User login
Canada approves Jivi for hemophilia A
Health Canada has approved Jivi® (antihemophilic factor [recombinant, B-domain deleted, PEGylated]) for use in patients with hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate developed by Bayer.
Health Canada has approved Jivi for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in previously treated hemophilia A patients age 12 and older.
Jivi is also approved for the control and prevention of episodic bleeding and for perioperative management of bleeding.
The recommended initial dosing for Jivi as prophylaxis is twice weekly, with the ability to dose every 5 days and further adjust dosing based on bleeding episodes.
Health Canada’s approval of Jivi is based on the PROTECT VIII trial. Results from this trial are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A. In part B, Jivi was used for perioperative management of bleeding.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
Health Canada has approved Jivi® (antihemophilic factor [recombinant, B-domain deleted, PEGylated]) for use in patients with hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate developed by Bayer.
Health Canada has approved Jivi for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in previously treated hemophilia A patients age 12 and older.
Jivi is also approved for the control and prevention of episodic bleeding and for perioperative management of bleeding.
The recommended initial dosing for Jivi as prophylaxis is twice weekly, with the ability to dose every 5 days and further adjust dosing based on bleeding episodes.
Health Canada’s approval of Jivi is based on the PROTECT VIII trial. Results from this trial are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A. In part B, Jivi was used for perioperative management of bleeding.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
Health Canada has approved Jivi® (antihemophilic factor [recombinant, B-domain deleted, PEGylated]) for use in patients with hemophilia A.
Jivi (formerly BAY94-9027) is a DNA-derived, factor VIII concentrate developed by Bayer.
Health Canada has approved Jivi for use as routine prophylaxis to prevent or reduce the frequency of bleeding episodes in previously treated hemophilia A patients age 12 and older.
Jivi is also approved for the control and prevention of episodic bleeding and for perioperative management of bleeding.
The recommended initial dosing for Jivi as prophylaxis is twice weekly, with the ability to dose every 5 days and further adjust dosing based on bleeding episodes.
Health Canada’s approval of Jivi is based on the PROTECT VIII trial. Results from this trial are available in the U.S. prescribing information for Jivi.
PROTECT VIII enrolled previously treated adults and adolescents (ages 12 to 65) with severe hemophilia A.
In part A, researchers evaluated different dosing regimens for Jivi used as prophylaxis and on-demand treatment. An optional extension study was available to patients who completed part A. In part B, Jivi was used for perioperative management of bleeding.
Efficacy
In part A, there were 132 patients in the intent‐to‐treat population—112 in the prophylaxis group and 20 in the on-demand group.
Patients received Jivi for 36 weeks. For the first 10 weeks, patients in the prophylaxis group received twice-weekly dosing at 25 IU/kg.
Patients with more than one bleed during this time went on to receive 30–40 IU/kg twice weekly. Patients with one or fewer bleeds were eligible for randomization to dosing every 5 days (45–60 IU/kg) or every 7 days (60 IU/kg).
The median annualized bleeding rate (ABR) was 4.1 for the patients who were treated twice weekly and were not eligible for randomization (n=13) and 1.9 for patients who were eligible for randomization but continued on twice-weekly treatment (n=11).
The median ABR was 1.9 for patients who were randomized to treatment every 5 days (n=43) and 0.96 for patients who completed prophylaxis with dosing every 7 days (32/43).
The median ABR for patients treated on demand was 24.1.
There were 388 treated bleeds in the on-demand group and 317 treated bleeds in the prophylaxis group. Overall, 73.3% of responses to treatment were considered “excellent” or “good,” 23.3% were considered “moderate,” and 3.3% were considered “poor.”
There were 17 patients who underwent 20 major surgeries in part B or the extension study and 10 patients who underwent minor surgeries in part A. Jivi provided “good” or “excellent” hemostatic control during all surgeries.
Safety
Safety data are available for 148 patients age 12 and older.
Adverse events in these patients included abdominal pain (3%), nausea (5%), vomiting (3%), injection site reactions (1%), pyrexia (5%), hypersensitivity (2%), dizziness (2%), headache (14%), insomnia (3%), cough (7%), erythema (1%), pruritus (1%), rash (2%), and flushing (1%).
A factor VIII inhibitor was reported in one adult patient, but repeat testing did not confirm the report.
One adult with asthma had a clinical hypersensitivity reaction and a transient increase of IgM anti-PEG antibody titer, which was negative upon retesting.
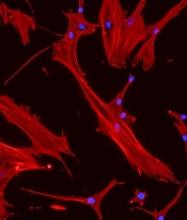
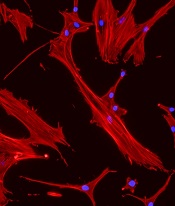
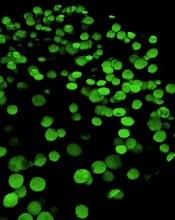
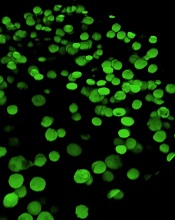
‘Mechanoprimed’ MSCs aid hematopoietic recovery
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Specially grown mesenchymal stromal cells (MSCs) can improve hematopoietic recovery, according to preclinical research published in Stem Cell Research and Therapy.
Researchers grew MSCs on a surface with mechanical properties similar to those of bone marrow, which prompted the MSCs to secrete growth factors that aid hematopoietic recovery.
When implanted in irradiated mice, these “mechanoprimed” MSCs sped recovery of all hematopoietic lineages and improved the animals’ survival.
“[MSCs] act like drug factories,” explained study author Krystyn Van Vliet, PhD, of the Massachusetts Institute of Technology in Cambridge.
“They can become tissue lineage cells, but they also pump out a lot of factors that change the environment that the hematopoietic stem cells are operating in.”
Dr. Van Vliet and her colleagues noted that MSCs play an important role in supporting, maintaining, and expanding hematopoietic stem and progenitor cells (HSPCs). However, in a given population of MSCs, usually only about 20% of the cells produce the factors needed to stimulate hematopoietic recovery.
In an earlier study, Dr. Van Vliet and her colleagues showed they could sort MSCs with a microfluidic device that can identify the 20% of cells that promote hematopoietic recovery.
However, the researchers wanted to improve on that by finding a way to stimulate an entire population of MSCs to produce the necessary factors. To do that, they first had to discover which factors were the most important.
Analyses in mice suggested eight factors were associated with improved survival after irradiation—IL-6, IL-8, BMP2, EGF, FGF1, RANTES, VEGF-A, and ANG-1.
Mechanopriming
Having identified factors associated with hematopoietic recovery, Dr. Van Vliet and her colleagues explored the idea of mechanopriming MSCs so they would produce more of these factors.
Over the past decade, researchers have shown that varying the mechanical properties of surfaces on which stem cells are grown can affect their differentiation into mature cell types. However, in this study, the researchers showed that mechanical properties can also affect the factors stem cells secrete before committing to a specific lineage.
For the growth surface, Dr. Van Vliet and her colleagues tested a polymer called polydimethylsiloxane (PDMS). The team varied the mechanical stiffness of the PDMS surface to see how this would affect the MSCs.
MSCs grown on the least stiff PDMS surface produced the greatest number of factors necessary to induce differentiation in HSPCs. These MSCs were able to promote hematopoiesis in an in vitro co-culture model with HSPCs.
Testing in mice
The researchers then tested the mechanoprimed MSCs by implanting them into irradiated mice.
The mechanoprimed MSCs quickly repopulated the animals’ blood cells and helped them recover more quickly than mice treated with MSCs grown on traditional glass surfaces.
Mice that received mechanoprimed MSCs also recovered faster than mice treated with factor-producing MSCs selected by the microfluidic sorting device.
Dr. Van Vliet’s lab is now performing more animal studies in hopes of developing a combination treatment of MSCs and HSPCs that could be tested in humans.
The current research was funded by the National Institutes of Health and the BioSystems and Micromechanics Interdisciplinary Research Group of the Singapore-MIT Alliance for Research and Technology through the Singapore National Research Foundation.
The researchers said they had no competing interests.
Three gene types drive MM disparity in African Americans
Researchers say they may have determined why African Americans have a two- to three-fold increased risk of multiple myeloma (MM) compared to European Americans.
The team genotyped 881 MM samples from various racial groups and identified three gene subtypes—t(11;14), t(14;16), and t(14;20)—that explain the racial disparity.
They found that patients with African ancestry of 80% or more had a significantly higher occurrence of these subtypes compared to individuals with African ancestry less than 0.1%.
And these subtypes are driving the disparity in MM diagnoses between the populations.
The researchers state that previous attempts to explain the disparity relied on self-reported race rather than quantitatively measured genetic ancestry, which could result in bias.
“A major new aspect of this study is that we identified the ancestry of each patient through DNA sequencing, which allowed us to determine ancestry more accurately,” said study author Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
He and his colleagues reported their findings in Blood Cancer Journal.
All 881 samples had abnormal plasma cell FISH, 851 had a normal chromosome study, and 30 had an abnormal study.
Median age for the entire group was 64 (range, 26–90), with 35.4% in the 60–69 age category. More samples were from men (n=478, 54.3%) than women (n=403, 45.7%).
Researchers observed no significant difference between men and women in the proportion of primary cytogenetic abnormalities.
Of the 881 samples, the median African ancestry was 2.3%, the median European ancestry was 64.7%, and Northern European ancestry was 26.6%.
Thirty percent of the entire cohort had less than 0.1% African ancestry, and 13.6% had 80% or greater African ancestry.
Using a logistic regression model, the researchers determined that a 10% increase in the percentage of African ancestry was associated with a 6% increase in the odds of detecting t(11;14), t(14;16), or t(14;20) (odds ratio=1.06, 95% CI: 1.02–1.11; P=0.05).
The researchers plotted the probability of observing these cytogenetic abnormalities with the percentage of African ancestry and found the differences were most striking in the extreme populations—individuals with ≥80.0% African ancestry and individuals with <0.1% African ancestry.
Upon further analysis, the team found a significantly higher prevalence of t(11;14), t(14;16), and t(14;20) in the group of patients with the greatest proportion of African ancestry (P=0.008) compared to the European cohort.
The researchers said the differences only emerged in the highest (n=120 individuals) and lowest (n=235 individuals) cohorts. Most patients (n=526, 60%) were not included in these extreme populations because they had mixed ancestry.
The team observed no significant differences when the cutoff of African ancestry was greater than 50%.
“Our findings provide important information that will help us determine the mechanism by which myeloma is more common in African Americans, as well as help us in our quest to find out what causes myeloma in the first place,” Dr. Rajkumar said.
The research was supported by the National Cancer Institute of the National Institutes of Health and the Mayo Clinic Department of Laboratory Medicine and Pathology and Center for Individualized Medicine. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
Researchers say they may have determined why African Americans have a two- to three-fold increased risk of multiple myeloma (MM) compared to European Americans.
The team genotyped 881 MM samples from various racial groups and identified three gene subtypes—t(11;14), t(14;16), and t(14;20)—that explain the racial disparity.
They found that patients with African ancestry of 80% or more had a significantly higher occurrence of these subtypes compared to individuals with African ancestry less than 0.1%.
And these subtypes are driving the disparity in MM diagnoses between the populations.
The researchers state that previous attempts to explain the disparity relied on self-reported race rather than quantitatively measured genetic ancestry, which could result in bias.
“A major new aspect of this study is that we identified the ancestry of each patient through DNA sequencing, which allowed us to determine ancestry more accurately,” said study author Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
He and his colleagues reported their findings in Blood Cancer Journal.
All 881 samples had abnormal plasma cell FISH, 851 had a normal chromosome study, and 30 had an abnormal study.
Median age for the entire group was 64 (range, 26–90), with 35.4% in the 60–69 age category. More samples were from men (n=478, 54.3%) than women (n=403, 45.7%).
Researchers observed no significant difference between men and women in the proportion of primary cytogenetic abnormalities.
Of the 881 samples, the median African ancestry was 2.3%, the median European ancestry was 64.7%, and Northern European ancestry was 26.6%.
Thirty percent of the entire cohort had less than 0.1% African ancestry, and 13.6% had 80% or greater African ancestry.
Using a logistic regression model, the researchers determined that a 10% increase in the percentage of African ancestry was associated with a 6% increase in the odds of detecting t(11;14), t(14;16), or t(14;20) (odds ratio=1.06, 95% CI: 1.02–1.11; P=0.05).
The researchers plotted the probability of observing these cytogenetic abnormalities with the percentage of African ancestry and found the differences were most striking in the extreme populations—individuals with ≥80.0% African ancestry and individuals with <0.1% African ancestry.
Upon further analysis, the team found a significantly higher prevalence of t(11;14), t(14;16), and t(14;20) in the group of patients with the greatest proportion of African ancestry (P=0.008) compared to the European cohort.
The researchers said the differences only emerged in the highest (n=120 individuals) and lowest (n=235 individuals) cohorts. Most patients (n=526, 60%) were not included in these extreme populations because they had mixed ancestry.
The team observed no significant differences when the cutoff of African ancestry was greater than 50%.
“Our findings provide important information that will help us determine the mechanism by which myeloma is more common in African Americans, as well as help us in our quest to find out what causes myeloma in the first place,” Dr. Rajkumar said.
The research was supported by the National Cancer Institute of the National Institutes of Health and the Mayo Clinic Department of Laboratory Medicine and Pathology and Center for Individualized Medicine. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
Researchers say they may have determined why African Americans have a two- to three-fold increased risk of multiple myeloma (MM) compared to European Americans.
The team genotyped 881 MM samples from various racial groups and identified three gene subtypes—t(11;14), t(14;16), and t(14;20)—that explain the racial disparity.
They found that patients with African ancestry of 80% or more had a significantly higher occurrence of these subtypes compared to individuals with African ancestry less than 0.1%.
And these subtypes are driving the disparity in MM diagnoses between the populations.
The researchers state that previous attempts to explain the disparity relied on self-reported race rather than quantitatively measured genetic ancestry, which could result in bias.
“A major new aspect of this study is that we identified the ancestry of each patient through DNA sequencing, which allowed us to determine ancestry more accurately,” said study author Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
He and his colleagues reported their findings in Blood Cancer Journal.
All 881 samples had abnormal plasma cell FISH, 851 had a normal chromosome study, and 30 had an abnormal study.
Median age for the entire group was 64 (range, 26–90), with 35.4% in the 60–69 age category. More samples were from men (n=478, 54.3%) than women (n=403, 45.7%).
Researchers observed no significant difference between men and women in the proportion of primary cytogenetic abnormalities.
Of the 881 samples, the median African ancestry was 2.3%, the median European ancestry was 64.7%, and Northern European ancestry was 26.6%.
Thirty percent of the entire cohort had less than 0.1% African ancestry, and 13.6% had 80% or greater African ancestry.
Using a logistic regression model, the researchers determined that a 10% increase in the percentage of African ancestry was associated with a 6% increase in the odds of detecting t(11;14), t(14;16), or t(14;20) (odds ratio=1.06, 95% CI: 1.02–1.11; P=0.05).
The researchers plotted the probability of observing these cytogenetic abnormalities with the percentage of African ancestry and found the differences were most striking in the extreme populations—individuals with ≥80.0% African ancestry and individuals with <0.1% African ancestry.
Upon further analysis, the team found a significantly higher prevalence of t(11;14), t(14;16), and t(14;20) in the group of patients with the greatest proportion of African ancestry (P=0.008) compared to the European cohort.
The researchers said the differences only emerged in the highest (n=120 individuals) and lowest (n=235 individuals) cohorts. Most patients (n=526, 60%) were not included in these extreme populations because they had mixed ancestry.
The team observed no significant differences when the cutoff of African ancestry was greater than 50%.
“Our findings provide important information that will help us determine the mechanism by which myeloma is more common in African Americans, as well as help us in our quest to find out what causes myeloma in the first place,” Dr. Rajkumar said.
The research was supported by the National Cancer Institute of the National Institutes of Health and the Mayo Clinic Department of Laboratory Medicine and Pathology and Center for Individualized Medicine. One study author reported relationships with Celgene, Takeda, Prothena, Janssen, Pfizer, Alnylam, and GSK. Two authors reported relationships with the DNA Diagnostics Center.
MDM2 inhibitors could treat resistant AML
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Preclinical research has revealed a potential treatment for chemotherapy-resistant acute myeloid leukemia (AML).
Researchers characterized a mechanism of chemotherapy resistance in AML and found that MDM2 is a key player in this dysregulated signaling pathway.
They tested MDM2 inhibitors and found these drugs could sensitize resistant AML to chemotherapy in vitro and in vivo.
In fact, mice with refractory AML responded to standard induction therapy when combined with an MDM2 inhibitor, showing no signs of disease and prolonged survival.
These results were published in Cancer Discovery.
“We were blown away when we saw the results,” said study author William Stanford, PhD, of Ottawa Hospital Research Institute in Ontario, Canada.
“If these findings hold up in clinical trials, we could have a new treatment for people who would almost certainly die of their disease today.”
Mechanism of resistance
Dr. Stanford’s research began with the protein MTF2. He and his colleagues previously found that MTF2 plays a role in erythropoiesis, and the team wanted to determine if MTF2 also plays a role in AML.
Using AML samples from patients treated at The Ottawa Hospital, the researchers found the mean survival was three times longer in patients with normal MTF2 activity than in patients with low MTF2 activity.
“Initially, we thought that MTF2 could be an important biomarker to identify patients who might benefit from experimental therapies,” Dr. Stanford said. “But then we started thinking that if we could understand what MTF2 was doing, maybe we could use this information to develop a new treatment.”
Dr. Stanford and his colleagues discovered that MTF2 represses MDM2, a protein that helps cells resist chemotherapy.
The team found that MTF2-deficient cells overexpress MDM2, which inhibits p53, and this leads to defects in cell-cycle regulation and apoptosis that enable resistance to chemotherapy.
Testing MDM2 inhibitors
Since MDM2 inhibitors are already being tested in clinical trials for other cancers, Dr. Stanford and his colleagues tested these inhibitors in vitro and in mouse models of chemotherapy-resistant AML.
The in vitro experiments included two MDM2 inhibitors—Nutlin3A and MI-773—combined with daunorubicin or cytarabine.
The researchers found that refractory, MTF2-deficient AML cells underwent apoptosis when treated with either daunorubicin or cytarabine in combination with Nutlin3A or MI-773. The effect was comparable to that observed in AML cells with normal MTF2.
The team found that Nutlin3A was more efficient at sensitizing refractory, MTF2-deficient AML cells to daunorubicin, so they used Nutlin3A in the in vivo experiments.
For these experiments, the researchers tested Nutlin3A in mice injected with either chemotherapy-responsive AML cells (with normal MTF2) or refractory, MTF2-deficient AML cells.
Once the mice had “a substantial leukemic burden” (≥ 20% CD45+CD33+ leukemic blasts in their peripheral blood), they were randomized to receive vehicle control, Nutlin3A, standard induction therapy, or induction plus Nutlin3A.
The mice engrafted with chemotherapy-responsive AML cells did not respond to vehicle control or Nutlin3A alone. However, they did respond to standard induction and induction plus Nutlin3A, surviving until the end of the experiment at 16 weeks.
Among the mice engrafted with refractory, MTF2-deficient AML cells, only those animals treated with induction plus Nutlin3A survived until the end of the experiment.
The researchers also noted a “dramatic loss” in the blast-containing CD45+CD33+ and CD34+CD38− populations in mice treated with induction plus Nutlin3A.
To assess residual disease, the researchers performed secondary transplants with cells from mice that had engrafted with refractory, MTF2-deficient AML cells but responded to induction plus Nutlin3A.
The recipient mice had no evidence of AML at 16 weeks after transplant when the experiment ended.
Dr. Stanford and his colleagues are now trying to obtain pharmaceutical-grade MDM2 inhibitors to conduct trials in AML patients at The Ottawa Hospital.
The researchers are also screening libraries of approved drugs to see if any of these can block MDM2, and they are working with a biotech company to develop a test to identify chemotherapy-resistant AML patients who would respond to MDM2 inhibitors.
The current research was supported by grants from the Canadian Cancer Society Research Institute, Canadian Institutes of Health Research, Cancer Research Society, National Institutes of Health, and a Tier 1 Canada Research Chair in Integrative Stem Cell Biology. One study author reported a relationship with Epicypher, Inc. No other conflicts of interest were reported.
Engineered MSCs could treat GVHD
LAUSANNE, SWITZERLAND—A mesenchymal stem cell (MSC) product could treat acute graft-versus-host disease (GVHD), according to preclinical research.
The product, apceth-201, improved symptoms of GVHD and prolonged survival in two mouse models.
Researchers described results with apceth-201 in a poster presentation at the Annual Congress of the European Society of Gene and Cell Therapy.
Most of the researchers involved in this study work for apceth Biopharma, the company developing apceth-201.
Apceth-201 consists of allogeneic human MSCs that have been genetically modified to express alpha-1 antitrypsin, a protease inhibitor exerting anti-inflammatory and tissue-protective functions.
In vitro assays showed that apceth-201 suppresses T-cell proliferation, polarizes macrophages to an anti-inflammatory M2 type, converts dendritic cells to a tolerogenic phenotype, and increases induction of regulatory T cells.
Apeceth-201 also demonstrated activity in vivo.
In a humanized GVHD model, treatment with apceth-201 improved bone marrow cellularity and reduced levels of inflammatory markers.
Additionally, mice treated with apceth-201 had double the median survival of mice treated with native MSCs.
Researchers also tested the effects of apceth-201 against GVHD in a model of haploidentical hematopoietic stem cell transplant.
All control mice succumbed to GVHD within 22 days, but more than half of mice treated with apceth-201 survived until the end of the experiment, which was 100 days.
All of the survivors had complete resolution of GVHD symptoms.
The researchers also said apceth-201 was safe. It did not impair T-cell-dependent antibody responses, and host resistance was similar in treated mice and controls.
“We are very excited with these results that clearly show the transformative therapeutic potential of apceth-201 in an immune-mediated disease with high unmet medical need and few treatment options,” said Christine Guenther, CEO of apceth Biopharma.
“Based on these promising and impressive data, we are currently preparing to initiate a phase 1/2 clinical study assessing the safety and efficacy of apceth-201 in adult steroid-refractory GVHD patients.”
LAUSANNE, SWITZERLAND—A mesenchymal stem cell (MSC) product could treat acute graft-versus-host disease (GVHD), according to preclinical research.
The product, apceth-201, improved symptoms of GVHD and prolonged survival in two mouse models.
Researchers described results with apceth-201 in a poster presentation at the Annual Congress of the European Society of Gene and Cell Therapy.
Most of the researchers involved in this study work for apceth Biopharma, the company developing apceth-201.
Apceth-201 consists of allogeneic human MSCs that have been genetically modified to express alpha-1 antitrypsin, a protease inhibitor exerting anti-inflammatory and tissue-protective functions.
In vitro assays showed that apceth-201 suppresses T-cell proliferation, polarizes macrophages to an anti-inflammatory M2 type, converts dendritic cells to a tolerogenic phenotype, and increases induction of regulatory T cells.
Apeceth-201 also demonstrated activity in vivo.
In a humanized GVHD model, treatment with apceth-201 improved bone marrow cellularity and reduced levels of inflammatory markers.
Additionally, mice treated with apceth-201 had double the median survival of mice treated with native MSCs.
Researchers also tested the effects of apceth-201 against GVHD in a model of haploidentical hematopoietic stem cell transplant.
All control mice succumbed to GVHD within 22 days, but more than half of mice treated with apceth-201 survived until the end of the experiment, which was 100 days.
All of the survivors had complete resolution of GVHD symptoms.
The researchers also said apceth-201 was safe. It did not impair T-cell-dependent antibody responses, and host resistance was similar in treated mice and controls.
“We are very excited with these results that clearly show the transformative therapeutic potential of apceth-201 in an immune-mediated disease with high unmet medical need and few treatment options,” said Christine Guenther, CEO of apceth Biopharma.
“Based on these promising and impressive data, we are currently preparing to initiate a phase 1/2 clinical study assessing the safety and efficacy of apceth-201 in adult steroid-refractory GVHD patients.”
LAUSANNE, SWITZERLAND—A mesenchymal stem cell (MSC) product could treat acute graft-versus-host disease (GVHD), according to preclinical research.
The product, apceth-201, improved symptoms of GVHD and prolonged survival in two mouse models.
Researchers described results with apceth-201 in a poster presentation at the Annual Congress of the European Society of Gene and Cell Therapy.
Most of the researchers involved in this study work for apceth Biopharma, the company developing apceth-201.
Apceth-201 consists of allogeneic human MSCs that have been genetically modified to express alpha-1 antitrypsin, a protease inhibitor exerting anti-inflammatory and tissue-protective functions.
In vitro assays showed that apceth-201 suppresses T-cell proliferation, polarizes macrophages to an anti-inflammatory M2 type, converts dendritic cells to a tolerogenic phenotype, and increases induction of regulatory T cells.
Apeceth-201 also demonstrated activity in vivo.
In a humanized GVHD model, treatment with apceth-201 improved bone marrow cellularity and reduced levels of inflammatory markers.
Additionally, mice treated with apceth-201 had double the median survival of mice treated with native MSCs.
Researchers also tested the effects of apceth-201 against GVHD in a model of haploidentical hematopoietic stem cell transplant.
All control mice succumbed to GVHD within 22 days, but more than half of mice treated with apceth-201 survived until the end of the experiment, which was 100 days.
All of the survivors had complete resolution of GVHD symptoms.
The researchers also said apceth-201 was safe. It did not impair T-cell-dependent antibody responses, and host resistance was similar in treated mice and controls.
“We are very excited with these results that clearly show the transformative therapeutic potential of apceth-201 in an immune-mediated disease with high unmet medical need and few treatment options,” said Christine Guenther, CEO of apceth Biopharma.
“Based on these promising and impressive data, we are currently preparing to initiate a phase 1/2 clinical study assessing the safety and efficacy of apceth-201 in adult steroid-refractory GVHD patients.”
Inhibitor could improve HSC donation and transplant
Preclinical research suggests a small-molecule inhibitor could potentially improve the donation and transplant of hematopoietic stem cells (HSCs).
One study showed that the inhibitor, CASIN, could improve HSC yields from murine transplant donors.
Another study showed that CASIN could be used as conditioning, with or without fludarabine, in murine transplant recipients.
Yi Zheng, PhD, of Cincinnati Children’s Cancer and Blood Diseases Institute in Ohio, and his colleagues conducted both studies and reported the results in Leukemia.
Previous research had shown that genetic ablation of CDC42 in HSCs mobilizes the cells without affecting their survival. CDC42 is a Rho family small GTPase that helps regulate HSC maintenance.
Dr. Zheng and his colleagues expanded upon this finding with two studies.
In the first study, the researchers identified CASIN, a small-molecule inhibitor of CDC42. The team tested CASIN in mice and found the inhibitor can effectively mobilize HSCs.
In fact, CASIN and plerixafor mobilized a similar number of phenotypic HSCs. However, HSCs harvested from CASIN-treated donor mice had better long-term reconstitution potential after transplant than HSCs harvested from plerixafor-treated mice.
In the second study, Dr. Zheng and his colleagues used CASIN to condition mice that were receiving HSC transplants.
The team found that CASIN reduced the number of phenotypic long-term HSCs in the bone marrow, starting 2 hours after CASIN administration and ending within 24 hours.
The researchers also found that CASIN synergized with fludarabine. Engraftment of transplanted HSCs was significantly higher in mice that received conditioning with CASIN and fludarabine than in mice that received either CASIN or fludarabine alone.
“Our data demonstrate that the new regimen of CASIN application has the potential to improve both sides of the transplant practice,” Dr. Zheng said. “It mobilizes higher quality donor HSCs during stem cell harvest, and it would condition transplant patients beforehand to increase engraftment efficiency.”
“This would be a major step forward, especially for the most vulnerable patients who cannot withstand the toxicity of chemotherapy conditioning regimens or are non-responsive to current [HSC] mobilization regimens.”
Both of these studies were funded, in part, by the National Institutes of Health. The authors declared no conflicts of interest.
Preclinical research suggests a small-molecule inhibitor could potentially improve the donation and transplant of hematopoietic stem cells (HSCs).
One study showed that the inhibitor, CASIN, could improve HSC yields from murine transplant donors.
Another study showed that CASIN could be used as conditioning, with or without fludarabine, in murine transplant recipients.
Yi Zheng, PhD, of Cincinnati Children’s Cancer and Blood Diseases Institute in Ohio, and his colleagues conducted both studies and reported the results in Leukemia.
Previous research had shown that genetic ablation of CDC42 in HSCs mobilizes the cells without affecting their survival. CDC42 is a Rho family small GTPase that helps regulate HSC maintenance.
Dr. Zheng and his colleagues expanded upon this finding with two studies.
In the first study, the researchers identified CASIN, a small-molecule inhibitor of CDC42. The team tested CASIN in mice and found the inhibitor can effectively mobilize HSCs.
In fact, CASIN and plerixafor mobilized a similar number of phenotypic HSCs. However, HSCs harvested from CASIN-treated donor mice had better long-term reconstitution potential after transplant than HSCs harvested from plerixafor-treated mice.
In the second study, Dr. Zheng and his colleagues used CASIN to condition mice that were receiving HSC transplants.
The team found that CASIN reduced the number of phenotypic long-term HSCs in the bone marrow, starting 2 hours after CASIN administration and ending within 24 hours.
The researchers also found that CASIN synergized with fludarabine. Engraftment of transplanted HSCs was significantly higher in mice that received conditioning with CASIN and fludarabine than in mice that received either CASIN or fludarabine alone.
“Our data demonstrate that the new regimen of CASIN application has the potential to improve both sides of the transplant practice,” Dr. Zheng said. “It mobilizes higher quality donor HSCs during stem cell harvest, and it would condition transplant patients beforehand to increase engraftment efficiency.”
“This would be a major step forward, especially for the most vulnerable patients who cannot withstand the toxicity of chemotherapy conditioning regimens or are non-responsive to current [HSC] mobilization regimens.”
Both of these studies were funded, in part, by the National Institutes of Health. The authors declared no conflicts of interest.
Preclinical research suggests a small-molecule inhibitor could potentially improve the donation and transplant of hematopoietic stem cells (HSCs).
One study showed that the inhibitor, CASIN, could improve HSC yields from murine transplant donors.
Another study showed that CASIN could be used as conditioning, with or without fludarabine, in murine transplant recipients.
Yi Zheng, PhD, of Cincinnati Children’s Cancer and Blood Diseases Institute in Ohio, and his colleagues conducted both studies and reported the results in Leukemia.
Previous research had shown that genetic ablation of CDC42 in HSCs mobilizes the cells without affecting their survival. CDC42 is a Rho family small GTPase that helps regulate HSC maintenance.
Dr. Zheng and his colleagues expanded upon this finding with two studies.
In the first study, the researchers identified CASIN, a small-molecule inhibitor of CDC42. The team tested CASIN in mice and found the inhibitor can effectively mobilize HSCs.
In fact, CASIN and plerixafor mobilized a similar number of phenotypic HSCs. However, HSCs harvested from CASIN-treated donor mice had better long-term reconstitution potential after transplant than HSCs harvested from plerixafor-treated mice.
In the second study, Dr. Zheng and his colleagues used CASIN to condition mice that were receiving HSC transplants.
The team found that CASIN reduced the number of phenotypic long-term HSCs in the bone marrow, starting 2 hours after CASIN administration and ending within 24 hours.
The researchers also found that CASIN synergized with fludarabine. Engraftment of transplanted HSCs was significantly higher in mice that received conditioning with CASIN and fludarabine than in mice that received either CASIN or fludarabine alone.
“Our data demonstrate that the new regimen of CASIN application has the potential to improve both sides of the transplant practice,” Dr. Zheng said. “It mobilizes higher quality donor HSCs during stem cell harvest, and it would condition transplant patients beforehand to increase engraftment efficiency.”
“This would be a major step forward, especially for the most vulnerable patients who cannot withstand the toxicity of chemotherapy conditioning regimens or are non-responsive to current [HSC] mobilization regimens.”
Both of these studies were funded, in part, by the National Institutes of Health. The authors declared no conflicts of interest.
Better PFS may not mean better HRQOL
Cancer treatments that prolong progression-free survival (PFS) may not improve health-related quality of life (HRQOL), researchers reported in JAMA Internal Medicine.
The researchers failed to find a significant association between PFS and HRQOL in an analysis of cancer clinical trials.
“There are only two reasons to use progression-free survival as a valid endpoint in oncology,” said study author Feng Xie, PhD, of McMaster University in Hamilton, Ontario, Canada.
“One is that it is a valid surrogate marker for overall survival. The second is the assumption that patients who live longer without disease progression will have better health-related quality of life, even without longer survival.”
“Given the increased use of progression-free survival as the primary outcome in new oncology drug trials, and uncertainty of overall survival, it remains possible that patients are receiving toxic and/or expensive treatments without experiencing important benefit.”
With this in mind, Dr. Xie and his colleagues conducted a review and meta-analysis of 52 articles reporting on 38 randomized clinical trials. The trials included 13,979 patients with 12 types of cancer, including 1 trial of patients with multiple myeloma.
The median follow-up in these trials ranged from 10.5 months to 66.0 months.
The median PFS of patients who received the trial interventions ranged from 1.8 months to 33.7 months.
For 28 of the trials (74%), patients who received the trial intervention had better PFS than patients who received the comparator. Overall, the mean difference in median PFS between the intervention and comparator arms was 1.91 months.
HRQOL was measured with 6 different instruments* across the trials, and the types of HRQOL measured varied. Thirty trials included global HRQOL, 20 included physical, and 13 included emotional HRQOL. The duration of reported or measured HRQOL ranged from 1 month to 34 months.
Improved global HRQOL was reported in 53% of trials (16/30), improved physical HRQOL was reported in 55% (11/20), and improved emotional HRQOL was reported in 62% (8/13). The mean difference in change of HRQOL adjusted to per-month values was −0.39 for global, 0.26 for physical, and 1.08 for emotional HRQOL.
The slope of the association between the difference in median PFS and the difference in HRQOL change was:
- 0.12 (95% confidence interval [CI], −0.27 to 0.52) for global HRQOL
- −0.20 (95% CI,−0.62 to 0.23) for physical HRQOL
- 0.78 (95% CI, −0.05 to 1.60) for emotional HRQOL.
Dr. Xie and his colleagues said these results suggest there is no significant association between PFS and HRQOL, so interventions prolonging PFS may not improve HRQOL.
“Therefore, to ensure patients are truly obtaining important benefit from cancer therapies, clinical trial investigators should measure health-related quality of life directly and accurately, ensuring adequate duration and follow-up, and publish it,” Dr. Xie said.
He also argued for the need to “revisit this issue of using surrogate outcomes to measure the safety and efficacy of new oncology drugs.”
Dr. Xie and his colleagues did not report any conflicts of interest. One study author (Marcin Waligora, PhD) reported funding from the National Science Centre in Poland.
*EORTC-QLQ-C30, FACT-G19, Lung Cancer Symptom Scale, EQ-5D, 8-item linear analog self-assessment (LASA) questionnaire, and clinician-reported Karnofsky score
Cancer treatments that prolong progression-free survival (PFS) may not improve health-related quality of life (HRQOL), researchers reported in JAMA Internal Medicine.
The researchers failed to find a significant association between PFS and HRQOL in an analysis of cancer clinical trials.
“There are only two reasons to use progression-free survival as a valid endpoint in oncology,” said study author Feng Xie, PhD, of McMaster University in Hamilton, Ontario, Canada.
“One is that it is a valid surrogate marker for overall survival. The second is the assumption that patients who live longer without disease progression will have better health-related quality of life, even without longer survival.”
“Given the increased use of progression-free survival as the primary outcome in new oncology drug trials, and uncertainty of overall survival, it remains possible that patients are receiving toxic and/or expensive treatments without experiencing important benefit.”
With this in mind, Dr. Xie and his colleagues conducted a review and meta-analysis of 52 articles reporting on 38 randomized clinical trials. The trials included 13,979 patients with 12 types of cancer, including 1 trial of patients with multiple myeloma.
The median follow-up in these trials ranged from 10.5 months to 66.0 months.
The median PFS of patients who received the trial interventions ranged from 1.8 months to 33.7 months.
For 28 of the trials (74%), patients who received the trial intervention had better PFS than patients who received the comparator. Overall, the mean difference in median PFS between the intervention and comparator arms was 1.91 months.
HRQOL was measured with 6 different instruments* across the trials, and the types of HRQOL measured varied. Thirty trials included global HRQOL, 20 included physical, and 13 included emotional HRQOL. The duration of reported or measured HRQOL ranged from 1 month to 34 months.
Improved global HRQOL was reported in 53% of trials (16/30), improved physical HRQOL was reported in 55% (11/20), and improved emotional HRQOL was reported in 62% (8/13). The mean difference in change of HRQOL adjusted to per-month values was −0.39 for global, 0.26 for physical, and 1.08 for emotional HRQOL.
The slope of the association between the difference in median PFS and the difference in HRQOL change was:
- 0.12 (95% confidence interval [CI], −0.27 to 0.52) for global HRQOL
- −0.20 (95% CI,−0.62 to 0.23) for physical HRQOL
- 0.78 (95% CI, −0.05 to 1.60) for emotional HRQOL.
Dr. Xie and his colleagues said these results suggest there is no significant association between PFS and HRQOL, so interventions prolonging PFS may not improve HRQOL.
“Therefore, to ensure patients are truly obtaining important benefit from cancer therapies, clinical trial investigators should measure health-related quality of life directly and accurately, ensuring adequate duration and follow-up, and publish it,” Dr. Xie said.
He also argued for the need to “revisit this issue of using surrogate outcomes to measure the safety and efficacy of new oncology drugs.”
Dr. Xie and his colleagues did not report any conflicts of interest. One study author (Marcin Waligora, PhD) reported funding from the National Science Centre in Poland.
*EORTC-QLQ-C30, FACT-G19, Lung Cancer Symptom Scale, EQ-5D, 8-item linear analog self-assessment (LASA) questionnaire, and clinician-reported Karnofsky score
Cancer treatments that prolong progression-free survival (PFS) may not improve health-related quality of life (HRQOL), researchers reported in JAMA Internal Medicine.
The researchers failed to find a significant association between PFS and HRQOL in an analysis of cancer clinical trials.
“There are only two reasons to use progression-free survival as a valid endpoint in oncology,” said study author Feng Xie, PhD, of McMaster University in Hamilton, Ontario, Canada.
“One is that it is a valid surrogate marker for overall survival. The second is the assumption that patients who live longer without disease progression will have better health-related quality of life, even without longer survival.”
“Given the increased use of progression-free survival as the primary outcome in new oncology drug trials, and uncertainty of overall survival, it remains possible that patients are receiving toxic and/or expensive treatments without experiencing important benefit.”
With this in mind, Dr. Xie and his colleagues conducted a review and meta-analysis of 52 articles reporting on 38 randomized clinical trials. The trials included 13,979 patients with 12 types of cancer, including 1 trial of patients with multiple myeloma.
The median follow-up in these trials ranged from 10.5 months to 66.0 months.
The median PFS of patients who received the trial interventions ranged from 1.8 months to 33.7 months.
For 28 of the trials (74%), patients who received the trial intervention had better PFS than patients who received the comparator. Overall, the mean difference in median PFS between the intervention and comparator arms was 1.91 months.
HRQOL was measured with 6 different instruments* across the trials, and the types of HRQOL measured varied. Thirty trials included global HRQOL, 20 included physical, and 13 included emotional HRQOL. The duration of reported or measured HRQOL ranged from 1 month to 34 months.
Improved global HRQOL was reported in 53% of trials (16/30), improved physical HRQOL was reported in 55% (11/20), and improved emotional HRQOL was reported in 62% (8/13). The mean difference in change of HRQOL adjusted to per-month values was −0.39 for global, 0.26 for physical, and 1.08 for emotional HRQOL.
The slope of the association between the difference in median PFS and the difference in HRQOL change was:
- 0.12 (95% confidence interval [CI], −0.27 to 0.52) for global HRQOL
- −0.20 (95% CI,−0.62 to 0.23) for physical HRQOL
- 0.78 (95% CI, −0.05 to 1.60) for emotional HRQOL.
Dr. Xie and his colleagues said these results suggest there is no significant association between PFS and HRQOL, so interventions prolonging PFS may not improve HRQOL.
“Therefore, to ensure patients are truly obtaining important benefit from cancer therapies, clinical trial investigators should measure health-related quality of life directly and accurately, ensuring adequate duration and follow-up, and publish it,” Dr. Xie said.
He also argued for the need to “revisit this issue of using surrogate outcomes to measure the safety and efficacy of new oncology drugs.”
Dr. Xie and his colleagues did not report any conflicts of interest. One study author (Marcin Waligora, PhD) reported funding from the National Science Centre in Poland.
*EORTC-QLQ-C30, FACT-G19, Lung Cancer Symptom Scale, EQ-5D, 8-item linear analog self-assessment (LASA) questionnaire, and clinician-reported Karnofsky score
Age limits restrict AYA participation in relevant trials
MUNICH—Age limits imposed in European countries can prevent adolescents and young adults (AYAs) from enrolling in appropriate clinical trials, a new study suggests.
Investigators reviewed phase 1 and 2 trials conducted over a 6-year period at a single center in France.
The results showed that adolescents were prevented from enrolling in potentially beneficial adult trials, and young adults were unable to enroll in potentially beneficial pediatric trials.
These results were presented at the ESMO 2018 Congress (abstract 424P_PR).
In Europe, the legal minimum age to participate in adult clinical trials is typically 18.
“We know, however, that certain girls will develop genetically driven breast cancers very early in life,” said Dr. Aurore Vozy, of Gustave Roussy Institut de Cancérologie in Villejuif, France.
“There are no pediatric trials for this disease, yet these patients are systematically barred from participating in the relevant adult trials. The situation is similar for some adolescents with lymphomas or sarcomas, whose tumors often resemble those of adults much more closely than those found in children.”
On the other hand, adults in their early twenties may be diagnosed with cancers most commonly seen in children. And pediatric clinical trials typically set an upper age limit of 18 or 21.
To assess the availability and accessibility of new treatments to AYA cancer patients, Dr. Vozy and her colleagues conducted a review of all phase 1 and 2 trials opened at Gustave Roussy from 2012 through 2017 for patients with solid tumors or lymphomas.
Over the 6-year period, 465 trials were open—403 adult trials and 62 pediatric trials.
Only 65 of the trials (14%) included patients between the ages of 12 and 17.
“In other words, patients in this age group had access to less than 15% of all the early phase trials at our institute,” Dr. Vozy said.
In all, there were 389 trials that were not open to adolescents, and the investigators found that 55% of these trials could have been relevant for underage patients. Twenty-eight of the trials targeted tumor types that are particularly common among teenagers.
“This means that patients have been denied access to innovative medicines which were available at the very center where they were being treated, and to which they may have had a better response than to conventional therapy,” Dr. Vozy said.
She and her colleagues also found that young adults were often unable to enroll in pediatric trials.
There were 62 pediatric trials open over the period studied, and more than half of them (n=36, 58%) did not recruit patients aged 19 to 25, even though 10 of these trials targeted tumor types that also occur in this age group.
“Raising the age bar in pediatric trials to 25 years would clearly make sense in certain cases,” Dr. Vozy said.
She argued, however, that the more pressing issue is the current age limit in adult trials.
“We know that the diseases, toxicities, and pharmacology seen in 12- to 17-year-olds are similar to what we find in adults, so it would be feasible to include these patients in adult trials at no additional risk to them,” Dr. Vozy said.
This has already been done successfully in the United States, where the minimum age for trial participation has been lowered to 12 years.
An additional measure to consider, Dr. Vozy said, is creating dedicated trial cohorts for adolescents within adult trials.
“In a context where, today, most phase 1 trials in oncology are launched with multiple study populations for different tumor types, it would be easy to cater to the specific needs of adolescents by including them in cohorts of their own,” she said.
“The main constraint is that trials which include underage patients should only be conducted in centers that also have pediatric services onsite. Adolescents may be affected by disease similarly to adults, but they still need to be treated and followed up on by pediatric specialists.”
One investigator involved in this study reported relationships with Amgen, Astellas, Astra Zeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. All other investigators declared no conflicts of interest.
MUNICH—Age limits imposed in European countries can prevent adolescents and young adults (AYAs) from enrolling in appropriate clinical trials, a new study suggests.
Investigators reviewed phase 1 and 2 trials conducted over a 6-year period at a single center in France.
The results showed that adolescents were prevented from enrolling in potentially beneficial adult trials, and young adults were unable to enroll in potentially beneficial pediatric trials.
These results were presented at the ESMO 2018 Congress (abstract 424P_PR).
In Europe, the legal minimum age to participate in adult clinical trials is typically 18.
“We know, however, that certain girls will develop genetically driven breast cancers very early in life,” said Dr. Aurore Vozy, of Gustave Roussy Institut de Cancérologie in Villejuif, France.
“There are no pediatric trials for this disease, yet these patients are systematically barred from participating in the relevant adult trials. The situation is similar for some adolescents with lymphomas or sarcomas, whose tumors often resemble those of adults much more closely than those found in children.”
On the other hand, adults in their early twenties may be diagnosed with cancers most commonly seen in children. And pediatric clinical trials typically set an upper age limit of 18 or 21.
To assess the availability and accessibility of new treatments to AYA cancer patients, Dr. Vozy and her colleagues conducted a review of all phase 1 and 2 trials opened at Gustave Roussy from 2012 through 2017 for patients with solid tumors or lymphomas.
Over the 6-year period, 465 trials were open—403 adult trials and 62 pediatric trials.
Only 65 of the trials (14%) included patients between the ages of 12 and 17.
“In other words, patients in this age group had access to less than 15% of all the early phase trials at our institute,” Dr. Vozy said.
In all, there were 389 trials that were not open to adolescents, and the investigators found that 55% of these trials could have been relevant for underage patients. Twenty-eight of the trials targeted tumor types that are particularly common among teenagers.
“This means that patients have been denied access to innovative medicines which were available at the very center where they were being treated, and to which they may have had a better response than to conventional therapy,” Dr. Vozy said.
She and her colleagues also found that young adults were often unable to enroll in pediatric trials.
There were 62 pediatric trials open over the period studied, and more than half of them (n=36, 58%) did not recruit patients aged 19 to 25, even though 10 of these trials targeted tumor types that also occur in this age group.
“Raising the age bar in pediatric trials to 25 years would clearly make sense in certain cases,” Dr. Vozy said.
She argued, however, that the more pressing issue is the current age limit in adult trials.
“We know that the diseases, toxicities, and pharmacology seen in 12- to 17-year-olds are similar to what we find in adults, so it would be feasible to include these patients in adult trials at no additional risk to them,” Dr. Vozy said.
This has already been done successfully in the United States, where the minimum age for trial participation has been lowered to 12 years.
An additional measure to consider, Dr. Vozy said, is creating dedicated trial cohorts for adolescents within adult trials.
“In a context where, today, most phase 1 trials in oncology are launched with multiple study populations for different tumor types, it would be easy to cater to the specific needs of adolescents by including them in cohorts of their own,” she said.
“The main constraint is that trials which include underage patients should only be conducted in centers that also have pediatric services onsite. Adolescents may be affected by disease similarly to adults, but they still need to be treated and followed up on by pediatric specialists.”
One investigator involved in this study reported relationships with Amgen, Astellas, Astra Zeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. All other investigators declared no conflicts of interest.
MUNICH—Age limits imposed in European countries can prevent adolescents and young adults (AYAs) from enrolling in appropriate clinical trials, a new study suggests.
Investigators reviewed phase 1 and 2 trials conducted over a 6-year period at a single center in France.
The results showed that adolescents were prevented from enrolling in potentially beneficial adult trials, and young adults were unable to enroll in potentially beneficial pediatric trials.
These results were presented at the ESMO 2018 Congress (abstract 424P_PR).
In Europe, the legal minimum age to participate in adult clinical trials is typically 18.
“We know, however, that certain girls will develop genetically driven breast cancers very early in life,” said Dr. Aurore Vozy, of Gustave Roussy Institut de Cancérologie in Villejuif, France.
“There are no pediatric trials for this disease, yet these patients are systematically barred from participating in the relevant adult trials. The situation is similar for some adolescents with lymphomas or sarcomas, whose tumors often resemble those of adults much more closely than those found in children.”
On the other hand, adults in their early twenties may be diagnosed with cancers most commonly seen in children. And pediatric clinical trials typically set an upper age limit of 18 or 21.
To assess the availability and accessibility of new treatments to AYA cancer patients, Dr. Vozy and her colleagues conducted a review of all phase 1 and 2 trials opened at Gustave Roussy from 2012 through 2017 for patients with solid tumors or lymphomas.
Over the 6-year period, 465 trials were open—403 adult trials and 62 pediatric trials.
Only 65 of the trials (14%) included patients between the ages of 12 and 17.
“In other words, patients in this age group had access to less than 15% of all the early phase trials at our institute,” Dr. Vozy said.
In all, there were 389 trials that were not open to adolescents, and the investigators found that 55% of these trials could have been relevant for underage patients. Twenty-eight of the trials targeted tumor types that are particularly common among teenagers.
“This means that patients have been denied access to innovative medicines which were available at the very center where they were being treated, and to which they may have had a better response than to conventional therapy,” Dr. Vozy said.
She and her colleagues also found that young adults were often unable to enroll in pediatric trials.
There were 62 pediatric trials open over the period studied, and more than half of them (n=36, 58%) did not recruit patients aged 19 to 25, even though 10 of these trials targeted tumor types that also occur in this age group.
“Raising the age bar in pediatric trials to 25 years would clearly make sense in certain cases,” Dr. Vozy said.
She argued, however, that the more pressing issue is the current age limit in adult trials.
“We know that the diseases, toxicities, and pharmacology seen in 12- to 17-year-olds are similar to what we find in adults, so it would be feasible to include these patients in adult trials at no additional risk to them,” Dr. Vozy said.
This has already been done successfully in the United States, where the minimum age for trial participation has been lowered to 12 years.
An additional measure to consider, Dr. Vozy said, is creating dedicated trial cohorts for adolescents within adult trials.
“In a context where, today, most phase 1 trials in oncology are launched with multiple study populations for different tumor types, it would be easy to cater to the specific needs of adolescents by including them in cohorts of their own,” she said.
“The main constraint is that trials which include underage patients should only be conducted in centers that also have pediatric services onsite. Adolescents may be affected by disease similarly to adults, but they still need to be treated and followed up on by pediatric specialists.”
One investigator involved in this study reported relationships with Amgen, Astellas, Astra Zeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. All other investigators declared no conflicts of interest.
Inhibitor receives orphan designation for ITP
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to PRN1008 for the treatment of patients with immune thrombocytopenia (ITP).
PRN1008 is an oral, reversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor being developed by Principia Biopharma, Inc.
Principia is conducting a phase 1/2 trial (NCT03395210) to evaluate the safety and efficacy of PRN1008 in patients with ITP.
Results of preclinical research with PRN1008 in ITP were presented at the 2017 ASH Annual Meeting.
There, researchers reported that PRN1008 significantly reduced platelet loss in a mouse model of ITP.
The team found the BTK inhibitor could diminish platelet loss in two ways:
- By reducing platelet destruction via inhibition of autoantibody/FcγR signaling in splenic macrophages
- By reducing autoantibody generation through inhibition of B-cell activation and maturation.
The researchers also assessed the effects of PRN1008 and ibrutinib on platelet function in samples from healthy volunteers and ITP patients.
Samples were treated with one of the two BTK inhibitors, and platelet aggregation was induced by platelet agonists.
Unlike ibrutinib, PRN1008 did not impact platelet aggregation in healthy volunteer or ITP patient samples.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to PRN1008 for the treatment of patients with immune thrombocytopenia (ITP).
PRN1008 is an oral, reversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor being developed by Principia Biopharma, Inc.
Principia is conducting a phase 1/2 trial (NCT03395210) to evaluate the safety and efficacy of PRN1008 in patients with ITP.
Results of preclinical research with PRN1008 in ITP were presented at the 2017 ASH Annual Meeting.
There, researchers reported that PRN1008 significantly reduced platelet loss in a mouse model of ITP.
The team found the BTK inhibitor could diminish platelet loss in two ways:
- By reducing platelet destruction via inhibition of autoantibody/FcγR signaling in splenic macrophages
- By reducing autoantibody generation through inhibition of B-cell activation and maturation.
The researchers also assessed the effects of PRN1008 and ibrutinib on platelet function in samples from healthy volunteers and ITP patients.
Samples were treated with one of the two BTK inhibitors, and platelet aggregation was induced by platelet agonists.
Unlike ibrutinib, PRN1008 did not impact platelet aggregation in healthy volunteer or ITP patient samples.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The U.S. Food and Drug Administration (FDA) has granted orphan drug designation to PRN1008 for the treatment of patients with immune thrombocytopenia (ITP).
PRN1008 is an oral, reversible, covalent Bruton’s tyrosine kinase (BTK) inhibitor being developed by Principia Biopharma, Inc.
Principia is conducting a phase 1/2 trial (NCT03395210) to evaluate the safety and efficacy of PRN1008 in patients with ITP.
Results of preclinical research with PRN1008 in ITP were presented at the 2017 ASH Annual Meeting.
There, researchers reported that PRN1008 significantly reduced platelet loss in a mouse model of ITP.
The team found the BTK inhibitor could diminish platelet loss in two ways:
- By reducing platelet destruction via inhibition of autoantibody/FcγR signaling in splenic macrophages
- By reducing autoantibody generation through inhibition of B-cell activation and maturation.
The researchers also assessed the effects of PRN1008 and ibrutinib on platelet function in samples from healthy volunteers and ITP patients.
Samples were treated with one of the two BTK inhibitors, and platelet aggregation was induced by platelet agonists.
Unlike ibrutinib, PRN1008 did not impact platelet aggregation in healthy volunteer or ITP patient samples.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the United States.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
CHMP recommends change for eptacog alfa
The European Medicine’s Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a change to the terms of the marketing authorization for the recombinant factor VIIa product eptacog alfa (NovoSeven).
The recommendation is to expand the approved use of eptacog alfa in patients with Glanzmann’s thrombasthenia.
Eptacog alfa is already approved by the European Commission (EC) for use in patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen who have past or present refractoriness to platelet transfusions.
Now, the CHMP has recommended expanding the use of eptacog alfa to include situations in which patients are not refractory to platelet transfusions but platelets are not readily available.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
If the EC follows the CHMP’s recommendation for eptacog alfa, the product will be approved for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- Patients with congenital hemophilia with inhibitors to coagulation factors VIII or IX > 5 Bethesda units
- Patients with congenital hemophilia who are expected to have a high anamnestic response to factor VIII or factor IX administration
- Patients with acquired hemophilia
- Patients with congenital FVII deficiency
- Patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen and past or present refractoriness to platelet transfusions, or where platelets are not readily available.
The European Medicine’s Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a change to the terms of the marketing authorization for the recombinant factor VIIa product eptacog alfa (NovoSeven).
The recommendation is to expand the approved use of eptacog alfa in patients with Glanzmann’s thrombasthenia.
Eptacog alfa is already approved by the European Commission (EC) for use in patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen who have past or present refractoriness to platelet transfusions.
Now, the CHMP has recommended expanding the use of eptacog alfa to include situations in which patients are not refractory to platelet transfusions but platelets are not readily available.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
If the EC follows the CHMP’s recommendation for eptacog alfa, the product will be approved for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- Patients with congenital hemophilia with inhibitors to coagulation factors VIII or IX > 5 Bethesda units
- Patients with congenital hemophilia who are expected to have a high anamnestic response to factor VIII or factor IX administration
- Patients with acquired hemophilia
- Patients with congenital FVII deficiency
- Patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen and past or present refractoriness to platelet transfusions, or where platelets are not readily available.
The European Medicine’s Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a change to the terms of the marketing authorization for the recombinant factor VIIa product eptacog alfa (NovoSeven).
The recommendation is to expand the approved use of eptacog alfa in patients with Glanzmann’s thrombasthenia.
Eptacog alfa is already approved by the European Commission (EC) for use in patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen who have past or present refractoriness to platelet transfusions.
Now, the CHMP has recommended expanding the use of eptacog alfa to include situations in which patients are not refractory to platelet transfusions but platelets are not readily available.
The CHMP’s recommendations are reviewed by the EC, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein. The EC usually makes a decision within 67 days of CHMP recommendations.
If the EC follows the CHMP’s recommendation for eptacog alfa, the product will be approved for the treatment of bleeding episodes and for the prevention of bleeding in those undergoing surgery or invasive procedures in the following patient groups:
- Patients with congenital hemophilia with inhibitors to coagulation factors VIII or IX > 5 Bethesda units
- Patients with congenital hemophilia who are expected to have a high anamnestic response to factor VIII or factor IX administration
- Patients with acquired hemophilia
- Patients with congenital FVII deficiency
- Patients with Glanzmann’s thrombasthenia with antibodies to glycoprotein IIb/IIIa and/or human leukocyte antigen and past or present refractoriness to platelet transfusions, or where platelets are not readily available.