User login
Resilience and mind-body interventions in late-life depression
Resilience has been defined as the ability to adapt and thrive in the face of adversity, acute stress, or trauma.1 Originally conceived as an inborn trait characteristic, resilience is now conceptualized as a dynamic, multidimensional capacity influenced by the interactions between internal factors (eg, personality, cognitive capacity, physical health) and environmental resources (eg, social status, financial stability).2,3 Resilience in older adults (typically defined as age ≥65) can improve the prognosis and outcomes for physical and mental conditions.4 The construct is closely aligned with “successful aging” and can be fostered in older adults, leading to improved physical and mental health and well-being.5
While initially resilience was conceptualized as the opposite of depressive states, recent research has identified resilience in the context of major depressive disorder (MDD) as the net effects of various psychosocial and biological variables that decrease the risk of onset, relapse, or depressive illness severity and increase the probability or speed of recovery.6 Late-life depression (LLD) in adults age >65 is a common and debilitating disease, often leading to decreased psychological well-being, increased cognitive decline, and excess mortality.7,8 LLD is associated with several factors, such as cerebrovascular disease, neurodegenerative disease, and inflammation, all of which could contribute to brain vulnerability and an increased risk of depression.9 Physical and cognitive engagement, physical activity, and high brain reserve have been shown to confer resilience to affective and cognitive changes in older adults, despite brain vulnerability.9
The greatest levels of resilience have been observed in individuals in their fifth decade of life and later,4,10 with high levels of resilience significantly contributing to longevity5; however, little is known about which factors contribute to heterogeneity in resilience characteristics and outcomes.4 Furthermore, the concept of resilience continues to raise numerous questions, including:
- how resilience should be measured or defined
- what factors promote or deter the development of resilience
- the effects of resilience on various health and psychological outcomes
- which interventions are effective in enhancing resilience in older adults.4
In this article, we describe resilience in older adults with LLD, its clinical and neurocognitive correlates, and underlying neurobiological and immunological biomarkers. We also examine resilience-building interventions, such as mind-body therapies (MBTs), that have been shown to enhance resilience by promoting positive perceptions of difficult experiences and challenges.
Clinical and neurocognitive correlates of resilience
Resilience varies substantially among older adults with LLD as well as across the lifespan of an individual.11 Identifying clinical components and predictors of resilience may usefully inform the development and testing of interventions to prevent and treat LLD.11 One tool widely used to measure resilience—the self-report Connor-Davidson Resilience Scale (CD-RISC)12— has been found to have clinically relevant characteristics.1,11 Using data from 337 older adults with LLD, Laird et al11 performed an exploratory factor analysis of the CD-RISC and found a 4-factor model:
- grit
- adaptive coping self-efficacy
- accommodative coping self-efficacy
- spirituality.1,11
Having a strong sense of purpose and not being easily discouraged by failure were items characteristic of grit.1,11 The preference to take the lead in problem-solving was typical of items loading on adaptive coping self-efficacy, while accommodative coping self-efficacy measured flexibility, cognitive reframing, a sense of humor, and acceptance in the face of uncontrollable stress.1,11 Finally, the belief that “things happen for a reason” and that “sometimes fate or God can help me” are characteristics of spirituality. 1,11 Using a multivariate model, the greatest variance in total resilience scores was explained by less depression, less apathy, higher quality of life, non-White race, and, somewhat counterintuitively, greater medical comorbidity.1,11 Thus, interventions designed to help older adults cultivate grit, active coping, accommodative coping, and spirituality may enhance resilience in LLD.
Resilience may also be positively associated with cognitive functioning and could be neuroprotective in LLD.13 Laird et al13 investigated associations between baseline resilience and several domains of neurocognitive functioning in 288 older adults with LLD. Several positive associations were found between measured language performance and total resilience, active coping, and accommodative coping.13 Additionally, total resilience and accommodative coping were significantly associated with a lower self-reported frequency of forgetfulness, a subjective measure of memory used in this study.13 Together, these results suggest that interventions targeting language might be useful to improve coping in LLD.13 Another interesting finding was that the resilience subdomain of spirituality was negatively associated with memory, language, and executive functioning performance.13 A distinction must be made between religious attendance (eg, regular attendance at religious institutions) vs religious beliefs, which may account for the previously reported associations between spirituality and improved cognition.13
Continue to: Self-reported resilience...
Self-reported resilience may also predict greater responsivity to antidepressant medication in patients with LLD.14 Older adults with LLD and greater self-reported baseline resilience were more likely to experience improvement or remission from depression with antidepressant treatment.14 This is congruent with conceptualizations of resilience as “the ability to adapt to and recover from stress.”14,15 Of the 4 identified resilience factors (grit, adaptive coping, accommodative coping, and spirituality), it appears that accommodative coping predicts LLD treatment response and remission.14 The unique ability to accommodate is associated with better mental health outcomes in the face of uncontrollable stress.14,16-18 Older adults appear to engage in more accommodative coping due to frequent uncontrollable stress and aging-related physiological changes (eg, sleep changes, chronic pain, declining cognition). This could make accommodative coping especially important in this population.14,19
The Figure, adapted from Weisenbach et al,9 exhibits factors that contribute to LLD, including cerebrovascular disease, neurodegeneration, and chronic inflammation, all of which can lead to a vulnerable aging brain that is at higher risk for depression, particularly within the context of stress. Clinical and neurocognitive factors associated with resilience can help buffer vulnerable brains from developing depression.
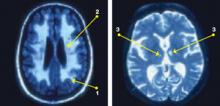
Neurobiological biomarkers of resilience in
Gross anatomical indicators: Findings from neuroimaging
The neurobiology underlying psychological resilience involves brain networks associated with stress response, negative affect, and emotional control.19 Increased amygdala reactivity and amygdala frontal connectivity are often implicated in neurobiological models of resilience.20 Leaver et al20 correlated psychological resilience measures with amygdala function in 48 depressed vs nondepressed individuals using functional magnetic resonance imaging. Specifically, they targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala while comparing the 2 groups based on resilience scores (CD-RISC), depressive symptom severity, and depression status.20 A significant correlation was identified between resilience and connectivity between the superficial group of amygdala nuclei and the ventral default mode network (VDMN).20 High levels of psychological resilience were associated with lower basal amygdala activity and decreased connectivity between amygdala nuclei and the VDMN.20 Additionally, lower depressive symptoms were associated with higher connectivity between the amygdalae and the dorsal frontal networks.20 These results suggest a complex relationship between amygdala activity, dorsal frontal regions, resilience, and LLD.20
Vlasova et al21 further addressed the multifactorial character of psychological resilience. The associations between the 4 factors of resilience and the regional integrity of white matter in older adults with LLD were examined using diffusion-weighted MRI.21 Grit was found to be associated with greater white matter integrity in the genu of the corpus callosum and cingulum bundle in LLD.21 There was also a positive association between grit and fractional anisotropy (FA) in the callosal region connecting the prefrontal cortex and FA in the cingulum fibers.21 However, results regarding the FA in the cingulum fibers did not survive correction for multiple comparisons and should be considered with caution, pending further research.21
Continue to: Stress response biomarkers of resilience
Stress response biomarkers of resilience
Stress response biomarkers include endocrine, immune, and inflammatory indices. Stress has been identified as a factor in inflammatory responses. Stress-related overstimulation of the HPA axis may increase the risk of LLD.22 Numerous studies have demonstrated an association between increased levels of peripheral proinflammatory cytokines and depressive symptoms in older adults.23 Interleukin-6 (IL-6) has been increasingly linked with depressive symptoms and poor memory performance in older adults.9 There also appears to be an interaction of inflammatory and vascular processes predisposing to LLD, as increased levels of IL-6 and C-reactive protein have been associated with higher white matter pathology.9 Additionally, proinflammatory cytokines impact monoamine neurotransmitter pathways, leading to a reduction in tryptophan and serotonin synthesis, disruption of glucocorticoid receptors, and a decrease in hippocampal neurotrophic support.9 Alexopoulos et al24 further explain that a prolonged CNS immune response can affect emotional and cognitive network functions related to LLD and has a role in the etiology of depressive symptoms in older adults.
Cardiovascular comorbidity and autonomic nervous system dysfunction
Many studies have revealed evidence of a bidirectional association between cardiovascular disease and depression.25 Dysregulation of the autonomic nervous system (ANS) is an underlying mechanism that could explain the link between cardiovascular risk and MDD via heart rate variability (HRV), though research examining age-related capacities provide conflicting data.25,26 HRV is a surrogate index of resting cardiac vagal outflow that represents the ability of the ANS to adapt to psychological, social, and physical environmental changes.27 Higher overall HRV is associated with greater self-regulating capacity, including behavioral, cognitive, and emotional control.28 Additionally, higher HRV may serve as a biomarker of resilience to the development of stress-related disorders such as MDD. Recent studies have shown an overall reduction in HRV in older adults with LLD.29 When high- and low-frequency HRV were investigated separately, only low-frequency HRV was significantly reduced in patients with depression.29 One explanation is that older adults with depression have impaired or reduced baroreflex sensitivity and gain, which is often associated with an increased risk of mortality following cardiac events.30 More research is needed to examine the complex processes required to better characterize the correlation between resilience in cardiovascular disease and autonomic dysfunction.
The Box6,31,32 describes the relationship between markers of cellular health and resilience.
Box
Among the biomarkers of resilience, telomere length and telomerase activity serve as biomarkers of biological aging that can differ from the chronological age and mark successful anti-aging, stress-reducing strategies.31 Telomerase, the cellular enzyme that regulates the health of cells when they reproduce (preserving the telomeres, repetitive DNA strands at the ends of chromosomes), is associated with overall cell health and cellular biological age.31 When telomerase is reduced, the telomeres in a cell are clipped, causing the cells to age more rapidly as the telomeres get shorter through the process of cellular reproduction.31 Psychological stress may play a significant role in telomerase production and subsequent telomere length.32 Lavretsky et al32 evaluated the effect of brief daily yogic meditation on depressive symptoms and immune cell telomerase activity in a family of dementia caregivers with mild depressive symptoms. Brief daily meditation practice led to significant lower levels of depressive symptoms that was accompanied by an increase in telomerase activity, suggesting improvement in stress-induced cellular aging.6,32
Mind-body therapies
There is increasing interest in improving older adults’ physical and emotional well-being while promoting resilience through stress-reducing lifestyle interventions such as MBTs.33 Because MBTs are often considered a natural and safer option compared to conventional medicine, these interventions are rapidly gaining popularity in the United States.33,34 According to a 2017 National Health Survey, there were 5% to 10% increases in the use of yoga, meditation, and chiropractic care from 2012 to 2017, with growing evidence supporting MBTs as minimally invasive, cost-effective approaches for managing stress and neurocognitive disorders.35 In contrast to pharmacologic approaches, MBTs can be used to train individuals to self-regulate in the face of adversity and stress, thus increasing their resilience.
MBTs can be divided into mindful movement exercises and meditative practices. Mindful movement exercises include yoga, tai chi, and qigong. Meditative practices that do not include movement include progressive relaxation, mindfulness, meditation, and acceptance therapies. On average, both mindful movement exercise (eg, yoga) and multicomponent mindfulness-based interventions (eg, mindfulness-based cognitive therapy, mindfulness-based stress reduction [MBSR], and mindfulness-based relapse prevention) can be as effective as other active treatments for psychiatric disorders such as MDD, anxiety, and substance use disorders.36,37 MBSR specifically has been shown to increase empathy, self-control, self-compassion, relationship quality, mindfulness, and spirituality as well as decrease rumination in healthy older adults.38 This suggests that MBSR can help strengthen the 4 factors of resilience.
Continue to: Research has also begun...
Research has also begun to evaluate the neurobiological mechanisms by which meditative therapies enhance resilience in mental health disorders, and several promising mechanistic domains (neural, hormonal, immune, cellular, and cardiovascular) have been identified.39 The physical yoga discipline includes asanas (postures), pranayama (breathing techniques), and dhyana (meditation). With the inclusion of mindfulness training, yoga involves the practice of meditation as well as the dynamic combination of proprioceptive and interoceptive awareness, resulting in both attention and profound focus.40 Dedicated yoga practice allows an individual to develop skills to withdraw the senses (pratyahara), concentrate the mind (dharana), and establish unwavering awareness (dhyana).41 The physical and cognitive benefits associated with yoga and mindfulness may be due to mechanisms including pranayama and activation of the parasympathetic nervous system; meditative or contemplative practices; increased body perception; stronger functional connectivity within the basal ganglia; or neuroplastic effects of increased grey matter volume and amygdala with regional enlargement.41 The new learning aspect of yoga practice may contribute to enhancing or improving various aspects of cognition, although the mechanisms are yet to be clarified.
Continued research in this area will promote the integration of MBTs into mainstream clinical practice and help alleviate the increased chronic health burden of an aging population. In the face of the COVID-19 pandemic, public interest in improving resilience and mental health42 can be supported by MBTs that can improve coping with the stress of the pandemic and enhance critical organ function (eg, lungs, heart, brain).43,44 As a result of these limitations, many resources and health care services have used telehealth and virtual platforms to adapt to these challenges and continue offering MBTs.45
Enhancing resilience to improve clinical outcomes
Increasing our understanding of clinical, neurocognitive, and neurobiological markers of resilience in older adults with and without depression could inform the development of interventions that treat and prevent mood and cognitive disorders of aging. Furthermore, stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, leading to greater resilience. Complementary use of MBTs combined with standard antidepressant treatment may allow for additional improvement in clinical outcomes of LLD, including resilience, quality of life, general health, and cognitive function. Additional research testing the efficacy of those interventions designed to improve resilience in older adults with mood and mental disorders is needed.
Bottom Line
Identifying the clinical, neurocognitive, and neurobiological biomarkers of resilience in late-life depression could aid in the development of targeted interventions that treat and prevent mood and cognitive disorders of aging. Mind-body interventions can help boost resilience and improve outcomes in geriatric patients with mood and cognitive disorders.
Related Resources
- Lavretsky H. Resilience and Aging: Research and Practice. Johns Hopkins University Press; 2014.
- Lavretsky H, Sajatovic M, Reynolds CF, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2016.
- Eyre HA, Berk M, Lavretsky H, et al, eds. Convergence Mental Health: A Transdisciplinary Approach to Innovation. Oxford University Press; 2021.
- UCLA Jane & Terry Semel Institute for Neuroscience & Human Behavior. Late-life Depression, Stress, and Wellness Research Program. https://www.semel.ucla.edu/latelife
1. Reynolds CF. Promoting resilience, reducing depression in older adults. Int Psychogeriatr. 2019;31(2):169-171.
2. Windle G. What is resilience? A review and concept analysis. Rev Clin Gerontol. 2011;21(2):152-169.
3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79-82.
4. Dunn LB, Predescu I. Resilience: a rich concept in need of research comment on: “Neurocognitive correlates of resilience in late-life depression” (by Laird et al.). Am J Geriatr Psychiatry. 2019;27(1):18-20.
5. Harmell AL, Kamat R, Jeste DV, et al. Resilience-building interventions for successful and positive aging. In: Lavretsky H, Sajatovic M, Reynolds C III, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2015:305-316.
6. Laird KT, Krause B, Funes C, et al. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9(1):88.
7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
8. Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13(11):746-752.
9. Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463.
10. Southwick SM, Litz BT, Charney D, et al. Resilience and Mental Health: Challenges Across the Lifespan. Cambridge University Press; 2011.
11. Laird KT, Lavretsky H, Paholpak P, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31(2):193-202.
12. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Laird KT, Lavretsky H, Wu P, et al. Neurocognitive correlates of resilience in late-life depression. Am J Geriatr Psychiatry. 2019;27(1):12-17.
14. Laird KT, Lavretsky H, St Cyr N, et al. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33(12):1596-1603.
15. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev. 2015;41:49-60.
16. Boerner K. Adaptation to disability among middle-aged and older adults: the role of assimilative and accommodative coping. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P35-P42.
17. Zakowski SG, Hall MH, Klein LC, et al. Appraised control, coping, and stress in a community sample: a test of the goodness-of-fit hypothesis. Ann Behav Med. 2001;23(3):158-165.
18. Cheng C, Lau HB, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140(6):1582-1607.
19. Stokes SA, Gordon SE. Common stressors experienced by the well elderly. Clinical implications. J Gerontol Nurs. 2003;29(5):38-46.
20. Leaver AM, Yang H, Siddarth P, et al. Resilience and amygdala function in older healthy and depressed adults. J Affect Disord. 2018;237:27-34.
21. Vlasova RM, Siddarth P, Krause B, et al. Resilience and white matter integrity in geriatric depression. Am J Geriatr Psychiatry. 2018;26(8):874-883.
22. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15(4):379-400.
23. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022.
24. Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109-1118.
25. Carney RM, Freedland KE, Sheline YI, et al. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20(3):196-200.
26. Carney RM, Freedland KE, Steinmeyer BC, et al. Nighttime heart rate predicts response to depression treatment in patients with coronary heart disease. J Affect Disord. 2016;200:165-171.
27. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psych. 2006;10(3):229-240.
28. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233-255.
29. Brown L, Karmakar C, Gray R, et al. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456-466.
30. La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(1901):478-484.
31. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306-322.
32. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
33. Siddiqui MJ, Min CS, Verma RK, et al. Role of complementary and alternative medicine in geriatric care: a mini review. Pharmacogn Rev. 2014;8(16):81-87.
34. Nguyen SA, Lavretsky H. Emerging complementary and integrative therapies for geriatric mental health. Curr Treat Options Psychiatry. 2020;7(4):447-470.
35. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
36. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749.
37. Ramadas E, de Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133.
38. Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593-600.
39. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
40. Chobe S, Chobe M, Metri K, et al. Impact of yoga on cognition and mental health among elderly: a systematic review. Complement Ther Med. 2020;52:102421.
41. Brunner D, Abramovitch A, Etherton J. A yoga program for cognitive enhancement. PLoS One. 2017;12(8):e0182366.
42. Dai J, Sang X, Menhas R, et al. The influence of COVID-19 pandemic on physical health-psychological health, physical activity, and overall well-being: the mediating role of emotional regulation. Front Psychol. 2021;12:667461.
43. Grolli RE, Mingoti MED, Bertollo AG, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol Neurobiol. 2021;58(5):1905-1916.
44. Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658.
45. Pandya SP. Older women and wellbeing through the pandemic: examining the effect of daily online yoga lessons. Health Care Women Int. 2021;42(11):1255-1278.
Resilience has been defined as the ability to adapt and thrive in the face of adversity, acute stress, or trauma.1 Originally conceived as an inborn trait characteristic, resilience is now conceptualized as a dynamic, multidimensional capacity influenced by the interactions between internal factors (eg, personality, cognitive capacity, physical health) and environmental resources (eg, social status, financial stability).2,3 Resilience in older adults (typically defined as age ≥65) can improve the prognosis and outcomes for physical and mental conditions.4 The construct is closely aligned with “successful aging” and can be fostered in older adults, leading to improved physical and mental health and well-being.5
While initially resilience was conceptualized as the opposite of depressive states, recent research has identified resilience in the context of major depressive disorder (MDD) as the net effects of various psychosocial and biological variables that decrease the risk of onset, relapse, or depressive illness severity and increase the probability or speed of recovery.6 Late-life depression (LLD) in adults age >65 is a common and debilitating disease, often leading to decreased psychological well-being, increased cognitive decline, and excess mortality.7,8 LLD is associated with several factors, such as cerebrovascular disease, neurodegenerative disease, and inflammation, all of which could contribute to brain vulnerability and an increased risk of depression.9 Physical and cognitive engagement, physical activity, and high brain reserve have been shown to confer resilience to affective and cognitive changes in older adults, despite brain vulnerability.9
The greatest levels of resilience have been observed in individuals in their fifth decade of life and later,4,10 with high levels of resilience significantly contributing to longevity5; however, little is known about which factors contribute to heterogeneity in resilience characteristics and outcomes.4 Furthermore, the concept of resilience continues to raise numerous questions, including:
- how resilience should be measured or defined
- what factors promote or deter the development of resilience
- the effects of resilience on various health and psychological outcomes
- which interventions are effective in enhancing resilience in older adults.4
In this article, we describe resilience in older adults with LLD, its clinical and neurocognitive correlates, and underlying neurobiological and immunological biomarkers. We also examine resilience-building interventions, such as mind-body therapies (MBTs), that have been shown to enhance resilience by promoting positive perceptions of difficult experiences and challenges.
Clinical and neurocognitive correlates of resilience
Resilience varies substantially among older adults with LLD as well as across the lifespan of an individual.11 Identifying clinical components and predictors of resilience may usefully inform the development and testing of interventions to prevent and treat LLD.11 One tool widely used to measure resilience—the self-report Connor-Davidson Resilience Scale (CD-RISC)12— has been found to have clinically relevant characteristics.1,11 Using data from 337 older adults with LLD, Laird et al11 performed an exploratory factor analysis of the CD-RISC and found a 4-factor model:
- grit
- adaptive coping self-efficacy
- accommodative coping self-efficacy
- spirituality.1,11
Having a strong sense of purpose and not being easily discouraged by failure were items characteristic of grit.1,11 The preference to take the lead in problem-solving was typical of items loading on adaptive coping self-efficacy, while accommodative coping self-efficacy measured flexibility, cognitive reframing, a sense of humor, and acceptance in the face of uncontrollable stress.1,11 Finally, the belief that “things happen for a reason” and that “sometimes fate or God can help me” are characteristics of spirituality. 1,11 Using a multivariate model, the greatest variance in total resilience scores was explained by less depression, less apathy, higher quality of life, non-White race, and, somewhat counterintuitively, greater medical comorbidity.1,11 Thus, interventions designed to help older adults cultivate grit, active coping, accommodative coping, and spirituality may enhance resilience in LLD.
Resilience may also be positively associated with cognitive functioning and could be neuroprotective in LLD.13 Laird et al13 investigated associations between baseline resilience and several domains of neurocognitive functioning in 288 older adults with LLD. Several positive associations were found between measured language performance and total resilience, active coping, and accommodative coping.13 Additionally, total resilience and accommodative coping were significantly associated with a lower self-reported frequency of forgetfulness, a subjective measure of memory used in this study.13 Together, these results suggest that interventions targeting language might be useful to improve coping in LLD.13 Another interesting finding was that the resilience subdomain of spirituality was negatively associated with memory, language, and executive functioning performance.13 A distinction must be made between religious attendance (eg, regular attendance at religious institutions) vs religious beliefs, which may account for the previously reported associations between spirituality and improved cognition.13
Continue to: Self-reported resilience...
Self-reported resilience may also predict greater responsivity to antidepressant medication in patients with LLD.14 Older adults with LLD and greater self-reported baseline resilience were more likely to experience improvement or remission from depression with antidepressant treatment.14 This is congruent with conceptualizations of resilience as “the ability to adapt to and recover from stress.”14,15 Of the 4 identified resilience factors (grit, adaptive coping, accommodative coping, and spirituality), it appears that accommodative coping predicts LLD treatment response and remission.14 The unique ability to accommodate is associated with better mental health outcomes in the face of uncontrollable stress.14,16-18 Older adults appear to engage in more accommodative coping due to frequent uncontrollable stress and aging-related physiological changes (eg, sleep changes, chronic pain, declining cognition). This could make accommodative coping especially important in this population.14,19
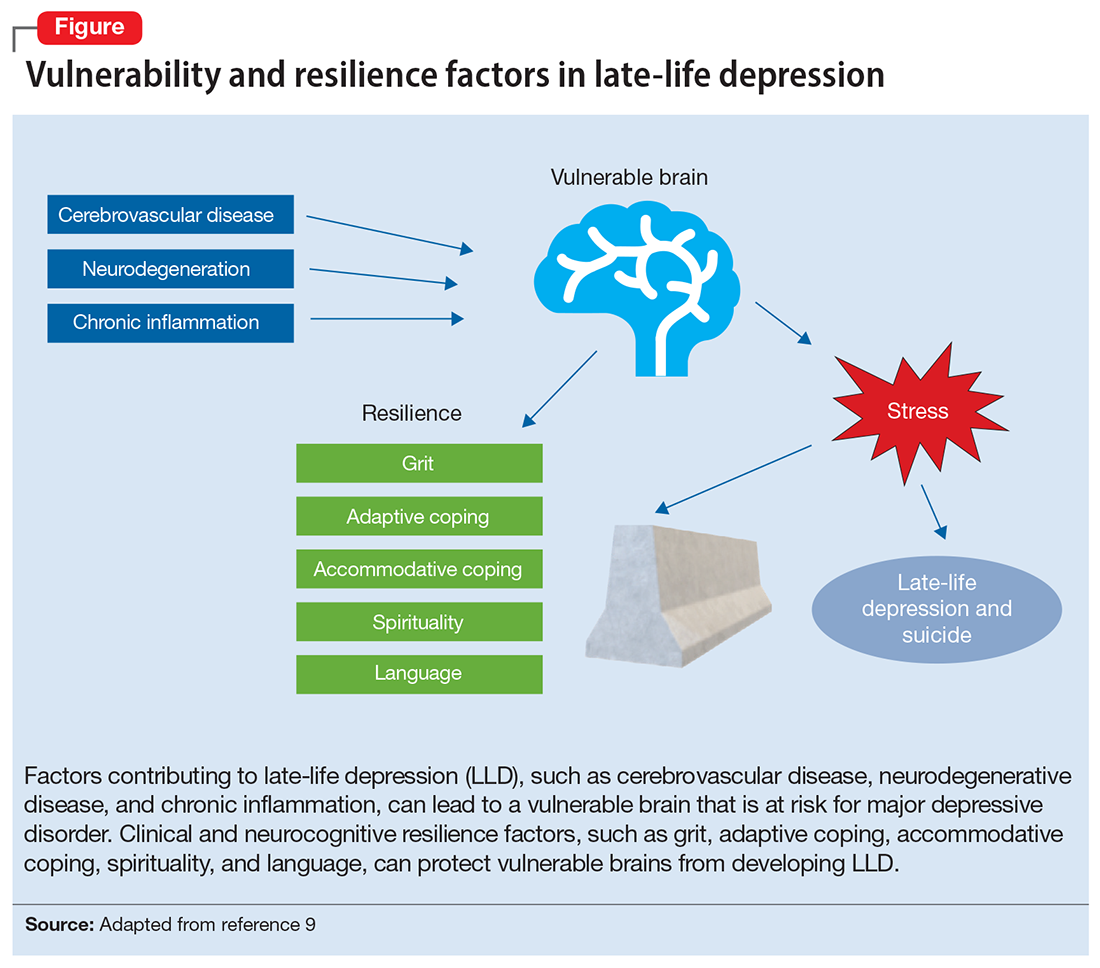
The Figure, adapted from Weisenbach et al,9 exhibits factors that contribute to LLD, including cerebrovascular disease, neurodegeneration, and chronic inflammation, all of which can lead to a vulnerable aging brain that is at higher risk for depression, particularly within the context of stress. Clinical and neurocognitive factors associated with resilience can help buffer vulnerable brains from developing depression.

Neurobiological biomarkers of resilience in
Gross anatomical indicators: Findings from neuroimaging
The neurobiology underlying psychological resilience involves brain networks associated with stress response, negative affect, and emotional control.19 Increased amygdala reactivity and amygdala frontal connectivity are often implicated in neurobiological models of resilience.20 Leaver et al20 correlated psychological resilience measures with amygdala function in 48 depressed vs nondepressed individuals using functional magnetic resonance imaging. Specifically, they targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala while comparing the 2 groups based on resilience scores (CD-RISC), depressive symptom severity, and depression status.20 A significant correlation was identified between resilience and connectivity between the superficial group of amygdala nuclei and the ventral default mode network (VDMN).20 High levels of psychological resilience were associated with lower basal amygdala activity and decreased connectivity between amygdala nuclei and the VDMN.20 Additionally, lower depressive symptoms were associated with higher connectivity between the amygdalae and the dorsal frontal networks.20 These results suggest a complex relationship between amygdala activity, dorsal frontal regions, resilience, and LLD.20
Vlasova et al21 further addressed the multifactorial character of psychological resilience. The associations between the 4 factors of resilience and the regional integrity of white matter in older adults with LLD were examined using diffusion-weighted MRI.21 Grit was found to be associated with greater white matter integrity in the genu of the corpus callosum and cingulum bundle in LLD.21 There was also a positive association between grit and fractional anisotropy (FA) in the callosal region connecting the prefrontal cortex and FA in the cingulum fibers.21 However, results regarding the FA in the cingulum fibers did not survive correction for multiple comparisons and should be considered with caution, pending further research.21
Continue to: Stress response biomarkers of resilience
Stress response biomarkers of resilience
Stress response biomarkers include endocrine, immune, and inflammatory indices. Stress has been identified as a factor in inflammatory responses. Stress-related overstimulation of the HPA axis may increase the risk of LLD.22 Numerous studies have demonstrated an association between increased levels of peripheral proinflammatory cytokines and depressive symptoms in older adults.23 Interleukin-6 (IL-6) has been increasingly linked with depressive symptoms and poor memory performance in older adults.9 There also appears to be an interaction of inflammatory and vascular processes predisposing to LLD, as increased levels of IL-6 and C-reactive protein have been associated with higher white matter pathology.9 Additionally, proinflammatory cytokines impact monoamine neurotransmitter pathways, leading to a reduction in tryptophan and serotonin synthesis, disruption of glucocorticoid receptors, and a decrease in hippocampal neurotrophic support.9 Alexopoulos et al24 further explain that a prolonged CNS immune response can affect emotional and cognitive network functions related to LLD and has a role in the etiology of depressive symptoms in older adults.
Cardiovascular comorbidity and autonomic nervous system dysfunction
Many studies have revealed evidence of a bidirectional association between cardiovascular disease and depression.25 Dysregulation of the autonomic nervous system (ANS) is an underlying mechanism that could explain the link between cardiovascular risk and MDD via heart rate variability (HRV), though research examining age-related capacities provide conflicting data.25,26 HRV is a surrogate index of resting cardiac vagal outflow that represents the ability of the ANS to adapt to psychological, social, and physical environmental changes.27 Higher overall HRV is associated with greater self-regulating capacity, including behavioral, cognitive, and emotional control.28 Additionally, higher HRV may serve as a biomarker of resilience to the development of stress-related disorders such as MDD. Recent studies have shown an overall reduction in HRV in older adults with LLD.29 When high- and low-frequency HRV were investigated separately, only low-frequency HRV was significantly reduced in patients with depression.29 One explanation is that older adults with depression have impaired or reduced baroreflex sensitivity and gain, which is often associated with an increased risk of mortality following cardiac events.30 More research is needed to examine the complex processes required to better characterize the correlation between resilience in cardiovascular disease and autonomic dysfunction.
The Box6,31,32 describes the relationship between markers of cellular health and resilience.
Box
Among the biomarkers of resilience, telomere length and telomerase activity serve as biomarkers of biological aging that can differ from the chronological age and mark successful anti-aging, stress-reducing strategies.31 Telomerase, the cellular enzyme that regulates the health of cells when they reproduce (preserving the telomeres, repetitive DNA strands at the ends of chromosomes), is associated with overall cell health and cellular biological age.31 When telomerase is reduced, the telomeres in a cell are clipped, causing the cells to age more rapidly as the telomeres get shorter through the process of cellular reproduction.31 Psychological stress may play a significant role in telomerase production and subsequent telomere length.32 Lavretsky et al32 evaluated the effect of brief daily yogic meditation on depressive symptoms and immune cell telomerase activity in a family of dementia caregivers with mild depressive symptoms. Brief daily meditation practice led to significant lower levels of depressive symptoms that was accompanied by an increase in telomerase activity, suggesting improvement in stress-induced cellular aging.6,32
Mind-body therapies
There is increasing interest in improving older adults’ physical and emotional well-being while promoting resilience through stress-reducing lifestyle interventions such as MBTs.33 Because MBTs are often considered a natural and safer option compared to conventional medicine, these interventions are rapidly gaining popularity in the United States.33,34 According to a 2017 National Health Survey, there were 5% to 10% increases in the use of yoga, meditation, and chiropractic care from 2012 to 2017, with growing evidence supporting MBTs as minimally invasive, cost-effective approaches for managing stress and neurocognitive disorders.35 In contrast to pharmacologic approaches, MBTs can be used to train individuals to self-regulate in the face of adversity and stress, thus increasing their resilience.
MBTs can be divided into mindful movement exercises and meditative practices. Mindful movement exercises include yoga, tai chi, and qigong. Meditative practices that do not include movement include progressive relaxation, mindfulness, meditation, and acceptance therapies. On average, both mindful movement exercise (eg, yoga) and multicomponent mindfulness-based interventions (eg, mindfulness-based cognitive therapy, mindfulness-based stress reduction [MBSR], and mindfulness-based relapse prevention) can be as effective as other active treatments for psychiatric disorders such as MDD, anxiety, and substance use disorders.36,37 MBSR specifically has been shown to increase empathy, self-control, self-compassion, relationship quality, mindfulness, and spirituality as well as decrease rumination in healthy older adults.38 This suggests that MBSR can help strengthen the 4 factors of resilience.
Continue to: Research has also begun...
Research has also begun to evaluate the neurobiological mechanisms by which meditative therapies enhance resilience in mental health disorders, and several promising mechanistic domains (neural, hormonal, immune, cellular, and cardiovascular) have been identified.39 The physical yoga discipline includes asanas (postures), pranayama (breathing techniques), and dhyana (meditation). With the inclusion of mindfulness training, yoga involves the practice of meditation as well as the dynamic combination of proprioceptive and interoceptive awareness, resulting in both attention and profound focus.40 Dedicated yoga practice allows an individual to develop skills to withdraw the senses (pratyahara), concentrate the mind (dharana), and establish unwavering awareness (dhyana).41 The physical and cognitive benefits associated with yoga and mindfulness may be due to mechanisms including pranayama and activation of the parasympathetic nervous system; meditative or contemplative practices; increased body perception; stronger functional connectivity within the basal ganglia; or neuroplastic effects of increased grey matter volume and amygdala with regional enlargement.41 The new learning aspect of yoga practice may contribute to enhancing or improving various aspects of cognition, although the mechanisms are yet to be clarified.
Continued research in this area will promote the integration of MBTs into mainstream clinical practice and help alleviate the increased chronic health burden of an aging population. In the face of the COVID-19 pandemic, public interest in improving resilience and mental health42 can be supported by MBTs that can improve coping with the stress of the pandemic and enhance critical organ function (eg, lungs, heart, brain).43,44 As a result of these limitations, many resources and health care services have used telehealth and virtual platforms to adapt to these challenges and continue offering MBTs.45
Enhancing resilience to improve clinical outcomes
Increasing our understanding of clinical, neurocognitive, and neurobiological markers of resilience in older adults with and without depression could inform the development of interventions that treat and prevent mood and cognitive disorders of aging. Furthermore, stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, leading to greater resilience. Complementary use of MBTs combined with standard antidepressant treatment may allow for additional improvement in clinical outcomes of LLD, including resilience, quality of life, general health, and cognitive function. Additional research testing the efficacy of those interventions designed to improve resilience in older adults with mood and mental disorders is needed.
Bottom Line
Identifying the clinical, neurocognitive, and neurobiological biomarkers of resilience in late-life depression could aid in the development of targeted interventions that treat and prevent mood and cognitive disorders of aging. Mind-body interventions can help boost resilience and improve outcomes in geriatric patients with mood and cognitive disorders.
Related Resources
- Lavretsky H. Resilience and Aging: Research and Practice. Johns Hopkins University Press; 2014.
- Lavretsky H, Sajatovic M, Reynolds CF, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2016.
- Eyre HA, Berk M, Lavretsky H, et al, eds. Convergence Mental Health: A Transdisciplinary Approach to Innovation. Oxford University Press; 2021.
- UCLA Jane & Terry Semel Institute for Neuroscience & Human Behavior. Late-life Depression, Stress, and Wellness Research Program. https://www.semel.ucla.edu/latelife
Resilience has been defined as the ability to adapt and thrive in the face of adversity, acute stress, or trauma.1 Originally conceived as an inborn trait characteristic, resilience is now conceptualized as a dynamic, multidimensional capacity influenced by the interactions between internal factors (eg, personality, cognitive capacity, physical health) and environmental resources (eg, social status, financial stability).2,3 Resilience in older adults (typically defined as age ≥65) can improve the prognosis and outcomes for physical and mental conditions.4 The construct is closely aligned with “successful aging” and can be fostered in older adults, leading to improved physical and mental health and well-being.5
While initially resilience was conceptualized as the opposite of depressive states, recent research has identified resilience in the context of major depressive disorder (MDD) as the net effects of various psychosocial and biological variables that decrease the risk of onset, relapse, or depressive illness severity and increase the probability or speed of recovery.6 Late-life depression (LLD) in adults age >65 is a common and debilitating disease, often leading to decreased psychological well-being, increased cognitive decline, and excess mortality.7,8 LLD is associated with several factors, such as cerebrovascular disease, neurodegenerative disease, and inflammation, all of which could contribute to brain vulnerability and an increased risk of depression.9 Physical and cognitive engagement, physical activity, and high brain reserve have been shown to confer resilience to affective and cognitive changes in older adults, despite brain vulnerability.9
The greatest levels of resilience have been observed in individuals in their fifth decade of life and later,4,10 with high levels of resilience significantly contributing to longevity5; however, little is known about which factors contribute to heterogeneity in resilience characteristics and outcomes.4 Furthermore, the concept of resilience continues to raise numerous questions, including:
- how resilience should be measured or defined
- what factors promote or deter the development of resilience
- the effects of resilience on various health and psychological outcomes
- which interventions are effective in enhancing resilience in older adults.4
In this article, we describe resilience in older adults with LLD, its clinical and neurocognitive correlates, and underlying neurobiological and immunological biomarkers. We also examine resilience-building interventions, such as mind-body therapies (MBTs), that have been shown to enhance resilience by promoting positive perceptions of difficult experiences and challenges.
Clinical and neurocognitive correlates of resilience
Resilience varies substantially among older adults with LLD as well as across the lifespan of an individual.11 Identifying clinical components and predictors of resilience may usefully inform the development and testing of interventions to prevent and treat LLD.11 One tool widely used to measure resilience—the self-report Connor-Davidson Resilience Scale (CD-RISC)12— has been found to have clinically relevant characteristics.1,11 Using data from 337 older adults with LLD, Laird et al11 performed an exploratory factor analysis of the CD-RISC and found a 4-factor model:
- grit
- adaptive coping self-efficacy
- accommodative coping self-efficacy
- spirituality.1,11
Having a strong sense of purpose and not being easily discouraged by failure were items characteristic of grit.1,11 The preference to take the lead in problem-solving was typical of items loading on adaptive coping self-efficacy, while accommodative coping self-efficacy measured flexibility, cognitive reframing, a sense of humor, and acceptance in the face of uncontrollable stress.1,11 Finally, the belief that “things happen for a reason” and that “sometimes fate or God can help me” are characteristics of spirituality. 1,11 Using a multivariate model, the greatest variance in total resilience scores was explained by less depression, less apathy, higher quality of life, non-White race, and, somewhat counterintuitively, greater medical comorbidity.1,11 Thus, interventions designed to help older adults cultivate grit, active coping, accommodative coping, and spirituality may enhance resilience in LLD.
Resilience may also be positively associated with cognitive functioning and could be neuroprotective in LLD.13 Laird et al13 investigated associations between baseline resilience and several domains of neurocognitive functioning in 288 older adults with LLD. Several positive associations were found between measured language performance and total resilience, active coping, and accommodative coping.13 Additionally, total resilience and accommodative coping were significantly associated with a lower self-reported frequency of forgetfulness, a subjective measure of memory used in this study.13 Together, these results suggest that interventions targeting language might be useful to improve coping in LLD.13 Another interesting finding was that the resilience subdomain of spirituality was negatively associated with memory, language, and executive functioning performance.13 A distinction must be made between religious attendance (eg, regular attendance at religious institutions) vs religious beliefs, which may account for the previously reported associations between spirituality and improved cognition.13
Continue to: Self-reported resilience...
Self-reported resilience may also predict greater responsivity to antidepressant medication in patients with LLD.14 Older adults with LLD and greater self-reported baseline resilience were more likely to experience improvement or remission from depression with antidepressant treatment.14 This is congruent with conceptualizations of resilience as “the ability to adapt to and recover from stress.”14,15 Of the 4 identified resilience factors (grit, adaptive coping, accommodative coping, and spirituality), it appears that accommodative coping predicts LLD treatment response and remission.14 The unique ability to accommodate is associated with better mental health outcomes in the face of uncontrollable stress.14,16-18 Older adults appear to engage in more accommodative coping due to frequent uncontrollable stress and aging-related physiological changes (eg, sleep changes, chronic pain, declining cognition). This could make accommodative coping especially important in this population.14,19
The Figure, adapted from Weisenbach et al,9 exhibits factors that contribute to LLD, including cerebrovascular disease, neurodegeneration, and chronic inflammation, all of which can lead to a vulnerable aging brain that is at higher risk for depression, particularly within the context of stress. Clinical and neurocognitive factors associated with resilience can help buffer vulnerable brains from developing depression.

Neurobiological biomarkers of resilience in
Gross anatomical indicators: Findings from neuroimaging
The neurobiology underlying psychological resilience involves brain networks associated with stress response, negative affect, and emotional control.19 Increased amygdala reactivity and amygdala frontal connectivity are often implicated in neurobiological models of resilience.20 Leaver et al20 correlated psychological resilience measures with amygdala function in 48 depressed vs nondepressed individuals using functional magnetic resonance imaging. Specifically, they targeted the basolateral, centromedial, and superficial nuclei groups of the amygdala while comparing the 2 groups based on resilience scores (CD-RISC), depressive symptom severity, and depression status.20 A significant correlation was identified between resilience and connectivity between the superficial group of amygdala nuclei and the ventral default mode network (VDMN).20 High levels of psychological resilience were associated with lower basal amygdala activity and decreased connectivity between amygdala nuclei and the VDMN.20 Additionally, lower depressive symptoms were associated with higher connectivity between the amygdalae and the dorsal frontal networks.20 These results suggest a complex relationship between amygdala activity, dorsal frontal regions, resilience, and LLD.20
Vlasova et al21 further addressed the multifactorial character of psychological resilience. The associations between the 4 factors of resilience and the regional integrity of white matter in older adults with LLD were examined using diffusion-weighted MRI.21 Grit was found to be associated with greater white matter integrity in the genu of the corpus callosum and cingulum bundle in LLD.21 There was also a positive association between grit and fractional anisotropy (FA) in the callosal region connecting the prefrontal cortex and FA in the cingulum fibers.21 However, results regarding the FA in the cingulum fibers did not survive correction for multiple comparisons and should be considered with caution, pending further research.21
Continue to: Stress response biomarkers of resilience
Stress response biomarkers of resilience
Stress response biomarkers include endocrine, immune, and inflammatory indices. Stress has been identified as a factor in inflammatory responses. Stress-related overstimulation of the HPA axis may increase the risk of LLD.22 Numerous studies have demonstrated an association between increased levels of peripheral proinflammatory cytokines and depressive symptoms in older adults.23 Interleukin-6 (IL-6) has been increasingly linked with depressive symptoms and poor memory performance in older adults.9 There also appears to be an interaction of inflammatory and vascular processes predisposing to LLD, as increased levels of IL-6 and C-reactive protein have been associated with higher white matter pathology.9 Additionally, proinflammatory cytokines impact monoamine neurotransmitter pathways, leading to a reduction in tryptophan and serotonin synthesis, disruption of glucocorticoid receptors, and a decrease in hippocampal neurotrophic support.9 Alexopoulos et al24 further explain that a prolonged CNS immune response can affect emotional and cognitive network functions related to LLD and has a role in the etiology of depressive symptoms in older adults.
Cardiovascular comorbidity and autonomic nervous system dysfunction
Many studies have revealed evidence of a bidirectional association between cardiovascular disease and depression.25 Dysregulation of the autonomic nervous system (ANS) is an underlying mechanism that could explain the link between cardiovascular risk and MDD via heart rate variability (HRV), though research examining age-related capacities provide conflicting data.25,26 HRV is a surrogate index of resting cardiac vagal outflow that represents the ability of the ANS to adapt to psychological, social, and physical environmental changes.27 Higher overall HRV is associated with greater self-regulating capacity, including behavioral, cognitive, and emotional control.28 Additionally, higher HRV may serve as a biomarker of resilience to the development of stress-related disorders such as MDD. Recent studies have shown an overall reduction in HRV in older adults with LLD.29 When high- and low-frequency HRV were investigated separately, only low-frequency HRV was significantly reduced in patients with depression.29 One explanation is that older adults with depression have impaired or reduced baroreflex sensitivity and gain, which is often associated with an increased risk of mortality following cardiac events.30 More research is needed to examine the complex processes required to better characterize the correlation between resilience in cardiovascular disease and autonomic dysfunction.
The Box6,31,32 describes the relationship between markers of cellular health and resilience.
Box
Among the biomarkers of resilience, telomere length and telomerase activity serve as biomarkers of biological aging that can differ from the chronological age and mark successful anti-aging, stress-reducing strategies.31 Telomerase, the cellular enzyme that regulates the health of cells when they reproduce (preserving the telomeres, repetitive DNA strands at the ends of chromosomes), is associated with overall cell health and cellular biological age.31 When telomerase is reduced, the telomeres in a cell are clipped, causing the cells to age more rapidly as the telomeres get shorter through the process of cellular reproduction.31 Psychological stress may play a significant role in telomerase production and subsequent telomere length.32 Lavretsky et al32 evaluated the effect of brief daily yogic meditation on depressive symptoms and immune cell telomerase activity in a family of dementia caregivers with mild depressive symptoms. Brief daily meditation practice led to significant lower levels of depressive symptoms that was accompanied by an increase in telomerase activity, suggesting improvement in stress-induced cellular aging.6,32
Mind-body therapies
There is increasing interest in improving older adults’ physical and emotional well-being while promoting resilience through stress-reducing lifestyle interventions such as MBTs.33 Because MBTs are often considered a natural and safer option compared to conventional medicine, these interventions are rapidly gaining popularity in the United States.33,34 According to a 2017 National Health Survey, there were 5% to 10% increases in the use of yoga, meditation, and chiropractic care from 2012 to 2017, with growing evidence supporting MBTs as minimally invasive, cost-effective approaches for managing stress and neurocognitive disorders.35 In contrast to pharmacologic approaches, MBTs can be used to train individuals to self-regulate in the face of adversity and stress, thus increasing their resilience.
MBTs can be divided into mindful movement exercises and meditative practices. Mindful movement exercises include yoga, tai chi, and qigong. Meditative practices that do not include movement include progressive relaxation, mindfulness, meditation, and acceptance therapies. On average, both mindful movement exercise (eg, yoga) and multicomponent mindfulness-based interventions (eg, mindfulness-based cognitive therapy, mindfulness-based stress reduction [MBSR], and mindfulness-based relapse prevention) can be as effective as other active treatments for psychiatric disorders such as MDD, anxiety, and substance use disorders.36,37 MBSR specifically has been shown to increase empathy, self-control, self-compassion, relationship quality, mindfulness, and spirituality as well as decrease rumination in healthy older adults.38 This suggests that MBSR can help strengthen the 4 factors of resilience.
Continue to: Research has also begun...
Research has also begun to evaluate the neurobiological mechanisms by which meditative therapies enhance resilience in mental health disorders, and several promising mechanistic domains (neural, hormonal, immune, cellular, and cardiovascular) have been identified.39 The physical yoga discipline includes asanas (postures), pranayama (breathing techniques), and dhyana (meditation). With the inclusion of mindfulness training, yoga involves the practice of meditation as well as the dynamic combination of proprioceptive and interoceptive awareness, resulting in both attention and profound focus.40 Dedicated yoga practice allows an individual to develop skills to withdraw the senses (pratyahara), concentrate the mind (dharana), and establish unwavering awareness (dhyana).41 The physical and cognitive benefits associated with yoga and mindfulness may be due to mechanisms including pranayama and activation of the parasympathetic nervous system; meditative or contemplative practices; increased body perception; stronger functional connectivity within the basal ganglia; or neuroplastic effects of increased grey matter volume and amygdala with regional enlargement.41 The new learning aspect of yoga practice may contribute to enhancing or improving various aspects of cognition, although the mechanisms are yet to be clarified.
Continued research in this area will promote the integration of MBTs into mainstream clinical practice and help alleviate the increased chronic health burden of an aging population. In the face of the COVID-19 pandemic, public interest in improving resilience and mental health42 can be supported by MBTs that can improve coping with the stress of the pandemic and enhance critical organ function (eg, lungs, heart, brain).43,44 As a result of these limitations, many resources and health care services have used telehealth and virtual platforms to adapt to these challenges and continue offering MBTs.45
Enhancing resilience to improve clinical outcomes
Increasing our understanding of clinical, neurocognitive, and neurobiological markers of resilience in older adults with and without depression could inform the development of interventions that treat and prevent mood and cognitive disorders of aging. Furthermore, stress reduction, decreased inflammation, and improved emotional regulation may have direct neuroplastic effects on the brain, leading to greater resilience. Complementary use of MBTs combined with standard antidepressant treatment may allow for additional improvement in clinical outcomes of LLD, including resilience, quality of life, general health, and cognitive function. Additional research testing the efficacy of those interventions designed to improve resilience in older adults with mood and mental disorders is needed.
Bottom Line
Identifying the clinical, neurocognitive, and neurobiological biomarkers of resilience in late-life depression could aid in the development of targeted interventions that treat and prevent mood and cognitive disorders of aging. Mind-body interventions can help boost resilience and improve outcomes in geriatric patients with mood and cognitive disorders.
Related Resources
- Lavretsky H. Resilience and Aging: Research and Practice. Johns Hopkins University Press; 2014.
- Lavretsky H, Sajatovic M, Reynolds CF, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2016.
- Eyre HA, Berk M, Lavretsky H, et al, eds. Convergence Mental Health: A Transdisciplinary Approach to Innovation. Oxford University Press; 2021.
- UCLA Jane & Terry Semel Institute for Neuroscience & Human Behavior. Late-life Depression, Stress, and Wellness Research Program. https://www.semel.ucla.edu/latelife
1. Reynolds CF. Promoting resilience, reducing depression in older adults. Int Psychogeriatr. 2019;31(2):169-171.
2. Windle G. What is resilience? A review and concept analysis. Rev Clin Gerontol. 2011;21(2):152-169.
3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79-82.
4. Dunn LB, Predescu I. Resilience: a rich concept in need of research comment on: “Neurocognitive correlates of resilience in late-life depression” (by Laird et al.). Am J Geriatr Psychiatry. 2019;27(1):18-20.
5. Harmell AL, Kamat R, Jeste DV, et al. Resilience-building interventions for successful and positive aging. In: Lavretsky H, Sajatovic M, Reynolds C III, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2015:305-316.
6. Laird KT, Krause B, Funes C, et al. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9(1):88.
7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
8. Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13(11):746-752.
9. Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463.
10. Southwick SM, Litz BT, Charney D, et al. Resilience and Mental Health: Challenges Across the Lifespan. Cambridge University Press; 2011.
11. Laird KT, Lavretsky H, Paholpak P, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31(2):193-202.
12. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Laird KT, Lavretsky H, Wu P, et al. Neurocognitive correlates of resilience in late-life depression. Am J Geriatr Psychiatry. 2019;27(1):12-17.
14. Laird KT, Lavretsky H, St Cyr N, et al. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33(12):1596-1603.
15. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev. 2015;41:49-60.
16. Boerner K. Adaptation to disability among middle-aged and older adults: the role of assimilative and accommodative coping. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P35-P42.
17. Zakowski SG, Hall MH, Klein LC, et al. Appraised control, coping, and stress in a community sample: a test of the goodness-of-fit hypothesis. Ann Behav Med. 2001;23(3):158-165.
18. Cheng C, Lau HB, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140(6):1582-1607.
19. Stokes SA, Gordon SE. Common stressors experienced by the well elderly. Clinical implications. J Gerontol Nurs. 2003;29(5):38-46.
20. Leaver AM, Yang H, Siddarth P, et al. Resilience and amygdala function in older healthy and depressed adults. J Affect Disord. 2018;237:27-34.
21. Vlasova RM, Siddarth P, Krause B, et al. Resilience and white matter integrity in geriatric depression. Am J Geriatr Psychiatry. 2018;26(8):874-883.
22. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15(4):379-400.
23. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022.
24. Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109-1118.
25. Carney RM, Freedland KE, Sheline YI, et al. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20(3):196-200.
26. Carney RM, Freedland KE, Steinmeyer BC, et al. Nighttime heart rate predicts response to depression treatment in patients with coronary heart disease. J Affect Disord. 2016;200:165-171.
27. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psych. 2006;10(3):229-240.
28. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233-255.
29. Brown L, Karmakar C, Gray R, et al. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456-466.
30. La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(1901):478-484.
31. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306-322.
32. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
33. Siddiqui MJ, Min CS, Verma RK, et al. Role of complementary and alternative medicine in geriatric care: a mini review. Pharmacogn Rev. 2014;8(16):81-87.
34. Nguyen SA, Lavretsky H. Emerging complementary and integrative therapies for geriatric mental health. Curr Treat Options Psychiatry. 2020;7(4):447-470.
35. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
36. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749.
37. Ramadas E, de Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133.
38. Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593-600.
39. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
40. Chobe S, Chobe M, Metri K, et al. Impact of yoga on cognition and mental health among elderly: a systematic review. Complement Ther Med. 2020;52:102421.
41. Brunner D, Abramovitch A, Etherton J. A yoga program for cognitive enhancement. PLoS One. 2017;12(8):e0182366.
42. Dai J, Sang X, Menhas R, et al. The influence of COVID-19 pandemic on physical health-psychological health, physical activity, and overall well-being: the mediating role of emotional regulation. Front Psychol. 2021;12:667461.
43. Grolli RE, Mingoti MED, Bertollo AG, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol Neurobiol. 2021;58(5):1905-1916.
44. Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658.
45. Pandya SP. Older women and wellbeing through the pandemic: examining the effect of daily online yoga lessons. Health Care Women Int. 2021;42(11):1255-1278.
1. Reynolds CF. Promoting resilience, reducing depression in older adults. Int Psychogeriatr. 2019;31(2):169-171.
2. Windle G. What is resilience? A review and concept analysis. Rev Clin Gerontol. 2011;21(2):152-169.
3. Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79-82.
4. Dunn LB, Predescu I. Resilience: a rich concept in need of research comment on: “Neurocognitive correlates of resilience in late-life depression” (by Laird et al.). Am J Geriatr Psychiatry. 2019;27(1):18-20.
5. Harmell AL, Kamat R, Jeste DV, et al. Resilience-building interventions for successful and positive aging. In: Lavretsky H, Sajatovic M, Reynolds C III, eds. Complementary and Integrative Therapies for Mental Health and Aging. Oxford University Press; 2015:305-316.
6. Laird KT, Krause B, Funes C, et al. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9(1):88.
7. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331.
8. Callahan CM, Wolinsky FD, Stump TE, et al. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med. 1998;13(11):746-752.
9. Weisenbach SL, Kumar A. Current understanding of the neurobiology and longitudinal course of geriatric depression. Curr Psychiatry Rep. 2014;16(9):463.
10. Southwick SM, Litz BT, Charney D, et al. Resilience and Mental Health: Challenges Across the Lifespan. Cambridge University Press; 2011.
11. Laird KT, Lavretsky H, Paholpak P, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31(2):193-202.
12. Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82.
13. Laird KT, Lavretsky H, Wu P, et al. Neurocognitive correlates of resilience in late-life depression. Am J Geriatr Psychiatry. 2019;27(1):12-17.
14. Laird KT, Lavretsky H, St Cyr N, et al. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33(12):1596-1603.
15. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev. 2015;41:49-60.
16. Boerner K. Adaptation to disability among middle-aged and older adults: the role of assimilative and accommodative coping. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P35-P42.
17. Zakowski SG, Hall MH, Klein LC, et al. Appraised control, coping, and stress in a community sample: a test of the goodness-of-fit hypothesis. Ann Behav Med. 2001;23(3):158-165.
18. Cheng C, Lau HB, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140(6):1582-1607.
19. Stokes SA, Gordon SE. Common stressors experienced by the well elderly. Clinical implications. J Gerontol Nurs. 2003;29(5):38-46.
20. Leaver AM, Yang H, Siddarth P, et al. Resilience and amygdala function in older healthy and depressed adults. J Affect Disord. 2018;237:27-34.
21. Vlasova RM, Siddarth P, Krause B, et al. Resilience and white matter integrity in geriatric depression. Am J Geriatr Psychiatry. 2018;26(8):874-883.
22. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opin Ther Targets. 2011;15(4):379-400.
23. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022.
24. Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26(11):1109-1118.
25. Carney RM, Freedland KE, Sheline YI, et al. Depression and coronary heart disease: a review for cardiologists. Clin Cardiol. 1997;20(3):196-200.
26. Carney RM, Freedland KE, Steinmeyer BC, et al. Nighttime heart rate predicts response to depression treatment in patients with coronary heart disease. J Affect Disord. 2016;200:165-171.
27. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psych. 2006;10(3):229-240.
28. Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233-255.
29. Brown L, Karmakar C, Gray R, et al. Heart rate variability alterations in late life depression: a meta-analysis. J Affect Disord. 2018;235:456-466.
30. La Rovere MT, Bigger JT Jr, Marcus FI, et al. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351(1901):478-484.
31. Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. 2021;184(2):306-322.
32. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
33. Siddiqui MJ, Min CS, Verma RK, et al. Role of complementary and alternative medicine in geriatric care: a mini review. Pharmacogn Rev. 2014;8(16):81-87.
34. Nguyen SA, Lavretsky H. Emerging complementary and integrative therapies for geriatric mental health. Curr Treat Options Psychiatry. 2020;7(4):447-470.
35. Clarke TC, Barnes PM, Black LI, et al. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1-8.
36. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749.
37. Ramadas E, de Lima MP, Caetano T, et al. Effectiveness of mindfulness-based relapse prevention in individuals with substance use disorders: a systematic review. Behav Sci (Basel). 2021;11(10):133.
38. Chiesa A, Serretti A. Mindfulness-based stress reduction for stress management in healthy people: a review and meta-analysis. J Altern Complement Med. 2009;15(5):593-600.
39. Strauss C, Cavanagh K, Oliver A, et al. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110.
40. Chobe S, Chobe M, Metri K, et al. Impact of yoga on cognition and mental health among elderly: a systematic review. Complement Ther Med. 2020;52:102421.
41. Brunner D, Abramovitch A, Etherton J. A yoga program for cognitive enhancement. PLoS One. 2017;12(8):e0182366.
42. Dai J, Sang X, Menhas R, et al. The influence of COVID-19 pandemic on physical health-psychological health, physical activity, and overall well-being: the mediating role of emotional regulation. Front Psychol. 2021;12:667461.
43. Grolli RE, Mingoti MED, Bertollo AG, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol Neurobiol. 2021;58(5):1905-1916.
44. Johansson A, Mohamed MS, Moulin TC, et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J Neuroimmunol. 2021;358:577658.
45. Pandya SP. Older women and wellbeing through the pandemic: examining the effect of daily online yoga lessons. Health Care Women Int. 2021;42(11):1255-1278.
Late-life depression: Managing mood in patients with vascular disease
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
Newly diagnosed major depressive disorder (MDD) in patients age ≥65 often has a vascular component. Concomitant cerebrovascular disease (CVD) does not substantially alter the management of late-life depression, but it may affect presenting symptoms, complicate the diagnosis, and influence treatment outcomes.
The relationship between depression and CVD progression remains to be fully explained, and no disease-specific interventions exist to address vascular depression’s pathophysiology. When planning treatment, however, one can draw inferences from existing studies. This article reviews the evidence on late-life depression accompanied by CVD and vascular risk factors, the “vascular depression” concept, and approaches to primary and secondary prevention and treatment.
CVD etiology of depression
Vascular depression constitutes a subgroup of late-life depression, usually associated with neuroimaging abnormalities in the basal ganglia and white matter on MRI.1 The cause of the structural brain changes is thought to be sclerosis in the small arterioles.2 These end-artery vessels may be particularly susceptible to pulse-wave changes caused by arterial rigidity or hypertension.
Alexopoulos et al1 and Krishnan et al3 proposed the concept of vascular depression on the premise that CVD may be etiologically related to geriatric depressive syndromes. Krishnan et al3 examined clinical and demographic characteristics of depressed elderly patients with vascular lesions on brain MRI. Those with clinically defined vascular depression experienced greater cognitive dysfunction, disability, and psychomotor retardation but less agitation and guilt feelings than patients with nonvascular depression.
Clinically, vascular depression resembles a medial frontal lobe syndrome, with prominent psychomotor retardation, apathy, and pronounced disability.4 Depression with vascular stigmata or cerebrovascular lesions on neuroimaging is characterized by poor outcomes, including persistent depressive symptoms, unstable remission, and increased risk for dementia.5,6 Patients with depression and subcortical vascular lesions have been shown to respond poorly to antidepressants.6
Impaired brain function also may predispose to geriatric depression, described by Alexopoulos as “depression-executive dysfunction syndrome of late life.”7 This common syndrome’s presentation—psychomotor retardation, lack of interest, limited depressive ideation and insight, and prominent disability—is consistent with its underlying abnormalities.5 Executive dysfunction also predicts limited response to antidepressants.8 Thus, the presentation and course of depression-executive dysfunction syndrome are consistent with those of subcortical ischemic depression.
Neuroimaging support
The vascular depression hypothesis is supported by observations related to MRI hyperintensities (HI):
- CT and MRI studies identify HI in persons with late-life depression.
- HI are associated with age and cerebrovascular risk factors.
- Pathophysiologic evidence indicates that HI are associated with widespread diminution in cerebral perfusion.9
Neuropathologic correlates of HI are diverse and represent ischemic changes, together with demyelination, edema, and gliosis.9-11 The putative link between HI and vascular disease is central to the vascular theory of depression.
In a study of 56 patients age ≥50 meeting DSM-III-R criteria for MDD, Fujikawa et al12 reported “silent cerebral infarctions” on MRI in 60% of patients. High rates of abnormalities consistently have been observed on MRIs of older adults with MDD,10,11 and these can be classified into 3 types (Figure):
- Periventricular HI are halos or rims adjacent to ventricles that in severe forms may invade surrounding deep white matter.
- Deep white matter HI are single, patchy, or confluent foci observed in subcortical white matter.
- Deep gray matter HI may be found, particularly in the basal ganglia, thalamus, and pons.9
These leukoaraiosis (or encephalomalacia) occur more frequently in patients with geriatric depression than in normal controls13 or patients with Alzheimer’s disease14 and may be comparable to the rate associated with vascular dementia.15 Observations in older adults11 suggest that diminished brain volume (especially in frontal regions) and HI may provide additive, albeit autonomous, pathways to late-life MDD. Vascular and nonvascular medical comorbidity contribute to HI, which in turn facilitate MDD.
Figure: Subcortical cerebrovascular disease in late-life depression
Structural MRIs of elderly adults with major depressive disorder consistently show high rates of brain abnormalities. Subcortical white matter abnormalities manifest as (1) periventricular hyperintensities [halos or rims adjacent to ventricles] and (2) deep white matter hyperintensities [single, patchy, or confluent foci]. Strategic subcortical gray matter infarctions (3) are observed, particularly in the basal ganglia, thalamus, and pons.
Bidirectional relationship
The relationship between depression and cardiovascular disease appears to be bidirectional:
- Depression may be the first clinical expression of an underlying cardiovascular disease, which is expressed as an increased risk for ischemic events.
- Depression itself, whether or not contributed by a silent cardiovascular disease, increases the risk of vascular damage, which in turn further promotes depression.
- Vascular pathogenesis affecting heart and brain is likely to increase the risk for depression through a variety of mechanisms.
Post-stroke depression (PSD) occurs within 12 to 24 months after a cerebrovascular accident.13 DSM-IV-TR categorizes PSD as a “mood disorder due to a general medical condition with the specifiers of (a) depressive features, (b) major depressive-like episodes, or (c) mixed features.”
Although important in depression’s pathophysiology, the location of stroke lesions is not the exclusive etiologic factor. Personal diathesis for depression, psychosocial factors, and physical and social impairment related to post-stroke neurologic deficits also may contribute to PSD.16
PSD patients with right-sided lesions often have family histories of depressive illness.17 Different serotonergic mechanisms might be responsible for depressive illness associated with right-sided vs left-sided lesions. This notion is supported by observed lateralized changes in serotonin type-2 (5-HT2) receptors18 and the influence of lateralized lesions on prolactin responsivity to d-fenfluramine challenge in PSD.19 Damage closer to the frontal lobes is likely to affect catecholamine-mediated brain activity.
The 8-year Framingham study20 examined the risk of developing cerebrovascular events in persons age ≤65 vs those age >65. Subjects age ≤65 with significant depressive symptoms—Center for Epidemiologic Studies Depression scale score >1621—were 4 times more likely to develop stroke or transient ischemic attack compared with the same age group without depression. Another study found a link between depression and stroke risk across the adult age range.22 Mechanisms by which depressive symptoms may predispose to stroke are not fully known, but depression has been shown to affect autonomic function and platelet activation.23
CHD and depression. In the United States, approximately 20% of coronary heart disease (CHD) patients have clinically significant depressive symptoms.24 A history of depression also has been shown to increase the relative risk of developing CHD by >80%.25
The association between depression and CHD is unclear but likely includes:
- direct biological mechanisms such as autonomic dysfunction and dysregulated inflammation
- behavioral factors such as smoking or poor self-care (Table 1).
A recent analysis of 13 cross-sectional studies26 suggests that reduced heart rate variability (HRV) related to autonomic dysfunction may be the link between depression and CHD risk. The studies’ effect sizes were small, however, and their methodologies varied considerably.
C-reactive protein (CRP), interleukin-6, tumor necrosis factor-α (TNF-α), and fibrinogen are inflammatory markers. In a 2-year follow-up study, Frasure-Smith et al27 investigated the relationship between depression and inflammatory markers in 741 patients (602 male) with acute coronary syndrome. Two months after an acute coronary event, depressive symptoms and elevated CRP levels were overlapping risk factors for future cardiac events in men.
Carney et al28 showed that fibrinogen was most associated with altered heart rate variability in depressed CHD patients and proposed deficits in parasympathetic modulation of immunity and coagulation as the cause. In contrast, Whooley et al29 found no association between major depression and inflammatory markers—including CRP, fibrinogen, and interleukin-6—in 984 outpatients with CHD. Differences in assessment scales and sample heterogeneity may have contributed to these disparate findings.
Diabetes and depression. As with CHD, a bidirectional relationship exists between depression and diabetes mellitus, although depression is only a modest risk factor for diabetes.30 Possible explanations include hypercortisolemia and increased inflammation resulting in increased insulin resistance and metabolic syndrome.
Table 1
Shared risk factors for depression and heart disease
| Decreased heart rate variability |
| Vascular inflammation (increased interleukin-6 and C-reactive protein) |
| Endothelial dysfunction |
| Platelet dysfunction |
| Atherosclerosis |
| Dyslipidemia |
| Smoking |
| Source: References 26-29 |
Diagnosis of vascular depression
Vascular depression is characterized by a clinical diagnosis of DSM-IV-TR-defined MDD, dysthymia, or depression not-otherwise-specified, accompanied by:
- evidence of CVD or
- known vascular risk factors (hypertension, diabetes, hyperlipidemia, stroke, heart failure, etc.).
In performing thorough neurologic, neuropsychiatric, and neuropsychological examinations, look for soft neurologic signs with regional weakness, apathy, and executive dysfunction. Useful bedside scales include the clock-drawing test, word list generation, brief dementia screens, and the Apathy Evaluation Scale.31
CT or MRI can provide supportive evidence by demonstrating signs of subcortical or cortical stroke. Neuroimaging studies may not be necessary, however, when depression onset is temporally associated with strong physical evidence of a stroke (such as falling, peripheral muscle weakness, or incontinence).
Treating depression symptoms
When treating vascular depression, clinical goals are to ameliorate affective symptoms, improve quality of life, and help patients perform activities of daily living (Table 2).
Psychosocial interventions. When depression is less than severe, consider psychosocial interventions as first-line treatment. Investigate environmental factors such as financial and marital problems or loneliness in patients’ depressive symptoms, and develop corresponding interventions—such as education, nutrition, exercise, socialization, or pain and stress management. Cognitive rehabilitation training and cognitive-behavioral therapy can reduce cognitive impairment and associated depression.
Antidepressants. A trial of antidepressant therapy is advisable for moderate-to-severe, chronic vascular depression, even though comorbid CVD may diminish the antidepressant response. In elderly patients, start with one-third to one-half the usual adult antidepressant dosage and increase while balancing efficacy and tolerability.
Match the medication’s side-effect profile with the patient’s target symptoms (such as anxiety vs apathy).32 Selective serotonin reuptake inhibitors are probably first-line, but bupropion, venlafaxine, duloxetine, or mirtazapine may be more appropriate for some patients (Table 3).
In PSD, nortriptyline has shown a significantly greater response rate than fluoxetine or placebo in improving anxiety symptoms and recovery of activities of daily living.33 Tricyclic antidepressants’ anticholinergic properties are a safety concern in patients with heart disease, however. In general, avoid agents with substantial anticholinergic effects in elderly patients to minimize the risk of cognitive impairment and other side effects, such as urinary retention or worsening of glaucoma.
Because of the substantial risk of postural hypotension, nonselective monoamine oxidase inhibitors are probably appropriate only for geriatric patients with highly treatment-refractory depression. Dopaminergic agents such as methylphenidate in a relatively moderate dose (such as 5 to 20 mg/d) may improve apathy and social withdrawal, but research into their use in vascular depression is lacking.
Other options. Clinical experience suggests that electroconvulsive therapy (ECT) is effective for patients who do not respond to antidepressants. ECT appears quite safe in older patients, especially if not used in the first 6 months post-stroke. Strategies to reduce the risk of cognitive side effects include:
- 2 rather than 3 weekly treatments
- unilateral or bifrontal rather than bilateral treatments
- frontal lead placement.34
In the only study of transcranial magnetic stimulation (TMS) for geriatric patients with depression (N=92), those with treatment-resistant vascular depression showed higher remission rates with TMS (27.3%) compared with sham TMS (3.5%). Response rates to TMS were negatively correlated with advancing age and positively correlated with higher frontal gray matter volumes.35
Fish oil or vitamin B complex may be used to manage hyperlipidemia or nutritional deficiencies.36 Herbal preparations such as St. John’s wort (Hypericum perforatum) or S-adenosyl-L-methionine (SAMe) have shown some efficacy in adults with MDD, but further study is needed.
Table 2
Clinical management of late-life vascular depression
| Decision point | Assessment/intervention |
|---|---|
| Diagnosis | Apply DSM-IV-TR diagnostic criteria based on results of comprehensive assessment (neuropsychiatric, neuropsychological, structural neuroimaging, vascular and genetic risk factors) |
| Prevention | Identify and treat modifiable risk factors for the development or worsening of cerebrovascular disease, especially in high-risk populations (Table 4) |
| Treatment goals | Target 1: Achieve remission of depressive symptoms, improved cognition and function Target 2: Maintain remission and prevent relapse |
| Managing psychological and behavioral symptoms | Step 1: Consider psychotherapy addressing existing stressors and environmental management in patients with mild-to-moderate depression Step 2: If depression is severe or Step 1 is ineffective, an antidepressant trial* is highly recommended (Table 3); consider ECT or TMS in severe cases |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | |
| ECT: electroconvulsive therapy; TMS: transcranial magnetic stimulation | |
Table 3
Recommended antidepressant dosing
for elderly patients with vascular depression*
| Drug | Starting daily dosage (usual therapeutic range) | Side effect profile (patient characteristics) |
|---|---|---|
| SSRIs | ||
| Escitalopram | 5 mg (10 to 20 mg) | Nausea, headaches, GI upset, insomnia, anxiety |
| Fluoxetine | 10 mg (10 to 60 mg) | |
| Paroxetine | 10 mg (10 to 30 mg) | |
| Sertraline | 25 mg (50 to 150 mg) | |
| Others | ||
| Bupropion | 75 mg (75 to 300 mg) | GI upset, anxiety (may be useful for patients with high apathy) |
| Mirtazapine | 7.5 mg (15 to 45 mg) | Sedation, weight gain (may be useful for patients with severe insomnia or anorexia) |
| Venlafaxine | 37.5 mg (75 to 300 mg) | Nausea, headaches, anxiety, blood pressure elevation, insomnia (may be useful for patients with chronic pain) |
| Duloxetine | 20 mg (30 to 120 mg) | |
| *Avoid medications that could worsen cognition or motor functioning, such as tricyclic antidepressants or neuroleptics | ||
| GI: gastrointestinal; SSRIs: selective serotonin reuptake inhibitors | ||
Treating vascular factors
In addition to treating your patients’ depressive symptoms, collaborate with their primary care physicians to modify physiologic and behavioral factors that increase the risk for vascular injury—such as hypertension, diabetes mellitus, cigarette smoking, and hyperlipidemia. All can be controlled in presymptomatic or mildly symptomatic stages (Table 4).
Anticoagulation. In appropriate patients, anticoagulation can prevent thromboembolic strokes, although risks such as increased hemorrhagic complications must be considered.37 In elderly adults, base treatment decisions on individual risk factors, goals of treatment, and quality-of-life expectancy. In a study of low-dose aspirin (81 mg/d) and low-intensity oral anticoagulation in men at risk of cardiovascular disease, verbal fluency and mental flexibility were significantly better in men taking antithrombotic medications (especially aspirin) than in those taking placebo.38
Antihypertensives and statins. Patients with vascular depression may benefit from calcium channel blockers or angiotensin-converting enzyme (ACE) inhibitors for hypertension and HMG-CoA reductase inhibitors (statins) for hyperlipidemia. Statins seem to decrease the generation of amyloid precursor protein, the neuronal secretion of β-amyloid, and cholesterol synthesis.39 Some epidemiologic studies suggest an association between statin use for cholesterol reduction and reduced prevalence of Alzheimer’s disease and vascular dementia.40
Potential preventive strategies are not without controversy, however:
- Beta blockers and ACE inhibitors have been linked to depression, although the evidence has been conflicting.
- Lipid-lowering therapies and calcium-channel blockers have been linked to an increased risk of suicide.41
- A more recent population-based study did not support an association between an increased risk of suicide and cardiovascular drugs (except perhaps for angiotensin-receptor antagonists).42
Table 4
Preventing vascular causes of late-life depression
| Decision point | Assessment/intervention | Comment |
|---|---|---|
| Primary, secondary prevention of stroke, vascular depression, and cognitive impairment | Identify and treat modifiable risk factors (hypertension, alcohol use, smoking, hyperlipidemia, diabetes mellitus), especially in high-risk patients | Consider as high-risk patients having ≥1 of these features: age >50; male gender; Asian, Hispanic, or African-American heritage; low educational achievement; concurrent vascular risk factors |
| Tertiary prevention of worsened illness in patients with established vascular disease | Intensively treat vascular risk factors | Collaborate with primary care physician to manage arterial hypertension, myocardial infarction, atrial fibrillation, coronary heart disease, diabetes, atherosclerosis, hyperlipidemia, obesity, and smoking |
| Rapidly identify and treat acute stroke to limit ischemic brain changes and promote recovery | ||
| Prevent stroke recurrence by aggressively treating vascular risk factors | Let CVD etiology guide treatment | |
| CVD: cerebrovascular disease | ||
| Source: Adapted from Lavretsky H. Diagnosis and treatment of vascular dementia. Directions in Psychiatry. 2006;26(1):49-68 | ||
Related resources
- Lavretsky H, Chui H. Vascular dementia. In: Agronin ME, Maletta GJ, eds. Principles and practice of geriatric psychiatry. New York, NY: Lippincott, Williams, and Wilkins; 2005: 301-310.
- Baldwin RC, O’Brien J. Vascular basis of late-onset depressive disorder. Br J Psychiatry. 2002;180:157-160.
- Kendler KS, Gardner CO, Fiske A, et al. Major depression and coronary heart disease in the Swedish twin registry. Arch Gen Psychiatry. 2008;66(8):857-863.
Drug brand names
- Bupropion • Wellbutrin
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Methylphenidate • Ritalin, Concerta, others
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Lavretsky receives grant/research support from Forest Research Institute and is a consultant to Forest Laboratories, Myriad Pharmaceuticals, and Accera, Inc.
Dr. Meeks reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work was supported by National Institute of Health grants R01 MH077650 and R-21 AT003480 (Dr. Lavretsky), the U.S. Department of Health and Human Services, Health Resources and Services Administration (Geriatric Academic Career Award), and the Sam and Rose Stein Institute for Research on Aging (Dr. Meeks).
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
1. Alexopoulos GS, Meyers BS, Young RC, et al. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565.
2. Newberg AR, Davydow DS, Lee HB. Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. Int Rev Psychiatry. 2006;18:433-441.
3. Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501.
4. Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;5(4):390-397.
5. Alexopoulos GS, Kiosses DN, Klimstra S, et al. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-102.
6. Taylor WD, Steffens DC, Krishnan KR. Psychiatric disease in the twenty-first century: the case for subcortical ischemic depression. Biol Psychiatry. 2006;60(12):1299-1303.
7. Alexopoulos GS. The depression-executive dysfunction syndrome of late life: a specific target for D3 receptor agonists? Am J Geriatr Psychiatry. 2001;9:1-8.
8. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961-1970.
9. Sackeim H. Brain structure and function in late-life depression. In: Morihisa JM, ed. Advances in brain imaging. Arlington, VA: American Psychiatric Publishing, Inc.; 2001:83–122.
10. Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264-274.
11. Kumar A, Mintz J, Bilker W, et al. Autonomous neurobiological pathways to late-life major depressive disorder: clinical and pathophysiological implications. Neuropsychopharmacology. 2002;26:229-236.
12. Fujikawa T, Yamawaki S, Fujita Y, et al. [Clinical study of correlation pre-senile, senile depressive state with silent cerebral infarction—MRI findings and its distribution]. Seishin Shinkeigaku Zasshi. 1992;94(9):851-863.
13. Kumar A, Cummings J. Depression in neurodegenerative disorders and related conditions in Alzheimer’s disease and related conditions. In: Gothier S, Cummings J, eds. Alzheimer’s disease and related disorders. London, UK: Martin Dunitz; 2001:123-141.
14. Erkinjuntti T, Gao F, Lee DH, et al. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994;51:260-268.
15. Zubenko G, Sullivan P, Nelson J, et al. Brain imaging abnormalities in mental disorders of late life. Arch Neurol. 1990;47:1107-1111.
16. Birkett DP. The psychiatry of stroke. Arlington, VA: American Psychiatric Publishing, Inc.; 1996.
17. Robinson PG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1-14.
18. Mayberg HS, Parikh RM, Morris PL, et al. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80-83.
19. Ramasubbu R, Flint A, Brown G, et al. A neuroendocrine study of serotonin function in depressed stroke patients compared to nondepressed stroke patients and healthy controls. J Affect Disord. 1999;52:121-133.
20. Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke. The Framingham study. Stroke. 2007;38:16-21.
21. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385-401.
22. Jonas BS, Mussolino ME. Symptoms of depression as a prospective risk factor for stroke. Psychosom Med. 2000;62:463-471.
23. Whyte EM, Pollock BG, Wagner WR, et al. Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. Am J Psychiatry. 2001;158(12):2074-2076.
24. Amin AA, Jones AM, Nugnet K, et al. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J. 2006;152:928-934.
25. Nicholson A, Kuper H, Hemingway H. Depression as an aetiolgic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763-2774.
26. Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200-211.
27. Frasure-Smith N, Lesperance F, Irwin MR, et al. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62:302-308.
28. Carney RM, Freedland KE, Stein PK, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosomatic Res. 2007;62:463-467.
29. Whooley MA, Caska CM, Hendrickson BE, et al. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314-320.
30. Hill Golden S, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751-2759.
31. Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30-33.
32. Roose S. Treatment of depression in patients with heart disease. Biol Psychiatry. 2003;54:262-268.
33. Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157(3):351-359.
34. Katz IR. Diagnosis and treatment of depression in patients with Alzheimer’s disease and other dementias. J Clin Psychiatry. 1998;59(9):38-44.
35. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268-276.
36. Lavretsky H. The use of complementary and alternative medicine for treatment of late-life neuropsychiatric disorders. J Aging Health. 2009;5(1):61-78.
37. Pantoni L, Inzitari D. New clinical relevance of leukoaraiosis. European force on age-related white-matter changes. Stroke. 1998;29(2):543.-
38. Richards M, Meade TW, Peart S, et al. Is there any evidence for a protective effect of antithrombotic medication on cognitive function in men at risk of cardiovascular disease? Some preliminary findings. J Neurol Neurosurg Psychiatry. 1997;62(3):269-272.
39. Lutjohann D, Papassotiropoulos A, Bjorkhem I, et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res. 2000;41(2):195-198.
40. Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356(9242):1627-1631.
41. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
42. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.