User login
Patellofemoral Instability in the Skeletally Immature Patient: A Review and Technical Description of Medial Patellofemoral Ligament Reconstruction in Patients with Open Physes
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
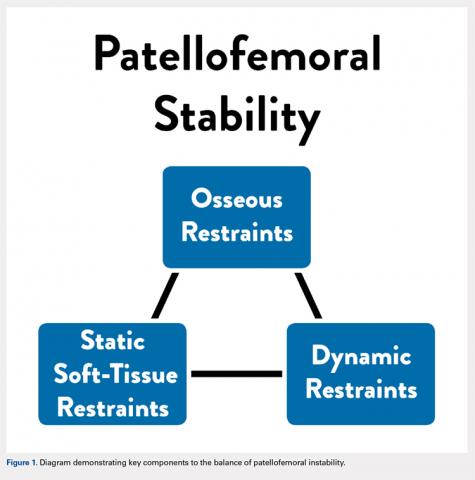
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
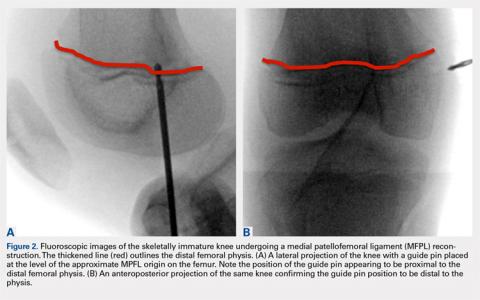
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
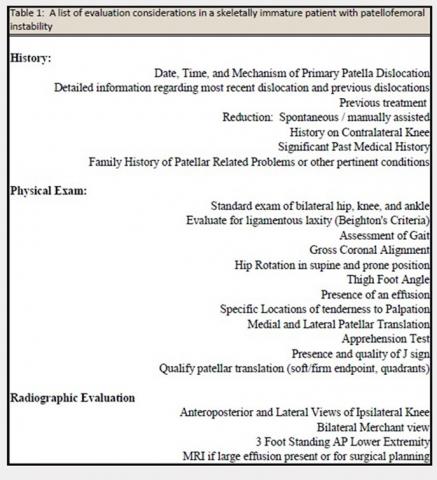
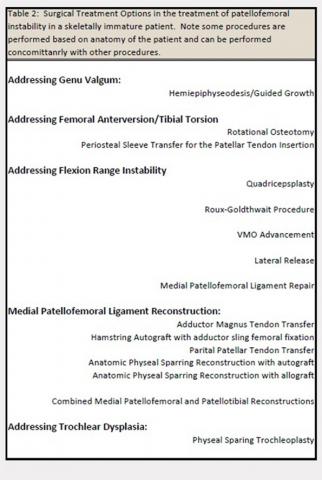
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
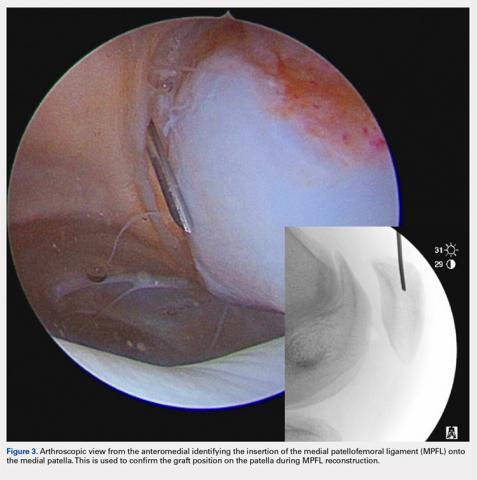
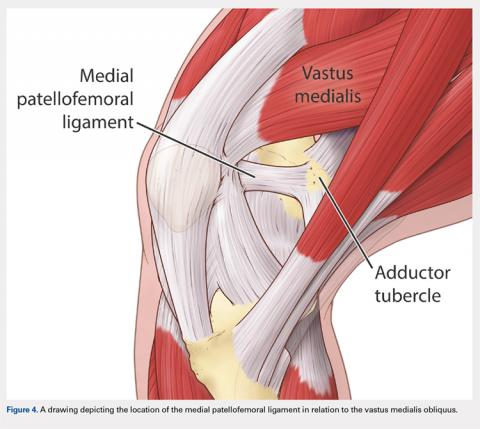
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
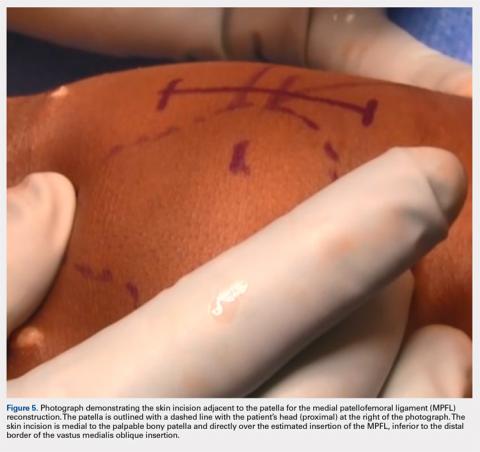
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
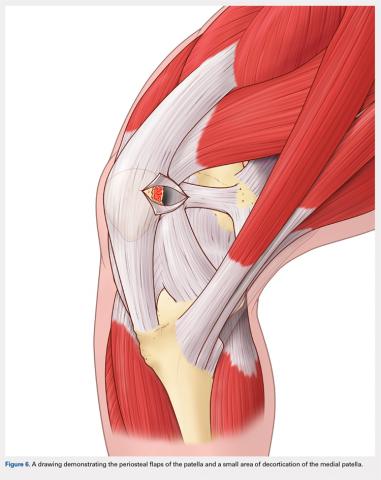
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
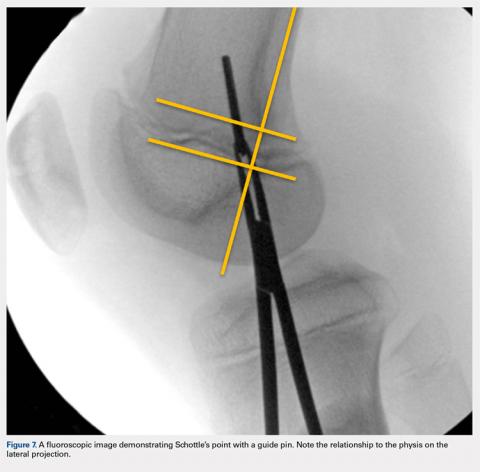
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
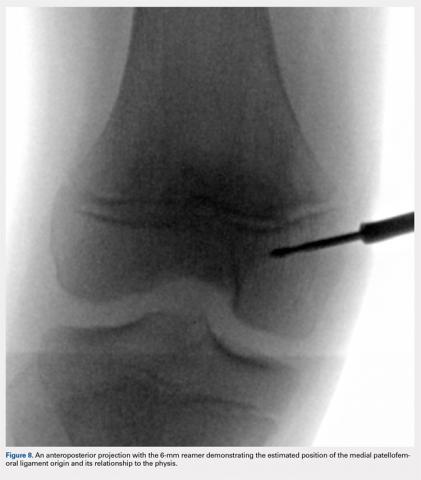
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
ABSTRACT
Patellofemoral instability commonly occurs in the young patient, and, often, skeletal immaturity may be a risk factor for possible recurrence. Treatment considerations, including operative and nonoperative management, are based on anatomic factors. A medial patellofemoral ligament (MPFL) reconstruction is a treatment option for a skeletally immature patient with recurrent instability or for patients with a high risk of patellofemoral instability recurrence. A physeal-sparing MPFL reconstruction technique that considers the origin of the MPFL to be distal to the distal femoral physis may be employed.
Continue to: Patellofemoral instability (PFI)...
Patellofemoral instability (PFI), 1 of the most common patellofemoral disorders observed in skeletally immature patients,1-4 has a reported incidence of 43 per 100,000 skeletally immature patients.3 The incidence is even higher in patients 9 to 15 years, with dislocations occurring in 107 per 100,000 individuals.5,6 In recent years, there has been an increasing interest in studying PFI in skeletally immature pediatric patients, who are particularly susceptible to recurrent dislocations. Studies have indicated that children <16 years are at the highest risk for recurrence.7 Lewallen and colleagues8 noted a 69% failure rate and a 3-fold increase in the probability of recurrent dislocation in skeletally immature patients treated nonoperatively.
Anatomic factors that contribute to PFI include ligament laxity, trochlear dysplasia, patella alta, excessive femoral anteversion or tibial torsion, genu valgum, and increased tibial tubercle-to-trochlear groove distance.1,3,8-11 When considering surgical treatment for PFI, all anatomic factors should be considered, with an emphasis on, and understanding of, the role of residual growth and development. One must also consider balancing static soft tissue, dynamic soft tissue, and osseous constraints of the patellofemoral joint to optimize the overall health and balance of the patella (Figure 1).
CHALLENGES IN TREATING PEDIATRIC PFI
A primary challenge in the treatment of PFI in pediatric patients is accounting for the impact of anatomic changes occurring secondary to growth and maturity. From birth to adulthood, the collagen composition of soft tissue changes from an elastic type III collagen to a stiffer type I collagen.12 These physiologic changes may influence the rigidity of the soft tissue restraints around the patellofemoral joint during periods of rapid growth. Longitudinal growth and rotational changes can also occur at the distal femoral and proximal tibial physes. The position of the tibial tubercle and the magnitude of femoral anteversion may also change after growth in adolescents.
Developing effective technical analogs of surgical procedures performed in mature patients with PFI for use in skeletally immature patients has been a second challenge. For example, a varus-producing distal femoral osteotomy to address genu valgum and PFI13 is contraindicated in immature patients, when a hemiepiphysiodesis for guided growth should be considered.14 Similarly, a periosteal sleeve medialization of the insertion of the patellar tendon may be used instead of a tibial tubercle transfer.15
If a medial patellofemoral ligament (MPFL) reconstruction is considered in immature patients, careful consideration of the position of the MPFL in relation to the distal femoral physis is paramount. Shea and colleagues16 originally described the position of the MPFL, based on Schottle’s point17 on lateral radiographs, to be proximal to the distal femoral physis (Figure 2). However, due to the undulation of the physis, the lateral projection may falsely demonstrate Schottle’s point to be on or just proximal to the physis. Other researchers have evaluated the position of the distal femoral physis to be proximal to the origin of the MPFL by a range of 2.9 mm to 8.5 mm on AP radiograph and MRI, respectively.18,19 More recent cadaveric studies have demonstrated the origin of the MPFL in pediatric specimens in relation to the physis to be variable.20 Although we believe that a relative anatomic femoral position of the MPFL origin can be achieved without disrupting the physis using Schottle’s point, some have indicated concerns that this may produce a nonisometric position, which has not been our experience.21
Continue to: TREATMENT OPTIONS...
TREATMENT OPTIONS
The evaluation of a skeletally immature patient may differ from that of an adult patient. (Table 1) represents a standard evaluation of a skeletally immature patient with PFI. The injury, physical examination findings, activity level, presence of an osteochondral fracture, and severity of bony dysplasia may indicate surgical treatment and influence selection of the surgical technique. Indications for surgical treatment include recurrent patellar instability, symptomatic patellar instability, the presence of a chondral or an osteochondral fracture, and a primary patella dislocation in patients with a high risk for recurrent dislocations. Table 2 represents a list of the possible surgical treatment options for the skeletally immature patient with PFI.21,22 Variable outcomes and recurrence rates are noted with each procedure.21,22
MPFL REPAIR VS RECONSTRUCTION
Although there is increased consensus for restoring normal anatomy, continued controversy exists regarding the utility of an MPFL repair versus reconstruction. An MPFL repair to the patella has been reported in a large series and was noted to have a 72% success rate.23 Although a perceived benefit of the procedure is the absence of risk to the physis, concerns exist regarding the unacceptable rate of continued patellar instability after a repair of native tissue.
Several studies have demonstrated that reconstruction of the MPFL yields lower rates of recurrent dislocation, improved knee function, and an ability to return to prior levels of activity with little to no functional impairment.1,2,24-26 Studies have also shown that MPFL reconstruction can be performed safely in skeletally immature patients, with little risk of growth impairment, good to excellent results, and low re-rupture rates.27,28
We propose that MPFL reconstruction for the skeletally immature patient with PFI should be the primary surgical treatment. Any patient with atypical or severe bony dysplasia may warrant additional intervention. Additional correction of valgus, in the form of guided growth, should be considered in conjunction with an MPFL reconstruction if the patient demonstrates a valgus of grade >2 or a lateral distal femoral angle of <84°.
GRAFT OPTIONS
When an MPFL reconstruction is indicated, graft options include autologous hamstring, quadriceps, adductor, or patellar tendon grafts. Allograft tendon may be an acceptable choice, and use of synthetic graft has also been described. A recent systematic review29 concluded that there was no difference in recurrence rates or outcomes based on graft type. However, in this study, the overall complication rates were higher in patients who had autologous graft than in those who had allograft. Although the use of allograft has not been specifically reported in a pediatric cohort, allografts have been successfully used in this age group. Obviating graft harvest eliminates an additional or extended incision, limits postoperative weakness, and may speed early recovery.
Continue to: PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT...
PREFERRED SURGICAL TECHNIQUE FOR MPFL RECONSTRUCTION WITH ALLOGRAFT
Surgery should be scheduled and performed as an outpatient procedure. Preoperative instructions may include crutch training, when indicated, and postoperative education regarding expected early therapy and pain management strategy may be employed. Chlorhexidine wipes are provided to patients preoperatively with instruction to use daily starting 3 days prior to surgery. A home exercise program with a focus on quadriceps activation and range of motion is provided and requested to be performed postoperatively.
A standard operating room setup for knee arthroscopy is employed (preference list noted in Table 3; items bolded may be unique to an MPFL reconstruction). Regional, single-shot anesthesia is employed using a sensory-only adductor canal block. A universal surgical timeout is performed before beginning the surgery to include verification of prophylactic antibiotics (Ancef or clindamycin in penicillin-allergic patients) and consideration of the use of a sequential compression device in children aged >10 years when ≥1 venous thromboembolic risk factors are detected in preoperative screening.
Nonirradiated allografts (semitendinosus or tibialis anterior), preferably from a donor <30 years, are our preferred graft choice. The minimum length of the graft should be 240 mm and the doubled thickness should be 5 to 6 mm. After thawing, the graft is lavaged with a mixture of antibiotic saline consisting of 50,000 U of bacitracin in 1 l of normal saline. Tension is then placed on the graft using the graft preparation board, and a whip stitch is placed on each side of the graft using a #2 FiberWire (Arthrex). The graft is sized with care to ensure that it is not thicker than 6.5 mm with an optimal goal of 6 mm.
A standard knee arthroscopy is performed with an emphasis on evaluating the patellofemoral anatomy. Although insufflation can cause distortion and, often, lateralize the patella, the surgeon should consider an estimate of when the patella engages the trochlea during knee flexion. This position of knee flexion will determine the appropriate position of the knee during graft fixation. A bipolar device (90°Arthrocare wand) may be used to debride synovial folds (ie, plica) when affecting the patellar tracking. To maximize visualization of the patella within the knee, we recommend switching the arthroscope from the anterolateral portal to the anteromedial portal. This allows for improved visualization of the lateral femoral condyle and the inferomedial patellar facet, common locations for chondral and osteochondral damage, as well as optimal visualization of patellar engagement within the trochlea. During an arthroscopic dynamic exam of the patella tracking, a lateralized patella may be observed. If the patella tilts upon manual medial translation toward the trochlear groove during the dynamic exam, the lateral retinaculum may be tight or constraining the patella laterally. If this occurs, a partial or complete lateral release may be indicated with a bipolar wand. Visualization of the posteromedial and the posterolateral compartments is required as loose chondral bodies may be present in these locations.
Any osteochondral or chondral injuries are addressed during the arthroscopy. Large osteochondral fractures of the lateral femoral condyle or the patella are repaired using headless compression screws (Acutrak Screws, Acumed) or headed low-profile screws (1.5-mm screws, DePuy Synthes). Headless screws can be buried 2 to 3 mm below the chondral surface when the fragment has an appropriate thickness, whereas thin fragments may necessitate headed screws for adequate fixation and subsequent implant removal in 8 to 10 weeks. Defects in the patella most often require an open arthrotomy to evert the patella 90° to allow adequate exposure for treatment. Chronic chondral fragmentation may be debrided using the motorized chondrotome or removed and indicated for microfracture (<2 cm2 in surface area and <4 mm in depth) or other types of chondral replacement. Chondral lesions over the inferior medial facet are typically not symptomatic and often require minimal treatment.
Continue to: There are 3 methods to identify...
There are 3 methods to identify the insertion of the MPFL into the patella. During the diagnostic arthroscopy, an 18-gauge needle can be used to mark the insertion of the MPFL as visualization of the ligament arthroscopically is often possible (Figure 3). Another useful technique is to follow the inferior aspect of the distal insertion of the vastus medialis oblique (VMO) into the patella. The typical insertion point of the MPFL is immediately distal to the insertion of the inferior aspect of the VMO (Figure 4). It is also helpful to note that the center point of the insertion of the MPFL is at the junction of the proximal one-third and distal two-thirds of the palpable osseous patella. The MPFL origin has been noted to be at the exact midpoint of the chondral surface of the patella or 5 mm proximal (41% of the length of the patella) to the midpoint of the osseous patella.30
Following arthroscopic examination and treatment, a linear incision is made at the superior two-thirds of the patella, 1-finger breadth medial to the patella (Figure 5). During the subcutaneous dissection, the goal is to visualize the fascia overlying the VMO. Once this is identified, the dissection, in this layer, is carried over to expose the anterior and central surface of the patella. Army/navy retractors are used to retract the skin, and the assistant will place manual pressure on the patella to stabilize it for preparation of the patellar surface.
A bovie cautery or a knife is used to mark the insertion of the MPFL, which is immediately distal to the inferior border of the VMO. An incision over the medial surface of the patella creates a T-incision with elevation of the subsequent flaps proximal and distal. This allows exposure of the superficial and medial surface of the patella. During medial exposure of the patella, care is taken to avoid an arthrotomy by leaving the synovial lining attachment. A rongeur is used to decorticate the medial patella and the superficial surface (Figure 6). If an MPFL avulsion is present, it is often embedded within the soft tissues adjacent to the medial patellar. The MPFL avulsion can be exposed and removed if small. During an MPFL reconstruction, a repair of the avulsion is typically not performed. A double-loaded suture anchor is inserted at the site identified as the insertion of the native MPFL (3.0 Biocomposite SutureTak, Arthrex). A single suture anchor is used instead of an interosseous tunnel or double tunnels to avoid creating a large defect that may increase the risk of fracture in a small, skeletally immature patella.31
A hemostat is used to identify the layer between the medial retinaculum and the synovium over the medial soft tissue of the knee. The MPFL is a well-defined thickening of the medial retinaculum, and the ideal placement of the reconstruction is immediately inferior to this layer. Once this layer has been identified, a blunt hemostat is inserted to mark the end of a blind pouch that is apparent in this layer. This blind pouch is marked on the surface of the skin as the origin of the MPFL.
Fluoroscopy is now used to identify the origin of the MPFL on the medial femoral condyle. A perfect lateral is obtained by lining up the posterior condyle. Often, existent trochlear dysplasia will modify the normal appearance of the anterior structures of the condyles. Schottle’s point17 is USED to locate the origin of the MPFL. This is defined by drawing a line from the posterior cortex distally through the condyles (Figure 7). Two perpendicular lines are drawn, one to the extension of the posterior cortex at the level of the posterior extent of Blumensaat’s line and a second at the metaphyseal flare. Schottle’s point is located just anterior to the posterior cortex and in between these perpendicular lines. In a skeletally immature patient, this point appears to be at the level of the physis, on the lateral projection.
Continue to: An anteroposterior and/or a notch view projection...
An anteroposterior and/or a notch view projection is then performed to confirm the location of Schottle’s point, distal to the physis. Because the origin of the MPFL is posterior to the condyle, the notch view should be used to visualize Schottle’s point in relation to the physis in the coronal plane. This location has been confirmed to approximate the origin of the MPFL by published anatomic studies. If the graft measures 6 mm, an ideal distance of 5 to 8 mm distal to the physis will ensure a tunnel location with a proximal margin 2 to 4 mm distal to the physis. In our experience, Schottle’s point is approximately 5 to 7 mm distal to the physis, and rarely do we adjust our position. The guide wire is placed on the medial femoral condyle at this point and then angled 30° proximal to distal to allow tunnel trajectory distal to the undulating physis (Figure 8).
A 6-mm reamer is used over the guide wire to a depth of 20 mm. The newly created tunnel is now exposed and visualized. The graft is folded over at the center aspect and marked 20 mm from the tip of a Bio-tenodesis screwdriver (Arthrex). A 6.25 × 15 mm Bio-tenodesis screw is used. The graft and the screw are inserted into the condyle, and the appropriate graft fixation is confirmed by longitudinal tension placed upon the graft.
The hemostat or the passing suture previously placed into the blind pouch below the native MPFL is used to pass the graft immediately superficial to the synovial lining, in an extra-articular location. The ends of the graft are now exposed through the incision adjacent to the patella. The knee is confirmed to be flexed to approximately 45° on the surgical positioning triangleor at the flexion position determined during diagnostic arthroscopy at which the patella engages the trochlea. The graft is set to length alongside the medial surface of the patella at the position of the suture anchor, and a single stitch is placed through both ends of the graft at this adjacent position using a free needle (Davis Tonsil ½ Circle Taper Point; Vessel Medical). A hemostat is placed on the single suture throw to provisionally secure the graft in this trial position. This allows the surgeon to trial and examine the MPFL graft in extension, 45° of flexion, and terminal flexion. The goal is to provide a check rein to lateral translation of the patella and provide a firm endpoint to avoid further dislocation without overtension of the graft that may lead to increased contact pressures in the patellofemoral joint.
Once appropriate graft position is confirmed, using the double-loaded suture, we prefer to secure each limb separately to the decorticated medial patella. One end of the suture is threaded through the graft once and will act as a post. The other end of the suture is threaded through the graft 3 times in a modified Mason-Allen stitch. With the knee in that same position of flexion, the knot is tied and the graft is secured to the medial side of the patella. For secondary fixation, the remaining ends of the graft are passed below the periosteum on the anterior surface of the patella. Using a 0 Vicryl (Ethicon US), the periosteal flaps are sutured on top of the graft, incorporating the residual graft. The ends are cut and a repeat dynamic examination is performed to confirm the position of the patella and the patellar tracking and to ensure that overtensioning did not occur. Following irrigation, a standard closure is performed. The patient is placed in a hinged knee brace and cryotherapy is applied (Polar Care, Breg Inc).
POSTOPERATIVE PROTOCOL
For the initial or the acute postoperative phases, an emphasis on edema control, early quadriceps activation, and range of motion is recommended. We recommend weight-bearing as tolerated with the leg locked in extension until adequate quadriceps control is achieved. The patient must be able to perform 10 straight leg raises without an extension loss to be cleared to weight-bear as tolerated without motion restriction in the brace. Full motion is allowed immediately.
OUTCOMES
In our experience using isolated MPFL reconstruction in the skeletally immature patient, we have had no evidence of physeal arrest, leg-length inequality, or angular deformity, and only11.4% of patients have had recurrent instability. The mean Kujala score in this cohort was 90.4, with a mean Tegner activity of 7, after the procedure. All failures in our cohort had had severe trochlear dysplasia.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
1. Chotel F, Berard J, Raux S. Patellar instability in children and adolescents. Orthop Traumatol Surg Res. 2014;100(1 Suppl.):S125-S137. doi: 10.1016/j.otsr.2013.06.014.
2. Gao B, Dwivedi S, Fabricant PD, Cruz AI Jr. Patterns in outcomes reporting of operatively managed pediatric patellofemoral instability: a systematic review and meta-analysis. Am J Sports Med. 2018;2:36354651876515. doi: 10.1177/0363546518765152.
3. Hennrikus W, Pylawka T. Patellofemoral instability in skeletally immature athletes. Instr Course Lect. 2013;62:445-453.
4. Vavken P, Wimmer MD, Camathias C, Quidde J, Valderrabano V, Pagenstert G. Treating patella instability in skeletally immature patients. Arthroscopy. 2013;29(8):1410-1422. doi: 10.1016/j.arthro.2013.03.075.
5. Askenberger M, Ekstrom W, Finnbogason T, Janarv PM. Occult intra-articular knee injuries in children With hemarthrosis. Am J Sports Med. 2014;42(7):1600-1606. doi: 10.1177/0363546514529639.
6. Seeley MA, Knesek M, Vanderhave KL. Osteochondral injury after acute patellar dislocation in children and adolescents. J Pediatr Orthop. 2013;33(5):511-518. doi: 10.1097/BPO.0b013e318288b7a0.
7. Cruz AI Jr, Milewski MD. Patellofemoral instability and other common knee issues in the skeletally immature knee (other knee pathology). In: Miller MD, ed. Orthopaedic Knowledge Update: Sports Medicine 5. 5th ed. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2015.
8. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581. doi: 10.1177/0363546512472873.
9. Arshi A, Cohen JR, Wang JC, Hame SL, McAllister DR, Jones KJ. Operative management of patellar instability in the United States: an evaluation of national practice patterns, surgical trends, and complications. Orthop J Sports Med. 2016;4(8):2325967116662873. doi: 10.1177/2325967116662873.
10. Camathias C, Studer K, Kiapour A, Rutz E, Vavken P. Trochleoplasty as a solitary treatment for recurrent patellar dislocation results in good clinical outcome in adolescents. Am J Sports Med. 2016;44(11):2855-2863. doi: 10.1177/0363546516652894.
11. Jaquith BP, Parikh SN. Predictors of recurrent patellar iInstability in children and adolescents after first-time dislocation. J Pediatr Orthop. 2017;37(7):484-490. doi: 10.1097/BPO.0000000000000674.
12. Rong YH, Zhang GA, Wang C, Ning FG. [Quantification of type I and III collagen content in normal human skin in different age groups]. Zhonghua Shao Shang Za Zhi. 2008;24(1):51-53.
13. Wilson PL, Black SR, Ellis HB, Podeszwa DA. Distal femoral valgus and recurrent traumatic patellar instability: is an isolated varus producing distal femoral osteotomy a treatment option? J Pediatr Orthop. 2018;38(3):e162-e167. doi: 10.1097/BPO.0000000000001128.
14. Kearney SP, Mosca VS. Selective hemiepiphyseodesis for patellar instability with associated genu valgum. J Orthop. 2015;12(1):17-22. doi: 10.1016/j.jor.2015.01.005.
15. Kraus T, Lidder S, Svehlik M, Rippel K, Schneider F, Eberl R, Linhart W. Patella re-alignment in children with a modified Grammont technique. Acta Orthop. 2012;83(5):504-510. doi: 10.3109/17453674.2012.736168.
16. Shea KG, Grimm NL, Belzer J, Burks RT, Pfeiffer R. The relation of the femoral physis and the medial patellofemoral ligament. Arthroscopy. 2010;26(8):1083-1087. doi: 10.1016/j.arthro.2009.12.020.
17. Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi: 10.1177/0363546506296415.
18. Kepler CK, Bogner EA, Hammoud S, Malcolmson G, Potter HG, Green DW. Zone of injury of the medial patellofemoral ligament after acute patellar dislocation in children and adolescents. Am J Sports Med. 2011;39(7):1444-1449. doi: 10.1177/0363546510397174.
19. Nelitz M, Dornacher D, Dreyhaupt J, Reichel H, Lippacher S. The relation of the distal femoral physis and the medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2067-2071. doi: 10.1007/s00167-011-1548-3.
20. Shea KG, Styhl AC, Jacobs JC Jr, Ganley TJ, Milewski MD, Cannamela PC, et al. The relationship of the femoral physis and the medial patellofemoral ligament in children: A Cadaveric Study. Am J Sports Med. 2016;44(11):2833-2837. doi: 10.1177/0363546516656366.
21. Popkin CA, Bayomy AF, Trupia EP, Chan CM, Redler LH. Patellar instability in the skeletally immature. Curr Rev Musculoskelet Med. 2018;11(2):172-181. doi: 10.1007/s12178-018-9472-5.
22. Keyes S, Price M, Green DW, Parikh SN. Special considerations for pediatric patellar instability. Am J Orthop Belle Mead NJ. 2018;47(3). doi: 10.12788/ajo.2018.0017.
23. Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38(11):2248-2254. doi: 10.1177/0363546510376230.
24. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of Patella alta in children. Knee. 2014;21(6):1180-1184. doi: 10.1016/j.knee.2014.07.023.
25. Matic GT, Magnussen RA, Kolovich GP, Flanigan DC. Return to activity after medial patellofemoral ligament repair or reconstruction. Arthroscopy. 2014;30(8):1018-1025. doi: 10.1016/j.arthro.2014.02.044.
26. Mostrom EB, Mikkelsen C, Weidenhielm L, Janarv PM. Long-term follow-up of nonoperatively and operatively treated acute primary patellar dislocation in skeletally immature patients. Sciworldj. 2014;2014:473281. doi: 10.1155/2014/473281.
27. Antinolfi P, Bartoli M, Placella G, Speziali A, Pace V, Delcogliano M, Mazzola C. Acute patellofemoral instability in children and adolescents. Joints. 2016;4(1):47-51. doi: 10.11138/jts/2016.4.1.047.
28. Nelitz M, Dreyhaupt J, Reichel H, Woelfle J, Lippacher S. Anatomic reconstruction of the medial patellofemoral ligament in children and adolescents with open growth plates: surgical technique and clinical outcome. Am J Sports Med. 2013;41(1):58-63. doi: 10.1177/0363546512463683.
29. McNeilan RJ, Everhart JS, Mescher PK, Abouljoud M, Magnussen RA, Flanigan DC. Graft choice in isolated medial patellofemoral ligament reconstruction: A systematic review with meta-analysis of rates of recurrent instability and patient-reported outcomes for autograft, allograft, and synthetic options. Arthroscopy. 2018;34(4):1340-1354. doi: 10.1016/j.arthro.2017.11.027.
30. Shea KG, Polousky JD, Jacobs JC Jr, Ganley TJ, Aoki SK, Grimm NL, Parikh SN. The patellar insertion of the medial patellofemoral ligament in children: a cadaveric study. J Pediatr Orthop. 2015;35(4):e31-e35. doi: 10.1097/BPO.0000000000000399.
31. Parikh SN, Wall EJ. Patellar fracture after medial patellofemoral ligament surgery: a report of five cases. J Bone Joint Surg, (Am.). 2011;93(17):e97(1-8). doi: 10.2106/JBJS.J.01558.
TAKE-HOME POINTS
- Patellofemoral instability is common in the skeletally immature age group.
- Many treatment options exist for patellofemoral instability; however, a medial patellofemoral ligament (MPFL) reconstruction is an option in a skeletally immature patient.
- Allograft or autografts may be utilized for the MPFL reconstruction.
- Three options exist to confirm location of the MPFL origin on the patella, but only one drill tunnel is recommended in pediatrics.
- During identification of MPFL origin, special views in the coronal plane should be considered to avoid injury or damage to the growth plate.