User login
In reply: Menopause, vitamin D, and oral health
In Reply: Dr. Mascitelli and colleagues bring up an excellent point regarding the role of vitamin D. Vitamin D deficiency (and insufficiency) is such a widespead problem that it deserves attention in both dental and medical circles, and to be fair, it deserves an article of its own. Low vitamin D has been associated with bone loss and an increased risk for certain cancers and other chronic diseases.1 The literature also suggests that low levels of vitamin D are associated with periodontal disease,2 and that supplementation with vitamin D (and calcium) leads to better periodontal health.3,4 However, since vitamin D supplementation is not a recognized way to treat periodontitis, mentioning it with therapies adjudicated as treatment modalities (such as removal of biofilm, which we stressed in our paper) risks misinterpretation by clinicians less versed in periodontal and dental conditions in general.
Nevertheless, the comment brings to light that medical, dental, and nutritional colleagues are very interested in learning more about the pathophysiologic commonalities in the diseases we treat and in a common postmenopausal patient cohort. Our paper focused more closely on what periodontitis is, and on the more primary etiologic pathophysiology—what common resorptive pathways it shares with osteoporosis in the postmenopausal cohort, and biofilm, the primary etiology of periodontitis. But there is need for more discussion and research into bone development (during childhood and adolescence as well) and the role of nutrition during all stages of life.
- Holick M. Vitamin D deficiency. N Eng J Med 2007; 357:266–281.
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80:108–113.
- Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin d and calcium supplementation effects on chronic periodontitis. J Periodontol 2009; 80:1433–1439.
- Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci 2009; 51:11–20.
In Reply: Dr. Mascitelli and colleagues bring up an excellent point regarding the role of vitamin D. Vitamin D deficiency (and insufficiency) is such a widespead problem that it deserves attention in both dental and medical circles, and to be fair, it deserves an article of its own. Low vitamin D has been associated with bone loss and an increased risk for certain cancers and other chronic diseases.1 The literature also suggests that low levels of vitamin D are associated with periodontal disease,2 and that supplementation with vitamin D (and calcium) leads to better periodontal health.3,4 However, since vitamin D supplementation is not a recognized way to treat periodontitis, mentioning it with therapies adjudicated as treatment modalities (such as removal of biofilm, which we stressed in our paper) risks misinterpretation by clinicians less versed in periodontal and dental conditions in general.
Nevertheless, the comment brings to light that medical, dental, and nutritional colleagues are very interested in learning more about the pathophysiologic commonalities in the diseases we treat and in a common postmenopausal patient cohort. Our paper focused more closely on what periodontitis is, and on the more primary etiologic pathophysiology—what common resorptive pathways it shares with osteoporosis in the postmenopausal cohort, and biofilm, the primary etiology of periodontitis. But there is need for more discussion and research into bone development (during childhood and adolescence as well) and the role of nutrition during all stages of life.
In Reply: Dr. Mascitelli and colleagues bring up an excellent point regarding the role of vitamin D. Vitamin D deficiency (and insufficiency) is such a widespead problem that it deserves attention in both dental and medical circles, and to be fair, it deserves an article of its own. Low vitamin D has been associated with bone loss and an increased risk for certain cancers and other chronic diseases.1 The literature also suggests that low levels of vitamin D are associated with periodontal disease,2 and that supplementation with vitamin D (and calcium) leads to better periodontal health.3,4 However, since vitamin D supplementation is not a recognized way to treat periodontitis, mentioning it with therapies adjudicated as treatment modalities (such as removal of biofilm, which we stressed in our paper) risks misinterpretation by clinicians less versed in periodontal and dental conditions in general.
Nevertheless, the comment brings to light that medical, dental, and nutritional colleagues are very interested in learning more about the pathophysiologic commonalities in the diseases we treat and in a common postmenopausal patient cohort. Our paper focused more closely on what periodontitis is, and on the more primary etiologic pathophysiology—what common resorptive pathways it shares with osteoporosis in the postmenopausal cohort, and biofilm, the primary etiology of periodontitis. But there is need for more discussion and research into bone development (during childhood and adolescence as well) and the role of nutrition during all stages of life.
- Holick M. Vitamin D deficiency. N Eng J Med 2007; 357:266–281.
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80:108–113.
- Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin d and calcium supplementation effects on chronic periodontitis. J Periodontol 2009; 80:1433–1439.
- Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci 2009; 51:11–20.
- Holick M. Vitamin D deficiency. N Eng J Med 2007; 357:266–281.
- Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr 2004; 80:108–113.
- Miley DD, Garcia MN, Hildebolt CF, et al. Cross-sectional study of vitamin d and calcium supplementation effects on chronic periodontitis. J Periodontol 2009; 80:1433–1439.
- Amano Y, Komiyama K, Makishima M. Vitamin D and periodontal disease. J Oral Sci 2009; 51:11–20.
How menopause affects oral health, and what we can do about it
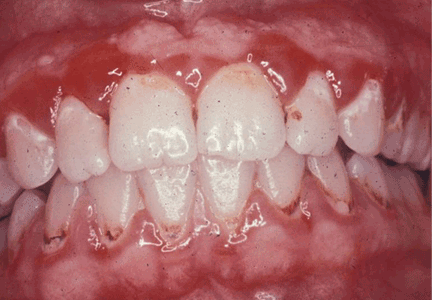
Menopause can bring oral health problems that physicians ought to keep in mind. The same processes that lead to loss of bone in the spine and hips can also lead to loss of the alveolar bone of the jaws, resulting in periodontal disease, loose teeth, and tooth loss. Although the mouth is traditionally the dentist’s responsibility, patients may need encouragement from their physicians to practice good oral hygiene and to see their dentists, and should be referred to a periodontist at the first sign of periodontal disease.
Moreover, bisphosphonates, the class of drugs most often prescribed for osteoporosis, have been linked by case reports (unfairly, we believe) to osteonecrosis of the jaw. This low-evidence-level information, its far-reaching interpretation, and misinformation in the lay media about hormonal changes associated with menopause have led to confusion among women; for clarification and reliable information, they are driven to ask their physicians challenging questions related to oral health.
This article reviews the published studies of the association between menopause and periodontal disease, specifically, the effects of hormonal changes, osteoporosis, and bisphosphonate use on the periodontal status of postmenopausal women. We will highlight the interrelationship of dental health and postmenopausal health and underscore the need for cross-communication and patient referral between physicians and dentists.
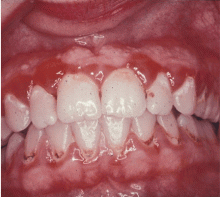
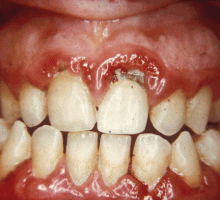
GINGIVITIS CAN PROGRESS TO PERIODONTITIS
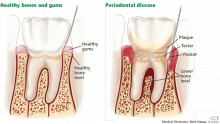
AS ESTROGEN DECLINES, SO DO THE BONES AND, MAYBE, THE TEETH
The rate of bone loss in postmenopausal women predicts tooth loss—for every 1%-per-year decrease in whole-body bone mineral density, the risk of tooth loss increases more than four times.2 In fact, Kribbs3 found that women with severe osteoporosis were three times more likely than healthy, age-matched controls to be edentulous (ie, to have fewer teeth).
Although a number of studies have found that the density of the alveolar bone in the mandible correlated with the density of the bone in the rest of the skeleton and that generalized bone loss may render the jaw susceptible to accelerated alveolar bone resorption,3–11 these findings are not universal. In a longitudinal study, Famili et al12 found no association between systemic bone loss, periodontal disease, and edentulism. This shows that the relationship between alveolar bone loss and systemic bone loss is multifactorial and not yet fully understood.13
Nevertheless, the American Academy of Periodontology considers osteoporosis to be a risk factor for periodontal disease.10 In fact, alveolar bone loss has been related not only to osteoporosis but also to osteopenia.14
Bone mineral density has also been studied in relation to the loss of periodontal ligament—the collagenous attachment of tooth to bone. Klemetti et al15 found that healthy postmenopausal women with high bone mineral density seemed to retain teeth more readily than those with low bone density or those with osteoporosis, even if they had deep periodontal pockets (a sign of periodontal disease). These findings were reiterated when osteoporotic women were found to have significantly greater loss of attachment compared with nonosteoporotic women.7
However, Hildebolt16 reported that loss of tooth attachment correlated with tooth loss but not with the density of the vertebrae or the proximal femur. This study called into question the findings of the previous studies and provoked debate.
Tezal et al17 found that low bone mineral density was related to the loss of interproximal alveolar bone (the alveolar bone between adjacent teeth) and, to a lesser extent, ligamentous attachment loss. These data implicated osteoporosis as a possible risk indicator for periodontal disease in white women. (This study was limited to white women because of different demographics in the incidence of osteoporosis.)
Another study showed only a weak correlation between changes in alveolar bone height (in periodontal disease, bone height decreases) and attachment levels. Although a correlation might be present, the relationship was complex and required further examination. The authors found no clear association between clinical attachment levels and bone mineral density in the lumbar spine, but they recognized that attachment loss often precedes the loss of alveolar bone by a significant time period.13
Several studies have found a possible relationship between the bone density in the jaw and the density in the rest of the skeleton. It appears that loss of bone mineral density in the hip, wrist, and lumbar areas is correlated with low density in the mandible. Taguchi et al18 reported that the density in the lumbar spine correlated with the density of the mandibular cortex in early menopause, and with the density of both the cortex and cancellous bone in later menopause.
But whatever the statistical measurement, the susceptibility to progressive periodontitis increases after menopause, and the primary cause is bacterial plaque. The best hedge against this increased susceptibility is regular dental care to remove bacterial plaque biofilm under the gum-line.
HORMONE THERAPY PRESERVES BONE IN THE JAW
Hormones have long been recognized as having some role in periodontal disease.
Payne et al19 reported that postmenopausal women who were estrogen-deficient had a higher frequency of sites with a net loss of alveolar bone density at follow-up. Furthermore, estrogen-deficient women undergoing supportive periodontal therapy following treatment of moderate to severe periodontitis had three times as many sites losing more than 0.4 mm of interproximal alveolar bone height. Patients who had sufficient estrogen levels did not lose bone during 1 year of follow-up.20
Estrogen replacement improves bone density in postmenopausal women. In a 3-year randomized trial in postmenopausal women with moderate or advanced periodontal disease, estrogen therapy significantly increased alveolar bone mass compared with placebo (P = .04), and it increased bone density in the femur but not the lumbar spine.21 Furthermore, women receiving hormonal therapy had significantly less gingival inflammation, lower plaque scores, and less loss of attachment.
On the other hand, a report by Albandar and Kingman22 suggested that women who comply with hormonal therapy also comply with oral hygiene instructions. This compliance could explain the lower gingival inflammation scores, lower plaque scores, and lesser loss of attachment.
Norderyd et al,23 in a cross-sectional study, found less periodontal disease in postmenopausal women who were on estrogen therapy than in those who were not, although the difference was not statistically significant.
In a 5-year longitudinal study of 69 postmenopausal women receiving estrogen, a moderate but significant relationship was found between bone mineral density of the lumbar spine and the mandible, and estrogen replacement therapy had a positive effect on the mandibular bone mass.24
In a longitudinal study of 24 postmenopausal women, estrogen-deficient women had a mean net loss of alveolar bone density over time, while estrogen-sufficient women had a mean net gain, suggesting that estrogen deficiency may be a risk factor for alveolar bone loss.20 More-recent studies had similar findings. A cross-sectional study by Meisel et al25 found that hormone therapy significantly reduced the extent of clinical attachment loss and, hence, periodontal disease.
The findings of these studies are generally consistent, suggesting that estrogen builds up mandibular bone mass and attenuates the severity of periodontal disease in postmenopausal women.26
DOES ESTROGEN THERAPY PROTECT THE TEETH?
Studies of the Leisure World,27 Framingham,28 and Nurses Health Study29 cohorts suggest that hormone therapy protects against tooth loss in postmenopausal women.
On the other hand, Taguchi et al30 evaluated more than 300 postmenopausal Japanese women and found no significant difference in the total number of teeth between estrogen users and nonusers. The population in this study was younger than in the other studies mentioned above,27–29 which may explain the negative finding. However, the duration of estrogen use was significantly associated with the total number of teeth remaining, independent of age.30 Meisel et al25 reported that women receiving hormonal therapy had more teeth, though the difference was not significant.
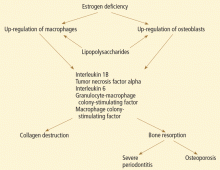
CYTOKINES, PERIODONTITIS, AND SKELETAL BONE LOSS
Studies suggest that low estrogen production after menopause is associated with increased production of interleukin 1 (IL-1), IL-6, IL-8, IL-10, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, which stimulate mature osteoclasts, modulate bone cell proliferation, and induce resorption of both skeletal and alveolar bone.31–34
In this regard, osteoporosis and periodontitis appear to be mediated by common cytokines. Managing osteoporosis, removing bacterial plaque biofilm with good oral hygiene, and regular dental visits are important in avoiding periodontitis in susceptible women.
BISPHOSPHONATES PROTECT BONE
In the skeleton
Bisphosphonates, the most commonly prescribed therapy for osteoporosis, inhibit systemic bone resorption and reduce the incidence of vertebral and nonvertebral fractures. Among the bisphosphonates, alendronate (Fosamax), risedronate (Actonel), and intravenous zoledronic acid (Reclast) have been shown to reduce the risk of both hip and vertebral fractures, whereas ibandronate (Boniva) has only been shown to decrease the risk of vertebral fracture.36 Specific findings:
- In the Fracture Intervention Trial,37 alendronate reduced the risk of vertebral fracture by 47% and hip fracture by 51% in women with low bone mineral density and previous vertebral fractures.
- In the Hip Intervention Program,38 risedronate decreased the risk of hip fracture by 40% in postmenopausal women 70 to 79 years old with osteoporosis, but not in those 80 years and older, who are at high risk of falls. Risedronate also reduced vertebral fracture risk by 49% after 3 years of treatment.39
- In the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Recurrent Fracture Trial,40 annual infusion of zoledronic acid after a hip fracture reduced the rates of new clinical vertebral and nonvertebral fractures and death from all causes.
In the jaw
Not surprisingly, recent studies suggest that bisphosphonates slow the resorption of alveolar bone of the maxilla and mandible as well. Alendronate and risedronate, in particular, have been noted to improve periodontal status.41–43 Findings:
- In a cross-sectional study by Palomo et al,41 postmenopausal women with low bone density using risedronate for at least 3 months showed significantly less plaque accumulation, less gingival inflammation, lower probing-depth measurments, less periodontal attachment loss, and greater alveolar bone levels.
- In a double-blind, controlled, prospective study by Rocha et al,42 6 months of alendronate therapy significantly improved periodontal disease as assessed radiographically and clinically in 40 postmenopausal women with established periodontal disease.
- Jeffcoat et al43 reported that 2 years of alendronate treatment significantly reduced alveolar bone loss relative to placebo in patients with low mandibular bone mineral density at baseline but not in those with normal baseline mandibular bone mineral density.
DO BISPHOSPHONATES CAUSE OSTEONECROSIS OF THE JAW?
The intravenous bisphosphonates most commonly used to treat hypercalcemia of malignancy, multiple myeloma, or metastatic bone disease are47:
- Pamidronate (Aredia) 90 mg infused over 2 to 24 hours every 3 to 4 weeks
- Zoledronic acid (Zometa) 4 mg infused over 15 minutes monthly.
The doses of bisphosphonates indicated for the treatment of osteoporosis are much lower,1 eg:
- Alendronate 70 mg by mouth once a week
- Risedronate 35 mg by mouth once a week or 150 mg once a month
- Ibandronate 150 mg by mouth once a month
- Ibandronate 3 mg intravenously every 3 months
- Zoledronic acid 5 mg intravenously once a year.
Moreover, less than 1% of an oral dose is absorbed by the gastrointestinal tract,49 whereas more than 50% of the dose of bisphosphonates given intravenously is bioavailable,50 which may account for the lower incidence of jaw ostenonecrosis with oral agents.
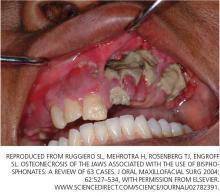
Osteonecrosis of the jaw can occur spontaneously but is more often associated with dental procedures that traumatize bone, such as tooth extraction.51 In a systematic review,45 patients with multiple myeloma and metastatic cancer to the bone who were receiving intravenous bisphosphonates accounted for 94% of published cases. Sixty percent of cases were preceded by dental surgical procedures, and in 39% of cases that occurred spontaneously the lesions were located on bony exostoses, a possible source of trauma. Of 63 cases reported by Ruggiero et al,47 56 patients were receiving intravenous bisphosphonates and 7 were receiving oral bisphosphonates. Older age (> 65 years), chronic systemic steroid use, periodontitis, and prolonged use of bisphosphonates have also been associated with a higher risk of osteonecrosis of the jaw.51
The risk of developing osteonecrosis of the jaw in people taking bisphosphonates in doses recommended by the US Food and Drug Administration for treating osteoporosis is very low (the incidence is calculated at 0.7 per 100,000 person-years of exposure to alendronate).51,52 In a 3-year prospective study in more than 7,000 women with post-menopausal osteoporosis, the incidence of osteonecrosis of the jaw was no different in those treated with zoledronic acid 5 mg intravenously than in those receiving placebo.53 In a randomized, placebo-controlled study of the effect of 2 years of alendronate treatment on alveolar bone loss involving 335 patients with periodontal disease, no cases of osteonecrosis of the jaw were reported.43
The American Dental Association (ADA) released a statement noting that osteonecrosis of the jaw can occur with or without bisphosphonate use.51 To date, a true cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate use has not been established. Further studies are needed to fully explore this relationship. Our group is currently exploring novel periodontal assessments comparing the oral health of postmenopausal women with osteoporosis who are on no bone therapy vs postmenopausal women with osteoporosis treated with bisphosphonates for 2 or more years.
Discussion of treatment for bisphosphonate-associated osteonecrosis of the jaw is beyond the scope of this article.
REGULAR DENTAL CARE IS ESSENTIAL
Regardless of whether the patient is receiving a bisphosphonate drug, physicians caring for postmenopausal women should be vigilant and encourage their patients to seek regular dental evaluation for prevention and early management of oral disorders. Conversely, dentists should be aware of the potential effects of menopause and its treatments on bone and dental health.
Questions from postmenopausal women can be managed, in part, by returning to the basics suggested by the ADA:
- Regular dental examinations; regular professional cleaning to remove bacterial plaque biofilm under the gum-line where a toothbrush will not reach
- Daily oral hygiene practices to remove biofilm at and above the gum-line including brushing twice daily with an ADA-accepted toothpaste
- Replacing the toothbrush every 3 to 4 months (or sooner if the bristles begin to look frayed)
- Cleaning interproximally (between teeth) with floss or interdental cleaner
- Maintaining a balanced diet
- No smoking.
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd ed; 2007.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 1996; 59:433–437.
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222.
- Kribbs PJ, Chesnut CH, Ott SM, Kilcoyne RF. Relationship between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707.
- Kribbs PJ, Chestnut CH, Ott SM, Kilcyne RE. Relationship between mandibular and skeletal bone in a population of normal women. J Prosthet Dent 1990; 63:86–89.
- Kribbs PJ, Smith DE, Chestnut CH. Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724.
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138.
- Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Peridontol 1996; 67(suppl 10):1076–1084.
- Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from the NHANES III. J Clin Periodontol 2000; 27:778–86.
- American Dental Association Council on Access, Prevention and Interprofessional Relations. Women’s Oral Health Issues. November 2006.
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000 2000; 23:94–102.
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15.
- Pilgram TK, Hildebolt CF, Yokoyama N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol 1999; 70:829–833.
- Jeffcoat MK, Lewis CE, Reddy MS, et al. Oral bone loss and systemic osteopenia, osteoporosis. InMarcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York Academic Press 1996:969–990.
- Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeletal and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188.
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol 1997; 26:3–15.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498.
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25:130–135.
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40.
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 1997; 68:24–31.
- Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement: a randomized, double blind, placebo-controlled trial. Arch Intern Med 2002; 162:1409–1415.
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999; 70:30–43.
- Norderyd OM, Grossi SG, Machtel EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol 1993; 64:957–962.
- Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996; 104:10–16.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause 2008; 15:270–275.
- Genco RJ, Grossi SG. Is estrogen deficiency a risk factor for periodontal disease? Compend Contin Educ Dent Suppl 1998; 22:S23–S29.
- Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med 1995; 155:2325–2329.
- Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Post-menopausal estrogen replacement and tooth retention. Am J Med 1997; 102:536–542.
- Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc 1996; 127:370–377.
- Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause 2004; 11:556–562.
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–1051.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int 1992; 50:295–299.
- Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest 1992; 89:883–891.
- Pacifici R, Brown C, Pusheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 1991; 88:5134–5138.
- Brennan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol 2007; 78:1051–1061.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in post-menopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- McClung MR, Geusen P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333–340.
- Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Palomo L, Bissada N, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: innocent association or significant risk? Cleve Clin J Med 2008; 75:871–879.
- Woo SB, Hellstein JW, Kalamare JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Dodson TB, Raje NS, Caruso PA, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 9–2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N Engl J Med 2008; 358:1283–1291.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 2004; 62:527–534.
- Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother 2007; 8:309–315.
- Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev 2000; 42:175–195.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 1997; 37:285–290.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofacial Surg 2007; 65:369–376.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
Menopause can bring oral health problems that physicians ought to keep in mind. The same processes that lead to loss of bone in the spine and hips can also lead to loss of the alveolar bone of the jaws, resulting in periodontal disease, loose teeth, and tooth loss. Although the mouth is traditionally the dentist’s responsibility, patients may need encouragement from their physicians to practice good oral hygiene and to see their dentists, and should be referred to a periodontist at the first sign of periodontal disease.
Moreover, bisphosphonates, the class of drugs most often prescribed for osteoporosis, have been linked by case reports (unfairly, we believe) to osteonecrosis of the jaw. This low-evidence-level information, its far-reaching interpretation, and misinformation in the lay media about hormonal changes associated with menopause have led to confusion among women; for clarification and reliable information, they are driven to ask their physicians challenging questions related to oral health.
This article reviews the published studies of the association between menopause and periodontal disease, specifically, the effects of hormonal changes, osteoporosis, and bisphosphonate use on the periodontal status of postmenopausal women. We will highlight the interrelationship of dental health and postmenopausal health and underscore the need for cross-communication and patient referral between physicians and dentists.
GINGIVITIS CAN PROGRESS TO PERIODONTITIS
AS ESTROGEN DECLINES, SO DO THE BONES AND, MAYBE, THE TEETH
The rate of bone loss in postmenopausal women predicts tooth loss—for every 1%-per-year decrease in whole-body bone mineral density, the risk of tooth loss increases more than four times.2 In fact, Kribbs3 found that women with severe osteoporosis were three times more likely than healthy, age-matched controls to be edentulous (ie, to have fewer teeth).
Although a number of studies have found that the density of the alveolar bone in the mandible correlated with the density of the bone in the rest of the skeleton and that generalized bone loss may render the jaw susceptible to accelerated alveolar bone resorption,3–11 these findings are not universal. In a longitudinal study, Famili et al12 found no association between systemic bone loss, periodontal disease, and edentulism. This shows that the relationship between alveolar bone loss and systemic bone loss is multifactorial and not yet fully understood.13
Nevertheless, the American Academy of Periodontology considers osteoporosis to be a risk factor for periodontal disease.10 In fact, alveolar bone loss has been related not only to osteoporosis but also to osteopenia.14
Bone mineral density has also been studied in relation to the loss of periodontal ligament—the collagenous attachment of tooth to bone. Klemetti et al15 found that healthy postmenopausal women with high bone mineral density seemed to retain teeth more readily than those with low bone density or those with osteoporosis, even if they had deep periodontal pockets (a sign of periodontal disease). These findings were reiterated when osteoporotic women were found to have significantly greater loss of attachment compared with nonosteoporotic women.7
However, Hildebolt16 reported that loss of tooth attachment correlated with tooth loss but not with the density of the vertebrae or the proximal femur. This study called into question the findings of the previous studies and provoked debate.
Tezal et al17 found that low bone mineral density was related to the loss of interproximal alveolar bone (the alveolar bone between adjacent teeth) and, to a lesser extent, ligamentous attachment loss. These data implicated osteoporosis as a possible risk indicator for periodontal disease in white women. (This study was limited to white women because of different demographics in the incidence of osteoporosis.)
Another study showed only a weak correlation between changes in alveolar bone height (in periodontal disease, bone height decreases) and attachment levels. Although a correlation might be present, the relationship was complex and required further examination. The authors found no clear association between clinical attachment levels and bone mineral density in the lumbar spine, but they recognized that attachment loss often precedes the loss of alveolar bone by a significant time period.13
Several studies have found a possible relationship between the bone density in the jaw and the density in the rest of the skeleton. It appears that loss of bone mineral density in the hip, wrist, and lumbar areas is correlated with low density in the mandible. Taguchi et al18 reported that the density in the lumbar spine correlated with the density of the mandibular cortex in early menopause, and with the density of both the cortex and cancellous bone in later menopause.
But whatever the statistical measurement, the susceptibility to progressive periodontitis increases after menopause, and the primary cause is bacterial plaque. The best hedge against this increased susceptibility is regular dental care to remove bacterial plaque biofilm under the gum-line.
HORMONE THERAPY PRESERVES BONE IN THE JAW
Hormones have long been recognized as having some role in periodontal disease.
Payne et al19 reported that postmenopausal women who were estrogen-deficient had a higher frequency of sites with a net loss of alveolar bone density at follow-up. Furthermore, estrogen-deficient women undergoing supportive periodontal therapy following treatment of moderate to severe periodontitis had three times as many sites losing more than 0.4 mm of interproximal alveolar bone height. Patients who had sufficient estrogen levels did not lose bone during 1 year of follow-up.20
Estrogen replacement improves bone density in postmenopausal women. In a 3-year randomized trial in postmenopausal women with moderate or advanced periodontal disease, estrogen therapy significantly increased alveolar bone mass compared with placebo (P = .04), and it increased bone density in the femur but not the lumbar spine.21 Furthermore, women receiving hormonal therapy had significantly less gingival inflammation, lower plaque scores, and less loss of attachment.
On the other hand, a report by Albandar and Kingman22 suggested that women who comply with hormonal therapy also comply with oral hygiene instructions. This compliance could explain the lower gingival inflammation scores, lower plaque scores, and lesser loss of attachment.
Norderyd et al,23 in a cross-sectional study, found less periodontal disease in postmenopausal women who were on estrogen therapy than in those who were not, although the difference was not statistically significant.
In a 5-year longitudinal study of 69 postmenopausal women receiving estrogen, a moderate but significant relationship was found between bone mineral density of the lumbar spine and the mandible, and estrogen replacement therapy had a positive effect on the mandibular bone mass.24
In a longitudinal study of 24 postmenopausal women, estrogen-deficient women had a mean net loss of alveolar bone density over time, while estrogen-sufficient women had a mean net gain, suggesting that estrogen deficiency may be a risk factor for alveolar bone loss.20 More-recent studies had similar findings. A cross-sectional study by Meisel et al25 found that hormone therapy significantly reduced the extent of clinical attachment loss and, hence, periodontal disease.
The findings of these studies are generally consistent, suggesting that estrogen builds up mandibular bone mass and attenuates the severity of periodontal disease in postmenopausal women.26
DOES ESTROGEN THERAPY PROTECT THE TEETH?
Studies of the Leisure World,27 Framingham,28 and Nurses Health Study29 cohorts suggest that hormone therapy protects against tooth loss in postmenopausal women.
On the other hand, Taguchi et al30 evaluated more than 300 postmenopausal Japanese women and found no significant difference in the total number of teeth between estrogen users and nonusers. The population in this study was younger than in the other studies mentioned above,27–29 which may explain the negative finding. However, the duration of estrogen use was significantly associated with the total number of teeth remaining, independent of age.30 Meisel et al25 reported that women receiving hormonal therapy had more teeth, though the difference was not significant.
CYTOKINES, PERIODONTITIS, AND SKELETAL BONE LOSS
Studies suggest that low estrogen production after menopause is associated with increased production of interleukin 1 (IL-1), IL-6, IL-8, IL-10, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, which stimulate mature osteoclasts, modulate bone cell proliferation, and induce resorption of both skeletal and alveolar bone.31–34
In this regard, osteoporosis and periodontitis appear to be mediated by common cytokines. Managing osteoporosis, removing bacterial plaque biofilm with good oral hygiene, and regular dental visits are important in avoiding periodontitis in susceptible women.
BISPHOSPHONATES PROTECT BONE
In the skeleton
Bisphosphonates, the most commonly prescribed therapy for osteoporosis, inhibit systemic bone resorption and reduce the incidence of vertebral and nonvertebral fractures. Among the bisphosphonates, alendronate (Fosamax), risedronate (Actonel), and intravenous zoledronic acid (Reclast) have been shown to reduce the risk of both hip and vertebral fractures, whereas ibandronate (Boniva) has only been shown to decrease the risk of vertebral fracture.36 Specific findings:
- In the Fracture Intervention Trial,37 alendronate reduced the risk of vertebral fracture by 47% and hip fracture by 51% in women with low bone mineral density and previous vertebral fractures.
- In the Hip Intervention Program,38 risedronate decreased the risk of hip fracture by 40% in postmenopausal women 70 to 79 years old with osteoporosis, but not in those 80 years and older, who are at high risk of falls. Risedronate also reduced vertebral fracture risk by 49% after 3 years of treatment.39
- In the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Recurrent Fracture Trial,40 annual infusion of zoledronic acid after a hip fracture reduced the rates of new clinical vertebral and nonvertebral fractures and death from all causes.
In the jaw
Not surprisingly, recent studies suggest that bisphosphonates slow the resorption of alveolar bone of the maxilla and mandible as well. Alendronate and risedronate, in particular, have been noted to improve periodontal status.41–43 Findings:
- In a cross-sectional study by Palomo et al,41 postmenopausal women with low bone density using risedronate for at least 3 months showed significantly less plaque accumulation, less gingival inflammation, lower probing-depth measurments, less periodontal attachment loss, and greater alveolar bone levels.
- In a double-blind, controlled, prospective study by Rocha et al,42 6 months of alendronate therapy significantly improved periodontal disease as assessed radiographically and clinically in 40 postmenopausal women with established periodontal disease.
- Jeffcoat et al43 reported that 2 years of alendronate treatment significantly reduced alveolar bone loss relative to placebo in patients with low mandibular bone mineral density at baseline but not in those with normal baseline mandibular bone mineral density.
DO BISPHOSPHONATES CAUSE OSTEONECROSIS OF THE JAW?
The intravenous bisphosphonates most commonly used to treat hypercalcemia of malignancy, multiple myeloma, or metastatic bone disease are47:
- Pamidronate (Aredia) 90 mg infused over 2 to 24 hours every 3 to 4 weeks
- Zoledronic acid (Zometa) 4 mg infused over 15 minutes monthly.
The doses of bisphosphonates indicated for the treatment of osteoporosis are much lower,1 eg:
- Alendronate 70 mg by mouth once a week
- Risedronate 35 mg by mouth once a week or 150 mg once a month
- Ibandronate 150 mg by mouth once a month
- Ibandronate 3 mg intravenously every 3 months
- Zoledronic acid 5 mg intravenously once a year.
Moreover, less than 1% of an oral dose is absorbed by the gastrointestinal tract,49 whereas more than 50% of the dose of bisphosphonates given intravenously is bioavailable,50 which may account for the lower incidence of jaw ostenonecrosis with oral agents.
Osteonecrosis of the jaw can occur spontaneously but is more often associated with dental procedures that traumatize bone, such as tooth extraction.51 In a systematic review,45 patients with multiple myeloma and metastatic cancer to the bone who were receiving intravenous bisphosphonates accounted for 94% of published cases. Sixty percent of cases were preceded by dental surgical procedures, and in 39% of cases that occurred spontaneously the lesions were located on bony exostoses, a possible source of trauma. Of 63 cases reported by Ruggiero et al,47 56 patients were receiving intravenous bisphosphonates and 7 were receiving oral bisphosphonates. Older age (> 65 years), chronic systemic steroid use, periodontitis, and prolonged use of bisphosphonates have also been associated with a higher risk of osteonecrosis of the jaw.51
The risk of developing osteonecrosis of the jaw in people taking bisphosphonates in doses recommended by the US Food and Drug Administration for treating osteoporosis is very low (the incidence is calculated at 0.7 per 100,000 person-years of exposure to alendronate).51,52 In a 3-year prospective study in more than 7,000 women with post-menopausal osteoporosis, the incidence of osteonecrosis of the jaw was no different in those treated with zoledronic acid 5 mg intravenously than in those receiving placebo.53 In a randomized, placebo-controlled study of the effect of 2 years of alendronate treatment on alveolar bone loss involving 335 patients with periodontal disease, no cases of osteonecrosis of the jaw were reported.43
The American Dental Association (ADA) released a statement noting that osteonecrosis of the jaw can occur with or without bisphosphonate use.51 To date, a true cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate use has not been established. Further studies are needed to fully explore this relationship. Our group is currently exploring novel periodontal assessments comparing the oral health of postmenopausal women with osteoporosis who are on no bone therapy vs postmenopausal women with osteoporosis treated with bisphosphonates for 2 or more years.
Discussion of treatment for bisphosphonate-associated osteonecrosis of the jaw is beyond the scope of this article.
REGULAR DENTAL CARE IS ESSENTIAL
Regardless of whether the patient is receiving a bisphosphonate drug, physicians caring for postmenopausal women should be vigilant and encourage their patients to seek regular dental evaluation for prevention and early management of oral disorders. Conversely, dentists should be aware of the potential effects of menopause and its treatments on bone and dental health.
Questions from postmenopausal women can be managed, in part, by returning to the basics suggested by the ADA:
- Regular dental examinations; regular professional cleaning to remove bacterial plaque biofilm under the gum-line where a toothbrush will not reach
- Daily oral hygiene practices to remove biofilm at and above the gum-line including brushing twice daily with an ADA-accepted toothpaste
- Replacing the toothbrush every 3 to 4 months (or sooner if the bristles begin to look frayed)
- Cleaning interproximally (between teeth) with floss or interdental cleaner
- Maintaining a balanced diet
- No smoking.
Menopause can bring oral health problems that physicians ought to keep in mind. The same processes that lead to loss of bone in the spine and hips can also lead to loss of the alveolar bone of the jaws, resulting in periodontal disease, loose teeth, and tooth loss. Although the mouth is traditionally the dentist’s responsibility, patients may need encouragement from their physicians to practice good oral hygiene and to see their dentists, and should be referred to a periodontist at the first sign of periodontal disease.
Moreover, bisphosphonates, the class of drugs most often prescribed for osteoporosis, have been linked by case reports (unfairly, we believe) to osteonecrosis of the jaw. This low-evidence-level information, its far-reaching interpretation, and misinformation in the lay media about hormonal changes associated with menopause have led to confusion among women; for clarification and reliable information, they are driven to ask their physicians challenging questions related to oral health.
This article reviews the published studies of the association between menopause and periodontal disease, specifically, the effects of hormonal changes, osteoporosis, and bisphosphonate use on the periodontal status of postmenopausal women. We will highlight the interrelationship of dental health and postmenopausal health and underscore the need for cross-communication and patient referral between physicians and dentists.
GINGIVITIS CAN PROGRESS TO PERIODONTITIS
AS ESTROGEN DECLINES, SO DO THE BONES AND, MAYBE, THE TEETH
The rate of bone loss in postmenopausal women predicts tooth loss—for every 1%-per-year decrease in whole-body bone mineral density, the risk of tooth loss increases more than four times.2 In fact, Kribbs3 found that women with severe osteoporosis were three times more likely than healthy, age-matched controls to be edentulous (ie, to have fewer teeth).
Although a number of studies have found that the density of the alveolar bone in the mandible correlated with the density of the bone in the rest of the skeleton and that generalized bone loss may render the jaw susceptible to accelerated alveolar bone resorption,3–11 these findings are not universal. In a longitudinal study, Famili et al12 found no association between systemic bone loss, periodontal disease, and edentulism. This shows that the relationship between alveolar bone loss and systemic bone loss is multifactorial and not yet fully understood.13
Nevertheless, the American Academy of Periodontology considers osteoporosis to be a risk factor for periodontal disease.10 In fact, alveolar bone loss has been related not only to osteoporosis but also to osteopenia.14
Bone mineral density has also been studied in relation to the loss of periodontal ligament—the collagenous attachment of tooth to bone. Klemetti et al15 found that healthy postmenopausal women with high bone mineral density seemed to retain teeth more readily than those with low bone density or those with osteoporosis, even if they had deep periodontal pockets (a sign of periodontal disease). These findings were reiterated when osteoporotic women were found to have significantly greater loss of attachment compared with nonosteoporotic women.7
However, Hildebolt16 reported that loss of tooth attachment correlated with tooth loss but not with the density of the vertebrae or the proximal femur. This study called into question the findings of the previous studies and provoked debate.
Tezal et al17 found that low bone mineral density was related to the loss of interproximal alveolar bone (the alveolar bone between adjacent teeth) and, to a lesser extent, ligamentous attachment loss. These data implicated osteoporosis as a possible risk indicator for periodontal disease in white women. (This study was limited to white women because of different demographics in the incidence of osteoporosis.)
Another study showed only a weak correlation between changes in alveolar bone height (in periodontal disease, bone height decreases) and attachment levels. Although a correlation might be present, the relationship was complex and required further examination. The authors found no clear association between clinical attachment levels and bone mineral density in the lumbar spine, but they recognized that attachment loss often precedes the loss of alveolar bone by a significant time period.13
Several studies have found a possible relationship between the bone density in the jaw and the density in the rest of the skeleton. It appears that loss of bone mineral density in the hip, wrist, and lumbar areas is correlated with low density in the mandible. Taguchi et al18 reported that the density in the lumbar spine correlated with the density of the mandibular cortex in early menopause, and with the density of both the cortex and cancellous bone in later menopause.
But whatever the statistical measurement, the susceptibility to progressive periodontitis increases after menopause, and the primary cause is bacterial plaque. The best hedge against this increased susceptibility is regular dental care to remove bacterial plaque biofilm under the gum-line.
HORMONE THERAPY PRESERVES BONE IN THE JAW
Hormones have long been recognized as having some role in periodontal disease.
Payne et al19 reported that postmenopausal women who were estrogen-deficient had a higher frequency of sites with a net loss of alveolar bone density at follow-up. Furthermore, estrogen-deficient women undergoing supportive periodontal therapy following treatment of moderate to severe periodontitis had three times as many sites losing more than 0.4 mm of interproximal alveolar bone height. Patients who had sufficient estrogen levels did not lose bone during 1 year of follow-up.20
Estrogen replacement improves bone density in postmenopausal women. In a 3-year randomized trial in postmenopausal women with moderate or advanced periodontal disease, estrogen therapy significantly increased alveolar bone mass compared with placebo (P = .04), and it increased bone density in the femur but not the lumbar spine.21 Furthermore, women receiving hormonal therapy had significantly less gingival inflammation, lower plaque scores, and less loss of attachment.
On the other hand, a report by Albandar and Kingman22 suggested that women who comply with hormonal therapy also comply with oral hygiene instructions. This compliance could explain the lower gingival inflammation scores, lower plaque scores, and lesser loss of attachment.
Norderyd et al,23 in a cross-sectional study, found less periodontal disease in postmenopausal women who were on estrogen therapy than in those who were not, although the difference was not statistically significant.
In a 5-year longitudinal study of 69 postmenopausal women receiving estrogen, a moderate but significant relationship was found between bone mineral density of the lumbar spine and the mandible, and estrogen replacement therapy had a positive effect on the mandibular bone mass.24
In a longitudinal study of 24 postmenopausal women, estrogen-deficient women had a mean net loss of alveolar bone density over time, while estrogen-sufficient women had a mean net gain, suggesting that estrogen deficiency may be a risk factor for alveolar bone loss.20 More-recent studies had similar findings. A cross-sectional study by Meisel et al25 found that hormone therapy significantly reduced the extent of clinical attachment loss and, hence, periodontal disease.
The findings of these studies are generally consistent, suggesting that estrogen builds up mandibular bone mass and attenuates the severity of periodontal disease in postmenopausal women.26
DOES ESTROGEN THERAPY PROTECT THE TEETH?
Studies of the Leisure World,27 Framingham,28 and Nurses Health Study29 cohorts suggest that hormone therapy protects against tooth loss in postmenopausal women.
On the other hand, Taguchi et al30 evaluated more than 300 postmenopausal Japanese women and found no significant difference in the total number of teeth between estrogen users and nonusers. The population in this study was younger than in the other studies mentioned above,27–29 which may explain the negative finding. However, the duration of estrogen use was significantly associated with the total number of teeth remaining, independent of age.30 Meisel et al25 reported that women receiving hormonal therapy had more teeth, though the difference was not significant.
CYTOKINES, PERIODONTITIS, AND SKELETAL BONE LOSS
Studies suggest that low estrogen production after menopause is associated with increased production of interleukin 1 (IL-1), IL-6, IL-8, IL-10, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor, which stimulate mature osteoclasts, modulate bone cell proliferation, and induce resorption of both skeletal and alveolar bone.31–34
In this regard, osteoporosis and periodontitis appear to be mediated by common cytokines. Managing osteoporosis, removing bacterial plaque biofilm with good oral hygiene, and regular dental visits are important in avoiding periodontitis in susceptible women.
BISPHOSPHONATES PROTECT BONE
In the skeleton
Bisphosphonates, the most commonly prescribed therapy for osteoporosis, inhibit systemic bone resorption and reduce the incidence of vertebral and nonvertebral fractures. Among the bisphosphonates, alendronate (Fosamax), risedronate (Actonel), and intravenous zoledronic acid (Reclast) have been shown to reduce the risk of both hip and vertebral fractures, whereas ibandronate (Boniva) has only been shown to decrease the risk of vertebral fracture.36 Specific findings:
- In the Fracture Intervention Trial,37 alendronate reduced the risk of vertebral fracture by 47% and hip fracture by 51% in women with low bone mineral density and previous vertebral fractures.
- In the Hip Intervention Program,38 risedronate decreased the risk of hip fracture by 40% in postmenopausal women 70 to 79 years old with osteoporosis, but not in those 80 years and older, who are at high risk of falls. Risedronate also reduced vertebral fracture risk by 49% after 3 years of treatment.39
- In the Health Outcomes and Reduced Incidence With Zoledronic Acid Once Yearly Recurrent Fracture Trial,40 annual infusion of zoledronic acid after a hip fracture reduced the rates of new clinical vertebral and nonvertebral fractures and death from all causes.
In the jaw
Not surprisingly, recent studies suggest that bisphosphonates slow the resorption of alveolar bone of the maxilla and mandible as well. Alendronate and risedronate, in particular, have been noted to improve periodontal status.41–43 Findings:
- In a cross-sectional study by Palomo et al,41 postmenopausal women with low bone density using risedronate for at least 3 months showed significantly less plaque accumulation, less gingival inflammation, lower probing-depth measurments, less periodontal attachment loss, and greater alveolar bone levels.
- In a double-blind, controlled, prospective study by Rocha et al,42 6 months of alendronate therapy significantly improved periodontal disease as assessed radiographically and clinically in 40 postmenopausal women with established periodontal disease.
- Jeffcoat et al43 reported that 2 years of alendronate treatment significantly reduced alveolar bone loss relative to placebo in patients with low mandibular bone mineral density at baseline but not in those with normal baseline mandibular bone mineral density.
DO BISPHOSPHONATES CAUSE OSTEONECROSIS OF THE JAW?
The intravenous bisphosphonates most commonly used to treat hypercalcemia of malignancy, multiple myeloma, or metastatic bone disease are47:
- Pamidronate (Aredia) 90 mg infused over 2 to 24 hours every 3 to 4 weeks
- Zoledronic acid (Zometa) 4 mg infused over 15 minutes monthly.
The doses of bisphosphonates indicated for the treatment of osteoporosis are much lower,1 eg:
- Alendronate 70 mg by mouth once a week
- Risedronate 35 mg by mouth once a week or 150 mg once a month
- Ibandronate 150 mg by mouth once a month
- Ibandronate 3 mg intravenously every 3 months
- Zoledronic acid 5 mg intravenously once a year.
Moreover, less than 1% of an oral dose is absorbed by the gastrointestinal tract,49 whereas more than 50% of the dose of bisphosphonates given intravenously is bioavailable,50 which may account for the lower incidence of jaw ostenonecrosis with oral agents.
Osteonecrosis of the jaw can occur spontaneously but is more often associated with dental procedures that traumatize bone, such as tooth extraction.51 In a systematic review,45 patients with multiple myeloma and metastatic cancer to the bone who were receiving intravenous bisphosphonates accounted for 94% of published cases. Sixty percent of cases were preceded by dental surgical procedures, and in 39% of cases that occurred spontaneously the lesions were located on bony exostoses, a possible source of trauma. Of 63 cases reported by Ruggiero et al,47 56 patients were receiving intravenous bisphosphonates and 7 were receiving oral bisphosphonates. Older age (> 65 years), chronic systemic steroid use, periodontitis, and prolonged use of bisphosphonates have also been associated with a higher risk of osteonecrosis of the jaw.51
The risk of developing osteonecrosis of the jaw in people taking bisphosphonates in doses recommended by the US Food and Drug Administration for treating osteoporosis is very low (the incidence is calculated at 0.7 per 100,000 person-years of exposure to alendronate).51,52 In a 3-year prospective study in more than 7,000 women with post-menopausal osteoporosis, the incidence of osteonecrosis of the jaw was no different in those treated with zoledronic acid 5 mg intravenously than in those receiving placebo.53 In a randomized, placebo-controlled study of the effect of 2 years of alendronate treatment on alveolar bone loss involving 335 patients with periodontal disease, no cases of osteonecrosis of the jaw were reported.43
The American Dental Association (ADA) released a statement noting that osteonecrosis of the jaw can occur with or without bisphosphonate use.51 To date, a true cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate use has not been established. Further studies are needed to fully explore this relationship. Our group is currently exploring novel periodontal assessments comparing the oral health of postmenopausal women with osteoporosis who are on no bone therapy vs postmenopausal women with osteoporosis treated with bisphosphonates for 2 or more years.
Discussion of treatment for bisphosphonate-associated osteonecrosis of the jaw is beyond the scope of this article.
REGULAR DENTAL CARE IS ESSENTIAL
Regardless of whether the patient is receiving a bisphosphonate drug, physicians caring for postmenopausal women should be vigilant and encourage their patients to seek regular dental evaluation for prevention and early management of oral disorders. Conversely, dentists should be aware of the potential effects of menopause and its treatments on bone and dental health.
Questions from postmenopausal women can be managed, in part, by returning to the basics suggested by the ADA:
- Regular dental examinations; regular professional cleaning to remove bacterial plaque biofilm under the gum-line where a toothbrush will not reach
- Daily oral hygiene practices to remove biofilm at and above the gum-line including brushing twice daily with an ADA-accepted toothpaste
- Replacing the toothbrush every 3 to 4 months (or sooner if the bristles begin to look frayed)
- Cleaning interproximally (between teeth) with floss or interdental cleaner
- Maintaining a balanced diet
- No smoking.
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd ed; 2007.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 1996; 59:433–437.
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222.
- Kribbs PJ, Chesnut CH, Ott SM, Kilcoyne RF. Relationship between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707.
- Kribbs PJ, Chestnut CH, Ott SM, Kilcyne RE. Relationship between mandibular and skeletal bone in a population of normal women. J Prosthet Dent 1990; 63:86–89.
- Kribbs PJ, Smith DE, Chestnut CH. Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724.
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138.
- Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Peridontol 1996; 67(suppl 10):1076–1084.
- Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from the NHANES III. J Clin Periodontol 2000; 27:778–86.
- American Dental Association Council on Access, Prevention and Interprofessional Relations. Women’s Oral Health Issues. November 2006.
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000 2000; 23:94–102.
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15.
- Pilgram TK, Hildebolt CF, Yokoyama N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol 1999; 70:829–833.
- Jeffcoat MK, Lewis CE, Reddy MS, et al. Oral bone loss and systemic osteopenia, osteoporosis. InMarcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York Academic Press 1996:969–990.
- Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeletal and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188.
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol 1997; 26:3–15.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498.
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25:130–135.
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40.
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 1997; 68:24–31.
- Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement: a randomized, double blind, placebo-controlled trial. Arch Intern Med 2002; 162:1409–1415.
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999; 70:30–43.
- Norderyd OM, Grossi SG, Machtel EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol 1993; 64:957–962.
- Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996; 104:10–16.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause 2008; 15:270–275.
- Genco RJ, Grossi SG. Is estrogen deficiency a risk factor for periodontal disease? Compend Contin Educ Dent Suppl 1998; 22:S23–S29.
- Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med 1995; 155:2325–2329.
- Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Post-menopausal estrogen replacement and tooth retention. Am J Med 1997; 102:536–542.
- Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc 1996; 127:370–377.
- Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause 2004; 11:556–562.
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–1051.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int 1992; 50:295–299.
- Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest 1992; 89:883–891.
- Pacifici R, Brown C, Pusheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 1991; 88:5134–5138.
- Brennan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol 2007; 78:1051–1061.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in post-menopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- McClung MR, Geusen P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333–340.
- Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Palomo L, Bissada N, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: innocent association or significant risk? Cleve Clin J Med 2008; 75:871–879.
- Woo SB, Hellstein JW, Kalamare JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Dodson TB, Raje NS, Caruso PA, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 9–2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N Engl J Med 2008; 358:1283–1291.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 2004; 62:527–534.
- Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother 2007; 8:309–315.
- Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev 2000; 42:175–195.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 1997; 37:285–290.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofacial Surg 2007; 65:369–376.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
- North American Menopause Society. Menopause Practice: A Clinician’s Guide. 3rd ed; 2007.
- Krall EA, Garcia RI, Dawson-Hughes B. Increased risk of tooth loss is related to bone loss at the whole body, hip and spine. Calcif Tissue Int 1996; 59:433–437.
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222.
- Kribbs PJ, Chesnut CH, Ott SM, Kilcoyne RF. Relationship between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707.
- Kribbs PJ, Chestnut CH, Ott SM, Kilcyne RE. Relationship between mandibular and skeletal bone in a population of normal women. J Prosthet Dent 1990; 63:86–89.
- Kribbs PJ, Smith DE, Chestnut CH. Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724.
- von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol 1994; 65:1134–1138.
- Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Peridontol 1996; 67(suppl 10):1076–1084.
- Ronderos M, Jacobs DR, Himes JH, Pihlstrom BL. Associations of periodontal disease with femoral bone mineral density and estrogen replacement therapy: cross-sectional evaluation of US adults from the NHANES III. J Clin Periodontol 2000; 27:778–86.
- American Dental Association Council on Access, Prevention and Interprofessional Relations. Women’s Oral Health Issues. November 2006.
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000 2000; 23:94–102.
- Famili P, Cauley J, Suzuki JB, Weyant R. Longitudinal study of periodontal disease and edentulism with rates of bone loss in older women. J Periodontol 2005; 76:11–15.
- Pilgram TK, Hildebolt CF, Yokoyama N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol 1999; 70:829–833.
- Jeffcoat MK, Lewis CE, Reddy MS, et al. Oral bone loss and systemic osteopenia, osteoporosis. InMarcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York Academic Press 1996:969–990.
- Klemetti E, Collin HL, Forss H, Markkanen H, Lassila V. Mineral status of skeletal and advanced periodontal disease. J Clin Periodontol 1994; 21:184–188.
- Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol 1997; 26:3–15.
- Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498.
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac Radiol 1996; 25:130–135.
- Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40.
- Payne JB, Zachs NR, Reinhardt RA, Nummikoski PV, Patil K. The association between estrogen status and alveolar bone density changes in postmenopausal women with a history of periodontitis. J Periodontol 1997; 68:24–31.
- Civitelli R, Pilgram TK, Dotson M, et al. Alveolar and postcranial bone density in postmenopausal women receiving hormone/estrogen replacement: a randomized, double blind, placebo-controlled trial. Arch Intern Med 2002; 162:1409–1415.
- Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol 1999; 70:30–43.
- Norderyd OM, Grossi SG, Machtel EE, et al. Periodontal status of women taking postmenopausal estrogen supplementation. J Periodontol 1993; 64:957–962.
- Jacobs R, Ghyselen J, Koninckx P, van Steenberghe D. Long-term bone mass evaluation of mandible and lumbar spine in a group of women receiving hormone replacement therapy. Eur J Oral Sci 1996; 104:10–16.
- Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause 2008; 15:270–275.
- Genco RJ, Grossi SG. Is estrogen deficiency a risk factor for periodontal disease? Compend Contin Educ Dent Suppl 1998; 22:S23–S29.
- Paganini-Hill A. The benefits of estrogen replacement therapy on oral health. The Leisure World cohort. Arch Intern Med 1995; 155:2325–2329.
- Krall EA, Dawson-Hughes B, Hannan MT, Wilson PW, Kiel DP. Post-menopausal estrogen replacement and tooth retention. Am J Med 1997; 102:536–542.
- Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc 1996; 127:370–377.
- Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause 2004; 11:556–562.
- Pacifici R. Estrogen, cytokines and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996; 11:1043–1051.
- Pacifici R. Is there a causal role for IL-1 in postmenopausal bone loss? Calcif Tissue Int 1992; 50:295–299.
- Girasole G, Jilka RL, Passeri G, et al. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest 1992; 89:883–891.
- Pacifici R, Brown C, Pusheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA 1991; 88:5134–5138.
- Brennan RM, Genco RJ, Wilding GE, Hovey KM, Trevisan M, Wactawski-Wende J. Bacterial species in subgingival plaque and oral bone loss in postmenopausal women. J Periodontol 2007; 78:1051–1061.
- Chestnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in post-menopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Cummings SR, Karpf DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- McClung MR, Geusen P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333–340.
- Reginster JY, Minne HW, Sorensen OH, et al. Randomized trial of effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Palomo L, Bissada N, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Carey JJ, Palomo L. Bisphosphonates and osteonecrosis of the jaw: innocent association or significant risk? Cleve Clin J Med 2008; 75:871–879.
- Woo SB, Hellstein JW, Kalamare JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Dodson TB, Raje NS, Caruso PA, Rosenberg AE. Case records of the Massachusetts General Hospital. Case 9–2008. A 65-year-old woman with a nonhealing ulcer of the jaw. N Engl J Med 2008; 358:1283–1291.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofacial Surg 2004; 62:527–534.
- Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother 2007; 8:309–315.
- Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev 2000; 42:175–195.
- Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol 1997; 37:285–290.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofacial Surg 2007; 65:369–376.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
KEY POINTS
- Physicians should be vigilant for dental problems and should encourage their patients to practice good oral hygiene and to seek regular dental care.
- Available information suggests that hormone therapy and bisphosphonate drugs may be developed to protect against alveolar bone loss and perhaps slow the progression of periodontal disease.
- Bisphosphonate-associated osteonecrosis of the jaw is rare, and most of the reported cases have been in cancer patients who received high doses of bisphosphonates intravenously and who had other risk factors for it.
Bisphosphonates and osteonecrosis of the jaw: Innocent association or significant risk?
Recent case reports have linked bisphosphonate drugs to osteonecrosis of the jaw, and these reports have been widely publicized. Many patients receiving these drugs are asking their dentists and doctors whether the drugs do more harm than good, and some have even stopped taking them against medical advice. Health care professionals may be unsure what to tell patients and may be fearful of litigation.
However, most of the cases reported were in cancer patients, who are at significantly higher risk of osteonecrosis of the jaw for several reasons, and who receive much higher doses of bisphosphonates than do patients with osteoporosis or Paget disease of bone.
Moreover, although case reports have clearly documented an association between these drugs and osteonecrosis of the jaw, there is a lack of robust scientific evidence to support a cause-and-effect relationship. In fact, wellcontrolled clinical studies have not shown an increased risk of this complication in patients with osteoporosis or Paget disease of bone who were exposed to these agents, nor have they elucidated definite pathogenic mechanisms by which it might occur.
For these reasons, we believe that patients with osteoporosis should be advised of:
- Their risk of fracture
- The significant risk of morbidity and death following such a fracture
- The effectiveness and excellent safety of bisphosphonate therapy in preventing fractures
- The evidence that such therapy for osteoporosis and Paget disease poses little or no risk of osteonecrosis of the jaw
- The need for further research.
WHAT IS OSTEONECROSIS OF THE JAW?
Osteonecrosis—a general loss of bone tissue as a result of cell death1—can occur at any skeletal site, but it typically involves the long bones, ie, the femur, tibia, and humerus.
Osteonecrosis of the jaw is a rare disorder characterized by exposure and loss of bone in the maxillofacial complex. It can result in significant morbidity and can be resistant or refractory to conventional therapy.
This condition is not new, having been described in 19th century factory workers exposed to white phosphorus used in matchstick manufacturing. Known then as “phossy jaw,” it was associated with poor dentition and often resulted in severe disfigurement, disease, and death. Use of white phosphorus, and matches containing it, were subsequently banned in many countries.2
In the early 20th century, radiation therapy for cancers of the head and neck area came into vogue, but its side effects included damage to the skeleton, or osteoradionecrosis.3 In 1950, LaDow4 described a case of osteoradionecrosis of the jaw and reviewed the literature available at that time. He concluded that there were three main causes of osteonecrosis of the jaw, namely, radiation therapy, trauma, and infection.
Although many such cases have since been reported in association with radiation therapy, chemotherapy, or both, and involvement of other skeletal sites is well described,5–8 the actual incidence of osteoradionecrosis in the general population remains unclear because no large epidemiologic studies to elucidate accurate numbers have been published.
BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS OF THE JAW
Bisphosphonate-associated osteonecrosis of the jaw is a relatively new condition, having been first reported in three case series9–11 published in 2003 and 2004. The patients had exposure of areas of alveolar bone, mostly after oral surgery, eg, mucogingival flap elevation procedures (such as tooth extraction), that did not respond or were refractory to conventional treatment. All had received a bisphosphonate drug.
After these articles were published, the number of reported cases rose dramatically, including a case presented by one of us.12 By the end of January 2008, more than 500 papers on this condition were listed in PubMed. More than 60% had been printed since 2003, and approximately 85% concerned the association between osteonecrosis of the jaw and bisphosphonate use (search terms: “osteonecrosis of the jaw” and “bisphosphonate”).13
Although some dentists and oral surgeons claim to have seen many patients with this disorder, physicians who specialize in osteoporosis and metabolic bone disease do not. The medical literature and popular press have suggested that bisphosphonates are the cause of this malady. However, such articles are more perspective than evidence, as they are not scientific studies but rather reports of cases or series, or reviews of these. High-impact journals have given such articles prominent positions, highlighting the issue further, rather than balancing what is known and what is not known.
Thus, medicine safety boards, physicians, dentists, and oral surgeons have become increasingly concerned about the possible risk of this disorder in their patients on long-term bisphosphonate therapy, prompting organizations to issue management guidelines for this disorder and regulatory bodies to mandate warning labels on all drugs in this class about the possible risk.14–18 Funding agencies have highlighted this as an area in need of further investigation.17
However, robust evidence of a causal relationship is lacking. Contributing to the problem, other disorders can have similar presentations.
As a result, the diagnosis requires a dental examination and dental imaging, which are often impossible or impractical in a medical setting. Well-designed studies have relied on blinded panels of dental specialists using clinical and imaging data to adjudicate cases as osteonecrosis of the jaw before including them in published reports; case reports, however, often do not.
HOW IS OSTEONECROSIS OF THE JAW DIAGNOSED AND MANAGED?
A working definition of osteonecrosis of the jaw has recently emerged, and it will likely continue to evolve as results of further investigation become available.
A confirmed case is defined as an area of exposed bone in the maxillofacial region that does not heal within 8 weeks after being identified by a health care provider, in a patient who is currently receiving or has been exposed to a bisphosphonate and who has not had radiation therapy to the craniofacial region.14,17 This 8-week duration is consistent with the time frame in which soft tissue would be expected to close and exposed bone would be expected to heal under normal conditions after oral surgery such as dental extraction or a flap elevation procedure.
Patients may have no symptoms at the time of presentation. However, symptoms can include oral or jaw pain, difficulty chewing, evidence of infection, and dental loss. Bone loss is often apparent radiographically, and it may be focal or generalized. Other imaging studies such as cone beam computed tomography provide greater detail on the extent and nature of the lesions, and thus provide a better assessment.
Histologically, there is evidence of necrosis, cell death, and, usually, concomitant infection.9–12,17
Management can be difficult
Osteonecrosis of the jaw can be difficult to manage, and extensive guidelines have been published.14–17 Its treatment is complicated because resection of the necrotic area often only makes the necrotic area bigger. Unlike in osteoradionecrosis, surgical removal of the affected area often results in necrosis at the margins of resected bone. This creates a potential situation of “chasing” affected bone in procedure after procedure, which results in significant morbidity.
A preventive protocol for cancer patients
Most of the cases reported so far have been in cancer patients receiving long-term treatment with potent bisphosphonates in high intravenous doses (12 times the usual dose for osteoporosis) after a mucoperiosteal flap elevation dental procedure (many of which were performed on an emergency basis).9–12,14–20 Authors have thus concluded that a preventive protocol should be followed for all patients being considered for intensive bisphosphonate treatment, similar to that adopted for patients receiving head and neck radiation.
Specifically, all chronic dental and periodontal conditions should be identified and stabilized before starting intensive bisphosphonate therapy. Experts today believe that controlling all chronic dental problems before starting intensive intravenous bisphosphonate therapy may be the best method to avoid dental surgery after bisphosphonate therapy has begun, particularly since the washout period (time to elimination of the drug) for bisphosphonates in alveolar bone is unknown.14–20
Although authors seem to agree that such a preventive protocol is prudent for intensive intravenous therapy, it does not appear to be necessary for patients without cancer.14,17 Indeed, such an approach is impractical, given the huge numbers involved and the lack of evidence to support it.
WHAT ARE BISPHOSPHONATES, AND WHY THE CONCERN?
Bisphosphonates are analogues of pyrophosphates, inorganic compounds developed to remove calcium carbonate from water in industrial pipes and laundry machines. Pyrophosphate use in humans arose from their affinity for calcium phosphate, which proved beneficial in scintigraphic imaging studies and in preventing tartar build-up, resulting in their incorporation into toothpastes. Modifications of the pyrophosphate molecule led to the development of diphosphonate compounds (later known as bisphosphonates), which have gained widespread use in treating a variety of disorders of the skeleton and of calcium metabolism.
These drugs prevent bone resorption by selectively inhibiting osteoclastic activity through several mechanisms (depending on the compound), thus helping prevent bone loss, bone pain, and hypercalcemia in diseases of the skeleton.
Bisphosphonates are widely used
Today, oral and intravenous bisphosphonates are widely prescribed for several skeletal disorders, including metastatic disease, malignant hypercalcemia, Paget disease of bone, and prevention and treatment of osteoporosis.21–23
More than 10 million Americans and more than 200 million people worldwide may have osteoporosis, which results in more than 1 million fractures each year. The lifetime risk of fracture for a postmenopausal white woman today is approximately 40% (approximately 15% for a 50-year-old man), and her annual risk of fracture is greater than her combined risk of stroke, heart attack, and breast cancer.22 Several bisphosphonates have been shown to safely and significantly reduce the risk of fracture in patients with osteoporosis and to be effective therapies for Paget disease of bone.24–31
Bisphosphonates are the most widely prescribed drugs for osteoporosis,22,23,29 with almost 200 million prescriptions for oral bisphosphonates worldwide. As of 2004, exposure to alendronate (Fosamax) was estimated to be about 20 million patient-years.32 Noncompliance limits their effectiveness in practice, due in part to concerns about adverse effects.
Since bisphosphonates are so widely prescribed, concern has been raised that they may be causing a new epidemic of osteonecrosis of the jaw.9 However, most reported cases have been in cancer patients, who are known to be at increased risk of this condition and who receive doses of bisphosphonates up to 12 times higher than in patients with osteoporosis or Paget disease of bone.9–12,33–38
The optimal duration of bisphosphonate therapy for these diseases to obtain the maximum benefit and minimize cost and harm remains unclear. Although a recent report suggests a bisphosphonate “drug holiday” may be an option when treating postmenopausal osteoporosis, larger, more robust studies of longer duration are needed.39 Outcomes of osteonecrosis of the jaw related to drug holidays have not been investigated.
‘IF I TAKE THIS TO STOP BONE LOSS, WILL IT HURT MY JAWS?’
The recently described association between bisphosphonates and osteonecrosis of the jaw has received considerable attention. Guidelines have been drawn up, some based on the assumption that bisphosphonates cause the osteonecrosis, but not based on scientific research.14–18 More than 90% of reported cases have been in cancer patients, a group known to be at increased risk of osteonecrosis of the jaw and other skeletal sites, for reasons that include radiation therapy, chemotherapy, corticosteroid use, and increased risk of infections.4,6,9–12,33–38 Nevertheless, it has been assumed that these patients are the same as osteoporosis patients, and sometimes that causation is beyond dispute. This is problematic for two main reasons:
- Since noncompliance and lack of adherence (due to lack of knowledge about the dangers posed by osteoporosis, cost of the drugs, difficulty with dosing regimens, and fear of adverse effects) limit the effectiveness of these therapies in clinical practice, such attention has already persuaded patients to discontinue or refuse therapy (J.J. Carey, personal experience and communications from colleagues); and
- Patients with osteoporosis and osteoporotic fractures have increased rates of morbidity and mortality and significantly higher fracture risk, which can be prevented with these agents if they are willing to take them.
Association does not prove causation
However, association does not prove causation. A relationship between a drug and a disease may be due to chance alone or to confounding factors.40 To judge the exact nature of this relationship, several issues need to be considered when reviewing the available evidence.
Substantiating that an agent causes a disease requires careful consideration of several aspects of their relationship: temporality, strength, dose-response, reversibility, consistency, biologic plausibility, and specificity.41 Correct interpretation of the strength of the evidence should also incorporate an evaluation of the study design, size, and reporting mechanism. Accordingly, case reports and case series are considered to constitute the weakest evidence, while randomized controlled trials and meta-analyses are usually considered the strongest.
When a true cause-and-effect relationship does exist, the situation can be a simple one in which only a single agent is involved. However, the issue can be decidedly more complex when the cause is an effect-modifier, requiring the interaction of additional factors.
When a cause has been assumed, demonstration of the dose-response relationship is also important: whether the risk is related in a continuous fashion to dose and duration of therapy (all patients), is seen only with particular doses or regimens (such as frequent use of high doses of potent bisphosphonates), or exists only in people who have passed a certain threshold value (for example, it may only occur in those who have received 0.5 g of an intravenous or 10 g of an oral bisphosphonate). Bearing in mind these considerations, the nature of the relationship between an agent and a disease can be better understood.40–43
A cause-and-effect relationship has not been established
A cause-and-effect relationship between bisphosphonates and osteonecrosis of the jaw has not been clearly established.14,17 Although case series highlight a relationship between the two, large controlled trials evaluating the occurrence of osteonecrosis of the jaw as the primary outcome have not been conducted. To date, most cases have been reported as uncontrolled case series, generally considered the weakest form of evidence.43
Most cases have been in cancer patients
Most cases of osteonecrosis of the jaw were in patients with cancer (particularly breast cancer and multiple myeloma) receiving potent intravenous bisphosphonates in high doses, most of whom had other documented risk factors, including recent dental procedures such as tooth extraction.9–12,15–19,33–38
One of the most compelling studies supporting causation examined the prevalence of osteonecrosis of the jaw in a cohort of 303 myeloma patients from 1991 to 2003. Osteonecrosis of the jaw developed only in those taking bisphosphonates (28 of 254), and the risk appeared greatest in those treated with both zoledronic acid (Zometa) and thalidomide (Thalomid). The importance of additional chemotherapies, concomitant diseases, and baseline dental pathology was not described.35 Biases, including channeling bias (in which patients who appear at increased risk of this rare condition also appear to be most likely to receive this medication), referral bias, and survivor bias, were not addressed in this paper or in others claiming that the risk is related to the type of bisphosphonate used and the duration of its use.15,33–38
A review of all cases of osteonecrosis of the jaw over a 5-year period in one institution (N = 163) found that only 17 (10%) were associated with bisphosphonate use, and all 17 patients had other risk factors, such as concomitant therapy for malignancy and recent dental surgery.34 The authors’ concern that longer follow-up may have shown a higher incidence of this problem is supported by the temporal relationship seen in other reports in which cancer patients with osteonecrosis of the jaw appear to have had higher cumulative doses of intravenous bisphosphonates than those without.9–12,15,34,35,37,38 Unfortunately, only one study had a control group to highlight the incidence of osteonecrosis of the jaw in similar patients not treated with bisphosphonates.35 The incidence in cancer patients treated with intravenous bisphosphonates has been reported as between 0% and 11%, and the incidence is higher following dental procedures and with a greater duration of drug exposure.11,14,15,17,35,38,44
Interestingly, in a recent survey of oncologists prescribing bisphosphonate medications for metastatic indications, two-thirds said they believe their patients probably have undiagnosed chronic oral conditions that could increase the risk of osteonecrosis of the jaw following bisphosphonate therapy and dental surgery procedures. A similar number reported that their patients receive routine dental care (access to and cost of dental care and the difficulty in physician prescreening are cited as obstacles), but only about one-third actually refer their patients to dentists before starting bisphosphonate therapy.45
What recent studies in osteoporosis and Paget disease showed
Controlled scientific studies in osteoporosis and Paget disease of bone have not shown osteonecrosis of the jaw to emerge, even after years of treatment with bisphosphonate drugs.24–31,46–49 To date, more than 50,000 patients have been treated with oral bisphosphonates— more than 100,000 patient-years for each drug: alendronate, risedronate (Actonel), and ibandronate (Boniva)—in clinical trials, and there has not been a single case of bisphosphonate-associated osteonecrosis in any of these studies.48
Recent publications have addressed the results of clinical trials comparing zoledronic acid (the drug most often associated with this condition in published case series) and risedronate in more than 300 patients with Paget disease of bone,31 and with placebo in postmenopausal women with osteoporosis and persons over 50 years of age suffering a hip fracture treated for up to 3 years following their fracture.29,30
In the largest trial, almost 4,000 osteoporotic women were treated with 5 mg of zoledronic acid annually for 3 years, and a similar number received placebo. Despite a rigorous search for any potential cases of bisphosphonate-associated osteonecrosis of the jaw—adjudicated by a blinded panel of ex on the basis of clinical and dental diagnostic imaging—only two possible cases were found: one in the placebo group and one in the treatment group (a case of osteomyelitis that preceded any treatment with zoledronic acid). Both patients recovered following a course of oral antibiotics and debridement. There was no increase in osteonecrosis at other skeletal sites.29,49
Observational studies have yielded conflicting results. An Australian postal survey of oral surgeons and dentists combined with drug adverse events data suggested the frequency of osteonecrosis of the jaw was 1:2,260 to 1:8,470 in patients on weekly alendronate treatment for osteoporosis, and 1:56 to 1:380 in patients with Paget disease. Following dental extractions, this rose to 1:296 to 1:1,130 and 1:7.4 to 1:48, respectively. Results in patients with malignancy were similar to those in other studies.44 The study raises issues similar to those in other studies: lack of an appropriate control group, reporting bias, and the possibility of multiple reportings of the same patients.
Unpublished information from pharmaceutical companies has suggested the incidence of unconfirmed cases of osteonecrosis of the jaw in persons taking alendronate is 0.7/100,000 person-years.14,17 One study using administrative claims data did not find evidence of increased bisphosphonate use in patients undergoing jaw surgery (used as a surrogate for osteonecrosis of the jaw),50 while another actually found that oral bisphosphonates had a protective effect against osteonecrosis of the jaw, inflammatory conditions of the jaw, and need for major jaw surgery.51
The risk, if any, is probably very small
This information suggests that if these drugs, used at the recommended dose, really do pose a risk, it is probably very small: less than 1 case in 100,000 patient-years if taking an oral bisphosphonate such as alendronate.14,17 This is significantly less than the risk of fracture in these patients (which may be higher than 1 in 10), the risk of death following such a fracture,22–30 or the risk of death from drowning, house fire, or motor vehicle accident.52
The cases of osteonecrosis of the jaw that we have personally seen—all in cancer patients treated with chemotherapy and highdose bisphosphonates—all showed histologic evidence of necrosis and concomitant infections, suggesting the actual diagnosis was osteomyelitis. Bone biopsies from affected but macroscopically normal mandibles at the time of surgical debridement for osteonecrosis of the jaw showed normal or increased osteoclastic activity, in contrast to what one would expect if there were oversuppression of bone turnover (unpublished data, J. Christian, J. Carey, Cleveland Clinic).
Recently, this family of drugs has shown some promise in limiting the progression of alveolar bone loss in periodontal disease (though they are not approved for this indication).53–55 Finally, published studies suggest bisphosphonate therapy may even be beneficial in animals and humans with osteonecrosis,56–58 and in conditions that mimic osteonecrosis such as SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis) of the mandible, in which the histologic appearance may resemble that of osteonecrosis.59
WHAT SHOULD WE TELL OUR PATIENTS?
Several things are worth emphasizing from the published data and guidelines:
- Many things are unknown about osteonecrosis of the jaw and the risk in people taking bisphosphonates.
- The best evidence today does not support a cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate therapy.
- If bisphosphonates are causative, the risk appears very low in patients without cancer.
- It is important to distinguish between cancer and noncancer patients because of different risk factors, the markedly higher doses of bisphosphonates used in cancer patients, and the much greater incidence of osteonecrosis of the jaw seen in cancer patients irrespective of the cause.
- The higher risk in cancer patients is likely modified or confounded by additional risk factors, possibly including long-term use of high-dose intravenous bisphosphonates.
- About 90% of cases of bisphosphonate-associated osteonecrosis of the jaw have been in cancer patients, in whom a substantial temporal relationship to bisphosphonate therapy has been seen.9–12,15–17,19,49,54–57
- Prevention will likely be the most effective management strategy because of the significant morbidity associated with and the refractory nature of osteonecrosis of the jaw.
- Prophylactic dental examinations and any needed repair work are probably best done before starting bisphosphonate therapy in cancer patients; however, studies supporting such a strategy are needed.
- There is no evidence to support routine dental examinations before starting such therapy for disorders other than cancer, or for stopping such therapy before, during, or after dental surgery. Whether this is true for patients who have been taking these drugs for several years or more is unclear.
- Good communication between patients and their physicians, dentists, periodontists, and surgeons will help provide them with the best possible care.
Clearly, much further research is needed on the causes, risks, diagnosis, and management of this disorder to optimize patient outcomes.
- Thomas CL. Taber’s Cyclopedic Medical Dictionary, 17th ed. Philadelphia, FA Davis, 1993.
- Donoghue AM. Bisphosphonates and osteonecrosis: analogy to phossy jaw. Med J Aust 2005; 183:163–164.
- Watson WL, Scarborough JE. Osteoradionecrosis in intraoral cancer. Am J Roentgenol 1938; 40:524–534.
- LaDow CS. Osteoradionecrosis of the jaw. Oral Surg Oral Med Oral Pathol 1950; 3:582–590.
- Topazian DS. Prevention of osteoradionecrosis of the jaws. Oral Surg Oral Med Oral Pathol 1959; 21:530–538.
- Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983; 41:283–288.
- Rossleigh MA, Smith J, Straus DJ, Engel IA. Osteonecrosis in patients with malignant lymphoma. A review of 31 cases. Cancer 1986; 58:1112–1116.
- Cook AM, Dzik-Jurasz AS, Padhani AR, Norman A, Huddart RA. The prevalence of avascular necrosis in patients treated with chemotherapy for testicular tumors. Br J Cancer 2001; 85:1624–1626.
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61:1115–1117.
- Migliorati CA. Bisphosphonates and oral cavity avascular bone necrosis. J Clin Oncol 2003; 21:4253–4254.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62:527–534.
- Wright MM, Wright BM, Christian J, Carey JJ. A case series of osteonecrosis of the jaw associated with the use of bisphosphonates. Arthritis Rheum 2005; ( suppl Sept): 1984.
- National Center for Biotechnology Information. PubMed. www.ncbi.nlm.nih.gov/PubMed. Accessed 10/1/2008.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2007; 65:369–376.
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bishosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479–1491.
- Ruggiero S, Gralow J, Marx R, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract 2006; 2:7–14.
- Wang HL, Weber D, McCauley LK. Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. J Periodontol 2007; 78:584–594.
- Freiberger JJ, Padilla-Burgos R, Chhoeu AH, et al. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg 2007; 65:1321–1327.
- Fleisch H. Bisphosphonates in Bone Disease. Fourth ed. San Diego, CA: Academic Press; 2000.
- Bone Health and Osteoporosis: A Report of the Surgeon General. www.surgeongeneral.gov/library/bonehealth. Accessed 10/1/2008.
- Carey JJ. What is a ‘failure’ of bisphosphonate therapy for osteoporosis? Cleve Clin J Med 2005; 72:1033–1039.
- Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333:1437–1443.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282:1344–1352.
- McClung MR, Geusens P, Miller PD, et al; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001; 344:333–340.
- Chesnut CH, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 2005; 353:898–908.
- Bone HG, Santora AC. Authors Reply. N Engl J Med 2004; 351:191–192.
- Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004; 9(suppl 4):28–37.
- Walter C, Grötz KA, Kunkel M, Al-Nawas B. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 2007; 15:197–202.
- Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaws in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol 2006; 134:620–623.
- Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: presentation of seven cases and literature review. Laryngoscope 2007; 117:30–34.
- Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006; 24:945–952.
- Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates (letter). N Engl J Med 2005; 353:99–100.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Gordis L. Epidemiology, 3rd ed. Philadelphia, Elsevier Saunders 2004:205.
- Fletcher RH, Fletcher SW, Wagner EH. Clinical Epidemiology. The Essentials. 3rd ed. Baltimore, MD; Lippincott, Williams & Wilkins, 1996:245.
- Sim J, Wright C. Research in Health Care, 1st ed. Cheltenham, England; Nelson Thornes, 2002.
- US Preventive Services Task Force Ratings. Strength of recommendations and quality of evidence. www.ahrq.gov/clinic/3rduspstf/ratings.htm. Accessed 10/1/2008.
- Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007; 65:415–423.
- Gibbs AE, Kherani A, Weitzel K, et al. Bisphosphonate-associated osteonecrosis: survey of oncologists. J Dent Res 2008; 87(special issue A):abstract #0639.
- Bone HG, Hosking D, Devogelaer JP, et al; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350:1189–1199.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462–468.
- Bilezekian JP, Gold DT, Goldring S, et al. Discussions in Osteoporosis Issue 5, Feb 2006 5–7. Adelphia Inc.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
- Pazianas M, Blumentals WA, Miller PD. Lack of association between oral bisphosphonates and osteonecrosis using jaw surgery as a surrogate marker. Osteoporos Int 2007; Epub Nov 13.
- Cartsos VM, Zhu S, Zavras AI. Bisphosphonate use and the risk of adverse jaw outcomes. J Am Dent Assoc 2008; 139:23–30.
- National Safety Council. The odds of dying from... www.nsc.org/lrs/statinfo/odds.htm. Accessed 10/1/2008.
- Palomo L, Bissada NF, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Little DG, Peat RA, Mcevoy A, Williams PR, Smith EJ, Baldock PA. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res 2003; 18:2016–2022.
- Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxf) 2005; 44:352–359.
- Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am 2007; 89:1727–1734.
- Kopterides P, Pikazis D, Koufos C. Successful treatment of SAPHO syndrome with zoledronic acid. Arthritis Rheum 2004; 50:2970–2973.
Recent case reports have linked bisphosphonate drugs to osteonecrosis of the jaw, and these reports have been widely publicized. Many patients receiving these drugs are asking their dentists and doctors whether the drugs do more harm than good, and some have even stopped taking them against medical advice. Health care professionals may be unsure what to tell patients and may be fearful of litigation.
However, most of the cases reported were in cancer patients, who are at significantly higher risk of osteonecrosis of the jaw for several reasons, and who receive much higher doses of bisphosphonates than do patients with osteoporosis or Paget disease of bone.
Moreover, although case reports have clearly documented an association between these drugs and osteonecrosis of the jaw, there is a lack of robust scientific evidence to support a cause-and-effect relationship. In fact, wellcontrolled clinical studies have not shown an increased risk of this complication in patients with osteoporosis or Paget disease of bone who were exposed to these agents, nor have they elucidated definite pathogenic mechanisms by which it might occur.
For these reasons, we believe that patients with osteoporosis should be advised of:
- Their risk of fracture
- The significant risk of morbidity and death following such a fracture
- The effectiveness and excellent safety of bisphosphonate therapy in preventing fractures
- The evidence that such therapy for osteoporosis and Paget disease poses little or no risk of osteonecrosis of the jaw
- The need for further research.
WHAT IS OSTEONECROSIS OF THE JAW?
Osteonecrosis—a general loss of bone tissue as a result of cell death1—can occur at any skeletal site, but it typically involves the long bones, ie, the femur, tibia, and humerus.
Osteonecrosis of the jaw is a rare disorder characterized by exposure and loss of bone in the maxillofacial complex. It can result in significant morbidity and can be resistant or refractory to conventional therapy.
This condition is not new, having been described in 19th century factory workers exposed to white phosphorus used in matchstick manufacturing. Known then as “phossy jaw,” it was associated with poor dentition and often resulted in severe disfigurement, disease, and death. Use of white phosphorus, and matches containing it, were subsequently banned in many countries.2
In the early 20th century, radiation therapy for cancers of the head and neck area came into vogue, but its side effects included damage to the skeleton, or osteoradionecrosis.3 In 1950, LaDow4 described a case of osteoradionecrosis of the jaw and reviewed the literature available at that time. He concluded that there were three main causes of osteonecrosis of the jaw, namely, radiation therapy, trauma, and infection.
Although many such cases have since been reported in association with radiation therapy, chemotherapy, or both, and involvement of other skeletal sites is well described,5–8 the actual incidence of osteoradionecrosis in the general population remains unclear because no large epidemiologic studies to elucidate accurate numbers have been published.
BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS OF THE JAW
Bisphosphonate-associated osteonecrosis of the jaw is a relatively new condition, having been first reported in three case series9–11 published in 2003 and 2004. The patients had exposure of areas of alveolar bone, mostly after oral surgery, eg, mucogingival flap elevation procedures (such as tooth extraction), that did not respond or were refractory to conventional treatment. All had received a bisphosphonate drug.
After these articles were published, the number of reported cases rose dramatically, including a case presented by one of us.12 By the end of January 2008, more than 500 papers on this condition were listed in PubMed. More than 60% had been printed since 2003, and approximately 85% concerned the association between osteonecrosis of the jaw and bisphosphonate use (search terms: “osteonecrosis of the jaw” and “bisphosphonate”).13
Although some dentists and oral surgeons claim to have seen many patients with this disorder, physicians who specialize in osteoporosis and metabolic bone disease do not. The medical literature and popular press have suggested that bisphosphonates are the cause of this malady. However, such articles are more perspective than evidence, as they are not scientific studies but rather reports of cases or series, or reviews of these. High-impact journals have given such articles prominent positions, highlighting the issue further, rather than balancing what is known and what is not known.
Thus, medicine safety boards, physicians, dentists, and oral surgeons have become increasingly concerned about the possible risk of this disorder in their patients on long-term bisphosphonate therapy, prompting organizations to issue management guidelines for this disorder and regulatory bodies to mandate warning labels on all drugs in this class about the possible risk.14–18 Funding agencies have highlighted this as an area in need of further investigation.17
However, robust evidence of a causal relationship is lacking. Contributing to the problem, other disorders can have similar presentations.
As a result, the diagnosis requires a dental examination and dental imaging, which are often impossible or impractical in a medical setting. Well-designed studies have relied on blinded panels of dental specialists using clinical and imaging data to adjudicate cases as osteonecrosis of the jaw before including them in published reports; case reports, however, often do not.
HOW IS OSTEONECROSIS OF THE JAW DIAGNOSED AND MANAGED?
A working definition of osteonecrosis of the jaw has recently emerged, and it will likely continue to evolve as results of further investigation become available.
A confirmed case is defined as an area of exposed bone in the maxillofacial region that does not heal within 8 weeks after being identified by a health care provider, in a patient who is currently receiving or has been exposed to a bisphosphonate and who has not had radiation therapy to the craniofacial region.14,17 This 8-week duration is consistent with the time frame in which soft tissue would be expected to close and exposed bone would be expected to heal under normal conditions after oral surgery such as dental extraction or a flap elevation procedure.
Patients may have no symptoms at the time of presentation. However, symptoms can include oral or jaw pain, difficulty chewing, evidence of infection, and dental loss. Bone loss is often apparent radiographically, and it may be focal or generalized. Other imaging studies such as cone beam computed tomography provide greater detail on the extent and nature of the lesions, and thus provide a better assessment.
Histologically, there is evidence of necrosis, cell death, and, usually, concomitant infection.9–12,17
Management can be difficult
Osteonecrosis of the jaw can be difficult to manage, and extensive guidelines have been published.14–17 Its treatment is complicated because resection of the necrotic area often only makes the necrotic area bigger. Unlike in osteoradionecrosis, surgical removal of the affected area often results in necrosis at the margins of resected bone. This creates a potential situation of “chasing” affected bone in procedure after procedure, which results in significant morbidity.
A preventive protocol for cancer patients
Most of the cases reported so far have been in cancer patients receiving long-term treatment with potent bisphosphonates in high intravenous doses (12 times the usual dose for osteoporosis) after a mucoperiosteal flap elevation dental procedure (many of which were performed on an emergency basis).9–12,14–20 Authors have thus concluded that a preventive protocol should be followed for all patients being considered for intensive bisphosphonate treatment, similar to that adopted for patients receiving head and neck radiation.
Specifically, all chronic dental and periodontal conditions should be identified and stabilized before starting intensive bisphosphonate therapy. Experts today believe that controlling all chronic dental problems before starting intensive intravenous bisphosphonate therapy may be the best method to avoid dental surgery after bisphosphonate therapy has begun, particularly since the washout period (time to elimination of the drug) for bisphosphonates in alveolar bone is unknown.14–20
Although authors seem to agree that such a preventive protocol is prudent for intensive intravenous therapy, it does not appear to be necessary for patients without cancer.14,17 Indeed, such an approach is impractical, given the huge numbers involved and the lack of evidence to support it.
WHAT ARE BISPHOSPHONATES, AND WHY THE CONCERN?
Bisphosphonates are analogues of pyrophosphates, inorganic compounds developed to remove calcium carbonate from water in industrial pipes and laundry machines. Pyrophosphate use in humans arose from their affinity for calcium phosphate, which proved beneficial in scintigraphic imaging studies and in preventing tartar build-up, resulting in their incorporation into toothpastes. Modifications of the pyrophosphate molecule led to the development of diphosphonate compounds (later known as bisphosphonates), which have gained widespread use in treating a variety of disorders of the skeleton and of calcium metabolism.
These drugs prevent bone resorption by selectively inhibiting osteoclastic activity through several mechanisms (depending on the compound), thus helping prevent bone loss, bone pain, and hypercalcemia in diseases of the skeleton.
Bisphosphonates are widely used
Today, oral and intravenous bisphosphonates are widely prescribed for several skeletal disorders, including metastatic disease, malignant hypercalcemia, Paget disease of bone, and prevention and treatment of osteoporosis.21–23
More than 10 million Americans and more than 200 million people worldwide may have osteoporosis, which results in more than 1 million fractures each year. The lifetime risk of fracture for a postmenopausal white woman today is approximately 40% (approximately 15% for a 50-year-old man), and her annual risk of fracture is greater than her combined risk of stroke, heart attack, and breast cancer.22 Several bisphosphonates have been shown to safely and significantly reduce the risk of fracture in patients with osteoporosis and to be effective therapies for Paget disease of bone.24–31
Bisphosphonates are the most widely prescribed drugs for osteoporosis,22,23,29 with almost 200 million prescriptions for oral bisphosphonates worldwide. As of 2004, exposure to alendronate (Fosamax) was estimated to be about 20 million patient-years.32 Noncompliance limits their effectiveness in practice, due in part to concerns about adverse effects.
Since bisphosphonates are so widely prescribed, concern has been raised that they may be causing a new epidemic of osteonecrosis of the jaw.9 However, most reported cases have been in cancer patients, who are known to be at increased risk of this condition and who receive doses of bisphosphonates up to 12 times higher than in patients with osteoporosis or Paget disease of bone.9–12,33–38
The optimal duration of bisphosphonate therapy for these diseases to obtain the maximum benefit and minimize cost and harm remains unclear. Although a recent report suggests a bisphosphonate “drug holiday” may be an option when treating postmenopausal osteoporosis, larger, more robust studies of longer duration are needed.39 Outcomes of osteonecrosis of the jaw related to drug holidays have not been investigated.
‘IF I TAKE THIS TO STOP BONE LOSS, WILL IT HURT MY JAWS?’
The recently described association between bisphosphonates and osteonecrosis of the jaw has received considerable attention. Guidelines have been drawn up, some based on the assumption that bisphosphonates cause the osteonecrosis, but not based on scientific research.14–18 More than 90% of reported cases have been in cancer patients, a group known to be at increased risk of osteonecrosis of the jaw and other skeletal sites, for reasons that include radiation therapy, chemotherapy, corticosteroid use, and increased risk of infections.4,6,9–12,33–38 Nevertheless, it has been assumed that these patients are the same as osteoporosis patients, and sometimes that causation is beyond dispute. This is problematic for two main reasons:
- Since noncompliance and lack of adherence (due to lack of knowledge about the dangers posed by osteoporosis, cost of the drugs, difficulty with dosing regimens, and fear of adverse effects) limit the effectiveness of these therapies in clinical practice, such attention has already persuaded patients to discontinue or refuse therapy (J.J. Carey, personal experience and communications from colleagues); and
- Patients with osteoporosis and osteoporotic fractures have increased rates of morbidity and mortality and significantly higher fracture risk, which can be prevented with these agents if they are willing to take them.
Association does not prove causation
However, association does not prove causation. A relationship between a drug and a disease may be due to chance alone or to confounding factors.40 To judge the exact nature of this relationship, several issues need to be considered when reviewing the available evidence.
Substantiating that an agent causes a disease requires careful consideration of several aspects of their relationship: temporality, strength, dose-response, reversibility, consistency, biologic plausibility, and specificity.41 Correct interpretation of the strength of the evidence should also incorporate an evaluation of the study design, size, and reporting mechanism. Accordingly, case reports and case series are considered to constitute the weakest evidence, while randomized controlled trials and meta-analyses are usually considered the strongest.
When a true cause-and-effect relationship does exist, the situation can be a simple one in which only a single agent is involved. However, the issue can be decidedly more complex when the cause is an effect-modifier, requiring the interaction of additional factors.
When a cause has been assumed, demonstration of the dose-response relationship is also important: whether the risk is related in a continuous fashion to dose and duration of therapy (all patients), is seen only with particular doses or regimens (such as frequent use of high doses of potent bisphosphonates), or exists only in people who have passed a certain threshold value (for example, it may only occur in those who have received 0.5 g of an intravenous or 10 g of an oral bisphosphonate). Bearing in mind these considerations, the nature of the relationship between an agent and a disease can be better understood.40–43
A cause-and-effect relationship has not been established
A cause-and-effect relationship between bisphosphonates and osteonecrosis of the jaw has not been clearly established.14,17 Although case series highlight a relationship between the two, large controlled trials evaluating the occurrence of osteonecrosis of the jaw as the primary outcome have not been conducted. To date, most cases have been reported as uncontrolled case series, generally considered the weakest form of evidence.43
Most cases have been in cancer patients
Most cases of osteonecrosis of the jaw were in patients with cancer (particularly breast cancer and multiple myeloma) receiving potent intravenous bisphosphonates in high doses, most of whom had other documented risk factors, including recent dental procedures such as tooth extraction.9–12,15–19,33–38
One of the most compelling studies supporting causation examined the prevalence of osteonecrosis of the jaw in a cohort of 303 myeloma patients from 1991 to 2003. Osteonecrosis of the jaw developed only in those taking bisphosphonates (28 of 254), and the risk appeared greatest in those treated with both zoledronic acid (Zometa) and thalidomide (Thalomid). The importance of additional chemotherapies, concomitant diseases, and baseline dental pathology was not described.35 Biases, including channeling bias (in which patients who appear at increased risk of this rare condition also appear to be most likely to receive this medication), referral bias, and survivor bias, were not addressed in this paper or in others claiming that the risk is related to the type of bisphosphonate used and the duration of its use.15,33–38
A review of all cases of osteonecrosis of the jaw over a 5-year period in one institution (N = 163) found that only 17 (10%) were associated with bisphosphonate use, and all 17 patients had other risk factors, such as concomitant therapy for malignancy and recent dental surgery.34 The authors’ concern that longer follow-up may have shown a higher incidence of this problem is supported by the temporal relationship seen in other reports in which cancer patients with osteonecrosis of the jaw appear to have had higher cumulative doses of intravenous bisphosphonates than those without.9–12,15,34,35,37,38 Unfortunately, only one study had a control group to highlight the incidence of osteonecrosis of the jaw in similar patients not treated with bisphosphonates.35 The incidence in cancer patients treated with intravenous bisphosphonates has been reported as between 0% and 11%, and the incidence is higher following dental procedures and with a greater duration of drug exposure.11,14,15,17,35,38,44
Interestingly, in a recent survey of oncologists prescribing bisphosphonate medications for metastatic indications, two-thirds said they believe their patients probably have undiagnosed chronic oral conditions that could increase the risk of osteonecrosis of the jaw following bisphosphonate therapy and dental surgery procedures. A similar number reported that their patients receive routine dental care (access to and cost of dental care and the difficulty in physician prescreening are cited as obstacles), but only about one-third actually refer their patients to dentists before starting bisphosphonate therapy.45
What recent studies in osteoporosis and Paget disease showed
Controlled scientific studies in osteoporosis and Paget disease of bone have not shown osteonecrosis of the jaw to emerge, even after years of treatment with bisphosphonate drugs.24–31,46–49 To date, more than 50,000 patients have been treated with oral bisphosphonates— more than 100,000 patient-years for each drug: alendronate, risedronate (Actonel), and ibandronate (Boniva)—in clinical trials, and there has not been a single case of bisphosphonate-associated osteonecrosis in any of these studies.48
Recent publications have addressed the results of clinical trials comparing zoledronic acid (the drug most often associated with this condition in published case series) and risedronate in more than 300 patients with Paget disease of bone,31 and with placebo in postmenopausal women with osteoporosis and persons over 50 years of age suffering a hip fracture treated for up to 3 years following their fracture.29,30
In the largest trial, almost 4,000 osteoporotic women were treated with 5 mg of zoledronic acid annually for 3 years, and a similar number received placebo. Despite a rigorous search for any potential cases of bisphosphonate-associated osteonecrosis of the jaw—adjudicated by a blinded panel of ex on the basis of clinical and dental diagnostic imaging—only two possible cases were found: one in the placebo group and one in the treatment group (a case of osteomyelitis that preceded any treatment with zoledronic acid). Both patients recovered following a course of oral antibiotics and debridement. There was no increase in osteonecrosis at other skeletal sites.29,49
Observational studies have yielded conflicting results. An Australian postal survey of oral surgeons and dentists combined with drug adverse events data suggested the frequency of osteonecrosis of the jaw was 1:2,260 to 1:8,470 in patients on weekly alendronate treatment for osteoporosis, and 1:56 to 1:380 in patients with Paget disease. Following dental extractions, this rose to 1:296 to 1:1,130 and 1:7.4 to 1:48, respectively. Results in patients with malignancy were similar to those in other studies.44 The study raises issues similar to those in other studies: lack of an appropriate control group, reporting bias, and the possibility of multiple reportings of the same patients.
Unpublished information from pharmaceutical companies has suggested the incidence of unconfirmed cases of osteonecrosis of the jaw in persons taking alendronate is 0.7/100,000 person-years.14,17 One study using administrative claims data did not find evidence of increased bisphosphonate use in patients undergoing jaw surgery (used as a surrogate for osteonecrosis of the jaw),50 while another actually found that oral bisphosphonates had a protective effect against osteonecrosis of the jaw, inflammatory conditions of the jaw, and need for major jaw surgery.51
The risk, if any, is probably very small
This information suggests that if these drugs, used at the recommended dose, really do pose a risk, it is probably very small: less than 1 case in 100,000 patient-years if taking an oral bisphosphonate such as alendronate.14,17 This is significantly less than the risk of fracture in these patients (which may be higher than 1 in 10), the risk of death following such a fracture,22–30 or the risk of death from drowning, house fire, or motor vehicle accident.52
The cases of osteonecrosis of the jaw that we have personally seen—all in cancer patients treated with chemotherapy and highdose bisphosphonates—all showed histologic evidence of necrosis and concomitant infections, suggesting the actual diagnosis was osteomyelitis. Bone biopsies from affected but macroscopically normal mandibles at the time of surgical debridement for osteonecrosis of the jaw showed normal or increased osteoclastic activity, in contrast to what one would expect if there were oversuppression of bone turnover (unpublished data, J. Christian, J. Carey, Cleveland Clinic).
Recently, this family of drugs has shown some promise in limiting the progression of alveolar bone loss in periodontal disease (though they are not approved for this indication).53–55 Finally, published studies suggest bisphosphonate therapy may even be beneficial in animals and humans with osteonecrosis,56–58 and in conditions that mimic osteonecrosis such as SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis) of the mandible, in which the histologic appearance may resemble that of osteonecrosis.59
WHAT SHOULD WE TELL OUR PATIENTS?
Several things are worth emphasizing from the published data and guidelines:
- Many things are unknown about osteonecrosis of the jaw and the risk in people taking bisphosphonates.
- The best evidence today does not support a cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate therapy.
- If bisphosphonates are causative, the risk appears very low in patients without cancer.
- It is important to distinguish between cancer and noncancer patients because of different risk factors, the markedly higher doses of bisphosphonates used in cancer patients, and the much greater incidence of osteonecrosis of the jaw seen in cancer patients irrespective of the cause.
- The higher risk in cancer patients is likely modified or confounded by additional risk factors, possibly including long-term use of high-dose intravenous bisphosphonates.
- About 90% of cases of bisphosphonate-associated osteonecrosis of the jaw have been in cancer patients, in whom a substantial temporal relationship to bisphosphonate therapy has been seen.9–12,15–17,19,49,54–57
- Prevention will likely be the most effective management strategy because of the significant morbidity associated with and the refractory nature of osteonecrosis of the jaw.
- Prophylactic dental examinations and any needed repair work are probably best done before starting bisphosphonate therapy in cancer patients; however, studies supporting such a strategy are needed.
- There is no evidence to support routine dental examinations before starting such therapy for disorders other than cancer, or for stopping such therapy before, during, or after dental surgery. Whether this is true for patients who have been taking these drugs for several years or more is unclear.
- Good communication between patients and their physicians, dentists, periodontists, and surgeons will help provide them with the best possible care.
Clearly, much further research is needed on the causes, risks, diagnosis, and management of this disorder to optimize patient outcomes.
Recent case reports have linked bisphosphonate drugs to osteonecrosis of the jaw, and these reports have been widely publicized. Many patients receiving these drugs are asking their dentists and doctors whether the drugs do more harm than good, and some have even stopped taking them against medical advice. Health care professionals may be unsure what to tell patients and may be fearful of litigation.
However, most of the cases reported were in cancer patients, who are at significantly higher risk of osteonecrosis of the jaw for several reasons, and who receive much higher doses of bisphosphonates than do patients with osteoporosis or Paget disease of bone.
Moreover, although case reports have clearly documented an association between these drugs and osteonecrosis of the jaw, there is a lack of robust scientific evidence to support a cause-and-effect relationship. In fact, wellcontrolled clinical studies have not shown an increased risk of this complication in patients with osteoporosis or Paget disease of bone who were exposed to these agents, nor have they elucidated definite pathogenic mechanisms by which it might occur.
For these reasons, we believe that patients with osteoporosis should be advised of:
- Their risk of fracture
- The significant risk of morbidity and death following such a fracture
- The effectiveness and excellent safety of bisphosphonate therapy in preventing fractures
- The evidence that such therapy for osteoporosis and Paget disease poses little or no risk of osteonecrosis of the jaw
- The need for further research.
WHAT IS OSTEONECROSIS OF THE JAW?
Osteonecrosis—a general loss of bone tissue as a result of cell death1—can occur at any skeletal site, but it typically involves the long bones, ie, the femur, tibia, and humerus.
Osteonecrosis of the jaw is a rare disorder characterized by exposure and loss of bone in the maxillofacial complex. It can result in significant morbidity and can be resistant or refractory to conventional therapy.
This condition is not new, having been described in 19th century factory workers exposed to white phosphorus used in matchstick manufacturing. Known then as “phossy jaw,” it was associated with poor dentition and often resulted in severe disfigurement, disease, and death. Use of white phosphorus, and matches containing it, were subsequently banned in many countries.2
In the early 20th century, radiation therapy for cancers of the head and neck area came into vogue, but its side effects included damage to the skeleton, or osteoradionecrosis.3 In 1950, LaDow4 described a case of osteoradionecrosis of the jaw and reviewed the literature available at that time. He concluded that there were three main causes of osteonecrosis of the jaw, namely, radiation therapy, trauma, and infection.
Although many such cases have since been reported in association with radiation therapy, chemotherapy, or both, and involvement of other skeletal sites is well described,5–8 the actual incidence of osteoradionecrosis in the general population remains unclear because no large epidemiologic studies to elucidate accurate numbers have been published.
BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS OF THE JAW
Bisphosphonate-associated osteonecrosis of the jaw is a relatively new condition, having been first reported in three case series9–11 published in 2003 and 2004. The patients had exposure of areas of alveolar bone, mostly after oral surgery, eg, mucogingival flap elevation procedures (such as tooth extraction), that did not respond or were refractory to conventional treatment. All had received a bisphosphonate drug.
After these articles were published, the number of reported cases rose dramatically, including a case presented by one of us.12 By the end of January 2008, more than 500 papers on this condition were listed in PubMed. More than 60% had been printed since 2003, and approximately 85% concerned the association between osteonecrosis of the jaw and bisphosphonate use (search terms: “osteonecrosis of the jaw” and “bisphosphonate”).13
Although some dentists and oral surgeons claim to have seen many patients with this disorder, physicians who specialize in osteoporosis and metabolic bone disease do not. The medical literature and popular press have suggested that bisphosphonates are the cause of this malady. However, such articles are more perspective than evidence, as they are not scientific studies but rather reports of cases or series, or reviews of these. High-impact journals have given such articles prominent positions, highlighting the issue further, rather than balancing what is known and what is not known.
Thus, medicine safety boards, physicians, dentists, and oral surgeons have become increasingly concerned about the possible risk of this disorder in their patients on long-term bisphosphonate therapy, prompting organizations to issue management guidelines for this disorder and regulatory bodies to mandate warning labels on all drugs in this class about the possible risk.14–18 Funding agencies have highlighted this as an area in need of further investigation.17
However, robust evidence of a causal relationship is lacking. Contributing to the problem, other disorders can have similar presentations.
As a result, the diagnosis requires a dental examination and dental imaging, which are often impossible or impractical in a medical setting. Well-designed studies have relied on blinded panels of dental specialists using clinical and imaging data to adjudicate cases as osteonecrosis of the jaw before including them in published reports; case reports, however, often do not.
HOW IS OSTEONECROSIS OF THE JAW DIAGNOSED AND MANAGED?
A working definition of osteonecrosis of the jaw has recently emerged, and it will likely continue to evolve as results of further investigation become available.
A confirmed case is defined as an area of exposed bone in the maxillofacial region that does not heal within 8 weeks after being identified by a health care provider, in a patient who is currently receiving or has been exposed to a bisphosphonate and who has not had radiation therapy to the craniofacial region.14,17 This 8-week duration is consistent with the time frame in which soft tissue would be expected to close and exposed bone would be expected to heal under normal conditions after oral surgery such as dental extraction or a flap elevation procedure.
Patients may have no symptoms at the time of presentation. However, symptoms can include oral or jaw pain, difficulty chewing, evidence of infection, and dental loss. Bone loss is often apparent radiographically, and it may be focal or generalized. Other imaging studies such as cone beam computed tomography provide greater detail on the extent and nature of the lesions, and thus provide a better assessment.
Histologically, there is evidence of necrosis, cell death, and, usually, concomitant infection.9–12,17
Management can be difficult
Osteonecrosis of the jaw can be difficult to manage, and extensive guidelines have been published.14–17 Its treatment is complicated because resection of the necrotic area often only makes the necrotic area bigger. Unlike in osteoradionecrosis, surgical removal of the affected area often results in necrosis at the margins of resected bone. This creates a potential situation of “chasing” affected bone in procedure after procedure, which results in significant morbidity.
A preventive protocol for cancer patients
Most of the cases reported so far have been in cancer patients receiving long-term treatment with potent bisphosphonates in high intravenous doses (12 times the usual dose for osteoporosis) after a mucoperiosteal flap elevation dental procedure (many of which were performed on an emergency basis).9–12,14–20 Authors have thus concluded that a preventive protocol should be followed for all patients being considered for intensive bisphosphonate treatment, similar to that adopted for patients receiving head and neck radiation.
Specifically, all chronic dental and periodontal conditions should be identified and stabilized before starting intensive bisphosphonate therapy. Experts today believe that controlling all chronic dental problems before starting intensive intravenous bisphosphonate therapy may be the best method to avoid dental surgery after bisphosphonate therapy has begun, particularly since the washout period (time to elimination of the drug) for bisphosphonates in alveolar bone is unknown.14–20
Although authors seem to agree that such a preventive protocol is prudent for intensive intravenous therapy, it does not appear to be necessary for patients without cancer.14,17 Indeed, such an approach is impractical, given the huge numbers involved and the lack of evidence to support it.
WHAT ARE BISPHOSPHONATES, AND WHY THE CONCERN?
Bisphosphonates are analogues of pyrophosphates, inorganic compounds developed to remove calcium carbonate from water in industrial pipes and laundry machines. Pyrophosphate use in humans arose from their affinity for calcium phosphate, which proved beneficial in scintigraphic imaging studies and in preventing tartar build-up, resulting in their incorporation into toothpastes. Modifications of the pyrophosphate molecule led to the development of diphosphonate compounds (later known as bisphosphonates), which have gained widespread use in treating a variety of disorders of the skeleton and of calcium metabolism.
These drugs prevent bone resorption by selectively inhibiting osteoclastic activity through several mechanisms (depending on the compound), thus helping prevent bone loss, bone pain, and hypercalcemia in diseases of the skeleton.
Bisphosphonates are widely used
Today, oral and intravenous bisphosphonates are widely prescribed for several skeletal disorders, including metastatic disease, malignant hypercalcemia, Paget disease of bone, and prevention and treatment of osteoporosis.21–23
More than 10 million Americans and more than 200 million people worldwide may have osteoporosis, which results in more than 1 million fractures each year. The lifetime risk of fracture for a postmenopausal white woman today is approximately 40% (approximately 15% for a 50-year-old man), and her annual risk of fracture is greater than her combined risk of stroke, heart attack, and breast cancer.22 Several bisphosphonates have been shown to safely and significantly reduce the risk of fracture in patients with osteoporosis and to be effective therapies for Paget disease of bone.24–31
Bisphosphonates are the most widely prescribed drugs for osteoporosis,22,23,29 with almost 200 million prescriptions for oral bisphosphonates worldwide. As of 2004, exposure to alendronate (Fosamax) was estimated to be about 20 million patient-years.32 Noncompliance limits their effectiveness in practice, due in part to concerns about adverse effects.
Since bisphosphonates are so widely prescribed, concern has been raised that they may be causing a new epidemic of osteonecrosis of the jaw.9 However, most reported cases have been in cancer patients, who are known to be at increased risk of this condition and who receive doses of bisphosphonates up to 12 times higher than in patients with osteoporosis or Paget disease of bone.9–12,33–38
The optimal duration of bisphosphonate therapy for these diseases to obtain the maximum benefit and minimize cost and harm remains unclear. Although a recent report suggests a bisphosphonate “drug holiday” may be an option when treating postmenopausal osteoporosis, larger, more robust studies of longer duration are needed.39 Outcomes of osteonecrosis of the jaw related to drug holidays have not been investigated.
‘IF I TAKE THIS TO STOP BONE LOSS, WILL IT HURT MY JAWS?’
The recently described association between bisphosphonates and osteonecrosis of the jaw has received considerable attention. Guidelines have been drawn up, some based on the assumption that bisphosphonates cause the osteonecrosis, but not based on scientific research.14–18 More than 90% of reported cases have been in cancer patients, a group known to be at increased risk of osteonecrosis of the jaw and other skeletal sites, for reasons that include radiation therapy, chemotherapy, corticosteroid use, and increased risk of infections.4,6,9–12,33–38 Nevertheless, it has been assumed that these patients are the same as osteoporosis patients, and sometimes that causation is beyond dispute. This is problematic for two main reasons:
- Since noncompliance and lack of adherence (due to lack of knowledge about the dangers posed by osteoporosis, cost of the drugs, difficulty with dosing regimens, and fear of adverse effects) limit the effectiveness of these therapies in clinical practice, such attention has already persuaded patients to discontinue or refuse therapy (J.J. Carey, personal experience and communications from colleagues); and
- Patients with osteoporosis and osteoporotic fractures have increased rates of morbidity and mortality and significantly higher fracture risk, which can be prevented with these agents if they are willing to take them.
Association does not prove causation
However, association does not prove causation. A relationship between a drug and a disease may be due to chance alone or to confounding factors.40 To judge the exact nature of this relationship, several issues need to be considered when reviewing the available evidence.
Substantiating that an agent causes a disease requires careful consideration of several aspects of their relationship: temporality, strength, dose-response, reversibility, consistency, biologic plausibility, and specificity.41 Correct interpretation of the strength of the evidence should also incorporate an evaluation of the study design, size, and reporting mechanism. Accordingly, case reports and case series are considered to constitute the weakest evidence, while randomized controlled trials and meta-analyses are usually considered the strongest.
When a true cause-and-effect relationship does exist, the situation can be a simple one in which only a single agent is involved. However, the issue can be decidedly more complex when the cause is an effect-modifier, requiring the interaction of additional factors.
When a cause has been assumed, demonstration of the dose-response relationship is also important: whether the risk is related in a continuous fashion to dose and duration of therapy (all patients), is seen only with particular doses or regimens (such as frequent use of high doses of potent bisphosphonates), or exists only in people who have passed a certain threshold value (for example, it may only occur in those who have received 0.5 g of an intravenous or 10 g of an oral bisphosphonate). Bearing in mind these considerations, the nature of the relationship between an agent and a disease can be better understood.40–43
A cause-and-effect relationship has not been established
A cause-and-effect relationship between bisphosphonates and osteonecrosis of the jaw has not been clearly established.14,17 Although case series highlight a relationship between the two, large controlled trials evaluating the occurrence of osteonecrosis of the jaw as the primary outcome have not been conducted. To date, most cases have been reported as uncontrolled case series, generally considered the weakest form of evidence.43
Most cases have been in cancer patients
Most cases of osteonecrosis of the jaw were in patients with cancer (particularly breast cancer and multiple myeloma) receiving potent intravenous bisphosphonates in high doses, most of whom had other documented risk factors, including recent dental procedures such as tooth extraction.9–12,15–19,33–38
One of the most compelling studies supporting causation examined the prevalence of osteonecrosis of the jaw in a cohort of 303 myeloma patients from 1991 to 2003. Osteonecrosis of the jaw developed only in those taking bisphosphonates (28 of 254), and the risk appeared greatest in those treated with both zoledronic acid (Zometa) and thalidomide (Thalomid). The importance of additional chemotherapies, concomitant diseases, and baseline dental pathology was not described.35 Biases, including channeling bias (in which patients who appear at increased risk of this rare condition also appear to be most likely to receive this medication), referral bias, and survivor bias, were not addressed in this paper or in others claiming that the risk is related to the type of bisphosphonate used and the duration of its use.15,33–38
A review of all cases of osteonecrosis of the jaw over a 5-year period in one institution (N = 163) found that only 17 (10%) were associated with bisphosphonate use, and all 17 patients had other risk factors, such as concomitant therapy for malignancy and recent dental surgery.34 The authors’ concern that longer follow-up may have shown a higher incidence of this problem is supported by the temporal relationship seen in other reports in which cancer patients with osteonecrosis of the jaw appear to have had higher cumulative doses of intravenous bisphosphonates than those without.9–12,15,34,35,37,38 Unfortunately, only one study had a control group to highlight the incidence of osteonecrosis of the jaw in similar patients not treated with bisphosphonates.35 The incidence in cancer patients treated with intravenous bisphosphonates has been reported as between 0% and 11%, and the incidence is higher following dental procedures and with a greater duration of drug exposure.11,14,15,17,35,38,44
Interestingly, in a recent survey of oncologists prescribing bisphosphonate medications for metastatic indications, two-thirds said they believe their patients probably have undiagnosed chronic oral conditions that could increase the risk of osteonecrosis of the jaw following bisphosphonate therapy and dental surgery procedures. A similar number reported that their patients receive routine dental care (access to and cost of dental care and the difficulty in physician prescreening are cited as obstacles), but only about one-third actually refer their patients to dentists before starting bisphosphonate therapy.45
What recent studies in osteoporosis and Paget disease showed
Controlled scientific studies in osteoporosis and Paget disease of bone have not shown osteonecrosis of the jaw to emerge, even after years of treatment with bisphosphonate drugs.24–31,46–49 To date, more than 50,000 patients have been treated with oral bisphosphonates— more than 100,000 patient-years for each drug: alendronate, risedronate (Actonel), and ibandronate (Boniva)—in clinical trials, and there has not been a single case of bisphosphonate-associated osteonecrosis in any of these studies.48
Recent publications have addressed the results of clinical trials comparing zoledronic acid (the drug most often associated with this condition in published case series) and risedronate in more than 300 patients with Paget disease of bone,31 and with placebo in postmenopausal women with osteoporosis and persons over 50 years of age suffering a hip fracture treated for up to 3 years following their fracture.29,30
In the largest trial, almost 4,000 osteoporotic women were treated with 5 mg of zoledronic acid annually for 3 years, and a similar number received placebo. Despite a rigorous search for any potential cases of bisphosphonate-associated osteonecrosis of the jaw—adjudicated by a blinded panel of ex on the basis of clinical and dental diagnostic imaging—only two possible cases were found: one in the placebo group and one in the treatment group (a case of osteomyelitis that preceded any treatment with zoledronic acid). Both patients recovered following a course of oral antibiotics and debridement. There was no increase in osteonecrosis at other skeletal sites.29,49
Observational studies have yielded conflicting results. An Australian postal survey of oral surgeons and dentists combined with drug adverse events data suggested the frequency of osteonecrosis of the jaw was 1:2,260 to 1:8,470 in patients on weekly alendronate treatment for osteoporosis, and 1:56 to 1:380 in patients with Paget disease. Following dental extractions, this rose to 1:296 to 1:1,130 and 1:7.4 to 1:48, respectively. Results in patients with malignancy were similar to those in other studies.44 The study raises issues similar to those in other studies: lack of an appropriate control group, reporting bias, and the possibility of multiple reportings of the same patients.
Unpublished information from pharmaceutical companies has suggested the incidence of unconfirmed cases of osteonecrosis of the jaw in persons taking alendronate is 0.7/100,000 person-years.14,17 One study using administrative claims data did not find evidence of increased bisphosphonate use in patients undergoing jaw surgery (used as a surrogate for osteonecrosis of the jaw),50 while another actually found that oral bisphosphonates had a protective effect against osteonecrosis of the jaw, inflammatory conditions of the jaw, and need for major jaw surgery.51
The risk, if any, is probably very small
This information suggests that if these drugs, used at the recommended dose, really do pose a risk, it is probably very small: less than 1 case in 100,000 patient-years if taking an oral bisphosphonate such as alendronate.14,17 This is significantly less than the risk of fracture in these patients (which may be higher than 1 in 10), the risk of death following such a fracture,22–30 or the risk of death from drowning, house fire, or motor vehicle accident.52
The cases of osteonecrosis of the jaw that we have personally seen—all in cancer patients treated with chemotherapy and highdose bisphosphonates—all showed histologic evidence of necrosis and concomitant infections, suggesting the actual diagnosis was osteomyelitis. Bone biopsies from affected but macroscopically normal mandibles at the time of surgical debridement for osteonecrosis of the jaw showed normal or increased osteoclastic activity, in contrast to what one would expect if there were oversuppression of bone turnover (unpublished data, J. Christian, J. Carey, Cleveland Clinic).
Recently, this family of drugs has shown some promise in limiting the progression of alveolar bone loss in periodontal disease (though they are not approved for this indication).53–55 Finally, published studies suggest bisphosphonate therapy may even be beneficial in animals and humans with osteonecrosis,56–58 and in conditions that mimic osteonecrosis such as SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, and osteitis) of the mandible, in which the histologic appearance may resemble that of osteonecrosis.59
WHAT SHOULD WE TELL OUR PATIENTS?
Several things are worth emphasizing from the published data and guidelines:
- Many things are unknown about osteonecrosis of the jaw and the risk in people taking bisphosphonates.
- The best evidence today does not support a cause-and-effect relationship between osteonecrosis of the jaw and bisphosphonate therapy.
- If bisphosphonates are causative, the risk appears very low in patients without cancer.
- It is important to distinguish between cancer and noncancer patients because of different risk factors, the markedly higher doses of bisphosphonates used in cancer patients, and the much greater incidence of osteonecrosis of the jaw seen in cancer patients irrespective of the cause.
- The higher risk in cancer patients is likely modified or confounded by additional risk factors, possibly including long-term use of high-dose intravenous bisphosphonates.
- About 90% of cases of bisphosphonate-associated osteonecrosis of the jaw have been in cancer patients, in whom a substantial temporal relationship to bisphosphonate therapy has been seen.9–12,15–17,19,49,54–57
- Prevention will likely be the most effective management strategy because of the significant morbidity associated with and the refractory nature of osteonecrosis of the jaw.
- Prophylactic dental examinations and any needed repair work are probably best done before starting bisphosphonate therapy in cancer patients; however, studies supporting such a strategy are needed.
- There is no evidence to support routine dental examinations before starting such therapy for disorders other than cancer, or for stopping such therapy before, during, or after dental surgery. Whether this is true for patients who have been taking these drugs for several years or more is unclear.
- Good communication between patients and their physicians, dentists, periodontists, and surgeons will help provide them with the best possible care.
Clearly, much further research is needed on the causes, risks, diagnosis, and management of this disorder to optimize patient outcomes.
- Thomas CL. Taber’s Cyclopedic Medical Dictionary, 17th ed. Philadelphia, FA Davis, 1993.
- Donoghue AM. Bisphosphonates and osteonecrosis: analogy to phossy jaw. Med J Aust 2005; 183:163–164.
- Watson WL, Scarborough JE. Osteoradionecrosis in intraoral cancer. Am J Roentgenol 1938; 40:524–534.
- LaDow CS. Osteoradionecrosis of the jaw. Oral Surg Oral Med Oral Pathol 1950; 3:582–590.
- Topazian DS. Prevention of osteoradionecrosis of the jaws. Oral Surg Oral Med Oral Pathol 1959; 21:530–538.
- Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983; 41:283–288.
- Rossleigh MA, Smith J, Straus DJ, Engel IA. Osteonecrosis in patients with malignant lymphoma. A review of 31 cases. Cancer 1986; 58:1112–1116.
- Cook AM, Dzik-Jurasz AS, Padhani AR, Norman A, Huddart RA. The prevalence of avascular necrosis in patients treated with chemotherapy for testicular tumors. Br J Cancer 2001; 85:1624–1626.
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61:1115–1117.
- Migliorati CA. Bisphosphonates and oral cavity avascular bone necrosis. J Clin Oncol 2003; 21:4253–4254.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62:527–534.
- Wright MM, Wright BM, Christian J, Carey JJ. A case series of osteonecrosis of the jaw associated with the use of bisphosphonates. Arthritis Rheum 2005; ( suppl Sept): 1984.
- National Center for Biotechnology Information. PubMed. www.ncbi.nlm.nih.gov/PubMed. Accessed 10/1/2008.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2007; 65:369–376.
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bishosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479–1491.
- Ruggiero S, Gralow J, Marx R, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract 2006; 2:7–14.
- Wang HL, Weber D, McCauley LK. Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. J Periodontol 2007; 78:584–594.
- Freiberger JJ, Padilla-Burgos R, Chhoeu AH, et al. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg 2007; 65:1321–1327.
- Fleisch H. Bisphosphonates in Bone Disease. Fourth ed. San Diego, CA: Academic Press; 2000.
- Bone Health and Osteoporosis: A Report of the Surgeon General. www.surgeongeneral.gov/library/bonehealth. Accessed 10/1/2008.
- Carey JJ. What is a ‘failure’ of bisphosphonate therapy for osteoporosis? Cleve Clin J Med 2005; 72:1033–1039.
- Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333:1437–1443.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282:1344–1352.
- McClung MR, Geusens P, Miller PD, et al; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001; 344:333–340.
- Chesnut CH, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 2005; 353:898–908.
- Bone HG, Santora AC. Authors Reply. N Engl J Med 2004; 351:191–192.
- Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004; 9(suppl 4):28–37.
- Walter C, Grötz KA, Kunkel M, Al-Nawas B. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 2007; 15:197–202.
- Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaws in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol 2006; 134:620–623.
- Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: presentation of seven cases and literature review. Laryngoscope 2007; 117:30–34.
- Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006; 24:945–952.
- Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates (letter). N Engl J Med 2005; 353:99–100.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Gordis L. Epidemiology, 3rd ed. Philadelphia, Elsevier Saunders 2004:205.
- Fletcher RH, Fletcher SW, Wagner EH. Clinical Epidemiology. The Essentials. 3rd ed. Baltimore, MD; Lippincott, Williams & Wilkins, 1996:245.
- Sim J, Wright C. Research in Health Care, 1st ed. Cheltenham, England; Nelson Thornes, 2002.
- US Preventive Services Task Force Ratings. Strength of recommendations and quality of evidence. www.ahrq.gov/clinic/3rduspstf/ratings.htm. Accessed 10/1/2008.
- Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007; 65:415–423.
- Gibbs AE, Kherani A, Weitzel K, et al. Bisphosphonate-associated osteonecrosis: survey of oncologists. J Dent Res 2008; 87(special issue A):abstract #0639.
- Bone HG, Hosking D, Devogelaer JP, et al; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350:1189–1199.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462–468.
- Bilezekian JP, Gold DT, Goldring S, et al. Discussions in Osteoporosis Issue 5, Feb 2006 5–7. Adelphia Inc.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
- Pazianas M, Blumentals WA, Miller PD. Lack of association between oral bisphosphonates and osteonecrosis using jaw surgery as a surrogate marker. Osteoporos Int 2007; Epub Nov 13.
- Cartsos VM, Zhu S, Zavras AI. Bisphosphonate use and the risk of adverse jaw outcomes. J Am Dent Assoc 2008; 139:23–30.
- National Safety Council. The odds of dying from... www.nsc.org/lrs/statinfo/odds.htm. Accessed 10/1/2008.
- Palomo L, Bissada NF, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Little DG, Peat RA, Mcevoy A, Williams PR, Smith EJ, Baldock PA. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res 2003; 18:2016–2022.
- Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxf) 2005; 44:352–359.
- Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am 2007; 89:1727–1734.
- Kopterides P, Pikazis D, Koufos C. Successful treatment of SAPHO syndrome with zoledronic acid. Arthritis Rheum 2004; 50:2970–2973.
- Thomas CL. Taber’s Cyclopedic Medical Dictionary, 17th ed. Philadelphia, FA Davis, 1993.
- Donoghue AM. Bisphosphonates and osteonecrosis: analogy to phossy jaw. Med J Aust 2005; 183:163–164.
- Watson WL, Scarborough JE. Osteoradionecrosis in intraoral cancer. Am J Roentgenol 1938; 40:524–534.
- LaDow CS. Osteoradionecrosis of the jaw. Oral Surg Oral Med Oral Pathol 1950; 3:582–590.
- Topazian DS. Prevention of osteoradionecrosis of the jaws. Oral Surg Oral Med Oral Pathol 1959; 21:530–538.
- Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983; 41:283–288.
- Rossleigh MA, Smith J, Straus DJ, Engel IA. Osteonecrosis in patients with malignant lymphoma. A review of 31 cases. Cancer 1986; 58:1112–1116.
- Cook AM, Dzik-Jurasz AS, Padhani AR, Norman A, Huddart RA. The prevalence of avascular necrosis in patients treated with chemotherapy for testicular tumors. Br J Cancer 2001; 85:1624–1626.
- Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61:1115–1117.
- Migliorati CA. Bisphosphonates and oral cavity avascular bone necrosis. J Clin Oncol 2003; 21:4253–4254.
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62:527–534.
- Wright MM, Wright BM, Christian J, Carey JJ. A case series of osteonecrosis of the jaw associated with the use of bisphosphonates. Arthritis Rheum 2005; ( suppl Sept): 1984.
- National Center for Biotechnology Information. PubMed. www.ncbi.nlm.nih.gov/PubMed. Accessed 10/1/2008.
- American Dental Association Council on Scientific Affairs. Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc 2006; 137:1144–1150.
- Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 2006; 144:753–761.
- Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 2007; 65:369–376.
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bishosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479–1491.
- Ruggiero S, Gralow J, Marx R, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract 2006; 2:7–14.
- Wang HL, Weber D, McCauley LK. Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. J Periodontol 2007; 78:584–594.
- Freiberger JJ, Padilla-Burgos R, Chhoeu AH, et al. Hyperbaric oxygen treatment and bisphosphonate-induced osteonecrosis of the jaw: a case series. J Oral Maxillofac Surg 2007; 65:1321–1327.
- Fleisch H. Bisphosphonates in Bone Disease. Fourth ed. San Diego, CA: Academic Press; 2000.
- Bone Health and Osteoporosis: A Report of the Surgeon General. www.surgeongeneral.gov/library/bonehealth. Accessed 10/1/2008.
- Carey JJ. What is a ‘failure’ of bisphosphonate therapy for osteoporosis? Cleve Clin J Med 2005; 72:1033–1039.
- Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333:1437–1443.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348:1535–1541.
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282:1344–1352.
- McClung MR, Geusens P, Miller PD, et al; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001; 344:333–340.
- Chesnut CH, Skag A, Christiansen C, et al; Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19:1241–1249.
- Black DM, Delmas PD, Eastell R, et al; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356:1809–1822.
- Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357:1799–1809.
- Reid IR, Miller P, Lyles K, et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 2005; 353:898–908.
- Bone HG, Santora AC. Authors Reply. N Engl J Med 2004; 351:191–192.
- Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004; 9(suppl 4):28–37.
- Walter C, Grötz KA, Kunkel M, Al-Nawas B. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 2007; 15:197–202.
- Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaws in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol 2006; 134:620–623.
- Mortensen M, Lawson W, Montazem A. Osteonecrosis of the jaw associated with bisphosphonate use: presentation of seven cases and literature review. Laryngoscope 2007; 117:30–34.
- Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006; 24:945–952.
- Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates (letter). N Engl J Med 2005; 353:99–100.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Gordis L. Epidemiology, 3rd ed. Philadelphia, Elsevier Saunders 2004:205.
- Fletcher RH, Fletcher SW, Wagner EH. Clinical Epidemiology. The Essentials. 3rd ed. Baltimore, MD; Lippincott, Williams & Wilkins, 1996:245.
- Sim J, Wright C. Research in Health Care, 1st ed. Cheltenham, England; Nelson Thornes, 2002.
- US Preventive Services Task Force Ratings. Strength of recommendations and quality of evidence. www.ahrq.gov/clinic/3rduspstf/ratings.htm. Accessed 10/1/2008.
- Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007; 65:415–423.
- Gibbs AE, Kherani A, Weitzel K, et al. Bisphosphonate-associated osteonecrosis: survey of oncologists. J Dent Res 2008; 87(special issue A):abstract #0639.
- Bone HG, Hosking D, Devogelaer JP, et al; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350:1189–1199.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462–468.
- Bilezekian JP, Gold DT, Goldring S, et al. Discussions in Osteoporosis Issue 5, Feb 2006 5–7. Adelphia Inc.
- Grbic JT, Landesberg R, Lin SQ, et al; Health Outcomes and Reduced Incidence with Zoledronic Acid Once yearly Pivotal Fracture Trial Research Group. Incidence of osteonecrosis of the jaw in women with postmenopausal osteoporosis in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial. J Am Dent Assoc 2008; 139:32–40.
- Pazianas M, Blumentals WA, Miller PD. Lack of association between oral bisphosphonates and osteonecrosis using jaw surgery as a surrogate marker. Osteoporos Int 2007; Epub Nov 13.
- Cartsos VM, Zhu S, Zavras AI. Bisphosphonate use and the risk of adverse jaw outcomes. J Am Dent Assoc 2008; 139:23–30.
- National Safety Council. The odds of dying from... www.nsc.org/lrs/statinfo/odds.htm. Accessed 10/1/2008.
- Palomo L, Bissada NF, Liu J. Periodontal assessment of postmenopausal women receiving risedronate. Menopause 2005; 12:685–690.
- Rocha ML, Malacara JM, Sánchez-Marin FJ, Vazquez de la Torre CJ, Fajardo ME. Effect of alendronate on periodontal disease in postmenopausal women: a randomized placebo-controlled trial. J Periodontol 2004; 75:1579–1585.
- Jeffcoat MK, Cizza G, Shih WJ, Genco R, Lombardi A. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol 2007; 9:70–76.
- Little DG, Peat RA, Mcevoy A, Williams PR, Smith EJ, Baldock PA. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res 2003; 18:2016–2022.
- Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology (Oxf) 2005; 44:352–359.
- Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am 2007; 89:1727–1734.
- Kopterides P, Pikazis D, Koufos C. Successful treatment of SAPHO syndrome with zoledronic acid. Arthritis Rheum 2004; 50:2970–2973.
KEY POINTS
- Recently published data do not support the hypothesis that these drugs cause osteonecrosis of the jaw.
- There is no evidence to support routine dental examinations for all patients before starting bisphosphonate therapy for osteoporosis or Paget disease, but heightened concern seems warranted for cancer patients.
- Clinical experience suggests that dental work by experienced dentists and surgeons can be carried out safely with very little risk to patients taking bisphosphonates.