User login
Risk-Prediction Model for Recurrent Clostridium difficile Infection
Clinical question: What are the risk factors identified at the onset of illness that are associated with recurrent C. diff infection?
Background: After initial infection, 10%-25% of patients experience recurrent C. diff infection (CDI). The identification of patients at high risk of recurrence would be beneficial for therapeutic decision making.
Study design: Retrospective cohort study.
Setting: Large, urban, academic medical center.
Synopsis: Authors included 4,196 patients with an initial infection, defined by a positive C. diff toxin assay and unformed stools. A repeat positive toxin within 42 days of completing treatment for the initial infection represented recurrent CDI. Multiple characteristics were examined to identify risks of recurrent infection, including demographics and those related to acute and chronic disease. A logistic regression model was used to identify risk factors for recurrence.
Four hundred twenty-five patients (10.1%) had recurrent infection. Age, fluoroquinolone and high-risk antibiotic use, community-acquired healthcare-associated infection, multiple hospitalizations, and gastric acid suppression were found to predict recurrent infection through multivariate analysis.
Limitations of the study included potential confounding, use of observational data, and generalizability, given the urban academic medical center setting. This prediction model differs from previously developed models in that it identifies factors present at the onset of infection.
Bottom line: Multiple factors identified at the onset of illness can predict CDI recurrence.
Citation: Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med. 2014;9(7):418-423.
Clinical question: What are the risk factors identified at the onset of illness that are associated with recurrent C. diff infection?
Background: After initial infection, 10%-25% of patients experience recurrent C. diff infection (CDI). The identification of patients at high risk of recurrence would be beneficial for therapeutic decision making.
Study design: Retrospective cohort study.
Setting: Large, urban, academic medical center.
Synopsis: Authors included 4,196 patients with an initial infection, defined by a positive C. diff toxin assay and unformed stools. A repeat positive toxin within 42 days of completing treatment for the initial infection represented recurrent CDI. Multiple characteristics were examined to identify risks of recurrent infection, including demographics and those related to acute and chronic disease. A logistic regression model was used to identify risk factors for recurrence.
Four hundred twenty-five patients (10.1%) had recurrent infection. Age, fluoroquinolone and high-risk antibiotic use, community-acquired healthcare-associated infection, multiple hospitalizations, and gastric acid suppression were found to predict recurrent infection through multivariate analysis.
Limitations of the study included potential confounding, use of observational data, and generalizability, given the urban academic medical center setting. This prediction model differs from previously developed models in that it identifies factors present at the onset of infection.
Bottom line: Multiple factors identified at the onset of illness can predict CDI recurrence.
Citation: Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med. 2014;9(7):418-423.
Clinical question: What are the risk factors identified at the onset of illness that are associated with recurrent C. diff infection?
Background: After initial infection, 10%-25% of patients experience recurrent C. diff infection (CDI). The identification of patients at high risk of recurrence would be beneficial for therapeutic decision making.
Study design: Retrospective cohort study.
Setting: Large, urban, academic medical center.
Synopsis: Authors included 4,196 patients with an initial infection, defined by a positive C. diff toxin assay and unformed stools. A repeat positive toxin within 42 days of completing treatment for the initial infection represented recurrent CDI. Multiple characteristics were examined to identify risks of recurrent infection, including demographics and those related to acute and chronic disease. A logistic regression model was used to identify risk factors for recurrence.
Four hundred twenty-five patients (10.1%) had recurrent infection. Age, fluoroquinolone and high-risk antibiotic use, community-acquired healthcare-associated infection, multiple hospitalizations, and gastric acid suppression were found to predict recurrent infection through multivariate analysis.
Limitations of the study included potential confounding, use of observational data, and generalizability, given the urban academic medical center setting. This prediction model differs from previously developed models in that it identifies factors present at the onset of infection.
Bottom line: Multiple factors identified at the onset of illness can predict CDI recurrence.
Citation: Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Development and validation of a recurrent Clostridium difficile risk-prediction model. J Hosp Med. 2014;9(7):418-423.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Neutral head position safe for internal jugular vein cannulation
- Thrombolysis decreases mortality in unstable patients with acute PE
- Rectal indomethacin decreases incidence of post-ERCP pancreatitis
- CHADS2-VASc and HAS-BLED as predictors in afib patients
- No readmission, mortality decreases with self-supported COPD management
- Medicare Premier P4P initiatives do not decrease mortality
- In-hospital rate of DVT/PE after hip and knee arthroplasty
- Sodium chloride prevents contrast-induced nephropathy
Neutral Head Position Is Safe for Internal Jugular Vein Cannulation
Clinical question: Is there a difference in the complication rate between neutral head position and 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation?
Background: Cannulation of the internal jugular vein using ultrasound decreases the rate of major complications (carotid artery puncture, pneumothorax, and hemothorax). The relative positions of the internal jugular vein and the carotid artery change based on degree of neck rotation. The optimal position for ultrasound-guided vein puncture has not been shown.
Study design: Prospective, randomized, controlled, non-blinded study.
Setting: Tertiary neurosurgical center in Milan, Italy.
Synopsis: One thousand, three hundred thirty-two patients undergoing major neurosurgical procedures who needed central venous catheter placement were randomized to a neutral head position (NH) or a 45-degree neck rotation (HT) during ultrasound-guided internal jugular vein cannulation. Exclusion criteria were consent refusal, age <12 years, and coagulopathy. Six experienced anesthesiologists performed the procedures; blinding was not possible.
There was no difference in the rate of major complications (carotid artery puncture, pneumothorax, or hemothorax) based on head position (0.9% in NH vs. 0.6% in HT). Minor complications (multiple skin punctures, multiple vein punctures, difficulty inserting the guidewire) were similar in the two groups (13.2% in NG vs. 12.6% in HT). Neck rotation was not associated with operator-reported difficulty or vascular access time.
Limitations of the study include the inability to blind the operator. Additionally, the study involved six experienced anesthesiologists at one center who performed the procedure on patients needing an elective central line. The ability to generalize the findings to other settings, less experienced providers, and patients who need an emergency line is not certain.
Bottom line: Neutral head position is as safe as 45-degree neck rotation for elective ultrasound-guided internal jugular vein cannulation.
Citation: Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114:777-784.
Thrombolysis Decreases Mortality in Unstable Patients with Acute Pulmonary Embolism
Clinical question: Does thrombolytic therapy decrease mortality in unstable patients with acute pulmonary embolism (PE)?
Background: PE is a common problem; associated mortality is high. Despite this fact, the data supporting thrombolytic therapy in hemodynamically unstable patients are not robust, and randomized, controlled trials are unlikely to be performed.
Study design: Retrospective cohort study.
Setting: One thousand nonfederal, short-term U.S. hospitals.
Synopsis: Using data from the Nationwide Inpatient Sample database from 1999-2008, investigators found that thrombolysis decreased both all-cause and PE-specific mortality for unstable patients, defined as those either in shock or on a ventilator. Specifically for all-cause mortality, 15% of patients who received thrombolysis died vs. 47% of those who did not (RR 0.31, 95% CI 0.30-0.32). Placement of an inferior vena cava (IVC) filter further reduced mortality, to only 7.6% in patients who received both IVC filter and thrombolysis.
For PE-specific mortality, patients who received thrombolysis also had decreased rates, from 42% to 8.4% (RR 0.20; 95% CI 0.19-0.22). Across all age groups, patients who received thrombolysis had decreased all-cause and PE-specific mortality. Patients who did not receive thrombolysis had additional comorbidities.
As this study is retrospective, it might be affected by unknown confounding. In addition, it relies on coding data to identify patient stability and treatment. Despite this limitation, a randomized, controlled trial is unlikely to be performed at this stage. This study provides evidence to support use of thrombolysis in unstable patients.
Bottom line: Thrombolysis might reduce mortality in unstable patients with acute PE. In combination with IVC filters, the mortality reduction might be even greater.
Citation: Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012;125(5):465-470.
Rectal Indomethacin Decreases Incidence of Post-ERCP Pancreatitis
Clinical question: Does rectal indomethacin reduce the incidence of pancreatitis after ERCP?
Background: Acute pancreatitis is the most common complication from endoscopic retrograde cholangiopancreatography (ERCP). No pharmacologic treatment has proven to reduce the incidence of pancreatitis. Preliminary studies, including a meta-analysis, suggest that the use of NSAIDs might reduce the incidence of post-ERCP pancreatitis.
Study design: Randomized, placebo-controlled, double-blind trial.
Setting: Multicenter trial at four university-affiliated medical centers in the U.S.
Synopsis: More than 600 patients at high risk for post-ERCP pancreatitis were randomized to receive either two 50-mg indomethacin suppositories or two identical-appearing placebo suppositories. Patients were considered at high risk for pancreatitis based on previously identified patient- and procedure-related risk factors. Most of the participants had sphincter of Oddi dysfunction (84.4% of the indomethacin group and 80.5% of the placebo group). Exclusion criteria included elevated creatinine and active peptic ulcer disease. The indomethacin or placebo suppositories were given immediately following the ERCP.
Post-ERCP pancreatitis, defined by upper abdominal pain, elevation of pancreatic enzymes, and hospitalization for at least two nights, was significantly higher in the placebo group compared with the indomethacin group (16.9% vs. 9.2%, P=0.005). Moderate or severe post-ERCP pancreatitis was significantly higher in the placebo group compared with the indomethacin group (8.8% vs. 4.4%, P=0.03).
There were no significant differences in the rates of clinically significant bleeding or acute renal failure between the two groups. The ability to generalize these findings to patients without risk factors for post-ERCP pancreatitis is not clear.
Bottom line: Rectal indomethacin decreases the rate of post-ERCP pancreatitis in patients who are at high risk for this complication.
Citation: Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366(15):1414-1422.
CHADS2-VASc and HAS-BLED Can Predict Thromboembolism and Bleeding Risk in Afib Patients
Clinical question: What are the risk factors for stroke/thromboembolism and bleeding from atrial fibrillation (afib), and how well do the CHADS2-VASc and HAS-BLED stroke and bleeding risk-assessment tools perform against other published stroke and bleeding risk-assessment tools (CHADS2 and HEMORR2HAGES) for patients with afib?
Background: In afib patients, the CHADS2-VASc tool might offer more comprehensive stroke assessment over the CHADS2 by identifying truly-low-risk patients with afib who might not even need antiplatelet therapy. HAS-BLED, a newer bleeding-risk-assessment tool, has been validated in previous trials and is more user-friendly than others. Use of CHADS2-VASc and HAS-BLED are recommended by the European Society of Cardiology to assess stroke and bleeding risks for patients with afib.
Study design: Prospective cohort study.
Setting: All hospitals in Sweden.
Synopsis: Investigators identified 182,678 afib patients via ICD-10 data from Sweden’s National Hospital Discharge Registry ICD-10 from 2005 to 2008. Approximately half the patients were not taking anticoagulants. Analysis assessed risk factors for stroke and bleeding and the performance of CHADS2-VASc and HAS-BLED against CHADS2 and HEMORR2HAGES stroke and bleeding risk-assessment tools.
Risk of composite thromboembolism (unspecified stroke, TIA, systemic embolism) was significantly higher in patients with increased age, peripheral arterial disease, prior myocardial infarction (MI), prior coronary artery bypass grafting (CABG), female gender, renal failure, and aspirin use, as well as hypertension, diabetes, prior thromboembolic event, or prior intracranial hemorrhage (ICH). Interestingly, a statistically increased risk was seen with aspirin use.
Conversely, history of heart failure, thyroid disease, and obesity were not associated with increased composite thromboembolic risk. The use of CHADS2-VASc was marginally better than CHADS2 in predicting stroke risk.
ICH risk was increased in patients with older age, prior ischemic stroke, prior ICH, and hypertension. Risk of composite bleeding (from ICH or other major bleeding) was significantly higher in patients with these risk factors, as well as renal failure, liver disease, anemia, dysfunctional platelets, alcohol use, and cancer. Ischemic heart disease was associated with a statistically significant lower risk of ICH, but not of composite bleeding risk.
HAS-BLED usage was as good as, and easier to use than, HEMORR2HAGES in predicting bleeding risk.
Bottom line: CHADS2-VASc might be better than CHADS2 in predicting truly-low-risk patients with nonvalvular afib; HAS-BLED is just as good as, and easier to use than, HEMORR2HAGES in predicting bleeding risk for patients with nonvalvular afib who are to receive antithrombotic therapy.
Citation: Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500-10 [Epub 2012 Jan 13].
Supported Self-Management of COPD Does Not Decrease Readmission or Mortality Rates
Clinical question: Does supported self-management of patients with chronic obstructive pulmonary disease (COPD) decrease COPD-related hospital readmission or death?
Clinical background: Supported self-management has benefited patients with such chronic diseases as heart failure and asthma. Evidence to support such a strategy for patients with COPD is relatively lacking.
Study design: Randomized, controlled trial.
Setting: Community-based care following urban hospitalization in western Scotland.
Synopsis: From June 2007 to May 2009, and following hospitalization for COPD exacerbation, 464 patients were randomized to receive routine community-based care with or without 12 months of support and training to detect, and promptly treat, recurrent exacerbations. Independent of disease severity or demographics, investigators found no difference in combined readmission (48% vs. 47%, 95% confidence interval [CI] 0.80-1.38) or death (10% vs. 7%, 95% CI 0.71-2.61).
Based on review of appropriateness of self-management strategies used by the intervention group, unplanned exploratory subgroup analysis classified a minority of the intervention group as “successful” (42%) supported self-managers, and demonstrated decreased COPD readmissions and death (27% vs. 49%, 95% CI 0.25-0.76, P=0.003) vs. “unsuccessful” self-managers. This successful group was younger and tended to live with others. Further research to define characteristics of patients who benefit from self-management is needed.
Bottom line: Supported self-management of COPD does not reduce COPD-related readmission or death in a large population.
Citation: Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060 [Epub ahead of print].
Medicare Premier P4P Initiatives Do Not Decrease Mortality
Clinical question: Has the Medicare Premier Hospital Quality Incentive Demonstration (HQID) resulted in lower mortality?
Background: The Centers for Medicare & Medicaid Services’ (CMS) value-based purchasing program will expand to include 30-day mortality in 2013, but do pay-for-performance (P4P) initiatives result in improved mortality? Studies have demonstrated improvement in process of care but have not demonstrated mortality benefit thus far.
Study design: Cohort study.
Setting: Two hundred fifty-two hospitals participating in the Premier HQID compared with 3,363 control hospitals participating in the Hospital Compare program.
Synopsis: Researchers examined 30-day mortality for patients admitted with acute myocardial infarction, congestive heart failure, pneumonia, and for coronary artery bypass grafting (CABG) from 2003 to 2009. Results showed no difference in 30-day mortality rates over the six-year span of the program for any of the conditions studied in the Premier hospitals (participating in the voluntary pay-for-performance program) vs. non-Premier hospitals (11.82% vs. 11.74%). This held true for each condition measured individually, with a higher mortality rate for patients undergoing CABG at the Premier hospitals.
In addition, in the hospitals that underperformed initially, there was no difference with respect to rate of improvement when comparing Premier vs. non-Premier hospitals. Furthermore, mortality rate trends did not differ between those conditions that were incentivized (acute MI and CABG) compared with those that were not (congestive heart failure and pneumonia).
The authors concluded that there was “little evidence” that the Premier HQID pay-for-performance program resulted in reduced 30-day mortality.
Bottom line: Programs participating in the Medicare Premier HQID pay-for-performance program had similar 30-day mortality compared with nonparticipating programs.
Citation: Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366:1606-1615.
In-Hospital Rate of DVT/PE After Hip and Knee Arthroplasty with Guideline-Recommended Prophylaxis
Clinical question: What is the rate of symptomatic DVT/PE after total or partial hip (TPHA) or knee (TPKA) arthroplasty using currently recommended prophylaxis?
Background: The rate of postoperative DVT/PE after TPHA/TPKA has dropped dramatically with use of pharmacologic prophylaxis. However, the current rate of symptomatic DVT/PE using current pharmacologic prophylaxis is not known. Such rates are needed for informed patient consent and development of patient safety benchmarks.
Study design: Systematic review.
Setting: Randomized clinical trials (RCTs) and observational studies worldwide of adult inpatients undergoing TPHA and/or TPKA from 1996 to 2011.
Synopsis: Forty-seven studies were included, of which 41 were RCTs and six were observational studies. Twenty-one studies evaluated rates after TPHA, 20 after TPKA, and six after both. More than 44,000 patients were included, with ages ranging from 58 to 74 years. The mean duration of prophylaxis was eight days, with a mean follow-up of 13 days.
In-hospital rates of symptomatic venous thromboembolism (VTE) were higher in patients undergoing TPKA than TPHA (1.09% vs. 0.53% for VTE, 0.63% vs. 0.26% for DVT, and 0.27% vs. 0.14% for PE). This is in contrast to the higher rates of VTE observed after TPHA when the post-discharge period is included. The pooled incidence of VTE was lower with use of direct inhibitors of Factors Xa or IIa when compared to low-molecular-weight heparin, although a direct efficacy comparison could not be made.
Because the majority of studies were RCTs with restrictive inclusion criteria, rates of DVT/PE in actual practice might be higher.
Bottom line: When informing patients of postoperative VTE risk, or establishing benchmarks to evaluate patient safety, one can anticipate an in-hospital VTE rate of 0.5% following TPHA and 1% following TPKA.
Citation: Januel JM, Chen G, Ruffieux C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307(3):294-303.
Sodium Chloride Prevents Contrast-Induced Nephropathy
Clinical question: Is sodium chloride more effective than sodium bicarbonate for preventing contrast-induced nephropathy?
Background: Contrast-induced nephropathy can be prevented with precontrast hydration. Study authors sought to compare sodium chloride administered over 24 hours with sodium bicarbonate administered over seven hours, and with sodium bicarbonate administered orally.
Study design: Randomized, open-label, controlled trial.
Setting: Three European medical centers.
Synopsis: The study examined 258 patients with an estimated glomerular filtration rate <60mL/min/1.73m2 undergoing intravenous or intra-arterial contrast procedure. Patients with Class III or IV heart failure were excluded. The remaining patients were randomized to receive one of three regimens: sodium chloride at 1 ml/kg/hr for 12 hours prior to and 12 hours following the procedure; intravenous sodium bicarbonate for one hour prior to and six hours following the procedure; or intravenous and oral sodium bicarbonate 20 minutes prior to the procedure.
The group that received saline had a lower incidence of contrast-induced nephropathy than the bicarbonate groups (1% vs. 9% vs. 10%). The oral bicarbonate strategy was noninferior to the seven-hour intravenous bicarbonate strategy.
The authors postulated that saline should be used for high-risk patients, but that given the overall low incidence of contrast-induced nephropathy, the short-term bicarbonate strategy (intravenous followed by oral) is a viable alternative for low-risk patients.
Bottom line: Sodium chloride is more effective than sodium bicarbonate for preventing contrast-induced nephropathy, but in light of the low incidence, a short course of sodium bicarbonate is a possible convenient alternative for low-risk patients.
Citation: Klima T, Christ A, Marana I, et al. Sodium chloride vs. sodium bicarbonate for the prevention of contrast medium-induced nephropathy: a randomized controlled trial. Eur Heart J. 2012 Jan 19 [Epub ahead of print].
In This Edition
Literature At A Glance
A guide to this month’s studies
- Neutral head position safe for internal jugular vein cannulation
- Thrombolysis decreases mortality in unstable patients with acute PE
- Rectal indomethacin decreases incidence of post-ERCP pancreatitis
- CHADS2-VASc and HAS-BLED as predictors in afib patients
- No readmission, mortality decreases with self-supported COPD management
- Medicare Premier P4P initiatives do not decrease mortality
- In-hospital rate of DVT/PE after hip and knee arthroplasty
- Sodium chloride prevents contrast-induced nephropathy
Neutral Head Position Is Safe for Internal Jugular Vein Cannulation
Clinical question: Is there a difference in the complication rate between neutral head position and 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation?
Background: Cannulation of the internal jugular vein using ultrasound decreases the rate of major complications (carotid artery puncture, pneumothorax, and hemothorax). The relative positions of the internal jugular vein and the carotid artery change based on degree of neck rotation. The optimal position for ultrasound-guided vein puncture has not been shown.
Study design: Prospective, randomized, controlled, non-blinded study.
Setting: Tertiary neurosurgical center in Milan, Italy.
Synopsis: One thousand, three hundred thirty-two patients undergoing major neurosurgical procedures who needed central venous catheter placement were randomized to a neutral head position (NH) or a 45-degree neck rotation (HT) during ultrasound-guided internal jugular vein cannulation. Exclusion criteria were consent refusal, age <12 years, and coagulopathy. Six experienced anesthesiologists performed the procedures; blinding was not possible.
There was no difference in the rate of major complications (carotid artery puncture, pneumothorax, or hemothorax) based on head position (0.9% in NH vs. 0.6% in HT). Minor complications (multiple skin punctures, multiple vein punctures, difficulty inserting the guidewire) were similar in the two groups (13.2% in NG vs. 12.6% in HT). Neck rotation was not associated with operator-reported difficulty or vascular access time.
Limitations of the study include the inability to blind the operator. Additionally, the study involved six experienced anesthesiologists at one center who performed the procedure on patients needing an elective central line. The ability to generalize the findings to other settings, less experienced providers, and patients who need an emergency line is not certain.
Bottom line: Neutral head position is as safe as 45-degree neck rotation for elective ultrasound-guided internal jugular vein cannulation.
Citation: Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114:777-784.
Thrombolysis Decreases Mortality in Unstable Patients with Acute Pulmonary Embolism
Clinical question: Does thrombolytic therapy decrease mortality in unstable patients with acute pulmonary embolism (PE)?
Background: PE is a common problem; associated mortality is high. Despite this fact, the data supporting thrombolytic therapy in hemodynamically unstable patients are not robust, and randomized, controlled trials are unlikely to be performed.
Study design: Retrospective cohort study.
Setting: One thousand nonfederal, short-term U.S. hospitals.
Synopsis: Using data from the Nationwide Inpatient Sample database from 1999-2008, investigators found that thrombolysis decreased both all-cause and PE-specific mortality for unstable patients, defined as those either in shock or on a ventilator. Specifically for all-cause mortality, 15% of patients who received thrombolysis died vs. 47% of those who did not (RR 0.31, 95% CI 0.30-0.32). Placement of an inferior vena cava (IVC) filter further reduced mortality, to only 7.6% in patients who received both IVC filter and thrombolysis.
For PE-specific mortality, patients who received thrombolysis also had decreased rates, from 42% to 8.4% (RR 0.20; 95% CI 0.19-0.22). Across all age groups, patients who received thrombolysis had decreased all-cause and PE-specific mortality. Patients who did not receive thrombolysis had additional comorbidities.
As this study is retrospective, it might be affected by unknown confounding. In addition, it relies on coding data to identify patient stability and treatment. Despite this limitation, a randomized, controlled trial is unlikely to be performed at this stage. This study provides evidence to support use of thrombolysis in unstable patients.
Bottom line: Thrombolysis might reduce mortality in unstable patients with acute PE. In combination with IVC filters, the mortality reduction might be even greater.
Citation: Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012;125(5):465-470.
Rectal Indomethacin Decreases Incidence of Post-ERCP Pancreatitis
Clinical question: Does rectal indomethacin reduce the incidence of pancreatitis after ERCP?
Background: Acute pancreatitis is the most common complication from endoscopic retrograde cholangiopancreatography (ERCP). No pharmacologic treatment has proven to reduce the incidence of pancreatitis. Preliminary studies, including a meta-analysis, suggest that the use of NSAIDs might reduce the incidence of post-ERCP pancreatitis.
Study design: Randomized, placebo-controlled, double-blind trial.
Setting: Multicenter trial at four university-affiliated medical centers in the U.S.
Synopsis: More than 600 patients at high risk for post-ERCP pancreatitis were randomized to receive either two 50-mg indomethacin suppositories or two identical-appearing placebo suppositories. Patients were considered at high risk for pancreatitis based on previously identified patient- and procedure-related risk factors. Most of the participants had sphincter of Oddi dysfunction (84.4% of the indomethacin group and 80.5% of the placebo group). Exclusion criteria included elevated creatinine and active peptic ulcer disease. The indomethacin or placebo suppositories were given immediately following the ERCP.
Post-ERCP pancreatitis, defined by upper abdominal pain, elevation of pancreatic enzymes, and hospitalization for at least two nights, was significantly higher in the placebo group compared with the indomethacin group (16.9% vs. 9.2%, P=0.005). Moderate or severe post-ERCP pancreatitis was significantly higher in the placebo group compared with the indomethacin group (8.8% vs. 4.4%, P=0.03).
There were no significant differences in the rates of clinically significant bleeding or acute renal failure between the two groups. The ability to generalize these findings to patients without risk factors for post-ERCP pancreatitis is not clear.
Bottom line: Rectal indomethacin decreases the rate of post-ERCP pancreatitis in patients who are at high risk for this complication.
Citation: Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366(15):1414-1422.
CHADS2-VASc and HAS-BLED Can Predict Thromboembolism and Bleeding Risk in Afib Patients
Clinical question: What are the risk factors for stroke/thromboembolism and bleeding from atrial fibrillation (afib), and how well do the CHADS2-VASc and HAS-BLED stroke and bleeding risk-assessment tools perform against other published stroke and bleeding risk-assessment tools (CHADS2 and HEMORR2HAGES) for patients with afib?
Background: In afib patients, the CHADS2-VASc tool might offer more comprehensive stroke assessment over the CHADS2 by identifying truly-low-risk patients with afib who might not even need antiplatelet therapy. HAS-BLED, a newer bleeding-risk-assessment tool, has been validated in previous trials and is more user-friendly than others. Use of CHADS2-VASc and HAS-BLED are recommended by the European Society of Cardiology to assess stroke and bleeding risks for patients with afib.
Study design: Prospective cohort study.
Setting: All hospitals in Sweden.
Synopsis: Investigators identified 182,678 afib patients via ICD-10 data from Sweden’s National Hospital Discharge Registry ICD-10 from 2005 to 2008. Approximately half the patients were not taking anticoagulants. Analysis assessed risk factors for stroke and bleeding and the performance of CHADS2-VASc and HAS-BLED against CHADS2 and HEMORR2HAGES stroke and bleeding risk-assessment tools.
Risk of composite thromboembolism (unspecified stroke, TIA, systemic embolism) was significantly higher in patients with increased age, peripheral arterial disease, prior myocardial infarction (MI), prior coronary artery bypass grafting (CABG), female gender, renal failure, and aspirin use, as well as hypertension, diabetes, prior thromboembolic event, or prior intracranial hemorrhage (ICH). Interestingly, a statistically increased risk was seen with aspirin use.
Conversely, history of heart failure, thyroid disease, and obesity were not associated with increased composite thromboembolic risk. The use of CHADS2-VASc was marginally better than CHADS2 in predicting stroke risk.
ICH risk was increased in patients with older age, prior ischemic stroke, prior ICH, and hypertension. Risk of composite bleeding (from ICH or other major bleeding) was significantly higher in patients with these risk factors, as well as renal failure, liver disease, anemia, dysfunctional platelets, alcohol use, and cancer. Ischemic heart disease was associated with a statistically significant lower risk of ICH, but not of composite bleeding risk.
HAS-BLED usage was as good as, and easier to use than, HEMORR2HAGES in predicting bleeding risk.
Bottom line: CHADS2-VASc might be better than CHADS2 in predicting truly-low-risk patients with nonvalvular afib; HAS-BLED is just as good as, and easier to use than, HEMORR2HAGES in predicting bleeding risk for patients with nonvalvular afib who are to receive antithrombotic therapy.
Citation: Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500-10 [Epub 2012 Jan 13].
Supported Self-Management of COPD Does Not Decrease Readmission or Mortality Rates
Clinical question: Does supported self-management of patients with chronic obstructive pulmonary disease (COPD) decrease COPD-related hospital readmission or death?
Clinical background: Supported self-management has benefited patients with such chronic diseases as heart failure and asthma. Evidence to support such a strategy for patients with COPD is relatively lacking.
Study design: Randomized, controlled trial.
Setting: Community-based care following urban hospitalization in western Scotland.
Synopsis: From June 2007 to May 2009, and following hospitalization for COPD exacerbation, 464 patients were randomized to receive routine community-based care with or without 12 months of support and training to detect, and promptly treat, recurrent exacerbations. Independent of disease severity or demographics, investigators found no difference in combined readmission (48% vs. 47%, 95% confidence interval [CI] 0.80-1.38) or death (10% vs. 7%, 95% CI 0.71-2.61).
Based on review of appropriateness of self-management strategies used by the intervention group, unplanned exploratory subgroup analysis classified a minority of the intervention group as “successful” (42%) supported self-managers, and demonstrated decreased COPD readmissions and death (27% vs. 49%, 95% CI 0.25-0.76, P=0.003) vs. “unsuccessful” self-managers. This successful group was younger and tended to live with others. Further research to define characteristics of patients who benefit from self-management is needed.
Bottom line: Supported self-management of COPD does not reduce COPD-related readmission or death in a large population.
Citation: Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060 [Epub ahead of print].
Medicare Premier P4P Initiatives Do Not Decrease Mortality
Clinical question: Has the Medicare Premier Hospital Quality Incentive Demonstration (HQID) resulted in lower mortality?
Background: The Centers for Medicare & Medicaid Services’ (CMS) value-based purchasing program will expand to include 30-day mortality in 2013, but do pay-for-performance (P4P) initiatives result in improved mortality? Studies have demonstrated improvement in process of care but have not demonstrated mortality benefit thus far.
Study design: Cohort study.
Setting: Two hundred fifty-two hospitals participating in the Premier HQID compared with 3,363 control hospitals participating in the Hospital Compare program.
Synopsis: Researchers examined 30-day mortality for patients admitted with acute myocardial infarction, congestive heart failure, pneumonia, and for coronary artery bypass grafting (CABG) from 2003 to 2009. Results showed no difference in 30-day mortality rates over the six-year span of the program for any of the conditions studied in the Premier hospitals (participating in the voluntary pay-for-performance program) vs. non-Premier hospitals (11.82% vs. 11.74%). This held true for each condition measured individually, with a higher mortality rate for patients undergoing CABG at the Premier hospitals.
In addition, in the hospitals that underperformed initially, there was no difference with respect to rate of improvement when comparing Premier vs. non-Premier hospitals. Furthermore, mortality rate trends did not differ between those conditions that were incentivized (acute MI and CABG) compared with those that were not (congestive heart failure and pneumonia).
The authors concluded that there was “little evidence” that the Premier HQID pay-for-performance program resulted in reduced 30-day mortality.
Bottom line: Programs participating in the Medicare Premier HQID pay-for-performance program had similar 30-day mortality compared with nonparticipating programs.
Citation: Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366:1606-1615.
In-Hospital Rate of DVT/PE After Hip and Knee Arthroplasty with Guideline-Recommended Prophylaxis
Clinical question: What is the rate of symptomatic DVT/PE after total or partial hip (TPHA) or knee (TPKA) arthroplasty using currently recommended prophylaxis?
Background: The rate of postoperative DVT/PE after TPHA/TPKA has dropped dramatically with use of pharmacologic prophylaxis. However, the current rate of symptomatic DVT/PE using current pharmacologic prophylaxis is not known. Such rates are needed for informed patient consent and development of patient safety benchmarks.
Study design: Systematic review.
Setting: Randomized clinical trials (RCTs) and observational studies worldwide of adult inpatients undergoing TPHA and/or TPKA from 1996 to 2011.
Synopsis: Forty-seven studies were included, of which 41 were RCTs and six were observational studies. Twenty-one studies evaluated rates after TPHA, 20 after TPKA, and six after both. More than 44,000 patients were included, with ages ranging from 58 to 74 years. The mean duration of prophylaxis was eight days, with a mean follow-up of 13 days.
In-hospital rates of symptomatic venous thromboembolism (VTE) were higher in patients undergoing TPKA than TPHA (1.09% vs. 0.53% for VTE, 0.63% vs. 0.26% for DVT, and 0.27% vs. 0.14% for PE). This is in contrast to the higher rates of VTE observed after TPHA when the post-discharge period is included. The pooled incidence of VTE was lower with use of direct inhibitors of Factors Xa or IIa when compared to low-molecular-weight heparin, although a direct efficacy comparison could not be made.
Because the majority of studies were RCTs with restrictive inclusion criteria, rates of DVT/PE in actual practice might be higher.
Bottom line: When informing patients of postoperative VTE risk, or establishing benchmarks to evaluate patient safety, one can anticipate an in-hospital VTE rate of 0.5% following TPHA and 1% following TPKA.
Citation: Januel JM, Chen G, Ruffieux C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307(3):294-303.
Sodium Chloride Prevents Contrast-Induced Nephropathy
Clinical question: Is sodium chloride more effective than sodium bicarbonate for preventing contrast-induced nephropathy?
Background: Contrast-induced nephropathy can be prevented with precontrast hydration. Study authors sought to compare sodium chloride administered over 24 hours with sodium bicarbonate administered over seven hours, and with sodium bicarbonate administered orally.
Study design: Randomized, open-label, controlled trial.
Setting: Three European medical centers.
Synopsis: The study examined 258 patients with an estimated glomerular filtration rate <60mL/min/1.73m2 undergoing intravenous or intra-arterial contrast procedure. Patients with Class III or IV heart failure were excluded. The remaining patients were randomized to receive one of three regimens: sodium chloride at 1 ml/kg/hr for 12 hours prior to and 12 hours following the procedure; intravenous sodium bicarbonate for one hour prior to and six hours following the procedure; or intravenous and oral sodium bicarbonate 20 minutes prior to the procedure.
The group that received saline had a lower incidence of contrast-induced nephropathy than the bicarbonate groups (1% vs. 9% vs. 10%). The oral bicarbonate strategy was noninferior to the seven-hour intravenous bicarbonate strategy.
The authors postulated that saline should be used for high-risk patients, but that given the overall low incidence of contrast-induced nephropathy, the short-term bicarbonate strategy (intravenous followed by oral) is a viable alternative for low-risk patients.
Bottom line: Sodium chloride is more effective than sodium bicarbonate for preventing contrast-induced nephropathy, but in light of the low incidence, a short course of sodium bicarbonate is a possible convenient alternative for low-risk patients.
Citation: Klima T, Christ A, Marana I, et al. Sodium chloride vs. sodium bicarbonate for the prevention of contrast medium-induced nephropathy: a randomized controlled trial. Eur Heart J. 2012 Jan 19 [Epub ahead of print].
In This Edition
Literature At A Glance
A guide to this month’s studies
- Neutral head position safe for internal jugular vein cannulation
- Thrombolysis decreases mortality in unstable patients with acute PE
- Rectal indomethacin decreases incidence of post-ERCP pancreatitis
- CHADS2-VASc and HAS-BLED as predictors in afib patients
- No readmission, mortality decreases with self-supported COPD management
- Medicare Premier P4P initiatives do not decrease mortality
- In-hospital rate of DVT/PE after hip and knee arthroplasty
- Sodium chloride prevents contrast-induced nephropathy
Neutral Head Position Is Safe for Internal Jugular Vein Cannulation
Clinical question: Is there a difference in the complication rate between neutral head position and 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation?
Background: Cannulation of the internal jugular vein using ultrasound decreases the rate of major complications (carotid artery puncture, pneumothorax, and hemothorax). The relative positions of the internal jugular vein and the carotid artery change based on degree of neck rotation. The optimal position for ultrasound-guided vein puncture has not been shown.
Study design: Prospective, randomized, controlled, non-blinded study.
Setting: Tertiary neurosurgical center in Milan, Italy.
Synopsis: One thousand, three hundred thirty-two patients undergoing major neurosurgical procedures who needed central venous catheter placement were randomized to a neutral head position (NH) or a 45-degree neck rotation (HT) during ultrasound-guided internal jugular vein cannulation. Exclusion criteria were consent refusal, age <12 years, and coagulopathy. Six experienced anesthesiologists performed the procedures; blinding was not possible.
There was no difference in the rate of major complications (carotid artery puncture, pneumothorax, or hemothorax) based on head position (0.9% in NH vs. 0.6% in HT). Minor complications (multiple skin punctures, multiple vein punctures, difficulty inserting the guidewire) were similar in the two groups (13.2% in NG vs. 12.6% in HT). Neck rotation was not associated with operator-reported difficulty or vascular access time.
Limitations of the study include the inability to blind the operator. Additionally, the study involved six experienced anesthesiologists at one center who performed the procedure on patients needing an elective central line. The ability to generalize the findings to other settings, less experienced providers, and patients who need an emergency line is not certain.
Bottom line: Neutral head position is as safe as 45-degree neck rotation for elective ultrasound-guided internal jugular vein cannulation.
Citation: Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114:777-784.
Thrombolysis Decreases Mortality in Unstable Patients with Acute Pulmonary Embolism
Clinical question: Does thrombolytic therapy decrease mortality in unstable patients with acute pulmonary embolism (PE)?
Background: PE is a common problem; associated mortality is high. Despite this fact, the data supporting thrombolytic therapy in hemodynamically unstable patients are not robust, and randomized, controlled trials are unlikely to be performed.
Study design: Retrospective cohort study.
Setting: One thousand nonfederal, short-term U.S. hospitals.
Synopsis: Using data from the Nationwide Inpatient Sample database from 1999-2008, investigators found that thrombolysis decreased both all-cause and PE-specific mortality for unstable patients, defined as those either in shock or on a ventilator. Specifically for all-cause mortality, 15% of patients who received thrombolysis died vs. 47% of those who did not (RR 0.31, 95% CI 0.30-0.32). Placement of an inferior vena cava (IVC) filter further reduced mortality, to only 7.6% in patients who received both IVC filter and thrombolysis.
For PE-specific mortality, patients who received thrombolysis also had decreased rates, from 42% to 8.4% (RR 0.20; 95% CI 0.19-0.22). Across all age groups, patients who received thrombolysis had decreased all-cause and PE-specific mortality. Patients who did not receive thrombolysis had additional comorbidities.
As this study is retrospective, it might be affected by unknown confounding. In addition, it relies on coding data to identify patient stability and treatment. Despite this limitation, a randomized, controlled trial is unlikely to be performed at this stage. This study provides evidence to support use of thrombolysis in unstable patients.
Bottom line: Thrombolysis might reduce mortality in unstable patients with acute PE. In combination with IVC filters, the mortality reduction might be even greater.
Citation: Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012;125(5):465-470.
Rectal Indomethacin Decreases Incidence of Post-ERCP Pancreatitis
Clinical question: Does rectal indomethacin reduce the incidence of pancreatitis after ERCP?
Background: Acute pancreatitis is the most common complication from endoscopic retrograde cholangiopancreatography (ERCP). No pharmacologic treatment has proven to reduce the incidence of pancreatitis. Preliminary studies, including a meta-analysis, suggest that the use of NSAIDs might reduce the incidence of post-ERCP pancreatitis.
Study design: Randomized, placebo-controlled, double-blind trial.
Setting: Multicenter trial at four university-affiliated medical centers in the U.S.
Synopsis: More than 600 patients at high risk for post-ERCP pancreatitis were randomized to receive either two 50-mg indomethacin suppositories or two identical-appearing placebo suppositories. Patients were considered at high risk for pancreatitis based on previously identified patient- and procedure-related risk factors. Most of the participants had sphincter of Oddi dysfunction (84.4% of the indomethacin group and 80.5% of the placebo group). Exclusion criteria included elevated creatinine and active peptic ulcer disease. The indomethacin or placebo suppositories were given immediately following the ERCP.
Post-ERCP pancreatitis, defined by upper abdominal pain, elevation of pancreatic enzymes, and hospitalization for at least two nights, was significantly higher in the placebo group compared with the indomethacin group (16.9% vs. 9.2%, P=0.005). Moderate or severe post-ERCP pancreatitis was significantly higher in the placebo group compared with the indomethacin group (8.8% vs. 4.4%, P=0.03).
There were no significant differences in the rates of clinically significant bleeding or acute renal failure between the two groups. The ability to generalize these findings to patients without risk factors for post-ERCP pancreatitis is not clear.
Bottom line: Rectal indomethacin decreases the rate of post-ERCP pancreatitis in patients who are at high risk for this complication.
Citation: Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366(15):1414-1422.
CHADS2-VASc and HAS-BLED Can Predict Thromboembolism and Bleeding Risk in Afib Patients
Clinical question: What are the risk factors for stroke/thromboembolism and bleeding from atrial fibrillation (afib), and how well do the CHADS2-VASc and HAS-BLED stroke and bleeding risk-assessment tools perform against other published stroke and bleeding risk-assessment tools (CHADS2 and HEMORR2HAGES) for patients with afib?
Background: In afib patients, the CHADS2-VASc tool might offer more comprehensive stroke assessment over the CHADS2 by identifying truly-low-risk patients with afib who might not even need antiplatelet therapy. HAS-BLED, a newer bleeding-risk-assessment tool, has been validated in previous trials and is more user-friendly than others. Use of CHADS2-VASc and HAS-BLED are recommended by the European Society of Cardiology to assess stroke and bleeding risks for patients with afib.
Study design: Prospective cohort study.
Setting: All hospitals in Sweden.
Synopsis: Investigators identified 182,678 afib patients via ICD-10 data from Sweden’s National Hospital Discharge Registry ICD-10 from 2005 to 2008. Approximately half the patients were not taking anticoagulants. Analysis assessed risk factors for stroke and bleeding and the performance of CHADS2-VASc and HAS-BLED against CHADS2 and HEMORR2HAGES stroke and bleeding risk-assessment tools.
Risk of composite thromboembolism (unspecified stroke, TIA, systemic embolism) was significantly higher in patients with increased age, peripheral arterial disease, prior myocardial infarction (MI), prior coronary artery bypass grafting (CABG), female gender, renal failure, and aspirin use, as well as hypertension, diabetes, prior thromboembolic event, or prior intracranial hemorrhage (ICH). Interestingly, a statistically increased risk was seen with aspirin use.
Conversely, history of heart failure, thyroid disease, and obesity were not associated with increased composite thromboembolic risk. The use of CHADS2-VASc was marginally better than CHADS2 in predicting stroke risk.
ICH risk was increased in patients with older age, prior ischemic stroke, prior ICH, and hypertension. Risk of composite bleeding (from ICH or other major bleeding) was significantly higher in patients with these risk factors, as well as renal failure, liver disease, anemia, dysfunctional platelets, alcohol use, and cancer. Ischemic heart disease was associated with a statistically significant lower risk of ICH, but not of composite bleeding risk.
HAS-BLED usage was as good as, and easier to use than, HEMORR2HAGES in predicting bleeding risk.
Bottom line: CHADS2-VASc might be better than CHADS2 in predicting truly-low-risk patients with nonvalvular afib; HAS-BLED is just as good as, and easier to use than, HEMORR2HAGES in predicting bleeding risk for patients with nonvalvular afib who are to receive antithrombotic therapy.
Citation: Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182,678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500-10 [Epub 2012 Jan 13].
Supported Self-Management of COPD Does Not Decrease Readmission or Mortality Rates
Clinical question: Does supported self-management of patients with chronic obstructive pulmonary disease (COPD) decrease COPD-related hospital readmission or death?
Clinical background: Supported self-management has benefited patients with such chronic diseases as heart failure and asthma. Evidence to support such a strategy for patients with COPD is relatively lacking.
Study design: Randomized, controlled trial.
Setting: Community-based care following urban hospitalization in western Scotland.
Synopsis: From June 2007 to May 2009, and following hospitalization for COPD exacerbation, 464 patients were randomized to receive routine community-based care with or without 12 months of support and training to detect, and promptly treat, recurrent exacerbations. Independent of disease severity or demographics, investigators found no difference in combined readmission (48% vs. 47%, 95% confidence interval [CI] 0.80-1.38) or death (10% vs. 7%, 95% CI 0.71-2.61).
Based on review of appropriateness of self-management strategies used by the intervention group, unplanned exploratory subgroup analysis classified a minority of the intervention group as “successful” (42%) supported self-managers, and demonstrated decreased COPD readmissions and death (27% vs. 49%, 95% CI 0.25-0.76, P=0.003) vs. “unsuccessful” self-managers. This successful group was younger and tended to live with others. Further research to define characteristics of patients who benefit from self-management is needed.
Bottom line: Supported self-management of COPD does not reduce COPD-related readmission or death in a large population.
Citation: Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060 [Epub ahead of print].
Medicare Premier P4P Initiatives Do Not Decrease Mortality
Clinical question: Has the Medicare Premier Hospital Quality Incentive Demonstration (HQID) resulted in lower mortality?
Background: The Centers for Medicare & Medicaid Services’ (CMS) value-based purchasing program will expand to include 30-day mortality in 2013, but do pay-for-performance (P4P) initiatives result in improved mortality? Studies have demonstrated improvement in process of care but have not demonstrated mortality benefit thus far.
Study design: Cohort study.
Setting: Two hundred fifty-two hospitals participating in the Premier HQID compared with 3,363 control hospitals participating in the Hospital Compare program.
Synopsis: Researchers examined 30-day mortality for patients admitted with acute myocardial infarction, congestive heart failure, pneumonia, and for coronary artery bypass grafting (CABG) from 2003 to 2009. Results showed no difference in 30-day mortality rates over the six-year span of the program for any of the conditions studied in the Premier hospitals (participating in the voluntary pay-for-performance program) vs. non-Premier hospitals (11.82% vs. 11.74%). This held true for each condition measured individually, with a higher mortality rate for patients undergoing CABG at the Premier hospitals.
In addition, in the hospitals that underperformed initially, there was no difference with respect to rate of improvement when comparing Premier vs. non-Premier hospitals. Furthermore, mortality rate trends did not differ between those conditions that were incentivized (acute MI and CABG) compared with those that were not (congestive heart failure and pneumonia).
The authors concluded that there was “little evidence” that the Premier HQID pay-for-performance program resulted in reduced 30-day mortality.
Bottom line: Programs participating in the Medicare Premier HQID pay-for-performance program had similar 30-day mortality compared with nonparticipating programs.
Citation: Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. N Engl J Med. 2012;366:1606-1615.
In-Hospital Rate of DVT/PE After Hip and Knee Arthroplasty with Guideline-Recommended Prophylaxis
Clinical question: What is the rate of symptomatic DVT/PE after total or partial hip (TPHA) or knee (TPKA) arthroplasty using currently recommended prophylaxis?
Background: The rate of postoperative DVT/PE after TPHA/TPKA has dropped dramatically with use of pharmacologic prophylaxis. However, the current rate of symptomatic DVT/PE using current pharmacologic prophylaxis is not known. Such rates are needed for informed patient consent and development of patient safety benchmarks.
Study design: Systematic review.
Setting: Randomized clinical trials (RCTs) and observational studies worldwide of adult inpatients undergoing TPHA and/or TPKA from 1996 to 2011.
Synopsis: Forty-seven studies were included, of which 41 were RCTs and six were observational studies. Twenty-one studies evaluated rates after TPHA, 20 after TPKA, and six after both. More than 44,000 patients were included, with ages ranging from 58 to 74 years. The mean duration of prophylaxis was eight days, with a mean follow-up of 13 days.
In-hospital rates of symptomatic venous thromboembolism (VTE) were higher in patients undergoing TPKA than TPHA (1.09% vs. 0.53% for VTE, 0.63% vs. 0.26% for DVT, and 0.27% vs. 0.14% for PE). This is in contrast to the higher rates of VTE observed after TPHA when the post-discharge period is included. The pooled incidence of VTE was lower with use of direct inhibitors of Factors Xa or IIa when compared to low-molecular-weight heparin, although a direct efficacy comparison could not be made.
Because the majority of studies were RCTs with restrictive inclusion criteria, rates of DVT/PE in actual practice might be higher.
Bottom line: When informing patients of postoperative VTE risk, or establishing benchmarks to evaluate patient safety, one can anticipate an in-hospital VTE rate of 0.5% following TPHA and 1% following TPKA.
Citation: Januel JM, Chen G, Ruffieux C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307(3):294-303.
Sodium Chloride Prevents Contrast-Induced Nephropathy
Clinical question: Is sodium chloride more effective than sodium bicarbonate for preventing contrast-induced nephropathy?
Background: Contrast-induced nephropathy can be prevented with precontrast hydration. Study authors sought to compare sodium chloride administered over 24 hours with sodium bicarbonate administered over seven hours, and with sodium bicarbonate administered orally.
Study design: Randomized, open-label, controlled trial.
Setting: Three European medical centers.
Synopsis: The study examined 258 patients with an estimated glomerular filtration rate <60mL/min/1.73m2 undergoing intravenous or intra-arterial contrast procedure. Patients with Class III or IV heart failure were excluded. The remaining patients were randomized to receive one of three regimens: sodium chloride at 1 ml/kg/hr for 12 hours prior to and 12 hours following the procedure; intravenous sodium bicarbonate for one hour prior to and six hours following the procedure; or intravenous and oral sodium bicarbonate 20 minutes prior to the procedure.
The group that received saline had a lower incidence of contrast-induced nephropathy than the bicarbonate groups (1% vs. 9% vs. 10%). The oral bicarbonate strategy was noninferior to the seven-hour intravenous bicarbonate strategy.
The authors postulated that saline should be used for high-risk patients, but that given the overall low incidence of contrast-induced nephropathy, the short-term bicarbonate strategy (intravenous followed by oral) is a viable alternative for low-risk patients.
Bottom line: Sodium chloride is more effective than sodium bicarbonate for preventing contrast-induced nephropathy, but in light of the low incidence, a short course of sodium bicarbonate is a possible convenient alternative for low-risk patients.
Citation: Klima T, Christ A, Marana I, et al. Sodium chloride vs. sodium bicarbonate for the prevention of contrast medium-induced nephropathy: a randomized controlled trial. Eur Heart J. 2012 Jan 19 [Epub ahead of print].
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Does supported self-management of patients with chronic obstructive pulmonary disease (COPD) decrease COPD-related hospital readmission or death?
Clinical background: Supported self-management has benefited patients with such chronic diseases as heart failure and asthma. Evidence to support such a strategy for patients with COPD is relatively lacking.
Study design: Randomized, controlled trial.
Setting: Community-based care following urban hospitalization in western Scotland.
Synopsis: From June 2007 to May 2009, and following hospitalization for COPD exacerbation, 464 patients were randomized to receive routine community-based care with or without 12 months of support and training to detect, and promptly treat, recurrent exacerbations. Independent of disease severity or demographics, investigators found no difference in combined readmission rates (48% vs. 47%, 95% confidence interval [CI] 0.80-1.38) or death (10% vs. 7%, 95% CI 0.71-2.61).
Based on review of appropriateness of self-management strategies used by the intervention group, unplanned exploratory subgroup analysis classified a minority of the intervention group as “successful” (42%) supported self-managers, and demonstrated decreased COPD readmissions and deaths (27% vs. 49%, 95% CI 0.25-0.76, P=0.003) vs. “unsuccessful” self-managers. This successful group was younger and tended to live with others. Further research to define characteristics of patients who benefit from self-management is needed.
Bottom line: Supported self-management of COPD does not reduce COPD-related readmissions or deaths in a large population.
Citation: Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060.
Check out more physician reviews of HM-relevant research.
Clinical question: Does supported self-management of patients with chronic obstructive pulmonary disease (COPD) decrease COPD-related hospital readmission or death?
Clinical background: Supported self-management has benefited patients with such chronic diseases as heart failure and asthma. Evidence to support such a strategy for patients with COPD is relatively lacking.
Study design: Randomized, controlled trial.
Setting: Community-based care following urban hospitalization in western Scotland.
Synopsis: From June 2007 to May 2009, and following hospitalization for COPD exacerbation, 464 patients were randomized to receive routine community-based care with or without 12 months of support and training to detect, and promptly treat, recurrent exacerbations. Independent of disease severity or demographics, investigators found no difference in combined readmission rates (48% vs. 47%, 95% confidence interval [CI] 0.80-1.38) or death (10% vs. 7%, 95% CI 0.71-2.61).
Based on review of appropriateness of self-management strategies used by the intervention group, unplanned exploratory subgroup analysis classified a minority of the intervention group as “successful” (42%) supported self-managers, and demonstrated decreased COPD readmissions and deaths (27% vs. 49%, 95% CI 0.25-0.76, P=0.003) vs. “unsuccessful” self-managers. This successful group was younger and tended to live with others. Further research to define characteristics of patients who benefit from self-management is needed.
Bottom line: Supported self-management of COPD does not reduce COPD-related readmissions or deaths in a large population.
Citation: Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060.
Check out more physician reviews of HM-relevant research.
Clinical question: Does supported self-management of patients with chronic obstructive pulmonary disease (COPD) decrease COPD-related hospital readmission or death?
Clinical background: Supported self-management has benefited patients with such chronic diseases as heart failure and asthma. Evidence to support such a strategy for patients with COPD is relatively lacking.
Study design: Randomized, controlled trial.
Setting: Community-based care following urban hospitalization in western Scotland.
Synopsis: From June 2007 to May 2009, and following hospitalization for COPD exacerbation, 464 patients were randomized to receive routine community-based care with or without 12 months of support and training to detect, and promptly treat, recurrent exacerbations. Independent of disease severity or demographics, investigators found no difference in combined readmission rates (48% vs. 47%, 95% confidence interval [CI] 0.80-1.38) or death (10% vs. 7%, 95% CI 0.71-2.61).
Based on review of appropriateness of self-management strategies used by the intervention group, unplanned exploratory subgroup analysis classified a minority of the intervention group as “successful” (42%) supported self-managers, and demonstrated decreased COPD readmissions and deaths (27% vs. 49%, 95% CI 0.25-0.76, P=0.003) vs. “unsuccessful” self-managers. This successful group was younger and tended to live with others. Further research to define characteristics of patients who benefit from self-management is needed.
Bottom line: Supported self-management of COPD does not reduce COPD-related readmissions or deaths in a large population.
Citation: Bucknall CE, Miller G, Lloyd SM, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ. 2012;344:e1060.
Check out more physician reviews of HM-relevant research.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Do oral fluoroquinolones increase the risk of retinal detachment?
Background: Fluoroquinolones are increasingly used in both inpatient and outpatient settings, given their broad antimicrobial coverage. However, adverse effects, including those related to connective tissue and the eye, are increasingly reported. Whether that also includes retinal detachment is not yet known.
Study design: Nested case control study.
Setting: Canadian province.
Synopsis: Using data from administrative databases to identify patients who visited ophthalmologists in British Columbia between 2000 and 2007, the investigators identified 4,384 cases of retinal detachment, and matched those cases to controls at a rate of 10:1. Current, recent, and past fluoroquinolone usage was the exposure of interest.
Patients actively taking a fluoroquinolone had a higher risk of retinal detachment compared with those not taking the drug (adjusted RR of 4.5, 95% CI of 3.56-5.70). Prior or recent use of a fluoroquinolone did not increase the rate of retinal detachment. The patients were more likely to be male, myopic, diabetic, and have a prior history of cataract surgery. Ciprofloxacin was the drug most frequently involved, but this is not adjusted by frequency of prescription. Despite this association, the actual outcome is quite rare (approximately 1,440 cases per year in the U.S.).
This study has the benefit of a large amount of data and captures prescription data well. It relied on coding to identify the cases and might have missed or inappropriately categorized some cases. Despite these caveats, this study adds to the concerning adverse events due to the increasing use of fluoroquinolone therapy, and hospitalists should use appropriate clinical judgment when prescribing and educating patients about the risks and benefits.
Bottom line: Fluoroquinolone use might increase the rate of retinal detachment in patients, but the absolute risk of the event is low.
Citation: Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
Clinical question: Do oral fluoroquinolones increase the risk of retinal detachment?
Background: Fluoroquinolones are increasingly used in both inpatient and outpatient settings, given their broad antimicrobial coverage. However, adverse effects, including those related to connective tissue and the eye, are increasingly reported. Whether that also includes retinal detachment is not yet known.
Study design: Nested case control study.
Setting: Canadian province.
Synopsis: Using data from administrative databases to identify patients who visited ophthalmologists in British Columbia between 2000 and 2007, the investigators identified 4,384 cases of retinal detachment, and matched those cases to controls at a rate of 10:1. Current, recent, and past fluoroquinolone usage was the exposure of interest.
Patients actively taking a fluoroquinolone had a higher risk of retinal detachment compared with those not taking the drug (adjusted RR of 4.5, 95% CI of 3.56-5.70). Prior or recent use of a fluoroquinolone did not increase the rate of retinal detachment. The patients were more likely to be male, myopic, diabetic, and have a prior history of cataract surgery. Ciprofloxacin was the drug most frequently involved, but this is not adjusted by frequency of prescription. Despite this association, the actual outcome is quite rare (approximately 1,440 cases per year in the U.S.).
This study has the benefit of a large amount of data and captures prescription data well. It relied on coding to identify the cases and might have missed or inappropriately categorized some cases. Despite these caveats, this study adds to the concerning adverse events due to the increasing use of fluoroquinolone therapy, and hospitalists should use appropriate clinical judgment when prescribing and educating patients about the risks and benefits.
Bottom line: Fluoroquinolone use might increase the rate of retinal detachment in patients, but the absolute risk of the event is low.
Citation: Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
Clinical question: Do oral fluoroquinolones increase the risk of retinal detachment?
Background: Fluoroquinolones are increasingly used in both inpatient and outpatient settings, given their broad antimicrobial coverage. However, adverse effects, including those related to connective tissue and the eye, are increasingly reported. Whether that also includes retinal detachment is not yet known.
Study design: Nested case control study.
Setting: Canadian province.
Synopsis: Using data from administrative databases to identify patients who visited ophthalmologists in British Columbia between 2000 and 2007, the investigators identified 4,384 cases of retinal detachment, and matched those cases to controls at a rate of 10:1. Current, recent, and past fluoroquinolone usage was the exposure of interest.
Patients actively taking a fluoroquinolone had a higher risk of retinal detachment compared with those not taking the drug (adjusted RR of 4.5, 95% CI of 3.56-5.70). Prior or recent use of a fluoroquinolone did not increase the rate of retinal detachment. The patients were more likely to be male, myopic, diabetic, and have a prior history of cataract surgery. Ciprofloxacin was the drug most frequently involved, but this is not adjusted by frequency of prescription. Despite this association, the actual outcome is quite rare (approximately 1,440 cases per year in the U.S.).
This study has the benefit of a large amount of data and captures prescription data well. It relied on coding to identify the cases and might have missed or inappropriately categorized some cases. Despite these caveats, this study adds to the concerning adverse events due to the increasing use of fluoroquinolone therapy, and hospitalists should use appropriate clinical judgment when prescribing and educating patients about the risks and benefits.
Bottom line: Fluoroquinolone use might increase the rate of retinal detachment in patients, but the absolute risk of the event is low.
Citation: Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. Oral fluoroquinolones and the risk of retinal detachment. JAMA. 2012;307:1414-1419.
Guidelines for Management of Atrial Fibrillation
Background
Atrial fibrillation (AF) is a common condition, affecting more than 2 million Americans.1 Hospital admissions due to AF have increased 66% in the past two decades. Hospitalization accounts for 52% of the cost of AF management, and the mortality rate of patients with this arrhythmia is twice that of patients in sinus rhythm.1
AF management decisions include choices for rate control, rhythm control, and prevention of thromboembolism. The benefits of a rhythm-control versus a rate-control strategy continue to be evaluated, along with consideration regarding an appropriate heart-rate goal. The modifiable risk factor of stroke in atrial fibrillation also continues to be a target for intervention as atrial fibrillation accounts for one-sixth of all strokes.
Guideline Update
The American College of Cardiology Foundation and American Heart Association (ACC/AHA), in conjunction with the European Society of Cardiology, released practice guidelines on the management of patients with atrial fibrillation in 2006. The ACCF/AHA, writing with the Heart Rhythm Society, released focused updates in early 2011 to be incorporated into the previous guidelines, given new data from major clinical trials and the FDA approval of new medications with indications for AF treatment.2,3
The new recommendations address components of all three major management decisions for AF: rate control, rhythm control, and prevention of thromboembolism.
When managing AF with a rate-control strategy, new guidelines no longer recommend the goal of a resting heart rate of <80 bpm or <115 bpm with activity. This is based on data from the RACE II trial that show no difference in meaningful outcomes with a more aggressive heart-rate goal. Achieving a resting heart rate of 110 bpm was deemed a reasonable approach, as long as the patient has stable ventricular function and acceptable symptoms.
The new drug dronedarone has been introduced in the algorithm for maintenance of sinus rhythm strategy, based on the DIONYSOS, ATHENA, and ANDROMEDA studies. The new algorithm excluded the use of dronedarone in patients with left ventricular hypertrophy, decompensated heart failure, or Class IV heart failure because it was shown to increase mortality in these groups. The guidelines also recommend that it should also be used with caution in patients with bradycardia, prolonged QT interval, increased creatinine, and in patients on agents that moderate CYP3A4 function.
The risks of interventions to decrease thromboembolism against bleeding risk continue to be evaluated in specified patient populations. Although dabigatran did not have FDA approval prior to submission of the 2011 updated guidelines, the 2011 “focused update” incorporated the results of the RE-LY trial. Publication of RE-LY resulted in a Class 1 recommendation for dabigatran as a useful alternative to warfarin in patients with nonvalvular AF without severe renal failure or advanced liver disease.3 However, there is no specific antidote, and dabigatran use is associated with higher rates of dyspepsia and a nonsignificant increase in rates of myocardial infarction. In patients for whom oral anticoagulation with warfarin is considered unsuitable, aspirin with clopidogrel may be considered, although warfarin therapy continues to be a superior therapy to this dual antiplatelet regimen based on the ACTIVE-W and ACTIVE-A studies.2
Established Guideline Analysis
Apart from the listed updates, the management of AF has not changed considerably in the past decade. Rate control continues to be the recommended strategy for older patients along with appropriate symptom control, particularly if they have hypertension or heart disease. Rhythm control is a frequent strategy in AF management, but several studies have not found any difference in quality of life, development or progression of heart failure, or stroke rates in patients for whom a rhythm-control strategy was chosen.
Additionally, these patients still require anticoagulation, and the side effects of anti-arrhythmic drugs might offset the benefits of sinus rhythm. Therefore, rate control is an appropriate strategy. The stroke rate and side-effect risks with anti-arrhythmics are considerably lower in younger patients or those with paroxysmal lone AF, and so a rhythm-control strategy in these groups is reasonable.
Stroke rate in AF increases with known high-risk factors (prior thromboembolism or rheumatic mitral stenosis) and moderate-risk factors (heart failure, hypertension, age over 75, and diabetes). Less validated risk factors include female gender, age 65-74, thyrotoxicosis, and the presence of coronary artery disease.
There are well-defined recommendations for how to anticoagulate specific subgroups that pose clinical challenges not directly addressed in studies, but the guidelines do assist with:
- Patients who have a stroke with a therapeutic INR: Rather than adding antiplatelet agents, INR goal can be raised to 3-3.5;
- Patients >75 years old who are at a high risk for bleeding: A target INR of 2.0 (target range 1.6-2.5) seems reasonable;1 and
- Patients with stable coronary artery disease and AF: Warfarin anticoagulation alone should provide satisfactory antithrombotic prophylaxis against cerebrovascular and coronary atheroembolic events.1
Decisions involving perioperative management of anticoagulation in patients with AF frequently arise. Per the guidelines, in patients with nonvalvular AF, anticoagulation can be stopped for up to one week without bridging for surgical or diagnostic procedures, but bridging should be considered in high-risk patients.
HM Takeaways
Hospitalists are likely to manage AF, whether alone or in conjunction with cardiology consultation. These new comprehensive guidelines deal with rate control, rhythm control, and prevention of thromboembolism. Hospitalists should take particular interest in the guidelines regarding lenient rate control, dronedarone for rhythm control, and dabigatran as a new alternative for anticoagulation in appropriate populations.
Drs. Farrell and Carbo are hospitalists at Beth Israel Deaconess Medical Center in Boston.
References
- Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101-98.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8(1):157-76.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011;57(11):1330-7.
Background
Atrial fibrillation (AF) is a common condition, affecting more than 2 million Americans.1 Hospital admissions due to AF have increased 66% in the past two decades. Hospitalization accounts for 52% of the cost of AF management, and the mortality rate of patients with this arrhythmia is twice that of patients in sinus rhythm.1
AF management decisions include choices for rate control, rhythm control, and prevention of thromboembolism. The benefits of a rhythm-control versus a rate-control strategy continue to be evaluated, along with consideration regarding an appropriate heart-rate goal. The modifiable risk factor of stroke in atrial fibrillation also continues to be a target for intervention as atrial fibrillation accounts for one-sixth of all strokes.
Guideline Update
The American College of Cardiology Foundation and American Heart Association (ACC/AHA), in conjunction with the European Society of Cardiology, released practice guidelines on the management of patients with atrial fibrillation in 2006. The ACCF/AHA, writing with the Heart Rhythm Society, released focused updates in early 2011 to be incorporated into the previous guidelines, given new data from major clinical trials and the FDA approval of new medications with indications for AF treatment.2,3
The new recommendations address components of all three major management decisions for AF: rate control, rhythm control, and prevention of thromboembolism.
When managing AF with a rate-control strategy, new guidelines no longer recommend the goal of a resting heart rate of <80 bpm or <115 bpm with activity. This is based on data from the RACE II trial that show no difference in meaningful outcomes with a more aggressive heart-rate goal. Achieving a resting heart rate of 110 bpm was deemed a reasonable approach, as long as the patient has stable ventricular function and acceptable symptoms.
The new drug dronedarone has been introduced in the algorithm for maintenance of sinus rhythm strategy, based on the DIONYSOS, ATHENA, and ANDROMEDA studies. The new algorithm excluded the use of dronedarone in patients with left ventricular hypertrophy, decompensated heart failure, or Class IV heart failure because it was shown to increase mortality in these groups. The guidelines also recommend that it should also be used with caution in patients with bradycardia, prolonged QT interval, increased creatinine, and in patients on agents that moderate CYP3A4 function.
The risks of interventions to decrease thromboembolism against bleeding risk continue to be evaluated in specified patient populations. Although dabigatran did not have FDA approval prior to submission of the 2011 updated guidelines, the 2011 “focused update” incorporated the results of the RE-LY trial. Publication of RE-LY resulted in a Class 1 recommendation for dabigatran as a useful alternative to warfarin in patients with nonvalvular AF without severe renal failure or advanced liver disease.3 However, there is no specific antidote, and dabigatran use is associated with higher rates of dyspepsia and a nonsignificant increase in rates of myocardial infarction. In patients for whom oral anticoagulation with warfarin is considered unsuitable, aspirin with clopidogrel may be considered, although warfarin therapy continues to be a superior therapy to this dual antiplatelet regimen based on the ACTIVE-W and ACTIVE-A studies.2
Established Guideline Analysis
Apart from the listed updates, the management of AF has not changed considerably in the past decade. Rate control continues to be the recommended strategy for older patients along with appropriate symptom control, particularly if they have hypertension or heart disease. Rhythm control is a frequent strategy in AF management, but several studies have not found any difference in quality of life, development or progression of heart failure, or stroke rates in patients for whom a rhythm-control strategy was chosen.
Additionally, these patients still require anticoagulation, and the side effects of anti-arrhythmic drugs might offset the benefits of sinus rhythm. Therefore, rate control is an appropriate strategy. The stroke rate and side-effect risks with anti-arrhythmics are considerably lower in younger patients or those with paroxysmal lone AF, and so a rhythm-control strategy in these groups is reasonable.
Stroke rate in AF increases with known high-risk factors (prior thromboembolism or rheumatic mitral stenosis) and moderate-risk factors (heart failure, hypertension, age over 75, and diabetes). Less validated risk factors include female gender, age 65-74, thyrotoxicosis, and the presence of coronary artery disease.
There are well-defined recommendations for how to anticoagulate specific subgroups that pose clinical challenges not directly addressed in studies, but the guidelines do assist with:
- Patients who have a stroke with a therapeutic INR: Rather than adding antiplatelet agents, INR goal can be raised to 3-3.5;
- Patients >75 years old who are at a high risk for bleeding: A target INR of 2.0 (target range 1.6-2.5) seems reasonable;1 and
- Patients with stable coronary artery disease and AF: Warfarin anticoagulation alone should provide satisfactory antithrombotic prophylaxis against cerebrovascular and coronary atheroembolic events.1
Decisions involving perioperative management of anticoagulation in patients with AF frequently arise. Per the guidelines, in patients with nonvalvular AF, anticoagulation can be stopped for up to one week without bridging for surgical or diagnostic procedures, but bridging should be considered in high-risk patients.
HM Takeaways
Hospitalists are likely to manage AF, whether alone or in conjunction with cardiology consultation. These new comprehensive guidelines deal with rate control, rhythm control, and prevention of thromboembolism. Hospitalists should take particular interest in the guidelines regarding lenient rate control, dronedarone for rhythm control, and dabigatran as a new alternative for anticoagulation in appropriate populations.
Drs. Farrell and Carbo are hospitalists at Beth Israel Deaconess Medical Center in Boston.
References
- Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101-98.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8(1):157-76.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011;57(11):1330-7.
Background
Atrial fibrillation (AF) is a common condition, affecting more than 2 million Americans.1 Hospital admissions due to AF have increased 66% in the past two decades. Hospitalization accounts for 52% of the cost of AF management, and the mortality rate of patients with this arrhythmia is twice that of patients in sinus rhythm.1
AF management decisions include choices for rate control, rhythm control, and prevention of thromboembolism. The benefits of a rhythm-control versus a rate-control strategy continue to be evaluated, along with consideration regarding an appropriate heart-rate goal. The modifiable risk factor of stroke in atrial fibrillation also continues to be a target for intervention as atrial fibrillation accounts for one-sixth of all strokes.
Guideline Update
The American College of Cardiology Foundation and American Heart Association (ACC/AHA), in conjunction with the European Society of Cardiology, released practice guidelines on the management of patients with atrial fibrillation in 2006. The ACCF/AHA, writing with the Heart Rhythm Society, released focused updates in early 2011 to be incorporated into the previous guidelines, given new data from major clinical trials and the FDA approval of new medications with indications for AF treatment.2,3
The new recommendations address components of all three major management decisions for AF: rate control, rhythm control, and prevention of thromboembolism.
When managing AF with a rate-control strategy, new guidelines no longer recommend the goal of a resting heart rate of <80 bpm or <115 bpm with activity. This is based on data from the RACE II trial that show no difference in meaningful outcomes with a more aggressive heart-rate goal. Achieving a resting heart rate of 110 bpm was deemed a reasonable approach, as long as the patient has stable ventricular function and acceptable symptoms.
The new drug dronedarone has been introduced in the algorithm for maintenance of sinus rhythm strategy, based on the DIONYSOS, ATHENA, and ANDROMEDA studies. The new algorithm excluded the use of dronedarone in patients with left ventricular hypertrophy, decompensated heart failure, or Class IV heart failure because it was shown to increase mortality in these groups. The guidelines also recommend that it should also be used with caution in patients with bradycardia, prolonged QT interval, increased creatinine, and in patients on agents that moderate CYP3A4 function.
The risks of interventions to decrease thromboembolism against bleeding risk continue to be evaluated in specified patient populations. Although dabigatran did not have FDA approval prior to submission of the 2011 updated guidelines, the 2011 “focused update” incorporated the results of the RE-LY trial. Publication of RE-LY resulted in a Class 1 recommendation for dabigatran as a useful alternative to warfarin in patients with nonvalvular AF without severe renal failure or advanced liver disease.3 However, there is no specific antidote, and dabigatran use is associated with higher rates of dyspepsia and a nonsignificant increase in rates of myocardial infarction. In patients for whom oral anticoagulation with warfarin is considered unsuitable, aspirin with clopidogrel may be considered, although warfarin therapy continues to be a superior therapy to this dual antiplatelet regimen based on the ACTIVE-W and ACTIVE-A studies.2
Established Guideline Analysis
Apart from the listed updates, the management of AF has not changed considerably in the past decade. Rate control continues to be the recommended strategy for older patients along with appropriate symptom control, particularly if they have hypertension or heart disease. Rhythm control is a frequent strategy in AF management, but several studies have not found any difference in quality of life, development or progression of heart failure, or stroke rates in patients for whom a rhythm-control strategy was chosen.
Additionally, these patients still require anticoagulation, and the side effects of anti-arrhythmic drugs might offset the benefits of sinus rhythm. Therefore, rate control is an appropriate strategy. The stroke rate and side-effect risks with anti-arrhythmics are considerably lower in younger patients or those with paroxysmal lone AF, and so a rhythm-control strategy in these groups is reasonable.
Stroke rate in AF increases with known high-risk factors (prior thromboembolism or rheumatic mitral stenosis) and moderate-risk factors (heart failure, hypertension, age over 75, and diabetes). Less validated risk factors include female gender, age 65-74, thyrotoxicosis, and the presence of coronary artery disease.
There are well-defined recommendations for how to anticoagulate specific subgroups that pose clinical challenges not directly addressed in studies, but the guidelines do assist with:
- Patients who have a stroke with a therapeutic INR: Rather than adding antiplatelet agents, INR goal can be raised to 3-3.5;
- Patients >75 years old who are at a high risk for bleeding: A target INR of 2.0 (target range 1.6-2.5) seems reasonable;1 and
- Patients with stable coronary artery disease and AF: Warfarin anticoagulation alone should provide satisfactory antithrombotic prophylaxis against cerebrovascular and coronary atheroembolic events.1
Decisions involving perioperative management of anticoagulation in patients with AF frequently arise. Per the guidelines, in patients with nonvalvular AF, anticoagulation can be stopped for up to one week without bridging for surgical or diagnostic procedures, but bridging should be considered in high-risk patients.
HM Takeaways
Hospitalists are likely to manage AF, whether alone or in conjunction with cardiology consultation. These new comprehensive guidelines deal with rate control, rhythm control, and prevention of thromboembolism. Hospitalists should take particular interest in the guidelines regarding lenient rate control, dronedarone for rhythm control, and dabigatran as a new alternative for anticoagulation in appropriate populations.
Drs. Farrell and Carbo are hospitalists at Beth Israel Deaconess Medical Center in Boston.
References
- Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11):e101-98.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm. 2011;8(1):157-76.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011;57(11):1330-7.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: With the current use of warfarin for stroke prophylaxis in patients with nonvalvular atrial fibrillation, what do the most recent data show with regard to time spent in the therapeutic window, stroke risk, and bleeding risk?
Background: Historically, warfarin has been shown to decrease stroke risk in nonvalvular atrial fibrillation by 62% compared with placebo, balanced by a significant risk of bleeding. Despite the availability of multiple new antithrombotic agents, warfarin will likely continue to be widely used given its lower cost. As a result, physicians need an accurate estimate of warfarin’s efficacy and safety as currently used in practice.
Study design: Meta-analysis of randomized controlled trials (RCTs).
Setting: RCTs comparing warfarin to an alternative antithrombotic agent from 2001 to 2011.
Synopsis: Eight RCTs of nonvalvular atrial fibrillation were included, yielding data on 32,053 patients with a mean age range of 70 to 82 years and widely variable CHADS2 scores. The time spent at a therapeutic INR was found to be improved when compared to historical rates, ranging from 55% to 68%. The rate of stroke or non-central-nervous-system embolism ranged from 1.2% to 2.3% per year, with a pooled event rate of 1.66% per year, compared with 2.09% per year in earlier trials.
Major bleeding was defined differently across studies, with a reported incidence of 1.4% to 3.4% per year, a pooled event rate of intracranial hemorrhage of 0.61%, and a cumulative adverse event rate of 3.0% to 7.64%. Stroke rates were highest in patients older than 75 years, women, those with a history of transient ischemic attack or stroke, those new to warfarin, and those with higher CHADS2 scores.
Bottom line: Warfarin as currently used is associated with an annual rate of stroke or systemic embolism of 1.66% and an annual rate of major bleeding ranging from 1.4% to 3.4%.
Citation: Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med. 2012;172:623-631.
Clinical question: With the current use of warfarin for stroke prophylaxis in patients with nonvalvular atrial fibrillation, what do the most recent data show with regard to time spent in the therapeutic window, stroke risk, and bleeding risk?
Background: Historically, warfarin has been shown to decrease stroke risk in nonvalvular atrial fibrillation by 62% compared with placebo, balanced by a significant risk of bleeding. Despite the availability of multiple new antithrombotic agents, warfarin will likely continue to be widely used given its lower cost. As a result, physicians need an accurate estimate of warfarin’s efficacy and safety as currently used in practice.
Study design: Meta-analysis of randomized controlled trials (RCTs).
Setting: RCTs comparing warfarin to an alternative antithrombotic agent from 2001 to 2011.
Synopsis: Eight RCTs of nonvalvular atrial fibrillation were included, yielding data on 32,053 patients with a mean age range of 70 to 82 years and widely variable CHADS2 scores. The time spent at a therapeutic INR was found to be improved when compared to historical rates, ranging from 55% to 68%. The rate of stroke or non-central-nervous-system embolism ranged from 1.2% to 2.3% per year, with a pooled event rate of 1.66% per year, compared with 2.09% per year in earlier trials.
Major bleeding was defined differently across studies, with a reported incidence of 1.4% to 3.4% per year, a pooled event rate of intracranial hemorrhage of 0.61%, and a cumulative adverse event rate of 3.0% to 7.64%. Stroke rates were highest in patients older than 75 years, women, those with a history of transient ischemic attack or stroke, those new to warfarin, and those with higher CHADS2 scores.
Bottom line: Warfarin as currently used is associated with an annual rate of stroke or systemic embolism of 1.66% and an annual rate of major bleeding ranging from 1.4% to 3.4%.
Citation: Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med. 2012;172:623-631.
Clinical question: With the current use of warfarin for stroke prophylaxis in patients with nonvalvular atrial fibrillation, what do the most recent data show with regard to time spent in the therapeutic window, stroke risk, and bleeding risk?
Background: Historically, warfarin has been shown to decrease stroke risk in nonvalvular atrial fibrillation by 62% compared with placebo, balanced by a significant risk of bleeding. Despite the availability of multiple new antithrombotic agents, warfarin will likely continue to be widely used given its lower cost. As a result, physicians need an accurate estimate of warfarin’s efficacy and safety as currently used in practice.
Study design: Meta-analysis of randomized controlled trials (RCTs).
Setting: RCTs comparing warfarin to an alternative antithrombotic agent from 2001 to 2011.
Synopsis: Eight RCTs of nonvalvular atrial fibrillation were included, yielding data on 32,053 patients with a mean age range of 70 to 82 years and widely variable CHADS2 scores. The time spent at a therapeutic INR was found to be improved when compared to historical rates, ranging from 55% to 68%. The rate of stroke or non-central-nervous-system embolism ranged from 1.2% to 2.3% per year, with a pooled event rate of 1.66% per year, compared with 2.09% per year in earlier trials.
Major bleeding was defined differently across studies, with a reported incidence of 1.4% to 3.4% per year, a pooled event rate of intracranial hemorrhage of 0.61%, and a cumulative adverse event rate of 3.0% to 7.64%. Stroke rates were highest in patients older than 75 years, women, those with a history of transient ischemic attack or stroke, those new to warfarin, and those with higher CHADS2 scores.
Bottom line: Warfarin as currently used is associated with an annual rate of stroke or systemic embolism of 1.66% and an annual rate of major bleeding ranging from 1.4% to 3.4%.
Citation: Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med. 2012;172:623-631.
Embedding a Discharge Facilitator
Recent studies have shown that a patient's discharge from the hospital is a vulnerable period for patient safety.14 With the reduction in length of stay (LOS) and the increase in patient acuity over the past decade, patients are discharged from acute care settings quicker and sicker, resulting in management of ongoing illness in a less‐monitored environment.5, 6 In addition, in teaching hospitals, residents are supervised by hospital‐based physicians who are rarely the primary care physician (PCP) for the residents' patients, which creates discontinuity of care.
One in 5 medical discharges is complicated by an adverse event believed, in part, to be due to poor communication between caregivers during this transition time.2 Discharge summaries, a key form of that communication, are not always done in a timely fashion and may lack key pieces of information.7, 8 For approximately 68% of patient discharges, the PCP will not have a discharge summary available for the patient's first follow‐up visit.911 In a survey of PCPs whose patients were in the hospital, only 23% reported direct communication with the hospital care team.12 This leaves PCPs unaware of pending test results or recommended follow‐up evaluations.10, 11, 13, 14 All of these factors are believed to contribute to adverse events, emergency department (ED) visits, and readmissions.
A recently published consensus statement on transitions of care by 6 major medical societies emphasizes the need for timely communication and transfer of information.15 These important processes are especially challenging to meet at academic medical centers, where discharge summaries and transition communication are done by residents in a hectic and challenging work environment, with multiple simultaneous and competing demands including outpatient clinic and required conferences.12 Residents have little formal training in how to write an effective discharge summary or how to systematically approach discharge planning. One study found higher error rates in discharge summaries written by residents compared with attending physicians.16 While the Accreditation Council for Graduate Medical Education (ACGME) limits the number of admissions per intern for both patient safety and educational reasons, the number of discharges per day is not limited despite the considerable amount of time required for appropriate discharge planning and communication.
Many interventions have been tried to improve the discharge process and reduce patient adverse events.17 Arranging early follow‐up appointments to reduce emergency department visits and readmissions has shown mixed results.13, 1820 Interventions that focus on specific populations, such as the elderly or patients with congestive heart failure, have been more successful.2123 Some interventions employed additional resources, such as a discharge form, transition coach, or discharge advocate, again with varying impact on results.18, 2427 A recent study by Jack et al. used nurse discharge advocates (DAs) to help with discharge planning and communication at an academic medical center.25 These DAs were independent of the care team, and focused on patient education and follow‐up plans, and reduced hospital reutilization in a selected population.
No studies have assessed the potential benefit of helping residents with the physician components of the discharge process. Prior studies have mainly focused on patient communication and follow‐up appointments, yet safe transitions also involve timely discharge summaries, physician‐to‐physician communication, physician‐to‐nurse communication, and medication reconciliation. Without support and time, these tasks can be very challenging for resident physicians with work‐hour limitations. We undertook a randomized, controlled trial to evaluate the impact on the discharge process of embedding a discharge facilitator in a resident medical team to help with the physician discharge process. We studied the effect for all the patients discharged from the resident team, rather than focusing on a select group or patients with a single diagnosis.
METHODS
Study Setting and Participants
This study was conducted on 2 of the 5 resident general medical teams on the inpatient teaching service at Massachusetts General Hospital (MGH), Boston, Massachusettsa large, 907‐bed, urban hospital. The residents' teams are regionalized and each care for approximately 20 patients on a single floor. Each of the study teams consists of a junior resident, 4 interns, and 1 to 2 attendings who rotate on the floor for 2‐week or 4‐week blocks. Attending rounds, which occur 10 AM to 12 PM weekdays, are for new patient presentations and discussion of plans. Interdisciplinary rounds occur 9:30 AM to 10 AM. Sign‐out rounds occur in the afternoon whenever all work is complete. The junior resident is responsible for all the discharge orders and communication with PCPs, and the discharge summaries for patients going to facilities. The interns are responsible for discharge summaries for patients discharged home; these summaries are not mandatory at the time of discharge. The majority of patients were admitted under the team attending(s). Patients were assigned to the teams by the admitting office, based on bed availability. All patients discharged from both resident medical teams over a 5‐month period were included in this study. Those who were not discharged from the hospital by the study teams (ie, transfers to intensive care units or deaths) were excluded. These exclusions accounted for less than 12% of all team patients. Partners Healthcare System Institutional Review Board approved all study activities.
Intervention
We randomly assigned a discharge facilitator (DF), a master's level nurse practitioner with prior inpatient medicine experience, to 1 of the 5 resident medical teams. She had no prior experience on this specific floor. A similar resident team, on a different floor, served as the control. For the intervention team, the DF attended daily resident work rounds and interdisciplinary discharge rounds. The resident and DF collaborated in identifying patients being discharged in the next 1 to 3 days, and the DF scheduled all follow‐up appointments and tests. The DF performed medication reconciliation, wrote prescriptions and faxed them to pharmacies, and arranged all anticoagulation services. In collaboration with the resident, the DF called PCPs' offices with discharge information and faxed discharge summaries to PCPs' offices outside the Partners Healthcare System. The DF wrote part or all of the computer discharge orders and discharge summaries at the request of the resident and interns. All discharge summaries still needed to be reviewed, edited, and signed by the resident or interns. The DF also noted pending tests and studies at time of discharge, and followed up on these tests for the team. The DF met with all patients to answer any questions about their discharge plan, medications, and appointments; while residents are encouraged to do this, it is not done as consistently. She provided her business card for any questions after their discharge. Follow‐up patient calls to the DF were either answered by her or triaged to the appropriate person. The DF also communicated with the patient's nurse about the discharge plans. For all patients discharged over a weekend, the DF would arrange the follow‐up appointments on Mondays and call the patients at home.
For both teams, residents received letters at the start of their rotation notifying them of the study and asking them to complete discharge summaries within 24 hours. All residents in the program were expected to do an online discharge tutorial and attend a didactic lecture on discharge summaries. The residents on the intervention team received a 5‐minute orientation on how best to work with the DF. Residents were given the autonomy to decide how much to use the DF's services. The scheduling of follow‐up appointments on the control team was the responsibility of the team resident as per usual care. The nursing component of the discharge process, including patient discharge education, was the same on both teams. Nurses on both floors are identically trained on these aspects of care. The nurses on both teams were surveyed about perception of the discharge process prior to the intervention and after the intervention. A research assistant (RA) called patients discharged home on both teams, 1 week after discharge, to ask about satisfaction with the discharge process, to determine if the patients had any questions, and to verify patient knowledge regarding whom they should contact for problems. The RA also noted the end time of attending rounds each day and the start time of resident sign‐out.
Outcome Measures and Follow‐Up
At the time of discharge, the RA collected baseline data on all patients discharged from both teams, including the number of follow‐up appointments scheduled. Patients were tracked through electronic medical records to see if and when they attended their follow‐up appointments, whether they changed the appointment, and whether patients returned to a hospital emergency department or were readmitted to MGH or an affiliated Partners hospital within 30 days. For patients outside the MGHPartners system, the research assistant contacted primary care physician offices to document follow‐up. The remaining patient data was obtained through the MGHPartners computerized information system.
The primary outcomes of the study were length of stay, time of discharge, number of emergency department visits, hospital readmissions, numbers of discharge summaries completed in 24 hours, time from discharge to discharge summary completion, and whether the discharge summary was completed before follow‐up. Secondary outcomes were number of follow‐up PCP appointments made at time of discharge, percentage of follow‐up appointments attended and time from discharge to attending a follow‐up appointment, patient phone survey results, and nursing perception of the discharge process, as well as the percentage of attending rounds that ended on time and the time of resident sign‐out.
Statistical Analyses
Patient characteristics were compared between intervention and control teams using 2‐sample t tests or Wilcoxon rank sum tests for continuous variables, and chi‐square tests for categorical variables. Hours to discharge summary completion and hospital length of stay were summarized using median and interquartiles (IQR), and compared between the 2 teams using Wilcoxon rank sum tests. Categorical outcomes were compared using chi‐square tests. Two‐sided P values 0.05 were considered statistically significant. SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
Study Sample
During the 5‐month intervention (November 12, 2008 to April 14, 2009), a combined total of 999 patients were admitted to the intervention and control general medical teams. We excluded 96 patients who were not discharged but transferred to another service or intensive care units, and 24 patients who died. We also excluded 7 patients who were discharged from both teams the first day of the study, because the DF was not involved with the patients' discharge planning. That left 872 patients discharged to either home, a facility, or having left against medical advice (AMA) included in the study: 440 patients on the intervention team and 432 patients on the control team (Figure 1). Baseline patient demographic and clinical characteristics were similar across both teams with only gender being significantly different (Table 1). The mean age was 63 years (range, 1896) and the mean comorbidity score was 2.3 (range, 012). Of note, about a quarter of patients were discharged to facilities, about half were Medicare recipients, and approximately 80% had a PCP. The DF participated in the discharge process for nearly all of the intervention patients; she reported contributing approximately 50% of the content to the discharge summaries.
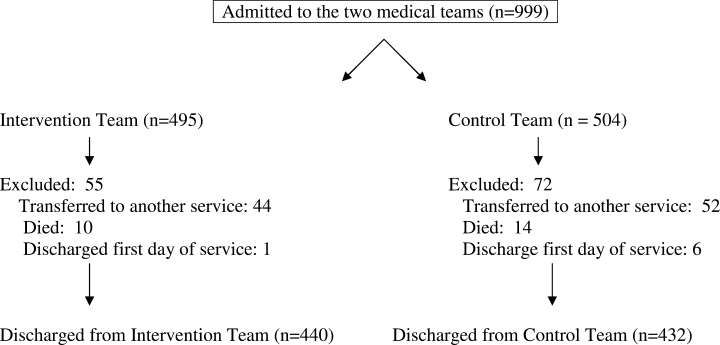
| Characteristics | Intervention Team | Control Team |
|---|---|---|
| n = 440 | n = 432 | |
| ||
| Mean age (SD), year | 63 (18) | 63 (18) |
| Women, n (%)* | 181 (41) | 207 (48) |
| Race, n (%) | ||
| White non‐Hispanic | 267 (61) | 243 (56) |
| Black non‐Hispanic | 24 (5) | 33 (8) |
| Hispanic | 21 (5) | 17 (4) |
| Unknown/other | 128 (29) | 139 (32) |
| Health insurance, n (%) | ||
| Medicare | 213 (48) | 226 (52) |
| Medicaid | 85 (19) | 81 (19) |
| Private | 110 (25) | 91 (21) |
| Other | 32 (7) | 34 (8) |
| PCP on admission, n (%) | 370 (84) | 356 (82) |
| Discharge disposition, n (%) | ||
| AMA | 12 (3) | 14 (3) |
| Home | 305 (69) | 315 (73) |
| Facility | 123 (28) | 103 (24) |
| Mean comorbidity index score (SD) | 2.3 (2.4) | 2.3 (2.4) |
| Diagnoses | ||
| Congestive heart failure | 30 (6%) | 27 (5%) |
| COPD/asthma | 34 (7%) | 47 (9%) |
| Cardiovascular disease | 54 (11%) | 50 (8%) |
| Alcohol/substance abuse | 29 (6%) | 34 (7%) |
| Gastrointestinal bleeds/ulcers | 38 (8%) | 41 (8%) |
| Hepatobiliary disease | 30 (6%) | 36 (7%) |
| Renal failure/kidney disease | 33 (7%) | 37 (7%) |
| Pneumonia | 36 (7%) | 22 (4%) |
| Musculoskeletal disease | 26 (5%) | 23 (5%) |
| Neurologic disease | 22 (4%) | 25 (5%) |
| Other | 163 (33%) | 172 (35%) |
Primary Outcomes
Primary outcomes from the 2 medical teams are listed in Table 2. In the intervention group, significantly more discharge summaries were completed within 24 hours compared to the control group (293 [67%] vs 207 [48%]; P < 0.0001). Since nearly all patients discharged to facilities must have a discharge summary at the time of discharge, the overall difference in completion rates came mainly from patients discharged home or having left AMA from the intervention team (177 [56%] vs 112 [34%]; P < 0.0001). For all discharge summaries, the median time to completion on the intervention team was 18.9 hours compared with 73.1 hours on the control team (P < 0.0001). More discharge summaries were completed before the first follow‐up appointment on the intervention team (393 [89%] vs 330 [76%]; P < 0.001). The DF intervention had no effect on 30‐day readmission or emergency department visits. For patients on the DF team, 88 (20%) were readmitted within 30 days of discharge, as compared with 79 (18%) on the control team (P = 0.55). Similarly, 40 (9%) of the intervention team patients, as compared with 39 (9%) of the control team patients, visited the emergency department at least once within 30 days (P = 1.0). There was no difference in length of stay (LOS) between the 2 teams (median 4.0 days for both teams, P = 0.84).
| Intervention Team | Control Team | ||
|---|---|---|---|
| Variables | n = 440 | n = 432 | P Value |
| |||
| Discharge summaries completed 24 hr, n (%) | 293 (67) | 207 (48) | <0.0001 |
| Discharges to facilities | 116 (94) | 95 (92) | 0.60 |
| Discharges to home/AMA | 177 (56) | 112 (34) | <0.0001 |
| Median hours to discharge summary completion for discharges to home/AMA (IQR) | 18.9 (0138) | 73.1 (4.3286) | <0.0001 |
| Discharge summary complete before time of follow‐up appointment. | 393 (89) | 330 (76) | <0.0001 |
| Emergency department visits in 30 days, n (%) | 40 (9) | 39 (9) | 1.0 |
| Readmissions in 30 days, n (%) | 88 (20) | 79 (18) | 0.55 |
| Median length of stay, days (IQR) | 4.0 (37) | 4.0 (28) | 0.84 |
| Discharges to facilities | 6.0 (511) | 8.0 (513) | 0.17 |
| Discharges to home/AMA | 4.0 (26) | 3.0 (26) | 0.61 |
| Discharged by noon, n (%) | 38 (9) | 42 (10) | 0.64 |
Secondary Outcomes
Table 3 shows secondary outcomes from the 2 medical teams. Among the patients discharged from the DF team, 264 (62%) had scheduled follow‐up appointments with PCPs compared to the control team 151 (36%) (P < 0.0001). (Many patients going to rehabilitation hospitals are not given PCP appointments at the time of discharge.) Despite having more scheduled appointments, patients' actual follow‐up with PCPs was similar during the 5‐month study period among both intervention and control group (234 [65%] vs 223 [63%]; P = 0.58). However, there was earlier follow‐up with the primary provider in the first 2 or 4 weeks in the intervention group. At 2 weeks, 129 (36%) patients in the intervention group saw their provider compared to 81 (23%) patients in the control group (P < 0.0002), and at 4 weeks, 159 (44%) of the intervention group was seen compared to 99 (28%) of the control group (P < 0.0001). Of note, among the 415 patients on both teams discharged with scheduled appointments, only 53 (13%) of patients did not show up for the scheduled appointment and this no‐show rate was the same on both teams.
| Variables | Intervention Team | Control Team | P Value |
|---|---|---|---|
| |||
| No. of eligible patients* | 428 | 418 | |
| Patients with follow‐up appointments to primary providers, n (%) | 264 (62) | 151 (36) | <0.0001 |
| No. of eligible patients | 359 | 354 | |
| Attended follow‐up appointment with primary provider during study, n (%) | 234 (65) | 223 (63) | 0.58 |
| Within 2 weeks of discharge | 129 (36) | 81 (23) | 0.0002 |
| Within 4 weeks of discharge | 159 (44) | 99 (28) | <0.0001 |
| No. of days round times were recorded | 100 | 99 | |
| No. of attending rounds ending by 12 PM | 45 (45%) | 31 (31%) | 0.058 |
| Mean start time of sign‐out rounds | 16:38 | 17:24 | 0.0007 |
Attending rounds ended on time (12 PM) 45% of the time in the intervention group compared to 31% in the control group (P = 0.058). Mean start time of resident sign‐out rounds was 1638 hours on the intervention team and 1724 hours on the control team (P = 0.0007).
We obtained patient reported outcome data by telephone within 2 to 4 weeks of discharge. Of the 620 patients discharged to home, 6 died or were readmitted to the hospital before being reached by phone. For the remaining 614 patients, we were able to contact 444 (72%). Of those, 321 (52%) agreed to participate in the phone interview. We surveyed similar proportions of intervention and control group patients (158 [52%] vs 163 [52%]) (Table 4). Both groups reported similar rates of having questions about their hospital stay after discharge (43 [27%] vs 49 [30%]; P = 0.62). The intervention group could better identify whom to call with questions (150 [95%] vs 138 [85%]; P = 0.003). The intervention group reported better understanding of their follow‐up plans (157 [99%] vs 141 [87%]; P = 0.001) and better understanding of their discharge medications (152 [96%] vs 142 [87%]; P = 0.001). More patients in the intervention group were satisfied with the discharge process (153 [97%] vs 124 [76%]; P < 0.0001).
| Intervention Team | Control Team | P Value | |
|---|---|---|---|
| |||
| Patients discharged home* | 304 | 310 | |
| Patients contacted by phone after discharge, n (%) | 213 (70) | 231 (75) | 0.24 |
| Agreed to participate in phone interview, n (%) | 158 (52) | 163 (53) | 0.94 |
| Among those agreed to participate, n (%) | |||
| Did you have questions about your hospital stay? | 43 (27) | 49 (30) | 0.62 |
| Would you know who to call if you had questions after discharge? | 150 (95) | 138 (85) | 0.003 |
| Satisfied with the discharge process? | 153 (97) | 124 (76) | <0.0001 |
| Did you understand your follow‐up plans? | 157 (99) | 141 (87) | <0.0001 |
| Did you understand your medications? | 152 (96) | 142 (87) | 0.001 |
| Did you feel safe going home? | 153 (97) | 151 (92) | 0.07 |
Compared with nurses on the control team, nurses on the intervention team more often reported paperwork being completed in a timely fashion (56% vs 29%; P = 0.041) and being less worried about the discharge plan (44% vs 57%; P = 0.027). The intervention team nurses also reported fewer issues with medications/prescriptions (61% vs 82%) and being included more often in the discharge planning (50% vs 38%). However, neither of these results reached statistical significance (P = 0.81 and 0.50, respectively).
DISCUSSION
Our study embedded a nurse practitioner on a busy resident general medical team to help with all aspects of the discharge process for which physicians are responsible. Previous studies have been limited to patients with specific diagnoses, age, or disposition plans.1825 In this study, we included all general medical patients. Our intervention improved several important quality of care elements: the timeliness of completion of discharge summaries; and increased number of early follow‐up appointments, with more patients seen within 2 and 4 weeks after discharge. Patients reported better understanding of their follow‐up plans and more satisfaction with the discharge process. While not statistically significant, there was a trend towards better communication with nurses. For residents with work‐hour limitations, there was time savings with a trend towards finishing attending rounds on time and statistically significant earlier sign‐out rounds (46 minutes earlier). This intervention had no effect on patient length of stay, readmissions, or emergency department visits in the 30 days after discharge.
Despite improving many aspects of the discharge process and communication that have previously been raised as areas of concern for patient safety, there was no improvement in readmissions rates and ED utilization which are often used as the quality indicators for effective discharge planning. Similar types of interventions on general medical patients have generally also failed to show improvement in readmission rates.1820, 25 Weinberger et al. arranged follow‐up appointments within 1 week for patients discharged from a Veterans Administrative hospital; while patients were seen more often, the intervention actually increased readmission rates.20 Fitzgerald et al. had a case manager contact patients at home and encourage follow‐up, which increased follow‐up visits, but again had no effect on readmission.19 Einstadter et al. had a nurse case manager coordinate outpatient follow‐up on a resident team and also did not effect readmission rates or ED visits.18 Jack et al. in project reengineered discharge (RED) did show a significant reduction in combined hospital utilization measures. However, their study focused on a more limited patient population, and employed both a discharge advocate to arrange follow‐up and improve patient education, and a pharmacist to make postdischarge phone calls.25
So why did readmissions rates and ED visits not change in our study? It would be reasonable to think that having earlier follow‐up appointments, better and timely physician‐to‐physician communication, and a facilitator for patient questions should improve the quality of the discharge process. In a recent study, Jha et al. found there was no association between chart‐based measures of discharge quality and readmissions rates, and only a modest association for patient‐reported measures of discharge quality and readmission rates.28 The authors suggest readmission rates are driven by many factors beyond just improved discharge safety. Perhaps readmission rates are too complex a measure to use to assess discharge process improvement. For fiscal reasons, it is understandable that hospitals, insurance companies, and the Centers for Medicare and Medicaid want to reduce readmission rates and ED utilization. Jencks et al. noted the cost of readmissions in 2004 was 17.4 billion dollars.29 However, sweeping efforts to improve the discharge process for all general medical patients may not yield significant reductions in readmissions, as this study suggests. We may need to focus aggressive intervention on smaller target populations, as prior studies on focused groups suggest.2123
There are no evidence‐based studies to suggest when optimal follow‐up should occur after discharge.26 Several medical society guidelines recommend 2 weeks. More patients on the intervention team were seen within 2 weeks, but readmission rates were not affected. The University Health System Consortium recently reported that the majority of readmissions occurred within 6 days, with the average being about 2 to 3 days.30 In this study, the median days to readmit were 12 for the intervention team and 10 for the control. It is possible that even with our improved 2‐week follow‐up, this was not early enough to reduce readmissions. Follow‐up may need to be within 13 days of discharge for highly vulnerable patients, to significantly change readmission rates. Further studies focusing on this question would be helpful.
Finally, with ACGME limitation of work hours, many residency programs are looking for ways to reduce residents' workload and increase time for education. With a significant trend towards finishing attending rounds on time, it is likely that more residents on the intervention team were able to attend the noon‐time educational conferences. We speculate that this was due to fewer interruptions during rounds because the DF was available for nurses' questions. Sign‐out rounds occurred significantly earlier, possibly because of improved resident efficiency due to the DF's help with the discharge process. While residents may lose some educational experience from not performing all discharge tasks, they gain experience working in interdisciplinary teams, have increased time for education, and reduced work hours. Since the ACGME limits the number of residents per program and increasing the residency size is not an option, a DF should be considered as a possible solution to ACGME work‐hour restrictions.
This study had several limitations. First, the intervention team had 1 specific person embedded, and therefore the results of this study may have limited generalizability. Second, the limited number of residents working with the DF could have biased the intervention, as not all residents worked equally well with the DF. However, this may represent the real‐world experience on any teaching service, given variation in working styles and learning curves of residents over their training. Third, this study was done at 1 university‐affiliated urban Academic Medical Center, making it potentially less generalizable to resident teams in community hospitals. Fourth, we were not able to capture readmissions and ED visits at institutions outside the MGHPartners Healthcare System. However, given that patients were assigned at random to either team, this factor should have impacted both teams equally. Fifth, the study occurred during Massachusetts healthcare reform which requires everyone to have health insurance. This may have affected the rates of ED visits and readmission rates, especially with a shortage of primary care physicians and office visits. Finally, this intervention was not cost‐neutral. Paying for a nurse practitioner to help residents with the work of discharge and providing patients with additional services had many advantages, but this quality improvement project did not pay for itself through shorter LOS, or decreases in ED visits or readmissions.
While readmission rates and ED utilization are important patient outcomes, especially in the current healthcare climate, what determines readmissions and ED visits is likely complex and multifactorial. This study suggests that, in the nationwide effort to reduce readmissions, solely improving the discharge process for all general medical patients may not produce the hoped‐for financial savings. Improving the discharge process, however, is something valuable in its own right. Adding a DF to a resident team does improve some quality markers of the discharge process and decreases work hours for residents.
Acknowledgements
Sara Macchiano, RN for her help with the data gathering of this study.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20(4):317–323.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,, et al.Prospective payment system and impairment at discharge. The ‘quicker‐and‐sicker’ story revisited.JAMA.1990;264(15):1980–1983.
- .The incidence of adverse medical outcomes under prospective payment.Econometrica. 1995;63:29–50.
- ,,.Content of a discharge summary from a medical ward: Views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29(4):307–310.
- ,.Quality assessment of a discharge summary system.Can Med Assoc J.1995;152(9):1437–1442.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17(3):186–192.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.J Gen Intern Med.2009;24(3):381–386.
- ,,.Tying up loose ends: Discharging patients with unresolved medical issues.Arch Intern Med.2007;167(12):1305–1311.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370.
- ,,,.Prospective audit of discharge summary errors.Br J Surg.1996;83(6):788–790.
- ,.Lost in transition: Challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,.Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11(11):684–688.
- ,,,,.A case manager intervention to reduce readmissions.Arch Intern Med.1994;154(15):1721–1729.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–1447.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: A randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: The Care Transitions Intervention.J Am Geriatr Soc.2004;52(11):1817–1825.
- ,,,.The care transitions intervention: Results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: A randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,,.Redefining and redesigning hospital discharge to enhance patient care: A randomized controlled study.J Gen Intern Med.2008;23(8):1228–1233.
- ,,, et al.Effect of a nurse team coordinator on outcomes for hospitalized medicine patients.Am J Med.2005;118(10):1148–1153.
- ,,.Public reporting of discharge planning and rates of readmissions.N Engl J Med.2009;361(27):2637–2645.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- Consortium UHS. Reducing Readmissions SC22009. Available at: https://www.uhc.edu/1244.htm
Recent studies have shown that a patient's discharge from the hospital is a vulnerable period for patient safety.14 With the reduction in length of stay (LOS) and the increase in patient acuity over the past decade, patients are discharged from acute care settings quicker and sicker, resulting in management of ongoing illness in a less‐monitored environment.5, 6 In addition, in teaching hospitals, residents are supervised by hospital‐based physicians who are rarely the primary care physician (PCP) for the residents' patients, which creates discontinuity of care.
One in 5 medical discharges is complicated by an adverse event believed, in part, to be due to poor communication between caregivers during this transition time.2 Discharge summaries, a key form of that communication, are not always done in a timely fashion and may lack key pieces of information.7, 8 For approximately 68% of patient discharges, the PCP will not have a discharge summary available for the patient's first follow‐up visit.911 In a survey of PCPs whose patients were in the hospital, only 23% reported direct communication with the hospital care team.12 This leaves PCPs unaware of pending test results or recommended follow‐up evaluations.10, 11, 13, 14 All of these factors are believed to contribute to adverse events, emergency department (ED) visits, and readmissions.
A recently published consensus statement on transitions of care by 6 major medical societies emphasizes the need for timely communication and transfer of information.15 These important processes are especially challenging to meet at academic medical centers, where discharge summaries and transition communication are done by residents in a hectic and challenging work environment, with multiple simultaneous and competing demands including outpatient clinic and required conferences.12 Residents have little formal training in how to write an effective discharge summary or how to systematically approach discharge planning. One study found higher error rates in discharge summaries written by residents compared with attending physicians.16 While the Accreditation Council for Graduate Medical Education (ACGME) limits the number of admissions per intern for both patient safety and educational reasons, the number of discharges per day is not limited despite the considerable amount of time required for appropriate discharge planning and communication.
Many interventions have been tried to improve the discharge process and reduce patient adverse events.17 Arranging early follow‐up appointments to reduce emergency department visits and readmissions has shown mixed results.13, 1820 Interventions that focus on specific populations, such as the elderly or patients with congestive heart failure, have been more successful.2123 Some interventions employed additional resources, such as a discharge form, transition coach, or discharge advocate, again with varying impact on results.18, 2427 A recent study by Jack et al. used nurse discharge advocates (DAs) to help with discharge planning and communication at an academic medical center.25 These DAs were independent of the care team, and focused on patient education and follow‐up plans, and reduced hospital reutilization in a selected population.
No studies have assessed the potential benefit of helping residents with the physician components of the discharge process. Prior studies have mainly focused on patient communication and follow‐up appointments, yet safe transitions also involve timely discharge summaries, physician‐to‐physician communication, physician‐to‐nurse communication, and medication reconciliation. Without support and time, these tasks can be very challenging for resident physicians with work‐hour limitations. We undertook a randomized, controlled trial to evaluate the impact on the discharge process of embedding a discharge facilitator in a resident medical team to help with the physician discharge process. We studied the effect for all the patients discharged from the resident team, rather than focusing on a select group or patients with a single diagnosis.
METHODS
Study Setting and Participants
This study was conducted on 2 of the 5 resident general medical teams on the inpatient teaching service at Massachusetts General Hospital (MGH), Boston, Massachusettsa large, 907‐bed, urban hospital. The residents' teams are regionalized and each care for approximately 20 patients on a single floor. Each of the study teams consists of a junior resident, 4 interns, and 1 to 2 attendings who rotate on the floor for 2‐week or 4‐week blocks. Attending rounds, which occur 10 AM to 12 PM weekdays, are for new patient presentations and discussion of plans. Interdisciplinary rounds occur 9:30 AM to 10 AM. Sign‐out rounds occur in the afternoon whenever all work is complete. The junior resident is responsible for all the discharge orders and communication with PCPs, and the discharge summaries for patients going to facilities. The interns are responsible for discharge summaries for patients discharged home; these summaries are not mandatory at the time of discharge. The majority of patients were admitted under the team attending(s). Patients were assigned to the teams by the admitting office, based on bed availability. All patients discharged from both resident medical teams over a 5‐month period were included in this study. Those who were not discharged from the hospital by the study teams (ie, transfers to intensive care units or deaths) were excluded. These exclusions accounted for less than 12% of all team patients. Partners Healthcare System Institutional Review Board approved all study activities.
Intervention
We randomly assigned a discharge facilitator (DF), a master's level nurse practitioner with prior inpatient medicine experience, to 1 of the 5 resident medical teams. She had no prior experience on this specific floor. A similar resident team, on a different floor, served as the control. For the intervention team, the DF attended daily resident work rounds and interdisciplinary discharge rounds. The resident and DF collaborated in identifying patients being discharged in the next 1 to 3 days, and the DF scheduled all follow‐up appointments and tests. The DF performed medication reconciliation, wrote prescriptions and faxed them to pharmacies, and arranged all anticoagulation services. In collaboration with the resident, the DF called PCPs' offices with discharge information and faxed discharge summaries to PCPs' offices outside the Partners Healthcare System. The DF wrote part or all of the computer discharge orders and discharge summaries at the request of the resident and interns. All discharge summaries still needed to be reviewed, edited, and signed by the resident or interns. The DF also noted pending tests and studies at time of discharge, and followed up on these tests for the team. The DF met with all patients to answer any questions about their discharge plan, medications, and appointments; while residents are encouraged to do this, it is not done as consistently. She provided her business card for any questions after their discharge. Follow‐up patient calls to the DF were either answered by her or triaged to the appropriate person. The DF also communicated with the patient's nurse about the discharge plans. For all patients discharged over a weekend, the DF would arrange the follow‐up appointments on Mondays and call the patients at home.
For both teams, residents received letters at the start of their rotation notifying them of the study and asking them to complete discharge summaries within 24 hours. All residents in the program were expected to do an online discharge tutorial and attend a didactic lecture on discharge summaries. The residents on the intervention team received a 5‐minute orientation on how best to work with the DF. Residents were given the autonomy to decide how much to use the DF's services. The scheduling of follow‐up appointments on the control team was the responsibility of the team resident as per usual care. The nursing component of the discharge process, including patient discharge education, was the same on both teams. Nurses on both floors are identically trained on these aspects of care. The nurses on both teams were surveyed about perception of the discharge process prior to the intervention and after the intervention. A research assistant (RA) called patients discharged home on both teams, 1 week after discharge, to ask about satisfaction with the discharge process, to determine if the patients had any questions, and to verify patient knowledge regarding whom they should contact for problems. The RA also noted the end time of attending rounds each day and the start time of resident sign‐out.
Outcome Measures and Follow‐Up
At the time of discharge, the RA collected baseline data on all patients discharged from both teams, including the number of follow‐up appointments scheduled. Patients were tracked through electronic medical records to see if and when they attended their follow‐up appointments, whether they changed the appointment, and whether patients returned to a hospital emergency department or were readmitted to MGH or an affiliated Partners hospital within 30 days. For patients outside the MGHPartners system, the research assistant contacted primary care physician offices to document follow‐up. The remaining patient data was obtained through the MGHPartners computerized information system.
The primary outcomes of the study were length of stay, time of discharge, number of emergency department visits, hospital readmissions, numbers of discharge summaries completed in 24 hours, time from discharge to discharge summary completion, and whether the discharge summary was completed before follow‐up. Secondary outcomes were number of follow‐up PCP appointments made at time of discharge, percentage of follow‐up appointments attended and time from discharge to attending a follow‐up appointment, patient phone survey results, and nursing perception of the discharge process, as well as the percentage of attending rounds that ended on time and the time of resident sign‐out.
Statistical Analyses
Patient characteristics were compared between intervention and control teams using 2‐sample t tests or Wilcoxon rank sum tests for continuous variables, and chi‐square tests for categorical variables. Hours to discharge summary completion and hospital length of stay were summarized using median and interquartiles (IQR), and compared between the 2 teams using Wilcoxon rank sum tests. Categorical outcomes were compared using chi‐square tests. Two‐sided P values 0.05 were considered statistically significant. SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
Study Sample
During the 5‐month intervention (November 12, 2008 to April 14, 2009), a combined total of 999 patients were admitted to the intervention and control general medical teams. We excluded 96 patients who were not discharged but transferred to another service or intensive care units, and 24 patients who died. We also excluded 7 patients who were discharged from both teams the first day of the study, because the DF was not involved with the patients' discharge planning. That left 872 patients discharged to either home, a facility, or having left against medical advice (AMA) included in the study: 440 patients on the intervention team and 432 patients on the control team (Figure 1). Baseline patient demographic and clinical characteristics were similar across both teams with only gender being significantly different (Table 1). The mean age was 63 years (range, 1896) and the mean comorbidity score was 2.3 (range, 012). Of note, about a quarter of patients were discharged to facilities, about half were Medicare recipients, and approximately 80% had a PCP. The DF participated in the discharge process for nearly all of the intervention patients; she reported contributing approximately 50% of the content to the discharge summaries.

| Characteristics | Intervention Team | Control Team |
|---|---|---|
| n = 440 | n = 432 | |
| ||
| Mean age (SD), year | 63 (18) | 63 (18) |
| Women, n (%)* | 181 (41) | 207 (48) |
| Race, n (%) | ||
| White non‐Hispanic | 267 (61) | 243 (56) |
| Black non‐Hispanic | 24 (5) | 33 (8) |
| Hispanic | 21 (5) | 17 (4) |
| Unknown/other | 128 (29) | 139 (32) |
| Health insurance, n (%) | ||
| Medicare | 213 (48) | 226 (52) |
| Medicaid | 85 (19) | 81 (19) |
| Private | 110 (25) | 91 (21) |
| Other | 32 (7) | 34 (8) |
| PCP on admission, n (%) | 370 (84) | 356 (82) |
| Discharge disposition, n (%) | ||
| AMA | 12 (3) | 14 (3) |
| Home | 305 (69) | 315 (73) |
| Facility | 123 (28) | 103 (24) |
| Mean comorbidity index score (SD) | 2.3 (2.4) | 2.3 (2.4) |
| Diagnoses | ||
| Congestive heart failure | 30 (6%) | 27 (5%) |
| COPD/asthma | 34 (7%) | 47 (9%) |
| Cardiovascular disease | 54 (11%) | 50 (8%) |
| Alcohol/substance abuse | 29 (6%) | 34 (7%) |
| Gastrointestinal bleeds/ulcers | 38 (8%) | 41 (8%) |
| Hepatobiliary disease | 30 (6%) | 36 (7%) |
| Renal failure/kidney disease | 33 (7%) | 37 (7%) |
| Pneumonia | 36 (7%) | 22 (4%) |
| Musculoskeletal disease | 26 (5%) | 23 (5%) |
| Neurologic disease | 22 (4%) | 25 (5%) |
| Other | 163 (33%) | 172 (35%) |
Primary Outcomes
Primary outcomes from the 2 medical teams are listed in Table 2. In the intervention group, significantly more discharge summaries were completed within 24 hours compared to the control group (293 [67%] vs 207 [48%]; P < 0.0001). Since nearly all patients discharged to facilities must have a discharge summary at the time of discharge, the overall difference in completion rates came mainly from patients discharged home or having left AMA from the intervention team (177 [56%] vs 112 [34%]; P < 0.0001). For all discharge summaries, the median time to completion on the intervention team was 18.9 hours compared with 73.1 hours on the control team (P < 0.0001). More discharge summaries were completed before the first follow‐up appointment on the intervention team (393 [89%] vs 330 [76%]; P < 0.001). The DF intervention had no effect on 30‐day readmission or emergency department visits. For patients on the DF team, 88 (20%) were readmitted within 30 days of discharge, as compared with 79 (18%) on the control team (P = 0.55). Similarly, 40 (9%) of the intervention team patients, as compared with 39 (9%) of the control team patients, visited the emergency department at least once within 30 days (P = 1.0). There was no difference in length of stay (LOS) between the 2 teams (median 4.0 days for both teams, P = 0.84).
| Intervention Team | Control Team | ||
|---|---|---|---|
| Variables | n = 440 | n = 432 | P Value |
| |||
| Discharge summaries completed 24 hr, n (%) | 293 (67) | 207 (48) | <0.0001 |
| Discharges to facilities | 116 (94) | 95 (92) | 0.60 |
| Discharges to home/AMA | 177 (56) | 112 (34) | <0.0001 |
| Median hours to discharge summary completion for discharges to home/AMA (IQR) | 18.9 (0138) | 73.1 (4.3286) | <0.0001 |
| Discharge summary complete before time of follow‐up appointment. | 393 (89) | 330 (76) | <0.0001 |
| Emergency department visits in 30 days, n (%) | 40 (9) | 39 (9) | 1.0 |
| Readmissions in 30 days, n (%) | 88 (20) | 79 (18) | 0.55 |
| Median length of stay, days (IQR) | 4.0 (37) | 4.0 (28) | 0.84 |
| Discharges to facilities | 6.0 (511) | 8.0 (513) | 0.17 |
| Discharges to home/AMA | 4.0 (26) | 3.0 (26) | 0.61 |
| Discharged by noon, n (%) | 38 (9) | 42 (10) | 0.64 |
Secondary Outcomes
Table 3 shows secondary outcomes from the 2 medical teams. Among the patients discharged from the DF team, 264 (62%) had scheduled follow‐up appointments with PCPs compared to the control team 151 (36%) (P < 0.0001). (Many patients going to rehabilitation hospitals are not given PCP appointments at the time of discharge.) Despite having more scheduled appointments, patients' actual follow‐up with PCPs was similar during the 5‐month study period among both intervention and control group (234 [65%] vs 223 [63%]; P = 0.58). However, there was earlier follow‐up with the primary provider in the first 2 or 4 weeks in the intervention group. At 2 weeks, 129 (36%) patients in the intervention group saw their provider compared to 81 (23%) patients in the control group (P < 0.0002), and at 4 weeks, 159 (44%) of the intervention group was seen compared to 99 (28%) of the control group (P < 0.0001). Of note, among the 415 patients on both teams discharged with scheduled appointments, only 53 (13%) of patients did not show up for the scheduled appointment and this no‐show rate was the same on both teams.
| Variables | Intervention Team | Control Team | P Value |
|---|---|---|---|
| |||
| No. of eligible patients* | 428 | 418 | |
| Patients with follow‐up appointments to primary providers, n (%) | 264 (62) | 151 (36) | <0.0001 |
| No. of eligible patients | 359 | 354 | |
| Attended follow‐up appointment with primary provider during study, n (%) | 234 (65) | 223 (63) | 0.58 |
| Within 2 weeks of discharge | 129 (36) | 81 (23) | 0.0002 |
| Within 4 weeks of discharge | 159 (44) | 99 (28) | <0.0001 |
| No. of days round times were recorded | 100 | 99 | |
| No. of attending rounds ending by 12 PM | 45 (45%) | 31 (31%) | 0.058 |
| Mean start time of sign‐out rounds | 16:38 | 17:24 | 0.0007 |
Attending rounds ended on time (12 PM) 45% of the time in the intervention group compared to 31% in the control group (P = 0.058). Mean start time of resident sign‐out rounds was 1638 hours on the intervention team and 1724 hours on the control team (P = 0.0007).
We obtained patient reported outcome data by telephone within 2 to 4 weeks of discharge. Of the 620 patients discharged to home, 6 died or were readmitted to the hospital before being reached by phone. For the remaining 614 patients, we were able to contact 444 (72%). Of those, 321 (52%) agreed to participate in the phone interview. We surveyed similar proportions of intervention and control group patients (158 [52%] vs 163 [52%]) (Table 4). Both groups reported similar rates of having questions about their hospital stay after discharge (43 [27%] vs 49 [30%]; P = 0.62). The intervention group could better identify whom to call with questions (150 [95%] vs 138 [85%]; P = 0.003). The intervention group reported better understanding of their follow‐up plans (157 [99%] vs 141 [87%]; P = 0.001) and better understanding of their discharge medications (152 [96%] vs 142 [87%]; P = 0.001). More patients in the intervention group were satisfied with the discharge process (153 [97%] vs 124 [76%]; P < 0.0001).
| Intervention Team | Control Team | P Value | |
|---|---|---|---|
| |||
| Patients discharged home* | 304 | 310 | |
| Patients contacted by phone after discharge, n (%) | 213 (70) | 231 (75) | 0.24 |
| Agreed to participate in phone interview, n (%) | 158 (52) | 163 (53) | 0.94 |
| Among those agreed to participate, n (%) | |||
| Did you have questions about your hospital stay? | 43 (27) | 49 (30) | 0.62 |
| Would you know who to call if you had questions after discharge? | 150 (95) | 138 (85) | 0.003 |
| Satisfied with the discharge process? | 153 (97) | 124 (76) | <0.0001 |
| Did you understand your follow‐up plans? | 157 (99) | 141 (87) | <0.0001 |
| Did you understand your medications? | 152 (96) | 142 (87) | 0.001 |
| Did you feel safe going home? | 153 (97) | 151 (92) | 0.07 |
Compared with nurses on the control team, nurses on the intervention team more often reported paperwork being completed in a timely fashion (56% vs 29%; P = 0.041) and being less worried about the discharge plan (44% vs 57%; P = 0.027). The intervention team nurses also reported fewer issues with medications/prescriptions (61% vs 82%) and being included more often in the discharge planning (50% vs 38%). However, neither of these results reached statistical significance (P = 0.81 and 0.50, respectively).
DISCUSSION
Our study embedded a nurse practitioner on a busy resident general medical team to help with all aspects of the discharge process for which physicians are responsible. Previous studies have been limited to patients with specific diagnoses, age, or disposition plans.1825 In this study, we included all general medical patients. Our intervention improved several important quality of care elements: the timeliness of completion of discharge summaries; and increased number of early follow‐up appointments, with more patients seen within 2 and 4 weeks after discharge. Patients reported better understanding of their follow‐up plans and more satisfaction with the discharge process. While not statistically significant, there was a trend towards better communication with nurses. For residents with work‐hour limitations, there was time savings with a trend towards finishing attending rounds on time and statistically significant earlier sign‐out rounds (46 minutes earlier). This intervention had no effect on patient length of stay, readmissions, or emergency department visits in the 30 days after discharge.
Despite improving many aspects of the discharge process and communication that have previously been raised as areas of concern for patient safety, there was no improvement in readmissions rates and ED utilization which are often used as the quality indicators for effective discharge planning. Similar types of interventions on general medical patients have generally also failed to show improvement in readmission rates.1820, 25 Weinberger et al. arranged follow‐up appointments within 1 week for patients discharged from a Veterans Administrative hospital; while patients were seen more often, the intervention actually increased readmission rates.20 Fitzgerald et al. had a case manager contact patients at home and encourage follow‐up, which increased follow‐up visits, but again had no effect on readmission.19 Einstadter et al. had a nurse case manager coordinate outpatient follow‐up on a resident team and also did not effect readmission rates or ED visits.18 Jack et al. in project reengineered discharge (RED) did show a significant reduction in combined hospital utilization measures. However, their study focused on a more limited patient population, and employed both a discharge advocate to arrange follow‐up and improve patient education, and a pharmacist to make postdischarge phone calls.25
So why did readmissions rates and ED visits not change in our study? It would be reasonable to think that having earlier follow‐up appointments, better and timely physician‐to‐physician communication, and a facilitator for patient questions should improve the quality of the discharge process. In a recent study, Jha et al. found there was no association between chart‐based measures of discharge quality and readmissions rates, and only a modest association for patient‐reported measures of discharge quality and readmission rates.28 The authors suggest readmission rates are driven by many factors beyond just improved discharge safety. Perhaps readmission rates are too complex a measure to use to assess discharge process improvement. For fiscal reasons, it is understandable that hospitals, insurance companies, and the Centers for Medicare and Medicaid want to reduce readmission rates and ED utilization. Jencks et al. noted the cost of readmissions in 2004 was 17.4 billion dollars.29 However, sweeping efforts to improve the discharge process for all general medical patients may not yield significant reductions in readmissions, as this study suggests. We may need to focus aggressive intervention on smaller target populations, as prior studies on focused groups suggest.2123
There are no evidence‐based studies to suggest when optimal follow‐up should occur after discharge.26 Several medical society guidelines recommend 2 weeks. More patients on the intervention team were seen within 2 weeks, but readmission rates were not affected. The University Health System Consortium recently reported that the majority of readmissions occurred within 6 days, with the average being about 2 to 3 days.30 In this study, the median days to readmit were 12 for the intervention team and 10 for the control. It is possible that even with our improved 2‐week follow‐up, this was not early enough to reduce readmissions. Follow‐up may need to be within 13 days of discharge for highly vulnerable patients, to significantly change readmission rates. Further studies focusing on this question would be helpful.
Finally, with ACGME limitation of work hours, many residency programs are looking for ways to reduce residents' workload and increase time for education. With a significant trend towards finishing attending rounds on time, it is likely that more residents on the intervention team were able to attend the noon‐time educational conferences. We speculate that this was due to fewer interruptions during rounds because the DF was available for nurses' questions. Sign‐out rounds occurred significantly earlier, possibly because of improved resident efficiency due to the DF's help with the discharge process. While residents may lose some educational experience from not performing all discharge tasks, they gain experience working in interdisciplinary teams, have increased time for education, and reduced work hours. Since the ACGME limits the number of residents per program and increasing the residency size is not an option, a DF should be considered as a possible solution to ACGME work‐hour restrictions.
This study had several limitations. First, the intervention team had 1 specific person embedded, and therefore the results of this study may have limited generalizability. Second, the limited number of residents working with the DF could have biased the intervention, as not all residents worked equally well with the DF. However, this may represent the real‐world experience on any teaching service, given variation in working styles and learning curves of residents over their training. Third, this study was done at 1 university‐affiliated urban Academic Medical Center, making it potentially less generalizable to resident teams in community hospitals. Fourth, we were not able to capture readmissions and ED visits at institutions outside the MGHPartners Healthcare System. However, given that patients were assigned at random to either team, this factor should have impacted both teams equally. Fifth, the study occurred during Massachusetts healthcare reform which requires everyone to have health insurance. This may have affected the rates of ED visits and readmission rates, especially with a shortage of primary care physicians and office visits. Finally, this intervention was not cost‐neutral. Paying for a nurse practitioner to help residents with the work of discharge and providing patients with additional services had many advantages, but this quality improvement project did not pay for itself through shorter LOS, or decreases in ED visits or readmissions.
While readmission rates and ED utilization are important patient outcomes, especially in the current healthcare climate, what determines readmissions and ED visits is likely complex and multifactorial. This study suggests that, in the nationwide effort to reduce readmissions, solely improving the discharge process for all general medical patients may not produce the hoped‐for financial savings. Improving the discharge process, however, is something valuable in its own right. Adding a DF to a resident team does improve some quality markers of the discharge process and decreases work hours for residents.
Acknowledgements
Sara Macchiano, RN for her help with the data gathering of this study.
Recent studies have shown that a patient's discharge from the hospital is a vulnerable period for patient safety.14 With the reduction in length of stay (LOS) and the increase in patient acuity over the past decade, patients are discharged from acute care settings quicker and sicker, resulting in management of ongoing illness in a less‐monitored environment.5, 6 In addition, in teaching hospitals, residents are supervised by hospital‐based physicians who are rarely the primary care physician (PCP) for the residents' patients, which creates discontinuity of care.
One in 5 medical discharges is complicated by an adverse event believed, in part, to be due to poor communication between caregivers during this transition time.2 Discharge summaries, a key form of that communication, are not always done in a timely fashion and may lack key pieces of information.7, 8 For approximately 68% of patient discharges, the PCP will not have a discharge summary available for the patient's first follow‐up visit.911 In a survey of PCPs whose patients were in the hospital, only 23% reported direct communication with the hospital care team.12 This leaves PCPs unaware of pending test results or recommended follow‐up evaluations.10, 11, 13, 14 All of these factors are believed to contribute to adverse events, emergency department (ED) visits, and readmissions.
A recently published consensus statement on transitions of care by 6 major medical societies emphasizes the need for timely communication and transfer of information.15 These important processes are especially challenging to meet at academic medical centers, where discharge summaries and transition communication are done by residents in a hectic and challenging work environment, with multiple simultaneous and competing demands including outpatient clinic and required conferences.12 Residents have little formal training in how to write an effective discharge summary or how to systematically approach discharge planning. One study found higher error rates in discharge summaries written by residents compared with attending physicians.16 While the Accreditation Council for Graduate Medical Education (ACGME) limits the number of admissions per intern for both patient safety and educational reasons, the number of discharges per day is not limited despite the considerable amount of time required for appropriate discharge planning and communication.
Many interventions have been tried to improve the discharge process and reduce patient adverse events.17 Arranging early follow‐up appointments to reduce emergency department visits and readmissions has shown mixed results.13, 1820 Interventions that focus on specific populations, such as the elderly or patients with congestive heart failure, have been more successful.2123 Some interventions employed additional resources, such as a discharge form, transition coach, or discharge advocate, again with varying impact on results.18, 2427 A recent study by Jack et al. used nurse discharge advocates (DAs) to help with discharge planning and communication at an academic medical center.25 These DAs were independent of the care team, and focused on patient education and follow‐up plans, and reduced hospital reutilization in a selected population.
No studies have assessed the potential benefit of helping residents with the physician components of the discharge process. Prior studies have mainly focused on patient communication and follow‐up appointments, yet safe transitions also involve timely discharge summaries, physician‐to‐physician communication, physician‐to‐nurse communication, and medication reconciliation. Without support and time, these tasks can be very challenging for resident physicians with work‐hour limitations. We undertook a randomized, controlled trial to evaluate the impact on the discharge process of embedding a discharge facilitator in a resident medical team to help with the physician discharge process. We studied the effect for all the patients discharged from the resident team, rather than focusing on a select group or patients with a single diagnosis.
METHODS
Study Setting and Participants
This study was conducted on 2 of the 5 resident general medical teams on the inpatient teaching service at Massachusetts General Hospital (MGH), Boston, Massachusettsa large, 907‐bed, urban hospital. The residents' teams are regionalized and each care for approximately 20 patients on a single floor. Each of the study teams consists of a junior resident, 4 interns, and 1 to 2 attendings who rotate on the floor for 2‐week or 4‐week blocks. Attending rounds, which occur 10 AM to 12 PM weekdays, are for new patient presentations and discussion of plans. Interdisciplinary rounds occur 9:30 AM to 10 AM. Sign‐out rounds occur in the afternoon whenever all work is complete. The junior resident is responsible for all the discharge orders and communication with PCPs, and the discharge summaries for patients going to facilities. The interns are responsible for discharge summaries for patients discharged home; these summaries are not mandatory at the time of discharge. The majority of patients were admitted under the team attending(s). Patients were assigned to the teams by the admitting office, based on bed availability. All patients discharged from both resident medical teams over a 5‐month period were included in this study. Those who were not discharged from the hospital by the study teams (ie, transfers to intensive care units or deaths) were excluded. These exclusions accounted for less than 12% of all team patients. Partners Healthcare System Institutional Review Board approved all study activities.
Intervention
We randomly assigned a discharge facilitator (DF), a master's level nurse practitioner with prior inpatient medicine experience, to 1 of the 5 resident medical teams. She had no prior experience on this specific floor. A similar resident team, on a different floor, served as the control. For the intervention team, the DF attended daily resident work rounds and interdisciplinary discharge rounds. The resident and DF collaborated in identifying patients being discharged in the next 1 to 3 days, and the DF scheduled all follow‐up appointments and tests. The DF performed medication reconciliation, wrote prescriptions and faxed them to pharmacies, and arranged all anticoagulation services. In collaboration with the resident, the DF called PCPs' offices with discharge information and faxed discharge summaries to PCPs' offices outside the Partners Healthcare System. The DF wrote part or all of the computer discharge orders and discharge summaries at the request of the resident and interns. All discharge summaries still needed to be reviewed, edited, and signed by the resident or interns. The DF also noted pending tests and studies at time of discharge, and followed up on these tests for the team. The DF met with all patients to answer any questions about their discharge plan, medications, and appointments; while residents are encouraged to do this, it is not done as consistently. She provided her business card for any questions after their discharge. Follow‐up patient calls to the DF were either answered by her or triaged to the appropriate person. The DF also communicated with the patient's nurse about the discharge plans. For all patients discharged over a weekend, the DF would arrange the follow‐up appointments on Mondays and call the patients at home.
For both teams, residents received letters at the start of their rotation notifying them of the study and asking them to complete discharge summaries within 24 hours. All residents in the program were expected to do an online discharge tutorial and attend a didactic lecture on discharge summaries. The residents on the intervention team received a 5‐minute orientation on how best to work with the DF. Residents were given the autonomy to decide how much to use the DF's services. The scheduling of follow‐up appointments on the control team was the responsibility of the team resident as per usual care. The nursing component of the discharge process, including patient discharge education, was the same on both teams. Nurses on both floors are identically trained on these aspects of care. The nurses on both teams were surveyed about perception of the discharge process prior to the intervention and after the intervention. A research assistant (RA) called patients discharged home on both teams, 1 week after discharge, to ask about satisfaction with the discharge process, to determine if the patients had any questions, and to verify patient knowledge regarding whom they should contact for problems. The RA also noted the end time of attending rounds each day and the start time of resident sign‐out.
Outcome Measures and Follow‐Up
At the time of discharge, the RA collected baseline data on all patients discharged from both teams, including the number of follow‐up appointments scheduled. Patients were tracked through electronic medical records to see if and when they attended their follow‐up appointments, whether they changed the appointment, and whether patients returned to a hospital emergency department or were readmitted to MGH or an affiliated Partners hospital within 30 days. For patients outside the MGHPartners system, the research assistant contacted primary care physician offices to document follow‐up. The remaining patient data was obtained through the MGHPartners computerized information system.
The primary outcomes of the study were length of stay, time of discharge, number of emergency department visits, hospital readmissions, numbers of discharge summaries completed in 24 hours, time from discharge to discharge summary completion, and whether the discharge summary was completed before follow‐up. Secondary outcomes were number of follow‐up PCP appointments made at time of discharge, percentage of follow‐up appointments attended and time from discharge to attending a follow‐up appointment, patient phone survey results, and nursing perception of the discharge process, as well as the percentage of attending rounds that ended on time and the time of resident sign‐out.
Statistical Analyses
Patient characteristics were compared between intervention and control teams using 2‐sample t tests or Wilcoxon rank sum tests for continuous variables, and chi‐square tests for categorical variables. Hours to discharge summary completion and hospital length of stay were summarized using median and interquartiles (IQR), and compared between the 2 teams using Wilcoxon rank sum tests. Categorical outcomes were compared using chi‐square tests. Two‐sided P values 0.05 were considered statistically significant. SAS version 9.2 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
RESULTS
Study Sample
During the 5‐month intervention (November 12, 2008 to April 14, 2009), a combined total of 999 patients were admitted to the intervention and control general medical teams. We excluded 96 patients who were not discharged but transferred to another service or intensive care units, and 24 patients who died. We also excluded 7 patients who were discharged from both teams the first day of the study, because the DF was not involved with the patients' discharge planning. That left 872 patients discharged to either home, a facility, or having left against medical advice (AMA) included in the study: 440 patients on the intervention team and 432 patients on the control team (Figure 1). Baseline patient demographic and clinical characteristics were similar across both teams with only gender being significantly different (Table 1). The mean age was 63 years (range, 1896) and the mean comorbidity score was 2.3 (range, 012). Of note, about a quarter of patients were discharged to facilities, about half were Medicare recipients, and approximately 80% had a PCP. The DF participated in the discharge process for nearly all of the intervention patients; she reported contributing approximately 50% of the content to the discharge summaries.

| Characteristics | Intervention Team | Control Team |
|---|---|---|
| n = 440 | n = 432 | |
| ||
| Mean age (SD), year | 63 (18) | 63 (18) |
| Women, n (%)* | 181 (41) | 207 (48) |
| Race, n (%) | ||
| White non‐Hispanic | 267 (61) | 243 (56) |
| Black non‐Hispanic | 24 (5) | 33 (8) |
| Hispanic | 21 (5) | 17 (4) |
| Unknown/other | 128 (29) | 139 (32) |
| Health insurance, n (%) | ||
| Medicare | 213 (48) | 226 (52) |
| Medicaid | 85 (19) | 81 (19) |
| Private | 110 (25) | 91 (21) |
| Other | 32 (7) | 34 (8) |
| PCP on admission, n (%) | 370 (84) | 356 (82) |
| Discharge disposition, n (%) | ||
| AMA | 12 (3) | 14 (3) |
| Home | 305 (69) | 315 (73) |
| Facility | 123 (28) | 103 (24) |
| Mean comorbidity index score (SD) | 2.3 (2.4) | 2.3 (2.4) |
| Diagnoses | ||
| Congestive heart failure | 30 (6%) | 27 (5%) |
| COPD/asthma | 34 (7%) | 47 (9%) |
| Cardiovascular disease | 54 (11%) | 50 (8%) |
| Alcohol/substance abuse | 29 (6%) | 34 (7%) |
| Gastrointestinal bleeds/ulcers | 38 (8%) | 41 (8%) |
| Hepatobiliary disease | 30 (6%) | 36 (7%) |
| Renal failure/kidney disease | 33 (7%) | 37 (7%) |
| Pneumonia | 36 (7%) | 22 (4%) |
| Musculoskeletal disease | 26 (5%) | 23 (5%) |
| Neurologic disease | 22 (4%) | 25 (5%) |
| Other | 163 (33%) | 172 (35%) |
Primary Outcomes
Primary outcomes from the 2 medical teams are listed in Table 2. In the intervention group, significantly more discharge summaries were completed within 24 hours compared to the control group (293 [67%] vs 207 [48%]; P < 0.0001). Since nearly all patients discharged to facilities must have a discharge summary at the time of discharge, the overall difference in completion rates came mainly from patients discharged home or having left AMA from the intervention team (177 [56%] vs 112 [34%]; P < 0.0001). For all discharge summaries, the median time to completion on the intervention team was 18.9 hours compared with 73.1 hours on the control team (P < 0.0001). More discharge summaries were completed before the first follow‐up appointment on the intervention team (393 [89%] vs 330 [76%]; P < 0.001). The DF intervention had no effect on 30‐day readmission or emergency department visits. For patients on the DF team, 88 (20%) were readmitted within 30 days of discharge, as compared with 79 (18%) on the control team (P = 0.55). Similarly, 40 (9%) of the intervention team patients, as compared with 39 (9%) of the control team patients, visited the emergency department at least once within 30 days (P = 1.0). There was no difference in length of stay (LOS) between the 2 teams (median 4.0 days for both teams, P = 0.84).
| Intervention Team | Control Team | ||
|---|---|---|---|
| Variables | n = 440 | n = 432 | P Value |
| |||
| Discharge summaries completed 24 hr, n (%) | 293 (67) | 207 (48) | <0.0001 |
| Discharges to facilities | 116 (94) | 95 (92) | 0.60 |
| Discharges to home/AMA | 177 (56) | 112 (34) | <0.0001 |
| Median hours to discharge summary completion for discharges to home/AMA (IQR) | 18.9 (0138) | 73.1 (4.3286) | <0.0001 |
| Discharge summary complete before time of follow‐up appointment. | 393 (89) | 330 (76) | <0.0001 |
| Emergency department visits in 30 days, n (%) | 40 (9) | 39 (9) | 1.0 |
| Readmissions in 30 days, n (%) | 88 (20) | 79 (18) | 0.55 |
| Median length of stay, days (IQR) | 4.0 (37) | 4.0 (28) | 0.84 |
| Discharges to facilities | 6.0 (511) | 8.0 (513) | 0.17 |
| Discharges to home/AMA | 4.0 (26) | 3.0 (26) | 0.61 |
| Discharged by noon, n (%) | 38 (9) | 42 (10) | 0.64 |
Secondary Outcomes
Table 3 shows secondary outcomes from the 2 medical teams. Among the patients discharged from the DF team, 264 (62%) had scheduled follow‐up appointments with PCPs compared to the control team 151 (36%) (P < 0.0001). (Many patients going to rehabilitation hospitals are not given PCP appointments at the time of discharge.) Despite having more scheduled appointments, patients' actual follow‐up with PCPs was similar during the 5‐month study period among both intervention and control group (234 [65%] vs 223 [63%]; P = 0.58). However, there was earlier follow‐up with the primary provider in the first 2 or 4 weeks in the intervention group. At 2 weeks, 129 (36%) patients in the intervention group saw their provider compared to 81 (23%) patients in the control group (P < 0.0002), and at 4 weeks, 159 (44%) of the intervention group was seen compared to 99 (28%) of the control group (P < 0.0001). Of note, among the 415 patients on both teams discharged with scheduled appointments, only 53 (13%) of patients did not show up for the scheduled appointment and this no‐show rate was the same on both teams.
| Variables | Intervention Team | Control Team | P Value |
|---|---|---|---|
| |||
| No. of eligible patients* | 428 | 418 | |
| Patients with follow‐up appointments to primary providers, n (%) | 264 (62) | 151 (36) | <0.0001 |
| No. of eligible patients | 359 | 354 | |
| Attended follow‐up appointment with primary provider during study, n (%) | 234 (65) | 223 (63) | 0.58 |
| Within 2 weeks of discharge | 129 (36) | 81 (23) | 0.0002 |
| Within 4 weeks of discharge | 159 (44) | 99 (28) | <0.0001 |
| No. of days round times were recorded | 100 | 99 | |
| No. of attending rounds ending by 12 PM | 45 (45%) | 31 (31%) | 0.058 |
| Mean start time of sign‐out rounds | 16:38 | 17:24 | 0.0007 |
Attending rounds ended on time (12 PM) 45% of the time in the intervention group compared to 31% in the control group (P = 0.058). Mean start time of resident sign‐out rounds was 1638 hours on the intervention team and 1724 hours on the control team (P = 0.0007).
We obtained patient reported outcome data by telephone within 2 to 4 weeks of discharge. Of the 620 patients discharged to home, 6 died or were readmitted to the hospital before being reached by phone. For the remaining 614 patients, we were able to contact 444 (72%). Of those, 321 (52%) agreed to participate in the phone interview. We surveyed similar proportions of intervention and control group patients (158 [52%] vs 163 [52%]) (Table 4). Both groups reported similar rates of having questions about their hospital stay after discharge (43 [27%] vs 49 [30%]; P = 0.62). The intervention group could better identify whom to call with questions (150 [95%] vs 138 [85%]; P = 0.003). The intervention group reported better understanding of their follow‐up plans (157 [99%] vs 141 [87%]; P = 0.001) and better understanding of their discharge medications (152 [96%] vs 142 [87%]; P = 0.001). More patients in the intervention group were satisfied with the discharge process (153 [97%] vs 124 [76%]; P < 0.0001).
| Intervention Team | Control Team | P Value | |
|---|---|---|---|
| |||
| Patients discharged home* | 304 | 310 | |
| Patients contacted by phone after discharge, n (%) | 213 (70) | 231 (75) | 0.24 |
| Agreed to participate in phone interview, n (%) | 158 (52) | 163 (53) | 0.94 |
| Among those agreed to participate, n (%) | |||
| Did you have questions about your hospital stay? | 43 (27) | 49 (30) | 0.62 |
| Would you know who to call if you had questions after discharge? | 150 (95) | 138 (85) | 0.003 |
| Satisfied with the discharge process? | 153 (97) | 124 (76) | <0.0001 |
| Did you understand your follow‐up plans? | 157 (99) | 141 (87) | <0.0001 |
| Did you understand your medications? | 152 (96) | 142 (87) | 0.001 |
| Did you feel safe going home? | 153 (97) | 151 (92) | 0.07 |
Compared with nurses on the control team, nurses on the intervention team more often reported paperwork being completed in a timely fashion (56% vs 29%; P = 0.041) and being less worried about the discharge plan (44% vs 57%; P = 0.027). The intervention team nurses also reported fewer issues with medications/prescriptions (61% vs 82%) and being included more often in the discharge planning (50% vs 38%). However, neither of these results reached statistical significance (P = 0.81 and 0.50, respectively).
DISCUSSION
Our study embedded a nurse practitioner on a busy resident general medical team to help with all aspects of the discharge process for which physicians are responsible. Previous studies have been limited to patients with specific diagnoses, age, or disposition plans.1825 In this study, we included all general medical patients. Our intervention improved several important quality of care elements: the timeliness of completion of discharge summaries; and increased number of early follow‐up appointments, with more patients seen within 2 and 4 weeks after discharge. Patients reported better understanding of their follow‐up plans and more satisfaction with the discharge process. While not statistically significant, there was a trend towards better communication with nurses. For residents with work‐hour limitations, there was time savings with a trend towards finishing attending rounds on time and statistically significant earlier sign‐out rounds (46 minutes earlier). This intervention had no effect on patient length of stay, readmissions, or emergency department visits in the 30 days after discharge.
Despite improving many aspects of the discharge process and communication that have previously been raised as areas of concern for patient safety, there was no improvement in readmissions rates and ED utilization which are often used as the quality indicators for effective discharge planning. Similar types of interventions on general medical patients have generally also failed to show improvement in readmission rates.1820, 25 Weinberger et al. arranged follow‐up appointments within 1 week for patients discharged from a Veterans Administrative hospital; while patients were seen more often, the intervention actually increased readmission rates.20 Fitzgerald et al. had a case manager contact patients at home and encourage follow‐up, which increased follow‐up visits, but again had no effect on readmission.19 Einstadter et al. had a nurse case manager coordinate outpatient follow‐up on a resident team and also did not effect readmission rates or ED visits.18 Jack et al. in project reengineered discharge (RED) did show a significant reduction in combined hospital utilization measures. However, their study focused on a more limited patient population, and employed both a discharge advocate to arrange follow‐up and improve patient education, and a pharmacist to make postdischarge phone calls.25
So why did readmissions rates and ED visits not change in our study? It would be reasonable to think that having earlier follow‐up appointments, better and timely physician‐to‐physician communication, and a facilitator for patient questions should improve the quality of the discharge process. In a recent study, Jha et al. found there was no association between chart‐based measures of discharge quality and readmissions rates, and only a modest association for patient‐reported measures of discharge quality and readmission rates.28 The authors suggest readmission rates are driven by many factors beyond just improved discharge safety. Perhaps readmission rates are too complex a measure to use to assess discharge process improvement. For fiscal reasons, it is understandable that hospitals, insurance companies, and the Centers for Medicare and Medicaid want to reduce readmission rates and ED utilization. Jencks et al. noted the cost of readmissions in 2004 was 17.4 billion dollars.29 However, sweeping efforts to improve the discharge process for all general medical patients may not yield significant reductions in readmissions, as this study suggests. We may need to focus aggressive intervention on smaller target populations, as prior studies on focused groups suggest.2123
There are no evidence‐based studies to suggest when optimal follow‐up should occur after discharge.26 Several medical society guidelines recommend 2 weeks. More patients on the intervention team were seen within 2 weeks, but readmission rates were not affected. The University Health System Consortium recently reported that the majority of readmissions occurred within 6 days, with the average being about 2 to 3 days.30 In this study, the median days to readmit were 12 for the intervention team and 10 for the control. It is possible that even with our improved 2‐week follow‐up, this was not early enough to reduce readmissions. Follow‐up may need to be within 13 days of discharge for highly vulnerable patients, to significantly change readmission rates. Further studies focusing on this question would be helpful.
Finally, with ACGME limitation of work hours, many residency programs are looking for ways to reduce residents' workload and increase time for education. With a significant trend towards finishing attending rounds on time, it is likely that more residents on the intervention team were able to attend the noon‐time educational conferences. We speculate that this was due to fewer interruptions during rounds because the DF was available for nurses' questions. Sign‐out rounds occurred significantly earlier, possibly because of improved resident efficiency due to the DF's help with the discharge process. While residents may lose some educational experience from not performing all discharge tasks, they gain experience working in interdisciplinary teams, have increased time for education, and reduced work hours. Since the ACGME limits the number of residents per program and increasing the residency size is not an option, a DF should be considered as a possible solution to ACGME work‐hour restrictions.
This study had several limitations. First, the intervention team had 1 specific person embedded, and therefore the results of this study may have limited generalizability. Second, the limited number of residents working with the DF could have biased the intervention, as not all residents worked equally well with the DF. However, this may represent the real‐world experience on any teaching service, given variation in working styles and learning curves of residents over their training. Third, this study was done at 1 university‐affiliated urban Academic Medical Center, making it potentially less generalizable to resident teams in community hospitals. Fourth, we were not able to capture readmissions and ED visits at institutions outside the MGHPartners Healthcare System. However, given that patients were assigned at random to either team, this factor should have impacted both teams equally. Fifth, the study occurred during Massachusetts healthcare reform which requires everyone to have health insurance. This may have affected the rates of ED visits and readmission rates, especially with a shortage of primary care physicians and office visits. Finally, this intervention was not cost‐neutral. Paying for a nurse practitioner to help residents with the work of discharge and providing patients with additional services had many advantages, but this quality improvement project did not pay for itself through shorter LOS, or decreases in ED visits or readmissions.
While readmission rates and ED utilization are important patient outcomes, especially in the current healthcare climate, what determines readmissions and ED visits is likely complex and multifactorial. This study suggests that, in the nationwide effort to reduce readmissions, solely improving the discharge process for all general medical patients may not produce the hoped‐for financial savings. Improving the discharge process, however, is something valuable in its own right. Adding a DF to a resident team does improve some quality markers of the discharge process and decreases work hours for residents.
Acknowledgements
Sara Macchiano, RN for her help with the data gathering of this study.
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20(4):317–323.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,, et al.Prospective payment system and impairment at discharge. The ‘quicker‐and‐sicker’ story revisited.JAMA.1990;264(15):1980–1983.
- .The incidence of adverse medical outcomes under prospective payment.Econometrica. 1995;63:29–50.
- ,,.Content of a discharge summary from a medical ward: Views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29(4):307–310.
- ,.Quality assessment of a discharge summary system.Can Med Assoc J.1995;152(9):1437–1442.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17(3):186–192.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.J Gen Intern Med.2009;24(3):381–386.
- ,,.Tying up loose ends: Discharging patients with unresolved medical issues.Arch Intern Med.2007;167(12):1305–1311.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370.
- ,,,.Prospective audit of discharge summary errors.Br J Surg.1996;83(6):788–790.
- ,.Lost in transition: Challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,.Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11(11):684–688.
- ,,,,.A case manager intervention to reduce readmissions.Arch Intern Med.1994;154(15):1721–1729.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–1447.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: A randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: The Care Transitions Intervention.J Am Geriatr Soc.2004;52(11):1817–1825.
- ,,,.The care transitions intervention: Results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: A randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,,.Redefining and redesigning hospital discharge to enhance patient care: A randomized controlled study.J Gen Intern Med.2008;23(8):1228–1233.
- ,,, et al.Effect of a nurse team coordinator on outcomes for hospitalized medicine patients.Am J Med.2005;118(10):1148–1153.
- ,,.Public reporting of discharge planning and rates of readmissions.N Engl J Med.2009;361(27):2637–2645.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- Consortium UHS. Reducing Readmissions SC22009. Available at: https://www.uhc.edu/1244.htm
- ,,,,.Adverse drug events occurring following hospital discharge.J Gen Intern Med.2005;20(4):317–323.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital.Ann Intern Med.2003;138(3):161–167.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting.J Gen Intern Med.2003;18(8):646–651.
- ,,,.Posthospital medication discrepancies: Prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,, et al.Prospective payment system and impairment at discharge. The ‘quicker‐and‐sicker’ story revisited.JAMA.1990;264(15):1980–1983.
- .The incidence of adverse medical outcomes under prospective payment.Econometrica. 1995;63:29–50.
- ,,.Content of a discharge summary from a medical ward: Views of general practitioners and hospital doctors.J R Coll Physicians Lond.1995;29(4):307–310.
- ,.Quality assessment of a discharge summary system.Can Med Assoc J.1995;152(9):1437–1442.
- ,,.Dissemination of discharge summaries. Not reaching follow‐up physicians.Can Fam Physician.2002;48:737–742.
- ,,,.Effect of discharge summary availability during post‐discharge visits on hospital readmission.J Gen Intern Med.2002;17(3):186–192.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care.JAMA.2007;297(8):831–841.
- ,,, et al.Association of communication between hospital‐based physicians and primary care providers with patient outcomes.J Gen Intern Med.2009;24(3):381–386.
- ,,.Tying up loose ends: Discharging patients with unresolved medical issues.Arch Intern Med.2007;167(12):1305–1311.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143(2):121–128.
- ,,, et al.Transitions of care consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370.
- ,,,.Prospective audit of discharge summary errors.Br J Surg.1996;83(6):788–790.
- ,.Lost in transition: Challenges and opportunities for improving the quality of transitional care.Ann Intern Med.2004;141(7):533–536.
- ,,.Effect of a nurse case manager on postdischarge follow‐up.J Gen Intern Med.1996;11(11):684–688.
- ,,,,.A case manager intervention to reduce readmissions.Arch Intern Med.1994;154(15):1721–1729.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–1447.
- ,,,,,.Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta‐analysis.JAMA.2004;291(11):1358–1367.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: A randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: The Care Transitions Intervention.J Am Geriatr Soc.2004;52(11):1817–1825.
- ,,,.The care transitions intervention: Results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: A randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,,.Redefining and redesigning hospital discharge to enhance patient care: A randomized controlled study.J Gen Intern Med.2008;23(8):1228–1233.
- ,,, et al.Effect of a nurse team coordinator on outcomes for hospitalized medicine patients.Am J Med.2005;118(10):1148–1153.
- ,,.Public reporting of discharge planning and rates of readmissions.N Engl J Med.2009;361(27):2637–2645.
- ,,.Rehospitalizations among patients in the Medicare fee‐for‐service program.N Engl J Med.2009;360(14):1418–1428.
- Consortium UHS. Reducing Readmissions SC22009. Available at: https://www.uhc.edu/1244.htm
Copyright © 2011 Society of Hospital Medicine