than benefits for healthy postmenopausal women. WHI studied the use of estrogen plus progestin for prevention of coronary heart disease in 16,608 postmenopausal women age 50–79 years. After 5 years of follow-up, this arm of the study was stopped because of the adverse effects of the intervention. The researchers found that HRT increases the risk of several events:
- coronary heart disease events (number needed to harm [NNH]=1428)
- invasive breast cancer (NNH=1250)
- stroke (NNH=1250)
- venous thromboembolic events (NNH=555)
- pulmonary embolism (NNH=1250).
An ongoing arm of WHI is studying estrogen alone in postmenopausal women who have had a hysterectomy.
The Heart and Estrogen/progestin Replacement Study (HERS)2 examined the effects of HRT in postmenopausal women with coronary artery disease. HERS was a large randomized controlled trial of 2763 women with an average follow-up time of 4.1 years. It showed no statistically significant difference between the HRT (estrogen plus medroxyprog-esterone) group compared with the placebo group in either the primary outcomes (nonfatal myocardial infarction or coronary heart disease death) or in the secondary outcomes (coronary revascularization, unstable angina, congestive heart failure, resuscitated cardiac arrest, stroke or transient ischemic attack, and peripheral arterial disease). The findings of the WHI and HERS trials have been summarized in a recent meta-analysis done for the United States Preventive Services Task Force.3
Both the WHI and HERS trials demonstrated some benefits for HRT. WHI found a reduced risk of colorectal cancer (number needed to treat [NNT]=1667) and a decreased risk of any osteoporotic fracture (NNT=228). The HERS group found that HRT improved the quality of life of women with postmenopausal symptoms, particularly flushing.
Evidence indicates that women who take HRT for 3 years and then stop achieve as much protection from osteoporotic fractures as women who continue their HRT beyond 3 years.4
Continuing HRT beyond 5 years dramatically increases the risk of coronary heart disease, stroke, thromboembolic events, breast cancer, and cholecystitis.3
Recommendations from others
The American College of Obstetricians and Gynecologists has convened a multispecialty panel of experts to draft new recommendations for HRT in light of the WHI findings.
Laura Hansen, PharmD, BCPS
University of Colorado, Boulder
The WHI and HERS trials demonstrated that long-term use of HRT (>5 years) incurs significantly more risks than benefits for a postmenopausal woman who has not undergone hysterectomy. However, these trials did not evaluate postmenopausal symptoms or quality of life as primary endpoints.
Most women experience postmenopausal symptoms for more than 1 year but have resolution of symptoms within a few years after menopause. Since HRT remains the most effective therapy for hot flushes, short-term use of HRT (<5 years) may be offered to women experiencing postmenopausal symptoms.
Physicians may instruct women to attempt HRT discontinuation each year because the duration of symptoms can be variable. Discontinuation should be performed using gradual dose reductions to prevent rapid return of postmenopausal symptoms.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
3. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872-81.
4. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-72.
Recent studies have demonstrated a small but significant risk of adverse effects from combined hormone replacement therapy (HRT), including cardiovascular disease, thromboembolic disease, and breast cancer. Time-limited HRT will control intolerable menopausal symptoms and prevent risk of fractures in newly menopausal women. However, HRT achieves its maximum efficacy in 35 years, and the risk of adverse outcomes increases as time progresses. Women considering HRT, particularly those at higher risk for vascular disease and breast cancer, should be informed of the potential risks.
There is inadequate evidence to determine the extent of these risks in women who have had a hysterectomy and are taking unopposed estrogen (strength of recommendation: A, based on large randomized controlled trials).
Evidence summary
The Women’s Health Initiative (WHI),1 the largest randomized trial of HRT, showed that long-term use of HRT poses more risks than benefits for healthy postmenopausal women. WHI studied the use of estrogen plus progestin for prevention of coronary heart disease in 16,608 postmenopausal women age 50–79 years. After 5 years of follow-up, this arm of the study was stopped because of the adverse effects of the intervention. The researchers found that HRT increases the risk of several events:
- coronary heart disease events (number needed to harm [NNH]=1428)
- invasive breast cancer (NNH=1250)
- stroke (NNH=1250)
- venous thromboembolic events (NNH=555)
- pulmonary embolism (NNH=1250).
An ongoing arm of WHI is studying estrogen alone in postmenopausal women who have had a hysterectomy.
The Heart and Estrogen/progestin Replacement Study (HERS)2 examined the effects of HRT in postmenopausal women with coronary artery disease. HERS was a large randomized controlled trial of 2763 women with an average follow-up time of 4.1 years. It showed no statistically significant difference between the HRT (estrogen plus medroxyprog-esterone) group compared with the placebo group in either the primary outcomes (nonfatal myocardial infarction or coronary heart disease death) or in the secondary outcomes (coronary revascularization, unstable angina, congestive heart failure, resuscitated cardiac arrest, stroke or transient ischemic attack, and peripheral arterial disease). The findings of the WHI and HERS trials have been summarized in a recent meta-analysis done for the United States Preventive Services Task Force.3
Both the WHI and HERS trials demonstrated some benefits for HRT. WHI found a reduced risk of colorectal cancer (number needed to treat [NNT]=1667) and a decreased risk of any osteoporotic fracture (NNT=228). The HERS group found that HRT improved the quality of life of women with postmenopausal symptoms, particularly flushing.
Evidence indicates that women who take HRT for 3 years and then stop achieve as much protection from osteoporotic fractures as women who continue their HRT beyond 3 years.4
Continuing HRT beyond 5 years dramatically increases the risk of coronary heart disease, stroke, thromboembolic events, breast cancer, and cholecystitis.3
Recommendations from others
The American College of Obstetricians and Gynecologists has convened a multispecialty panel of experts to draft new recommendations for HRT in light of the WHI findings.
Laura Hansen, PharmD, BCPS
University of Colorado, Boulder
The WHI and HERS trials demonstrated that long-term use of HRT (>5 years) incurs significantly more risks than benefits for a postmenopausal woman who has not undergone hysterectomy. However, these trials did not evaluate postmenopausal symptoms or quality of life as primary endpoints.
Most women experience postmenopausal symptoms for more than 1 year but have resolution of symptoms within a few years after menopause. Since HRT remains the most effective therapy for hot flushes, short-term use of HRT (<5 years) may be offered to women experiencing postmenopausal symptoms.
Physicians may instruct women to attempt HRT discontinuation each year because the duration of symptoms can be variable. Discontinuation should be performed using gradual dose reductions to prevent rapid return of postmenopausal symptoms.
Recent studies have demonstrated a small but significant risk of adverse effects from combined hormone replacement therapy (HRT), including cardiovascular disease, thromboembolic disease, and breast cancer. Time-limited HRT will control intolerable menopausal symptoms and prevent risk of fractures in newly menopausal women. However, HRT achieves its maximum efficacy in 35 years, and the risk of adverse outcomes increases as time progresses. Women considering HRT, particularly those at higher risk for vascular disease and breast cancer, should be informed of the potential risks.
There is inadequate evidence to determine the extent of these risks in women who have had a hysterectomy and are taking unopposed estrogen (strength of recommendation: A, based on large randomized controlled trials).
Evidence summary
The Women’s Health Initiative (WHI),1 the largest randomized trial of HRT, showed that long-term use of HRT poses more risks than benefits for healthy postmenopausal women. WHI studied the use of estrogen plus progestin for prevention of coronary heart disease in 16,608 postmenopausal women age 50–79 years. After 5 years of follow-up, this arm of the study was stopped because of the adverse effects of the intervention. The researchers found that HRT increases the risk of several events:
- coronary heart disease events (number needed to harm [NNH]=1428)
- invasive breast cancer (NNH=1250)
- stroke (NNH=1250)
- venous thromboembolic events (NNH=555)
- pulmonary embolism (NNH=1250).
An ongoing arm of WHI is studying estrogen alone in postmenopausal women who have had a hysterectomy.
The Heart and Estrogen/progestin Replacement Study (HERS)2 examined the effects of HRT in postmenopausal women with coronary artery disease. HERS was a large randomized controlled trial of 2763 women with an average follow-up time of 4.1 years. It showed no statistically significant difference between the HRT (estrogen plus medroxyprog-esterone) group compared with the placebo group in either the primary outcomes (nonfatal myocardial infarction or coronary heart disease death) or in the secondary outcomes (coronary revascularization, unstable angina, congestive heart failure, resuscitated cardiac arrest, stroke or transient ischemic attack, and peripheral arterial disease). The findings of the WHI and HERS trials have been summarized in a recent meta-analysis done for the United States Preventive Services Task Force.3
Both the WHI and HERS trials demonstrated some benefits for HRT. WHI found a reduced risk of colorectal cancer (number needed to treat [NNT]=1667) and a decreased risk of any osteoporotic fracture (NNT=228). The HERS group found that HRT improved the quality of life of women with postmenopausal symptoms, particularly flushing.
Evidence indicates that women who take HRT for 3 years and then stop achieve as much protection from osteoporotic fractures as women who continue their HRT beyond 3 years.4
Continuing HRT beyond 5 years dramatically increases the risk of coronary heart disease, stroke, thromboembolic events, breast cancer, and cholecystitis.3
Recommendations from others
The American College of Obstetricians and Gynecologists has convened a multispecialty panel of experts to draft new recommendations for HRT in light of the WHI findings.
Laura Hansen, PharmD, BCPS
University of Colorado, Boulder
The WHI and HERS trials demonstrated that long-term use of HRT (>5 years) incurs significantly more risks than benefits for a postmenopausal woman who has not undergone hysterectomy. However, these trials did not evaluate postmenopausal symptoms or quality of life as primary endpoints.
Most women experience postmenopausal symptoms for more than 1 year but have resolution of symptoms within a few years after menopause. Since HRT remains the most effective therapy for hot flushes, short-term use of HRT (<5 years) may be offered to women experiencing postmenopausal symptoms.
Physicians may instruct women to attempt HRT discontinuation each year because the duration of symptoms can be variable. Discontinuation should be performed using gradual dose reductions to prevent rapid return of postmenopausal symptoms.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
3. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872-81.
4. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-72.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
2. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
3. Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002;288:872-81.
4. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med 2002;162:665-72.
Evidence-based answers from the Family Physicians Inquiries Network