User login
A 32-year-old man presents to the emergency department with excruciating abdominal pain associated with multiple episodes of vomiting for the past 2 days. He reports no fevers, headaches, diarrhea, constipation, hematochezia, melena, musculoskeletal symptoms, or weight loss. His abdominal pain is generalized and crampy. It does not radiate and has no precipitating factors. The pain is relieved only with intravenous narcotics.
He does not smoke, drink alcohol, or use illicit drugs. He has no known drug or food allergies. He says that his current condition causes him emotional stress that affects his performance at work.
About a year ago, after a complicated surgical procedure, he needed chronic high-dose narcotics. A few months later, he developed multiple bouts of abdominal pain and vomiting that required hospital visits. He now takes oral oxycodone 10–15 mg every 4–6 hours.
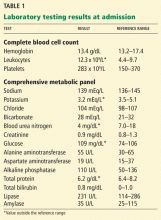
On admission, his vital signs are stable, but he is in excruciating pain. He is alert and oriented to person, place, and time. His sclera are anicteric, and the pupils are equal, round, and reactive to light. Lung and heart examinations are normal. The abdomen is soft and nondistended but tender in all four quadrants without guarding; the liver and spleen are not palpable, and no abdominal masses are detected. He has no skin rash, joint swelling or tenderness, or peripheral edema. The neurologic examination is normal. Computed tomography (CT) of the abdomen with contrast shows no signs of bowel obstruction, pancreatic calcifications or edema, cholecystitis, or hepatobiliary disease. Results of initial laboratory testing are shown in Table 1.
1. Based on the information available, which is the least likely cause of his symptoms?
- Acute pancreatitis
- Cyclic vomiting syndrome
- Acute intermittent porphyria
- Gastroparesis
Acute pancreatitis
Acute pancreatitis is the least likely cause of his symptoms. It is commonly caused by gallstones, alcohol, hypertriglyceridemia, and certain drugs.1 The associated abdominal pain is usually epigastric, radiates to the back, and is accompanied by nausea or vomiting, or both. The onset of pain is sudden and rapidly increases in severity within 30 minutes. CT shows enlargement of the pancreas with diffuse edema, heterogeneity of pancreatic parenchyma, peripancreatic stranding, and peripancreatic fluid collections.1 The diagnosis is based on two of the following three criteria: abdominal pain characteristic of acute pancreatitis; a serum amylase or lipase concentration three or more times the upper limit of normal; and characteristic findings of acute pancreatitis on CT.1
Cyclic vomiting syndrome
Cyclic vomiting syndrome is thought to be caused by episodic dysautonomia, mitochondrial DNA mutations, and hypothalamic emetic response oversensitivity,2–4 but the exact pathogenesis is unknown. The syndrome has been strongly linked to migraine and to the chronic excessive use of cannabinoids.5–9 The Rome III diagnostic criteria10 are the following: the vomiting episodes are stereotypical, ie, they are acute and last for less than 1 week; the patient has had three or more episodes in the previous year; and the patient has no nausea or vomiting between episodes. The patient must meet all three criteria. A history of migraine or a family history of migraine further supports the diagnosis.
Acute intermittent porphyria
Acute intermittent porphyria is characterized by neurovisceral symptoms such as convulsions, paresis, autonomic dysfunction, constipation, and diarrhea that result from the overproduction of porphyrin precursors and deficiency of porphobilinogen deaminase.11
Most patients have poorly localized, severe, steady abdominal pain that develops over hours to days and that may persist for days to weeks.11 Since the pain is neuropathic, abdominal tenderness is usually minimal during an acute attack. Other clues include signs of ileus, such as constipation, nausea, abdominal distention, or decreased bowel sounds; bladder dysfunction, eg, urinary retention, incontinence, or dysuria; reddish-brown urine; and sensory neuropathy of the chest, back, and extremities.11 Blistering skin lesions are usually not seen. The presence of porphobilinogen in the urine confirms the diagnosis.11
Gastroparesis
Gastroparesis is a result of discoordination between the sympathetic and parasympathetic nervous systems, neurons, and smooth muscles within the stomach, causing a decrease in gastric motility. Common causes are diabetes,12 scleroderma,13 and neurologic disorders.14 It can also be iatrogenic,15 resulting from visceral nerve injury and drug treatment with narcotics, calcium channel blockers, muscarinic cholinergic antagonists, or certain antidepressants. Symptoms are related to gastric stasis, ie, abdominal pain from gastric distention, bloating, vomiting, and early satiety.15 Abdominal pain may worsen after eating, and vomitus usually consists of recently ingested food. These patients may have abdominal distension or tenderness and succussion splash. After excluding possible mechanical obstruction, a gastric-emptying study may be necessary to make the diagnosis.15
CASE CONTINUED
A serum and urine drug screen in our patient is positive only for opioids. Urine measures of delta-aminolevulinic acid and porphobilinogen are normal. CT angiography of the abdomen shows no signs of mesenteric vascular occlusion. Esophagogastroduodenoscopy shows antral gastritis, but the esophagus and duodenum appear normal, and colonoscopy is normal as well. Histologic study of biopsy specimens obtained during endoscopy is unrevealing. A gastric-emptying study shows delayed emptying. The patient’s abdominal pain and vomiting persist with the initial dose of intravenous narcotic but resolve with escalating doses. When asked, the patient denies an excessive need for hot baths.
2. Which is the most likely diagnosis at this point?
- Narcotic bowel syndrome
- Opioid withdrawal
- Crohn disease
- Chronic pancreatitis
- Chronic mesenteric ischemia
- Cannabinoid hyperemesis
Narcotic bowel syndrome
Narcotic bowel syndrome is the most likely diagnosis. Grunkemeier et al16 described it as chronic or frequently recurring abdominal pain that is treated with narcotics, either chronically or acutely with high doses, and that includes all the following features16:
- The pain worsens or resolves incompletely with continued or increasing doses of narcotics
- The pain markedly worsens when the narcotic dose is decreased, and decreases when the drug is reinstituted (the “soarand-crash” effect)
- The frequency, duration, and intensity of the pain episodes gradually increase
- The nature of the pain and its intensity are not explained by a current or previous gastrointestinal diagnosis.16
This syndrome is common in patients who receive high doses of narcotics for postoperative pain or for other, nonmalignant causes of pain. Patients eventually become dependent on the drugs but are not aware that chronic use activates and facilitates areas in the brain that enhance the perception of pain.16 A study of a rat model of narcotic bowel syndrome17 showed that morphine-induced hyperalgesia depends on central sensitization involving the activation of spinal microglia. This eventually results in concomitant peripheral sensitization involving the colonic mucosal neuroimmune system, and also in central or peripheral activation of opioid kappa-receptors by dynorphin release.17
Patients tend to present with chronic or intermittent colicky abdominal pain that requires escalating doses of narcotics. Eventually, they develop tachyphylaxis and shortened pain-free periods and will require even higher doses of narcotics. This ultimately enhances the perception of pain and worsens opioid bowel symptoms, causing a vicious circle of pain and more narcotic use.16
Laboratory tests are usually normal, and imaging may show only ileus. Gastric emptying may be delayed in patients who have either narcotic bowel syndrome or gastroparesis, but since abdominal pain from narcotic bowel syndrome is a result of central and visceral hypersensitivity, these patients perceive more severe abdominal pain than patients with gastroparesis alone.
Opioid withdrawal
Symptoms of opioid withdrawal may appear as soon as 6 to 24 hours after cessation of the opioid in patients known to be dependent on opioids. These patients present with crampy abdominal pain with nausea.18 Other symptoms include agitation, rhinorrhea, lacrimation, excessive yawning, arthralgias, papillary dilation, and piloerection.18
Our patient did not have the typical signs of opioid withdrawal.
Crohn disease
Crohn disease is a multisystem disorder with specific clinical and pathologic features. It is characterized by focal, asymmetric, transmural, and occasionally granulomatous inflammation primarily affecting the gastrointestinal tract.19 Characteristic symptoms include abdominal pain, chronic diarrhea with or without rectal bleeding, and weight loss. Extraintestinal signs may include anemia and inflammatory changes in the eyes, skin, and joints. The diagnosis is based on endoscopic, radiographic, and pathologic findings.19
Our patient did not have diarrhea or signs of Crohn disease on CT, endoscopy, or histology.
Chronic pancreatitis
Chronic pancreatitis involves progressive inflammatory changes resulting in permanent structural damage to the pancreas and subsequent exocrine and endocrine dysfunction.20 Patients have epigastric abdominal pain that often radiates to the back20; it is associated with eating and is partly relieved with leaning forward. Symptoms of pancreatic insufficiency such as fat malabsorption (resulting in steatorrhea and fat-soluble vitamin deficiency) and diabetes are common. Calcifications within the pancreas on CT suggest chronic pancreatitis.20 Serum lipase and amylase levels may be normal or slightly elevated.20
Our patient’s abdominal pain was not typical of pancreatitis. He had no signs or symptoms of pancreatic insufficiency and no calcifications within the pancreas.
Chronic mesenteric ischemia
Chronic mesenteric ischemia (“intestinal angina”) is caused by a reduction in intestinal blood flow as a result of occlusion, vasospasm, or hypoperfusion of the mesenteric vasculature.21 It is commonly seen in patients who smoke or who have atherosclerotic vascular disease. These patients have chronic dull or crampy abdominal pain that usually occurs within 1 hour after eating.21 To avoid pain, patients avoid eating, resulting in weight loss.21 CT angiography with multi-detector CT is as effective as angiography (the gold standard) in depicting splanchnic arterial anatomy.22
Our patient is young and has no known risk factors for atherosclerosis such as smoking. His abdominal pain is more intermittent than chronic and is not associated with eating.
Cannabinoid hyperemesis
Cannabinoid hyperemesis should be considered in patients with long-term cannabis use presenting with cyclic vomiting, abdominal pain, compulsive use of hot showers, and improvement of symptoms with cannabis cessation.23 Although cannabinoids have been recognized for their antiemetic effects, long-term use may eventually cause autonomic instability and disturbances in the hypothalamic-pituitary-adrenal axis, resulting in cyclic vomiting and thermoregulatory impairment.23
Although our patient presented with multiple episodes of vomiting and abdominal pain, he denied using marijuana, he tested negative for tetrahydrocannabinol, and he did not associate any relief of his symptoms with hot baths.
CASE CONTINUED
Our patient receives intravenous hydration, antiemetics, and a narcotic in tapering intravenous doses, and his symptoms gradually improve. He is discharged from the hospital. However, a few weeks later he is readmitted with the same symptoms of abdominal pain and nausea.
3. What is the cornerstone of treatment for narcotic bowel syndrome?
- Establishing a therapeutic relationship
- Detoxification
- Supportive management with intravenous fluids, antiemetics, and stool-softeners
- Medical management with a short-acting narcotic, clonidine, lorazepam, and desipramine
MANAGEMENT OF NARCOTIC BOWEL SYNDROME
An effective therapeutic relationship with the patient is the cornerstone of treatment and should be established before starting detoxification.17 The physician must first learn to accept that the patient’s condition is real and must show genuine empathy as well as provide information about the pathophysiologic basis of the condition, the rationale for withholding narcotics, and the detrimental role narcotics play in the vicious circle of pain.
Detoxification involves gradually withdrawing the narcotic and substituting a nonnarcotic such as an antidepressant for pain control, as well as prescribing a drug such as a benzodiazepine or clonidine to prevent withdrawal symptoms and a laxative to prevent constipation.17,24 The physician must reassure the patient that he or she will not be abandoned in pain and that all medications will be continuously adjusted as needed to keep him or her comfortable throughout the detoxification process.17,24 The physician must continuously gauge the patient’s willingness to continue with treatment and must also be readily available to address the patient’s concerns in a timely manner.17,24 Involving family members and friends may provide additional support to the patient. Referral to a functional gastrointestinal motility program, a pain specialist, and a psychologist may also be considered.17,24 Follow-up care is essential, even after the withdrawal program has ended.17,24
BACK TO THE PATIENT
After successfully establishing a therapeutic relationship and discussing the treatment plan with our patient, we started him on the same dosage of narcotic that he had been receiving, calculated in intravenous morphine equivalents to achieve maximal comfort, and then decreased the dosage by 10% to 33% daily until he was completely off narcotics. An antidepressant and a benzodiazepine were given simultaneously with narcotic tapering. Oral clonidine (0.1–0.4 mg/day) was given after the narcotic dosage was reduced to about half, and polyethylene glycol was given as needed for constipation. The total duration of detoxification was 7 days.
The patient was referred to a psychologist for cognitive-behavioral and relaxation therapy, as well as for encouragement and support. At 6 months, he had had no recurrence of symptoms.
TAKE-HOME MESSAGE
In the United States, the number of patients taking a narcotic for nonmalignant pain is increasing, 25 and physicians should be more aware of complications such as narcotic bowel syndrome.
Narcotic bowel syndrome should be suspected in any patient with prolonged narcotic use presenting with multiple recurrent episodes of abdominal pain after other causes are ruled out.
Establishing a good therapeutic relationship with the patient is the cornerstone of successful treatment. Patients who understand their condition and are willing to be treated tend to have better outcomes.
Supportive treatment, symptom relief, and emotional support during detoxification increase compliance.
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Boles RG, Adams K, Ito M, Li BU. Maternal inheritance in cyclic vomiting syndrome with neuromuscular disease. Am J Med Genet A 2003; 120A:474–482.
- Wang Q, Ito M, Adams K, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A 2004; 131:50–58.
- Taché Y. Cyclic vomiting syndrome: the corticotropinreleasing-factor hypothesis. Dig Dis Sci 1999; 44(suppl 8):79S–86S.
- Withers GD, Silburn SR, Forbes DA. Precipitants and aetiology of cyclic vomiting syndrome. Acta Paediatr 1998; 87:272–277.
- Whitney HB. Cyclic vomiting. A brief review of this affection as illustrated by a typical case. Arch Pediatr 1898; 15:839–845.
- Stickler GB. Relationship between cyclic vomiting syndrome and migraine. Clin Pediatr (Phila) 2005; 44:505–508.
- Li BU, Murray RD, Heitlinger LA, Robbins JL, Hayes JR. Is cyclic vomiting syndrome related to migraine? J Pediatr 1999; 134:567–572.
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570.
- Rome Foundation. Rome III disorders and diagnostic criteria. http://www.romecriteria.org/criteria/. Accessed February 27, 2013.
- Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 2005; 142:439–450.
- Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med 2007; 356:820–829.
- Maddern GJ, Horowitz M, Jamieson GG, Chatterton BE, Collins PJ, Roberts-Thomson P. Abnormalities of esophageal and gastric emptying in progressive systemic sclerosis. Gastroenterology 1984; 87:922–926.
- Jost WH. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol Sci 2010; 289:69–73.
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127:1592–1622.
- Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5:1126–1139.
- Agostini S, Eutamene H, Cartier C, et al. Evidence of central and peripheral sensitization in a rat model of narcotic bowel-like syndrome. Gastroenterology 2010; 139:553–563,563.e1–e5.
- Nicholls L, Bragaw L, Ruetsch C. Opioid dependence treatment and guidelines. J Manag Care Pharm 2010; 16(1 suppl B):S14–S21.
- Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol 2009; 104:465–483.
- Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med 1995; 332:1482–1490.
- American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology 2000; 118:951–953.
- Savastano S, Teso S, Corrà S, Fantozzi O, Miotto D. Multislice CT angiography of the celiac and superior mesenteric arteries: comparison with arteriographic findings. Radiol Med 2002; 103:456–463.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87:114–119.
- Drossman DA, Morris CB, Edwards H, et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol 2012; 107:1426–1440.
- Trescot AM, Boswell MV, Atluri SL, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician 2006; 9:1–39.
A 32-year-old man presents to the emergency department with excruciating abdominal pain associated with multiple episodes of vomiting for the past 2 days. He reports no fevers, headaches, diarrhea, constipation, hematochezia, melena, musculoskeletal symptoms, or weight loss. His abdominal pain is generalized and crampy. It does not radiate and has no precipitating factors. The pain is relieved only with intravenous narcotics.
He does not smoke, drink alcohol, or use illicit drugs. He has no known drug or food allergies. He says that his current condition causes him emotional stress that affects his performance at work.
About a year ago, after a complicated surgical procedure, he needed chronic high-dose narcotics. A few months later, he developed multiple bouts of abdominal pain and vomiting that required hospital visits. He now takes oral oxycodone 10–15 mg every 4–6 hours.
On admission, his vital signs are stable, but he is in excruciating pain. He is alert and oriented to person, place, and time. His sclera are anicteric, and the pupils are equal, round, and reactive to light. Lung and heart examinations are normal. The abdomen is soft and nondistended but tender in all four quadrants without guarding; the liver and spleen are not palpable, and no abdominal masses are detected. He has no skin rash, joint swelling or tenderness, or peripheral edema. The neurologic examination is normal. Computed tomography (CT) of the abdomen with contrast shows no signs of bowel obstruction, pancreatic calcifications or edema, cholecystitis, or hepatobiliary disease. Results of initial laboratory testing are shown in Table 1.
1. Based on the information available, which is the least likely cause of his symptoms?
- Acute pancreatitis
- Cyclic vomiting syndrome
- Acute intermittent porphyria
- Gastroparesis
Acute pancreatitis
Acute pancreatitis is the least likely cause of his symptoms. It is commonly caused by gallstones, alcohol, hypertriglyceridemia, and certain drugs.1 The associated abdominal pain is usually epigastric, radiates to the back, and is accompanied by nausea or vomiting, or both. The onset of pain is sudden and rapidly increases in severity within 30 minutes. CT shows enlargement of the pancreas with diffuse edema, heterogeneity of pancreatic parenchyma, peripancreatic stranding, and peripancreatic fluid collections.1 The diagnosis is based on two of the following three criteria: abdominal pain characteristic of acute pancreatitis; a serum amylase or lipase concentration three or more times the upper limit of normal; and characteristic findings of acute pancreatitis on CT.1
Cyclic vomiting syndrome
Cyclic vomiting syndrome is thought to be caused by episodic dysautonomia, mitochondrial DNA mutations, and hypothalamic emetic response oversensitivity,2–4 but the exact pathogenesis is unknown. The syndrome has been strongly linked to migraine and to the chronic excessive use of cannabinoids.5–9 The Rome III diagnostic criteria10 are the following: the vomiting episodes are stereotypical, ie, they are acute and last for less than 1 week; the patient has had three or more episodes in the previous year; and the patient has no nausea or vomiting between episodes. The patient must meet all three criteria. A history of migraine or a family history of migraine further supports the diagnosis.
Acute intermittent porphyria
Acute intermittent porphyria is characterized by neurovisceral symptoms such as convulsions, paresis, autonomic dysfunction, constipation, and diarrhea that result from the overproduction of porphyrin precursors and deficiency of porphobilinogen deaminase.11
Most patients have poorly localized, severe, steady abdominal pain that develops over hours to days and that may persist for days to weeks.11 Since the pain is neuropathic, abdominal tenderness is usually minimal during an acute attack. Other clues include signs of ileus, such as constipation, nausea, abdominal distention, or decreased bowel sounds; bladder dysfunction, eg, urinary retention, incontinence, or dysuria; reddish-brown urine; and sensory neuropathy of the chest, back, and extremities.11 Blistering skin lesions are usually not seen. The presence of porphobilinogen in the urine confirms the diagnosis.11
Gastroparesis
Gastroparesis is a result of discoordination between the sympathetic and parasympathetic nervous systems, neurons, and smooth muscles within the stomach, causing a decrease in gastric motility. Common causes are diabetes,12 scleroderma,13 and neurologic disorders.14 It can also be iatrogenic,15 resulting from visceral nerve injury and drug treatment with narcotics, calcium channel blockers, muscarinic cholinergic antagonists, or certain antidepressants. Symptoms are related to gastric stasis, ie, abdominal pain from gastric distention, bloating, vomiting, and early satiety.15 Abdominal pain may worsen after eating, and vomitus usually consists of recently ingested food. These patients may have abdominal distension or tenderness and succussion splash. After excluding possible mechanical obstruction, a gastric-emptying study may be necessary to make the diagnosis.15
CASE CONTINUED
A serum and urine drug screen in our patient is positive only for opioids. Urine measures of delta-aminolevulinic acid and porphobilinogen are normal. CT angiography of the abdomen shows no signs of mesenteric vascular occlusion. Esophagogastroduodenoscopy shows antral gastritis, but the esophagus and duodenum appear normal, and colonoscopy is normal as well. Histologic study of biopsy specimens obtained during endoscopy is unrevealing. A gastric-emptying study shows delayed emptying. The patient’s abdominal pain and vomiting persist with the initial dose of intravenous narcotic but resolve with escalating doses. When asked, the patient denies an excessive need for hot baths.
2. Which is the most likely diagnosis at this point?
- Narcotic bowel syndrome
- Opioid withdrawal
- Crohn disease
- Chronic pancreatitis
- Chronic mesenteric ischemia
- Cannabinoid hyperemesis
Narcotic bowel syndrome
Narcotic bowel syndrome is the most likely diagnosis. Grunkemeier et al16 described it as chronic or frequently recurring abdominal pain that is treated with narcotics, either chronically or acutely with high doses, and that includes all the following features16:
- The pain worsens or resolves incompletely with continued or increasing doses of narcotics
- The pain markedly worsens when the narcotic dose is decreased, and decreases when the drug is reinstituted (the “soarand-crash” effect)
- The frequency, duration, and intensity of the pain episodes gradually increase
- The nature of the pain and its intensity are not explained by a current or previous gastrointestinal diagnosis.16
This syndrome is common in patients who receive high doses of narcotics for postoperative pain or for other, nonmalignant causes of pain. Patients eventually become dependent on the drugs but are not aware that chronic use activates and facilitates areas in the brain that enhance the perception of pain.16 A study of a rat model of narcotic bowel syndrome17 showed that morphine-induced hyperalgesia depends on central sensitization involving the activation of spinal microglia. This eventually results in concomitant peripheral sensitization involving the colonic mucosal neuroimmune system, and also in central or peripheral activation of opioid kappa-receptors by dynorphin release.17
Patients tend to present with chronic or intermittent colicky abdominal pain that requires escalating doses of narcotics. Eventually, they develop tachyphylaxis and shortened pain-free periods and will require even higher doses of narcotics. This ultimately enhances the perception of pain and worsens opioid bowel symptoms, causing a vicious circle of pain and more narcotic use.16
Laboratory tests are usually normal, and imaging may show only ileus. Gastric emptying may be delayed in patients who have either narcotic bowel syndrome or gastroparesis, but since abdominal pain from narcotic bowel syndrome is a result of central and visceral hypersensitivity, these patients perceive more severe abdominal pain than patients with gastroparesis alone.
Opioid withdrawal
Symptoms of opioid withdrawal may appear as soon as 6 to 24 hours after cessation of the opioid in patients known to be dependent on opioids. These patients present with crampy abdominal pain with nausea.18 Other symptoms include agitation, rhinorrhea, lacrimation, excessive yawning, arthralgias, papillary dilation, and piloerection.18
Our patient did not have the typical signs of opioid withdrawal.
Crohn disease
Crohn disease is a multisystem disorder with specific clinical and pathologic features. It is characterized by focal, asymmetric, transmural, and occasionally granulomatous inflammation primarily affecting the gastrointestinal tract.19 Characteristic symptoms include abdominal pain, chronic diarrhea with or without rectal bleeding, and weight loss. Extraintestinal signs may include anemia and inflammatory changes in the eyes, skin, and joints. The diagnosis is based on endoscopic, radiographic, and pathologic findings.19
Our patient did not have diarrhea or signs of Crohn disease on CT, endoscopy, or histology.
Chronic pancreatitis
Chronic pancreatitis involves progressive inflammatory changes resulting in permanent structural damage to the pancreas and subsequent exocrine and endocrine dysfunction.20 Patients have epigastric abdominal pain that often radiates to the back20; it is associated with eating and is partly relieved with leaning forward. Symptoms of pancreatic insufficiency such as fat malabsorption (resulting in steatorrhea and fat-soluble vitamin deficiency) and diabetes are common. Calcifications within the pancreas on CT suggest chronic pancreatitis.20 Serum lipase and amylase levels may be normal or slightly elevated.20
Our patient’s abdominal pain was not typical of pancreatitis. He had no signs or symptoms of pancreatic insufficiency and no calcifications within the pancreas.
Chronic mesenteric ischemia
Chronic mesenteric ischemia (“intestinal angina”) is caused by a reduction in intestinal blood flow as a result of occlusion, vasospasm, or hypoperfusion of the mesenteric vasculature.21 It is commonly seen in patients who smoke or who have atherosclerotic vascular disease. These patients have chronic dull or crampy abdominal pain that usually occurs within 1 hour after eating.21 To avoid pain, patients avoid eating, resulting in weight loss.21 CT angiography with multi-detector CT is as effective as angiography (the gold standard) in depicting splanchnic arterial anatomy.22
Our patient is young and has no known risk factors for atherosclerosis such as smoking. His abdominal pain is more intermittent than chronic and is not associated with eating.
Cannabinoid hyperemesis
Cannabinoid hyperemesis should be considered in patients with long-term cannabis use presenting with cyclic vomiting, abdominal pain, compulsive use of hot showers, and improvement of symptoms with cannabis cessation.23 Although cannabinoids have been recognized for their antiemetic effects, long-term use may eventually cause autonomic instability and disturbances in the hypothalamic-pituitary-adrenal axis, resulting in cyclic vomiting and thermoregulatory impairment.23
Although our patient presented with multiple episodes of vomiting and abdominal pain, he denied using marijuana, he tested negative for tetrahydrocannabinol, and he did not associate any relief of his symptoms with hot baths.
CASE CONTINUED
Our patient receives intravenous hydration, antiemetics, and a narcotic in tapering intravenous doses, and his symptoms gradually improve. He is discharged from the hospital. However, a few weeks later he is readmitted with the same symptoms of abdominal pain and nausea.
3. What is the cornerstone of treatment for narcotic bowel syndrome?
- Establishing a therapeutic relationship
- Detoxification
- Supportive management with intravenous fluids, antiemetics, and stool-softeners
- Medical management with a short-acting narcotic, clonidine, lorazepam, and desipramine
MANAGEMENT OF NARCOTIC BOWEL SYNDROME
An effective therapeutic relationship with the patient is the cornerstone of treatment and should be established before starting detoxification.17 The physician must first learn to accept that the patient’s condition is real and must show genuine empathy as well as provide information about the pathophysiologic basis of the condition, the rationale for withholding narcotics, and the detrimental role narcotics play in the vicious circle of pain.
Detoxification involves gradually withdrawing the narcotic and substituting a nonnarcotic such as an antidepressant for pain control, as well as prescribing a drug such as a benzodiazepine or clonidine to prevent withdrawal symptoms and a laxative to prevent constipation.17,24 The physician must reassure the patient that he or she will not be abandoned in pain and that all medications will be continuously adjusted as needed to keep him or her comfortable throughout the detoxification process.17,24 The physician must continuously gauge the patient’s willingness to continue with treatment and must also be readily available to address the patient’s concerns in a timely manner.17,24 Involving family members and friends may provide additional support to the patient. Referral to a functional gastrointestinal motility program, a pain specialist, and a psychologist may also be considered.17,24 Follow-up care is essential, even after the withdrawal program has ended.17,24
BACK TO THE PATIENT
After successfully establishing a therapeutic relationship and discussing the treatment plan with our patient, we started him on the same dosage of narcotic that he had been receiving, calculated in intravenous morphine equivalents to achieve maximal comfort, and then decreased the dosage by 10% to 33% daily until he was completely off narcotics. An antidepressant and a benzodiazepine were given simultaneously with narcotic tapering. Oral clonidine (0.1–0.4 mg/day) was given after the narcotic dosage was reduced to about half, and polyethylene glycol was given as needed for constipation. The total duration of detoxification was 7 days.
The patient was referred to a psychologist for cognitive-behavioral and relaxation therapy, as well as for encouragement and support. At 6 months, he had had no recurrence of symptoms.
TAKE-HOME MESSAGE
In the United States, the number of patients taking a narcotic for nonmalignant pain is increasing, 25 and physicians should be more aware of complications such as narcotic bowel syndrome.
Narcotic bowel syndrome should be suspected in any patient with prolonged narcotic use presenting with multiple recurrent episodes of abdominal pain after other causes are ruled out.
Establishing a good therapeutic relationship with the patient is the cornerstone of successful treatment. Patients who understand their condition and are willing to be treated tend to have better outcomes.
Supportive treatment, symptom relief, and emotional support during detoxification increase compliance.
A 32-year-old man presents to the emergency department with excruciating abdominal pain associated with multiple episodes of vomiting for the past 2 days. He reports no fevers, headaches, diarrhea, constipation, hematochezia, melena, musculoskeletal symptoms, or weight loss. His abdominal pain is generalized and crampy. It does not radiate and has no precipitating factors. The pain is relieved only with intravenous narcotics.
He does not smoke, drink alcohol, or use illicit drugs. He has no known drug or food allergies. He says that his current condition causes him emotional stress that affects his performance at work.
About a year ago, after a complicated surgical procedure, he needed chronic high-dose narcotics. A few months later, he developed multiple bouts of abdominal pain and vomiting that required hospital visits. He now takes oral oxycodone 10–15 mg every 4–6 hours.
On admission, his vital signs are stable, but he is in excruciating pain. He is alert and oriented to person, place, and time. His sclera are anicteric, and the pupils are equal, round, and reactive to light. Lung and heart examinations are normal. The abdomen is soft and nondistended but tender in all four quadrants without guarding; the liver and spleen are not palpable, and no abdominal masses are detected. He has no skin rash, joint swelling or tenderness, or peripheral edema. The neurologic examination is normal. Computed tomography (CT) of the abdomen with contrast shows no signs of bowel obstruction, pancreatic calcifications or edema, cholecystitis, or hepatobiliary disease. Results of initial laboratory testing are shown in Table 1.
1. Based on the information available, which is the least likely cause of his symptoms?
- Acute pancreatitis
- Cyclic vomiting syndrome
- Acute intermittent porphyria
- Gastroparesis
Acute pancreatitis
Acute pancreatitis is the least likely cause of his symptoms. It is commonly caused by gallstones, alcohol, hypertriglyceridemia, and certain drugs.1 The associated abdominal pain is usually epigastric, radiates to the back, and is accompanied by nausea or vomiting, or both. The onset of pain is sudden and rapidly increases in severity within 30 minutes. CT shows enlargement of the pancreas with diffuse edema, heterogeneity of pancreatic parenchyma, peripancreatic stranding, and peripancreatic fluid collections.1 The diagnosis is based on two of the following three criteria: abdominal pain characteristic of acute pancreatitis; a serum amylase or lipase concentration three or more times the upper limit of normal; and characteristic findings of acute pancreatitis on CT.1
Cyclic vomiting syndrome
Cyclic vomiting syndrome is thought to be caused by episodic dysautonomia, mitochondrial DNA mutations, and hypothalamic emetic response oversensitivity,2–4 but the exact pathogenesis is unknown. The syndrome has been strongly linked to migraine and to the chronic excessive use of cannabinoids.5–9 The Rome III diagnostic criteria10 are the following: the vomiting episodes are stereotypical, ie, they are acute and last for less than 1 week; the patient has had three or more episodes in the previous year; and the patient has no nausea or vomiting between episodes. The patient must meet all three criteria. A history of migraine or a family history of migraine further supports the diagnosis.
Acute intermittent porphyria
Acute intermittent porphyria is characterized by neurovisceral symptoms such as convulsions, paresis, autonomic dysfunction, constipation, and diarrhea that result from the overproduction of porphyrin precursors and deficiency of porphobilinogen deaminase.11
Most patients have poorly localized, severe, steady abdominal pain that develops over hours to days and that may persist for days to weeks.11 Since the pain is neuropathic, abdominal tenderness is usually minimal during an acute attack. Other clues include signs of ileus, such as constipation, nausea, abdominal distention, or decreased bowel sounds; bladder dysfunction, eg, urinary retention, incontinence, or dysuria; reddish-brown urine; and sensory neuropathy of the chest, back, and extremities.11 Blistering skin lesions are usually not seen. The presence of porphobilinogen in the urine confirms the diagnosis.11
Gastroparesis
Gastroparesis is a result of discoordination between the sympathetic and parasympathetic nervous systems, neurons, and smooth muscles within the stomach, causing a decrease in gastric motility. Common causes are diabetes,12 scleroderma,13 and neurologic disorders.14 It can also be iatrogenic,15 resulting from visceral nerve injury and drug treatment with narcotics, calcium channel blockers, muscarinic cholinergic antagonists, or certain antidepressants. Symptoms are related to gastric stasis, ie, abdominal pain from gastric distention, bloating, vomiting, and early satiety.15 Abdominal pain may worsen after eating, and vomitus usually consists of recently ingested food. These patients may have abdominal distension or tenderness and succussion splash. After excluding possible mechanical obstruction, a gastric-emptying study may be necessary to make the diagnosis.15
CASE CONTINUED
A serum and urine drug screen in our patient is positive only for opioids. Urine measures of delta-aminolevulinic acid and porphobilinogen are normal. CT angiography of the abdomen shows no signs of mesenteric vascular occlusion. Esophagogastroduodenoscopy shows antral gastritis, but the esophagus and duodenum appear normal, and colonoscopy is normal as well. Histologic study of biopsy specimens obtained during endoscopy is unrevealing. A gastric-emptying study shows delayed emptying. The patient’s abdominal pain and vomiting persist with the initial dose of intravenous narcotic but resolve with escalating doses. When asked, the patient denies an excessive need for hot baths.
2. Which is the most likely diagnosis at this point?
- Narcotic bowel syndrome
- Opioid withdrawal
- Crohn disease
- Chronic pancreatitis
- Chronic mesenteric ischemia
- Cannabinoid hyperemesis
Narcotic bowel syndrome
Narcotic bowel syndrome is the most likely diagnosis. Grunkemeier et al16 described it as chronic or frequently recurring abdominal pain that is treated with narcotics, either chronically or acutely with high doses, and that includes all the following features16:
- The pain worsens or resolves incompletely with continued or increasing doses of narcotics
- The pain markedly worsens when the narcotic dose is decreased, and decreases when the drug is reinstituted (the “soarand-crash” effect)
- The frequency, duration, and intensity of the pain episodes gradually increase
- The nature of the pain and its intensity are not explained by a current or previous gastrointestinal diagnosis.16
This syndrome is common in patients who receive high doses of narcotics for postoperative pain or for other, nonmalignant causes of pain. Patients eventually become dependent on the drugs but are not aware that chronic use activates and facilitates areas in the brain that enhance the perception of pain.16 A study of a rat model of narcotic bowel syndrome17 showed that morphine-induced hyperalgesia depends on central sensitization involving the activation of spinal microglia. This eventually results in concomitant peripheral sensitization involving the colonic mucosal neuroimmune system, and also in central or peripheral activation of opioid kappa-receptors by dynorphin release.17
Patients tend to present with chronic or intermittent colicky abdominal pain that requires escalating doses of narcotics. Eventually, they develop tachyphylaxis and shortened pain-free periods and will require even higher doses of narcotics. This ultimately enhances the perception of pain and worsens opioid bowel symptoms, causing a vicious circle of pain and more narcotic use.16
Laboratory tests are usually normal, and imaging may show only ileus. Gastric emptying may be delayed in patients who have either narcotic bowel syndrome or gastroparesis, but since abdominal pain from narcotic bowel syndrome is a result of central and visceral hypersensitivity, these patients perceive more severe abdominal pain than patients with gastroparesis alone.
Opioid withdrawal
Symptoms of opioid withdrawal may appear as soon as 6 to 24 hours after cessation of the opioid in patients known to be dependent on opioids. These patients present with crampy abdominal pain with nausea.18 Other symptoms include agitation, rhinorrhea, lacrimation, excessive yawning, arthralgias, papillary dilation, and piloerection.18
Our patient did not have the typical signs of opioid withdrawal.
Crohn disease
Crohn disease is a multisystem disorder with specific clinical and pathologic features. It is characterized by focal, asymmetric, transmural, and occasionally granulomatous inflammation primarily affecting the gastrointestinal tract.19 Characteristic symptoms include abdominal pain, chronic diarrhea with or without rectal bleeding, and weight loss. Extraintestinal signs may include anemia and inflammatory changes in the eyes, skin, and joints. The diagnosis is based on endoscopic, radiographic, and pathologic findings.19
Our patient did not have diarrhea or signs of Crohn disease on CT, endoscopy, or histology.
Chronic pancreatitis
Chronic pancreatitis involves progressive inflammatory changes resulting in permanent structural damage to the pancreas and subsequent exocrine and endocrine dysfunction.20 Patients have epigastric abdominal pain that often radiates to the back20; it is associated with eating and is partly relieved with leaning forward. Symptoms of pancreatic insufficiency such as fat malabsorption (resulting in steatorrhea and fat-soluble vitamin deficiency) and diabetes are common. Calcifications within the pancreas on CT suggest chronic pancreatitis.20 Serum lipase and amylase levels may be normal or slightly elevated.20
Our patient’s abdominal pain was not typical of pancreatitis. He had no signs or symptoms of pancreatic insufficiency and no calcifications within the pancreas.
Chronic mesenteric ischemia
Chronic mesenteric ischemia (“intestinal angina”) is caused by a reduction in intestinal blood flow as a result of occlusion, vasospasm, or hypoperfusion of the mesenteric vasculature.21 It is commonly seen in patients who smoke or who have atherosclerotic vascular disease. These patients have chronic dull or crampy abdominal pain that usually occurs within 1 hour after eating.21 To avoid pain, patients avoid eating, resulting in weight loss.21 CT angiography with multi-detector CT is as effective as angiography (the gold standard) in depicting splanchnic arterial anatomy.22
Our patient is young and has no known risk factors for atherosclerosis such as smoking. His abdominal pain is more intermittent than chronic and is not associated with eating.
Cannabinoid hyperemesis
Cannabinoid hyperemesis should be considered in patients with long-term cannabis use presenting with cyclic vomiting, abdominal pain, compulsive use of hot showers, and improvement of symptoms with cannabis cessation.23 Although cannabinoids have been recognized for their antiemetic effects, long-term use may eventually cause autonomic instability and disturbances in the hypothalamic-pituitary-adrenal axis, resulting in cyclic vomiting and thermoregulatory impairment.23
Although our patient presented with multiple episodes of vomiting and abdominal pain, he denied using marijuana, he tested negative for tetrahydrocannabinol, and he did not associate any relief of his symptoms with hot baths.
CASE CONTINUED
Our patient receives intravenous hydration, antiemetics, and a narcotic in tapering intravenous doses, and his symptoms gradually improve. He is discharged from the hospital. However, a few weeks later he is readmitted with the same symptoms of abdominal pain and nausea.
3. What is the cornerstone of treatment for narcotic bowel syndrome?
- Establishing a therapeutic relationship
- Detoxification
- Supportive management with intravenous fluids, antiemetics, and stool-softeners
- Medical management with a short-acting narcotic, clonidine, lorazepam, and desipramine
MANAGEMENT OF NARCOTIC BOWEL SYNDROME
An effective therapeutic relationship with the patient is the cornerstone of treatment and should be established before starting detoxification.17 The physician must first learn to accept that the patient’s condition is real and must show genuine empathy as well as provide information about the pathophysiologic basis of the condition, the rationale for withholding narcotics, and the detrimental role narcotics play in the vicious circle of pain.
Detoxification involves gradually withdrawing the narcotic and substituting a nonnarcotic such as an antidepressant for pain control, as well as prescribing a drug such as a benzodiazepine or clonidine to prevent withdrawal symptoms and a laxative to prevent constipation.17,24 The physician must reassure the patient that he or she will not be abandoned in pain and that all medications will be continuously adjusted as needed to keep him or her comfortable throughout the detoxification process.17,24 The physician must continuously gauge the patient’s willingness to continue with treatment and must also be readily available to address the patient’s concerns in a timely manner.17,24 Involving family members and friends may provide additional support to the patient. Referral to a functional gastrointestinal motility program, a pain specialist, and a psychologist may also be considered.17,24 Follow-up care is essential, even after the withdrawal program has ended.17,24
BACK TO THE PATIENT
After successfully establishing a therapeutic relationship and discussing the treatment plan with our patient, we started him on the same dosage of narcotic that he had been receiving, calculated in intravenous morphine equivalents to achieve maximal comfort, and then decreased the dosage by 10% to 33% daily until he was completely off narcotics. An antidepressant and a benzodiazepine were given simultaneously with narcotic tapering. Oral clonidine (0.1–0.4 mg/day) was given after the narcotic dosage was reduced to about half, and polyethylene glycol was given as needed for constipation. The total duration of detoxification was 7 days.
The patient was referred to a psychologist for cognitive-behavioral and relaxation therapy, as well as for encouragement and support. At 6 months, he had had no recurrence of symptoms.
TAKE-HOME MESSAGE
In the United States, the number of patients taking a narcotic for nonmalignant pain is increasing, 25 and physicians should be more aware of complications such as narcotic bowel syndrome.
Narcotic bowel syndrome should be suspected in any patient with prolonged narcotic use presenting with multiple recurrent episodes of abdominal pain after other causes are ruled out.
Establishing a good therapeutic relationship with the patient is the cornerstone of successful treatment. Patients who understand their condition and are willing to be treated tend to have better outcomes.
Supportive treatment, symptom relief, and emotional support during detoxification increase compliance.
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Boles RG, Adams K, Ito M, Li BU. Maternal inheritance in cyclic vomiting syndrome with neuromuscular disease. Am J Med Genet A 2003; 120A:474–482.
- Wang Q, Ito M, Adams K, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A 2004; 131:50–58.
- Taché Y. Cyclic vomiting syndrome: the corticotropinreleasing-factor hypothesis. Dig Dis Sci 1999; 44(suppl 8):79S–86S.
- Withers GD, Silburn SR, Forbes DA. Precipitants and aetiology of cyclic vomiting syndrome. Acta Paediatr 1998; 87:272–277.
- Whitney HB. Cyclic vomiting. A brief review of this affection as illustrated by a typical case. Arch Pediatr 1898; 15:839–845.
- Stickler GB. Relationship between cyclic vomiting syndrome and migraine. Clin Pediatr (Phila) 2005; 44:505–508.
- Li BU, Murray RD, Heitlinger LA, Robbins JL, Hayes JR. Is cyclic vomiting syndrome related to migraine? J Pediatr 1999; 134:567–572.
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570.
- Rome Foundation. Rome III disorders and diagnostic criteria. http://www.romecriteria.org/criteria/. Accessed February 27, 2013.
- Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 2005; 142:439–450.
- Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med 2007; 356:820–829.
- Maddern GJ, Horowitz M, Jamieson GG, Chatterton BE, Collins PJ, Roberts-Thomson P. Abnormalities of esophageal and gastric emptying in progressive systemic sclerosis. Gastroenterology 1984; 87:922–926.
- Jost WH. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol Sci 2010; 289:69–73.
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127:1592–1622.
- Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5:1126–1139.
- Agostini S, Eutamene H, Cartier C, et al. Evidence of central and peripheral sensitization in a rat model of narcotic bowel-like syndrome. Gastroenterology 2010; 139:553–563,563.e1–e5.
- Nicholls L, Bragaw L, Ruetsch C. Opioid dependence treatment and guidelines. J Manag Care Pharm 2010; 16(1 suppl B):S14–S21.
- Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol 2009; 104:465–483.
- Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med 1995; 332:1482–1490.
- American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology 2000; 118:951–953.
- Savastano S, Teso S, Corrà S, Fantozzi O, Miotto D. Multislice CT angiography of the celiac and superior mesenteric arteries: comparison with arteriographic findings. Radiol Med 2002; 103:456–463.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87:114–119.
- Drossman DA, Morris CB, Edwards H, et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol 2012; 107:1426–1440.
- Trescot AM, Boswell MV, Atluri SL, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician 2006; 9:1–39.
- Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006; 101:2379–2400.
- Boles RG, Adams K, Ito M, Li BU. Maternal inheritance in cyclic vomiting syndrome with neuromuscular disease. Am J Med Genet A 2003; 120A:474–482.
- Wang Q, Ito M, Adams K, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A 2004; 131:50–58.
- Taché Y. Cyclic vomiting syndrome: the corticotropinreleasing-factor hypothesis. Dig Dis Sci 1999; 44(suppl 8):79S–86S.
- Withers GD, Silburn SR, Forbes DA. Precipitants and aetiology of cyclic vomiting syndrome. Acta Paediatr 1998; 87:272–277.
- Whitney HB. Cyclic vomiting. A brief review of this affection as illustrated by a typical case. Arch Pediatr 1898; 15:839–845.
- Stickler GB. Relationship between cyclic vomiting syndrome and migraine. Clin Pediatr (Phila) 2005; 44:505–508.
- Li BU, Murray RD, Heitlinger LA, Robbins JL, Hayes JR. Is cyclic vomiting syndrome related to migraine? J Pediatr 1999; 134:567–572.
- Allen JH, de Moore GM, Heddle R, Twartz JC. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53:1566–1570.
- Rome Foundation. Rome III disorders and diagnostic criteria. http://www.romecriteria.org/criteria/. Accessed February 27, 2013.
- Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med 2005; 142:439–450.
- Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med 2007; 356:820–829.
- Maddern GJ, Horowitz M, Jamieson GG, Chatterton BE, Collins PJ, Roberts-Thomson P. Abnormalities of esophageal and gastric emptying in progressive systemic sclerosis. Gastroenterology 1984; 87:922–926.
- Jost WH. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol Sci 2010; 289:69–73.
- Parkman HP, Hasler WL, Fisher RS; American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004; 127:1592–1622.
- Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5:1126–1139.
- Agostini S, Eutamene H, Cartier C, et al. Evidence of central and peripheral sensitization in a rat model of narcotic bowel-like syndrome. Gastroenterology 2010; 139:553–563,563.e1–e5.
- Nicholls L, Bragaw L, Ruetsch C. Opioid dependence treatment and guidelines. J Manag Care Pharm 2010; 16(1 suppl B):S14–S21.
- Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol 2009; 104:465–483.
- Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med 1995; 332:1482–1490.
- American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology 2000; 118:951–953.
- Savastano S, Teso S, Corrà S, Fantozzi O, Miotto D. Multislice CT angiography of the celiac and superior mesenteric arteries: comparison with arteriographic findings. Radiol Med 2002; 103:456–463.
- Simonetto DA, Oxentenko AS, Herman ML, Szostek JH. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87:114–119.
- Drossman DA, Morris CB, Edwards H, et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am J Gastroenterol 2012; 107:1426–1440.
- Trescot AM, Boswell MV, Atluri SL, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician 2006; 9:1–39.