User login
Management of Rodenticide Poisoning Associated with Synthetic Cannabinoids
Between March 7, 2018, and May 9, 2018, at least 164 people in Illinois were sickened by synthetic cannabinoids laced with rodenticides. The Illinois Department of Public Health has reported 4 deaths connected with the use of synthetic cannabinoids (sold under names such as Spice, K2, Legal Weed, etc).1 Synthetic cannabinoids are mind-altering chemicals that are sprayed on dried plant material and often sold at convenience stores. Some users have reported smoking these substances because they are generally not detected by standard urine toxicology tests.
Recreational use of synthetic cannabinoids can lead to serious and, at times, deadly complications. Chemicals found in rat poison have contaminated batches of synthetic cannabinoids, leading to coagulopathy and severe bleeding. Affected patients have reported hemoptysis, hematuria, severe epistaxis, bleeding gums, conjunctival hemorrhages, and gastrointestinal bleeding. The following case is of a patient who presented to an emergency department (ED) with severe coagulopathy and cardiotoxicity after using an adulterated synthetic cannabinoid product.
Case Presentation
A 65-year-old man presented to the ED reporting hematochezia, hematuria, and hemoptysis. He reported that these symptoms began about 1 day after he had smoked a synthetic cannabinoid called K2. The patient stated that some of his friends who used the same product were experiencing similar symptoms. He reported mild generalized abdominal pain but reported no chest pain, dyspnea, headache, fevers, chills, or dysuria.
The patient’s past medical history included hypertension, dyslipidemia, chronic lower back pain, and vitamin D deficiency. His past surgical history was notable for an exploratory laparotomy after a stab wound to the abdomen. The patient reported taking the following medications: morphine SA 30 mg bid, meloxicam 15 mg daily, amitriptyline 100 mg qhs, amlodipine 5 mg daily, hydrocodone/acetaminophen 5/325 mg q12h prn, atorvastatin 20 mg qhs, omeprazole 20 mg qam, senna 187 mg daily prn, psyllium 1 packet dissolved in water daily prn, and cholecalciferol 1,000 IU daily.
The patient’s temperature was 98o F, blood pressure, 144/80 mm Hg; pulse, 131 beats per minute; respiratory rate, 18 breaths per minute; and O2 saturation, 98% (ambient air). A physical examination revealed no acute distress; he was coughing up blood; clear lungs; heart sounds were tachycardic and irregularly irregular; soft, nondistended, mild generalized tenderness in the abdomen with no guarding and no rebound. The pertinent laboratory tests were international normalized ratio (INR), > 20; prothrombin time, > 150 seconds; prothrombin thromboplastin time, 157 seconds; hemoglobin, 13.3 g/dL; platelet count, 195 k/uL; white blood count, 11.3 k/uL; creatinine, 0.57mg/dL; potassium, 3.8 mmol/L, D-dimertest, 0.87 ug/mL fibrinogen equivalent units; fibrinogen level, 624 mg/dL; troponin, < 0.04 ng/mL; lactic acid, 1.3 mmol/L; total bilirubin, 0.8 mg/dL; alanine aminotransferase, 22 U/L, aspartate aminotransferase, 22 U/L; alkaline phosphatase, 89 U/L; urinalysis with > 50 red blood cells/high power field; large blood, negative leukocyte esterase, negative nitrite. The patient’s urine toxicology was negative for cannabinoids, methadone, amphetamines, cocaine, and benzodiazepines; but was positive for opiates. An anticoagulant poisoning panel also was ordered.
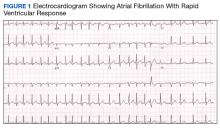
An electrocardiogram (ECG) and imaging studies were ordered. The ECG showed atrial fibrillation (AF) with rapid ventricular response (Figure 1). A chest X-ray indicated bibasilar consolidations that were worse on the right side. A noncontrast computed tomography (CT) of the head did not show intracranial bleeding. An abdomen/pelvis CT showed bilateral diffuse patchy peribronchovascular ground-glass opacities in the lung bases that could represent pulmonary hemorrhage, but no peritoneal or retroperitoneal bleeding.
Treatment
In the ED, the case was discussed with the Illinois Poison Control Center. The patient was diagnosed with coagulopathy likely due to anticoagulant poisoning. He was immediately treated with 10 mg of IV vitamin K, a fixed dose of 2,000 units of 4-factor prothrombin complex concentrate, and 4 units of fresh frozen plasma. His INR improved to 1.42 within several hours. He received 5 mg of IV metoprolol for uncontrolled AF and was admitted to the intensive care unit (ICU) for further care.
In the ICU the patient was started on oral vitamin K 50 mg tid for ongoing treatment of coagulopathy due to concern for possible rodenticide poisoning associated with very long half-life. This dose was then decreased to 50 mg bid. He was given IV fluid resuscitation with normal saline and started on rate control for AF with oral metoprolol. His heart rate improved. An echocardiogram showed new cardiomyopathy with an ejection fraction of 25% to 30%. Given basilar infiltrates and 1 episode of low-grade fever, he was started on ceftriaxone for possible community-acquired pneumonia. The patient was started on cholestyramine to help with washout of the possible rodenticide. No endoscopic interventions were performed.
The patient was transferred to an inpatient telemetry floor 24 hours after admission to the ICU once his tachycardia and bleeding improved. He did not require transfusion of packed red blood cells. In the ICU his INR had ranged between 1.62 and 2.46 (down from > 20 in the ED). His hemoglobin dropped from 13.3 g/dL on admission to 12 g/dL on transfer from the ICU, before stabilizing around 11 g/dL on the floor. The patient’s heart rate required better control, so metoprolol was increased to a total daily dose of 200 mg on the telemetry floor. Oral digoxin was then added after a digoxin load for additional rate control, as the patient remained tachycardic. Twice a day the patient continued to take 50 mg vitamin K. Cholestyramine and ceftriaxone were initially continued, but when the INR started increasing again, the cholestyramine was stopped to allow for an increase to more frequent 3-times daily vitamin 50 mg K administration (cholestyramine can interfere with vitamin K absorption). According to the toxicology service, there was only weak evidence to support use of cholestyramine in this setting.
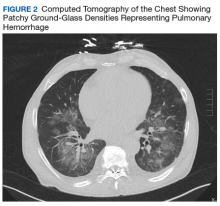
Given his ongoing mild hemoptysis, the patient received first 1 unit, and then another 4 units of FFP when the INR increased to 3.96 despite oral vitamin K. After FFP, the INR decreased to 1.93 and subsequently to 1.52. A CT of the chest showed patchy ground-glass densities throughout the lungs, predominantly at the lung bases and to a lesser extent in the upper lobes. The findings were felt to represent pulmonary hemorrhage given the patient’s history of hemoptysis (Figure 2).
The patient’s heart rate control improved, and he remained hemodynamically stable. A thyroid function test was within normal limits. Lisinopril was added to metoprolol and digoxin given his newly diagnosed cardiomyopathy. The patient was observed for a total of 4 days on the inpatient floor and discharged after his INR stabilized around 1.5 on twice daily 50 mg vitamin K. The patient’s hematuria and hematochezia completely resolved, and hemoptysis was much improved at the time of discharge. His hemoglobin remained stable. The anticoagulant poisoning panel came back positive for
The patient has remained in AF at all follow-up visits. The INR normalized by 6 weeks after hospital discharge, and the dose of vitamin K slowly was tapered with close monitoring of the INR. Vitamin K was tapered for about 6 months after his initial presentation, and the patient was started on a direct oral anticoagulant (DOAC) for anticoagulation when the INR remained stable off vitamin K. He subsequently underwent a transesophageal echocardiogram followed by an attempt at direct current (DC) cardioversion; however, he did not remain in sinus rhythm, and is being continued on anticoagulation and rate control for his AF.
Discussion
Users generally smoke synthetic cannabinoids, which produce cannabis-like effects. However, atypical intoxication effects with worse complications often occur.2 These products typically contain dried shredded plant material that is soaked in or sprayed with several synthetic cannabinoids, varying in dosage and combination.3 Synthetic cannabinoids have been associated with serious adverse effects (AEs), including drowsiness, light-headedness, and fast or irregular heartbeat.4 More severe clinical features such as psychosis, delirium, cardiotoxicity, seizures, rhabdomyolysis, acute kidney injury, hyperthermia, myocardial ischemia, ischemic strokes, and death have also been noted.4
It is not known how some batches of synthetic cannabinoids came to be contaminated with rat poison or how commonly such an adulteration is found across the country. Several different guidelines provide pathways for the treatment of acute bleeding in the setting of coagulopathy due to vitamin K antagonists.5,6 Each guideline divides the indications for reversal into either severity of bleeding or the criticality of the bleeding based on location.5,6 All guidelines recommend the use of vitamin K (either oral or IV) followed by FFP or 4-factor prothrombin complex concentrate (PCC) for more severe bleeding.5,6 However, recommendations regarding the use of PCC vary in dosing for vitamin K antagonists (in contrast to treatment of coagulopathy due to DOACs). Recent studies and guidelines suggest that fixed-dose (rather than weight-based dose) PCC is effective for the reversal of coagulopathy due to vitamin K antagonists.6,7 Using fixed rather than weight-based dosing decreases cost and may decrease the possibility of thrombotic AEs.7 In this patient, a fixed-dose of 2,000 units of PCC was given based on data that were extrapolated from warfarin reversal using PCC.7
The vitamin K antagonists that adulterated this patient’s synthetic cannabinoid were difenacoum and brodifacoum, which are 4-hydroxycoumarin derivatives. These are second-generation long-acting anticoagulant rodenticides (LAARs) that are about 100 times more potent than warfarin.8 As the name implies, LAARs have a longer duration of action in the body of any organism that ingests the poison, which is due to the highly lipophilic groups that have been added to the warfarin molecule to combat resistance in rodents.9
As a result of the deposition in the tissues, there have been reports of the duration of action of brodifacoum ranging from 51 days to 9 months after ingestion, with the latter caused by an intentional overdose in a human.9-12 Reports suggest that coagulopathy is not likely to occur when the serum brodifacoum concentration is < 10 ng/mL.13,14 Animal models show difenacoum has a tissue half-life of about 62 days.15 Reports of difenacoum poisoning in humans have shown variable lengths of treatment, ranging from 30 to 47 days.16-18 The length of treatment for either brodifacoum or difenacoum will depend on the amount of poison exposure.
The long duration of action and treatment duration may lead to problems with drug procurement, especially in the early phase of treatment in which IV vitamin K is used. The supply of IV vitamin K recently has been limited for at least some manufacturers. According to the American Society of Health System Pharmacists Current Drug Shortage List, the increased demand is thought to be due to increased use of synthetic inhaled cannabinoids laced with anticoagulant.19 IV vitamin K products are available from suppliers such as Amphastar (Rancho Cucamonga, CA) and Hospira (Lake Forest, IL).
The American College of Chest Physicians recommends IV vitamin K administration in patients with major bleeding secondary to vitamin K antagonists.20 The oral route is thought to be more effective than a subcutaneous route in the treatment of nonbleeding patients with rodenticide-associated coagulopathy. Due to erratic and unpredictable absorption, the subcutaneous route of administration has fallen out of favor. Oral vitamin K products were not affected by the recent shortage. However, large doses of oral vitamin K can be costly. Due to the long half-life of LAAR, many patients are discharged with a prescription for oral vitamin K. Although vitamin K is found in most over-the-counter (OTC) multivitamins, the strength is insufficient. Most OTC formulations are ≤ 100 μg, whereas the prescription strength is 5 mg, but patients being treated for rodenticide poisoning require much larger doses.
Commercial insurance carriers and Medicare Part D usually do not cover vitamins and minerals unless it is for a medically accepted indication or is an indication supported by citation in either the American Hospital Formulary System, United States Pharmacopeia drug information book, or an electronic information resource that is supported by evidence such as Micromedex.21 For a patient without insurance coverage being treated with high-dose vitamin K therapy for rodenticide poisoning outside of a federal health care system, the cost could be as high as $500 to $1,000 per day, depending on the dose of vitamin K needed to maintain an acceptable INR.
Conclusion
In addition to bleeding as a result of coagulopathy, this patient presented with new onset of AF with rapid ventricular response and a newly diagnosed cardiomyopathy. Although the patient had other cardiovascular risk factors, such as hypertension, dyslipidemia, and a remote history of cocaine use, it is likely that the use of the synthetic cannabinoids contributed to the development and/or worsening of this arrhythmia and cardiomyopathy. The patient remained in AF 6 weeks after hospital discharge with a controlled ventricular rate on metoprolol and digoxin. An interval echocardiogram 6 weeks after hospital discharge showed a recovered ejection fraction. In cases of tachycardia-induced cardiomyopathy, the ejection fraction often recovers with control of the tachycardia. The patient was weaned off vitamin K about 6 months after his initial presentation and started on a DOAC for anticoagulation. He subsequently underwent a transesophageal echocardiogram followed by an attempt at DC cardioversion; however, he did not remain in sinus rhythm and is being continued on anticoagulation and rate control for his AF.
Although unclear how synthetic cannabinoids became adulterated with a potent vitamin K antagonist, health care practitioners should consider this if a patient presents with unexplained coagulopathy and widespread bleeding. Fixed-dose PCC should be considered as an alternative to weight-based dosing in these cases. Physicians and pharmacy personnel should anticipate a need for long-term high doses of vitamin K in order to begin work early to obtain sufficient supplies to treat presenting patients.
1. Illinois Department of Public Health. Synthetic cannabinoids. http://dph.illinois.gov/topics-services/prevention-wellness/medical-cannabis/synthetic-cannabinoids. Updated May 30, 2018. Accessed April 8, 2019.
2. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: update 2015. Subst Abus. 2017;38(3):344-366.
3. United Nations Office on Drugs and Crime. Global SMART update. https://www.unodc.org/documents/scientific/Global_SMART_Update_13_web.pdf. Published March 2015. Accessed April 8, 2019.
4. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235-242.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
6. Cushman M, Lim W, Zakai NA. 2011 Clinical Practice guide on anticoagulant dosing and management of anticoagulant-associated bleeding complications in adults. http://www.hematology.org/Clinicians/Guidelines-Quality/Quick-Ref/525.aspx. Published 2011. Accessed April 8, 2019.
7. Klein L, Peters J, Miner J, Gorlin J. Evaluation of fixed dose 4-factor prothrombin complex concentrate for emergent warfarin reversal. Am J Emerg Med. 2015;33(9):1213-1218.
8. Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281-288.
9. Lipton RA, Klass EM. Human ingestion of ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004-3005.
10. Chong LL, Chau WK, Ho CH. A case of ‘superwarfarin’ poisoning. Scand J Haematol. 1986;36(3):314-331.
11. Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
12. Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15(1):126-130.
13. Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med. 1993;153(16):1925-1928.
14. Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36(3):262-267.
15. Vandenbrouke V, Bousquet-Meloua A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31(5):437-445.
16. Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed). 1982;285(6341):541.
17. Katona B, Wason S. Superwarfarin poisoning. J Emerg Med. 1989;7(6):627-631.
18. Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol. 1992;11(6):553-554.
19. American Society of Health System Pharmacists. Current drug shortages. Vitamin K (phytonadione) injection. https://www.ashp.org/drug-shortages/current-shortages/Drug-Shortage-Detail.aspx?id=100. Updated July 5, 2018. Accessed April 8, 2019.
20. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
21. Centers for Medicare and Medicaid Services. Part D Excluded Drugs. https://www.medicareadvocacy.org/old-site/News/Archives/PartD_ExcludedDrugsByState.htm. Accessed on August 23, 2018.
Between March 7, 2018, and May 9, 2018, at least 164 people in Illinois were sickened by synthetic cannabinoids laced with rodenticides. The Illinois Department of Public Health has reported 4 deaths connected with the use of synthetic cannabinoids (sold under names such as Spice, K2, Legal Weed, etc).1 Synthetic cannabinoids are mind-altering chemicals that are sprayed on dried plant material and often sold at convenience stores. Some users have reported smoking these substances because they are generally not detected by standard urine toxicology tests.
Recreational use of synthetic cannabinoids can lead to serious and, at times, deadly complications. Chemicals found in rat poison have contaminated batches of synthetic cannabinoids, leading to coagulopathy and severe bleeding. Affected patients have reported hemoptysis, hematuria, severe epistaxis, bleeding gums, conjunctival hemorrhages, and gastrointestinal bleeding. The following case is of a patient who presented to an emergency department (ED) with severe coagulopathy and cardiotoxicity after using an adulterated synthetic cannabinoid product.
Case Presentation
A 65-year-old man presented to the ED reporting hematochezia, hematuria, and hemoptysis. He reported that these symptoms began about 1 day after he had smoked a synthetic cannabinoid called K2. The patient stated that some of his friends who used the same product were experiencing similar symptoms. He reported mild generalized abdominal pain but reported no chest pain, dyspnea, headache, fevers, chills, or dysuria.
The patient’s past medical history included hypertension, dyslipidemia, chronic lower back pain, and vitamin D deficiency. His past surgical history was notable for an exploratory laparotomy after a stab wound to the abdomen. The patient reported taking the following medications: morphine SA 30 mg bid, meloxicam 15 mg daily, amitriptyline 100 mg qhs, amlodipine 5 mg daily, hydrocodone/acetaminophen 5/325 mg q12h prn, atorvastatin 20 mg qhs, omeprazole 20 mg qam, senna 187 mg daily prn, psyllium 1 packet dissolved in water daily prn, and cholecalciferol 1,000 IU daily.
The patient’s temperature was 98o F, blood pressure, 144/80 mm Hg; pulse, 131 beats per minute; respiratory rate, 18 breaths per minute; and O2 saturation, 98% (ambient air). A physical examination revealed no acute distress; he was coughing up blood; clear lungs; heart sounds were tachycardic and irregularly irregular; soft, nondistended, mild generalized tenderness in the abdomen with no guarding and no rebound. The pertinent laboratory tests were international normalized ratio (INR), > 20; prothrombin time, > 150 seconds; prothrombin thromboplastin time, 157 seconds; hemoglobin, 13.3 g/dL; platelet count, 195 k/uL; white blood count, 11.3 k/uL; creatinine, 0.57mg/dL; potassium, 3.8 mmol/L, D-dimertest, 0.87 ug/mL fibrinogen equivalent units; fibrinogen level, 624 mg/dL; troponin, < 0.04 ng/mL; lactic acid, 1.3 mmol/L; total bilirubin, 0.8 mg/dL; alanine aminotransferase, 22 U/L, aspartate aminotransferase, 22 U/L; alkaline phosphatase, 89 U/L; urinalysis with > 50 red blood cells/high power field; large blood, negative leukocyte esterase, negative nitrite. The patient’s urine toxicology was negative for cannabinoids, methadone, amphetamines, cocaine, and benzodiazepines; but was positive for opiates. An anticoagulant poisoning panel also was ordered.
An electrocardiogram (ECG) and imaging studies were ordered. The ECG showed atrial fibrillation (AF) with rapid ventricular response (Figure 1). A chest X-ray indicated bibasilar consolidations that were worse on the right side. A noncontrast computed tomography (CT) of the head did not show intracranial bleeding. An abdomen/pelvis CT showed bilateral diffuse patchy peribronchovascular ground-glass opacities in the lung bases that could represent pulmonary hemorrhage, but no peritoneal or retroperitoneal bleeding.
Treatment
In the ED, the case was discussed with the Illinois Poison Control Center. The patient was diagnosed with coagulopathy likely due to anticoagulant poisoning. He was immediately treated with 10 mg of IV vitamin K, a fixed dose of 2,000 units of 4-factor prothrombin complex concentrate, and 4 units of fresh frozen plasma. His INR improved to 1.42 within several hours. He received 5 mg of IV metoprolol for uncontrolled AF and was admitted to the intensive care unit (ICU) for further care.
In the ICU the patient was started on oral vitamin K 50 mg tid for ongoing treatment of coagulopathy due to concern for possible rodenticide poisoning associated with very long half-life. This dose was then decreased to 50 mg bid. He was given IV fluid resuscitation with normal saline and started on rate control for AF with oral metoprolol. His heart rate improved. An echocardiogram showed new cardiomyopathy with an ejection fraction of 25% to 30%. Given basilar infiltrates and 1 episode of low-grade fever, he was started on ceftriaxone for possible community-acquired pneumonia. The patient was started on cholestyramine to help with washout of the possible rodenticide. No endoscopic interventions were performed.
The patient was transferred to an inpatient telemetry floor 24 hours after admission to the ICU once his tachycardia and bleeding improved. He did not require transfusion of packed red blood cells. In the ICU his INR had ranged between 1.62 and 2.46 (down from > 20 in the ED). His hemoglobin dropped from 13.3 g/dL on admission to 12 g/dL on transfer from the ICU, before stabilizing around 11 g/dL on the floor. The patient’s heart rate required better control, so metoprolol was increased to a total daily dose of 200 mg on the telemetry floor. Oral digoxin was then added after a digoxin load for additional rate control, as the patient remained tachycardic. Twice a day the patient continued to take 50 mg vitamin K. Cholestyramine and ceftriaxone were initially continued, but when the INR started increasing again, the cholestyramine was stopped to allow for an increase to more frequent 3-times daily vitamin 50 mg K administration (cholestyramine can interfere with vitamin K absorption). According to the toxicology service, there was only weak evidence to support use of cholestyramine in this setting.
Given his ongoing mild hemoptysis, the patient received first 1 unit, and then another 4 units of FFP when the INR increased to 3.96 despite oral vitamin K. After FFP, the INR decreased to 1.93 and subsequently to 1.52. A CT of the chest showed patchy ground-glass densities throughout the lungs, predominantly at the lung bases and to a lesser extent in the upper lobes. The findings were felt to represent pulmonary hemorrhage given the patient’s history of hemoptysis (Figure 2).
The patient’s heart rate control improved, and he remained hemodynamically stable. A thyroid function test was within normal limits. Lisinopril was added to metoprolol and digoxin given his newly diagnosed cardiomyopathy. The patient was observed for a total of 4 days on the inpatient floor and discharged after his INR stabilized around 1.5 on twice daily 50 mg vitamin K. The patient’s hematuria and hematochezia completely resolved, and hemoptysis was much improved at the time of discharge. His hemoglobin remained stable. The anticoagulant poisoning panel came back positive for
The patient has remained in AF at all follow-up visits. The INR normalized by 6 weeks after hospital discharge, and the dose of vitamin K slowly was tapered with close monitoring of the INR. Vitamin K was tapered for about 6 months after his initial presentation, and the patient was started on a direct oral anticoagulant (DOAC) for anticoagulation when the INR remained stable off vitamin K. He subsequently underwent a transesophageal echocardiogram followed by an attempt at direct current (DC) cardioversion; however, he did not remain in sinus rhythm, and is being continued on anticoagulation and rate control for his AF.
Discussion
Users generally smoke synthetic cannabinoids, which produce cannabis-like effects. However, atypical intoxication effects with worse complications often occur.2 These products typically contain dried shredded plant material that is soaked in or sprayed with several synthetic cannabinoids, varying in dosage and combination.3 Synthetic cannabinoids have been associated with serious adverse effects (AEs), including drowsiness, light-headedness, and fast or irregular heartbeat.4 More severe clinical features such as psychosis, delirium, cardiotoxicity, seizures, rhabdomyolysis, acute kidney injury, hyperthermia, myocardial ischemia, ischemic strokes, and death have also been noted.4
It is not known how some batches of synthetic cannabinoids came to be contaminated with rat poison or how commonly such an adulteration is found across the country. Several different guidelines provide pathways for the treatment of acute bleeding in the setting of coagulopathy due to vitamin K antagonists.5,6 Each guideline divides the indications for reversal into either severity of bleeding or the criticality of the bleeding based on location.5,6 All guidelines recommend the use of vitamin K (either oral or IV) followed by FFP or 4-factor prothrombin complex concentrate (PCC) for more severe bleeding.5,6 However, recommendations regarding the use of PCC vary in dosing for vitamin K antagonists (in contrast to treatment of coagulopathy due to DOACs). Recent studies and guidelines suggest that fixed-dose (rather than weight-based dose) PCC is effective for the reversal of coagulopathy due to vitamin K antagonists.6,7 Using fixed rather than weight-based dosing decreases cost and may decrease the possibility of thrombotic AEs.7 In this patient, a fixed-dose of 2,000 units of PCC was given based on data that were extrapolated from warfarin reversal using PCC.7
The vitamin K antagonists that adulterated this patient’s synthetic cannabinoid were difenacoum and brodifacoum, which are 4-hydroxycoumarin derivatives. These are second-generation long-acting anticoagulant rodenticides (LAARs) that are about 100 times more potent than warfarin.8 As the name implies, LAARs have a longer duration of action in the body of any organism that ingests the poison, which is due to the highly lipophilic groups that have been added to the warfarin molecule to combat resistance in rodents.9
As a result of the deposition in the tissues, there have been reports of the duration of action of brodifacoum ranging from 51 days to 9 months after ingestion, with the latter caused by an intentional overdose in a human.9-12 Reports suggest that coagulopathy is not likely to occur when the serum brodifacoum concentration is < 10 ng/mL.13,14 Animal models show difenacoum has a tissue half-life of about 62 days.15 Reports of difenacoum poisoning in humans have shown variable lengths of treatment, ranging from 30 to 47 days.16-18 The length of treatment for either brodifacoum or difenacoum will depend on the amount of poison exposure.
The long duration of action and treatment duration may lead to problems with drug procurement, especially in the early phase of treatment in which IV vitamin K is used. The supply of IV vitamin K recently has been limited for at least some manufacturers. According to the American Society of Health System Pharmacists Current Drug Shortage List, the increased demand is thought to be due to increased use of synthetic inhaled cannabinoids laced with anticoagulant.19 IV vitamin K products are available from suppliers such as Amphastar (Rancho Cucamonga, CA) and Hospira (Lake Forest, IL).
The American College of Chest Physicians recommends IV vitamin K administration in patients with major bleeding secondary to vitamin K antagonists.20 The oral route is thought to be more effective than a subcutaneous route in the treatment of nonbleeding patients with rodenticide-associated coagulopathy. Due to erratic and unpredictable absorption, the subcutaneous route of administration has fallen out of favor. Oral vitamin K products were not affected by the recent shortage. However, large doses of oral vitamin K can be costly. Due to the long half-life of LAAR, many patients are discharged with a prescription for oral vitamin K. Although vitamin K is found in most over-the-counter (OTC) multivitamins, the strength is insufficient. Most OTC formulations are ≤ 100 μg, whereas the prescription strength is 5 mg, but patients being treated for rodenticide poisoning require much larger doses.
Commercial insurance carriers and Medicare Part D usually do not cover vitamins and minerals unless it is for a medically accepted indication or is an indication supported by citation in either the American Hospital Formulary System, United States Pharmacopeia drug information book, or an electronic information resource that is supported by evidence such as Micromedex.21 For a patient without insurance coverage being treated with high-dose vitamin K therapy for rodenticide poisoning outside of a federal health care system, the cost could be as high as $500 to $1,000 per day, depending on the dose of vitamin K needed to maintain an acceptable INR.
Conclusion
In addition to bleeding as a result of coagulopathy, this patient presented with new onset of AF with rapid ventricular response and a newly diagnosed cardiomyopathy. Although the patient had other cardiovascular risk factors, such as hypertension, dyslipidemia, and a remote history of cocaine use, it is likely that the use of the synthetic cannabinoids contributed to the development and/or worsening of this arrhythmia and cardiomyopathy. The patient remained in AF 6 weeks after hospital discharge with a controlled ventricular rate on metoprolol and digoxin. An interval echocardiogram 6 weeks after hospital discharge showed a recovered ejection fraction. In cases of tachycardia-induced cardiomyopathy, the ejection fraction often recovers with control of the tachycardia. The patient was weaned off vitamin K about 6 months after his initial presentation and started on a DOAC for anticoagulation. He subsequently underwent a transesophageal echocardiogram followed by an attempt at DC cardioversion; however, he did not remain in sinus rhythm and is being continued on anticoagulation and rate control for his AF.
Although unclear how synthetic cannabinoids became adulterated with a potent vitamin K antagonist, health care practitioners should consider this if a patient presents with unexplained coagulopathy and widespread bleeding. Fixed-dose PCC should be considered as an alternative to weight-based dosing in these cases. Physicians and pharmacy personnel should anticipate a need for long-term high doses of vitamin K in order to begin work early to obtain sufficient supplies to treat presenting patients.
Between March 7, 2018, and May 9, 2018, at least 164 people in Illinois were sickened by synthetic cannabinoids laced with rodenticides. The Illinois Department of Public Health has reported 4 deaths connected with the use of synthetic cannabinoids (sold under names such as Spice, K2, Legal Weed, etc).1 Synthetic cannabinoids are mind-altering chemicals that are sprayed on dried plant material and often sold at convenience stores. Some users have reported smoking these substances because they are generally not detected by standard urine toxicology tests.
Recreational use of synthetic cannabinoids can lead to serious and, at times, deadly complications. Chemicals found in rat poison have contaminated batches of synthetic cannabinoids, leading to coagulopathy and severe bleeding. Affected patients have reported hemoptysis, hematuria, severe epistaxis, bleeding gums, conjunctival hemorrhages, and gastrointestinal bleeding. The following case is of a patient who presented to an emergency department (ED) with severe coagulopathy and cardiotoxicity after using an adulterated synthetic cannabinoid product.
Case Presentation
A 65-year-old man presented to the ED reporting hematochezia, hematuria, and hemoptysis. He reported that these symptoms began about 1 day after he had smoked a synthetic cannabinoid called K2. The patient stated that some of his friends who used the same product were experiencing similar symptoms. He reported mild generalized abdominal pain but reported no chest pain, dyspnea, headache, fevers, chills, or dysuria.
The patient’s past medical history included hypertension, dyslipidemia, chronic lower back pain, and vitamin D deficiency. His past surgical history was notable for an exploratory laparotomy after a stab wound to the abdomen. The patient reported taking the following medications: morphine SA 30 mg bid, meloxicam 15 mg daily, amitriptyline 100 mg qhs, amlodipine 5 mg daily, hydrocodone/acetaminophen 5/325 mg q12h prn, atorvastatin 20 mg qhs, omeprazole 20 mg qam, senna 187 mg daily prn, psyllium 1 packet dissolved in water daily prn, and cholecalciferol 1,000 IU daily.
The patient’s temperature was 98o F, blood pressure, 144/80 mm Hg; pulse, 131 beats per minute; respiratory rate, 18 breaths per minute; and O2 saturation, 98% (ambient air). A physical examination revealed no acute distress; he was coughing up blood; clear lungs; heart sounds were tachycardic and irregularly irregular; soft, nondistended, mild generalized tenderness in the abdomen with no guarding and no rebound. The pertinent laboratory tests were international normalized ratio (INR), > 20; prothrombin time, > 150 seconds; prothrombin thromboplastin time, 157 seconds; hemoglobin, 13.3 g/dL; platelet count, 195 k/uL; white blood count, 11.3 k/uL; creatinine, 0.57mg/dL; potassium, 3.8 mmol/L, D-dimertest, 0.87 ug/mL fibrinogen equivalent units; fibrinogen level, 624 mg/dL; troponin, < 0.04 ng/mL; lactic acid, 1.3 mmol/L; total bilirubin, 0.8 mg/dL; alanine aminotransferase, 22 U/L, aspartate aminotransferase, 22 U/L; alkaline phosphatase, 89 U/L; urinalysis with > 50 red blood cells/high power field; large blood, negative leukocyte esterase, negative nitrite. The patient’s urine toxicology was negative for cannabinoids, methadone, amphetamines, cocaine, and benzodiazepines; but was positive for opiates. An anticoagulant poisoning panel also was ordered.
An electrocardiogram (ECG) and imaging studies were ordered. The ECG showed atrial fibrillation (AF) with rapid ventricular response (Figure 1). A chest X-ray indicated bibasilar consolidations that were worse on the right side. A noncontrast computed tomography (CT) of the head did not show intracranial bleeding. An abdomen/pelvis CT showed bilateral diffuse patchy peribronchovascular ground-glass opacities in the lung bases that could represent pulmonary hemorrhage, but no peritoneal or retroperitoneal bleeding.
Treatment
In the ED, the case was discussed with the Illinois Poison Control Center. The patient was diagnosed with coagulopathy likely due to anticoagulant poisoning. He was immediately treated with 10 mg of IV vitamin K, a fixed dose of 2,000 units of 4-factor prothrombin complex concentrate, and 4 units of fresh frozen plasma. His INR improved to 1.42 within several hours. He received 5 mg of IV metoprolol for uncontrolled AF and was admitted to the intensive care unit (ICU) for further care.
In the ICU the patient was started on oral vitamin K 50 mg tid for ongoing treatment of coagulopathy due to concern for possible rodenticide poisoning associated with very long half-life. This dose was then decreased to 50 mg bid. He was given IV fluid resuscitation with normal saline and started on rate control for AF with oral metoprolol. His heart rate improved. An echocardiogram showed new cardiomyopathy with an ejection fraction of 25% to 30%. Given basilar infiltrates and 1 episode of low-grade fever, he was started on ceftriaxone for possible community-acquired pneumonia. The patient was started on cholestyramine to help with washout of the possible rodenticide. No endoscopic interventions were performed.
The patient was transferred to an inpatient telemetry floor 24 hours after admission to the ICU once his tachycardia and bleeding improved. He did not require transfusion of packed red blood cells. In the ICU his INR had ranged between 1.62 and 2.46 (down from > 20 in the ED). His hemoglobin dropped from 13.3 g/dL on admission to 12 g/dL on transfer from the ICU, before stabilizing around 11 g/dL on the floor. The patient’s heart rate required better control, so metoprolol was increased to a total daily dose of 200 mg on the telemetry floor. Oral digoxin was then added after a digoxin load for additional rate control, as the patient remained tachycardic. Twice a day the patient continued to take 50 mg vitamin K. Cholestyramine and ceftriaxone were initially continued, but when the INR started increasing again, the cholestyramine was stopped to allow for an increase to more frequent 3-times daily vitamin 50 mg K administration (cholestyramine can interfere with vitamin K absorption). According to the toxicology service, there was only weak evidence to support use of cholestyramine in this setting.
Given his ongoing mild hemoptysis, the patient received first 1 unit, and then another 4 units of FFP when the INR increased to 3.96 despite oral vitamin K. After FFP, the INR decreased to 1.93 and subsequently to 1.52. A CT of the chest showed patchy ground-glass densities throughout the lungs, predominantly at the lung bases and to a lesser extent in the upper lobes. The findings were felt to represent pulmonary hemorrhage given the patient’s history of hemoptysis (Figure 2).
The patient’s heart rate control improved, and he remained hemodynamically stable. A thyroid function test was within normal limits. Lisinopril was added to metoprolol and digoxin given his newly diagnosed cardiomyopathy. The patient was observed for a total of 4 days on the inpatient floor and discharged after his INR stabilized around 1.5 on twice daily 50 mg vitamin K. The patient’s hematuria and hematochezia completely resolved, and hemoptysis was much improved at the time of discharge. His hemoglobin remained stable. The anticoagulant poisoning panel came back positive for
The patient has remained in AF at all follow-up visits. The INR normalized by 6 weeks after hospital discharge, and the dose of vitamin K slowly was tapered with close monitoring of the INR. Vitamin K was tapered for about 6 months after his initial presentation, and the patient was started on a direct oral anticoagulant (DOAC) for anticoagulation when the INR remained stable off vitamin K. He subsequently underwent a transesophageal echocardiogram followed by an attempt at direct current (DC) cardioversion; however, he did not remain in sinus rhythm, and is being continued on anticoagulation and rate control for his AF.
Discussion
Users generally smoke synthetic cannabinoids, which produce cannabis-like effects. However, atypical intoxication effects with worse complications often occur.2 These products typically contain dried shredded plant material that is soaked in or sprayed with several synthetic cannabinoids, varying in dosage and combination.3 Synthetic cannabinoids have been associated with serious adverse effects (AEs), including drowsiness, light-headedness, and fast or irregular heartbeat.4 More severe clinical features such as psychosis, delirium, cardiotoxicity, seizures, rhabdomyolysis, acute kidney injury, hyperthermia, myocardial ischemia, ischemic strokes, and death have also been noted.4
It is not known how some batches of synthetic cannabinoids came to be contaminated with rat poison or how commonly such an adulteration is found across the country. Several different guidelines provide pathways for the treatment of acute bleeding in the setting of coagulopathy due to vitamin K antagonists.5,6 Each guideline divides the indications for reversal into either severity of bleeding or the criticality of the bleeding based on location.5,6 All guidelines recommend the use of vitamin K (either oral or IV) followed by FFP or 4-factor prothrombin complex concentrate (PCC) for more severe bleeding.5,6 However, recommendations regarding the use of PCC vary in dosing for vitamin K antagonists (in contrast to treatment of coagulopathy due to DOACs). Recent studies and guidelines suggest that fixed-dose (rather than weight-based dose) PCC is effective for the reversal of coagulopathy due to vitamin K antagonists.6,7 Using fixed rather than weight-based dosing decreases cost and may decrease the possibility of thrombotic AEs.7 In this patient, a fixed-dose of 2,000 units of PCC was given based on data that were extrapolated from warfarin reversal using PCC.7
The vitamin K antagonists that adulterated this patient’s synthetic cannabinoid were difenacoum and brodifacoum, which are 4-hydroxycoumarin derivatives. These are second-generation long-acting anticoagulant rodenticides (LAARs) that are about 100 times more potent than warfarin.8 As the name implies, LAARs have a longer duration of action in the body of any organism that ingests the poison, which is due to the highly lipophilic groups that have been added to the warfarin molecule to combat resistance in rodents.9
As a result of the deposition in the tissues, there have been reports of the duration of action of brodifacoum ranging from 51 days to 9 months after ingestion, with the latter caused by an intentional overdose in a human.9-12 Reports suggest that coagulopathy is not likely to occur when the serum brodifacoum concentration is < 10 ng/mL.13,14 Animal models show difenacoum has a tissue half-life of about 62 days.15 Reports of difenacoum poisoning in humans have shown variable lengths of treatment, ranging from 30 to 47 days.16-18 The length of treatment for either brodifacoum or difenacoum will depend on the amount of poison exposure.
The long duration of action and treatment duration may lead to problems with drug procurement, especially in the early phase of treatment in which IV vitamin K is used. The supply of IV vitamin K recently has been limited for at least some manufacturers. According to the American Society of Health System Pharmacists Current Drug Shortage List, the increased demand is thought to be due to increased use of synthetic inhaled cannabinoids laced with anticoagulant.19 IV vitamin K products are available from suppliers such as Amphastar (Rancho Cucamonga, CA) and Hospira (Lake Forest, IL).
The American College of Chest Physicians recommends IV vitamin K administration in patients with major bleeding secondary to vitamin K antagonists.20 The oral route is thought to be more effective than a subcutaneous route in the treatment of nonbleeding patients with rodenticide-associated coagulopathy. Due to erratic and unpredictable absorption, the subcutaneous route of administration has fallen out of favor. Oral vitamin K products were not affected by the recent shortage. However, large doses of oral vitamin K can be costly. Due to the long half-life of LAAR, many patients are discharged with a prescription for oral vitamin K. Although vitamin K is found in most over-the-counter (OTC) multivitamins, the strength is insufficient. Most OTC formulations are ≤ 100 μg, whereas the prescription strength is 5 mg, but patients being treated for rodenticide poisoning require much larger doses.
Commercial insurance carriers and Medicare Part D usually do not cover vitamins and minerals unless it is for a medically accepted indication or is an indication supported by citation in either the American Hospital Formulary System, United States Pharmacopeia drug information book, or an electronic information resource that is supported by evidence such as Micromedex.21 For a patient without insurance coverage being treated with high-dose vitamin K therapy for rodenticide poisoning outside of a federal health care system, the cost could be as high as $500 to $1,000 per day, depending on the dose of vitamin K needed to maintain an acceptable INR.
Conclusion
In addition to bleeding as a result of coagulopathy, this patient presented with new onset of AF with rapid ventricular response and a newly diagnosed cardiomyopathy. Although the patient had other cardiovascular risk factors, such as hypertension, dyslipidemia, and a remote history of cocaine use, it is likely that the use of the synthetic cannabinoids contributed to the development and/or worsening of this arrhythmia and cardiomyopathy. The patient remained in AF 6 weeks after hospital discharge with a controlled ventricular rate on metoprolol and digoxin. An interval echocardiogram 6 weeks after hospital discharge showed a recovered ejection fraction. In cases of tachycardia-induced cardiomyopathy, the ejection fraction often recovers with control of the tachycardia. The patient was weaned off vitamin K about 6 months after his initial presentation and started on a DOAC for anticoagulation. He subsequently underwent a transesophageal echocardiogram followed by an attempt at DC cardioversion; however, he did not remain in sinus rhythm and is being continued on anticoagulation and rate control for his AF.
Although unclear how synthetic cannabinoids became adulterated with a potent vitamin K antagonist, health care practitioners should consider this if a patient presents with unexplained coagulopathy and widespread bleeding. Fixed-dose PCC should be considered as an alternative to weight-based dosing in these cases. Physicians and pharmacy personnel should anticipate a need for long-term high doses of vitamin K in order to begin work early to obtain sufficient supplies to treat presenting patients.
1. Illinois Department of Public Health. Synthetic cannabinoids. http://dph.illinois.gov/topics-services/prevention-wellness/medical-cannabis/synthetic-cannabinoids. Updated May 30, 2018. Accessed April 8, 2019.
2. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: update 2015. Subst Abus. 2017;38(3):344-366.
3. United Nations Office on Drugs and Crime. Global SMART update. https://www.unodc.org/documents/scientific/Global_SMART_Update_13_web.pdf. Published March 2015. Accessed April 8, 2019.
4. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235-242.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
6. Cushman M, Lim W, Zakai NA. 2011 Clinical Practice guide on anticoagulant dosing and management of anticoagulant-associated bleeding complications in adults. http://www.hematology.org/Clinicians/Guidelines-Quality/Quick-Ref/525.aspx. Published 2011. Accessed April 8, 2019.
7. Klein L, Peters J, Miner J, Gorlin J. Evaluation of fixed dose 4-factor prothrombin complex concentrate for emergent warfarin reversal. Am J Emerg Med. 2015;33(9):1213-1218.
8. Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281-288.
9. Lipton RA, Klass EM. Human ingestion of ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004-3005.
10. Chong LL, Chau WK, Ho CH. A case of ‘superwarfarin’ poisoning. Scand J Haematol. 1986;36(3):314-331.
11. Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
12. Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15(1):126-130.
13. Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med. 1993;153(16):1925-1928.
14. Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36(3):262-267.
15. Vandenbrouke V, Bousquet-Meloua A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31(5):437-445.
16. Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed). 1982;285(6341):541.
17. Katona B, Wason S. Superwarfarin poisoning. J Emerg Med. 1989;7(6):627-631.
18. Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol. 1992;11(6):553-554.
19. American Society of Health System Pharmacists. Current drug shortages. Vitamin K (phytonadione) injection. https://www.ashp.org/drug-shortages/current-shortages/Drug-Shortage-Detail.aspx?id=100. Updated July 5, 2018. Accessed April 8, 2019.
20. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
21. Centers for Medicare and Medicaid Services. Part D Excluded Drugs. https://www.medicareadvocacy.org/old-site/News/Archives/PartD_ExcludedDrugsByState.htm. Accessed on August 23, 2018.
1. Illinois Department of Public Health. Synthetic cannabinoids. http://dph.illinois.gov/topics-services/prevention-wellness/medical-cannabis/synthetic-cannabinoids. Updated May 30, 2018. Accessed April 8, 2019.
2. Tournebize J, Gibaja V, Kahn JP. Acute effects of synthetic cannabinoids: update 2015. Subst Abus. 2017;38(3):344-366.
3. United Nations Office on Drugs and Crime. Global SMART update. https://www.unodc.org/documents/scientific/Global_SMART_Update_13_web.pdf. Published March 2015. Accessed April 8, 2019.
4. Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med. 2017;376(3):235-242.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-3067.
6. Cushman M, Lim W, Zakai NA. 2011 Clinical Practice guide on anticoagulant dosing and management of anticoagulant-associated bleeding complications in adults. http://www.hematology.org/Clinicians/Guidelines-Quality/Quick-Ref/525.aspx. Published 2011. Accessed April 8, 2019.
7. Klein L, Peters J, Miner J, Gorlin J. Evaluation of fixed dose 4-factor prothrombin complex concentrate for emergent warfarin reversal. Am J Emerg Med. 2015;33(9):1213-1218.
8. Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27(5):281-288.
9. Lipton RA, Klass EM. Human ingestion of ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004-3005.
10. Chong LL, Chau WK, Ho CH. A case of ‘superwarfarin’ poisoning. Scand J Haematol. 1986;36(3):314-331.
11. Jones EC, Growe GH, Naiman SC. Prolonged anticoagulation in rat poisoning. JAMA. 1984;252(21):3005-3007.
12. Babcock J, Hartman K, Pedersen A, Murphy M, Alving B. Rodenticide-induced coagulopathy in a young child. A case of Munchausen syndrome by proxy. Am J Pediatr Hematol Oncol. 1993;15(1):126-130.
13. Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Arch Intern Med. 1993;153(16):1925-1928.
14. Bruno GR, Howland MA, McMeeking A, Hoffman RS. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36(3):262-267.
15. Vandenbrouke V, Bousquet-Meloua A, De Backer P, Croubels S. Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther. 2008;31(5):437-445.
16. Barlow AM, Gay AL, Park BK. Difenacoum (Neosorexa) poisoning. Br Med J (Clin Res Ed). 1982;285(6341):541.
17. Katona B, Wason S. Superwarfarin poisoning. J Emerg Med. 1989;7(6):627-631.
18. Butcher GP, Shearer MJ, MacNicoll AD, Kelly MJ, Ind PW. Difenacoum poisoning as a cause of haematuria. Hum Exp Toxicol. 1992;11(6):553-554.
19. American Society of Health System Pharmacists. Current drug shortages. Vitamin K (phytonadione) injection. https://www.ashp.org/drug-shortages/current-shortages/Drug-Shortage-Detail.aspx?id=100. Updated July 5, 2018. Accessed April 8, 2019.
20. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
21. Centers for Medicare and Medicaid Services. Part D Excluded Drugs. https://www.medicareadvocacy.org/old-site/News/Archives/PartD_ExcludedDrugsByState.htm. Accessed on August 23, 2018.