User login
Do abnormal fetal kick counts predict intrauterine death in average-risk pregnancies?
No. Structured daily monitoring of fetal movement doesn’t decrease the rate of all-cause antenatal death in average-risk pregnancies (strength of recommendation [SOR]: B, single good-quality, randomized controlled trial [RCT]). Although maternal perception of decreased fetal movement may herald fetal death, it isn’t specific for poor neonatal outcome (SOR: B, single good-quality, diagnostic cohort study). Monitoring fetal movement increases the frequency of non-stress-test monitoring (SOR: B, single good-quality RCT).
A rare tragedy that monitoring can’t prevent
Johanna Warren, MD
Oregon Health and Sciences University, Portland
Fetal movement is a marker of well-being. We draw on our experience with fetal monitoring to know that in healthy fetuses, movement increases sympathetic response and accelerates heart rate. Fetuses with severe acid-base disorders can’t oxygenate their muscles adequately and don’t move. Fetal movement, therefore, is a relatively simple indirect means of fetal assessment that indicates a lack of significant acidosis.
Intrauterine fetal demise (IUFD) is a rare but devastating event in an uncomplicated term pregnancy; it occurs in about 5000 of nearly 4 million us births each year (0.125). As the authors of this Clinical Inquiry state, nearly half of term IUFDs are unexpected and unexplained. Although it may be a logical extension to apply our knowledge of fetal physiology in an attempt to prevent IUFD, no conclusive evidence suggests that daily monitoring of fetal movement improves fetal or neonatal outcomes. We can hope that, with more accurate dating methods and more aggressive control of hypertension, diabetes, and anemia in pregnancy, the number of term IUFDs will continue to fall.
Evidence summary
Nearly 50% of late-pregnancy IUFDs have no associated risk factors. Fetal demise, however, may be heralded by decreased fetal movement followed by cessation of movement at least 12 hours before death.1 Maternal monitoring of fetal movement by kick counts has been proposed as a method to verify fetal well-being and decrease the rate of IUFD in the general obstetric population.
Counting doesn’t reduce antenatal death, large study shows
A well-done RCT randomized 68,654 women to either usual care or structured, daily monitoring of fetal movement using the count-to-10 method—daily maternal documentation of the amount of time it takes to perceive 10 fetal movements. Usual care was comprised of a query about fetal movement at antenatal visits and instruction to perform fetal movement monitoring at the provider’s discretion. Mothers were told to visit their health-care provider for evaluation if they felt no movement in 24 hours or fewer than 10 movements in 10 hours during a 48-hour period. The trial showed no benefit from monitoring in reducing the rate of antenatal death from all causes.
The rate of all fetal deaths in the counting group was 2.9 per 1000 normally formed, live, singleton births; the rate in the control group was 2.67 (absolute risk reduction=0.24; 95% confidence interval [CI], –0.5 to 0.98). Women in the counting group spent an average of 160 hours counting during pregnancy and had a statistically significant increase in fetal non-stress-test (NST) monitoring (odds ratio [OR]=1.39; 95% CI, 1.31-1.49; number needed to harm [NNH]=50 to cause 1 additional NST). A statistically insignificant trend toward increased antepartum admissions was also noted in the counting group.2
Maternal perception of less movement not linked to fetal outcome
A retrospective cohort study of 6793 patients compared pregnancy outcomes of 463 women who presented for evaluation of decreased fetal movement with outcomes among the general obstetric population. The study excluded women who reported complete cessation of fetal movement.
Pregnancies evaluated for decreased fetal movement were less likely to have an Apgar score <7 at 5 minutes (relative risk [RR]=0.56; 95% CI, 0.29-0.96; P=.05) and less likely to be preterm (RR=0.68; 95% CI, 0.48-0.94; P=.02). No significant difference in cesarean section for fetal distress or admission to the neonatal intensive care unit was noted between the study and control groups. The study suggests that maternal perception of decreased fetal movement is not associated with poor fetal outcome.3
A recent rigorous systematic review yielded no significant outcome effect related to fetal kick counts.4 A prospective cohort study of 4383 births in California, using historical controls, found a drop in fetal mortality from 8.7 to 2.1 deaths/1000. The historical control rate was higher than statewide data from the same time period, however. The overall weaker design of the study and probable effect of regression to the mean significantly limit the interpretation of outcomes.5
Recommendations
The American College of Obstetrics and Gynecology (ACOG) makes no recommendation for or against assessing daily fetal movement in routine pregnancies. ACOG notes that no consistent evidence suggests that formal assessment of fetal movement decreases IUFD.6
The Institute for Clinical Systems Improvement recommends instructing patients on “daily identification of fetal movement at the 28-week visit.” The institute doesn’t recommend specific criteria for evaluating fetal movements or offer recommendations for follow-up of a maternal report of decreased fetal movement.1 The National Institute for Clinical Excellence in Great Britain recommends against routine formal fetal-movement counting.7
1. Institute for Clinical systems Improvement. Routine Prenatal Care. 12th ed. August 2008. Available at: http://www.icsi.org/prenatal_care_4/prenatal_care__routine__full_version__2.html. Accessed november 7, 2008.
2. Grant A, Elbourne D, Valentin L, et al. Routine formal fetal movement counting and risk of antepartum late death in normally formed singletons. Lancet. 1989;2:345-349.
3. Harrington K, Thompson O, Jordan L, et al. Obstetric outcome in women who present with a reduction in fetal movements in the third trimester of pregnancy. J Perinat Med. 1998;26:77-82.
4. Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2007;(1):CD004909.-
5. Moore TR, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1989;160:1075-1080.
6. ACOG. Antepartum Fetal Surveillance. ACOG Practice Bulletin, Number 9. Washington, DC: American College of Obstetrics and Gynecology; October 1999.
7. National Institute for Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. Clinical Guideline 62. London: National Institute for Health and Clinical Excellence; March 2008.
No. Structured daily monitoring of fetal movement doesn’t decrease the rate of all-cause antenatal death in average-risk pregnancies (strength of recommendation [SOR]: B, single good-quality, randomized controlled trial [RCT]). Although maternal perception of decreased fetal movement may herald fetal death, it isn’t specific for poor neonatal outcome (SOR: B, single good-quality, diagnostic cohort study). Monitoring fetal movement increases the frequency of non-stress-test monitoring (SOR: B, single good-quality RCT).
A rare tragedy that monitoring can’t prevent
Johanna Warren, MD
Oregon Health and Sciences University, Portland
Fetal movement is a marker of well-being. We draw on our experience with fetal monitoring to know that in healthy fetuses, movement increases sympathetic response and accelerates heart rate. Fetuses with severe acid-base disorders can’t oxygenate their muscles adequately and don’t move. Fetal movement, therefore, is a relatively simple indirect means of fetal assessment that indicates a lack of significant acidosis.
Intrauterine fetal demise (IUFD) is a rare but devastating event in an uncomplicated term pregnancy; it occurs in about 5000 of nearly 4 million us births each year (0.125). As the authors of this Clinical Inquiry state, nearly half of term IUFDs are unexpected and unexplained. Although it may be a logical extension to apply our knowledge of fetal physiology in an attempt to prevent IUFD, no conclusive evidence suggests that daily monitoring of fetal movement improves fetal or neonatal outcomes. We can hope that, with more accurate dating methods and more aggressive control of hypertension, diabetes, and anemia in pregnancy, the number of term IUFDs will continue to fall.
Evidence summary
Nearly 50% of late-pregnancy IUFDs have no associated risk factors. Fetal demise, however, may be heralded by decreased fetal movement followed by cessation of movement at least 12 hours before death.1 Maternal monitoring of fetal movement by kick counts has been proposed as a method to verify fetal well-being and decrease the rate of IUFD in the general obstetric population.
Counting doesn’t reduce antenatal death, large study shows
A well-done RCT randomized 68,654 women to either usual care or structured, daily monitoring of fetal movement using the count-to-10 method—daily maternal documentation of the amount of time it takes to perceive 10 fetal movements. Usual care was comprised of a query about fetal movement at antenatal visits and instruction to perform fetal movement monitoring at the provider’s discretion. Mothers were told to visit their health-care provider for evaluation if they felt no movement in 24 hours or fewer than 10 movements in 10 hours during a 48-hour period. The trial showed no benefit from monitoring in reducing the rate of antenatal death from all causes.
The rate of all fetal deaths in the counting group was 2.9 per 1000 normally formed, live, singleton births; the rate in the control group was 2.67 (absolute risk reduction=0.24; 95% confidence interval [CI], –0.5 to 0.98). Women in the counting group spent an average of 160 hours counting during pregnancy and had a statistically significant increase in fetal non-stress-test (NST) monitoring (odds ratio [OR]=1.39; 95% CI, 1.31-1.49; number needed to harm [NNH]=50 to cause 1 additional NST). A statistically insignificant trend toward increased antepartum admissions was also noted in the counting group.2
Maternal perception of less movement not linked to fetal outcome
A retrospective cohort study of 6793 patients compared pregnancy outcomes of 463 women who presented for evaluation of decreased fetal movement with outcomes among the general obstetric population. The study excluded women who reported complete cessation of fetal movement.
Pregnancies evaluated for decreased fetal movement were less likely to have an Apgar score <7 at 5 minutes (relative risk [RR]=0.56; 95% CI, 0.29-0.96; P=.05) and less likely to be preterm (RR=0.68; 95% CI, 0.48-0.94; P=.02). No significant difference in cesarean section for fetal distress or admission to the neonatal intensive care unit was noted between the study and control groups. The study suggests that maternal perception of decreased fetal movement is not associated with poor fetal outcome.3
A recent rigorous systematic review yielded no significant outcome effect related to fetal kick counts.4 A prospective cohort study of 4383 births in California, using historical controls, found a drop in fetal mortality from 8.7 to 2.1 deaths/1000. The historical control rate was higher than statewide data from the same time period, however. The overall weaker design of the study and probable effect of regression to the mean significantly limit the interpretation of outcomes.5
Recommendations
The American College of Obstetrics and Gynecology (ACOG) makes no recommendation for or against assessing daily fetal movement in routine pregnancies. ACOG notes that no consistent evidence suggests that formal assessment of fetal movement decreases IUFD.6
The Institute for Clinical Systems Improvement recommends instructing patients on “daily identification of fetal movement at the 28-week visit.” The institute doesn’t recommend specific criteria for evaluating fetal movements or offer recommendations for follow-up of a maternal report of decreased fetal movement.1 The National Institute for Clinical Excellence in Great Britain recommends against routine formal fetal-movement counting.7
No. Structured daily monitoring of fetal movement doesn’t decrease the rate of all-cause antenatal death in average-risk pregnancies (strength of recommendation [SOR]: B, single good-quality, randomized controlled trial [RCT]). Although maternal perception of decreased fetal movement may herald fetal death, it isn’t specific for poor neonatal outcome (SOR: B, single good-quality, diagnostic cohort study). Monitoring fetal movement increases the frequency of non-stress-test monitoring (SOR: B, single good-quality RCT).
A rare tragedy that monitoring can’t prevent
Johanna Warren, MD
Oregon Health and Sciences University, Portland
Fetal movement is a marker of well-being. We draw on our experience with fetal monitoring to know that in healthy fetuses, movement increases sympathetic response and accelerates heart rate. Fetuses with severe acid-base disorders can’t oxygenate their muscles adequately and don’t move. Fetal movement, therefore, is a relatively simple indirect means of fetal assessment that indicates a lack of significant acidosis.
Intrauterine fetal demise (IUFD) is a rare but devastating event in an uncomplicated term pregnancy; it occurs in about 5000 of nearly 4 million us births each year (0.125). As the authors of this Clinical Inquiry state, nearly half of term IUFDs are unexpected and unexplained. Although it may be a logical extension to apply our knowledge of fetal physiology in an attempt to prevent IUFD, no conclusive evidence suggests that daily monitoring of fetal movement improves fetal or neonatal outcomes. We can hope that, with more accurate dating methods and more aggressive control of hypertension, diabetes, and anemia in pregnancy, the number of term IUFDs will continue to fall.
Evidence summary
Nearly 50% of late-pregnancy IUFDs have no associated risk factors. Fetal demise, however, may be heralded by decreased fetal movement followed by cessation of movement at least 12 hours before death.1 Maternal monitoring of fetal movement by kick counts has been proposed as a method to verify fetal well-being and decrease the rate of IUFD in the general obstetric population.
Counting doesn’t reduce antenatal death, large study shows
A well-done RCT randomized 68,654 women to either usual care or structured, daily monitoring of fetal movement using the count-to-10 method—daily maternal documentation of the amount of time it takes to perceive 10 fetal movements. Usual care was comprised of a query about fetal movement at antenatal visits and instruction to perform fetal movement monitoring at the provider’s discretion. Mothers were told to visit their health-care provider for evaluation if they felt no movement in 24 hours or fewer than 10 movements in 10 hours during a 48-hour period. The trial showed no benefit from monitoring in reducing the rate of antenatal death from all causes.
The rate of all fetal deaths in the counting group was 2.9 per 1000 normally formed, live, singleton births; the rate in the control group was 2.67 (absolute risk reduction=0.24; 95% confidence interval [CI], –0.5 to 0.98). Women in the counting group spent an average of 160 hours counting during pregnancy and had a statistically significant increase in fetal non-stress-test (NST) monitoring (odds ratio [OR]=1.39; 95% CI, 1.31-1.49; number needed to harm [NNH]=50 to cause 1 additional NST). A statistically insignificant trend toward increased antepartum admissions was also noted in the counting group.2
Maternal perception of less movement not linked to fetal outcome
A retrospective cohort study of 6793 patients compared pregnancy outcomes of 463 women who presented for evaluation of decreased fetal movement with outcomes among the general obstetric population. The study excluded women who reported complete cessation of fetal movement.
Pregnancies evaluated for decreased fetal movement were less likely to have an Apgar score <7 at 5 minutes (relative risk [RR]=0.56; 95% CI, 0.29-0.96; P=.05) and less likely to be preterm (RR=0.68; 95% CI, 0.48-0.94; P=.02). No significant difference in cesarean section for fetal distress or admission to the neonatal intensive care unit was noted between the study and control groups. The study suggests that maternal perception of decreased fetal movement is not associated with poor fetal outcome.3
A recent rigorous systematic review yielded no significant outcome effect related to fetal kick counts.4 A prospective cohort study of 4383 births in California, using historical controls, found a drop in fetal mortality from 8.7 to 2.1 deaths/1000. The historical control rate was higher than statewide data from the same time period, however. The overall weaker design of the study and probable effect of regression to the mean significantly limit the interpretation of outcomes.5
Recommendations
The American College of Obstetrics and Gynecology (ACOG) makes no recommendation for or against assessing daily fetal movement in routine pregnancies. ACOG notes that no consistent evidence suggests that formal assessment of fetal movement decreases IUFD.6
The Institute for Clinical Systems Improvement recommends instructing patients on “daily identification of fetal movement at the 28-week visit.” The institute doesn’t recommend specific criteria for evaluating fetal movements or offer recommendations for follow-up of a maternal report of decreased fetal movement.1 The National Institute for Clinical Excellence in Great Britain recommends against routine formal fetal-movement counting.7
1. Institute for Clinical systems Improvement. Routine Prenatal Care. 12th ed. August 2008. Available at: http://www.icsi.org/prenatal_care_4/prenatal_care__routine__full_version__2.html. Accessed november 7, 2008.
2. Grant A, Elbourne D, Valentin L, et al. Routine formal fetal movement counting and risk of antepartum late death in normally formed singletons. Lancet. 1989;2:345-349.
3. Harrington K, Thompson O, Jordan L, et al. Obstetric outcome in women who present with a reduction in fetal movements in the third trimester of pregnancy. J Perinat Med. 1998;26:77-82.
4. Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2007;(1):CD004909.-
5. Moore TR, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1989;160:1075-1080.
6. ACOG. Antepartum Fetal Surveillance. ACOG Practice Bulletin, Number 9. Washington, DC: American College of Obstetrics and Gynecology; October 1999.
7. National Institute for Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. Clinical Guideline 62. London: National Institute for Health and Clinical Excellence; March 2008.
1. Institute for Clinical systems Improvement. Routine Prenatal Care. 12th ed. August 2008. Available at: http://www.icsi.org/prenatal_care_4/prenatal_care__routine__full_version__2.html. Accessed november 7, 2008.
2. Grant A, Elbourne D, Valentin L, et al. Routine formal fetal movement counting and risk of antepartum late death in normally formed singletons. Lancet. 1989;2:345-349.
3. Harrington K, Thompson O, Jordan L, et al. Obstetric outcome in women who present with a reduction in fetal movements in the third trimester of pregnancy. J Perinat Med. 1998;26:77-82.
4. Mangesi L, Hofmeyr GJ. Fetal movement counting for assessment of fetal wellbeing. Cochrane Database Syst Rev. 2007;(1):CD004909.-
5. Moore TR, Piacquadio K. A prospective evaluation of fetal movement screening to reduce the incidence of antepartum fetal death. Am J Obstet Gynecol. 1989;160:1075-1080.
6. ACOG. Antepartum Fetal Surveillance. ACOG Practice Bulletin, Number 9. Washington, DC: American College of Obstetrics and Gynecology; October 1999.
7. National Institute for Clinical Excellence. Antenatal Care: Routine Care for the Healthy Pregnant Woman. Clinical Guideline 62. London: National Institute for Health and Clinical Excellence; March 2008.
Evidence-based answers from the Family Physicians Inquiries Network
Hyperpigmentation and vesicles after beach vacation
A 21-year-old woman traveled to Florida for spring break, accompanied by her fiancé and friends. While there, she developed a raised, pruritic 10-cm×30-cm lesion on her right lateral chest wall. A local pharmacist thought she was having an allergic reaction and recommended topical hydrocortisone cream and oral diphenhydramine (Benadryl). In spite of this therapy the rash progressed.
Five days after returning home, she presented to the emergency department, where she was diagnosed with herpes zoster. The rash was described as multiple vesicles in a dermatomal distribution on the right side of her torso. She was started on famciclovir (Famvir) and given a prescription for hydrocodone with acetaminophen (Vicodin).
Four days later she presented to her primary care physician because she continued to develop areas of hyperpigmentation around her mouth, on the chin, upper chest and breasts, thighs, and forearms. The original lesion on the right lateral thorax had dried, crusted, and was beginning to peel. It was no longer pruritic. The rash had never been painful and she had not taken any hydrocodone. She was most concerned about the disfigurement caused by the rash and wondered if she was having a reaction to the famciclovir. She had suffered no injuries or trauma, and did not engage in substance abuse.
While in Florida she did sun on the beach with friends, but she used sunblock regularly. She had used the same skin products and sunblock for many years. Upon questioning, she recalled drinking citrus beverages while out in the sun. Her history was otherwise unremarkable. Her only prescription medication before onset of the rash was montelukast (Singulair) prescribed for allergic rhinitis.
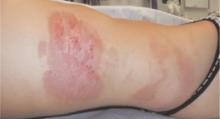
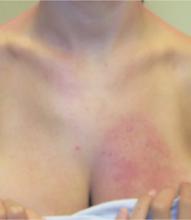
Examination revealed a bizarre pattern of hyperpigmentation with small punctate lesions on the upper chest, linear lesions on the posterior right arm and thighs, and broad areas of darkened skin on the lower face and chin (FIGURE 1). There was a broad patch of dense hyperpigmentation with desquamation on that right lateral chest wall that appeared to be healing (FIGURE 2).
FIGURE 1
Vesicles on the trunk…
FIGURE 2
…and anterior chest
What is your diagnosis?
Diagnosis: Phytophotodermatitis
This patient has phytophotodermatitis, a phototoxic dermatologic reaction that occurs with exposure to ultraviolet light after contact with certain plants. Hyperpigmentation and vesicle or bullae formation are hallmarks of the process.
The sun-sensitizing chemicals are furocoumarins, found in many plants (TABLE).1 Psoralen, the same substance used in PUVA treatments, is one of the furocoumarins. These agents contact the skin when juice is released from the fruit or stem or by direct contact with leaves.2
This patient remembered eating fresh limes on the beach and posing for a photograph next to her fiancé, when he placed his right hand around her back and on her right thorax (FIGURE 1). Limes have a high concentration of psoralens and are often the culprit in phytophotodermatitis.1,3 It is therefore incumbent on the clinician to know this and ask about lime and plant exposure when suspecting this diagnosis.
Erythema typically develops within 24 hours of sun exposure and contact with furocoumarin-containing plants. Vesicles or bulla may develop within 72 hours. Hyperpigmentation occurs over 1 to 2 weeks and can persist for 6 to 12 months. Hyperpigmentation can appear in bizarre streaks or patterns depending on where the chemicals contacted sun-exposed skin. Areas that have formed bulla typically exfoliate within 10 to 14 days.3
TABLE
Common furocoumarin-containing plants1
| Bitter orange | Lime |
| Carrot | Parsley |
| Celery | Parsnip |
| Fig |
Differential diagnosis
The differential diagnosis for this process includes herpes zoster, atopic dermatitis, allergic contact dermatitis, porphyria cutanea tarda, chemical or thermal burn, and abuse.2,4 While chemical or thermal burn and abuse are suspected with the appropriate history, the remainder of the diagnoses have defining characteristics allowing for proper identification.
Herpes zoster is caused by the reactivation of latent varicella zoster virus. Itching or burning precedes cutaneous eruption of red swollen plaques with vesicles of various sizes, which umbilicate or rupture. These lesions occur in a discreet dermatomal distribution.5
Atopic dermatitis has poorly understood pathophysiology. It typically starts in infancy and causes pruritus, eczematous lesions, xerosis, and lichenification of the skin.5
Allergic contact dermatitis is a delayed hypersensitivity reaction that requires prior sensitization. It is classically described as pruritic papules on an erythematous base.5
Porphyria cutanea tarda is a caused by an inborn enzyme deficiency in the heme biosynthetic pathway that results in mechanical fragility, subepidermal bullae, hypertrichosis, and pigmentation.5
Pathophysiology of phytophotodermatitis
In the presence of ultraviolet A (UVA) irradiation, psoralens cause DNA cross-linkage in the skin. This stimulates increased melanin production with resultant hyper-pigmentation. It may also cause the development of radicals, which damage cell membranes and intracellular contents, resulting in edema, erythema, and the formation of vesicles or bullae.3
Several factors have been implicated in the severity of the phototoxic reaction. Increased humidity, concentration of furocoumarins in plant juice, and UVA intensity all worsened the severity of reactions in one study. The concentration of furocoumarin is variable between species and between different parts of the same plant.2
Diagnosis and treatment
Consider phytophotodermatitis when patients present with hyperpigmentation, often in bizarre streaks on sun-exposed areas, or with vesicles in a nondermatomal distribution. It is a clinical diagnosis based on history and physical examination. Clues in this patient included recent vacation to Florida and consumption of citrus beverages along with the bizarre pattern of rash and sparing of the skin of the upper chest covered by her bathing suit. Any laboratory data obtained are used to exclude other diseases in the differential diagnosis. For example, porphyrin levels may be obtained to rule out porphyria cutanea tarda. If the clinical diagnosis remains in question, a skin biopsy may be performed to confirm the diagnosis.
Treatment involves use of topical steroids such as triamcinolone 0.1% cream applied to the skin two to three times per day, and the application of cool compresses for comfort. Advise patients to use sunscreen to prevent further hyperpigmentation. A topical bleaching agent such as hydroquinone may help reduce hyperpigmentation, although most lesions fade with time1 (strength of recommendation: C, based on usual practice or opinion).
CORRESPONDENCE
Andrea L. Darby-Stewart, MD, Assistant Professor of Family Medicine, Mayo Thunderbird Family Medicine, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: darbystewart.andrea@mayo.edu
1. Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other “lime” disease. J Emer Med 1999;17:235-237.
2. Knudson EA, Kroon S. In vitro and in vivo phototoxicity of furocoumarin-containing plants. Clin Exp Dermatol 1988;13:92-96.
3. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furocoumarins in limes. Am J Contact Dermatitis 2002;13:10-14.
4. Solis RR, Dotson DA, Trizna Z. Phytophotodermatitis: a sometimes difficult diagnosis. Arch Fam Med 2000;9:1195-1196.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St Louis, Mo: Mosby; 2004.
A 21-year-old woman traveled to Florida for spring break, accompanied by her fiancé and friends. While there, she developed a raised, pruritic 10-cm×30-cm lesion on her right lateral chest wall. A local pharmacist thought she was having an allergic reaction and recommended topical hydrocortisone cream and oral diphenhydramine (Benadryl). In spite of this therapy the rash progressed.
Five days after returning home, she presented to the emergency department, where she was diagnosed with herpes zoster. The rash was described as multiple vesicles in a dermatomal distribution on the right side of her torso. She was started on famciclovir (Famvir) and given a prescription for hydrocodone with acetaminophen (Vicodin).
Four days later she presented to her primary care physician because she continued to develop areas of hyperpigmentation around her mouth, on the chin, upper chest and breasts, thighs, and forearms. The original lesion on the right lateral thorax had dried, crusted, and was beginning to peel. It was no longer pruritic. The rash had never been painful and she had not taken any hydrocodone. She was most concerned about the disfigurement caused by the rash and wondered if she was having a reaction to the famciclovir. She had suffered no injuries or trauma, and did not engage in substance abuse.
While in Florida she did sun on the beach with friends, but she used sunblock regularly. She had used the same skin products and sunblock for many years. Upon questioning, she recalled drinking citrus beverages while out in the sun. Her history was otherwise unremarkable. Her only prescription medication before onset of the rash was montelukast (Singulair) prescribed for allergic rhinitis.
Examination revealed a bizarre pattern of hyperpigmentation with small punctate lesions on the upper chest, linear lesions on the posterior right arm and thighs, and broad areas of darkened skin on the lower face and chin (FIGURE 1). There was a broad patch of dense hyperpigmentation with desquamation on that right lateral chest wall that appeared to be healing (FIGURE 2).
FIGURE 1
Vesicles on the trunk…
FIGURE 2
…and anterior chest
What is your diagnosis?
Diagnosis: Phytophotodermatitis
This patient has phytophotodermatitis, a phototoxic dermatologic reaction that occurs with exposure to ultraviolet light after contact with certain plants. Hyperpigmentation and vesicle or bullae formation are hallmarks of the process.
The sun-sensitizing chemicals are furocoumarins, found in many plants (TABLE).1 Psoralen, the same substance used in PUVA treatments, is one of the furocoumarins. These agents contact the skin when juice is released from the fruit or stem or by direct contact with leaves.2
This patient remembered eating fresh limes on the beach and posing for a photograph next to her fiancé, when he placed his right hand around her back and on her right thorax (FIGURE 1). Limes have a high concentration of psoralens and are often the culprit in phytophotodermatitis.1,3 It is therefore incumbent on the clinician to know this and ask about lime and plant exposure when suspecting this diagnosis.
Erythema typically develops within 24 hours of sun exposure and contact with furocoumarin-containing plants. Vesicles or bulla may develop within 72 hours. Hyperpigmentation occurs over 1 to 2 weeks and can persist for 6 to 12 months. Hyperpigmentation can appear in bizarre streaks or patterns depending on where the chemicals contacted sun-exposed skin. Areas that have formed bulla typically exfoliate within 10 to 14 days.3
TABLE
Common furocoumarin-containing plants1
| Bitter orange | Lime |
| Carrot | Parsley |
| Celery | Parsnip |
| Fig |
Differential diagnosis
The differential diagnosis for this process includes herpes zoster, atopic dermatitis, allergic contact dermatitis, porphyria cutanea tarda, chemical or thermal burn, and abuse.2,4 While chemical or thermal burn and abuse are suspected with the appropriate history, the remainder of the diagnoses have defining characteristics allowing for proper identification.
Herpes zoster is caused by the reactivation of latent varicella zoster virus. Itching or burning precedes cutaneous eruption of red swollen plaques with vesicles of various sizes, which umbilicate or rupture. These lesions occur in a discreet dermatomal distribution.5
Atopic dermatitis has poorly understood pathophysiology. It typically starts in infancy and causes pruritus, eczematous lesions, xerosis, and lichenification of the skin.5
Allergic contact dermatitis is a delayed hypersensitivity reaction that requires prior sensitization. It is classically described as pruritic papules on an erythematous base.5
Porphyria cutanea tarda is a caused by an inborn enzyme deficiency in the heme biosynthetic pathway that results in mechanical fragility, subepidermal bullae, hypertrichosis, and pigmentation.5
Pathophysiology of phytophotodermatitis
In the presence of ultraviolet A (UVA) irradiation, psoralens cause DNA cross-linkage in the skin. This stimulates increased melanin production with resultant hyper-pigmentation. It may also cause the development of radicals, which damage cell membranes and intracellular contents, resulting in edema, erythema, and the formation of vesicles or bullae.3
Several factors have been implicated in the severity of the phototoxic reaction. Increased humidity, concentration of furocoumarins in plant juice, and UVA intensity all worsened the severity of reactions in one study. The concentration of furocoumarin is variable between species and between different parts of the same plant.2
Diagnosis and treatment
Consider phytophotodermatitis when patients present with hyperpigmentation, often in bizarre streaks on sun-exposed areas, or with vesicles in a nondermatomal distribution. It is a clinical diagnosis based on history and physical examination. Clues in this patient included recent vacation to Florida and consumption of citrus beverages along with the bizarre pattern of rash and sparing of the skin of the upper chest covered by her bathing suit. Any laboratory data obtained are used to exclude other diseases in the differential diagnosis. For example, porphyrin levels may be obtained to rule out porphyria cutanea tarda. If the clinical diagnosis remains in question, a skin biopsy may be performed to confirm the diagnosis.
Treatment involves use of topical steroids such as triamcinolone 0.1% cream applied to the skin two to three times per day, and the application of cool compresses for comfort. Advise patients to use sunscreen to prevent further hyperpigmentation. A topical bleaching agent such as hydroquinone may help reduce hyperpigmentation, although most lesions fade with time1 (strength of recommendation: C, based on usual practice or opinion).
CORRESPONDENCE
Andrea L. Darby-Stewart, MD, Assistant Professor of Family Medicine, Mayo Thunderbird Family Medicine, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: darbystewart.andrea@mayo.edu
A 21-year-old woman traveled to Florida for spring break, accompanied by her fiancé and friends. While there, she developed a raised, pruritic 10-cm×30-cm lesion on her right lateral chest wall. A local pharmacist thought she was having an allergic reaction and recommended topical hydrocortisone cream and oral diphenhydramine (Benadryl). In spite of this therapy the rash progressed.
Five days after returning home, she presented to the emergency department, where she was diagnosed with herpes zoster. The rash was described as multiple vesicles in a dermatomal distribution on the right side of her torso. She was started on famciclovir (Famvir) and given a prescription for hydrocodone with acetaminophen (Vicodin).
Four days later she presented to her primary care physician because she continued to develop areas of hyperpigmentation around her mouth, on the chin, upper chest and breasts, thighs, and forearms. The original lesion on the right lateral thorax had dried, crusted, and was beginning to peel. It was no longer pruritic. The rash had never been painful and she had not taken any hydrocodone. She was most concerned about the disfigurement caused by the rash and wondered if she was having a reaction to the famciclovir. She had suffered no injuries or trauma, and did not engage in substance abuse.
While in Florida she did sun on the beach with friends, but she used sunblock regularly. She had used the same skin products and sunblock for many years. Upon questioning, she recalled drinking citrus beverages while out in the sun. Her history was otherwise unremarkable. Her only prescription medication before onset of the rash was montelukast (Singulair) prescribed for allergic rhinitis.
Examination revealed a bizarre pattern of hyperpigmentation with small punctate lesions on the upper chest, linear lesions on the posterior right arm and thighs, and broad areas of darkened skin on the lower face and chin (FIGURE 1). There was a broad patch of dense hyperpigmentation with desquamation on that right lateral chest wall that appeared to be healing (FIGURE 2).
FIGURE 1
Vesicles on the trunk…
FIGURE 2
…and anterior chest
What is your diagnosis?
Diagnosis: Phytophotodermatitis
This patient has phytophotodermatitis, a phototoxic dermatologic reaction that occurs with exposure to ultraviolet light after contact with certain plants. Hyperpigmentation and vesicle or bullae formation are hallmarks of the process.
The sun-sensitizing chemicals are furocoumarins, found in many plants (TABLE).1 Psoralen, the same substance used in PUVA treatments, is one of the furocoumarins. These agents contact the skin when juice is released from the fruit or stem or by direct contact with leaves.2
This patient remembered eating fresh limes on the beach and posing for a photograph next to her fiancé, when he placed his right hand around her back and on her right thorax (FIGURE 1). Limes have a high concentration of psoralens and are often the culprit in phytophotodermatitis.1,3 It is therefore incumbent on the clinician to know this and ask about lime and plant exposure when suspecting this diagnosis.
Erythema typically develops within 24 hours of sun exposure and contact with furocoumarin-containing plants. Vesicles or bulla may develop within 72 hours. Hyperpigmentation occurs over 1 to 2 weeks and can persist for 6 to 12 months. Hyperpigmentation can appear in bizarre streaks or patterns depending on where the chemicals contacted sun-exposed skin. Areas that have formed bulla typically exfoliate within 10 to 14 days.3
TABLE
Common furocoumarin-containing plants1
| Bitter orange | Lime |
| Carrot | Parsley |
| Celery | Parsnip |
| Fig |
Differential diagnosis
The differential diagnosis for this process includes herpes zoster, atopic dermatitis, allergic contact dermatitis, porphyria cutanea tarda, chemical or thermal burn, and abuse.2,4 While chemical or thermal burn and abuse are suspected with the appropriate history, the remainder of the diagnoses have defining characteristics allowing for proper identification.
Herpes zoster is caused by the reactivation of latent varicella zoster virus. Itching or burning precedes cutaneous eruption of red swollen plaques with vesicles of various sizes, which umbilicate or rupture. These lesions occur in a discreet dermatomal distribution.5
Atopic dermatitis has poorly understood pathophysiology. It typically starts in infancy and causes pruritus, eczematous lesions, xerosis, and lichenification of the skin.5
Allergic contact dermatitis is a delayed hypersensitivity reaction that requires prior sensitization. It is classically described as pruritic papules on an erythematous base.5
Porphyria cutanea tarda is a caused by an inborn enzyme deficiency in the heme biosynthetic pathway that results in mechanical fragility, subepidermal bullae, hypertrichosis, and pigmentation.5
Pathophysiology of phytophotodermatitis
In the presence of ultraviolet A (UVA) irradiation, psoralens cause DNA cross-linkage in the skin. This stimulates increased melanin production with resultant hyper-pigmentation. It may also cause the development of radicals, which damage cell membranes and intracellular contents, resulting in edema, erythema, and the formation of vesicles or bullae.3
Several factors have been implicated in the severity of the phototoxic reaction. Increased humidity, concentration of furocoumarins in plant juice, and UVA intensity all worsened the severity of reactions in one study. The concentration of furocoumarin is variable between species and between different parts of the same plant.2
Diagnosis and treatment
Consider phytophotodermatitis when patients present with hyperpigmentation, often in bizarre streaks on sun-exposed areas, or with vesicles in a nondermatomal distribution. It is a clinical diagnosis based on history and physical examination. Clues in this patient included recent vacation to Florida and consumption of citrus beverages along with the bizarre pattern of rash and sparing of the skin of the upper chest covered by her bathing suit. Any laboratory data obtained are used to exclude other diseases in the differential diagnosis. For example, porphyrin levels may be obtained to rule out porphyria cutanea tarda. If the clinical diagnosis remains in question, a skin biopsy may be performed to confirm the diagnosis.
Treatment involves use of topical steroids such as triamcinolone 0.1% cream applied to the skin two to three times per day, and the application of cool compresses for comfort. Advise patients to use sunscreen to prevent further hyperpigmentation. A topical bleaching agent such as hydroquinone may help reduce hyperpigmentation, although most lesions fade with time1 (strength of recommendation: C, based on usual practice or opinion).
CORRESPONDENCE
Andrea L. Darby-Stewart, MD, Assistant Professor of Family Medicine, Mayo Thunderbird Family Medicine, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: darbystewart.andrea@mayo.edu
1. Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other “lime” disease. J Emer Med 1999;17:235-237.
2. Knudson EA, Kroon S. In vitro and in vivo phototoxicity of furocoumarin-containing plants. Clin Exp Dermatol 1988;13:92-96.
3. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furocoumarins in limes. Am J Contact Dermatitis 2002;13:10-14.
4. Solis RR, Dotson DA, Trizna Z. Phytophotodermatitis: a sometimes difficult diagnosis. Arch Fam Med 2000;9:1195-1196.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St Louis, Mo: Mosby; 2004.
1. Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other “lime” disease. J Emer Med 1999;17:235-237.
2. Knudson EA, Kroon S. In vitro and in vivo phototoxicity of furocoumarin-containing plants. Clin Exp Dermatol 1988;13:92-96.
3. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furocoumarins in limes. Am J Contact Dermatitis 2002;13:10-14.
4. Solis RR, Dotson DA, Trizna Z. Phytophotodermatitis: a sometimes difficult diagnosis. Arch Fam Med 2000;9:1195-1196.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St Louis, Mo: Mosby; 2004.