User login
Causes of peripheral neuropathy: Diabetes and beyond
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
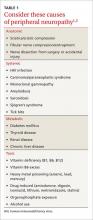
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
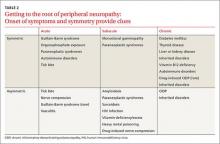
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
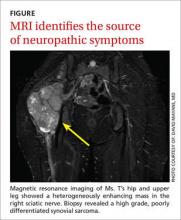
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; lmayans@kumc.edu.
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; lmayans@kumc.edu.
› When evaluating a patient with lower extremity numbness and tingling, order fasting blood glucose, vitamin B12 level with methylmalonic acid, and either serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE) because these test have a high diagnostic yield. C
› Obtain SPEP or IFE when evaluating all patients over age 60 with lower extremity paresthesias. C
› Consider prescribing pregabalin for treating painful paresthesias because strong evidence supports its use; the evidence for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine is moderate. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Sally G, age 46, has been experiencing paresthesias for the past 3 months. She says that when she is cycling, the air on her legs feels much cooler than normal, with a similar feeling in her hands. Whenever her hands or legs are in cool water, she says it feels as if she’s dipped them into an ice bucket. Summer heat makes her skin feel as if it's on fire, and she’s noticed increased sweating on her lower legs. She complains of itching (although she has no rash) and she’s had intermittent tingling and burning in her toes. On neurologic exam, she demonstrates normal strength, sensation, reflexes, coordination, and cranial nerve function.
Case 2 › Jessica T, age 25, comes in to see her family physician because she’s been experiencing numbness in her right leg. It had begun with numbness of the right great toe about a year ago. Subsequently, the numbness extended up her foot to the lateral aspect of the lower leg with an accompanying burning sensation. Three months prior to this visit, she developed weakness in her right foot and toes. She denies any symptoms in her left leg, upper extremities, or face.
A neurologic exam of the upper extremities is normal. Ms. T also has normal cranial nerve function, and normal strength, sensation, and reflexes in the left leg. A motor exam of the right leg reveals normal strength in the hip flexors, hip adductors, hip abductors, and quadriceps. On the Medical Research Council scale, she has 4/5 strength in the hamstrings, 0/5 in the ankle dorsiflexors, 1/5 in the posterior tibialis, and 3/5 in the gastrocnemius. She has a normal right patellar reflex, and an ankle jerk reflex and Babinski sign are absent. She has reduced sensation on the posterior and lateral portions of the right leg and the entire foot. Sensation is preserved on the medial side of the right lower leg and anterior thigh. She has right-sided steppage gait.
If these 2 women were your patients, how would you proceed with their care?
Paresthesias such as numbness and tingling in the lower extremities are common complaints in family medicine. These symptoms can be challenging to evaluate because they have multiple potential etiologies with varied clinical presentations.1
A well-honed understanding of lower extremity anatomy and the location and characteristics of common complaints is essential to making an accurate diagnosis and treatment plan. This article discusses the tests to use when evaluating a patient who presents with lower extremity numbness and pain. It also describes the typical presentation and findings of several types of peripheral neuropathy, and how to manage them.
Parasthesias are often the result of peripheral neuropathy
While paresthesias can arise from disorders of the central or peripheral nervous system, this article focuses on paresthesias that are the result of peripheral neuropathy. Peripheral neuropathy can be classified as mononeuropathy, multiple mononeuropathy, or polyneuropathy:
- Mononeuropathy is focal involvement of a single nerve resulting from a localized process such as compression or entrapment, as in carpal tunnel syndrome.1
- Multiple mononeuropathy (mononeuritis multiplex) results from damage to multiple noncontiguous nerves that can occur simultaneously or sequentially, as in vasculitic causes of neuropathy.1
- Polyneuropathy involves 2 or more contiguous nerves, usually symmetric and length-dependent, creating a “stocking-glove” pattern of paresthesias.1 Polyneuropathy affects longer nerves first, and thus, patients will initially complain of symptoms in their feet and legs, and later their hands. Polyneuropathy is most commonly seen in diabetes.
Possible causes of peripheral neuropathy include numerous anatomic, systemic, metabolic, and toxic conditions (TABLE 1).1,2
What's causing the neuropathy? The search for telltale clues
While obtaining the history, ask the patient about the presence of positive, negative, or autonomic neuropathic symptoms. Positive symptoms, which usually present first, are due to excess or inappropriate nerve activity and include cramping, twitching, burning, and tingling.3 Negative symptoms are due to reduced nerve activity and include numbness, weakness, decreased balance, and poor sensation. Autonomic symptoms include early satiety, constipation or diarrhea, impotence, sweating abnormalities, and orthostasis.3 The timing of onset, progression, and duration of such symptoms can give important diagnostic clues. For example, an acute onset of painful foot drop may indicate an inflammatory cause such as vasculitis, whereas slowly progressive numbness in both feet points toward a distal sensorimotor polyneuropathy, likely from a metabolic cause. Symmetry or asymmetry at presentation, as well as speed of progression of symptoms, can also significantly narrow the differential (TABLE 2).
Determining the exact location of symptoms is important and usually requires prompting. For example, when a patient refers to “the legs,” he could mean anywhere from the foot to the hip. The presence of radiating pain can also help localize the lesion, generally pointing to a radiculopathy (disease at the root of a nerve). Bowel or bladder involvement could suggest involvement of the spinal cord or autonomic nervous system.
A thorough social history can help identify potentially treatable causes of neuropathy. The probability of a toxic, infectious, or vitamin deficiency etiology can be ascertained by inquiring about a patient’s occupation, sexual history, dietary habits, and drug, alcohol, and tobacco history.3 Personal and family medical history can suggest possible genetic or endocrine causes of neuropathy. A personal or family history of childhood “clumsiness” (suggestive of a hereditary neuropathy, such as Charcot-Marie-Tooth disease), diabetes mellitus, or thyroid, renal, hepatic, or autoimmune diseases would be significant. A personal or family history of cancer is also an important diagnostic clue.3
These tests help narrow the diagnostic possibilities
Motor and sensory testing are essential, as is testing of coordination and reflexes. Motor examination involves manual muscle testing. In many patients, pain can limit effort, so encourage patients to try hard during testing so you can determine the true severity of weakness. Sensory testing should include pinprick, temperature differentiation, vibration, and proprioception. Also examine the cranial nerves and upper extremities because abnormal findings could suggest a central nervous system (CNS) lesion or proximal progression of disease, with the patient unaware of subtle symptom worsening or spreading. The pattern of deficits as well as predominance of motor vs sensory nerve involvement can further narrow the differential. For example, unilateral symptoms typically suggest either a structural lesion or inflammatory lesion as the cause, while unilateral weakness without numbness could be significant for the onset of amyotrophic lateral sclerosis.1 A careful skin, hair, and mucous membrane exam is useful because many infectious, toxic, autoimmune, and genetic causes of peripheral neuropathy also cause changes in these areas. High arches, hammer toes, and inverted champagne bottle legs suggest a hereditary neuropathy.3
In addition to the history and examination, electrodiagnostic testing (EDX) is often helpful, and judicious laboratory testing can further narrow diagnostic possibilities. (See “How best to use EDX and lab testing to evaluate peripheral neuropathy”.1-3)
So what type of neuropathy are you dealing with?
The details of your patient’s history and findings from the exam and testing will point you toward any one of a number of different types of neuropathies. The list below covers a range—from the common (distal sensorimotor polyneuropathy) to the more rare (paraneoplastic neuropathies).
Distal sensorimotor polyneuropathy (DSP)
DSP is the most common type of neuropathy.4 The typical presentation of DSP is chronic, distal, symmetric, and predominantly sensory.5 Any variation on this suggests an atypical neuropathy.5 Patients with DSP present with loss of function (loss of sensation to pinprick, temperature, vibration, proprioception) and/or tingling, burning, and pain starting symmetrically in the lower extremities. Over the course of years, paresthesias move up the legs to the knees before symptoms begin in the arms.
While the disorder can be quite painful, it is not usually functionally limiting unless the loss of sensation is severe enough to cause falls from sensory ataxia. Weakness is rare. When it occurs, it is usually a mild weakness of the distal leg with foot atrophy.
The most common cause of DSP is diabetes or impaired glucose tolerance. Other common causes are vitamin deficiencies (vitamin B1, B6, B12), folate deficiency, paraproteinemia, and hypo/hyperthyroidism. Also consider alcohol abuse, human immunodeficiency virus (HIV) infection, gastric bypass, chemotherapy, chronic kidney disease, and autoimmune conditions such as Sjögren’s syndrome, lupus, and rheumatoid arthritis.1
Testing. EDX can help confirm a diagnosis of DSP. A 2009 American Academy of Neurology review of lab testing for DSP found the tests with the highest diagnostic yield were fasting blood glucose, vitamin B12 level with methylmalonic acid, and serum protein electrophoresis and immunofixation electrophoresis (IFE).4 If the initial screen with a fasting blood sugar or hemoglobin A1c (HbA1c) is negative, further testing with a glucose tolerance test is recommended.
Treatment of DSP depends on the underlying etiology. Vitamin deficiencies should be corrected and metabolic or autoimmune etiologies addressed as appropriate. There are multiple pharmacologic options for treating persistent pain or discomfort. Best evidence (Level A) exists for pregabalin.6 Moderate evidence of effectiveness (Level B) exists for gabapentin, sodium valproate, amitriptyline, venlafaxine, and duloxetine.6
After taking a detailed history and performing a physical exam on a patient with lower extremity numbness and tingling, electrodiagnostic testing (EDX) and laboratory testing can help further elucidate the diagnosis.
EDX can be considered an extension of the physical exam. It can assess and characterize the proportion of motor vs sensory involvement, the severity of symptoms, and distribution of deficits and dysfunction.3 EDX studies consist of both electromyography (EMG) and nerve conduction studies (NCS). These tests are complementary and should be performed together. They have essentially no contraindications, although they are usually not performed on patients who have open sores or cellulitis. EMG is avoided in certain muscles in anticoagulated patients, primarily the paraspinal muscles and tibialis anterior, to avoid either an epidural hematoma or compartment syndrome.
A systematic evaluation of sequential muscles and nerves can identify polyneuropathy, entrapment neuropathy, plexopathy, or radiculopathy. Even a normal study can be informative. For example, EDX can provide information only on large fiber nerves; small nerve fibers cannot be tested. Therefore, a normal EDX in certain clinical scenarios suggests a small fiber neuropathy, which can be confirmed by skin biopsy.
Laboratory testing is a useful adjunct because the possible causes of peripheral neuropathy are vast. According to expert opinion, lab work that should be ordered routinely when evaluating lower extremity peripheral neuropathy includes a complete blood cell count, erythrocyte sedimentation rate, fasting blood glucose (and possibly hemoglobin A1c), thyroid studies, renal function studies, and vitamin B12 level.1-3 If a patient’s B12 level is <400 pg/mL, also test methylmalonic acid and homocysteine levels due to their greater diagnostic yield.3
Serum protein electrophoresis or serum immunofixation electrophoresis are also recommended in patients over age 60, because monoclonal gammopathy is a common cause of peripheral neuropathy in this age group.1,3 If the history and physical warrant, laboratory tests for paraneoplastic, autoimmune, infectious, or toxic etiologies can be performed.
Small fiber neuropathy
Small fiber neuropathy can present similarly to DSP, with distal painful paresthesias, but can spread to the upper extremities within a few weeks or months from onset, while DSP spreads to the hands years after onset. Small fiber neuropathy is also associated with early autonomic dysfunction. Examination usually reveals decreased sensation distally, but reflexes and strength are normal.
Common causes of small fiber neuropathy are diabetes, glucose intolerance, metabolic syndrome, hypo/hyperthyroidism, monoclonal gammopathy, alcohol abuse, vitamin B12 deficiency, and hypertriglyceridemia.7 Less common causes include Sjögren’s syndrome, HIV, Lyme disease, sarcoidosis, heavy metal toxicity, amyloidosis, and celiac disease.7
Testing and treatment. Skin biopsy is used to confirm the diagnosis of small fiber neuropathy.7 (EDX results are normal.7) Persistent pain can be treated with the same agents discussed above for treating DSP.
Acquired demyelinating neuropathy
Acquired demyelinating neuropathy is a rare condition, but one in which prompt recognition and treatment can prevent significant neurologic decline. There are both acute and chronic types of acquired demyelinating neuropathies.
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyradiculoneuropathy. Nearly two-thirds of patients with GBS report a previous respiratory or gastrointestinal illness; cytomegalovirus and Campylobacter jejuni are the most frequently associated infections.8
The onset of GBS often involves pain in the back or limbs, followed by a rapid progression of sensory loss and weakness (over days to a few weeks) that typically starts in the feet and moves upward.8 Though the typical presentation of GBS is “ascending,” there are frequent exceptions to this pattern.8 Physical exam shows weakness, sensory loss, and absent reflexes. Severe cases can result in complete paralysis, even of extraocular movements. Autonomic dysfunction is common.
Testing. EDX and lumbar puncture are needed to accurately diagnose GBS.8 EDX initially may be unremarkable, but over time, areas of demyelination become apparent. Lumbar puncture shows albuminocytologic dissociation (no white cells, elevated protein).
Treatment. Patients with GBS are initially managed as inpatients because 33% of cases lead to respiratory failure.9 Treatments include intravenous immunoglobulin (IVIg) or plasmapheresis; both have similar outcomes, speeding neurologic recovery time but not affecting overall long-term prognosis.10 Response to treatment is often not immediate, and some patients continue to worsen after treatment.8 Still, long-term prognosis is good, even for severely affected patients, as long as they receive good supportive care. The relapse rate is between 2% and 6%.8
In chronic inflammatory demyelinating polyneuropathy (CIDP), patients develop stepwise nerve dysfunction over many weeks to months. One nerve is affected, then another, usually in a different limb. There is generally no antecedent illness, and pain is infrequent.8 Progressive limb weakness is by far the most common presentation, and manifests as a foot drop or wrist drop. Patients may report difficulty getting up from a chair, walking up stairs, or opening jars.8 Facial or extraocular nerve involvement is uncommon, as is respiratory involvement.8 Neurologic exam shows absent reflexes, weakness, and loss of sensation in the distribution of a particular nerve or nerves.
Testing and treatment. Diagnosis of CIDP is made by a combination of EDX that shows demyelination and lumbar puncture that demonstrates albuminocytologic dissociation. Treatments include long-term immunosuppression with oral prednisone, IVIg, plasmapheresis, and rarely, agents such as mycophenolate mofetil, azathioprine, cyclosporine, and rituximab.9
Entrapment neuropathy
This is the result of compression or entrapment of a nerve by another anatomic structure. It can be caused by internal or external factors, including fluid retention.11 Damage from compression or entrapment progresses in stages and, over time, can result in demyelination and distal degeneration of the nerve.11 More interior nerve fibers, such as pain nerve fibers, are often the last to be affected.11 Therefore, patients often first experience loss of motor function or loss of sensation to light touch.
Common fibular nerve (formerly known as common peroneal nerve) entrapment at the fibular head is the most common entrapment neuropathy in the lower extremities. It’s usually the result of direct trauma, such as prolonged positioning in debilitated patients or surgical patients, habitual leg-crossing, tight boots, or tight casts.11,12 Uncoordinated gait due to poor dorsiflexion of the foot at the ankle (foot drop) is common while plantar flexion is preserved. Pain and sensory loss depend on the degree of compression and the exact location of compression.
Testing and treatment. EDX is useful for identifying the location of compression or entrapment and can guide further imaging, if needed. Conservative treatments aimed at modifying or correcting the underlying etiology, such as removing a tight-fitting cast or brace or instructing a patient to stop leg crossing, can be effective. Occasionally, surgery is required.
Anterior tarsal tunnel syndrome is compression of the deep fibular nerve as it passes through the inferior extensor retinaculum of the distal lower leg. Characteristic symptoms include pain and burning over the dorsum of the foot.11 Paresthesias in the first dorsal web space are also common.11 This can be seen in athletes who perform repetitive ankle plantar flexion, such as ballet dancers, soccer players, and runners.12 It can also be caused by recurrent ankle sprains, ganglion cysts, and tight-fitting shoes or boots.11,12 Chronic cases can result in toe extensor weakness or atrophy of the extensor digitorum brevis muscle.
Testing and treatment. Again, EDX is very useful in identifying the exact area of compression and involved nerve segments. Management requires correcting the underlying etiology, which can usually be done conservatively. Surgical decompression may be needed.
Paraneoplastic neuropathies
Paraneoplastic neuropathies are exceptionally rare but often develop before cancer is diagnosed. Therefore, early suspicion and recognition can greatly affect cancer prognosis.13 Certain characteristics should increase suspicion of a paraneoplastic process. For example, symptoms with a subacute progressive onset that involve the upper extremities early in the disease are characteristic of a paraneoplastic process.13
Coexisting CNS symptoms and/or constitutional symptoms of malignancy should also increase suspicion.13 Consider a paraneoplastic process in patients who have a past history of cancer or significant cancer risk factors, such as smoking.
Testing. When you suspect a paraneoplastic process, the work-up should include antibody testing for the most common or likely cancers according to patient characteristics. Panels of the most common paraneoplastic antibodies are available from many commercial labs. Obtain imaging to identify a possible underlying malignancy.
That said, it’s also important to perform a basic work-up for the more common causes of neuropathy in patients you suspect may have cancer. The reason: Paraneoplastic neuropathies are rare, and not all neuropathies in patients with cancer are paraneoplastic.13
CASE 1 › Ms. G describes diffuse paresthesias that are worse in her lower extremities, but she has a normal neurologic exam. Her physician suspects a neuropathic cause, and a normal exam makes small fiber neuropathy more likely. EDX is normal. The initial work-up includes an HbA1c, thyroid-stimulating hormone, vitamin B12 level, antinuclear antibody, erythrocyte sedimentation rate, IFE, and free light chain assay.
Testing reveals that Ms. G has a high free light chain ratio, which suggests a monoclonal gammopathy is the most likely etiology. Skin biopsy demonstrates decreased nerve fiber density consistent with a small fiber neuropathy. Her physician refers her to Hematology for bone marrow biopsy, and also prescribes gabapentin 300 mg/d at bedtime for symptomatic relief. Ms. G is currently being closely monitored for conversion to multiple myeloma.
CASE 2 › In Ms. T’s case, the exam helps localize the lesion. Areas supplied by the common fibular nerve, tibial nerve, and sural nerve are affected, while the area innervated by the femoral nerve and saphenous nerve and the proximal hip muscles are spared. This localizes a lesion to the sciatic nerve. EDX confirms a proximal sciatic lesion, but not the underlying etiology. Since the lesion had been precisely localized, her physician orders imaging.
Magnetic resonance imaging of Ms. T’s hip and upper leg shows a 10.7 cm x 7.8 cm x 13 cm heterogeneously enhancing mass in the expected location of the right sciatic nerve (FIGURE). Biopsy reveals a high grade, poorly differentiated synovial sarcoma. Her physician refers her to an oncologist for initiation of chemotherapy, radiation, and debulking surgery.
CORRESPONDENCE
Laura C. Mayans, MD, Department of Family and Community Medicine, University of Kansas School of Medicine-Wichita, 1010 N. Kansas, Wichita, KS 67214; lmayans@kumc.edu.
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.
1. Katirji B, Koontz D. Disorders of peripheral nerves. In: Daroff R, ed. Bradley’s Neurology in Clinical Practice. 6th ed. Philadelphia, PA: Elsevier; 2012:1915-1983.
2. Azhary H, Farooq MU, Bhanushali M, et al. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887-892.
3. Alport AR, Sander HW. Clinical approach to peripheral neuropathy: anatomic localization and diagnostic testing. Continuum (Minneap Minn). 2012;18:13-38.
4. England JD, Gronseth GS, Franklin G, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Neurology. 2009;72:185-192.
5. Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn). 2012;18:60-84.
6. Shenoy AM. Guidelines in practice: treatment of painful diabetic neuropathy. Continuum (Minneap Minn). 2012;18:192-198.
7. Gibbons CH. Small fiber neuropathies. Continuum (Minneap Minn). 2014;20:1398-1412.
8. So YT. Immune-mediated neuropathies. Continuum (Minneap Minn). 2012;18:85-105.
9. Dimachkie MM, Saperstein DS. Acquired immune demyelinating neuropathies. Continuum (Minneap Minn). 2014;20:1241-1260.
10. Patwa HS, Chaudhry V, Katzberg H, et al. Evidence-based guideline: intravenous immunogloblin in the treatment of neuromuscular disorders. Neurology. 2012;78:1009-1015.
11. Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle and foot. Foot Ankle Clin. 2011;16:255-274.
12. Meadows JR, Finnoff JT. Lower extremity nerve entrapments in athletes. Curr Sports Med Rep. 2014;13:299-306.
13. Muppidi A, Vernino S. Paraneoplastic neuropathies. Continuum (Minneap Minn). 2014;20:1359-1372.