User login
How to identify and manage cesarean-scar pregnancy
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
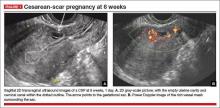
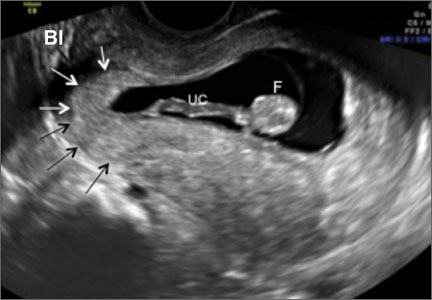
- empty uterine cavity and cervical canal (FIGURE 1A)
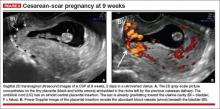
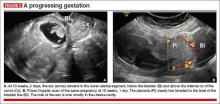
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
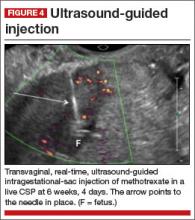
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
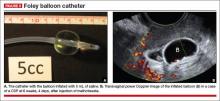
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
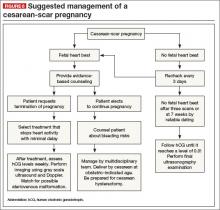
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: rbarbieri@frontlinemedcom.com Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: rbarbieri@frontlinemedcom.com Please include the city and state in which you practice.
Few ObGyn clinicians have faced a patient with a cesarean-scar pregnancy (CSP). Those few were confronted with a management dilemma. Continue the gestation, which would expose the mother to an elevated risk of heavy bleeding? Or terminate the pregnancy? And if termination is the patient’s choice, what is the most effective method?
The literature contains more than 750 reports of CSP, ranging from a single sporadic case to a series of one to two dozen cases. It is impossible to make sense of the numerous treatments used in the past, which were “tested” on extremely small numbers of patients (sometimes as few as one). In this article, we formulate a management plan for the diagnosis and treatment of CSP based on an in-depth review of the published literature and our personal experience in treating more than four dozen patients with CSP.
We’re all familiar with the “epidemic” of cesarean deliveries in this country, including late consequences of cesarean such as placenta previa and morbidly adherent placenta. One of the long-term consequences of cesarean delivery—the first-trimester CSP—is less well known and documented.
Our in-depth review of 751 CSP cases found no less than 30 published therapeutic approaches.1 No consensus exists as to management guidelines. We have formulated this clinical guide, based on the literature and our experience managing CSP, for clinicians who encounter this dangerous form of pregnancy.2
DIAGNOSIS REQUIRES TRANSVAGINAL SONOGRAPHY
Transvaginal sonography (TVS) is thought to be the best and first-line diagnostic tool, with magnetic resonance imaging (MRI) reserved for cases in which there is a diagnostic problem.
In making a diagnosis, consider two main differential diagnoses:
- Cervical pregnancy—This type of gestation is more likely to occur in women with no history of cesarean delivery
- Spontaneous miscarriage in progress—In a number of cases, the miscarriage happened to be caught on imaging as it passed the area where the CSP usually resides. Because there is no live embryo or fetus in spontaneous miscarriage, a heartbeat cannot be documented.
Components of diagnosis by TVS
Accurate identification of CSP depends on the following sonographic criteria:
- empty uterine cavity and cervical canal (FIGURE 1A)
- close proximity of the gestational sac and the placenta to the anterior uterine surface within the scar or niche of the previous cesarean delivery (FIGURES 1B, 2A, and 2B)
- color flow signals between the posterior bladder wall and the gestation within the placenta (FIGURES 1B, 2B, and 3B)
- abundant blood flow around the gestational sac, at times morphing into an arteriovenous malformation with a high peak systolic velocity blood flow demonstrable on pulsed Doppler.
Our analysis of 751 cases of CSP found that almost a third—30%—were misdiagnosed, contributing to a large number of treatment complications. Most of these complications could have been avoided if diagnosis had been early and correct. The earlier the diagnosis, the better the outcome seemed to be. This was true even when treatment modalities with slightly higher complication rates were used in very early gestation.
Related articles:
• Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy? Andrew M. Kaunitz, MD (Examining the Evidence; February 2012)
• Can a single progesterone test distinguish viable and nonviable pregnancies accurately in women with pain or bleeding? Linda R. Chambliss, MD, MPH (Examining the Evidence; March 2013)
THOROUGH COUNSELING OF THE PATIENT IS PARAMOUNT
Once a diagnosis of CSP has been established, the patient should be counseled about her options. The presence of a live CSP requires immediate and decisive action to prevent further growth of the embryo or fetus. Literature from the past decade, particularly from the past several years, makes evidence-based counseling possible.
In general, treatment should be individualized, based on the patient’s age, number of previous cesarean deliveries, number of children, and the expertise of the clinicians managing her care. Options include:
- termination of the pregnancy
- continuation of the pregnancy with the possibility of delivering a live offspring, provided the patient understands that a morbidly adherent placenta may occur, often necessitating emergency hysterectomy.3,4
MANAGEMENT APPROACHES
Most treatment regimens and combinations thereof can be classified as one of the following:
- Surgical—requiring general anesthesia and either laparotomy with excision or hysterectomy, or laparoscopic or hysteroscopic excision followed by dilation and curettage (D&C).
- Minimally invasive—involving local injection of methotrexate or potassium chloride or systemic intervention, involving a major procedure such as uterine artery embolization in combination with a less complicated one: intramuscular injection of methotrexate in a single or a multidose regimen.
A variety of simultaneous as well as sequential combination treatments also were used. More recently, an ingenious adjunct to treatment is gaining attention: insertion and inflation of a Foley balloon catheter to prevent or tamponade bleeding.
A large number of treatments described in the literature—and their different combinations—have been reported as relatively small case series. Gynecologic surgeons generally perform D&C, laparoscopy, and hysteroscopy or laparotomy as the first-line approach. Obstetricians, radiologists, and in vitro fertilization specialists usually prefer systemic, parenteral administration of methotrexate or ultrasound-guided local methotrexate (or potassium chloride) as an injection into the gestational sac. On occasion, the help of an interventional radiologist was requested to embolize the area of the CSP through the uterine arteries.
POTENTIAL COMPLICATIONS
In our analysis of 751 cases of CSP, we used a rigorous definition of complication, which included an immediate or delayed need for a secondary treatment for blood loss exceeding 200 mL or requiring blood transfusion. If general anesthesia or major surgery was required, we classified that need as a complication.
Utilizing these criteria, we observed an overall complication rate of 44.1% (331 of 751 cases).1
Complications occurred most often when the following treatment modalities were used alone:
- single systemic dose of methotrexate
- D&C
- uterine artery embolization.
Of the 751 cases reviewed, 21.8% resulted in major surgery or interventional radiology procedures (primary or emergency). The total planned primary (nonemergency) interventions performed were 66 (8.7%), which included 3 hysterectomies, 14 laparotomies, and 49 uterine artery embolizations or ligations. There were 98 (13.0%) emergency interventions, which included 36 hysterectomies, 40 laparotomies, and 22 uterine artery embolizations or ligations.1
Related article: Eight tools for improving obstetric patient safety and unit performance. Henry M. Lerner, MD (Professional Liability; March 2014)
NINE TREATMENTS AND THEIR COMPLICATIONS
1. Systemic, single-dose methotrexate
The usual protocols were 1 mg/kg of body weight or 50 mg/m2 of body surface area. This treatment was associated with a complication rate of 64.6%, mostly because it required a second treatment when the fetal heart beat did not cease after several days.1
We speculate that the high failure rate with this treatment may be caused by its slow action and questionable ability to stop cardiac activity and placental expansion. The expected result can take days, and all the while the gestational sac, the embryo or fetus, and its vascularity are growing. Secondary treatment has to address a larger gestation with more abundant vascularization.
2. Systemic, multidose, sequential methotrexate
In this regimen, the amounts of methotrexate injected are similar to the dose for the single-dose regimen. Two to three intramuscular injections (1 mg/kg of body weight or 50 mg/mm2 of surface area) are given at an interval of 2 or 3 days over the course of a week. Be aware of the cumulative adverse effects of this drug on the liver and bone marrow—and the fact that even multidose treatment can fail.1
We found it impossible to assess the complication rate associated with this approach because it was often used in conjunction with another “first-line” treatment or after it. However, it is clear that methotrexate can be combined with other, mostly nonsurgical treatments.
3. Suction aspiration or D&C, alone or in combination
This option requires general anesthesia. The 305 cases involving this treatment had a mean complication rate of about 62% (range, 29%–86%).1 This approach caused the greatest number of bleeding complications, necessitating a third-line treatment that almost always was surgical.
At delivery or the time of spontaneous abortion, the multilayered myometrial grid in the uterine body is able to contain bleeding vessels after placental separation. However, in CSP, the exposed vessels in the cervical scar tissue bleed because there is no muscle grid to contract and contain the profuse bleeding.
If you choose D&C or aspiration, have blood products available and a Foley balloon catheter handy! In several reports, a Foley balloon catheter was used as backup after significant bleeding occurred following curettage.5,6
In one of the series involving 45 cases treated by methotrexate followed by suction curettage, mean blood loss was significant at 707 mL (standard deviation, 642 mL; range, 100–2,000 mL), and treatment failed in three patients despite insertion of a Foley balloon catheter.
4. Uterine artery embolization, alone or in combination
This treatment requires general anesthesia. The complication rate was 47% among the 64 cases described in the literature.1 Uterine artery embolization appeared to work better when it was combined with other noninvasive treatments. It probably is not the best first-line treatment because the delay between treatment and effect allows the gestation to grow and vascularity to increase. And if uterine artery embolization fails, the clinician must contend with a larger gestation.
5. Excision by laparotomy, alone or in combination with hysteroscopy
General anesthesia is required. Of the 18 cases described in the literature, only five complications were reported—and only when used in an emergency situation.1
6. Laparoscopic excision
Again, general anesthesia is required. Fifteen of the 49 cases (30.6%) described in the literature involved complications, but only one of five cases (20%) experienced complications if hysteroscopy and laparoscopy were combined. Small numbers may not allow meaningful evaluation of the latter approach.1
7. Operative hysteroscopy, alone or in combination
General anesthesia is required. The overall complication rate for 108 cases was 13.8%. However, if hysteroscopy was combined with transabdominal ultrasound guidance (as it was in nine cases), no complications were noted. If hysteroscopy was combined with mifepristone, the complication rate was 17%.1 It appears that, when it is performed by an experienced clinician with ultrasound guidance, hysteroscopy may be a reasonable operative solution to CSP.
8. Intragestational-sac injection of methotrexate or potassium chloride, with ultrasound guidance
No anesthesia is required. This approach (FIGURE 4) had the fewest and least-involved complications. Of 83 cases, only 9 (10.8%) involved complications.
Cases performed with transabdominal sonography guidance had a slighter higher complication rate (15%) than those using TVS guidance.1
Because local injections are performed without general anesthesia and provide a final treatment by stopping heart activity, they appear to be the most effective intervention and may be especially useful when future fertility is desired.
9. Use of a Foley balloon catheter
Inserting a Foley balloon catheter and inflating it at the site of the CSP is an ingenious, relatively new approach.1,2,5–7 The catheters come with balloons of different capacity (FIGURE 5A). They can be used alone (usually in gestations of 5–7 weeks) in the hope of stopping the evolution of the pregnancy by placing pressure on a small gestational sac. Even so, this approach is almost always used in a planned fashion in conjunction with another treatment or as backup if bleeding occurs.
Our impression of the value of the balloon catheters is positive. We suggest the French-12 size 10-mL silicone balloon catheter (prices range from $2 to $20), although we used a French-14 catheter with a 30-mL balloon successfully in a case of an 8-week CSP.
Insert the catheter using real-time transabdominal sonographic guidance when the patient has a comfortably full bladder. One also can switch to TVS guidance to allow for more precise placement and assess the pressure, avoiding overinflation of the balloon (FIGURE 5B).
There is no information in the literature about how long such a catheter should be kept in place. In our experience, 24 to 48 hours is the preferred duration, with the outer end of the catheter fastened to the patient’s thigh. We also provide antibiotic coverage and reevaluate the effect in 2 days or as needed. General anesthesia is not required.
KEY TAKEAWAYS
Is there any single and effective treatment protocol? Probably not. Our management approach is presented as an algorithm (FIGURE 6).
We also offer the following guidelines:
- Do not confuse CSP with ectopic pregnancy. Such nomenclature has caused some referring physicians to simply use methotrexate protocols developed on “garden variety” tubal ectopic pregnancies, which not only failed but yielded disastrous results.
- Early diagnosis matters. TVS is the most effective and preferred diagnostic tool. Delay in the diagnosis delays treatment, increasing the possibility of complications.
- Cervical pregnancy is rare. In a patient who has had a cesarean delivery, a low chorionic sac is almost always a CSP.
- A key first step: Determine whether heart activity is present, and avoid methotrexate if no heart activity is observed.
- Counsel the patient. If heart activity is documented, provide evidence-based counseling about the patient’s options.
- Act fast. If continuation of the pregnancy is not desired, provide a reliable treatment that stops the embryonic or fetal heart beat without delay. Early treatment minimizes complications.
- Avoid single treatments unlikely to be effective, including D&C, suction curettage, single-dose intramuscular methotrexate, and uterine artery embolization applied alone.
- Keep a catheter at hand. Foley balloon tamponade to prevent or treat bleeding is a useful adjunct to have within easy reach.
- Consider combination treatments, as they may provide the best results.
- Fully inform the patient of the risks of pregnancy continuation. If a patient elects to continue the pregnancy, schedule an additional counseling session in which a more detailed overview of the anticipated clinical road is thoroughly explained.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your letter to the Editor to: rbarbieri@frontlinemedcom.com Please include the city and state in which you practice.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.
- Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. A review. [published correction appears in Am J Obstet Gynecol. 2014;210(4):371–374.] Am J Obstet Gynecol. 2012;207(1):14–29.
- Timor-Tritsch IE, Monteagudo A, Santos R, Tsymbal T, Pineda G, Arslan AA. The diagnosis, treatment, and follow-up of cesarean scar pregnancy. Am J Obstet Gynecol. 2012;207(1):44.e1–e13.
- Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–1841.
- Timor-Tritsch IE, Monteagudo A, Cali P, et al. Cesarean scar pregnancy and early placenta accreta share a common histology. Ultrasound Obstet Gynecol. 2014;43(4):383–395.
- Yu XL, Zhang N, Zuo WL. Cesarean scar pregnancy: An analysis of 100 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2011;91(45):3186–3189.
- Jiang T, Liu G, Huang L, Ma H, Zhang S. Methotrexate therapy followed by suction curettage followed by Foley tamponade for cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):209–211.
- Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2012. Natl Vital Stat Rep. 2013;62(3):1–20.