User login
Eosinophilic esophagitis: Frequently asked questions (and answers) for the early-career gastroenterologist
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).
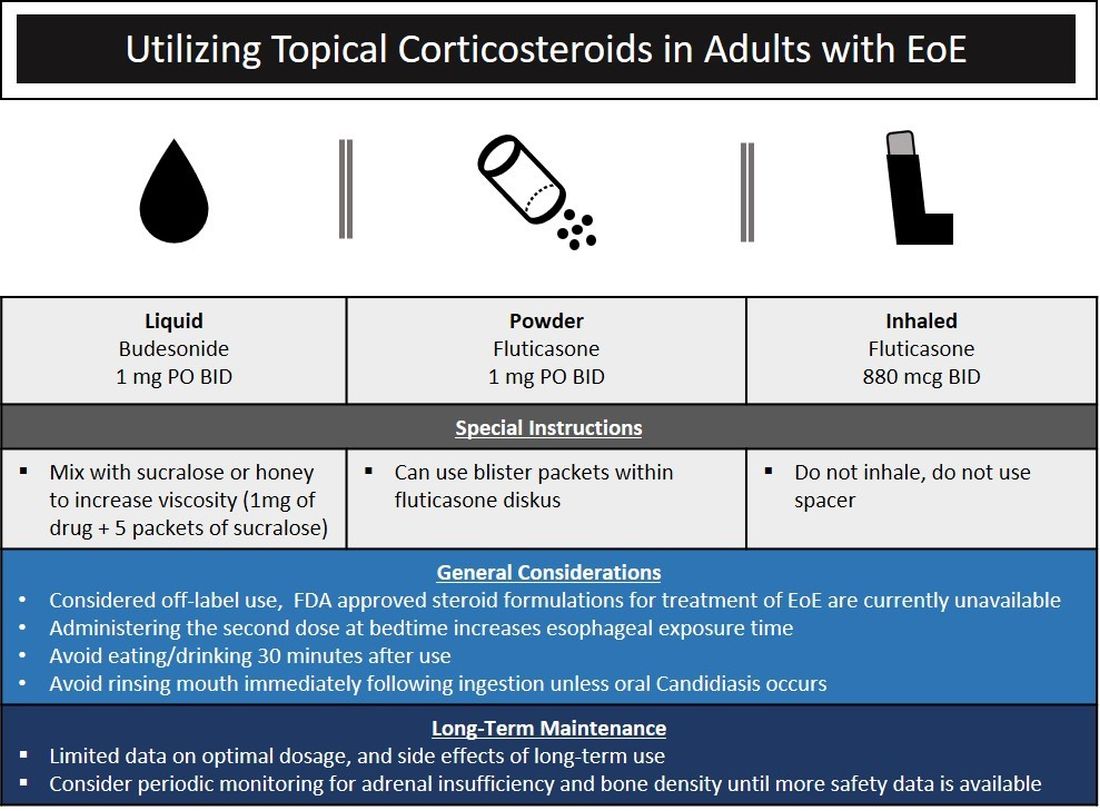
Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).
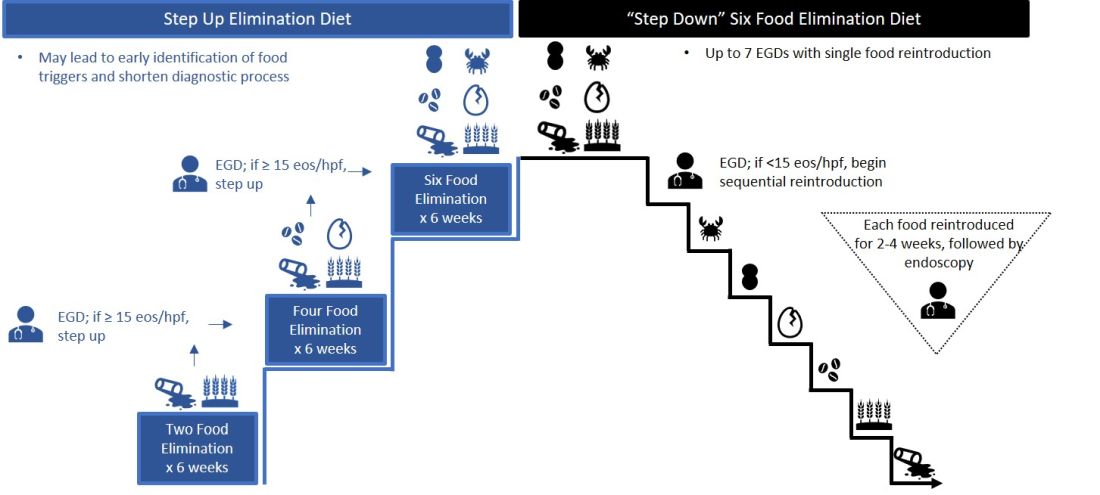
Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
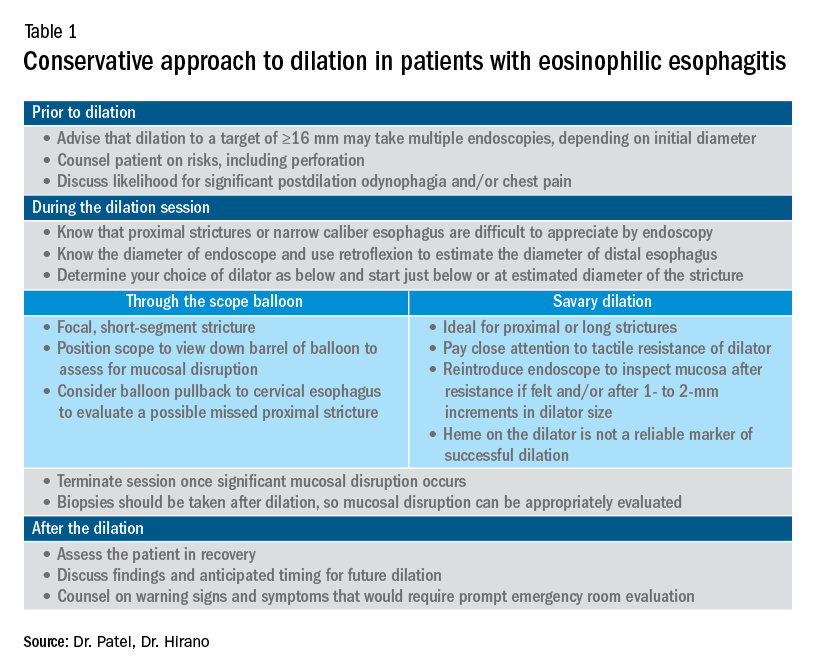
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12
When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Introduction
Eosinophilic esophagitis (EoE) has transformed over the past 3 decades from a rarely encountered entity to one of the most common causes of dysphagia in adults.1 Given the marked rise in prevalence, the early-career gastroenterologist will undoubtedly be involved with managing this disease.2 The typical presentation includes a young, atopic male presenting with dysphagia in the outpatient setting or, more acutely, with a food impaction when on call. As every fellow is keenly aware, the calls often come late at night as patients commonly have meat impactions while consuming dinner. Current management focuses on symptomatic, histologic, and endoscopic improvement with medication, dietary, and mechanical (i.e., dilation) modalities.
EoE is defined by the presence of esophageal dysfunction and esophageal eosinophilic inflammation with ≥15 eosinophils/high-powered field (eos/hpf) required for the diagnosis. With better understanding of the pathogenesis of EoE involving the complex interaction of environmental, host, and genetic factors, advancements have been made as it relates to the diagnostic criteria, endoscopic evaluation, and therapeutic options. In this article, we review the current management of adult patients with EoE and offer practical guidance to key questions for the young gastroenterologist as well as insights into future areas of interest.
What should I consider when diagnosing EoE?
Symptoms are central to the diagnosis and clinical presentation of EoE. In assessing symptoms, clinicians should be aware of adaptive “IMPACT” strategies patients often subconsciously develop in response to their chronic and progressive condition: Imbibing fluids with meals, modifying foods by cutting or pureeing, prolonging meal times, avoiding harder texture foods, chewing excessively, and turning away tablets/pills.3 Failure to query such adaptive behaviors may lead to an underestimation of disease activity and severity.
An important aspect to confirming the diagnosis of EoE is to exclude other causes of esophageal eosinophilia. Gastroesophageal reflux disease (GERD) is known to cause esophageal eosinophilia and historically has been viewed as a distinct disease process. In fact, initial guidelines included lack of response to a proton pump inhibitor (PPI) trial or normal esophageal pH monitoring as diagnostic criteria.4 However, as experience was garnered, it became clear that PPI therapy was effective at improving inflammation in 30%-50% of patients with clinical presentations and histologic features consistent with EoE. As such, the concept of PPI–responsive esophageal eosinophilia (PPI-REE) was introduced in 2011.5 Further investigation then highlighted that PPI-REE and EoE had nearly identical clinical, endoscopic, and histologic features as well as eosinophil biomarker and gene expression profiles. Hence, recent international guidelines no longer necessitate a PPI trial to establish a diagnosis of EoE.6
The young gastroenterologist should also be mindful of other issues related to the initial diagnosis of EoE. EoE may present concomitantly with other disease entities including GERD, “extra-esophageal” eosinophilic gastrointestinal diseases, concomitant IgE-mediated food allergy, hypereosinophilic syndromes, connective tissue disorders, autoimmune diseases, celiac disease, and inflammatory bowel disease.3 It has been speculated that some of these disorders share common aspects of genetic and environmental predisposing factors as well as shared pathogenesis. Careful history taking should include a full review of atopic conditions and GI-related symptoms and endoscopy should carefully inspect not only the esophagus, but also gastric and duodenal mucosa. The endoscopic features almost always reveal edema, rings, exudates, furrows, and strictures and can be assessed using the EoE Endoscopic Reference Scoring system (EREFS).7 EREFS allows for systematic identification of abnormalities that can inform decisions regarding treatment efficacy and decisions on the need for esophageal dilation. When the esophageal mucosa is evaluated for biopsies, furrows and exudates should be targeted, if present, and multiple biopsies (minimum of five to six) should be taken throughout the esophagus given the patchy nature of the disease.
How do I choose an initial therapy?
The choice of initial therapy considers patient preferences, medication availability, disease severity, impact on quality of life, and need for repeated endoscopies. While there are many novel agents currently being investigated in phase 2 and 3 clinical trials, the current mainstays of treatment include PPI therapy, topical steroids, dietary therapy, and dilation. Of note, there have been no head-to-head trials comparing these different modalities. A recent systematic review reported that PPIs can induce histologic remission in 42% of patients.8 The ease of use and availability of PPI therapy make this an attractive first choice for patients. Pooled estimates show that topical steroids can induce remission in 66% of patients.8 It is important to note that there is currently no Food and Drug Administration–approved formulation of steroids for the treatment of EoE. As such, there are several practical aspects to consider when instructing patients to use agents not designed for esophageal delivery (Figure 1).

Source: Dr. Patel, Dr. Hirano
Lack of insurance coverage for topical steroids can make cost of a prescription a deterrent to use. While topical steroids are well tolerated, concerns for candidiasis and adrenal insufficiency are being monitored in prospective, long-term clinical trials. Concomitant use of steroids with PPI would be appropriate for EoE patients with coexisting GERD (severe heartburn, erosive esophagitis, Barrett’s esophagus). In addition, we often combine steroids with PPI therapy for EoE patients who demonstrate a convincing but incomplete response to PPI monotherapy (i.e., reduction of baseline inflammation from 75 eos/hpf to 20 eos/hpf).
Diet therapy is a popular choice for management of EoE by patients, given the ability to remove food triggers that initiate the immune dysregulation and to avoid chronic medication use. Three dietary options have been described including an elemental, amino acid–based diet which eliminates all common food allergens, allergy testing–directed elimination diet, and an empiric elimination diet. Though elemental diets have shown the most efficacy, practical aspects of implementing, maintaining, and identifying triggers restrict their adoption by most patients and clinicians.9 Allergy-directed elimination diets, where allergens are eliminated based on office-based allergy testing, initially seemed promising, though studies have shown limited histologic remission, compared with other diet therapies as well as the inability to identify true food triggers. Advancement of office-based testing to identify food triggers is needed to streamline this dietary approach. In the adult patient, the empiric elimination diet remains an attractive choice of the available dietary therapies. In this dietary approach, which has shown efficacy in both children and adults, the most common food allergens (milk, wheat, soy, egg, nuts, and seafood) are eliminated.9
How do I make dietary therapy work in clinical practice?
Before dietary therapy is initiated, it is important that your practice is situated to support this approach and that patients fully understand the process. A multidisciplinary approach optimizes dietary therapy. Dietitians provide expert guidance on eliminating trigger foods, maintaining nutrition, and avoiding inadvertent cross-contamination. Patient questions may include the safety of consumption of non–cow-based cheese/milk, alcoholic beverages, wheat alternatives, and restaurant food. Allergists address concerns for a concomitant IgE food allergy based on a clinical history or previous testing. Patients should be informed that identifying a food trigger often takes several months and multiple endoscopies. Clinicians should be aware of potential food cost and accessibility issues as well as the reported, albeit uncommon, development of de novo IgE-mediated food allergy during reintroduction. Timing of diet therapy is also a factor in success. Patients should avoid starting diets during major holidays, family celebrations, college years, and busy travel months.
Particularly empiric elimination diets, frequently used in adults, several approaches have been described (Figure 2).

Source: Dr. Patel, Dr. Hirano
Initially, a step-down approach was described, with patients pursuing a six-food elimination diet (SFED), which eliminates the six most common triggers: milk, wheat, soy/legumes, egg, nuts, and seafood. Once in histologic remission, patients then systematically reintroduce foods in order to identify a causative trigger. Given that many patients have only one or two identified food triggers, other approaches were created including a single-food elimination diet eliminating milk, the two-food elimination diet (TFED) eliminating milk and wheat, and the four-food elimination diet (FFED) eliminating milk, wheat, soy/legumes, and eggs. A novel step-up approach has also now been described where patients start with the TFED and progress to the FFED and then potentially SFED based on histologic response.10 This approach has the potential to more readily identify triggers, decrease diagnostic time, and reduce endoscopic interventions. There are pros and cons to each elimination diet approach that should be discussed with patients. Many patients may find a one- or two-food elimination diet more feasible than a full SFED.
What should I consider when performing dilation?
Esophageal dilation is frequently used to address the fibrostenotic complications of EoE that do not as readily respond to PPI, steroid, or diet therapy. The majority of patients note symptomatic improvement following dilation, though dilation alone does not address the inflammatory component of disease.8 With a conservative approach, the complication rates of esophageal dilation in EoE are similar to that of benign, esophageal strictures. Endoscopists should be aware that endoscopy alone can miss strictures and consider both practical and technical aspects when performing dilations (Table 1).11,12

When should an allergist be consulted?
The role of the allergist in the management of patients with EoE varies by patient and practice. IgE serologic or skin testing have limited accuracy in identifying food triggers for EoE. Nevertheless, the majority of patients with EoE have an atopic condition which may include asthma, allergic rhinitis, atopic dermatitis, or IgE-mediated food allergy. Although EoE is thought to primarily occur from an immune response to ingested oral allergens, aeroallergens may exacerbate disease as evidenced by the seasonal variation in EoE symptoms in some patients. The allergist provides treatment for these “extraesophageal” atopic conditions which may, in turn, have synergistic effects on the treatment of EoE. Furthermore, allergists may prescribe biologic therapies that are FDA approved for the treatment of atopic dermatitis, asthma, and allergic rhinitis. While not approved for EoE, several of these agents have shown efficacy in phase 2 clinical trials in EoE. In some practice settings, allergists primarily manage EoE patients with the assistance of gastroenterologists for periodic endoscopic activity assessment.
What are the key aspects of maintenance therapy?
The goals of treatment focus on symptomatic, histologic, and endoscopic improvement, and the prevention of future or ongoing fibrostenotic complications.2 Because of the adaptive eating behaviors discussed above, symptom response may not reliably correlate with histologic and/or endoscopic improvement. Moreover, dysphagia is related to strictures that often do not resolve in spite of resolution of mucosal inflammation. As such, histology and endoscopy are more objective and reliable targets of a successful response to therapy. Though studies have used variable esophageal density levels for response, using a cutoff of <15 eos/hpf as a therapeutic endpoint is reasonable for both initial response to therapy and long-term monitoring.13 We advocate for standardization of reporting endoscopic findings to better track change over time using the EREFS scoring system.7 While inflammatory features improve, the fibrostenotic features may persist despite improvement in histology. Dilation is often performed in these situations, especially for symptomatic individuals.
During clinical follow-up, the frequency of monitoring as it relates to symptom and endoscopic assessment is not well defined. It is reasonable to repeat endoscopic intervention following changes in therapy (i.e., reduction in steroid dosing or reintroduction of putative food triggers) or in symptoms.13 It is unclear if patients benefit from repeated endoscopies at set intervals without symptom change and after histologic response has been confirmed. In our practice, endoscopies are often considered on an annual basis. This interval is increased for patients with demonstrated stability of disease.
For patients who opt for dietary therapy and have one or two food triggers identified, long-term maintenance therapy can be straightforward with ongoing food avoidance. Limited data exist regarding long-term effectiveness of dietary therapy but loss of initial response has been reported that is often attributed to problems with adherence. Use of “diet holidays” or “planned cheats” to allow for intermittent consumption of trigger foods, often under the cover of short-term use of steroids, may improve the long-term feasibility of diet approaches.
In the recent American Gastroenterological Association guidelines, continuation of swallowed, topical steroids is recommended following remission with short-term treatment. The recurrence of both symptoms and inflammation following medication withdrawal supports this practice. Furthermore, natural history studies demonstrate progression of esophageal strictures with untreated disease.
There are no clear guidelines for long-term dosage and use of PPI or topical steroid therapy. Our practice is to down-titrate the dose of PPI or steroid following remission with short-term therapy, often starting with a reduction from twice a day to daily dosing. Although topical steroid therapy has fewer side effects, compared with systemic steroids, patients should be aware of the potential for adrenal suppression especially in an atopic population who may be exposed to multiple forms of topical steroids. Shared decision-making between patients and providers is recommended to determine comfort level with long-term use of prescription medications and dosage.
What’s on the horizon?
Several areas of development are underway to better assess and manage EoE. Novel histologic scoring tools now assess characteristics on pathology beyond eosinophil density, office-based testing modalities have been developed to assess inflammatory activity and thereby obviate the need for endoscopy, new technology can provide measures of esophageal remodeling and provide assessment of disease severity, and several biologic agents are being studied that target specific allergic mediators of the immune response in EoE.3,14-18 These novel tools, technologies, and therapies will undoubtedly change the management approach to EoE. Referral of patients into ongoing clinical trials will help inform advances in the field.
Conclusion
As an increasingly prevalent disease with a high degree of upper GI morbidity, EoE has transitioned from a rare entity to a commonly encountered disease. The new gastroenterologist will confront both straightforward as well as complex patients with EoE, and we offer several practical aspects on management. In the years ahead, the care of patients with EoE will continue to evolve to a more streamlined, effective, and personalized approach.
References
1. Kidambi T et al. World J Gastroenterol. 2012;18:4335-41.
2. Dellon ES et al. Gastroenterology. 2018;154:319-32 e3.
3. Hirano I et al. Gastroenterology. 2020;158:840-51.
4. Furuta GT et al. Gastroenterology. 2007;133:1342-63.
5. Liacouras CA et al. J Allergy Clin Immunol. 2011;128:3-20 e6; quiz 1-2.
6. Dellon ES et al. Gastroenterology. 2018;155:1022-33 e10.
7. Hirano I et al. Gut. 2013;62:489-95.
8. Rank MA et al. Gastroenterology. 2020;158:1789-810 e15.
9. Arias A et al. Gastroenterology. 2014;146:1639-48.
10. Molina-Infante J et al. J Allergy Clin Immunol. 2018;141:1365-72.
11. Gentile N et al. Aliment Pharmacol Ther. 2014;40:1333-40.
12. Hirano I. Gastroenterology. 2018;155:601-6.
13. Hirano I et al. Gastroenterology. 2020;158:1776-86.
14. Collins MH et al. Dis Esophagus. 2017;30:1-8.
15. Furuta GT et al. Gut. 2013;62:1395-405.
16. Katzka DA et al. Clin Gastroenterol Hepatol. 2015;13:77-83 e2.
17. Kwiatek MA et al. Gastroenterology. 2011;140:82-90.
18. Nicodeme F et al. Clin Gastroenterol Hepatol. 2013;11:1101-7 e1.
Eosinophilic esophagitis: Faces and facets of a new disease
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.
A dramatic rise in the recognition of eosinophilic esophagitis (EoE) has followed the case series by Stephen Attwood, MD, and Alex Straumann, MD, which first characterized the disease 25 years ago. While still a young disease, EoE has evolved from esoterica to a leading cause of dysphagia and food impaction worldwide (Gastroenterology. 2018 Jan;154[2]:319-32.). The typical face of EoE is a 30- to 40-year-old white man, but EoE afflicts both men and women of all ages and ethnic groups.
Guidelines prior to 2017 excluded proton pump inhibitor–responsive esophageal eosinophilia (PPIREE) from a formal diagnosis of EoE. The last decade, however, has witnessed the rise of fall of PPIREE, which was first reported in 2006 in a case series of three pediatric patients with presentations consistent with EoE, but symptom and histologic resolution after treatment with omeprazole. At the time, these cases were viewed as rare curiosities. Subsequent to a prospective series by Javier Molina-Infante, MD, in 2011, however, multiple studies have demonstrated that 30%-50% of patients suspected of having EoE respond to proton pump inhibitor (PPI). Clearly, PPIREE is not rare. Clinical and translational studies have investigated the phenomenon of PPIREE, noting that EoE and PPIREE share demographic, symptom, endoscopic, and pathologic features as well as biomarker expression and gene profiles that are distinct from gastroesophageal reflux disease (GERD). Furthermore, studies have identified intriguing, acid-independent properties of PPIs that inhibit allergic inflammation in cultured EoE cell lines. Together, these clinical and translational studies led to a 2016 European task force recommendation to remove the PPI trial from the diagnostic criteria for EoE (Gut 2016 Mar;65[3]:524-31). At Digestive Disease Week 2017®, an international consortium sponsored by the International Gastrointestinal Eosinophil Researchers (TIGERS) convened in Chicago to review this controversy. The consensus from this meeting was in line with the European position statement. For patients with a clinical presentation suggestive of EoE and esophageal eosinophilia, clinicians should carefully consider non-EoE causes of esophageal eosinophilia but would not be required to use PPIs to establish a diagnosis of EoE.
Assessment of disease activity in EoE has largely focused on counting eosinophils on esophageal biopsies, but the mucosa may be the tip of the EoE iceberg. There is increasing evidence that the inflammation and remodeling aspects of EoE extend beneath the mucosa. If you “dig a little deeper” and sample the subepithelial space, a different face of EoE emerges, with eosinophilic inflammation and fibrosis in EoE that are distinct from GERD. This subepithelial remodeling forms the basis for the strictures and narrow caliber esophagus that are major complications of EoE.
Treatment of EoE involves a multifaceted approach that includes medications, dietary therapy, and esophageal dilation. No drugs have yet been approved by the Food and Drug Administration for EoE. Off-label use of topical corticosteroids are a mainstay of therapy, with 10 double-blind, placebo-controlled randomized trials demonstrating efficacy for both histology and symptoms. Novel therapeutic approaches to EoE are targeting allergic cytokine mediators including interleukin-4, 5, and 13 with promising results. The role of biologic therapies in the management of EoE is yet undefined but the increasing recognition of steroid-refractory patients as well as potential effects on esophageal remodeling are unmet needs. Diet therapy continues to be an important, first-line option for motivated patients and clinicians, with removal of the six most common food allergens associated with a 70% histologic response in both pediatric and adult studies. Less-restrictive diets have been devised to reduce the need for repeated endoscopies. At the same time, several office-based tests of disease activity are undergoing validation, including the esophageal string test, Cytosponge, mucosal impedance, transnasal endoscopy, and confocal microscopy capsule. These technologies will lead to fewer endoscopies and may shift EoE management to the primary care or allergist’s office.
Finally, it is important to acknowledge that EoE is not a “GI disease,” but one that is best managed by a multifaceted approach that integrates allergists, immunologists, pathologists, radiologists, dietitians, patient advocacy, and epidemiologists who are confronting this new disease. The Consortium of Eosinophilic Gastrointestinal Disease Researchers, funded by the National Institutes of Health and the Rare Diseases Clinical Research Network, is an example of a multidisciplinary collaboration that addresses fundamental questions regarding the natural history and optimal management of EoE.
Dr. Hirano is a professor of medicine, division of gastroenterology, Northwestern University, Chicago. He has received grant support from the NIH Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR, U54 AI117804). CEGIR is also supported by patient advocacy groups including the American Partnership for Eosinophilic Disorders, the CURED Foundation, and the Eosinophilic Family Coalition. Dr. Hirano has received consulting fees and research funding from Celgene, Regeneron, and Shire among others. Dr. Hirano made his comments during the AGA Institute Presidential Plenary at the Annual Digestive Disease Week.



