User login
Adult ADHD: 6 studies of nonpharmacologic interventions
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
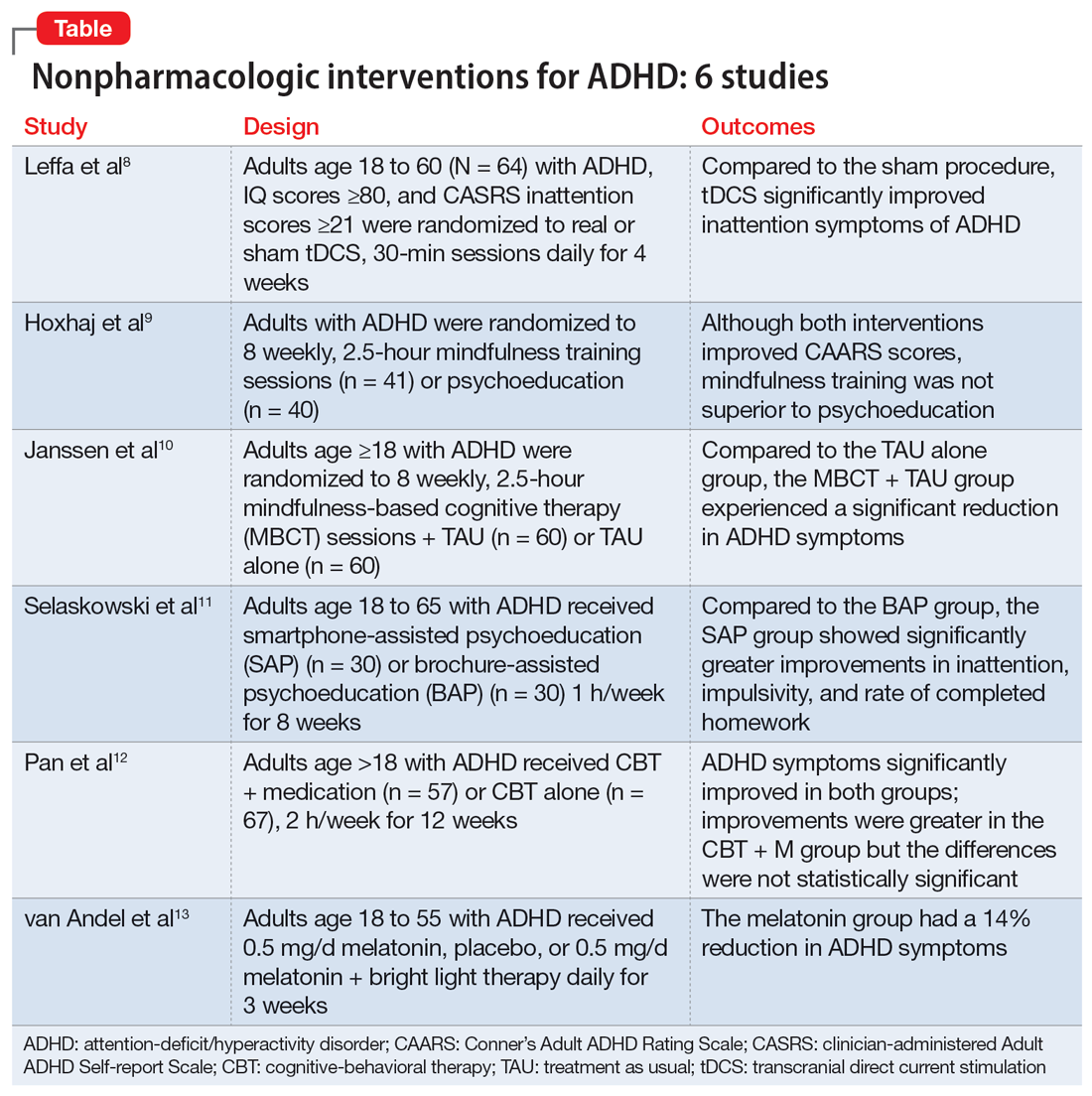
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).
1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
SECOND OF 2 PARTS
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional impairment.1 ADHD begins in childhood, continues into adulthood, and has negative consequences in many facets of adult patients’ lives, including their careers, daily functioning, and interpersonal relationships.2 According to the National Institute of Health and Care Excellence’s recommendations, both pharmacotherapy and psychotherapy are advised for patients with ADHD.3 Although various pharmacotherapies are advised as first-line treatments for ADHD, they are frequently linked to unfavorable adverse effects, partial responses, chronic residual symptoms, high dropout rates, and issues with addiction.4 As a result, there is a need for evidence-based nonpharmacologic therapies.
In a systematic review, Nimmo-Smith et al5 found that certain nonpharmacologic treatments can be effective in helping patients with ADHD manage their illness. In clinical and cognitive assessments of ADHD, a recent meta-analysis found that noninvasive brain stimulation had a small but significant effect.6 Some evidence suggests that in addition to noninvasive brain stimulation, other nonpharmacologic interventions, including psychoeducation (PE), mindfulness, cognitive-behavioral therapy (CBT), and chronotherapy, can be effective as an adjunct treatment to pharmacotherapy, and possibly as monotherapy.
Part 1 of this 2-part article reviewed 6 randomized controlled trials (RCTs) of pharmacologic interventions for adult ADHD published within the last 5 years.7 Part 2 analyzes 6 RCTs of nonpharmacologic treatments for adult ADHD published within the last 5 years (Table8-13).

1. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
Transcranial direct current stimulation (tDCS) uses noninvasive, low-intensity electrical current on the scalp to affect underlying cortical activity.14 This form of neurostimulation offers an alternative treatment option for when medications fail or are not tolerated, and can be used at home without the direct involvement of a clinician.14 tDCS as a treatment for ADHD has been increasingly researched, though many studies have been limited by short treatment periods and varied methodological approaches. In a meta-analysis, Westwood et al6 found a trend toward improvement on the function of processing speed but not on attention. Leffa et al8 examined the efficacy and safety of a 4-week course of home-based tDCS in adult patients with ADHD, specifically looking at reduction in inattention symptoms.
Study design
- This randomized, double-blind, parallel, sham-controlled clinical trial evaluated 64 participants age 18 to 60 from a single center in Brazil who met DSM-5 criteria for combined or primarily inattentive ADHD.
- Inclusion criteria included an inattention score ≥21 on the clinician-administered Adult ADHD Self-report Scale version 1.1 (CASRS). This scale assesses both inattentive symptoms (CASRS-I) and hyperactive-impulsive symptoms (CASRS-HI). Participants were not being treated with stimulants or agreed to undergo a 30-day washout of stimulants prior to the study.
- Exclusion criteria included current moderate to severe depression (Beck Depression Inventory-II [BDI] score >21), current moderate to severe anxiety (Beck Anxiety Inventory [BAI] score ≥21), diagnosis of bipolar disorder (BD) with either a manic or depressive episode in the year prior to study, diagnosis of a psychotic disorder, diagnosis of autism spectrum disorder (ASD), positive screen for substance use, unstable medical condition resulting in poor functionality, pregnant or planning on becoming pregnant within 3 months of the study, not able to use home-based equipment, history of neurosurgery, presence of ferromagnetic metal in the head or presence of implanted medical devices in head/neck region, or history of epilepsy with reported seizures in the year prior to the study.
- Participants were randomized to self-administer real or sham tDCS; the devices looked the same. Participants underwent daily 30-minute sessions using a 2-mA direct constant current for a total of 28 sessions. Sham treatment involved a 30-second ramp-up to 2-mA and a 30-second ramp-down sensation at the beginning, middle, and end of each respective session.
- The primary outcome was a change in symptoms of inattention per CASRS-I. Secondary outcomes were scores on the CASRS-HI, BDI, BAI, and Behavior Rating Inventory of Executive Functions-Adult (BRIEF-A), which evaluates executive function.
Outcomes
- A total of 53 participants used stimulant medications prior to the study and 8 required a washout. The average age was 38.3, and 53% of participants were male.
- For the 55 participants who completed 4 weeks of treatment, the mean number of sessions was 25.2 in the tDCS group and 24.8 in the sham group.
- At the end of Week 4, there was a statistically significant treatment by time interaction in CASRS-I scores in the tDCS group compared to the sham group (18.88 vs 23.63 on final CASRS-I scores; P < .001).
- There were no statistically significant differences in any of the secondary outcomes.
Conclusions/limitations
- This study showed the benefits of 4 weeks of home-based tDCS for managing inattentive symptoms in adults with ADHD. The authors noted that extended treatment of tDCS may incur greater benefit, as this study used a longer treatment course compared to others that have used a shorter duration of treatment (ie, days instead of weeks). Additionally, this study placed the anodal electrode over the right dorsolateral prefrontal cortex (DLPFC) vs over the left DLPFC, because there may be a decrease in activation in the right DLPFC in adults with ADHD undergoing attention tasks.15
- This study also showed that home-based tDCS can be an easier and more accessible way for patients to receive treatment, as opposed to needing to visit a health care facility.
- Limitations: The dropout rate (although only 2 of 7 participants who dropped out of the active group withdrew due to adverse events), lack of remote monitoring of patients, and restrictive inclusion criteria limit the generalizability of these findings. Additionally, 3 patients in the tDCS group and 7 in the sham group were taking psychotropic medications for anxiety or depression.
Continue to: #2
2. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
Previous research has shown that using mindfulness-based approaches can improve ADHD symptoms.16,17 Hoxhaj et al9 looked at the effectiveness of mindfulness awareness practices (MAP) for alleviating ADHD symptoms.
Study design
- This RCT enrolled 81 adults from a German medical center who met DSM-IV criteria for ADHD, were not taking any ADHD medications, and had not undergone any psychotherapeutic treatments in the last 3 months. Participants were randomized to receive MAP (n = 41) or PE (n = 40).
- Exclusion criteria included having a previous diagnosis of schizophrenia, BD I, active substance dependence, ASD, suicidality, self-injurious behavior, or neurologic disorders.
- The MAP group underwent 8 weekly 2.5-hour sessions, plus homework involving meditation and other exercises. The PE group was given information regarding ADHD and management options, including organization and stress management skills.
- Patients were assessed 2 weeks before treatment (T1), at the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- The primary outcome was the change in the blind-observer rated Conner’s Adult ADHD Rating Scales (CAARS) inattention/memory scales from T1 to T2.
- Secondary outcomes included the other CAARS subscales, the Brief Symptom Inventory (BSI), the BDI, the 36-item Short Form Health Survey, and the Five Facet Mindfulness Questionnaire (FFMQ).
Outcomes
- Baseline demographics did not differ between groups other than the MAP group having a significantly higher IQ than the PE group. However, this difference resolved after the final sample was analyzed, as there were 2 dropouts and 7 participants lost to follow-up in the MAP group and 4 dropouts and 4 participants lost to follow-up in the PE group.
- There was no significant difference between the groups in the primary outcome of observer-rated CAARS inattention/memory subscale scores, or other ADHD symptoms per the CAARS.
- However, there was a significant difference within each group on all ADHD subscales of the observer-rated CAARS at T2. Persistent, significant differences were noted for the observer-rated CAARS subscales of self-concept and DSM-IV Inattentive Symptoms, and all CAARS self-report scales to T3.
- Compared to the PE group, there was a significantly larger improvement in the MAP group on scores of the mindfulness parameters of observation and nonreactivity to inner experience.
- There were significant improvements regarding depression per the BDI and global severity per the BSI in both treatment groups, with no differences between the groups.
- At T3, in the MAP group, 3 patients received methylphenidate, 1 received atomoxetine, and 1 received antidepressant medication. In the PE group, 2 patients took methylphenidate, and 2 participants took antidepressants.
- There was a significant difference regarding sex and response, with men experiencing less overall improvement than women.
Conclusions/limitations
- MAP was not superior to PE in terms of changes on CAARS scores, although within each group, both therapies showed improvement over time.
- While there may be gender-specific differences in processing information and coping strategies, future research should examine the differences between men and women with different therapeutic approaches.
- Limitations: This study did not employ a true placebo but instead had 2 active arms. Generalizability is limited due to a lack of certain comorbidities and use of medications.
Continue to: #3
3. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
Mindfulness-based cognitive therapy (MBCT) is a form of psychotherapy that combines mindfulness with the principles of CBT. Hepark et al18 found benefits of MBCT for reducing ADHD symptoms. In a larger, multicenter, single-blind RCT, Janssen et al10 reviewed the efficacy of MBCT compared to treatment as usual (TAU).
Study design
- A total of 120 participants age ≥18 who met DSM-IV criteria for ADHD were recruited from Dutch clinics and advertisements and randomized to receive MBCT plus TAU (n = 60) or TAU alone (n = 60). There were no significant demographic differences between groups at baseline.
- Exclusion criteria included active depression with psychosis or suicidality, active manic episode, tic disorder with vocal tics, ASD, learning or other cognitive impairments, borderline or antisocial personality disorder, substance dependence, or previous participation in MBCT or other mindfulness-based interventions. Participants also had to be able to complete the questionnaires in Dutch.
- Blinded evaluations were conducted at baseline (T0), at the completion of therapy (T1), 3 months after the completion of therapy (T2), and 6 months after the completion of therapy (T3).
- MBCT included 8 weekly, 2.5-hour sessions and a 6-hour silent session between the sixth and seventh sessions. Patients participated in various meditation techniques with the addition of PE, CBT, and group discussions. They were also instructed to practice guided exercises 6 days/week, for approximately 30 minutes/day.
- The primary outcome was change in ADHD symptoms as assessed by the investigator-rated CAARS (CAARS-INV) at T1.
- Secondary outcomes included change in scores on the CAARS: Screening Version (CAARS-S:SV), BRIEF-A, Five Facet Mindfulness Questionnaire-Short Form (FFMQ-SF), Self-Compassion Scale-Short Form (SCS-SF), Mental Health Continuum-Short Form (MHC-SF), and Outcome Questionnaire (OQ 45.2).
Outcomes
- In the MBCT group, participants who dropped out (n = 9) were less likely to be using ADHD medication at baseline than those who completed the study.
- At T1, the MBCT plus TAU group had significantly less ADHD symptoms on CAARS-INV compared to TAU (d = 0.41, P = .004), with more participants in the MBCT plus TAU group experiencing a symptom reduction ≥30% (24% vs 7%, P = .001) and remission (P = .039).
- The MBCT plus TAU group also had a significant reduction in scores on CAARS-S:SV as well as significant improvement on self-compassion per SCS-SF, mindfulness skills per FFMQ-SF, and positive mental health per MHC-SF, but not on executive functioning per BRIEF-A or general functioning per OQ 45.2.
- Over 6-month follow-up, there continued to be significant improvement in CAARS-INV, CAARS-S:SV, mindfulness skills, self-compassion, and positive mental health in the MBCT plus TAU group compared to TAU. The difference in executive functioning (BRIEF-A) also became significant over time.
Conclusions/limitations
- MBCT plus TAU appears to be effective for reducing ADHD symptoms, both from a clinician-rated and self-reported perspective, with improvements lasting up to 6 months.
- There were also improvements in mindfulness, self-compassion, and positive mental health posttreatment in the MBCT plus TAU group, with improvement in executive functioning seen over the follow-up periods.
- Limitations: The sample was drawn solely from a Dutch population and did not assess the success of blinding.
Continue to: #4
4. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi:10.1016/j.psychres.2022.114802
Managing adult ADHD can include PE, but few studies have reviewed the effectiveness of formal clinical PE. PE is “systemic, didactic-psychotherapeutic interventions, which are adequate for informing patients and their relatives about the illness and its treatment, facilitating both an understanding and personally responsible handling of the illness and supporting those afflicted in coping with the disorder.”19 Selaskowski et al11 investigated the feasibility of using smartphone-assisted PE (SAP) for adults diagnosed with ADHD.
Study design
- Participants were 60 adults age 18 to 65 who met DSM-5 diagnostic criteria for ADHD. They were required to have a working comprehension of the German language and access to an Android-powered smartphone.
- Exclusion criteria included a diagnosis of schizophrenia or other psychotic disorder, antisocial personality disorder, substance use disorder, severe affective disorder, severe neurologic disorder, or initial use or dose change of ADHD medications 2 weeks prior to baseline.
- Participants were randomized to SAP (n = 30) or brochure-assisted PE (BAP) (n = 30). The demographics at baseline were mostly balanced between the groups except for substance abuse (5 in the SAP group vs 0 in the BAP group; P = .022).
- The primary outcome was severity of total ADHD symptoms, which was assessed by blinded evaluations conducted at baseline (T0) and after 8 weekly PE sessions (T1).
- Secondary outcomes included dropout rates, improvement in depressive symptoms as measured by the German BDI-II, improvement in functional impairment as measured by the Weiss Functional Impairment Scale (WFIRS), homework performed, attendance, and obtained PE knowledge.
- Both groups attended 8 weekly 1-hour PE group sessions led by 2 therapists and comprised of 10 participants.
Outcomes
- Only 43 of the 60 initial participants completed the study; 24 in the SAP group and 19 in the BAP group.
- The SAP group experienced a significant symptom improvement of 33.4% from T0 to T1 compared to the BAP group, which experienced a symptom improvement of 17.3% (P = .019).
- ADHD core symptoms considerably decreased in both groups. There was no significant difference between groups (P = .74).
- SAP dramatically improved inattention (P = .019), improved impulsivity (P = .03), and increased completed homework (P < .001), compared to the BAP group.
- There was no significant difference in correctly answered quiz questions or in BDI-II or WFIRS scores.
Conclusions/limitations
- Both SAP and BAP appear to be effective methods for PE, but patients who participated in SAP showed greater improvements than those who participated in BAP.
- Limitations: This study lacked a control intervention that was substantially different from SAP and lacked follow-up. The sample was a mostly German population, participants were required to have smartphone access beforehand, and substance abuse was more common in the SAP group.
Continue to: #5
5. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
CBT has demonstrated long-term benefit for the core symptoms of ADHD, comorbid symptoms (anxiety and depression), and social functioning. For ADHD, pharmacotherapies have a bottom-up effect where they increase neurotransmitter concentration, leading to an effect in the prefrontal lobe, whereas psychotherapies affect behavior-related brain activity in the prefrontal lobes, leading to the release of neurotransmitters. Pan et al12 compared the benefits of CBT plus medication (CBT + M) to CBT alone on core ADHD symptoms, social functioning, and comorbid symptoms.
Study design
- The sample consisted of 124 participants age >18 who had received a diagnosis of adult ADHD according to DSM-IV via Conner’s Adult ADHD Diagnostic Interview and were either outpatients at Peking University Sixth Hospital or participants in a previous RCT (Huang et al20).
- Exclusion criteria included organic mental disorders, high suicide risk in those with major depressive disorder, acute BD episode requiring medication or severe panic disorder or psychotic disorder requiring medication, pervasive developmental disorder, previous or current involvement in other psychological therapies, IQ <90, unstable physical conditions requiring medical treatment, attending <7 CBT sessions, or having serious adverse effects from medication.
- Participants received CBT + M (n = 57) or CBT alone (n = 67); 40 (70.18%) participants in the CBT + M group received methylphenidate hydrochloride controlled-release tablets (average dose 27.45 ± 9.97 mg) and 17 (29.82%) received atomoxetine hydrochloride (average dose 46.35 ± 20.09 mg). There were no significant demographic differences between groups.
- CBT consisted of 12 weekly 2-hour sessions (8 to 12 participants in each group) that were led by 2 trained psychiatrist therapists and focused on behavioral and cognitive strategies.
- Participants in the CBT alone group were drug-naïve and those in CBT + M group were stable on medications.
- The primary outcome was change in ADHD Rating Scale (ADHD-RS) score from baseline to Week 12.
- Secondary outcomes included Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Self-Esteem Scale (SES), executive functioning (BRIEF-A), and quality of life (World Health Organization Quality of Life-Brief version [WHOQOL-BREF]).
Outcomes
- ADHD-RS total, impulsiveness-hyperactivity subscale, and inattention subscale scores significantly improved in both groups (P < .01). The improvements were greater in the CBT + M group compared to the CBT-only group, but the differences were not statistically significant.
- There was no significant difference between groups in remission rate (P < .689).
- There was a significant improvement in SAS, SES, and SDS scores in both groups (P < .01).
- In terms of the WHOQOL-BREF, the CBT + M group experienced improvements only in the psychological and environmental domains, while the CBT-only group significantly improved across the board. The CBT-only group experienced greater improvement in the physical domain (P < .01).
- Both groups displayed considerable improvements in the Metacognition Index and Global Executive Composite for BRIEF-A. The shift, self-monitor, initiate, working memory, plan/organize, task monitor, and material organization skills significantly improved in the CBT + M group. The only areas where the CBT group significantly improved were initiate, material organization, and working memory. No significant differences in BRIEF-A effectiveness were discovered.
Conclusions/limitations
- CBT is an effective treatment for improving core ADHD symptoms.
- This study was unable to establish that CBT alone was preferable to CBT + M, particularly in terms of core symptoms, emotional symptoms, or self-esteem.
- CBT + M could lead to a greater improvement in executive function than CBT alone.
- Limitations: This study used previous databases rather than RCTs. There was no placebo in the CBT-only group. The findings may not be generalizable because participants had high education levels and IQ. The study lacked follow-up after 12 weeks.
Continue to: #6
6. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
Most individuals with ADHD have a delayed circadian rhythm.21 Delayed sleep phase syndrome (DSPS) is diagnosed when a persistently delayed circadian rhythm is not brought on by other diseases or medications. ADHD symptoms and circadian rhythm may both benefit from DSPS treatment. A 3-armed randomized clinical parallel-group trial by van Andel et al13 investigated the effects of chronotherapy on ADHD symptoms and circadian rhythm.
Study design
- Participants were Dutch-speaking individuals age 18 to 55 who were diagnosed with ADHD and DSPS. They were randomized to receive melatonin 0.5 mg/d (n = 17), placebo (n = 17), or melatonin 0.5 mg/d plus 30 minutes of timed morning bright light therapy (BLT) (n = 15) daily for 3 weeks. There were no significant differences in baseline characteristics between groups except that the melatonin plus BLT group had higher use of oral contraceptives (P = .007).
- This study was completed in the Netherlands with participants from an outpatient adult ADHD clinic.
- Exclusion criteria included epilepsy, psychotic disorders, anxiety or depression requiring acute treatment, alcohol intake >15 units/week in women or >21 units/week in men, ADHD medications, medications affecting sleep, use of drugs, mental retardation, amnestic disorder, dementia, cognitive dysfunction, crossed >2 time zones in the 2 weeks prior to the study, shift work within the previous month, having children disturbing sleep, glaucoma, retinopathy, having BLT within the previous month, pregnancy, lactation, or trying to conceive.
- The study consisted of 3-armed placebo-controlled parallel groups in which 2 were double-blind (melatonin group and placebo group).
- During the first week of treatment, medication was taken 3 hours before dim-light melatonin onset (DLMO) and later advanced to 4 and 5 hours in Week 2 and Week 3, respectively. BLT was used at 20 cm from the eyes for 30 minutes every morning between 7
am and 8am . - The primary outcome was DLMO in which radioimmunoassay was used to determine melatonin concentrations. DLMO was used as a marker for internal circadian rhythm.
- The secondary outcome was ADHD symptoms using the Dutch version of the ADHD Rating Scale-IV.
- Evaluations were conducted at baseline (T0), the conclusion of treatment (T1), and 2 weeks after the end of treatment (T2).
Outcomes
- Out of 51 participants, 2 dropped out of the melatonin plus BLT group before baseline, and 3 dropped out of the placebo group before T1.
- At baseline, the average DLMO was 11:43
pm ± 1 hour and 46 minutes, with 77% of participants experiencing DLMO after 11pm . Melatonin advanced DLMO by 1 hour and 28 minutes (P = .001) and melatonin plus BLT had an advance of 1 hour and 58 minutes (P < .001). DLMO was unaffected by placebo. - The melatonin group experienced a 14% reduction in ADHD symptoms (P = .038); the placebo and melatonin plus BLT groups did not experience a reduction.
- DLMO and ADHD symptoms returned to baseline 2 weeks after therapy ended.
Conclusions/limitations
- In patients with DSPS and ADHD, low-dose melatonin can improve internal circadian rhythm and decrease ADHD symptoms.
- Melatonin plus BLT was not effective in improving ADHD symptoms or advancing DLMO.
- Limitations: This study used self-reported measures for ADHD symptoms. The generalizability of the findings is limited because the exclusion criteria led to minimal comorbidity. The sample was comprised of a mostly Dutch population.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Goodman DW. The consequences of attention-deficit/hyperactivity disorder in adults. J Psychiatr Pract. 2007;13(5):318-327. doi:10.1097/01.pra.0000290670.87236.18
3. National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
4. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
5. Nimmo-Smith V, Merwood A, Hank D, et al. Non-pharmacological interventions for adult ADHD: a systematic review. Psychol Med. 2020;50(4):529-541. doi:10.1017/S0033291720000069
6. Westwood SJ, Radua J, Rubia K. Noninvasive brain stimulation in children and adults with attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Psychiatry Neurosci. 2021;46(1):E14-E33. doi:10.1503/jpn.190179
7. Santos MG, Majarwitz DJ, Saeed SA. Adult ADHD: 6 studies of pharmacologic interventions. Current Psychiatry. 2023;22(4):17-27. doi:10.12788/cp.0344
8. Leffa DT, Grevet EH, Bau CHD, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79(9):847-856. doi:10.1001/jamapsychiatry.2022.2055
9. Hoxhaj E, Sadohara C, Borel P, et al. Mindfulness vs psychoeducation in adult ADHD: a randomized controlled trial. Eur Arch Psychiatry Clin Neurosci. 2018;268(4):321-335. doi:10.1007/s00406-018-0868-4
10. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65. doi:10.1017/S0033291718000429
11. Selaskowski B, Steffens M, Schulze M, et al. Smartphone-assisted psychoeducation in adult attention-deficit/hyperactivity disorder: a randomized controlled trial. Psychiatry Res. 2022;317:114802. doi: 10.1016/j.psychres.2022.114802
12. Pan MR, Huang F, Zhao MJ, et al. A comparison of efficacy between cognitive behavioral therapy (CBT) and CBT combined with medication in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2019;279:23-33. doi:10.1016/j.psychres.2019.06.040
13. van Andel E, Bijlenga D, Vogel SWN, et al. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: a randomized clinical trial. Chronobiol Int. 2021;38(2):260-269. doi:10.1080/07420528.2020.1835943
14. Philip NS, Nelson B, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry. 2017;174(7):628-639. doi:10.1176/appi.ajp.2017.16090996
15. Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185-198. doi:10.1001/jamapsychiatry.2013.277
16. Zylowska L, Ackerman DL, Yang MH, et al. Mindfulness meditation training in adults and adolescents with ADHD: a feasibility study. J Atten Disord. 2008;11(6):737-746. doi:10.1177/1087054707308502
17. Mitchell JT, McIntyre EM, English JS, et al. A pilot trial of mindfulness meditation training for ADHD in adulthood: impact on core symptoms, executive functioning, and emotion dysregulation. J Atten Disord. 2017;21(13):1105-1120. doi:10.1177/1087054713513328
18. Hepark S, Janssen L, de Vries A, et al. The efficacy of adapted MBCT on core symptoms and executive functioning in adults with ADHD: a preliminary randomized controlled trial. J Atten Disord. 2019;23(4):351-362. Doi:10.1177/1087054715613587
19. Bäuml J, Froböse T, Kraemer S, et al. Psychoeducation: a basic psychotherapeutic intervention for patients with schizophrenia and their families. Schizophr Bull. 2006;32 Suppl 1 (Suppl 1):S1-S9. doi:10.1093/schbul/sbl017
20. Huang F, Tang Y, Zhao M, et al. Cognitive-behavioral therapy for adult ADHD: a randomized clinical trial in China. J Atten Disord. 2019;23(9):1035-1046. doi:10.1177/1087054717725874
21. Van Veen MM, Kooij JJS, Boonstra AM, et al. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry. 2010;67(11):1091-1096. doi:10.1016/j.biopsych.2009.12.032
Neuropsychiatric aspects of Parkinson’s disease: Practical considerations
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).
Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32
Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36
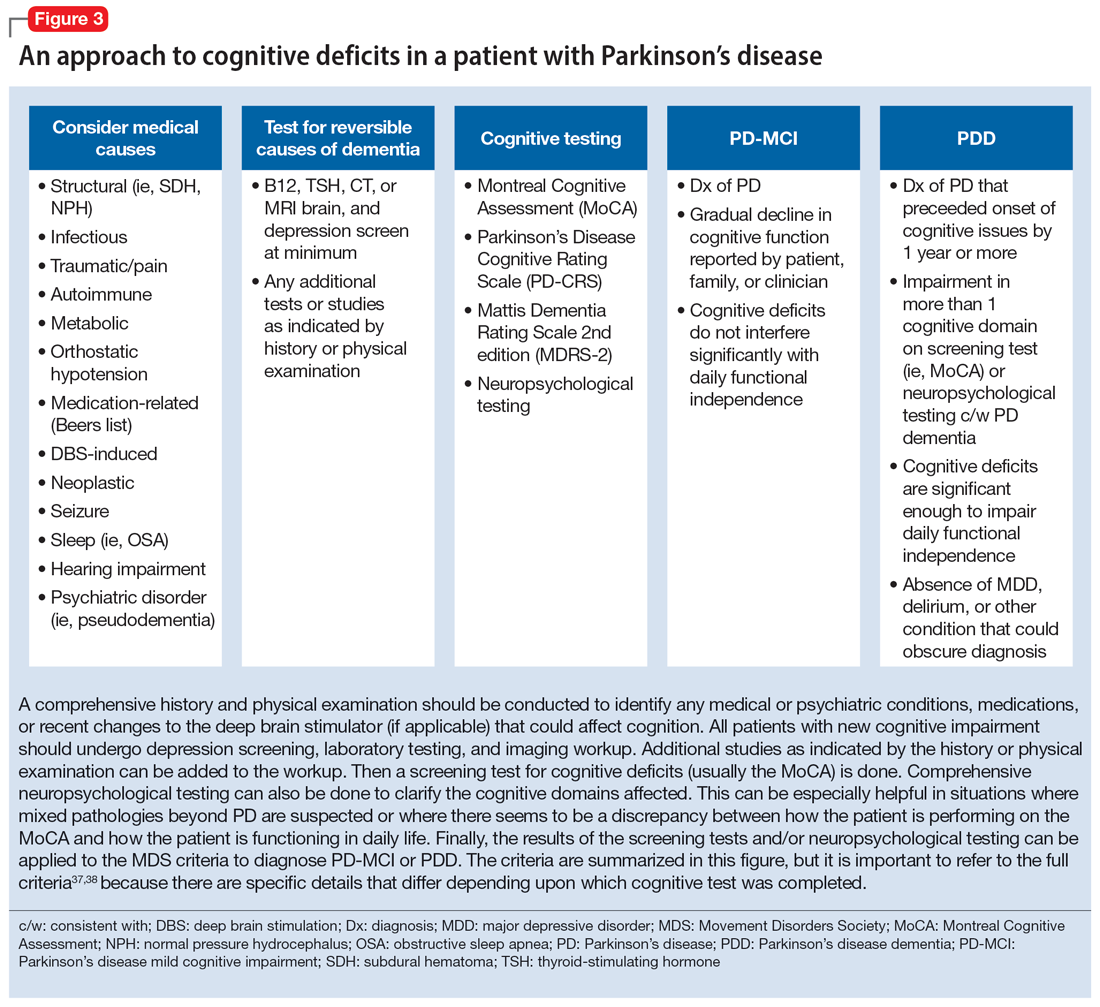
Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42
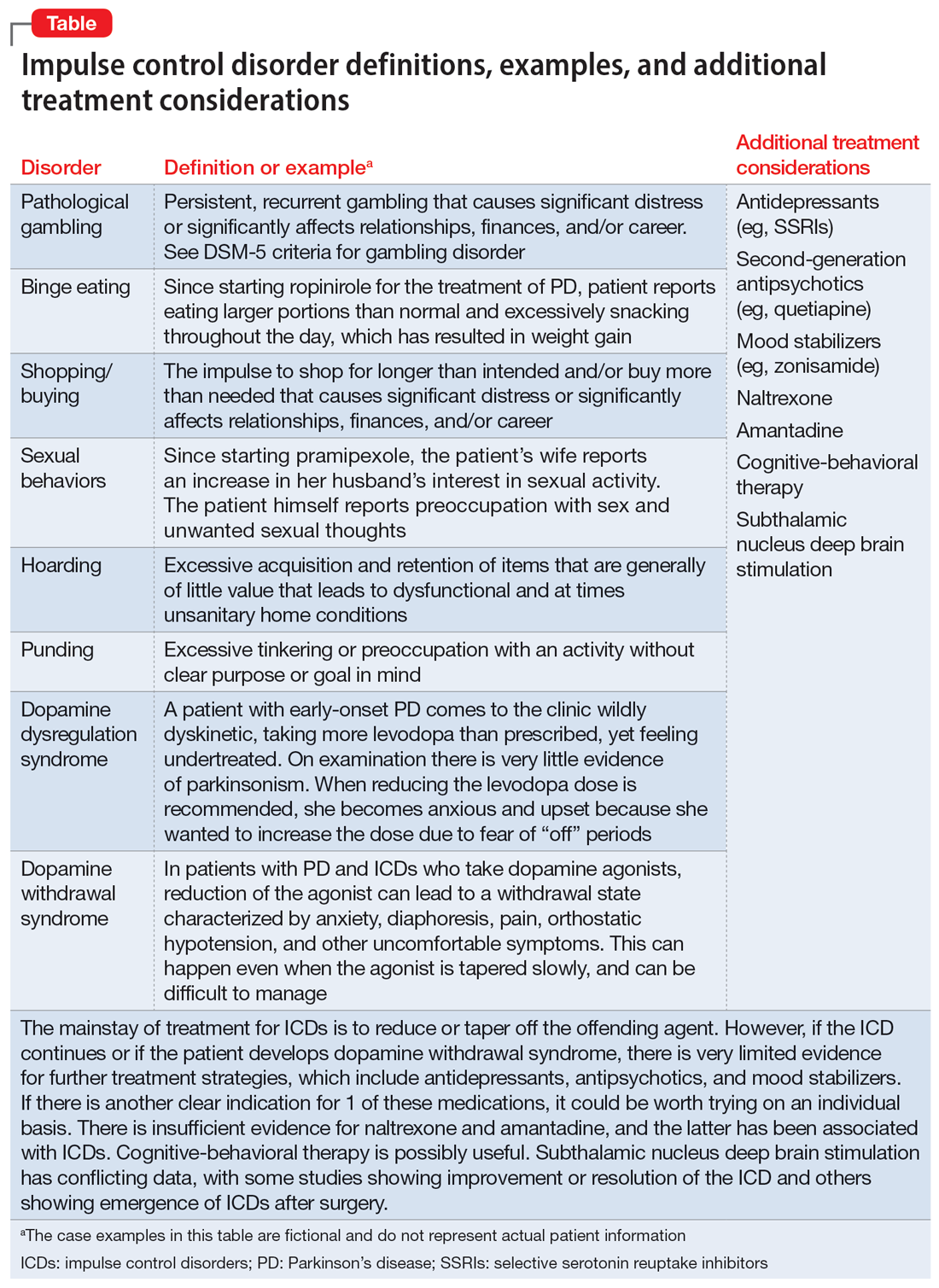
The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
Parkinson’s disease (PD) is a neurodegenerative condition diagnosed pathologically by alpha synuclein–containing Lewy bodies and dopaminergic cell loss in the substantia nigra pars compacta of the midbrain. Loss of dopaminergic input to the caudate and putamen disrupts the direct and indirect basal ganglia pathways for motor control and contributes to the motor symptoms of PD.1 According to the Movement Disorder Society criteria, PD is diagnosed clinically by bradykinesia (slowness of movement) plus resting tremor and/or rigidity in the presence of supportive criteria, such as levodopa responsiveness and hyposmia, and in the absence of exclusion criteria and red flags that would suggest atypical parkinsonism or an alternative diagnosis.2
Although the diagnosis and treatment of PD focus heavily on the motor symptoms, nonmotor symptoms can arise decades before the onset of motor symptoms and continue throughout the lifespan. Nonmotor symptoms affect patients from head (ie, cognition and mood) to toe (ie, striatal toe pain) and multiple organ systems in between, including the olfactory, integumentary, cardiovascular, gastrointestinal, genitourinary, and autonomic nervous systems. Thus, it is not surprising that nonmotor symptoms of PD impact health-related quality of life more substantially than motor symptoms.3 A helpful analogy is to consider the motor symptoms of PD as the tip of the iceberg and the nonmotor symptoms as the larger, submerged portions of the iceberg.4
Nonmotor symptoms can negatively impact the treatment of motor symptoms. For example, imagine a patient who is very rigid and dyscoordinated in the arms and legs, which limits their ability to dress and walk. If this patient also suffers from nonmotor symptoms of orthostatic hypotension and psychosis—both of which can be exacerbated by levodopa—dose escalation of levodopa for the rigidity and dyscoordination could be compromised, rendering the patient undertreated and less mobile.
In this review, we focus on identifying and managing nonmotor symptoms of PD that are relevant to psychiatric practice, including mood and motivational disorders, anxiety disorders, psychosis, cognitive disorders, and disorders related to the pharmacologic and surgical treatment of PD (Figure 1).

Mood and motivational disorders
Depression
Depression is a common symptom in PD that can occur in the prodromal period years to decades before the onset of motor symptoms, as well as throughout the disease course.5 The prevalence of depression in PD varies from 3% to 90%, depending on the methods of assessment, clinical setting of assessment, motor symptom severity, and other factors; clinically significant depression likely affects approximately 35% to 38% of patients.5,6 How depression in patients with PD differs from depression in the general population is not entirely understood, but there does seem to be less guilt and suicidal ideation and a substantial component of negative affect, including dysphoria and anxiety.7 Practically speaking, depression is treated similarly in PD and general populations, with a few considerations.
Despite limited randomized controlled trials (RCTs) for efficacy specifically in patients with PD, selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are generally considered first-line treatments. There is also evidence for tricyclic antidepressants (TCAs), but due to potential worsening of orthostatic hypotension and cognition, TCAs may not be a favorable option for certain patients with PD.8,9 All antidepressants have the potential to worsen tremor. Theoretically, SNRIs, with noradrenergic activity, may be less tolerable than SSRIs in patients with PD. However, worsening tremor generally has not been a clinically significant adverse event reported in PD depression clinical trials, although it was seen in 17% of patients receiving paroxetine and 21% of patients receiving venlafaxine compared to 7% of patients receiving placebo.9-11 If tremor worsens, mirtazapine could be considered because it has been reported to cause less tremor than SSRIs or TCAs.12
Among medications for PD, pramipexole, a dopamine agonist, may have a beneficial effect on depression.13 Additionally, some evidence supports rasagiline, a monoamine oxidase type B inhibitor, as an adjunctive medication for depression in PD.14 Nevertheless, antidepressant medications remain the standard pharmacologic treatment for PD depression.
Continue to: In terms of nonpharmacologic options...
In terms of nonpharmacologic options, cognitive-behavioral therapy (CBT) is likely efficacious, exercise (especially yoga) is likely efficacious, and repetitive transcranial magnetic stimulation may be efficacious.15,16 While further high-quality trials are needed, these treatments are low-risk and can be considered, especially for patients who cannot tolerate medications.
Apathy
Apathy—a loss of motivation and goal-directed behavior—can occur in up to 30% of patients during the prodromal period of PD, and in up to 70% of patients throughout the disease course.17 Apathy can coexist with depression, which can make apathy difficult to diagnose.17 Given the time constraints of a clinic visit, a practical approach would be to first screen for depression and cognitive impairment. If there is continued suspicion of apathy, the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part I question (“In the past week have you felt indifferent to doing activities or being with people?”) can be used to screen for apathy, and more detailed scales, such as the Apathy Scale (AS) or Lille Apathy Rating Scale (LARS), could be used if indicated.18
There are limited high-quality positive trials of apathy-specific treatments in PD. In an RCT of patients with PD who did not have depression or dementia, rivastigmine improved LARS scores compared to placebo.15 Piribedil, a D2/D3 receptor agonist, improved apathy in patients who underwent subthalamic nucleus deep brain stimulation (STN DBS).15 Exercise such as individualized physical therapy programs, dance, and Nordic walking as well as mindfulness interventions were shown to significantly reduce apathy scale scores.19 SSRIs, SNRIs, and rotigotine showed a trend toward reducing AS scores in RCTs.10,20
Larger, high-quality studies are needed to clarify the treatment of apathy in PD. In the meantime, a reasonable approach is to first treat any comorbid psychiatric or cognitive disorders, since apathy can be associated with these conditions, and to optimize antiparkinsonian medications for motor symptoms, motor fluctuations, and nonmotor fluctuations. Then, the investigational apathy treatments described in this section could be considered on an individual basis.
Anxiety disorders
Anxiety is seen throughout the disease course of PD in approximately 30% to 50% of patients.21 It can manifest as generalized anxiety disorder, panic disorder, and other anxiety disorders. There are no high-quality RCTs of pharmacologic treatments of anxiety specifically in patients with PD, except for a negative safety and tolerability study of buspirone in which one-half of patients experienced worsening motor symptoms.15,22 Thus, the treatment of anxiety in patients with PD is similar to treatments in the general population. SSRIs and SNRIs are typically considered first-line, benzodiazepines are sometimes used with caution (although cognitive adverse effects and fall risk need to be considered), and nonpharmacologic treatments such as mindfulness yoga, exercise, CBT, and psychotherapy can be effective.16,21,23
Continue to: Because there is the lack...
Because there is the lack of evidence-based treatments for anxiety in PD, we highlight 2 PD-specific anxiety disorders: internal tremor, and nonmotor “off” anxiety.
Internal tremor
Internal tremor is a sense of vibration in the axial and/or appendicular muscles that cannot be seen externally by the patient or examiner. It is not yet fully understood if this phenomenon is sensory, anxiety-related, related to subclinical tremor, or the result of a combination of these factors (ie, sensory awareness of a subclinical tremor that triggers or is worsened by anxiety). There is some evidence for subclinical tremor on electromyography, but internal tremor does not respond to antiparkinsonian medications in 70% of patients.24 More electrophysiological research is needed to clarify this phenomenon. Internal tremor has been associated with anxiety in 64% of patients and often improves with anxiolytic therapies.24
Although poorly understood, internal tremor is a documented phenomenon in 33% to 44% of patients with PD, and in some cases, it may be an initial symptom that motivates a patient to seek medical attention for the first time.24,25 Internal tremor has also been reported in patients with essential tremor and multiple sclerosis.25 Therefore, physicians should be aware of internal tremor because this symptom could herald an underlying neurological disease.
Nonmotor ‘off’ anxiety
Patients with PD are commonly prescribed carbidopa-levodopa, a dopamine precursor, at least 3 times daily. Initially, this medication controls motor symptoms well from 1 dose to the next. However, as the disease progresses, some patients report motor fluctuations in which an individual dose of carbidopa-levodopa may wear off early, take longer than usual to take effect, or not take effect at all. Patients describe these periods as an “off” state in which they do not feel their medications are working. Such motor fluctuations can lead to anxiety and avoidance behaviors, because patients fear being in public at times when the medication does not adequately control their motor symptoms.
In addition to these motor symptom fluctuations and related anxiety, patients can also experience nonmotor symptom fluctuations. A wide variety of nonmotor symptoms, such as mood, cognitive, and behavioral symptoms, have been reported to fluctuate in parallel with motor symptoms.26,27 One study reported fluctuating restlessness in 39% of patients with PD, excessive worry in 17%, shortness of breath in 13%, excessive sweating and fear in 12%, and palpitations in 10%.27 A patient with fluctuating shortness of breath, sweating, and palpitations (for example) may repeatedly present to the emergency department with a negative cardiac workup and eventually be diagnosed with panic disorder, whereas the patient is truly experiencing nonmotor “off” symptoms. Thus, it is important to be aware of nonmotor fluctuations so this diagnosis can be made and the symptoms appropriately treated. The first step in treating nonmotor fluctuations is to optimize the antiparkinsonian regimen to minimize fluctuations. If “off” anxiety symptoms persist, anxiolytic medications can be prescribed.21
Continue to: Psychosis
Psychosis
Psychosis can occur in prodromal and early PD but is most common in advanced PD.28 One study reported that 60% of patients developed hallucinations or delusions after 12 years of follow-up.29 Disease duration, disease severity, dementia, and rapid eye movement sleep behavior disorder are significant risk factors for psychosis in PD.30 Well-formed visual hallucinations are the most common manifestation of psychosis in patients with PD. Auditory hallucinations and delusions are less common. Delusions are usually seen in patients with dementia and are often paranoid delusions, such as of spousal infidelity.30 Sensory hallucinations can occur, but should not be mistaken with formication, a central pain syndrome in PD that can represent a nonmotor “off” symptom that may respond to dopaminergic medication.31 Other more mild psychotic symptoms include illusions or misinterpretation of stimuli, false sense of presence, and passage hallucinations of fleeting figures in the peripheral vision.30
The pathophysiology of PD psychosis is not entirely understood but differs from psychosis in other disorders. It can occur in the absence of antiparkinsonian medication exposure and is thought to be a consequence of the underlying disease process of PD involving neurodegeneration in certain brain regions and aberrant neurotransmission of not only dopamine but also serotonin, acetylcholine, and glutamate.30
Figure 2 outlines the management of psychosis in PD. After addressing medical and medication-related causes, it is important to determine if the psychotic symptom is sufficiently bothersome to and/or potentially dangerous for the patient to warrant treatment. If treatment is indicated, pimavanserin and clozapine are efficacious for psychosis in PD without worsening motor symptoms, and quetiapine is possibly efficacious with a low risk of worsening motor symptoms.15 Other antipsychotics, such as olanzapine, risperidone, and haloperidol, can substantially worsen motor symptoms.15 Both second-generation antipsychotics and pimavanserin have an FDA black-box warning for a higher risk of all-cause mortality in older patients with dementia; however, because psychosis is associated with early mortality in PD, the risk/benefit ratio should be discussed with the patient and family for shared decision-making.30 If the patient also has dementia, rivastigmine—which is FDA-approved for PD dementia (PDD)—may also improve hallucinations.32

Cognitive disorders
This section focuses on PD mild cognitive impairment (PD-MCI) and PDD. When a patient with PD reports cognitive concerns, the approach outlined in Figure 3 can be used to diagnose the cognitive disorder. A detailed history, medication review, and physical examination can identify any medical or psychiatric conditions that could affect cognition. The American Academy of Neurology recommends screening for depression, obtaining blood levels of vitamin B12 and thyroid-stimulating hormone, and obtaining a CT or MRI of the brain to rule out reversible causes of dementia.33 A validated screening test such as the Montreal Cognitive Assessment, which has higher sensitivity for PD-MCI than the Mini-Mental State Examination, is used to identify and quantify cognitive impairment.34 Neuropsychological testing is the gold standard and can be used to confirm and/or better quantify the degree and domains of cognitive impairment.35 Typically, cognitive deficits in PD affect executive function, attention, and/or visuospatial domains more than memory and language early on, and deficits in visuospatial and language domains have the highest sensitivity for predicting progression to PDD.36

Once reversible causes of dementia are addressed or ruled out and cognitive testing is completed, the Movement Disorder Society (MDS) criteria for PD-MCI and PDD summarized in Figure 3 can be used to diagnose the cognitive disorder.37,38 The MDS criteria for PDD require a diagnosis of PD for ≥1 year prior to the onset of dementia to differentiate PDD from dementia with Lewy bodies (DLB). If the dementia starts within 1 year of the onset of parkinsonism, the diagnosis would be DLB. PDD and DLB are on the spectrum of Lewy body dementia, with the same Lewy body pathology in different temporal and spatial distributions in the brain.38
Continue to: PD-MCI is present in...
PD-MCI is present in approximately 25% of patients.35 PD-MCI does not always progress to dementia but increases the risk of dementia 6-fold. The prevalence of PDD increases with disease duration; it is present in approximately 50% of patients at 10 years and 80% of patients at 20 years of disease.35 Rivastigmine is the only FDA-approved medication to slow progression of PDD. There is insufficient evidence for other acetylcholinesterase inhibitors and memantine.15 Unfortunately, RCTs of pharmacotherapy for PD-MCI have failed to show efficacy. However, exercise, cognitive rehabilitation, and neuromodulation are being studied. In the meantime, addressing modifiable risk factors (such as vascular risk factors and alcohol consumption) and treating comorbid orthostatic hypotension, obstructive sleep apnea, and depression may improve cognition.35,39
Treatment-related disorders
Impulse control disorders
Impulse control disorders (ICDs) are an important medication-related consideration in patients with PD. The ICDs seen in PD include pathological gambling, binge eating, excessive shopping, hypersexual behaviors, and dopamine dysregulation syndrome (Table). These disorders are more common in younger patients with a history of impulsive personality traits and addictive behaviors (eg, history of tobacco or alcohol abuse), and are most strongly associated with dopaminergic therapies, particularly the dopamine agonists.40,41 In the DOMINION study, the odds of ICDs were 2- to 3.5-fold higher in patients taking dopamine agonists.42 This is mainly thought to be due to stimulation of D2/D3 receptors in the mesolimbic system.40 High doses of levodopa, monoamine oxidase inhibitors, and amantadine are also associated with ICDs.40-42

The first step in managing ICDs is diagnosing them, which can be difficult because patients often are not forthcoming about these problems due to embarrassment or failure to recognize that the ICD is related to PD medications. If a family member accompanies the patient at the visit, the patient may not want to disclose the amount of money they spend or the extent to which the behavior is a problem. Thus, a screening questionnaire, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) can be a helpful way for patients to alert the clinician to the issue.41 Education for the patient and family is crucial before the ICD causes significant financial, health, or relationship problems.
The mainstay of treatment is to reduce or taper off the dopamine agonist or other offending agent while monitoring for worsening motor symptoms and dopamine withdrawal syndrome. If this is unsuccessful, there is very limited evidence for further treatment strategies (Table), including antidepressants, antipsychotics, and mood stabilizers.40,43,44 There is insufficient evidence for naltrexone based on an RCT that failed to meet its primary endpoint, although naltrexone did significantly reduce QUIP scores.15,44 There is also insufficient evidence for amantadine, which showed benefit in some studies but was associated with ICDs in the DOMINION study.15,40,42 In terms of nonpharmacologic treatments, CBT is likely efficacious.15,40 There are mixed results for STN DBS. Some studies showed improvement in the ICD, due at least in part to dopaminergic medication reduction postoperatively, but this treatment has also been reported to increase impulsivity.40,45
Deep brain stimulation–related disorders
For patients with PD, the ideal lead location for STN DBS is the dorsolateral aspect of the STN, as this is the motor region of the nucleus. The STN functions in indirect and hyperdirect pathways to put the brake on certain motor programs so only the desired movement can be executed. Its function is clinically demonstrated by patients with STN stroke who develop excessive ballistic movements. Adjacent to the motor region of the STN is a centrally located associative region and a medially located limbic region. Thus, when stimulating the dorsolateral STN, current can spread to those regions as well, and the STN’s ability to put the brake on behavioral and emotional programs can be affected.46 Stimulation of the STN has been associated with mania, euphoria, new-onset ICDs, decreased verbal fluency, and executive dysfunction. Depression, apathy, and anxiety can also occur, but more commonly result from rapid withdrawal of antiparkinsonian medications after DBS surgery.46,47 Therefore, for PD patients with DBS with new or worsening psychiatric or cognitive symptoms, it is important to inquire about any recent programming sessions with neurology as well as recent self-increases in stimulation by the patient using their controller. Collaboration with neurology is important to troubleshoot whether stimulation could be contributing to the patient’s psychiatric or cognitive symptoms.
Continue to: Bottom Line
Bottom Line
Mood, anxiety, psychotic, and cognitive symptoms and disorders are common psychiatric manifestations associated with Parkinson’s disease (PD). In addition, patients with PD may experience impulsive control disorders and other symptoms related to treatments they receive for PD. Careful assessment and collaboration with neurology is crucial to alleviating the effects of these conditions.
Related Resources
- Weintraub D, Aarsland D, Chaudhuri KR, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurology. 2022;21(1):89-102. doi:10.1016/S1474-4422(21)00330-6
- Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurologic Clinics. 2020;38(2):269-292. doi:10.1016/j.ncl.2019.12.003
- Castrioto A, Lhommee E, Moro E et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurology. 2014;13(3):287-305. doi:10.1016/ S1474-4422(13)70294-1
Drug Brand Names
Amantadine • Gocovri
Carbidopa-levodopa • Sinemet
Clozapine • Clozaril
Haloperidol • Haldol
Memantine • Namenda
Mirtazapine • Remeron
Naltrexone • Vivitrol
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimavanserin • Nuplazid
Piribedil • Pronoran
Pramipexole • Mirapex
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zonisamide • Zonegran
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
1. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet Neurology. 2021;397(10291):2284-2303.
2. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement Disorders. 2015;30(12):1591-1601.
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtiz MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord. 2011;26(3):399-406.
4. Langston WJ. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59(4):591-596.
5. Cong S, Xiang C, Zhang S, et al. Prevalence and clinical aspects of depression in Parkinson’s disease: a systematic review and meta‑analysis of 129 studies. Neurosci Biobehav Rev. 2022;141:104749. doi:10.1016/j.neubiorev.2022.104749
6. Reijnders JS, Ehrt U, Weber WE, et al. A systematic review of prevalence studies in depression in Parkinson’s disease. Mov Disord. 2008;23(2):183-189.
7. Zahodne LB, Marsiske M, Okun MS, et al. Components of depression in Parkinson disease. J Geriatr Psychiatry Neurol. 2012;25(3):131-137.
8. Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurology. 2010;10:49. doi:10.1186/1471-2377-10-49
9. Richard IH, McDermott MP, Kurlan R, et al; SAD-PD Study Group. A randomized, double-blind placebo-controlled trial of antidepressants in Parkinson’s disease. Neurology. 2012;78(16):1229-1236.
10. Takahashi M, Tabu H, Ozaki A, et al. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: a multicenter randomized study. Intern Med. 2019;58(3):361-368.
11. Bonuccelli U, Mecco G, Fabrini G, et al. A non-comparative assessment of tolerability and efficacy of duloxetine in the treatment of depressed patients with Parkinson’s disease. Expert Opin Pharmacother. 2012;13(16):2269-2280.
12. Wantanabe N, Omorio IM, Nakagawa A, et al; MANGA (Meta-Analysis of New Generation Antidepressants) Study Group. Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression. CNS Drugs. 2010;24(1):35-53.
13. Barone P, Scarzella L, Marconi R, et al; Depression/Parkinson Italian Study Group. Pramipexole versus sertraline in the treatment of depression in Parkinson’s disease: a national multicenter parallel-group randomized study. J Neurol. 2006;253(5):601-607.
14. Smith KM, Eyal E, Weintraub D, et al; ADAGIO Investigators. Combined rasagiline and anti-depressant use in Parkinson’s disease in the ADAGIO study: effects on non-motor symptoms and tolerability. JAMA Neurology. 2015;72(1):88-95.
15. Seppi K, Chaudhuri R, Coelho M, et al; the collaborators of the Parkinson’s Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson’s disease--an evidence-based medicine review. Mov Disord. 2019;34(2):180-198.
16. Kwok JYY, Kwan JCY, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):755-763.
17. De Waele S, Cras P, Crosiers D. Apathy in Parkinson’s disease: defining the Park apathy subtype. Brain Sci. 2022;12(7):923.
18. Mele B, Van S, Holroyd-Leduc J, et al. Diagnosis, treatment and management of apathy in Parkinson’s disease: a scoping review. BMJ Open. 2020;10(9):037632. doi:10.1136/bmjopen-2020-037632
19. Mele B, Ismail Z, Goodarzi Z, et al. Non-pharmacological interventions to treat apathy in Parkinson’s disease: a realist review. Clin Park Relat Disord. 2021;4:100096. doi:10.1016/j.prdoa.2021.100096
20. Chung SJ, Asgharnejad M, Bauer L, et al. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson’s disease. Expert Opin Pharmacother. 2016;(17)11:1453-1461.
21. Goldman JG, Guerra CM. Treatment of nonmotor symptoms associated with Parkinson disease. Neurol Clin. 2020;38(2):269-292.
22. Schneider RB, Auinger P, Tarolli CG, et al. A trial of buspirone for anxiety in Parkinson’s disease: safety and tolerability. Parkinsonism Relat Disord. 2020;81:69-74.
23. Moonen AJH, Mulders AEP, Defebvre L, et al. Cognitive behavioral therapy for anxiety in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2021;36(11):2539-2548.
24. Shulman LM, Singer C, Bean JA, et al. Internal tremor in patient with Parkinson’s disease. Mov Disord. 1996;11(1):3-7.
25. Cochrane GD, Rizvi S, Abrantes A, et al. Internal tremor in Parkinson’s disease, multiple sclerosis, and essential tremor. Parkinsonism Relat Disord. 2015;21(10):1145-1147.
26. Del Prete E, Schmitt E, Meoni S, et al. Do neuropsychiatric fluctuations temporally match motor fluctuations in Parkinson’s disease? Neurol Sci. 2022;43(6):3641-3647.
27. Kleiner G, Fernandez HH, Chou KL, et al. Non-motor fluctuations in Parkinson’s disease: validation of the non-motor fluctuation assessment questionnaire. Mov Disord. 2021;36(6):1392-1400.
28. Pachi I, Maraki MI, Giagkou N, et al. Late life psychotic features in prodromal Parkinson’s disease. Parkinsonism Relat Disord. 2021;86:67-73.
29. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson’s disease. Arch Neurol. 2010;67(8):996-1001.
30. Chang A, Fox SH. Psychosis in Parkinson’s disease: epidemiology, pathophysiology, and management. Drugs. 2016;76(11):1093-1118.
31. Kasunich A, Kilbane C, Wiggins R. Movement disorders moment: pain and palliative care in movement disorders. Practical Neurology. 2021;20(4):63-67.
32. Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899-1907.
33. Tripathi M, Vibha D. Reversible dementias. Indian J Psychiatry. 2009; 51 Suppl 1(Suppl 1): S52-S55.
34. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717-1725.
35. Goldman J, Sieg, E. Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. 2020;36(2):365-377.
36. Gonzalez-Latapi P, Bayram E, Litvan I, et al. Cognitive impairment in Parkinson’s disease: epidemiology, clinical profile, protective and risk factors. Behav Sci (Basel). 2021;11(5):74.
37. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force Guidelines. Mov Disord. 2012;27(3):349-356.
38. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314-2324.
39. Aarsland D, Batzu L, Halliday GM, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47. doi:10.1038/s41572-021-00280-3
40. Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson’s disease. Int Rev Neurobiol. 2017;133:679-717.
41. Vilas D, Pont-Sunyer C, Tolosa E. Impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S80-S84.
42. Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589-595.
43. Faouzi J, Corvol JC, Mariani LL. Impulse control disorders and related behaviors in Parkinson’s disease: risk factors, clinical and genetic aspects, and management. Curr Opin Neurol. 2021;34(4):547-555.
44. Samuel M, Rodriguez-Oroz M, Antonini A, et al. Impulse control disorders in Parkinson’s disease: management, controversies, and potential approaches. Mov Disord. 2015;30(2):150-159.
45. Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation and medication in Parkinsonism. Science. 2007;318(5854):1309-1312.
46. Jahanshahi M, Obeso I, Baunez C, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128-140.
47. Castrioto A, Lhommée E, Moro E, et al. Mood and behavioral effects of subthalamic stimulation in Parkinson’s disease. Lancet Neurol. 2014;13(3):287-305.
ADHD in older adults: A closer look
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).
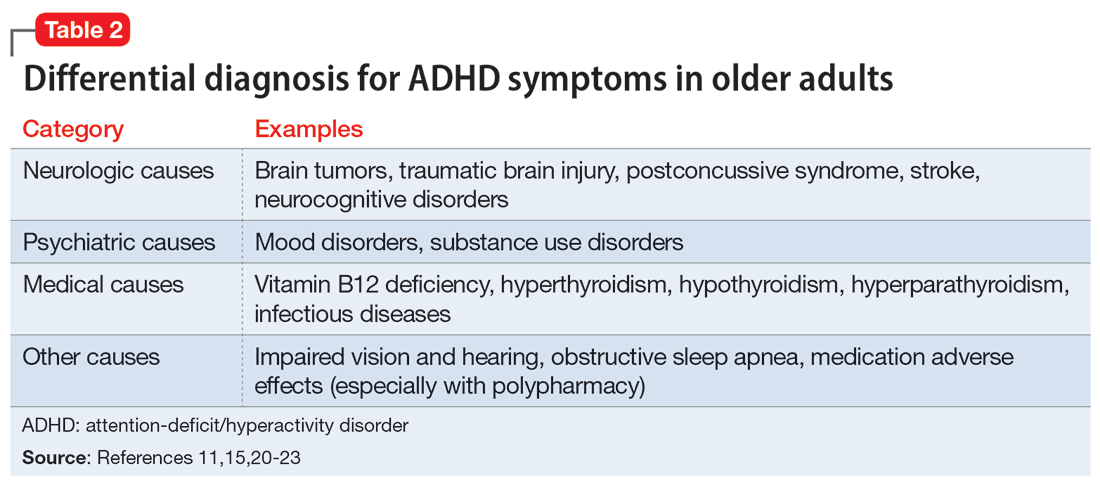
In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21
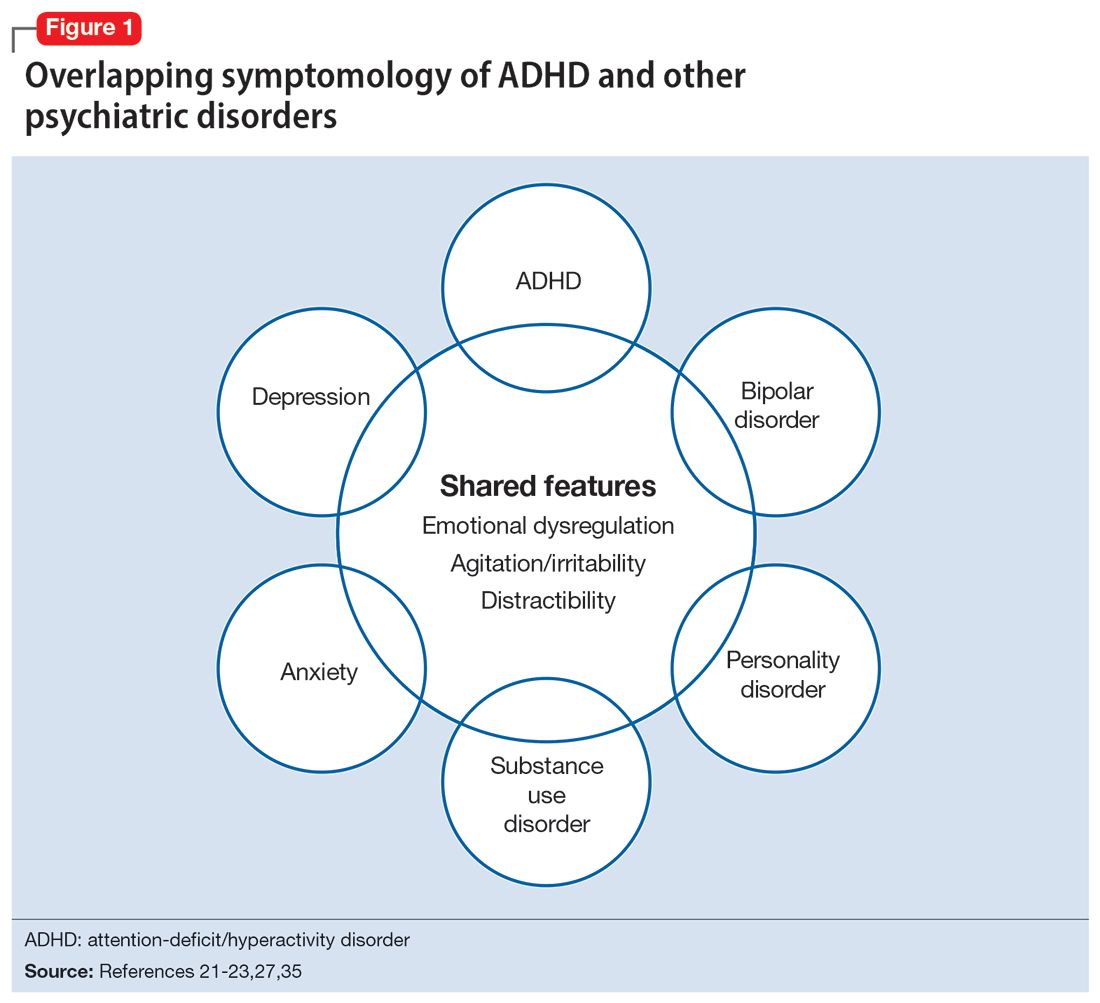
Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.
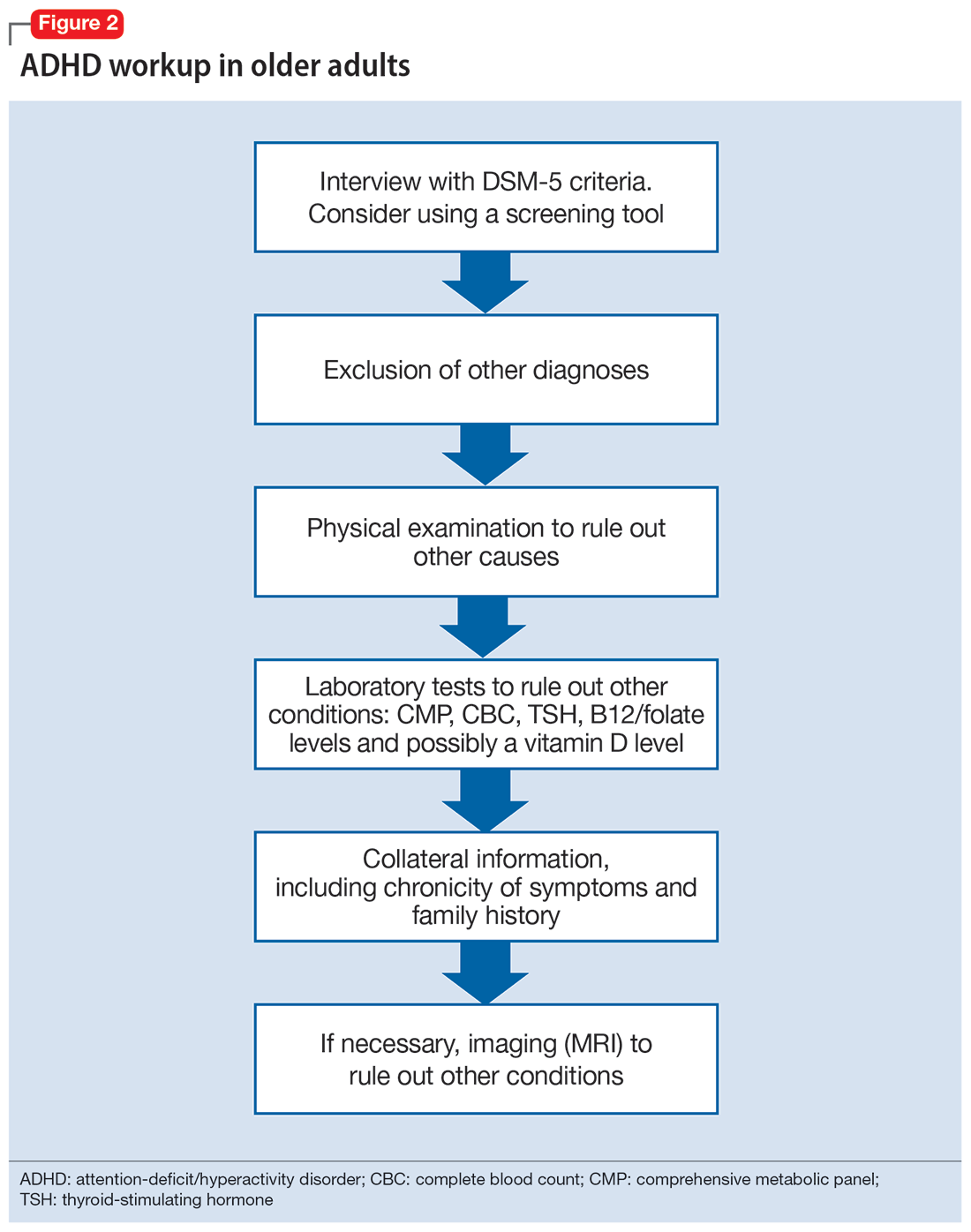
Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
Climate change and mental illness: What psychiatrists can do
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).
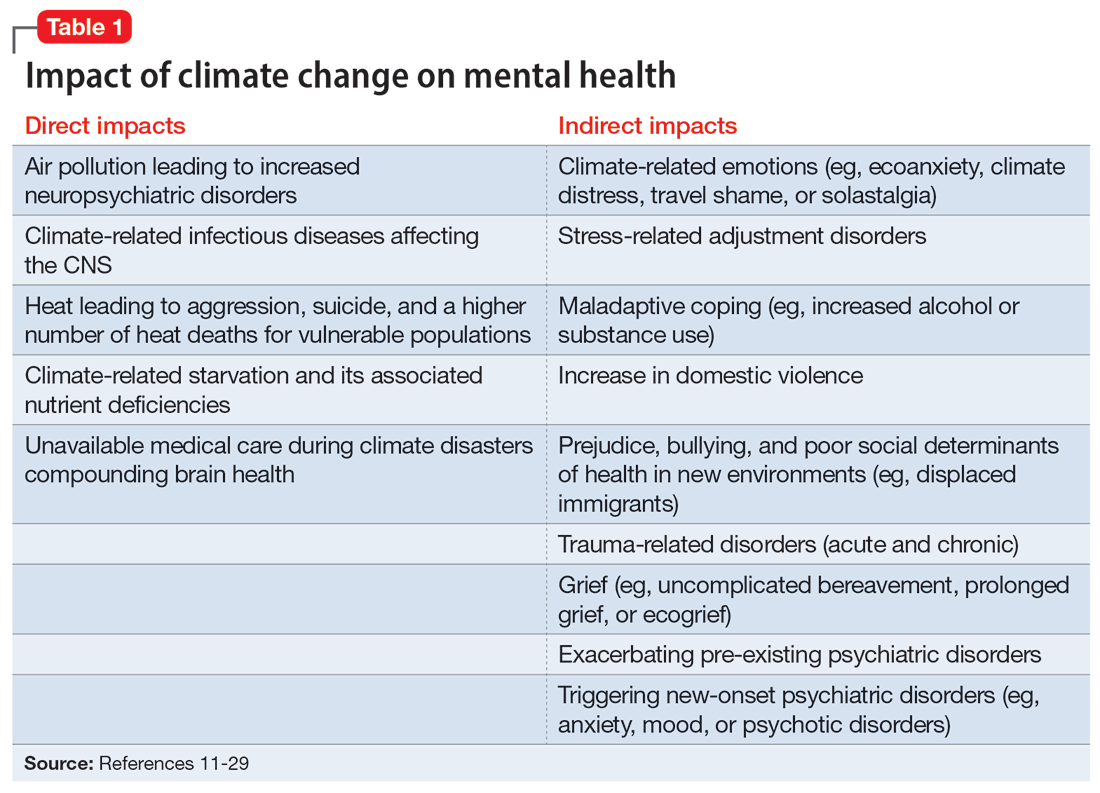
Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).
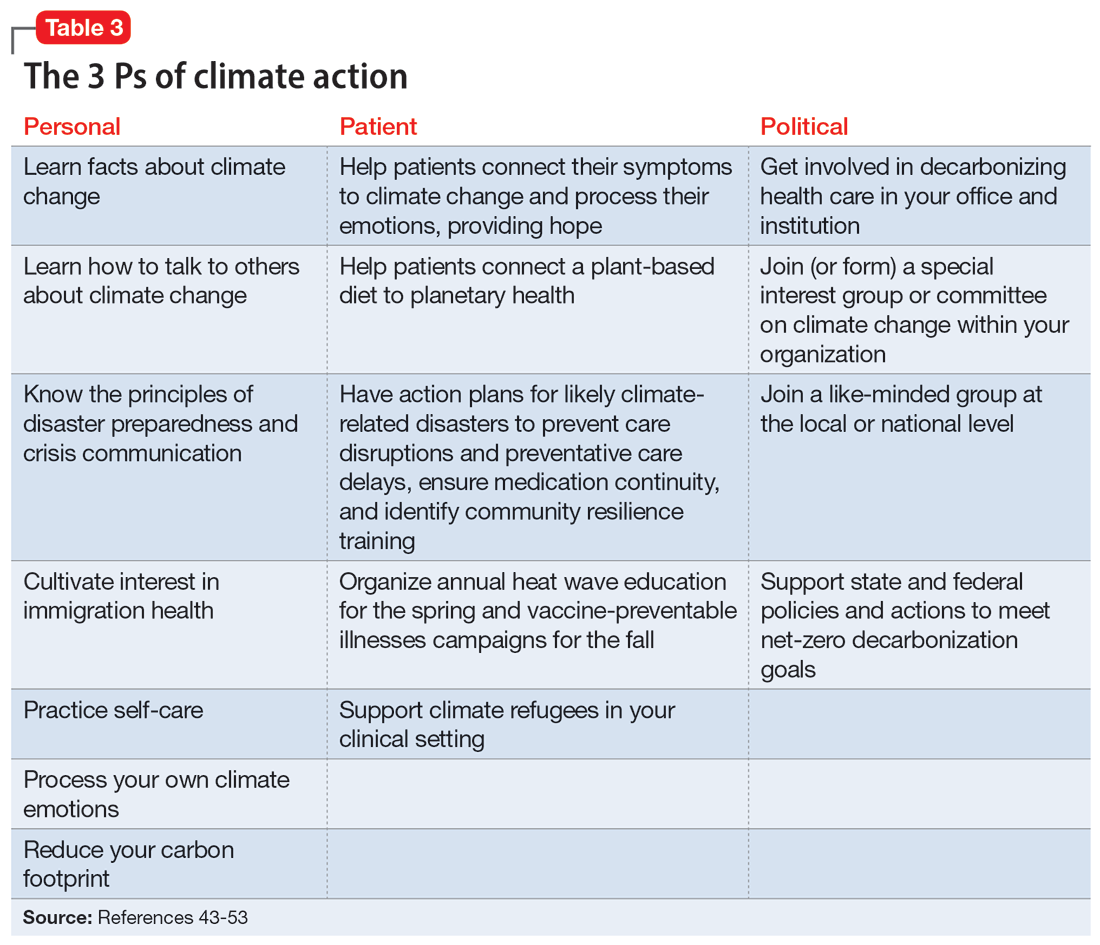
Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
“ Hope is engagement with the act of mapping our destinies.” 1
—Valerie Braithwaite
Why should psychiatrists care about climate change and try to mitigate its effects? First, we are tasked by society with managing the psychological and neuropsychiatric sequelae from disasters, which include climate change. The American Psychiatric Association’s position statement on climate change includes it as a legitimate focus for our specialty.2 Second, as physicians, we are morally obligated to do no harm. Since the health care sector contributes significantly to climate change (8.5% of national carbon emissions stem from health care) and causes demonstrable health impacts,3 managing these impacts and decarbonizing the health care industry is morally imperative.4 And third, psychiatric clinicians have transferrable skills that can address fears of climate change, challenge climate change denialism,5 motivate people to adopt more pro-environmental behaviors, and help communities not only endure the emotional impact of climate change but become more psychologically resilient.6
Most psychiatrists, however, did not receive formal training on climate change and the related field of disaster preparedness. For example, Harvard Medical School did not include a course on climate change in their medical student curriculum until 2023.7 In this article, we provide a basic framework of climate change and its impact on mental health, with particular focus on patients with serious mental illness (SMI). We offer concrete steps clinicians can take to prevent or mitigate harm from climate change for their patients, prepare for disasters at the level of individual patient encounters, and strengthen their clinics and communities. We also encourage clinicians to take active leadership roles in their professional organizations to be part of climate solutions, building on the trust patients continue to have in their physicians.8 Even if clinicians do not view climate change concerns under their conceived clinical care mandate, having a working knowledge about it is important because patients, paraprofessional staff, or medical trainees are likely to bring it up.9
Climate change and mental health
Climate change is harmful to human health, including mental health.10 It can impact mental health directly via its impact on brain function and neuropsychiatric sequelae, and indirectly via climate-related disasters leading to acute or chronic stress, losses, and displacement with psychiatric and psychological sequelae (Table 111-29).

Direct impact
The effects of air pollution, heat, infections, and starvation are examples of how climate change directly impacts mental health. Air pollution and brain health are a concern for psychiatry, given the well-described effects of air deterioration on the developing brain.11 In animal models, airborne pollutants lead to widespread neuroinflammation and cell loss via a multitude of mechanisms.12 This is consistent with worse cognitive and behavioral functions across a wide range of cognitive domains seen in children exposed to pollution compared to those who grew up in environments with healthy air.13 Even low-level exposure to air pollution increases the risk for later onset of depression, suicide, and anxiety.14 Hippocampal atrophy observed in patients with first-episode psychosis may also be partially attributable to air pollution.15 An association between heat and suicide (and to a lesser extent, aggression) has also been reported.16
Worse physical health (eg, strokes) due to excessive heat can further compound mental health via elevated rates of depression. Data from the United States and Mexico show that for each degree Celsius increase in ambient temperature, suicide rates may increase by approximately 1%.17 A meta-analysis by Frangione et al18 similarly concluded that each degree Celsius increase results in an overall risk ratio of 1.016 (95% CI, 1.012 to 1.019) for deaths by suicide and suicide attempts. Additionally, global warming is shifting the endemic areas for many infectious agents, particularly vector-borne diseases,19 to regions in which they had hitherto been unknown, increasing the risk for future outbreaks and even pandemics.20 These infectious illnesses often carry neuropsychiatric morbidity, with seizures, encephalopathy with incomplete recovery, and psychiatric syndromes occurring in many cases. Crop failure can lead to starvation during pregnancy and childhood, which has wide-ranging consequences for brain development and later physical and psychological health in adults.21,22 Mothers affected by starvation also experience negative impacts on childbearing and childrearing.23
Indirect impact
Climate change’s indirect impact on mental health can stem from the stress of living through a disaster such as an extreme weather event; from losses, including the death of friends and family members; and from becoming temporarily displaced.24 Some climate change–driven disasters can be viewed as slow-moving, such as drought and the rising of sea levels, where displacement becomes permanent. Managing mass migration from internally or externally displaced people who must abandon their communities because of climate change will have significant repercussions for all societies.25 The term “climate refugee” is not (yet) included in the United Nations’ official definition of refugees; it defines refugees as individuals who have fled their countries because of war, violence, or persecution.26 These and other bureaucratic issues can come up when clinicians are trying to help migrants with immigration-related paperwork.
Continue to: As the inevitability of climate change...
As the inevitability of climate change sinks in, its long-term ramifications have introduced a new lexicon of psychological suffering related to the crisis.27 Common terms for such distress include ecoanxiety (fear of what is happening and will happen with climate change), ecogrief (sadness about the destruction of species and natural habitats), solastalgia28 (the nostalgia an individual feels for emotionally treasured landscapes that have changed), and terrafuria or ecorage (the reaction to betrayal and inaction by governments and leaders).29 Climate-related emotions can lead to pessimism about the future and a nihilistic outlook on an individual’s ability to effect change and have agency over their life’s outcomes.
The categories of direct and indirect impacts are not mutually exclusive. A child may be starving due to weather-related crop failure as the family is forced to move to another country, then have to contend with prejudice and bullying as an immigrant, and later become anxiously preoccupied with climate change and its ability to cause further distress.
Effect on individuals with serious mental illness
Patients with SMI are particularly vulnerable to the impact of climate change. They are less resilient to climate change–related events, such as heat waves or temporary displacement from flooding, both at the personal level due to illness factors (eg, negative symptoms or cognitive impairment) and at the community level due to social factors (eg, weaker social support or poverty).
Recognizing the increased vulnerability to heat waves and preparing for them is particularly important for patients with SMI because they are at an increased risk for heat-related illnesses.30 For example, patients may not appreciate the danger from heat and live in conditions that put them at risk (ie, not having air conditioning in their home or living alone). Their illness alone impairs heat regulation31; patients with depression and anxiety also dissipate heat less effectively.32,33 Additionally, many psychiatric medications, particularly antipsychotics, impair key mechanisms of heat dissipation.34,35 Antipsychotics render organisms more poikilothermic (susceptible to environmental temperature, like cold-blooded animals) and can be anticholinergic, which impedes sweating. A recent analysis of heat-related deaths during a period of extreme and prolonged heat in British Columbia in 2021 affirmed these concerns, reporting that patients with schizophrenia had the highest odds of death during this heat-related event.36
COVID-19 has shown that flexible models of care are needed to prevent disengagement from medical and psychiatric care37 and assure continued treatment with essential medications such as clozapine38 and long-acting injectable antipsychotics39 during periods of social change, as with climate change. While telehealth was critical during the COVID-19 pandemic40 and is here to stay, it alone may be insufficient given the digital divide (patients with SMI may be less likely to have access to or be proficient in the use of digital technologies). The pandemic has shown the importance of public health efforts, including benefits from targeted outreach, with regards to vaccinations for this patient group.41,42Table 2 summarizes things clinicians should consider when preparing patients with SMI for the effects of climate change.

Continue to: The psychiatrist's role
The psychiatrist’s role
There are many ways a psychiatrist can professionally get involved in addressing climate change. Table 343-53 outlines the 3 Ps of climate action (taking actions to mitigate the effects of climate change): personal, patient (and clinic), and political (advocacy).

Personal
Even if clinicians believe climate change is important for their clinical work, they may still feel overwhelmed and unsure what to do in the context of competing responsibilities. A necessary first step is overcoming paralysis from the enormity of the problem, including the need to shift away from an expanding consumption model to environmental sustainability in a short period of time.
A good starting point is to get educated on the facts of climate change and how to discuss it in an office setting as well as in your personal life. A basic principle of climate change communication is that constructive hope (progress achieved despite everything) coupled with constructive doubt (the reality of the threat) can mobilize people towards action, whereas false hope or fatalistic doubt impedes action.43 The importance of optimal public health messaging cannot be overstated; well-meaning campaigns to change behavior can fail if they emphasize the wrong message. For example, in a study examining COVID-19 messaging in >80 countries, Dorison et al44 found that negatively framed messages mostly increased anxiety but had no benefit with regard to shifting people toward desired behaviors.
In addition, clinicians can learn how to confront climate disavowal and difficult emotions in themselves and even plan to shift to carbon neutrality, such as purchasing carbon offsets or green sources of energy and transportation. They may not be familiar with principles of disaster preparedness or crisis communication.46 Acquiring those professional skills may suggest next steps for action. Being familiar with the challenges and resources for immigrants, including individuals displaced due to climate change, may be necessary.47 Finally, to reduce the risk of burnout, it is important to practice self-care, including strategies to reduce feelings of being overwhelmed.
Patient
In clinical encounters, clinicians can be proactive in helping patients understand their climate-related anxieties around an uncertain future, including identifying barriers to climate action.48
Continue to: Clinics must prepare for disasters...
Clinics must prepare for disasters in their communities to prevent disruption of psychiatric care by having an action plan, including the provision of medications. Such action plans should be prioritized for the most likely scenarios in an individual’s setting (eg, heat waves, wildfires, hurricanes, or flooding).
It is important to educate clinic staff and include them in planning for emergencies, because an all-hands approach and buy-in from all team members is critical. Clinicians should review how patients would continue to receive services, particularly medications, in the event of a disaster. In some cases, providing a 90-day medication supply will suffice, while in others (eg, patients receiving long-acting antipsychotics or clozapine) more preparation is necessary. Some events are predictable and can be organized annually, such as clinicians becoming vaccine ambassadors and organizing vaccine campaigns every fall50; winter-related disaster preparation every fall; and heat wave education every spring (leaflets for patients, staff, and family members; review of safety of medications during heat waves). Plan for, monitor, and coordinate medical care and services for climate refugees and other populations that may otherwise delay medical care and impede illness prevention. Finally, support climate refugees, including connecting them to services or providing trauma-informed care.
Political
Some clinicians may feel compelled to become politically active to advocate for changes within the health care system. Two initiatives related to decarbonizing the health care sector are My Green Doctor51 and Health Care Without Harm,52 which offer help in shifting your office, clinic, or hospital towards carbon neutrality.
Climate change unevenly affects people and will continue to exacerbate inequalities in society, including individuals with mental illness.53 To work toward climate justice on behalf of their patients, clinicians could join (or form) climate committees of special interest groups in their professional organizations or setting. Joining like-minded groups working on climate change at the local or national level prevents an omission of a psychiatric voice and counteracts burnout. It is important to stay focused on the root causes of the problem during activism: doing something to reduce fossil fuel use is ultimately most important.54 The concrete goal of reaching the Paris 1.5-degree Celsius climate goal is a critical benchmark against which any other action can be measured.54
Planning for the future
Over the course of history, societies have always faced difficult periods in which they needed to rebuild after natural disasters or self-inflicted catastrophes such as terrorist attacks or wars. Since the advent of the nuclear age, people have lived under the existential threat of nuclear war. The Anthropocene is a proposed geological term that reflects the enormous and possibly disastrous impact human activity has had on our planet.55 While not yet formally adopted, this term has heuristic value, directing attention and reflection to our role and its now undisputed consequences. In the future, historians will debate if the scale of our current climate crisis has been different. It is, however, not controversial that humanity will be faced with the effects of climate change for the foreseeable future.10 Already, even “normal” weather events are fueled by energy in overcharged and altered weather systems due to global warming, leading to weather events ranging from droughts to floods and storms that are more severe, more frequent, and have longer-lasting effects on communities.56
Continue to: As physicians, we are tasked...
As physicians, we are tasked by society to create and maintain a health care system that addresses the needs of our patients and the communities in which they live. Increasingly, we are forced to contend with an addition to the traditional 5 phases of acute disaster management (prevention, mitigation, preparedness, response, and recovery) to manage prolonged or even parallel disasters, where a series of disasters occurs before the community has recovered and healed. We must grapple with a sense of an “extended period of insecurity and instability” (permacrisis) and must better prepare for and prevent the polycrisis (many simultaneous crises) or the metacrisis of our “age of turmoil”57 in which we must limit global warming, mitigate its damage, and increase community resilience to adapt.
Leading by personal example and providing hope may be what some patients need, as the reality of climate change contributes to the general uneasiness about the future and doomsday scenarios to which many fall victim. At the level of professional societies, many are calling for leadership, including from mental health organizations, to bolster the “social climate,” to help us strengthen our emotional resilience and social bonds to better withstand climate change together.58 It is becoming harder to justify standing on the sidelines,59 and it may be better for both our world and a clinician’s own sanity to be engaged in professional and private hopeful action1 to address climate change. Without ecological or planetary health, there can be no mental health.
Bottom Line
Clinicians can prepare their patients for climate-related disruptions and manage the impact climate change has on their mental health. Addressing climate change at clinical and political levels is consistent with the leadership roles and professional ethics clinicians face in daily practice.
Related Resources
- Lim C, MacLaurin S, Freudenreich O. Preparing patients with serious mental illness for extreme HEAT. Current Psychiatry. 2022;21(9):27-28. doi:10.12788/cp.0287
- My Green Doctor. https://mygreendoctor.org/
- The Climate Resilience for Frontline Clinics Toolkit from Americares. https://www.americares.org/what-we-do/community-health/climate-resilient-health-clinics
- Climate Psychiatry Alliance. https://www.climatepsychiatry.org/
Drug Brand Names
Clozapine • Clozaril
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
1. Kretz L. Hope in environmental philosophy. J Agricult Environ Ethics. 2013;26:925-944. doi:10.1007/s10806-012-9425-8
2. Ursano RJ, Morganstein JC, Cooper R. Position statement on mental health and climate change. American Psychiatric Association. March 2023. Accessed August 6, 2023. https://www.psychiatry.org/getattachment/0ce71f37-61a6-44d0-8fcd-c752b7e935fd/Position-Mental-Health-Climate-Change.pdf
3. Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
4. Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. health sector - a call to action. N Engl J Med. 2021;385(23):2117-2119. doi:10.1056/NEJMp2115675
5. Haase E, Augustinavicius JH, K. Climate change and psychiatry. In: Tasman A, Riba MB, Alarcón RD, et al, eds. Tasman’s Psychiatry. 5th ed. Springer; 2023.
6. Belkin G. Mental health and the global race to resilience. Psychiatr Times. 2023;40(3):26.
7. Hu SR, Yang JQ. Harvard Medical School will integrate climate change into M.D. curriculum. The Harvard Crimson. February 3, 2023. Accessed August 6, 2023. https://www.thecrimson.com/article/2023/2/3/hms-climate-curriculum/#:~:text=The%20new%20climate%20change%20curriculum,in%20arriving%20at%20climate%20solutions
8. Funk C, Gramlich J. Amid coronavirus threat, Americans generally have a high level of trust in medical doctors. Pew Research Center. March 13, 2020. Accessed August 6, 2023. https://www.pewresearch.org/fact-tank/2020/03/13/amid-coronavirus-threat-americans-generally-have-a-high-level-of-trust-in-medical-doctors/
9. Coverdale J, Balon R, Beresin EV, et al. Climate change: a call to action for the psychiatric profession. Acad Psychiatry. 2018;42(3):317-323. doi:10.1007/s40596-018-0885-7
10. Intergovernmental Panel on Climate Change. AR6 synthesis report: climate change 2023. Accessed August 6, 2023. https://www.ipcc.ch/report/sixth-assessment-report-cycle/
11. Perera FP. Multiple threats to child health from fossil fuel combustion: impacts of air pollution and climate change. Environ Health Perspect. 2017;125(2):141-148. doi:10.1289/EHP299
12. Hahad O, Lelieveldz J, Birklein F, et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int J Mol Sci. 2020;21(12):4306. doi:10.3390/ijms21124306
13. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational Neurosci. 2016;7(1):24-30. doi:10.1515/tnsci-2016-0005
14. Yang T, Wang J, Huang J, et al. Long-term exposure to multiple ambient air pollutants and association with incident depression and anxiety. JAMA Psychiatry. 2023;80:305-313. doi:10.1001/jamapsychiatry.2022.4812
15. Worthington MA, Petkova E, Freudenreich O, et al. Air pollution and hippocampal atrophy in first episode schizophrenia. Schizophr Res. 2020;218:63-69. doi:10.1016/j.schres.2020.03.001
16. Dumont C, Haase E, Dolber T, et al. Climate change and risk of completed suicide. J Nerv Ment Dis. 2020;208(7):559-565. doi:10.1097/NMD.0000000000001162
17. Burke M, Gonzales F, Bayis P, et al. Higher temperatures increase suicide rates in the United States and Mexico. Nat Climate Change. 2018;8:723-729. doi:10.1038/s41558-018-0222-x
18. Frangione B, Villamizar LAR, Lang JJ, et al. Short-term changes in meteorological conditions and suicide: a systematic review and meta-analysis. Environ Res. 2022;207:112230. doi:10.1016/j.envres.2021.112230
19. Rocklov J, Dubrow R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat Immunol. 2020;21(5):479-483. doi:10.1038/s41590-020-0648-y
20. Carlson CJ, Albery GF, Merow C, et al. Climate change increases cross-species viral transmission risk. Nature. 2022;607(7919):555-562. doi:10.1038/s41586-022-04788-w
21. Roseboom TJ, Painter RC, van Abeelen AFM, et al. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70(2):141-145. doi:10.1016/j.maturitas.2011.06.017
22. Liu Y, Diao L, Xu L. The impact of childhood experience of starvations on the health of older adults: evidence from China. Int J Health Plann Manage. 2021;36(2):515-531. doi:10.1002/hpm.3099
23. Rothschild J, Haase E. The mental health of women and climate change: direct neuropsychiatric impacts and associated psychological concerns. Int J Gynaecol Obstet. 2023;160(2):405-413. doi:10.1002/ijgo.14479
24. Cianconi P, Betro S, Janiri L. The impact of climate change on mental health: a systematic descriptive review. Frontiers Psychiatry. 2020;11:74. doi:10.3389/fpsyt.2020.00074
25. World Economic Forum. Climate refugees – the world’s forgotten victims. June 18, 2021. Accessed August 6, 2023. https://www.weforum.org/agenda/2021/06/climate-refugees-the-world-s-forgotten-victims
26. Climate Refugees. Accessed August 6, 2023. https://www.climate-refugees.org/why
27. Pihkala P. Anxiety and the ecological crisis: an analysis of eco-anxiety and climate anxiety. Sustainability. 2020;12(19):7836. doi:10.3390/su12197836
28. Galway LP, Beery T, Jones-Casey K, et al. Mapping the solastalgia literature: a scoping review study. Int J Environ Res Public Health. 2019;16(15):2662. doi:10.3390/ijerph16152662
29. Albrecht GA. Earth Emotions. New Words for a New World. Cornell University Press; 2019.
30. Sorensen C, Hess J. Treatment and prevention of heat-related illness. N Engl J Med. 2022;387(15):1404-1413. doi:10.1056/NEJMcp2210623
31. Chong TWH, Castle DJ. Layer upon layer: thermoregulation in schizophrenia. Schizophr Res. 2004;69(2-3):149-157. doi:10.1016/s0920-9964(03)00222-6
32. von Salis S, Ehlert U, Fischer S. Altered experienced thermoregulation in depression--no evidence for an effect of early life stress. Front Psychiatry. 2021;12:620656. doi:10.3389/fpsyt.2021.620656
33. Sarchiapone M, Gramaglia C, Iosue M, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: a systematic review and narrative synthesis. BMC Psychiatry. 2018;18(1):22. doi:10.1186/s12888-017-1551-4
34. Martin-Latry K, Goumy MP, Latry P, et al. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry. 2007;22(6):335-338. doi:10.1016/j.eurpsy.2007.03.007
35. Ebi KL, Capon A, Berry P, et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698-708. doi:10.1016/S0140-6736(21)01208-3
36. Lee MJ, McLean KE, Kuo M, et al. Chronic diseases associated with mortality in British Columbia, Canada during the 2021 Western North America extreme heat event. Geohealth. 2023;7(3):e2022GH000729. doi:10.1029/2022GH000729
37. Busch AB, Huskamp HA, Raja P, et al. Disruptions in care for Medicare beneficiaries with severe mental illness during the COVID-19 pandemic. JAMA Netw Open. 2022;5(1):e2145677. doi:10.1001/jamanetworkopen.2021.45677
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223. doi:10.1503/jpn.200061
39. MacLaurin SA, Mulligan C, Van Alphen MU, et al. Optimal long-acting injectable antipsychotic management during COVID-19. J Clin Psychiatry. 2021;82(1): 20l13730. doi:10.4088/JCP.20l13730
40. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness. Psychiatr Serv. 2020;71(10):1078-1081. doi:10.1176/appi.ps.202000244
41. Van Alphen MU, Lim C, Freudenreich O. Mobile vaccine clinics for patients with serious mental illness and health care workers in outpatient mental health clinics. Psychiatr Serv. February 8, 2023. doi:10.1176/appi.ps.20220460
42. Lim C, Van Alphen MU, Maclaurin S, et al. Increasing COVID-19 vaccination rates among patients with serious mental illness: a pilot intervention study. Psychiatr Serv. 2022;73(11):1274-1277. doi:10.1176/appi.ps.202100702
43. Marlon JR, Bloodhart B, Ballew MT, et al. How hope and doubt affect climate change mobilization. Front Commun. May 21, 2019. doi:10.3389/fcomm.2019.00020
44. Dorison CA, Lerner JS, Heller BH, et al. In COVID-19 health messaging, loss framing increases anxiety with little-to-no concomitant benefits: experimental evidence from 84 countries. Affective Sci. 2022;3(3):577-602. doi:10.1007/s42761-022-00128-3
45. Maibach E. Increasing public awareness and facilitating behavior change: two guiding heuristics. George Mason University, Center for Climate Change Communication. September 2015. Accessed August 6, 2023. https://www.climatechangecommunication.org/wp-content/uploads/2018/06/Maibach-Two-hueristics-September-2015-revised.pdf
46. Koh KA, Raviola G, Stoddard FJ Jr. Psychiatry and crisis communication during COVID-19: a view from the trenches. Psychiatr Serv. 2021;72(5):615. doi:10.1176/appi.ps.202000912
47. Velez G, Adam B, Shadid O, et al. The clock is ticking: are we prepared for mass climate migration? Psychiatr News. March 24, 2023. Accessed August 6, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2023.04.4.3
48. Ingle HE, Mikulewicz M. Mental health and climate change: tackling invisible injustice. Lancet Planet Health. 2020;4:e128-e130. doi:10.1016/S2542-5196(20)30081-4
49. Shah UA, Merlo G. Personal and planetary health--the connection with dietary choices. JAMA. 2023;329(21):1823-1824. doi:10.1001/jama.2023.6118
50. Lim C, Van Alphen MU, Freudenreich O. Becoming vaccine ambassadors: a new role for psychiatrists. Current Psychiatry. 2021;20(8):10-11,17-21,26-28,38. doi:10.12788/cp.0155
51. My Green Doctor. Accessed August 6, 2023. https://mygreendoctor.org/
52. Healthcare Without Harm. Accessed August 6, 2023. https://noharm.org/
53. Levy BS, Patz JA. Climate change, human rights, and social justice. Ann Glob Health. 2015;81:310-322.
54. Intergovernmental Panel on Climate Change. Global warming of 1.5° C 2018. Accessed August 6, 2023. https://www.ipcc.ch/sr15/
55. Steffen W, Crutzen J, McNeill JR. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 2007;36(8):614-621. doi:10.1579/0044-7447(2007)36[614:taahno]2.0.co;2
56. American Meteorological Society. Explaining extreme events from a climate perspective. Accessed August 6, 2023. https://www.ametsoc.org/ams/index.cfm/publications/bulletin-of-the-american-meteorological-society-bams/explaining-extreme-events-from-a-climate-perspective/
57. Nierenberg AA. Coping in the age of turmoil. Psychiatr Ann. 2022;52(7):263. July 1, 2022. doi:10.3928/23258160-20220701-01
58. Belkin G. Leadership for the social climate. N Engl J Med. 2020;382(21):1975-1977. doi:10.1056/NEJMp2001507
59. Skinner JR. Doctors and climate change: first do no harm. J Paediatr Child Health. 2021;57(11):1754-1758. doi:10.1111/jpc.15658
Off-label medications for addictive disorders
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52
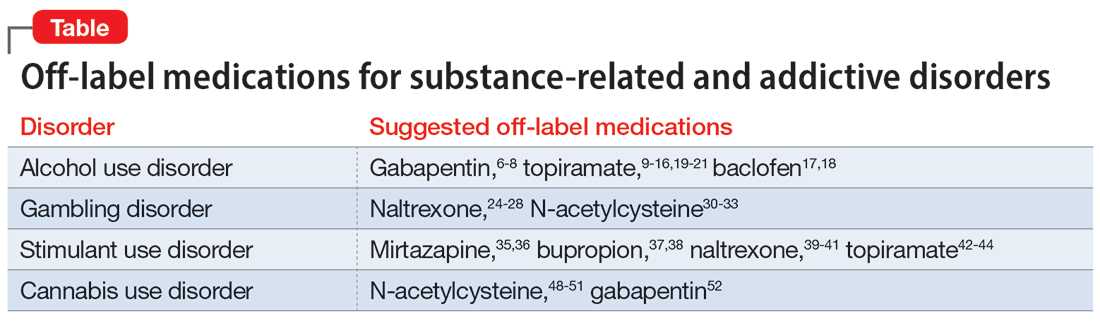
The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Burnout among surgeons: Lessons for psychiatrists
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.
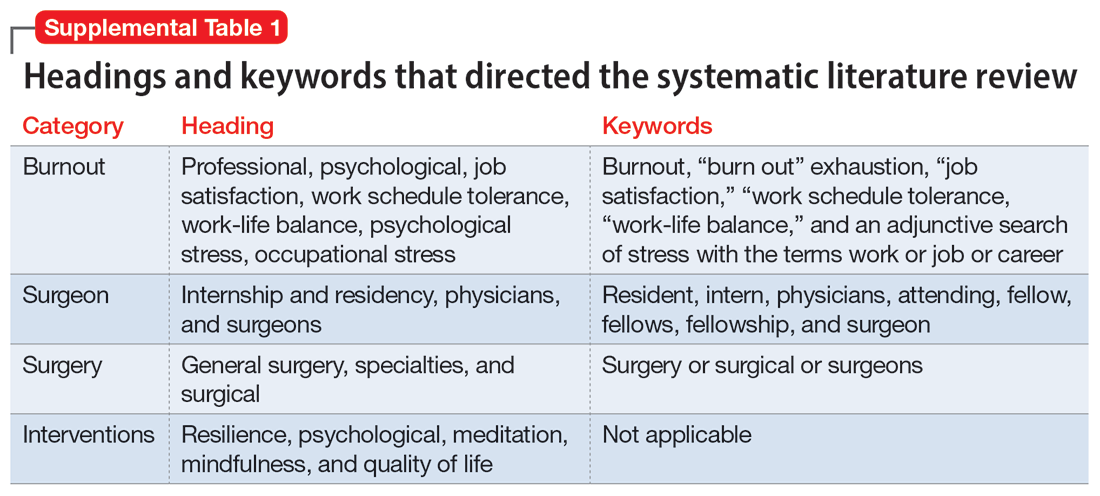
Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.
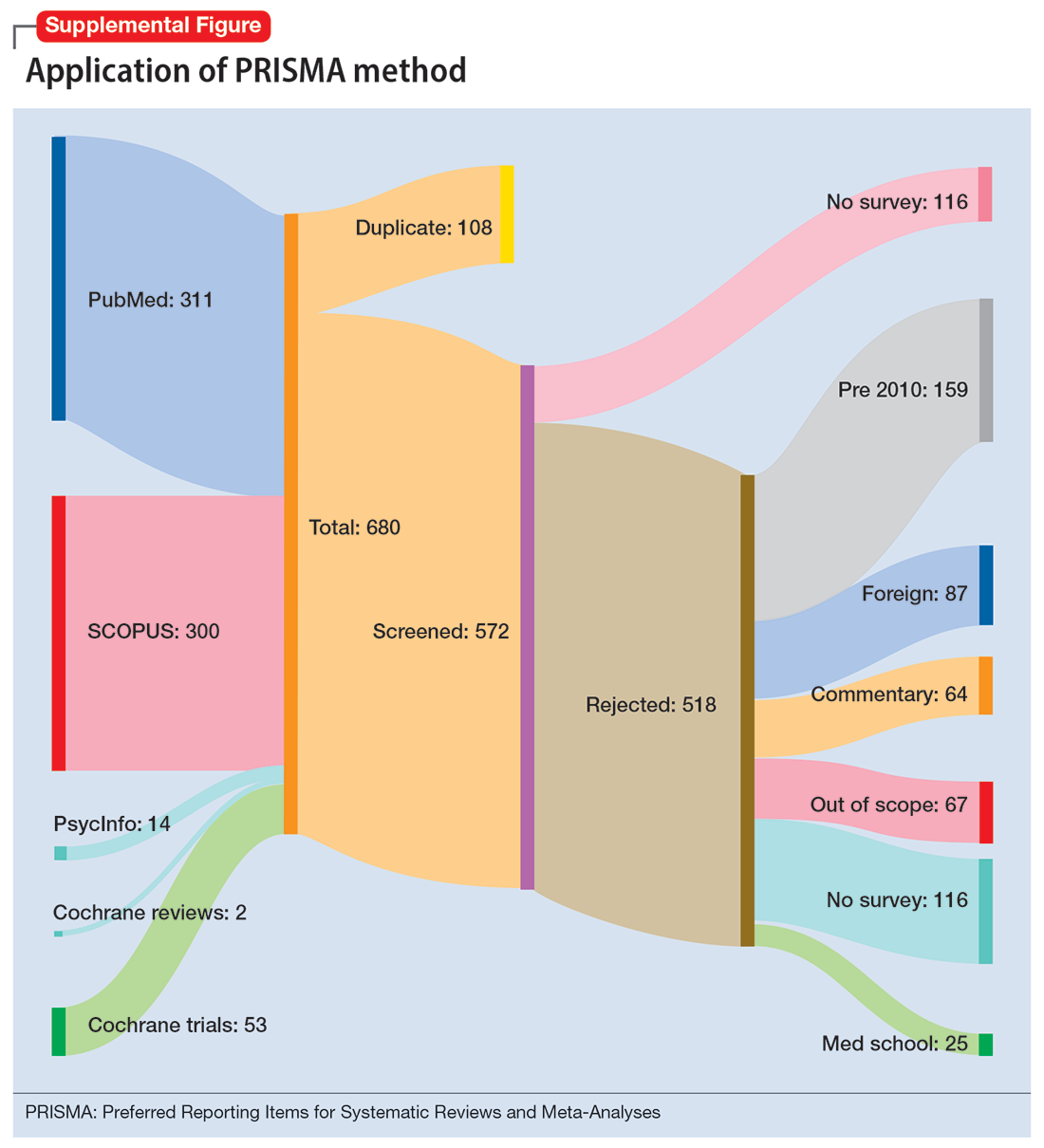
Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.
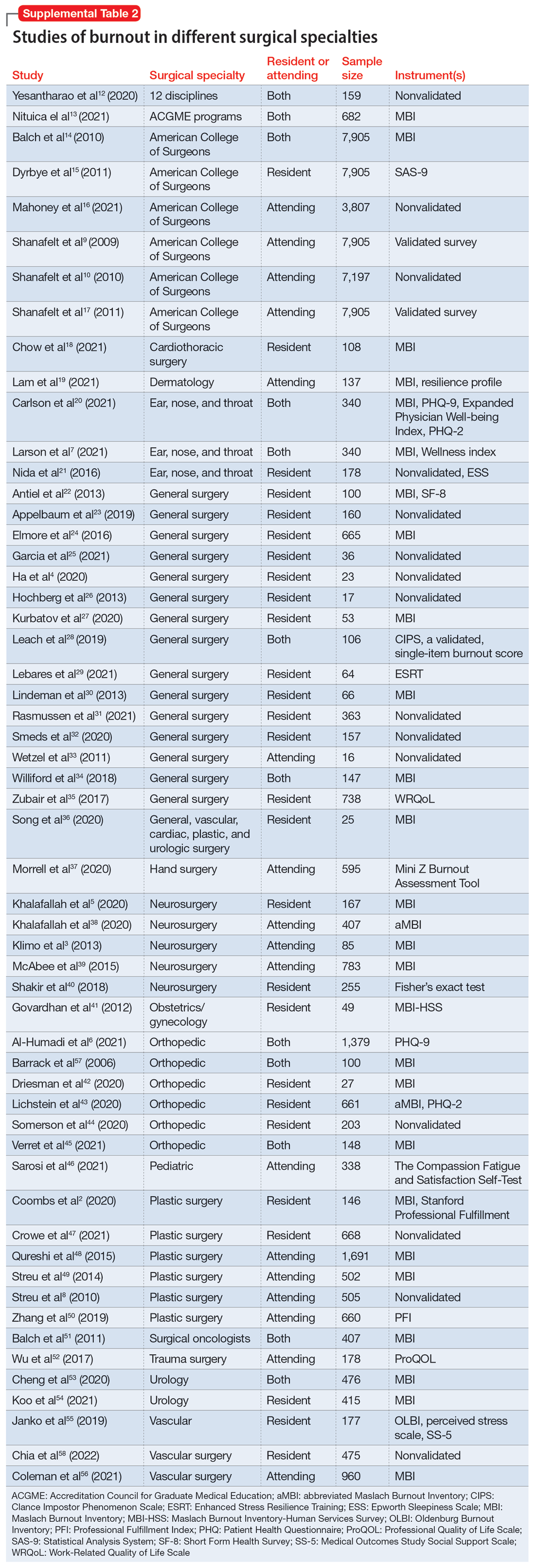
Work/life balance factors
Fifteen studies
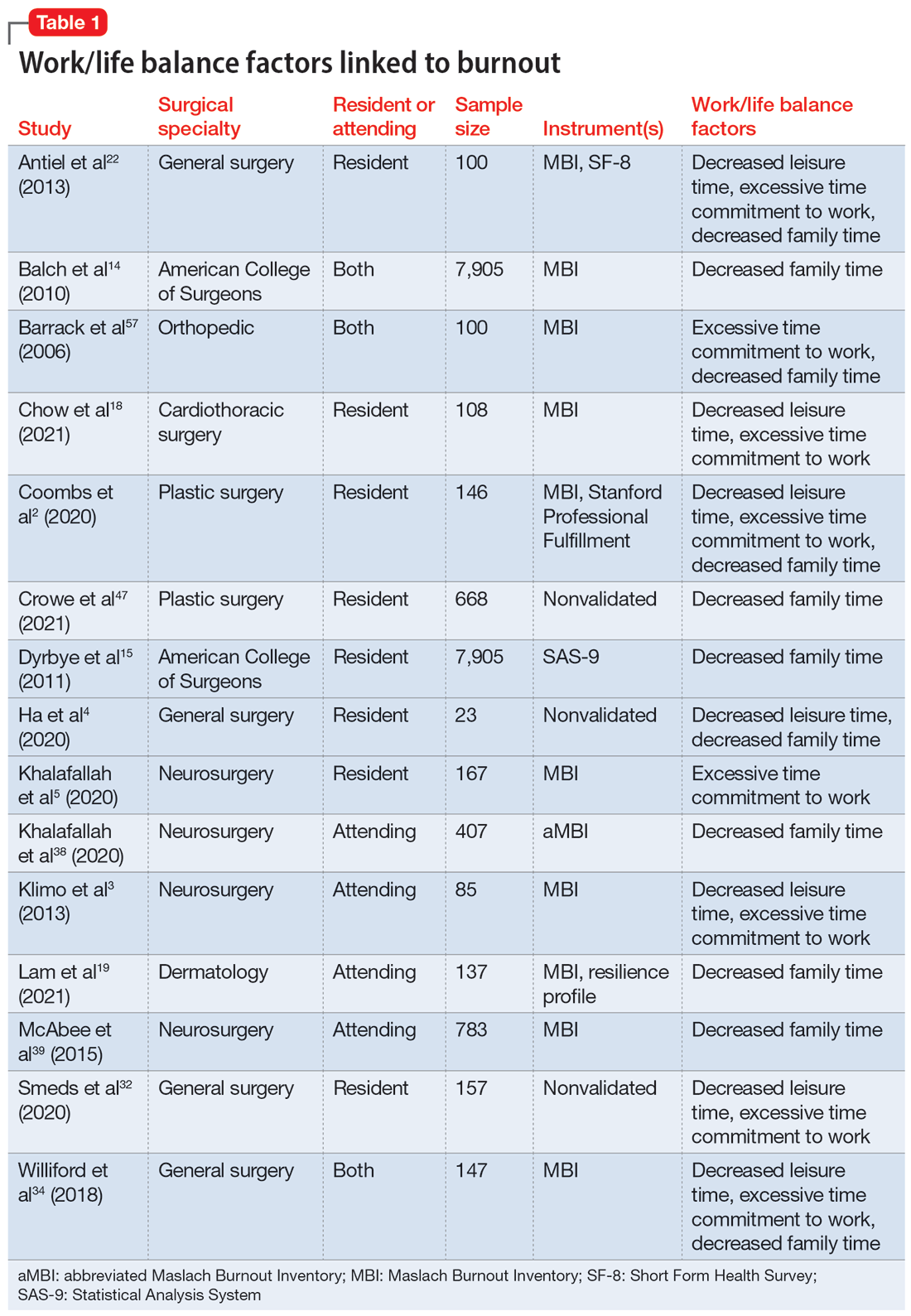
Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies
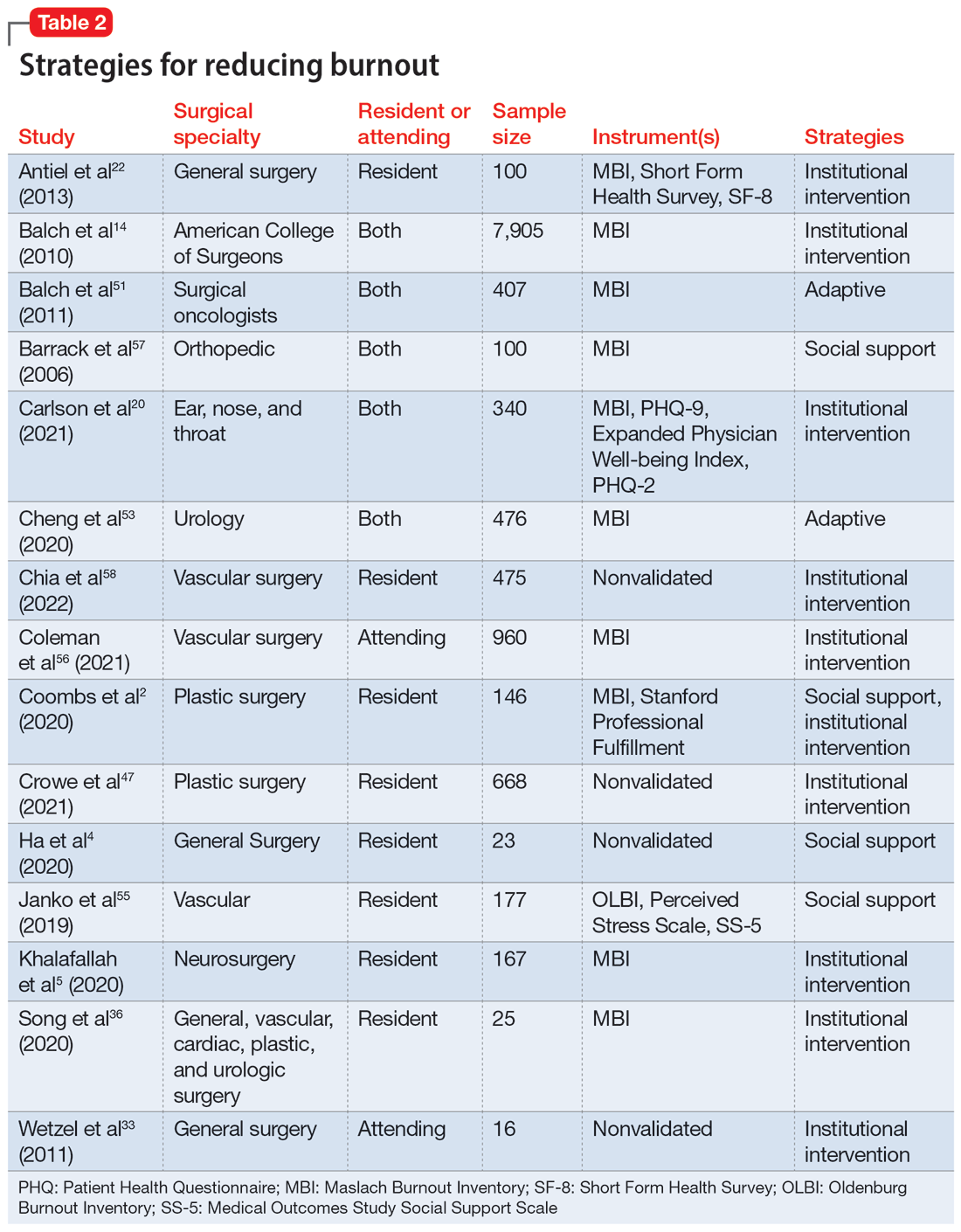
Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Risk Evaluation and Mitigation Strategy programs: How they can be improved
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).
Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.

Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.
Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.
Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration