User login
American Heart Association (AHA): Scientific Sessions 2013
In utero exposure to dyslipidemia magnifies LDL risk in offspring
DALLAS – Elevated LDL cholesterol in women before childbirth was associated with a fivefold increased risk of elevated LDL 2 decades later in their young adult offspring in a new analysis from the Framingham Heart Study.
In contrast, paternal elevation of LDL pre pregnancy was not associated with increased likelihood of hyperlipidemia in children at age 20 years.
In utero exposure to maternal dyslipidemia appears to have lasting adverse consequences in terms of cardiovascular disease risk. If confirmed, the implications of the findings are huge, given that an estimated 25% of American women of childbearing age have elevated LDL, according to National Health and Nutrition Examination Survey data, Dr. Michael M. Mendelson noted at the American Heart Association scientific sessions.
"We postulate that identifying young women of childbearing age with dyslipidemia and reducing abnormal LDL with lipid-specific healthy lifestyle interventions may further reduce the transgenerational cycle of dyslipidemia and cardiovascular disease risk," declared Dr. Mendelson of Boston Children’s Hospital.
He presented prospectively acquired data on 343 maternal-child pairs enrolled in the Framingham Heart Study. Parental serum lipids were measured roughly 3 years prior to childbirth and again 20 years later, when the now young-adult offspring also had their serum lipids measured as part of their first comprehensive assessment as Framingham participants.
Women with an LDL level greater than 130 mg/dL at their prebirth assessment were fivefold more likely to have young-adult offspring with an elevated LDL in a multivariate analysis adjusted for maternal age and offspring gender. With further adjustment for prepregnancy maternal body mass index, smoking status, and genetic variants known to be associated with LDL level – most notably familial hypercholesterolemia – maternal dyslipidemia pre pregnancy remained associated with a highly significant 3.7-fold increased risk of dyslipidemia in young-adult offspring (P = .004).
In contrast, high paternal LDL pre pregnancy was not associated with increased odds of adult dyslipidemia in the offspring. And neither high maternal nor paternal LDL measured 20 years after childbirth was linked to increased likelihood of dyslipidemia in 20-year-old children in the fully adjusted multivariate logistic regression analysis.
The Framingham Heart Study is funded by the National Institutes of Health. Dr. Mendelson reported having no financial conflicts of interest.
DALLAS – Elevated LDL cholesterol in women before childbirth was associated with a fivefold increased risk of elevated LDL 2 decades later in their young adult offspring in a new analysis from the Framingham Heart Study.
In contrast, paternal elevation of LDL pre pregnancy was not associated with increased likelihood of hyperlipidemia in children at age 20 years.
In utero exposure to maternal dyslipidemia appears to have lasting adverse consequences in terms of cardiovascular disease risk. If confirmed, the implications of the findings are huge, given that an estimated 25% of American women of childbearing age have elevated LDL, according to National Health and Nutrition Examination Survey data, Dr. Michael M. Mendelson noted at the American Heart Association scientific sessions.
"We postulate that identifying young women of childbearing age with dyslipidemia and reducing abnormal LDL with lipid-specific healthy lifestyle interventions may further reduce the transgenerational cycle of dyslipidemia and cardiovascular disease risk," declared Dr. Mendelson of Boston Children’s Hospital.
He presented prospectively acquired data on 343 maternal-child pairs enrolled in the Framingham Heart Study. Parental serum lipids were measured roughly 3 years prior to childbirth and again 20 years later, when the now young-adult offspring also had their serum lipids measured as part of their first comprehensive assessment as Framingham participants.
Women with an LDL level greater than 130 mg/dL at their prebirth assessment were fivefold more likely to have young-adult offspring with an elevated LDL in a multivariate analysis adjusted for maternal age and offspring gender. With further adjustment for prepregnancy maternal body mass index, smoking status, and genetic variants known to be associated with LDL level – most notably familial hypercholesterolemia – maternal dyslipidemia pre pregnancy remained associated with a highly significant 3.7-fold increased risk of dyslipidemia in young-adult offspring (P = .004).
In contrast, high paternal LDL pre pregnancy was not associated with increased odds of adult dyslipidemia in the offspring. And neither high maternal nor paternal LDL measured 20 years after childbirth was linked to increased likelihood of dyslipidemia in 20-year-old children in the fully adjusted multivariate logistic regression analysis.
The Framingham Heart Study is funded by the National Institutes of Health. Dr. Mendelson reported having no financial conflicts of interest.
DALLAS – Elevated LDL cholesterol in women before childbirth was associated with a fivefold increased risk of elevated LDL 2 decades later in their young adult offspring in a new analysis from the Framingham Heart Study.
In contrast, paternal elevation of LDL pre pregnancy was not associated with increased likelihood of hyperlipidemia in children at age 20 years.
In utero exposure to maternal dyslipidemia appears to have lasting adverse consequences in terms of cardiovascular disease risk. If confirmed, the implications of the findings are huge, given that an estimated 25% of American women of childbearing age have elevated LDL, according to National Health and Nutrition Examination Survey data, Dr. Michael M. Mendelson noted at the American Heart Association scientific sessions.
"We postulate that identifying young women of childbearing age with dyslipidemia and reducing abnormal LDL with lipid-specific healthy lifestyle interventions may further reduce the transgenerational cycle of dyslipidemia and cardiovascular disease risk," declared Dr. Mendelson of Boston Children’s Hospital.
He presented prospectively acquired data on 343 maternal-child pairs enrolled in the Framingham Heart Study. Parental serum lipids were measured roughly 3 years prior to childbirth and again 20 years later, when the now young-adult offspring also had their serum lipids measured as part of their first comprehensive assessment as Framingham participants.
Women with an LDL level greater than 130 mg/dL at their prebirth assessment were fivefold more likely to have young-adult offspring with an elevated LDL in a multivariate analysis adjusted for maternal age and offspring gender. With further adjustment for prepregnancy maternal body mass index, smoking status, and genetic variants known to be associated with LDL level – most notably familial hypercholesterolemia – maternal dyslipidemia pre pregnancy remained associated with a highly significant 3.7-fold increased risk of dyslipidemia in young-adult offspring (P = .004).
In contrast, high paternal LDL pre pregnancy was not associated with increased odds of adult dyslipidemia in the offspring. And neither high maternal nor paternal LDL measured 20 years after childbirth was linked to increased likelihood of dyslipidemia in 20-year-old children in the fully adjusted multivariate logistic regression analysis.
The Framingham Heart Study is funded by the National Institutes of Health. Dr. Mendelson reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: With further adjustment for prepregnancy maternal body mass index, smoking status, and genetic variants known to be associated with LDL level – most notably familial hypercholesterolemia – maternal dyslipidemia before pregnancy remained associated with a highly significant 3.7-fold increased risk of dyslipidemia in young-adult offspring (P = .004).
Data source: Prospectively acquired data on 343 maternal-child pairs enrolled in the Framingham Heart Study.
Disclosures: The study is funded by the National Institutes of Health. The presenter reported having no financial disclosures.
Routinely screen relatives when thoracic aortic aneurysm disease presents before age 60
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: One in seven patients presenting with thoracic aortic disease in a large series had thoracic aortic aneurysm and dissection, or TAAD, an under-recognized, asymptomatic condition characterized by cystic medial necrosis and a strong genetic component.
Data source: This was a review of 1,276 patients who presented to a tertiary center with thoracic aortic disease. Systemic screening of first-degree relatives of patients with familial TAAD turned up two additional cases per proband.
Disclosures: The study was conducted free of commercial support. The presenter reported having no financial conflicts.
Genotyping adds little to optimized warfarin dosing
DALLAS – Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we’ve seen, I don’t believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don’t think we’ll see another genetics trial in warfarin treatment. I think this is it."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients’ international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it’s more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment. Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient’s genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients’ average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial’s primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non–African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non–African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden. The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment. In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study’s primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting. The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1311386]).
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market – dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) – are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it’s what you know versus what you don’t know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you’d see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don’t encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn’t change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
DALLAS – Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we’ve seen, I don’t believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don’t think we’ll see another genetics trial in warfarin treatment. I think this is it."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients’ international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it’s more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment. Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient’s genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients’ average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial’s primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non–African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non–African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden. The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment. In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study’s primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting. The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1311386]).
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market – dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) – are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it’s what you know versus what you don’t know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you’d see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don’t encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn’t change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
DALLAS – Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we’ve seen, I don’t believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don’t think we’ll see another genetics trial in warfarin treatment. I think this is it."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients’ international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it’s more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment. Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient’s genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients’ average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial’s primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non–African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non–African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden. The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment. In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study’s primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting. The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1311386]).
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market – dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) – are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it’s what you know versus what you don’t know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you’d see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don’t encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn’t change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Patients starting warfarin with dosing based on a formula that took into account seven clinical and demographic factors averaged 45% of the time in therapeutic range regardless of whether the starting dosage was adjusted based on genotype results.
Data source: COAG, a randomized trial with 1,015 patients starting warfarin therapy at 18 U.S. centers.
Disclosures: The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Opportunities to Boost Cardiovascular Disease Awareness
DALLAS – A mere 28% of 1,013 surveyed New York–area women correctly identified cardiovascular disease as the No. 1 killer of women. And two-thirds of those women said their primary care provider is an obstetrician-gynecologist who does not discuss heart health as part of preventive care, Dr. Allison J. Brusati reported at the American Heart Association scientific sessions.
"In a population with extremely low levels of awareness of cardiovascular disease and a high reliance on ob.gyn. care – and particularly young, reproductive-age women – ob.gyns. are not only well poised but [also are as] obligated as primary care clinicians to provide heart-health education to their patients. Without adding much time to a patient’s annual visit, an ob.gyn. can discuss a woman’s risk factors and advise lifestyle changes that may prove essential in preventing heart disease as well as other chronic diseases," asserted Dr. Brusati of Albert Einstein College of Medicine in New York.
The 28% rate of awareness of heart disease as the top killer of women in this New York survey is exactly half the rate found in a recent AHA-sponsored national survey of women (Circulation 2013;127:1254-63). The survey was conducted at five ob.gyn. clinics affiliated with Montefiore Medical Center, four situated in largely impoverished sections of the Bronx and one in wealthy Westchester County. The survey population was 40% Hispanic, 31% black, and 20% white. Only 21% of non-white women, compared with 55% of white women, were able to identify cardiovascular disease as the top killer of women.
"Younger women with lower income levels and without a higher degree of education were more likely to be unaware of their risk of heart disease," said Dr. Brusati. Still, education was no guarantee of being heart-health savvy. Of survey respondents who had a college degree or higher, 44% identified cardiovascular disease as the top cause of mortality in women, 42% thought cancer was the top cause of death in women, and 20% had no idea of the No. 1 cause of death.
The survey also found that most of the ob.gyns. who served as primary care providers for the New York women also failed to discuss other key aspects of primary prevention beyond heart health. Smoking was discussed by 51% of ob.gyns, exercise by 42%, diet by 38%, and colonoscopy by 32%. Mammography was discussed by 75% of ob.gyns.
Dr. Brusati’s coinvestigator in this unfunded study was Dr. Mary L. Rosser, an ob.gyn. at Montefiore Medical Center. They reported having no financial conflicts.
DALLAS – A mere 28% of 1,013 surveyed New York–area women correctly identified cardiovascular disease as the No. 1 killer of women. And two-thirds of those women said their primary care provider is an obstetrician-gynecologist who does not discuss heart health as part of preventive care, Dr. Allison J. Brusati reported at the American Heart Association scientific sessions.
"In a population with extremely low levels of awareness of cardiovascular disease and a high reliance on ob.gyn. care – and particularly young, reproductive-age women – ob.gyns. are not only well poised but [also are as] obligated as primary care clinicians to provide heart-health education to their patients. Without adding much time to a patient’s annual visit, an ob.gyn. can discuss a woman’s risk factors and advise lifestyle changes that may prove essential in preventing heart disease as well as other chronic diseases," asserted Dr. Brusati of Albert Einstein College of Medicine in New York.
The 28% rate of awareness of heart disease as the top killer of women in this New York survey is exactly half the rate found in a recent AHA-sponsored national survey of women (Circulation 2013;127:1254-63). The survey was conducted at five ob.gyn. clinics affiliated with Montefiore Medical Center, four situated in largely impoverished sections of the Bronx and one in wealthy Westchester County. The survey population was 40% Hispanic, 31% black, and 20% white. Only 21% of non-white women, compared with 55% of white women, were able to identify cardiovascular disease as the top killer of women.
"Younger women with lower income levels and without a higher degree of education were more likely to be unaware of their risk of heart disease," said Dr. Brusati. Still, education was no guarantee of being heart-health savvy. Of survey respondents who had a college degree or higher, 44% identified cardiovascular disease as the top cause of mortality in women, 42% thought cancer was the top cause of death in women, and 20% had no idea of the No. 1 cause of death.
The survey also found that most of the ob.gyns. who served as primary care providers for the New York women also failed to discuss other key aspects of primary prevention beyond heart health. Smoking was discussed by 51% of ob.gyns, exercise by 42%, diet by 38%, and colonoscopy by 32%. Mammography was discussed by 75% of ob.gyns.
Dr. Brusati’s coinvestigator in this unfunded study was Dr. Mary L. Rosser, an ob.gyn. at Montefiore Medical Center. They reported having no financial conflicts.
DALLAS – A mere 28% of 1,013 surveyed New York–area women correctly identified cardiovascular disease as the No. 1 killer of women. And two-thirds of those women said their primary care provider is an obstetrician-gynecologist who does not discuss heart health as part of preventive care, Dr. Allison J. Brusati reported at the American Heart Association scientific sessions.
"In a population with extremely low levels of awareness of cardiovascular disease and a high reliance on ob.gyn. care – and particularly young, reproductive-age women – ob.gyns. are not only well poised but [also are as] obligated as primary care clinicians to provide heart-health education to their patients. Without adding much time to a patient’s annual visit, an ob.gyn. can discuss a woman’s risk factors and advise lifestyle changes that may prove essential in preventing heart disease as well as other chronic diseases," asserted Dr. Brusati of Albert Einstein College of Medicine in New York.
The 28% rate of awareness of heart disease as the top killer of women in this New York survey is exactly half the rate found in a recent AHA-sponsored national survey of women (Circulation 2013;127:1254-63). The survey was conducted at five ob.gyn. clinics affiliated with Montefiore Medical Center, four situated in largely impoverished sections of the Bronx and one in wealthy Westchester County. The survey population was 40% Hispanic, 31% black, and 20% white. Only 21% of non-white women, compared with 55% of white women, were able to identify cardiovascular disease as the top killer of women.
"Younger women with lower income levels and without a higher degree of education were more likely to be unaware of their risk of heart disease," said Dr. Brusati. Still, education was no guarantee of being heart-health savvy. Of survey respondents who had a college degree or higher, 44% identified cardiovascular disease as the top cause of mortality in women, 42% thought cancer was the top cause of death in women, and 20% had no idea of the No. 1 cause of death.
The survey also found that most of the ob.gyns. who served as primary care providers for the New York women also failed to discuss other key aspects of primary prevention beyond heart health. Smoking was discussed by 51% of ob.gyns, exercise by 42%, diet by 38%, and colonoscopy by 32%. Mammography was discussed by 75% of ob.gyns.
Dr. Brusati’s coinvestigator in this unfunded study was Dr. Mary L. Rosser, an ob.gyn. at Montefiore Medical Center. They reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Opportunities to boost cardiovascular disease awareness
DALLAS – A mere 28% of 1,013 surveyed New York–area women correctly identified cardiovascular disease as the No. 1 killer of women. And two-thirds of those women said their primary care provider is an obstetrician-gynecologist who does not discuss heart health as part of preventive care, Dr. Allison J. Brusati reported at the American Heart Association scientific sessions.
"In a population with extremely low levels of awareness of cardiovascular disease and a high reliance on ob.gyn. care – and particularly young, reproductive-age women – ob.gyns. are not only well poised but [also are as] obligated as primary care physicians to provide heart-health education to their patients. Without adding much time to a patient’s annual visit, an ob.gyn. can discuss a woman’s risk factors and advise lifestyle changes that may prove essential in preventing heart disease as well as other chronic diseases," asserted Dr. Brusati of Albert Einstein College of Medicine in New York.
The 28% rate of awareness of heart disease as the top killer of women in this New York survey is exactly half the rate found in a recent AHA-sponsored national survey of women (Circulation 2013;127:1254-63). The survey was conducted at five ob.gyn. clinics affiliated with Montefiore Medical Center, four situated in largely impoverished sections of the Bronx and one in wealthy Westchester County. The survey population was 40% Hispanic, 31% black, and 20% white. Only 21% of non-white women, compared with 55% of white women, were able to identify cardiovascular disease as the top killer of women.
"Younger women with lower income levels and without a higher degree of education were more likely to be unaware of their risk of heart disease," said Dr. Brusati. Still, education was no guarantee of being heart-health savvy. Of survey respondents who had a college degree or higher, 44% identified cardiovascular disease as the top cause of mortality in women, 42% thought cancer was the top cause of death in women, and 20% had no idea of the No. 1 cause of death.
The survey also found that most of the ob.gyns. who served as primary care providers for the New York women also failed to discuss other key aspects of primary prevention beyond heart health. Smoking was discussed by 51% of ob.gyns, exercise by 42%, diet by 38%, and colonoscopy by 32%. Mammography was discussed by 75% of ob.gyns.
Dr. Brusati’s coinvestigator in this unfunded study was Dr. Mary L. Rosser, an ob.gyn. at Montefiore Medical Center. They reported having no financial conflicts.
DALLAS – A mere 28% of 1,013 surveyed New York–area women correctly identified cardiovascular disease as the No. 1 killer of women. And two-thirds of those women said their primary care provider is an obstetrician-gynecologist who does not discuss heart health as part of preventive care, Dr. Allison J. Brusati reported at the American Heart Association scientific sessions.
"In a population with extremely low levels of awareness of cardiovascular disease and a high reliance on ob.gyn. care – and particularly young, reproductive-age women – ob.gyns. are not only well poised but [also are as] obligated as primary care physicians to provide heart-health education to their patients. Without adding much time to a patient’s annual visit, an ob.gyn. can discuss a woman’s risk factors and advise lifestyle changes that may prove essential in preventing heart disease as well as other chronic diseases," asserted Dr. Brusati of Albert Einstein College of Medicine in New York.
The 28% rate of awareness of heart disease as the top killer of women in this New York survey is exactly half the rate found in a recent AHA-sponsored national survey of women (Circulation 2013;127:1254-63). The survey was conducted at five ob.gyn. clinics affiliated with Montefiore Medical Center, four situated in largely impoverished sections of the Bronx and one in wealthy Westchester County. The survey population was 40% Hispanic, 31% black, and 20% white. Only 21% of non-white women, compared with 55% of white women, were able to identify cardiovascular disease as the top killer of women.
"Younger women with lower income levels and without a higher degree of education were more likely to be unaware of their risk of heart disease," said Dr. Brusati. Still, education was no guarantee of being heart-health savvy. Of survey respondents who had a college degree or higher, 44% identified cardiovascular disease as the top cause of mortality in women, 42% thought cancer was the top cause of death in women, and 20% had no idea of the No. 1 cause of death.
The survey also found that most of the ob.gyns. who served as primary care providers for the New York women also failed to discuss other key aspects of primary prevention beyond heart health. Smoking was discussed by 51% of ob.gyns, exercise by 42%, diet by 38%, and colonoscopy by 32%. Mammography was discussed by 75% of ob.gyns.
Dr. Brusati’s coinvestigator in this unfunded study was Dr. Mary L. Rosser, an ob.gyn. at Montefiore Medical Center. They reported having no financial conflicts.
DALLAS – A mere 28% of 1,013 surveyed New York–area women correctly identified cardiovascular disease as the No. 1 killer of women. And two-thirds of those women said their primary care provider is an obstetrician-gynecologist who does not discuss heart health as part of preventive care, Dr. Allison J. Brusati reported at the American Heart Association scientific sessions.
"In a population with extremely low levels of awareness of cardiovascular disease and a high reliance on ob.gyn. care – and particularly young, reproductive-age women – ob.gyns. are not only well poised but [also are as] obligated as primary care physicians to provide heart-health education to their patients. Without adding much time to a patient’s annual visit, an ob.gyn. can discuss a woman’s risk factors and advise lifestyle changes that may prove essential in preventing heart disease as well as other chronic diseases," asserted Dr. Brusati of Albert Einstein College of Medicine in New York.
The 28% rate of awareness of heart disease as the top killer of women in this New York survey is exactly half the rate found in a recent AHA-sponsored national survey of women (Circulation 2013;127:1254-63). The survey was conducted at five ob.gyn. clinics affiliated with Montefiore Medical Center, four situated in largely impoverished sections of the Bronx and one in wealthy Westchester County. The survey population was 40% Hispanic, 31% black, and 20% white. Only 21% of non-white women, compared with 55% of white women, were able to identify cardiovascular disease as the top killer of women.
"Younger women with lower income levels and without a higher degree of education were more likely to be unaware of their risk of heart disease," said Dr. Brusati. Still, education was no guarantee of being heart-health savvy. Of survey respondents who had a college degree or higher, 44% identified cardiovascular disease as the top cause of mortality in women, 42% thought cancer was the top cause of death in women, and 20% had no idea of the No. 1 cause of death.
The survey also found that most of the ob.gyns. who served as primary care providers for the New York women also failed to discuss other key aspects of primary prevention beyond heart health. Smoking was discussed by 51% of ob.gyns, exercise by 42%, diet by 38%, and colonoscopy by 32%. Mammography was discussed by 75% of ob.gyns.
Dr. Brusati’s coinvestigator in this unfunded study was Dr. Mary L. Rosser, an ob.gyn. at Montefiore Medical Center. They reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Two-thirds of the women whose primary care provider was an ob.gyn. said their physician does not discuss heart health with them.
Data source: This was a survey of 1,013 women attending five ob.gyn. clinics affiliated with Montefiore Medical Center in New York.
Disclosures: The presenter of this unfunded study reported having no financial conflicts.
No link found between high-potency statins andacute kidney injury
DALLAS – High-potency statin therapy given post acute coronary syndrome did not raise serum creatinine or cause more risk of acute kidney injury than low-potency statin regimens did in a new analysis of two published landmark randomized clinical trials.
"Considering the recently updated AHA/American College of Cardiology lipid guidelines, which call for the use of high-potency statins in millions more patients, these findings provide important reassurance that a high-potency statin regimen will not increase the incidence of adverse renal events," Dr. Amy Sarma said in presenting the study results at the American Heart Association scientific sessions.
She noted that a recent Canadian observational study utilizing Canadian and U.S. administrative databases totaling more than 2 million patients over age 40 who were newly placed on statin therapy showed an adjusted 1.34-fold increased rate of hospitalization for acute kidney injury within the first 120 days in those on a high- as compared to a low-potency regimen, and a 1.11-fold increased risk beyond 120 days through the end of the first year (BMJ 2013;346:f880). Both risk elevations were statistically significant.
However, observational studies such as this are prone to bias in the form of potentially crucial differences between the patients given a prescription for statins and those who aren’t. For this reason, Dr. Sarma and her coinvestigators turned for guidance to two randomized trials of high- versus low-dose statins, since this study design obviates the risks of confounding. The trials were PROVE IT-TIMI 22 (N. Engl. J. Med. 2004;350:1495-504) and the A-to-Z trial (JAMA 2004;292:1307-16).
PROVE IT included 4,122 patients randomized within 10 days post ACS to standard background therapy plus either pravastatin at 40 mg/day or atorvastatin at 80 mg/day. A-to-Z involved 4,497 patients who were placed on simvastatin at either 20 or 80 mg/day within 5 days post ACS. Both trials had a median follow-up of 2 years, and both featured serial measurements of serum creatinine. Two-thirds of subjects in PROVE IT had a baseline estimated glomerular filtration rate below 90 mL/min per 1.73 m2, while two-thirds of those in A-to-Z had a baseline eGFR less than 60, noted Dr. Sarma of Brigham and Women’s Hospital, Boston.
In both studies, mean serum creatinine rose equally during the first 30 days of statin therapy, regardless of treatment potency, and then levels declined. In PROVE IT, for example, serum creatinine in the pravastatin and atorvastatin arms rose by 0.96% and 0.97% above baseline, respectively, at 30 days. Values then dropped by 2.88% and 3.85% from baseline at 4 months, and by 3.88% and 5.83% at 16 months.
In both studies, there was no difference between the high- and low-potency statin groups in the incidence of any increase in serum creatinine of at least 1.5-fold, 2.0-fold, or 3.0-fold greater than baseline. In other words, there was no hint of a safety signal, she added.
Dr. Sarma reported having no financial conflicts of interest.
I’m not convinced by the PROVE IT and A-to-Z data. In the combined analysis, the high-potency statin group had a 15% greater risk of acute kidney injury compared with the low-potency statin group during the first 4 months of treatment. Although that wasn’t a statistically significant difference due to limited patient numbers and broad confidence intervals, it was quite similar to the long-term 11% increased risk seen in the Canadian observational database study with 2 million patients.
Moreover, an even bigger recent observational study led by investigators at the University of North Carolina, Chapel Hill, involving 3.9 million U.S. statin initiators found a 42% greater rate of acute kidney injury during the first year on high- as compared to lower-dose simvastatin in commercially insured patients and a 24% increased risk in the Medicare population. Both risk increases were statistically significant due to the huge patient numbers (Pharmacoepidemiol. Drug Saf. 2013;22:1061-70).
 |
|
Also, PROVE IT and A-to-Z featured patient populations who were of a younger average age and less likely to be male and to have diabetes than the general American population of acute MI patients as depicted in the National Cardiovascular Data Registry (J. Am. Coll. Cardiol. 2013;62:1931-47). Advancing age, diabetes, and male gender are predisposing factors for acute kidney disease.
The Canadian observational study defined high-potency statin therapy as at least 20 mg/day of atorvastatin or 40 mg or simvastatin. The University of North Carolina study also defined high-potency simvastatin as at least 40 mg/day as opposed to the 80 mg/day employed in the A-to-Z trial. One wonders whether they would have seen an even larger magnitude of association had they used a higher threshold to define high potency.
I don’t necessarily disagree with Dr. Sarma’s conclusion that for most people after ACS a high-potency statin will be warranted. I just think we need to be cautious and think about the individual patient when we consider the risks and benefits.
It may well be the case that high-potency statin therapy means fewer cardiovascular events at the cost of a greater risk of acute kidney injury and other adverse events. With lower-potency statins, the trade-off may be more cardiovascular events but a lower acute kidney injury risk.
Dr. Tara Chang is a nephrologist at Stanford (Calif.) University. She was the discussant of the paper at the meeting. Dr. Chang disclosed having no financial conflicts.
I’m not convinced by the PROVE IT and A-to-Z data. In the combined analysis, the high-potency statin group had a 15% greater risk of acute kidney injury compared with the low-potency statin group during the first 4 months of treatment. Although that wasn’t a statistically significant difference due to limited patient numbers and broad confidence intervals, it was quite similar to the long-term 11% increased risk seen in the Canadian observational database study with 2 million patients.
Moreover, an even bigger recent observational study led by investigators at the University of North Carolina, Chapel Hill, involving 3.9 million U.S. statin initiators found a 42% greater rate of acute kidney injury during the first year on high- as compared to lower-dose simvastatin in commercially insured patients and a 24% increased risk in the Medicare population. Both risk increases were statistically significant due to the huge patient numbers (Pharmacoepidemiol. Drug Saf. 2013;22:1061-70).
 |
|
Also, PROVE IT and A-to-Z featured patient populations who were of a younger average age and less likely to be male and to have diabetes than the general American population of acute MI patients as depicted in the National Cardiovascular Data Registry (J. Am. Coll. Cardiol. 2013;62:1931-47). Advancing age, diabetes, and male gender are predisposing factors for acute kidney disease.
The Canadian observational study defined high-potency statin therapy as at least 20 mg/day of atorvastatin or 40 mg or simvastatin. The University of North Carolina study also defined high-potency simvastatin as at least 40 mg/day as opposed to the 80 mg/day employed in the A-to-Z trial. One wonders whether they would have seen an even larger magnitude of association had they used a higher threshold to define high potency.
I don’t necessarily disagree with Dr. Sarma’s conclusion that for most people after ACS a high-potency statin will be warranted. I just think we need to be cautious and think about the individual patient when we consider the risks and benefits.
It may well be the case that high-potency statin therapy means fewer cardiovascular events at the cost of a greater risk of acute kidney injury and other adverse events. With lower-potency statins, the trade-off may be more cardiovascular events but a lower acute kidney injury risk.
Dr. Tara Chang is a nephrologist at Stanford (Calif.) University. She was the discussant of the paper at the meeting. Dr. Chang disclosed having no financial conflicts.
I’m not convinced by the PROVE IT and A-to-Z data. In the combined analysis, the high-potency statin group had a 15% greater risk of acute kidney injury compared with the low-potency statin group during the first 4 months of treatment. Although that wasn’t a statistically significant difference due to limited patient numbers and broad confidence intervals, it was quite similar to the long-term 11% increased risk seen in the Canadian observational database study with 2 million patients.
Moreover, an even bigger recent observational study led by investigators at the University of North Carolina, Chapel Hill, involving 3.9 million U.S. statin initiators found a 42% greater rate of acute kidney injury during the first year on high- as compared to lower-dose simvastatin in commercially insured patients and a 24% increased risk in the Medicare population. Both risk increases were statistically significant due to the huge patient numbers (Pharmacoepidemiol. Drug Saf. 2013;22:1061-70).
 |
|
Also, PROVE IT and A-to-Z featured patient populations who were of a younger average age and less likely to be male and to have diabetes than the general American population of acute MI patients as depicted in the National Cardiovascular Data Registry (J. Am. Coll. Cardiol. 2013;62:1931-47). Advancing age, diabetes, and male gender are predisposing factors for acute kidney disease.
The Canadian observational study defined high-potency statin therapy as at least 20 mg/day of atorvastatin or 40 mg or simvastatin. The University of North Carolina study also defined high-potency simvastatin as at least 40 mg/day as opposed to the 80 mg/day employed in the A-to-Z trial. One wonders whether they would have seen an even larger magnitude of association had they used a higher threshold to define high potency.
I don’t necessarily disagree with Dr. Sarma’s conclusion that for most people after ACS a high-potency statin will be warranted. I just think we need to be cautious and think about the individual patient when we consider the risks and benefits.
It may well be the case that high-potency statin therapy means fewer cardiovascular events at the cost of a greater risk of acute kidney injury and other adverse events. With lower-potency statins, the trade-off may be more cardiovascular events but a lower acute kidney injury risk.
Dr. Tara Chang is a nephrologist at Stanford (Calif.) University. She was the discussant of the paper at the meeting. Dr. Chang disclosed having no financial conflicts.
DALLAS – High-potency statin therapy given post acute coronary syndrome did not raise serum creatinine or cause more risk of acute kidney injury than low-potency statin regimens did in a new analysis of two published landmark randomized clinical trials.
"Considering the recently updated AHA/American College of Cardiology lipid guidelines, which call for the use of high-potency statins in millions more patients, these findings provide important reassurance that a high-potency statin regimen will not increase the incidence of adverse renal events," Dr. Amy Sarma said in presenting the study results at the American Heart Association scientific sessions.
She noted that a recent Canadian observational study utilizing Canadian and U.S. administrative databases totaling more than 2 million patients over age 40 who were newly placed on statin therapy showed an adjusted 1.34-fold increased rate of hospitalization for acute kidney injury within the first 120 days in those on a high- as compared to a low-potency regimen, and a 1.11-fold increased risk beyond 120 days through the end of the first year (BMJ 2013;346:f880). Both risk elevations were statistically significant.
However, observational studies such as this are prone to bias in the form of potentially crucial differences between the patients given a prescription for statins and those who aren’t. For this reason, Dr. Sarma and her coinvestigators turned for guidance to two randomized trials of high- versus low-dose statins, since this study design obviates the risks of confounding. The trials were PROVE IT-TIMI 22 (N. Engl. J. Med. 2004;350:1495-504) and the A-to-Z trial (JAMA 2004;292:1307-16).
PROVE IT included 4,122 patients randomized within 10 days post ACS to standard background therapy plus either pravastatin at 40 mg/day or atorvastatin at 80 mg/day. A-to-Z involved 4,497 patients who were placed on simvastatin at either 20 or 80 mg/day within 5 days post ACS. Both trials had a median follow-up of 2 years, and both featured serial measurements of serum creatinine. Two-thirds of subjects in PROVE IT had a baseline estimated glomerular filtration rate below 90 mL/min per 1.73 m2, while two-thirds of those in A-to-Z had a baseline eGFR less than 60, noted Dr. Sarma of Brigham and Women’s Hospital, Boston.
In both studies, mean serum creatinine rose equally during the first 30 days of statin therapy, regardless of treatment potency, and then levels declined. In PROVE IT, for example, serum creatinine in the pravastatin and atorvastatin arms rose by 0.96% and 0.97% above baseline, respectively, at 30 days. Values then dropped by 2.88% and 3.85% from baseline at 4 months, and by 3.88% and 5.83% at 16 months.
In both studies, there was no difference between the high- and low-potency statin groups in the incidence of any increase in serum creatinine of at least 1.5-fold, 2.0-fold, or 3.0-fold greater than baseline. In other words, there was no hint of a safety signal, she added.
Dr. Sarma reported having no financial conflicts of interest.
DALLAS – High-potency statin therapy given post acute coronary syndrome did not raise serum creatinine or cause more risk of acute kidney injury than low-potency statin regimens did in a new analysis of two published landmark randomized clinical trials.
"Considering the recently updated AHA/American College of Cardiology lipid guidelines, which call for the use of high-potency statins in millions more patients, these findings provide important reassurance that a high-potency statin regimen will not increase the incidence of adverse renal events," Dr. Amy Sarma said in presenting the study results at the American Heart Association scientific sessions.
She noted that a recent Canadian observational study utilizing Canadian and U.S. administrative databases totaling more than 2 million patients over age 40 who were newly placed on statin therapy showed an adjusted 1.34-fold increased rate of hospitalization for acute kidney injury within the first 120 days in those on a high- as compared to a low-potency regimen, and a 1.11-fold increased risk beyond 120 days through the end of the first year (BMJ 2013;346:f880). Both risk elevations were statistically significant.
However, observational studies such as this are prone to bias in the form of potentially crucial differences between the patients given a prescription for statins and those who aren’t. For this reason, Dr. Sarma and her coinvestigators turned for guidance to two randomized trials of high- versus low-dose statins, since this study design obviates the risks of confounding. The trials were PROVE IT-TIMI 22 (N. Engl. J. Med. 2004;350:1495-504) and the A-to-Z trial (JAMA 2004;292:1307-16).
PROVE IT included 4,122 patients randomized within 10 days post ACS to standard background therapy plus either pravastatin at 40 mg/day or atorvastatin at 80 mg/day. A-to-Z involved 4,497 patients who were placed on simvastatin at either 20 or 80 mg/day within 5 days post ACS. Both trials had a median follow-up of 2 years, and both featured serial measurements of serum creatinine. Two-thirds of subjects in PROVE IT had a baseline estimated glomerular filtration rate below 90 mL/min per 1.73 m2, while two-thirds of those in A-to-Z had a baseline eGFR less than 60, noted Dr. Sarma of Brigham and Women’s Hospital, Boston.
In both studies, mean serum creatinine rose equally during the first 30 days of statin therapy, regardless of treatment potency, and then levels declined. In PROVE IT, for example, serum creatinine in the pravastatin and atorvastatin arms rose by 0.96% and 0.97% above baseline, respectively, at 30 days. Values then dropped by 2.88% and 3.85% from baseline at 4 months, and by 3.88% and 5.83% at 16 months.
In both studies, there was no difference between the high- and low-potency statin groups in the incidence of any increase in serum creatinine of at least 1.5-fold, 2.0-fold, or 3.0-fold greater than baseline. In other words, there was no hint of a safety signal, she added.
Dr. Sarma reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Patients randomized to a high-potency statin regimen shortly after an acute coronary syndrome did not have higher serum creatinine levels or a greater risk of acute kidney injury than those randomized to a low-potency statin.
Data source: PROVE IT-TIMI 22 and the A-to-Z trial were randomized, double-blind clinical trials in which a total of 8,619 patients with a recent acute coronary syndrome were assigned to high- or lower-potency statin therapy and prospectively followed for a median of 2 years.
Disclosures: Dr. Sarma reported having no financial conflicts of interest.
Cardiac stress-imaging in stable angina patients
No one knows whether patients with stable ischemic heart disease and moderate or severe inducible ischemia benefit from revascularization when added to optimal medical therapy. A major, federally-funded trial named ISCHEMIA is underway to answer this question.
Main results from ISCHEMIA are still several years off, but the study has already produced an interesting finding about current cardiology practice and the way that cardiac stress-imaging studies are ordered.
Based on first-year enrollment data from the ISCHEMIA trial, the vast majority of both U.S. and European patients currently referred for stress-imaging assessment of ischemia have little or no inducible ischemia, Dr. Judith S. Hochman, head of the study, said during a talk at the American Heart Association’s Scientific Sessions in November.
Stable angina patients enrolled into ISCHEMIA need to have at least moderate inducible ischemia, defined as involving at least 10% of the left ventricle, in a cardiac imaging study read by a core laboratory. Since the trial began in July 2012, fewer than 700 patients had been enrolled based on imaging studies from about 15,000 patients. In the United States, about 3% of imaged patients had moderate or severe induced ischemia; the other 97% of patients referred for assessment had mild or no induced ischemia. In Europe, the rate with moderate or severe induced ischemia was slightly higher at 5%.
This observation made Dr. Hochman, a New York University cardiologist, ask why the prevalence of moderate or severe ischemia is so low in patients referred for stress imaging. She also wondered which patients with stable angina are undergoing revascularization today if so few qualify with moderate or severe inducible ischemia.
Another surprising fact she highlighted is how cardiologists manage patients found to have moderate or severe inducible ischemia. "Most of us think that all these patients with moderate to severe inducible ischemia are referred for catheterization," but that’s not what study results showed. She cited U.S. data from the mid- and late 2000s documenting that about one-third to two-thirds of these patients undergo cardiac catheterization.
This finding shows that there is "clinical equipoise" on how to manage these patients, and the ISCHEMIA trial will address that issue.
But until the results arrive in 2020, will as many stress-imaging studies continue for patients with stable angina when so many referred patients turn out to be negative for more advanced coronary disease? And, as Dr. Tracy Y. Wang, a cardiologist at Duke University, Durham, N.C., asked after hearing about the equivocal use of catheterization for patients with worse inducible ischemia: Why do physicians order these tests if they don’t intend to catheterize patients found to have moderate-to-severe inducible ischemia?
On Twitter @mitchelzoler
No one knows whether patients with stable ischemic heart disease and moderate or severe inducible ischemia benefit from revascularization when added to optimal medical therapy. A major, federally-funded trial named ISCHEMIA is underway to answer this question.
Main results from ISCHEMIA are still several years off, but the study has already produced an interesting finding about current cardiology practice and the way that cardiac stress-imaging studies are ordered.
Based on first-year enrollment data from the ISCHEMIA trial, the vast majority of both U.S. and European patients currently referred for stress-imaging assessment of ischemia have little or no inducible ischemia, Dr. Judith S. Hochman, head of the study, said during a talk at the American Heart Association’s Scientific Sessions in November.
Stable angina patients enrolled into ISCHEMIA need to have at least moderate inducible ischemia, defined as involving at least 10% of the left ventricle, in a cardiac imaging study read by a core laboratory. Since the trial began in July 2012, fewer than 700 patients had been enrolled based on imaging studies from about 15,000 patients. In the United States, about 3% of imaged patients had moderate or severe induced ischemia; the other 97% of patients referred for assessment had mild or no induced ischemia. In Europe, the rate with moderate or severe induced ischemia was slightly higher at 5%.
This observation made Dr. Hochman, a New York University cardiologist, ask why the prevalence of moderate or severe ischemia is so low in patients referred for stress imaging. She also wondered which patients with stable angina are undergoing revascularization today if so few qualify with moderate or severe inducible ischemia.
Another surprising fact she highlighted is how cardiologists manage patients found to have moderate or severe inducible ischemia. "Most of us think that all these patients with moderate to severe inducible ischemia are referred for catheterization," but that’s not what study results showed. She cited U.S. data from the mid- and late 2000s documenting that about one-third to two-thirds of these patients undergo cardiac catheterization.
This finding shows that there is "clinical equipoise" on how to manage these patients, and the ISCHEMIA trial will address that issue.
But until the results arrive in 2020, will as many stress-imaging studies continue for patients with stable angina when so many referred patients turn out to be negative for more advanced coronary disease? And, as Dr. Tracy Y. Wang, a cardiologist at Duke University, Durham, N.C., asked after hearing about the equivocal use of catheterization for patients with worse inducible ischemia: Why do physicians order these tests if they don’t intend to catheterize patients found to have moderate-to-severe inducible ischemia?
On Twitter @mitchelzoler
No one knows whether patients with stable ischemic heart disease and moderate or severe inducible ischemia benefit from revascularization when added to optimal medical therapy. A major, federally-funded trial named ISCHEMIA is underway to answer this question.
Main results from ISCHEMIA are still several years off, but the study has already produced an interesting finding about current cardiology practice and the way that cardiac stress-imaging studies are ordered.
Based on first-year enrollment data from the ISCHEMIA trial, the vast majority of both U.S. and European patients currently referred for stress-imaging assessment of ischemia have little or no inducible ischemia, Dr. Judith S. Hochman, head of the study, said during a talk at the American Heart Association’s Scientific Sessions in November.
Stable angina patients enrolled into ISCHEMIA need to have at least moderate inducible ischemia, defined as involving at least 10% of the left ventricle, in a cardiac imaging study read by a core laboratory. Since the trial began in July 2012, fewer than 700 patients had been enrolled based on imaging studies from about 15,000 patients. In the United States, about 3% of imaged patients had moderate or severe induced ischemia; the other 97% of patients referred for assessment had mild or no induced ischemia. In Europe, the rate with moderate or severe induced ischemia was slightly higher at 5%.
This observation made Dr. Hochman, a New York University cardiologist, ask why the prevalence of moderate or severe ischemia is so low in patients referred for stress imaging. She also wondered which patients with stable angina are undergoing revascularization today if so few qualify with moderate or severe inducible ischemia.
Another surprising fact she highlighted is how cardiologists manage patients found to have moderate or severe inducible ischemia. "Most of us think that all these patients with moderate to severe inducible ischemia are referred for catheterization," but that’s not what study results showed. She cited U.S. data from the mid- and late 2000s documenting that about one-third to two-thirds of these patients undergo cardiac catheterization.
This finding shows that there is "clinical equipoise" on how to manage these patients, and the ISCHEMIA trial will address that issue.
But until the results arrive in 2020, will as many stress-imaging studies continue for patients with stable angina when so many referred patients turn out to be negative for more advanced coronary disease? And, as Dr. Tracy Y. Wang, a cardiologist at Duke University, Durham, N.C., asked after hearing about the equivocal use of catheterization for patients with worse inducible ischemia: Why do physicians order these tests if they don’t intend to catheterize patients found to have moderate-to-severe inducible ischemia?
On Twitter @mitchelzoler
First-in-man bioengineered graft proves enduring for vascular access
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.
 |
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.
 |
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.
 |
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: The 6-month enduring patency rate of an investigational tissue-engineered vascular graft for hemodialysis access was 100%, markedly better than rates achievable with synthetic PTFE grafts, the current benchmark technology.
Data source: An initial report from an ongoing prospective first-in-man study in which, to date, 28 patients with end-stage renal disease have been implanted with a novel tissue-engineered vascular graft for use as a hemodialysis access.
Disclosures: The study was funded by the Department of Defense and Humacyte. The presenter is a consultant to the company.
Meta-analysis: Statins beneficial, even after age 75
DALLAS – Reassurance regarding the cardiovascular benefits of statin therapy in the elderly, even in those above age 75, is provided by a new meta-analysis by the international Cholesterol Treatment Trialists’ Collaboration.
The meta-analysis, which included 174,099 participants in 27 major, published, randomized controlled trials with a median follow-up of 4.9 years, should go a long way toward banishing physician and patient uncertainty about the appropriateness of statin therapy in the elderly. It’s evident that such uncertainty is widespread from recent studies indicating only about half of patients over age 65 are on statin therapy post myocardial infarction (MI). Moreover, the controversial new prevention guidelines don’t address the use of statins in patients over age 75, citing a lack of persuasive evidence because such patients were often excluded from participation in the major statin trials (J. Am. Coll. Cardiol. 2013 [doi: 10.1016/j.jacc.2013.11.002]).
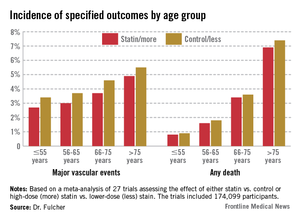
Yet in the new meta-analysis by the University of Oxford–based Cholesterol Treatment Trialist's Collaboration, 7% of all participants – that’s nearly 13,000 patients – were over age 75. That’s a large enough number to be able to draw tentative conclusions. In addition, another 33% of subjects in the meta-analysis were aged 66-75 years, Dr. Jordan Fulcher observed in presenting the results at the American Heart Association scientific sessions.
Dividing the nearly 175,000 subjects into four age groups – 55 and younger, 56-65, 66-75, and over 75 – it quickly became apparent to the investigators that while statin therapy significantly reduced nonfatal MI, cardiovascular death, all-cause mortality, and major vascular events in each of the four age groups, there was also a significant trend for smaller relative risk reductions with advancing age.
For example, the incidence of nonfatal MI or coronary heart disease (CHD) death in statin-treated patients aged 55 years or younger was 1.1%, compared with 1.5% in controls, for a 31% relative risk reduction per 39 mg/dL decrease in low-density lipoprotein (LDL) cholesterol, while in the over-75 group the rates were 2.8% versus 3.3%, for a less robust 24% relative risk reduction, reported Dr. Jordan Fulcher of the University of Sydney, Australia.
Looking at major vascular events, which is a broader composite outcome composed of nonfatal MI, CHD death, stroke, or coronary revascularization, patients aged 55 years or less who achieved a 39-mg/dL reduction in LDL had a 25% reduction compared with controls. This relative risk reduction was whittled down to 15% in the over-75s after adjustment for baseline differences in hypertension, gender, diabetes, prior cardiovascular disease, smoking, and creatinine clearance.
Nonetheless – and this is a key study finding – because of the increasing absolute risk of major events with advancing age, the number needed to treat in order to prevent one additional event is "almost identical" across all age groups, according to Dr. Fulcher.
"We therefore conclude that elderly patients at risk should be considered equally as younger patients for statin therapy," he said.
The meta-analysis showed no evidence of favorable or adverse effects of statin therapy on cancer incidence or mortality or on nonvascular mortality in any age group.
Session chair Dr. Lori Mosca said that "as a card-carrying epidemiologist," she thinks it’s important to emphasize for the benefit of nonepidemiologists the truism that relative risk reductions for effective therapies get smaller as people get older because the background rate of disease goes up.
"Relative risk is used for etiology. Absolute risk is used for treatment decisions. I don’t really care if the relative risk [reduction] gets lower as we get older. That has nothing to do with the importance of statins in older patients. It could potentially be more important to treat the elderly despite a lower relative risk" reduction, said Dr. Mosca, professor of medicine at Columbia University, New York, and director of preventive cardiology at New York–Presbyterian Hospital.
The Cholesterol Treatment Trialists’ Collaboration was established back in 1994 in early recognition that no single statin megatrial would have sufficient size and statistical power to answer all the key future clinical issues to arise. The trialists’ database includes virtually all the landmark statin trials whose acronyms are household names within medicine.
Funding for the trialists’ ongoing work is provided by the U.K. Medical Research Council and other national health research organizations. Dr. Fulcher reported having no financial conflicts of interest.
DALLAS – Reassurance regarding the cardiovascular benefits of statin therapy in the elderly, even in those above age 75, is provided by a new meta-analysis by the international Cholesterol Treatment Trialists’ Collaboration.
The meta-analysis, which included 174,099 participants in 27 major, published, randomized controlled trials with a median follow-up of 4.9 years, should go a long way toward banishing physician and patient uncertainty about the appropriateness of statin therapy in the elderly. It’s evident that such uncertainty is widespread from recent studies indicating only about half of patients over age 65 are on statin therapy post myocardial infarction (MI). Moreover, the controversial new prevention guidelines don’t address the use of statins in patients over age 75, citing a lack of persuasive evidence because such patients were often excluded from participation in the major statin trials (J. Am. Coll. Cardiol. 2013 [doi: 10.1016/j.jacc.2013.11.002]).

Yet in the new meta-analysis by the University of Oxford–based Cholesterol Treatment Trialist's Collaboration, 7% of all participants – that’s nearly 13,000 patients – were over age 75. That’s a large enough number to be able to draw tentative conclusions. In addition, another 33% of subjects in the meta-analysis were aged 66-75 years, Dr. Jordan Fulcher observed in presenting the results at the American Heart Association scientific sessions.
Dividing the nearly 175,000 subjects into four age groups – 55 and younger, 56-65, 66-75, and over 75 – it quickly became apparent to the investigators that while statin therapy significantly reduced nonfatal MI, cardiovascular death, all-cause mortality, and major vascular events in each of the four age groups, there was also a significant trend for smaller relative risk reductions with advancing age.
For example, the incidence of nonfatal MI or coronary heart disease (CHD) death in statin-treated patients aged 55 years or younger was 1.1%, compared with 1.5% in controls, for a 31% relative risk reduction per 39 mg/dL decrease in low-density lipoprotein (LDL) cholesterol, while in the over-75 group the rates were 2.8% versus 3.3%, for a less robust 24% relative risk reduction, reported Dr. Jordan Fulcher of the University of Sydney, Australia.
Looking at major vascular events, which is a broader composite outcome composed of nonfatal MI, CHD death, stroke, or coronary revascularization, patients aged 55 years or less who achieved a 39-mg/dL reduction in LDL had a 25% reduction compared with controls. This relative risk reduction was whittled down to 15% in the over-75s after adjustment for baseline differences in hypertension, gender, diabetes, prior cardiovascular disease, smoking, and creatinine clearance.
Nonetheless – and this is a key study finding – because of the increasing absolute risk of major events with advancing age, the number needed to treat in order to prevent one additional event is "almost identical" across all age groups, according to Dr. Fulcher.
"We therefore conclude that elderly patients at risk should be considered equally as younger patients for statin therapy," he said.
The meta-analysis showed no evidence of favorable or adverse effects of statin therapy on cancer incidence or mortality or on nonvascular mortality in any age group.
Session chair Dr. Lori Mosca said that "as a card-carrying epidemiologist," she thinks it’s important to emphasize for the benefit of nonepidemiologists the truism that relative risk reductions for effective therapies get smaller as people get older because the background rate of disease goes up.
"Relative risk is used for etiology. Absolute risk is used for treatment decisions. I don’t really care if the relative risk [reduction] gets lower as we get older. That has nothing to do with the importance of statins in older patients. It could potentially be more important to treat the elderly despite a lower relative risk" reduction, said Dr. Mosca, professor of medicine at Columbia University, New York, and director of preventive cardiology at New York–Presbyterian Hospital.
The Cholesterol Treatment Trialists’ Collaboration was established back in 1994 in early recognition that no single statin megatrial would have sufficient size and statistical power to answer all the key future clinical issues to arise. The trialists’ database includes virtually all the landmark statin trials whose acronyms are household names within medicine.
Funding for the trialists’ ongoing work is provided by the U.K. Medical Research Council and other national health research organizations. Dr. Fulcher reported having no financial conflicts of interest.
DALLAS – Reassurance regarding the cardiovascular benefits of statin therapy in the elderly, even in those above age 75, is provided by a new meta-analysis by the international Cholesterol Treatment Trialists’ Collaboration.
The meta-analysis, which included 174,099 participants in 27 major, published, randomized controlled trials with a median follow-up of 4.9 years, should go a long way toward banishing physician and patient uncertainty about the appropriateness of statin therapy in the elderly. It’s evident that such uncertainty is widespread from recent studies indicating only about half of patients over age 65 are on statin therapy post myocardial infarction (MI). Moreover, the controversial new prevention guidelines don’t address the use of statins in patients over age 75, citing a lack of persuasive evidence because such patients were often excluded from participation in the major statin trials (J. Am. Coll. Cardiol. 2013 [doi: 10.1016/j.jacc.2013.11.002]).

Yet in the new meta-analysis by the University of Oxford–based Cholesterol Treatment Trialist's Collaboration, 7% of all participants – that’s nearly 13,000 patients – were over age 75. That’s a large enough number to be able to draw tentative conclusions. In addition, another 33% of subjects in the meta-analysis were aged 66-75 years, Dr. Jordan Fulcher observed in presenting the results at the American Heart Association scientific sessions.
Dividing the nearly 175,000 subjects into four age groups – 55 and younger, 56-65, 66-75, and over 75 – it quickly became apparent to the investigators that while statin therapy significantly reduced nonfatal MI, cardiovascular death, all-cause mortality, and major vascular events in each of the four age groups, there was also a significant trend for smaller relative risk reductions with advancing age.
For example, the incidence of nonfatal MI or coronary heart disease (CHD) death in statin-treated patients aged 55 years or younger was 1.1%, compared with 1.5% in controls, for a 31% relative risk reduction per 39 mg/dL decrease in low-density lipoprotein (LDL) cholesterol, while in the over-75 group the rates were 2.8% versus 3.3%, for a less robust 24% relative risk reduction, reported Dr. Jordan Fulcher of the University of Sydney, Australia.
Looking at major vascular events, which is a broader composite outcome composed of nonfatal MI, CHD death, stroke, or coronary revascularization, patients aged 55 years or less who achieved a 39-mg/dL reduction in LDL had a 25% reduction compared with controls. This relative risk reduction was whittled down to 15% in the over-75s after adjustment for baseline differences in hypertension, gender, diabetes, prior cardiovascular disease, smoking, and creatinine clearance.
Nonetheless – and this is a key study finding – because of the increasing absolute risk of major events with advancing age, the number needed to treat in order to prevent one additional event is "almost identical" across all age groups, according to Dr. Fulcher.
"We therefore conclude that elderly patients at risk should be considered equally as younger patients for statin therapy," he said.
The meta-analysis showed no evidence of favorable or adverse effects of statin therapy on cancer incidence or mortality or on nonvascular mortality in any age group.
Session chair Dr. Lori Mosca said that "as a card-carrying epidemiologist," she thinks it’s important to emphasize for the benefit of nonepidemiologists the truism that relative risk reductions for effective therapies get smaller as people get older because the background rate of disease goes up.
"Relative risk is used for etiology. Absolute risk is used for treatment decisions. I don’t really care if the relative risk [reduction] gets lower as we get older. That has nothing to do with the importance of statins in older patients. It could potentially be more important to treat the elderly despite a lower relative risk" reduction, said Dr. Mosca, professor of medicine at Columbia University, New York, and director of preventive cardiology at New York–Presbyterian Hospital.
The Cholesterol Treatment Trialists’ Collaboration was established back in 1994 in early recognition that no single statin megatrial would have sufficient size and statistical power to answer all the key future clinical issues to arise. The trialists’ database includes virtually all the landmark statin trials whose acronyms are household names within medicine.
Funding for the trialists’ ongoing work is provided by the U.K. Medical Research Council and other national health research organizations. Dr. Fulcher reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: While the relative risk reduction provided by statin therapy in patients over age 75 is smaller than in younger age groups, the number needed to treat in order to prevent one major vascular event is virtually identical across the full age spectrum due to the increasing absolute risk of vascular disease with advancing age.
Data source: A meta-analysis of 27 major randomized controlled clinical trials of statin therapy with 174,099 participants.
Disclosures: Funding for the trialists’ ongoing work is provided by the U.K. Medical Research Council and other national health research organizations. Dr. Fulcher reported having no financial conflicts of interest.
Statin in childhood reduces CHD risk in familial hypercholesterolemia
DALLAS – Roughly 10 years after going on statin therapy as children or adolescents, a group of Dutch patients with familial hypercholesterolemia already has a significantly lower cardiovascular event rate than their affected parents did at the same age.
"Statin therapy in childhood seems to be effective in reducing CHD [coronary heart disease] risk in individuals with FH [familial hypercholesterolemia], although longer follow-up is needed to confirm these results," Dr. Marjet J.A.M. Braamskamp declared at the American Heart Association scientific sessions.
The 214 Dutch youths with FH started on statin therapy at age 8-18 years as part of a randomized clinical trial. None has experienced a cardiovascular event by age 30. In contrast, their affected parents, for whom statins weren’t available until they were well into adulthood, already had a 7% CHD event rate by age 30, a statistically significant difference, reported Dr. Braamskamp of the Academic Medical Center, Amsterdam.
In an interview, she said the plan is to continue to follow this cohort of young adults who started statin therapy in childhood at least until age 50. By that age, 55% of their fathers with FH and 24% of their mothers with FH had known CHD. While none of the FH mothers died of CHD before age 60, 16% of the FH fathers did, at an average age of 35.9 years.
Current European and American guidelines recommend statin therapy starting at age 8 or 10 years in children with the molecular or clinical diagnosis of FH and elevated cholesterol levels. Those guidelines were issued based upon recognition that children with FH have elevated cholesterol levels from birth onward, coupled with evidence from short-term randomized trials demonstrating that statins are safe and effective for lipid-lowering in such patients. The Dutch follow-up study provides evidence to suggest this early-treatment strategy also pays off in terms of reduced CHD risk.
The study was supported by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.
DALLAS – Roughly 10 years after going on statin therapy as children or adolescents, a group of Dutch patients with familial hypercholesterolemia already has a significantly lower cardiovascular event rate than their affected parents did at the same age.
"Statin therapy in childhood seems to be effective in reducing CHD [coronary heart disease] risk in individuals with FH [familial hypercholesterolemia], although longer follow-up is needed to confirm these results," Dr. Marjet J.A.M. Braamskamp declared at the American Heart Association scientific sessions.
The 214 Dutch youths with FH started on statin therapy at age 8-18 years as part of a randomized clinical trial. None has experienced a cardiovascular event by age 30. In contrast, their affected parents, for whom statins weren’t available until they were well into adulthood, already had a 7% CHD event rate by age 30, a statistically significant difference, reported Dr. Braamskamp of the Academic Medical Center, Amsterdam.
In an interview, she said the plan is to continue to follow this cohort of young adults who started statin therapy in childhood at least until age 50. By that age, 55% of their fathers with FH and 24% of their mothers with FH had known CHD. While none of the FH mothers died of CHD before age 60, 16% of the FH fathers did, at an average age of 35.9 years.
Current European and American guidelines recommend statin therapy starting at age 8 or 10 years in children with the molecular or clinical diagnosis of FH and elevated cholesterol levels. Those guidelines were issued based upon recognition that children with FH have elevated cholesterol levels from birth onward, coupled with evidence from short-term randomized trials demonstrating that statins are safe and effective for lipid-lowering in such patients. The Dutch follow-up study provides evidence to suggest this early-treatment strategy also pays off in terms of reduced CHD risk.
The study was supported by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.
DALLAS – Roughly 10 years after going on statin therapy as children or adolescents, a group of Dutch patients with familial hypercholesterolemia already has a significantly lower cardiovascular event rate than their affected parents did at the same age.
"Statin therapy in childhood seems to be effective in reducing CHD [coronary heart disease] risk in individuals with FH [familial hypercholesterolemia], although longer follow-up is needed to confirm these results," Dr. Marjet J.A.M. Braamskamp declared at the American Heart Association scientific sessions.
The 214 Dutch youths with FH started on statin therapy at age 8-18 years as part of a randomized clinical trial. None has experienced a cardiovascular event by age 30. In contrast, their affected parents, for whom statins weren’t available until they were well into adulthood, already had a 7% CHD event rate by age 30, a statistically significant difference, reported Dr. Braamskamp of the Academic Medical Center, Amsterdam.
In an interview, she said the plan is to continue to follow this cohort of young adults who started statin therapy in childhood at least until age 50. By that age, 55% of their fathers with FH and 24% of their mothers with FH had known CHD. While none of the FH mothers died of CHD before age 60, 16% of the FH fathers did, at an average age of 35.9 years.
Current European and American guidelines recommend statin therapy starting at age 8 or 10 years in children with the molecular or clinical diagnosis of FH and elevated cholesterol levels. Those guidelines were issued based upon recognition that children with FH have elevated cholesterol levels from birth onward, coupled with evidence from short-term randomized trials demonstrating that statins are safe and effective for lipid-lowering in such patients. The Dutch follow-up study provides evidence to suggest this early-treatment strategy also pays off in terms of reduced CHD risk.
The study was supported by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: At age 30, the cardiovascular event rate in 214 Dutch patients with FH who began statin therapy as children or adolescents was zero, significantly lower than the 7% rate in their affected parents at the same age, who didn’t have access to statin therapy.
Data source: This was a follow-up report on 214 young adults with familial hypercholesterolemia.
Disclosures: The study was funded by the Dutch Heart Foundation. Dr. Braamskamp reported having no financial conflicts.


















