User login
Radiation therapy: Managing GI tract complications
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
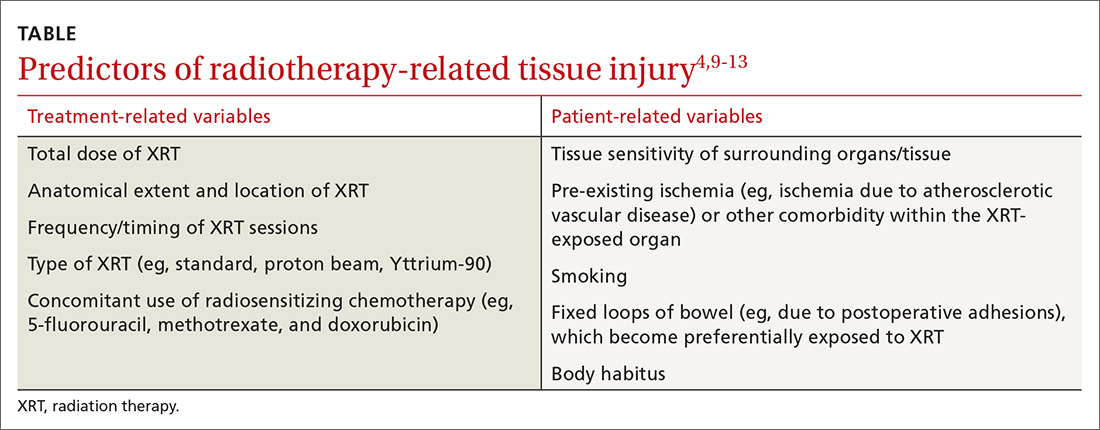
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.
Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; JTabibian@dhs.lacounty.gov.
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.
Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; JTabibian@dhs.lacounty.gov.
CASE A 57-year-old man presented for evaluation of painless, intermittent passage of bright red blood per rectum for several months. His bowel habits were otherwise unchanged, averaging 2 soft bowel movements daily without straining. His medical history was significant for radiation therapy for prostate cancer 18 months earlier and a recent finding of mild microcytic anemia. A colonoscopy 7 years ago was negative for polyps, diverticula, or other lesions. He denied any family history of colon cancer or other gastrointestinal disorders. He wanted to know what he could do to stop the bleeding or if further testing would be needed.
Next steps?
Radiation therapy and its effect on the GI tract
In 1895, Dr. Wilhelm Roentgen first introduced the use of x-rays for diagnostic radiographic purposes. A year later, Dr. Emil Gruble made the first attempt to use radiation therapy (XRT) to treat cancer. In 1897, Dr. David Walsh described the first case of XRT-induced tissue injury in the British Medical Journal.1
Since then, XRT has been used extensively to treat cancer, and its delivery techniques have improved and diversified. Like chemotherapy, XRT has its greatest effect on rapidly dividing cells, but as a result, the adverse effects of therapy are also greatest on rapidly dividing normal tissues, as well as others in the radiation field.
A large proportion of cancer patients will receive XRT, yet XRT-related costs account for less than 5% of total cancer care expenditure, suggesting cost effectiveness.2,3 However, even with the great progress achieved in the delivery of XRT, it continues to have its share of acute and chronic complications, among the most common of which is gastrointestinal (GI) tract toxicity. These adverse effects are often first reported to, diagnosed, or treated by the primary care provider, who frequently remains pivotally involved in the patient’s longitudinal care.
Approximately 50% to 75% of patients undergoing XRT will have some degree of GI symptoms of acute injury, but the majority will recover fully within a few weeks following completion of treatment.4-6 However, in about 5% of patients,4-6 there will be long-term consequences of varying degrees that may develop as soon as one year or as long as 10 years after XRT. These can pose substantial challenges for patients, as well as both the primary care provider and consulting specialists.
In the review that follows, we detail the potential acute and chronic complications of XRT on the GI tract and how best to manage them. But first, a word about the related terminology.
Getting a handle on XRT-related injury terminology
The preferred terms used to describe injury to normal tissue as a result of XRT include “XRT-related injury” or “pelvic radiation disease” (when the injury is confined to intrapelvic tissues); organ-specific descriptors such as “radiation enteropathy” or “XRT-induced esophageal stricture” are also used and are acceptable.4,7,8
Terms such as “radiation enteritis” or “radiation proctitis” are considered misnomers since there is no significant histologic inflammation. Indeed, as we will discuss, acute injury is largely due to epithelial cellular injury and cell death (necrosis), while chronic injury is primarily the consequence of ongoing tissue ischemia, fibrosis, and other pathophysiologic processes.
Acute vs chronic XRT-related tissue injury
From a pathobiologic and clinical perspective, XRT-related injury can be categorized as either acute or chronic.8-12 Acute XRT-related injury involves direct cellular necrosis of the epithelial cells and damage (eg, irreparable DNA alterations) to stem cells. This acute injury prevents appropriate cellular regeneration, which results in denuded mucosa, mucosal ulcerations, and even perforation in severe cases.10 Acute injury starts 2 to 3 weeks after initiating XRT and typically resolves within 2 to 3 months following completion of treatment.
Chronic XRT toxicity is pathophysiologically complex and multifactorial.10-12 It includes: obliterative endarteritis of submucosal arterioles with chronic tissue ischemia, eosinophil infiltration, fibroblast proliferation and pathologic fibrosis, neovascularization with friable telangiectasia formation, and bowel serosal injury that promotes formation of dense adhesions.13 Its pathogenesis remains incompletely understood.
Several treatment- and patient-related variables can impact the occurrence and nature of tissue injury secondary to XRT and are summarized in the Table.4,9-13 Newer forms of radiotherapy such as proton beam and Yttrium-90 radioembolization may also cause radiation injury,14 but to a lesser degree than conventional external beam XRT, in part because of improved dose targeting. We will not discuss these modalities in this review.
Can’t something be done to prevent injury in the first place?
There are no convincing evidence-based preventive or therapeutic treatments that address the underlying mechanisms of either the acute or chronic phases of XRT-related GI tract injury, although hyperbaric oxygen (which we’ll discuss in greater detail shortly) may be a promising option.8,11,12,15-17 It’s believed that hyperbaric oxygen may prove useful by facilitating angiogenesis and improving tissue oxygenation.8,11,15-17 Unfortunately, this treatment is not widely available, and the frequency and duration required for optimal results is unclear.
Numerous pharmacologic radioprotectants have been suggested or evaluated in small studies, but none have an established role in addressing XRT-related injury. Given these voids, emphasis on symptom management and empathic, supportive care is essential.18
A look at injuries and Tx options by organs affected
The esophagus
Injurious effects on the esophagus are seen following XRT for lung, mediastinal, hypopharyngeal, or esophageal cancers.19,20 The total XRT dose and regimen may vary, but a typical course may involve 10 gray (ie, 1000 rads) per week (2 gray per day) for 5 weeks. The maximum tolerated dose by the esophagus is approximately 6 gray, above which most patients will have long-term complications; however, some patients may experience toxicity at even lower doses.
Acute complications of esophageal XRT-related injury include mucosal ulcerations, which can present as chest pain and odynophagia. The mucosal pathology can cause dysmotility, which results in dysphagia for both liquids and solids.19-21
If severe symptoms develop during treatment, the dose per session can be reduced and/or the sessions can be delayed. Some patients require temporary gastrostomy feeding tubes until symptoms resolve. Mucosal ulcerations can become a chronic issue as well. The mainstay of treatment is symptomatic relief with topical anesthetics and anti-acid medications.
Chronic symptoms are more varied and can be difficult to manage14,15 and include the following:
- Strictures. Esophageal dysphagia develops in nearly two-thirds of patients postradiation and, in many cases, is due to stricture formation.22 Symptoms may range from mild dysphagia with solids to complete esophageal obstruction.23 Barium esophagography can be helpful to delineate esophageal stricture morphology and determine treatment options.
For the majority of patients, serial endoscopic dilation with a balloon catheter or bougie (or other endoscopic techniques) achieves adequate esophageal patency to alleviate symptoms; this may need to be repeated periodically to maintain patency, as nearly one-third of patients will experience recurrent stricturing.21,23
- Tracheo-esophageal fistulae. This complication can lead to pneumonia and generally has a poor prognosis.
Fistulae are chiefly treated endoscopically with esophageal, and occasionally, tracheobronchial stent placement. As with esophageal strictures, barium imaging can help plan the therapeutic approach. Percutaneous feeding may be required in some patients as a bridge or when fistula closure cannot be achieved.
- Secondary esophageal carcinogenesis. This dreaded complication develops in up to 2% to 3% of patients at 10 years post-XRT.19
Pharmacologic therapy for esophageal symptoms is generally unsuccessful, although acid suppression therapy may help as an adjuvant treatment to endoscopic dilation for esophageal strictures. Surgery is seldom attempted because of the fibrotic/ischemic tissues and high postoperative morbidity/mortality.
The stomach
The stomach is relatively resistant to XRT injury. Although XRT therapy can cause a transient decrease in acid output, there are rarely significant short- or long-term consequences with conventional therapeutic dosing (less than 50 gray).11
The liver
Hepatic resistance to radiation is relatively high; however, liver toxicity has been reported at low doses, an effect that is seen largely following bone marrow transplantation.24 Acute histologic XRT-related liver injury changes consist of severe pan-lobar congestion leading to hemorrhagic necrosis, cell atrophy, and perivascular fibrosis, as well as sclerosis of central and sublobular hepatic veins. The majority of patients will show reversal of the histologic changes within 3 months; however, approximately 25% to 40% of patients,25 depending on total XRT dose to the liver and other technical factors, will experience progressive and chronic changes resulting in liver atrophy, severe perivascular injury, and fibrosis of the portal vein or bile ducts.
The clinical symptoms of acute liver injury may include right upper quadrant pain, ascites, jaundice, veno-occlusive disease, or Budd-Chiari syndrome.25 The major chronic complication of XRT-related liver injury is progressive fibrosis, which may advance to cirrhosis.
Small bowel
The small bowel is the most radiosensitive GI tract organ due to high cell turnover, which makes it very susceptible to XRT-related injury.4,8,10,26-28 Under 3 gray, ≤20% of patients will develop radiation enteropathy, while at >5 gray, the incidence rises progressively with dose, and a majority of patients will be symptomatic.29 The degree to which the bowel is healthy before XRT can be an important factor in developing enteropathy. Parenthetically, treatment with a full bladder may also help displace some of the loops from the field of XRT and decrease injury.
Acute XRT-related injury of the small bowel includes mucosal necrosis (ie, direct cell death) and ulcerations that may present as diarrhea, pain, malabsorption, weight loss, bleeding, and perforation.4,8,10,26-28 Fortunately, in most patients, these are self-limited and can be managed symptomatically. Loperamide is the first-line medication for diarrhea, although Lomotil (diphenoxylate/atropine) may also be used if necessary.4,8,10,26-28 Nutrition may be challenging in severe cases, and if dietary modifications and supplementation do not prove sufficient, home parenteral nutrition is required.
Over time, chronic small bowel pathology may develop, including strictures in 3% to 15%, fistulae in 0.6% to 4.8%, secondary neoplasia in up to 10%, dysmotility- or adhesion-related small intestinal bacterial overgrowth in up to 45%, and malabsorption with associated nutritional deficiency in up to 63%.26-28 Other common XRT-related complications are chronic pain, which could be due to adhesions or ischemia, small intestinal bacterial overgrowth, or partial bowel obstruction, and telangiectasias that result with acute or chronic blood loss.13
Imaging of small bowel disease to diagnose the various manifestations of radiation enteropathy is challenging. Conventional X-rays may be difficult to interpret. Therefore, computerized tomography or magnetic resonance enterography, capsule endoscopy, or balloon-assisted enteroscopy is preferred—depending on availability, local expertise, and the suspected pre-procedure diagnosis.
Telangiectasias are not seen on cross-sectional imaging but can be seen with capsule endoscopy (which should not be ordered if stricture is suspected unless a patency capsule has been tried). Single or double balloon enteroscopy (specialized endoscopes intended for reaching the mid and distal ileum), which has been used to treat strictures or telangiectasia in healthy tissues,29 can be difficult or impossible in post-XRT patients because adhesions may limit progress of the scope to the area of interest, and forceful advancement of the scope increases the risk of perforation.
Small bowel telangiectasias can cause chronic occult blood loss, which often requires iron supplementation; acute bleeding may require blood transfusion and hospitalization. Of note, choosing an iron formulation that is well tolerated is critical to avoid (additional) unpleasant GI tract adverse effects. We typically recommend elemental iron with Vitamin C to augment absorption or ferrous gluconate; some patients will require intravenous iron infusion.
Surgery may be advisable to address complications such as fistulous tracts, complex strictures, or bowel obstruction; how-ever, operating on radiated abdominal tissues and ischemic bowel is associated with high morbidity and mortality.4,25,28,30 The surgeon may encounter dense adhesions that make an otherwise “simple” surgery problematic.
For example, it may be difficult to access the desired region and determine the borders of healthy tissue; wide excisions are, thus, often performed, which may result in small bowel failure (ie, short gut syndrome) and a mortality rate in excess of 30%.31 In addition, the ischemic post-XRT tissues may not heal well even if the intended surgery is completed; indeed, anastomotic leaks, failures, and infections are not uncommon. Moreover, another 30% will have other postoperative complications, 40% to 60% may require more than one laparotomy, and 50% of those who recover from the initial surgery will develop recurrence of the fistulous tract or stricture.4,25,28,30
No drug therapy has proven effective for prevention or mechanistically-driven treatment of XRT-induced small bowel injury. Hyperbaric oxygen therapy may be the most promising medical treatment, with early response in 53% of cases and long-term response of 66% to 73% for global symptomatic relief.32 It has been used successfully for treatment of pain, diarrhea, malabsorption, and hemorrhage from mucosal ulcerations, stenosis, and fistulous tracts. When available, it should be considered as a potential therapeutic intervention.
Colon
Injury to the colon is seen in 10% to 20% of patients following XRT for prostate, bladder, cervical, or uterine cancer.33 The maximum tolerated dose of the colon is slightly higher than for the small intestine.34 The rectosigmoid area is the area most commonly implicated, but depending on the field of radiation, injury can be more extensive/proximal.
Acute XRT injury of the colon produces acute mucosal necrosis, which may manifest as bowel dysmotility, diarrhea, cramps, tenesmus, or hematochezia. Sigmoidoscopy or colonoscopy will show mucosal edema, erosions, and ulcerations with a purplish/red discoloration. A barium enema will show spasm of the affected area with so-called “thumbprinting,” which indicates mucosal edema. The onset of symptoms is generally within 3 weeks of XRT initiation; symptoms are self-limited in most cases. Management is centered on symptom relief; loperamide and Lomotil are first-line agents for diarrheal symptoms.
Chronic XRT-related colopathy is the result of chronic tissue ischemia and fibrosis. This may lead to dysmotility resulting in abnormal bowel habits (ranging from constipation to diarrhea) or sigmoid stenosis/stricture resulting in an inability to evacuate the bowel. For the latter, it is important to note that fiber supplementation may not be optimal, since increasing the fecal caliber makes it more difficult to pass through the stenotic, colonic segment.
Emollients such as small doses of mineral oil will not increase the fecal caliber, but will soften fecal matter so that it can be passed with greater ease. MiraLAX may be effective, as well, but can increase the sense of urgency and contribute to incontinence in some. Lactulose can be effective, but it causes excessive gassiness/bloating that may result in abdominal pain and episodes of incontinence.
Bleeding from telangiectasias is another chronic complication of XRT-related colonic injury. Argon plasma coagulation (APC) via flexible sigmoidoscopy or colonoscopy is typically the primary therapeutic approach, reported to have a success rate of up to 90% in healthy tissues.33,35 Even with endoscopic treatment, as mentioned earlier in the context of small bowel XRT-related telangiectasias, iron supplementation is often needed to replete stores, and choice of iron agent is important.
Furthermore, it is essential to recognize that repeat endoscopic sessions may be needed to fully treat telangiectasias, and recrudescence of bleeding months or years later should raise suspicion for recurrent telangiectasia formation (and need for repeat treatment). As with other organs, there may be a role for hyperbaric oxygen, even in difficult-to-treat cases.36,37
Colonic fibrosis/stenosis and fistulous tract formation, as in the small bowel, are also seen in this population of patients. Endoscopic dilation can be considered, and stenting may be reasonable for short and/or distal strictures. Surgical approaches for fistulous tracts and strictures can be high-risk and associated with poor outcomes, mostly because of the underlying chronic tissue ischemia and fibrosis,4,8,27,30,34 as discussed in the small bowel section.
Rectum
The rectum has tolerance to XRT similar to the colon,38 but because of its anatomical location, rectal radiation injury is more common, and is typically seen after XRT for prostate, bladder, cervical, or uterine cancer. Acute rectal radiation injury is seen in 50% to 78% of patients,36 and symptoms are similar to that of injury to the sigmoid (eg, tenesmus, loose evacuations, hematochezia), all of which are consequences of direct radiation injury to the mucosa.
Chronic rectal radiation injury may present in a variety of ways. Tenesmus and incontinence are seen in 8% to 20% of patients, frequent defecation in 50%, urgency in 47%, and rectal cancer in up to 2% to 3% after 10 years.36,37 Other complications include anorectal strictures, fissures, fistulae, and bleeding from rectal telangiectasias. While anoscopy can diagnose many of these, flexible sigmoidoscopy is needed to examine more proximal rectal sites as well as for treatment. Treatment of these chronic complications of XRT is analogous to those of the colon7 with the following exceptions:
- Anorectal strictures. In contrast to sigmoid strictures, these are generally more amenable to dilatation. If symptoms recur frequently, patients may be instructed on self-dilatations at home.
- Bleeding from rectal telangiectasias. In the rare cases where endoscopic APC is not feasible or successful, an alternative treatment would be radiofrequency ablation or the application of 2% to 10% formalin intra-rectally. This is reported to have up to a 93% success rate;37 however, because formalin can also cause rectal pain, spasm, ulcerations, or stenosis, it is not a first-line therapy.
- Tenesmus, urgency, and incontinence. These represent a therapeutic challenge, often with no satisfactory outcomes. An array of empiric treatments may be used for symptomatic relief, including but not limited to, a trial of loperamide or fiber supplementation, which may be helpful for frequent evacuation.
- Fistulous tracts associated with rectal radiation. Endoscopic clip closure of XRT-related and other fistulous tracts is an option. This has been attempted via a variety of techniques, but results depend on the size and location of the fistulous tract, as well as other characteristics of the fistula and its surrounding tissue.7,38,39 Use of mesenchymal stem cells has also been described for rectal and other fistulae,40 but its indications have yet to be elucidated, and current use is mostly experimental.
CASE The patient’s recent-onset symptoms and clinical history were most suggestive of radiation proctopathy; a shared decision was made to pursue endoscopic evaluation with possible therapeutic intervention.
Given that data were not available about the quality of the colon preparation during the exam 7 years earlier, and to rule out a more proximal colonic lesion, the patient was scheduled for colonoscopy. This revealed numerous telangiectasias and moderate friability involving the distal third of the rectum, consistent with radiation proctopathy. The telangiectasias were treated with APC. Follow-up flexible sigmoidoscopy 2 months later showed a few remaining scattered telangiectasias, which were also treated with APC.
The patient has been clinically well, without evidence of bleeding for 6 months and with resolution of anemia.
CORRESPONDENCE
James H. Tabibian, Division of Gastroenterology, Department of Medicine, 14445 Olive View Dr., 2B-182, Sylmar, CA 91342; JTabibian@dhs.lacounty.gov.
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
1. Walsh D. Deep tissue traumatism from roentgen ray exposure. Brit Med J. 1897;2:272-273.
2. Paravati AJ, Boero IJ, Triplett DP, et al. Variation in the cost of radiation therapy among Medicare patients with cancer. J Oncol Pract. 2015;11:403-409.
3. Leung HWC, Chan ALF. Direct medical cost of radiation therapy for cancer patients in Taiwan. SciRes. 2013;5:989-993.
4. Andreyev HJ. GI consequences of cancer treatment: a clinical perspective. Radiat Res. 2016;185:341-348.
5. Olopade FA, Norman A, Blake P, et al. A modified inflammatory bowel disease questionnaire and the Vaizey incontinence questionnaire are simple ways to identify patients with significant gastrointestinal symptoms after pelvic radiotherapy. Br J Cancer. 2005;92:1663-1670.
6. Lawrie TA, Kulier R, Nardin JM. Techniques for the interruption of tubal patency for female sterilization. Cochrane Database Syst Rev. 2016 Aug 5;8:CD003034.
7. ASGE. The role of endoscopy in patients with anorectal disorders. Gastrointest Endosc. 2010;72:1117-1123.
8. Stacey R, Green JT. Radiation-induced small bowel disease: latest developments and clinical guidance. Ther Adv Chronic Dis. 2014:5:15-29.
9. Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136-143.
10. Theiss VS, Sripadam R, Ramani V, et al. Chronic radiation enteritis. Clin Oncol (R Coll Radiol). 2010;22:70-83.
11. DeCosse JJ, Rhodes RS, Wentz WB, et al. The natural history of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369-384.
12. Shadad AK, Sullivan FJ, Martin JD, et al. Gastrointestinal radiation injury: symptoms, risk factors and mechanisms. World J Gastroenterol. 2013;19:185-198.
13. Tabibian N, Swehli E, Boyd A, et al. Abdominal adhesions: a practical review of an often overlooked entity. Am Med Surg (Lond). 2017;15:9-13.
14. Baumann J, Lin M, Patel C. An unusual case of gastritis and duodenitis after yttrium 90-microsphere selective internal radiation. Clin Gastroenterol Hepatol. 2015;13:xxiii-xxiv.
15. Bennett MH, Feldmeier J, Hampson NB, et al. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016 Apr 28;4:CD005005.
16. Berbée M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care. 2012;6:54-59.
17. Marshall GT, Thirlby RC, Bredfelt JE, et al. Treatment of gastrointestinal radiation injury with hyperbaric oxygen. Undersea Hyperb Med. 2007;34:35-42.
18. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. 2013;7:467-473.
19. Chowhan NM. Injurious effects of radiation on the esophagus. Am J Gastroenterol. 1990;85:115-120.
20. Vanagunas A, Jacob P, Olinger E. Radiation-induced esophageal injury: a spectrum from esophagitis to cancer. Am J Gastroenterol. 1990;85:808-812.
21. Agarwalla A, Small AJ, Mendelson AH, et al. Risk of recurrent or refractory strictures and outcome of endoscopic dilation for radiation-induced esophageal strictures. Surg Endosc. 2015;29:1903-1912.
22. Kaasa S, Mastekaasa A, Thorud E. Toxicity, physical function and everyday activity reported by patients with inoperable non-small cell lung cancer in a randomized trial (chemotherapy versus radiotherapy). Acta Oncol. 1988;27:343-349.
23. Maple JT, Petersen BT, Baron TH, et al. Endoscopic management of radiation-induced complete upper esophageal obstruction with an antegrade-retrograde rendezvous technique. Gastrointest Endosc. 2006;64:822-828.
24. Lewin K, Mills RR. Human radiation hepatitis. A morphologic study with emphasis on the late changes. Arch Pathol. 1973;96:21-26.
25. Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128-134.
26. Bismar MM, Sinicrope FA. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361-365.
27. Andreyev HJ, Vlavianos P, Blake P, et al. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Phys. 2005;62:1464-1471.
28. Zimmer T, Böcker U, Wang F, et al. Medical prevention and treatment of acute and chronic radiation induced enteritis—is there any proven therapy? A short review. Z Gastroenterol. 2008;46:441-448.
29. Kita H, Yamamoto H, Yano T, et al. Double balloon endoscopy in two hundred fifty cases for the diagnosis and treatment of small bowel intestinal disorders. Inflammopharmacology. 2007;15:74-77.
30. Girvent M, Carlson GL, Anderson I, et al. Intestinal failure after surgery for complicated radiation enteritis. Ann R Coll Surg Engl. 2000;82:198-201.
31. Thompson JS, DiBaise JK, Iyer KR, et al. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201:85-89.
32. Hampson NB, Holm JR, Wreford-Brown CE, et al. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860-3868.
33. Chun M, Kang S, Kil HJ, et al. Rectal bleeding and its management after irradiation for uterine cervical cancer. Int J Radiat Oncol Phys. 2004;58:98-105.
34. Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85-91.
35. Villavicencia RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70-74.
36. Dall’Era MA, Hampson NB, His RA, et al. Hyperbaric oxygen therapy for radiation-induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87-90.
37. Henson C. Chronic radiation proctitis: issues surrounding delayed bowel dysfunction post-pelvic radiotherapy and an update on medical treatment. Therap Adv Gastroenterol. 2010;3:359-365.
38. Gilinsky NH, Kottler RE. Idiopathic obstructive eosinophilic enteritis with raised IgE: response to oral disodium cromoglycate. Postgrad Med J. 1982;58:239-243.
39. Tabibian JH, Kochman ML. Over-the-wire technique to facilitate over-the-scope clip closure of fistulae. Gastrointest Endosc. 2017;85:454-455.
40. Nicolay NH, Lopez Perez R, Debus J, et al. Mesenchymal stem cells — a new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015;366:133-140.
PRACTICE RECOMMENDATIONS
› Correlate the patient’s symptoms with the radiation therapy history to determine if the onset, anatomical location, and nature of the symptoms suggest a (causal) relationship. B
› Refer patients for radiographic, endoscopic, or other diagnostic modalities according to the suspected pathology and treat (eg, pharmacologically, endoscopically, or surgically) when possible. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series