User login
Acute pain management in hospitalized adult patients with opioid dependence: a narrative review and guide for clinicians
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
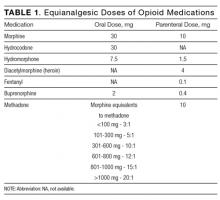
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
© 2017 Society of Hospital Medicine
Alcohol withdrawal syndrome in medical patients
Deprived of alcohol while in the hospital, up to 80% of patients who are alcohol-dependent risk developing alcohol withdrawal syndrome,1 a potentially life-threatening condition. Clinicians should anticipate the syndrome and be ready to treat and prevent its complications.
Because alcoholism is common, nearly every provider will encounter its complications and withdrawal symptoms. Each year, an estimated 1.2 million hospital admissions are related to alcohol abuse, and about 500,000 episodes of withdrawal symptoms are severe enough to require clinical attention.1–3 Nearly 50% of patients with alcohol withdrawal syndrome are middle-class, highly functional individuals, making withdrawal difficult to recognize.1
While acute trauma patients or those with alcohol withdrawal delirium are often admitted directly to an intensive care unit (ICU), many others are at risk for or develop alcohol withdrawal syndrome and are managed initially or wholly on the acute medical unit. While specific statistics have not been published on non-ICU patients with alcohol withdrawal syndrome, they are an important group of patients who need to be well managed to prevent the progression of alcohol withdrawal syndrome to alcohol withdrawal delirium, alcohol withdrawal-induced seizure, and other complications.
This article reviews how to identify and manage alcohol withdrawal symptoms in noncritical, acutely ill medical patients, with practical recommendations for diagnosis and management.
CAN LEAD TO DELIRIUM TREMENS
In people who are physiologically dependent on alcohol, symptoms of withdrawal usually occur after abrupt cessation.4 If not addressed early in the hospitalization, alcohol withdrawal syndrome can progress to alcohol withdrawal delirium (also known as delirium tremens or DTs), in which the mortality rate is 5% to 10%.5,6 Potential mechanisms of DTs include increased dopamine release and dopamine receptor activity, hypersensitivity to N-methyl-d-aspartate, and reduced levels of gamma-aminobutyric acid (GABA).7
Long-term changes are thought to occur in neurons after repeated detoxification from alcohol, a phenomenon called “kindling.” After each detoxification, alcohol craving and obsessive thoughts increase,8 and subsequent episodes of alcohol withdrawal tend to be progressively worse.
Withdrawal symptoms
Alcohol withdrawal syndrome encompasses a spectrum of symptoms and conditions, from minor (eg, insomnia, tremulousness) to severe (seizures, DTs).2 The symptoms typically depend on the amount of alcohol consumed, the time since the last drink, and the number of previous detoxifications.9
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,1 states that to establish a diagnosis of alcohol withdrawal syndrome, a patient must meet four criteria:
- The patient must have ceased or reduced alcohol intake after heavy or prolonged use.
- Two or more of the following must develop within a few hours to a few days: autonomic hyperactivity (sweating or pulse greater than 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; grand mal seizure.
- The above symptoms must cause significant distress or functional impairment.
- The symptoms must not be related to another medical condition.
Some of the symptoms described in the second criterion above can occur while the patient still has a measurable blood alcohol level, usually within 6 hours of cessation of drinking.10 Table 1 describes the timetable of onset of symptoms and their severity.2
The elderly may be affected more severely
While the progression of the symptoms described above is commonly used for medical inpatients, the timeline may be different in an elderly patient. Compared with younger patients, elderly patients may have higher blood alcohol concentrations owing to lower total body water, so small amounts of alcohol can produce significant effects.11,12 Brower et al12 found that elderly patients experienced more withdrawal symptoms, especially cognitive impairment, weakness, and high blood pressure, and for 3 days longer.
In the elderly, alcohol may have a greater impact on the central nervous system because of increased permeability of the blood-brain barrier. And importantly, elderly patients tend to have more concomitant diseases and take more medications, all of which can affect alcohol metabolism.
ASSESSMENT SCALES FOR ALCOHOL WITHDRAWAL SYNDROME
A number of clinical scales for evaluating alcohol withdrawal have been developed. Early ones such as the 30-item Total Severity Assessment (TSA) scale and the 11-item Selected Severity Assessment (SSA) scale were limited because they were extremely detailed, burdensome to nursing staff to administer, and contained items such as daily “sleep disturbances” that were not acute enough to meet specific monitoring needs or to guide drug therapy.13,14
The Clinical Institute Withdrawal Assessment for alcohol (CIWA-A) scale, with 15 items, was derived from the SSA scale and includes acute items for assessment as often as every half-hour.15
The CIWA-Ar scale (Table 2) was developed from the CIWA-A scale by Sullivan et al.15 Using both observation and interview, it focuses on 10 areas: nausea and vomiting, tremor, paroxysmal sweats, anxiety, agitation, headache, disorientation, tactile disturbances, auditory disturbances, and visual disturbances. Scores can range from 0 to 67; a higher score indicates worse withdrawal symptoms and outcomes and therefore necessitates escalation of treatment.
The CIWA-Ar scale is now the one most commonly used in clinical trials16–20 and, we believe, in practice. Other scales, including the CIWA-AD and the Alcohol Withdrawal Scale have been validated but are not widely used in practice.14,21
BASELINE ASSESSMENT AND EARLY SUPPORTIVE CARE
A thorough history and physical examination should be performed on admission in patients known to be or suspected of being alcohol-dependent to assess the patient’s affected body systems. The time elapsed since the patient’s last alcohol drink helps predict the onset of withdrawal complications.
Baseline laboratory tests for most patients with suspected alcohol withdrawal syndrome should include a basic blood chemistry panel, complete blood cell count, and possibly an alcohol and toxicology screen, depending on the patient’s history and presentation.
Hydration and nutritional support are important in patients presenting with alcohol withdrawal syndrome. Severe disturbances in electrolytes can lead to serious complications, including cardiac arrhythmia. Close monitoring and electrolyte replacement as needed are recommended for hospitalized alcoholic patients and should follow hospital protocols.22
Thiamine and folic acid status deserve special attention, since long-standing malnutrition is common in alcoholic patients. Thiamine deficiency can result in Wernicke encephalopathy and Korsakoff syndrome, characterized by delirium, ataxia, vision changes, and amnesia. Alcohol withdrawal guidelines recommend giving thiamine intravenously for the first 2 to 5 days after admission.23 In addition, thiamine must be given before any intravenous glucose product, as thiamine is a cofactor in carbohydrate metabolism.23 Folic acid should also be supplemented, as chronic deficiencies may lead to megaloblastic or macrocytic anemia.
CIWA-Ar scale. To provide consistent monitoring and ongoing treatment, clinicians and institutions are encouraged to use a simple assessment scale that detects and quantifies alcohol withdrawal syndrome and that can be used for reassessment after an intervention.21 The CIWA-Ar scale should be used to facilitate “symptom-triggered therapy” in which, depending on the score, the patient receives pharmacologic treatment followed by a scheduled reevaluation.23,24 Most patients with a CIWA-Ar score of 8 or higher benefit from benzodiazepine therapy.16,18,19
PRIMARY DRUG THERAPIES FOR MEDICAL INPATIENTS
Benzodiazepines are the first-line agents
Benzodiazepines are the first-line agents recommended for preventing and treating alcohol withdrawal syndrome.23 Their various pharmacokinetic profiles, wide therapeutic indices, and safety compared with older sedative hypnotics make them the preferred class.23,25 No single benzodiazepine is preferred over the others for treating alcohol withdrawal syndrome: studies have shown benefits using short-acting, intermediate-acting, and long-acting agents. The choice of drug is variable and patient-specific.16,18,26
Benzodiazepines promote and enhance binding of the inhibitory neurotransmitter GABA to GABAA receptors in the central nervous system.27 As a class, benzodiazepines are all structurally related and produce the same effects—namely, sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity.27
The most studied benzodiazepines for treating and preventing alcohol withdrawal syndrome are chlordiazepoxide, oxazepam, and lorazepam,16–20 whereas diazepam was used in older studies.23
Diazepam and chlordiazepoxide are metabolized by oxidation, each sharing the long-acting active metabolite desmethyldiazepam (half-life 72 hours), and short-acting metabolite oxazepam (half-life 8 hours).27 In addition, the parent drugs also have varying pharmacokinetic profiles: diazepam has a half-life of more than 30 hours and chlordiazepoxide a half-life of about 8 hours. Chlordiazepoxide and diazepam’s combination of both long- and short-acting benzodiazepine activity provides long-term efficacy in attenuating withdrawal symptoms, but chlordiazepoxide’s shorter parent half-life allows more frequent dosing.
Lorazepam (half-life 10–20 hours) and oxazepam (half-life 5–20 hours) undergo glucuronide conjugation and do not have metabolites.27,28 Table 3 provides a pharmacokinetic summary.27,28
Various dosage regimens are used in giving benzodiazepines, the most common being symptom-triggered therapy, governed by assessment scales, and scheduled around-the-clock therapy.29 Current evidence supports symptom-triggered therapy in most inpatients who are not critically ill, as it can reduce both benzodiazepine use and adverse drug events and can reduce the length of stay.16,19
Trials of symptom-triggered benzodiazepine therapy
Most inpatient trials of symptom-triggered therapy (Table 4)3,16–20 used the CIWA-Ar scale for monitoring. In some of the studies, benzodiazepines were given if the score was 8 or higher, but others used cut points as high as 15 or higher. Doses:
- Chlordiazepoxide (first dose 25–100 mg)
- Lorazepam (first dose 0.5–2 mg)
- Oxazepam (30 mg).
After each dose, patients were reevaluated at intervals of 30 minutes to 8 hours.
Most of these trials showed no difference in rates of adverse drug events such as seizures, hallucinations, and lethargy with symptom-triggered therapy compared with scheduled therapy.16,18,20 They also found either no difference in the incidence of delirium tremens, or a lower incidence of delirium tremens with symptom-triggered therapy than with scheduled therapy.16–18,20
Weaver et al19 found no difference in length of stay between scheduled therapy and symptom-triggered therapy, but Saitz et al16 reported a median benzodiazepine treatment duration of 9 hours with symptom-triggered therapy vs 68 hours with fixed dosing. Thus, the study by Saitz et al suggests that hospitalization might be shorter with symptom-triggered therapy.
Many of the trials had notable limitations related to the diversity of patients enrolled and the protocols for both symptom-triggered therapy and fixed dosing. Some trials enrolled only inpatients in detoxification programs; others focused on inpatients with acute medical illness. The inpatient alcohol treatment trials16,18 excluded medically ill patients and those with concurrent psychiatric illness,16,18 and one excluded patients with seizures.16 One of the inpatient alcohol treatment program trials16 excluded patients on beta-blockers or clonidine because of concern that these drugs could mask withdrawal symptoms, whereas trials in medically ill patients allowed these same drugs.17,19,20
Most of the patients were men (approximately 75%, but ranging from 74% to 100%), and therefore the study results may not be as applicable to women.16–20 Most participants were middle-aged, with average ages in all studies between 46 and 55. Finally, the studies used a wide range of medications and dosing, with patient monitoring intervals ranging from every 30 minutes to every 8 hours.16–20
In a 2010 Cochrane analysis, Amato et al29 concluded that the limited evidence available favors symptom-triggered regimens over fixed-dosing regimens, but that differences in isolated trials should be interpreted very cautiously.
Therapeutic ethanol
Aside from the lack of evidence to support its use in alcohol withdrawal syndrome, prescribing oral ethanol to alcoholic patients clearly poses an ethical dilemma. However, giving ethanol intravenously has been studied, mostly in surgical trauma patients.30
Early reports described giving intravenous ethanol on a gram-to-gram basis to match the patient’s consumption before admission to prevent alcohol withdrawal syndrome. But later studies reported prevention of alcohol withdrawal syndrome with very small amounts of intravenous ethanol.30,31 While clinical trials have been limited to ICU patients, ethanol infusion at an initial rate of 2.5 to 5 g per hour and titrated up to 10 g per hour has appeared to be safe and effective for preventing alcohol withdrawal syndrome.30,31 The initial infusion rate of 2.5 to 5 g per hour is equivalent to 4 to 10 alcoholic beverages per 24 hours.
Nevertheless, ethanol infusion carries the potential for toxicities (eg, gastric irritation, precipitation of acute hepatic failure, hypoglycemia, pancreatitis, bone marrow suppression, prolonged wound healing) and drug interactions (eg, with anticoagulants and anticonvulsants). Thus, ethanol is neither widely used nor recommended.25,31
ADJUNCTIVE THERAPIES
Many medications are used adjunctively in the acute setting, both for symptoms of alcohol withdrawal syndrome and for agitation.
Haloperidol
No clinical trial has yet examined haloperidol monotherapy in patients with alcohol withdrawal syndrome in either general medical units or intensive care units. Yet haloperidol remains important and is recommended as an adjunct therapy for agitation.23,32 Dosing of haloperidol in protocols for surgical patients ranged from 2 to 5 mg intravenously every 0.5 to 2 hours, with a maximum dosage of 0.5 mg per kg per 24 hours.7,33
Alpha-2 agonists
Alpha-2 agonists are thought to reduce sympathetic overdrive and the autonomic symptoms associated with alcohol withdrawal syndrome, and these agents (primarily clonidine) have been studied in the treatment of alcohol withdrawal syndrome.34,35
Clonidine. In a Swedish study,34 26 men ages 20 to 55 who presented with the tremor, sweating, dysphoria, tension, anxiety, and tachycardia associated with alcohol withdrawal syndrome received clonidine 4 µg per kg twice daily or carbamazepine 200 mg three to four times daily in addition to an antiepileptic. Adjunctive use of a benzodiazepine was allowed at night in both groups. No statistically significant difference in symptom reduction was noted between the two groups, and there was no difference in total benzodiazepine use.
Dexmedetomidine, given intravenously, has been tested as an adjunct to benzodiazepine treatment in severe alcohol withdrawal syndrome. It has been shown to decrease the amount of total benzodiazepine needed compared with benzodiazepine therapy alone, but no differences have been seen in length of hospital stay.36–39 However, research on this drug so far is limited to ICU patients.
Beta-blockers
Beta-blockers have been used in inpatients with alcohol withdrawal syndrome to reduce heart rate and potentially reduce alcohol craving. However, the data are limited and conflicting.
Atenolol 50 to 100 mg daily, in a study in 120 patients, reduced length of stay (4 vs 5 days), reduced benzodiazepine use, and improved vital signs and behavior compared with placebo.40
Propranolol 40 mg every 6 hours reduced arrhythmias but increased hallucinations when used alone in a study in 47 patients.41 When used in combination with chlordiazepoxide, no benefit was seen in arrhythmia reduction.41
Barbiturates and other antiepileptics
Data continue to emerge on antiepileptics as both monotherapy and adjunctive therapy for alcohol withdrawal syndrome. Barbiturates as monotherapy were largely replaced by benzodiazepines in view of the narrow therapeutic index of barbiturates and their full agonist effect on the GABA receptor complex. However, phenobarbital has been evaluated in patients presenting with severe alcohol withdrawal syndrome or resistant alcohol withdrawal (ie, symptoms despite large or repeated doses of benzodiazepines) as an adjunct to benzodiazepines.42,43
In addition, a newer trial44 involved giving a single dose of phenobarbital in the emergency department in combination with a CIWA-Ar–based benzodiazepine protocol, compared with the benzodiazepine protocol alone. The group that received phenobarbital had fewer ICU admissions; its evaluation is ongoing.
The three other medications with the most data are carbamazepine, valproic acid, and gabapentin.45,46 However, the studies were small and the benefit was modest. Although these agents are possible alternatives in protracted alcohol withdrawal syndrome, no definite conclusion can be made regarding their place in therapy.46
RECOMMENDATIONS FOR DRUG THERAPY AND SUPPORTIVE CARE
Which benzodiazepine to use?
No specific benzodiazepine is recommended over the others for managing alcohol withdrawal syndrome, but studies best support the long-acting agent chlordiazepoxide.16,17,20 Other benzodiazepines such as lorazepam and oxazepam have proved to be beneficial, but drugs should be selected on the basis of patient characteristics and drug metabolism.18,19,27
Patients with severe liver dysfunction and the elderly may have slower oxidative metabolism, so the effects of medications that are primarily oxidized, such as chlordiazepoxide and diazepam, may be prolonged. Therefore, lorazepam and oxazepam would be preferred in these groups.47 While most patients with alcohol withdrawal syndrome and liver dysfunction do not have advanced cirrhosis, we recommend liver function testing (serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) and screening for liver disease, given the drug metabolism and package insert caution for use in those with impaired hepatic function.48
Patients with end-stage renal disease (stage 5 chronic kidney disease) or acute kidney injury should not receive parenteral diazepam or lorazepam. The rationale is the potential accumulation of propylene glycol, the solvent used in these formulations.
In the elderly, the Beers list of drugs to avoid in older adults includes benzodiazepines, not differentiating individual benzodiazepines in terms of risk.49 However, chlordiazepoxide may be preferable to diazepam due to its shorter parent half-life and lower lipophilicity.27 Few studies have been done using benzodiazepines in elderly patients with alcohol withdrawal syndrome, but those published have shown either equivalent dosing required compared with younger patients or more severe withdrawal for which they received greater amounts of chlordiazepoxide.9,12 Lorazepam and oxazepam have less potential to accumulate in the elderly compared with the nonelderly due to the drugs’ metabolic profiles; lorazepam is the preferred agent because of its faster onset of action.47 Ultimately, the choice of benzodiazepine in elderly patients with alcohol withdrawal syndrome should be based on patient-specific characteristics.
How should benzodiazepines be dosed?
While the CIWA-Ar thresholds and subsequent dosing of benzodiazepines varied in different studies, we recommend starting benzodiazepine therapy at a CIWA-Ar score of 8 or higher, with subsequent dosing based on score reassessment. Starting doses of benzodiazepines should be chlordiazepoxide 25 to 50 mg, lorazepam 1 to 2 mg, or oxazepam 15 mg.16–20
Subsequent doses should be titrated upward, increasing by 1.5 to 2 times the previous dose and monitored at least every 1 to 2 hours after dose adjustments. Once a patient is stable and the CIWA-Ar score is less than 8, monitoring intervals can be extended to every 4 to 8 hours. If the CIWA-Ar score is more than 20, studies suggest the need for patient reevaluation for transfer to the ICU; however, some health systems have a lower threshold for this intervention.7,14,50
Dosing algorithms and CIWA-Ar goals may vary slightly from institution to institution, but it has been shown that symptom-triggered therapy works best when hospitals have a protocol for it and staff are adequately trained to assess patients with alcohol withdrawal syndrome.7,50,51 Suggestions for dose ranges and symptom-triggered therapy are shown in Table 5.
In case of benzodiazepine overdose or potential benzodiazepine-induced delirium, flumazenil could be considered.52
Patients who should not receive symptom-triggered therapy include immediate postoperative patients in whom clinicians cannot properly assess withdrawal symptoms and patients with a history of DTs.51 While controversy exists regarding the use of symptom-triggered therapy in patients with complicated medical comorbidities, there are data to support symptom-triggered therapy in some ICU patients, as it has resulted in less benzodiazepine use and reduced mechanical ventilation.53,54
There are limited data to support phenobarbital in treating resistant alcohol withdrawal syndrome, either alone or concurrently with benzodiazepines, in escalating doses ranging from 65 to 260 mg, with a maximum daily dose of 520 mg.42,55,56
Haloperidol
For patients exhibiting agitation despite benzodiazepine therapy, giving haloperidol adjunctively can be beneficial.
Haloperidol can be used in medical patients as an adjunctive therapy for agitation, but caution is advised because of the potential for a lowering of the seizure threshold, extrapyramidal effects, and risk of QTc prolongation leading to arrhythmias. Patients considered at highest risk for torsades de pointes may have a QTc of 500 msec or greater.57
Patients should also be screened for factors that have been shown to be independent predictors of QTc prolongation (female sex, diagnosis of myocardial infarction, septic shock or left ventricular dysfunction, other QT-prolonging drugs, age > 68, baseline QTc ≥ 450 msec, and hypokalemia).58 If combined predictors have been identified, it is recommended that haloperidol be avoided.
If haloperidol is to be given, a baseline electrocardiogram and electrolyte panel should be obtained, with daily electrocardiograms thereafter, as well as ongoing review of the patient’s medications to minimize drug interactions that could further increase the risk for QTc prolongation.
Suggested haloperidol dosing is 2 to 5 mg intravenously every 0.5 to 2 hours with a maximum dose of 0.5 mg/kg/24 hours.8,33 A maximum of 35 mg of intravenous haloperidol should be used in a 24-hour period to avoid QTc prolongation.57
Antihypertensive therapy
Many patients receive symptomatic relief of autonomic hyperreactivity with benzodiazepines. However, some may require additional antihypertensive therapy for cardiac adrenergic symptoms (hypertension, tachycardia) if symptoms do not resolve by treating other medical problems commonly seen in patients with alcohol withdrawal syndrome, such as dehydration and electrolyte imbalances.7
Published protocols suggest giving clonidine 0.1 mg orally every hour up to three times as needed until systolic blood pressure is less than 140 mm Hg (less than 160 mm Hg if the patient is over age 60) and diastolic pressure is less than 90 mm Hg.51 Once the patient is stabilized, the dosing can be scheduled to a maximum of 2.4 mg daily.59 However, we believe that the use of clonidine should be restricted to patients who have a substantial increase in blood pressure over baseline or are nearing a hypertensive urgency or emergency (pressures > 180/120 mm Hg) and should not be used to treat other general symptoms associated with alcohol withdrawal syndrome.42
In addition, based on limited evidence, we recommend using beta-blockers only in patients with symptomatic tachycardia or as an adjunct in hypertension management.40,41
Therapies to avoid in acutely ill medical patients
Ethanol is not recommended. Instead, intravenous benzodiazepines should be given in patients presenting with severe alcohol withdrawal syndrome.
Antiepileptics, including valproic acid, carbamazepine, and pregabalin, lack benefit in these patients either as monotherapy or as adjunctive therapy and so are not recommended.45,60–62
Magnesium supplementation (in patients with normal serum magnesium levels) should not be given, as no clinical benefit has been shown.63
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013:501.
- Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician 2004; 69:1443–1450.
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348:1786–1795.
- Isbell H, Fraser HF, Wilker A, Bellevile RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol 1955; 16:1–33.
- Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med 2008; 15:788–790.
- Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol 2010; 45:151–158.
- Stanley KM, Amabile CM, Simpson KN, Couillard D, Norcross ED, Worrall CL. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy 2003; 23:843–854.
- Brousse G, Arnaud B, Vorspan F, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol 2012; 47:501–508.
- Liskow BI, Rinck C, Campbell J, DeSouza C. Alcohol withdrawal in the elderly. J Stud Alcohol 1989; 50:414–421.
- Etherington JM. Emergency management of acute alcohol problems. Part 1: uncomplicated withdrawal. Can Fam Physician 1996; 42:2186–2190.
- Letizia M, Reinbolz M. Identifying and managing acute alcohol withdrawal in the elderly. Geriatr Nurs 2005; 26:176–183.
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res 1994; 18:196–201.
- Williams D, Lewis J, McBride A. A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108.
- Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict 2006; 15:85–93.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357.
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA 1994; 272:519–523.
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc 2001; 76:695–701.
- Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med 2002; 162:1117–1121.
- Weaver MF, Hoffman HJ, Johnson RE, Mauck K. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis 2006; 25:17–24.
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar). Am J Addict 2000; 9:135–144.
- Wetterling T, Kanitz RD, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale). Alcohol Alcohol 1997; 32:753–760.
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World 1998; 22:38–43.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Sellers EM, Sullivan JT, Somer G, Sykora K. Characterization of DSM-III-R criteria for uncomplicated alcohol withdrawal provides an empirical basis for DSM-IV. Arch Gen Psychiatry 1991; 48:442–447.
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med 2010; 38(suppl):S494–S501.
- Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs 2009; 70:467–474.
- Bird RD, Makela EH. Alcohol withdrawal: what is the benzodiazepine of choice? Ann Pharmacother 1994; 28:67–71.
- Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs 2014; 28:401–410.
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010; 3:CD005063.
- Weinberg JA, Magnotti LJ, Fischer PE, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma 2008; 64:99–104.
- Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994; 87:47–54.
- Ungur LA, Neuner B, John S, Wernecke K, Spies C. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res 2013; 37:675–686.
- Lansford CD, Guerriero CH, Kocan MJ, et al. Improved outcomes in patients with head and neck cancer using a standardized care protocol for postoperative alcohol withdrawal. Arch Otolaryngol Head Neck Surg 2008; 134:865–872.
- Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend 1981; 8:345–348.
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother 2011; 45:649–657.
- Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy 2014; 34:910–917.
- VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med 2014. [Epub ahead of print October 16, 2014]
- Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care 2012; 2:12.
- Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs 2013; 27:913–920.
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med 1985; 313:905–909.
- Zilm DH, Jacob MS, MacLeod SM, Sellers EM, Ti TY. Propranolol and chlordiazepoxide effects on cardiac arrhythmias during alcohol withdrawal. Alcohol Clin Exp Res 1980; 4:400–405.
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60.
- Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy 2009; 29:875–878.
- Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med 2013; 44:592–598.e2.
- Prince V, Turpin KR. Treatment of alcohol withdrawal syndrome with carbamazepine, gabapentin, and nitrous oxide. Am J Health Syst Pharm 2008; 65:1039–1047.
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1106–1117.
- Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16:49–57.
- Valeant Pharmaceuticals North America LLC. Librium—chlordiazepoxide hydrochloride capsule, gelatin coated. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=125207. Accessed November 20, 2015.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc 2008; 83:274–279.
- Manasco A, Chang S, Larriviere J, Hamm LL, Glass M. Alcohol withdrawal. South Med J 2012; 105:607–612.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 2014; 10:126–132.
- Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics 2004; 45:256–261.
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest 2010; 138:994–1003.
- Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med 1987; 16:847–850.
- Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med 2011; 29:382–385.
- Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998; 81:238–240.
- Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6:479–487.
- Boehringer Ingelheim Pharmaceuticals, Inc. Product Information: Catapres oral tablets, clonidine HCl oral tablets, 2012.
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res 2001; 25:1324–1329.
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 1989; 146:617–621.
- Förg A, Hein J, Volkmar K, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol 2012; 47:149–155.
- Wilson A, Vulcano B. A double-blind, placebo-controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcohol Clin Exp Res 1984; 8:542–545.
Deprived of alcohol while in the hospital, up to 80% of patients who are alcohol-dependent risk developing alcohol withdrawal syndrome,1 a potentially life-threatening condition. Clinicians should anticipate the syndrome and be ready to treat and prevent its complications.
Because alcoholism is common, nearly every provider will encounter its complications and withdrawal symptoms. Each year, an estimated 1.2 million hospital admissions are related to alcohol abuse, and about 500,000 episodes of withdrawal symptoms are severe enough to require clinical attention.1–3 Nearly 50% of patients with alcohol withdrawal syndrome are middle-class, highly functional individuals, making withdrawal difficult to recognize.1
While acute trauma patients or those with alcohol withdrawal delirium are often admitted directly to an intensive care unit (ICU), many others are at risk for or develop alcohol withdrawal syndrome and are managed initially or wholly on the acute medical unit. While specific statistics have not been published on non-ICU patients with alcohol withdrawal syndrome, they are an important group of patients who need to be well managed to prevent the progression of alcohol withdrawal syndrome to alcohol withdrawal delirium, alcohol withdrawal-induced seizure, and other complications.
This article reviews how to identify and manage alcohol withdrawal symptoms in noncritical, acutely ill medical patients, with practical recommendations for diagnosis and management.
CAN LEAD TO DELIRIUM TREMENS
In people who are physiologically dependent on alcohol, symptoms of withdrawal usually occur after abrupt cessation.4 If not addressed early in the hospitalization, alcohol withdrawal syndrome can progress to alcohol withdrawal delirium (also known as delirium tremens or DTs), in which the mortality rate is 5% to 10%.5,6 Potential mechanisms of DTs include increased dopamine release and dopamine receptor activity, hypersensitivity to N-methyl-d-aspartate, and reduced levels of gamma-aminobutyric acid (GABA).7
Long-term changes are thought to occur in neurons after repeated detoxification from alcohol, a phenomenon called “kindling.” After each detoxification, alcohol craving and obsessive thoughts increase,8 and subsequent episodes of alcohol withdrawal tend to be progressively worse.
Withdrawal symptoms
Alcohol withdrawal syndrome encompasses a spectrum of symptoms and conditions, from minor (eg, insomnia, tremulousness) to severe (seizures, DTs).2 The symptoms typically depend on the amount of alcohol consumed, the time since the last drink, and the number of previous detoxifications.9
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,1 states that to establish a diagnosis of alcohol withdrawal syndrome, a patient must meet four criteria:
- The patient must have ceased or reduced alcohol intake after heavy or prolonged use.
- Two or more of the following must develop within a few hours to a few days: autonomic hyperactivity (sweating or pulse greater than 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; grand mal seizure.
- The above symptoms must cause significant distress or functional impairment.
- The symptoms must not be related to another medical condition.
Some of the symptoms described in the second criterion above can occur while the patient still has a measurable blood alcohol level, usually within 6 hours of cessation of drinking.10 Table 1 describes the timetable of onset of symptoms and their severity.2
The elderly may be affected more severely
While the progression of the symptoms described above is commonly used for medical inpatients, the timeline may be different in an elderly patient. Compared with younger patients, elderly patients may have higher blood alcohol concentrations owing to lower total body water, so small amounts of alcohol can produce significant effects.11,12 Brower et al12 found that elderly patients experienced more withdrawal symptoms, especially cognitive impairment, weakness, and high blood pressure, and for 3 days longer.
In the elderly, alcohol may have a greater impact on the central nervous system because of increased permeability of the blood-brain barrier. And importantly, elderly patients tend to have more concomitant diseases and take more medications, all of which can affect alcohol metabolism.
ASSESSMENT SCALES FOR ALCOHOL WITHDRAWAL SYNDROME
A number of clinical scales for evaluating alcohol withdrawal have been developed. Early ones such as the 30-item Total Severity Assessment (TSA) scale and the 11-item Selected Severity Assessment (SSA) scale were limited because they were extremely detailed, burdensome to nursing staff to administer, and contained items such as daily “sleep disturbances” that were not acute enough to meet specific monitoring needs or to guide drug therapy.13,14
The Clinical Institute Withdrawal Assessment for alcohol (CIWA-A) scale, with 15 items, was derived from the SSA scale and includes acute items for assessment as often as every half-hour.15
The CIWA-Ar scale (Table 2) was developed from the CIWA-A scale by Sullivan et al.15 Using both observation and interview, it focuses on 10 areas: nausea and vomiting, tremor, paroxysmal sweats, anxiety, agitation, headache, disorientation, tactile disturbances, auditory disturbances, and visual disturbances. Scores can range from 0 to 67; a higher score indicates worse withdrawal symptoms and outcomes and therefore necessitates escalation of treatment.
The CIWA-Ar scale is now the one most commonly used in clinical trials16–20 and, we believe, in practice. Other scales, including the CIWA-AD and the Alcohol Withdrawal Scale have been validated but are not widely used in practice.14,21
BASELINE ASSESSMENT AND EARLY SUPPORTIVE CARE
A thorough history and physical examination should be performed on admission in patients known to be or suspected of being alcohol-dependent to assess the patient’s affected body systems. The time elapsed since the patient’s last alcohol drink helps predict the onset of withdrawal complications.
Baseline laboratory tests for most patients with suspected alcohol withdrawal syndrome should include a basic blood chemistry panel, complete blood cell count, and possibly an alcohol and toxicology screen, depending on the patient’s history and presentation.
Hydration and nutritional support are important in patients presenting with alcohol withdrawal syndrome. Severe disturbances in electrolytes can lead to serious complications, including cardiac arrhythmia. Close monitoring and electrolyte replacement as needed are recommended for hospitalized alcoholic patients and should follow hospital protocols.22
Thiamine and folic acid status deserve special attention, since long-standing malnutrition is common in alcoholic patients. Thiamine deficiency can result in Wernicke encephalopathy and Korsakoff syndrome, characterized by delirium, ataxia, vision changes, and amnesia. Alcohol withdrawal guidelines recommend giving thiamine intravenously for the first 2 to 5 days after admission.23 In addition, thiamine must be given before any intravenous glucose product, as thiamine is a cofactor in carbohydrate metabolism.23 Folic acid should also be supplemented, as chronic deficiencies may lead to megaloblastic or macrocytic anemia.
CIWA-Ar scale. To provide consistent monitoring and ongoing treatment, clinicians and institutions are encouraged to use a simple assessment scale that detects and quantifies alcohol withdrawal syndrome and that can be used for reassessment after an intervention.21 The CIWA-Ar scale should be used to facilitate “symptom-triggered therapy” in which, depending on the score, the patient receives pharmacologic treatment followed by a scheduled reevaluation.23,24 Most patients with a CIWA-Ar score of 8 or higher benefit from benzodiazepine therapy.16,18,19
PRIMARY DRUG THERAPIES FOR MEDICAL INPATIENTS
Benzodiazepines are the first-line agents
Benzodiazepines are the first-line agents recommended for preventing and treating alcohol withdrawal syndrome.23 Their various pharmacokinetic profiles, wide therapeutic indices, and safety compared with older sedative hypnotics make them the preferred class.23,25 No single benzodiazepine is preferred over the others for treating alcohol withdrawal syndrome: studies have shown benefits using short-acting, intermediate-acting, and long-acting agents. The choice of drug is variable and patient-specific.16,18,26
Benzodiazepines promote and enhance binding of the inhibitory neurotransmitter GABA to GABAA receptors in the central nervous system.27 As a class, benzodiazepines are all structurally related and produce the same effects—namely, sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity.27
The most studied benzodiazepines for treating and preventing alcohol withdrawal syndrome are chlordiazepoxide, oxazepam, and lorazepam,16–20 whereas diazepam was used in older studies.23
Diazepam and chlordiazepoxide are metabolized by oxidation, each sharing the long-acting active metabolite desmethyldiazepam (half-life 72 hours), and short-acting metabolite oxazepam (half-life 8 hours).27 In addition, the parent drugs also have varying pharmacokinetic profiles: diazepam has a half-life of more than 30 hours and chlordiazepoxide a half-life of about 8 hours. Chlordiazepoxide and diazepam’s combination of both long- and short-acting benzodiazepine activity provides long-term efficacy in attenuating withdrawal symptoms, but chlordiazepoxide’s shorter parent half-life allows more frequent dosing.
Lorazepam (half-life 10–20 hours) and oxazepam (half-life 5–20 hours) undergo glucuronide conjugation and do not have metabolites.27,28 Table 3 provides a pharmacokinetic summary.27,28
Various dosage regimens are used in giving benzodiazepines, the most common being symptom-triggered therapy, governed by assessment scales, and scheduled around-the-clock therapy.29 Current evidence supports symptom-triggered therapy in most inpatients who are not critically ill, as it can reduce both benzodiazepine use and adverse drug events and can reduce the length of stay.16,19
Trials of symptom-triggered benzodiazepine therapy
Most inpatient trials of symptom-triggered therapy (Table 4)3,16–20 used the CIWA-Ar scale for monitoring. In some of the studies, benzodiazepines were given if the score was 8 or higher, but others used cut points as high as 15 or higher. Doses:
- Chlordiazepoxide (first dose 25–100 mg)
- Lorazepam (first dose 0.5–2 mg)
- Oxazepam (30 mg).
After each dose, patients were reevaluated at intervals of 30 minutes to 8 hours.
Most of these trials showed no difference in rates of adverse drug events such as seizures, hallucinations, and lethargy with symptom-triggered therapy compared with scheduled therapy.16,18,20 They also found either no difference in the incidence of delirium tremens, or a lower incidence of delirium tremens with symptom-triggered therapy than with scheduled therapy.16–18,20
Weaver et al19 found no difference in length of stay between scheduled therapy and symptom-triggered therapy, but Saitz et al16 reported a median benzodiazepine treatment duration of 9 hours with symptom-triggered therapy vs 68 hours with fixed dosing. Thus, the study by Saitz et al suggests that hospitalization might be shorter with symptom-triggered therapy.
Many of the trials had notable limitations related to the diversity of patients enrolled and the protocols for both symptom-triggered therapy and fixed dosing. Some trials enrolled only inpatients in detoxification programs; others focused on inpatients with acute medical illness. The inpatient alcohol treatment trials16,18 excluded medically ill patients and those with concurrent psychiatric illness,16,18 and one excluded patients with seizures.16 One of the inpatient alcohol treatment program trials16 excluded patients on beta-blockers or clonidine because of concern that these drugs could mask withdrawal symptoms, whereas trials in medically ill patients allowed these same drugs.17,19,20
Most of the patients were men (approximately 75%, but ranging from 74% to 100%), and therefore the study results may not be as applicable to women.16–20 Most participants were middle-aged, with average ages in all studies between 46 and 55. Finally, the studies used a wide range of medications and dosing, with patient monitoring intervals ranging from every 30 minutes to every 8 hours.16–20
In a 2010 Cochrane analysis, Amato et al29 concluded that the limited evidence available favors symptom-triggered regimens over fixed-dosing regimens, but that differences in isolated trials should be interpreted very cautiously.
Therapeutic ethanol
Aside from the lack of evidence to support its use in alcohol withdrawal syndrome, prescribing oral ethanol to alcoholic patients clearly poses an ethical dilemma. However, giving ethanol intravenously has been studied, mostly in surgical trauma patients.30
Early reports described giving intravenous ethanol on a gram-to-gram basis to match the patient’s consumption before admission to prevent alcohol withdrawal syndrome. But later studies reported prevention of alcohol withdrawal syndrome with very small amounts of intravenous ethanol.30,31 While clinical trials have been limited to ICU patients, ethanol infusion at an initial rate of 2.5 to 5 g per hour and titrated up to 10 g per hour has appeared to be safe and effective for preventing alcohol withdrawal syndrome.30,31 The initial infusion rate of 2.5 to 5 g per hour is equivalent to 4 to 10 alcoholic beverages per 24 hours.
Nevertheless, ethanol infusion carries the potential for toxicities (eg, gastric irritation, precipitation of acute hepatic failure, hypoglycemia, pancreatitis, bone marrow suppression, prolonged wound healing) and drug interactions (eg, with anticoagulants and anticonvulsants). Thus, ethanol is neither widely used nor recommended.25,31
ADJUNCTIVE THERAPIES
Many medications are used adjunctively in the acute setting, both for symptoms of alcohol withdrawal syndrome and for agitation.
Haloperidol
No clinical trial has yet examined haloperidol monotherapy in patients with alcohol withdrawal syndrome in either general medical units or intensive care units. Yet haloperidol remains important and is recommended as an adjunct therapy for agitation.23,32 Dosing of haloperidol in protocols for surgical patients ranged from 2 to 5 mg intravenously every 0.5 to 2 hours, with a maximum dosage of 0.5 mg per kg per 24 hours.7,33
Alpha-2 agonists
Alpha-2 agonists are thought to reduce sympathetic overdrive and the autonomic symptoms associated with alcohol withdrawal syndrome, and these agents (primarily clonidine) have been studied in the treatment of alcohol withdrawal syndrome.34,35
Clonidine. In a Swedish study,34 26 men ages 20 to 55 who presented with the tremor, sweating, dysphoria, tension, anxiety, and tachycardia associated with alcohol withdrawal syndrome received clonidine 4 µg per kg twice daily or carbamazepine 200 mg three to four times daily in addition to an antiepileptic. Adjunctive use of a benzodiazepine was allowed at night in both groups. No statistically significant difference in symptom reduction was noted between the two groups, and there was no difference in total benzodiazepine use.
Dexmedetomidine, given intravenously, has been tested as an adjunct to benzodiazepine treatment in severe alcohol withdrawal syndrome. It has been shown to decrease the amount of total benzodiazepine needed compared with benzodiazepine therapy alone, but no differences have been seen in length of hospital stay.36–39 However, research on this drug so far is limited to ICU patients.
Beta-blockers
Beta-blockers have been used in inpatients with alcohol withdrawal syndrome to reduce heart rate and potentially reduce alcohol craving. However, the data are limited and conflicting.
Atenolol 50 to 100 mg daily, in a study in 120 patients, reduced length of stay (4 vs 5 days), reduced benzodiazepine use, and improved vital signs and behavior compared with placebo.40
Propranolol 40 mg every 6 hours reduced arrhythmias but increased hallucinations when used alone in a study in 47 patients.41 When used in combination with chlordiazepoxide, no benefit was seen in arrhythmia reduction.41
Barbiturates and other antiepileptics
Data continue to emerge on antiepileptics as both monotherapy and adjunctive therapy for alcohol withdrawal syndrome. Barbiturates as monotherapy were largely replaced by benzodiazepines in view of the narrow therapeutic index of barbiturates and their full agonist effect on the GABA receptor complex. However, phenobarbital has been evaluated in patients presenting with severe alcohol withdrawal syndrome or resistant alcohol withdrawal (ie, symptoms despite large or repeated doses of benzodiazepines) as an adjunct to benzodiazepines.42,43
In addition, a newer trial44 involved giving a single dose of phenobarbital in the emergency department in combination with a CIWA-Ar–based benzodiazepine protocol, compared with the benzodiazepine protocol alone. The group that received phenobarbital had fewer ICU admissions; its evaluation is ongoing.
The three other medications with the most data are carbamazepine, valproic acid, and gabapentin.45,46 However, the studies were small and the benefit was modest. Although these agents are possible alternatives in protracted alcohol withdrawal syndrome, no definite conclusion can be made regarding their place in therapy.46
RECOMMENDATIONS FOR DRUG THERAPY AND SUPPORTIVE CARE
Which benzodiazepine to use?
No specific benzodiazepine is recommended over the others for managing alcohol withdrawal syndrome, but studies best support the long-acting agent chlordiazepoxide.16,17,20 Other benzodiazepines such as lorazepam and oxazepam have proved to be beneficial, but drugs should be selected on the basis of patient characteristics and drug metabolism.18,19,27
Patients with severe liver dysfunction and the elderly may have slower oxidative metabolism, so the effects of medications that are primarily oxidized, such as chlordiazepoxide and diazepam, may be prolonged. Therefore, lorazepam and oxazepam would be preferred in these groups.47 While most patients with alcohol withdrawal syndrome and liver dysfunction do not have advanced cirrhosis, we recommend liver function testing (serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) and screening for liver disease, given the drug metabolism and package insert caution for use in those with impaired hepatic function.48
Patients with end-stage renal disease (stage 5 chronic kidney disease) or acute kidney injury should not receive parenteral diazepam or lorazepam. The rationale is the potential accumulation of propylene glycol, the solvent used in these formulations.
In the elderly, the Beers list of drugs to avoid in older adults includes benzodiazepines, not differentiating individual benzodiazepines in terms of risk.49 However, chlordiazepoxide may be preferable to diazepam due to its shorter parent half-life and lower lipophilicity.27 Few studies have been done using benzodiazepines in elderly patients with alcohol withdrawal syndrome, but those published have shown either equivalent dosing required compared with younger patients or more severe withdrawal for which they received greater amounts of chlordiazepoxide.9,12 Lorazepam and oxazepam have less potential to accumulate in the elderly compared with the nonelderly due to the drugs’ metabolic profiles; lorazepam is the preferred agent because of its faster onset of action.47 Ultimately, the choice of benzodiazepine in elderly patients with alcohol withdrawal syndrome should be based on patient-specific characteristics.
How should benzodiazepines be dosed?
While the CIWA-Ar thresholds and subsequent dosing of benzodiazepines varied in different studies, we recommend starting benzodiazepine therapy at a CIWA-Ar score of 8 or higher, with subsequent dosing based on score reassessment. Starting doses of benzodiazepines should be chlordiazepoxide 25 to 50 mg, lorazepam 1 to 2 mg, or oxazepam 15 mg.16–20
Subsequent doses should be titrated upward, increasing by 1.5 to 2 times the previous dose and monitored at least every 1 to 2 hours after dose adjustments. Once a patient is stable and the CIWA-Ar score is less than 8, monitoring intervals can be extended to every 4 to 8 hours. If the CIWA-Ar score is more than 20, studies suggest the need for patient reevaluation for transfer to the ICU; however, some health systems have a lower threshold for this intervention.7,14,50
Dosing algorithms and CIWA-Ar goals may vary slightly from institution to institution, but it has been shown that symptom-triggered therapy works best when hospitals have a protocol for it and staff are adequately trained to assess patients with alcohol withdrawal syndrome.7,50,51 Suggestions for dose ranges and symptom-triggered therapy are shown in Table 5.
In case of benzodiazepine overdose or potential benzodiazepine-induced delirium, flumazenil could be considered.52
Patients who should not receive symptom-triggered therapy include immediate postoperative patients in whom clinicians cannot properly assess withdrawal symptoms and patients with a history of DTs.51 While controversy exists regarding the use of symptom-triggered therapy in patients with complicated medical comorbidities, there are data to support symptom-triggered therapy in some ICU patients, as it has resulted in less benzodiazepine use and reduced mechanical ventilation.53,54
There are limited data to support phenobarbital in treating resistant alcohol withdrawal syndrome, either alone or concurrently with benzodiazepines, in escalating doses ranging from 65 to 260 mg, with a maximum daily dose of 520 mg.42,55,56
Haloperidol
For patients exhibiting agitation despite benzodiazepine therapy, giving haloperidol adjunctively can be beneficial.
Haloperidol can be used in medical patients as an adjunctive therapy for agitation, but caution is advised because of the potential for a lowering of the seizure threshold, extrapyramidal effects, and risk of QTc prolongation leading to arrhythmias. Patients considered at highest risk for torsades de pointes may have a QTc of 500 msec or greater.57
Patients should also be screened for factors that have been shown to be independent predictors of QTc prolongation (female sex, diagnosis of myocardial infarction, septic shock or left ventricular dysfunction, other QT-prolonging drugs, age > 68, baseline QTc ≥ 450 msec, and hypokalemia).58 If combined predictors have been identified, it is recommended that haloperidol be avoided.
If haloperidol is to be given, a baseline electrocardiogram and electrolyte panel should be obtained, with daily electrocardiograms thereafter, as well as ongoing review of the patient’s medications to minimize drug interactions that could further increase the risk for QTc prolongation.
Suggested haloperidol dosing is 2 to 5 mg intravenously every 0.5 to 2 hours with a maximum dose of 0.5 mg/kg/24 hours.8,33 A maximum of 35 mg of intravenous haloperidol should be used in a 24-hour period to avoid QTc prolongation.57
Antihypertensive therapy
Many patients receive symptomatic relief of autonomic hyperreactivity with benzodiazepines. However, some may require additional antihypertensive therapy for cardiac adrenergic symptoms (hypertension, tachycardia) if symptoms do not resolve by treating other medical problems commonly seen in patients with alcohol withdrawal syndrome, such as dehydration and electrolyte imbalances.7
Published protocols suggest giving clonidine 0.1 mg orally every hour up to three times as needed until systolic blood pressure is less than 140 mm Hg (less than 160 mm Hg if the patient is over age 60) and diastolic pressure is less than 90 mm Hg.51 Once the patient is stabilized, the dosing can be scheduled to a maximum of 2.4 mg daily.59 However, we believe that the use of clonidine should be restricted to patients who have a substantial increase in blood pressure over baseline or are nearing a hypertensive urgency or emergency (pressures > 180/120 mm Hg) and should not be used to treat other general symptoms associated with alcohol withdrawal syndrome.42
In addition, based on limited evidence, we recommend using beta-blockers only in patients with symptomatic tachycardia or as an adjunct in hypertension management.40,41
Therapies to avoid in acutely ill medical patients
Ethanol is not recommended. Instead, intravenous benzodiazepines should be given in patients presenting with severe alcohol withdrawal syndrome.
Antiepileptics, including valproic acid, carbamazepine, and pregabalin, lack benefit in these patients either as monotherapy or as adjunctive therapy and so are not recommended.45,60–62
Magnesium supplementation (in patients with normal serum magnesium levels) should not be given, as no clinical benefit has been shown.63
Deprived of alcohol while in the hospital, up to 80% of patients who are alcohol-dependent risk developing alcohol withdrawal syndrome,1 a potentially life-threatening condition. Clinicians should anticipate the syndrome and be ready to treat and prevent its complications.
Because alcoholism is common, nearly every provider will encounter its complications and withdrawal symptoms. Each year, an estimated 1.2 million hospital admissions are related to alcohol abuse, and about 500,000 episodes of withdrawal symptoms are severe enough to require clinical attention.1–3 Nearly 50% of patients with alcohol withdrawal syndrome are middle-class, highly functional individuals, making withdrawal difficult to recognize.1
While acute trauma patients or those with alcohol withdrawal delirium are often admitted directly to an intensive care unit (ICU), many others are at risk for or develop alcohol withdrawal syndrome and are managed initially or wholly on the acute medical unit. While specific statistics have not been published on non-ICU patients with alcohol withdrawal syndrome, they are an important group of patients who need to be well managed to prevent the progression of alcohol withdrawal syndrome to alcohol withdrawal delirium, alcohol withdrawal-induced seizure, and other complications.
This article reviews how to identify and manage alcohol withdrawal symptoms in noncritical, acutely ill medical patients, with practical recommendations for diagnosis and management.
CAN LEAD TO DELIRIUM TREMENS
In people who are physiologically dependent on alcohol, symptoms of withdrawal usually occur after abrupt cessation.4 If not addressed early in the hospitalization, alcohol withdrawal syndrome can progress to alcohol withdrawal delirium (also known as delirium tremens or DTs), in which the mortality rate is 5% to 10%.5,6 Potential mechanisms of DTs include increased dopamine release and dopamine receptor activity, hypersensitivity to N-methyl-d-aspartate, and reduced levels of gamma-aminobutyric acid (GABA).7
Long-term changes are thought to occur in neurons after repeated detoxification from alcohol, a phenomenon called “kindling.” After each detoxification, alcohol craving and obsessive thoughts increase,8 and subsequent episodes of alcohol withdrawal tend to be progressively worse.
Withdrawal symptoms
Alcohol withdrawal syndrome encompasses a spectrum of symptoms and conditions, from minor (eg, insomnia, tremulousness) to severe (seizures, DTs).2 The symptoms typically depend on the amount of alcohol consumed, the time since the last drink, and the number of previous detoxifications.9
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,1 states that to establish a diagnosis of alcohol withdrawal syndrome, a patient must meet four criteria:
- The patient must have ceased or reduced alcohol intake after heavy or prolonged use.
- Two or more of the following must develop within a few hours to a few days: autonomic hyperactivity (sweating or pulse greater than 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; grand mal seizure.
- The above symptoms must cause significant distress or functional impairment.
- The symptoms must not be related to another medical condition.
Some of the symptoms described in the second criterion above can occur while the patient still has a measurable blood alcohol level, usually within 6 hours of cessation of drinking.10 Table 1 describes the timetable of onset of symptoms and their severity.2
The elderly may be affected more severely
While the progression of the symptoms described above is commonly used for medical inpatients, the timeline may be different in an elderly patient. Compared with younger patients, elderly patients may have higher blood alcohol concentrations owing to lower total body water, so small amounts of alcohol can produce significant effects.11,12 Brower et al12 found that elderly patients experienced more withdrawal symptoms, especially cognitive impairment, weakness, and high blood pressure, and for 3 days longer.
In the elderly, alcohol may have a greater impact on the central nervous system because of increased permeability of the blood-brain barrier. And importantly, elderly patients tend to have more concomitant diseases and take more medications, all of which can affect alcohol metabolism.
ASSESSMENT SCALES FOR ALCOHOL WITHDRAWAL SYNDROME
A number of clinical scales for evaluating alcohol withdrawal have been developed. Early ones such as the 30-item Total Severity Assessment (TSA) scale and the 11-item Selected Severity Assessment (SSA) scale were limited because they were extremely detailed, burdensome to nursing staff to administer, and contained items such as daily “sleep disturbances” that were not acute enough to meet specific monitoring needs or to guide drug therapy.13,14
The Clinical Institute Withdrawal Assessment for alcohol (CIWA-A) scale, with 15 items, was derived from the SSA scale and includes acute items for assessment as often as every half-hour.15
The CIWA-Ar scale (Table 2) was developed from the CIWA-A scale by Sullivan et al.15 Using both observation and interview, it focuses on 10 areas: nausea and vomiting, tremor, paroxysmal sweats, anxiety, agitation, headache, disorientation, tactile disturbances, auditory disturbances, and visual disturbances. Scores can range from 0 to 67; a higher score indicates worse withdrawal symptoms and outcomes and therefore necessitates escalation of treatment.
The CIWA-Ar scale is now the one most commonly used in clinical trials16–20 and, we believe, in practice. Other scales, including the CIWA-AD and the Alcohol Withdrawal Scale have been validated but are not widely used in practice.14,21
BASELINE ASSESSMENT AND EARLY SUPPORTIVE CARE
A thorough history and physical examination should be performed on admission in patients known to be or suspected of being alcohol-dependent to assess the patient’s affected body systems. The time elapsed since the patient’s last alcohol drink helps predict the onset of withdrawal complications.
Baseline laboratory tests for most patients with suspected alcohol withdrawal syndrome should include a basic blood chemistry panel, complete blood cell count, and possibly an alcohol and toxicology screen, depending on the patient’s history and presentation.
Hydration and nutritional support are important in patients presenting with alcohol withdrawal syndrome. Severe disturbances in electrolytes can lead to serious complications, including cardiac arrhythmia. Close monitoring and electrolyte replacement as needed are recommended for hospitalized alcoholic patients and should follow hospital protocols.22
Thiamine and folic acid status deserve special attention, since long-standing malnutrition is common in alcoholic patients. Thiamine deficiency can result in Wernicke encephalopathy and Korsakoff syndrome, characterized by delirium, ataxia, vision changes, and amnesia. Alcohol withdrawal guidelines recommend giving thiamine intravenously for the first 2 to 5 days after admission.23 In addition, thiamine must be given before any intravenous glucose product, as thiamine is a cofactor in carbohydrate metabolism.23 Folic acid should also be supplemented, as chronic deficiencies may lead to megaloblastic or macrocytic anemia.
CIWA-Ar scale. To provide consistent monitoring and ongoing treatment, clinicians and institutions are encouraged to use a simple assessment scale that detects and quantifies alcohol withdrawal syndrome and that can be used for reassessment after an intervention.21 The CIWA-Ar scale should be used to facilitate “symptom-triggered therapy” in which, depending on the score, the patient receives pharmacologic treatment followed by a scheduled reevaluation.23,24 Most patients with a CIWA-Ar score of 8 or higher benefit from benzodiazepine therapy.16,18,19
PRIMARY DRUG THERAPIES FOR MEDICAL INPATIENTS
Benzodiazepines are the first-line agents
Benzodiazepines are the first-line agents recommended for preventing and treating alcohol withdrawal syndrome.23 Their various pharmacokinetic profiles, wide therapeutic indices, and safety compared with older sedative hypnotics make them the preferred class.23,25 No single benzodiazepine is preferred over the others for treating alcohol withdrawal syndrome: studies have shown benefits using short-acting, intermediate-acting, and long-acting agents. The choice of drug is variable and patient-specific.16,18,26
Benzodiazepines promote and enhance binding of the inhibitory neurotransmitter GABA to GABAA receptors in the central nervous system.27 As a class, benzodiazepines are all structurally related and produce the same effects—namely, sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia, and anticonvulsant activity.27
The most studied benzodiazepines for treating and preventing alcohol withdrawal syndrome are chlordiazepoxide, oxazepam, and lorazepam,16–20 whereas diazepam was used in older studies.23
Diazepam and chlordiazepoxide are metabolized by oxidation, each sharing the long-acting active metabolite desmethyldiazepam (half-life 72 hours), and short-acting metabolite oxazepam (half-life 8 hours).27 In addition, the parent drugs also have varying pharmacokinetic profiles: diazepam has a half-life of more than 30 hours and chlordiazepoxide a half-life of about 8 hours. Chlordiazepoxide and diazepam’s combination of both long- and short-acting benzodiazepine activity provides long-term efficacy in attenuating withdrawal symptoms, but chlordiazepoxide’s shorter parent half-life allows more frequent dosing.
Lorazepam (half-life 10–20 hours) and oxazepam (half-life 5–20 hours) undergo glucuronide conjugation and do not have metabolites.27,28 Table 3 provides a pharmacokinetic summary.27,28
Various dosage regimens are used in giving benzodiazepines, the most common being symptom-triggered therapy, governed by assessment scales, and scheduled around-the-clock therapy.29 Current evidence supports symptom-triggered therapy in most inpatients who are not critically ill, as it can reduce both benzodiazepine use and adverse drug events and can reduce the length of stay.16,19
Trials of symptom-triggered benzodiazepine therapy
Most inpatient trials of symptom-triggered therapy (Table 4)3,16–20 used the CIWA-Ar scale for monitoring. In some of the studies, benzodiazepines were given if the score was 8 or higher, but others used cut points as high as 15 or higher. Doses:
- Chlordiazepoxide (first dose 25–100 mg)
- Lorazepam (first dose 0.5–2 mg)
- Oxazepam (30 mg).
After each dose, patients were reevaluated at intervals of 30 minutes to 8 hours.
Most of these trials showed no difference in rates of adverse drug events such as seizures, hallucinations, and lethargy with symptom-triggered therapy compared with scheduled therapy.16,18,20 They also found either no difference in the incidence of delirium tremens, or a lower incidence of delirium tremens with symptom-triggered therapy than with scheduled therapy.16–18,20
Weaver et al19 found no difference in length of stay between scheduled therapy and symptom-triggered therapy, but Saitz et al16 reported a median benzodiazepine treatment duration of 9 hours with symptom-triggered therapy vs 68 hours with fixed dosing. Thus, the study by Saitz et al suggests that hospitalization might be shorter with symptom-triggered therapy.
Many of the trials had notable limitations related to the diversity of patients enrolled and the protocols for both symptom-triggered therapy and fixed dosing. Some trials enrolled only inpatients in detoxification programs; others focused on inpatients with acute medical illness. The inpatient alcohol treatment trials16,18 excluded medically ill patients and those with concurrent psychiatric illness,16,18 and one excluded patients with seizures.16 One of the inpatient alcohol treatment program trials16 excluded patients on beta-blockers or clonidine because of concern that these drugs could mask withdrawal symptoms, whereas trials in medically ill patients allowed these same drugs.17,19,20
Most of the patients were men (approximately 75%, but ranging from 74% to 100%), and therefore the study results may not be as applicable to women.16–20 Most participants were middle-aged, with average ages in all studies between 46 and 55. Finally, the studies used a wide range of medications and dosing, with patient monitoring intervals ranging from every 30 minutes to every 8 hours.16–20
In a 2010 Cochrane analysis, Amato et al29 concluded that the limited evidence available favors symptom-triggered regimens over fixed-dosing regimens, but that differences in isolated trials should be interpreted very cautiously.
Therapeutic ethanol
Aside from the lack of evidence to support its use in alcohol withdrawal syndrome, prescribing oral ethanol to alcoholic patients clearly poses an ethical dilemma. However, giving ethanol intravenously has been studied, mostly in surgical trauma patients.30
Early reports described giving intravenous ethanol on a gram-to-gram basis to match the patient’s consumption before admission to prevent alcohol withdrawal syndrome. But later studies reported prevention of alcohol withdrawal syndrome with very small amounts of intravenous ethanol.30,31 While clinical trials have been limited to ICU patients, ethanol infusion at an initial rate of 2.5 to 5 g per hour and titrated up to 10 g per hour has appeared to be safe and effective for preventing alcohol withdrawal syndrome.30,31 The initial infusion rate of 2.5 to 5 g per hour is equivalent to 4 to 10 alcoholic beverages per 24 hours.
Nevertheless, ethanol infusion carries the potential for toxicities (eg, gastric irritation, precipitation of acute hepatic failure, hypoglycemia, pancreatitis, bone marrow suppression, prolonged wound healing) and drug interactions (eg, with anticoagulants and anticonvulsants). Thus, ethanol is neither widely used nor recommended.25,31
ADJUNCTIVE THERAPIES
Many medications are used adjunctively in the acute setting, both for symptoms of alcohol withdrawal syndrome and for agitation.
Haloperidol
No clinical trial has yet examined haloperidol monotherapy in patients with alcohol withdrawal syndrome in either general medical units or intensive care units. Yet haloperidol remains important and is recommended as an adjunct therapy for agitation.23,32 Dosing of haloperidol in protocols for surgical patients ranged from 2 to 5 mg intravenously every 0.5 to 2 hours, with a maximum dosage of 0.5 mg per kg per 24 hours.7,33
Alpha-2 agonists
Alpha-2 agonists are thought to reduce sympathetic overdrive and the autonomic symptoms associated with alcohol withdrawal syndrome, and these agents (primarily clonidine) have been studied in the treatment of alcohol withdrawal syndrome.34,35
Clonidine. In a Swedish study,34 26 men ages 20 to 55 who presented with the tremor, sweating, dysphoria, tension, anxiety, and tachycardia associated with alcohol withdrawal syndrome received clonidine 4 µg per kg twice daily or carbamazepine 200 mg three to four times daily in addition to an antiepileptic. Adjunctive use of a benzodiazepine was allowed at night in both groups. No statistically significant difference in symptom reduction was noted between the two groups, and there was no difference in total benzodiazepine use.
Dexmedetomidine, given intravenously, has been tested as an adjunct to benzodiazepine treatment in severe alcohol withdrawal syndrome. It has been shown to decrease the amount of total benzodiazepine needed compared with benzodiazepine therapy alone, but no differences have been seen in length of hospital stay.36–39 However, research on this drug so far is limited to ICU patients.
Beta-blockers
Beta-blockers have been used in inpatients with alcohol withdrawal syndrome to reduce heart rate and potentially reduce alcohol craving. However, the data are limited and conflicting.
Atenolol 50 to 100 mg daily, in a study in 120 patients, reduced length of stay (4 vs 5 days), reduced benzodiazepine use, and improved vital signs and behavior compared with placebo.40
Propranolol 40 mg every 6 hours reduced arrhythmias but increased hallucinations when used alone in a study in 47 patients.41 When used in combination with chlordiazepoxide, no benefit was seen in arrhythmia reduction.41
Barbiturates and other antiepileptics
Data continue to emerge on antiepileptics as both monotherapy and adjunctive therapy for alcohol withdrawal syndrome. Barbiturates as monotherapy were largely replaced by benzodiazepines in view of the narrow therapeutic index of barbiturates and their full agonist effect on the GABA receptor complex. However, phenobarbital has been evaluated in patients presenting with severe alcohol withdrawal syndrome or resistant alcohol withdrawal (ie, symptoms despite large or repeated doses of benzodiazepines) as an adjunct to benzodiazepines.42,43
In addition, a newer trial44 involved giving a single dose of phenobarbital in the emergency department in combination with a CIWA-Ar–based benzodiazepine protocol, compared with the benzodiazepine protocol alone. The group that received phenobarbital had fewer ICU admissions; its evaluation is ongoing.
The three other medications with the most data are carbamazepine, valproic acid, and gabapentin.45,46 However, the studies were small and the benefit was modest. Although these agents are possible alternatives in protracted alcohol withdrawal syndrome, no definite conclusion can be made regarding their place in therapy.46
RECOMMENDATIONS FOR DRUG THERAPY AND SUPPORTIVE CARE
Which benzodiazepine to use?
No specific benzodiazepine is recommended over the others for managing alcohol withdrawal syndrome, but studies best support the long-acting agent chlordiazepoxide.16,17,20 Other benzodiazepines such as lorazepam and oxazepam have proved to be beneficial, but drugs should be selected on the basis of patient characteristics and drug metabolism.18,19,27
Patients with severe liver dysfunction and the elderly may have slower oxidative metabolism, so the effects of medications that are primarily oxidized, such as chlordiazepoxide and diazepam, may be prolonged. Therefore, lorazepam and oxazepam would be preferred in these groups.47 While most patients with alcohol withdrawal syndrome and liver dysfunction do not have advanced cirrhosis, we recommend liver function testing (serum aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels) and screening for liver disease, given the drug metabolism and package insert caution for use in those with impaired hepatic function.48
Patients with end-stage renal disease (stage 5 chronic kidney disease) or acute kidney injury should not receive parenteral diazepam or lorazepam. The rationale is the potential accumulation of propylene glycol, the solvent used in these formulations.
In the elderly, the Beers list of drugs to avoid in older adults includes benzodiazepines, not differentiating individual benzodiazepines in terms of risk.49 However, chlordiazepoxide may be preferable to diazepam due to its shorter parent half-life and lower lipophilicity.27 Few studies have been done using benzodiazepines in elderly patients with alcohol withdrawal syndrome, but those published have shown either equivalent dosing required compared with younger patients or more severe withdrawal for which they received greater amounts of chlordiazepoxide.9,12 Lorazepam and oxazepam have less potential to accumulate in the elderly compared with the nonelderly due to the drugs’ metabolic profiles; lorazepam is the preferred agent because of its faster onset of action.47 Ultimately, the choice of benzodiazepine in elderly patients with alcohol withdrawal syndrome should be based on patient-specific characteristics.
How should benzodiazepines be dosed?
While the CIWA-Ar thresholds and subsequent dosing of benzodiazepines varied in different studies, we recommend starting benzodiazepine therapy at a CIWA-Ar score of 8 or higher, with subsequent dosing based on score reassessment. Starting doses of benzodiazepines should be chlordiazepoxide 25 to 50 mg, lorazepam 1 to 2 mg, or oxazepam 15 mg.16–20
Subsequent doses should be titrated upward, increasing by 1.5 to 2 times the previous dose and monitored at least every 1 to 2 hours after dose adjustments. Once a patient is stable and the CIWA-Ar score is less than 8, monitoring intervals can be extended to every 4 to 8 hours. If the CIWA-Ar score is more than 20, studies suggest the need for patient reevaluation for transfer to the ICU; however, some health systems have a lower threshold for this intervention.7,14,50
Dosing algorithms and CIWA-Ar goals may vary slightly from institution to institution, but it has been shown that symptom-triggered therapy works best when hospitals have a protocol for it and staff are adequately trained to assess patients with alcohol withdrawal syndrome.7,50,51 Suggestions for dose ranges and symptom-triggered therapy are shown in Table 5.
In case of benzodiazepine overdose or potential benzodiazepine-induced delirium, flumazenil could be considered.52
Patients who should not receive symptom-triggered therapy include immediate postoperative patients in whom clinicians cannot properly assess withdrawal symptoms and patients with a history of DTs.51 While controversy exists regarding the use of symptom-triggered therapy in patients with complicated medical comorbidities, there are data to support symptom-triggered therapy in some ICU patients, as it has resulted in less benzodiazepine use and reduced mechanical ventilation.53,54
There are limited data to support phenobarbital in treating resistant alcohol withdrawal syndrome, either alone or concurrently with benzodiazepines, in escalating doses ranging from 65 to 260 mg, with a maximum daily dose of 520 mg.42,55,56
Haloperidol
For patients exhibiting agitation despite benzodiazepine therapy, giving haloperidol adjunctively can be beneficial.
Haloperidol can be used in medical patients as an adjunctive therapy for agitation, but caution is advised because of the potential for a lowering of the seizure threshold, extrapyramidal effects, and risk of QTc prolongation leading to arrhythmias. Patients considered at highest risk for torsades de pointes may have a QTc of 500 msec or greater.57
Patients should also be screened for factors that have been shown to be independent predictors of QTc prolongation (female sex, diagnosis of myocardial infarction, septic shock or left ventricular dysfunction, other QT-prolonging drugs, age > 68, baseline QTc ≥ 450 msec, and hypokalemia).58 If combined predictors have been identified, it is recommended that haloperidol be avoided.
If haloperidol is to be given, a baseline electrocardiogram and electrolyte panel should be obtained, with daily electrocardiograms thereafter, as well as ongoing review of the patient’s medications to minimize drug interactions that could further increase the risk for QTc prolongation.
Suggested haloperidol dosing is 2 to 5 mg intravenously every 0.5 to 2 hours with a maximum dose of 0.5 mg/kg/24 hours.8,33 A maximum of 35 mg of intravenous haloperidol should be used in a 24-hour period to avoid QTc prolongation.57
Antihypertensive therapy
Many patients receive symptomatic relief of autonomic hyperreactivity with benzodiazepines. However, some may require additional antihypertensive therapy for cardiac adrenergic symptoms (hypertension, tachycardia) if symptoms do not resolve by treating other medical problems commonly seen in patients with alcohol withdrawal syndrome, such as dehydration and electrolyte imbalances.7
Published protocols suggest giving clonidine 0.1 mg orally every hour up to three times as needed until systolic blood pressure is less than 140 mm Hg (less than 160 mm Hg if the patient is over age 60) and diastolic pressure is less than 90 mm Hg.51 Once the patient is stabilized, the dosing can be scheduled to a maximum of 2.4 mg daily.59 However, we believe that the use of clonidine should be restricted to patients who have a substantial increase in blood pressure over baseline or are nearing a hypertensive urgency or emergency (pressures > 180/120 mm Hg) and should not be used to treat other general symptoms associated with alcohol withdrawal syndrome.42
In addition, based on limited evidence, we recommend using beta-blockers only in patients with symptomatic tachycardia or as an adjunct in hypertension management.40,41
Therapies to avoid in acutely ill medical patients
Ethanol is not recommended. Instead, intravenous benzodiazepines should be given in patients presenting with severe alcohol withdrawal syndrome.
Antiepileptics, including valproic acid, carbamazepine, and pregabalin, lack benefit in these patients either as monotherapy or as adjunctive therapy and so are not recommended.45,60–62
Magnesium supplementation (in patients with normal serum magnesium levels) should not be given, as no clinical benefit has been shown.63
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013:501.
- Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician 2004; 69:1443–1450.
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348:1786–1795.
- Isbell H, Fraser HF, Wilker A, Bellevile RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol 1955; 16:1–33.
- Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med 2008; 15:788–790.
- Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol 2010; 45:151–158.
- Stanley KM, Amabile CM, Simpson KN, Couillard D, Norcross ED, Worrall CL. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy 2003; 23:843–854.
- Brousse G, Arnaud B, Vorspan F, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol 2012; 47:501–508.
- Liskow BI, Rinck C, Campbell J, DeSouza C. Alcohol withdrawal in the elderly. J Stud Alcohol 1989; 50:414–421.
- Etherington JM. Emergency management of acute alcohol problems. Part 1: uncomplicated withdrawal. Can Fam Physician 1996; 42:2186–2190.
- Letizia M, Reinbolz M. Identifying and managing acute alcohol withdrawal in the elderly. Geriatr Nurs 2005; 26:176–183.
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res 1994; 18:196–201.
- Williams D, Lewis J, McBride A. A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108.
- Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict 2006; 15:85–93.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357.
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA 1994; 272:519–523.
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc 2001; 76:695–701.
- Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med 2002; 162:1117–1121.
- Weaver MF, Hoffman HJ, Johnson RE, Mauck K. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis 2006; 25:17–24.
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar). Am J Addict 2000; 9:135–144.
- Wetterling T, Kanitz RD, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale). Alcohol Alcohol 1997; 32:753–760.
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World 1998; 22:38–43.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Sellers EM, Sullivan JT, Somer G, Sykora K. Characterization of DSM-III-R criteria for uncomplicated alcohol withdrawal provides an empirical basis for DSM-IV. Arch Gen Psychiatry 1991; 48:442–447.
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med 2010; 38(suppl):S494–S501.
- Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs 2009; 70:467–474.
- Bird RD, Makela EH. Alcohol withdrawal: what is the benzodiazepine of choice? Ann Pharmacother 1994; 28:67–71.
- Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs 2014; 28:401–410.
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010; 3:CD005063.
- Weinberg JA, Magnotti LJ, Fischer PE, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma 2008; 64:99–104.
- Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994; 87:47–54.
- Ungur LA, Neuner B, John S, Wernecke K, Spies C. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res 2013; 37:675–686.
- Lansford CD, Guerriero CH, Kocan MJ, et al. Improved outcomes in patients with head and neck cancer using a standardized care protocol for postoperative alcohol withdrawal. Arch Otolaryngol Head Neck Surg 2008; 134:865–872.
- Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend 1981; 8:345–348.
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother 2011; 45:649–657.
- Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy 2014; 34:910–917.
- VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med 2014. [Epub ahead of print October 16, 2014]
- Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care 2012; 2:12.
- Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs 2013; 27:913–920.
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med 1985; 313:905–909.
- Zilm DH, Jacob MS, MacLeod SM, Sellers EM, Ti TY. Propranolol and chlordiazepoxide effects on cardiac arrhythmias during alcohol withdrawal. Alcohol Clin Exp Res 1980; 4:400–405.
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60.
- Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy 2009; 29:875–878.
- Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med 2013; 44:592–598.e2.
- Prince V, Turpin KR. Treatment of alcohol withdrawal syndrome with carbamazepine, gabapentin, and nitrous oxide. Am J Health Syst Pharm 2008; 65:1039–1047.
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1106–1117.
- Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16:49–57.
- Valeant Pharmaceuticals North America LLC. Librium—chlordiazepoxide hydrochloride capsule, gelatin coated. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=125207. Accessed November 20, 2015.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc 2008; 83:274–279.
- Manasco A, Chang S, Larriviere J, Hamm LL, Glass M. Alcohol withdrawal. South Med J 2012; 105:607–612.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 2014; 10:126–132.
- Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics 2004; 45:256–261.
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest 2010; 138:994–1003.
- Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med 1987; 16:847–850.
- Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med 2011; 29:382–385.
- Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998; 81:238–240.
- Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6:479–487.
- Boehringer Ingelheim Pharmaceuticals, Inc. Product Information: Catapres oral tablets, clonidine HCl oral tablets, 2012.
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res 2001; 25:1324–1329.
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 1989; 146:617–621.
- Förg A, Hein J, Volkmar K, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol 2012; 47:149–155.
- Wilson A, Vulcano B. A double-blind, placebo-controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcohol Clin Exp Res 1984; 8:542–545.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association, 2013:501.
- Bayard M, McIntyre J, Hill KR, Woodside J Jr. Alcohol withdrawal syndrome. Am Fam Physician 2004; 69:1443–1450.
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med 2003; 348:1786–1795.
- Isbell H, Fraser HF, Wilker A, Bellevile RE, Eisenman AJ. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol 1955; 16:1–33.
- Khan A, Levy P, DeHorn S, Miller W, Compton S. Predictors of mortality in patients with delirium tremens. Acad Emerg Med 2008; 15:788–790.
- Monte R, Rabuñal R, Casariego E, López-Agreda H, Mateos A, Pértega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol 2010; 45:151–158.
- Stanley KM, Amabile CM, Simpson KN, Couillard D, Norcross ED, Worrall CL. Impact of an alcohol withdrawal syndrome practice guideline on surgical patient outcomes. Pharmacotherapy 2003; 23:843–854.
- Brousse G, Arnaud B, Vorspan F, et al. Alteration of glutamate/GABA balance during acute alcohol withdrawal in emergency department: a prospective analysis. Alcohol Alcohol 2012; 47:501–508.
- Liskow BI, Rinck C, Campbell J, DeSouza C. Alcohol withdrawal in the elderly. J Stud Alcohol 1989; 50:414–421.
- Etherington JM. Emergency management of acute alcohol problems. Part 1: uncomplicated withdrawal. Can Fam Physician 1996; 42:2186–2190.
- Letizia M, Reinbolz M. Identifying and managing acute alcohol withdrawal in the elderly. Geriatr Nurs 2005; 26:176–183.
- Brower KJ, Mudd S, Blow FC, Young JP, Hill EM. Severity and treatment of alcohol withdrawal in elderly versus younger patients. Alcohol Clin Exp Res 1994; 18:196–201.
- Williams D, Lewis J, McBride A. A comparison of rating scales for the alcohol-withdrawal syndrome. Alcohol Alcohol 2001; 36:104–108.
- Reoux JP, Oreskovich MR. A comparison of two versions of the Clinical Institute Withdrawal Assessment for Alcohol: the CIWA-Ar and CIWA-AD. Am J Addict 2006; 15:85–93.
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised Clinical Institute Withdrawal Assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357.
- Saitz R, Mayo-Smith MF, Roberts MS, Redmond HA, Bernard DR, Calkins DR. Individualized treatment for alcohol withdrawal. A randomized double-blind controlled trial. JAMA 1994; 272:519–523.
- Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc 2001; 76:695–701.
- Daeppen JB, Gache P, Landry U, et al. Symptom-triggered vs fixed-schedule doses of benzodiazepine for alcohol withdrawal: a randomized treatment trial. Arch Intern Med 2002; 162:1117–1121.
- Weaver MF, Hoffman HJ, Johnson RE, Mauck K. Alcohol withdrawal pharmacotherapy for inpatients with medical comorbidity. J Addict Dis 2006; 25:17–24.
- Reoux JP, Miller K. Routine hospital alcohol detoxification practice compared to symptom triggered management with an Objective Withdrawal Scale (CIWA-Ar). Am J Addict 2000; 9:135–144.
- Wetterling T, Kanitz RD, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS scale). Alcohol Alcohol 1997; 32:753–760.
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World 1998; 22:38–43.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Sellers EM, Sullivan JT, Somer G, Sykora K. Characterization of DSM-III-R criteria for uncomplicated alcohol withdrawal provides an empirical basis for DSM-IV. Arch Gen Psychiatry 1991; 48:442–447.
- Sarff M, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med 2010; 38(suppl):S494–S501.
- Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs 2009; 70:467–474.
- Bird RD, Makela EH. Alcohol withdrawal: what is the benzodiazepine of choice? Ann Pharmacother 1994; 28:67–71.
- Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs 2014; 28:401–410.
- Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev 2010; 3:CD005063.
- Weinberg JA, Magnotti LJ, Fischer PE, et al. Comparison of intravenous ethanol versus diazepam for alcohol withdrawal prophylaxis in the trauma ICU: results of a randomized trial. J Trauma 2008; 64:99–104.
- Craft PP, Foil MB, Cunningham PR, Patselas PC, Long-Snyder BM, Collier MS. Intravenous ethanol for alcohol detoxification in trauma patients. South Med J 1994; 87:47–54.
- Ungur LA, Neuner B, John S, Wernecke K, Spies C. Prevention and therapy of alcohol withdrawal on intensive care units: systematic review of controlled trials. Alcohol Clin Exp Res 2013; 37:675–686.
- Lansford CD, Guerriero CH, Kocan MJ, et al. Improved outcomes in patients with head and neck cancer using a standardized care protocol for postoperative alcohol withdrawal. Arch Otolaryngol Head Neck Surg 2008; 134:865–872.
- Walinder J, Balldin J, Bokstrom K, Karlsson I, Lundstrom B, Svensson TH. Clonidine suppression of the alcohol withdrawal syndrome. Drug Alcohol Depend 1981; 8:345–348.
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother 2011; 45:649–657.
- Crispo AL, Daley MJ, Pepin JL, Harford PH, Brown CV. Comparison of clinical outcomes in nonintubated patients with severe alcohol withdrawal syndrome treated with continuous-infusion sedatives: dexmedetomidine versus benzodiazepines. Pharmacotherapy 2014; 34:910–917.
- VanderWeide LA, Foster CJ, MacLaren R, Kiser TH, Fish DN, Mueller SW. Evaluation of early dexmedetomidine addition to the standard of care for severe alcohol withdrawal in the ICU: a retrospective controlled cohort study. J Intensive Care Med 2014. [Epub ahead of print October 16, 2014]
- Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensive Care 2012; 2:12.
- Muzyk AJ, Kerns S, Brudney S, Gagliardi JP. Dexmedetomidine for the treatment of alcohol withdrawal syndrome: rationale and current status of research. CNS Drugs 2013; 27:913–920.
- Kraus ML, Gottlieb LD, Horwitz RI, Anscher M. Randomized clinical trial of atenolol in patients with alcohol withdrawal. N Engl J Med 1985; 313:905–909.
- Zilm DH, Jacob MS, MacLeod SM, Sellers EM, Ti TY. Propranolol and chlordiazepoxide effects on cardiac arrhythmias during alcohol withdrawal. Alcohol Clin Exp Res 1980; 4:400–405.
- Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol 2006; 2:55–60.
- Hayner CE, Wuestefeld NL, Bolton PJ. Phenobarbital treatment in a patient with resistant alcohol withdrawal syndrome. Pharmacotherapy 2009; 29:875–878.
- Rosenson J, Clements C, Simon B, et al. Phenobarbital for acute alcohol withdrawal: a prospective randomized double-blind placebo-controlled study. J Emerg Med 2013; 44:592–598.e2.
- Prince V, Turpin KR. Treatment of alcohol withdrawal syndrome with carbamazepine, gabapentin, and nitrous oxide. Am J Health Syst Pharm 2008; 65:1039–1047.
- Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome. A focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1106–1117.
- Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16:49–57.
- Valeant Pharmaceuticals North America LLC. Librium—chlordiazepoxide hydrochloride capsule, gelatin coated. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=125207. Accessed November 20, 2015.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60:616–631.
- Hecksel KA, Bostwick JM, Jaeger TM, Cha SS. Inappropriate use of symptom-triggered therapy for alcohol withdrawal in the general hospital. Mayo Clin Proc 2008; 83:274–279.
- Manasco A, Chang S, Larriviere J, Hamm LL, Glass M. Alcohol withdrawal. South Med J 2012; 105:607–612.
- Moore PW, Donovan JW, Burkhart KK, et al. Safety and efficacy of flumazenil for reversal of iatrogenic benzodiazepine-associated delirium toxicity during treatment of alcohol withdrawal, a retrospective review at one center. J Med Toxicol 2014; 10:126–132.
- Bostwick JM, Lapid MI. False positives on the clinical institute withdrawal assessment for alcohol-revised: is this scale appropriate for use in the medically ill? Psychosomatics 2004; 45:256–261.
- de Wit M, Jones DG, Sessler CN, Zilberberg MD, Weaver MF. Alcohol-use disorders in the critically ill patient. Chest 2010; 138:994–1003.
- Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med 1987; 16:847–850.
- Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med 2011; 29:382–385.
- Sharma ND, Rosman HS, Padhi ID, Tisdale JE. Torsades de pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol 1998; 81:238–240.
- Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 6:479–487.
- Boehringer Ingelheim Pharmaceuticals, Inc. Product Information: Catapres oral tablets, clonidine HCl oral tablets, 2012.
- Reoux JP, Saxon AJ, Malte CA, Baer JS, Sloan KL. Divalproex sodium in alcohol withdrawal: a randomized double-blind placebo-controlled clinical trial. Alcohol Clin Exp Res 2001; 25:1324–1329.
- Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry 1989; 146:617–621.
- Förg A, Hein J, Volkmar K, et al. Efficacy and safety of pregabalin in the treatment of alcohol withdrawal syndrome: a randomized placebo-controlled trial. Alcohol Alcohol 2012; 47:149–155.
- Wilson A, Vulcano B. A double-blind, placebo-controlled trial of magnesium sulfate in the ethanol withdrawal syndrome. Alcohol Clin Exp Res 1984; 8:542–545.
KEY POINTS
- Patients diagnosed with or suspected of having alcohol withdrawal syndrome need a thorough history and physical examination, appropriate laboratory tests, and monitoring using the revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) or a similar scale.
- For most patients, benzodiazepines should be given in a symptom-triggered fashion, using the CIWA-Ar score as a monitoring tool. Alternatively, scheduled benzodiazepine dosing should be considered for patients with a history of alcohol withdrawal delirium or for patients in whom withdrawal symptoms cannot be easily assessed.
- The choice of benzodiazepine should be individualized, based on the half-life of the drug, comorbid diseases, and monitoring plans.
- Many patients with alcohol withdrawal syndrome require fluid and electrolyte replacement, as well as adjunctive therapies such as haloperidol for delirium and antihypertensives for cardiac or adrenergic symptoms. No standard currently exists for drug dosing, administration, and assessment protocols in these patients. Therefore, clinicians are adapting study designs and assessment scales to meet patients’ individual needs.