User login
A 67-year old man with an abdominal aortic aneurysm
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
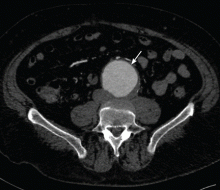
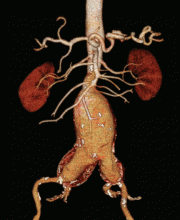
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
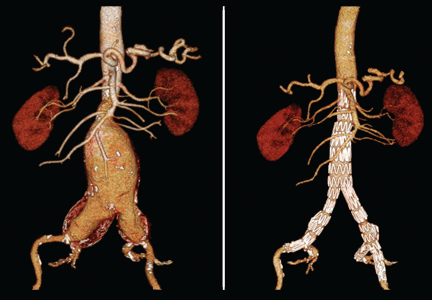
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
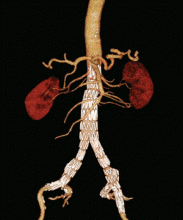
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
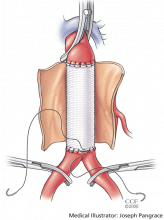
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
A 67-year-old man presented for evaluation of an abdominal aortic aneurysm, noted 1 month previously after his primary care physician ordered screening ultrasonography as part of a routine annual physical examination. The man was experiencing no symptoms.
He had type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. He smoked two packs of cigarettes a day. He had never had surgery. His current medications included diltiazem, fenofibrate, niacin, and aspirin; because he had chronic obstructive pulmonary disease, he was not on a beta-blocker.
His father had died suddenly at the age of 77; his death was attributed to a cardiac cause, but no formal autopsy was performed. Neither the patient’s siblings nor his children were screened for aneurysms.
On physical examination, he was comfortable and in no acute distress. His blood pressure was 156/71 mm Hg, pulse 60, temperature 36.1°C (97.0°F), and body mass index 30.15 kg/m2, which is in the obese range.
He had no jugular venous distention, no carotid bruits, and no lymphadenopathy. The cardiac examination was unremarkable, with regular rate, normal sinus rhythm, and no murmurs. On pulmonary examination, inspiratory and expiratory wheezes were noted in all lung fields.
His abdomen was obese but not tender to palpation. The aneurysm was not palpable. His pedal pulses were normal. The remainder of the examination was normal.
WHO SHOULD BE SCREENED?
1. For which of the following groups does the United States Preventive Services Task Force (USPSTF) strongly recommend screening for abdominal aortic aneurysms?
- Men and women over age 65
- Men and women who have ever smoked and are over age 65
- Men over age 75 and men over age 65 who smoke
- Men age 65 to 75 who have ever smoked
In 2005, the USPSTF recommended one-time screening ultrasonography for all men age 65 to 75 who have ever smoked. On the basis of evidence available at the time, it made no recommendation for men age 65 to 75 who have never smoked, and it recommended against screening women.1
ANEURYSMS ARE COMMON, OFTEN ASYMPTOMATIC, UNTIL THEY RUPTURE
Abdominal aortic aneurysms are relatively common in older adults, with a prevalence of 1.4% in the US population age 50 to 84 years.2 In four randomized controlled trials of aneurysm screening in Europe and Australia, the prevalence of any aneurysm (not just abdominal aortic aneurysms) in men was 6% (95% confidence interval 5–6).3–6
Fewer studies are available on the prevalence in women. One study found a prevalence of 0.7% in 10,012 US women, compared with 3.9% in men.7
In a recent report of the aneurysm screening program in the United Kingdom, the incidence of aneurysms had decreased from historically reported estimates.8,9
In the year 2000, abdominal aortic aneurysms caused 15,000 deaths in the United States and were the 10th leading cause of death in white men age 65 to 74.10 The actual number of deaths may be larger, since some people may die suddenly of an aneurysm with no evaluation for attributable cause.11
Aortic aneurysms are often asymptomatic until they rupture, making them difficult to detect without a focused screening program. The goal of treatment is to avoid spontaneous rupture and death. When aneurysms rupture, the estimated death rate is 80%.6
EVIDENCE IN FAVOR OF SCREENING
Ultrasonography is nearly 100% sensitive and specific in detecting abdominal aortic aneurysms in patients without symptoms.12 In comparison, abdominal palpation is 68% sensitive and 75% specific.13
The larger the aneurysm, the higher the risk of rupture.14–16 The annual risk of rupture is:
- 0.5% with aneurysms smaller than 4.0 cm
- 1.0% with aneurysms 4.0–4.9 cm
- 11% with aneurysms 5.0–5.9 cm
- 26% with aneurysms 6.0–6.9 cm.
Several large randomized controlled trials in men over age 65 evaluated the effect of screening programs for abdominal aortic aneurysms on the rate of deaths from this cause.3–6,17 A meta-analysis of these trials found a relative risk of 0.60 in favor of screening—ie, men over age 65 who were screened had a 40% lower risk of dying of an abdominal aortic aneurysm than men who were not screened.18 In long-term follow-up, the rate continued to be about 50% lower with screening than without.19,20 The absolute reduction in risk of death was 0.13%.21
Absolute risk reduction and number needed to screen
2. If screening offers an absolute risk reduction in the death rate of 0.13%, how many need to be screened to prevent one death?
- 769
- 856
- 1,300
- 13,000
The number of patients that need to be screened to prevent one death is called the number needed to screen.22 It is calculated as 1 divided by the absolute risk reduction. Therefore, in screening for abdominal aortic aneurysms, the number needed to screen is 1/0.0013, or 769. Recall that these numbers are from men over age 65, with no upper limit in age. If we consider only men age 65 to 75, the absolute risk reduction is 0.16%, which corresponds to a number needed to screen of 625.
To put this in perspective, the number of people who need to be screened using fecal occult blood testing to prevent one death from colon cancer is 808, and the number of women who need to undergo mammography to prevent one breast cancer death is 1,887.21,22
Criteria for a good screening test
3. Which of the following is not one of the World Health Organization’s guiding principles for adopting a screening test?
- The disease must be common, or it must have grave consequences if it is not detected
- The disease must be detectable in a latent or early stage
- A screening test must exist that is acceptable to patients
- A treatment must exist that affects the natural history of the disease and its prognosis
- The cost of screening must be reasonable
- The screening test must have high sensitivity and specificity
In 1968, the World Health Organization published guidelines that continue to be used to determine the acceptability of screening tests.23 These principles state that for a screening test to be acceptable, the disease must be highly prevalent or result in grave consequences if not detected. The disease must have a latent or early stage in which it can be detected, and treatment must be available at that stage that affects the natural history and prognosis of the illness. The test must also be acceptable to patients physically, and the cost of it should be balanced in relation to possible expenditure on medical care as a whole.
As discussed previously, abdominal aortic aneurysms are common, and the consequences of rupture are grave. If the condition is detected early, treatment is available that can be lifesaving. Additionally, abdominal ultrasonography is noninvasive and inexpensive (costing roughly a few hundred dollars).24 Therefore, all of the World Health Organization criteria are satisfied. Improved outcomes with newer endovascular techniques for repair23 will likely also improve the value of screening.
Although high sensitivity and specificity are not required to satisfy the criteria, abdominal ultrasonography is nearly 100% sensitive and specific for detecting abdominal aortic aneurysms in patients without symptoms.12
Given the prevalence of the disease, by one estimate, if current USPSTF guidelines are followed (ie, if we screen only men age 65 to 75 who have ever smoked), for every 20 men we screen, we would detect one abdominal aortic aneurysm, and we would detect 29.5% of all of these aneurysms.2 If we screen all patients age 50 to 84, 74 people would need to be screened to detect one abdominal aortic aneurysm, but a much greater percentage of all of these aneurysms would be detected.
SHOULD OTHER GROUPS BE SCREENED?
4. The patient has a 40-year-old daughter who has hypertension and a 20-pack-year history of smoking. Should she be screened for an abdominal aortic aneurysm?
- Yes
- No
The 2005 USPSTF report recommends onetime ultrasonographic screening for all men age 65 to 75 who have ever smoked.1
The American Heart Association made a similar recommendation in 2005 in conjunction with the Society for Vascular Surgery, the American Association of Vascular Surgery, the Society for Vascular Medicine and Biology, and others.25 However, these groups also support screening men age 60 and older who are siblings or children of patients with abdominal aortic aneurysms, using physical examination and abdominal ultrasonography.
Both of the guidelines exclude women (who account for 41% of all deaths from this disease by one estimate) and nonsmokers (who account for 22%).2
The USPSTF makes no recommendation about nonsmokers, but it specifically recommends against screening women, stating that women have a low prevalence of large abdominal aortic aneurysms and that few women die of this disease. Therefore, according to the USPSTF, the risks of early treatment in women—including morbidity and death with surgical treatment and associated psychological harms—are not worth the benefits.1
However, a study of 3.1 million Americans found that women who have multiple cardiovascular risk factors such as smoking, hypertension, hyperlipidemia, and a family history of abdominal aortic aneurysm are at as great or greater risk of abdominal aortic aneurysm as men who fit the USPSTF criteria.2 Additionally, a positive family history of abdominal aortic aneurysm was among the strongest predictors of a diagnosis of abdominal aortic aneurysm on screening.2
Since 2005, newer guidelines have been released that broaden the recommendations for who should be screened. The Society for Vascular Surgery12 recommends screening:
- All men age 65 and older
- Men age 55 and older and women age 65 and older who have a family history of abdominal aortic aneurysm
- Women age 65 and older who have ever smoked.
A recent Swedish study demonstrated that the prevalence of abdominal aortic aneurysms in siblings of patients known to have this condition is significantly higher than in the general population; of the siblings who were screened, 11% had an abdominal aortic aneurysm, as did 17% of brothers and 6% of sisters.26
Nevertheless, broadened screening remains controversial, and more investigations of family history-based screening are ongoing.
WHEN DOES AN ABDOMINAL AORTIC ANEURYSM NEED SURGERY ?
Our patient was diagnosed with an infrarenal abdominal aortic aneurysm 6.5 cm in diameter and with bilateral common iliac artery aneurysms measuring 3.8 cm on the left and 5.2 cm on the right.
Computed tomography (CT) was done for preoperative planning (Figures 1 and 2), as it can define the aneurysm better for surgical intervention. Ultrasonography, while nearly 99% sensitive and specific for finding abdominal aortic aneurysms,12 does not provide the view of the abdominal anatomy that may be needed in surgical planning. The patient was seen by a vascular surgeon, and appropriate preoperative testing was done; the results showed that his risk during an open surgical procedure would be slightly above average.
The decision that needed to be made in this case was whether the patient should undergo surgery (either open or endovascular) or only medical intervention. In two randomized controlled trials comparing immediate intervention vs ongoing surveillance, the best threshold for surgical intervention was an aneurysm larger than 5.5 cm.27–29 Both trials found no benefit in terms of survival with surgical repair of aneurysms 4.0 to 5.4 cm: there was no long-term difference in the rate of survival in patients who underwent early surgical intervention compared with surveillance until the aneurysm was larger than 5.5 cm.
But this was with open surgery. What about endovascular repair? More recent studies that evaluated endovascular repair of small aneurysms (4.0–5.0 cm) found no improvement in end points, including time to aneurysm rupture and rate of aneurysm-related death, compared with surveillance.30,31
Treat risk factors
Medical therapy currently focuses on reducing risk factors for aneurysm growth and rupture, including hypertension, hyperlipidemia, and smoking, but research is focusing on angiotensin-converting enzyme inhibitors and experimental agents such as metalloproteinase inhibitors.32,33
Smoking is a major risk factor in the development, growth, and rupture of abdominal aortic aneurysms,34 and the 2005 joint guidelines of the American College of Cardiology and the American Heart Association (ACC/AHA) recommend that everyone with an abdominal aortic aneurysm or a family history of it be advised to stop smoking.25 This is especially important in light of data that show a higher risk of abdominal aortic aneurysm with a higher volume of smoking (total pack-years) and a decrease in risk with time since quitting.2
Medical management also includes treating other associated cardiovascular risk factors, including hypertension and dyslipidemia. The ACC/AHA guidelines recommend that patients with abdominal aortic aneurysms be treated similarly to patients with atherosclerotic disease or a coronary artery disease equivalent, including giving them a statin and an antiplatelet drug such as aspirin.
The ACC/AHA guidelines also recommend that patients who are managed medically and have an aneurysm of 3.0 to 4.0 cm undergo ultrasonographic monitoring every 2 to 3 years, and those with an aneurysm of 4.0 to 5.4 cm undergo monitoring with ultrasonography or CT every 6 to 12 months.25
5. Which of the following is the treatment of choice for our patient’s high blood pressure?
- Propranolol
- Lisinopril
- Hydralazine
- Hydrochlorothiazide
The recommended agents for blood pressure control in this patient population are betablockers, such as propranolol. In a small study of patients with infrarenal aortic aneurysms, beta-blockers reduced the mean expansion rate from 0.68 cm/year to 0.36 cm/year, although larger trials have not yet confirmed this benefit.35,36 The 2005 ACC/AHA guidelines recommend beta-blockers for patients who are being managed medically.25 Other antihypertensive drugs can be added to achieve optimal blood pressure control after the addition of a beta-blocker.
Open vs endovascular repair
If a patient has an abdominal aortic aneurysm larger than 5.5 cm or if the benefits of surgery are determined to outweigh the risks, a surgical plan should be developed. Patients should be evaluated for surgical risk factors, and this should guide the choice of surgical approach—ie, open repair or endovascular repair.
Compared with open repair, endovascular repair has been increasing in popularity. It has a lower rate of complications, including a significantly lower rate of perioperative death, even though patients who undergo endovascular repair are on average significantly older than those who undergo open repair.37–39
Endovascular repair is performed with open or percutaneous access of the common femoral artery. An endograft, which is packed into an introductory sheath, is introduced into the aorta and expands upon unsheathing. It is positioned to “land” in sealing zones of normal-caliber aorta, where it seals to exclude the aneurysm from circulatory flow (Figure 3).
This is different from the open approach in that it avoids the large incision and aortic cross-clamping necessary in open repair. In open repair, a large incision is made in the patient’s abdomen and the aorta is cross-clamped to stop blood flow. The aneurysm is then incised and a graft is sutured into place to protect the vessel wall from stress (Figure 4).
CASE CONCLUDED
Our patient elected to undergo endovascular repair of his aneurysm with a bifurcated graft (Figure 3). He was able to walk the day after his procedure, and he was sent home that same day. According to the guidelines of the Society for Vascular Surgery,40 he will have surveillance CT angiography at 1 and 12 months to detect “endoleak” or aneurysm enlargement. If these are not seen, he will then undergo routine surveillance with abdominal duplex ultrasonography.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.
- US Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005; 142:198–202.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010; 52:539–548.
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ 2005; 330:750.
- Ashton HA, Buxton MJ, Day NE, et al; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360:1531–1539.
- Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004; 329:1259.
- Vardulaki KA, Walker NM, Couto E, et al. Late results concerning feasibility and compliance from a randomized trial of ultrasonographic screening for abdominal aortic aneurysm. Br J Surg 2002; 89:861–864.
- Derubertis BG, Trocciola SM, Ryer EJ, et al. Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 2007; 46:630–635.
- Sandiford P, Mosquera D, Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011; 98:645–651.
- Anjum A, Powell JT. Is the incidence of abdominal aortic aneurysm declining in the 21st century? Mortality and hospital admissions for England & Wales and Scotland. Eur J Vasc Endovasc Surg 2012; 43:161–166.
- Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep 2002; 50:1–85.
- Kent KC, Zwolak RM, Jaff MR, et al; Society for Vascular Surgery; American Association of Vascular Surgery; Society for Vascular Medicine and Biology. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39:267–269.
- Chaikof EL, Brewster DC, Dalman RL, et al; Society for Vascular Surgery. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg 2009; 50(suppl 4):S2–S49.
- Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med 2000; 160:833–836.
- Reed WW, Hallett JW, Damiano MA, Ballard DJ. Learning from the last ultrasound. A population-based study of patients with abdominal aortic aneurysm. Arch Intern Med 1997; 157:2064–2068.
- Bernstein EF, Dilley RB, Goldberger LE, Gosink BB, Leopold GR. Growth rates of small abdominal aortic aneurysms. Surgery 1976; 80:765–773.
- Cronenwett JL, Sargent SK, Wall MH, et al. Variables that affect the expansion rate and outcome of small abdominal aortic aneurysms. J Vasc Surg 1990; 11:260–268.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002; 89:283–285.
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the US Preventive Services Task Force. Ann Intern Med 2005; 142:203–211.
- Lindholt JS, Sørensen J, Søgaard R, Henneberg EW. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br J Surg 2010; 97:826–834.
- Thompson SG, Ashton HA, Gao L, Scott RA; Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised Multicentre Aneurysm Screening Study. BMJ 2009; 338:b2307.
- Mastracci TM, Cina CS. Regarding Screening for abdominal aortic aneurysm reduces both aneurysm-related and all-cause mortality (letter). J Vasc Surg 2007; 46:1312.
- Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ 1998; 317:307–312.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. World Health Organization. Public Health Papers #34.
- Lee TY, Korn P, Heller JA, et al. The cost-effectiveness of a “quickscreen” program for abdominal aortic aneurysms. Surgery 2002; 132:399–407.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113:e463–e654.
- Linné A, Lindström D, Hultgren R. High prevalence of abdominal aortic aneurysms in brothers and sisters of patients despite a low prevalence in the population. J Vasc Surg 2012; 56:305–310.
- The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet 1998; 352:1649–1655.
- Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126:441–449.
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS; Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003; 37:1106–117.
- Ouriel K, Clair DG, Kent KC, Zarins CK; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg 2010; 51:1081–1087.
- De Rango P, Verzini F, Parlani G; Comparison of surveillance vs Aortic Endografting for Small Aneurysm Repair (CAESAR) Investigators. Quality of life in patients with small abdominal aortic aneurysm: the effect of early endovascular repair versus surveillance in the CAESAR trial. Eur J Vasc Endovasc Surg 2011; 41:324–331.
- Antoniou GA, Lazarides MK, Patera S, et al. Assessment of insertion/deletion polymorphism of the angiotensin-converting enzyme gene in abdominal aortic aneurysm and inguinal hernia. Vascular 2012; Epub ahead of print.
- Ogata T, Shibamura H, Tromp G, et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J Vasc Surg 2005; 41:1036–1042.
- Powell JT, Greenhalgh RM. Clinical practice. Small abdominal aortic aneurysms. N Engl J Med 2003; 348:1895–1901.
- Gadowski GR, Pilcher DB, Ricci MA. Abdominal aortic aneurysm expansion rate: effect of size and beta-adrenergic blockade. J Vasc Surg 1994; 19:727–731.
- Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg 2002; 35:72–79.
- Jackson RS, Chang DC, Freischlag JA. Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries. JAMA 2012; 307:1621–1628.
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg 2006; 43:446–451.
- Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009; 49:543–550.
- Chaikof EL, Blankensteijn JD, Harris PL, et al; Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002; 35:1048–1060.