User login
Is yearly chest x-ray screening helpful in reducing mortality for smokers?
For current and former smokers, the evidence does not support yearly chest x-rays to decrease lung cancer mortality (strength of recommendation [SOR]: A, based on multiple randomized controlledtrials). Even with the addition of sputum cytology and more frequent chest x-rays, lung cancer mortality was unchanged (SOR: A).
Reduce morbidity and mortality by helping patients quit smoking
The bottom line is that morbidity and mortality are not reduced when we use chest x-rays, sputum cytology, or a combination of the 2 in screening for lung cancer. One thing we can do for our patients is counsel them about the ill effects of tobacco use and support them in their smoking cessation efforts. Although there is no guarantee that those who quit will not get lung cancer, cessation certainly reduces the risk and brings other health and financial benefits.
Of interest is the ongoing National Lung Screening trial, which compares screening spiral CT scans with chest x-rays in the detection of lung cancer. This large trial, sponsored by the NCI, will compare both modalities over 8 years and should help determine if either test is better at reducing morbidity and mortality from this disease.
Evidence summary
Five randomized controlled trials have examined lung cancer mortality after screening chest x-rays. In the first trial—the only one that included former as well as current smokers and nonsmokers—subjects were randomized to undergo chest x-ray studies every 6 months, or at baseline and again at the end of the 3-year study. After 3 years, there was no statistically significant mortality difference with more frequent chest x-rays.1,2
Another trial involved male smokers who were randomized to undergo chest x-ray and sputum cytology either every 6 months or after 3 years. After 3 years, both groups were screened annually with chest x-ray alone for an additional 3 years. There was no significant difference in lung cancer mortality at any point, including at a 15-year post-trial follow-up.3Both studies showed earlier detection and longer survivorship of lung cancer among screened vs nonscreened groups due to lead-time bias (because the cancer was detected earlier from screening vs clinical diagnosis, it falsely appears to prolong survival). Overall mortality was the same in both groups.
The National Cancer Institute (NCI) sponsored 3 randomized controlled trials on lung cancer screening for male smokers involving 3 major medical centers. The studies were designed to determine the incremental benefit of adding sputum cytology to chest x-ray screening. In 2 of the NCI studies, participants were randomly assigned to receive annual chest x-ray only or a dual screen with annual chest x-ray and sputum cytologies every 4 months. In both studies, there was no statistical difference in lung cancer mortality between the 2 groups.4-6The third NCI study randomized participants to chest x-ray and sputum cytology either every 4 months or annually. Again, there was no significant difference in lung cancer mortality,4even after an extended follow-up of 20.5 years.7Adding sputum cytology to chest x-ray only improved lung cancer detection rates over chest x-ray alone.
A significant limitation of the 5 studies presented is that no true control or non-screening groups determined the real efficacy of screening chest x-rays vs no screening. The goal of a study of a screening program is to detect a disease early enough so that treatment can alter mortality. These uncontrolled studies of routine screening chest x-rays, no matter how frequently performed, do not meet this criteria for current and former smokers.
Recommendations from others
The US Preventive Services Task Force does not recommend for or against screening asymptomatic or high-risk persons for lung cancer with either low-dose computed tomography (CT), chest x-ray, sputum cytology, or a combination of these tests.8 The American Cancer Society and American Academy of Family Physicians recommend against the use of chest x-ray or sputum cytology in asymptomatic high-risk persons.9,10The American College of Chest Physicians recommends against the use of serial chest x-rays for individuals without symptoms or without a history of cancer.11 They do not comment about high-risk groups—that is, current or former smokers.
1. Humphrey LL, Teautsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: An update for the US Preventive Task Force. Ann Intern Med 2004;140:740-755.
2. Brett GZ. The value of six-monthly chest radiographs. Thorax 1968;23:414-420.
3. Kubik AK, Parkin DM, Zatloukal P. Czech study on lung cancer screening: Post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer 2000;89:2363-2368.
4. Bach PB, Kelley MJ, Tate RC, McCrory DC. Screening for lung cancer: A review of the current literature. Chest 2003;123:72S-82S.
5. Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer: Results of the Memorial Sloan-Kettering Study in New York. Czest 1984;86:44-53.
6. Tockman MS. Survival and mortality from lung cancer in a screened population: The John Hopkins Study. Chest 1986;89:342S-325S.
7. Marcus PM, Bergstralh EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-1316.
8. US Preventive Services Task Force. Lung Cancer Screening: Recommendation Statement. Ann Intern Med 2004;140:738-739.
9. Smith RA, Mettlin CJ, Davis KJ, Eyre H. American Cancer Society Guidelines for the Early Detection of Cancer. Available at: www.cancer.org/docroot/PUB/content/PUB_3_8X_American_Cancer_Society_Guideli nes_for_the_Early_Detection_of_Cancer_update_2001.asp. Accessed on August 12, 2005.
10. Summary of Policy Recommendations for Periodic Health Exams. AAFP Policy Action 2004. Available at: www.aafp.org/x24974.xml. Accessed on August 12, 2005.
11. Bach PB, Niewoehner DE, Black WC. Screening for Lung Cancer: The Guidelines. Chest 2003;123:83S-88S.
For current and former smokers, the evidence does not support yearly chest x-rays to decrease lung cancer mortality (strength of recommendation [SOR]: A, based on multiple randomized controlledtrials). Even with the addition of sputum cytology and more frequent chest x-rays, lung cancer mortality was unchanged (SOR: A).
Reduce morbidity and mortality by helping patients quit smoking
The bottom line is that morbidity and mortality are not reduced when we use chest x-rays, sputum cytology, or a combination of the 2 in screening for lung cancer. One thing we can do for our patients is counsel them about the ill effects of tobacco use and support them in their smoking cessation efforts. Although there is no guarantee that those who quit will not get lung cancer, cessation certainly reduces the risk and brings other health and financial benefits.
Of interest is the ongoing National Lung Screening trial, which compares screening spiral CT scans with chest x-rays in the detection of lung cancer. This large trial, sponsored by the NCI, will compare both modalities over 8 years and should help determine if either test is better at reducing morbidity and mortality from this disease.
Evidence summary
Five randomized controlled trials have examined lung cancer mortality after screening chest x-rays. In the first trial—the only one that included former as well as current smokers and nonsmokers—subjects were randomized to undergo chest x-ray studies every 6 months, or at baseline and again at the end of the 3-year study. After 3 years, there was no statistically significant mortality difference with more frequent chest x-rays.1,2
Another trial involved male smokers who were randomized to undergo chest x-ray and sputum cytology either every 6 months or after 3 years. After 3 years, both groups were screened annually with chest x-ray alone for an additional 3 years. There was no significant difference in lung cancer mortality at any point, including at a 15-year post-trial follow-up.3Both studies showed earlier detection and longer survivorship of lung cancer among screened vs nonscreened groups due to lead-time bias (because the cancer was detected earlier from screening vs clinical diagnosis, it falsely appears to prolong survival). Overall mortality was the same in both groups.
The National Cancer Institute (NCI) sponsored 3 randomized controlled trials on lung cancer screening for male smokers involving 3 major medical centers. The studies were designed to determine the incremental benefit of adding sputum cytology to chest x-ray screening. In 2 of the NCI studies, participants were randomly assigned to receive annual chest x-ray only or a dual screen with annual chest x-ray and sputum cytologies every 4 months. In both studies, there was no statistical difference in lung cancer mortality between the 2 groups.4-6The third NCI study randomized participants to chest x-ray and sputum cytology either every 4 months or annually. Again, there was no significant difference in lung cancer mortality,4even after an extended follow-up of 20.5 years.7Adding sputum cytology to chest x-ray only improved lung cancer detection rates over chest x-ray alone.
A significant limitation of the 5 studies presented is that no true control or non-screening groups determined the real efficacy of screening chest x-rays vs no screening. The goal of a study of a screening program is to detect a disease early enough so that treatment can alter mortality. These uncontrolled studies of routine screening chest x-rays, no matter how frequently performed, do not meet this criteria for current and former smokers.
Recommendations from others
The US Preventive Services Task Force does not recommend for or against screening asymptomatic or high-risk persons for lung cancer with either low-dose computed tomography (CT), chest x-ray, sputum cytology, or a combination of these tests.8 The American Cancer Society and American Academy of Family Physicians recommend against the use of chest x-ray or sputum cytology in asymptomatic high-risk persons.9,10The American College of Chest Physicians recommends against the use of serial chest x-rays for individuals without symptoms or without a history of cancer.11 They do not comment about high-risk groups—that is, current or former smokers.
For current and former smokers, the evidence does not support yearly chest x-rays to decrease lung cancer mortality (strength of recommendation [SOR]: A, based on multiple randomized controlledtrials). Even with the addition of sputum cytology and more frequent chest x-rays, lung cancer mortality was unchanged (SOR: A).
Reduce morbidity and mortality by helping patients quit smoking
The bottom line is that morbidity and mortality are not reduced when we use chest x-rays, sputum cytology, or a combination of the 2 in screening for lung cancer. One thing we can do for our patients is counsel them about the ill effects of tobacco use and support them in their smoking cessation efforts. Although there is no guarantee that those who quit will not get lung cancer, cessation certainly reduces the risk and brings other health and financial benefits.
Of interest is the ongoing National Lung Screening trial, which compares screening spiral CT scans with chest x-rays in the detection of lung cancer. This large trial, sponsored by the NCI, will compare both modalities over 8 years and should help determine if either test is better at reducing morbidity and mortality from this disease.
Evidence summary
Five randomized controlled trials have examined lung cancer mortality after screening chest x-rays. In the first trial—the only one that included former as well as current smokers and nonsmokers—subjects were randomized to undergo chest x-ray studies every 6 months, or at baseline and again at the end of the 3-year study. After 3 years, there was no statistically significant mortality difference with more frequent chest x-rays.1,2
Another trial involved male smokers who were randomized to undergo chest x-ray and sputum cytology either every 6 months or after 3 years. After 3 years, both groups were screened annually with chest x-ray alone for an additional 3 years. There was no significant difference in lung cancer mortality at any point, including at a 15-year post-trial follow-up.3Both studies showed earlier detection and longer survivorship of lung cancer among screened vs nonscreened groups due to lead-time bias (because the cancer was detected earlier from screening vs clinical diagnosis, it falsely appears to prolong survival). Overall mortality was the same in both groups.
The National Cancer Institute (NCI) sponsored 3 randomized controlled trials on lung cancer screening for male smokers involving 3 major medical centers. The studies were designed to determine the incremental benefit of adding sputum cytology to chest x-ray screening. In 2 of the NCI studies, participants were randomly assigned to receive annual chest x-ray only or a dual screen with annual chest x-ray and sputum cytologies every 4 months. In both studies, there was no statistical difference in lung cancer mortality between the 2 groups.4-6The third NCI study randomized participants to chest x-ray and sputum cytology either every 4 months or annually. Again, there was no significant difference in lung cancer mortality,4even after an extended follow-up of 20.5 years.7Adding sputum cytology to chest x-ray only improved lung cancer detection rates over chest x-ray alone.
A significant limitation of the 5 studies presented is that no true control or non-screening groups determined the real efficacy of screening chest x-rays vs no screening. The goal of a study of a screening program is to detect a disease early enough so that treatment can alter mortality. These uncontrolled studies of routine screening chest x-rays, no matter how frequently performed, do not meet this criteria for current and former smokers.
Recommendations from others
The US Preventive Services Task Force does not recommend for or against screening asymptomatic or high-risk persons for lung cancer with either low-dose computed tomography (CT), chest x-ray, sputum cytology, or a combination of these tests.8 The American Cancer Society and American Academy of Family Physicians recommend against the use of chest x-ray or sputum cytology in asymptomatic high-risk persons.9,10The American College of Chest Physicians recommends against the use of serial chest x-rays for individuals without symptoms or without a history of cancer.11 They do not comment about high-risk groups—that is, current or former smokers.
1. Humphrey LL, Teautsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: An update for the US Preventive Task Force. Ann Intern Med 2004;140:740-755.
2. Brett GZ. The value of six-monthly chest radiographs. Thorax 1968;23:414-420.
3. Kubik AK, Parkin DM, Zatloukal P. Czech study on lung cancer screening: Post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer 2000;89:2363-2368.
4. Bach PB, Kelley MJ, Tate RC, McCrory DC. Screening for lung cancer: A review of the current literature. Chest 2003;123:72S-82S.
5. Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer: Results of the Memorial Sloan-Kettering Study in New York. Czest 1984;86:44-53.
6. Tockman MS. Survival and mortality from lung cancer in a screened population: The John Hopkins Study. Chest 1986;89:342S-325S.
7. Marcus PM, Bergstralh EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-1316.
8. US Preventive Services Task Force. Lung Cancer Screening: Recommendation Statement. Ann Intern Med 2004;140:738-739.
9. Smith RA, Mettlin CJ, Davis KJ, Eyre H. American Cancer Society Guidelines for the Early Detection of Cancer. Available at: www.cancer.org/docroot/PUB/content/PUB_3_8X_American_Cancer_Society_Guideli nes_for_the_Early_Detection_of_Cancer_update_2001.asp. Accessed on August 12, 2005.
10. Summary of Policy Recommendations for Periodic Health Exams. AAFP Policy Action 2004. Available at: www.aafp.org/x24974.xml. Accessed on August 12, 2005.
11. Bach PB, Niewoehner DE, Black WC. Screening for Lung Cancer: The Guidelines. Chest 2003;123:83S-88S.
1. Humphrey LL, Teautsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: An update for the US Preventive Task Force. Ann Intern Med 2004;140:740-755.
2. Brett GZ. The value of six-monthly chest radiographs. Thorax 1968;23:414-420.
3. Kubik AK, Parkin DM, Zatloukal P. Czech study on lung cancer screening: Post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer 2000;89:2363-2368.
4. Bach PB, Kelley MJ, Tate RC, McCrory DC. Screening for lung cancer: A review of the current literature. Chest 2003;123:72S-82S.
5. Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer: Results of the Memorial Sloan-Kettering Study in New York. Czest 1984;86:44-53.
6. Tockman MS. Survival and mortality from lung cancer in a screened population: The John Hopkins Study. Chest 1986;89:342S-325S.
7. Marcus PM, Bergstralh EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst 2000;92:1308-1316.
8. US Preventive Services Task Force. Lung Cancer Screening: Recommendation Statement. Ann Intern Med 2004;140:738-739.
9. Smith RA, Mettlin CJ, Davis KJ, Eyre H. American Cancer Society Guidelines for the Early Detection of Cancer. Available at: www.cancer.org/docroot/PUB/content/PUB_3_8X_American_Cancer_Society_Guideli nes_for_the_Early_Detection_of_Cancer_update_2001.asp. Accessed on August 12, 2005.
10. Summary of Policy Recommendations for Periodic Health Exams. AAFP Policy Action 2004. Available at: www.aafp.org/x24974.xml. Accessed on August 12, 2005.
11. Bach PB, Niewoehner DE, Black WC. Screening for Lung Cancer: The Guidelines. Chest 2003;123:83S-88S.
Evidence-based answers from the Family Physicians Inquiries Network
Are breast self-exams or clinical exams effective for screening breast cancer?
Breast self-examination has little or no impact on breast cancer mortality and cannot be recommended for cancer screening (strength of recommendation [SOR]: A, based on a systematic review of high-quality randomized, controlled trials [RCTs]). Clinical breast examination is an important means of averting some deaths from breast cancer, but demands careful attention to technique and thoroughness (SOR: B, extrapolating from a high-quality RCT).
We might better serve our patients by improving our examination skills than by urging self-exams
We should inform women who choose to practice breast self-examination that they run a higher risk of having a breast biopsy that does not reveal a cancer and that it is not known whether self-examination reduces a woman’s chance of dying from breast cancer.1 Mammography is neither perfectly sensitive nor universally available, and many women detect breast cancer themselves; it remains important for women to know how their breasts look and feel in order to recognize and report any anomalies. But we might better serve our patients by improving our clinical breast examination skills than by urging them to perform regular self-exams; clinicians who spend 3 minutes per breast and use proper technique (vertical strip search pattern, thoroughness, varying palpation pressure, 3 fingers, circular motion, finger pads) have significantly better sensitivity and specificity than those who do not.2
Evidence summary
Breast cancer is the second leading cause of cancer death among American women; 1 in 8 women will be diagnosed with breast cancer in her lifetime, and 1 in 30 will die of it.3 Breast cancer screening and mammography have become almost synonymous. But physical examinations by clinicians or women themselves remain important methods of screening to consider.
Breast self-examination is appealing as a patient-centered, inexpensive, noninvasive procedure that empowers women and is universally available. However, a recent Cochrane review found no evidence of benefit from self-screening.
Two large RCTs, conducted in St Petersburg, Russia (122,471 women) and Shanghai, China (266,064 women), were found. Both studies used cluster randomization (by worksite) and involved large numbers of women who were meticulously trained in proper breast self-examination technique and had numerousreinforcement sessions. Study compliance and follow-up were excellent. Outcomes assessment was explicitly blinded in the Shanghai study. Neither trial demonstrated a reduction in breast cancer mortality or improvement in the number or stage of cancers detected during 9 to 11 years of follow-up, but there is evidence for harm: a nearly 2-fold increase in false-positive results, physician visits, and biopsies for benign disease.4
No trials comparing screening clinical breast examinations alone to no screening have been reported, but good indirect evidence of efficacy comes from the results of the Canadian National Breast Screening Study-2 (CNBSS-2).5 A total of 39,405 women aged 50 to 59 years were randomized to screening with clinical exams plus mammography or clinical exams alone. Other large RCTs have shown a consistent benefit to mammography screening for women of this age (in-depth independent reviews of recent criticism of the trials have concluded that their flaws do not negate mammography’s efficacy in reducing breast cancer mortality).3,6 The CNBSS-2 trial showed no mortality advantage when mammography was added to an annual, standardized 10- to 15-minute breast examination, implying that careful, detailed, annual clinical breast examinations may be as effective as a mammography screening program.3
Recommendations from others
The US Preventive Services Task Force found insufficient evidence to recommend for or against routine clinical exams alone to screen for breast cancer, or to recommend for or against teaching or performing routine breast self-examination.3 The Canadian Task Force on Preventive Health Services recommends against teaching self-examination to women aged 40 to 69 years due to “fair evidence of no benefit and good evidence of harm.”7,8
The American Cancer Society continues to recommend periodic clinical exams,6 and women who choose to do self-examination should receive instruction and have their technique reviewed during periodic health examinations; it is acceptable for women to choose not to do self-examinations. The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against breast self-examination.9 The American College of Obstetricians and Gynecologists recommends both.10
1. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-1457.
2. Barton MB, Harris R, Fletcher SW. Th erational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA 1999;282:1270-1280.
3. Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347-360.
4. Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.-
5. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study 2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-1499.
6. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA 2005;293:1245-1256.
7. Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837-1846.
8. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003;53:141-169.
9. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examinations. Revision 5.6, August 2004. Leawood, Kansas: AAFP; 2004.
10. American College of Obstetricians and Gynecologists. Breast cancer screening. ACOG practice bulletin No. 42). Washington, DC:ACOG, 2003.
Breast self-examination has little or no impact on breast cancer mortality and cannot be recommended for cancer screening (strength of recommendation [SOR]: A, based on a systematic review of high-quality randomized, controlled trials [RCTs]). Clinical breast examination is an important means of averting some deaths from breast cancer, but demands careful attention to technique and thoroughness (SOR: B, extrapolating from a high-quality RCT).
We might better serve our patients by improving our examination skills than by urging self-exams
We should inform women who choose to practice breast self-examination that they run a higher risk of having a breast biopsy that does not reveal a cancer and that it is not known whether self-examination reduces a woman’s chance of dying from breast cancer.1 Mammography is neither perfectly sensitive nor universally available, and many women detect breast cancer themselves; it remains important for women to know how their breasts look and feel in order to recognize and report any anomalies. But we might better serve our patients by improving our clinical breast examination skills than by urging them to perform regular self-exams; clinicians who spend 3 minutes per breast and use proper technique (vertical strip search pattern, thoroughness, varying palpation pressure, 3 fingers, circular motion, finger pads) have significantly better sensitivity and specificity than those who do not.2
Evidence summary
Breast cancer is the second leading cause of cancer death among American women; 1 in 8 women will be diagnosed with breast cancer in her lifetime, and 1 in 30 will die of it.3 Breast cancer screening and mammography have become almost synonymous. But physical examinations by clinicians or women themselves remain important methods of screening to consider.
Breast self-examination is appealing as a patient-centered, inexpensive, noninvasive procedure that empowers women and is universally available. However, a recent Cochrane review found no evidence of benefit from self-screening.
Two large RCTs, conducted in St Petersburg, Russia (122,471 women) and Shanghai, China (266,064 women), were found. Both studies used cluster randomization (by worksite) and involved large numbers of women who were meticulously trained in proper breast self-examination technique and had numerousreinforcement sessions. Study compliance and follow-up were excellent. Outcomes assessment was explicitly blinded in the Shanghai study. Neither trial demonstrated a reduction in breast cancer mortality or improvement in the number or stage of cancers detected during 9 to 11 years of follow-up, but there is evidence for harm: a nearly 2-fold increase in false-positive results, physician visits, and biopsies for benign disease.4
No trials comparing screening clinical breast examinations alone to no screening have been reported, but good indirect evidence of efficacy comes from the results of the Canadian National Breast Screening Study-2 (CNBSS-2).5 A total of 39,405 women aged 50 to 59 years were randomized to screening with clinical exams plus mammography or clinical exams alone. Other large RCTs have shown a consistent benefit to mammography screening for women of this age (in-depth independent reviews of recent criticism of the trials have concluded that their flaws do not negate mammography’s efficacy in reducing breast cancer mortality).3,6 The CNBSS-2 trial showed no mortality advantage when mammography was added to an annual, standardized 10- to 15-minute breast examination, implying that careful, detailed, annual clinical breast examinations may be as effective as a mammography screening program.3
Recommendations from others
The US Preventive Services Task Force found insufficient evidence to recommend for or against routine clinical exams alone to screen for breast cancer, or to recommend for or against teaching or performing routine breast self-examination.3 The Canadian Task Force on Preventive Health Services recommends against teaching self-examination to women aged 40 to 69 years due to “fair evidence of no benefit and good evidence of harm.”7,8
The American Cancer Society continues to recommend periodic clinical exams,6 and women who choose to do self-examination should receive instruction and have their technique reviewed during periodic health examinations; it is acceptable for women to choose not to do self-examinations. The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against breast self-examination.9 The American College of Obstetricians and Gynecologists recommends both.10
Breast self-examination has little or no impact on breast cancer mortality and cannot be recommended for cancer screening (strength of recommendation [SOR]: A, based on a systematic review of high-quality randomized, controlled trials [RCTs]). Clinical breast examination is an important means of averting some deaths from breast cancer, but demands careful attention to technique and thoroughness (SOR: B, extrapolating from a high-quality RCT).
We might better serve our patients by improving our examination skills than by urging self-exams
We should inform women who choose to practice breast self-examination that they run a higher risk of having a breast biopsy that does not reveal a cancer and that it is not known whether self-examination reduces a woman’s chance of dying from breast cancer.1 Mammography is neither perfectly sensitive nor universally available, and many women detect breast cancer themselves; it remains important for women to know how their breasts look and feel in order to recognize and report any anomalies. But we might better serve our patients by improving our clinical breast examination skills than by urging them to perform regular self-exams; clinicians who spend 3 minutes per breast and use proper technique (vertical strip search pattern, thoroughness, varying palpation pressure, 3 fingers, circular motion, finger pads) have significantly better sensitivity and specificity than those who do not.2
Evidence summary
Breast cancer is the second leading cause of cancer death among American women; 1 in 8 women will be diagnosed with breast cancer in her lifetime, and 1 in 30 will die of it.3 Breast cancer screening and mammography have become almost synonymous. But physical examinations by clinicians or women themselves remain important methods of screening to consider.
Breast self-examination is appealing as a patient-centered, inexpensive, noninvasive procedure that empowers women and is universally available. However, a recent Cochrane review found no evidence of benefit from self-screening.
Two large RCTs, conducted in St Petersburg, Russia (122,471 women) and Shanghai, China (266,064 women), were found. Both studies used cluster randomization (by worksite) and involved large numbers of women who were meticulously trained in proper breast self-examination technique and had numerousreinforcement sessions. Study compliance and follow-up were excellent. Outcomes assessment was explicitly blinded in the Shanghai study. Neither trial demonstrated a reduction in breast cancer mortality or improvement in the number or stage of cancers detected during 9 to 11 years of follow-up, but there is evidence for harm: a nearly 2-fold increase in false-positive results, physician visits, and biopsies for benign disease.4
No trials comparing screening clinical breast examinations alone to no screening have been reported, but good indirect evidence of efficacy comes from the results of the Canadian National Breast Screening Study-2 (CNBSS-2).5 A total of 39,405 women aged 50 to 59 years were randomized to screening with clinical exams plus mammography or clinical exams alone. Other large RCTs have shown a consistent benefit to mammography screening for women of this age (in-depth independent reviews of recent criticism of the trials have concluded that their flaws do not negate mammography’s efficacy in reducing breast cancer mortality).3,6 The CNBSS-2 trial showed no mortality advantage when mammography was added to an annual, standardized 10- to 15-minute breast examination, implying that careful, detailed, annual clinical breast examinations may be as effective as a mammography screening program.3
Recommendations from others
The US Preventive Services Task Force found insufficient evidence to recommend for or against routine clinical exams alone to screen for breast cancer, or to recommend for or against teaching or performing routine breast self-examination.3 The Canadian Task Force on Preventive Health Services recommends against teaching self-examination to women aged 40 to 69 years due to “fair evidence of no benefit and good evidence of harm.”7,8
The American Cancer Society continues to recommend periodic clinical exams,6 and women who choose to do self-examination should receive instruction and have their technique reviewed during periodic health examinations; it is acceptable for women to choose not to do self-examinations. The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against breast self-examination.9 The American College of Obstetricians and Gynecologists recommends both.10
1. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-1457.
2. Barton MB, Harris R, Fletcher SW. Th erational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA 1999;282:1270-1280.
3. Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347-360.
4. Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.-
5. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study 2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-1499.
6. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA 2005;293:1245-1256.
7. Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837-1846.
8. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003;53:141-169.
9. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examinations. Revision 5.6, August 2004. Leawood, Kansas: AAFP; 2004.
10. American College of Obstetricians and Gynecologists. Breast cancer screening. ACOG practice bulletin No. 42). Washington, DC:ACOG, 2003.
1. Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-1457.
2. Barton MB, Harris R, Fletcher SW. Th erational clinical examination. Does this patient have breast cancer? The screening clinical breast examination: Should it be done? How? JAMA 1999;282:1270-1280.
3. Humphrey LL, Helfand M, Chan BKS, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:347-360.
4. Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev 2003;(2):CD003373.-
5. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study 2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-1499.
6. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA 2005;293:1245-1256.
7. Baxter N. Canadian Task Force on Preventive Health Care. Preventive health care, 2001 update: Should women be routinely taught breast self-examination to screen for breast cancer? CMAJ 2001;164:1837-1846.
8. Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003;53:141-169.
9. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examinations. Revision 5.6, August 2004. Leawood, Kansas: AAFP; 2004.
10. American College of Obstetricians and Gynecologists. Breast cancer screening. ACOG practice bulletin No. 42). Washington, DC:ACOG, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
Should we recommend universal neonatal hearing screening?
Universal neonatal hearing screening leads to both earlier detection and earlier treatment of infants with hearing loss (strength of recommendation [SOR]: A, based on a systematic review). Available evidence suggests early identification and intervention may improve language outcomes (SOR: C, based on retrospective cohort studies).
Despite lack of evidence, early intervention could aid future language skills
Despite the lack of hard outcomes data to support neonatal hearing screening, it seems reasonable that early intervention will aid future language skills. Hopefully, future evidence will support the notion that early treatment leads to tangible school performance improvement. For most, however, the decision to universally screen neonates will be guided by state law rather than clinical evidence alone; 38 states currently have mandated screening programs with legislation pending in others.
Evidence summary
In the United States, approximately 5000 infants with moderate-to-profound hearing loss are born annually.1 Affected children graduate high school averaging 4th-grade academic performance skills.2 Efforts to reduce the impact on these children have focused on early diagnosis and treatment.
A systematic review gathered studies comparing universal hearing screening with selective screening.1 Most included studies used a 2-stage universal screening protocol. Infants who failed initial testing were retested within 12 weeks. Testing methods included otoacoustic emissions (OAE) and auditory brainstem response (ABR). Infants who failed the second test were referred for audiological evaluation. Using these data, a hypothetical model was created, which found that 1441 newborns would need to be screened to diagnose 1 additional case of moderate-to-profound permanent hearing loss before 10 months of age (at cost of 200 extra referrals for false-positives). Sensitivity and specificity of the hypothetical model’s 2-stage screening was 85% and 97%, respectively. The estimated positive predictive value was 6.7%.1,3
Individually, OAE and ABR accurately diagnose neonatal hearing loss. One multicenter cohort of 2995 infants measured test performance of OAE and ABR against the gold standard (visual reinforcement audiometry performed at 8–12 months).4 The authors used a receiver operating characteristics (ROC) curve to plot speech awareness thresholds for both tests. When middle-ear pathology and progressive hearing loss were excluded, the area under the ROC curves for ABR and OAE were 0.91 and 0.94, respectively, indication that both tests had excellent test accuracy (a perfect test would have an area under the curve of 1.0).
Strategies based on selective screening of high-risk infants fails to identify permanent hearing loss in many affected infants. In a cohort study of more than 10,000 infants, only 43% of infants with permanent hearing loss were identified with selective versus universal screening. Most affected infants would have been missed using risk-based criteria.5
Limited evidence suggests that early identification of infants with permanent hearing loss improves language skills. In a retrospective cohort study of 150 infants examining language outcomes, participants were grouped according to age at identification of hearing loss.6 All participants received comprehensive in-home language intervention services plus amplification devices.
Of the 85 children with normal cognitive ability, the mean receptive and expressive language quotients at 13 to 36 months were higher in the early-identified group vs the late-identified group (receptive language quotients, 79.6 vs 64.6, P<.001; expressive language quotients, 78.3 vs 63.1, P<.001). Total language quotient was also higher in the early group (language quotients, 79 vs 64; P<.001).
The conclusions were limited by multiple factors: retrospective study design, cohort selection drawn from different hospitals during different time periods, unblinded participant selection, and unblended outcome assessments. Other published studies have inconclusive outcome data. The Cochrane Collaboration published a systematic review in which no studies were found that fulfilled the inclusion criteria to evaluate the effectiveness of universal hearing screening.7
Recommendations from others
The Joint Committee on Infant Hearing recommended universal neonatal hearing screening during hospital birth admission in their Year 2000 Position Statement.8 For infants whose hearing is impaired on re-screening, the committee recommends audiology referral and medical evaluation to rule out associated conditions before age 3 months. They further recommend interventional services begin before age 6 months for infants with confirmed hearing loss.
The US Preventive Services Task Force does not recommend for or against universal hearing screening, citing insufficient outcomes data.9
1. Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening, summary of evidence. JAMA 2001;256:200-010.
2. Holt JA. Stanford Achievement Test—8th edition: reading comprehension subgroup results. Am Ann Deaf 1993;138:17-75.
3. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment. Wessex Universal Neonatal Hearing Screening Trial Group. Lancet 1998;352:195-964.
4. Norton SJ, Gorga MP, Widen SJ, et al. Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brainstem response test performance. Ear Hear 2000;21:50-28.
5. Watkin PM, Baldwin M, McEnery G. Neonatal at risk screening and the identification of deafness. Arch Dis Child 1991;66(10 Spec No):113-135.
6. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early-and later-identified children with hearing loss. Pediatrics 1998;102:116-171.
7. Puig T, Municio A, Medà C. Universal neonatal hearing screening versus selective screening as part of the management of childhood deafness. Cochrane Database Syst Rev 2005;(2):CD003731.-
8. Joint Committee on Infant Hearing, American Academy of Audiology, American Academy of Pediatrics, American Speech-Language-Hearing Association, Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2000;106:79-17.
9. US Preventive Services Task Force. Newborn Hearing Screening: Recommendations and Rationale. October 2001. Agency for Healthcare Research and Quality, Rockville, Md. Available at: www.ahrq.gov/clinic/3rduspstf/newbornscreen/newhearrr.htm. Accessed on July 6, 2005.
Universal neonatal hearing screening leads to both earlier detection and earlier treatment of infants with hearing loss (strength of recommendation [SOR]: A, based on a systematic review). Available evidence suggests early identification and intervention may improve language outcomes (SOR: C, based on retrospective cohort studies).
Despite lack of evidence, early intervention could aid future language skills
Despite the lack of hard outcomes data to support neonatal hearing screening, it seems reasonable that early intervention will aid future language skills. Hopefully, future evidence will support the notion that early treatment leads to tangible school performance improvement. For most, however, the decision to universally screen neonates will be guided by state law rather than clinical evidence alone; 38 states currently have mandated screening programs with legislation pending in others.
Evidence summary
In the United States, approximately 5000 infants with moderate-to-profound hearing loss are born annually.1 Affected children graduate high school averaging 4th-grade academic performance skills.2 Efforts to reduce the impact on these children have focused on early diagnosis and treatment.
A systematic review gathered studies comparing universal hearing screening with selective screening.1 Most included studies used a 2-stage universal screening protocol. Infants who failed initial testing were retested within 12 weeks. Testing methods included otoacoustic emissions (OAE) and auditory brainstem response (ABR). Infants who failed the second test were referred for audiological evaluation. Using these data, a hypothetical model was created, which found that 1441 newborns would need to be screened to diagnose 1 additional case of moderate-to-profound permanent hearing loss before 10 months of age (at cost of 200 extra referrals for false-positives). Sensitivity and specificity of the hypothetical model’s 2-stage screening was 85% and 97%, respectively. The estimated positive predictive value was 6.7%.1,3
Individually, OAE and ABR accurately diagnose neonatal hearing loss. One multicenter cohort of 2995 infants measured test performance of OAE and ABR against the gold standard (visual reinforcement audiometry performed at 8–12 months).4 The authors used a receiver operating characteristics (ROC) curve to plot speech awareness thresholds for both tests. When middle-ear pathology and progressive hearing loss were excluded, the area under the ROC curves for ABR and OAE were 0.91 and 0.94, respectively, indication that both tests had excellent test accuracy (a perfect test would have an area under the curve of 1.0).
Strategies based on selective screening of high-risk infants fails to identify permanent hearing loss in many affected infants. In a cohort study of more than 10,000 infants, only 43% of infants with permanent hearing loss were identified with selective versus universal screening. Most affected infants would have been missed using risk-based criteria.5
Limited evidence suggests that early identification of infants with permanent hearing loss improves language skills. In a retrospective cohort study of 150 infants examining language outcomes, participants were grouped according to age at identification of hearing loss.6 All participants received comprehensive in-home language intervention services plus amplification devices.
Of the 85 children with normal cognitive ability, the mean receptive and expressive language quotients at 13 to 36 months were higher in the early-identified group vs the late-identified group (receptive language quotients, 79.6 vs 64.6, P<.001; expressive language quotients, 78.3 vs 63.1, P<.001). Total language quotient was also higher in the early group (language quotients, 79 vs 64; P<.001).
The conclusions were limited by multiple factors: retrospective study design, cohort selection drawn from different hospitals during different time periods, unblinded participant selection, and unblended outcome assessments. Other published studies have inconclusive outcome data. The Cochrane Collaboration published a systematic review in which no studies were found that fulfilled the inclusion criteria to evaluate the effectiveness of universal hearing screening.7
Recommendations from others
The Joint Committee on Infant Hearing recommended universal neonatal hearing screening during hospital birth admission in their Year 2000 Position Statement.8 For infants whose hearing is impaired on re-screening, the committee recommends audiology referral and medical evaluation to rule out associated conditions before age 3 months. They further recommend interventional services begin before age 6 months for infants with confirmed hearing loss.
The US Preventive Services Task Force does not recommend for or against universal hearing screening, citing insufficient outcomes data.9
Universal neonatal hearing screening leads to both earlier detection and earlier treatment of infants with hearing loss (strength of recommendation [SOR]: A, based on a systematic review). Available evidence suggests early identification and intervention may improve language outcomes (SOR: C, based on retrospective cohort studies).
Despite lack of evidence, early intervention could aid future language skills
Despite the lack of hard outcomes data to support neonatal hearing screening, it seems reasonable that early intervention will aid future language skills. Hopefully, future evidence will support the notion that early treatment leads to tangible school performance improvement. For most, however, the decision to universally screen neonates will be guided by state law rather than clinical evidence alone; 38 states currently have mandated screening programs with legislation pending in others.
Evidence summary
In the United States, approximately 5000 infants with moderate-to-profound hearing loss are born annually.1 Affected children graduate high school averaging 4th-grade academic performance skills.2 Efforts to reduce the impact on these children have focused on early diagnosis and treatment.
A systematic review gathered studies comparing universal hearing screening with selective screening.1 Most included studies used a 2-stage universal screening protocol. Infants who failed initial testing were retested within 12 weeks. Testing methods included otoacoustic emissions (OAE) and auditory brainstem response (ABR). Infants who failed the second test were referred for audiological evaluation. Using these data, a hypothetical model was created, which found that 1441 newborns would need to be screened to diagnose 1 additional case of moderate-to-profound permanent hearing loss before 10 months of age (at cost of 200 extra referrals for false-positives). Sensitivity and specificity of the hypothetical model’s 2-stage screening was 85% and 97%, respectively. The estimated positive predictive value was 6.7%.1,3
Individually, OAE and ABR accurately diagnose neonatal hearing loss. One multicenter cohort of 2995 infants measured test performance of OAE and ABR against the gold standard (visual reinforcement audiometry performed at 8–12 months).4 The authors used a receiver operating characteristics (ROC) curve to plot speech awareness thresholds for both tests. When middle-ear pathology and progressive hearing loss were excluded, the area under the ROC curves for ABR and OAE were 0.91 and 0.94, respectively, indication that both tests had excellent test accuracy (a perfect test would have an area under the curve of 1.0).
Strategies based on selective screening of high-risk infants fails to identify permanent hearing loss in many affected infants. In a cohort study of more than 10,000 infants, only 43% of infants with permanent hearing loss were identified with selective versus universal screening. Most affected infants would have been missed using risk-based criteria.5
Limited evidence suggests that early identification of infants with permanent hearing loss improves language skills. In a retrospective cohort study of 150 infants examining language outcomes, participants were grouped according to age at identification of hearing loss.6 All participants received comprehensive in-home language intervention services plus amplification devices.
Of the 85 children with normal cognitive ability, the mean receptive and expressive language quotients at 13 to 36 months were higher in the early-identified group vs the late-identified group (receptive language quotients, 79.6 vs 64.6, P<.001; expressive language quotients, 78.3 vs 63.1, P<.001). Total language quotient was also higher in the early group (language quotients, 79 vs 64; P<.001).
The conclusions were limited by multiple factors: retrospective study design, cohort selection drawn from different hospitals during different time periods, unblinded participant selection, and unblended outcome assessments. Other published studies have inconclusive outcome data. The Cochrane Collaboration published a systematic review in which no studies were found that fulfilled the inclusion criteria to evaluate the effectiveness of universal hearing screening.7
Recommendations from others
The Joint Committee on Infant Hearing recommended universal neonatal hearing screening during hospital birth admission in their Year 2000 Position Statement.8 For infants whose hearing is impaired on re-screening, the committee recommends audiology referral and medical evaluation to rule out associated conditions before age 3 months. They further recommend interventional services begin before age 6 months for infants with confirmed hearing loss.
The US Preventive Services Task Force does not recommend for or against universal hearing screening, citing insufficient outcomes data.9
1. Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening, summary of evidence. JAMA 2001;256:200-010.
2. Holt JA. Stanford Achievement Test—8th edition: reading comprehension subgroup results. Am Ann Deaf 1993;138:17-75.
3. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment. Wessex Universal Neonatal Hearing Screening Trial Group. Lancet 1998;352:195-964.
4. Norton SJ, Gorga MP, Widen SJ, et al. Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brainstem response test performance. Ear Hear 2000;21:50-28.
5. Watkin PM, Baldwin M, McEnery G. Neonatal at risk screening and the identification of deafness. Arch Dis Child 1991;66(10 Spec No):113-135.
6. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early-and later-identified children with hearing loss. Pediatrics 1998;102:116-171.
7. Puig T, Municio A, Medà C. Universal neonatal hearing screening versus selective screening as part of the management of childhood deafness. Cochrane Database Syst Rev 2005;(2):CD003731.-
8. Joint Committee on Infant Hearing, American Academy of Audiology, American Academy of Pediatrics, American Speech-Language-Hearing Association, Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2000;106:79-17.
9. US Preventive Services Task Force. Newborn Hearing Screening: Recommendations and Rationale. October 2001. Agency for Healthcare Research and Quality, Rockville, Md. Available at: www.ahrq.gov/clinic/3rduspstf/newbornscreen/newhearrr.htm. Accessed on July 6, 2005.
1. Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M. Universal newborn hearing screening, summary of evidence. JAMA 2001;256:200-010.
2. Holt JA. Stanford Achievement Test—8th edition: reading comprehension subgroup results. Am Ann Deaf 1993;138:17-75.
3. Controlled trial of universal neonatal screening for early identification of permanent childhood hearing impairment. Wessex Universal Neonatal Hearing Screening Trial Group. Lancet 1998;352:195-964.
4. Norton SJ, Gorga MP, Widen SJ, et al. Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brainstem response test performance. Ear Hear 2000;21:50-28.
5. Watkin PM, Baldwin M, McEnery G. Neonatal at risk screening and the identification of deafness. Arch Dis Child 1991;66(10 Spec No):113-135.
6. Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early-and later-identified children with hearing loss. Pediatrics 1998;102:116-171.
7. Puig T, Municio A, Medà C. Universal neonatal hearing screening versus selective screening as part of the management of childhood deafness. Cochrane Database Syst Rev 2005;(2):CD003731.-
8. Joint Committee on Infant Hearing, American Academy of Audiology, American Academy of Pediatrics, American Speech-Language-Hearing Association, Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2000;106:79-17.
9. US Preventive Services Task Force. Newborn Hearing Screening: Recommendations and Rationale. October 2001. Agency for Healthcare Research and Quality, Rockville, Md. Available at: www.ahrq.gov/clinic/3rduspstf/newbornscreen/newhearrr.htm. Accessed on July 6, 2005.
Evidence-based answers from the Family Physicians Inquiries Network
What are the indications for bariatric surgery?
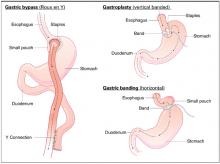
No studies evaluate the commonly used indications for bariatric surgery. Consensus guidelines suggest that the surgical treatment of obesity should be reserved for patients with a body-mass index (BMI) >40 kg/m2 or with BMI >35 kg/m2 and 1 or more significant comorbid conditions, when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality (strength of recommendation: C, based on consensus guidelines).
Evidence summary
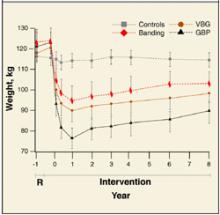
Because of the nature of major surgery, there are practical and ethical barriers to true randomized controlled trials (RCTs) comparing bariatric surgery with placebo or to no intervention. However, multiple RCTs have compared the weight-reducing effects of different bariatric surgical techniques against each other.1 All studies included patients who had a BMI >40 kg/m2, or a BMI >35 kg/m2 with at least 1 comorbidity, such as cardiovascular disease, sleep apnea, uncontrolled type 2 diabetes, or weight-induced physical problems interfering with performance of daily life activities. It is these study inclusion criteria that, by default, have become widely accepted indications for bariatric surgery. Weight loss in all RCTs was substantial, ranging from 50 to 100 kg over 6 months to 1 year. Comorbid factors associated with obesity showed either resolution or improvement after surgery in 91% of patients.
Patients with a BMI >40 have substantially more serious health consequences and a reduced life expectancy. Obesity significantly impairs quality of life, and the risk of morbidity and mortality increases with the degree of obesity.2 Those who are extremely obese often do not have sustained benefit from more conservative treatment. The benefits of nonsurgical treatment are significantly limited by the failure to maintain reduced body weight.
A large literature of controlled and uncontrolled cohort studies show that surgery has produced the longest period of sustained weight loss.3 A recent meta-analysis proved bariatric surgery not only efficacious for weight loss, but showed that a substantial majority of patients with diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea experienced complete resolution or significant improvement of their comorbid condition after surgery.4
The possibility of significant adverse effects remains. The postoperative mortality rate for bariatric surgery is approximately 0.2%. Reoperation is required for up to 25% of patients within 5 years. Other complications are wound infection, staple failure, vitamin deficiency, diarrhea, and hemorrhage.3 The long-term health effects of bariatric surgery are not well known.
Recommendations from others
The NIH statement “Gastrointestinal Surgery for Severe Obesity” concluded that the benefits outweigh the risks and that surgical treatment is reasonable in those who strongly desire substantial weight loss and have life-threatening comorbid conditions.2
Clinical guidelines developed by the National Heart, Lung, and Blood Institute Expert Panel on the identification, evaluation, and treatment of obesity for adults recommend that bariatric surgery be an option for carefully selected patients with clinically severe obesity (BMI >40 or >35 with comorbid conditions) when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality.1
The American Gastroenterological Association (AGA) medical position statement on obesity finds surgical therapy to be the most effective approach for achieving long-term weight loss. The AGA recommends surgery for patients with a BMI >40, or those with BMI >35 and 1 or more severe obesity-related medical complication (eg, hypertension, heart failure, or sleep apnea) if they have been unable to achieve or maintain weight loss with conventional therapy, have acceptable operative risks, and are able to comply with long-term treatment and follow-up.5
The American College of Preventive Medicine, in its policy statement on weight management counseling, recommends limiting surgical therapy for obesity to severely obese patients, defined as BMI >40.6
Assessing perioperative risk and long-term complications is critical
National data indicate that more than 5 million Americans have a BMI >35. Thus the implications of recommending bariatric surgery are enormous. Patients who have undergone surgical treatment for obesity require lifelong monitoring and often nutritional supplementation, and the lifelong severe dietary restriction that follows bariatric surgery can be psychologically devastating. Psychological and behavioral factors must be carefully considered in presurgical evaluation. No standardized protocol exists for this assessment and few empiric data specify which factors predict successful surgical outcomes.
Great progress has been made in developing safer and more effective surgical procedures for promoting weight loss, yet the possibility of significant adverse effects remain. Assessing both perioperative risk and long-term complications is critical and requires a risk/benefit analysis in each case.
1. NHLBI Obesity Education Initiative. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults: The Evidence Report. NIH Publication No. 98-4083. Bethesda, Md: National Heart, Lung, and Blood Institute; 1998.
2. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Int Med 1991;115:956-961.
3. US Preventive Services Task Force. Screening for Obesity in Adults: Recommendations and Rationale. Rockville, Md: Agency for Healthcare Research and Quality, 2003.
4. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-1737.
5. American Gastroenterological Association. American Gastroenterology Association medical position statement on Obesity. Gastroenterology 2002;123:879-881.
6. Nawaz H, Katz D. ACPM Practice Policy Statement. Weight management counseling of overweight adults. Am J Prev Med 2001;21:73-78.
No studies evaluate the commonly used indications for bariatric surgery. Consensus guidelines suggest that the surgical treatment of obesity should be reserved for patients with a body-mass index (BMI) >40 kg/m2 or with BMI >35 kg/m2 and 1 or more significant comorbid conditions, when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality (strength of recommendation: C, based on consensus guidelines).
Evidence summary
Because of the nature of major surgery, there are practical and ethical barriers to true randomized controlled trials (RCTs) comparing bariatric surgery with placebo or to no intervention. However, multiple RCTs have compared the weight-reducing effects of different bariatric surgical techniques against each other.1 All studies included patients who had a BMI >40 kg/m2, or a BMI >35 kg/m2 with at least 1 comorbidity, such as cardiovascular disease, sleep apnea, uncontrolled type 2 diabetes, or weight-induced physical problems interfering with performance of daily life activities. It is these study inclusion criteria that, by default, have become widely accepted indications for bariatric surgery. Weight loss in all RCTs was substantial, ranging from 50 to 100 kg over 6 months to 1 year. Comorbid factors associated with obesity showed either resolution or improvement after surgery in 91% of patients.
Patients with a BMI >40 have substantially more serious health consequences and a reduced life expectancy. Obesity significantly impairs quality of life, and the risk of morbidity and mortality increases with the degree of obesity.2 Those who are extremely obese often do not have sustained benefit from more conservative treatment. The benefits of nonsurgical treatment are significantly limited by the failure to maintain reduced body weight.
A large literature of controlled and uncontrolled cohort studies show that surgery has produced the longest period of sustained weight loss.3 A recent meta-analysis proved bariatric surgery not only efficacious for weight loss, but showed that a substantial majority of patients with diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea experienced complete resolution or significant improvement of their comorbid condition after surgery.4
The possibility of significant adverse effects remains. The postoperative mortality rate for bariatric surgery is approximately 0.2%. Reoperation is required for up to 25% of patients within 5 years. Other complications are wound infection, staple failure, vitamin deficiency, diarrhea, and hemorrhage.3 The long-term health effects of bariatric surgery are not well known.
Recommendations from others
The NIH statement “Gastrointestinal Surgery for Severe Obesity” concluded that the benefits outweigh the risks and that surgical treatment is reasonable in those who strongly desire substantial weight loss and have life-threatening comorbid conditions.2
Clinical guidelines developed by the National Heart, Lung, and Blood Institute Expert Panel on the identification, evaluation, and treatment of obesity for adults recommend that bariatric surgery be an option for carefully selected patients with clinically severe obesity (BMI >40 or >35 with comorbid conditions) when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality.1
The American Gastroenterological Association (AGA) medical position statement on obesity finds surgical therapy to be the most effective approach for achieving long-term weight loss. The AGA recommends surgery for patients with a BMI >40, or those with BMI >35 and 1 or more severe obesity-related medical complication (eg, hypertension, heart failure, or sleep apnea) if they have been unable to achieve or maintain weight loss with conventional therapy, have acceptable operative risks, and are able to comply with long-term treatment and follow-up.5
The American College of Preventive Medicine, in its policy statement on weight management counseling, recommends limiting surgical therapy for obesity to severely obese patients, defined as BMI >40.6
Assessing perioperative risk and long-term complications is critical
National data indicate that more than 5 million Americans have a BMI >35. Thus the implications of recommending bariatric surgery are enormous. Patients who have undergone surgical treatment for obesity require lifelong monitoring and often nutritional supplementation, and the lifelong severe dietary restriction that follows bariatric surgery can be psychologically devastating. Psychological and behavioral factors must be carefully considered in presurgical evaluation. No standardized protocol exists for this assessment and few empiric data specify which factors predict successful surgical outcomes.
Great progress has been made in developing safer and more effective surgical procedures for promoting weight loss, yet the possibility of significant adverse effects remain. Assessing both perioperative risk and long-term complications is critical and requires a risk/benefit analysis in each case.
No studies evaluate the commonly used indications for bariatric surgery. Consensus guidelines suggest that the surgical treatment of obesity should be reserved for patients with a body-mass index (BMI) >40 kg/m2 or with BMI >35 kg/m2 and 1 or more significant comorbid conditions, when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality (strength of recommendation: C, based on consensus guidelines).
Evidence summary
Because of the nature of major surgery, there are practical and ethical barriers to true randomized controlled trials (RCTs) comparing bariatric surgery with placebo or to no intervention. However, multiple RCTs have compared the weight-reducing effects of different bariatric surgical techniques against each other.1 All studies included patients who had a BMI >40 kg/m2, or a BMI >35 kg/m2 with at least 1 comorbidity, such as cardiovascular disease, sleep apnea, uncontrolled type 2 diabetes, or weight-induced physical problems interfering with performance of daily life activities. It is these study inclusion criteria that, by default, have become widely accepted indications for bariatric surgery. Weight loss in all RCTs was substantial, ranging from 50 to 100 kg over 6 months to 1 year. Comorbid factors associated with obesity showed either resolution or improvement after surgery in 91% of patients.
Patients with a BMI >40 have substantially more serious health consequences and a reduced life expectancy. Obesity significantly impairs quality of life, and the risk of morbidity and mortality increases with the degree of obesity.2 Those who are extremely obese often do not have sustained benefit from more conservative treatment. The benefits of nonsurgical treatment are significantly limited by the failure to maintain reduced body weight.
A large literature of controlled and uncontrolled cohort studies show that surgery has produced the longest period of sustained weight loss.3 A recent meta-analysis proved bariatric surgery not only efficacious for weight loss, but showed that a substantial majority of patients with diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea experienced complete resolution or significant improvement of their comorbid condition after surgery.4
The possibility of significant adverse effects remains. The postoperative mortality rate for bariatric surgery is approximately 0.2%. Reoperation is required for up to 25% of patients within 5 years. Other complications are wound infection, staple failure, vitamin deficiency, diarrhea, and hemorrhage.3 The long-term health effects of bariatric surgery are not well known.
Recommendations from others
The NIH statement “Gastrointestinal Surgery for Severe Obesity” concluded that the benefits outweigh the risks and that surgical treatment is reasonable in those who strongly desire substantial weight loss and have life-threatening comorbid conditions.2
Clinical guidelines developed by the National Heart, Lung, and Blood Institute Expert Panel on the identification, evaluation, and treatment of obesity for adults recommend that bariatric surgery be an option for carefully selected patients with clinically severe obesity (BMI >40 or >35 with comorbid conditions) when less invasive methods of weight loss have failed and the patient is at high risk for obesity-associated morbidity and mortality.1
The American Gastroenterological Association (AGA) medical position statement on obesity finds surgical therapy to be the most effective approach for achieving long-term weight loss. The AGA recommends surgery for patients with a BMI >40, or those with BMI >35 and 1 or more severe obesity-related medical complication (eg, hypertension, heart failure, or sleep apnea) if they have been unable to achieve or maintain weight loss with conventional therapy, have acceptable operative risks, and are able to comply with long-term treatment and follow-up.5
The American College of Preventive Medicine, in its policy statement on weight management counseling, recommends limiting surgical therapy for obesity to severely obese patients, defined as BMI >40.6
Assessing perioperative risk and long-term complications is critical
National data indicate that more than 5 million Americans have a BMI >35. Thus the implications of recommending bariatric surgery are enormous. Patients who have undergone surgical treatment for obesity require lifelong monitoring and often nutritional supplementation, and the lifelong severe dietary restriction that follows bariatric surgery can be psychologically devastating. Psychological and behavioral factors must be carefully considered in presurgical evaluation. No standardized protocol exists for this assessment and few empiric data specify which factors predict successful surgical outcomes.
Great progress has been made in developing safer and more effective surgical procedures for promoting weight loss, yet the possibility of significant adverse effects remain. Assessing both perioperative risk and long-term complications is critical and requires a risk/benefit analysis in each case.
1. NHLBI Obesity Education Initiative. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults: The Evidence Report. NIH Publication No. 98-4083. Bethesda, Md: National Heart, Lung, and Blood Institute; 1998.
2. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Int Med 1991;115:956-961.
3. US Preventive Services Task Force. Screening for Obesity in Adults: Recommendations and Rationale. Rockville, Md: Agency for Healthcare Research and Quality, 2003.
4. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-1737.
5. American Gastroenterological Association. American Gastroenterology Association medical position statement on Obesity. Gastroenterology 2002;123:879-881.
6. Nawaz H, Katz D. ACPM Practice Policy Statement. Weight management counseling of overweight adults. Am J Prev Med 2001;21:73-78.
1. NHLBI Obesity Education Initiative. Clinical Guidelines on the Identification, Evaluation and Treatment of Overweight and Obesity in Adults: The Evidence Report. NIH Publication No. 98-4083. Bethesda, Md: National Heart, Lung, and Blood Institute; 1998.
2. NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Int Med 1991;115:956-961.
3. US Preventive Services Task Force. Screening for Obesity in Adults: Recommendations and Rationale. Rockville, Md: Agency for Healthcare Research and Quality, 2003.
4. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-1737.
5. American Gastroenterological Association. American Gastroenterology Association medical position statement on Obesity. Gastroenterology 2002;123:879-881.
6. Nawaz H, Katz D. ACPM Practice Policy Statement. Weight management counseling of overweight adults. Am J Prev Med 2001;21:73-78.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best way to distinguish type 1 and 2 diabetes?
No clinical characteristic or diagnostic test is available to readily distinguish type 1 from type 2 diabetes mellitus. Although C-peptide levels, autoantibodies, and adiponectin-to-leptin ratios show some utility, they do not yet have a standard diagnostic role; research on the pathophysiology of diabetes suggests that the classic type 1 and type 2 distinctions may not be appropriate for all patients1 (strength of recommendation: C, based on expert opinion).
Evidence summary
Onset of diabetes in childhood with ketoacidosis and insulin dependency has traditionally been sufficient to diagnose type 1 diabetes, while onset in older, obese patients with primary insulin resistance suggested type 2 diabetes. Unfortunately, features of type 1 and type 2 diabetes may be present in the same patient, making differentiation difficult. No diagnostic studies in the literature were identified that definitively demonstrate how to separate type 1 from type 2 diabetes.
A patient’s age may suggest, but does not reliably distinguish, diabetes types. A study of 569 new-onset type 1 and type 2 diabetic children and adolescents showed that older age was only weakly associated with type 2 diagnosis (odds ratio [OR]= 1.4 for each 1-year increment in age; 95% confidence interval [CI], 1.3–1.6).2 In fact, newly diagnosed 12-year-old children have an equal incidence of type 1 as type 2 diabetes. Likewise, adults with type 2 phenotype (no initial insulin requirement) can present with positive autoantibodies typically found in younger type 1 patients. Older patients who fit this profile have been classified as type 1.5 diabetes or latent autoimmune disease in adults (LADA).3
A history of diabetic ketoacidosis (DKA) also does not reliably distinguish between types 1 and 2. A retrospective chart review gathered data on adults over 18 years of age who were admitted for DKA in a urban US hospital. Many patients with DKA were subsequently diagnosed with type 2 diabetes. Rates of type 2 diabetes in patients with DKA varied by race: 47% of Hispanics, 44% of African Americans, and 17% of Caucasians had type 2 diabetes.4
The overlapping presence of autoantibodies in both types of diabetes limits their use (TABLE). Autoantibodies do predict an earlier need for insulin. One prevalence study of 101 type 2 adult patients found 20% were positive for glutamic acid decarboxylase autoantibody (GADAb), which was positively associated with insulin dependence at 4 years postdiagnosis (OR=5.8; 95% CI, 1.8–18.9).5 Eighty percent of patients with autoantibodies required insulin compared with 41% of patients without autoantibodies. Another study in young adults with type 2 or unclassified diabetes from Sweden found 93% of patients who were GADAb+ required insulin at 3 years, compared with 51% who were GADAb–(OR=18.8; 95% CI, 1.8–191).6
One motivation to study autoantibody testing is a potential benefit in preserving pancreatic function. Kobayashi proposed treating those with autoantibody-positive diabetes (presumed type 1 or type 1.5) with insulin immediately, while initiating oral medications in those who test negative (presumed type 2 diabetes). This approach lacks significant patient-oriented outcome data, but his small RCT of 55 patients was encouraging. With a 3-year follow-up rate of 89%, early insulin use in GADAb+ patients preserved C-peptide levels and possibly prolonged pancreatic beta cell survival.7 Insulin dependency, defined as needing insulin for survival, occurred in 47% of controls (who received oral sulfonylureas) and only 13% of patients receiving insulin (number needed to treat [NNT]= 4; P=.043).7 The theoretical benefit is that if beta cell exhaustion can be delayed, endogenous insulin production could be maintained to assist prevention of damaging postprandial glucose spikes.
Although daily variation in serum insulin levels limits its use, C-peptide levels show more promise. Random C-peptide levels were superior to fasting or glucagon stimulated levels in 1093 patients, who were followed for 3 years to confirm insulin requirements. Using a receiver operating characteristic (ROC) curve, the area under the curve for random C-peptide levels to distinguish diabetes types was 0.98 (95% CI, 0.97–0.99).8 For patients under the optimal cutoff of 0.5 nmol/L, the positive predictive value was 96% for diagnosing type 1 and the likelihood ratio was 22.5.
Finally, the ratio of adiponectin to leptin hormone may show diagnostic merit. Adipocytes secrete adiponectin which acts as an insulin sensitizer, antiatherogenic and anti-inflammatory agent. Obesity and type 2 phenotype correlate with lower levels of adiponectin, but are associated with higher levels of leptin hormone, another molecule secreted by adipocytes. A recent case-control study of children aged 6 to 21 years analyzed adiponectin and leptin hormone levels in patients with classical type 1 and 2 diabetes, as determined by 2 pediatric endocrinologists; interestingly, 29% of the type 1 patients were autoantibody negative.9 After plotting a ROC curve, they found the area under the curve was 0.97 (95% CI, 0.93–1.00). At an adiponectin-to-leptin ratio cutoff less than 0.7, they found the sensitivity to diagnose type 2 was 88% (95% CI, 64–99%), the specificity was 90% (95% CI, 77–97), and the likelihood ratio for a positive test was 8.8.9
TABLE 1
Antibody markers and diabetes type
| PREVALENCE OF ANY AUTOANTIBODY MARKER | PERCENT |
|---|---|
| Newly diagnosed type 1 (Caucasian) | 73–90 |
| Newly diagnosed type 1 (African or Asian) | 50 |
| Newly diagnosed type 2 (Caucasian) | 3–22 |
| Healthy individuals | 1–2 |
| Source: Wingfield et al 20041 and Maron et al 1996.3 | |
Recommendations from others
The National Academy of Clinical Biochemistry and the American Association of Clinical Endocrinologists recommend against routine testing of insulin, C-peptide, autoantibodies and genetic markers.1,10 Guidelines from the American Diabetes Association admit that many diabetic individuals do not easily fit into a distinct diagnostic category; however, they only provide criteria for the general diagnosis of diabetes, not specific criteria to distinguish type 1 from type 2.11
Focus on attaining optimal diabetes control goals as recommended by the ADA
Vincent Lo, MD
St. Elizabeth Family Medicine Residency Program/SUNY Upstate Medical University, Utica, New York
Not long ago, clinicians were advised to avoid the terms type 1 and type 2 diabetes, because they were not very helpful in clinical management of our patients. Instead, it was suggested that we use insulin-dependent or non-insulin-dependent. The rationale is that for patients with diabetes, there is an absolute insulin deficiency due to premature beta-cell failure in type 1 diabetes, as well as a relative insulin deficiency due to insulin resistance in type 2. In addition, studies also suggest that a majority of patients with type 2 diabetes would require some form of exogenous insulin therapy after a duration of 8 to 10 years of their disease. Therefore, distinguishing between types 1 and 2 is neither clinically helpful nor cost-effective, as suggested by current review of the literature. Instead, clinicians should focus on attaining optimal diabetic control goals as recommended by the practice guidelines of management of diabetes mellitus from the ADA. Furthermore, it was also recognized that one of the hurdles of failure to reach the target goal of HbA1C <7.0, among patients with type 2 diabetes is the delayed use of exogenous insulin therapy. Therefore, it is imperative for clinicians to discuss with each patient with a new diagnosis of diabetes, the natural progression of its disease process and its potential need and benefit of exogenous insulin therapy in the near future.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the US Air Force medical department or the US Air Force at large.
1. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436-472.
2. Macaluso CJ, Bauer UE, Deeb LC, et al. Type 2 diabetes mellitus among Florida children and adolescents, 1994 through 1998. Public Health Rep 2002;117:373-379.
3. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care 2001;24:1460-1467.
4. Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs type 2 diabetics and the effect of ethnicity. Arch Int Med 1999;159:2317-2322.
5. Grasso YZ, Reddy SK, Rosenfeld CR, et al. Autoantibodies to IA-2 and GAD65 in patients with type 2 diabetes mellitus of varied duration: prevalence and correlation with clinical features. Endocr Pract 2001;7:339-345.
6. Torn C, Landin-Olsson M, Ostman J, et al. Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as Type 1 diabetes on clinical grounds. Diabetes Metab Res Rev 2000;16:442-447.
7. Kobayashi T, Maruyama T, Shimada A, et al. Insulin intervention to preserve beta cells in slowly progressive insulin-dependent (type 1) diabetes mellitus. Ann NY Acad Sci 2002;958:117-130.
8. Berger B, Stenstrom G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest 2000;60:687-693.
9. Morales A, Wasserfall C, Brusko T, et al. Adiponectin and leptin concentrations may aid in discriminating disease forms in children and adolescents with type 1 and type 2 diabetes. Diabetes Care 2004;27:2010-2014.
10. The American Association of Clinical Endocrinologists. The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE system of intensive diabetes self-management—2000 Update. Endocr Pract 2000;6:43-84.
11. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160-3167.
No clinical characteristic or diagnostic test is available to readily distinguish type 1 from type 2 diabetes mellitus. Although C-peptide levels, autoantibodies, and adiponectin-to-leptin ratios show some utility, they do not yet have a standard diagnostic role; research on the pathophysiology of diabetes suggests that the classic type 1 and type 2 distinctions may not be appropriate for all patients1 (strength of recommendation: C, based on expert opinion).
Evidence summary
Onset of diabetes in childhood with ketoacidosis and insulin dependency has traditionally been sufficient to diagnose type 1 diabetes, while onset in older, obese patients with primary insulin resistance suggested type 2 diabetes. Unfortunately, features of type 1 and type 2 diabetes may be present in the same patient, making differentiation difficult. No diagnostic studies in the literature were identified that definitively demonstrate how to separate type 1 from type 2 diabetes.
A patient’s age may suggest, but does not reliably distinguish, diabetes types. A study of 569 new-onset type 1 and type 2 diabetic children and adolescents showed that older age was only weakly associated with type 2 diagnosis (odds ratio [OR]= 1.4 for each 1-year increment in age; 95% confidence interval [CI], 1.3–1.6).2 In fact, newly diagnosed 12-year-old children have an equal incidence of type 1 as type 2 diabetes. Likewise, adults with type 2 phenotype (no initial insulin requirement) can present with positive autoantibodies typically found in younger type 1 patients. Older patients who fit this profile have been classified as type 1.5 diabetes or latent autoimmune disease in adults (LADA).3
A history of diabetic ketoacidosis (DKA) also does not reliably distinguish between types 1 and 2. A retrospective chart review gathered data on adults over 18 years of age who were admitted for DKA in a urban US hospital. Many patients with DKA were subsequently diagnosed with type 2 diabetes. Rates of type 2 diabetes in patients with DKA varied by race: 47% of Hispanics, 44% of African Americans, and 17% of Caucasians had type 2 diabetes.4
The overlapping presence of autoantibodies in both types of diabetes limits their use (TABLE). Autoantibodies do predict an earlier need for insulin. One prevalence study of 101 type 2 adult patients found 20% were positive for glutamic acid decarboxylase autoantibody (GADAb), which was positively associated with insulin dependence at 4 years postdiagnosis (OR=5.8; 95% CI, 1.8–18.9).5 Eighty percent of patients with autoantibodies required insulin compared with 41% of patients without autoantibodies. Another study in young adults with type 2 or unclassified diabetes from Sweden found 93% of patients who were GADAb+ required insulin at 3 years, compared with 51% who were GADAb–(OR=18.8; 95% CI, 1.8–191).6
One motivation to study autoantibody testing is a potential benefit in preserving pancreatic function. Kobayashi proposed treating those with autoantibody-positive diabetes (presumed type 1 or type 1.5) with insulin immediately, while initiating oral medications in those who test negative (presumed type 2 diabetes). This approach lacks significant patient-oriented outcome data, but his small RCT of 55 patients was encouraging. With a 3-year follow-up rate of 89%, early insulin use in GADAb+ patients preserved C-peptide levels and possibly prolonged pancreatic beta cell survival.7 Insulin dependency, defined as needing insulin for survival, occurred in 47% of controls (who received oral sulfonylureas) and only 13% of patients receiving insulin (number needed to treat [NNT]= 4; P=.043).7 The theoretical benefit is that if beta cell exhaustion can be delayed, endogenous insulin production could be maintained to assist prevention of damaging postprandial glucose spikes.
Although daily variation in serum insulin levels limits its use, C-peptide levels show more promise. Random C-peptide levels were superior to fasting or glucagon stimulated levels in 1093 patients, who were followed for 3 years to confirm insulin requirements. Using a receiver operating characteristic (ROC) curve, the area under the curve for random C-peptide levels to distinguish diabetes types was 0.98 (95% CI, 0.97–0.99).8 For patients under the optimal cutoff of 0.5 nmol/L, the positive predictive value was 96% for diagnosing type 1 and the likelihood ratio was 22.5.
Finally, the ratio of adiponectin to leptin hormone may show diagnostic merit. Adipocytes secrete adiponectin which acts as an insulin sensitizer, antiatherogenic and anti-inflammatory agent. Obesity and type 2 phenotype correlate with lower levels of adiponectin, but are associated with higher levels of leptin hormone, another molecule secreted by adipocytes. A recent case-control study of children aged 6 to 21 years analyzed adiponectin and leptin hormone levels in patients with classical type 1 and 2 diabetes, as determined by 2 pediatric endocrinologists; interestingly, 29% of the type 1 patients were autoantibody negative.9 After plotting a ROC curve, they found the area under the curve was 0.97 (95% CI, 0.93–1.00). At an adiponectin-to-leptin ratio cutoff less than 0.7, they found the sensitivity to diagnose type 2 was 88% (95% CI, 64–99%), the specificity was 90% (95% CI, 77–97), and the likelihood ratio for a positive test was 8.8.9
TABLE 1
Antibody markers and diabetes type
| PREVALENCE OF ANY AUTOANTIBODY MARKER | PERCENT |
|---|---|
| Newly diagnosed type 1 (Caucasian) | 73–90 |
| Newly diagnosed type 1 (African or Asian) | 50 |
| Newly diagnosed type 2 (Caucasian) | 3–22 |
| Healthy individuals | 1–2 |
| Source: Wingfield et al 20041 and Maron et al 1996.3 | |
Recommendations from others
The National Academy of Clinical Biochemistry and the American Association of Clinical Endocrinologists recommend against routine testing of insulin, C-peptide, autoantibodies and genetic markers.1,10 Guidelines from the American Diabetes Association admit that many diabetic individuals do not easily fit into a distinct diagnostic category; however, they only provide criteria for the general diagnosis of diabetes, not specific criteria to distinguish type 1 from type 2.11
Focus on attaining optimal diabetes control goals as recommended by the ADA
Vincent Lo, MD
St. Elizabeth Family Medicine Residency Program/SUNY Upstate Medical University, Utica, New York
Not long ago, clinicians were advised to avoid the terms type 1 and type 2 diabetes, because they were not very helpful in clinical management of our patients. Instead, it was suggested that we use insulin-dependent or non-insulin-dependent. The rationale is that for patients with diabetes, there is an absolute insulin deficiency due to premature beta-cell failure in type 1 diabetes, as well as a relative insulin deficiency due to insulin resistance in type 2. In addition, studies also suggest that a majority of patients with type 2 diabetes would require some form of exogenous insulin therapy after a duration of 8 to 10 years of their disease. Therefore, distinguishing between types 1 and 2 is neither clinically helpful nor cost-effective, as suggested by current review of the literature. Instead, clinicians should focus on attaining optimal diabetic control goals as recommended by the practice guidelines of management of diabetes mellitus from the ADA. Furthermore, it was also recognized that one of the hurdles of failure to reach the target goal of HbA1C <7.0, among patients with type 2 diabetes is the delayed use of exogenous insulin therapy. Therefore, it is imperative for clinicians to discuss with each patient with a new diagnosis of diabetes, the natural progression of its disease process and its potential need and benefit of exogenous insulin therapy in the near future.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the US Air Force medical department or the US Air Force at large.
No clinical characteristic or diagnostic test is available to readily distinguish type 1 from type 2 diabetes mellitus. Although C-peptide levels, autoantibodies, and adiponectin-to-leptin ratios show some utility, they do not yet have a standard diagnostic role; research on the pathophysiology of diabetes suggests that the classic type 1 and type 2 distinctions may not be appropriate for all patients1 (strength of recommendation: C, based on expert opinion).
Evidence summary
Onset of diabetes in childhood with ketoacidosis and insulin dependency has traditionally been sufficient to diagnose type 1 diabetes, while onset in older, obese patients with primary insulin resistance suggested type 2 diabetes. Unfortunately, features of type 1 and type 2 diabetes may be present in the same patient, making differentiation difficult. No diagnostic studies in the literature were identified that definitively demonstrate how to separate type 1 from type 2 diabetes.
A patient’s age may suggest, but does not reliably distinguish, diabetes types. A study of 569 new-onset type 1 and type 2 diabetic children and adolescents showed that older age was only weakly associated with type 2 diagnosis (odds ratio [OR]= 1.4 for each 1-year increment in age; 95% confidence interval [CI], 1.3–1.6).2 In fact, newly diagnosed 12-year-old children have an equal incidence of type 1 as type 2 diabetes. Likewise, adults with type 2 phenotype (no initial insulin requirement) can present with positive autoantibodies typically found in younger type 1 patients. Older patients who fit this profile have been classified as type 1.5 diabetes or latent autoimmune disease in adults (LADA).3
A history of diabetic ketoacidosis (DKA) also does not reliably distinguish between types 1 and 2. A retrospective chart review gathered data on adults over 18 years of age who were admitted for DKA in a urban US hospital. Many patients with DKA were subsequently diagnosed with type 2 diabetes. Rates of type 2 diabetes in patients with DKA varied by race: 47% of Hispanics, 44% of African Americans, and 17% of Caucasians had type 2 diabetes.4
The overlapping presence of autoantibodies in both types of diabetes limits their use (TABLE). Autoantibodies do predict an earlier need for insulin. One prevalence study of 101 type 2 adult patients found 20% were positive for glutamic acid decarboxylase autoantibody (GADAb), which was positively associated with insulin dependence at 4 years postdiagnosis (OR=5.8; 95% CI, 1.8–18.9).5 Eighty percent of patients with autoantibodies required insulin compared with 41% of patients without autoantibodies. Another study in young adults with type 2 or unclassified diabetes from Sweden found 93% of patients who were GADAb+ required insulin at 3 years, compared with 51% who were GADAb–(OR=18.8; 95% CI, 1.8–191).6
One motivation to study autoantibody testing is a potential benefit in preserving pancreatic function. Kobayashi proposed treating those with autoantibody-positive diabetes (presumed type 1 or type 1.5) with insulin immediately, while initiating oral medications in those who test negative (presumed type 2 diabetes). This approach lacks significant patient-oriented outcome data, but his small RCT of 55 patients was encouraging. With a 3-year follow-up rate of 89%, early insulin use in GADAb+ patients preserved C-peptide levels and possibly prolonged pancreatic beta cell survival.7 Insulin dependency, defined as needing insulin for survival, occurred in 47% of controls (who received oral sulfonylureas) and only 13% of patients receiving insulin (number needed to treat [NNT]= 4; P=.043).7 The theoretical benefit is that if beta cell exhaustion can be delayed, endogenous insulin production could be maintained to assist prevention of damaging postprandial glucose spikes.
Although daily variation in serum insulin levels limits its use, C-peptide levels show more promise. Random C-peptide levels were superior to fasting or glucagon stimulated levels in 1093 patients, who were followed for 3 years to confirm insulin requirements. Using a receiver operating characteristic (ROC) curve, the area under the curve for random C-peptide levels to distinguish diabetes types was 0.98 (95% CI, 0.97–0.99).8 For patients under the optimal cutoff of 0.5 nmol/L, the positive predictive value was 96% for diagnosing type 1 and the likelihood ratio was 22.5.
Finally, the ratio of adiponectin to leptin hormone may show diagnostic merit. Adipocytes secrete adiponectin which acts as an insulin sensitizer, antiatherogenic and anti-inflammatory agent. Obesity and type 2 phenotype correlate with lower levels of adiponectin, but are associated with higher levels of leptin hormone, another molecule secreted by adipocytes. A recent case-control study of children aged 6 to 21 years analyzed adiponectin and leptin hormone levels in patients with classical type 1 and 2 diabetes, as determined by 2 pediatric endocrinologists; interestingly, 29% of the type 1 patients were autoantibody negative.9 After plotting a ROC curve, they found the area under the curve was 0.97 (95% CI, 0.93–1.00). At an adiponectin-to-leptin ratio cutoff less than 0.7, they found the sensitivity to diagnose type 2 was 88% (95% CI, 64–99%), the specificity was 90% (95% CI, 77–97), and the likelihood ratio for a positive test was 8.8.9
TABLE 1
Antibody markers and diabetes type
| PREVALENCE OF ANY AUTOANTIBODY MARKER | PERCENT |
|---|---|
| Newly diagnosed type 1 (Caucasian) | 73–90 |
| Newly diagnosed type 1 (African or Asian) | 50 |
| Newly diagnosed type 2 (Caucasian) | 3–22 |
| Healthy individuals | 1–2 |
| Source: Wingfield et al 20041 and Maron et al 1996.3 | |
Recommendations from others
The National Academy of Clinical Biochemistry and the American Association of Clinical Endocrinologists recommend against routine testing of insulin, C-peptide, autoantibodies and genetic markers.1,10 Guidelines from the American Diabetes Association admit that many diabetic individuals do not easily fit into a distinct diagnostic category; however, they only provide criteria for the general diagnosis of diabetes, not specific criteria to distinguish type 1 from type 2.11
Focus on attaining optimal diabetes control goals as recommended by the ADA
Vincent Lo, MD
St. Elizabeth Family Medicine Residency Program/SUNY Upstate Medical University, Utica, New York
Not long ago, clinicians were advised to avoid the terms type 1 and type 2 diabetes, because they were not very helpful in clinical management of our patients. Instead, it was suggested that we use insulin-dependent or non-insulin-dependent. The rationale is that for patients with diabetes, there is an absolute insulin deficiency due to premature beta-cell failure in type 1 diabetes, as well as a relative insulin deficiency due to insulin resistance in type 2. In addition, studies also suggest that a majority of patients with type 2 diabetes would require some form of exogenous insulin therapy after a duration of 8 to 10 years of their disease. Therefore, distinguishing between types 1 and 2 is neither clinically helpful nor cost-effective, as suggested by current review of the literature. Instead, clinicians should focus on attaining optimal diabetic control goals as recommended by the practice guidelines of management of diabetes mellitus from the ADA. Furthermore, it was also recognized that one of the hurdles of failure to reach the target goal of HbA1C <7.0, among patients with type 2 diabetes is the delayed use of exogenous insulin therapy. Therefore, it is imperative for clinicians to discuss with each patient with a new diagnosis of diabetes, the natural progression of its disease process and its potential need and benefit of exogenous insulin therapy in the near future.
Acknowledgments
The opinions and assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting the views of the US Air Force medical department or the US Air Force at large.
1. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436-472.
2. Macaluso CJ, Bauer UE, Deeb LC, et al. Type 2 diabetes mellitus among Florida children and adolescents, 1994 through 1998. Public Health Rep 2002;117:373-379.
3. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care 2001;24:1460-1467.
4. Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs type 2 diabetics and the effect of ethnicity. Arch Int Med 1999;159:2317-2322.
5. Grasso YZ, Reddy SK, Rosenfeld CR, et al. Autoantibodies to IA-2 and GAD65 in patients with type 2 diabetes mellitus of varied duration: prevalence and correlation with clinical features. Endocr Pract 2001;7:339-345.
6. Torn C, Landin-Olsson M, Ostman J, et al. Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as Type 1 diabetes on clinical grounds. Diabetes Metab Res Rev 2000;16:442-447.
7. Kobayashi T, Maruyama T, Shimada A, et al. Insulin intervention to preserve beta cells in slowly progressive insulin-dependent (type 1) diabetes mellitus. Ann NY Acad Sci 2002;958:117-130.
8. Berger B, Stenstrom G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest 2000;60:687-693.
9. Morales A, Wasserfall C, Brusko T, et al. Adiponectin and leptin concentrations may aid in discriminating disease forms in children and adolescents with type 1 and type 2 diabetes. Diabetes Care 2004;27:2010-2014.
10. The American Association of Clinical Endocrinologists. The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE system of intensive diabetes self-management—2000 Update. Endocr Pract 2000;6:43-84.
11. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160-3167.
1. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436-472.
2. Macaluso CJ, Bauer UE, Deeb LC, et al. Type 2 diabetes mellitus among Florida children and adolescents, 1994 through 1998. Public Health Rep 2002;117:373-379.
3. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care 2001;24:1460-1467.
4. Balasubramanyam A, Zern JW, Hyman DJ, Pavlik V. New profiles of diabetic ketoacidosis: type 1 vs type 2 diabetics and the effect of ethnicity. Arch Int Med 1999;159:2317-2322.
5. Grasso YZ, Reddy SK, Rosenfeld CR, et al. Autoantibodies to IA-2 and GAD65 in patients with type 2 diabetes mellitus of varied duration: prevalence and correlation with clinical features. Endocr Pract 2001;7:339-345.
6. Torn C, Landin-Olsson M, Ostman J, et al. Glutamic acid decarboxylase antibodies (GADA) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as Type 1 diabetes on clinical grounds. Diabetes Metab Res Rev 2000;16:442-447.
7. Kobayashi T, Maruyama T, Shimada A, et al. Insulin intervention to preserve beta cells in slowly progressive insulin-dependent (type 1) diabetes mellitus. Ann NY Acad Sci 2002;958:117-130.
8. Berger B, Stenstrom G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest 2000;60:687-693.
9. Morales A, Wasserfall C, Brusko T, et al. Adiponectin and leptin concentrations may aid in discriminating disease forms in children and adolescents with type 1 and type 2 diabetes. Diabetes Care 2004;27:2010-2014.
10. The American Association of Clinical Endocrinologists. The American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: the AACE system of intensive diabetes self-management—2000 Update. Endocr Pract 2000;6:43-84.
11. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160-3167.
Evidence-based answers from the Family Physicians Inquiries Network
Does digoxin decrease morbidity for those in sinus rhythm with heart failure?
In patients with congestive heart failure due to systolic dysfunction who are in normal sinus rhythm, digoxin therapy reduces rates of hospitalization, as well as clinical deterioration, defined as worsening New York Heart Association (NYHA) classification or an increase in clinical signs and symptoms (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCT]).1 These benefits appear to be more pronounced for men.2
Patients treated with digoxin are at increased risk of developing supraventricular dysrhythmias and second- or third-degree atrioventricular block (SOR: A, large RCT).3 It is unclear if patients with diastolic dysfunction experience similar benefits or harms (SOR: A, systematic review of RCTs).1 Digoxin has not been shown to have any effect on mortality for men with congestive heart failure in sinus rhythm (SOR: A, systematic review of RCTs).1 Digoxin use for women may be associated with an increased risk of mortality2 (SOR: B, extrapolation from RCT).
Evidence summary
A recent Cochrane systematic review summarizes the clinical effects of digoxin when used for patients with heart failure in normal sinus rhythm. Thirteen studies including 7896 participants, most of whom had systolic dysfunction, met the criteria for inclusion. Ninety-four percent of all study participants came from a single large randomized placebo-controlled trial.3 Because the studies did not all measure the same outcomes, subgroup analyses were performed.
Four studies with 1096 participants contributed to the findings on clinical status, 12 studies with 7262 participants contributed to the findings of hospitalization and 8 studies including 7755 patients contributed to the data on mortality. Patients receiving digoxin experienced reduced rates of hospitalization due to worsening heart failure (odds ratio [OR]=0.68; 95% confidence interval [CI], 0.61–0.75; number needed to treat [NNT]=13–17) and less clinical deterioration (OR=0.31; 95% CI, 0.21–0.43; NNT=3–61). The wide range in NNT for the reduction in clinical deterioration reflects varying baseline rates of worsening clinical status found among the 12 studies for patients receiving placebo. The narrow CI associated with the odds ratio for reduced rates of clinical deterioration reflects the fact that the majority of patients whose clinical status was evaluated as an outcome came from a single large study, the DIG trial.3 This trial followed 6800 patients with NYHA classifications I to III. Ninety-four percent of patients in this trial were additionally on angiotensin-converting enzyme (ACE) inhibitors and 82% were taking diuretics. Patients were followed for a mean of 37 months.
A subgroup analysis of 988 patients with diastolic dysfunction (ejection fraction >45%) in this study3 suggested no clear benefits or harms when digoxin was used in combination with other therapies vs placebo; however, it did show a positive trend towards the combined outcome of reduced hospitalizations and less clinical deterioration (relative risk [RR]=0.82; 95% CI, 0.63–1.07). Increased rates of supraventricular dysrhythmias (RR=2.08; 95% CI, 1.44–2.99; number needed to harm [NNH]=77) and second- and third-degree heart block were demonstrated for patients receiving digoxin (RR=2.93; 95% CI, 1.61–5.34; NNH=125). There was no difference in mortality between patients receiving digoxin or those receiving placebo (OR=0.98; 95% CI, 0.89–1.09).1
A post-hoc subgroup analysis focusing only on sex-based differences in the DIG trial suggested women benefit less than men from reduced hospitalizations: –4.2% (95% CI, –8.9 to 0.5) vs –8.9% (95% CI, –11.4 to –6.5) (P=.053).2 When a multivariable analysis was performed, digoxin use for women was associated with a higher risk of mortality (adjusted hazard ratio vs placebo=1.23; 95% CI, 1.02–1.47).2
Two randomized controlled withdrawal studies, in which patients who were being treated with digoxin had it discontinued, were also included in the systematic review. These patients’ clinical outcomes were then compared with persons who had continued to receive digoxin for the duration of the trial. Six parallel design studies, in which patients taking digoxin underwent a washout period before being randomized to either digoxin or placebo, were also included in the evaluation of digoxin’s effect on clinical status. Because these patients had already demonstrated the ability to tolerate digoxin, these studies may have been biased in favor of digoxin.4,5
Recommendations from others
The American College of Cardiology/ American Heart Association6 and Heart Failure Society of America7 guidelines both recommend that digoxin be used in NYHA class II–III patients in sinus rhythm who remain symptomatic on standard therapy (described as ACE inhibitors, diuretics, and beta-blockers). Guidelines from the Scottish Intercollegiate Society,8 the European Society of Cardiology,9 and the American Medical Directors Association10 all offer similar recommendations.
Digoxin unlikely to benefit most patients with mild heart failure
It is clear that ACE inhibitors, diuretics, and beta-blockers should all be the first drugs chosen for therapy for patients with CHF. They have not only been shown to improve mortality and reduce symptoms but they do not carry any of the significant risks associated with digoxin toxicity.
Digoxin is unlikely to benefit patients with Class I heart failure, as their risk of clinical deterioration and hospitalizations are low. However, for patients who cannot tolerate any of the first-line drugs or who remain symptomatic while taking them, digoxin carefully dosed and monitored is a useful adjunct in practice.
While it is true that these patients need periodic laboratory monitoring, by the time they require digoxin therapy, their visits for care are already frequent and they would likely require few, if any, additional visits.
1. Hood WB, Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJV. Digitalis for treatment of congestive heart failure in patients in sinus rhythm. Cochrane Database Syst Rev 2004;(2):CD002901.-
2. Rathore SS, Wang Y, Krumholz HM. Sex based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 2002;347:1403-1411.
3. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med 1997;336:525-333.
4. Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol 1993;22:955-962.
5. Packer M, Gheoghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med 1993;329:1-7.
6. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Bethesda, Md: American College of Cardiology Foundation; 2001.
7. Heart Failure Society of America guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction-pharmacological management. J Card Fail 1999;5:357-382.
8. Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. Edinburgh: Scottish Intercollegiate Guidelines Network; 1999. Available at: www.sign.ac.uk/guidelines/fulltext/35/index.html.
9. Remme WJ, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure. European Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527-1560.
10. American Medical Directors Association. Heart failure. Columbia, Md: American Medical Directors Association (AMDA); 2002.
In patients with congestive heart failure due to systolic dysfunction who are in normal sinus rhythm, digoxin therapy reduces rates of hospitalization, as well as clinical deterioration, defined as worsening New York Heart Association (NYHA) classification or an increase in clinical signs and symptoms (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCT]).1 These benefits appear to be more pronounced for men.2
Patients treated with digoxin are at increased risk of developing supraventricular dysrhythmias and second- or third-degree atrioventricular block (SOR: A, large RCT).3 It is unclear if patients with diastolic dysfunction experience similar benefits or harms (SOR: A, systematic review of RCTs).1 Digoxin has not been shown to have any effect on mortality for men with congestive heart failure in sinus rhythm (SOR: A, systematic review of RCTs).1 Digoxin use for women may be associated with an increased risk of mortality2 (SOR: B, extrapolation from RCT).
Evidence summary
A recent Cochrane systematic review summarizes the clinical effects of digoxin when used for patients with heart failure in normal sinus rhythm. Thirteen studies including 7896 participants, most of whom had systolic dysfunction, met the criteria for inclusion. Ninety-four percent of all study participants came from a single large randomized placebo-controlled trial.3 Because the studies did not all measure the same outcomes, subgroup analyses were performed.
Four studies with 1096 participants contributed to the findings on clinical status, 12 studies with 7262 participants contributed to the findings of hospitalization and 8 studies including 7755 patients contributed to the data on mortality. Patients receiving digoxin experienced reduced rates of hospitalization due to worsening heart failure (odds ratio [OR]=0.68; 95% confidence interval [CI], 0.61–0.75; number needed to treat [NNT]=13–17) and less clinical deterioration (OR=0.31; 95% CI, 0.21–0.43; NNT=3–61). The wide range in NNT for the reduction in clinical deterioration reflects varying baseline rates of worsening clinical status found among the 12 studies for patients receiving placebo. The narrow CI associated with the odds ratio for reduced rates of clinical deterioration reflects the fact that the majority of patients whose clinical status was evaluated as an outcome came from a single large study, the DIG trial.3 This trial followed 6800 patients with NYHA classifications I to III. Ninety-four percent of patients in this trial were additionally on angiotensin-converting enzyme (ACE) inhibitors and 82% were taking diuretics. Patients were followed for a mean of 37 months.
A subgroup analysis of 988 patients with diastolic dysfunction (ejection fraction >45%) in this study3 suggested no clear benefits or harms when digoxin was used in combination with other therapies vs placebo; however, it did show a positive trend towards the combined outcome of reduced hospitalizations and less clinical deterioration (relative risk [RR]=0.82; 95% CI, 0.63–1.07). Increased rates of supraventricular dysrhythmias (RR=2.08; 95% CI, 1.44–2.99; number needed to harm [NNH]=77) and second- and third-degree heart block were demonstrated for patients receiving digoxin (RR=2.93; 95% CI, 1.61–5.34; NNH=125). There was no difference in mortality between patients receiving digoxin or those receiving placebo (OR=0.98; 95% CI, 0.89–1.09).1
A post-hoc subgroup analysis focusing only on sex-based differences in the DIG trial suggested women benefit less than men from reduced hospitalizations: –4.2% (95% CI, –8.9 to 0.5) vs –8.9% (95% CI, –11.4 to –6.5) (P=.053).2 When a multivariable analysis was performed, digoxin use for women was associated with a higher risk of mortality (adjusted hazard ratio vs placebo=1.23; 95% CI, 1.02–1.47).2
Two randomized controlled withdrawal studies, in which patients who were being treated with digoxin had it discontinued, were also included in the systematic review. These patients’ clinical outcomes were then compared with persons who had continued to receive digoxin for the duration of the trial. Six parallel design studies, in which patients taking digoxin underwent a washout period before being randomized to either digoxin or placebo, were also included in the evaluation of digoxin’s effect on clinical status. Because these patients had already demonstrated the ability to tolerate digoxin, these studies may have been biased in favor of digoxin.4,5
Recommendations from others
The American College of Cardiology/ American Heart Association6 and Heart Failure Society of America7 guidelines both recommend that digoxin be used in NYHA class II–III patients in sinus rhythm who remain symptomatic on standard therapy (described as ACE inhibitors, diuretics, and beta-blockers). Guidelines from the Scottish Intercollegiate Society,8 the European Society of Cardiology,9 and the American Medical Directors Association10 all offer similar recommendations.
Digoxin unlikely to benefit most patients with mild heart failure
It is clear that ACE inhibitors, diuretics, and beta-blockers should all be the first drugs chosen for therapy for patients with CHF. They have not only been shown to improve mortality and reduce symptoms but they do not carry any of the significant risks associated with digoxin toxicity.
Digoxin is unlikely to benefit patients with Class I heart failure, as their risk of clinical deterioration and hospitalizations are low. However, for patients who cannot tolerate any of the first-line drugs or who remain symptomatic while taking them, digoxin carefully dosed and monitored is a useful adjunct in practice.
While it is true that these patients need periodic laboratory monitoring, by the time they require digoxin therapy, their visits for care are already frequent and they would likely require few, if any, additional visits.
In patients with congestive heart failure due to systolic dysfunction who are in normal sinus rhythm, digoxin therapy reduces rates of hospitalization, as well as clinical deterioration, defined as worsening New York Heart Association (NYHA) classification or an increase in clinical signs and symptoms (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCT]).1 These benefits appear to be more pronounced for men.2
Patients treated with digoxin are at increased risk of developing supraventricular dysrhythmias and second- or third-degree atrioventricular block (SOR: A, large RCT).3 It is unclear if patients with diastolic dysfunction experience similar benefits or harms (SOR: A, systematic review of RCTs).1 Digoxin has not been shown to have any effect on mortality for men with congestive heart failure in sinus rhythm (SOR: A, systematic review of RCTs).1 Digoxin use for women may be associated with an increased risk of mortality2 (SOR: B, extrapolation from RCT).
Evidence summary
A recent Cochrane systematic review summarizes the clinical effects of digoxin when used for patients with heart failure in normal sinus rhythm. Thirteen studies including 7896 participants, most of whom had systolic dysfunction, met the criteria for inclusion. Ninety-four percent of all study participants came from a single large randomized placebo-controlled trial.3 Because the studies did not all measure the same outcomes, subgroup analyses were performed.
Four studies with 1096 participants contributed to the findings on clinical status, 12 studies with 7262 participants contributed to the findings of hospitalization and 8 studies including 7755 patients contributed to the data on mortality. Patients receiving digoxin experienced reduced rates of hospitalization due to worsening heart failure (odds ratio [OR]=0.68; 95% confidence interval [CI], 0.61–0.75; number needed to treat [NNT]=13–17) and less clinical deterioration (OR=0.31; 95% CI, 0.21–0.43; NNT=3–61). The wide range in NNT for the reduction in clinical deterioration reflects varying baseline rates of worsening clinical status found among the 12 studies for patients receiving placebo. The narrow CI associated with the odds ratio for reduced rates of clinical deterioration reflects the fact that the majority of patients whose clinical status was evaluated as an outcome came from a single large study, the DIG trial.3 This trial followed 6800 patients with NYHA classifications I to III. Ninety-four percent of patients in this trial were additionally on angiotensin-converting enzyme (ACE) inhibitors and 82% were taking diuretics. Patients were followed for a mean of 37 months.
A subgroup analysis of 988 patients with diastolic dysfunction (ejection fraction >45%) in this study3 suggested no clear benefits or harms when digoxin was used in combination with other therapies vs placebo; however, it did show a positive trend towards the combined outcome of reduced hospitalizations and less clinical deterioration (relative risk [RR]=0.82; 95% CI, 0.63–1.07). Increased rates of supraventricular dysrhythmias (RR=2.08; 95% CI, 1.44–2.99; number needed to harm [NNH]=77) and second- and third-degree heart block were demonstrated for patients receiving digoxin (RR=2.93; 95% CI, 1.61–5.34; NNH=125). There was no difference in mortality between patients receiving digoxin or those receiving placebo (OR=0.98; 95% CI, 0.89–1.09).1
A post-hoc subgroup analysis focusing only on sex-based differences in the DIG trial suggested women benefit less than men from reduced hospitalizations: –4.2% (95% CI, –8.9 to 0.5) vs –8.9% (95% CI, –11.4 to –6.5) (P=.053).2 When a multivariable analysis was performed, digoxin use for women was associated with a higher risk of mortality (adjusted hazard ratio vs placebo=1.23; 95% CI, 1.02–1.47).2
Two randomized controlled withdrawal studies, in which patients who were being treated with digoxin had it discontinued, were also included in the systematic review. These patients’ clinical outcomes were then compared with persons who had continued to receive digoxin for the duration of the trial. Six parallel design studies, in which patients taking digoxin underwent a washout period before being randomized to either digoxin or placebo, were also included in the evaluation of digoxin’s effect on clinical status. Because these patients had already demonstrated the ability to tolerate digoxin, these studies may have been biased in favor of digoxin.4,5
Recommendations from others
The American College of Cardiology/ American Heart Association6 and Heart Failure Society of America7 guidelines both recommend that digoxin be used in NYHA class II–III patients in sinus rhythm who remain symptomatic on standard therapy (described as ACE inhibitors, diuretics, and beta-blockers). Guidelines from the Scottish Intercollegiate Society,8 the European Society of Cardiology,9 and the American Medical Directors Association10 all offer similar recommendations.
Digoxin unlikely to benefit most patients with mild heart failure
It is clear that ACE inhibitors, diuretics, and beta-blockers should all be the first drugs chosen for therapy for patients with CHF. They have not only been shown to improve mortality and reduce symptoms but they do not carry any of the significant risks associated with digoxin toxicity.
Digoxin is unlikely to benefit patients with Class I heart failure, as their risk of clinical deterioration and hospitalizations are low. However, for patients who cannot tolerate any of the first-line drugs or who remain symptomatic while taking them, digoxin carefully dosed and monitored is a useful adjunct in practice.
While it is true that these patients need periodic laboratory monitoring, by the time they require digoxin therapy, their visits for care are already frequent and they would likely require few, if any, additional visits.
1. Hood WB, Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJV. Digitalis for treatment of congestive heart failure in patients in sinus rhythm. Cochrane Database Syst Rev 2004;(2):CD002901.-
2. Rathore SS, Wang Y, Krumholz HM. Sex based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 2002;347:1403-1411.
3. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med 1997;336:525-333.
4. Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol 1993;22:955-962.
5. Packer M, Gheoghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med 1993;329:1-7.
6. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Bethesda, Md: American College of Cardiology Foundation; 2001.
7. Heart Failure Society of America guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction-pharmacological management. J Card Fail 1999;5:357-382.
8. Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. Edinburgh: Scottish Intercollegiate Guidelines Network; 1999. Available at: www.sign.ac.uk/guidelines/fulltext/35/index.html.
9. Remme WJ, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure. European Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527-1560.
10. American Medical Directors Association. Heart failure. Columbia, Md: American Medical Directors Association (AMDA); 2002.
1. Hood WB, Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJV. Digitalis for treatment of congestive heart failure in patients in sinus rhythm. Cochrane Database Syst Rev 2004;(2):CD002901.-
2. Rathore SS, Wang Y, Krumholz HM. Sex based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med 2002;347:1403-1411.
3. The effect of digoxin on mortality and morbidity in patients with heart failure. The Digitalis Investigation Group. N Engl J Med 1997;336:525-333.
4. Uretsky BF, Young JB, Shahidi FE, Yellen LG, Harrison MC, Jolly MK. Randomized study assessing the effect of digoxin withdrawal in patients with mild to moderate chronic congestive heart failure: results of the PROVED trial. PROVED Investigative Group. J Am Coll Cardiol 1993;22:955-962.
5. Packer M, Gheoghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting-enzyme inhibitors. RADIANCE Study. N Engl J Med 1993;329:1-7.
6. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Bethesda, Md: American College of Cardiology Foundation; 2001.
7. Heart Failure Society of America guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction-pharmacological management. J Card Fail 1999;5:357-382.
8. Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. Edinburgh: Scottish Intercollegiate Guidelines Network; 1999. Available at: www.sign.ac.uk/guidelines/fulltext/35/index.html.
9. Remme WJ, Swedberg K. Task Force for the Diagnosis and Treatment of Chronic Heart Failure. European Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527-1560.
10. American Medical Directors Association. Heart failure. Columbia, Md: American Medical Directors Association (AMDA); 2002.
Evidence-based answers from the Family Physicians Inquiries Network
How does tissue adhesive compare with suturing for superficial lacerations?
Tissue adhesives are effective and yield results comparable to those with conventional suturing of superficial, linear, and low-tension lacerations. The cosmetic outcome is similar; wound complications, such as infection and dehiscence, may be lower with tissue adhesives. Wound closure of superficial lacerations by tissue adhesives is quicker and less painful compared with conventional suturing (strength of recommendation: A, systematic reviews of randomized trials).
Evidence summary
Multiple studies and reviews have compared tissue adhesives with sutures or adhesive strips for wound closure. A Cochrane review found 10 studies, which included 970 patients in the emergency-room setting. Review of these articles found no significant difference in cosmetic appearance between tissue adhesive closure and standard suture closure with a 3-month follow-up period in acute, linear wounds under low tension. Wound erythema (number needed to treat [NNT]=10) and dehiscence rates (NNT=25) were lower for tissue adhesives.1 In the 6 studies that reported time data, treatment with tissue adhesive took 4.7 fewer minutes. In all 6 studies that reported patients’ perception of pain, pain was significantly less with tissue adhesive (weighted mean difference=13.7 mm [on 100-mm scale]; 95% confidence interval [CI], –20.0 to –6.9).
A multicenter, randomized trial studied 924 wounds (383 traumatic, 541 surgical) and reported no difference in cosmetic appearance upon grading by both a clinician and the patients themselves.2 This study was not included in the Cochrane review because of the inclusion of surgical wounds. In a clinical trial reported after the Cochrane review, Holger and colleagues3 studied tissue adhesives against standard wound closure using either nylon or absorbable gut sutures. The study included 145 patients, 84 of whom had at least a 9-month follow-up. No significant difference was noted in a visual analog grading scale, with a 10- to 15-mm difference (out of 100 mm) considered significant.3 Tissue adhesive closure was, on average, 5.7 minutes faster than standard wound closure with sutures for superficial lacerations. Pain outcomes in the studies showed that closure with tissue adhesive was less painful due to the lack of a need for anesthesia.4
Recommendations from others
No major guidelines were found regarding the use of skin adhesives for wound closure.
Skin adhesives offer reduced pain and less time spent closing the wound
Skin adhesives should be considered for closure of superficial cuts because skin adhesives are comparable to sutures in both cosmetic outcome and complication rates. Additionally, skin adhesives offer the patient benefits of reduced pain and less time spent in closing the wound. Although the cost of the tissue adhesives is higher than conventional sutures, follow-up visits for suture removal are not needed, reducing medical service time during the wound check visit.
1. Farion K, Osmond MH, Hartling L, Russell K, Klassen T, Crumley E, Wiebe N. Tissue adhesives for traumatic lacerations in children and adults. Cochrane Database Syst Rev 2004;(3).-
2. Singer AJ, Quinn JV, Clark RE, Hollander JE. TraumaSeal StudyGroup. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery 2002;131:270-276.
3. Holger JS, Wandersee SC, Hale DB. Cosmetic outcomes of facial lacerations repaired with tissue-adhesive, absorbable, and non-absorbablesutures. Am J Emerg Med 2004;22:254-257.
4. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surgery 2004;187:238-248.
Tissue adhesives are effective and yield results comparable to those with conventional suturing of superficial, linear, and low-tension lacerations. The cosmetic outcome is similar; wound complications, such as infection and dehiscence, may be lower with tissue adhesives. Wound closure of superficial lacerations by tissue adhesives is quicker and less painful compared with conventional suturing (strength of recommendation: A, systematic reviews of randomized trials).
Evidence summary
Multiple studies and reviews have compared tissue adhesives with sutures or adhesive strips for wound closure. A Cochrane review found 10 studies, which included 970 patients in the emergency-room setting. Review of these articles found no significant difference in cosmetic appearance between tissue adhesive closure and standard suture closure with a 3-month follow-up period in acute, linear wounds under low tension. Wound erythema (number needed to treat [NNT]=10) and dehiscence rates (NNT=25) were lower for tissue adhesives.1 In the 6 studies that reported time data, treatment with tissue adhesive took 4.7 fewer minutes. In all 6 studies that reported patients’ perception of pain, pain was significantly less with tissue adhesive (weighted mean difference=13.7 mm [on 100-mm scale]; 95% confidence interval [CI], –20.0 to –6.9).
A multicenter, randomized trial studied 924 wounds (383 traumatic, 541 surgical) and reported no difference in cosmetic appearance upon grading by both a clinician and the patients themselves.2 This study was not included in the Cochrane review because of the inclusion of surgical wounds. In a clinical trial reported after the Cochrane review, Holger and colleagues3 studied tissue adhesives against standard wound closure using either nylon or absorbable gut sutures. The study included 145 patients, 84 of whom had at least a 9-month follow-up. No significant difference was noted in a visual analog grading scale, with a 10- to 15-mm difference (out of 100 mm) considered significant.3 Tissue adhesive closure was, on average, 5.7 minutes faster than standard wound closure with sutures for superficial lacerations. Pain outcomes in the studies showed that closure with tissue adhesive was less painful due to the lack of a need for anesthesia.4
Recommendations from others
No major guidelines were found regarding the use of skin adhesives for wound closure.
Skin adhesives offer reduced pain and less time spent closing the wound
Skin adhesives should be considered for closure of superficial cuts because skin adhesives are comparable to sutures in both cosmetic outcome and complication rates. Additionally, skin adhesives offer the patient benefits of reduced pain and less time spent in closing the wound. Although the cost of the tissue adhesives is higher than conventional sutures, follow-up visits for suture removal are not needed, reducing medical service time during the wound check visit.
Tissue adhesives are effective and yield results comparable to those with conventional suturing of superficial, linear, and low-tension lacerations. The cosmetic outcome is similar; wound complications, such as infection and dehiscence, may be lower with tissue adhesives. Wound closure of superficial lacerations by tissue adhesives is quicker and less painful compared with conventional suturing (strength of recommendation: A, systematic reviews of randomized trials).
Evidence summary
Multiple studies and reviews have compared tissue adhesives with sutures or adhesive strips for wound closure. A Cochrane review found 10 studies, which included 970 patients in the emergency-room setting. Review of these articles found no significant difference in cosmetic appearance between tissue adhesive closure and standard suture closure with a 3-month follow-up period in acute, linear wounds under low tension. Wound erythema (number needed to treat [NNT]=10) and dehiscence rates (NNT=25) were lower for tissue adhesives.1 In the 6 studies that reported time data, treatment with tissue adhesive took 4.7 fewer minutes. In all 6 studies that reported patients’ perception of pain, pain was significantly less with tissue adhesive (weighted mean difference=13.7 mm [on 100-mm scale]; 95% confidence interval [CI], –20.0 to –6.9).
A multicenter, randomized trial studied 924 wounds (383 traumatic, 541 surgical) and reported no difference in cosmetic appearance upon grading by both a clinician and the patients themselves.2 This study was not included in the Cochrane review because of the inclusion of surgical wounds. In a clinical trial reported after the Cochrane review, Holger and colleagues3 studied tissue adhesives against standard wound closure using either nylon or absorbable gut sutures. The study included 145 patients, 84 of whom had at least a 9-month follow-up. No significant difference was noted in a visual analog grading scale, with a 10- to 15-mm difference (out of 100 mm) considered significant.3 Tissue adhesive closure was, on average, 5.7 minutes faster than standard wound closure with sutures for superficial lacerations. Pain outcomes in the studies showed that closure with tissue adhesive was less painful due to the lack of a need for anesthesia.4
Recommendations from others
No major guidelines were found regarding the use of skin adhesives for wound closure.
Skin adhesives offer reduced pain and less time spent closing the wound
Skin adhesives should be considered for closure of superficial cuts because skin adhesives are comparable to sutures in both cosmetic outcome and complication rates. Additionally, skin adhesives offer the patient benefits of reduced pain and less time spent in closing the wound. Although the cost of the tissue adhesives is higher than conventional sutures, follow-up visits for suture removal are not needed, reducing medical service time during the wound check visit.
1. Farion K, Osmond MH, Hartling L, Russell K, Klassen T, Crumley E, Wiebe N. Tissue adhesives for traumatic lacerations in children and adults. Cochrane Database Syst Rev 2004;(3).-
2. Singer AJ, Quinn JV, Clark RE, Hollander JE. TraumaSeal StudyGroup. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery 2002;131:270-276.
3. Holger JS, Wandersee SC, Hale DB. Cosmetic outcomes of facial lacerations repaired with tissue-adhesive, absorbable, and non-absorbablesutures. Am J Emerg Med 2004;22:254-257.
4. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surgery 2004;187:238-248.
1. Farion K, Osmond MH, Hartling L, Russell K, Klassen T, Crumley E, Wiebe N. Tissue adhesives for traumatic lacerations in children and adults. Cochrane Database Syst Rev 2004;(3).-
2. Singer AJ, Quinn JV, Clark RE, Hollander JE. TraumaSeal StudyGroup. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery 2002;131:270-276.
3. Holger JS, Wandersee SC, Hale DB. Cosmetic outcomes of facial lacerations repaired with tissue-adhesive, absorbable, and non-absorbablesutures. Am J Emerg Med 2004;22:254-257.
4. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surgery 2004;187:238-248.
Evidence-based answers from the Family Physicians Inquiries Network
How useful is ultrasound to evaluate patients with postmenopausal bleeding?
Using a threshold of ≤5 mm, transvaginal ultrasound (TVUS) can be used to identify those patients with postmenopausal bleeding who are at low risk for endometrial cancer, polyps, or atypical hyperplasia at a sensitivity comparable with that of endometrial biopsy and dilatation and curettage (D&C) (strength of recommendation: B, based on systematic reviews of consistent exploratory cohort studies.)
Evidence summary
A 1998 meta-analysis of 35 exploratory cohort studies published between 1966 and 1996 included a total of 5892 women with postmenopausal bleeding.1 TVUS evaluations were followed by endometrial tissue sampling and results were compared. Using endometrial thickness of ≤5 mm as the threshold, ultrasound was very accurate at ruling out patients with endometrial cancer but only fair at diagnosing cancer (likelihood ratio for a positive test [LR+]=2.5; LR for a negative test [LR–]=0.06). In addition, the 5-mm threshold was accurate at ruling out any endometrial abnormality (cancer, polyp, atypical hyperplasia: LR– = 0.01). The authors suggested that TVUS can reliably rule out significant endometrial disease among postmenopausal women with vaginal bleeding.
A 2002 meta-analysis of 57 cohort studies, without consistently applied reference standards, published between 1966 and 2000 included a total of 9031 women with postmenopausal bleeding.2 Because many of the studies were felt to use inadequately stringent criteria for diagnosis, the authors limited their final analysis to only 4 studies. They concluded that a negative result using a 5-mm threshold rules out endometrial pathology with fair certainty (LR– = 0.21).
Recommendations from others
A Consensus Conference Statement from the Society of Radiologists in Ultrasound recommended that either TVUS or endometrial biopsy could be used in the initial evaluation of patients with postmenopausal bleeding.3 Using a threshold of >5 mm as abnormal, they concluded that the sensitivities of TVUS and endometrial biopsy are comparable when “sufficient tissue” is obtained with endometrial biopsy. They felt that data was currently insufficient to clearly state which technique is more effective.
TVUS is an effective, relatively noninvasive way to rule out significant pathology
Postmenopausal women need accurate diagnostic evaluation when they have abnormal bleeding. While the majority have a benign cause of bleeding, such as atrophic endometrium, many have significant pathology, including cancer (Table). Many older patients are reluctant to undergo invasive sampling studies. Cervical stenosis, a common occurrence in this age group, further complicates matters. Evidence suggests that TVUS with a full endometrial thickness of 5 mm or less, full visualization of the cavity, and no other abnormal findings, can identify patients at low risk for significant abnormalities. The false negative rate for TVUS (8%) compares quite favorably with endometrial biopsy (5%–15%) and even D&C (2%–6%).1 TVUS is an effective and relatively noninvasive strategy for ruling out significant pathology. Given the false negative rates of these techniques, all patients with postmenopausal bleeding require close follow-up.
TABLE
Differential diagnosis of postmenopausal bleeding
| Histologic diagnosis | Incidence (n=1138) |
|---|---|
| Atrophy | 59% |
| Endometrial polyp | 12% |
| Hyperplasia | 10% |
| Endometrial cancer | 10% |
| Hormonal effect | 7% |
| Cervical cancer | 2% |
| Other | <1% |
| Source: Karlsson et al 1995.4 | |
1. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998;280:1510-1517.
2. Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand 2002;81:799-816.
3. Evaluation of the woman with postmenopausal bleeding. Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med 2001;20:1025-1036.
4. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol 1995;172:1488-1494.
Using a threshold of ≤5 mm, transvaginal ultrasound (TVUS) can be used to identify those patients with postmenopausal bleeding who are at low risk for endometrial cancer, polyps, or atypical hyperplasia at a sensitivity comparable with that of endometrial biopsy and dilatation and curettage (D&C) (strength of recommendation: B, based on systematic reviews of consistent exploratory cohort studies.)
Evidence summary
A 1998 meta-analysis of 35 exploratory cohort studies published between 1966 and 1996 included a total of 5892 women with postmenopausal bleeding.1 TVUS evaluations were followed by endometrial tissue sampling and results were compared. Using endometrial thickness of ≤5 mm as the threshold, ultrasound was very accurate at ruling out patients with endometrial cancer but only fair at diagnosing cancer (likelihood ratio for a positive test [LR+]=2.5; LR for a negative test [LR–]=0.06). In addition, the 5-mm threshold was accurate at ruling out any endometrial abnormality (cancer, polyp, atypical hyperplasia: LR– = 0.01). The authors suggested that TVUS can reliably rule out significant endometrial disease among postmenopausal women with vaginal bleeding.
A 2002 meta-analysis of 57 cohort studies, without consistently applied reference standards, published between 1966 and 2000 included a total of 9031 women with postmenopausal bleeding.2 Because many of the studies were felt to use inadequately stringent criteria for diagnosis, the authors limited their final analysis to only 4 studies. They concluded that a negative result using a 5-mm threshold rules out endometrial pathology with fair certainty (LR– = 0.21).
Recommendations from others
A Consensus Conference Statement from the Society of Radiologists in Ultrasound recommended that either TVUS or endometrial biopsy could be used in the initial evaluation of patients with postmenopausal bleeding.3 Using a threshold of >5 mm as abnormal, they concluded that the sensitivities of TVUS and endometrial biopsy are comparable when “sufficient tissue” is obtained with endometrial biopsy. They felt that data was currently insufficient to clearly state which technique is more effective.
TVUS is an effective, relatively noninvasive way to rule out significant pathology
Postmenopausal women need accurate diagnostic evaluation when they have abnormal bleeding. While the majority have a benign cause of bleeding, such as atrophic endometrium, many have significant pathology, including cancer (Table). Many older patients are reluctant to undergo invasive sampling studies. Cervical stenosis, a common occurrence in this age group, further complicates matters. Evidence suggests that TVUS with a full endometrial thickness of 5 mm or less, full visualization of the cavity, and no other abnormal findings, can identify patients at low risk for significant abnormalities. The false negative rate for TVUS (8%) compares quite favorably with endometrial biopsy (5%–15%) and even D&C (2%–6%).1 TVUS is an effective and relatively noninvasive strategy for ruling out significant pathology. Given the false negative rates of these techniques, all patients with postmenopausal bleeding require close follow-up.
TABLE
Differential diagnosis of postmenopausal bleeding
| Histologic diagnosis | Incidence (n=1138) |
|---|---|
| Atrophy | 59% |
| Endometrial polyp | 12% |
| Hyperplasia | 10% |
| Endometrial cancer | 10% |
| Hormonal effect | 7% |
| Cervical cancer | 2% |
| Other | <1% |
| Source: Karlsson et al 1995.4 | |
Using a threshold of ≤5 mm, transvaginal ultrasound (TVUS) can be used to identify those patients with postmenopausal bleeding who are at low risk for endometrial cancer, polyps, or atypical hyperplasia at a sensitivity comparable with that of endometrial biopsy and dilatation and curettage (D&C) (strength of recommendation: B, based on systematic reviews of consistent exploratory cohort studies.)
Evidence summary
A 1998 meta-analysis of 35 exploratory cohort studies published between 1966 and 1996 included a total of 5892 women with postmenopausal bleeding.1 TVUS evaluations were followed by endometrial tissue sampling and results were compared. Using endometrial thickness of ≤5 mm as the threshold, ultrasound was very accurate at ruling out patients with endometrial cancer but only fair at diagnosing cancer (likelihood ratio for a positive test [LR+]=2.5; LR for a negative test [LR–]=0.06). In addition, the 5-mm threshold was accurate at ruling out any endometrial abnormality (cancer, polyp, atypical hyperplasia: LR– = 0.01). The authors suggested that TVUS can reliably rule out significant endometrial disease among postmenopausal women with vaginal bleeding.
A 2002 meta-analysis of 57 cohort studies, without consistently applied reference standards, published between 1966 and 2000 included a total of 9031 women with postmenopausal bleeding.2 Because many of the studies were felt to use inadequately stringent criteria for diagnosis, the authors limited their final analysis to only 4 studies. They concluded that a negative result using a 5-mm threshold rules out endometrial pathology with fair certainty (LR– = 0.21).
Recommendations from others
A Consensus Conference Statement from the Society of Radiologists in Ultrasound recommended that either TVUS or endometrial biopsy could be used in the initial evaluation of patients with postmenopausal bleeding.3 Using a threshold of >5 mm as abnormal, they concluded that the sensitivities of TVUS and endometrial biopsy are comparable when “sufficient tissue” is obtained with endometrial biopsy. They felt that data was currently insufficient to clearly state which technique is more effective.
TVUS is an effective, relatively noninvasive way to rule out significant pathology
Postmenopausal women need accurate diagnostic evaluation when they have abnormal bleeding. While the majority have a benign cause of bleeding, such as atrophic endometrium, many have significant pathology, including cancer (Table). Many older patients are reluctant to undergo invasive sampling studies. Cervical stenosis, a common occurrence in this age group, further complicates matters. Evidence suggests that TVUS with a full endometrial thickness of 5 mm or less, full visualization of the cavity, and no other abnormal findings, can identify patients at low risk for significant abnormalities. The false negative rate for TVUS (8%) compares quite favorably with endometrial biopsy (5%–15%) and even D&C (2%–6%).1 TVUS is an effective and relatively noninvasive strategy for ruling out significant pathology. Given the false negative rates of these techniques, all patients with postmenopausal bleeding require close follow-up.
TABLE
Differential diagnosis of postmenopausal bleeding
| Histologic diagnosis | Incidence (n=1138) |
|---|---|
| Atrophy | 59% |
| Endometrial polyp | 12% |
| Hyperplasia | 10% |
| Endometrial cancer | 10% |
| Hormonal effect | 7% |
| Cervical cancer | 2% |
| Other | <1% |
| Source: Karlsson et al 1995.4 | |
1. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998;280:1510-1517.
2. Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand 2002;81:799-816.
3. Evaluation of the woman with postmenopausal bleeding. Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med 2001;20:1025-1036.
4. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol 1995;172:1488-1494.
1. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998;280:1510-1517.
2. Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand 2002;81:799-816.
3. Evaluation of the woman with postmenopausal bleeding. Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med 2001;20:1025-1036.
4. Karlsson B, Granberg S, Wikland M, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding—a Nordic multicenter study. Am J Obstet Gynecol 1995;172:1488-1494.
Evidence-based answers from the Family Physicians Inquiries Network
Is antibiotic prophylaxis effective for recurrent acute otitis media?
For children who have recurrent episodes of clinically diagnosed acute otitis media (AOM), antibiotic prophylaxis significantly reduces recurrence, although the effect is not large (strength of recommendation: A–, based on 1 systematic review of randomized controlled trials [RCTs] with below-average quality and 1 subsequent RCT with conflicting results). Evidence is insufficient to suggest which antibiotic is most appropriate, the optimal length of prophylaxis, or the number of episodes of AOM needed to justify prophylactic treatment. Possible harms of antibiotics include vomiting, diarrhea, rash, and infection with antibiotic-resistant organisms.
Evidence summary
A systematic review of antibiotic prophylaxis for recurrent AOM examined 9 RCTs with a total of 958 children. Recurrent AOM was defined as 3 or more episodes per 6 to 18 months. The studies were low to moderate in quality (mean methodologic quality score of 11.8 out of 29 possible points). The most commonly used antibiotics were amoxicillin, cotrimoxazole, and sulfamethoxazole, given for 3 to 24 months (dosing not reported).
Children taking antibiotics had 0.11 (95% confidence interval [CI], 0.03–0.19) fewer episodes of recurrent AOM per patient-month than those taking placebo. The rate in the control group was 0.19 (95% CI, 0.13–0.26). Nine children would have to be treated per month to prevent 1 ear infection (NNT=9; 95% CI, 5–33). Only 2 of the 9 studies had statistically significant results; both used sulfisoxazole for 10 to 12 weeks and were of similar methodologic quality (12.5 out 29 points).
A trend towards a better outcome in studies that used sulfisoxazole did not reach significance compared with those using other medications (ie, ampicillin, amoxicillin, cotrimoxazole). Shorter treatment intervals (<6 months) trended toward being more effective than longer intervals, but this also did not reach significance. Children with more frequent episodes of AOM did no better than those with less frequent episodes.1
Since that review was published, another study of prophylaxis for ear infections had been published. This randomized, double blind, placebo-controlled study enrolled 194 children aged 3 months to 6 years with at least 3 documented AOM episodes in the preceding 6 months. The children were given amoxicillin (20 mg/kg/d) either once daily (n=55) or divided twice daily (n=44) or placebo (n=59). Excluding 36 noncompliant subjects, the percentages without a recurrent episode were 63% for the placebo group, 64% for the once-daily amoxicillin group, and 61% for the twice-daily amoxicillin group. There was no significant difference in the incidence of new AOM episodes among the children in the 3 groups.2
A review article states: “Many children with acute otitis media do not benefit from antimicrobial therapy because the cause of their illness is not bacterial or the infection is cleared by the immune system without use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for AOM.”3 The lack of criteria for determining which children need antibiotic therapy for AOM makes it more difficult to select children for antibiotic prophylaxis against recurrent AOM.
Recommendations from others
The American Academy of Pediatrics and the American Academy of Family Physicians do not address antibiotic prophylaxis for recurrent episodes of otitis media in their guidelines. Both groups recommend modification of risk factors to decrease recurrent AOM, including promoting breastfeeding during the first 6 months, avoiding bottle-propping, reducing or eliminating pacifier use in the second 6 months of life, and eliminating exposure to secondhand smoke.
They also recommend pneumococcal conjugate vaccine to reduce vaccine-serotype pneumococcal otitis and live-attenuated influenza vaccine during respiratory virus season for children aged >2 years.
Treatment options include observation, antibiotic prophylaxis, tympanostomy tubes; no option is ideal for all
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax, Va
Treatment options for children with recurrent acute otitis media include observation with treatment of recurrences, antibiotic prophylaxis, or tympanostomy tubes. No option is ideal for all children.
Multiple factors can be weighed to choose more or less aggressive treatment including frequency and severity of infections, exposure to secondhand smoke, day care enrollment, sibling history, parental comfort and anxiety, presence of serous otitis media between episodes, time of year, and effect on overall hearing. Measures to prevent otitis media and reserving the diagnosis of acute otitis media for “true” purulent infections can help limit the number of children diagnosed with recurrent disease.
1. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 1993;270:1344-1351.
2. Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr Infect Dis 1997;16:376-381.
3. Pichichero ME. Acute otitis media: part II. Treatment in an era of increasing antibiotic resistance. Am Fam Physician 2000;61:2410-2416.
4. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451-1465.
For children who have recurrent episodes of clinically diagnosed acute otitis media (AOM), antibiotic prophylaxis significantly reduces recurrence, although the effect is not large (strength of recommendation: A–, based on 1 systematic review of randomized controlled trials [RCTs] with below-average quality and 1 subsequent RCT with conflicting results). Evidence is insufficient to suggest which antibiotic is most appropriate, the optimal length of prophylaxis, or the number of episodes of AOM needed to justify prophylactic treatment. Possible harms of antibiotics include vomiting, diarrhea, rash, and infection with antibiotic-resistant organisms.
Evidence summary
A systematic review of antibiotic prophylaxis for recurrent AOM examined 9 RCTs with a total of 958 children. Recurrent AOM was defined as 3 or more episodes per 6 to 18 months. The studies were low to moderate in quality (mean methodologic quality score of 11.8 out of 29 possible points). The most commonly used antibiotics were amoxicillin, cotrimoxazole, and sulfamethoxazole, given for 3 to 24 months (dosing not reported).
Children taking antibiotics had 0.11 (95% confidence interval [CI], 0.03–0.19) fewer episodes of recurrent AOM per patient-month than those taking placebo. The rate in the control group was 0.19 (95% CI, 0.13–0.26). Nine children would have to be treated per month to prevent 1 ear infection (NNT=9; 95% CI, 5–33). Only 2 of the 9 studies had statistically significant results; both used sulfisoxazole for 10 to 12 weeks and were of similar methodologic quality (12.5 out 29 points).
A trend towards a better outcome in studies that used sulfisoxazole did not reach significance compared with those using other medications (ie, ampicillin, amoxicillin, cotrimoxazole). Shorter treatment intervals (<6 months) trended toward being more effective than longer intervals, but this also did not reach significance. Children with more frequent episodes of AOM did no better than those with less frequent episodes.1
Since that review was published, another study of prophylaxis for ear infections had been published. This randomized, double blind, placebo-controlled study enrolled 194 children aged 3 months to 6 years with at least 3 documented AOM episodes in the preceding 6 months. The children were given amoxicillin (20 mg/kg/d) either once daily (n=55) or divided twice daily (n=44) or placebo (n=59). Excluding 36 noncompliant subjects, the percentages without a recurrent episode were 63% for the placebo group, 64% for the once-daily amoxicillin group, and 61% for the twice-daily amoxicillin group. There was no significant difference in the incidence of new AOM episodes among the children in the 3 groups.2
A review article states: “Many children with acute otitis media do not benefit from antimicrobial therapy because the cause of their illness is not bacterial or the infection is cleared by the immune system without use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for AOM.”3 The lack of criteria for determining which children need antibiotic therapy for AOM makes it more difficult to select children for antibiotic prophylaxis against recurrent AOM.
Recommendations from others
The American Academy of Pediatrics and the American Academy of Family Physicians do not address antibiotic prophylaxis for recurrent episodes of otitis media in their guidelines. Both groups recommend modification of risk factors to decrease recurrent AOM, including promoting breastfeeding during the first 6 months, avoiding bottle-propping, reducing or eliminating pacifier use in the second 6 months of life, and eliminating exposure to secondhand smoke.
They also recommend pneumococcal conjugate vaccine to reduce vaccine-serotype pneumococcal otitis and live-attenuated influenza vaccine during respiratory virus season for children aged >2 years.
Treatment options include observation, antibiotic prophylaxis, tympanostomy tubes; no option is ideal for all
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax, Va
Treatment options for children with recurrent acute otitis media include observation with treatment of recurrences, antibiotic prophylaxis, or tympanostomy tubes. No option is ideal for all children.
Multiple factors can be weighed to choose more or less aggressive treatment including frequency and severity of infections, exposure to secondhand smoke, day care enrollment, sibling history, parental comfort and anxiety, presence of serous otitis media between episodes, time of year, and effect on overall hearing. Measures to prevent otitis media and reserving the diagnosis of acute otitis media for “true” purulent infections can help limit the number of children diagnosed with recurrent disease.
For children who have recurrent episodes of clinically diagnosed acute otitis media (AOM), antibiotic prophylaxis significantly reduces recurrence, although the effect is not large (strength of recommendation: A–, based on 1 systematic review of randomized controlled trials [RCTs] with below-average quality and 1 subsequent RCT with conflicting results). Evidence is insufficient to suggest which antibiotic is most appropriate, the optimal length of prophylaxis, or the number of episodes of AOM needed to justify prophylactic treatment. Possible harms of antibiotics include vomiting, diarrhea, rash, and infection with antibiotic-resistant organisms.
Evidence summary
A systematic review of antibiotic prophylaxis for recurrent AOM examined 9 RCTs with a total of 958 children. Recurrent AOM was defined as 3 or more episodes per 6 to 18 months. The studies were low to moderate in quality (mean methodologic quality score of 11.8 out of 29 possible points). The most commonly used antibiotics were amoxicillin, cotrimoxazole, and sulfamethoxazole, given for 3 to 24 months (dosing not reported).
Children taking antibiotics had 0.11 (95% confidence interval [CI], 0.03–0.19) fewer episodes of recurrent AOM per patient-month than those taking placebo. The rate in the control group was 0.19 (95% CI, 0.13–0.26). Nine children would have to be treated per month to prevent 1 ear infection (NNT=9; 95% CI, 5–33). Only 2 of the 9 studies had statistically significant results; both used sulfisoxazole for 10 to 12 weeks and were of similar methodologic quality (12.5 out 29 points).
A trend towards a better outcome in studies that used sulfisoxazole did not reach significance compared with those using other medications (ie, ampicillin, amoxicillin, cotrimoxazole). Shorter treatment intervals (<6 months) trended toward being more effective than longer intervals, but this also did not reach significance. Children with more frequent episodes of AOM did no better than those with less frequent episodes.1
Since that review was published, another study of prophylaxis for ear infections had been published. This randomized, double blind, placebo-controlled study enrolled 194 children aged 3 months to 6 years with at least 3 documented AOM episodes in the preceding 6 months. The children were given amoxicillin (20 mg/kg/d) either once daily (n=55) or divided twice daily (n=44) or placebo (n=59). Excluding 36 noncompliant subjects, the percentages without a recurrent episode were 63% for the placebo group, 64% for the once-daily amoxicillin group, and 61% for the twice-daily amoxicillin group. There was no significant difference in the incidence of new AOM episodes among the children in the 3 groups.2
A review article states: “Many children with acute otitis media do not benefit from antimicrobial therapy because the cause of their illness is not bacterial or the infection is cleared by the immune system without use of a drug. At present, we do not have clinical criteria for distinguishing which children are in need of antibiotic therapy for AOM.”3 The lack of criteria for determining which children need antibiotic therapy for AOM makes it more difficult to select children for antibiotic prophylaxis against recurrent AOM.
Recommendations from others
The American Academy of Pediatrics and the American Academy of Family Physicians do not address antibiotic prophylaxis for recurrent episodes of otitis media in their guidelines. Both groups recommend modification of risk factors to decrease recurrent AOM, including promoting breastfeeding during the first 6 months, avoiding bottle-propping, reducing or eliminating pacifier use in the second 6 months of life, and eliminating exposure to secondhand smoke.
They also recommend pneumococcal conjugate vaccine to reduce vaccine-serotype pneumococcal otitis and live-attenuated influenza vaccine during respiratory virus season for children aged >2 years.
Treatment options include observation, antibiotic prophylaxis, tympanostomy tubes; no option is ideal for all
Alex Krist, MD
Fairfax Family Practice Residency, Virginia Commonwealth University, Fairfax, Va
Treatment options for children with recurrent acute otitis media include observation with treatment of recurrences, antibiotic prophylaxis, or tympanostomy tubes. No option is ideal for all children.
Multiple factors can be weighed to choose more or less aggressive treatment including frequency and severity of infections, exposure to secondhand smoke, day care enrollment, sibling history, parental comfort and anxiety, presence of serous otitis media between episodes, time of year, and effect on overall hearing. Measures to prevent otitis media and reserving the diagnosis of acute otitis media for “true” purulent infections can help limit the number of children diagnosed with recurrent disease.
1. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 1993;270:1344-1351.
2. Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr Infect Dis 1997;16:376-381.
3. Pichichero ME. Acute otitis media: part II. Treatment in an era of increasing antibiotic resistance. Am Fam Physician 2000;61:2410-2416.
4. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451-1465.
1. Williams RL, Chalmers TC, Stange KC, Chalmers FT, Bowlin SJ. Use of antibiotics in preventing recurrent acute otitis media and in treating otitis media with effusion. A meta-analytic attempt to resolve the brouhaha. JAMA 1993;270:1344-1351.
2. Roark R, Berman S. Continuous twice daily or once daily amoxicillin prophylaxis compared with placebo for children with recurrent acute otitis media. Pediatr Infect Dis 1997;16:376-381.
3. Pichichero ME. Acute otitis media: part II. Treatment in an era of increasing antibiotic resistance. Am Fam Physician 2000;61:2410-2416.
4. American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113:1451-1465.
Evidence-based answers from the Family Physicians Inquiries Network
How effective is gastric bypass for weight loss?
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
Gastric bypass results in weight loss of approximately 33% at 2 years and 25% at 8 years (strength of recommendation [SOR]: B, based on a cohort study). Gastric bypass is one type of bariatric surgery, which also includes gastroplasty and gastric banding procedures ( Figure 1 ). These procedures all can produce enough weight loss to measurably improve health, but they differ in the amount of long-term weight loss, as well as side effects, which can be serious.
Gastric bypass is more effective than gastroplasty for weight loss and is associated with fewer revisions, but it has more side effects (SOR: A, based on a systematic review). Limited evidence suggests that gastric bypass produces more weight loss than gastric banding (SOR: B, based on a cohort study).
Bariatric surgery, including gastric bypass, improves conditions comorbid with obesity, including diabetes, abnormal lipid profiles, and low quality-of-life scores. It decreases the incidence of hypertension at 2 years after surgery, but whether this effect is sustained is unclear (SOR: B, based on a cohort study and multiple case series). Bariatric surgery also improves obstructive sleep apnea, obesity hypoventilation syndrome, menstrual irregularity, and female urinary stress incontinence (SOR: C, based on multiple case series). Bariatric surgery has a complication rate of 13% and a mortality rate of 0.2% (SOR: B, based on 1 cohort study).
FIGURE 1
Bariatric surgical techniques for weight loss
Evidence summary
A systematic review comparing bariatric surgery with conventional medical therapy for obesity included 1 randomized controlled trial and the Swedish Obesity Study, a large cohort study with matched controls. Surgery produced 23 to 28 kg more weight loss at 2 years.1 The study demonstrated 33% ± 10% weight loss for gastric bypass and 0% for medical therapy (not described) at 2 years,2 and 25% ± 6% loss vs 0.9% gain at 8 years.3 Among bariatric surgical techniques, patients undergoing gastric bypass lost more weight than those with gastroplasty (using staples to partition the stomach, either horizontally or vertically ( Figure 1 ) (P=.057, not significant) or gastric banding (placing a constricting ring around the stomach) (P<.05) at 8 years.3
The same systematic review assessed multiple randomized controlled trials comparing gastric bypass with gastroplasty and found greater weight loss, fewer revisions, and more side effects from gastric bypass ( Figure 2 ).1 Five trials comparing gastric bypass with horizontal gastroplasty demonstrated significantly greater weight loss from gastric bypass. Five other trials comparing weight loss from gastric bypass with vertical gastroplasty produced mixed results, with 3 trials favoring gastric bypass and 2 showing no difference.1 Fewer patients required revision after gastric bypass (0%–4%) compared with vertical gastroplasty (9%) or horizontal gastroplasty (19%–40%). One included trial found that postoperative dumping syndrome (28% vs 0%, P<0.05) and heartburn (59% vs 32%, P<.05) were more common with gastric bypass than with gastroplasty.1
Bariatric surgery, including gastric bypass, improves a variety of obesity-related comorbid conditions. Diabetes prevalence decreased among gastric bypass patients at 2 years (0.0% vs 4.7%, P<0.005) and 8 years (3.6% vs 18.5%, P<.0005) compared with those receiving medical therapy.2,3 In a case series involving 154 diabetic gastric bypass patients, diabetes resolved for 83% by 1 year, and for 86% at 5 to 7 years.4 In several case series, most patients became euglycemic and discontinued insulin or oral agents.
In the Swedish Obesity Study, hypertriglyceridemia decreased postoperatively but hypercholesterolemia did not.5 In a case series, bariatric surgery reduced triglycerides (50%) as well as total cholesterol (15%) (P<.05 for both) at 6 months and significantly increased high-density lipoprotein cholesterol levels at 1 and 5 years.6
Bariatric surgery significantly lowered the incidence of hypertension at 2 years (3.2%) compared with conventional treatment (9.9%), but after 8 years this difference disappeared.2,3,5 However, in multiple large case series with morbidly obese patients, hypertension resolved or improved. The largest study showed resolution of hypertension for 69% at 1 to 2 years (91% follow-up), 66% at 5 to 7 years (50% follow-up), and 51% at 10 to 12 years (37% follow-up).4
Bariatric surgery improved obstructive sleep apnea and obesity hypoventilation syndrome in 2 case series. In one, Epworth Sleepiness Scale scores, minimum O2 saturation, and other measures improved significantly (P<.001) by 3 to 21 months after surgery.7
In another case series, menstrual irregularities decreased from 40.4% to 4.6% following surgery (P<.001) among women who lost 50% of their excess weight.8 The incidence of urinary stress incontinence also decreased significantly (61.2% to 11.6%, P<.001 in this study8 ). The Swedish Obesity Study found significant improvements in Health-Related Quality of Life scores at 2 years with surgery vs conventional treatment.9
Bariatric surgery, including gastric bypass, has significant postoperative morbidity and mortality. Thirteen percent of patients in the Swedish Obesity Study experienced peri-operative complications, including pulmonary symptoms (6.2%), abdominal infection (2.1%), wound complications (1.8%), bleeding (0.9%), thromboembolic events (0.8%), and other miscellaneous complications (4.8%). Postoperative complications required reoperation for 2.2% of surgical patients, and there were 4 postoperative deaths (0.2% of the operative patients; 3 due to leakage, and 1 due to a technical laparoscopic error).2
Nutritional and vitamin deficiencies are common following gastric bypass, including deficiencies of vitamin B12, iron, folate, and calcium. Lifelong nutritional supplementation is generally necessary following this procedure.10
FIGURE 2
Long-term weight loss with bariatric surgery
Long-term weight loss with bariatric surgery: comparison of controls, horizontal gastric banding (Banding), vertical band-ed gastroplasty (VPG), and gastric bypass (GBP). Source: Sjostrom et al 2000. 3
Recommendations from others
A 1991 National Institutes of Health consensus conference suggested consideration of obesity surgery for patients with a body-mass index ≥40, or ≥35 plus severe obesity-related medical comorbidities (such as severe sleep apnea, obesity hypoventilation syndrome, obesity-related cardiomyopathy, or severe diabetes) who have not been successfully treated with non-surgical attempts at weight reduction.
Selected patients should be well-informed and motivated, with acceptable operative risk. A multidisciplinary team with medical, surgical, psychiatric, and nutritional expertise should evaluate patients who are candidates for surgery. An experienced surgeon, working in a clinical setting with adequate support for all aspects of management and assessment, should perform the surgery.
Lifelong medical surveillance is necessary after surgery, and patients should be selected who are likely to comply with this.11
Bariatric surgery is an important option for select patients
Tim Mott, MD
Family Practice Staff, Navy Hospital, Pensacola, Fla
The lack of successful interventions for obesity is frustrating. This is accentuated as obesity is increasingly recognized as the proverbial forest in which we find ourselves hacking at the “trees” of diabetes, hypertension, dyslipidemia, and many other diseases. As we focus on this, the second-leading preventable cause of death, we find ourselves uniquely skilled as family physicians to offer balanced advice and advocacy.12
Bariatric surgery is an important option for select patients. For such a patient, I continuously advocate for lifestyle changes, document all non-surgical measures pursued (important for third-party review), discuss realistic expectations and risks, and direct the patient to a trusted bariatric surgery center. For the postsurgical patient, I reinforce the lifestyle commitments, ensure ongoing vitamin and mineral supplementation, and help monitor for possible complications.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
1. Colquitt J, Clegg A, Sidhu M, Royle P. Surgery for morbid obesity (Cochrane Review). In: The Cochrane Library, Issue 4, 2003; Chichester, UK: John Wiley & Sons, Ltd.
2. Torgerson JS, Sjostrom L. The Swedish Obese Subjects (SOS) study—rationale and results. Int J Obes Relat Metab Disord 2001;25 Supp1:S2-S4.
3. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25.
4. Sugerman HJ, Wolfe LG, Sica DA, Clore JN. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-758.
5. Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-484.
6. Brolin RE, Bradley LJ, Wilson AC, Cody RP. Lipid risk profile and weight stability after gastric restrictive operations for morbid obesity. J Gastrointest Surg 2000;4:464-469.
7. Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg 2003;13:58-61.
8. Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr 1988;7:147-153.
9. Karlsson J, Sjostrom L, Sullivan M. Swedish obese subjects (SOS)- an intervention study of obesity. Two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord 1998;22:113-126.
10. Kushner R. Managing the obese patient after bariatric surgery: A case report of severe malnutrition and review of the literature. J Parenteral Enteral Nutrition 2000;24:126-132.
11. NIH conference: Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med 1991;115:956-961.
12. Flegal K, Carroll M, Ogden C, et al. Prevalence trends in obesity among US adults, 1999–2000. JAMA 2002;288:1723-1727.
Evidence-based answers from the Family Physicians Inquiries Network