User login
Managing Diabetes in Women of Childbearing Age
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
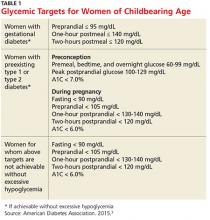
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
Managing Thyroid Disease in Pregnancy
Management of thyroid disease during pregnancy presents unique challenges due to physiologic changes that occur. These include
• Serum levels of thyroxine-binding globulin (TBG) increase along with estrogen; in turn, total thyroxine (T4) and triiodothyronine (T3) levels increase.
• Human chorionic gonadotropin (hCG) stimulates the thyroid stimulating hormone (TSH) receptors.1
Since hCG and TSH share similar glycoprotein subunits, a transient suppression of TSH—especially around weeks 10 to 12, when hCG concentrations peak—is considered a physiologic finding. Interpretation of thyroid function testing should be made in relation to the hCG-mediated decrease in serum TSH levels.2
The following four cases will help guide your clinical management of thyroid disease in both preconception and pregnancy. Inadequately controlled thyroid dysfunction can lead to poor pregnancy outcomes for both mother and child, which will be further discussed.
Continue for Case 1: Stable Hypothyroidism >>
CASE 1: STABLE HYPOTHYROIDISM
A 29-year-old woman with stable primary hypothyroidism calls your office to report that she is pregnant. She has taken levothyroxine (100 mg) for the past three years, and her TSH level was 1.21 mIU/L at last measurement. She denies any symptoms of hyperthyroidism or hypothyroidism. What is your next step in her management?
Recommendation
The American Thyroid Association recommends monitoring serum TSH every four weeks during the first half of pregnancy and at least once per trimester thereafter, with frequency depending on symptoms and TSH levels.3 Most women will require higher doses of levothyroxine supplementation to maintain therapeutic TSH levels.
Prior to 18 weeks’ gestation, the fetus is dependent on maternal thyroid hormone. When pregnancy is confirmed, there is support in the literature for having the patient take two additional doses of levothyroxine per week until TSH can be tested.4 However, many endocrinology practices opt to check TSH and total T4 as soon as pregnancy is confirmed.
Since free T4 results may be unreliable during pregnancy (due to the effect of TBG), free thyroxine index (FTI) or total T4 should be monitored instead. FTI mathematically corrects free T4 for TBG levels, making it a useful marker. If total T4 is measured, it is important to remember that results will be approximately 1.5x the nonpregnancy value; thus, the reference range must be multiplied by 1.5 to calculate appropriate high and low parameters for pregnant patients.
Ideally, all women of childbearing age should be encouraged to plan pregnancy, to ensure TSH is at target prior to conception. Maintaining a euthyroid state throughout pregnancy (starting at conception) is important to decrease risk for such adverse outcomes as spontaneous abortion, placental abruption, and gestational hypertension.2 Low birth weight and respiratory distress are potential complications for newborns whose mothers have inadequately controlled hypothyroidism.
Patients should be counseled against simultaneous dosing of prenatal vitamins and levothyroxine. Prenatal vitamins contain iron, which reduces absorption of levothyroxine; therefore, it is recommended that the levothyroxine be taken four hours or more apart from prenatal vitamins.
The Endocrine Society recommends a TSH no higher than 2.5 mIU/L for hypothyroidism diagnosed prior to pregnancy.2,3 After delivery, levothyroxine doses should be reduced to prepregnancy levels, with close monitoring of TSH.2
Next page: Case 2 >>
CASE 2: HISTORY OF SPONTANEOUS ABORTION
A 36-year-old G3P0 woman visits your office for a work-up after her third spontaneous abortion at 16 weeks. The patient denies history of thyroid disease but notes her maternal grandmother has Hashimoto disease. She denies symptoms of hyperthyroidism or hypothyroidism.
Recommendation
Both hyperthyroidism and hypothyroidism are associated with an increase in spontaneous abortion, premature labor, and low birth weight. Negro et al observed an increased risk for fetal loss, small-for-gestational-age fetus, premature delivery, and premature mortality in women who were TPO-antibody-positive, even if they were euthyroid. Improved fetal outcomes occurred when TPO-antibody-positive mothers received supplemental levothyroxine.5
However, the American Thyroid Association and the Endocrine Society state there is currently insufficient evidence to recommend universal screening of thyroid antibodies during pregnancy.2,3 Obtaining thyroid function studies and TPO-antibody tests could be considered as part of a work-up for women who experience multiple spontaneous abortions or have a personal or family history of autoimmune diseases.
Continue for Case 3: Cardiovascular Symptoms >>
CASE 3: CARDIOVASCULAR SYMPTOMS
A 24-year-old primigravida woman presents with complaints of palpitations and increased anxiety. She is currently 28 weeks pregnant. Her TSH level is undetectable (< 0.01 mIU/L), and her free T4 is 2.1 mg/dL (reference range, 0.5-1.6 mg/dL). An ECG performed at your office shows sinus tachycardia with a rate of 127 beats/min.
Recommendation
Maternal hyperthyroidism increases risk for maternal congestive heart failure, uncontrolled hypertension, atrial fibrillation, and thyroid storm. Additionally, fetal hyperthyroidism can occur, especially if the mother has Graves disease. Since thyroid-stimulating immunoglobulins (TSI) can permeate the placental barrier, poor fetal growth, cardiac failure, and fetal thyrotoxicosis are severe adverse effects of in-utero TSI exposure.
To prevent further complications, antithyroid medications should be started in this case. Methimazole (MMI) carries a risk for a rare birth defect, aplasia cutis, in the first trimester and is best avoided during this time. Propylthiouracil (PTU) should be given in the first trimester and then switched to MMI in the second trimester to decrease the risk for hepatotoxicity associated with PTU. Breastfeeding mothers should be assured that low-dose MMI is generally considered safe for breastfed infants but should be taken after feeding in divided doses if possible.1
For symptom relief, b-blockers can be used, although they do come with some risks. As pregnancy Category C drugs, b-blockers are associated with neonatal growth retardation, hypoglycemia, hypoxia, lower Apgar scores, and neonatal respiratory distress.6 Consider giving the lowest dose possible for the duration of the patient’s symptoms.
Radioactive iodine (I-131) should not be given to patients who plan to become pregnant or who are pregnant.2,3 The Endocrine Society recommends that if a woman inadvertently becomes pregnant, she should be counseled on the risks of radiation to the fetus, which include thyroid destruction if treatment occurs/continues after the 12th week of pregnancy.2 Furthermore, pregnancy should be avoided for the first six months after thyroid ablation to allow sufficient time to obtain the target maternal serum TSH level of 0.3 to 2.5 mIU/L.
Next page: Case 4 >>
CASE 4: PRECONCEPTION SCREENING
A 39-year-old G0P0 woman presents for preconception counseling. She denies family or personal history of thyroid disease and symptoms of thyroid disease. Should she be screened?
Recommendation
There is no consensus or guideline regarding preconception laboratory screening for thyroid disease. Current guidelines by the American Thyroid Association, the American College of Obstetricians and Gynecologists, and The Endocrine Society recommend targeted, not universal, screening.2,3,7
The American Thyroid Association and the Endocrine Society recommend screening TSH in women who are pregnant or intend to become pregnant and
• Have a personal or family history of thyroid disease
• Are older than 30
• Demonstrate symptoms of thyroid dysfunction
• Have goiter
• Are TPO-antibody positive
• Have type 1 diabetes or other autoimmune disorders
• Have a history of miscarriage or preterm delivery
• Have a history of head or neck radiation or thyroid surgery
• Are morbidly obese (BMI > 40)
• Use amiodarone or lithium or were exposed to iodinated radiologic contrast
• Are infertile
• Live in an area with moderate to severe iodine insufficiency.2,3
Rationale for targeted screening of asymptomatic women: Large-scale research has not demonstrated significantly better outcomes in those with subclinical hypothyroidism who receive treatment.7 Small studies2 have demonstrated improved fetal outcomes when subclinical hypothyroidism is treated, but for large bodies (eg, the US Preventive Services Task Force) to recommend screening, a clear improvement in health outcomes must be established via controlled studies. Future research should evaluate the effect of treating subclinical hypothyroidism during pregnancy.
REFERENCES
1. Ballabio, M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73(4):824.
2. The Endocrine Society. Management of thyroid disease in pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:2543–2565.
3. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
4. Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
5. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Clin Endocrinol Metab. 2006;91(7):2587-2591.
6. Cooper DH. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
7. American College of Obstetricians and Gynecologists. Routine thyroid screening not recommended for pregnant women. J Obstet Gynecol. 2007;110:959-960.
Management of thyroid disease during pregnancy presents unique challenges due to physiologic changes that occur. These include
• Serum levels of thyroxine-binding globulin (TBG) increase along with estrogen; in turn, total thyroxine (T4) and triiodothyronine (T3) levels increase.
• Human chorionic gonadotropin (hCG) stimulates the thyroid stimulating hormone (TSH) receptors.1
Since hCG and TSH share similar glycoprotein subunits, a transient suppression of TSH—especially around weeks 10 to 12, when hCG concentrations peak—is considered a physiologic finding. Interpretation of thyroid function testing should be made in relation to the hCG-mediated decrease in serum TSH levels.2
The following four cases will help guide your clinical management of thyroid disease in both preconception and pregnancy. Inadequately controlled thyroid dysfunction can lead to poor pregnancy outcomes for both mother and child, which will be further discussed.
Continue for Case 1: Stable Hypothyroidism >>
CASE 1: STABLE HYPOTHYROIDISM
A 29-year-old woman with stable primary hypothyroidism calls your office to report that she is pregnant. She has taken levothyroxine (100 mg) for the past three years, and her TSH level was 1.21 mIU/L at last measurement. She denies any symptoms of hyperthyroidism or hypothyroidism. What is your next step in her management?
Recommendation
The American Thyroid Association recommends monitoring serum TSH every four weeks during the first half of pregnancy and at least once per trimester thereafter, with frequency depending on symptoms and TSH levels.3 Most women will require higher doses of levothyroxine supplementation to maintain therapeutic TSH levels.
Prior to 18 weeks’ gestation, the fetus is dependent on maternal thyroid hormone. When pregnancy is confirmed, there is support in the literature for having the patient take two additional doses of levothyroxine per week until TSH can be tested.4 However, many endocrinology practices opt to check TSH and total T4 as soon as pregnancy is confirmed.
Since free T4 results may be unreliable during pregnancy (due to the effect of TBG), free thyroxine index (FTI) or total T4 should be monitored instead. FTI mathematically corrects free T4 for TBG levels, making it a useful marker. If total T4 is measured, it is important to remember that results will be approximately 1.5x the nonpregnancy value; thus, the reference range must be multiplied by 1.5 to calculate appropriate high and low parameters for pregnant patients.
Ideally, all women of childbearing age should be encouraged to plan pregnancy, to ensure TSH is at target prior to conception. Maintaining a euthyroid state throughout pregnancy (starting at conception) is important to decrease risk for such adverse outcomes as spontaneous abortion, placental abruption, and gestational hypertension.2 Low birth weight and respiratory distress are potential complications for newborns whose mothers have inadequately controlled hypothyroidism.
Patients should be counseled against simultaneous dosing of prenatal vitamins and levothyroxine. Prenatal vitamins contain iron, which reduces absorption of levothyroxine; therefore, it is recommended that the levothyroxine be taken four hours or more apart from prenatal vitamins.
The Endocrine Society recommends a TSH no higher than 2.5 mIU/L for hypothyroidism diagnosed prior to pregnancy.2,3 After delivery, levothyroxine doses should be reduced to prepregnancy levels, with close monitoring of TSH.2
Next page: Case 2 >>
CASE 2: HISTORY OF SPONTANEOUS ABORTION
A 36-year-old G3P0 woman visits your office for a work-up after her third spontaneous abortion at 16 weeks. The patient denies history of thyroid disease but notes her maternal grandmother has Hashimoto disease. She denies symptoms of hyperthyroidism or hypothyroidism.
Recommendation
Both hyperthyroidism and hypothyroidism are associated with an increase in spontaneous abortion, premature labor, and low birth weight. Negro et al observed an increased risk for fetal loss, small-for-gestational-age fetus, premature delivery, and premature mortality in women who were TPO-antibody-positive, even if they were euthyroid. Improved fetal outcomes occurred when TPO-antibody-positive mothers received supplemental levothyroxine.5
However, the American Thyroid Association and the Endocrine Society state there is currently insufficient evidence to recommend universal screening of thyroid antibodies during pregnancy.2,3 Obtaining thyroid function studies and TPO-antibody tests could be considered as part of a work-up for women who experience multiple spontaneous abortions or have a personal or family history of autoimmune diseases.
Continue for Case 3: Cardiovascular Symptoms >>
CASE 3: CARDIOVASCULAR SYMPTOMS
A 24-year-old primigravida woman presents with complaints of palpitations and increased anxiety. She is currently 28 weeks pregnant. Her TSH level is undetectable (< 0.01 mIU/L), and her free T4 is 2.1 mg/dL (reference range, 0.5-1.6 mg/dL). An ECG performed at your office shows sinus tachycardia with a rate of 127 beats/min.
Recommendation
Maternal hyperthyroidism increases risk for maternal congestive heart failure, uncontrolled hypertension, atrial fibrillation, and thyroid storm. Additionally, fetal hyperthyroidism can occur, especially if the mother has Graves disease. Since thyroid-stimulating immunoglobulins (TSI) can permeate the placental barrier, poor fetal growth, cardiac failure, and fetal thyrotoxicosis are severe adverse effects of in-utero TSI exposure.
To prevent further complications, antithyroid medications should be started in this case. Methimazole (MMI) carries a risk for a rare birth defect, aplasia cutis, in the first trimester and is best avoided during this time. Propylthiouracil (PTU) should be given in the first trimester and then switched to MMI in the second trimester to decrease the risk for hepatotoxicity associated with PTU. Breastfeeding mothers should be assured that low-dose MMI is generally considered safe for breastfed infants but should be taken after feeding in divided doses if possible.1
For symptom relief, b-blockers can be used, although they do come with some risks. As pregnancy Category C drugs, b-blockers are associated with neonatal growth retardation, hypoglycemia, hypoxia, lower Apgar scores, and neonatal respiratory distress.6 Consider giving the lowest dose possible for the duration of the patient’s symptoms.
Radioactive iodine (I-131) should not be given to patients who plan to become pregnant or who are pregnant.2,3 The Endocrine Society recommends that if a woman inadvertently becomes pregnant, she should be counseled on the risks of radiation to the fetus, which include thyroid destruction if treatment occurs/continues after the 12th week of pregnancy.2 Furthermore, pregnancy should be avoided for the first six months after thyroid ablation to allow sufficient time to obtain the target maternal serum TSH level of 0.3 to 2.5 mIU/L.
Next page: Case 4 >>
CASE 4: PRECONCEPTION SCREENING
A 39-year-old G0P0 woman presents for preconception counseling. She denies family or personal history of thyroid disease and symptoms of thyroid disease. Should she be screened?
Recommendation
There is no consensus or guideline regarding preconception laboratory screening for thyroid disease. Current guidelines by the American Thyroid Association, the American College of Obstetricians and Gynecologists, and The Endocrine Society recommend targeted, not universal, screening.2,3,7
The American Thyroid Association and the Endocrine Society recommend screening TSH in women who are pregnant or intend to become pregnant and
• Have a personal or family history of thyroid disease
• Are older than 30
• Demonstrate symptoms of thyroid dysfunction
• Have goiter
• Are TPO-antibody positive
• Have type 1 diabetes or other autoimmune disorders
• Have a history of miscarriage or preterm delivery
• Have a history of head or neck radiation or thyroid surgery
• Are morbidly obese (BMI > 40)
• Use amiodarone or lithium or were exposed to iodinated radiologic contrast
• Are infertile
• Live in an area with moderate to severe iodine insufficiency.2,3
Rationale for targeted screening of asymptomatic women: Large-scale research has not demonstrated significantly better outcomes in those with subclinical hypothyroidism who receive treatment.7 Small studies2 have demonstrated improved fetal outcomes when subclinical hypothyroidism is treated, but for large bodies (eg, the US Preventive Services Task Force) to recommend screening, a clear improvement in health outcomes must be established via controlled studies. Future research should evaluate the effect of treating subclinical hypothyroidism during pregnancy.
REFERENCES
1. Ballabio, M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73(4):824.
2. The Endocrine Society. Management of thyroid disease in pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:2543–2565.
3. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
4. Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
5. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Clin Endocrinol Metab. 2006;91(7):2587-2591.
6. Cooper DH. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
7. American College of Obstetricians and Gynecologists. Routine thyroid screening not recommended for pregnant women. J Obstet Gynecol. 2007;110:959-960.
Management of thyroid disease during pregnancy presents unique challenges due to physiologic changes that occur. These include
• Serum levels of thyroxine-binding globulin (TBG) increase along with estrogen; in turn, total thyroxine (T4) and triiodothyronine (T3) levels increase.
• Human chorionic gonadotropin (hCG) stimulates the thyroid stimulating hormone (TSH) receptors.1
Since hCG and TSH share similar glycoprotein subunits, a transient suppression of TSH—especially around weeks 10 to 12, when hCG concentrations peak—is considered a physiologic finding. Interpretation of thyroid function testing should be made in relation to the hCG-mediated decrease in serum TSH levels.2
The following four cases will help guide your clinical management of thyroid disease in both preconception and pregnancy. Inadequately controlled thyroid dysfunction can lead to poor pregnancy outcomes for both mother and child, which will be further discussed.
Continue for Case 1: Stable Hypothyroidism >>
CASE 1: STABLE HYPOTHYROIDISM
A 29-year-old woman with stable primary hypothyroidism calls your office to report that she is pregnant. She has taken levothyroxine (100 mg) for the past three years, and her TSH level was 1.21 mIU/L at last measurement. She denies any symptoms of hyperthyroidism or hypothyroidism. What is your next step in her management?
Recommendation
The American Thyroid Association recommends monitoring serum TSH every four weeks during the first half of pregnancy and at least once per trimester thereafter, with frequency depending on symptoms and TSH levels.3 Most women will require higher doses of levothyroxine supplementation to maintain therapeutic TSH levels.
Prior to 18 weeks’ gestation, the fetus is dependent on maternal thyroid hormone. When pregnancy is confirmed, there is support in the literature for having the patient take two additional doses of levothyroxine per week until TSH can be tested.4 However, many endocrinology practices opt to check TSH and total T4 as soon as pregnancy is confirmed.
Since free T4 results may be unreliable during pregnancy (due to the effect of TBG), free thyroxine index (FTI) or total T4 should be monitored instead. FTI mathematically corrects free T4 for TBG levels, making it a useful marker. If total T4 is measured, it is important to remember that results will be approximately 1.5x the nonpregnancy value; thus, the reference range must be multiplied by 1.5 to calculate appropriate high and low parameters for pregnant patients.
Ideally, all women of childbearing age should be encouraged to plan pregnancy, to ensure TSH is at target prior to conception. Maintaining a euthyroid state throughout pregnancy (starting at conception) is important to decrease risk for such adverse outcomes as spontaneous abortion, placental abruption, and gestational hypertension.2 Low birth weight and respiratory distress are potential complications for newborns whose mothers have inadequately controlled hypothyroidism.
Patients should be counseled against simultaneous dosing of prenatal vitamins and levothyroxine. Prenatal vitamins contain iron, which reduces absorption of levothyroxine; therefore, it is recommended that the levothyroxine be taken four hours or more apart from prenatal vitamins.
The Endocrine Society recommends a TSH no higher than 2.5 mIU/L for hypothyroidism diagnosed prior to pregnancy.2,3 After delivery, levothyroxine doses should be reduced to prepregnancy levels, with close monitoring of TSH.2
Next page: Case 2 >>
CASE 2: HISTORY OF SPONTANEOUS ABORTION
A 36-year-old G3P0 woman visits your office for a work-up after her third spontaneous abortion at 16 weeks. The patient denies history of thyroid disease but notes her maternal grandmother has Hashimoto disease. She denies symptoms of hyperthyroidism or hypothyroidism.
Recommendation
Both hyperthyroidism and hypothyroidism are associated with an increase in spontaneous abortion, premature labor, and low birth weight. Negro et al observed an increased risk for fetal loss, small-for-gestational-age fetus, premature delivery, and premature mortality in women who were TPO-antibody-positive, even if they were euthyroid. Improved fetal outcomes occurred when TPO-antibody-positive mothers received supplemental levothyroxine.5
However, the American Thyroid Association and the Endocrine Society state there is currently insufficient evidence to recommend universal screening of thyroid antibodies during pregnancy.2,3 Obtaining thyroid function studies and TPO-antibody tests could be considered as part of a work-up for women who experience multiple spontaneous abortions or have a personal or family history of autoimmune diseases.
Continue for Case 3: Cardiovascular Symptoms >>
CASE 3: CARDIOVASCULAR SYMPTOMS
A 24-year-old primigravida woman presents with complaints of palpitations and increased anxiety. She is currently 28 weeks pregnant. Her TSH level is undetectable (< 0.01 mIU/L), and her free T4 is 2.1 mg/dL (reference range, 0.5-1.6 mg/dL). An ECG performed at your office shows sinus tachycardia with a rate of 127 beats/min.
Recommendation
Maternal hyperthyroidism increases risk for maternal congestive heart failure, uncontrolled hypertension, atrial fibrillation, and thyroid storm. Additionally, fetal hyperthyroidism can occur, especially if the mother has Graves disease. Since thyroid-stimulating immunoglobulins (TSI) can permeate the placental barrier, poor fetal growth, cardiac failure, and fetal thyrotoxicosis are severe adverse effects of in-utero TSI exposure.
To prevent further complications, antithyroid medications should be started in this case. Methimazole (MMI) carries a risk for a rare birth defect, aplasia cutis, in the first trimester and is best avoided during this time. Propylthiouracil (PTU) should be given in the first trimester and then switched to MMI in the second trimester to decrease the risk for hepatotoxicity associated with PTU. Breastfeeding mothers should be assured that low-dose MMI is generally considered safe for breastfed infants but should be taken after feeding in divided doses if possible.1
For symptom relief, b-blockers can be used, although they do come with some risks. As pregnancy Category C drugs, b-blockers are associated with neonatal growth retardation, hypoglycemia, hypoxia, lower Apgar scores, and neonatal respiratory distress.6 Consider giving the lowest dose possible for the duration of the patient’s symptoms.
Radioactive iodine (I-131) should not be given to patients who plan to become pregnant or who are pregnant.2,3 The Endocrine Society recommends that if a woman inadvertently becomes pregnant, she should be counseled on the risks of radiation to the fetus, which include thyroid destruction if treatment occurs/continues after the 12th week of pregnancy.2 Furthermore, pregnancy should be avoided for the first six months after thyroid ablation to allow sufficient time to obtain the target maternal serum TSH level of 0.3 to 2.5 mIU/L.
Next page: Case 4 >>
CASE 4: PRECONCEPTION SCREENING
A 39-year-old G0P0 woman presents for preconception counseling. She denies family or personal history of thyroid disease and symptoms of thyroid disease. Should she be screened?
Recommendation
There is no consensus or guideline regarding preconception laboratory screening for thyroid disease. Current guidelines by the American Thyroid Association, the American College of Obstetricians and Gynecologists, and The Endocrine Society recommend targeted, not universal, screening.2,3,7
The American Thyroid Association and the Endocrine Society recommend screening TSH in women who are pregnant or intend to become pregnant and
• Have a personal or family history of thyroid disease
• Are older than 30
• Demonstrate symptoms of thyroid dysfunction
• Have goiter
• Are TPO-antibody positive
• Have type 1 diabetes or other autoimmune disorders
• Have a history of miscarriage or preterm delivery
• Have a history of head or neck radiation or thyroid surgery
• Are morbidly obese (BMI > 40)
• Use amiodarone or lithium or were exposed to iodinated radiologic contrast
• Are infertile
• Live in an area with moderate to severe iodine insufficiency.2,3
Rationale for targeted screening of asymptomatic women: Large-scale research has not demonstrated significantly better outcomes in those with subclinical hypothyroidism who receive treatment.7 Small studies2 have demonstrated improved fetal outcomes when subclinical hypothyroidism is treated, but for large bodies (eg, the US Preventive Services Task Force) to recommend screening, a clear improvement in health outcomes must be established via controlled studies. Future research should evaluate the effect of treating subclinical hypothyroidism during pregnancy.
REFERENCES
1. Ballabio, M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73(4):824.
2. The Endocrine Society. Management of thyroid disease in pregnancy and postpartum. J Clin Endocrinol Metab. 2012;97:2543–2565.
3. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081-1125.
4. Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241-249.
5. Negro R, Formoso G, Mangieri T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. Clin Endocrinol Metab. 2006;91(7):2587-2591.
6. Cooper DH. Antithyroid drugs. N Engl J Med. 2005;352:905-917.
7. American College of Obstetricians and Gynecologists. Routine thyroid screening not recommended for pregnant women. J Obstet Gynecol. 2007;110:959-960.