User login
Evolving strategies to prevent stroke and thromboembolism in nonvalvular atrial fibrillation
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
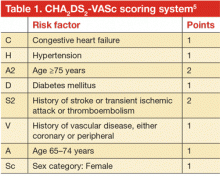
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
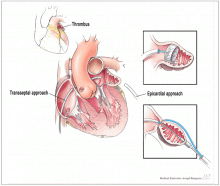
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
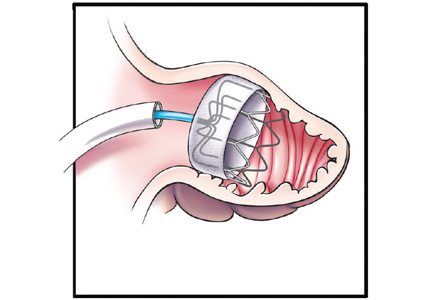
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
Atrial fibrillation (AF), the most common cardiac arrhythmia, has become a major public health problem. In the United States, the prevalence of AF was estimated at 2.7 to 6.1 million in 2010, and it is expected to rise to between 5.6 and 12 million by 2050.1 The arrhythmia is associated with impaired quality of life and increased morbidity and mortality.1,2 Stroke remains the most devastating consequence of AF.
The clinical management of patients with AF typically targets two main goals: prevention of stroke or thromboembolism and control of symptoms. This article addresses the evolving pharmacologic and nonpharmacologic strategies in stroke prevention in nonvalvular AF; reviews clinical trials evaluating medical and procedural strategies, including the novel oral anticoagulants and left atrial appendage (LAA) exclusion devices; and assesses the impact of these novel strategies on clinical practice.
RISK OF STROKE AND THROMBOEMBOLISM IN NONVALVULAR AF
Stroke occurrence from AF is primarily caused by thrombi formation in the left atrium, most commonly in the LAA. It is important to recognize that the cardiovascular risk factors for AF are also risk factors for atheroembolism; therefore, specific risk factor management is as important as anticoagulation when addressing stroke risk.
The incidence of all-cause stroke in patients with AF is 5%, and it is believed that AF causes approximately 15% of all strokes in the United States.1 This risk appears to be more significant in older patients who are more vulnerable to ischemic strokes. Estimates are that AF independently increases the risk of stroke by fivefold throughout all ages, with a steep increase in percentage of strokes attributed to AF from 1.5% at ages 50 to 59 to 23.5% at ages 80 to 89.1 Importantly, the clinical course of ischemic stroke associated with AF is often more severe than for strokes of other causes,3 further emphasizing the need for stroke prevention.
Assessment of stroke risk/thromboembolism
Multiple risk estimation scores have been developed based on epidemiologic data. Until recently, the CHADS2 score4 was the most commonly used, but it has been superseded by the CHA2DS2-VASc score.5 The point system for this scoring system is shown in Table 1. In contrast with CHADS2, this updated system assigns 2 points for age over 75 years and accounts for stroke risk in the relatively younger group of patients (age 65–75) and in females, neither of whom were included in CHADS2. The CHA2DS2-VASc score ranges between 0 and 9 with a respective estimated stroke risk of 0 to 15.2% per year. Note that for females who are younger than 65 years, no points are given for sex. The major advantage of the CHA2DS2-VASc score over the CHADS2 score is that it is more accurate for lower-risk categories. It has been adopted in most of the recent guidelines that address stroke risk in AF.
In clinical practice, practitioners use these scores to define three primary stroke risk categories: low, intermediate, or high. In our practice, we use a 2% per year cut-off to identify high-risk patients in whom the risk of stroke significantly outweighs the risk of bleeding on anticoagulants. In general, patients with a CHA2DS2-VASc score equal to or greater than 2 have a greater than 2% stroke risk per year and are most likely to benefit from antithrombotic therapies.
In male patients with a CHA2DS2-VASc score of 0 and in most patients with a score of 1, the stroke risk is less than 1% per year. These patients are not likely to derive benefit from anticoagulant therapy. They are usually approached on a case-by-case basis with careful assessment of bleeding risk and discussion of risks and benefits of anticoagulant strategies.
Assessment of bleeding risk
Any general approach to thromboembolism risk assessment in patients with AF should include an analysis that weighs the benefits of anticoagulant therapies against the risks of bleeding. Although no precise tools exist to predict bleeding risk, the HAS-BLED score is increasingly used.6 This score assigns 1 point to each of the following:
- systolic blood pressure greater than 160 mm Hg
- abnormal renal function
- abnormal liver function
- age older than 65
- prior cerebrovascular event
- prior bleeding
- history of labile international normalized ratios (INR)
- alcohol intake (> 8 U/week)
- drug use, especially antiplatelet agents or nonsteroidal anti-inflammatory drugs (NSAIDs).
In general, a HAS-BLED score of 3 or greater indicates increased 1-year risk of intracranial bleed, bleeding requiring hospitalization, drop in hemoglobin of at least 2 g/dL, or need for transfusion.
One problem with the bleeding risk scores is that they were derived from studies that included bleeding events of differing severity. Most bleeding events do not lead to death or severe disability with the exception of intracranial bleeding, which is, therefore, the primary concern when assessing bleeding risk.
The estimated bleeding risk with anticoagulant therapy ranges from 0.2% to 0.4% per year but could be much higher in patients with prior severe bleeding, intracranial hemorrhage, thrombocytopenia, coagulopathies, recent surgery, or ongoing bleeding, aortic dissection, malignant hypertension, and in those receiving a combination of anticoagulant and antiplatelet agents.
MEDICAL THERAPIES TO PREVENT STROKE AND THROMBOEMBOLISM IN AF
In general, anticoagulation reduces the risk of ischemic stroke and thromboembolic events by approximately two-thirds, regardless of baseline risk. Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
Warfarin
Warfarin has been used for decades for stroke prevention. It remains the only acceptable anticoagulant in patients with valvular AF. Multiple randomized clinical trials have assessed the efficacy of warfarin for stroke prevention in patients with nonvalvular AF.7 These trials demonstrated that warfarin significantly reduces stroke risk, stroke severity, and 30-day mortality compared with no anticoagulant therapy.7,8
Although warfarin is one of the most efficacious drugs to prevent stroke in AF, it has several key limitations. The most important is the need for dose adjustment to keep the INR in a narrow window (2.0 to 3.0) in which net clinical benefit is achieved without increased bleeding risk. The need for continuous monitoring is an inconvenience to patients and often leads to drug discontinuation and nonadherence. A meta-analysis found that patients are only in the therapeutic INR about half of the time.9 Importantly, the time spent in therapeutic INR range cor- relates significantly with the reduction in stroke risk.10 Furthermore, patients who spend less than 40% of the time in the therapeutic INR range are at a higher stroke risk than those not taking warfarin.10
Another limitation with the medication is the dietary restriction on intake of vitamin K-rich green vegetables, which are emphasized as healthy food choices especially in patients with heart disease. Higher warfarin doses are required in patients who consume greens and salads. It is important that patients be consistent in their intake of vitamin K-rich foods to avoid labile INRs, a difficult task for most patients.
Finally, there are several drugs that might interact with warfarin and potentially interfere with its safety or efficacy. These drugs include amiodarone, statins including simvastatin and rosuvastatin (not atorvastatin or pravastatin), fibrates (fenofibrate, gemfibrozil), antibiotics (sulfamethoxazole/trimethoprim, metronidazole), and azole antifungals (fluconazole, miconazole, voriconazole). The use of drugs that induce the cytochrome P450 enzyme CYP2C9, such as rifampin, decrease warfarin effectiveness by reducing INR values. Other non-CYP2C9-dependent drug interactions exist as well.
Aspirin monotherapy or in combination with other agents
Aspirin monotherapy or aspirin plus clopidogrel both increase the risk of bleeding without appreciable benefit, and, as such, their use for stroke prevention in patients with AF is not well supported. The combination of aspirin plus low-dose warfarin was assessed in the SPAF-III trial11 that randomized AF patients at stroke risk to either aspirin plus low-dose warfarin or to dose-adjusted warfarin to target a therapeutic INR. In this trial, patients on aspirin plus low-dose warfarin had significantly higher morbidity and mortality than patients who took adjusted-dose warfarin alone. Thus, the combination of low-dose warfarin plus aspirin should not be used for stroke prevention in AF.
In contrast, the combination of aspirin plus full anticoagulation with warfarin has not been well studied. Limited post-hoc data from the SPORTIF trials suggest, however, that this combination does not reduce the risk of stroke or thromboembolism more than warfarin alone.12
Novel oral anticoagulants
Dabigatran. The value of dabigatran for prevention of stroke or thromboembolism in AF was tested in the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy).13 In this trial, 18,113 patients with AF at high risk for stroke were randomized to dabigatran (110 mg or 150 mg twice daily) or adjusted-dose warfarin (INR target 2.0–3.0). By intention-to-treat analysis, dabigatran 150 mg was superior to warfarin for stroke prevention. Importantly, the risk of intracranial or life-threatening bleeding was significantly lower for both dabigatran doses compared with warfarin. Of note, gastrointestinal bleeding was more common with dabigatran 150 mg than warfarin; rates were similar for dabigatran 110 mg versus warfarin.
Rivaroxaban. This factor-Xa inhibitor was assessed in ROCKET-AF (Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation).14 In this trial, 14,264 patients with AF and at risk for stroke were randomized to rivaroxaban (20 mg once daily) or warfarin (INR target 2.5). In the warfarin arm, INR was in the therapeutic range only 55% of the time. Results showed rivaroxaban was noninferior, but not superior, to warfarin for the prevention of stroke or systemic thromboembolism, the primary end points. From a safety standpoint, the overall bleeding rates were similar in the treatment arms with less life-threatening (fatal or intracranial) hemorrhage events with rivaroxaban.
Apixaban. This factor-Xa inhibitor was tested for stroke prevention in AF in two separate clinical trials.15,16 In the AVERROES trial (Apixaban Versus Acetylsalicylic Acid to Prevent Strokes),15 5,599 patients with AF deemed “unsuitable” for warfarin were randomized to apixaban (5 mg twice daily) or aspirin (81–324 mg daily). The trial was terminated early due to superiority of apixaban in achieving the primary end point: occurrence of stroke or systemic embolism. Importantly, the risk of major bleeding appeared to be similar with apixaban versus aspirin.
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial16 randomized 18,201 patients with AF and at least one additional risk factor for stroke to either apixaban 5 mg or warfarin (target INR 2.0–3.0). In this trial, apixaban was superior to warfarin for the prevention of stroke or systemic embolism, the primary efficacy end point. There also appeared to be a mortality benefit with apixaban versus warfarin. Importantly, the risk of major and intracranial bleeding, the primary safety outcomes, occurred at lower rates in the apixaban group.
All three of these oral anticoagulants require dose adjustment in patients with renal insufficiency and are contraindicated in patients with end-stage renal failure. They are not indicated in patients with valvular heart disease or a mechanical heart valves.
NONPHARMACOLOGIC INTERVENTIONS
Most atrial thrombi in patients with nonvalvular AF form in the LAA. Nonpharmacologic interventions have been developed to block the LAA to reduce the risk of stroke. These are especially valuable options for patients who are not candidates for chronic anticoagulation. In patients undergoing mitral valve surgery,17 ligation to close the LAA has become a standard practice at experienced centers. The introduction of less invasive catheter-based interventions to occlude the LAA has provided additional options.
Watchman device
The Watchman implant, a closure device that blocks the LAA, was recently approved by the US Food and Drug Administration (FDA) for stroke prevention in patients with nonvalvular AF who are at increased risk for stroke based on a CHA2DS2-VASc score of 2 or greater; candidates also must have an appropriate rationale for nonpharmacologic therapy. The expandable-cage device is surgically delivered into the LAA (Figure 1), which subsequently endothelializes and isolates the LAA. (Video 1 shows the delivery sheath positioned at the os of the appendage with a confirmatory contrast appendogram. Video 2 shows the delivery of the device into the appendage.) Therapeutic warfarin is required for a minimum of 45 days after implant followed by aspirin and clopidogrel for 6 months and then aspirin alone.
This device was initially tested in the PROTECT AF (Watchman Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial, a noninferiority trial that randomized patients to either device implant or warfarin.18 Device implant was successful in 91% of patients in whom it was attempted. Overall, the study showed noninferiority of the device to warfarin in terms of the primary efficacy standpoint, which included stroke, systemic embolism, and cardiovascular death; however, this came at the expense of higher incidence of procedure-related complications, which seemed to be dependent on a learning curve with the device.
In the PREVAIL (Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial,19 although noninferiority was not achieved, the event rates were low and the safety of the procedure was much improved. This was also demonstrated in registry data, which showed improved safety with device implantation with increased operator experience.
Of note, both of these trials included only patients who were eligible for warfarin. Another nonrandomized study20 assessed the use of this device in patients with a contraindication to long-term anticoagulation. Results showed a very low incidence of stroke, which was lower than CHADS2-matched controls taking either aspirin or clopidogrel. The FDA has approved this device for stroke prevention in AF.
Lariat system
The Lariat system is another percutaneous system for occlusion of the LAA. This device, which requires both atrial transseptal and epicardial access, ligates the appendage from the pericardial space. While some small studies have shown it safe and efficacious in patients with AF who cannot take anticoagulation,21 other reports have not been as encouraging.22 The device has been approved by the FDA for “soft tissue approximation”; it is not approved for stroke prevention.
SUMMARY
The most common serious complications of AF are stroke and thromboembolism. Medical and interventional therapies have been developed to prevent these complications. In patients with an estimated thromboembolic risk of greater than 1% to 2% per year, anticoagulation is warranted to reduce that risk. Warfarin remains one of the most studied and useful medications for this purpose, but its use is limited by the need for frequent monitoring, multiple drug interactions, dietary restrictions, and, most importantly, by the difficulty of consistently maintaining therapeutic INRs.
The recently introduced novel oral anticoagulants have been found to be at least noninferior to warfarin and for some agents to be superior to warfarin for the prevention of stroke and thromboembolism. Their main advantage is that they do not require monitoring and have fewer drug interactions and dietary restrictions. Most important, there appear to be fewer major and life-threatening bleeding events than with warfarin. However, use of these novel agents could be limited by patients’ renal function, which needs to be assessed when these agents are being considered. Another limitation with these agents (versus warfarin) is that patients are not therapeutically anticoagulated when they miss a dose, whereas missing a single dose of warfarin may not have the same effect.
In our practice, we have transitioned to use of these novel oral anticoagulants whenever possible using an approach that assesses the individual patient’s risks for both stroke and thromboembolism as well as the risk for bleeding. In patients who are at increased risk of bleeding but at risk of stroke in AF, percutaneous occlusion of the LAA using the Watchman device is offered. This device has been shown to be noninferior to warfarin for stroke prevention and may provide a survival benefit due to the reduction of life-threatening bleeding, which is an inherent risk with anticoagulants. One caveat is that there is a residual risk of stroke after undergoing LAA occlusion because the same risk factors for stroke in AF contribute to stroke from atherothrombosis and atheroembolism; also, thrombi can form in the body of the left atrium.
Clinical decision-making is often challenging in patients with AF who are at risk of stroke and bleeding. In fact, these risk factors often overlap. In our practice, we have established a multidisciplinary clinic for stroke prevention in AF that involves cardiologists, cardiac electrophysiologists, neurologists, gastroenterologists, and vascular medicine specialists. This model allows a multidisciplinary assessment of patients’ individual risks and ultimately facilitates clinical decision-making in terms of strategies to prevent stroke and thromboembolism in AF.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
- Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21):e1–76.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 2003; 22:118–123.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010; 137:263-272.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146:857–867.
- Johnsen SP, Svendsen ML, Hansen ML, Brandes A, Mehnert F, Husted SE. Preadmission oral anticoagulant treatment and clinical outcome among patients hospitalized with acute stroke and atrial fibrillation: a nationwide study. Stroke 2014; 45:168-175.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the united states. J Manage Care Pharm 2009; 15:244-252.
- Morgan CL, McEwan P, Tukiendorf A, Robinson PA, Clemens A, Plumb JM. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res 2009; 124:37–41.
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996; 348(9028):633–638.
- Flaker GC, Gruber M, Connolly SJ, et al. Risks and benefits of combining aspirin with anticoagulant therapy in patients with atrial fibrillation: an exploratory analysis of stroke prevention using an oral thrombin inhibitor in atrial fibrillation (SPORTIF) trials. Am Heart J 2006; 152:967–973.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al, and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374(9689):534–542.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J Am Coll Cardiol 2014; 64:1–12.
- Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Chatterjee S, Herrmann HC, Wilensky RL, et al. Safety and procedural success of left atrial appendage exclusion with the Lariat device: a systematic review of published reports and analytic review of the FDA MAUDE database. JAMA Intern Med 2015; 175(7):1104–9.
KEY POINTS
- Specific risk factor management is as important as anticoagulation when addressing stroke risk.
- The CHADS2 score has been superseded by the CHA2DS2-VASc score, which is more accurate for lower-risk categories.
- Anticoagulant options have increased substantially in the past few years with the introduction of novel oral anticoagulants, including the direct thrombin-inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban.
- Most atrial thrombi in patients with nonvalvular atrial fibrillation form in the left atrial appendage (LAA); nonpharmacologic interventions have been developed to block the LAA and reduce the risk of stroke.