User login
Should off-pump CABG be abandoned?
YES
I believe on-pump coronary artery bypass graft (CABG) surgery should be our primary operation, with off-pump CABG reserved only for certain situations.
In talking about these two surgical options, there are basically three main issues of interest: procedural outcomes, the quality of revascularization, and long-term effectiveness.
Procedural outcomes: In the 1990s, there was great excitement for the potential for off-pump CABG. We thought survival would be better, stroke less common, neurocognitive outcomes improved, etc., but after several randomized studies and a number of well-controlled observational studies, we found that, in terms of the important procedural outcomes – death, MI, stroke, and acute renal failure – there were actually no differences between off- and on-pump CABG. We saw this in ROOBY, with low-risk patients and less experienced surgeons; in the Canadian CORONARY trial, with experienced off-pump surgeons; and in the European GOPCABE trial, where the surgeons were very experienced and the patients were high risk.
There are some benefits with off-pump CABG in terms of what I call the "reversible" complications of surgery: fewer transfusions, less postoperative atrial fibrillation, fewer respiratory infections; but I believe these things have to be balanced against some of the negative effects of off-pump surgery in terms of its quality of revascularization and long-term effectiveness.
Probably one of the biggest disappointments we’ve had with off-pump CABG is that, in the trials, we didn’t see any major difference in neurocognitive dysfunction. What we learned is that cardiopulmonary bypass really is not that bad in terms of neurocognitive dysfunction after surgery.
One issue that tends to be forgotten is the risk associated with conversion. In a hemodynamically unstable patient, converting from off-pump to on-pump is associated with higher risks of death, bleeding, renal failure, stroke, respiratory failure, and GI complications. In CORONARY, with experienced surgeons, the conversion rate was almost 7.9%, and in GOPCABE, it was 9.7%. Even with experienced surgeons, conversions do occur, and they have negative consequences.
Quality of revascularization: We know that long-term survival is related to completeness of revascularization. The more ischemic myocardium that is left at risk after CABG, the more likely the patients is to have an MI or die, and this has been very clearly demonstrated in the surgical literature as well as the percutaneous coronary intervention literature. It has been shown multiple times that fewer grafts are done in off-pump patients, compared with on-pump patients. Indeed, in a recent Cochrane meta-analysis of more than 7,000 patients in 57 trials, there were significantly more grafts done in on-pump than off-pump cases.
Also in terms of graft patency, again, multiple studies have shown lower graft patency in off-pump patients, either directly or by means of showing a higher reintervention rate after off-pump as compared with on-pump surgery. In both CORONARY and GOPCABE, the repeat revascularization rate at 30 days was higher for off-pump CABG.
Long-term effectiveness: The long-term results from ROOBY are very sobering: significantly higher 1-year cardiac mortality in the off-pump arm and higher 1-year composite adverse events. Again, in the 2012 Cochrane meta-analysis, with more than 10,000 patients in 75 trials, mortality was significantly higher with off-pump patients, compared with on-pump, with a hazard ratio of 1.24 (95% CI, 1.01-1.53). When the authors of the Cochrane meta-analysis removed what they called the "studies with bias," this signal was even stronger. Also in observational studies, such as one published by Racz et al. in 2004 (J. Am. Coll. Cardiol. 2004; 43: 557-64), the best survival was found to be in patients who had on-pump CABG and the worst in those who had undergone off-pump CABG.
In summary, procedural outcomes with off-pump and on-pump CABG are similar, albeit with lower reversible complications in the off-pump patients, but a greater conversion risk, along with its associated complications. The quality of the revascularization is worse in off-pump patients, the completeness of revascularization is less and re-intervention rates are higher, and the bottom line, of course, it that there is higher long-term mortality after off-pump CABG.
"Should off-pump CABG be abandoned?" As our default procedure, yes. The vast majority of our patients are best served with on-pump revascularization.
Dr. Joseph Sabik is the chairman of the department of thoracic and cardiovascular surgery and the Sheik Hamdam Bin Rashid Al Maktoum Distinguished Chair at the Cleveland Clinic. He disclosed that he performs both off-pump and on-pump CABG.
References
Anesthesiology 2005;102:188-203
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Cochrane Database Syst. Rev. 2012; Mar 14. 3: CD007224 [doi:10.1002/14651858.CD007224.pub2])
J. Am. Coll. Cardiol. 2004;43:557-64
NO
Should off pump bypass be abandoned? Absolutely not, but let’s do it well.
The rationale for why off-pump CABG should be the preferred strategy is simple: cardiopulmonary bypass (CPB) entails extracorporeal circulation, aortic cannulation and clamping, global MI, hypothermia, and hemodilution, among other potentially deleterious phenomena. There are morbidities that can be attributed to these entities, and off-pump bypass avoids those effects by mechanically stabilizing each coronary artery target individually, while the rest of the heart beats and supports normal physiologic circulation. There is an important caveat to this, however, and that is if – and perhaps only if – a complete revascularization with precise anastomoses can be accomplished off pump, then the patient will in fact benefit.
At Emory University in Atlanta, we did a prospective, randomized trial on my own patients, which we called SMART (Surgical Management of Arterial Revascularization) trial. What we found was that in 200 unselected, consecutive patients undergoing either off- or on-pump CABG, we had lower myocardial enzyme release, fewer transfusions, more rapid extubation, and a shorter length of stay in hospital with off-pump CABG.
Completeness of revascularization is a very important issue. In SMART, the number of grafts per patient was exactly the same: 3.39 per patient with off-pump CABG and 3.4 with on-pump CABG. We coined the phrase "Index of Completeness of Revascularization," which we defined as the number of grafts we planned to do in examining the arteriogram prior to randomization and surgery divided by the number of grafts we actually did. We found no difference here, meaning we were able to do the operation we planned to do (1.00 vs. 1.01; P = not significant). Moreover, for the lateral wall, which is technically more difficult to reach in a beating heart, the number was similar in the off- and on-pump groups (0.97 vs. 0.98; P = not significant). We also used a similar percentage of arterial grafts in both groups.
CPB was an independent predictor of the need for transfusion by multivariate analysis with an odds ratio of 2.42 (P = .0073) and was associated with a longer length of stay by 1 day (5.1 days for off-pump and 6.1 for on-pump; P = .005 Wilcoxon).
Creatine phosphokinase of muscle band and troponin I release was about half as much in the off-pump group as in the on-pump group (P less than .001 Wilcoxon), and the rates of death, stroke, MI, angina, and reintervention were similar at both 30 days and 1 year, as was graft patency and quality of life. Off-pump CABG costs at 1 year were $1,955 less than on-pump, but this difference was not statistically significant (P = .08).
At 8 years, survival in SMART was still similar between groups (P = .33), as was graft patency in the small number of patients who had CT angiograms. PET scan results similarly showed no significant difference in ischemia between these two groups (P = .62). One patient in each group has had a percutaneous reintervention, and none have had a repeat coronary bypass in 10 years.
To see if these results could be replicated nationally, we turned to the STS database and looked at North American centers that performed more than 100 on-pump CABGs and more than 100 off-pump surgeries. This gave us 42,477 patients (16,245 off pump and 26,232 on pump) at 63 North American centers. We included the 2.2% of off-pump cases that were converted to on-pump cases in the off-pump group.
After risk adjustment for 32 variables, for the outcomes of death, stroke, MI, and major adverse cardiac events, off-pump bypass outperformed on-pump bypass in this huge cohort of patients from around the country. Looking at less-significant outcomes – renal failure, dialysis, sternal infection, reoperation, atrial fibrillation, prolonged ventilation, and length of stay greater than 14 days – all of them favored off-pump bypass.
We then looked at the Emory dataset (14,766 consecutive patients, 48% of whom had off-pump CABG and 52% on-pump) to see which patients benefitted more. For patients in the two lower quartiles of predicted risk, there was no difference in operative mortality. In the higher two risk quartiles, there was a mortality benefit with off-pump CABG, with a risk reduction for operative mortality of about 55% in the highest risk patients (P less than 0.001).
Logistic regression confirmed that there was an interaction between surgery type and predicted risk, and we now know that low-risk patients do not have the survival benefit of avoiding CPB. They do fine with on-pump CABG, but higher risk patients have a benefit from avoiding CPB and the higher the predicted risk, the greater the benefit to the patient.
We went back to the STS database and we looked at whether this applies only at some centers or all centers, some surgeons or all surgeons. We looked at almost a million cases, 210,469 of which were at sites that had a large off-pump CABG volume. With the usual adjustments, off-pump CABG was associated with significant reduction of risk of death, stroke, renal failure, any morbidity or mortality or prolonged length of stay, compared with on-pump bypass. This benefit was even more pronounced after adjustment for surgeon effect. Once again, the greater reduction was enjoyed by those patients who had the highest preoperative risk. In all predicted risk quartiles, off pump bypass reduced risk of death and stroke and that magnitude increased with increasing predicted risk of mortality. This was seen in large-volume centers and low-volume centers.
Similar results were seen in a recent multicenter, randomized, prospective trial by Lemma et al. that assigned 411 patients to either off- or on-pump coronary bypass (J. Thorac. Cardiovasc. Surg. 2012;143:625-31). There was reduced early mortality and morbidity among higher-risk patients. Interestingly, in this study, they used an experience-based randomization scheme, in which they had surgeons within each center who like to do off-pump CABG and those who like to do on-pump CABG, and each surgeon had hundreds of cases under his belt. For the primary endpoint, a composite of death, MI, stroke or TIA, renal failure, acute respiratory distress syndrome, or reoperation for bleeding, the rates were 5.8% of off-pump and 13.3% for on-pump patients (odds ratio, 2.5; P = .01).
I think the conclusions are clear, but not everyone has reached these same conclusions. In ROOBY, the results were different. Although the study was well conducted, it enrolled low-risk patients, in whom avoidance of CPB was unlikely to improve the expected excellent outcomes. And the operations were performed by residents, with supervising attendings who themselves only had to do 20 total career off-pump cases to be eligible. I think this lack of experience is well demonstrated by the 12.5% conversion rate from off-pump cases to on-pump in that trial. ROOBY enrolled the wrong patients and used the wrong surgeons.
In the CORONARY trial, conducted by Dr. Lamy in Canada but enrolling patients from 19 countries outside of Canada, there was no difference in the primary endpoint of death, stroke, MI, and renal failure at 30 days, but there was a decrease in transfusion, reoperation for bleeding, acute kidney injury, or respiratory complications. There wasn\'t a difference in stroke, interestingly, but the surgeons in this trial appropriately converted a hundred patients from on pump to off pump to avoid manipulating a calcified aorta. This was a good, well-conducted trial.
However, the primary outcome in CORONARY differed when assessed according to EuroSCORE. When the EuroSCORE was low, on pump outperformed off pump. When the EuroSCORE was high, off pump outperformed on pump.
These two trials offer important perspective: the ROOBY trial, enrolling low-risk patients, was actually in favor of on-pump CABG. The CORONARY trial, enrolling higher-risk patients, had a slight benefit in favor of off-pump CABG, and this was particularly evident in the Canadian cohort of 830 randomized patients, in which the primary outcome was, in fact, statistically significantly better in the off-pump group at 9.2% vs. 13.7%.
At the end of the day, I think it matters in whom you do off-pump CABG and how well you do it. It may not be for every patient or for every surgeon; off-pump CABG requires a focused and sustained effort to master a new set of physical and psychological skills to accomplish precise and complete revascularization. When we can do this, I think, we offer better outcomes for our patients.
Dr. John Puskas is the chairman of the department of cardiac surgery at Mount Sinai Beth Israel in New York. Dr. Puskas disclosed that he also does both on- and off-pump CABG. He received royalties from coronary surgical instruments marketed by Scanlan, as well.
References
JAMA 2004;291:1841-9
Ann. Thorac. Surg. 2009;88:1142-7
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2010;362:851
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Ann. Thorac. Surg. 2011;91:1836-42
J. Thorac. Cardiovasc. Surg. 2012;143:625-31
YES
I believe on-pump coronary artery bypass graft (CABG) surgery should be our primary operation, with off-pump CABG reserved only for certain situations.
In talking about these two surgical options, there are basically three main issues of interest: procedural outcomes, the quality of revascularization, and long-term effectiveness.
Procedural outcomes: In the 1990s, there was great excitement for the potential for off-pump CABG. We thought survival would be better, stroke less common, neurocognitive outcomes improved, etc., but after several randomized studies and a number of well-controlled observational studies, we found that, in terms of the important procedural outcomes – death, MI, stroke, and acute renal failure – there were actually no differences between off- and on-pump CABG. We saw this in ROOBY, with low-risk patients and less experienced surgeons; in the Canadian CORONARY trial, with experienced off-pump surgeons; and in the European GOPCABE trial, where the surgeons were very experienced and the patients were high risk.
There are some benefits with off-pump CABG in terms of what I call the "reversible" complications of surgery: fewer transfusions, less postoperative atrial fibrillation, fewer respiratory infections; but I believe these things have to be balanced against some of the negative effects of off-pump surgery in terms of its quality of revascularization and long-term effectiveness.
Probably one of the biggest disappointments we’ve had with off-pump CABG is that, in the trials, we didn’t see any major difference in neurocognitive dysfunction. What we learned is that cardiopulmonary bypass really is not that bad in terms of neurocognitive dysfunction after surgery.
One issue that tends to be forgotten is the risk associated with conversion. In a hemodynamically unstable patient, converting from off-pump to on-pump is associated with higher risks of death, bleeding, renal failure, stroke, respiratory failure, and GI complications. In CORONARY, with experienced surgeons, the conversion rate was almost 7.9%, and in GOPCABE, it was 9.7%. Even with experienced surgeons, conversions do occur, and they have negative consequences.
Quality of revascularization: We know that long-term survival is related to completeness of revascularization. The more ischemic myocardium that is left at risk after CABG, the more likely the patients is to have an MI or die, and this has been very clearly demonstrated in the surgical literature as well as the percutaneous coronary intervention literature. It has been shown multiple times that fewer grafts are done in off-pump patients, compared with on-pump patients. Indeed, in a recent Cochrane meta-analysis of more than 7,000 patients in 57 trials, there were significantly more grafts done in on-pump than off-pump cases.
Also in terms of graft patency, again, multiple studies have shown lower graft patency in off-pump patients, either directly or by means of showing a higher reintervention rate after off-pump as compared with on-pump surgery. In both CORONARY and GOPCABE, the repeat revascularization rate at 30 days was higher for off-pump CABG.
Long-term effectiveness: The long-term results from ROOBY are very sobering: significantly higher 1-year cardiac mortality in the off-pump arm and higher 1-year composite adverse events. Again, in the 2012 Cochrane meta-analysis, with more than 10,000 patients in 75 trials, mortality was significantly higher with off-pump patients, compared with on-pump, with a hazard ratio of 1.24 (95% CI, 1.01-1.53). When the authors of the Cochrane meta-analysis removed what they called the "studies with bias," this signal was even stronger. Also in observational studies, such as one published by Racz et al. in 2004 (J. Am. Coll. Cardiol. 2004; 43: 557-64), the best survival was found to be in patients who had on-pump CABG and the worst in those who had undergone off-pump CABG.
In summary, procedural outcomes with off-pump and on-pump CABG are similar, albeit with lower reversible complications in the off-pump patients, but a greater conversion risk, along with its associated complications. The quality of the revascularization is worse in off-pump patients, the completeness of revascularization is less and re-intervention rates are higher, and the bottom line, of course, it that there is higher long-term mortality after off-pump CABG.
"Should off-pump CABG be abandoned?" As our default procedure, yes. The vast majority of our patients are best served with on-pump revascularization.
Dr. Joseph Sabik is the chairman of the department of thoracic and cardiovascular surgery and the Sheik Hamdam Bin Rashid Al Maktoum Distinguished Chair at the Cleveland Clinic. He disclosed that he performs both off-pump and on-pump CABG.
References
Anesthesiology 2005;102:188-203
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Cochrane Database Syst. Rev. 2012; Mar 14. 3: CD007224 [doi:10.1002/14651858.CD007224.pub2])
J. Am. Coll. Cardiol. 2004;43:557-64
NO
Should off pump bypass be abandoned? Absolutely not, but let’s do it well.
The rationale for why off-pump CABG should be the preferred strategy is simple: cardiopulmonary bypass (CPB) entails extracorporeal circulation, aortic cannulation and clamping, global MI, hypothermia, and hemodilution, among other potentially deleterious phenomena. There are morbidities that can be attributed to these entities, and off-pump bypass avoids those effects by mechanically stabilizing each coronary artery target individually, while the rest of the heart beats and supports normal physiologic circulation. There is an important caveat to this, however, and that is if – and perhaps only if – a complete revascularization with precise anastomoses can be accomplished off pump, then the patient will in fact benefit.
At Emory University in Atlanta, we did a prospective, randomized trial on my own patients, which we called SMART (Surgical Management of Arterial Revascularization) trial. What we found was that in 200 unselected, consecutive patients undergoing either off- or on-pump CABG, we had lower myocardial enzyme release, fewer transfusions, more rapid extubation, and a shorter length of stay in hospital with off-pump CABG.
Completeness of revascularization is a very important issue. In SMART, the number of grafts per patient was exactly the same: 3.39 per patient with off-pump CABG and 3.4 with on-pump CABG. We coined the phrase "Index of Completeness of Revascularization," which we defined as the number of grafts we planned to do in examining the arteriogram prior to randomization and surgery divided by the number of grafts we actually did. We found no difference here, meaning we were able to do the operation we planned to do (1.00 vs. 1.01; P = not significant). Moreover, for the lateral wall, which is technically more difficult to reach in a beating heart, the number was similar in the off- and on-pump groups (0.97 vs. 0.98; P = not significant). We also used a similar percentage of arterial grafts in both groups.
CPB was an independent predictor of the need for transfusion by multivariate analysis with an odds ratio of 2.42 (P = .0073) and was associated with a longer length of stay by 1 day (5.1 days for off-pump and 6.1 for on-pump; P = .005 Wilcoxon).
Creatine phosphokinase of muscle band and troponin I release was about half as much in the off-pump group as in the on-pump group (P less than .001 Wilcoxon), and the rates of death, stroke, MI, angina, and reintervention were similar at both 30 days and 1 year, as was graft patency and quality of life. Off-pump CABG costs at 1 year were $1,955 less than on-pump, but this difference was not statistically significant (P = .08).
At 8 years, survival in SMART was still similar between groups (P = .33), as was graft patency in the small number of patients who had CT angiograms. PET scan results similarly showed no significant difference in ischemia between these two groups (P = .62). One patient in each group has had a percutaneous reintervention, and none have had a repeat coronary bypass in 10 years.
To see if these results could be replicated nationally, we turned to the STS database and looked at North American centers that performed more than 100 on-pump CABGs and more than 100 off-pump surgeries. This gave us 42,477 patients (16,245 off pump and 26,232 on pump) at 63 North American centers. We included the 2.2% of off-pump cases that were converted to on-pump cases in the off-pump group.
After risk adjustment for 32 variables, for the outcomes of death, stroke, MI, and major adverse cardiac events, off-pump bypass outperformed on-pump bypass in this huge cohort of patients from around the country. Looking at less-significant outcomes – renal failure, dialysis, sternal infection, reoperation, atrial fibrillation, prolonged ventilation, and length of stay greater than 14 days – all of them favored off-pump bypass.
We then looked at the Emory dataset (14,766 consecutive patients, 48% of whom had off-pump CABG and 52% on-pump) to see which patients benefitted more. For patients in the two lower quartiles of predicted risk, there was no difference in operative mortality. In the higher two risk quartiles, there was a mortality benefit with off-pump CABG, with a risk reduction for operative mortality of about 55% in the highest risk patients (P less than 0.001).
Logistic regression confirmed that there was an interaction between surgery type and predicted risk, and we now know that low-risk patients do not have the survival benefit of avoiding CPB. They do fine with on-pump CABG, but higher risk patients have a benefit from avoiding CPB and the higher the predicted risk, the greater the benefit to the patient.
We went back to the STS database and we looked at whether this applies only at some centers or all centers, some surgeons or all surgeons. We looked at almost a million cases, 210,469 of which were at sites that had a large off-pump CABG volume. With the usual adjustments, off-pump CABG was associated with significant reduction of risk of death, stroke, renal failure, any morbidity or mortality or prolonged length of stay, compared with on-pump bypass. This benefit was even more pronounced after adjustment for surgeon effect. Once again, the greater reduction was enjoyed by those patients who had the highest preoperative risk. In all predicted risk quartiles, off pump bypass reduced risk of death and stroke and that magnitude increased with increasing predicted risk of mortality. This was seen in large-volume centers and low-volume centers.
Similar results were seen in a recent multicenter, randomized, prospective trial by Lemma et al. that assigned 411 patients to either off- or on-pump coronary bypass (J. Thorac. Cardiovasc. Surg. 2012;143:625-31). There was reduced early mortality and morbidity among higher-risk patients. Interestingly, in this study, they used an experience-based randomization scheme, in which they had surgeons within each center who like to do off-pump CABG and those who like to do on-pump CABG, and each surgeon had hundreds of cases under his belt. For the primary endpoint, a composite of death, MI, stroke or TIA, renal failure, acute respiratory distress syndrome, or reoperation for bleeding, the rates were 5.8% of off-pump and 13.3% for on-pump patients (odds ratio, 2.5; P = .01).
I think the conclusions are clear, but not everyone has reached these same conclusions. In ROOBY, the results were different. Although the study was well conducted, it enrolled low-risk patients, in whom avoidance of CPB was unlikely to improve the expected excellent outcomes. And the operations were performed by residents, with supervising attendings who themselves only had to do 20 total career off-pump cases to be eligible. I think this lack of experience is well demonstrated by the 12.5% conversion rate from off-pump cases to on-pump in that trial. ROOBY enrolled the wrong patients and used the wrong surgeons.
In the CORONARY trial, conducted by Dr. Lamy in Canada but enrolling patients from 19 countries outside of Canada, there was no difference in the primary endpoint of death, stroke, MI, and renal failure at 30 days, but there was a decrease in transfusion, reoperation for bleeding, acute kidney injury, or respiratory complications. There wasn\'t a difference in stroke, interestingly, but the surgeons in this trial appropriately converted a hundred patients from on pump to off pump to avoid manipulating a calcified aorta. This was a good, well-conducted trial.
However, the primary outcome in CORONARY differed when assessed according to EuroSCORE. When the EuroSCORE was low, on pump outperformed off pump. When the EuroSCORE was high, off pump outperformed on pump.
These two trials offer important perspective: the ROOBY trial, enrolling low-risk patients, was actually in favor of on-pump CABG. The CORONARY trial, enrolling higher-risk patients, had a slight benefit in favor of off-pump CABG, and this was particularly evident in the Canadian cohort of 830 randomized patients, in which the primary outcome was, in fact, statistically significantly better in the off-pump group at 9.2% vs. 13.7%.
At the end of the day, I think it matters in whom you do off-pump CABG and how well you do it. It may not be for every patient or for every surgeon; off-pump CABG requires a focused and sustained effort to master a new set of physical and psychological skills to accomplish precise and complete revascularization. When we can do this, I think, we offer better outcomes for our patients.
Dr. John Puskas is the chairman of the department of cardiac surgery at Mount Sinai Beth Israel in New York. Dr. Puskas disclosed that he also does both on- and off-pump CABG. He received royalties from coronary surgical instruments marketed by Scanlan, as well.
References
JAMA 2004;291:1841-9
Ann. Thorac. Surg. 2009;88:1142-7
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2010;362:851
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Ann. Thorac. Surg. 2011;91:1836-42
J. Thorac. Cardiovasc. Surg. 2012;143:625-31
YES
I believe on-pump coronary artery bypass graft (CABG) surgery should be our primary operation, with off-pump CABG reserved only for certain situations.
In talking about these two surgical options, there are basically three main issues of interest: procedural outcomes, the quality of revascularization, and long-term effectiveness.
Procedural outcomes: In the 1990s, there was great excitement for the potential for off-pump CABG. We thought survival would be better, stroke less common, neurocognitive outcomes improved, etc., but after several randomized studies and a number of well-controlled observational studies, we found that, in terms of the important procedural outcomes – death, MI, stroke, and acute renal failure – there were actually no differences between off- and on-pump CABG. We saw this in ROOBY, with low-risk patients and less experienced surgeons; in the Canadian CORONARY trial, with experienced off-pump surgeons; and in the European GOPCABE trial, where the surgeons were very experienced and the patients were high risk.
There are some benefits with off-pump CABG in terms of what I call the "reversible" complications of surgery: fewer transfusions, less postoperative atrial fibrillation, fewer respiratory infections; but I believe these things have to be balanced against some of the negative effects of off-pump surgery in terms of its quality of revascularization and long-term effectiveness.
Probably one of the biggest disappointments we’ve had with off-pump CABG is that, in the trials, we didn’t see any major difference in neurocognitive dysfunction. What we learned is that cardiopulmonary bypass really is not that bad in terms of neurocognitive dysfunction after surgery.
One issue that tends to be forgotten is the risk associated with conversion. In a hemodynamically unstable patient, converting from off-pump to on-pump is associated with higher risks of death, bleeding, renal failure, stroke, respiratory failure, and GI complications. In CORONARY, with experienced surgeons, the conversion rate was almost 7.9%, and in GOPCABE, it was 9.7%. Even with experienced surgeons, conversions do occur, and they have negative consequences.
Quality of revascularization: We know that long-term survival is related to completeness of revascularization. The more ischemic myocardium that is left at risk after CABG, the more likely the patients is to have an MI or die, and this has been very clearly demonstrated in the surgical literature as well as the percutaneous coronary intervention literature. It has been shown multiple times that fewer grafts are done in off-pump patients, compared with on-pump patients. Indeed, in a recent Cochrane meta-analysis of more than 7,000 patients in 57 trials, there were significantly more grafts done in on-pump than off-pump cases.
Also in terms of graft patency, again, multiple studies have shown lower graft patency in off-pump patients, either directly or by means of showing a higher reintervention rate after off-pump as compared with on-pump surgery. In both CORONARY and GOPCABE, the repeat revascularization rate at 30 days was higher for off-pump CABG.
Long-term effectiveness: The long-term results from ROOBY are very sobering: significantly higher 1-year cardiac mortality in the off-pump arm and higher 1-year composite adverse events. Again, in the 2012 Cochrane meta-analysis, with more than 10,000 patients in 75 trials, mortality was significantly higher with off-pump patients, compared with on-pump, with a hazard ratio of 1.24 (95% CI, 1.01-1.53). When the authors of the Cochrane meta-analysis removed what they called the "studies with bias," this signal was even stronger. Also in observational studies, such as one published by Racz et al. in 2004 (J. Am. Coll. Cardiol. 2004; 43: 557-64), the best survival was found to be in patients who had on-pump CABG and the worst in those who had undergone off-pump CABG.
In summary, procedural outcomes with off-pump and on-pump CABG are similar, albeit with lower reversible complications in the off-pump patients, but a greater conversion risk, along with its associated complications. The quality of the revascularization is worse in off-pump patients, the completeness of revascularization is less and re-intervention rates are higher, and the bottom line, of course, it that there is higher long-term mortality after off-pump CABG.
"Should off-pump CABG be abandoned?" As our default procedure, yes. The vast majority of our patients are best served with on-pump revascularization.
Dr. Joseph Sabik is the chairman of the department of thoracic and cardiovascular surgery and the Sheik Hamdam Bin Rashid Al Maktoum Distinguished Chair at the Cleveland Clinic. He disclosed that he performs both off-pump and on-pump CABG.
References
Anesthesiology 2005;102:188-203
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Cochrane Database Syst. Rev. 2012; Mar 14. 3: CD007224 [doi:10.1002/14651858.CD007224.pub2])
J. Am. Coll. Cardiol. 2004;43:557-64
NO
Should off pump bypass be abandoned? Absolutely not, but let’s do it well.
The rationale for why off-pump CABG should be the preferred strategy is simple: cardiopulmonary bypass (CPB) entails extracorporeal circulation, aortic cannulation and clamping, global MI, hypothermia, and hemodilution, among other potentially deleterious phenomena. There are morbidities that can be attributed to these entities, and off-pump bypass avoids those effects by mechanically stabilizing each coronary artery target individually, while the rest of the heart beats and supports normal physiologic circulation. There is an important caveat to this, however, and that is if – and perhaps only if – a complete revascularization with precise anastomoses can be accomplished off pump, then the patient will in fact benefit.
At Emory University in Atlanta, we did a prospective, randomized trial on my own patients, which we called SMART (Surgical Management of Arterial Revascularization) trial. What we found was that in 200 unselected, consecutive patients undergoing either off- or on-pump CABG, we had lower myocardial enzyme release, fewer transfusions, more rapid extubation, and a shorter length of stay in hospital with off-pump CABG.
Completeness of revascularization is a very important issue. In SMART, the number of grafts per patient was exactly the same: 3.39 per patient with off-pump CABG and 3.4 with on-pump CABG. We coined the phrase "Index of Completeness of Revascularization," which we defined as the number of grafts we planned to do in examining the arteriogram prior to randomization and surgery divided by the number of grafts we actually did. We found no difference here, meaning we were able to do the operation we planned to do (1.00 vs. 1.01; P = not significant). Moreover, for the lateral wall, which is technically more difficult to reach in a beating heart, the number was similar in the off- and on-pump groups (0.97 vs. 0.98; P = not significant). We also used a similar percentage of arterial grafts in both groups.
CPB was an independent predictor of the need for transfusion by multivariate analysis with an odds ratio of 2.42 (P = .0073) and was associated with a longer length of stay by 1 day (5.1 days for off-pump and 6.1 for on-pump; P = .005 Wilcoxon).
Creatine phosphokinase of muscle band and troponin I release was about half as much in the off-pump group as in the on-pump group (P less than .001 Wilcoxon), and the rates of death, stroke, MI, angina, and reintervention were similar at both 30 days and 1 year, as was graft patency and quality of life. Off-pump CABG costs at 1 year were $1,955 less than on-pump, but this difference was not statistically significant (P = .08).
At 8 years, survival in SMART was still similar between groups (P = .33), as was graft patency in the small number of patients who had CT angiograms. PET scan results similarly showed no significant difference in ischemia between these two groups (P = .62). One patient in each group has had a percutaneous reintervention, and none have had a repeat coronary bypass in 10 years.
To see if these results could be replicated nationally, we turned to the STS database and looked at North American centers that performed more than 100 on-pump CABGs and more than 100 off-pump surgeries. This gave us 42,477 patients (16,245 off pump and 26,232 on pump) at 63 North American centers. We included the 2.2% of off-pump cases that were converted to on-pump cases in the off-pump group.
After risk adjustment for 32 variables, for the outcomes of death, stroke, MI, and major adverse cardiac events, off-pump bypass outperformed on-pump bypass in this huge cohort of patients from around the country. Looking at less-significant outcomes – renal failure, dialysis, sternal infection, reoperation, atrial fibrillation, prolonged ventilation, and length of stay greater than 14 days – all of them favored off-pump bypass.
We then looked at the Emory dataset (14,766 consecutive patients, 48% of whom had off-pump CABG and 52% on-pump) to see which patients benefitted more. For patients in the two lower quartiles of predicted risk, there was no difference in operative mortality. In the higher two risk quartiles, there was a mortality benefit with off-pump CABG, with a risk reduction for operative mortality of about 55% in the highest risk patients (P less than 0.001).
Logistic regression confirmed that there was an interaction between surgery type and predicted risk, and we now know that low-risk patients do not have the survival benefit of avoiding CPB. They do fine with on-pump CABG, but higher risk patients have a benefit from avoiding CPB and the higher the predicted risk, the greater the benefit to the patient.
We went back to the STS database and we looked at whether this applies only at some centers or all centers, some surgeons or all surgeons. We looked at almost a million cases, 210,469 of which were at sites that had a large off-pump CABG volume. With the usual adjustments, off-pump CABG was associated with significant reduction of risk of death, stroke, renal failure, any morbidity or mortality or prolonged length of stay, compared with on-pump bypass. This benefit was even more pronounced after adjustment for surgeon effect. Once again, the greater reduction was enjoyed by those patients who had the highest preoperative risk. In all predicted risk quartiles, off pump bypass reduced risk of death and stroke and that magnitude increased with increasing predicted risk of mortality. This was seen in large-volume centers and low-volume centers.
Similar results were seen in a recent multicenter, randomized, prospective trial by Lemma et al. that assigned 411 patients to either off- or on-pump coronary bypass (J. Thorac. Cardiovasc. Surg. 2012;143:625-31). There was reduced early mortality and morbidity among higher-risk patients. Interestingly, in this study, they used an experience-based randomization scheme, in which they had surgeons within each center who like to do off-pump CABG and those who like to do on-pump CABG, and each surgeon had hundreds of cases under his belt. For the primary endpoint, a composite of death, MI, stroke or TIA, renal failure, acute respiratory distress syndrome, or reoperation for bleeding, the rates were 5.8% of off-pump and 13.3% for on-pump patients (odds ratio, 2.5; P = .01).
I think the conclusions are clear, but not everyone has reached these same conclusions. In ROOBY, the results were different. Although the study was well conducted, it enrolled low-risk patients, in whom avoidance of CPB was unlikely to improve the expected excellent outcomes. And the operations were performed by residents, with supervising attendings who themselves only had to do 20 total career off-pump cases to be eligible. I think this lack of experience is well demonstrated by the 12.5% conversion rate from off-pump cases to on-pump in that trial. ROOBY enrolled the wrong patients and used the wrong surgeons.
In the CORONARY trial, conducted by Dr. Lamy in Canada but enrolling patients from 19 countries outside of Canada, there was no difference in the primary endpoint of death, stroke, MI, and renal failure at 30 days, but there was a decrease in transfusion, reoperation for bleeding, acute kidney injury, or respiratory complications. There wasn\'t a difference in stroke, interestingly, but the surgeons in this trial appropriately converted a hundred patients from on pump to off pump to avoid manipulating a calcified aorta. This was a good, well-conducted trial.
However, the primary outcome in CORONARY differed when assessed according to EuroSCORE. When the EuroSCORE was low, on pump outperformed off pump. When the EuroSCORE was high, off pump outperformed on pump.
These two trials offer important perspective: the ROOBY trial, enrolling low-risk patients, was actually in favor of on-pump CABG. The CORONARY trial, enrolling higher-risk patients, had a slight benefit in favor of off-pump CABG, and this was particularly evident in the Canadian cohort of 830 randomized patients, in which the primary outcome was, in fact, statistically significantly better in the off-pump group at 9.2% vs. 13.7%.
At the end of the day, I think it matters in whom you do off-pump CABG and how well you do it. It may not be for every patient or for every surgeon; off-pump CABG requires a focused and sustained effort to master a new set of physical and psychological skills to accomplish precise and complete revascularization. When we can do this, I think, we offer better outcomes for our patients.
Dr. John Puskas is the chairman of the department of cardiac surgery at Mount Sinai Beth Israel in New York. Dr. Puskas disclosed that he also does both on- and off-pump CABG. He received royalties from coronary surgical instruments marketed by Scanlan, as well.
References
JAMA 2004;291:1841-9
Ann. Thorac. Surg. 2009;88:1142-7
N. Engl. J. Med. 2009;361:1827-37
N. Engl. J. Med. 2010;362:851
N. Engl. J. Med. 2012;366:1489-97
N. Engl. J. Med. 2013;368:1179-88
Ann. Thorac. Surg. 2011;91:1836-42
J. Thorac. Cardiovasc. Surg. 2012;143:625-31
The FREEDOM trial: In appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
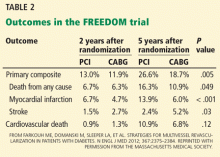
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
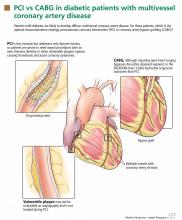
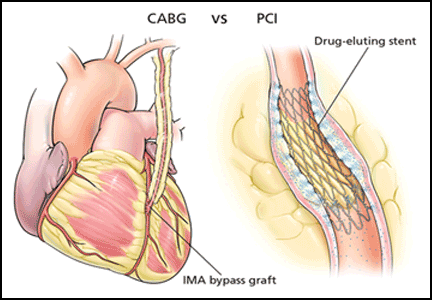
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
KEY POINTS
- Patients with diabetes have a higher prevalence of multivessel coronary artery disease and often have complex, diffuse lesions.
- Bypass surgery is the preferred method of revascularization in appropriately selected patients with diabetes and multivessel coronary artery disease.
- In the FREEDOM trial, only about 10% of the screened patients were eligible for the study, limiting its generalizability; however, this is comparable to exclusion rates in previous large randomized trials.
- When choosing a revascularization method, the physician team needs to discuss the options with the patient before performing diagnostic angiography. The team should include a cardiac surgeon and a cardiologist.