User login
Malpositioned IUDs: When you should intervene (and when you should not)
CASE Embedded IUD, currently asymptomatic patient
A 32-year-old G4, P3 presents 1 day after pelvic ultrasonography (US) is performed to evaluate a previous report of intermittent left lower quadrant pain. She is using a levonorgestrel-intrauterine system (LNG-IUS) for contraception, which was placed 1 year ago when she was 6-weeks postpartum. She previously had heavy menses but now has minimal bleeding and is happy with her intrauterine device (IUD). US showed that the IUD is in the lower uterine segment, with the left arm embedded in the myometrium (FIGURE 1). The patient’s pain resolved spontaneously 2 weeks ago, and she is now asymptomatic.
What were this patient’s risk factors for IUD malpositioning? How would you manage her at this time?
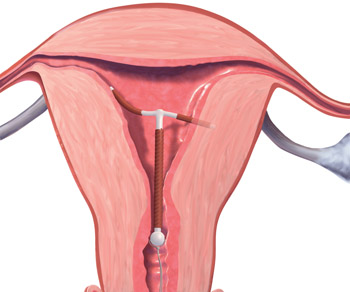
FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.IUDs are an increasingly common form of birth control, now used by 5.5% of contracepting women in the United States.1 With frequent use of pelvic US to evaluate gynecologic complaints, the discovery of malpositioned IUDs also has become an increasingly common occurrence. Clinicians often find themselves faced with dilemmas regarding how to manage a malpositioned IUD, especially in the setting of an asymptomatic patient.
In this article, we review: 1) what constitutes a malpositioned IUD, 2) the consequences of malpositioning, 3) how or if malpositioning can be avoided, and 4) how to manage a malpositioned IUD.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Update on Contraception
Tami Rowen, MD, MS; Mitchell D. Creinin, MD (August 2012)
What constitutes malpositioning?
A correctly positioned IUD should be located at the fundus of the uterus, with the arms fully expanded and extending toward the uterine cornua. The vertical portion of the “T” should extend straight down in the uterine corpus. When noted on US, malpositioned IUDs may be described as:
- located in the lower uterine segment or cervix
- rotated (FIGURE 2)
- embedded in the myometrium (one or both arms) (FIGURE 3)
- partially expelled (if an IUD is low enough in the cervix that the hub extends through the external os), or
- protruding through the uterine serosa or completely outside the uterus and within the abdominal cavity. (This is how a perforated or partially perforated IUD may be described.)
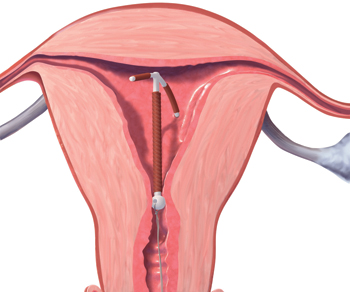
FIGURE 2 Rotated IUD
Copper intrauterine device rotated horizontally.
FIGURE 3 Embedded IUD
Three-dimensional sonogram of a 26-year-old patient, showing an intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.If US does not show an IUD that has been placed, x-ray should performed to explore for perforation (see algorithm.)
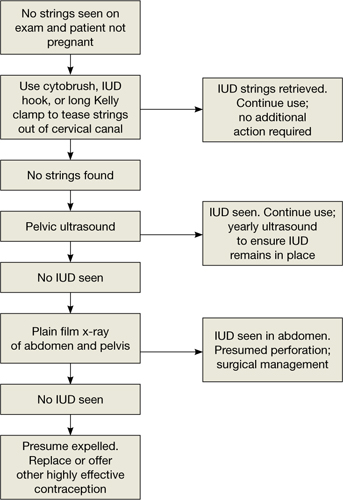
What damage can malpositioning cause?
For many women, a malpositioned IUD may have minimal or no adverse consequences. The most common negative sequelae of women with a malpositioned IUD, however, include an increase in bleeding or pain, compared with women with fundally positioned IUDs.
Bernacerraf and colleagues retrospectively reviewed the medical records of 167 consecutive women who had ultrasound examination with an IUD in place and found that 28 (16.8%) of them had malpositioned devices.2 Of these 28 women, 75% presented with either bleeding or pain, compared with 34.5% of women with normally positioned devices (P=.0001). Twenty of the 21 patients with a malpositioned device and symptoms reported improvement in their symptoms after IUD removal. In this study, the type of IUD was not specified.
Similar to Bernacerraf and colleagues, authors of a case-controlled study, in which women with malpositioned IUDs were compared with women with normally positioned IUDs, found a higher proportion of symptoms, including bleeding and pain, among women with malpositioned IUDs.3 This study included both copper and levonorgestrel IUDs.
The bowel can suffer, though on rare occasion. The rarest, though most serious form of IUD malpositioning, is the IUD that has perforated the uterine corpus and is intraperitoneally located (FIGURE 4). Studies suggest that approximately 15% of these perforated IUDs cause injury or damage to surrounding organs, primarily the bowel. Management of intraperitoneal IUDs generally involves laparoscopy or laparotomy for removal and exploration of the surrounding structures.4
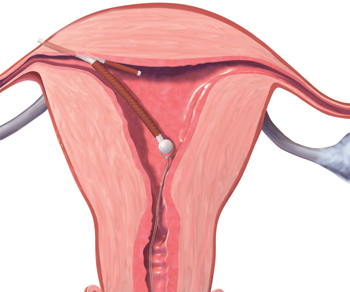
FIGURE 4 Perforation
A copper intrauterine device perforating the serosa.
What about risk of pregnancy?
For the asymptomatic patient, your biggest concern often is whether a malpositioned IUD poses an increased risk of pregnancy. Though data are limited, the available literature suggests that malpositioned, specifically cervically located, copper IUDs may pose an increased risk of pregnancy.
In a prospective study, the authors compared 97 women who had a Cu375 Multiload IUD inserted with 25 women in whom pregnancy was discovered with an IUD in place.5 They found a greater occurrence of intracervical IUDs among the pregnant women, with an odds ratio of 13.93 for pregnancy among women with cervically versus correctly positioned IUDs. Similarly, findings from a case-control study, in which 318 women with pregnancies with CuT380A IUDs in place were compared with 300 controls also using the CuT380A IUD, revealed a 64% rate of IUD malpositioning among the pregnant cases, compared with an 11% rate among the nonpregnant controls (P<.05).6
Does pregnancy or malpositioned IUD come first? None of these studies are able to clarify if it is low placement of the IUD that leads to increased risk of pregnancy or if the pregnancy itself causes malpositioning of the IUD. It is also not known if other types of malpositioning, such as arms extending into the myometrium, are associated with any greater risk of pregnancy. Finally, because there are no prospective studies that have followed a cohort of women with IUDs in situ and assessed pregnancy status according to IUD position, we do not have any data on the absolute risk of pregnancy with a malpositioned IUD in place, though it is likely very small.
IUD type makes a difference. The LNG-IUS does not appear to pose the same risk of pregnancy as copper IUDs if malpositioned. The LNG-IUS prevents pregnancy primarily through hormonal effects on the cervical mucus and endometrium. It seems that the local effects of levonorgestrel are likely adequate for contraception even if the device is not at the fundus, as long as it remains within the uterine cavity. This hypothesis is supported by a randomized clinical trial in which researchers compared the efficacy of an intracervical device that releases the same dose of levonorgestrel as the LNG-IUS, with the efficacy of an LNG-IUS placed at the fundus.7 This study demonstrated no difference in pregnancy rates between the intracervically and the fundally positioned devices.
You also may worry that a downwardly displaced IUD represents risk for expulsion. Although two small studies have suggested that IUDs positioned more than 3 mm from the fundus might have a higher risk of expulsion, most downwardly displaced IUDs are not expelled.8,9 Removal and replacement of downwardly displaced IUDs for the purpose of preventing expulsion would result in a large number of unnecessary removals. Also, studies have shown that not all downwardly displaced IUDs remain so. In fact, the vast majority of IUDs that are downwardly displaced shortly after insertion move to a fundal position within 3 months.10,11
Can malpositioning be avoided?
It is not clear to what extent prevention is possible. Risk factors for IUD malpositioning were examined in a recent case-controlled study. Its authors found that suspected adenomyosis increased the risk of IUD malpositioning and that prior vaginal delivery was protective. No effect of delayed postpartum insertion was seen. The authors also found that public or no insurance was associated with an increased risk of malpositioning; they suggest that this may be related to higher rates of insertion by trainees. Indeed, other studies have found that IUD complications, such as failed insertion and early removals due to pain or bleeding, are associated with insertion by less experienced providers12,13; more skilled providers experience lower rates of IUD malpositioning. Enhancing IUD insertion training may decrease the risk of malpositioning; however, a learning curve may remain.
Despite the fact that some women may be at higher risk for IUD malpositioning, it does not mean they are not IUD candidates. It may be prudent to consider US guidance for IUD insertion in cases of:
- a previous difficult insertion
- obesity precluding the accurate assessment of uterine position, or
- suspected abnormal or distorted uterine cavity.
Integrating evidence and experience
The greatest risk for pregnancy may be unnecessary removal of an IUD. In a recent case-controlled study, Braaten and colleagues compared 182 women with malpositioned IUDs noted on US with 182 women found to have normally positioned IUDs on US. An important finding of this study was that women initially found to have a malpositioned device had a higher rate of pregnancy in the subsequent 2 years. There were no pregnancies among women with malpositioned IUDs left in place; rather, the higher pregnancy rate was due to higher rates of IUD removals (approximately two-thirds of malpositioned IUDs were removed), without replacement with another highly effective method of contraception.
While findings from earlier studies suggest there may be a small increased risk of pregnancy with a malpositioned copper IUD left in situ, as compared with a fundally placed device, this study demonstrates that the real-life risk of pregnancy with removal of an IUD and use of less effective methods of contraception is significantly higher.
Clinicians should be cognizant of this risk prior to removal of a malpositioned IUD and try to ensure that, if a malpositioned IUD is removed, it is quickly replaced with another highly effective form of contraception, such as another IUD, subdermal implant, or sterilization.
Coding for insertion of intrauterine devices (IUDs) can be a hassle if you aren’t familiar with the right code combinations. Here is some advice you can use right now to ensure reimbursement for the usual and unusual situations.
If the purpose of the visit is insertion of an IUD, you only code for that insertion plus the supply. (Even if patient history is repeated at the visit, a separate significant E/M service is not warranted.) Coding is 58300 and J7300 for a copper IUD or J7302 for a levonorgestrel-containing IUD. Note, however, that Blue Cross/Blue Shield payers may require the HCPCS code S4989 (Contraceptive IUD [eg, Progestacert], including implants and supplies), rather than the CPT code.
If you require ultrasound guidance in placing the IUD, the code 76998 can be reported as well.
In some cases, the patient may have a stenotic cervix; if cervical dilation is performed that too can be billed using either 57800, Dilation of cervical canal, instrumental (separate procedure) or 59200, Insertion of cervical dilator (eg, laminaria, prostaglandin) (separate procedure). Because both of these codes are CPT “separate procedures,” a modifier -59 should be added to indicate that a distinct procedure was performed.
In cases in which the IUD is placed immediately following birth, 58300 can be billed but will require a modifier -51. When the IUD is placed 24 hours or more after birth, 58300 requires the addition of the modifier -79 (Unrelated procedure or service by the same physician during the postoperative period).
Sometimes the insertion does not go as planned. If insertion:
- fails due to cervical stenosis, report 58300 with a modifier -52 (Reduced services) since, after considerable work is performed, the decision is made to not insert the device.
- must be stopped because of an unexpected physical reaction by the patient (fainting or a sudden increase or drop in blood pressure), a modifier -53 (Discontinued procedure) is more appropriate.
- is successful but the IUD is expelled from the uterus, repeat insertion may be performed by adding a modifier -76 (Repeat procedure) to 58300.
- is successful but the IUD perforates the uterus to lodge in the abdominal cavity and laparoscopic surgery is required to remove it, the correct code is 49329 (Unlisted laparoscopy procedure, abdomen, peritoneum and omentum). Be sure to compare the work to code 49402, (Removal of peritoneal foreign body from peritoneal cavity) to ensure fair reimbursement.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Our recommendations
The management of malpositioned IUDs can be clinically challenging. Given the available evidence, we suggest the following:
- If patients present with symptoms that may be attributable to the malpositioned device, such as bleeding or pain, the device should be removed and the patient should be offered immediate replacement or immediate initiation of another form of highly effective contraception. Many women will show improvement in their symptoms if the malpositioned device is replaced with one that is correctly placed.
- The asymptomatic patient with a malpositioned LNG-IUS that is still in the uterine cavity can be expectantly managed. She may be offered replacement if she desires.
- An asymptomatic patient with a malpositioned copper IUD should be counseled that she is potentially at higher risk for pregnancy than she would be if her IUD were correctly positioned. This risk cannot be quantified easily, but it is likely lower than the risk of pregnancy associated with most forms of short-acting contraception. She should be counseled for IUD replacement or removal and immediate initiation of another form of highly effective contraception—but you and she also may opt for expectant management if initiating another highly effective form of contraception is not feasible.
CASE Conclusion
The patient is asymptomatic with an LNG-IUS in the lower uterine segment and embedded in the myometrium. She has no obvious risk factors for IUD malpositioning, but given that her menses were heavy prior to placement, she may have adenomyosis.
Given that she is currently asymptomatic with a hormone-containing IUD, she may be managed expectantly. Were she to become symptomatic, she should be offered IUD replacement (versus another form of highly effective contraception such as an implant or sterilization).
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
1. Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):1-44.
2. Bernacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110-115.
3. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014-1020.
4. Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81(1):112-114.
5. Inal MM, Ertopçu K, Özelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005;10(4):266-271.
6. Pakarinen P, Luukkainen T. Five years’ experience with a small intracervical/intrauterine levonorgestrel-releasing device. Contraception. 2005;72(5):342-325.
7. Tangtonpet O, Choktanasiri W, Patrachai S, Israngura Na, Ayudhya N. Intrauterine location and expulsion of intrauterine device. Thai J Obstet Gynecol. 2003;15:45-50.
8. Petta CA, Faúndes D, Pimentel E, Diaz J, Bahamondes L. The use of vaginal ultrasound to identify copper T IUDs at high risk of expulsion. Contraception. 1996;54(5):287-289.
9. Morales-Roselló J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430-431.
10. Faúndes D, Perdigão A, Faúndes A, Bahamondes L, Petta CA. T-shaped IUDs accommodate in their position during the first 3 months after insertion. Contraception. 2000;62(4):165-168.
11. Arslan A, Kanat-Pektas M, Yesilyurt H, Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstet. 2009;279(3):395-397.
12. Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003;29(4):227-231.
13. Zhang J. Factors associated with copper T IUD removal for bleeding/pain: a mulitvariate analysis. Contraception. 1993;48(1):13-21.
CASE Embedded IUD, currently asymptomatic patient
A 32-year-old G4, P3 presents 1 day after pelvic ultrasonography (US) is performed to evaluate a previous report of intermittent left lower quadrant pain. She is using a levonorgestrel-intrauterine system (LNG-IUS) for contraception, which was placed 1 year ago when she was 6-weeks postpartum. She previously had heavy menses but now has minimal bleeding and is happy with her intrauterine device (IUD). US showed that the IUD is in the lower uterine segment, with the left arm embedded in the myometrium (FIGURE 1). The patient’s pain resolved spontaneously 2 weeks ago, and she is now asymptomatic.
What were this patient’s risk factors for IUD malpositioning? How would you manage her at this time?

FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.IUDs are an increasingly common form of birth control, now used by 5.5% of contracepting women in the United States.1 With frequent use of pelvic US to evaluate gynecologic complaints, the discovery of malpositioned IUDs also has become an increasingly common occurrence. Clinicians often find themselves faced with dilemmas regarding how to manage a malpositioned IUD, especially in the setting of an asymptomatic patient.
In this article, we review: 1) what constitutes a malpositioned IUD, 2) the consequences of malpositioning, 3) how or if malpositioning can be avoided, and 4) how to manage a malpositioned IUD.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Update on Contraception
Tami Rowen, MD, MS; Mitchell D. Creinin, MD (August 2012)
What constitutes malpositioning?
A correctly positioned IUD should be located at the fundus of the uterus, with the arms fully expanded and extending toward the uterine cornua. The vertical portion of the “T” should extend straight down in the uterine corpus. When noted on US, malpositioned IUDs may be described as:
- located in the lower uterine segment or cervix
- rotated (FIGURE 2)
- embedded in the myometrium (one or both arms) (FIGURE 3)
- partially expelled (if an IUD is low enough in the cervix that the hub extends through the external os), or
- protruding through the uterine serosa or completely outside the uterus and within the abdominal cavity. (This is how a perforated or partially perforated IUD may be described.)

FIGURE 2 Rotated IUD
Copper intrauterine device rotated horizontally.
FIGURE 3 Embedded IUD
Three-dimensional sonogram of a 26-year-old patient, showing an intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.If US does not show an IUD that has been placed, x-ray should performed to explore for perforation (see algorithm.)

What damage can malpositioning cause?
For many women, a malpositioned IUD may have minimal or no adverse consequences. The most common negative sequelae of women with a malpositioned IUD, however, include an increase in bleeding or pain, compared with women with fundally positioned IUDs.
Bernacerraf and colleagues retrospectively reviewed the medical records of 167 consecutive women who had ultrasound examination with an IUD in place and found that 28 (16.8%) of them had malpositioned devices.2 Of these 28 women, 75% presented with either bleeding or pain, compared with 34.5% of women with normally positioned devices (P=.0001). Twenty of the 21 patients with a malpositioned device and symptoms reported improvement in their symptoms after IUD removal. In this study, the type of IUD was not specified.
Similar to Bernacerraf and colleagues, authors of a case-controlled study, in which women with malpositioned IUDs were compared with women with normally positioned IUDs, found a higher proportion of symptoms, including bleeding and pain, among women with malpositioned IUDs.3 This study included both copper and levonorgestrel IUDs.
The bowel can suffer, though on rare occasion. The rarest, though most serious form of IUD malpositioning, is the IUD that has perforated the uterine corpus and is intraperitoneally located (FIGURE 4). Studies suggest that approximately 15% of these perforated IUDs cause injury or damage to surrounding organs, primarily the bowel. Management of intraperitoneal IUDs generally involves laparoscopy or laparotomy for removal and exploration of the surrounding structures.4

FIGURE 4 Perforation
A copper intrauterine device perforating the serosa.
What about risk of pregnancy?
For the asymptomatic patient, your biggest concern often is whether a malpositioned IUD poses an increased risk of pregnancy. Though data are limited, the available literature suggests that malpositioned, specifically cervically located, copper IUDs may pose an increased risk of pregnancy.
In a prospective study, the authors compared 97 women who had a Cu375 Multiload IUD inserted with 25 women in whom pregnancy was discovered with an IUD in place.5 They found a greater occurrence of intracervical IUDs among the pregnant women, with an odds ratio of 13.93 for pregnancy among women with cervically versus correctly positioned IUDs. Similarly, findings from a case-control study, in which 318 women with pregnancies with CuT380A IUDs in place were compared with 300 controls also using the CuT380A IUD, revealed a 64% rate of IUD malpositioning among the pregnant cases, compared with an 11% rate among the nonpregnant controls (P<.05).6
Does pregnancy or malpositioned IUD come first? None of these studies are able to clarify if it is low placement of the IUD that leads to increased risk of pregnancy or if the pregnancy itself causes malpositioning of the IUD. It is also not known if other types of malpositioning, such as arms extending into the myometrium, are associated with any greater risk of pregnancy. Finally, because there are no prospective studies that have followed a cohort of women with IUDs in situ and assessed pregnancy status according to IUD position, we do not have any data on the absolute risk of pregnancy with a malpositioned IUD in place, though it is likely very small.
IUD type makes a difference. The LNG-IUS does not appear to pose the same risk of pregnancy as copper IUDs if malpositioned. The LNG-IUS prevents pregnancy primarily through hormonal effects on the cervical mucus and endometrium. It seems that the local effects of levonorgestrel are likely adequate for contraception even if the device is not at the fundus, as long as it remains within the uterine cavity. This hypothesis is supported by a randomized clinical trial in which researchers compared the efficacy of an intracervical device that releases the same dose of levonorgestrel as the LNG-IUS, with the efficacy of an LNG-IUS placed at the fundus.7 This study demonstrated no difference in pregnancy rates between the intracervically and the fundally positioned devices.
You also may worry that a downwardly displaced IUD represents risk for expulsion. Although two small studies have suggested that IUDs positioned more than 3 mm from the fundus might have a higher risk of expulsion, most downwardly displaced IUDs are not expelled.8,9 Removal and replacement of downwardly displaced IUDs for the purpose of preventing expulsion would result in a large number of unnecessary removals. Also, studies have shown that not all downwardly displaced IUDs remain so. In fact, the vast majority of IUDs that are downwardly displaced shortly after insertion move to a fundal position within 3 months.10,11
Can malpositioning be avoided?
It is not clear to what extent prevention is possible. Risk factors for IUD malpositioning were examined in a recent case-controlled study. Its authors found that suspected adenomyosis increased the risk of IUD malpositioning and that prior vaginal delivery was protective. No effect of delayed postpartum insertion was seen. The authors also found that public or no insurance was associated with an increased risk of malpositioning; they suggest that this may be related to higher rates of insertion by trainees. Indeed, other studies have found that IUD complications, such as failed insertion and early removals due to pain or bleeding, are associated with insertion by less experienced providers12,13; more skilled providers experience lower rates of IUD malpositioning. Enhancing IUD insertion training may decrease the risk of malpositioning; however, a learning curve may remain.
Despite the fact that some women may be at higher risk for IUD malpositioning, it does not mean they are not IUD candidates. It may be prudent to consider US guidance for IUD insertion in cases of:
- a previous difficult insertion
- obesity precluding the accurate assessment of uterine position, or
- suspected abnormal or distorted uterine cavity.
Integrating evidence and experience
The greatest risk for pregnancy may be unnecessary removal of an IUD. In a recent case-controlled study, Braaten and colleagues compared 182 women with malpositioned IUDs noted on US with 182 women found to have normally positioned IUDs on US. An important finding of this study was that women initially found to have a malpositioned device had a higher rate of pregnancy in the subsequent 2 years. There were no pregnancies among women with malpositioned IUDs left in place; rather, the higher pregnancy rate was due to higher rates of IUD removals (approximately two-thirds of malpositioned IUDs were removed), without replacement with another highly effective method of contraception.
While findings from earlier studies suggest there may be a small increased risk of pregnancy with a malpositioned copper IUD left in situ, as compared with a fundally placed device, this study demonstrates that the real-life risk of pregnancy with removal of an IUD and use of less effective methods of contraception is significantly higher.
Clinicians should be cognizant of this risk prior to removal of a malpositioned IUD and try to ensure that, if a malpositioned IUD is removed, it is quickly replaced with another highly effective form of contraception, such as another IUD, subdermal implant, or sterilization.
Coding for insertion of intrauterine devices (IUDs) can be a hassle if you aren’t familiar with the right code combinations. Here is some advice you can use right now to ensure reimbursement for the usual and unusual situations.
If the purpose of the visit is insertion of an IUD, you only code for that insertion plus the supply. (Even if patient history is repeated at the visit, a separate significant E/M service is not warranted.) Coding is 58300 and J7300 for a copper IUD or J7302 for a levonorgestrel-containing IUD. Note, however, that Blue Cross/Blue Shield payers may require the HCPCS code S4989 (Contraceptive IUD [eg, Progestacert], including implants and supplies), rather than the CPT code.
If you require ultrasound guidance in placing the IUD, the code 76998 can be reported as well.
In some cases, the patient may have a stenotic cervix; if cervical dilation is performed that too can be billed using either 57800, Dilation of cervical canal, instrumental (separate procedure) or 59200, Insertion of cervical dilator (eg, laminaria, prostaglandin) (separate procedure). Because both of these codes are CPT “separate procedures,” a modifier -59 should be added to indicate that a distinct procedure was performed.
In cases in which the IUD is placed immediately following birth, 58300 can be billed but will require a modifier -51. When the IUD is placed 24 hours or more after birth, 58300 requires the addition of the modifier -79 (Unrelated procedure or service by the same physician during the postoperative period).
Sometimes the insertion does not go as planned. If insertion:
- fails due to cervical stenosis, report 58300 with a modifier -52 (Reduced services) since, after considerable work is performed, the decision is made to not insert the device.
- must be stopped because of an unexpected physical reaction by the patient (fainting or a sudden increase or drop in blood pressure), a modifier -53 (Discontinued procedure) is more appropriate.
- is successful but the IUD is expelled from the uterus, repeat insertion may be performed by adding a modifier -76 (Repeat procedure) to 58300.
- is successful but the IUD perforates the uterus to lodge in the abdominal cavity and laparoscopic surgery is required to remove it, the correct code is 49329 (Unlisted laparoscopy procedure, abdomen, peritoneum and omentum). Be sure to compare the work to code 49402, (Removal of peritoneal foreign body from peritoneal cavity) to ensure fair reimbursement.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Our recommendations
The management of malpositioned IUDs can be clinically challenging. Given the available evidence, we suggest the following:
- If patients present with symptoms that may be attributable to the malpositioned device, such as bleeding or pain, the device should be removed and the patient should be offered immediate replacement or immediate initiation of another form of highly effective contraception. Many women will show improvement in their symptoms if the malpositioned device is replaced with one that is correctly placed.
- The asymptomatic patient with a malpositioned LNG-IUS that is still in the uterine cavity can be expectantly managed. She may be offered replacement if she desires.
- An asymptomatic patient with a malpositioned copper IUD should be counseled that she is potentially at higher risk for pregnancy than she would be if her IUD were correctly positioned. This risk cannot be quantified easily, but it is likely lower than the risk of pregnancy associated with most forms of short-acting contraception. She should be counseled for IUD replacement or removal and immediate initiation of another form of highly effective contraception—but you and she also may opt for expectant management if initiating another highly effective form of contraception is not feasible.
CASE Conclusion
The patient is asymptomatic with an LNG-IUS in the lower uterine segment and embedded in the myometrium. She has no obvious risk factors for IUD malpositioning, but given that her menses were heavy prior to placement, she may have adenomyosis.
Given that she is currently asymptomatic with a hormone-containing IUD, she may be managed expectantly. Were she to become symptomatic, she should be offered IUD replacement (versus another form of highly effective contraception such as an implant or sterilization).
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
CASE Embedded IUD, currently asymptomatic patient
A 32-year-old G4, P3 presents 1 day after pelvic ultrasonography (US) is performed to evaluate a previous report of intermittent left lower quadrant pain. She is using a levonorgestrel-intrauterine system (LNG-IUS) for contraception, which was placed 1 year ago when she was 6-weeks postpartum. She previously had heavy menses but now has minimal bleeding and is happy with her intrauterine device (IUD). US showed that the IUD is in the lower uterine segment, with the left arm embedded in the myometrium (FIGURE 1). The patient’s pain resolved spontaneously 2 weeks ago, and she is now asymptomatic.
What were this patient’s risk factors for IUD malpositioning? How would you manage her at this time?

FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.IUDs are an increasingly common form of birth control, now used by 5.5% of contracepting women in the United States.1 With frequent use of pelvic US to evaluate gynecologic complaints, the discovery of malpositioned IUDs also has become an increasingly common occurrence. Clinicians often find themselves faced with dilemmas regarding how to manage a malpositioned IUD, especially in the setting of an asymptomatic patient.
In this article, we review: 1) what constitutes a malpositioned IUD, 2) the consequences of malpositioning, 3) how or if malpositioning can be avoided, and 4) how to manage a malpositioned IUD.
Let’s increase our use of IUDs and improve contraceptive effectiveness in this country
Robert L. Barbieri, MD (Editorial, August 2012)
Update on Contraception
Tami Rowen, MD, MS; Mitchell D. Creinin, MD (August 2012)
What constitutes malpositioning?
A correctly positioned IUD should be located at the fundus of the uterus, with the arms fully expanded and extending toward the uterine cornua. The vertical portion of the “T” should extend straight down in the uterine corpus. When noted on US, malpositioned IUDs may be described as:
- located in the lower uterine segment or cervix
- rotated (FIGURE 2)
- embedded in the myometrium (one or both arms) (FIGURE 3)
- partially expelled (if an IUD is low enough in the cervix that the hub extends through the external os), or
- protruding through the uterine serosa or completely outside the uterus and within the abdominal cavity. (This is how a perforated or partially perforated IUD may be described.)

FIGURE 2 Rotated IUD
Copper intrauterine device rotated horizontally.
FIGURE 3 Embedded IUD
Three-dimensional sonogram of a 26-year-old patient, showing an intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium.If US does not show an IUD that has been placed, x-ray should performed to explore for perforation (see algorithm.)

What damage can malpositioning cause?
For many women, a malpositioned IUD may have minimal or no adverse consequences. The most common negative sequelae of women with a malpositioned IUD, however, include an increase in bleeding or pain, compared with women with fundally positioned IUDs.
Bernacerraf and colleagues retrospectively reviewed the medical records of 167 consecutive women who had ultrasound examination with an IUD in place and found that 28 (16.8%) of them had malpositioned devices.2 Of these 28 women, 75% presented with either bleeding or pain, compared with 34.5% of women with normally positioned devices (P=.0001). Twenty of the 21 patients with a malpositioned device and symptoms reported improvement in their symptoms after IUD removal. In this study, the type of IUD was not specified.
Similar to Bernacerraf and colleagues, authors of a case-controlled study, in which women with malpositioned IUDs were compared with women with normally positioned IUDs, found a higher proportion of symptoms, including bleeding and pain, among women with malpositioned IUDs.3 This study included both copper and levonorgestrel IUDs.
The bowel can suffer, though on rare occasion. The rarest, though most serious form of IUD malpositioning, is the IUD that has perforated the uterine corpus and is intraperitoneally located (FIGURE 4). Studies suggest that approximately 15% of these perforated IUDs cause injury or damage to surrounding organs, primarily the bowel. Management of intraperitoneal IUDs generally involves laparoscopy or laparotomy for removal and exploration of the surrounding structures.4

FIGURE 4 Perforation
A copper intrauterine device perforating the serosa.
What about risk of pregnancy?
For the asymptomatic patient, your biggest concern often is whether a malpositioned IUD poses an increased risk of pregnancy. Though data are limited, the available literature suggests that malpositioned, specifically cervically located, copper IUDs may pose an increased risk of pregnancy.
In a prospective study, the authors compared 97 women who had a Cu375 Multiload IUD inserted with 25 women in whom pregnancy was discovered with an IUD in place.5 They found a greater occurrence of intracervical IUDs among the pregnant women, with an odds ratio of 13.93 for pregnancy among women with cervically versus correctly positioned IUDs. Similarly, findings from a case-control study, in which 318 women with pregnancies with CuT380A IUDs in place were compared with 300 controls also using the CuT380A IUD, revealed a 64% rate of IUD malpositioning among the pregnant cases, compared with an 11% rate among the nonpregnant controls (P<.05).6
Does pregnancy or malpositioned IUD come first? None of these studies are able to clarify if it is low placement of the IUD that leads to increased risk of pregnancy or if the pregnancy itself causes malpositioning of the IUD. It is also not known if other types of malpositioning, such as arms extending into the myometrium, are associated with any greater risk of pregnancy. Finally, because there are no prospective studies that have followed a cohort of women with IUDs in situ and assessed pregnancy status according to IUD position, we do not have any data on the absolute risk of pregnancy with a malpositioned IUD in place, though it is likely very small.
IUD type makes a difference. The LNG-IUS does not appear to pose the same risk of pregnancy as copper IUDs if malpositioned. The LNG-IUS prevents pregnancy primarily through hormonal effects on the cervical mucus and endometrium. It seems that the local effects of levonorgestrel are likely adequate for contraception even if the device is not at the fundus, as long as it remains within the uterine cavity. This hypothesis is supported by a randomized clinical trial in which researchers compared the efficacy of an intracervical device that releases the same dose of levonorgestrel as the LNG-IUS, with the efficacy of an LNG-IUS placed at the fundus.7 This study demonstrated no difference in pregnancy rates between the intracervically and the fundally positioned devices.
You also may worry that a downwardly displaced IUD represents risk for expulsion. Although two small studies have suggested that IUDs positioned more than 3 mm from the fundus might have a higher risk of expulsion, most downwardly displaced IUDs are not expelled.8,9 Removal and replacement of downwardly displaced IUDs for the purpose of preventing expulsion would result in a large number of unnecessary removals. Also, studies have shown that not all downwardly displaced IUDs remain so. In fact, the vast majority of IUDs that are downwardly displaced shortly after insertion move to a fundal position within 3 months.10,11
Can malpositioning be avoided?
It is not clear to what extent prevention is possible. Risk factors for IUD malpositioning were examined in a recent case-controlled study. Its authors found that suspected adenomyosis increased the risk of IUD malpositioning and that prior vaginal delivery was protective. No effect of delayed postpartum insertion was seen. The authors also found that public or no insurance was associated with an increased risk of malpositioning; they suggest that this may be related to higher rates of insertion by trainees. Indeed, other studies have found that IUD complications, such as failed insertion and early removals due to pain or bleeding, are associated with insertion by less experienced providers12,13; more skilled providers experience lower rates of IUD malpositioning. Enhancing IUD insertion training may decrease the risk of malpositioning; however, a learning curve may remain.
Despite the fact that some women may be at higher risk for IUD malpositioning, it does not mean they are not IUD candidates. It may be prudent to consider US guidance for IUD insertion in cases of:
- a previous difficult insertion
- obesity precluding the accurate assessment of uterine position, or
- suspected abnormal or distorted uterine cavity.
Integrating evidence and experience
The greatest risk for pregnancy may be unnecessary removal of an IUD. In a recent case-controlled study, Braaten and colleagues compared 182 women with malpositioned IUDs noted on US with 182 women found to have normally positioned IUDs on US. An important finding of this study was that women initially found to have a malpositioned device had a higher rate of pregnancy in the subsequent 2 years. There were no pregnancies among women with malpositioned IUDs left in place; rather, the higher pregnancy rate was due to higher rates of IUD removals (approximately two-thirds of malpositioned IUDs were removed), without replacement with another highly effective method of contraception.
While findings from earlier studies suggest there may be a small increased risk of pregnancy with a malpositioned copper IUD left in situ, as compared with a fundally placed device, this study demonstrates that the real-life risk of pregnancy with removal of an IUD and use of less effective methods of contraception is significantly higher.
Clinicians should be cognizant of this risk prior to removal of a malpositioned IUD and try to ensure that, if a malpositioned IUD is removed, it is quickly replaced with another highly effective form of contraception, such as another IUD, subdermal implant, or sterilization.
Coding for insertion of intrauterine devices (IUDs) can be a hassle if you aren’t familiar with the right code combinations. Here is some advice you can use right now to ensure reimbursement for the usual and unusual situations.
If the purpose of the visit is insertion of an IUD, you only code for that insertion plus the supply. (Even if patient history is repeated at the visit, a separate significant E/M service is not warranted.) Coding is 58300 and J7300 for a copper IUD or J7302 for a levonorgestrel-containing IUD. Note, however, that Blue Cross/Blue Shield payers may require the HCPCS code S4989 (Contraceptive IUD [eg, Progestacert], including implants and supplies), rather than the CPT code.
If you require ultrasound guidance in placing the IUD, the code 76998 can be reported as well.
In some cases, the patient may have a stenotic cervix; if cervical dilation is performed that too can be billed using either 57800, Dilation of cervical canal, instrumental (separate procedure) or 59200, Insertion of cervical dilator (eg, laminaria, prostaglandin) (separate procedure). Because both of these codes are CPT “separate procedures,” a modifier -59 should be added to indicate that a distinct procedure was performed.
In cases in which the IUD is placed immediately following birth, 58300 can be billed but will require a modifier -51. When the IUD is placed 24 hours or more after birth, 58300 requires the addition of the modifier -79 (Unrelated procedure or service by the same physician during the postoperative period).
Sometimes the insertion does not go as planned. If insertion:
- fails due to cervical stenosis, report 58300 with a modifier -52 (Reduced services) since, after considerable work is performed, the decision is made to not insert the device.
- must be stopped because of an unexpected physical reaction by the patient (fainting or a sudden increase or drop in blood pressure), a modifier -53 (Discontinued procedure) is more appropriate.
- is successful but the IUD is expelled from the uterus, repeat insertion may be performed by adding a modifier -76 (Repeat procedure) to 58300.
- is successful but the IUD perforates the uterus to lodge in the abdominal cavity and laparoscopic surgery is required to remove it, the correct code is 49329 (Unlisted laparoscopy procedure, abdomen, peritoneum and omentum). Be sure to compare the work to code 49402, (Removal of peritoneal foreign body from peritoneal cavity) to ensure fair reimbursement.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Our recommendations
The management of malpositioned IUDs can be clinically challenging. Given the available evidence, we suggest the following:
- If patients present with symptoms that may be attributable to the malpositioned device, such as bleeding or pain, the device should be removed and the patient should be offered immediate replacement or immediate initiation of another form of highly effective contraception. Many women will show improvement in their symptoms if the malpositioned device is replaced with one that is correctly placed.
- The asymptomatic patient with a malpositioned LNG-IUS that is still in the uterine cavity can be expectantly managed. She may be offered replacement if she desires.
- An asymptomatic patient with a malpositioned copper IUD should be counseled that she is potentially at higher risk for pregnancy than she would be if her IUD were correctly positioned. This risk cannot be quantified easily, but it is likely lower than the risk of pregnancy associated with most forms of short-acting contraception. She should be counseled for IUD replacement or removal and immediate initiation of another form of highly effective contraception—but you and she also may opt for expectant management if initiating another highly effective form of contraception is not feasible.
CASE Conclusion
The patient is asymptomatic with an LNG-IUS in the lower uterine segment and embedded in the myometrium. She has no obvious risk factors for IUD malpositioning, but given that her menses were heavy prior to placement, she may have adenomyosis.
Given that she is currently asymptomatic with a hormone-containing IUD, she may be managed expectantly. Were she to become symptomatic, she should be offered IUD replacement (versus another form of highly effective contraception such as an implant or sterilization).
Click here to find 7 additional articles on contraception published in OBG Management in 2012.
We want to hear from you! Tell us what you think.
1. Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):1-44.
2. Bernacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110-115.
3. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014-1020.
4. Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81(1):112-114.
5. Inal MM, Ertopçu K, Özelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005;10(4):266-271.
6. Pakarinen P, Luukkainen T. Five years’ experience with a small intracervical/intrauterine levonorgestrel-releasing device. Contraception. 2005;72(5):342-325.
7. Tangtonpet O, Choktanasiri W, Patrachai S, Israngura Na, Ayudhya N. Intrauterine location and expulsion of intrauterine device. Thai J Obstet Gynecol. 2003;15:45-50.
8. Petta CA, Faúndes D, Pimentel E, Diaz J, Bahamondes L. The use of vaginal ultrasound to identify copper T IUDs at high risk of expulsion. Contraception. 1996;54(5):287-289.
9. Morales-Roselló J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430-431.
10. Faúndes D, Perdigão A, Faúndes A, Bahamondes L, Petta CA. T-shaped IUDs accommodate in their position during the first 3 months after insertion. Contraception. 2000;62(4):165-168.
11. Arslan A, Kanat-Pektas M, Yesilyurt H, Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstet. 2009;279(3):395-397.
12. Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003;29(4):227-231.
13. Zhang J. Factors associated with copper T IUD removal for bleeding/pain: a mulitvariate analysis. Contraception. 1993;48(1):13-21.
1. Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):1-44.
2. Bernacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110-115.
3. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors outcomes, and future pregnancies. Obstet Gynecol. 2011;118(5):1014-1020.
4. Anteby E, Revel A, Ben-Chetrit A, Rosen B, Tadmor O, Yagel S. Intrauterine device failure: relation to its location within the uterine cavity. Obstet Gynecol. 1993;81(1):112-114.
5. Inal MM, Ertopçu K, Özelmas I. The evaluation of 318 intrauterine pregnancy cases with an intrauterine device. Eur J Contracept Reprod Health Care. 2005;10(4):266-271.
6. Pakarinen P, Luukkainen T. Five years’ experience with a small intracervical/intrauterine levonorgestrel-releasing device. Contraception. 2005;72(5):342-325.
7. Tangtonpet O, Choktanasiri W, Patrachai S, Israngura Na, Ayudhya N. Intrauterine location and expulsion of intrauterine device. Thai J Obstet Gynecol. 2003;15:45-50.
8. Petta CA, Faúndes D, Pimentel E, Diaz J, Bahamondes L. The use of vaginal ultrasound to identify copper T IUDs at high risk of expulsion. Contraception. 1996;54(5):287-289.
9. Morales-Roselló J. Spontaneous upward movement of lowly placed T-shaped IUDs. Contraception. 2005;72(6):430-431.
10. Faúndes D, Perdigão A, Faúndes A, Bahamondes L, Petta CA. T-shaped IUDs accommodate in their position during the first 3 months after insertion. Contraception. 2000;62(4):165-168.
11. Arslan A, Kanat-Pektas M, Yesilyurt H, Bilge U. Colon penetration by a copper intrauterine device: a case report with literature review. Arch Gynecol Obstet. 2009;279(3):395-397.
12. Farmer M, Webb A. Intrauterine device insertion-related complications: can they be predicted? J Fam Plann Reprod Health Care. 2003;29(4):227-231.
13. Zhang J. Factors associated with copper T IUD removal for bleeding/pain: a mulitvariate analysis. Contraception. 1993;48(1):13-21.