User login
Child of The New Gastroenterologist
Update on the Management of Acute Pancreatitis and Its Complications
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
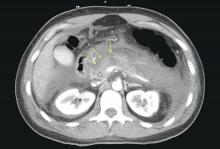
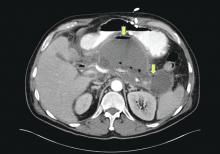
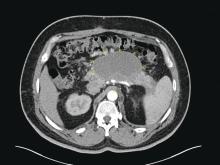
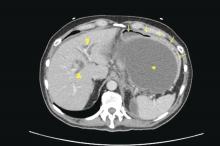
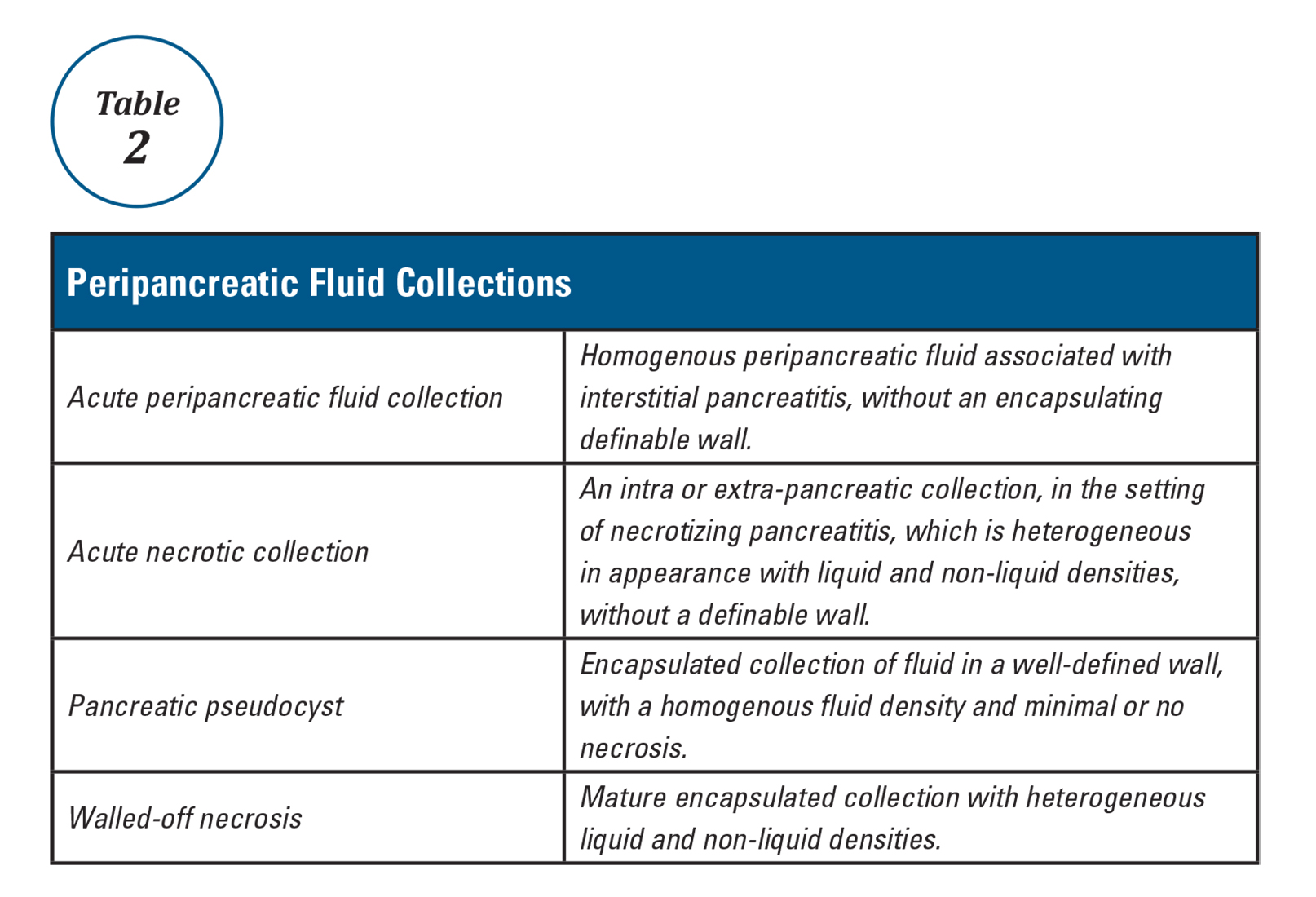
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.
Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.

Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.

Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.