User login
Treatment of Lactic Acidosis
Lactic acidosis (LA) is common in hospitalized patients and is associated with a high mortality.1, 2 Commonly, it is defined as a lactic acid concentration greater than 5 mmol/L with a pH less than 7.35.3 There are no evidence‐based guidelines for the treatment of LA despite progress in our understanding of its pathophysiology.36 This is not surprising, given the uncertainty regarding the impact of LA itself on clinical outcomes. In this regard, it is interesting to note that, despite its well‐recognized role as a marker of tissue hypoxia, lactate accumulation appears to have beneficial effects and may function as an adaptive mechanism. This raises the possibility that therapy directed at altering this adaptation may be detrimental. Pursuing correction of the pH in LA has been shown to have untoward physiologic effects. These and other ambiguities in the pathophysiology and treatment of LA are the focus of this review.
Lactate Metabolism
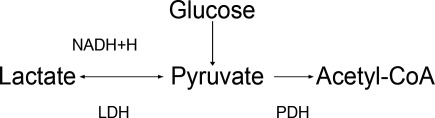
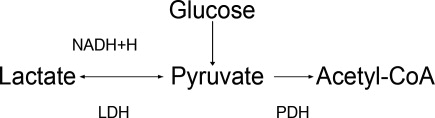
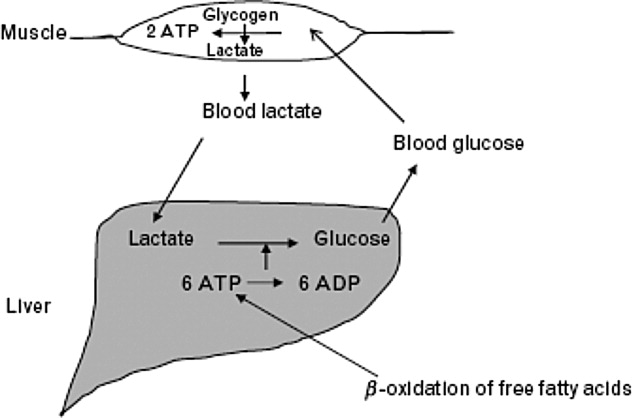
The body produces approximately 1400 mmol of lactate daily.7 Lactate is derived from the metabolism of pyruvate through an anaerobic reaction that occurs in all tissues (Figure 1). The liver is the primary site of lactate clearance and can metabolize up to 100 mmol per hour under normal conditions.8 There, lactate is converted to glucose to serve as an energy source during periods of hypoxia (Figure 2).9
Approximately 20% to 30% of the daily lactate load is metabolized by the kidneys.10, 11 Renal clearance is increased in acidosis12 and is maintained even in the presence of low renal perfusion.10, 12, 13 Renal lactate clearance is primarily through metabolism and not excretion.10, 14
LA Subtypes
Generally, lactic acid accumulation results from excess lactic acid production and not from reduced clearance.15 In cases of fulminant liver failure, it is due to a combination of decreased clearance and tissue hypoxia.16 In the setting of tissue hypoxia, an impairment of mitochondrial oxidative capacity results in the accumulation of pyruvate and generation of lactate. Lactic acid accumulation through this mechanism has historically been described as Type A LA.7 Hence, in critically ill patients lactate has traditionally been viewed as a marker of tissue hypoxia.15, 1721 Hyperlactatemia without tissue hypoxia has been referred to as type B LA. This is seen in a variety of circumstances. In sepsis, for example, several studies have shown lactic acid accumulation, despite adequate oxygen delivery.2224
Hyperlactatemia may also occur in cases of pure mitochondrial dysfunction, which can be induced by commonly prescribed medications such as the biguanides, nucleoside analog reverse‐transcriptase inhibitors (NRTIs), and linezolid.2527 Alternatively, lactate generation from metabolism of agents such as propylene glycol is possible. Finally, excessive lactate generation may occur following stress due to altered carbohydrate metabolism, or with respiratory alkalosis.2831
Lactate: A Metabolic Adaptation
Lactate was traditionally considered only as a marker of tissue hypoxia and anaerobic metabolism.17 This is certainly the case in situations of poor perfusion such as cardiogenic,15, 18 vasopressor‐resistant,19 or hypovolemic shock.20, 21
Alternative explanations for lactic acid accumulation, without tissue hypoperfusion, include catecholamine‐induced alterations in glycolysis,32, 33 mitochondrial disturbances,3436 and increased pyruvate production combined with increased glucose entry into cells.24, 37 In addition, the activity of an enzyme regulating lactate metabolism, pyruvate dehydrogenase kinase, increases in sepsis.38 This enzyme inactivates the pyruvate dehydrogenase (PDH) complex, which metabolizes pyruvate. Pyruvate and lactate may accumulate as a result. These changes partly explain the generation of LA in sepsis, independent of any effect of diminished tissue perfusion.
Recognizing the body's tendency toward homeostasis, it is appealing to speculate that lactate accumulation is adaptive.9 A number of findings support this. For example, lactate may act to shuttle energy between organs, or between cell types in the same organ. The astrocyteneuron lactate shuttle and the spermatogenic lactate shuttle are 2 examples of lactate's valuable effects on cellular metabolism.39 In the astrocyteneuron lactate shuttle, astrocytes support the increased metabolic demands of neurons through lactic acid production.40 Specifically, the neurotransmitter glutamate is released by the neurons and taken up by the astrocytes. Astrocytes produce lactate, which then moves back to the neuron to be used as an energy source. Glutamine, also released by the astrocytes, leads to the regeneration of glutamate and the potential to restart the cycle.39
Animal and human studies have suggested that, in periods of stress, lactate is the preferential energy substrate in the brain.4144 The usefulness of increased lactate production routinely seen in sepsis may thus represent multiple adaptive processes aimed primarily at improving the delivery of energy substrates. Thus, therapeutic strategies aimed specifically at lowering lactic acid levels may prove to have deleterious effects on cellular metabolism.
Impact of LA on Morbidity and Mortality
The poor prognosis in patients with LA is well recognized.2, 4548 For example, in a study of 126 patients with various causes of LA, the median survival was 38.5 hours and 30‐day survival was 17%.2
Studies have revealed that LA with low pH is associated with adverse effects on the cardiovascular system, particularly a decrease in cardiac contractility.49, 50 This effect is particularly prominent with a pH below 7.20. In contrast, acidosis in animal models has been shown to limit myocardial infarct size after reperfusion.51, 52 Variable effects of LA on cell death have been found. A worsening of apoptosis in myocytes has been noted;53 alternatively, protection from hypoxic injury in hepatocytes and myocardium has been observed.52, 54 Thus, although LA is associated with poor outcomes in human studies,2, 4547 it is still unclear to what extent lactic acid accumulation is a marker of severe illness, an independent effector of pathology, or a mechanism with the potential to serve a protective role.
Available data indicate that lactate itself is not harmful. Studies on infusion of lactate solutions to postoperative patients was shown to be safe.55 Also, the fact that lactate generation in states of respiratory alkalosis, stress, or altered carbohydrate metabolism without sepsis is not associated with worse outcomes supports the fact that lactic acid alone may not be maladaptive.2831
Similarly, low pH is not necessarily maladaptive. In the postictal state,56 diabetic ketoacidosis,57 spontaneous respiratory acidosis,58 or permissive hypercapnia,59 low blood pH is not deleterious.
In summary, LA is associated with poor outcomes, and indirect evidence suggests that it is the underlying causative condition rather than the low pH or the lactate that is responsible for the dire outcomes.
Treatment of LA with Sodium Bicarbonate
Since excessive lactic acid generation is accompanied by consumption of plasma bicarbonate and a fall in plasma pH, sodium bicarbonate has been long proposed as a treatment for LA. While theoretically appealing, this strategy has not been validated by studies in animals or humans. Indeed, bicarbonate administration in LA often has been shown to be detrimental.60, 61 The adverse effects of bicarbonate administration in LA, while initially paradoxical, have a number of possible explanations.
First, bicarbonate administration can induce a reduction in intracellular pH.60, 62, 63 The mechanism involves bicarbonate's effect to increase carbon dioxide (CO2) generation through mass action effect. Because the cell membrane is more permeable to CO2 than to bicarbonate, intracellular pH falls.64, 65 In sepsis, this intracellular/extracellular pH discrepancy may be more pronounced due to alterations in blood flow.66 Other reports on outcomes of intracellular pH with bicarbonate therapy show variable effects.6772
Second, to the extent that bicarbonate administration raises extracellular pH, it is associated with a reduction in ionized calcium concentration, since the binding of calcium to albumin is pH dependent.73 A sodium bicarbonate load administered to patients with LA was associated with a significant fall in ionized calcium concentration, whereas a sodium chloride load was not.1 This can affect cardiac function, as the latter varies proportionally with calcium levels.74
Third, bicarbonate administration may reduce tissue oxygen delivery since the affinity of hemoglobin for oxygen increases as pH rises (Bohr effect).75 The administration of bicarbonate worsened systemic oxygen consumption in one study76 and decreased oxygen delivery in another.75
Fourth, bicarbonate administration may indirectly increase intracellular calcium concentration. Low intracellular pH (see above) stimulates proton efflux by way of proton transporters and exchangers, increasing intracellular sodium content.77 A high cell sodium content then may increase intracellular calcium, through the Na/Ca exchanger, impairing cellular function.7779 Compounding this, the reduced function of the Na/H ATPase as a regulator of intracellular sodium in sepsis may not be adequate to limit cell swelling.77
Against this background of mechanistic concerns with the use of bicarbonate treatment, it is not surprising that clinical outcomes have been inconsistent at best. In animal models of LA, the use of sodium bicarbonate has either negative effects on cardiac output60, 72 or no significant hemodynamic effect when compared to sodium chloride infusion.67, 80, 81 One animal study did show some benefit with sodium bicarbonate compared to saline, though all animals subsequently died.50
In humans, sodium bicarbonate was studied in 2 randomized trials of sepsis‐induced LA.1, 82 In a study by Cooper et al.,1 14 critically‐ill patients received sequential infusions of sodium bicarbonate or sodium chloride. Neither solution was superior to the other in terms of hemodynamic improvement. No benefit was noted even when analysis was limited to those with very low pH (<7.2). Mathieu et al.82 randomized 10 critically‐ill patients to sequential infusion of either sodium bicarbonate or sodium chloride. Similarly, no significant difference in hemodynamic variables was noted.
When taken together, these studies evaluating sodium bicarbonate in LA fail to show convincing benefit and raise serious questions about its detrimental effects. Extracellular pH may be a misleading marker of success in the treatment of LA, given its direct influence by sodium bicarbonate administration.
Treatment of LA and Use of Other Buffers
Other buffers (Carbicarb, dichloroacetate, and tromethamine [THAM]) have been studied for treatment of LA. Human studies have not shown superiority of any of the buffers as far as improving pH,83, 84 hemodynamics, or survival.85
Treatment of LA by Renal Replacement Therapy
Renal replacement therapy (RRT; dialysis and its variants) has been studied for the treatment of severe acidosis. RRT has a number of theoretical advantages over purely medical therapies in the treatment of LA: it can deliver large quantities of base without contributing to volume overload; it can directly remove lactate from the plasma; and it can mitigate the effect of alkalinization on ionized calcium concentration by delivering calcium.
In critically ill patients with intact liver function, continuous venovenous hemofiltration (CVVH) appears to contribute very little (less then 3%) to overall lactate clearance.86 While outcome studies are limited, continuous dialysis modalities consistently show improved resolution of acidosis of various types when compared to intermittent modalities.87, 88 As described above, this is related to base administration and is not a surprising finding. There are no studies comparing RRT and medical therapy with respect to clinical outcomes in patients with LA.
Special Situations
Biguanides
Biguanide‐induced LA can be due to impairment of hepatic neoglucogenesis, in the case of metformin, or increasing hepatic oxidative phosphorylation, in the case of phenformin.89 This infrequent complication90, 91 is associated with a high mortality.92 Proposed therapy has included the use of sodium bicarbonate infusion.93 In this setting, it is unclear if the use of bicarbonate alone improves clinical outcomes.94
Renal replacement therapy in a wide variety of formats has been used to treat this condition.93, 95101 Metformin has a high clearance during dialysis due to its low molecular weight and lack of protein binding.97, 98, 102 Nonetheless, its high volume of distribution suggests a longer dialysis time would be more beneficial if the main goal is reducing metformin levels.97, 103 The limited prospective literature and lack of conclusive evidence about what levels of metformin induce LA makes generalized recommendations about duration of hemodialysis purely speculative.104
NRTIs
The use of NRTIs is associated with LA due to impairment of mitochondrial oxidative phosphorylation.105108 This uncommon complication, if not recognized early, is associated with a high mortality.101, 109 Investigations are ongoing into agents directed at improving mitochondrial function such as riboflavin, thiamine, and L‐carnitine.110112 As with biguanide‐associated LA, RRT decisions should be individualized based on metabolic circumstances.
Lorazepam
Many intravenous medications are formulated in the alcohol solvent, propylene glycol. Injectable lorazepam has the highest proportional amount of propylene glycol compared with other commonly used agents.113, 114 The kidney normally eliminates 12% to 50% of administered propylene glycol via proximal tubule secretion.115 The remainder is metabolized by the liver to form pyruvate and lactate.114, 116, 117
When propylene glycol accumulates, as in cases of reduced renal function, it results in hyperosmolarity, LA, and can even induce additional kidney injury (probably through proximal tubular cell necrosis).118
LA due to propylene glycol has been reported by many authors and its incidence with high dose intravenous (IV) lorazepam has been estimated to be as high as 19%.114, 116, 119, 120 This disorder can frequently go unrecognized, as many other factors that induce LA often coincide in such patients. But when identified and promptly addressed, its prognosis seems to be favorable.114
The best treatment is prevention, by avoiding the use of IV lorazepam in patients with impaired renal function. Once it is recognized, the drug should be promptly withdrawn. In addition, removal by hemodialysis can quickly lower propylene glycol levels since it is a small, highly water soluble, non‐protein‐bound molecule.121 As no rebound in the level is expected, intermittent dialysis should be an acceptable modality.117
Linezolid
Recently, Gram‐positive bacteria in general and methicillin‐resistant Staphylococcus aureus in particular have emerged as major causes of nosocomial and community‐acquired infections. Linezolid, an oxazolidinone, is increasingly used to treat such infections. Several cases of LA have been associated with linezolid.27, 122, 123 and a survey of the Infectious Diseases Society of America (IDSA) Emerging Infections Network members revealed that this complication was commonly encountered.124 Linezolid causes LA by mitochondrial toxicity125, 126 and risk factors include prolonged exposure and older age. Once the disorder is recognized, the clinician should stop the drug immediately. Chemistries should be monitored frequently in patients on long‐term therapy.
Conclusions
Many studies note the association between LA and adverse outcomes.2, 4547 Though metabolic acidosis from elevated lactate levels may negatively affect organ function, the evidence supporting therapy specifically aimed at increasing pH in these settings is consistently poor.3, 127 Limitations have included small numbers of subjects,1, 82 variable outcomes studied, and the inability to assess intracellular metabolic stability.1, 61 When taking these factors into account it is hard to justify aggressive treatment of LA with mechanisms aimed at raising pH. Literature on the treatment of patients with LA and very low pH (below 7.2) is even more limited.
Moreover, lactate elevations may not represent tissue hypoperfusion. Lactate may have an important role in improving energy metabolism. This represents 1 additional reason to be hesitant when attempting to normalize pH in LA; we may be disrupting the body's physiologic response to sepsis. A conflict for clinicians emerges, however, as lactate is often used to define tissue ischemia. Obviously, more specific markers of tissue hypoperfusion would be ideal.
Bicarbonate therapy is an understandably attractive means to improve the acidemia, but there are serious mechanistic concerns with it use. Moreover, neither animal nor human studies, limited as they may be, show a convincing benefit. LA in the setting of acute kidney injury may be best treated with renal replacement therapy with bicarbonate‐based buffers, but controlled trials are lacking.
A number of commonly used drugs can cause LA. A heightened awareness on the part of clinicians will lead to prompt recognition of these cases, and timely treatment.
- ,,,.Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study.Ann Intern Med.1990;112(7):492–498.
- ,,, et al.Natural history and course of acquired lactic acidosis in adults. DCA‐Lactic Acidosis Study Group.Am J Med.1994;97(1):47–54.
- ,.Sodium bicarbonate for the treatment of lactic acidosis.Chest.2000;117(1):260–267.
- ,.Management of life‐threatening acid‐base disorders. First of two parts.N Engl J Med.1998;338(1):26–34.
- .Indications for use of bicarbonate in patients with metabolic acidosis.Br J Anaesth.1991;67(2):165–177.
- ,.Bench‐to‐bedside review: treating acid‐base abnormalities in the intensive care unit—the role of buffers.Crit Care.2004;8(4):259–265.
- .Lactic acidosis update for critical care clinicians.J Am Soc Nephrol.2001;12(suppl 17):S15–S19.
- ,.Lactic acidosis: diagnosis and treatment.Clin Endocrinol Metab.1980;9(3):513–541.
- .Lactate and shock state: the metabolic view.Curr Opin Crit Care.2006;12(4):315–321.
- .Bench‐to‐bedside review: lactate and the kidney.Crit Care.2002;6(4):322–326.
- ,,,.The influence of renal function on lactate and glucose metabolism.Biochem J.1984;219(1):73–78.
- ,.The effect of acidosis on lactate removal by the perfused rat kidney.Clin Sci Mol Med.1976;50(3):185–194.
- ,,.Transvisceral lactate fluxes during early endotoxemia.Chest.1996;110(1):198–204.
- ,,,,.Metabolism of lactate by the intact functioning kidney of the dog.Am J Physiol.1973;224(6):1463–1467.
- ,,, et al.Lactate and glucose metabolism in severe sepsis and cardiogenic shock.Crit Care Med.2005;33(10):2235–2240.
- ,,,, et al.Lactic acidosis in fulminant hepatic failure. Some aspects of pathogenesis and prognosis.J Hepatol.1985;1(4):405–416.
- ,.Lactic acidosis in critical illness.Crit Care Med.1992;20(1):80–93.
- ,,, et al.Effects of cardiogenic shock on lactate and glucose metabolism after heart surgery.Crit Care Med.2000;28(12):3784–3791.
- ,,,,,.Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine‐treated septic shock.Crit Care Med.2000;28(1):114–119.
- ,,, et al.Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock.Crit Care Med.1991;19(2):231–243.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,,,.Skeletal muscle partial pressure of oxygen in patients with sepsis.Crit Care Med.1994;22(4):640–650.
- ,.Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis.JAMA.1992;267(11):1503–1510.
- ,,,.Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability.Ann Surg.1996;224(1):97–102.
- ,,,.Biguanide‐associated lactic acidosis. Case report and review of the literature.Arch Intern Med.1992;152(11):2333–2336.
- ,,,.Bench‐to‐bedside review: severe lactic acidosis in HIV patients treated with nucleoside analogue reverse transcriptase inhibitors.Crit Care.2003;7(3):226–232.
- ,,,.Lactic acidosis after treatment with linezolid.Infection.2007;35(4):278–281.
- ,,,.Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion.Surgery.1991;110(1):54–67.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98(1):75–84.
- .,,,.Lactic acid kinetics in respiratory alkalosis.Crit Care Med.1991;19(9):1120–1124.
- .Significance of hyperlactatemia without acidosis during hypermetabolic stress.Crit Care Med.1997;25(11):1780–1781.
- ,,,.Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis.Lancet.1999;354(9177):505–508.
- ,,,,.Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study.Lancet.2005;365(9462):871–875.
- ,.Sepsis induces diaphragm electron transport chain dysfunction and protein depletion.Am J Respir Crit Care Med.2005;172(7):861–868.
- ,,, et al.Association between mitochondrial dysfunction and severity and outcome of septic shock.Lancet.2002;360(9328):219–223.
- ,.Mitochondrial dysfunction in sepsis.Biochem Soc Symp.1999;66:149–166.
- ,,, et al.Altered glucose transporter mRNA abundance in a rat model of endotoxic shock.Biochem Biophys Res Commun.1991;176(1):535–540.
- .Increased pyruvate dehydrogenase kinase activity in response to sepsis.Am J Physiol.1991;260(5 Pt 1):E669–E674.
- .Lactate metabolism: a new paradigm for the third millennium.J Physiol.2004;558(Pt 1):5–30.
- ,,, et al.Evidence supporting the existence of an activity‐dependent astrocyte‐neuron lactate shuttle.Dev Neurosci.1998;20(4–5):291–299.
- ,,,.Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation.J Neurochem.1997;69(1):423–426.
- .Bench‐to‐bedside review: a possible resolution of the glucose paradox of cerebral ischemia.Crit Care.2002;6(4):330–334.
- ,,,,,.Intravenous lactate prevents cerebral dysfunction during hypoglycaemia in insulin‐dependent diabetes mellitus.Clin Sci (Lond).1998;94(2):157–163.
- ,,,.Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study.Brain Res.1997;744(1):105–111.
- ,,,,,.Clinical prognostic markers in patients with severe sepsis: a prospective analysis of 139 consecutive cases.J Infect.2003;47(4):300–306.
- ,,, et al.Serum lactate as a predictor of mortality in patients with infection.Intensive Care Med.2007;33(6):970–977.
- ,,,.Lactate versus non‐lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients.Crit Care.2006;10(1):R22.
- .Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients.Am J Med.1961;30:840–848.
- ,,,,.Effect of lactic acidosis on canine hemodynamics and left ventricular function.Am J Physiol.1990;258(4 Pt 2):H1193–H1199.
- ,,,.Alkali therapy extends the period of survival during hypoxia: studies in rats.Am J Physiol.1996;271(2 Pt 2):R381–R387.
- ,,.Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts.J Clin Invest.1988;82(3):920–927.
- ,,, et al.Effect of acidotic blood reperfusion on reperfusion injury after coronary artery occlusion in the dog heart.J Cardiovasc Pharmacol.1998;31(2):179–186.
- ,,,.Hypoxia and acidosis activate cardiac myocyte death through the Bcl‐2 family protein BNIP3.Proc Natl Acad Sci U S A.2002;99(20):12825–12830.
- ,,,,,.Extracellular acidosis delays onset of cell death in ATP‐depleted hepatocytes.Am J Physiol.1988;255(3 Pt 1):C315–C322.
- ,.Metabolic and hemodynamic effects of hypertonic solutions: sodium‐lactate versus sodium chloride infusion in postoperative patients.Shock.2002;18(4):306–310.
- .Medical complications of status epilepticus.Adv Neurol.1983;34:395–398.
- ,,,,,.Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial.Rev Invest Clin.1991;43(3):234–238.
- ,,.Supercarbia in children: clinical course and outcome.Crit Care Med.1990;18(2):166–168.
- ,,,.Low mortality rate in adult respiratory distress syndrome using low‐volume, pressure‐limited ventilation with permissive hypercapnia: a prospective study.Crit Care Med.1994;22(10):1568–1578.
- ,,,.Systemic effects of NaHCO3 in experimental lactic acidosis in dogs.Am J Physiol.1982;242(6):F586–F591.
- ,,,,.Lactic acidosis: effect of treatment on intracellular pH and energetics in living rat heart.Am J Physiol.1992;262(5 Pt 2):H1572–H1578.
- ,,,,,.Effect of sodium bicarbonate on intracellular pH under different buffering conditions.Kidney Int.1996;49(5):1262–1267.
- ,,.Hemodynamic and hepatic pH responses to sodium bicarbonate and Carbicarb during systemic acidosis.Magn Reson Med.1990;16(3):403–410.
- ,,, et al.The increase in CO2 production induced by NaHCO3 depends on blood albumin and hemoglobin concentrations.Intensive Care Med.2000;26(5):558–564.
- ,,, et al.Initial effect of sodium bicarbonate on intracellular pH depends on the extracellular nonbicarbonate buffering capacity.Crit Care Med.2001;29(5):1033–1039.
- ,,,,,.Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock.Crit Care Med.2000;28(9):3233–3241.
- ,,,,,.The effects of sodium bicarbonate and a mixture of sodium bicarbonate and carbonate (“Carbicarb”) on skeletal muscle pH and hemodynamic status in rats with hypovolemic shock.Metabolism.1994;43(4):518–522.
- ,,, et al.Haemodynamic and metabolic effects in diabetic ketoacidosis in rats of treatment with sodium bicarbonate or a mixture of sodium bicarbonate and sodium carbonate.Diabetologia.1995;38(8):889–898.
- ,,,,,.Effects of sodium bicarbonate on striated muscle metabolism and intracellular pH during endotoxic shock.Shock.1994;1(3):196–200.
- ,,,,.Carbicarb, sodium bicarbonate, and sodium chloride in hypoxic lactic acidosis. Effect on arterial blood gases, lactate concentrations, hemodynamic variables, and myocardial intracellular pH.Chest.1993;104(3):913–918.
- ,.Improved hemodynamic function during hypoxia with Carbicarb, a new agent for the management of acidosis.Circulation.1988;77(1):227–233.
- ,,.Metabolic effects of sodium bicarbonate in hypoxic lactic acidosis in dogs.Am J Physiol.1985;249(5 Pt 2):F630–F635.
- .Binding of calcium to serum albumin. II. Effect of pH via competitive hydrogen and calcium ion binding to the imidazole groups of albumin.Scand J Clin Lab Invest.1972;29(1):75–83.
- ,,,,.Left ventricular contractility varies directly with blood ionized calcium.Ann Intern Med.1988;108(4):524–529.
- ,,.Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis.J Clin Invest.1971;50(3):700–706.
- ,,.Metabolic and hemodynamic consequences of sodium bicarbonate administration in patients with heart disease.Am J Med.1989;87(1):7–14.
- ,,.Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury.J Clin Pharmacol.1998;38(10):887–897.
- ,.SEA0400: a novel sodium‐calcium exchange inhibitor with cardioprotective properties.Cardiovasc Drug Rev.2004;22(4):334–347.
- ,,,.Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart.Circ Res.1987;60(5):700–707.
- ,.Acute haemodynamic effects of sodium bicarbonate administration in respiratory and metabolic acidosis in anaesthetized dogs.Anaesth Intensive Care.1997;25(6):615–620.
- ,,,.Bicarbonate does not increase left ventricular contractility during L‐lactic acidemia in pigs.Am Rev Respir Dis.1993;148(2):317–322.
- ,,,,.Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study.Crit Care Med.1991;19(11):1352–1356.
- ,,, et al.Safety and efficacy of intravenous Carbicarb in patients undergoing surgery: comparison with sodium bicarbonate in the treatment of mild metabolic acidosis. SPI Research Group. Study of Perioperative Ischemia.Crit Care Med.1994;22(10):1540–1549.
- ,,, et al.Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis.J Nephrol.2005;18(3):303–307.
- ,,, et al.A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate‐Lactic Acidosis Study Group.N Engl J Med.1992;327(22):1564–1569.
- ,,,,,.Effect of continuous venovenous hemofiltration with dialysis on lactate clearance in critically ill patients.Crit Care Med.1997;25(1):58–62.
- ,,,,.A pilot randomised controlled comparison of continuous veno‐venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid‐base balance.Intensive Care Med.2007;33(5):830–835.
- ,,.Intermittent versus continuous renal replacement therapy in the ICU: impact on electrolyte and acid‐base balance.Intensive Care Med.2001;27(6):1037–1043.
- ,,,.Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta‐analysis.Arch Intern Med.2003;163(21):2594–2602.
- ,,,,.Severe acidosis in patients taking metformin—rapid reversal and survival despite high APACHE score.Diabet Med.2006;23(4):432–435.
- ,,.Incidence of lactic acidosis in metformin users.Diabetes Care.1999;22(6):925–927.
- ,.Lactic acidosis in metformin‐treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations.Drug Saf.1999;20(4):377–384.
- ,,,.The management of metformin overdose.Anaesthesia.1998;53(7):698–701.
- ,,.Lactic acidosis in biguanide‐treated diabetics: a review of 330 cases.Diabetologia.1978;14(2):75–87.
- ,,,,.Treatment of metformin‐associated lactic acidosis with closed recirculation bicarbonate‐buffered hemodialysis.Arch Intern Med.1984;144(1):203–205.
- ,,,.High anion gap metabolic acidosis in suicide: don't forget metformin intoxication—two patients' experiences.Ren Fail.2002;24(5):671–675.
- ,,, et al.Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: a study of metformin elimination.Int J Clin Pharmacol Ther Toxicol.1989;27(6):285–288.
- ,,.Bicarbonate haemodialysis as a treatment of metformin overdose.Nephrol Dial Transplant.1997;12(5):1046–1047.
- ,,,.Combination of intermittent haemodialysis and high‐volume continuous haemofiltration for the treatment of severe metformin‐induced lactic acidosis.Nephrol Dial Transplant.2004;19(8):2157–2158.
- ,,,.When a friend can become an enemy! Recognition and management of metformin‐associated lactic acidosis.Kidney Int.2007;72(9):1157–1160.
- .,, et al.Severe nucleoside‐associated lactic acidosis in human immunodeficiency virus‐infected patients: report of 12 cases and review of the literature.Clin Infect Dis.2002;34(6):838–846.
- ,,.Clearance of metformin by hemofiltration in overdose.J Toxicol Clin Toxicol.2002;40(2):177–180.
- .Metformin‐associated lactic acidosis.J Emerg Med.2001;20(3):267–272.
- ,.Contraindications to use of metformin. Blanket banning of metformin two days before surgery may not be a good idea.BMJ.2003;326(7392):762; author reply 762.
- ,,, et al.Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B.N Engl J Med.1995;333(17):1099–1105.
- ,,,.Zidovudine‐induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature.Crit Care Med.1997;25(8):1425–1430.
- ,.Mitochondrial toxicity of antiviral drugs.Nat Med.1995;1(5):417–422.
- ,.Mitochondrial toxicity of nucleoside analogue reverse transcriptase inhibitors: a looming obstacle for long‐term antiretroviral therapy?Curr Opin Infect Dis.2000;13(1):5–11.
- ,,, et al.Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society‐USA panel.JAMA.2006;296(7):827–843.
- ,,.Riboflavin to treat nucleoside analogue‐induced lactic acidosis.Lancet.1998;352(9124):291–292.
- ,,,,,.Severe lactic acidosis and thiamine administration in an HIV‐infected patient on HAART.Int J STD AIDS.2001;12(6):407–409.
- ,,, et al.Detecting life‐threatening lactic acidosis related to nucleoside‐analog treatment of human immunodeficiency virus‐infected patients, and treatment with L‐carnitine.Crit Care Med.2003;31(4):1042–1047.
- ,.Hyperosmolar metabolic acidosis and intravenous Lorazepam.N Engl J Med.2002;347(11):857–858; author reply 857–858.
- ,,,.Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study.Chest.2005;128(3):1674–1681.
- ,,, et al.Propylene glycol pharmacokinetics and effects after intravenous infusion in humans.Ther Drug Monit.1987;9(3):255–258.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacotherapy.2001;21(9):1140–1144.
- ,,.Recognition, treatment, and prevention of propylene glycol toxicity.Semin Dial.2007;20(3):217–219.
- ,,.Propylene glycol‐mediated cell injury in a primary culture of human proximal tubule cells.Toxicol Sci.1998;46(2):410–417.
- ,.Osmolar gap metabolic acidosis in a 60‐year‐old man treated for hypoxemic respiratory failure.Chest.2000;118(2):545–546.
- ,,,.Relationship of continuous infusion lorazepam to serum propylene glycol concentration in critically ill adults.Crit Care Med.2004;32(8):1709–1714.
- ,,,.Removal of propylene glycol and correction of increased osmolar gap by hemodialysis in a patient on high dose lorazepam infusion therapy.Intensive Care Med.2002;28(1):81–84.
- ,,.Linezolid use associated with lactic acidosis.Scand J Infect Dis.2005;37(2):153–154.
- ,.Linezolid‐induced lactic acidosis.N Engl J Med.2003;348(1):86–87.
- ,,.Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey.Diagn Microbiol Infect Dis.2008;62(4):407–410.
- ,,.Mitochondrial toxicity associated with linezolid.N Engl J Med.2005;353(21):2305–2306.
- ,,, et al.Linezolid‐induced inhibition of mitochondrial protein synthesis.Clin Infect Dis.2006;42(8):1111–1117.
- ,.Use of base in the treatment of severe acidemic states.Am J Kidney Dis.2001;38(4):703–727.
Lactic acidosis (LA) is common in hospitalized patients and is associated with a high mortality.1, 2 Commonly, it is defined as a lactic acid concentration greater than 5 mmol/L with a pH less than 7.35.3 There are no evidence‐based guidelines for the treatment of LA despite progress in our understanding of its pathophysiology.36 This is not surprising, given the uncertainty regarding the impact of LA itself on clinical outcomes. In this regard, it is interesting to note that, despite its well‐recognized role as a marker of tissue hypoxia, lactate accumulation appears to have beneficial effects and may function as an adaptive mechanism. This raises the possibility that therapy directed at altering this adaptation may be detrimental. Pursuing correction of the pH in LA has been shown to have untoward physiologic effects. These and other ambiguities in the pathophysiology and treatment of LA are the focus of this review.
Lactate Metabolism
The body produces approximately 1400 mmol of lactate daily.7 Lactate is derived from the metabolism of pyruvate through an anaerobic reaction that occurs in all tissues (Figure 1). The liver is the primary site of lactate clearance and can metabolize up to 100 mmol per hour under normal conditions.8 There, lactate is converted to glucose to serve as an energy source during periods of hypoxia (Figure 2).9


Approximately 20% to 30% of the daily lactate load is metabolized by the kidneys.10, 11 Renal clearance is increased in acidosis12 and is maintained even in the presence of low renal perfusion.10, 12, 13 Renal lactate clearance is primarily through metabolism and not excretion.10, 14
LA Subtypes
Generally, lactic acid accumulation results from excess lactic acid production and not from reduced clearance.15 In cases of fulminant liver failure, it is due to a combination of decreased clearance and tissue hypoxia.16 In the setting of tissue hypoxia, an impairment of mitochondrial oxidative capacity results in the accumulation of pyruvate and generation of lactate. Lactic acid accumulation through this mechanism has historically been described as Type A LA.7 Hence, in critically ill patients lactate has traditionally been viewed as a marker of tissue hypoxia.15, 1721 Hyperlactatemia without tissue hypoxia has been referred to as type B LA. This is seen in a variety of circumstances. In sepsis, for example, several studies have shown lactic acid accumulation, despite adequate oxygen delivery.2224
Hyperlactatemia may also occur in cases of pure mitochondrial dysfunction, which can be induced by commonly prescribed medications such as the biguanides, nucleoside analog reverse‐transcriptase inhibitors (NRTIs), and linezolid.2527 Alternatively, lactate generation from metabolism of agents such as propylene glycol is possible. Finally, excessive lactate generation may occur following stress due to altered carbohydrate metabolism, or with respiratory alkalosis.2831
Lactate: A Metabolic Adaptation
Lactate was traditionally considered only as a marker of tissue hypoxia and anaerobic metabolism.17 This is certainly the case in situations of poor perfusion such as cardiogenic,15, 18 vasopressor‐resistant,19 or hypovolemic shock.20, 21
Alternative explanations for lactic acid accumulation, without tissue hypoperfusion, include catecholamine‐induced alterations in glycolysis,32, 33 mitochondrial disturbances,3436 and increased pyruvate production combined with increased glucose entry into cells.24, 37 In addition, the activity of an enzyme regulating lactate metabolism, pyruvate dehydrogenase kinase, increases in sepsis.38 This enzyme inactivates the pyruvate dehydrogenase (PDH) complex, which metabolizes pyruvate. Pyruvate and lactate may accumulate as a result. These changes partly explain the generation of LA in sepsis, independent of any effect of diminished tissue perfusion.
Recognizing the body's tendency toward homeostasis, it is appealing to speculate that lactate accumulation is adaptive.9 A number of findings support this. For example, lactate may act to shuttle energy between organs, or between cell types in the same organ. The astrocyteneuron lactate shuttle and the spermatogenic lactate shuttle are 2 examples of lactate's valuable effects on cellular metabolism.39 In the astrocyteneuron lactate shuttle, astrocytes support the increased metabolic demands of neurons through lactic acid production.40 Specifically, the neurotransmitter glutamate is released by the neurons and taken up by the astrocytes. Astrocytes produce lactate, which then moves back to the neuron to be used as an energy source. Glutamine, also released by the astrocytes, leads to the regeneration of glutamate and the potential to restart the cycle.39
Animal and human studies have suggested that, in periods of stress, lactate is the preferential energy substrate in the brain.4144 The usefulness of increased lactate production routinely seen in sepsis may thus represent multiple adaptive processes aimed primarily at improving the delivery of energy substrates. Thus, therapeutic strategies aimed specifically at lowering lactic acid levels may prove to have deleterious effects on cellular metabolism.
Impact of LA on Morbidity and Mortality
The poor prognosis in patients with LA is well recognized.2, 4548 For example, in a study of 126 patients with various causes of LA, the median survival was 38.5 hours and 30‐day survival was 17%.2
Studies have revealed that LA with low pH is associated with adverse effects on the cardiovascular system, particularly a decrease in cardiac contractility.49, 50 This effect is particularly prominent with a pH below 7.20. In contrast, acidosis in animal models has been shown to limit myocardial infarct size after reperfusion.51, 52 Variable effects of LA on cell death have been found. A worsening of apoptosis in myocytes has been noted;53 alternatively, protection from hypoxic injury in hepatocytes and myocardium has been observed.52, 54 Thus, although LA is associated with poor outcomes in human studies,2, 4547 it is still unclear to what extent lactic acid accumulation is a marker of severe illness, an independent effector of pathology, or a mechanism with the potential to serve a protective role.
Available data indicate that lactate itself is not harmful. Studies on infusion of lactate solutions to postoperative patients was shown to be safe.55 Also, the fact that lactate generation in states of respiratory alkalosis, stress, or altered carbohydrate metabolism without sepsis is not associated with worse outcomes supports the fact that lactic acid alone may not be maladaptive.2831
Similarly, low pH is not necessarily maladaptive. In the postictal state,56 diabetic ketoacidosis,57 spontaneous respiratory acidosis,58 or permissive hypercapnia,59 low blood pH is not deleterious.
In summary, LA is associated with poor outcomes, and indirect evidence suggests that it is the underlying causative condition rather than the low pH or the lactate that is responsible for the dire outcomes.
Treatment of LA with Sodium Bicarbonate
Since excessive lactic acid generation is accompanied by consumption of plasma bicarbonate and a fall in plasma pH, sodium bicarbonate has been long proposed as a treatment for LA. While theoretically appealing, this strategy has not been validated by studies in animals or humans. Indeed, bicarbonate administration in LA often has been shown to be detrimental.60, 61 The adverse effects of bicarbonate administration in LA, while initially paradoxical, have a number of possible explanations.
First, bicarbonate administration can induce a reduction in intracellular pH.60, 62, 63 The mechanism involves bicarbonate's effect to increase carbon dioxide (CO2) generation through mass action effect. Because the cell membrane is more permeable to CO2 than to bicarbonate, intracellular pH falls.64, 65 In sepsis, this intracellular/extracellular pH discrepancy may be more pronounced due to alterations in blood flow.66 Other reports on outcomes of intracellular pH with bicarbonate therapy show variable effects.6772
Second, to the extent that bicarbonate administration raises extracellular pH, it is associated with a reduction in ionized calcium concentration, since the binding of calcium to albumin is pH dependent.73 A sodium bicarbonate load administered to patients with LA was associated with a significant fall in ionized calcium concentration, whereas a sodium chloride load was not.1 This can affect cardiac function, as the latter varies proportionally with calcium levels.74
Third, bicarbonate administration may reduce tissue oxygen delivery since the affinity of hemoglobin for oxygen increases as pH rises (Bohr effect).75 The administration of bicarbonate worsened systemic oxygen consumption in one study76 and decreased oxygen delivery in another.75
Fourth, bicarbonate administration may indirectly increase intracellular calcium concentration. Low intracellular pH (see above) stimulates proton efflux by way of proton transporters and exchangers, increasing intracellular sodium content.77 A high cell sodium content then may increase intracellular calcium, through the Na/Ca exchanger, impairing cellular function.7779 Compounding this, the reduced function of the Na/H ATPase as a regulator of intracellular sodium in sepsis may not be adequate to limit cell swelling.77
Against this background of mechanistic concerns with the use of bicarbonate treatment, it is not surprising that clinical outcomes have been inconsistent at best. In animal models of LA, the use of sodium bicarbonate has either negative effects on cardiac output60, 72 or no significant hemodynamic effect when compared to sodium chloride infusion.67, 80, 81 One animal study did show some benefit with sodium bicarbonate compared to saline, though all animals subsequently died.50
In humans, sodium bicarbonate was studied in 2 randomized trials of sepsis‐induced LA.1, 82 In a study by Cooper et al.,1 14 critically‐ill patients received sequential infusions of sodium bicarbonate or sodium chloride. Neither solution was superior to the other in terms of hemodynamic improvement. No benefit was noted even when analysis was limited to those with very low pH (<7.2). Mathieu et al.82 randomized 10 critically‐ill patients to sequential infusion of either sodium bicarbonate or sodium chloride. Similarly, no significant difference in hemodynamic variables was noted.
When taken together, these studies evaluating sodium bicarbonate in LA fail to show convincing benefit and raise serious questions about its detrimental effects. Extracellular pH may be a misleading marker of success in the treatment of LA, given its direct influence by sodium bicarbonate administration.
Treatment of LA and Use of Other Buffers
Other buffers (Carbicarb, dichloroacetate, and tromethamine [THAM]) have been studied for treatment of LA. Human studies have not shown superiority of any of the buffers as far as improving pH,83, 84 hemodynamics, or survival.85
Treatment of LA by Renal Replacement Therapy
Renal replacement therapy (RRT; dialysis and its variants) has been studied for the treatment of severe acidosis. RRT has a number of theoretical advantages over purely medical therapies in the treatment of LA: it can deliver large quantities of base without contributing to volume overload; it can directly remove lactate from the plasma; and it can mitigate the effect of alkalinization on ionized calcium concentration by delivering calcium.
In critically ill patients with intact liver function, continuous venovenous hemofiltration (CVVH) appears to contribute very little (less then 3%) to overall lactate clearance.86 While outcome studies are limited, continuous dialysis modalities consistently show improved resolution of acidosis of various types when compared to intermittent modalities.87, 88 As described above, this is related to base administration and is not a surprising finding. There are no studies comparing RRT and medical therapy with respect to clinical outcomes in patients with LA.
Special Situations
Biguanides
Biguanide‐induced LA can be due to impairment of hepatic neoglucogenesis, in the case of metformin, or increasing hepatic oxidative phosphorylation, in the case of phenformin.89 This infrequent complication90, 91 is associated with a high mortality.92 Proposed therapy has included the use of sodium bicarbonate infusion.93 In this setting, it is unclear if the use of bicarbonate alone improves clinical outcomes.94
Renal replacement therapy in a wide variety of formats has been used to treat this condition.93, 95101 Metformin has a high clearance during dialysis due to its low molecular weight and lack of protein binding.97, 98, 102 Nonetheless, its high volume of distribution suggests a longer dialysis time would be more beneficial if the main goal is reducing metformin levels.97, 103 The limited prospective literature and lack of conclusive evidence about what levels of metformin induce LA makes generalized recommendations about duration of hemodialysis purely speculative.104
NRTIs
The use of NRTIs is associated with LA due to impairment of mitochondrial oxidative phosphorylation.105108 This uncommon complication, if not recognized early, is associated with a high mortality.101, 109 Investigations are ongoing into agents directed at improving mitochondrial function such as riboflavin, thiamine, and L‐carnitine.110112 As with biguanide‐associated LA, RRT decisions should be individualized based on metabolic circumstances.
Lorazepam
Many intravenous medications are formulated in the alcohol solvent, propylene glycol. Injectable lorazepam has the highest proportional amount of propylene glycol compared with other commonly used agents.113, 114 The kidney normally eliminates 12% to 50% of administered propylene glycol via proximal tubule secretion.115 The remainder is metabolized by the liver to form pyruvate and lactate.114, 116, 117
When propylene glycol accumulates, as in cases of reduced renal function, it results in hyperosmolarity, LA, and can even induce additional kidney injury (probably through proximal tubular cell necrosis).118
LA due to propylene glycol has been reported by many authors and its incidence with high dose intravenous (IV) lorazepam has been estimated to be as high as 19%.114, 116, 119, 120 This disorder can frequently go unrecognized, as many other factors that induce LA often coincide in such patients. But when identified and promptly addressed, its prognosis seems to be favorable.114
The best treatment is prevention, by avoiding the use of IV lorazepam in patients with impaired renal function. Once it is recognized, the drug should be promptly withdrawn. In addition, removal by hemodialysis can quickly lower propylene glycol levels since it is a small, highly water soluble, non‐protein‐bound molecule.121 As no rebound in the level is expected, intermittent dialysis should be an acceptable modality.117
Linezolid
Recently, Gram‐positive bacteria in general and methicillin‐resistant Staphylococcus aureus in particular have emerged as major causes of nosocomial and community‐acquired infections. Linezolid, an oxazolidinone, is increasingly used to treat such infections. Several cases of LA have been associated with linezolid.27, 122, 123 and a survey of the Infectious Diseases Society of America (IDSA) Emerging Infections Network members revealed that this complication was commonly encountered.124 Linezolid causes LA by mitochondrial toxicity125, 126 and risk factors include prolonged exposure and older age. Once the disorder is recognized, the clinician should stop the drug immediately. Chemistries should be monitored frequently in patients on long‐term therapy.
Conclusions
Many studies note the association between LA and adverse outcomes.2, 4547 Though metabolic acidosis from elevated lactate levels may negatively affect organ function, the evidence supporting therapy specifically aimed at increasing pH in these settings is consistently poor.3, 127 Limitations have included small numbers of subjects,1, 82 variable outcomes studied, and the inability to assess intracellular metabolic stability.1, 61 When taking these factors into account it is hard to justify aggressive treatment of LA with mechanisms aimed at raising pH. Literature on the treatment of patients with LA and very low pH (below 7.2) is even more limited.
Moreover, lactate elevations may not represent tissue hypoperfusion. Lactate may have an important role in improving energy metabolism. This represents 1 additional reason to be hesitant when attempting to normalize pH in LA; we may be disrupting the body's physiologic response to sepsis. A conflict for clinicians emerges, however, as lactate is often used to define tissue ischemia. Obviously, more specific markers of tissue hypoperfusion would be ideal.
Bicarbonate therapy is an understandably attractive means to improve the acidemia, but there are serious mechanistic concerns with it use. Moreover, neither animal nor human studies, limited as they may be, show a convincing benefit. LA in the setting of acute kidney injury may be best treated with renal replacement therapy with bicarbonate‐based buffers, but controlled trials are lacking.
A number of commonly used drugs can cause LA. A heightened awareness on the part of clinicians will lead to prompt recognition of these cases, and timely treatment.
Lactic acidosis (LA) is common in hospitalized patients and is associated with a high mortality.1, 2 Commonly, it is defined as a lactic acid concentration greater than 5 mmol/L with a pH less than 7.35.3 There are no evidence‐based guidelines for the treatment of LA despite progress in our understanding of its pathophysiology.36 This is not surprising, given the uncertainty regarding the impact of LA itself on clinical outcomes. In this regard, it is interesting to note that, despite its well‐recognized role as a marker of tissue hypoxia, lactate accumulation appears to have beneficial effects and may function as an adaptive mechanism. This raises the possibility that therapy directed at altering this adaptation may be detrimental. Pursuing correction of the pH in LA has been shown to have untoward physiologic effects. These and other ambiguities in the pathophysiology and treatment of LA are the focus of this review.
Lactate Metabolism
The body produces approximately 1400 mmol of lactate daily.7 Lactate is derived from the metabolism of pyruvate through an anaerobic reaction that occurs in all tissues (Figure 1). The liver is the primary site of lactate clearance and can metabolize up to 100 mmol per hour under normal conditions.8 There, lactate is converted to glucose to serve as an energy source during periods of hypoxia (Figure 2).9


Approximately 20% to 30% of the daily lactate load is metabolized by the kidneys.10, 11 Renal clearance is increased in acidosis12 and is maintained even in the presence of low renal perfusion.10, 12, 13 Renal lactate clearance is primarily through metabolism and not excretion.10, 14
LA Subtypes
Generally, lactic acid accumulation results from excess lactic acid production and not from reduced clearance.15 In cases of fulminant liver failure, it is due to a combination of decreased clearance and tissue hypoxia.16 In the setting of tissue hypoxia, an impairment of mitochondrial oxidative capacity results in the accumulation of pyruvate and generation of lactate. Lactic acid accumulation through this mechanism has historically been described as Type A LA.7 Hence, in critically ill patients lactate has traditionally been viewed as a marker of tissue hypoxia.15, 1721 Hyperlactatemia without tissue hypoxia has been referred to as type B LA. This is seen in a variety of circumstances. In sepsis, for example, several studies have shown lactic acid accumulation, despite adequate oxygen delivery.2224
Hyperlactatemia may also occur in cases of pure mitochondrial dysfunction, which can be induced by commonly prescribed medications such as the biguanides, nucleoside analog reverse‐transcriptase inhibitors (NRTIs), and linezolid.2527 Alternatively, lactate generation from metabolism of agents such as propylene glycol is possible. Finally, excessive lactate generation may occur following stress due to altered carbohydrate metabolism, or with respiratory alkalosis.2831
Lactate: A Metabolic Adaptation
Lactate was traditionally considered only as a marker of tissue hypoxia and anaerobic metabolism.17 This is certainly the case in situations of poor perfusion such as cardiogenic,15, 18 vasopressor‐resistant,19 or hypovolemic shock.20, 21
Alternative explanations for lactic acid accumulation, without tissue hypoperfusion, include catecholamine‐induced alterations in glycolysis,32, 33 mitochondrial disturbances,3436 and increased pyruvate production combined with increased glucose entry into cells.24, 37 In addition, the activity of an enzyme regulating lactate metabolism, pyruvate dehydrogenase kinase, increases in sepsis.38 This enzyme inactivates the pyruvate dehydrogenase (PDH) complex, which metabolizes pyruvate. Pyruvate and lactate may accumulate as a result. These changes partly explain the generation of LA in sepsis, independent of any effect of diminished tissue perfusion.
Recognizing the body's tendency toward homeostasis, it is appealing to speculate that lactate accumulation is adaptive.9 A number of findings support this. For example, lactate may act to shuttle energy between organs, or between cell types in the same organ. The astrocyteneuron lactate shuttle and the spermatogenic lactate shuttle are 2 examples of lactate's valuable effects on cellular metabolism.39 In the astrocyteneuron lactate shuttle, astrocytes support the increased metabolic demands of neurons through lactic acid production.40 Specifically, the neurotransmitter glutamate is released by the neurons and taken up by the astrocytes. Astrocytes produce lactate, which then moves back to the neuron to be used as an energy source. Glutamine, also released by the astrocytes, leads to the regeneration of glutamate and the potential to restart the cycle.39
Animal and human studies have suggested that, in periods of stress, lactate is the preferential energy substrate in the brain.4144 The usefulness of increased lactate production routinely seen in sepsis may thus represent multiple adaptive processes aimed primarily at improving the delivery of energy substrates. Thus, therapeutic strategies aimed specifically at lowering lactic acid levels may prove to have deleterious effects on cellular metabolism.
Impact of LA on Morbidity and Mortality
The poor prognosis in patients with LA is well recognized.2, 4548 For example, in a study of 126 patients with various causes of LA, the median survival was 38.5 hours and 30‐day survival was 17%.2
Studies have revealed that LA with low pH is associated with adverse effects on the cardiovascular system, particularly a decrease in cardiac contractility.49, 50 This effect is particularly prominent with a pH below 7.20. In contrast, acidosis in animal models has been shown to limit myocardial infarct size after reperfusion.51, 52 Variable effects of LA on cell death have been found. A worsening of apoptosis in myocytes has been noted;53 alternatively, protection from hypoxic injury in hepatocytes and myocardium has been observed.52, 54 Thus, although LA is associated with poor outcomes in human studies,2, 4547 it is still unclear to what extent lactic acid accumulation is a marker of severe illness, an independent effector of pathology, or a mechanism with the potential to serve a protective role.
Available data indicate that lactate itself is not harmful. Studies on infusion of lactate solutions to postoperative patients was shown to be safe.55 Also, the fact that lactate generation in states of respiratory alkalosis, stress, or altered carbohydrate metabolism without sepsis is not associated with worse outcomes supports the fact that lactic acid alone may not be maladaptive.2831
Similarly, low pH is not necessarily maladaptive. In the postictal state,56 diabetic ketoacidosis,57 spontaneous respiratory acidosis,58 or permissive hypercapnia,59 low blood pH is not deleterious.
In summary, LA is associated with poor outcomes, and indirect evidence suggests that it is the underlying causative condition rather than the low pH or the lactate that is responsible for the dire outcomes.
Treatment of LA with Sodium Bicarbonate
Since excessive lactic acid generation is accompanied by consumption of plasma bicarbonate and a fall in plasma pH, sodium bicarbonate has been long proposed as a treatment for LA. While theoretically appealing, this strategy has not been validated by studies in animals or humans. Indeed, bicarbonate administration in LA often has been shown to be detrimental.60, 61 The adverse effects of bicarbonate administration in LA, while initially paradoxical, have a number of possible explanations.
First, bicarbonate administration can induce a reduction in intracellular pH.60, 62, 63 The mechanism involves bicarbonate's effect to increase carbon dioxide (CO2) generation through mass action effect. Because the cell membrane is more permeable to CO2 than to bicarbonate, intracellular pH falls.64, 65 In sepsis, this intracellular/extracellular pH discrepancy may be more pronounced due to alterations in blood flow.66 Other reports on outcomes of intracellular pH with bicarbonate therapy show variable effects.6772
Second, to the extent that bicarbonate administration raises extracellular pH, it is associated with a reduction in ionized calcium concentration, since the binding of calcium to albumin is pH dependent.73 A sodium bicarbonate load administered to patients with LA was associated with a significant fall in ionized calcium concentration, whereas a sodium chloride load was not.1 This can affect cardiac function, as the latter varies proportionally with calcium levels.74
Third, bicarbonate administration may reduce tissue oxygen delivery since the affinity of hemoglobin for oxygen increases as pH rises (Bohr effect).75 The administration of bicarbonate worsened systemic oxygen consumption in one study76 and decreased oxygen delivery in another.75
Fourth, bicarbonate administration may indirectly increase intracellular calcium concentration. Low intracellular pH (see above) stimulates proton efflux by way of proton transporters and exchangers, increasing intracellular sodium content.77 A high cell sodium content then may increase intracellular calcium, through the Na/Ca exchanger, impairing cellular function.7779 Compounding this, the reduced function of the Na/H ATPase as a regulator of intracellular sodium in sepsis may not be adequate to limit cell swelling.77
Against this background of mechanistic concerns with the use of bicarbonate treatment, it is not surprising that clinical outcomes have been inconsistent at best. In animal models of LA, the use of sodium bicarbonate has either negative effects on cardiac output60, 72 or no significant hemodynamic effect when compared to sodium chloride infusion.67, 80, 81 One animal study did show some benefit with sodium bicarbonate compared to saline, though all animals subsequently died.50
In humans, sodium bicarbonate was studied in 2 randomized trials of sepsis‐induced LA.1, 82 In a study by Cooper et al.,1 14 critically‐ill patients received sequential infusions of sodium bicarbonate or sodium chloride. Neither solution was superior to the other in terms of hemodynamic improvement. No benefit was noted even when analysis was limited to those with very low pH (<7.2). Mathieu et al.82 randomized 10 critically‐ill patients to sequential infusion of either sodium bicarbonate or sodium chloride. Similarly, no significant difference in hemodynamic variables was noted.
When taken together, these studies evaluating sodium bicarbonate in LA fail to show convincing benefit and raise serious questions about its detrimental effects. Extracellular pH may be a misleading marker of success in the treatment of LA, given its direct influence by sodium bicarbonate administration.
Treatment of LA and Use of Other Buffers
Other buffers (Carbicarb, dichloroacetate, and tromethamine [THAM]) have been studied for treatment of LA. Human studies have not shown superiority of any of the buffers as far as improving pH,83, 84 hemodynamics, or survival.85
Treatment of LA by Renal Replacement Therapy
Renal replacement therapy (RRT; dialysis and its variants) has been studied for the treatment of severe acidosis. RRT has a number of theoretical advantages over purely medical therapies in the treatment of LA: it can deliver large quantities of base without contributing to volume overload; it can directly remove lactate from the plasma; and it can mitigate the effect of alkalinization on ionized calcium concentration by delivering calcium.
In critically ill patients with intact liver function, continuous venovenous hemofiltration (CVVH) appears to contribute very little (less then 3%) to overall lactate clearance.86 While outcome studies are limited, continuous dialysis modalities consistently show improved resolution of acidosis of various types when compared to intermittent modalities.87, 88 As described above, this is related to base administration and is not a surprising finding. There are no studies comparing RRT and medical therapy with respect to clinical outcomes in patients with LA.
Special Situations
Biguanides
Biguanide‐induced LA can be due to impairment of hepatic neoglucogenesis, in the case of metformin, or increasing hepatic oxidative phosphorylation, in the case of phenformin.89 This infrequent complication90, 91 is associated with a high mortality.92 Proposed therapy has included the use of sodium bicarbonate infusion.93 In this setting, it is unclear if the use of bicarbonate alone improves clinical outcomes.94
Renal replacement therapy in a wide variety of formats has been used to treat this condition.93, 95101 Metformin has a high clearance during dialysis due to its low molecular weight and lack of protein binding.97, 98, 102 Nonetheless, its high volume of distribution suggests a longer dialysis time would be more beneficial if the main goal is reducing metformin levels.97, 103 The limited prospective literature and lack of conclusive evidence about what levels of metformin induce LA makes generalized recommendations about duration of hemodialysis purely speculative.104
NRTIs
The use of NRTIs is associated with LA due to impairment of mitochondrial oxidative phosphorylation.105108 This uncommon complication, if not recognized early, is associated with a high mortality.101, 109 Investigations are ongoing into agents directed at improving mitochondrial function such as riboflavin, thiamine, and L‐carnitine.110112 As with biguanide‐associated LA, RRT decisions should be individualized based on metabolic circumstances.
Lorazepam
Many intravenous medications are formulated in the alcohol solvent, propylene glycol. Injectable lorazepam has the highest proportional amount of propylene glycol compared with other commonly used agents.113, 114 The kidney normally eliminates 12% to 50% of administered propylene glycol via proximal tubule secretion.115 The remainder is metabolized by the liver to form pyruvate and lactate.114, 116, 117
When propylene glycol accumulates, as in cases of reduced renal function, it results in hyperosmolarity, LA, and can even induce additional kidney injury (probably through proximal tubular cell necrosis).118
LA due to propylene glycol has been reported by many authors and its incidence with high dose intravenous (IV) lorazepam has been estimated to be as high as 19%.114, 116, 119, 120 This disorder can frequently go unrecognized, as many other factors that induce LA often coincide in such patients. But when identified and promptly addressed, its prognosis seems to be favorable.114
The best treatment is prevention, by avoiding the use of IV lorazepam in patients with impaired renal function. Once it is recognized, the drug should be promptly withdrawn. In addition, removal by hemodialysis can quickly lower propylene glycol levels since it is a small, highly water soluble, non‐protein‐bound molecule.121 As no rebound in the level is expected, intermittent dialysis should be an acceptable modality.117
Linezolid
Recently, Gram‐positive bacteria in general and methicillin‐resistant Staphylococcus aureus in particular have emerged as major causes of nosocomial and community‐acquired infections. Linezolid, an oxazolidinone, is increasingly used to treat such infections. Several cases of LA have been associated with linezolid.27, 122, 123 and a survey of the Infectious Diseases Society of America (IDSA) Emerging Infections Network members revealed that this complication was commonly encountered.124 Linezolid causes LA by mitochondrial toxicity125, 126 and risk factors include prolonged exposure and older age. Once the disorder is recognized, the clinician should stop the drug immediately. Chemistries should be monitored frequently in patients on long‐term therapy.
Conclusions
Many studies note the association between LA and adverse outcomes.2, 4547 Though metabolic acidosis from elevated lactate levels may negatively affect organ function, the evidence supporting therapy specifically aimed at increasing pH in these settings is consistently poor.3, 127 Limitations have included small numbers of subjects,1, 82 variable outcomes studied, and the inability to assess intracellular metabolic stability.1, 61 When taking these factors into account it is hard to justify aggressive treatment of LA with mechanisms aimed at raising pH. Literature on the treatment of patients with LA and very low pH (below 7.2) is even more limited.
Moreover, lactate elevations may not represent tissue hypoperfusion. Lactate may have an important role in improving energy metabolism. This represents 1 additional reason to be hesitant when attempting to normalize pH in LA; we may be disrupting the body's physiologic response to sepsis. A conflict for clinicians emerges, however, as lactate is often used to define tissue ischemia. Obviously, more specific markers of tissue hypoperfusion would be ideal.
Bicarbonate therapy is an understandably attractive means to improve the acidemia, but there are serious mechanistic concerns with it use. Moreover, neither animal nor human studies, limited as they may be, show a convincing benefit. LA in the setting of acute kidney injury may be best treated with renal replacement therapy with bicarbonate‐based buffers, but controlled trials are lacking.
A number of commonly used drugs can cause LA. A heightened awareness on the part of clinicians will lead to prompt recognition of these cases, and timely treatment.
- ,,,.Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study.Ann Intern Med.1990;112(7):492–498.
- ,,, et al.Natural history and course of acquired lactic acidosis in adults. DCA‐Lactic Acidosis Study Group.Am J Med.1994;97(1):47–54.
- ,.Sodium bicarbonate for the treatment of lactic acidosis.Chest.2000;117(1):260–267.
- ,.Management of life‐threatening acid‐base disorders. First of two parts.N Engl J Med.1998;338(1):26–34.
- .Indications for use of bicarbonate in patients with metabolic acidosis.Br J Anaesth.1991;67(2):165–177.
- ,.Bench‐to‐bedside review: treating acid‐base abnormalities in the intensive care unit—the role of buffers.Crit Care.2004;8(4):259–265.
- .Lactic acidosis update for critical care clinicians.J Am Soc Nephrol.2001;12(suppl 17):S15–S19.
- ,.Lactic acidosis: diagnosis and treatment.Clin Endocrinol Metab.1980;9(3):513–541.
- .Lactate and shock state: the metabolic view.Curr Opin Crit Care.2006;12(4):315–321.
- .Bench‐to‐bedside review: lactate and the kidney.Crit Care.2002;6(4):322–326.
- ,,,.The influence of renal function on lactate and glucose metabolism.Biochem J.1984;219(1):73–78.
- ,.The effect of acidosis on lactate removal by the perfused rat kidney.Clin Sci Mol Med.1976;50(3):185–194.
- ,,.Transvisceral lactate fluxes during early endotoxemia.Chest.1996;110(1):198–204.
- ,,,,.Metabolism of lactate by the intact functioning kidney of the dog.Am J Physiol.1973;224(6):1463–1467.
- ,,, et al.Lactate and glucose metabolism in severe sepsis and cardiogenic shock.Crit Care Med.2005;33(10):2235–2240.
- ,,,, et al.Lactic acidosis in fulminant hepatic failure. Some aspects of pathogenesis and prognosis.J Hepatol.1985;1(4):405–416.
- ,.Lactic acidosis in critical illness.Crit Care Med.1992;20(1):80–93.
- ,,, et al.Effects of cardiogenic shock on lactate and glucose metabolism after heart surgery.Crit Care Med.2000;28(12):3784–3791.
- ,,,,,.Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine‐treated septic shock.Crit Care Med.2000;28(1):114–119.
- ,,, et al.Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock.Crit Care Med.1991;19(2):231–243.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,,,.Skeletal muscle partial pressure of oxygen in patients with sepsis.Crit Care Med.1994;22(4):640–650.
- ,.Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis.JAMA.1992;267(11):1503–1510.
- ,,,.Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability.Ann Surg.1996;224(1):97–102.
- ,,,.Biguanide‐associated lactic acidosis. Case report and review of the literature.Arch Intern Med.1992;152(11):2333–2336.
- ,,,.Bench‐to‐bedside review: severe lactic acidosis in HIV patients treated with nucleoside analogue reverse transcriptase inhibitors.Crit Care.2003;7(3):226–232.
- ,,,.Lactic acidosis after treatment with linezolid.Infection.2007;35(4):278–281.
- ,,,.Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion.Surgery.1991;110(1):54–67.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98(1):75–84.
- .,,,.Lactic acid kinetics in respiratory alkalosis.Crit Care Med.1991;19(9):1120–1124.
- .Significance of hyperlactatemia without acidosis during hypermetabolic stress.Crit Care Med.1997;25(11):1780–1781.
- ,,,.Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis.Lancet.1999;354(9177):505–508.
- ,,,,.Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study.Lancet.2005;365(9462):871–875.
- ,.Sepsis induces diaphragm electron transport chain dysfunction and protein depletion.Am J Respir Crit Care Med.2005;172(7):861–868.
- ,,, et al.Association between mitochondrial dysfunction and severity and outcome of septic shock.Lancet.2002;360(9328):219–223.
- ,.Mitochondrial dysfunction in sepsis.Biochem Soc Symp.1999;66:149–166.
- ,,, et al.Altered glucose transporter mRNA abundance in a rat model of endotoxic shock.Biochem Biophys Res Commun.1991;176(1):535–540.
- .Increased pyruvate dehydrogenase kinase activity in response to sepsis.Am J Physiol.1991;260(5 Pt 1):E669–E674.
- .Lactate metabolism: a new paradigm for the third millennium.J Physiol.2004;558(Pt 1):5–30.
- ,,, et al.Evidence supporting the existence of an activity‐dependent astrocyte‐neuron lactate shuttle.Dev Neurosci.1998;20(4–5):291–299.
- ,,,.Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation.J Neurochem.1997;69(1):423–426.
- .Bench‐to‐bedside review: a possible resolution of the glucose paradox of cerebral ischemia.Crit Care.2002;6(4):330–334.
- ,,,,,.Intravenous lactate prevents cerebral dysfunction during hypoglycaemia in insulin‐dependent diabetes mellitus.Clin Sci (Lond).1998;94(2):157–163.
- ,,,.Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study.Brain Res.1997;744(1):105–111.
- ,,,,,.Clinical prognostic markers in patients with severe sepsis: a prospective analysis of 139 consecutive cases.J Infect.2003;47(4):300–306.
- ,,, et al.Serum lactate as a predictor of mortality in patients with infection.Intensive Care Med.2007;33(6):970–977.
- ,,,.Lactate versus non‐lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients.Crit Care.2006;10(1):R22.
- .Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients.Am J Med.1961;30:840–848.
- ,,,,.Effect of lactic acidosis on canine hemodynamics and left ventricular function.Am J Physiol.1990;258(4 Pt 2):H1193–H1199.
- ,,,.Alkali therapy extends the period of survival during hypoxia: studies in rats.Am J Physiol.1996;271(2 Pt 2):R381–R387.
- ,,.Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts.J Clin Invest.1988;82(3):920–927.
- ,,, et al.Effect of acidotic blood reperfusion on reperfusion injury after coronary artery occlusion in the dog heart.J Cardiovasc Pharmacol.1998;31(2):179–186.
- ,,,.Hypoxia and acidosis activate cardiac myocyte death through the Bcl‐2 family protein BNIP3.Proc Natl Acad Sci U S A.2002;99(20):12825–12830.
- ,,,,,.Extracellular acidosis delays onset of cell death in ATP‐depleted hepatocytes.Am J Physiol.1988;255(3 Pt 1):C315–C322.
- ,.Metabolic and hemodynamic effects of hypertonic solutions: sodium‐lactate versus sodium chloride infusion in postoperative patients.Shock.2002;18(4):306–310.
- .Medical complications of status epilepticus.Adv Neurol.1983;34:395–398.
- ,,,,,.Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial.Rev Invest Clin.1991;43(3):234–238.
- ,,.Supercarbia in children: clinical course and outcome.Crit Care Med.1990;18(2):166–168.
- ,,,.Low mortality rate in adult respiratory distress syndrome using low‐volume, pressure‐limited ventilation with permissive hypercapnia: a prospective study.Crit Care Med.1994;22(10):1568–1578.
- ,,,.Systemic effects of NaHCO3 in experimental lactic acidosis in dogs.Am J Physiol.1982;242(6):F586–F591.
- ,,,,.Lactic acidosis: effect of treatment on intracellular pH and energetics in living rat heart.Am J Physiol.1992;262(5 Pt 2):H1572–H1578.
- ,,,,,.Effect of sodium bicarbonate on intracellular pH under different buffering conditions.Kidney Int.1996;49(5):1262–1267.
- ,,.Hemodynamic and hepatic pH responses to sodium bicarbonate and Carbicarb during systemic acidosis.Magn Reson Med.1990;16(3):403–410.
- ,,, et al.The increase in CO2 production induced by NaHCO3 depends on blood albumin and hemoglobin concentrations.Intensive Care Med.2000;26(5):558–564.
- ,,, et al.Initial effect of sodium bicarbonate on intracellular pH depends on the extracellular nonbicarbonate buffering capacity.Crit Care Med.2001;29(5):1033–1039.
- ,,,,,.Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock.Crit Care Med.2000;28(9):3233–3241.
- ,,,,,.The effects of sodium bicarbonate and a mixture of sodium bicarbonate and carbonate (“Carbicarb”) on skeletal muscle pH and hemodynamic status in rats with hypovolemic shock.Metabolism.1994;43(4):518–522.
- ,,, et al.Haemodynamic and metabolic effects in diabetic ketoacidosis in rats of treatment with sodium bicarbonate or a mixture of sodium bicarbonate and sodium carbonate.Diabetologia.1995;38(8):889–898.
- ,,,,,.Effects of sodium bicarbonate on striated muscle metabolism and intracellular pH during endotoxic shock.Shock.1994;1(3):196–200.
- ,,,,.Carbicarb, sodium bicarbonate, and sodium chloride in hypoxic lactic acidosis. Effect on arterial blood gases, lactate concentrations, hemodynamic variables, and myocardial intracellular pH.Chest.1993;104(3):913–918.
- ,.Improved hemodynamic function during hypoxia with Carbicarb, a new agent for the management of acidosis.Circulation.1988;77(1):227–233.
- ,,.Metabolic effects of sodium bicarbonate in hypoxic lactic acidosis in dogs.Am J Physiol.1985;249(5 Pt 2):F630–F635.
- .Binding of calcium to serum albumin. II. Effect of pH via competitive hydrogen and calcium ion binding to the imidazole groups of albumin.Scand J Clin Lab Invest.1972;29(1):75–83.
- ,,,,.Left ventricular contractility varies directly with blood ionized calcium.Ann Intern Med.1988;108(4):524–529.
- ,,.Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis.J Clin Invest.1971;50(3):700–706.
- ,,.Metabolic and hemodynamic consequences of sodium bicarbonate administration in patients with heart disease.Am J Med.1989;87(1):7–14.
- ,,.Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury.J Clin Pharmacol.1998;38(10):887–897.
- ,.SEA0400: a novel sodium‐calcium exchange inhibitor with cardioprotective properties.Cardiovasc Drug Rev.2004;22(4):334–347.
- ,,,.Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart.Circ Res.1987;60(5):700–707.
- ,.Acute haemodynamic effects of sodium bicarbonate administration in respiratory and metabolic acidosis in anaesthetized dogs.Anaesth Intensive Care.1997;25(6):615–620.
- ,,,.Bicarbonate does not increase left ventricular contractility during L‐lactic acidemia in pigs.Am Rev Respir Dis.1993;148(2):317–322.
- ,,,,.Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study.Crit Care Med.1991;19(11):1352–1356.
- ,,, et al.Safety and efficacy of intravenous Carbicarb in patients undergoing surgery: comparison with sodium bicarbonate in the treatment of mild metabolic acidosis. SPI Research Group. Study of Perioperative Ischemia.Crit Care Med.1994;22(10):1540–1549.
- ,,, et al.Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis.J Nephrol.2005;18(3):303–307.
- ,,, et al.A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate‐Lactic Acidosis Study Group.N Engl J Med.1992;327(22):1564–1569.
- ,,,,,.Effect of continuous venovenous hemofiltration with dialysis on lactate clearance in critically ill patients.Crit Care Med.1997;25(1):58–62.
- ,,,,.A pilot randomised controlled comparison of continuous veno‐venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid‐base balance.Intensive Care Med.2007;33(5):830–835.
- ,,.Intermittent versus continuous renal replacement therapy in the ICU: impact on electrolyte and acid‐base balance.Intensive Care Med.2001;27(6):1037–1043.
- ,,,.Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta‐analysis.Arch Intern Med.2003;163(21):2594–2602.
- ,,,,.Severe acidosis in patients taking metformin—rapid reversal and survival despite high APACHE score.Diabet Med.2006;23(4):432–435.
- ,,.Incidence of lactic acidosis in metformin users.Diabetes Care.1999;22(6):925–927.
- ,.Lactic acidosis in metformin‐treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations.Drug Saf.1999;20(4):377–384.
- ,,,.The management of metformin overdose.Anaesthesia.1998;53(7):698–701.
- ,,.Lactic acidosis in biguanide‐treated diabetics: a review of 330 cases.Diabetologia.1978;14(2):75–87.
- ,,,,.Treatment of metformin‐associated lactic acidosis with closed recirculation bicarbonate‐buffered hemodialysis.Arch Intern Med.1984;144(1):203–205.
- ,,,.High anion gap metabolic acidosis in suicide: don't forget metformin intoxication—two patients' experiences.Ren Fail.2002;24(5):671–675.
- ,,, et al.Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: a study of metformin elimination.Int J Clin Pharmacol Ther Toxicol.1989;27(6):285–288.
- ,,.Bicarbonate haemodialysis as a treatment of metformin overdose.Nephrol Dial Transplant.1997;12(5):1046–1047.
- ,,,.Combination of intermittent haemodialysis and high‐volume continuous haemofiltration for the treatment of severe metformin‐induced lactic acidosis.Nephrol Dial Transplant.2004;19(8):2157–2158.
- ,,,.When a friend can become an enemy! Recognition and management of metformin‐associated lactic acidosis.Kidney Int.2007;72(9):1157–1160.
- .,, et al.Severe nucleoside‐associated lactic acidosis in human immunodeficiency virus‐infected patients: report of 12 cases and review of the literature.Clin Infect Dis.2002;34(6):838–846.
- ,,.Clearance of metformin by hemofiltration in overdose.J Toxicol Clin Toxicol.2002;40(2):177–180.
- .Metformin‐associated lactic acidosis.J Emerg Med.2001;20(3):267–272.
- ,.Contraindications to use of metformin. Blanket banning of metformin two days before surgery may not be a good idea.BMJ.2003;326(7392):762; author reply 762.
- ,,, et al.Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B.N Engl J Med.1995;333(17):1099–1105.
- ,,,.Zidovudine‐induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature.Crit Care Med.1997;25(8):1425–1430.
- ,.Mitochondrial toxicity of antiviral drugs.Nat Med.1995;1(5):417–422.
- ,.Mitochondrial toxicity of nucleoside analogue reverse transcriptase inhibitors: a looming obstacle for long‐term antiretroviral therapy?Curr Opin Infect Dis.2000;13(1):5–11.
- ,,, et al.Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society‐USA panel.JAMA.2006;296(7):827–843.
- ,,.Riboflavin to treat nucleoside analogue‐induced lactic acidosis.Lancet.1998;352(9124):291–292.
- ,,,,,.Severe lactic acidosis and thiamine administration in an HIV‐infected patient on HAART.Int J STD AIDS.2001;12(6):407–409.
- ,,, et al.Detecting life‐threatening lactic acidosis related to nucleoside‐analog treatment of human immunodeficiency virus‐infected patients, and treatment with L‐carnitine.Crit Care Med.2003;31(4):1042–1047.
- ,.Hyperosmolar metabolic acidosis and intravenous Lorazepam.N Engl J Med.2002;347(11):857–858; author reply 857–858.
- ,,,.Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study.Chest.2005;128(3):1674–1681.
- ,,, et al.Propylene glycol pharmacokinetics and effects after intravenous infusion in humans.Ther Drug Monit.1987;9(3):255–258.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacotherapy.2001;21(9):1140–1144.
- ,,.Recognition, treatment, and prevention of propylene glycol toxicity.Semin Dial.2007;20(3):217–219.
- ,,.Propylene glycol‐mediated cell injury in a primary culture of human proximal tubule cells.Toxicol Sci.1998;46(2):410–417.
- ,.Osmolar gap metabolic acidosis in a 60‐year‐old man treated for hypoxemic respiratory failure.Chest.2000;118(2):545–546.
- ,,,.Relationship of continuous infusion lorazepam to serum propylene glycol concentration in critically ill adults.Crit Care Med.2004;32(8):1709–1714.
- ,,,.Removal of propylene glycol and correction of increased osmolar gap by hemodialysis in a patient on high dose lorazepam infusion therapy.Intensive Care Med.2002;28(1):81–84.
- ,,.Linezolid use associated with lactic acidosis.Scand J Infect Dis.2005;37(2):153–154.
- ,.Linezolid‐induced lactic acidosis.N Engl J Med.2003;348(1):86–87.
- ,,.Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey.Diagn Microbiol Infect Dis.2008;62(4):407–410.
- ,,.Mitochondrial toxicity associated with linezolid.N Engl J Med.2005;353(21):2305–2306.
- ,,, et al.Linezolid‐induced inhibition of mitochondrial protein synthesis.Clin Infect Dis.2006;42(8):1111–1117.
- ,.Use of base in the treatment of severe acidemic states.Am J Kidney Dis.2001;38(4):703–727.
- ,,,.Bicarbonate does not improve hemodynamics in critically ill patients who have lactic acidosis. A prospective, controlled clinical study.Ann Intern Med.1990;112(7):492–498.
- ,,, et al.Natural history and course of acquired lactic acidosis in adults. DCA‐Lactic Acidosis Study Group.Am J Med.1994;97(1):47–54.
- ,.Sodium bicarbonate for the treatment of lactic acidosis.Chest.2000;117(1):260–267.
- ,.Management of life‐threatening acid‐base disorders. First of two parts.N Engl J Med.1998;338(1):26–34.
- .Indications for use of bicarbonate in patients with metabolic acidosis.Br J Anaesth.1991;67(2):165–177.
- ,.Bench‐to‐bedside review: treating acid‐base abnormalities in the intensive care unit—the role of buffers.Crit Care.2004;8(4):259–265.
- .Lactic acidosis update for critical care clinicians.J Am Soc Nephrol.2001;12(suppl 17):S15–S19.
- ,.Lactic acidosis: diagnosis and treatment.Clin Endocrinol Metab.1980;9(3):513–541.
- .Lactate and shock state: the metabolic view.Curr Opin Crit Care.2006;12(4):315–321.
- .Bench‐to‐bedside review: lactate and the kidney.Crit Care.2002;6(4):322–326.
- ,,,.The influence of renal function on lactate and glucose metabolism.Biochem J.1984;219(1):73–78.
- ,.The effect of acidosis on lactate removal by the perfused rat kidney.Clin Sci Mol Med.1976;50(3):185–194.
- ,,.Transvisceral lactate fluxes during early endotoxemia.Chest.1996;110(1):198–204.
- ,,,,.Metabolism of lactate by the intact functioning kidney of the dog.Am J Physiol.1973;224(6):1463–1467.
- ,,, et al.Lactate and glucose metabolism in severe sepsis and cardiogenic shock.Crit Care Med.2005;33(10):2235–2240.
- ,,,, et al.Lactic acidosis in fulminant hepatic failure. Some aspects of pathogenesis and prognosis.J Hepatol.1985;1(4):405–416.
- ,.Lactic acidosis in critical illness.Crit Care Med.1992;20(1):80–93.
- ,,, et al.Effects of cardiogenic shock on lactate and glucose metabolism after heart surgery.Crit Care Med.2000;28(12):3784–3791.
- ,,,,,.Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine‐treated septic shock.Crit Care Med.2000;28(1):114–119.
- ,,, et al.Oxygen debt and metabolic acidemia as quantitative predictors of mortality and the severity of the ischemic insult in hemorrhagic shock.Crit Care Med.1991;19(2):231–243.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345(19):1368–1377.
- ,,,.Skeletal muscle partial pressure of oxygen in patients with sepsis.Crit Care Med.1994;22(4):640–650.
- ,.Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis.JAMA.1992;267(11):1503–1510.
- ,,,.Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability.Ann Surg.1996;224(1):97–102.
- ,,,.Biguanide‐associated lactic acidosis. Case report and review of the literature.Arch Intern Med.1992;152(11):2333–2336.
- ,,,.Bench‐to‐bedside review: severe lactic acidosis in HIV patients treated with nucleoside analogue reverse transcriptase inhibitors.Crit Care.2003;7(3):226–232.
- ,,,.Lactic acidosis after treatment with linezolid.Infection.2007;35(4):278–281.
- ,,,.Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion.Surgery.1991;110(1):54–67.
- .Alterations in carbohydrate metabolism during stress: a review of the literature.Am J Med.1995;98(1):75–84.
- .,,,.Lactic acid kinetics in respiratory alkalosis.Crit Care Med.1991;19(9):1120–1124.
- .Significance of hyperlactatemia without acidosis during hypermetabolic stress.Crit Care Med.1997;25(11):1780–1781.
- ,,,.Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis.Lancet.1999;354(9177):505–508.
- ,,,,.Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study.Lancet.2005;365(9462):871–875.
- ,.Sepsis induces diaphragm electron transport chain dysfunction and protein depletion.Am J Respir Crit Care Med.2005;172(7):861–868.
- ,,, et al.Association between mitochondrial dysfunction and severity and outcome of septic shock.Lancet.2002;360(9328):219–223.
- ,.Mitochondrial dysfunction in sepsis.Biochem Soc Symp.1999;66:149–166.
- ,,, et al.Altered glucose transporter mRNA abundance in a rat model of endotoxic shock.Biochem Biophys Res Commun.1991;176(1):535–540.
- .Increased pyruvate dehydrogenase kinase activity in response to sepsis.Am J Physiol.1991;260(5 Pt 1):E669–E674.
- .Lactate metabolism: a new paradigm for the third millennium.J Physiol.2004;558(Pt 1):5–30.
- ,,, et al.Evidence supporting the existence of an activity‐dependent astrocyte‐neuron lactate shuttle.Dev Neurosci.1998;20(4–5):291–299.
- ,,,.Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation.J Neurochem.1997;69(1):423–426.
- .Bench‐to‐bedside review: a possible resolution of the glucose paradox of cerebral ischemia.Crit Care.2002;6(4):330–334.
- ,,,,,.Intravenous lactate prevents cerebral dysfunction during hypoglycaemia in insulin‐dependent diabetes mellitus.Clin Sci (Lond).1998;94(2):157–163.
- ,,,.Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study.Brain Res.1997;744(1):105–111.
- ,,,,,.Clinical prognostic markers in patients with severe sepsis: a prospective analysis of 139 consecutive cases.J Infect.2003;47(4):300–306.
- ,,, et al.Serum lactate as a predictor of mortality in patients with infection.Intensive Care Med.2007;33(6):970–977.
- ,,,.Lactate versus non‐lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients.Crit Care.2006;10(1):R22.
- .Abnormal resting blood lactate. I. The significance of hyperlactatemia in hospitalized patients.Am J Med.1961;30:840–848.
- ,,,,.Effect of lactic acidosis on canine hemodynamics and left ventricular function.Am J Physiol.1990;258(4 Pt 2):H1193–H1199.
- ,,,.Alkali therapy extends the period of survival during hypoxia: studies in rats.Am J Physiol.1996;271(2 Pt 2):R381–R387.
- ,,.Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts.J Clin Invest.1988;82(3):920–927.
- ,,, et al.Effect of acidotic blood reperfusion on reperfusion injury after coronary artery occlusion in the dog heart.J Cardiovasc Pharmacol.1998;31(2):179–186.
- ,,,.Hypoxia and acidosis activate cardiac myocyte death through the Bcl‐2 family protein BNIP3.Proc Natl Acad Sci U S A.2002;99(20):12825–12830.
- ,,,,,.Extracellular acidosis delays onset of cell death in ATP‐depleted hepatocytes.Am J Physiol.1988;255(3 Pt 1):C315–C322.
- ,.Metabolic and hemodynamic effects of hypertonic solutions: sodium‐lactate versus sodium chloride infusion in postoperative patients.Shock.2002;18(4):306–310.
- .Medical complications of status epilepticus.Adv Neurol.1983;34:395–398.
- ,,,,,.Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial.Rev Invest Clin.1991;43(3):234–238.
- ,,.Supercarbia in children: clinical course and outcome.Crit Care Med.1990;18(2):166–168.
- ,,,.Low mortality rate in adult respiratory distress syndrome using low‐volume, pressure‐limited ventilation with permissive hypercapnia: a prospective study.Crit Care Med.1994;22(10):1568–1578.
- ,,,.Systemic effects of NaHCO3 in experimental lactic acidosis in dogs.Am J Physiol.1982;242(6):F586–F591.
- ,,,,.Lactic acidosis: effect of treatment on intracellular pH and energetics in living rat heart.Am J Physiol.1992;262(5 Pt 2):H1572–H1578.
- ,,,,,.Effect of sodium bicarbonate on intracellular pH under different buffering conditions.Kidney Int.1996;49(5):1262–1267.
- ,,.Hemodynamic and hepatic pH responses to sodium bicarbonate and Carbicarb during systemic acidosis.Magn Reson Med.1990;16(3):403–410.
- ,,, et al.The increase in CO2 production induced by NaHCO3 depends on blood albumin and hemoglobin concentrations.Intensive Care Med.2000;26(5):558–564.
- ,,, et al.Initial effect of sodium bicarbonate on intracellular pH depends on the extracellular nonbicarbonate buffering capacity.Crit Care Med.2001;29(5):1033–1039.
- ,,,,,.Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock.Crit Care Med.2000;28(9):3233–3241.
- ,,,,,.The effects of sodium bicarbonate and a mixture of sodium bicarbonate and carbonate (“Carbicarb”) on skeletal muscle pH and hemodynamic status in rats with hypovolemic shock.Metabolism.1994;43(4):518–522.
- ,,, et al.Haemodynamic and metabolic effects in diabetic ketoacidosis in rats of treatment with sodium bicarbonate or a mixture of sodium bicarbonate and sodium carbonate.Diabetologia.1995;38(8):889–898.
- ,,,,,.Effects of sodium bicarbonate on striated muscle metabolism and intracellular pH during endotoxic shock.Shock.1994;1(3):196–200.
- ,,,,.Carbicarb, sodium bicarbonate, and sodium chloride in hypoxic lactic acidosis. Effect on arterial blood gases, lactate concentrations, hemodynamic variables, and myocardial intracellular pH.Chest.1993;104(3):913–918.
- ,.Improved hemodynamic function during hypoxia with Carbicarb, a new agent for the management of acidosis.Circulation.1988;77(1):227–233.
- ,,.Metabolic effects of sodium bicarbonate in hypoxic lactic acidosis in dogs.Am J Physiol.1985;249(5 Pt 2):F630–F635.
- .Binding of calcium to serum albumin. II. Effect of pH via competitive hydrogen and calcium ion binding to the imidazole groups of albumin.Scand J Clin Lab Invest.1972;29(1):75–83.
- ,,,,.Left ventricular contractility varies directly with blood ionized calcium.Ann Intern Med.1988;108(4):524–529.
- ,,.Regulatory mechanisms of hemoglobin oxygen affinity in acidosis and alkalosis.J Clin Invest.1971;50(3):700–706.
- ,,.Metabolic and hemodynamic consequences of sodium bicarbonate administration in patients with heart disease.Am J Med.1989;87(1):7–14.
- ,,.Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury.J Clin Pharmacol.1998;38(10):887–897.
- ,.SEA0400: a novel sodium‐calcium exchange inhibitor with cardioprotective properties.Cardiovasc Drug Rev.2004;22(4):334–347.
- ,,,.Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart.Circ Res.1987;60(5):700–707.
- ,.Acute haemodynamic effects of sodium bicarbonate administration in respiratory and metabolic acidosis in anaesthetized dogs.Anaesth Intensive Care.1997;25(6):615–620.
- ,,,.Bicarbonate does not increase left ventricular contractility during L‐lactic acidemia in pigs.Am Rev Respir Dis.1993;148(2):317–322.
- ,,,,.Effects of bicarbonate therapy on hemodynamics and tissue oxygenation in patients with lactic acidosis: a prospective, controlled clinical study.Crit Care Med.1991;19(11):1352–1356.
- ,,, et al.Safety and efficacy of intravenous Carbicarb in patients undergoing surgery: comparison with sodium bicarbonate in the treatment of mild metabolic acidosis. SPI Research Group. Study of Perioperative Ischemia.Crit Care Med.1994;22(10):1540–1549.
- ,,, et al.Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis.J Nephrol.2005;18(3):303–307.
- ,,, et al.A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate‐Lactic Acidosis Study Group.N Engl J Med.1992;327(22):1564–1569.
- ,,,,,.Effect of continuous venovenous hemofiltration with dialysis on lactate clearance in critically ill patients.Crit Care Med.1997;25(1):58–62.
- ,,,,.A pilot randomised controlled comparison of continuous veno‐venous haemofiltration and extended daily dialysis with filtration: effect on small solutes and acid‐base balance.Intensive Care Med.2007;33(5):830–835.
- ,,.Intermittent versus continuous renal replacement therapy in the ICU: impact on electrolyte and acid‐base balance.Intensive Care Med.2001;27(6):1037–1043.
- ,,,.Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and meta‐analysis.Arch Intern Med.2003;163(21):2594–2602.
- ,,,,.Severe acidosis in patients taking metformin—rapid reversal and survival despite high APACHE score.Diabet Med.2006;23(4):432–435.
- ,,.Incidence of lactic acidosis in metformin users.Diabetes Care.1999;22(6):925–927.
- ,.Lactic acidosis in metformin‐treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations.Drug Saf.1999;20(4):377–384.
- ,,,.The management of metformin overdose.Anaesthesia.1998;53(7):698–701.
- ,,.Lactic acidosis in biguanide‐treated diabetics: a review of 330 cases.Diabetologia.1978;14(2):75–87.
- ,,,,.Treatment of metformin‐associated lactic acidosis with closed recirculation bicarbonate‐buffered hemodialysis.Arch Intern Med.1984;144(1):203–205.
- ,,,.High anion gap metabolic acidosis in suicide: don't forget metformin intoxication—two patients' experiences.Ren Fail.2002;24(5):671–675.
- ,,, et al.Hemodialysis in the treatment of lactic acidosis in diabetics treated by metformin: a study of metformin elimination.Int J Clin Pharmacol Ther Toxicol.1989;27(6):285–288.
- ,,.Bicarbonate haemodialysis as a treatment of metformin overdose.Nephrol Dial Transplant.1997;12(5):1046–1047.
- ,,,.Combination of intermittent haemodialysis and high‐volume continuous haemofiltration for the treatment of severe metformin‐induced lactic acidosis.Nephrol Dial Transplant.2004;19(8):2157–2158.
- ,,,.When a friend can become an enemy! Recognition and management of metformin‐associated lactic acidosis.Kidney Int.2007;72(9):1157–1160.
- .,, et al.Severe nucleoside‐associated lactic acidosis in human immunodeficiency virus‐infected patients: report of 12 cases and review of the literature.Clin Infect Dis.2002;34(6):838–846.
- ,,.Clearance of metformin by hemofiltration in overdose.J Toxicol Clin Toxicol.2002;40(2):177–180.
- .Metformin‐associated lactic acidosis.J Emerg Med.2001;20(3):267–272.
- ,.Contraindications to use of metformin. Blanket banning of metformin two days before surgery may not be a good idea.BMJ.2003;326(7392):762; author reply 762.
- ,,, et al.Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B.N Engl J Med.1995;333(17):1099–1105.
- ,,,.Zidovudine‐induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature.Crit Care Med.1997;25(8):1425–1430.
- ,.Mitochondrial toxicity of antiviral drugs.Nat Med.1995;1(5):417–422.
- ,.Mitochondrial toxicity of nucleoside analogue reverse transcriptase inhibitors: a looming obstacle for long‐term antiretroviral therapy?Curr Opin Infect Dis.2000;13(1):5–11.
- ,,, et al.Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society‐USA panel.JAMA.2006;296(7):827–843.
- ,,.Riboflavin to treat nucleoside analogue‐induced lactic acidosis.Lancet.1998;352(9124):291–292.
- ,,,,,.Severe lactic acidosis and thiamine administration in an HIV‐infected patient on HAART.Int J STD AIDS.2001;12(6):407–409.
- ,,, et al.Detecting life‐threatening lactic acidosis related to nucleoside‐analog treatment of human immunodeficiency virus‐infected patients, and treatment with L‐carnitine.Crit Care Med.2003;31(4):1042–1047.
- ,.Hyperosmolar metabolic acidosis and intravenous Lorazepam.N Engl J Med.2002;347(11):857–858; author reply 857–858.
- ,,,.Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines: a case series and prospective, observational pilot study.Chest.2005;128(3):1674–1681.
- ,,, et al.Propylene glycol pharmacokinetics and effects after intravenous infusion in humans.Ther Drug Monit.1987;9(3):255–258.
- .Short‐term lorazepam infusion and concern for propylene glycol toxicity: case report and review.Pharmacotherapy.2001;21(9):1140–1144.
- ,,.Recognition, treatment, and prevention of propylene glycol toxicity.Semin Dial.2007;20(3):217–219.
- ,,.Propylene glycol‐mediated cell injury in a primary culture of human proximal tubule cells.Toxicol Sci.1998;46(2):410–417.
- ,.Osmolar gap metabolic acidosis in a 60‐year‐old man treated for hypoxemic respiratory failure.Chest.2000;118(2):545–546.
- ,,,.Relationship of continuous infusion lorazepam to serum propylene glycol concentration in critically ill adults.Crit Care Med.2004;32(8):1709–1714.
- ,,,.Removal of propylene glycol and correction of increased osmolar gap by hemodialysis in a patient on high dose lorazepam infusion therapy.Intensive Care Med.2002;28(1):81–84.
- ,,.Linezolid use associated with lactic acidosis.Scand J Infect Dis.2005;37(2):153–154.
- ,.Linezolid‐induced lactic acidosis.N Engl J Med.2003;348(1):86–87.
- ,,.Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey.Diagn Microbiol Infect Dis.2008;62(4):407–410.
- ,,.Mitochondrial toxicity associated with linezolid.N Engl J Med.2005;353(21):2305–2306.
- ,,, et al.Linezolid‐induced inhibition of mitochondrial protein synthesis.Clin Infect Dis.2006;42(8):1111–1117.
- ,.Use of base in the treatment of severe acidemic states.Am J Kidney Dis.2001;38(4):703–727.