User login
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
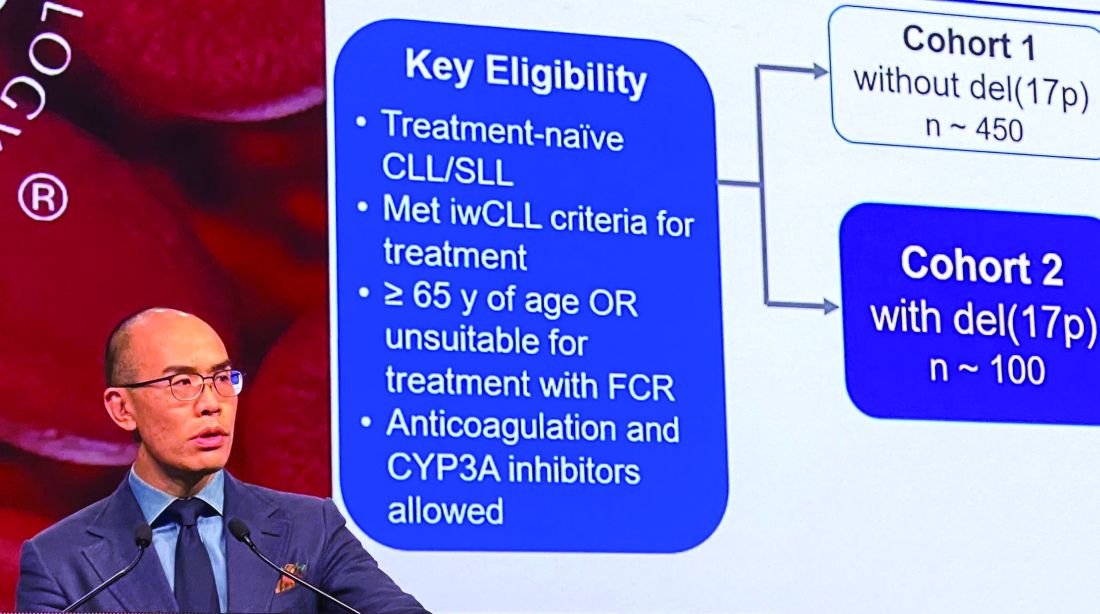
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
ORLANDO – Zanubrutinib has produced a high overall response rate in one the largest cohorts of patients with treatment-naive 17p-deletion chronic lymphocytic leukemia (CLL) studied to date.
An overall response rate of nearly 93% was seen in this 109-patient, high-risk cohort, enrolled as part of the phase 3 SEQUOIA study (BGB-3111-304), said Constantine S. Tam, MBBS, MD, of St. Vincent’s Hospital and Peter MacCallum Cancer Centre in Melbourne.
Tolerability of zanubrutinib was essentially consistent with previous reports of the agent as used in other B-cell malignancies, Dr. Tam said in an oral presentation of the results at the annual meeting of the American Society of Hematology.
Deletion of chromosome 17p13.1, or del(17p), is a marker of poor prognosis and poor response to chemotherapy in patients with CLL or small lymphocytic lymphoma (SLL). For patients with del(17p) CLL, the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become a standard of care, Dr. Tam said.
Zanubrutinib, a next-generation BTK inhibitor, was developed to improve BTK occupancy and minimize off-target inhibition of TEC and epidermal growth factor receptor kinases. “What this effectively means is that we are able to dose this drug at levels much higher than that achievable with ibrutinib, and not get intolerable side effects,” Dr. Tam said.
Zanubrutinib has been approved in the United States for previously treated mantle cell lymphoma, and generated durable responses among CLL/SLL patients with or without del(17p) in a phase 1/2 study, according to Dr. Tam.
In the present study, which exclusively enrolled patients with del(17p) CLL/SLL, patients received 160 mg twice daily of zanubrutinib, Dr. Tam said. Out of 109 patients enrolled, 10 (9.2%) had SLL. All patients were aged at least 65 years or were deemed unsuitable for treatment with the combination of fludarabine, cyclophosphamide, and rituximab.
Of 109 patients enrolled, 104 received on-study treatment. The median age was 70 years, Dr. Tam reported, and a number of patients had other high-risk markers beyond del(17p), including unmutated IgVH status in 61.5% of patients.
With a median follow-up of 10 months, the overall response rate was 92.7%, including 1.9% complete responses and 78.9% partial responses. “Only one patient had primary progressive disease after starting this drug,” Dr. Tam said.
Time to response was rapid, according to the investigator, at about 2.8 months; after 6 months, 95% of responders remained in response.
Further analysis showed that the response rate was consistent across subgroups. “There was not a single group that did not respond with a high response rate, including poor prognostic groups,” Dr. Tam said.
Most adverse events were grade 1-2 in severity, and the most common events included confusion and upper respiratory tract infection. The only common grade 3 event, according to Dr. Tam, was neutropenia. Rates of grade 3 major bleeding were low, he said, and the rate of grade 3 atrial fibrillation was 0.9%. One patient died due to pneumonia.
The ongoing SEQUOIA study, designed to compare zanubrutinib to the combination of bendamustine and rituximab in patients with previously untreated CLL or SLL, is sponsored by BeiGene. Dr. Tam reported disclosures related to Novartis, Pharmacyclics, AbbVie, BeiGene, Janssen, and Roche.
SOURCE: Tam C et al. ASH 2019, Abstract 499.
REPORTING FROM ASH 2019