User login
Parkinson disease: Not just a movement disorder
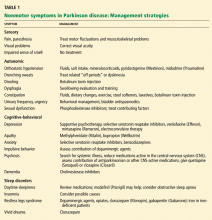
These nonmotor symptoms—sensory, autonomic, and behavioral—are important to recognize, as they can lead to even more serious complications and impair quality of life.
COMMON, TROUBLESOME, AND UNDERDIAGNOSED
Nonmotor symptoms are very common. In fact, up to 60% of patients suffer from more than one nonmotor symptom, and 25% have four or more,1 including autonomic dysfunction, sensory symptoms, and cognitive and behavioral problems.2
Nonmotor symptoms can be primary complaints and, for some patients and family members, can cause greater disability than motor symptoms.3,4 For instance, depression and cognitive problems contribute to a decline in quality of life regardless of the degree of motor impairment.
Yet these symptoms are often underdiagnosed. 5 A delay in diagnosis may reflect the tendency of clinicians, patients, and family members to focus on the more apparent motor features of Parkinson disease, a lack of awareness of the nonmotor symptoms, or both. Consequently, patient education is essential. Identifying and treating these symptoms requires a multidisciplinary clinical team approach and an ongoing dialogue with the patient and family.
VARIOUS SYMPTOMS, VARIOUS CAUSES
Some nonmotor symptoms (eg, impaired sense of smell, depression, anxiety, fatigue, and constipation) can precede the motor symptoms of Parkinson disease and may be symptoms of the disease itself. Perhaps accounting for these observations, recent pathologic studies described diffuse Lewy body deposition in areas outside of nigral dopaminergic neurons,6,7 and the olfactory bulb, medulla, and pontine tegmentum may be involved before the substantia nigra.
Other symptoms, such as orthostatic hypotension, sedation, psychosis, confusion, and impulsiveness, may be adverse effects of medical therapy or may worsen with it.
Nonmotor symptoms can also emerge as a “wearing-off” phenomenon with standard treatment.2,8 The “off period” is a term primarily used to describe the reemergence of motor symptoms as a dose of levodopa wears off and before the patient receives the next dose. However, nonmotor symptoms, in particular depression and anxiety, can also occur in this period.
To treat nonmotor symptoms, one needs to identify and treat the primary symptom or the comorbid illnesses that may worsen it (eg, confusion in the setting of dehydration and infection), assess the possibility of adverse drug effects (a particular problem with many anti-Parkinson drugs), and try to reduce off periods with changes in dopaminergic therapy.
SENSORY SYMPTOMS
Off-period pain, paresthesia
Pain that cannot be attributed to muscle spasms, dystonia, somatic disease, autonomic dysfunction, or peripheral nerve disease occurs in up to 38% of patients with Parkinson disease.9,10 The pain is often diffuse and aching. Paresthesia-like complaints include numbness, tingling, and change in temperature.
Some sensory symptoms occur mostly during off periods and may respond to dopaminergic therapy. Examples are limb paresthesia, truncal pain, trigeminal neuralgia-like pain, and vaginal or perineal pain.9–11
Impaired sense of smell, vision
Impaired sense of smell can precede motor symptoms and is being investigated as a possible screening symptom for early diagnosis.12,13
Altered vision is a less recognized symptom of Parkinson disease. Many patients have difficulty reading even if they have normal visual acuity. Part of their difficulty stems from oculomotor defects such as impairment in visual saccadic movements and muscle rigidity.14 Visual pathways can be affected, as evidenced by abnormal visual evoked potentials that correlate with disease severity and by impairment in contrast sensitivity, color perception, and judgment of line orientation.15
It is unclear how these visual abnormalities contribute to everyday symptoms in Parkinson disease. Certainly, visual scanning activities such as reading are impaired.
Patients also suffer from drug-induced visual illusions and hallucinations, which are often colorful. Diederich et al16 found that contrast discrimination and color perception were significantly more impaired in Parkinson patients who have visual hallucinations, which suggests that it is important to correct visual abnormalities.
AUTONOMIC SYMPTOMS
In general, autonomic problems increase with age, disease severity, medication use, postural instability, cognitive decline, and visual hallucinations.17,18
Orthostatic hypotension
Almost half of patients with Parkinson disease have orthostatic hypotension.19 Of concern, patients with postural instability are at greater risk of orthostatic hypotension, thereby further increasing their risk of falling and injuring themselves.20
Postprandial hypotension is more common in Parkinson disease and is more often associated with midday meals, perhaps owing to a higher carbohydrate content and its effect on insulin release21 (many patients reserve high-protein meals for the evening and eat a greater proportion of carbohydrates during the day).
Home blood pressure monitoring is especially helpful, since blood pressure can fluctuate significantly and office readings may not reveal the problem.
Orthostatic hypotension can be both a drug side effect and a manifestation of the disease. Although all dopaminergic drugs can worsen orthostatic hypotension, the motor benefits of these drugs should be reviewed in relation to this risk. Dopaminergic agonists and amantadine (Symmetrel) should be used with caution in patients with significant orthostatic hypotension.
Treatment should include fluids, a highsalt diet, elastic stockings, fludrocortisone (Florinef), pyridostigmine (Mestinon), and perhaps the selective alpha 1 agonist midodrine (Proamatine). However, midodrine can cause supine hypertension and must be used cautiously in patients with advanced disease who take daytime naps because of fatigue. If postprandial hypotension is a problem, altering the patient's diet to include smaller but more frequent meals may help.
Cold limbs, sweating
Complaints related to temperature regulation include cold limbs and excessive sweating. Off-period drenching sweats occur as an endof- dose symptom thought to be related to subtherapeutic plasma dopamine levels and may respond to dopaminergic therapy aimed at reducing motor fluctuations and off periods.22
Gastrointestinal symptoms
Dysphagia (difficulty swallowing), sialorrhea (excessive salivation), nausea, constipation, and defecatory dysfunction are more common in patients with Parkinson disease than in agematched controls, even after controlling for factors such as drugs, autonomic dysfunction, diet, and exercise.23 These problems can cause malnutrition (necessitating a gastrostomy tube), aspiration pneumonia, and difficulty in swallowing and retaining pills,24 all of which can lead to problems that are even more serious. Although gastrointestinal symptoms occur in all stages of Parkinson disease, patients with advanced disease are at greater risk.
Dysphagia. James Parkinson described dysphagia and sialorrhea in his original 1816 monograph.25 The prevalence of dysphagia increases with severity of disease.23 Dysphagia is usually due to altered pharyngeal contraction, resulting in difficulty propelling food into the pharynx and retention of food in the pyriform sinuses and valleculae, but esophageal dilatation and dysmotility, spasms, gastroesophageal reflux, and increased transit time also contribute.24,26,27
Constipation may affect more than half of patients.28 One study reported a higher risk of developing Parkinson disease in men with infrequent bowel movements.29
Constipation and defecatory dysfunction can be severe enough to result in colonic dilatation and pseudo-obstruction.30 Altered gastrointestinal transit time may contribute to erratic absorption of medications.
Causes of constipation include slow colonic transit, weak abdominal muscles, decreased phasic contraction, and a paradoxical increase in puborectalis muscle and anal sphincter activity with straining, consistent with pelvic muscle dystonia.31
Treatment includes reducing off periods, limiting anticholinergic agents, prescribing a proper bowel regimen, and encouraging fluids and exercise. In addition, daily stool softeners, fiber, and polyethylene glycol are effective. Botulinum toxin injection into the puborectalis muscle may improve outlet obstruction.32
Risk of aspiration. Dysphagia, in association with respiratory symptoms, should prompt an evaluation for aspiration. Thickening liquids and early referral to a swallowing specialist may lessen the risk of aspiration.
Drooling is bothersome and embarrassing for many patients. Treatment historically included antimuscarinic agents, but these can cause constipation and confusion in patients with advanced disease. More recently, botulinum toxin injections into the parotid and submandibular glands have been shown to be effective in patients with excessive drooling.33
Urinary problems
Urologic abnormalities can be divided into dysfunction of the bladder, dysfunction of the urethral sphincter, and other causes of outflow obstruction such as prostate enlargement in men. The most common complaint is nocturia, followed by frequency and urgency.34
Nocturnal polyuria and urinary hesitancy and urgency are embarrassing but treatable. Urinary incontinence is a common reason for nursing home placement, and nocturia is a common cause of falls, as patients attempt to get up to urinate.35,36
Treatment of urinary incontinence should begin with an assessment for urinary tract infection, stress incontinence in women, and prostate enlargement in men. Urinary urgency due to detrusor hyperreflexia or spastic bladder can improve with anticholinergic antispasmodic agents. However, these should be used with caution in patients who are experiencing hallucinations or cognitive problems.
Sexual dysfunction
Sexual dysfunction, including decreased libido and erectile dysfunction, is in part related to autonomic dysfunction.
Treatment is complex, as these problems are multifactorial. Contributing factors include physical disability, the stress of living with a progressive illness, drug effects, depression, pain, difficulty in communication, caregiver stress, and impact on intimacy. The phosphodiesterase inhibitor sildenafil (Viagra) can improve erectile dysfunction, but must be used cautiously in patients with orthostatic hypotension.37 Other drugs of this class are available but have not been tested in this population.
COGNITIVE-BEHAVIORAL PROBLEMS
Neuropsychiatric disorders are common in Parkinson disease and at times can be more distressing to the patient and family than the motor symptoms. These include mood disorders, apathy, anxiety, impulse control disorders, psychosis, and dementia.
Dopaminergic medications used to treat movement can precipitate or exacerbate these neuropsychiatric problems. Therefore, treatment requires a balance between motor and neuropsychiatric benefits, with close dialogue with the patient and family about primary goals of treatment.
Depression
Depression is among the most common neuropsychiatric symptoms in Parkinson disease, occurring to some degree in up to 50% of patients.38 Diagnosing it is critical, because it can worsen physical symptoms, cognitive status, quality of life, and caregiver distress.39
However, depression can be difficult to recognize, because many of its features (eg, fatigue, psychomotor slowing, flattened affect, sleep difficulties) can also be manifestations of Parkinson disease. One should specifically ascertain whether the patient has a depressed mood or loss of interest in pleasurable activities. The Beck Depression Inventory is sensitive for depression in Parkinson disease and thus may be a reasonable screening tool.40
Treatment. Psychotherapy may help and may even be a first-line treatment in patients who cannot tolerate antidepressant drugs. Practical recommendations include relaxation techniques, a sleep hygiene regimen, engaging in meaningful activities to achieve a sense of purpose, and caregiver education.41
We have little evidence-based guidance on drug treatment of depression in patients with Parkinson disease. A recent meta-analysis found only two placebo-controlled studies in the past 40 years that monitored outcome based on a standardized rating scale of depression in patients with Parkinson disease.42
In preliminary studies, dopamine agonists have shown some efficacy in treating depression without Parkinson disease.43 However, the antidepressant contributions of anti-Parkinson drugs have not been well established.
Selective serotonin reuptake inhibitors (SSRIs) appear to be safe and well tolerated.44 Venlafaxine (Effexor) and mirtazapine (Remeron) are also reasonable initial options. Tricyclic antidepressants should be used with caution because they can cause anticholinergic side effects, especially confusion, in this population.
All serotonergic agents should be used with caution when given in combination with monoamine oxidase inhibitors, which are often used to treat motor symptoms in Parkinson disease, because of the risk of serotonin syndrome, which is characterized by fever, altered mental status, myoclonus, tremor, hyperreflexia, and diaphoresis and may be fatal.
Electroconvulsive therapy can be reserved for the treatment of severe refractory depression in patients with Parkinson disease without complex medical issues.45
Apathy
Apathy is present in approximately 30% of patients with Parkinson disease.46
Apathy can be very difficult to differentiate from depression, as the two disorders can occur together. Apathy can also be present without signs or symptoms of depressed mood. Apathy is commonly conceptualized as involving three domains: cognitive (lack of interest), behavioral (lack of initiation and drive), and affective (lack of emotion). The possibility of a primary apathy syndrome should be considered if the patient does not respond to standard treatments for depression. This is critical, as the treatment of the two syndromes may differ.47
Although efficacy data are limited, bupropion (Wellbutrin) and methylphenidate (Ritalin)48 can be tried for their activating or stimulant properties.
Anxiety disorders
Anxiety disorders commonly accompany depression in Parkinson disease, but they can also occur independently. They most often present in the setting of wearing-off or on-off fluctuations associated with medication status. Anxiety disorders in patients with Parkinson disease include generalized anxiety and panic attacks, which are responsive to SSRIs and benzodiazepines.49 Benzodiazepines are effective, and clonazepam (Klonopin) may be preferred because it has a long half-life, thereby minimizing anxiety associated with wearingoff or unpredictable off periods. Conversely, a long half-life and depressive effects may limit its use in advanced age.
Classic obsessive-compulsive disorder is less common, but obsessive behaviors and impulse control difficulties can occur in up to 7% of patients with Parkinson disease and presumably reflect dopamine dysregulation, most often associated with dopamine agonists.50 Impulsive behavior can include gambling, hypersexuality, and bingeing.
One form of obsessive-compulsive disorder is punding, a behavior characterized by intense fascination with repetitive handling and examining of objects, most often mechanical objects.51 Behaviors can include assembling and disassembling, collecting, or sorting of objects.
Visual hallucinations
Many patients with Parkinson disease have visual hallucinations as a side effect of dopaminergic drugs. At first, the patient realizes that they are hallucinations, but this insight may be lost as the disease progresses. The clinician should also consider other potential causes such as dementia, systemic illness, or psychosocial stress.
If visual hallucinations present early in the course of the disease and are accompanied by loss of insight and by cognitive fluctuations, the patient may actually have Lewy body dementia.52 Its features include parkinsonian symptoms, visual hallucinations, a fluctuating level of consciousness, and neuroleptic sensitivity.
Psychosis
The first-line treatment for psychosis in patients with Parkinson disease should involve:
- Searching for a systemic illness such as urinary tract infection, aspiration pneumonia, or dehydration;
- Stopping or lowering the dose of drugs that act on the central nervous system; and
- If possible, stopping or lowering the dose of anti-Parkinson drugs that have the greatest risk of cognitive side effects. In many cases, this is not a realistic option, and adding an antipsychotic drug may be necessary.
Monitoring and treating psychosis is paramount, as it is a major risk factor for nursing home placement.53
Conventional neuroleptics such as haloperidol (Haldol) should be avoided because they can exacerbate parkinsonian symptoms.
Two atypical neuroleptics, clozapine (Clozaril) and quetiapine (Seroquel), appear to be the best tolerated in Parkinson disease patients. Clozapine is the only neuroleptic found to be more efficacious than placebo in patients with Parkinson disease.54 However, of the two, clozapine has a higher risk of side effects and requires frequent blood monitoring, making it difficult to use. Quetiapine has been shown to be as efficacious as clozapine,55 but it failed to show efficacy in a recent controlled study.56 Nevertheless, it is considered the first-line treatment option, since it is safer.40
Other atypical antipsychotics appear to exacerbate Parkinson disease or have not been adequately studied and should therefore be used with caution.
Dementia
Estimates of the prevalence of dementia in patients with Parkinson disease vary widely, most likely reflecting differences in populations studied and methods used. The best estimates indicate that 20% to 30% of patients with Parkinson disease develop dementia.57
Parkinson dementia is one of the classic subcortical dementias, characterized by slow thinking and by difficulties in working memory and problem-solving due to disruption of frontal-subcortical circuits.58 Its most common neuropsychiatric symptoms are hallucinations and depression, with less agitation, disinhibition, and irritability than in Alzheimer dementia.59
Anti-Parkinson drugs, especially anticholinergics, can exacerbate cognitive impairments in patients with Parkinson disease. An acute change in cognitive abilities or visual hallucinations is often associated with an un derlying medical illness such as infection (eg, a urinary tract infection or aspiration pneumonia) or dehydration.
Anticholinesterase inhibitors such as rivastigmine (Exelon) are indicated in patients with Parkinson dementia but may provide only a modest benefit.60
SLEEP DISORDERS
Excessive daytime sleepiness
Fatigue and hypersomnolence often impair quality of life.61 Daytime hypersomnolence is often multifactorial: it can be caused by the disease itself,62 by dopaminergic therapy,63,64 by a comorbid illness, or by nighttime sleep problems.
Sudden and uncontrollable episodes of sleep are an extreme form of hypersomnolence and are worrisome, especially with activities such as driving.65 Greater sleepiness (measured by the Epworth Sleepiness Scale), longer duration of Parkinson disease, and use of dopaminergic agonists increase the risk of such attacks.66 In general, very few patients experience such attacks without warning signs of sedation.
Treatment must include a comprehensive evaluation of current medications, the effect of dopaminergic agents (especially agonists), and nighttime factors that influence sleep (some of which are described below). Modafinil (Provigil), effective in narcolepsy, helped in some studies67 but not in others68; it could be tried in moderate to severe cases of excessive daytime sleepiness.
Sleep apnea contributes to daytime hypersomnolence. An evaluation for sleep apnea should be considered even in patients who are not overweight, as some evidence suggests that this disorder is common in Parkinson disease and correlates with disease severity.69
Insomnia
Sleep is impaired in up to 74% of Parkinson patients.70,71 A sleep study may show a low total sleep time, many awakenings, a short rapid-eye movement (REM) latency, and short slow-wave sleep (stages III and IV); the patient experiences the problem as light sleep with frequent awakenings.72 A variety of problems related to Parkinson disease can directly affect sleep patterns: eg, pain, stiffness, tremor, problems turning over in bed, dystonia, dementia, nocturia,73 depression, and anxiety.74
Restless legs syndrome
Restless legs syndrome is an uncomfortable, sometimes painful feeling in the legs or other body parts during rest (especially at night) that improves with movement.75 It may be more common in patients with Parkinson disease than in the general population76,77 and can precede the diagnosis of Parkinson disease.78
Iron supplementation with ferrous sulfate can help if iron deficiency (ferritin < 50 μg/L or iron saturation < 16%) is present but is ineffective in its absence. Levodopa and the dopaminergic agonists ropinirole (Requip)70 and pramipexole (Mirapex)80 are effective Parkinson disease treatments that also treat restless legs syndrome. In addition, opioids, clonazepam, and gabapentin (Gabarone) may help.81
Vivid dreams
Parkinson patients often describe very vivid, intense, frightening, and unpleasant dreams,73 which may be a precursor to psychosis.82
REM sleep behavior disorder is characterized by sustained phasic muscle activity in place of normal atonia during REM or dream sleep. The patient's bed partner may describe him or her behaving in an aggressive way as if acting out his or her dreams, ie, hitting, yelling, or kicking. Upon awakening, the patient's recall of the dream content is consistent with the nocturnal behavior.
REM sleep behavior disorder may actually precede the motor symptoms of Parkinson disease,83,84 and as many as 20% of patents with REM sleep behavior disorder eventually develop Parkinson disease.85 The possible link between the two disorders is strengthened by a case report describing a patient with REM sleep behavior disorder, no signs of Parkinson disease on examination, but brain pathology similar to that found in Parkinson disease.86
Clonazepam is an effective treatment for REM sleep behavior disorder and should be considered if sleep is disrupted or patient safety becomes a concern.87
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001; 16:507–510.
- Hillen ME, Sage JI. Nonmotor fluctuations in patients with Parkinson's disease. Neurology 1996; 47:1180–1183.
- Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 2002; 59:408–413.
- Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord 2005; 20(suppl 11):S23–S29.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002; 8:193–197.
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004; 318:121–134.
- Wolters ECh, Braak H. Parkinson's disease: premotor clinico-pathological correlations. J Neural Transmiss Suppl 2006; 70:309–319.
- Gunal DI, Nurichalichi K, Tuncer N, Bekiroglu N, Aktan S. The clinical profile of nonmotor fluctuations in Parkinson's disease patients. Can J Neurol Sci 2002; 29:61–64.
- Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology 1976; 26:423–429.
- Koller WC. Sensory symptoms in Parkinson's disease. Neurology 1984; 34:957–959.
- Ford B, Louis ED, Geene P, Fahn S. Oral and genital pain syndromes in Parkinson's disease. Mov Disord 1996: 11:421–426.
- Haehner A, Hummel T, Hummel C, et al. Olfactory loss may be the first sign of idiopathic Parkinson's disease. Mov Disord 2007; 22:839–842.
- Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis and prognosis of new onset Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 66:968–975.
- Hunt LA, Sadun AA, Bassi CJ. Review of the visual system in Parkinson's disease. Optom Vis Sci 1995; 72:92–99.
- Rodnitzky RL. Visual dysfunction in Parkinson's disease. Clin Neurosci 1998; 5:102–106.
- Diederich N, Goetz G, Pappert E. Primary deficits in visual discrimination is a risk factor for visual hallucinations in Parkinson's disease [abstract]. Neurology 1997; 48:A181.
- Oka H, Yoshioka M, Onouchi K, et al. Characteristics of orthostatic hypotension in Parkinson's disease. Brain 2007; 130:2425–2432.
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson's disease. Neurology 2007; 69:323–341.
- Allcock LdM, Ullyart K, Kenny RA, Burn DJ. Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2004; 75:1470–1471.
- Allcock LM, Kenny RA, Burn DJ. Clinical phenotype of subjects with Parkinson's disease and orthostatic hypotension: autonomic symptom and demographic comparison. Mov Disord 2006; 21:1851–1855.
- Micieli G, Martignoni E, Cavallini A, Sandrini G, Nappi G. Postprandial and orthostatic hypotension in Parkinson's disease. Neurology 1987; 37:386–393.
- Sage JI, Mark MH. Drenching sweats as an off phenomenon in Parkinson's disease: treatment and relationship to plasma levodopa profile. Ann Neurol 1995; 37:120–122.
- Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord 1991; 6:151–156.
- Bushman M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson's disease. Neurology 1989; 39:1309–1314.
- Parkinson J. An Essay on the Shaking Palsy. London: Whittingham and Roland, 1817.
- Gibberd FB, Gleeson JA, Gossage AA, Wilson RS. Oesophageal dilatation in Parkinson's disease. J Neurol Neurosurg Psychiatry 1974; 37:938–940.
- Bird MR, Woodword MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Ageing 1994; 23:251–254.
- Kaye J, Gage H, Kimber A, Storey L, Trend P. Excess burden of constipation in Parkinson's disease: a pilot study. Mov Disord 2006; 21:1270–1273.
- Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001; 57:456–462.
- Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson's disease. Am J Gastroenterol 1994; 89:15–25.
- Sakakibara R, Odaka T, Uchiyama T, et al. Colon transit time and rectoanal videomanometry in Parkinson's disease. J Neurol Neurosurg Psychiatry 2003; 74:268–272.
- Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G. Outlet type obstruction in Parkinson's disease: results of botulinum toxin treatment. Aliment Pharmacol Ther 2005; 22:997–1003.
- Molloy L. Treatment of sialorrhoea in patients with Parkinson's disease: best current evidence. Curr Opin Neurol 2007; 20:493–498.
- Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinson's disease. Neurourol Urodyn 2006; 25:116–122.
- Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson's disease: frequency, impact and identifying factors. J Neurol 2005; 252:1310–1315.
- Chutka DS, Fleming KC, Evans MP, Evans JM, Andrews KL. Urinary incontinence in the elderly population. Mayo Clin Proc 1996; 71:93–101.
- Hussain IF, Brady CM, Swinn MJ, Mathias CJ, Fowler CJ. Treatment of erectile dysfunction with sildenafil citrate (Viagra) in parkinsonism due to Parkinson's disease or multiple system atrophy with observations on orthostatic hypotension. J Neurol Neurosurg Psychiatry 2001; 71:371–374.
- Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease. A community-based study. Arch Neurol 1996; 53:175–179.
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson's disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999; 14:866–874.
- Miyasaki JM, Shannon K, Voon V, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 66:996–1002.
- Dobkin RD, Allen LA, Menza M. A cognitive-behavioral treatment package for depression in Parkinson's disease. Psychosomatics 2006; 47:259–263.
- Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005; 20:1161–1169.
- Cassano P, Lattanzi L, Soldani F, et al. Pramipexole in treatment-resistant depression: an extended follow-up. Depress Anxiety 2004; 20:131–138.
- Dell'Agnello G, Ceravolo R, Nuti A, et al. SSRIs do not worsen Parkinson's disease: evidence from an open-label, prospective study. Clin Neuropharmacol 2001; 24:221–227.
- Fall P, Granérus AK. Maintenance ECT in Parkinson's disease. J Neural Transm 1999; 106:737–741.
- Kirsch-Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology 2006; 67:33–38.
- van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci 2005; 17:7–19.
- Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2002; 14:461–462.
- Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005; 96:42–55.
- Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 2006; 63:969–973.
- Fernandez HH, Friedman JH. Punding on L-dopa. Mov Disord 1999; 14:836–838.
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006; 9(suppl 3):417–423.
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993; 43:2227–2229.
- Frieling H, Hillemacher T, Ziegenbein M, Neundörfer B, Bleich S. Treating dopamimetic psychosis in Parkinson's disease: structured review and meta-analysis. Eur Neuropsychopharmacol 2007; 17:165–171.
- Morgante L, Epifanio A, Spina E, et al. Quetiapine versus clozapine: a preliminary report of comparative effects on dopaminergic psychosis in patients with Parkinson's disease. Neurol Sci 2002; 23(suppl 2):S89–S90.
- Rabey JM, Prokhorov T, Moniovitz A, Dobronevsky E, Klein C. Effect of quetiapine in psychotic Parkinson's disease patients: a double-blind labeled study of 3 months' duration. Mov Disord 2007; 22:313–318.
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord 2005; 20:1255–1263.
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive nd behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 2003; 16:193–210.
- Aarsland D, Cummings JL, Larsen JP. Neuropsychiatric differences between Parkinson's disease with dementia and Alzheimer's disease. Int J Geriatr Psychiatry 2001; 16:184–191.
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004; 351:2509–2518.
- Martinez-Martin P, Catalan MJ, Benito-Leon J, et al. Impact of fatigue in Parkinson's disease: the Fatigue Impact Scale for Daily Use (D-FIS). Qual Life Res 2006; 15:597–606.
- Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson's disease and sleepiness: an integral part of Parkinson disease. Neurology 2002; 58:1019–1024.
- Ondo WG, Dat Vong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001; 57:1392–1396.
- O'Suilleabhain PE, Dewey RB. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson's disease. Arch Neurol 2002; 59:986–989.
- Fucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 1999; 52:1908–1910.
- Paus S, Brecht HM, Köster J, Seeger G, Klockgether T, Wüllner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003; 18:659–667.
- Adler CH, Caviness JN, Hentz JG, Lind M, Tiede J. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003; 18:287–293.
- Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005; 76:1636–1639.
- Maria B, Sophia S, Michalis M, et al. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med 2003; 97:1151–1157.
- Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson's disease. Clin Neuropharmacol 1988; 6:512–519.
- Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord 1998; 13:895–899.
- van Hilten J, Hoff JI, Middelkoop HA, et al. Sleep disruption in Parkinson's disease: assessment by continuous activity monitoring. Arch Neurol 1994; 51:922–928.
- van Hilten JJ, Weggeman M, van der Velde EA, Kerkhof GA, van Dijk JG, Roos RA. Sleep, excessive daytime sleepiness and fatigue in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1993; 5:235–244.
- Borek LL, Kohn R, Friedman JH. Mood and sleep in Parkinson's disease. J Clin Psychiatry 2006; 67:958–963.
- Walters AS, Hening W. Clinical presentation and neuropharmacology of restless legs syndrome. Clin Neuropharmacol 1987; 10:225–237.
- Krishnan PR, Bhatia M, Behari M. Restless leg syndrome in Parkinson's disease: a case-controlled study. Mov Disord 2003; 18:181–185.
- Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol 2002; 59:421–424.
- Lang AE. Restless legs syndrome and Parkinson's disease: insights into pathophysiology. Clin Neuropharmacol 1987; 10:476–478.
- Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY; TREAT RLS US Study Group. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc 2006; 81:17–27.
- Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology 2006; 67:1034–1039.
- Thorpy MJ. New paradigms in the treatment of restless legs syndrome. Neurology 2005; 64(supp 3):S28–S33.
- Moskovitz C, Hoses H, Klawans H. Levodopa-induced psychosis: a kindling phenomenon. Am J Psychiatry 1978; 135:669–675.
- Hickey MM, Demaerschalk BM, Casaelli RJ, Parish JM, Wingerchuk DM. “Idiopathic” rapid-eye-movement (REM) sleep behavior disorder is associated with future development of neurodegenerative diseases. Neurologist 2007; 13:98–101.
- Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson's disease with therapeutic response to levodopa. Mov Disord 1996; 11:214–216.
- Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 1996; 46:388–393.
- Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in patients with REM sleep behavior disorder. Neurology 1995; 45:709–712.
- Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and Parkinson disease. Neurology 2005; 65:247–252.
These nonmotor symptoms—sensory, autonomic, and behavioral—are important to recognize, as they can lead to even more serious complications and impair quality of life.
COMMON, TROUBLESOME, AND UNDERDIAGNOSED
Nonmotor symptoms are very common. In fact, up to 60% of patients suffer from more than one nonmotor symptom, and 25% have four or more,1 including autonomic dysfunction, sensory symptoms, and cognitive and behavioral problems.2
Nonmotor symptoms can be primary complaints and, for some patients and family members, can cause greater disability than motor symptoms.3,4 For instance, depression and cognitive problems contribute to a decline in quality of life regardless of the degree of motor impairment.
Yet these symptoms are often underdiagnosed. 5 A delay in diagnosis may reflect the tendency of clinicians, patients, and family members to focus on the more apparent motor features of Parkinson disease, a lack of awareness of the nonmotor symptoms, or both. Consequently, patient education is essential. Identifying and treating these symptoms requires a multidisciplinary clinical team approach and an ongoing dialogue with the patient and family.
VARIOUS SYMPTOMS, VARIOUS CAUSES
Some nonmotor symptoms (eg, impaired sense of smell, depression, anxiety, fatigue, and constipation) can precede the motor symptoms of Parkinson disease and may be symptoms of the disease itself. Perhaps accounting for these observations, recent pathologic studies described diffuse Lewy body deposition in areas outside of nigral dopaminergic neurons,6,7 and the olfactory bulb, medulla, and pontine tegmentum may be involved before the substantia nigra.
Other symptoms, such as orthostatic hypotension, sedation, psychosis, confusion, and impulsiveness, may be adverse effects of medical therapy or may worsen with it.
Nonmotor symptoms can also emerge as a “wearing-off” phenomenon with standard treatment.2,8 The “off period” is a term primarily used to describe the reemergence of motor symptoms as a dose of levodopa wears off and before the patient receives the next dose. However, nonmotor symptoms, in particular depression and anxiety, can also occur in this period.
To treat nonmotor symptoms, one needs to identify and treat the primary symptom or the comorbid illnesses that may worsen it (eg, confusion in the setting of dehydration and infection), assess the possibility of adverse drug effects (a particular problem with many anti-Parkinson drugs), and try to reduce off periods with changes in dopaminergic therapy.
SENSORY SYMPTOMS
Off-period pain, paresthesia
Pain that cannot be attributed to muscle spasms, dystonia, somatic disease, autonomic dysfunction, or peripheral nerve disease occurs in up to 38% of patients with Parkinson disease.9,10 The pain is often diffuse and aching. Paresthesia-like complaints include numbness, tingling, and change in temperature.
Some sensory symptoms occur mostly during off periods and may respond to dopaminergic therapy. Examples are limb paresthesia, truncal pain, trigeminal neuralgia-like pain, and vaginal or perineal pain.9–11
Impaired sense of smell, vision
Impaired sense of smell can precede motor symptoms and is being investigated as a possible screening symptom for early diagnosis.12,13
Altered vision is a less recognized symptom of Parkinson disease. Many patients have difficulty reading even if they have normal visual acuity. Part of their difficulty stems from oculomotor defects such as impairment in visual saccadic movements and muscle rigidity.14 Visual pathways can be affected, as evidenced by abnormal visual evoked potentials that correlate with disease severity and by impairment in contrast sensitivity, color perception, and judgment of line orientation.15
It is unclear how these visual abnormalities contribute to everyday symptoms in Parkinson disease. Certainly, visual scanning activities such as reading are impaired.
Patients also suffer from drug-induced visual illusions and hallucinations, which are often colorful. Diederich et al16 found that contrast discrimination and color perception were significantly more impaired in Parkinson patients who have visual hallucinations, which suggests that it is important to correct visual abnormalities.
AUTONOMIC SYMPTOMS
In general, autonomic problems increase with age, disease severity, medication use, postural instability, cognitive decline, and visual hallucinations.17,18
Orthostatic hypotension
Almost half of patients with Parkinson disease have orthostatic hypotension.19 Of concern, patients with postural instability are at greater risk of orthostatic hypotension, thereby further increasing their risk of falling and injuring themselves.20
Postprandial hypotension is more common in Parkinson disease and is more often associated with midday meals, perhaps owing to a higher carbohydrate content and its effect on insulin release21 (many patients reserve high-protein meals for the evening and eat a greater proportion of carbohydrates during the day).
Home blood pressure monitoring is especially helpful, since blood pressure can fluctuate significantly and office readings may not reveal the problem.
Orthostatic hypotension can be both a drug side effect and a manifestation of the disease. Although all dopaminergic drugs can worsen orthostatic hypotension, the motor benefits of these drugs should be reviewed in relation to this risk. Dopaminergic agonists and amantadine (Symmetrel) should be used with caution in patients with significant orthostatic hypotension.
Treatment should include fluids, a highsalt diet, elastic stockings, fludrocortisone (Florinef), pyridostigmine (Mestinon), and perhaps the selective alpha 1 agonist midodrine (Proamatine). However, midodrine can cause supine hypertension and must be used cautiously in patients with advanced disease who take daytime naps because of fatigue. If postprandial hypotension is a problem, altering the patient's diet to include smaller but more frequent meals may help.
Cold limbs, sweating
Complaints related to temperature regulation include cold limbs and excessive sweating. Off-period drenching sweats occur as an endof- dose symptom thought to be related to subtherapeutic plasma dopamine levels and may respond to dopaminergic therapy aimed at reducing motor fluctuations and off periods.22
Gastrointestinal symptoms
Dysphagia (difficulty swallowing), sialorrhea (excessive salivation), nausea, constipation, and defecatory dysfunction are more common in patients with Parkinson disease than in agematched controls, even after controlling for factors such as drugs, autonomic dysfunction, diet, and exercise.23 These problems can cause malnutrition (necessitating a gastrostomy tube), aspiration pneumonia, and difficulty in swallowing and retaining pills,24 all of which can lead to problems that are even more serious. Although gastrointestinal symptoms occur in all stages of Parkinson disease, patients with advanced disease are at greater risk.
Dysphagia. James Parkinson described dysphagia and sialorrhea in his original 1816 monograph.25 The prevalence of dysphagia increases with severity of disease.23 Dysphagia is usually due to altered pharyngeal contraction, resulting in difficulty propelling food into the pharynx and retention of food in the pyriform sinuses and valleculae, but esophageal dilatation and dysmotility, spasms, gastroesophageal reflux, and increased transit time also contribute.24,26,27
Constipation may affect more than half of patients.28 One study reported a higher risk of developing Parkinson disease in men with infrequent bowel movements.29
Constipation and defecatory dysfunction can be severe enough to result in colonic dilatation and pseudo-obstruction.30 Altered gastrointestinal transit time may contribute to erratic absorption of medications.
Causes of constipation include slow colonic transit, weak abdominal muscles, decreased phasic contraction, and a paradoxical increase in puborectalis muscle and anal sphincter activity with straining, consistent with pelvic muscle dystonia.31
Treatment includes reducing off periods, limiting anticholinergic agents, prescribing a proper bowel regimen, and encouraging fluids and exercise. In addition, daily stool softeners, fiber, and polyethylene glycol are effective. Botulinum toxin injection into the puborectalis muscle may improve outlet obstruction.32
Risk of aspiration. Dysphagia, in association with respiratory symptoms, should prompt an evaluation for aspiration. Thickening liquids and early referral to a swallowing specialist may lessen the risk of aspiration.
Drooling is bothersome and embarrassing for many patients. Treatment historically included antimuscarinic agents, but these can cause constipation and confusion in patients with advanced disease. More recently, botulinum toxin injections into the parotid and submandibular glands have been shown to be effective in patients with excessive drooling.33
Urinary problems
Urologic abnormalities can be divided into dysfunction of the bladder, dysfunction of the urethral sphincter, and other causes of outflow obstruction such as prostate enlargement in men. The most common complaint is nocturia, followed by frequency and urgency.34
Nocturnal polyuria and urinary hesitancy and urgency are embarrassing but treatable. Urinary incontinence is a common reason for nursing home placement, and nocturia is a common cause of falls, as patients attempt to get up to urinate.35,36
Treatment of urinary incontinence should begin with an assessment for urinary tract infection, stress incontinence in women, and prostate enlargement in men. Urinary urgency due to detrusor hyperreflexia or spastic bladder can improve with anticholinergic antispasmodic agents. However, these should be used with caution in patients who are experiencing hallucinations or cognitive problems.
Sexual dysfunction
Sexual dysfunction, including decreased libido and erectile dysfunction, is in part related to autonomic dysfunction.
Treatment is complex, as these problems are multifactorial. Contributing factors include physical disability, the stress of living with a progressive illness, drug effects, depression, pain, difficulty in communication, caregiver stress, and impact on intimacy. The phosphodiesterase inhibitor sildenafil (Viagra) can improve erectile dysfunction, but must be used cautiously in patients with orthostatic hypotension.37 Other drugs of this class are available but have not been tested in this population.
COGNITIVE-BEHAVIORAL PROBLEMS
Neuropsychiatric disorders are common in Parkinson disease and at times can be more distressing to the patient and family than the motor symptoms. These include mood disorders, apathy, anxiety, impulse control disorders, psychosis, and dementia.
Dopaminergic medications used to treat movement can precipitate or exacerbate these neuropsychiatric problems. Therefore, treatment requires a balance between motor and neuropsychiatric benefits, with close dialogue with the patient and family about primary goals of treatment.
Depression
Depression is among the most common neuropsychiatric symptoms in Parkinson disease, occurring to some degree in up to 50% of patients.38 Diagnosing it is critical, because it can worsen physical symptoms, cognitive status, quality of life, and caregiver distress.39
However, depression can be difficult to recognize, because many of its features (eg, fatigue, psychomotor slowing, flattened affect, sleep difficulties) can also be manifestations of Parkinson disease. One should specifically ascertain whether the patient has a depressed mood or loss of interest in pleasurable activities. The Beck Depression Inventory is sensitive for depression in Parkinson disease and thus may be a reasonable screening tool.40
Treatment. Psychotherapy may help and may even be a first-line treatment in patients who cannot tolerate antidepressant drugs. Practical recommendations include relaxation techniques, a sleep hygiene regimen, engaging in meaningful activities to achieve a sense of purpose, and caregiver education.41
We have little evidence-based guidance on drug treatment of depression in patients with Parkinson disease. A recent meta-analysis found only two placebo-controlled studies in the past 40 years that monitored outcome based on a standardized rating scale of depression in patients with Parkinson disease.42
In preliminary studies, dopamine agonists have shown some efficacy in treating depression without Parkinson disease.43 However, the antidepressant contributions of anti-Parkinson drugs have not been well established.
Selective serotonin reuptake inhibitors (SSRIs) appear to be safe and well tolerated.44 Venlafaxine (Effexor) and mirtazapine (Remeron) are also reasonable initial options. Tricyclic antidepressants should be used with caution because they can cause anticholinergic side effects, especially confusion, in this population.
All serotonergic agents should be used with caution when given in combination with monoamine oxidase inhibitors, which are often used to treat motor symptoms in Parkinson disease, because of the risk of serotonin syndrome, which is characterized by fever, altered mental status, myoclonus, tremor, hyperreflexia, and diaphoresis and may be fatal.
Electroconvulsive therapy can be reserved for the treatment of severe refractory depression in patients with Parkinson disease without complex medical issues.45
Apathy
Apathy is present in approximately 30% of patients with Parkinson disease.46
Apathy can be very difficult to differentiate from depression, as the two disorders can occur together. Apathy can also be present without signs or symptoms of depressed mood. Apathy is commonly conceptualized as involving three domains: cognitive (lack of interest), behavioral (lack of initiation and drive), and affective (lack of emotion). The possibility of a primary apathy syndrome should be considered if the patient does not respond to standard treatments for depression. This is critical, as the treatment of the two syndromes may differ.47
Although efficacy data are limited, bupropion (Wellbutrin) and methylphenidate (Ritalin)48 can be tried for their activating or stimulant properties.
Anxiety disorders
Anxiety disorders commonly accompany depression in Parkinson disease, but they can also occur independently. They most often present in the setting of wearing-off or on-off fluctuations associated with medication status. Anxiety disorders in patients with Parkinson disease include generalized anxiety and panic attacks, which are responsive to SSRIs and benzodiazepines.49 Benzodiazepines are effective, and clonazepam (Klonopin) may be preferred because it has a long half-life, thereby minimizing anxiety associated with wearingoff or unpredictable off periods. Conversely, a long half-life and depressive effects may limit its use in advanced age.
Classic obsessive-compulsive disorder is less common, but obsessive behaviors and impulse control difficulties can occur in up to 7% of patients with Parkinson disease and presumably reflect dopamine dysregulation, most often associated with dopamine agonists.50 Impulsive behavior can include gambling, hypersexuality, and bingeing.
One form of obsessive-compulsive disorder is punding, a behavior characterized by intense fascination with repetitive handling and examining of objects, most often mechanical objects.51 Behaviors can include assembling and disassembling, collecting, or sorting of objects.
Visual hallucinations
Many patients with Parkinson disease have visual hallucinations as a side effect of dopaminergic drugs. At first, the patient realizes that they are hallucinations, but this insight may be lost as the disease progresses. The clinician should also consider other potential causes such as dementia, systemic illness, or psychosocial stress.
If visual hallucinations present early in the course of the disease and are accompanied by loss of insight and by cognitive fluctuations, the patient may actually have Lewy body dementia.52 Its features include parkinsonian symptoms, visual hallucinations, a fluctuating level of consciousness, and neuroleptic sensitivity.
Psychosis
The first-line treatment for psychosis in patients with Parkinson disease should involve:
- Searching for a systemic illness such as urinary tract infection, aspiration pneumonia, or dehydration;
- Stopping or lowering the dose of drugs that act on the central nervous system; and
- If possible, stopping or lowering the dose of anti-Parkinson drugs that have the greatest risk of cognitive side effects. In many cases, this is not a realistic option, and adding an antipsychotic drug may be necessary.
Monitoring and treating psychosis is paramount, as it is a major risk factor for nursing home placement.53
Conventional neuroleptics such as haloperidol (Haldol) should be avoided because they can exacerbate parkinsonian symptoms.
Two atypical neuroleptics, clozapine (Clozaril) and quetiapine (Seroquel), appear to be the best tolerated in Parkinson disease patients. Clozapine is the only neuroleptic found to be more efficacious than placebo in patients with Parkinson disease.54 However, of the two, clozapine has a higher risk of side effects and requires frequent blood monitoring, making it difficult to use. Quetiapine has been shown to be as efficacious as clozapine,55 but it failed to show efficacy in a recent controlled study.56 Nevertheless, it is considered the first-line treatment option, since it is safer.40
Other atypical antipsychotics appear to exacerbate Parkinson disease or have not been adequately studied and should therefore be used with caution.
Dementia
Estimates of the prevalence of dementia in patients with Parkinson disease vary widely, most likely reflecting differences in populations studied and methods used. The best estimates indicate that 20% to 30% of patients with Parkinson disease develop dementia.57
Parkinson dementia is one of the classic subcortical dementias, characterized by slow thinking and by difficulties in working memory and problem-solving due to disruption of frontal-subcortical circuits.58 Its most common neuropsychiatric symptoms are hallucinations and depression, with less agitation, disinhibition, and irritability than in Alzheimer dementia.59
Anti-Parkinson drugs, especially anticholinergics, can exacerbate cognitive impairments in patients with Parkinson disease. An acute change in cognitive abilities or visual hallucinations is often associated with an un derlying medical illness such as infection (eg, a urinary tract infection or aspiration pneumonia) or dehydration.
Anticholinesterase inhibitors such as rivastigmine (Exelon) are indicated in patients with Parkinson dementia but may provide only a modest benefit.60
SLEEP DISORDERS
Excessive daytime sleepiness
Fatigue and hypersomnolence often impair quality of life.61 Daytime hypersomnolence is often multifactorial: it can be caused by the disease itself,62 by dopaminergic therapy,63,64 by a comorbid illness, or by nighttime sleep problems.
Sudden and uncontrollable episodes of sleep are an extreme form of hypersomnolence and are worrisome, especially with activities such as driving.65 Greater sleepiness (measured by the Epworth Sleepiness Scale), longer duration of Parkinson disease, and use of dopaminergic agonists increase the risk of such attacks.66 In general, very few patients experience such attacks without warning signs of sedation.
Treatment must include a comprehensive evaluation of current medications, the effect of dopaminergic agents (especially agonists), and nighttime factors that influence sleep (some of which are described below). Modafinil (Provigil), effective in narcolepsy, helped in some studies67 but not in others68; it could be tried in moderate to severe cases of excessive daytime sleepiness.
Sleep apnea contributes to daytime hypersomnolence. An evaluation for sleep apnea should be considered even in patients who are not overweight, as some evidence suggests that this disorder is common in Parkinson disease and correlates with disease severity.69
Insomnia
Sleep is impaired in up to 74% of Parkinson patients.70,71 A sleep study may show a low total sleep time, many awakenings, a short rapid-eye movement (REM) latency, and short slow-wave sleep (stages III and IV); the patient experiences the problem as light sleep with frequent awakenings.72 A variety of problems related to Parkinson disease can directly affect sleep patterns: eg, pain, stiffness, tremor, problems turning over in bed, dystonia, dementia, nocturia,73 depression, and anxiety.74
Restless legs syndrome
Restless legs syndrome is an uncomfortable, sometimes painful feeling in the legs or other body parts during rest (especially at night) that improves with movement.75 It may be more common in patients with Parkinson disease than in the general population76,77 and can precede the diagnosis of Parkinson disease.78
Iron supplementation with ferrous sulfate can help if iron deficiency (ferritin < 50 μg/L or iron saturation < 16%) is present but is ineffective in its absence. Levodopa and the dopaminergic agonists ropinirole (Requip)70 and pramipexole (Mirapex)80 are effective Parkinson disease treatments that also treat restless legs syndrome. In addition, opioids, clonazepam, and gabapentin (Gabarone) may help.81
Vivid dreams
Parkinson patients often describe very vivid, intense, frightening, and unpleasant dreams,73 which may be a precursor to psychosis.82
REM sleep behavior disorder is characterized by sustained phasic muscle activity in place of normal atonia during REM or dream sleep. The patient's bed partner may describe him or her behaving in an aggressive way as if acting out his or her dreams, ie, hitting, yelling, or kicking. Upon awakening, the patient's recall of the dream content is consistent with the nocturnal behavior.
REM sleep behavior disorder may actually precede the motor symptoms of Parkinson disease,83,84 and as many as 20% of patents with REM sleep behavior disorder eventually develop Parkinson disease.85 The possible link between the two disorders is strengthened by a case report describing a patient with REM sleep behavior disorder, no signs of Parkinson disease on examination, but brain pathology similar to that found in Parkinson disease.86
Clonazepam is an effective treatment for REM sleep behavior disorder and should be considered if sleep is disrupted or patient safety becomes a concern.87
These nonmotor symptoms—sensory, autonomic, and behavioral—are important to recognize, as they can lead to even more serious complications and impair quality of life.
COMMON, TROUBLESOME, AND UNDERDIAGNOSED
Nonmotor symptoms are very common. In fact, up to 60% of patients suffer from more than one nonmotor symptom, and 25% have four or more,1 including autonomic dysfunction, sensory symptoms, and cognitive and behavioral problems.2
Nonmotor symptoms can be primary complaints and, for some patients and family members, can cause greater disability than motor symptoms.3,4 For instance, depression and cognitive problems contribute to a decline in quality of life regardless of the degree of motor impairment.
Yet these symptoms are often underdiagnosed. 5 A delay in diagnosis may reflect the tendency of clinicians, patients, and family members to focus on the more apparent motor features of Parkinson disease, a lack of awareness of the nonmotor symptoms, or both. Consequently, patient education is essential. Identifying and treating these symptoms requires a multidisciplinary clinical team approach and an ongoing dialogue with the patient and family.
VARIOUS SYMPTOMS, VARIOUS CAUSES
Some nonmotor symptoms (eg, impaired sense of smell, depression, anxiety, fatigue, and constipation) can precede the motor symptoms of Parkinson disease and may be symptoms of the disease itself. Perhaps accounting for these observations, recent pathologic studies described diffuse Lewy body deposition in areas outside of nigral dopaminergic neurons,6,7 and the olfactory bulb, medulla, and pontine tegmentum may be involved before the substantia nigra.
Other symptoms, such as orthostatic hypotension, sedation, psychosis, confusion, and impulsiveness, may be adverse effects of medical therapy or may worsen with it.
Nonmotor symptoms can also emerge as a “wearing-off” phenomenon with standard treatment.2,8 The “off period” is a term primarily used to describe the reemergence of motor symptoms as a dose of levodopa wears off and before the patient receives the next dose. However, nonmotor symptoms, in particular depression and anxiety, can also occur in this period.
To treat nonmotor symptoms, one needs to identify and treat the primary symptom or the comorbid illnesses that may worsen it (eg, confusion in the setting of dehydration and infection), assess the possibility of adverse drug effects (a particular problem with many anti-Parkinson drugs), and try to reduce off periods with changes in dopaminergic therapy.
SENSORY SYMPTOMS
Off-period pain, paresthesia
Pain that cannot be attributed to muscle spasms, dystonia, somatic disease, autonomic dysfunction, or peripheral nerve disease occurs in up to 38% of patients with Parkinson disease.9,10 The pain is often diffuse and aching. Paresthesia-like complaints include numbness, tingling, and change in temperature.
Some sensory symptoms occur mostly during off periods and may respond to dopaminergic therapy. Examples are limb paresthesia, truncal pain, trigeminal neuralgia-like pain, and vaginal or perineal pain.9–11
Impaired sense of smell, vision
Impaired sense of smell can precede motor symptoms and is being investigated as a possible screening symptom for early diagnosis.12,13
Altered vision is a less recognized symptom of Parkinson disease. Many patients have difficulty reading even if they have normal visual acuity. Part of their difficulty stems from oculomotor defects such as impairment in visual saccadic movements and muscle rigidity.14 Visual pathways can be affected, as evidenced by abnormal visual evoked potentials that correlate with disease severity and by impairment in contrast sensitivity, color perception, and judgment of line orientation.15
It is unclear how these visual abnormalities contribute to everyday symptoms in Parkinson disease. Certainly, visual scanning activities such as reading are impaired.
Patients also suffer from drug-induced visual illusions and hallucinations, which are often colorful. Diederich et al16 found that contrast discrimination and color perception were significantly more impaired in Parkinson patients who have visual hallucinations, which suggests that it is important to correct visual abnormalities.
AUTONOMIC SYMPTOMS
In general, autonomic problems increase with age, disease severity, medication use, postural instability, cognitive decline, and visual hallucinations.17,18
Orthostatic hypotension
Almost half of patients with Parkinson disease have orthostatic hypotension.19 Of concern, patients with postural instability are at greater risk of orthostatic hypotension, thereby further increasing their risk of falling and injuring themselves.20
Postprandial hypotension is more common in Parkinson disease and is more often associated with midday meals, perhaps owing to a higher carbohydrate content and its effect on insulin release21 (many patients reserve high-protein meals for the evening and eat a greater proportion of carbohydrates during the day).
Home blood pressure monitoring is especially helpful, since blood pressure can fluctuate significantly and office readings may not reveal the problem.
Orthostatic hypotension can be both a drug side effect and a manifestation of the disease. Although all dopaminergic drugs can worsen orthostatic hypotension, the motor benefits of these drugs should be reviewed in relation to this risk. Dopaminergic agonists and amantadine (Symmetrel) should be used with caution in patients with significant orthostatic hypotension.
Treatment should include fluids, a highsalt diet, elastic stockings, fludrocortisone (Florinef), pyridostigmine (Mestinon), and perhaps the selective alpha 1 agonist midodrine (Proamatine). However, midodrine can cause supine hypertension and must be used cautiously in patients with advanced disease who take daytime naps because of fatigue. If postprandial hypotension is a problem, altering the patient's diet to include smaller but more frequent meals may help.
Cold limbs, sweating
Complaints related to temperature regulation include cold limbs and excessive sweating. Off-period drenching sweats occur as an endof- dose symptom thought to be related to subtherapeutic plasma dopamine levels and may respond to dopaminergic therapy aimed at reducing motor fluctuations and off periods.22
Gastrointestinal symptoms
Dysphagia (difficulty swallowing), sialorrhea (excessive salivation), nausea, constipation, and defecatory dysfunction are more common in patients with Parkinson disease than in agematched controls, even after controlling for factors such as drugs, autonomic dysfunction, diet, and exercise.23 These problems can cause malnutrition (necessitating a gastrostomy tube), aspiration pneumonia, and difficulty in swallowing and retaining pills,24 all of which can lead to problems that are even more serious. Although gastrointestinal symptoms occur in all stages of Parkinson disease, patients with advanced disease are at greater risk.
Dysphagia. James Parkinson described dysphagia and sialorrhea in his original 1816 monograph.25 The prevalence of dysphagia increases with severity of disease.23 Dysphagia is usually due to altered pharyngeal contraction, resulting in difficulty propelling food into the pharynx and retention of food in the pyriform sinuses and valleculae, but esophageal dilatation and dysmotility, spasms, gastroesophageal reflux, and increased transit time also contribute.24,26,27
Constipation may affect more than half of patients.28 One study reported a higher risk of developing Parkinson disease in men with infrequent bowel movements.29
Constipation and defecatory dysfunction can be severe enough to result in colonic dilatation and pseudo-obstruction.30 Altered gastrointestinal transit time may contribute to erratic absorption of medications.
Causes of constipation include slow colonic transit, weak abdominal muscles, decreased phasic contraction, and a paradoxical increase in puborectalis muscle and anal sphincter activity with straining, consistent with pelvic muscle dystonia.31
Treatment includes reducing off periods, limiting anticholinergic agents, prescribing a proper bowel regimen, and encouraging fluids and exercise. In addition, daily stool softeners, fiber, and polyethylene glycol are effective. Botulinum toxin injection into the puborectalis muscle may improve outlet obstruction.32
Risk of aspiration. Dysphagia, in association with respiratory symptoms, should prompt an evaluation for aspiration. Thickening liquids and early referral to a swallowing specialist may lessen the risk of aspiration.
Drooling is bothersome and embarrassing for many patients. Treatment historically included antimuscarinic agents, but these can cause constipation and confusion in patients with advanced disease. More recently, botulinum toxin injections into the parotid and submandibular glands have been shown to be effective in patients with excessive drooling.33
Urinary problems
Urologic abnormalities can be divided into dysfunction of the bladder, dysfunction of the urethral sphincter, and other causes of outflow obstruction such as prostate enlargement in men. The most common complaint is nocturia, followed by frequency and urgency.34
Nocturnal polyuria and urinary hesitancy and urgency are embarrassing but treatable. Urinary incontinence is a common reason for nursing home placement, and nocturia is a common cause of falls, as patients attempt to get up to urinate.35,36
Treatment of urinary incontinence should begin with an assessment for urinary tract infection, stress incontinence in women, and prostate enlargement in men. Urinary urgency due to detrusor hyperreflexia or spastic bladder can improve with anticholinergic antispasmodic agents. However, these should be used with caution in patients who are experiencing hallucinations or cognitive problems.
Sexual dysfunction
Sexual dysfunction, including decreased libido and erectile dysfunction, is in part related to autonomic dysfunction.
Treatment is complex, as these problems are multifactorial. Contributing factors include physical disability, the stress of living with a progressive illness, drug effects, depression, pain, difficulty in communication, caregiver stress, and impact on intimacy. The phosphodiesterase inhibitor sildenafil (Viagra) can improve erectile dysfunction, but must be used cautiously in patients with orthostatic hypotension.37 Other drugs of this class are available but have not been tested in this population.
COGNITIVE-BEHAVIORAL PROBLEMS
Neuropsychiatric disorders are common in Parkinson disease and at times can be more distressing to the patient and family than the motor symptoms. These include mood disorders, apathy, anxiety, impulse control disorders, psychosis, and dementia.
Dopaminergic medications used to treat movement can precipitate or exacerbate these neuropsychiatric problems. Therefore, treatment requires a balance between motor and neuropsychiatric benefits, with close dialogue with the patient and family about primary goals of treatment.
Depression
Depression is among the most common neuropsychiatric symptoms in Parkinson disease, occurring to some degree in up to 50% of patients.38 Diagnosing it is critical, because it can worsen physical symptoms, cognitive status, quality of life, and caregiver distress.39
However, depression can be difficult to recognize, because many of its features (eg, fatigue, psychomotor slowing, flattened affect, sleep difficulties) can also be manifestations of Parkinson disease. One should specifically ascertain whether the patient has a depressed mood or loss of interest in pleasurable activities. The Beck Depression Inventory is sensitive for depression in Parkinson disease and thus may be a reasonable screening tool.40
Treatment. Psychotherapy may help and may even be a first-line treatment in patients who cannot tolerate antidepressant drugs. Practical recommendations include relaxation techniques, a sleep hygiene regimen, engaging in meaningful activities to achieve a sense of purpose, and caregiver education.41
We have little evidence-based guidance on drug treatment of depression in patients with Parkinson disease. A recent meta-analysis found only two placebo-controlled studies in the past 40 years that monitored outcome based on a standardized rating scale of depression in patients with Parkinson disease.42
In preliminary studies, dopamine agonists have shown some efficacy in treating depression without Parkinson disease.43 However, the antidepressant contributions of anti-Parkinson drugs have not been well established.
Selective serotonin reuptake inhibitors (SSRIs) appear to be safe and well tolerated.44 Venlafaxine (Effexor) and mirtazapine (Remeron) are also reasonable initial options. Tricyclic antidepressants should be used with caution because they can cause anticholinergic side effects, especially confusion, in this population.
All serotonergic agents should be used with caution when given in combination with monoamine oxidase inhibitors, which are often used to treat motor symptoms in Parkinson disease, because of the risk of serotonin syndrome, which is characterized by fever, altered mental status, myoclonus, tremor, hyperreflexia, and diaphoresis and may be fatal.
Electroconvulsive therapy can be reserved for the treatment of severe refractory depression in patients with Parkinson disease without complex medical issues.45
Apathy
Apathy is present in approximately 30% of patients with Parkinson disease.46
Apathy can be very difficult to differentiate from depression, as the two disorders can occur together. Apathy can also be present without signs or symptoms of depressed mood. Apathy is commonly conceptualized as involving three domains: cognitive (lack of interest), behavioral (lack of initiation and drive), and affective (lack of emotion). The possibility of a primary apathy syndrome should be considered if the patient does not respond to standard treatments for depression. This is critical, as the treatment of the two syndromes may differ.47
Although efficacy data are limited, bupropion (Wellbutrin) and methylphenidate (Ritalin)48 can be tried for their activating or stimulant properties.
Anxiety disorders
Anxiety disorders commonly accompany depression in Parkinson disease, but they can also occur independently. They most often present in the setting of wearing-off or on-off fluctuations associated with medication status. Anxiety disorders in patients with Parkinson disease include generalized anxiety and panic attacks, which are responsive to SSRIs and benzodiazepines.49 Benzodiazepines are effective, and clonazepam (Klonopin) may be preferred because it has a long half-life, thereby minimizing anxiety associated with wearingoff or unpredictable off periods. Conversely, a long half-life and depressive effects may limit its use in advanced age.
Classic obsessive-compulsive disorder is less common, but obsessive behaviors and impulse control difficulties can occur in up to 7% of patients with Parkinson disease and presumably reflect dopamine dysregulation, most often associated with dopamine agonists.50 Impulsive behavior can include gambling, hypersexuality, and bingeing.
One form of obsessive-compulsive disorder is punding, a behavior characterized by intense fascination with repetitive handling and examining of objects, most often mechanical objects.51 Behaviors can include assembling and disassembling, collecting, or sorting of objects.
Visual hallucinations
Many patients with Parkinson disease have visual hallucinations as a side effect of dopaminergic drugs. At first, the patient realizes that they are hallucinations, but this insight may be lost as the disease progresses. The clinician should also consider other potential causes such as dementia, systemic illness, or psychosocial stress.
If visual hallucinations present early in the course of the disease and are accompanied by loss of insight and by cognitive fluctuations, the patient may actually have Lewy body dementia.52 Its features include parkinsonian symptoms, visual hallucinations, a fluctuating level of consciousness, and neuroleptic sensitivity.
Psychosis
The first-line treatment for psychosis in patients with Parkinson disease should involve:
- Searching for a systemic illness such as urinary tract infection, aspiration pneumonia, or dehydration;
- Stopping or lowering the dose of drugs that act on the central nervous system; and
- If possible, stopping or lowering the dose of anti-Parkinson drugs that have the greatest risk of cognitive side effects. In many cases, this is not a realistic option, and adding an antipsychotic drug may be necessary.
Monitoring and treating psychosis is paramount, as it is a major risk factor for nursing home placement.53
Conventional neuroleptics such as haloperidol (Haldol) should be avoided because they can exacerbate parkinsonian symptoms.
Two atypical neuroleptics, clozapine (Clozaril) and quetiapine (Seroquel), appear to be the best tolerated in Parkinson disease patients. Clozapine is the only neuroleptic found to be more efficacious than placebo in patients with Parkinson disease.54 However, of the two, clozapine has a higher risk of side effects and requires frequent blood monitoring, making it difficult to use. Quetiapine has been shown to be as efficacious as clozapine,55 but it failed to show efficacy in a recent controlled study.56 Nevertheless, it is considered the first-line treatment option, since it is safer.40
Other atypical antipsychotics appear to exacerbate Parkinson disease or have not been adequately studied and should therefore be used with caution.
Dementia
Estimates of the prevalence of dementia in patients with Parkinson disease vary widely, most likely reflecting differences in populations studied and methods used. The best estimates indicate that 20% to 30% of patients with Parkinson disease develop dementia.57
Parkinson dementia is one of the classic subcortical dementias, characterized by slow thinking and by difficulties in working memory and problem-solving due to disruption of frontal-subcortical circuits.58 Its most common neuropsychiatric symptoms are hallucinations and depression, with less agitation, disinhibition, and irritability than in Alzheimer dementia.59
Anti-Parkinson drugs, especially anticholinergics, can exacerbate cognitive impairments in patients with Parkinson disease. An acute change in cognitive abilities or visual hallucinations is often associated with an un derlying medical illness such as infection (eg, a urinary tract infection or aspiration pneumonia) or dehydration.
Anticholinesterase inhibitors such as rivastigmine (Exelon) are indicated in patients with Parkinson dementia but may provide only a modest benefit.60
SLEEP DISORDERS
Excessive daytime sleepiness
Fatigue and hypersomnolence often impair quality of life.61 Daytime hypersomnolence is often multifactorial: it can be caused by the disease itself,62 by dopaminergic therapy,63,64 by a comorbid illness, or by nighttime sleep problems.
Sudden and uncontrollable episodes of sleep are an extreme form of hypersomnolence and are worrisome, especially with activities such as driving.65 Greater sleepiness (measured by the Epworth Sleepiness Scale), longer duration of Parkinson disease, and use of dopaminergic agonists increase the risk of such attacks.66 In general, very few patients experience such attacks without warning signs of sedation.
Treatment must include a comprehensive evaluation of current medications, the effect of dopaminergic agents (especially agonists), and nighttime factors that influence sleep (some of which are described below). Modafinil (Provigil), effective in narcolepsy, helped in some studies67 but not in others68; it could be tried in moderate to severe cases of excessive daytime sleepiness.
Sleep apnea contributes to daytime hypersomnolence. An evaluation for sleep apnea should be considered even in patients who are not overweight, as some evidence suggests that this disorder is common in Parkinson disease and correlates with disease severity.69
Insomnia
Sleep is impaired in up to 74% of Parkinson patients.70,71 A sleep study may show a low total sleep time, many awakenings, a short rapid-eye movement (REM) latency, and short slow-wave sleep (stages III and IV); the patient experiences the problem as light sleep with frequent awakenings.72 A variety of problems related to Parkinson disease can directly affect sleep patterns: eg, pain, stiffness, tremor, problems turning over in bed, dystonia, dementia, nocturia,73 depression, and anxiety.74
Restless legs syndrome
Restless legs syndrome is an uncomfortable, sometimes painful feeling in the legs or other body parts during rest (especially at night) that improves with movement.75 It may be more common in patients with Parkinson disease than in the general population76,77 and can precede the diagnosis of Parkinson disease.78
Iron supplementation with ferrous sulfate can help if iron deficiency (ferritin < 50 μg/L or iron saturation < 16%) is present but is ineffective in its absence. Levodopa and the dopaminergic agonists ropinirole (Requip)70 and pramipexole (Mirapex)80 are effective Parkinson disease treatments that also treat restless legs syndrome. In addition, opioids, clonazepam, and gabapentin (Gabarone) may help.81
Vivid dreams
Parkinson patients often describe very vivid, intense, frightening, and unpleasant dreams,73 which may be a precursor to psychosis.82
REM sleep behavior disorder is characterized by sustained phasic muscle activity in place of normal atonia during REM or dream sleep. The patient's bed partner may describe him or her behaving in an aggressive way as if acting out his or her dreams, ie, hitting, yelling, or kicking. Upon awakening, the patient's recall of the dream content is consistent with the nocturnal behavior.
REM sleep behavior disorder may actually precede the motor symptoms of Parkinson disease,83,84 and as many as 20% of patents with REM sleep behavior disorder eventually develop Parkinson disease.85 The possible link between the two disorders is strengthened by a case report describing a patient with REM sleep behavior disorder, no signs of Parkinson disease on examination, but brain pathology similar to that found in Parkinson disease.86
Clonazepam is an effective treatment for REM sleep behavior disorder and should be considered if sleep is disrupted or patient safety becomes a concern.87
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001; 16:507–510.
- Hillen ME, Sage JI. Nonmotor fluctuations in patients with Parkinson's disease. Neurology 1996; 47:1180–1183.
- Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 2002; 59:408–413.
- Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord 2005; 20(suppl 11):S23–S29.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002; 8:193–197.
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004; 318:121–134.
- Wolters ECh, Braak H. Parkinson's disease: premotor clinico-pathological correlations. J Neural Transmiss Suppl 2006; 70:309–319.
- Gunal DI, Nurichalichi K, Tuncer N, Bekiroglu N, Aktan S. The clinical profile of nonmotor fluctuations in Parkinson's disease patients. Can J Neurol Sci 2002; 29:61–64.
- Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology 1976; 26:423–429.
- Koller WC. Sensory symptoms in Parkinson's disease. Neurology 1984; 34:957–959.
- Ford B, Louis ED, Geene P, Fahn S. Oral and genital pain syndromes in Parkinson's disease. Mov Disord 1996: 11:421–426.
- Haehner A, Hummel T, Hummel C, et al. Olfactory loss may be the first sign of idiopathic Parkinson's disease. Mov Disord 2007; 22:839–842.
- Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis and prognosis of new onset Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 66:968–975.
- Hunt LA, Sadun AA, Bassi CJ. Review of the visual system in Parkinson's disease. Optom Vis Sci 1995; 72:92–99.
- Rodnitzky RL. Visual dysfunction in Parkinson's disease. Clin Neurosci 1998; 5:102–106.
- Diederich N, Goetz G, Pappert E. Primary deficits in visual discrimination is a risk factor for visual hallucinations in Parkinson's disease [abstract]. Neurology 1997; 48:A181.
- Oka H, Yoshioka M, Onouchi K, et al. Characteristics of orthostatic hypotension in Parkinson's disease. Brain 2007; 130:2425–2432.
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson's disease. Neurology 2007; 69:323–341.
- Allcock LdM, Ullyart K, Kenny RA, Burn DJ. Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2004; 75:1470–1471.
- Allcock LM, Kenny RA, Burn DJ. Clinical phenotype of subjects with Parkinson's disease and orthostatic hypotension: autonomic symptom and demographic comparison. Mov Disord 2006; 21:1851–1855.
- Micieli G, Martignoni E, Cavallini A, Sandrini G, Nappi G. Postprandial and orthostatic hypotension in Parkinson's disease. Neurology 1987; 37:386–393.
- Sage JI, Mark MH. Drenching sweats as an off phenomenon in Parkinson's disease: treatment and relationship to plasma levodopa profile. Ann Neurol 1995; 37:120–122.
- Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord 1991; 6:151–156.
- Bushman M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson's disease. Neurology 1989; 39:1309–1314.
- Parkinson J. An Essay on the Shaking Palsy. London: Whittingham and Roland, 1817.
- Gibberd FB, Gleeson JA, Gossage AA, Wilson RS. Oesophageal dilatation in Parkinson's disease. J Neurol Neurosurg Psychiatry 1974; 37:938–940.
- Bird MR, Woodword MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Ageing 1994; 23:251–254.
- Kaye J, Gage H, Kimber A, Storey L, Trend P. Excess burden of constipation in Parkinson's disease: a pilot study. Mov Disord 2006; 21:1270–1273.
- Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001; 57:456–462.
- Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson's disease. Am J Gastroenterol 1994; 89:15–25.
- Sakakibara R, Odaka T, Uchiyama T, et al. Colon transit time and rectoanal videomanometry in Parkinson's disease. J Neurol Neurosurg Psychiatry 2003; 74:268–272.
- Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G. Outlet type obstruction in Parkinson's disease: results of botulinum toxin treatment. Aliment Pharmacol Ther 2005; 22:997–1003.
- Molloy L. Treatment of sialorrhoea in patients with Parkinson's disease: best current evidence. Curr Opin Neurol 2007; 20:493–498.
- Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinson's disease. Neurourol Urodyn 2006; 25:116–122.
- Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson's disease: frequency, impact and identifying factors. J Neurol 2005; 252:1310–1315.
- Chutka DS, Fleming KC, Evans MP, Evans JM, Andrews KL. Urinary incontinence in the elderly population. Mayo Clin Proc 1996; 71:93–101.
- Hussain IF, Brady CM, Swinn MJ, Mathias CJ, Fowler CJ. Treatment of erectile dysfunction with sildenafil citrate (Viagra) in parkinsonism due to Parkinson's disease or multiple system atrophy with observations on orthostatic hypotension. J Neurol Neurosurg Psychiatry 2001; 71:371–374.
- Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease. A community-based study. Arch Neurol 1996; 53:175–179.
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson's disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999; 14:866–874.
- Miyasaki JM, Shannon K, Voon V, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 66:996–1002.
- Dobkin RD, Allen LA, Menza M. A cognitive-behavioral treatment package for depression in Parkinson's disease. Psychosomatics 2006; 47:259–263.
- Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005; 20:1161–1169.
- Cassano P, Lattanzi L, Soldani F, et al. Pramipexole in treatment-resistant depression: an extended follow-up. Depress Anxiety 2004; 20:131–138.
- Dell'Agnello G, Ceravolo R, Nuti A, et al. SSRIs do not worsen Parkinson's disease: evidence from an open-label, prospective study. Clin Neuropharmacol 2001; 24:221–227.
- Fall P, Granérus AK. Maintenance ECT in Parkinson's disease. J Neural Transm 1999; 106:737–741.
- Kirsch-Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology 2006; 67:33–38.
- van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci 2005; 17:7–19.
- Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2002; 14:461–462.
- Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005; 96:42–55.
- Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 2006; 63:969–973.
- Fernandez HH, Friedman JH. Punding on L-dopa. Mov Disord 1999; 14:836–838.
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006; 9(suppl 3):417–423.
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993; 43:2227–2229.
- Frieling H, Hillemacher T, Ziegenbein M, Neundörfer B, Bleich S. Treating dopamimetic psychosis in Parkinson's disease: structured review and meta-analysis. Eur Neuropsychopharmacol 2007; 17:165–171.
- Morgante L, Epifanio A, Spina E, et al. Quetiapine versus clozapine: a preliminary report of comparative effects on dopaminergic psychosis in patients with Parkinson's disease. Neurol Sci 2002; 23(suppl 2):S89–S90.
- Rabey JM, Prokhorov T, Moniovitz A, Dobronevsky E, Klein C. Effect of quetiapine in psychotic Parkinson's disease patients: a double-blind labeled study of 3 months' duration. Mov Disord 2007; 22:313–318.
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord 2005; 20:1255–1263.
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive nd behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 2003; 16:193–210.
- Aarsland D, Cummings JL, Larsen JP. Neuropsychiatric differences between Parkinson's disease with dementia and Alzheimer's disease. Int J Geriatr Psychiatry 2001; 16:184–191.
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004; 351:2509–2518.
- Martinez-Martin P, Catalan MJ, Benito-Leon J, et al. Impact of fatigue in Parkinson's disease: the Fatigue Impact Scale for Daily Use (D-FIS). Qual Life Res 2006; 15:597–606.
- Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson's disease and sleepiness: an integral part of Parkinson disease. Neurology 2002; 58:1019–1024.
- Ondo WG, Dat Vong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001; 57:1392–1396.
- O'Suilleabhain PE, Dewey RB. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson's disease. Arch Neurol 2002; 59:986–989.
- Fucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 1999; 52:1908–1910.
- Paus S, Brecht HM, Köster J, Seeger G, Klockgether T, Wüllner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003; 18:659–667.
- Adler CH, Caviness JN, Hentz JG, Lind M, Tiede J. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003; 18:287–293.
- Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005; 76:1636–1639.
- Maria B, Sophia S, Michalis M, et al. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med 2003; 97:1151–1157.
- Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson's disease. Clin Neuropharmacol 1988; 6:512–519.
- Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord 1998; 13:895–899.
- van Hilten J, Hoff JI, Middelkoop HA, et al. Sleep disruption in Parkinson's disease: assessment by continuous activity monitoring. Arch Neurol 1994; 51:922–928.
- van Hilten JJ, Weggeman M, van der Velde EA, Kerkhof GA, van Dijk JG, Roos RA. Sleep, excessive daytime sleepiness and fatigue in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1993; 5:235–244.
- Borek LL, Kohn R, Friedman JH. Mood and sleep in Parkinson's disease. J Clin Psychiatry 2006; 67:958–963.
- Walters AS, Hening W. Clinical presentation and neuropharmacology of restless legs syndrome. Clin Neuropharmacol 1987; 10:225–237.
- Krishnan PR, Bhatia M, Behari M. Restless leg syndrome in Parkinson's disease: a case-controlled study. Mov Disord 2003; 18:181–185.
- Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol 2002; 59:421–424.
- Lang AE. Restless legs syndrome and Parkinson's disease: insights into pathophysiology. Clin Neuropharmacol 1987; 10:476–478.
- Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY; TREAT RLS US Study Group. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc 2006; 81:17–27.
- Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology 2006; 67:1034–1039.
- Thorpy MJ. New paradigms in the treatment of restless legs syndrome. Neurology 2005; 64(supp 3):S28–S33.
- Moskovitz C, Hoses H, Klawans H. Levodopa-induced psychosis: a kindling phenomenon. Am J Psychiatry 1978; 135:669–675.
- Hickey MM, Demaerschalk BM, Casaelli RJ, Parish JM, Wingerchuk DM. “Idiopathic” rapid-eye-movement (REM) sleep behavior disorder is associated with future development of neurodegenerative diseases. Neurologist 2007; 13:98–101.
- Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson's disease with therapeutic response to levodopa. Mov Disord 1996; 11:214–216.
- Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 1996; 46:388–393.
- Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in patients with REM sleep behavior disorder. Neurology 1995; 45:709–712.
- Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and Parkinson disease. Neurology 2005; 65:247–252.
- Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson's disease. Mov Disord 2001; 16:507–510.
- Hillen ME, Sage JI. Nonmotor fluctuations in patients with Parkinson's disease. Neurology 1996; 47:1180–1183.
- Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 2002; 59:408–413.
- Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord 2005; 20(suppl 11):S23–S29.
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson's disease. Parkinsonism Relat Disord 2002; 8:193–197.
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004; 318:121–134.
- Wolters ECh, Braak H. Parkinson's disease: premotor clinico-pathological correlations. J Neural Transmiss Suppl 2006; 70:309–319.
- Gunal DI, Nurichalichi K, Tuncer N, Bekiroglu N, Aktan S. The clinical profile of nonmotor fluctuations in Parkinson's disease patients. Can J Neurol Sci 2002; 29:61–64.
- Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology 1976; 26:423–429.
- Koller WC. Sensory symptoms in Parkinson's disease. Neurology 1984; 34:957–959.
- Ford B, Louis ED, Geene P, Fahn S. Oral and genital pain syndromes in Parkinson's disease. Mov Disord 1996: 11:421–426.
- Haehner A, Hummel T, Hummel C, et al. Olfactory loss may be the first sign of idiopathic Parkinson's disease. Mov Disord 2007; 22:839–842.
- Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis and prognosis of new onset Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 66:968–975.
- Hunt LA, Sadun AA, Bassi CJ. Review of the visual system in Parkinson's disease. Optom Vis Sci 1995; 72:92–99.
- Rodnitzky RL. Visual dysfunction in Parkinson's disease. Clin Neurosci 1998; 5:102–106.
- Diederich N, Goetz G, Pappert E. Primary deficits in visual discrimination is a risk factor for visual hallucinations in Parkinson's disease [abstract]. Neurology 1997; 48:A181.
- Oka H, Yoshioka M, Onouchi K, et al. Characteristics of orthostatic hypotension in Parkinson's disease. Brain 2007; 130:2425–2432.
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, van Hilten JJ. Patient-reported autonomic symptoms in Parkinson's disease. Neurology 2007; 69:323–341.
- Allcock LdM, Ullyart K, Kenny RA, Burn DJ. Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2004; 75:1470–1471.
- Allcock LM, Kenny RA, Burn DJ. Clinical phenotype of subjects with Parkinson's disease and orthostatic hypotension: autonomic symptom and demographic comparison. Mov Disord 2006; 21:1851–1855.
- Micieli G, Martignoni E, Cavallini A, Sandrini G, Nappi G. Postprandial and orthostatic hypotension in Parkinson's disease. Neurology 1987; 37:386–393.
- Sage JI, Mark MH. Drenching sweats as an off phenomenon in Parkinson's disease: treatment and relationship to plasma levodopa profile. Ann Neurol 1995; 37:120–122.
- Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M. Gastrointestinal symptoms in Parkinson's disease. Mov Disord 1991; 6:151–156.
- Bushman M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson's disease. Neurology 1989; 39:1309–1314.
- Parkinson J. An Essay on the Shaking Palsy. London: Whittingham and Roland, 1817.
- Gibberd FB, Gleeson JA, Gossage AA, Wilson RS. Oesophageal dilatation in Parkinson's disease. J Neurol Neurosurg Psychiatry 1974; 37:938–940.
- Bird MR, Woodword MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Ageing 1994; 23:251–254.
- Kaye J, Gage H, Kimber A, Storey L, Trend P. Excess burden of constipation in Parkinson's disease: a pilot study. Mov Disord 2006; 21:1270–1273.
- Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson's disease. Neurology 2001; 57:456–462.
- Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson's disease. Am J Gastroenterol 1994; 89:15–25.
- Sakakibara R, Odaka T, Uchiyama T, et al. Colon transit time and rectoanal videomanometry in Parkinson's disease. J Neurol Neurosurg Psychiatry 2003; 74:268–272.
- Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G. Outlet type obstruction in Parkinson's disease: results of botulinum toxin treatment. Aliment Pharmacol Ther 2005; 22:997–1003.
- Molloy L. Treatment of sialorrhoea in patients with Parkinson's disease: best current evidence. Curr Opin Neurol 2007; 20:493–498.
- Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinson's disease. Neurourol Urodyn 2006; 25:116–122.
- Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson's disease: frequency, impact and identifying factors. J Neurol 2005; 252:1310–1315.
- Chutka DS, Fleming KC, Evans MP, Evans JM, Andrews KL. Urinary incontinence in the elderly population. Mayo Clin Proc 1996; 71:93–101.
- Hussain IF, Brady CM, Swinn MJ, Mathias CJ, Fowler CJ. Treatment of erectile dysfunction with sildenafil citrate (Viagra) in parkinsonism due to Parkinson's disease or multiple system atrophy with observations on orthostatic hypotension. J Neurol Neurosurg Psychiatry 2001; 71:371–374.
- Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson's disease. A community-based study. Arch Neurol 1996; 53:175–179.
- Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson's disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999; 14:866–874.
- Miyasaki JM, Shannon K, Voon V, et al; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson's disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 66:996–1002.
- Dobkin RD, Allen LA, Menza M. A cognitive-behavioral treatment package for depression in Parkinson's disease. Psychosomatics 2006; 47:259–263.
- Weintraub D, Morales KH, Moberg PJ, et al. Antidepressant studies in Parkinson's disease: a review and meta-analysis. Mov Disord 2005; 20:1161–1169.
- Cassano P, Lattanzi L, Soldani F, et al. Pramipexole in treatment-resistant depression: an extended follow-up. Depress Anxiety 2004; 20:131–138.
- Dell'Agnello G, Ceravolo R, Nuti A, et al. SSRIs do not worsen Parkinson's disease: evidence from an open-label, prospective study. Clin Neuropharmacol 2001; 24:221–227.
- Fall P, Granérus AK. Maintenance ECT in Parkinson's disease. J Neural Transm 1999; 106:737–741.
- Kirsch-Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology 2006; 67:33–38.
- van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci 2005; 17:7–19.
- Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2002; 14:461–462.
- Richard IH. Anxiety disorders in Parkinson's disease. Adv Neurol 2005; 96:42–55.
- Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 2006; 63:969–973.
- Fernandez HH, Friedman JH. Punding on L-dopa. Mov Disord 1999; 14:836–838.
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 2006; 9(suppl 3):417–423.
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993; 43:2227–2229.
- Frieling H, Hillemacher T, Ziegenbein M, Neundörfer B, Bleich S. Treating dopamimetic psychosis in Parkinson's disease: structured review and meta-analysis. Eur Neuropsychopharmacol 2007; 17:165–171.
- Morgante L, Epifanio A, Spina E, et al. Quetiapine versus clozapine: a preliminary report of comparative effects on dopaminergic psychosis in patients with Parkinson's disease. Neurol Sci 2002; 23(suppl 2):S89–S90.
- Rabey JM, Prokhorov T, Moniovitz A, Dobronevsky E, Klein C. Effect of quetiapine in psychotic Parkinson's disease patients: a double-blind labeled study of 3 months' duration. Mov Disord 2007; 22:313–318.
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord 2005; 20:1255–1263.
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive nd behavioral sequelae of Parkinson's disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 2003; 16:193–210.
- Aarsland D, Cummings JL, Larsen JP. Neuropsychiatric differences between Parkinson's disease with dementia and Alzheimer's disease. Int J Geriatr Psychiatry 2001; 16:184–191.
- Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 2004; 351:2509–2518.
- Martinez-Martin P, Catalan MJ, Benito-Leon J, et al. Impact of fatigue in Parkinson's disease: the Fatigue Impact Scale for Daily Use (D-FIS). Qual Life Res 2006; 15:597–606.
- Arnulf I, Konofal E, Merino-Andreu M, et al. Parkinson's disease and sleepiness: an integral part of Parkinson disease. Neurology 2002; 58:1019–1024.
- Ondo WG, Dat Vong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 2001; 57:1392–1396.
- O'Suilleabhain PE, Dewey RB. Contributions of dopaminergic drugs and disease severity to daytime sleepiness in Parkinson's disease. Arch Neurol 2002; 59:986–989.
- Fucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 1999; 52:1908–1910.
- Paus S, Brecht HM, Köster J, Seeger G, Klockgether T, Wüllner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003; 18:659–667.
- Adler CH, Caviness JN, Hentz JG, Lind M, Tiede J. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson's disease. Mov Disord 2003; 18:287–293.
- Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005; 76:1636–1639.
- Maria B, Sophia S, Michalis M, et al. Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med 2003; 97:1151–1157.
- Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson's disease. Clin Neuropharmacol 1988; 6:512–519.
- Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson's disease. Mov Disord 1998; 13:895–899.
- van Hilten J, Hoff JI, Middelkoop HA, et al. Sleep disruption in Parkinson's disease: assessment by continuous activity monitoring. Arch Neurol 1994; 51:922–928.
- van Hilten JJ, Weggeman M, van der Velde EA, Kerkhof GA, van Dijk JG, Roos RA. Sleep, excessive daytime sleepiness and fatigue in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1993; 5:235–244.
- Borek LL, Kohn R, Friedman JH. Mood and sleep in Parkinson's disease. J Clin Psychiatry 2006; 67:958–963.
- Walters AS, Hening W. Clinical presentation and neuropharmacology of restless legs syndrome. Clin Neuropharmacol 1987; 10:225–237.
- Krishnan PR, Bhatia M, Behari M. Restless leg syndrome in Parkinson's disease: a case-controlled study. Mov Disord 2003; 18:181–185.
- Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol 2002; 59:421–424.
- Lang AE. Restless legs syndrome and Parkinson's disease: insights into pathophysiology. Clin Neuropharmacol 1987; 10:476–478.
- Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY; TREAT RLS US Study Group. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc 2006; 81:17–27.
- Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology 2006; 67:1034–1039.
- Thorpy MJ. New paradigms in the treatment of restless legs syndrome. Neurology 2005; 64(supp 3):S28–S33.
- Moskovitz C, Hoses H, Klawans H. Levodopa-induced psychosis: a kindling phenomenon. Am J Psychiatry 1978; 135:669–675.
- Hickey MM, Demaerschalk BM, Casaelli RJ, Parish JM, Wingerchuk DM. “Idiopathic” rapid-eye-movement (REM) sleep behavior disorder is associated with future development of neurodegenerative diseases. Neurologist 2007; 13:98–101.
- Tan A, Salgado M, Fahn S. Rapid eye movement sleep behavior disorder preceding Parkinson's disease with therapeutic response to levodopa. Mov Disord 1996; 11:214–216.
- Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology 1996; 46:388–393.
- Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in patients with REM sleep behavior disorder. Neurology 1995; 45:709–712.
- Iranzo A, Santamaria J, Rye DB, et al. Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and Parkinson disease. Neurology 2005; 65:247–252.
KEY POINTS
- Nonmotor symptoms can be due to the disease itself, to its treatment, or to on-off fluctuations in motor status as doses of medication wear off.
- Impaired sense of smell, depression, anxiety, fatigue, and constipation can precede the motor symptoms of Parkinson disease and may be symptoms of the disease itself.
- Orthostatic hypotension, sedation, psychosis, confusion, and impulsiveness may be adverse effects of medical therapy or may worsen with it.
- Depression occurs in up to 50% of patients with Parkinson disease, although it may be difficult to recognize because many of its physical features can also be manifestations of Parkinson disease itself.