User login
2015 Update on contraception
Unintended pregnancy remains a serious problem in the United States, and the rate continues to increase. Currently, the unintended pregnancy rate is at an all-time high, estimated at 51% of all pregnancies.1 Sobering statistics reveal that the United States has a significantly higher rate of unintended pregnancy than any other developed country.2,3
So the question remains: If we have been introducing new contraceptive methods, why do unintended pregnancy rates keep rising in the United States?
The answer: Key barriers prevent women from attaining their desired contraceptive—foremost among them, cost.
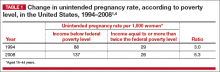
The unintended pregnancy rate is highest among women who are poor, young (aged 18–24 years), minorities, or cohabitating.1 The unintended pregnancy rate among poor women (income below the federal poverty level) of reproductive age has increased over the past few decades, whereas the rate among high-income women (more than twice the federal poverty level) has declined.1 The unintended pregnancy rate discrepancy between poor women and those with means has increased 77%, from a threefold difference in 1995 to a difference of more than fivefold in 2008 (TABLE 1).1,4
These data indicate that we are doing well providing contraception to women of means. However, as a society, we need to improve how we deliver contraceptives—especially highly effective methods—to poor women. As one might expect, given these numbers, low-income women have rates of abortion and unplanned birth that are nearly 6 times higher than their higher-income counterparts.1 The resultant cost to society is substantial, with 68% of unplanned births paid for by public insurance programs such as Medicaid, compared with 38% of planned births.5
As women’s health providers, we must work to improve these numbers and advocate for our patients to help them gain access to the contraceptives they need in accordance with their reproductive life plan. In this article, we hope to put this information in context as we:
- review reports and studies that evaluatethe trend of unintended pregnancy
- describe one state initiative that reduced the rates of unintended pregnancy, birth, and abortion
- introduce a new, highly effective levonorgestrel-releasing intrauterine system (Liletta) being marketed with the goal of reaching women who receive care from private physicians as well as public health clinics.
National and state snapshots reveal shifting proportions of intended, unintended pregnancies
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. https://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 29, 2015.
Recent studies have demonstrated trends in unplanned pregnancy over the past 2 decades on both a national and statewide level. Data from the National Survey of Family Growth have highlighted trends in abortion and miscarriage, and data from the National Center for Health Statistics have shed light on birth trends. Of 6 million births in 2008, 51% were unintended.1 Unintended pregnancy was defined as a gestation that was mistimed or unwanted. Intended pregnancy was defined as one that was desired at the time it occurred or sooner.
2008 data focus on the national level
Although the overall pregnancy rate for US women aged 15 to 44 years is relatively unchanged, there is a small change in whether or not the pregnancy was intended. For 2008, as the rate of intended pregnancy dropped slightly, from 54 to 51 pregnancies per 1,000 women, the unintended pregnancy rate increased by 10%, from 49 to 54 pregnancies per 1,000 women. Proportionally, unintended pregnancies that resulted in abortion declined from 47% to 40%, whereas the rate of unintended pregnancies ending in birth increased to 27 births per 1,000 women.1
2010 data focus on individual states
We now have state-specific data on trends in unintended pregnancy rates from 2002 to 2010 in the United States.6 In 28 of 50 states, more than half of all pregnancies were unintended, with rates ranging from 36% to 62%. The median rate was 47 unintended pregnancies per 1,000 women aged 15 to 44, with the lowest unintended pregnancy rate in New Hampshire at 32 per 1,000 women and highest rates in Delaware, Hawaii, and New York at 61 to 62 unintended pregnancies per 1,000 women.6
Between 2002 and 2010, unintended pregnancy rates fell 5% or more in 18 states and rose 5% or more in 4 states. In the remaining 12 states for which there are data, unintended pregnancy rates remained unchanged.
Interestingly, 16 states had increases in unintended pregnancy rates of 5% or more between 2002 and 2006. The trend reversed between 2006 and 2010, during which 28 of 41 states with available data experienced decreases of 5% or more and only 1 state experienced an increase of 5% or more. These latest numbers suggest we may be making some progress in reducing the overall rate of unintended pregnancy.
What this EVIDENCE means for practice
Although the rate of unintended pregnancy is declining in some states, the national rate is still increasing. This information emphasizes the need for all providers to consider initiating discussions about pregnancy intentions—a step that may be as important as obtaining blood pressure and weight. When women are seen for any health visit, they should be asked about their reproductive plans. The Centers for Disease Control and Prevention (CDC) has issued a helpful set of questions to guide the discussion of timing and planning pregnancy. It also provides useful information on ways to increase utilization of long-acting reversible contraceptives (LARCs) to help realize the goal of fewer unintended pregnancies. (For more on this discussion, see “How to motivate your patient to create a reproductive life plan,” below.)
How to motivate your patient to create a reproductive life plan
The Centers for Disease Control and Prevention (CDC) offers a tool for health care professionals to use to encourage patients to think about their reproductive goals and make a plan to facilitate those goals. It’s available at: http://www.cdc.gov/preconception/documents/rlphealthproviders.pdf.
Questions to ask your patient
- Do you plan to have any (more) children at any time in your future?
IF YES
- How many children would you like to have?
- How long would you like to wait until you or your partner become pregnant?
- What family planning method do you plan to use until you or your partner are ready to become pregnant?
- How sure are you that you will be able to use this method without any problems?
IF NO
- What family planning method will you use to avoid pregnancy?
- How sure are you that you will be able to use this method without any problems?
- People’s plans change. Is it possible that you or your partner could ever decide to become pregnant?
Action plan
Encourage your patient to make a plan and take action. Remind her that the plan doesn’t have to be set in stone.
How Colorado broke down barriers to highly effective contraception—and saved $42.5 million in 1 year
Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
The Contraceptive CHOICE Project in St. Louis County, Missouri, is an incredible success story. In it, clinicians made highly effective LARCs readily available and free of charge. When a large percentage of women chose a LARC method, the unintended pregnancy and abortion rates declined.7 In Colorado, providers put this study into practice, creating a successful statewide initiative to reduce the unintended pregnancy rate.8
The data behind LARC methods
LARC methods are backed by endorsements from the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the CDC, and the World Health Organization, which recommend them for adolescents because of their superiority to shorter-acting methods. With LARCs, failure rates are lower and compliance is greater, making them ideal for adolescents, who have high unintended pregnancy rates.1
However, based on 2011 data, only 2% of LARC users nationwide are aged 20 or younger. A number of barriers prevent young women from obtaining LARCs, including lack of education, limited access to and availability of the contraceptives, and, importantly, cost. Many state plans have adopted Medicaid expansions to reduce barriers to LARCs. However, this benefit is still not available in many states, Colorado being 1 of them.
How the Colorado initiative worked
In 2005, 40% of Colorado’s births were unintended, and 60% of those unintended births occurred in women aged 15 to 24 years. About three-quarters of women who were using a contraceptive method at the time of unintended pregnancy reported that it was a low-cost, high-failure method such as condoms or withdrawal.
In response, Colorado’s Department of Public Health and Environment and the Colorado Family Planning Initiative (CFPI) used private funds from an anonymous foundation to provide LARC products at no cost to the Title X–funded clinics in the state.
The initiative began in 2009 in clinics that served 95% of the state’s total population. The funding provided the products themselves (intrauterine device [IUD], implant), as well as training for providers and staff.
Before the initiative began, 52,645 clients received services in these clinics annually. In the third year of the initiative, that number had increased to 64,928 annually. About 55% of clients receiving services both preinitiative and postinitiative were younger than 25 years, and most (92% in 2011) had income below the poverty level.
LARC use increased fourfold over the 3 years of the funded program, from less than 4.5% to 19.4% in the third year. Contraceptive implant use increased tenfold, and IUD use increased by almost threefold. At the same time, oral contraceptive use declined 13%. Before the initiative, only 620 young, low-income women used a LARC method; afterward, 8,435 did.
These changes in contraceptive practice triggered a significant decline in pregnancy rates (TABLE 2) and abortion rates (TABLE 3). Abortion rates increased 8% among 20- to 24-year-olds who were not enrolled in the initiative and decreased 18% among those who were. The proportion of high-risk births (births to unmarried, low-income women with less than a high school education) dropped 24% after the initiative began. The proportion of high-risk births in counties not receiving CFPI funds stayed the same at 7%.
Colorado program saved $42.5 million in public funds
This Colorado program demonstrated that the CHOICE Project can be translated to a statewide initiative. Whereas CHOICE enrolled 9,256 women over 4 years, the Colorado initiative included more than 50,000 clients annually over 3 years. Colorado did not use any state funds for this project, which resulted in significant decreases in the unintended birth rate, abortion rate, and rate of high-risk births.
The Colorado governor’s office estimates that the CFPI saved $5.68 in Medicaid costs for Colorado for every dollar spent on contraceptives. In just 1 year (2010), the program saved approximately $42.5 million in public funds.
Ironically, despite the success of this project, the Colorado legislature denied further funding once the initial financial support ceased.
What this EVIDENCE means for practice
The Colorado program demonstrates that we all can provide LARC methods in practice, especially to young women. In this population, use of highly effective contraception resulted in fewer unintended pregnancies, births, and abortions statewide.
We also need to advocate for our patients, particularly those who have less means and rely on public assistance. Public funding of LARC methods clearly improves outcomes at an individual and population level.
A new, more affordable IUD enters the market
Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
Programs such as the Contraceptive CHOICE Project and the Colorado Family Planning Initiative relied on private foundations for financial support, largely because of the high cost of the IUDs and implants currently available in the United States. Even with the Affordable Care Act (ACA) reducing the costs of LARC products and other contraceptives for patients, there are still many women not covered by these programs. For example, “grandfathered” health insurance plans do not need to follow some aspects of the ACA.
Just as important, the high cost of LARC products takes a toll on providers and clinics that must finance the cost per unit to have stock on hand and then wait for months for reimbursement by insurance companies. As a result, some providers do not stock IUDs and implants and only order them as they are needed and approved by insurance for a particular patient. These barriers limit access to LARC methods and reduce the number of women who receive the products.8
Liletta is less expensive than other IUDs
Enter Liletta, a new levonorgestrel-releasing, 52-mg intrauterine system (IUS) that has been in clinical trials since 2009.9 ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an Intrauterine System) was initiated by Medicines360, a unique nonprofit pharmaceutical company committed to ensuring access to reproductive health products for all women (private and public sector).
ACCESS IUS is the largest IUD approval study ever performed exclusively in the United States. The Phase 3, open-label clinical trial was conducted at 29 sites around the United States, enrolling healthy, nonpregnant, sexually active women aged 16 to 45 years with regular menstrual cycles. Both nulliparous and parous women were included, with no weight or body mass index (BMI) restrictions applied. The study is ongoing and will continue for as long as 7 years. Eisenberg and colleagues published the data used for initial approval for 3 years of use in the United States and Europe.9
Details of the trial
A total of 1,600 women aged 16 to 35 years comprised the group in which efficacy was evaluated. An additional 151 women aged 36 to 45 years were evaluated for safety only. Of the enrolled women, 1,011 (58%) were nulliparous, making ACCESS IUS the largest product approval study of nulliparous women. In addition, 438 women (25.1%) were obese, and 5% of these women had a BMI greater than 40 kg/m2.
Liletta was placed successfully in 1,714 women (97.9%). Fifteen women did not have placement attempted due to uterine factors (the uterus could not be sounded, or the sound was <5.5 cm) or factors unrelated to the product or inserter. In women in whom placement was attempted, the success rate was 98.7%.
The first-year Pearl index for Liletta was 0.15. Life-table pregnancy rates were 0.14 through year 1 and 0.55 through year 3. Four of the 6 pregnancies reported through 3 years of use were ectopic. Adverse events and their incidence, occurring in more than 2% of users, were acne (6%), expulsion (3.5%), dyspareunia (2.8%), and mastalgia (2.0%). The most common adverse events leading to discontinuation were expulsion (3.5%), bleeding complaints (1.5%), acne (1.3%), and mood swings (1.3%).
Uterine perforation with Liletta was diagnosed in 2 participants (0.11%). Expulsion occurred in 62 users (3.5%) and was more frequent among parous than nulliparous women (5.6% vs 2.0%, respectively; P<.001). Most (80.6%) of the expulsions were reported in the first year of product use. Pelvic infection was reported in 10 participants (0.6%), and all cases resolved with outpatient antibiotic treatment.9
Keep in mind that this is an ongoing study—not all women have reached a full 3 years of use. Updates on efficacy and adverse events will be published in the future. This current publication demonstrates the high efficacy and safety of the product through 3 years of use, permitting its approval for contraception in the United States.
In Europe, the product is approved for both contraception and the treatment of heavy menstrual bleeding, based on a European study.10
What this EVIDENCE means for practice
Liletta is a branded product (not generic) designed to be similar to Mirena, with the same size, frame, hormone content, and hormone release rate.11 Medicines360 has entered a groundbreaking marketing partnership with Actavis to make Liletta widely available and affordable. For most public sector providers and clinics in the United States, Liletta costs only $50, significantly less than other LARC methods available in the United States. Actavis also has a program that ensures that any woman lacking insurance coverage for an IUD and not receiving care at a public sector clinic will not be charged more than $75 for her IUD. However, the price of the device is only one aspect of its overall cost, as women still need to pay for any office visit or insertion fees.
For society, this unique business partnership has to include providers and patients as well. Sales of Liletta in the private sector will support the very low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we can all help poor women access very affordable highly effective contraception.
For providers, Liletta is a lower-cost alternative to currently available hormonal IUDs and should perform well over the long term. The highly successful use of Liletta in nulliparous women demonstrates its safety in this population. The 3-year approval is the first step, as the Phase 3 study continues. In the future, Liletta is expected to be approved for 5 years or longer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
2. Guttmacher Institute. Fact Sheet: Unintended Pregnancy in the United States. http://www.guttmacher.org/pubs/FB-Unintended-Pregnancy-US.html. Published February 2015. Accessed June 29, 2015.
3. Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends and outcomes. Stud Fam Plann. 2010;41(4):241–250.
4. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspectives. 1998;30(1):24–29, 46.
5. Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care. National and States Estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed June 29, 2015.
6. Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. http://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 30, 2015.
7. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the ContraceptiveCHOICE Project in review. J Womens Health. 2015; 24(5):349–353.
8. Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
9. Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
10. Mawet M, Nollevaux F, Nizet D, et al. Impact of a new levonorgestrel intrauterine system, Levosert, on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contracept Reprod Health Care. 2014;19(3):169–179.
11. Gopalakrishnan M, Liu T, Gobburu J, Creinin MD. Levonorgestrel release rates with LNG20, a new levonorgestrel intrauterine system. Obstet Gynecol. 2015;125(suppl 1): 62S–63S.
Unintended pregnancy remains a serious problem in the United States, and the rate continues to increase. Currently, the unintended pregnancy rate is at an all-time high, estimated at 51% of all pregnancies.1 Sobering statistics reveal that the United States has a significantly higher rate of unintended pregnancy than any other developed country.2,3
So the question remains: If we have been introducing new contraceptive methods, why do unintended pregnancy rates keep rising in the United States?
The answer: Key barriers prevent women from attaining their desired contraceptive—foremost among them, cost.
The unintended pregnancy rate is highest among women who are poor, young (aged 18–24 years), minorities, or cohabitating.1 The unintended pregnancy rate among poor women (income below the federal poverty level) of reproductive age has increased over the past few decades, whereas the rate among high-income women (more than twice the federal poverty level) has declined.1 The unintended pregnancy rate discrepancy between poor women and those with means has increased 77%, from a threefold difference in 1995 to a difference of more than fivefold in 2008 (TABLE 1).1,4
These data indicate that we are doing well providing contraception to women of means. However, as a society, we need to improve how we deliver contraceptives—especially highly effective methods—to poor women. As one might expect, given these numbers, low-income women have rates of abortion and unplanned birth that are nearly 6 times higher than their higher-income counterparts.1 The resultant cost to society is substantial, with 68% of unplanned births paid for by public insurance programs such as Medicaid, compared with 38% of planned births.5
As women’s health providers, we must work to improve these numbers and advocate for our patients to help them gain access to the contraceptives they need in accordance with their reproductive life plan. In this article, we hope to put this information in context as we:
- review reports and studies that evaluatethe trend of unintended pregnancy
- describe one state initiative that reduced the rates of unintended pregnancy, birth, and abortion
- introduce a new, highly effective levonorgestrel-releasing intrauterine system (Liletta) being marketed with the goal of reaching women who receive care from private physicians as well as public health clinics.
National and state snapshots reveal shifting proportions of intended, unintended pregnancies
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. https://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 29, 2015.
Recent studies have demonstrated trends in unplanned pregnancy over the past 2 decades on both a national and statewide level. Data from the National Survey of Family Growth have highlighted trends in abortion and miscarriage, and data from the National Center for Health Statistics have shed light on birth trends. Of 6 million births in 2008, 51% were unintended.1 Unintended pregnancy was defined as a gestation that was mistimed or unwanted. Intended pregnancy was defined as one that was desired at the time it occurred or sooner.
2008 data focus on the national level
Although the overall pregnancy rate for US women aged 15 to 44 years is relatively unchanged, there is a small change in whether or not the pregnancy was intended. For 2008, as the rate of intended pregnancy dropped slightly, from 54 to 51 pregnancies per 1,000 women, the unintended pregnancy rate increased by 10%, from 49 to 54 pregnancies per 1,000 women. Proportionally, unintended pregnancies that resulted in abortion declined from 47% to 40%, whereas the rate of unintended pregnancies ending in birth increased to 27 births per 1,000 women.1
2010 data focus on individual states
We now have state-specific data on trends in unintended pregnancy rates from 2002 to 2010 in the United States.6 In 28 of 50 states, more than half of all pregnancies were unintended, with rates ranging from 36% to 62%. The median rate was 47 unintended pregnancies per 1,000 women aged 15 to 44, with the lowest unintended pregnancy rate in New Hampshire at 32 per 1,000 women and highest rates in Delaware, Hawaii, and New York at 61 to 62 unintended pregnancies per 1,000 women.6
Between 2002 and 2010, unintended pregnancy rates fell 5% or more in 18 states and rose 5% or more in 4 states. In the remaining 12 states for which there are data, unintended pregnancy rates remained unchanged.
Interestingly, 16 states had increases in unintended pregnancy rates of 5% or more between 2002 and 2006. The trend reversed between 2006 and 2010, during which 28 of 41 states with available data experienced decreases of 5% or more and only 1 state experienced an increase of 5% or more. These latest numbers suggest we may be making some progress in reducing the overall rate of unintended pregnancy.
What this EVIDENCE means for practice
Although the rate of unintended pregnancy is declining in some states, the national rate is still increasing. This information emphasizes the need for all providers to consider initiating discussions about pregnancy intentions—a step that may be as important as obtaining blood pressure and weight. When women are seen for any health visit, they should be asked about their reproductive plans. The Centers for Disease Control and Prevention (CDC) has issued a helpful set of questions to guide the discussion of timing and planning pregnancy. It also provides useful information on ways to increase utilization of long-acting reversible contraceptives (LARCs) to help realize the goal of fewer unintended pregnancies. (For more on this discussion, see “How to motivate your patient to create a reproductive life plan,” below.)
How to motivate your patient to create a reproductive life plan
The Centers for Disease Control and Prevention (CDC) offers a tool for health care professionals to use to encourage patients to think about their reproductive goals and make a plan to facilitate those goals. It’s available at: http://www.cdc.gov/preconception/documents/rlphealthproviders.pdf.
Questions to ask your patient
- Do you plan to have any (more) children at any time in your future?
IF YES
- How many children would you like to have?
- How long would you like to wait until you or your partner become pregnant?
- What family planning method do you plan to use until you or your partner are ready to become pregnant?
- How sure are you that you will be able to use this method without any problems?
IF NO
- What family planning method will you use to avoid pregnancy?
- How sure are you that you will be able to use this method without any problems?
- People’s plans change. Is it possible that you or your partner could ever decide to become pregnant?
Action plan
Encourage your patient to make a plan and take action. Remind her that the plan doesn’t have to be set in stone.
How Colorado broke down barriers to highly effective contraception—and saved $42.5 million in 1 year
Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
The Contraceptive CHOICE Project in St. Louis County, Missouri, is an incredible success story. In it, clinicians made highly effective LARCs readily available and free of charge. When a large percentage of women chose a LARC method, the unintended pregnancy and abortion rates declined.7 In Colorado, providers put this study into practice, creating a successful statewide initiative to reduce the unintended pregnancy rate.8
The data behind LARC methods
LARC methods are backed by endorsements from the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the CDC, and the World Health Organization, which recommend them for adolescents because of their superiority to shorter-acting methods. With LARCs, failure rates are lower and compliance is greater, making them ideal for adolescents, who have high unintended pregnancy rates.1
However, based on 2011 data, only 2% of LARC users nationwide are aged 20 or younger. A number of barriers prevent young women from obtaining LARCs, including lack of education, limited access to and availability of the contraceptives, and, importantly, cost. Many state plans have adopted Medicaid expansions to reduce barriers to LARCs. However, this benefit is still not available in many states, Colorado being 1 of them.
How the Colorado initiative worked
In 2005, 40% of Colorado’s births were unintended, and 60% of those unintended births occurred in women aged 15 to 24 years. About three-quarters of women who were using a contraceptive method at the time of unintended pregnancy reported that it was a low-cost, high-failure method such as condoms or withdrawal.
In response, Colorado’s Department of Public Health and Environment and the Colorado Family Planning Initiative (CFPI) used private funds from an anonymous foundation to provide LARC products at no cost to the Title X–funded clinics in the state.
The initiative began in 2009 in clinics that served 95% of the state’s total population. The funding provided the products themselves (intrauterine device [IUD], implant), as well as training for providers and staff.
Before the initiative began, 52,645 clients received services in these clinics annually. In the third year of the initiative, that number had increased to 64,928 annually. About 55% of clients receiving services both preinitiative and postinitiative were younger than 25 years, and most (92% in 2011) had income below the poverty level.
LARC use increased fourfold over the 3 years of the funded program, from less than 4.5% to 19.4% in the third year. Contraceptive implant use increased tenfold, and IUD use increased by almost threefold. At the same time, oral contraceptive use declined 13%. Before the initiative, only 620 young, low-income women used a LARC method; afterward, 8,435 did.
These changes in contraceptive practice triggered a significant decline in pregnancy rates (TABLE 2) and abortion rates (TABLE 3). Abortion rates increased 8% among 20- to 24-year-olds who were not enrolled in the initiative and decreased 18% among those who were. The proportion of high-risk births (births to unmarried, low-income women with less than a high school education) dropped 24% after the initiative began. The proportion of high-risk births in counties not receiving CFPI funds stayed the same at 7%.
Colorado program saved $42.5 million in public funds
This Colorado program demonstrated that the CHOICE Project can be translated to a statewide initiative. Whereas CHOICE enrolled 9,256 women over 4 years, the Colorado initiative included more than 50,000 clients annually over 3 years. Colorado did not use any state funds for this project, which resulted in significant decreases in the unintended birth rate, abortion rate, and rate of high-risk births.
The Colorado governor’s office estimates that the CFPI saved $5.68 in Medicaid costs for Colorado for every dollar spent on contraceptives. In just 1 year (2010), the program saved approximately $42.5 million in public funds.
Ironically, despite the success of this project, the Colorado legislature denied further funding once the initial financial support ceased.
What this EVIDENCE means for practice
The Colorado program demonstrates that we all can provide LARC methods in practice, especially to young women. In this population, use of highly effective contraception resulted in fewer unintended pregnancies, births, and abortions statewide.
We also need to advocate for our patients, particularly those who have less means and rely on public assistance. Public funding of LARC methods clearly improves outcomes at an individual and population level.
A new, more affordable IUD enters the market
Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
Programs such as the Contraceptive CHOICE Project and the Colorado Family Planning Initiative relied on private foundations for financial support, largely because of the high cost of the IUDs and implants currently available in the United States. Even with the Affordable Care Act (ACA) reducing the costs of LARC products and other contraceptives for patients, there are still many women not covered by these programs. For example, “grandfathered” health insurance plans do not need to follow some aspects of the ACA.
Just as important, the high cost of LARC products takes a toll on providers and clinics that must finance the cost per unit to have stock on hand and then wait for months for reimbursement by insurance companies. As a result, some providers do not stock IUDs and implants and only order them as they are needed and approved by insurance for a particular patient. These barriers limit access to LARC methods and reduce the number of women who receive the products.8
Liletta is less expensive than other IUDs
Enter Liletta, a new levonorgestrel-releasing, 52-mg intrauterine system (IUS) that has been in clinical trials since 2009.9 ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an Intrauterine System) was initiated by Medicines360, a unique nonprofit pharmaceutical company committed to ensuring access to reproductive health products for all women (private and public sector).
ACCESS IUS is the largest IUD approval study ever performed exclusively in the United States. The Phase 3, open-label clinical trial was conducted at 29 sites around the United States, enrolling healthy, nonpregnant, sexually active women aged 16 to 45 years with regular menstrual cycles. Both nulliparous and parous women were included, with no weight or body mass index (BMI) restrictions applied. The study is ongoing and will continue for as long as 7 years. Eisenberg and colleagues published the data used for initial approval for 3 years of use in the United States and Europe.9
Details of the trial
A total of 1,600 women aged 16 to 35 years comprised the group in which efficacy was evaluated. An additional 151 women aged 36 to 45 years were evaluated for safety only. Of the enrolled women, 1,011 (58%) were nulliparous, making ACCESS IUS the largest product approval study of nulliparous women. In addition, 438 women (25.1%) were obese, and 5% of these women had a BMI greater than 40 kg/m2.
Liletta was placed successfully in 1,714 women (97.9%). Fifteen women did not have placement attempted due to uterine factors (the uterus could not be sounded, or the sound was <5.5 cm) or factors unrelated to the product or inserter. In women in whom placement was attempted, the success rate was 98.7%.
The first-year Pearl index for Liletta was 0.15. Life-table pregnancy rates were 0.14 through year 1 and 0.55 through year 3. Four of the 6 pregnancies reported through 3 years of use were ectopic. Adverse events and their incidence, occurring in more than 2% of users, were acne (6%), expulsion (3.5%), dyspareunia (2.8%), and mastalgia (2.0%). The most common adverse events leading to discontinuation were expulsion (3.5%), bleeding complaints (1.5%), acne (1.3%), and mood swings (1.3%).
Uterine perforation with Liletta was diagnosed in 2 participants (0.11%). Expulsion occurred in 62 users (3.5%) and was more frequent among parous than nulliparous women (5.6% vs 2.0%, respectively; P<.001). Most (80.6%) of the expulsions were reported in the first year of product use. Pelvic infection was reported in 10 participants (0.6%), and all cases resolved with outpatient antibiotic treatment.9
Keep in mind that this is an ongoing study—not all women have reached a full 3 years of use. Updates on efficacy and adverse events will be published in the future. This current publication demonstrates the high efficacy and safety of the product through 3 years of use, permitting its approval for contraception in the United States.
In Europe, the product is approved for both contraception and the treatment of heavy menstrual bleeding, based on a European study.10
What this EVIDENCE means for practice
Liletta is a branded product (not generic) designed to be similar to Mirena, with the same size, frame, hormone content, and hormone release rate.11 Medicines360 has entered a groundbreaking marketing partnership with Actavis to make Liletta widely available and affordable. For most public sector providers and clinics in the United States, Liletta costs only $50, significantly less than other LARC methods available in the United States. Actavis also has a program that ensures that any woman lacking insurance coverage for an IUD and not receiving care at a public sector clinic will not be charged more than $75 for her IUD. However, the price of the device is only one aspect of its overall cost, as women still need to pay for any office visit or insertion fees.
For society, this unique business partnership has to include providers and patients as well. Sales of Liletta in the private sector will support the very low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we can all help poor women access very affordable highly effective contraception.
For providers, Liletta is a lower-cost alternative to currently available hormonal IUDs and should perform well over the long term. The highly successful use of Liletta in nulliparous women demonstrates its safety in this population. The 3-year approval is the first step, as the Phase 3 study continues. In the future, Liletta is expected to be approved for 5 years or longer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Unintended pregnancy remains a serious problem in the United States, and the rate continues to increase. Currently, the unintended pregnancy rate is at an all-time high, estimated at 51% of all pregnancies.1 Sobering statistics reveal that the United States has a significantly higher rate of unintended pregnancy than any other developed country.2,3
So the question remains: If we have been introducing new contraceptive methods, why do unintended pregnancy rates keep rising in the United States?
The answer: Key barriers prevent women from attaining their desired contraceptive—foremost among them, cost.
The unintended pregnancy rate is highest among women who are poor, young (aged 18–24 years), minorities, or cohabitating.1 The unintended pregnancy rate among poor women (income below the federal poverty level) of reproductive age has increased over the past few decades, whereas the rate among high-income women (more than twice the federal poverty level) has declined.1 The unintended pregnancy rate discrepancy between poor women and those with means has increased 77%, from a threefold difference in 1995 to a difference of more than fivefold in 2008 (TABLE 1).1,4
These data indicate that we are doing well providing contraception to women of means. However, as a society, we need to improve how we deliver contraceptives—especially highly effective methods—to poor women. As one might expect, given these numbers, low-income women have rates of abortion and unplanned birth that are nearly 6 times higher than their higher-income counterparts.1 The resultant cost to society is substantial, with 68% of unplanned births paid for by public insurance programs such as Medicaid, compared with 38% of planned births.5
As women’s health providers, we must work to improve these numbers and advocate for our patients to help them gain access to the contraceptives they need in accordance with their reproductive life plan. In this article, we hope to put this information in context as we:
- review reports and studies that evaluatethe trend of unintended pregnancy
- describe one state initiative that reduced the rates of unintended pregnancy, birth, and abortion
- introduce a new, highly effective levonorgestrel-releasing intrauterine system (Liletta) being marketed with the goal of reaching women who receive care from private physicians as well as public health clinics.
National and state snapshots reveal shifting proportions of intended, unintended pregnancies
Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. https://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 29, 2015.
Recent studies have demonstrated trends in unplanned pregnancy over the past 2 decades on both a national and statewide level. Data from the National Survey of Family Growth have highlighted trends in abortion and miscarriage, and data from the National Center for Health Statistics have shed light on birth trends. Of 6 million births in 2008, 51% were unintended.1 Unintended pregnancy was defined as a gestation that was mistimed or unwanted. Intended pregnancy was defined as one that was desired at the time it occurred or sooner.
2008 data focus on the national level
Although the overall pregnancy rate for US women aged 15 to 44 years is relatively unchanged, there is a small change in whether or not the pregnancy was intended. For 2008, as the rate of intended pregnancy dropped slightly, from 54 to 51 pregnancies per 1,000 women, the unintended pregnancy rate increased by 10%, from 49 to 54 pregnancies per 1,000 women. Proportionally, unintended pregnancies that resulted in abortion declined from 47% to 40%, whereas the rate of unintended pregnancies ending in birth increased to 27 births per 1,000 women.1
2010 data focus on individual states
We now have state-specific data on trends in unintended pregnancy rates from 2002 to 2010 in the United States.6 In 28 of 50 states, more than half of all pregnancies were unintended, with rates ranging from 36% to 62%. The median rate was 47 unintended pregnancies per 1,000 women aged 15 to 44, with the lowest unintended pregnancy rate in New Hampshire at 32 per 1,000 women and highest rates in Delaware, Hawaii, and New York at 61 to 62 unintended pregnancies per 1,000 women.6
Between 2002 and 2010, unintended pregnancy rates fell 5% or more in 18 states and rose 5% or more in 4 states. In the remaining 12 states for which there are data, unintended pregnancy rates remained unchanged.
Interestingly, 16 states had increases in unintended pregnancy rates of 5% or more between 2002 and 2006. The trend reversed between 2006 and 2010, during which 28 of 41 states with available data experienced decreases of 5% or more and only 1 state experienced an increase of 5% or more. These latest numbers suggest we may be making some progress in reducing the overall rate of unintended pregnancy.
What this EVIDENCE means for practice
Although the rate of unintended pregnancy is declining in some states, the national rate is still increasing. This information emphasizes the need for all providers to consider initiating discussions about pregnancy intentions—a step that may be as important as obtaining blood pressure and weight. When women are seen for any health visit, they should be asked about their reproductive plans. The Centers for Disease Control and Prevention (CDC) has issued a helpful set of questions to guide the discussion of timing and planning pregnancy. It also provides useful information on ways to increase utilization of long-acting reversible contraceptives (LARCs) to help realize the goal of fewer unintended pregnancies. (For more on this discussion, see “How to motivate your patient to create a reproductive life plan,” below.)
How to motivate your patient to create a reproductive life plan
The Centers for Disease Control and Prevention (CDC) offers a tool for health care professionals to use to encourage patients to think about their reproductive goals and make a plan to facilitate those goals. It’s available at: http://www.cdc.gov/preconception/documents/rlphealthproviders.pdf.
Questions to ask your patient
- Do you plan to have any (more) children at any time in your future?
IF YES
- How many children would you like to have?
- How long would you like to wait until you or your partner become pregnant?
- What family planning method do you plan to use until you or your partner are ready to become pregnant?
- How sure are you that you will be able to use this method without any problems?
IF NO
- What family planning method will you use to avoid pregnancy?
- How sure are you that you will be able to use this method without any problems?
- People’s plans change. Is it possible that you or your partner could ever decide to become pregnant?
Action plan
Encourage your patient to make a plan and take action. Remind her that the plan doesn’t have to be set in stone.
How Colorado broke down barriers to highly effective contraception—and saved $42.5 million in 1 year
Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
The Contraceptive CHOICE Project in St. Louis County, Missouri, is an incredible success story. In it, clinicians made highly effective LARCs readily available and free of charge. When a large percentage of women chose a LARC method, the unintended pregnancy and abortion rates declined.7 In Colorado, providers put this study into practice, creating a successful statewide initiative to reduce the unintended pregnancy rate.8
The data behind LARC methods
LARC methods are backed by endorsements from the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the CDC, and the World Health Organization, which recommend them for adolescents because of their superiority to shorter-acting methods. With LARCs, failure rates are lower and compliance is greater, making them ideal for adolescents, who have high unintended pregnancy rates.1
However, based on 2011 data, only 2% of LARC users nationwide are aged 20 or younger. A number of barriers prevent young women from obtaining LARCs, including lack of education, limited access to and availability of the contraceptives, and, importantly, cost. Many state plans have adopted Medicaid expansions to reduce barriers to LARCs. However, this benefit is still not available in many states, Colorado being 1 of them.
How the Colorado initiative worked
In 2005, 40% of Colorado’s births were unintended, and 60% of those unintended births occurred in women aged 15 to 24 years. About three-quarters of women who were using a contraceptive method at the time of unintended pregnancy reported that it was a low-cost, high-failure method such as condoms or withdrawal.
In response, Colorado’s Department of Public Health and Environment and the Colorado Family Planning Initiative (CFPI) used private funds from an anonymous foundation to provide LARC products at no cost to the Title X–funded clinics in the state.
The initiative began in 2009 in clinics that served 95% of the state’s total population. The funding provided the products themselves (intrauterine device [IUD], implant), as well as training for providers and staff.
Before the initiative began, 52,645 clients received services in these clinics annually. In the third year of the initiative, that number had increased to 64,928 annually. About 55% of clients receiving services both preinitiative and postinitiative were younger than 25 years, and most (92% in 2011) had income below the poverty level.
LARC use increased fourfold over the 3 years of the funded program, from less than 4.5% to 19.4% in the third year. Contraceptive implant use increased tenfold, and IUD use increased by almost threefold. At the same time, oral contraceptive use declined 13%. Before the initiative, only 620 young, low-income women used a LARC method; afterward, 8,435 did.
These changes in contraceptive practice triggered a significant decline in pregnancy rates (TABLE 2) and abortion rates (TABLE 3). Abortion rates increased 8% among 20- to 24-year-olds who were not enrolled in the initiative and decreased 18% among those who were. The proportion of high-risk births (births to unmarried, low-income women with less than a high school education) dropped 24% after the initiative began. The proportion of high-risk births in counties not receiving CFPI funds stayed the same at 7%.
Colorado program saved $42.5 million in public funds
This Colorado program demonstrated that the CHOICE Project can be translated to a statewide initiative. Whereas CHOICE enrolled 9,256 women over 4 years, the Colorado initiative included more than 50,000 clients annually over 3 years. Colorado did not use any state funds for this project, which resulted in significant decreases in the unintended birth rate, abortion rate, and rate of high-risk births.
The Colorado governor’s office estimates that the CFPI saved $5.68 in Medicaid costs for Colorado for every dollar spent on contraceptives. In just 1 year (2010), the program saved approximately $42.5 million in public funds.
Ironically, despite the success of this project, the Colorado legislature denied further funding once the initial financial support ceased.
What this EVIDENCE means for practice
The Colorado program demonstrates that we all can provide LARC methods in practice, especially to young women. In this population, use of highly effective contraception resulted in fewer unintended pregnancies, births, and abortions statewide.
We also need to advocate for our patients, particularly those who have less means and rely on public assistance. Public funding of LARC methods clearly improves outcomes at an individual and population level.
A new, more affordable IUD enters the market
Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
Programs such as the Contraceptive CHOICE Project and the Colorado Family Planning Initiative relied on private foundations for financial support, largely because of the high cost of the IUDs and implants currently available in the United States. Even with the Affordable Care Act (ACA) reducing the costs of LARC products and other contraceptives for patients, there are still many women not covered by these programs. For example, “grandfathered” health insurance plans do not need to follow some aspects of the ACA.
Just as important, the high cost of LARC products takes a toll on providers and clinics that must finance the cost per unit to have stock on hand and then wait for months for reimbursement by insurance companies. As a result, some providers do not stock IUDs and implants and only order them as they are needed and approved by insurance for a particular patient. These barriers limit access to LARC methods and reduce the number of women who receive the products.8
Liletta is less expensive than other IUDs
Enter Liletta, a new levonorgestrel-releasing, 52-mg intrauterine system (IUS) that has been in clinical trials since 2009.9 ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an Intrauterine System) was initiated by Medicines360, a unique nonprofit pharmaceutical company committed to ensuring access to reproductive health products for all women (private and public sector).
ACCESS IUS is the largest IUD approval study ever performed exclusively in the United States. The Phase 3, open-label clinical trial was conducted at 29 sites around the United States, enrolling healthy, nonpregnant, sexually active women aged 16 to 45 years with regular menstrual cycles. Both nulliparous and parous women were included, with no weight or body mass index (BMI) restrictions applied. The study is ongoing and will continue for as long as 7 years. Eisenberg and colleagues published the data used for initial approval for 3 years of use in the United States and Europe.9
Details of the trial
A total of 1,600 women aged 16 to 35 years comprised the group in which efficacy was evaluated. An additional 151 women aged 36 to 45 years were evaluated for safety only. Of the enrolled women, 1,011 (58%) were nulliparous, making ACCESS IUS the largest product approval study of nulliparous women. In addition, 438 women (25.1%) were obese, and 5% of these women had a BMI greater than 40 kg/m2.
Liletta was placed successfully in 1,714 women (97.9%). Fifteen women did not have placement attempted due to uterine factors (the uterus could not be sounded, or the sound was <5.5 cm) or factors unrelated to the product or inserter. In women in whom placement was attempted, the success rate was 98.7%.
The first-year Pearl index for Liletta was 0.15. Life-table pregnancy rates were 0.14 through year 1 and 0.55 through year 3. Four of the 6 pregnancies reported through 3 years of use were ectopic. Adverse events and their incidence, occurring in more than 2% of users, were acne (6%), expulsion (3.5%), dyspareunia (2.8%), and mastalgia (2.0%). The most common adverse events leading to discontinuation were expulsion (3.5%), bleeding complaints (1.5%), acne (1.3%), and mood swings (1.3%).
Uterine perforation with Liletta was diagnosed in 2 participants (0.11%). Expulsion occurred in 62 users (3.5%) and was more frequent among parous than nulliparous women (5.6% vs 2.0%, respectively; P<.001). Most (80.6%) of the expulsions were reported in the first year of product use. Pelvic infection was reported in 10 participants (0.6%), and all cases resolved with outpatient antibiotic treatment.9
Keep in mind that this is an ongoing study—not all women have reached a full 3 years of use. Updates on efficacy and adverse events will be published in the future. This current publication demonstrates the high efficacy and safety of the product through 3 years of use, permitting its approval for contraception in the United States.
In Europe, the product is approved for both contraception and the treatment of heavy menstrual bleeding, based on a European study.10
What this EVIDENCE means for practice
Liletta is a branded product (not generic) designed to be similar to Mirena, with the same size, frame, hormone content, and hormone release rate.11 Medicines360 has entered a groundbreaking marketing partnership with Actavis to make Liletta widely available and affordable. For most public sector providers and clinics in the United States, Liletta costs only $50, significantly less than other LARC methods available in the United States. Actavis also has a program that ensures that any woman lacking insurance coverage for an IUD and not receiving care at a public sector clinic will not be charged more than $75 for her IUD. However, the price of the device is only one aspect of its overall cost, as women still need to pay for any office visit or insertion fees.
For society, this unique business partnership has to include providers and patients as well. Sales of Liletta in the private sector will support the very low price in the public sector. As a health care community, even if we do not directly care for women in public-sector settings, we can all help poor women access very affordable highly effective contraception.
For providers, Liletta is a lower-cost alternative to currently available hormonal IUDs and should perform well over the long term. The highly successful use of Liletta in nulliparous women demonstrates its safety in this population. The 3-year approval is the first step, as the Phase 3 study continues. In the future, Liletta is expected to be approved for 5 years or longer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
2. Guttmacher Institute. Fact Sheet: Unintended Pregnancy in the United States. http://www.guttmacher.org/pubs/FB-Unintended-Pregnancy-US.html. Published February 2015. Accessed June 29, 2015.
3. Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends and outcomes. Stud Fam Plann. 2010;41(4):241–250.
4. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspectives. 1998;30(1):24–29, 46.
5. Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care. National and States Estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed June 29, 2015.
6. Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. http://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 30, 2015.
7. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the ContraceptiveCHOICE Project in review. J Womens Health. 2015; 24(5):349–353.
8. Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
9. Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
10. Mawet M, Nollevaux F, Nizet D, et al. Impact of a new levonorgestrel intrauterine system, Levosert, on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contracept Reprod Health Care. 2014;19(3):169–179.
11. Gopalakrishnan M, Liu T, Gobburu J, Creinin MD. Levonorgestrel release rates with LNG20, a new levonorgestrel intrauterine system. Obstet Gynecol. 2015;125(suppl 1): 62S–63S.
1. Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001–2008. Am J Public Health. 2014;104(suppl 1):s43–s48.
2. Guttmacher Institute. Fact Sheet: Unintended Pregnancy in the United States. http://www.guttmacher.org/pubs/FB-Unintended-Pregnancy-US.html. Published February 2015. Accessed June 29, 2015.
3. Singh S, Sedgh G, Hussain R. Unintended pregnancy: worldwide levels, trends and outcomes. Stud Fam Plann. 2010;41(4):241–250.
4. Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspectives. 1998;30(1):24–29, 46.
5. Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care. National and States Estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed June 29, 2015.
6. Kost K. Unintended pregnancy rates at the state level: estimates for 2010 and trends since 2002. Guttmacher Institute. http://www.guttmacher.org/pubs/StateUP10.pdf. Published January 2015. Accessed June 30, 2015.
7. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing unintended pregnancy: the ContraceptiveCHOICE Project in review. J Womens Health. 2015; 24(5):349–353.
8. Ricketts S, Klinger G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline of births among young, low-income women. Perspect Sexual Reprod Health. 2014;46(3):125–132.
9. Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
10. Mawet M, Nollevaux F, Nizet D, et al. Impact of a new levonorgestrel intrauterine system, Levosert, on heavy menstrual bleeding: results of a one-year randomised controlled trial. Eur J Contracept Reprod Health Care. 2014;19(3):169–179.
11. Gopalakrishnan M, Liu T, Gobburu J, Creinin MD. Levonorgestrel release rates with LNG20, a new levonorgestrel intrauterine system. Obstet Gynecol. 2015;125(suppl 1): 62S–63S.
In this Article
- How Colorado broke down barriers to highly effective contraception
- How to motivate your patient to create a reproductive life plan
- A more affordable IUD enters the market