User login
Abuse-Deterrent Opioids: What Practitioners Need to Know
Opioid Abuse-Deterrent Formulations
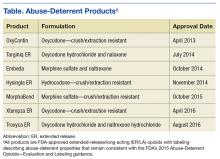
The meaning of the term abuse-deterrent is often misunderstood to mean abuse-proof. The FDA defines abuse-deterrent properties as those properties expected to meaningfully deter abuse even if they do not fully prevent abuse. Abuse-deterrent properties make certain types of abuse, such as crushing in order to snort or dissolving in order to inject, more difficult or less rewarding. However, this does not mean that the product is impossible to abuse or that these properties will necessarily prevent addiction, overdose, or death.
Of note, currently marketed abuse-deterrent formulation technologies do not effectively deter one of the most common forms of opioid abuse—simply swallowing a number of intact tablets or capsules. Abuse-deterrent opioids do not reduce the risk for opioid addiction, and they carry the same warnings about the risk for addiction as do conventional opioids.
Abuse and Misuse Data
The FDA is encouraging pharmaceutical industry efforts to develop pain medicines that are more difficult to abuse and to prioritize the need for data and study methods that will help evaluate the impact of abuse-deterrent opioids on misuse and abuse in the community. To collect this important information, the FDA requires that all companies that have brand-name opioids with labeling describing abuse-deterrent properties conduct postmarketing studies to determine the impact of abuse-deterrent formulation technologies in the real world. Each company is given a time line to which they must adhere. These types of studies take several years to conduct and analyze. Data collected will include the amount prescribed for each product; adverse events related to the use, abuse, and misuse of the products; and epidemiologic data on the rates of abuse and misuse and their consequences (addiction, overdose, and death). These studies should allow the FDA to assess the impact in the community, if any, attributable to the abuse-deterrent properties.
The science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies ar
Key Points for Practitioners
The FDA’s work to facilitate the safe use of opioids is taking place within a larger policy framework aimed at addressing opioid abuse while ensuring appropriate access to pain treatment. The FDA has undertaken several efforts helpful to clinicians. The FDA’s Extended-Release and Long-Acting Opioid Analgesics Risk Evaluation and Mitigation Strategy (ER/LA REMS) Program is required for all companies who make these products. The program’s goal is to reduce serious adverse outcomes of inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. Adverse outcomes of concern include addiction, unintentional overdose, and death.
As part of the REMS, all ER/LA opioid analgesic pharmaceutical companies must provide education for prescribers of their medications through accredited continuing education activities that are supported by independent educational grants. Companies must also provide information that prescribers can use when counseling patients about the risks and benefits associated with ER/LA opioid analgesic use.
The FDA has developed core messages that are communicated to prescribers in the Blueprint for Prescriber Education. The Blueprint is directed to prescribers of ER/LA opioid analgesics but also may be relevant for other health care professionals (eg, pharmacists). Companies involved in the ER/LA Opioid Analgesics REMS Program have collaborated to implement a single shared REMS. This group provides a list of REMS-compliant continuing education activities, which can be found at http://www.er-la-opioidrems.com.
It is important for practitioners to understand that all currently approved abuse-deterrent opioid products still can be abused, and as scheduled controlled substances, they are addictive. The abuse-deterrent properties are expected to deter but do not wholly prevent abuse. Because in the end opioid medications must be able to deliver the opioid to the patient, there probably always will be potential for abuse of these products. Consequently, practitioners should counsel their patients on the following:
- Keep medicines in a secure location out of the reach and out of sight of children and pets. Put away medicines after every use. Accidental exposure to medicine in the home is a major source of unintentional poisonings in the U.S.
- If medicines are no longer needed, dispose of them properly. Disposing of all unused opioid analgesics reduces access to these medications by family members and household guests seeking opioids for abuse.
- The FDA recommends returning most prescription medications through a local or U.S. Drug Enforcement Administration (DEA)-sponsored take-back program or DEA-authorized collector. For opioid analgesics, the FDA recommends immediate removal from the home by flushing them down the toilet or sink.
Opioids Action Plan
In February 2016, FDA Commissioner Robert Califf (then the deputy commissioner for medical products and tobacco) announced the FDA Opioids Action Plan. The plan focuses on policies aimed at reversing the opioid epidemic while still providing patients in pain access to effective pain relief. The FDA actions include:
- Convening an expert advisory committee before approving any new drug application for an opioid that does not have abuse-deterrent properties;
- Consulting with the Pediatric Advisory Committee about a framework for pediatric opioid labeling before any new labeling is approved;
- Updating the REMS requirements for ER/LA opioid analgesics after considering the advisory committee’s recommendations from a meeting held in May 2016 and reviewing existing requirements;
- Improving access to naloxone (by facilitating the development of an over-the-counter version of naloxone, which is currently available only by prescription, thereby making it more accessible to treat opioid overdose), and medication-assisted treatment options for patients with opioid use disorders; and
- Supporting better pain management options, including alternative, nonaddictive treatments for pain.
The FDA is conducting research on pain measurements for conditions such as chronic low back pain, osteoarthritis, diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. The FDA is also working to support the development of nonopioid options for these patients.
Consistent with the plan, in March 2016, the FDA announced that it was requiring changes to the labeling on immediate-release opioids, including additional warnings and safety information that incorporate elements similar to the ER/LA opioid analgesics labeling. Furthermore, among other steps, the FDA has contracted with the National Academy of Medicine to provide advice on how to incorporate current evidence about the public health impact of opioid use (for patients who are prescribed opioids as well as for nonpatients) into regulatory activities concerning opioids.
The FDA shares the responsibility of keeping patients safe. Working with the health care community and federal and state partners to help reduce opioid misuse and abuse and improve appropriate opioid prescribing while ensuring that patients in pain continue to have appropriate access to opioid analgesics is a top priority for the FDA and part of the targeted approach of the HHS focused on prevention, treatment, and intervention.
Opioid Abuse-Deterrent Formulations
The meaning of the term abuse-deterrent is often misunderstood to mean abuse-proof. The FDA defines abuse-deterrent properties as those properties expected to meaningfully deter abuse even if they do not fully prevent abuse. Abuse-deterrent properties make certain types of abuse, such as crushing in order to snort or dissolving in order to inject, more difficult or less rewarding. However, this does not mean that the product is impossible to abuse or that these properties will necessarily prevent addiction, overdose, or death.
Of note, currently marketed abuse-deterrent formulation technologies do not effectively deter one of the most common forms of opioid abuse—simply swallowing a number of intact tablets or capsules. Abuse-deterrent opioids do not reduce the risk for opioid addiction, and they carry the same warnings about the risk for addiction as do conventional opioids.
Abuse and Misuse Data
The FDA is encouraging pharmaceutical industry efforts to develop pain medicines that are more difficult to abuse and to prioritize the need for data and study methods that will help evaluate the impact of abuse-deterrent opioids on misuse and abuse in the community. To collect this important information, the FDA requires that all companies that have brand-name opioids with labeling describing abuse-deterrent properties conduct postmarketing studies to determine the impact of abuse-deterrent formulation technologies in the real world. Each company is given a time line to which they must adhere. These types of studies take several years to conduct and analyze. Data collected will include the amount prescribed for each product; adverse events related to the use, abuse, and misuse of the products; and epidemiologic data on the rates of abuse and misuse and their consequences (addiction, overdose, and death). These studies should allow the FDA to assess the impact in the community, if any, attributable to the abuse-deterrent properties.
The science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies ar
Key Points for Practitioners
The FDA’s work to facilitate the safe use of opioids is taking place within a larger policy framework aimed at addressing opioid abuse while ensuring appropriate access to pain treatment. The FDA has undertaken several efforts helpful to clinicians. The FDA’s Extended-Release and Long-Acting Opioid Analgesics Risk Evaluation and Mitigation Strategy (ER/LA REMS) Program is required for all companies who make these products. The program’s goal is to reduce serious adverse outcomes of inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. Adverse outcomes of concern include addiction, unintentional overdose, and death.
As part of the REMS, all ER/LA opioid analgesic pharmaceutical companies must provide education for prescribers of their medications through accredited continuing education activities that are supported by independent educational grants. Companies must also provide information that prescribers can use when counseling patients about the risks and benefits associated with ER/LA opioid analgesic use.
The FDA has developed core messages that are communicated to prescribers in the Blueprint for Prescriber Education. The Blueprint is directed to prescribers of ER/LA opioid analgesics but also may be relevant for other health care professionals (eg, pharmacists). Companies involved in the ER/LA Opioid Analgesics REMS Program have collaborated to implement a single shared REMS. This group provides a list of REMS-compliant continuing education activities, which can be found at http://www.er-la-opioidrems.com.
It is important for practitioners to understand that all currently approved abuse-deterrent opioid products still can be abused, and as scheduled controlled substances, they are addictive. The abuse-deterrent properties are expected to deter but do not wholly prevent abuse. Because in the end opioid medications must be able to deliver the opioid to the patient, there probably always will be potential for abuse of these products. Consequently, practitioners should counsel their patients on the following:
- Keep medicines in a secure location out of the reach and out of sight of children and pets. Put away medicines after every use. Accidental exposure to medicine in the home is a major source of unintentional poisonings in the U.S.
- If medicines are no longer needed, dispose of them properly. Disposing of all unused opioid analgesics reduces access to these medications by family members and household guests seeking opioids for abuse.
- The FDA recommends returning most prescription medications through a local or U.S. Drug Enforcement Administration (DEA)-sponsored take-back program or DEA-authorized collector. For opioid analgesics, the FDA recommends immediate removal from the home by flushing them down the toilet or sink.
Opioids Action Plan
In February 2016, FDA Commissioner Robert Califf (then the deputy commissioner for medical products and tobacco) announced the FDA Opioids Action Plan. The plan focuses on policies aimed at reversing the opioid epidemic while still providing patients in pain access to effective pain relief. The FDA actions include:
- Convening an expert advisory committee before approving any new drug application for an opioid that does not have abuse-deterrent properties;
- Consulting with the Pediatric Advisory Committee about a framework for pediatric opioid labeling before any new labeling is approved;
- Updating the REMS requirements for ER/LA opioid analgesics after considering the advisory committee’s recommendations from a meeting held in May 2016 and reviewing existing requirements;
- Improving access to naloxone (by facilitating the development of an over-the-counter version of naloxone, which is currently available only by prescription, thereby making it more accessible to treat opioid overdose), and medication-assisted treatment options for patients with opioid use disorders; and
- Supporting better pain management options, including alternative, nonaddictive treatments for pain.
The FDA is conducting research on pain measurements for conditions such as chronic low back pain, osteoarthritis, diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. The FDA is also working to support the development of nonopioid options for these patients.
Consistent with the plan, in March 2016, the FDA announced that it was requiring changes to the labeling on immediate-release opioids, including additional warnings and safety information that incorporate elements similar to the ER/LA opioid analgesics labeling. Furthermore, among other steps, the FDA has contracted with the National Academy of Medicine to provide advice on how to incorporate current evidence about the public health impact of opioid use (for patients who are prescribed opioids as well as for nonpatients) into regulatory activities concerning opioids.
The FDA shares the responsibility of keeping patients safe. Working with the health care community and federal and state partners to help reduce opioid misuse and abuse and improve appropriate opioid prescribing while ensuring that patients in pain continue to have appropriate access to opioid analgesics is a top priority for the FDA and part of the targeted approach of the HHS focused on prevention, treatment, and intervention.
Opioid Abuse-Deterrent Formulations
The meaning of the term abuse-deterrent is often misunderstood to mean abuse-proof. The FDA defines abuse-deterrent properties as those properties expected to meaningfully deter abuse even if they do not fully prevent abuse. Abuse-deterrent properties make certain types of abuse, such as crushing in order to snort or dissolving in order to inject, more difficult or less rewarding. However, this does not mean that the product is impossible to abuse or that these properties will necessarily prevent addiction, overdose, or death.
Of note, currently marketed abuse-deterrent formulation technologies do not effectively deter one of the most common forms of opioid abuse—simply swallowing a number of intact tablets or capsules. Abuse-deterrent opioids do not reduce the risk for opioid addiction, and they carry the same warnings about the risk for addiction as do conventional opioids.
Abuse and Misuse Data
The FDA is encouraging pharmaceutical industry efforts to develop pain medicines that are more difficult to abuse and to prioritize the need for data and study methods that will help evaluate the impact of abuse-deterrent opioids on misuse and abuse in the community. To collect this important information, the FDA requires that all companies that have brand-name opioids with labeling describing abuse-deterrent properties conduct postmarketing studies to determine the impact of abuse-deterrent formulation technologies in the real world. Each company is given a time line to which they must adhere. These types of studies take several years to conduct and analyze. Data collected will include the amount prescribed for each product; adverse events related to the use, abuse, and misuse of the products; and epidemiologic data on the rates of abuse and misuse and their consequences (addiction, overdose, and death). These studies should allow the FDA to assess the impact in the community, if any, attributable to the abuse-deterrent properties.
The science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies ar
Key Points for Practitioners
The FDA’s work to facilitate the safe use of opioids is taking place within a larger policy framework aimed at addressing opioid abuse while ensuring appropriate access to pain treatment. The FDA has undertaken several efforts helpful to clinicians. The FDA’s Extended-Release and Long-Acting Opioid Analgesics Risk Evaluation and Mitigation Strategy (ER/LA REMS) Program is required for all companies who make these products. The program’s goal is to reduce serious adverse outcomes of inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. Adverse outcomes of concern include addiction, unintentional overdose, and death.
As part of the REMS, all ER/LA opioid analgesic pharmaceutical companies must provide education for prescribers of their medications through accredited continuing education activities that are supported by independent educational grants. Companies must also provide information that prescribers can use when counseling patients about the risks and benefits associated with ER/LA opioid analgesic use.
The FDA has developed core messages that are communicated to prescribers in the Blueprint for Prescriber Education. The Blueprint is directed to prescribers of ER/LA opioid analgesics but also may be relevant for other health care professionals (eg, pharmacists). Companies involved in the ER/LA Opioid Analgesics REMS Program have collaborated to implement a single shared REMS. This group provides a list of REMS-compliant continuing education activities, which can be found at http://www.er-la-opioidrems.com.
It is important for practitioners to understand that all currently approved abuse-deterrent opioid products still can be abused, and as scheduled controlled substances, they are addictive. The abuse-deterrent properties are expected to deter but do not wholly prevent abuse. Because in the end opioid medications must be able to deliver the opioid to the patient, there probably always will be potential for abuse of these products. Consequently, practitioners should counsel their patients on the following:
- Keep medicines in a secure location out of the reach and out of sight of children and pets. Put away medicines after every use. Accidental exposure to medicine in the home is a major source of unintentional poisonings in the U.S.
- If medicines are no longer needed, dispose of them properly. Disposing of all unused opioid analgesics reduces access to these medications by family members and household guests seeking opioids for abuse.
- The FDA recommends returning most prescription medications through a local or U.S. Drug Enforcement Administration (DEA)-sponsored take-back program or DEA-authorized collector. For opioid analgesics, the FDA recommends immediate removal from the home by flushing them down the toilet or sink.
Opioids Action Plan
In February 2016, FDA Commissioner Robert Califf (then the deputy commissioner for medical products and tobacco) announced the FDA Opioids Action Plan. The plan focuses on policies aimed at reversing the opioid epidemic while still providing patients in pain access to effective pain relief. The FDA actions include:
- Convening an expert advisory committee before approving any new drug application for an opioid that does not have abuse-deterrent properties;
- Consulting with the Pediatric Advisory Committee about a framework for pediatric opioid labeling before any new labeling is approved;
- Updating the REMS requirements for ER/LA opioid analgesics after considering the advisory committee’s recommendations from a meeting held in May 2016 and reviewing existing requirements;
- Improving access to naloxone (by facilitating the development of an over-the-counter version of naloxone, which is currently available only by prescription, thereby making it more accessible to treat opioid overdose), and medication-assisted treatment options for patients with opioid use disorders; and
- Supporting better pain management options, including alternative, nonaddictive treatments for pain.
The FDA is conducting research on pain measurements for conditions such as chronic low back pain, osteoarthritis, diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. The FDA is also working to support the development of nonopioid options for these patients.
Consistent with the plan, in March 2016, the FDA announced that it was requiring changes to the labeling on immediate-release opioids, including additional warnings and safety information that incorporate elements similar to the ER/LA opioid analgesics labeling. Furthermore, among other steps, the FDA has contracted with the National Academy of Medicine to provide advice on how to incorporate current evidence about the public health impact of opioid use (for patients who are prescribed opioids as well as for nonpatients) into regulatory activities concerning opioids.
The FDA shares the responsibility of keeping patients safe. Working with the health care community and federal and state partners to help reduce opioid misuse and abuse and improve appropriate opioid prescribing while ensuring that patients in pain continue to have appropriate access to opioid analgesics is a top priority for the FDA and part of the targeted approach of the HHS focused on prevention, treatment, and intervention.