User login
Out of Sight, Not Out of Mind
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
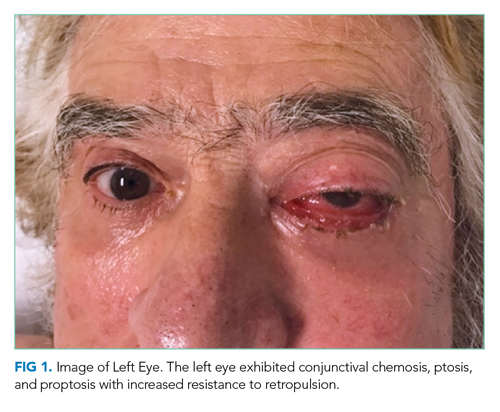
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
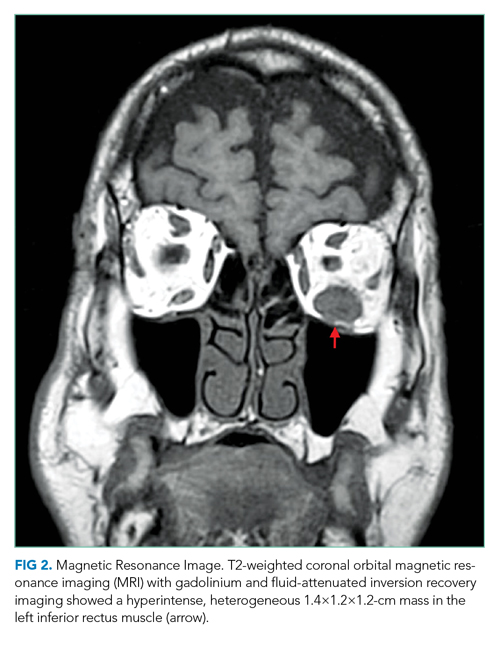
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
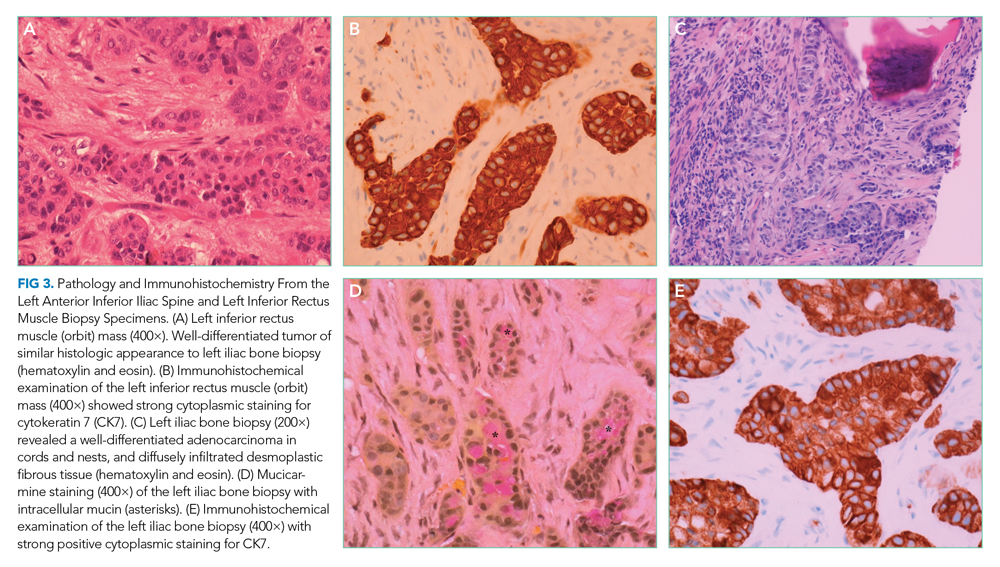
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
A 73-year-old man presented to clinic with 6 weeks of headache. He occasionally experienced generalized headaches throughout his life that resolved with naproxen. His new headache was characterized by a progressively worsening sensation of left-eye pressure with radiation to the left temple. Over the previous week, he had intermittent diplopia, left ptosis, and left lacrimation. He denied head trauma, fever, vision loss, photophobia, dysphagia, dysarthria, nausea, vomiting, or jaw claudication.
Primary headaches include tension type, migraine, and trigeminal autonomic cephalalgias (eg, cluster headache). A new headache in an older patient, particularly if protracted and progressive, prioritizes consideration of a secondary headache, which may reflect pathology within the brain parenchyma (eg, intracranial mass), blood vessels (eg, giant cell arteritis), meninges (eg, meningitis), or ventricles (eg, intraventricular cyst). Eye pain may arise from ocular and extraocular disease. Corneal abrasions, infectious keratitis, scleritis, uveitis, or acute angle-closure glaucoma are painful, although the latter is less likely given the prolonged duration of symptoms. Thyroid eye disease or other infiltrative disorders of the orbit can also cause eye discomfort.
Ptosis commonly results from degeneration of the levator aponeurosis. Other causes include third cranial nerve palsy and myasthenia gravis. Interruption of sympathetic innervation of the eyelid by lesions in the brain stem, spinal cord, lung (eg, Pancoast tumor), or cavernous sinus also can result in ptosis.
Whether the patient has monocular or binocular diplopia is uncertain. Monocular diplopia persists with only one eye open and can arise from uncorrected refractive error, corneal irregularities, lenticular opacities, or unilateral macular disease. Binocular diplopia develops from ocular misalignment due to neuromuscular weakness, extraocular muscle entrapment, or an orbital mass displacing the globe. An orbital mass would also explain the unilateral headache and unilateral ptosis.
His medical history included coronary artery disease, seronegative rheumatoid arthritis, osteoporosis, benign prostatic hypertrophy, and ureteral strictures from chronic nephrolithiasis. Following a cholecystectomy for gallstone pancreatitis 13 years earlier, he was hospitalized five more times for pancreatitis. The last episode was 6 years prior to this presentation. At that time, magnetic resonance cholangiopancreatography (MRCP) did not reveal pancreatic divisum, annular pancreas, biliary strictures, or a pancreatic mass. Esophagogastroduodenoscopy peformed during the same hospitalization showed mild gastritis. His recurrent pancreatitis was deemed idiopathic.
His medications were folic acid, cholecalciferol, lisinopril, metoprolol, omeprazole, simvastatin, aspirin, and weekly methotrexate. His sister had breast and ovarian cancer, and his brother had gastric cancer. He had two subcentimeter tubular adenomas removed during a screening colonoscopy 3 years prior. He had a 30 pack-year smoking history and quit 28 years earlier. He did not use alcohol or drugs. He was a retired chemical plant worker.
Choledocholithiasis (as discrete stones or biliary sludge) can trigger pancreatitis despite a cholecystectomy, but the recurrent episodes and negative MRCP should prompt consideration of other causes, such as alcohol. Hypercalcemia, hypertriglyceridemia, and medications are infrequent causes of pancreatic inflammation. IgG4-related disease (IgG4-RD) causes autoimmune pancreatitis and can infiltrate the eyelids, lacrimal glands, extraocular muscles, or orbital connective tissue. Malignancy of the pancreas or ampulla can trigger pancreatitis by causing pancreatic duct obstruction but would not go undetected for 13 years.
The patient was evaluated by an ophthalmologist and a neurologist. His heart rate was 52 beats per minute and blood pressure, 174/70 mm Hg; other vital signs were normal. He had conjunctival chemosis, ptosis, and nonpulsatile proptosis of the left eye with tenderness and increased resistance to retropulsion compared to the right eye (Figure 1). Visual acuity was 20/25 for the right eye and hand motions only in the left eye. The pupils were reactive and symmetric without afferent pupillary defect. There was no optic nerve swelling or pallor. Abduction, adduction, and elevation of the left eye were restricted and associated with diplopia. Movement of the right eye was unrestricted. There was no other facial asymmetry. Facial sensation was normal. Corneal reflexes were intact. Shoulder shrug strength was equal and symmetric. Tongue protrusion was midline. Olfaction and hearing were not assessed. Strength, sensation, and deep tendon reflexes were normal in all extremities. The plantar response was flexor bilaterally.
Unilateral ptosis, chemosis, proptosis, ophthalmoplegia, eye tenderness, and visual loss collectively point to a space-occupying orbital disease. Orbital masses are caused by cancers, infections such as mucormycosis (usually in an immunocompromised host), and inflammatory disorders such as thyroid orbitopathy, sarcoidosis, IgG4-related orbitopathy, granulomatosis with polyangiitis, and orbital pseudotumor (idiopathic inflammation of the orbit). Chemosis reflects edema of the conjunctiva, which can arise from direct conjunctival injury (eg, allergy, infection, or trauma), interruption of the venous drainage of the conjunctiva by vascular disorders (eg, cavernous sinus thrombosis or carotid-cavernous fistula), or space-occupying diseases of the orbit. Monocular visual loss arises from a prechiasmal lesion, and acute monocular visual loss is more commonly caused by posterior ocular pathology (eg, retina or optic nerve) than anterior disease (eg, keratitis). Visual loss in the presence of an orbital process suggests a compressive or infiltrative disease of the optic nerve.
Complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and thyroid function tests were normal. Interferon-gamma release assay, HIV antibody, rapid plasma reagin, Lyme antibody, antinuclear antibody, and antineutrophil cytoplasmic antibody (ANCA) tests were negative. A noncontrast computed tomography (CT) scan of the head revealed thickening of the left inferior rectus muscle. Orbital magnetic resonance imaging (MRI) with gadolinium and fluid-attenuated inversion recovery imaging demonstrated a T2 hyperintense, heterogeneous 1.4-cm mass in the left inferior rectus muscle (Figure 2). There was no carotid-cavernous fistula, brain mass, or meningeal enhancement.
An isolated mass in one ocular muscle raises the probability of a cancer. The most common malignant orbital tumor is B-cell lymphoma. Metastatic cancer to the eye is rare; breast, prostate, and lung cancer account for the majority of cases. The family history of breast and ovarian cancer raises the possibility of a BRCA mutation, which is also associated with gastric, pancreatic, and prostate malignancies. Granulomatosis with polyangiitis may be ANCA negative in localized sino-orbital disease. Biopsy of the orbital mass is the next step.
The patient underwent transconjunctival orbitotomy with excision of the left inferior rectus mass. Two days later, he presented to the emergency department with acute onset epigastric pain, nausea, and vomiting. A comprehensive review of systems, which had not been performed until this visit, revealed an unintentional 20-lb weight loss over the previous 3 months. He had a progressive ache in the left anterior groin that was dull, tender, nonradiating, and worse with weight bearing. He denied melena or hematochezia.
His temperature was 37 °C; heart rate, 98 beats per minute; and blood pressure, 128/63 mm Hg. He had midepigastric tenderness and point tenderness over the anterior iliac spine. White blood cell count was 12,600/μL; hemo globin, 14.5 g/dL; and platelet count, 158,000/μL. Serum lipase was 7,108 U/L. Serum creatinine, calcium, and triglyceride levels were normal. Alkaline phosphatase was 117 U/L (normal, 34-104 U/L); total bilirubin, 1.1 mg/dL; alanine aminotransferase (ALT), 119 U/L (normal, 7-52 U/L); and aspartate aminotransferase (AST), 236 U/L (normal, 13-39 U/L). Troponin I was undetectable, and an electrocardiogram demonstrated sinus tachycardia. Urinalysis was normal.
Concomitant pancreatitis and hepatitis with an elevated AST-to-ALT ratio should prompt evaluation of recurrent choledocholithiasis and a repeat inquiry about alcohol use. His medications should be reviewed for an association with pancreatitis. Anterior groin discomfort usually reflects osteoarthritis of the hip joint, inguinal hernia, or inguinal lymphadenopathy. Groin pain may be referred from spinal nerve root compression, aortoiliac occlusion, or nephrolithiasis. Weight loss in the presence of an inferior rectus mass suggests one of the aforementioned systemic diseases with orbital manifestations. Pancreatitis and groin discomfort may be important clues, but the chronicity of the recurrent pancreatitis and the high prevalence of hip osteoarthritis make it equally likely that they are unrelated to the eye disease.
CT scan of the abdomen and pelvis with contrast showed peripancreatic edema with fat stranding but no pancreatic or hepatobiliary mass. The common bile duct was normal. A 2.2×1.3-cm mass in the right posterior subphrenic space, a lytic lesion in the left anterior inferior iliac spine, and right nonobstructive nephrolithiasis were identified. CT scan of the chest with contrast showed multiple subpleural nodules and innumerable parenchymal nodules. Subcentimeter hilar, mediastinal, and prevascular lymphadenopathy were present, as well as multiple sclerotic lesions in the right fourth and sixth ribs. Prostate-specific antigen was 0.7 ng/mL (normal, ≤ 4.0 ng/mL). Cancer antigen 19-9 level was 5.5 U/mL (normal, < 37.0 U/mL), and carcinoembryonic antigen (CEA) was 100.1 ng/mL (normal, 0-3 U/mL).
Widespread pulmonary nodules, diffuse lymphadenopathy, and bony lesions raise concern for a metastatic malignancy. There is no evidence of a primary carcinoma. The lack of hepatic involvement reduces the likelihood of a gastrointestinal tumor, although a rectal cancer, which may drain directly into the inferior vena cava and bypass the portal circulation, could present as lung metastases on CT imaging. Lymphoma is plausible given the diffuse lymphadenopathy and orbital mass. Sarcoidosis and histiocytic disorders (eg, Langerhans cell histiocytosis) also cause orbital disease, pulmonary nodules, lymphadenopathy, and bone lesions, although a subphrenic mass would be atypical for both disorders; furthermore, the majority of patients with adult Langerhans cell histiocytosis smoke cigarettes. The elevated CEA makes a metastatic solid tumor more likely than lymphoma but does not specify the location of the primary tumor.
Pathology of the inferior rectus muscle mass showed well-differentiated adenocarcinoma (Figure 3A and 3B). A CT-guided biopsy of the left anterior inferior iliac spine revealed well-differentiated adenocarcinoma (Figure 3C). Adenocarcinoma of unknown primary wasdiagnosed.
Subsequent immunohistochemical (IHC) staining was positive for cytokeratin 7 (CK7) and mucicarmine (Figure 3D and 3E) and negative for cytokeratin 20 (CK20) and thyroid transcription factor 1 (TTF1). This IHC profile suggested pancreatic or upper gastrointestinal tract lineage. Positron emission tomography–CT (PET-CT) scan was aborted because of dyspnea and chest pressure following contrast administration. He declined further imaging or endoscopy. He received palliative radiation and three cycles of paclitaxel and gemcitabine for cancer of unknown primary (CUP). Two months later, he developed bilateral upper-arm weakness due to C7 and T2 cord compression from vertebral and epidural metastases; his symptoms progressed despite salvage chemotherapy. He was transitioned to comfort care and died at home 9 months after diagnosis.
DISCUSSION
This patient’s new headache and ocular abnormalities led to the discovery of an inferior rectus muscle mass. Initially unrecognized unintentional weight loss and hip pain recast a localized orbital syndrome as a systemic disease with pancreatic, ocular, pulmonary, lymph node, and skeletal pathology. Biopsies of the orbital rectus muscle and iliac bone demonstrated metastatic adenocarcinoma. Imaging studies did not identify a primary cancer, but IHC analysis suggested carcinoma of upper gastrointestinal or pancreatic origin.
Acute and chronic pancreatitis are both associated with pancreatic cancer.1 Chronic pancreatitis is associated with an increasing cumulative risk of pancreatic cancer; a potential mechanism is chronic inflammation with malignant transformation.2,3 There is also a 20-fold increased risk of pancreatic cancer in the first 2 years following an episode of acute pancreatitis,4 which may develop from malignant pancreatic duct obstruction. Although the post–acute pancreatitis risk of pancreatic cancer attenuates over time, a two-fold increased risk of pancreatic cancer remains after 10 years,4 which suggests that acute pancreatitis (particularly when idiopathic) either contributes to or shares pathogenesis with pancreatic adenocarcinoma. In elderly patients without gallstones or alcohol use, an abdominal CT scan or MRI shortly after resolution of the acute pancreatitis may be considered to assess for an underlying pancreatic tumor.5
CUP is a histologically defined malignancy without a known primary anatomic site despite an extensive evaluation. CUP accounts for up to 10% of all cancer diagnoses.6 CUP is ascribed to a primary cancer that remains too small to be detected or spontaneous regression of the primary cancer.7 Approximately 70% of autopsies of patients with CUP identify the primary tumor, which most commonly originates in the lung, gastrointestinal tract, breast, or pancreas.8
When a metastatic focus of cancer is found but the initial diagnostic evaluation (including CT scan of the chest, abdomen, and pelvis) fails to locate a primary cancer, the next step in searching for the tissue of origin is an IHC analysis of the tumor specimen. IHC analysis is a multistep staining process that can identify major categories of cancer, including carcinoma (adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma) and poorly or undifferentiated neoplasms (including carcinoma, lymphoma, sarcoma, or melanoma). Eighty-five percent of CUP cases are adenocarcinoma, 10% are squamous cell carcinoma, and the remaining 5% are undifferentiated neoplasms.9
There are no consensus guidelines for imaging in patients with CUP who have already undergone a CT scan of the chest, abdomen, and pelvis. Mammography is indicated in women with metastatic adenocarcinoma or axillary lymphadenopathy.7 MRI of the breast is obtained when mammography is nondiagnostic and the suspicion for breast cancer is high. Small clinical studies and meta-analyses support the use of PET-CT scans,7 although one study found that a PET-CT scan was not superior to CT imaging in identifying the primary tumor site in CUP.10 Endoscopy of the upper airway or gastrointestinal tract is rarely diagnostic in the absence of referable symptoms or a suggestive IHC profile (eg, CK7−, CK20+ suggestive of colon cancer).6
Molecular cancer classification has emerged as a useful diagnostic technique in CUP. Cancer cells retain gene expression patterns based on cellular origin, and a tumor’s profile can be compared with a reference database of known cancers, aiding in the identification of the primary tumor type. Molecular cancer classifier assays that use gene expression profiling can accurately determine a primary site11 and have been shown to be concordant with IHC testing.12 Molecular cancer classification is distinct from genetic assays that identify mutations for which there are approved therapies. Serum tumor markers are generally not useful in establishing the primary tumor and should be considered based on the clinical presentation (eg, prostate-specific antigen testing in a man with adenocarcinoma of unknown primary and osteoblastic metastases).
CUP is classified as favorable or unfavorable based on the IHC, pattern of spread, and serum markers in certain cases.6 Approximately 20% of CUP patients can be categorized into favorable subsets, such as adenocarcinoma in a single axillary lymph node in a female patient suggestive of a breast primary cancer, or squamous cell carcinoma in a cervical lymph node suggestive of a head or neck primary cancer.7 The remaining 80% of cases are categorized as unfavorable CUP and often have multiple metastases. Our patient’s pattern of spread and limited response to chemotherapy is characteristic of the unfavorable subset of CUP. The median survival of this group is 9 months, and only 25% of patients survive longer than 1 year.13
Biomarker-driven treatment of specific molecular targets independent of the tissue of origin (tissue-agnostic therapy) has shown promising results in the treatment of skin, lung, thyroid, colorectal, and gastric cancers.14 Pembrolizumab was the first drug approved by the US Food and Drug Administration based on a tumor’s biomarker without regard to its primary location. Data to support this approach for treating CUP are evolving and offer hope for patients with specific molecular targets.
Following the focused neuro-ophthalmologic evaluations, with focused examination and imaging, the hospitalist’s review of systems at the time of the final admission for pancreatitis set in motion an evaluation that led to a diagnosis of metastatic cancer. The risk factor of recurrent pancreatitis and IHC results suggested that pancreatic adenocarcinoma was the most likely primary tumor. As the focus of cancer treatment shifts away from the tissue of origin and toward molecular and genetic profiles, the search for the primary site may decrease in importance. In the future, even when we do not know the cancer’s origin, we may still know precisely what to do. But for now, as in this patient, our treatments continue to be based on a tumor that is out of sight, but not out of mind.
KEY TEACHING POINTS
- Acute and chronic pancreatitis are associated with an increased risk of pancreatic adenocarcinoma.
- CUP is a cancer in which diagnostic testing does not identify a primary tumor site. Immunohistochemistry and molecular analysis, imaging, and endoscopy are utilized selectively to identify a primary tumor type.
- Treatment of CUP currently depends on the suspected tissue of origin and pattern of spread.
- Tissue-agnostic therapy could allow for treatment for CUP patients independent of the tissue of origin.
Acknowledgments
We thank Andrew Mick, OD, for his review of an earlier version of this manuscript and Peter Phillips, MD, for his interpretation of the pathologic images.
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
1. Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(111):1711-1719. https://doi.org/10.1038/s41395-018-0255-9
2. Duell EJ, Lucenteforte E, Olson SH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2012;23(11):2964-2970. https://doi.org/10.1093/annonc/mds140
3. Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329(20):1502-1503. https://doi.org/10.1056/NEJM199311113292016
4. Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute pancreatitis and pancreatic cancer risk: a nationwide matched-cohort study in Denmark. Gastroenterology. 2018;154(156):1729-1736. https://doi.org/10.1053/j.gastro.2018.02.011
5. Frampas E, Morla O, Regenet N, Eugene T, Dupas B, Meurette G. A solid pancreatic mass: tumour or inflammation? Diagn Interv Imaging. 2013;94(7-8):741-755. https://doi.org/10.1016/j.diii.2013.03.013
6. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. https://doi.org/10.1056/NEJMra1303917
7. Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2017. 2018;35(3):199-206. https://doi.org//10.1053/j.semdp.2017.11.013
8. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-2036. https://doi.org/10.1016/j.ejca.2007.06.023
9. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-278. https://doi.org/10.1016/j.critrevonc.2008.09.005
10. Moller AK, Loft A, Berthelsen AK, et al. A prospective comparison of 18F-FDG PET/CT and CT as diagnostic tools to identify the primary tumor site in patients with extracervical carcinoma of unknown primary site. Oncologist. 2012;17(9):1146-1154. https://doi.org/10.1634/theoncologist.2011-0449
11. Economopoulou P, Mountzios G, Pavlidis N, Pentheroudakis G. Cancer of unknown primary origin in the genomic era: elucidating the dark box of cancer. Cancer Treat Rev. 2015;41(7):598-604. https://doi.org/10.1016/j.ctrv.2015.05.010
12. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642. https://doi.org/10.1007/s11864-013-0257-1
13. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-710. https://doi.org/10.1038/nrclinonc.2011.158
14. Luoh SW, Flaherty KT. When tissue is no longer the issue: tissue-agnostic cancer therapy comes of age. Ann Intern Med. 2018;169(4):233-239. https://doi.org/10.7326/M17-2832
© 2021 Society of Hospital Medicine
A Protean Protein
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
© 2019 Society of Hospital Medicine