User login
CATIE phase 2 offers insights on efficacy an tolerability
After nearly 3 out of 4 phase 1 patients stopped taking their assigned antipsychotics within 18 months, researchers in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) braced themselves for phase 2.February 2006)
CATIE’s eligibility criteria are broad and include schizophrenia patients with comorbid conditions such as substance abuse and mood disorders. The primary outcome measure is all-cause treatment discontinuation, which incorporates efficacy, safety, tolerability, patient choice, and clinician choice (Table 1).
Phase 1 compared the efficacy and safety of four second-generation antipsychotics (SGA) and one first-generation antipsychotic (FGA).3 Nasrallah concluded that—despite the high discontinuation rate in that phase—there were “no winners or losers” among the five antipsychotics. The results, Nasrallah concluded:
- provide a compelling rationale for clinicians to match medication profiles to individual patients
- support the need for clinicians to have choices among medications when treating patients with schizophrenia.4
Table 1
Drug discontinuation patterns in CATIE phase 1
| Measures | Findings after 18 months | |
|---|---|---|
| % of patients who discontinued medication for any reason | Olanzapine (64%) | Ziprasidone (79%) |
| Risperidone (74%) | Quetiapine (82%) | |
| Perphenazine (75%) | ||
| Time to discontinuation for any reason | Longest (most favorable) with olanzapine, but not statistically longer with olanzapine than with ziprasidone or perphenazine | |
| No statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | ||
| Time to discontinuation for lack of efficacy* | Longer with olanzapine; no statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | |
| Time to discontinuation for intolerable side effects | No statistical difference among agents | |
| Rate of discontinuation for intolerable side effects | Highest (19%) with olanzapine (primarily because of weight gain or metabolic effects with this medication) | |
| Rate of discontinuation for extrapyramidal effects | Highest (8%) with perphenazine | |
| Rate of discontinuation for intolerability (overall) | Lowest with risperidone (10%) | |
| * Nonequivalent dosing in CATIE phase 1 is an ongoing debate. | ||
What to do next?
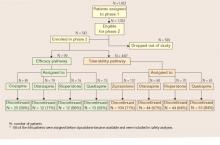
When an initial antipsychotic proves inadequate or causes intolerable side effects, how do you choose a more efficacious or tolerable medication? Phase 2 offered CATIE patients and their clinicians two choices—an efficacy and a tolerability pathway (Figure).1,2
CATIE phase 2: Distribution of patients in efficacy and tolerability pathways
Efficacy pathway. Patients who chose the efficacy pathway were randomly assigned to clozapine (50%) or olanzapine, risperidone, or quetiapine.1 Researchers selected clozapine as the major efficacy comparator because of its robust effects in treatment-refractory schizophrenia. Clozapine was given open-label because of its safety monitoring requirements; other treatments were double-blind.
As in phase 1, the primary outcome measure was time until discontinuation for any reason. Secondary outcome measures included time to discontinuation because of side effects, patient choice, or lack of efficacy.
Tolerability pathway. Patients who chose the tolerability pathway were randomly assigned to double-blind treatment with ziprasidone, olanzapine, risperidone, or quetiapine.2 Ziprasidone was the major comparator because of clinical data showing a favorable tolerability profile.
The primary outcome measure was time to discontinuation for any reason. Secondary outcomes included reason for discontinuation (as determined by the study clinician), symptomatic ratings, and evaluations of adverse effects.
Trial duration. No patients in either pathway received the same antipsychotics they had taken in phase 1. All patients could continue treatment through the 18 months of the CATIE trial or until they completed 6 months in phase 2.
Efficacy pathway results
Discontinuation. Consistent with literature about its efficacy in treatment-refractory schizophrenia, clozapine showed a robust clinical effect. Overall, more patients receiving clozapine stayed on treatment and for longer periods, compared with patients receiving olanzapine, risperidone, or quetiapine (Table 2).
On secondary measures, discontinuation for lack of efficacy was significantly lower with clozapine (11%) than with:
- olanzapine (35%)
- risperidone or quetiapine (each at 43%).
Discontinuation rates because of adverse effects or by patient choice were the same across all medications (Table 3). Patients on clozapine achieved better ratings in overall psychotic symptoms, positive symptoms, and general function, but not in negative symptoms.
Weight gain. On average, patients gained more weight while taking olanzapine (+1.1 lb/mo) than with:
- risperidone (+0.5 lb/mo)
- clozapine (+0.5 lb/mo)
- quetiapine (+0.5 lb/mo)
Differences in weight gain—or in metabolic parameters or other adverse effects—were not statistically significant, however.
Table 2
Phase 2 efficacy pathway: Discontinuation for any reason
| Measure | Clozapine | Olanzapine | Risperidone | Quetiapine |
|---|---|---|---|---|
| How many patients discontinued | 25 of 49 (56%) | 12 of 19 (71%) | 12 of 16 (86%) | 13 of 15 (93%) |
| Median time to discontinuation | 10.5 months | 2.7 months | 2.8 months | 3.3 months |
Table 3
Reasons patients stopped taking their medications in CATIE phase 2
| Reason | Efficacy pathway | Tolerability pathway |
|---|---|---|
| All cause | 69% | 74% |
| Lack of efficacy | 26% | 29% |
| Lack of tolerability | 10% | 15% |
| Patient choice | 26% | 24% |
Tolerability pathway results
Discontinuation. Patients in the tolerability pathway took olanzapine or risperidone significantly longer—median 6.3 and 7 months, respectively— compared with ziprasidone (4 months) or quetiapine (2.8 months).
- Time to discontinuation during phase 2 was the same across all drugs among patients who entered phase 2 because of intolerable side effects in phase 1.
- Time to discontinuation because of side effects also was similar whether patients discontinued phase 1 for lack of efficacy or intolerable side effects. Patients stopped treatment in the efficacy and tolerability pathways for similar reasons (Table 3).
Weight gain. Patients taking olanzapine gained more weight (average +1.3 lb/mo) than did those taking the other drugs. Patients taking ziprasidone lost weight (average –1.7 lb/mo). Among 61 patients who gained weight during phase 1, 42% of those switched to ziprasidone lost weight in phase 2, as did:
- 20% of those switched to risperidone
- 7% of those switched to quetiapine.
Among those switched to olanzapine in phase 2, no one lost weight and 2% gained weight.
Metabolic effects. Some parameters changed, depending on drug assignment:
- prolactin increased in patients switched to risperidone
- cholesterol and triglycerides increased in patients switched to olanzapine or quetiapine but decreased in those switched to risperidone or ziprasidone
- QTc interval measurements showed no difference across all drugs.
Methodologic caveats
When considering how CATIE’s phase 2 findings might apply to clinical practice, keep in mind four caveats about the study’s design.
Clozapine was given open-label, yet quetiapine, olanzapine, and risperidone were given double-blind in the efficacy pathway. This pathway’s findings are consistent with what we know about clozapine and other SGAs in treatment-refractory schizophrenia, but how the open-label design affected clozapine therapy outcomes is unclear.
Were patients who knew they were taking clozapine more willing to “stay the course” than were patients in the pathway’s double-blind arm?
Discontinuation rates remained high. The 74% “overall discontinuation rate” in phase 1 surprised many psychiatrists because of the perceived high rate at which patients did not adhere to the first medications they received. To some extent, the word “discontinuation” is imprecise, however, because this group includes patients who did not drop out of treatment altogether but chose to move on to phase 2.
It is important to note, however, that nearly one-half of phase 1 patients who were eligible to enter phase 2 (509 of 1,052) did not. This group represents the true drop-out rate, which is substantial. The high rates of discontinuation seen in phase 1 also occurred in both phase 2 pathways (Table 3).
Few patients entered the efficacy pathway. In an approach designed to reflect routine clinical practice, the researchers recommended the efficacy pathway to patients who discontinued phase 1 because of lack of efficacy and the tolerability pathway to those who discontinued phase 1 because of intolerability. Many patients did not follow the recommendations, however, and seemed to choose their pathways based on whether they wanted a chance to receive clozapine or ziprasidone in phase 2.
Thus, among the 543 phase 1 patients who enrolled in phase 2, 99 (18%) entered the efficacy pathway, and 444 (82%) entered the tolerability pathway. The efficacy pathway included 85 patients who discontinued phase 1 for lack of efficacy and 5 for lack of tolerability. The tolerability pathway included 184 patients who discontinued phase 1 for lack of efficacy and 168 for lack of tolerability.
Dosages may not have been equivalent. SGAs’ dosing equivalency is unknown,5,6 which impedes our ability to interpret comparative studies such as CATIE. The study’s designers developed the its dosing ranges by careful consideration, including recommendations from each SGA’s manufacturer. As Nasrallah described,4 the trial’s dosages were not universally consistent with FDA-approved ranges or usual clinical practice (Table 4). In phase 2, for example, ziprasidone dosages were less than psychiatrists usually use, and quetiapine dosages were greater than usual.
Fortunately, studies are underway to determine each SGA’s optimum dosing. This work will help us understand what we can expect when we increase an antipsychotic’s dosage—a key step towards understanding dosing equivalency.
Table 4
Mean modal antipsychotic dosages (mg/d) in CATIE phase 2 pathways*
| Clozapine | Ziprasidone | Olanzapine | Risperidone | Quetiapine | |
|---|---|---|---|---|---|
| Efficacy pathway | 332 | — | 23.4 | 4.8 | 642.9 |
| Tolerability pathway | — | 115.9 | 20.5 | 4.15 | 65.2 |
| * 800 mg/d of quetiapine and 160 mg/d of ziprasidone are generally regarded as therapeutically equivalent to 20 mg/d of olanzapine. | |||||
What clinicians can expect
A recent analysis helps put CATIE’s findings in perspective. Citrome and Stroup7 quantified the results of phase 1 and 2 with respect to:
- number needed to treat (NNT)—how many patients a clinician needs to treat with drug A to see one additional benefit, compared with drug B
- number needed to harm (NNH)—how many patients a clinician needs to treat with drug A to see a given adverse effect, compared with drug B.
In this analysis, the NNT for olanzapine (5.5 to 10) was lowest among the drugs compared in phase 1, and the NNT for clozapine (3) was lowest among those compared in phase 2. A lower number means that, overall, clinicians can expect a more robust treatment response.
On the other hand, the NNH for olanzapine in weight gain and metabolic disturbances (12.4 to 17.7) was the lowest in phase 1, indicating that clinicians can expect more weight gain and metabolic effects with olanzapine than with other SGAs. Ziprasidone had the highest NNH (106 to 208) among the agents in phase 2 for avoiding discontinuation because of weight gain or metabolic disturbances. In other words, ziprasidone appears less likely than other SGAs to cause metabolic problems.
These risk-attribution measures demonstrate the dilemma clinicians face when trying to match schizophrenia patients with antipsychotics. CATIE was “an N of 1,493” subjects, whereas each patient we see in clinical practice is “an N of 1.” One patient may need a more-robust response; another may need improved tolerability.
We strive for balance, seeking to optimize efficacy—often by raising the dosage—while minimizing adverse effects.
What to tell patients
CATIE phases 1 and 2 provide a compelling rationale for individualized treatment, which should be standard clinical practice for schizophrenia:
- All drugs used in phases 1 and 2 worked.
- All showed noteworthy adverse effects that were different for each drug.
- Different patients responded differently to each drug.
Using our clinical judgment and available information, we must match—as best we can—the individual patient’s characteristics with the antipsychotics’ risk: benefit profiles. CATIE phases 1 and 2 provide independent information on the comparative efficacy and tolerability of each medication.
The CATIE investigators and NIMH have done a great service to our field in providing a rich repository of timely information to inform clinical practice. But the CATIE study was not designed to answer all our questions about treating schizophrenia.8,9 Clinicians and patients need to look elsewhere for guidance on the roles of:
- psychosocial treatments
- recovery and the therapeutic alliance in maximizing outcomes
- long-acting SGA formulations
- aripiprazole (addressed in CATIE phase 3)
- SGAs in first-episode schizophrenia
- FGAs when a patient does not adequately respond to an initial SGA.
Related resources
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). www.CATIE.unc.edu.
- Lieberman JA. What the CATIE study means in clinical practice. Psychiatr Serv 2006;57(8):1075.
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Buckley receives research/grant support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, Pfizer, and Solvay Pharmaceuticals, and is a consultant to Abbott Laboratories, Alamo Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, Merck & Co., and Pfizer.
Acknowledgement
The author thanks Del Miller, MD, for comments given on a draft of this paper.
1. Stroup TS, Lieberman JA, McEvoy JP, et al for the CATIE investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
2. McEvoy JP, Lieberman JA, Stroup TS, et al for the CATIE investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. Nasrallah HA. CATIE’s surprises: In antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):52-65.
5. Buckley PF. Dosing equivalency of second-generation antipsychotics. J Clin Psychopharmacol 2005;25(5):501-2.
6. Davis JM. The choice of drugs for schizophrenia. N Engl J Med 2006;354(5):518-20.
7. Citrome L, Stroup TS. Schizophrenia clinical antipsychotic trials intervention effectiveness and number needed to treat: How can CATIE inform clinicians? Int J Clin Pract 2006 (in press).
8. Ragins M. Should the CATIE study be a wake-up call? Psychiatr Serv 2005;56:1489.-
9. Lieberman JA, Hsiao J. Interpreting the results of the CATIE study. Psychiatr Serv 2006;57:139.-
CATIE phase 2 offers insights on efficacy an tolerability
After nearly 3 out of 4 phase 1 patients stopped taking their assigned antipsychotics within 18 months, researchers in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) braced themselves for phase 2.February 2006)
CATIE’s eligibility criteria are broad and include schizophrenia patients with comorbid conditions such as substance abuse and mood disorders. The primary outcome measure is all-cause treatment discontinuation, which incorporates efficacy, safety, tolerability, patient choice, and clinician choice (Table 1).
Phase 1 compared the efficacy and safety of four second-generation antipsychotics (SGA) and one first-generation antipsychotic (FGA).3 Nasrallah concluded that—despite the high discontinuation rate in that phase—there were “no winners or losers” among the five antipsychotics. The results, Nasrallah concluded:
- provide a compelling rationale for clinicians to match medication profiles to individual patients
- support the need for clinicians to have choices among medications when treating patients with schizophrenia.4
Table 1
Drug discontinuation patterns in CATIE phase 1
| Measures | Findings after 18 months | |
|---|---|---|
| % of patients who discontinued medication for any reason | Olanzapine (64%) | Ziprasidone (79%) |
| Risperidone (74%) | Quetiapine (82%) | |
| Perphenazine (75%) | ||
| Time to discontinuation for any reason | Longest (most favorable) with olanzapine, but not statistically longer with olanzapine than with ziprasidone or perphenazine | |
| No statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | ||
| Time to discontinuation for lack of efficacy* | Longer with olanzapine; no statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | |
| Time to discontinuation for intolerable side effects | No statistical difference among agents | |
| Rate of discontinuation for intolerable side effects | Highest (19%) with olanzapine (primarily because of weight gain or metabolic effects with this medication) | |
| Rate of discontinuation for extrapyramidal effects | Highest (8%) with perphenazine | |
| Rate of discontinuation for intolerability (overall) | Lowest with risperidone (10%) | |
| * Nonequivalent dosing in CATIE phase 1 is an ongoing debate. | ||
What to do next?
When an initial antipsychotic proves inadequate or causes intolerable side effects, how do you choose a more efficacious or tolerable medication? Phase 2 offered CATIE patients and their clinicians two choices—an efficacy and a tolerability pathway (Figure).1,2
CATIE phase 2: Distribution of patients in efficacy and tolerability pathways
Efficacy pathway. Patients who chose the efficacy pathway were randomly assigned to clozapine (50%) or olanzapine, risperidone, or quetiapine.1 Researchers selected clozapine as the major efficacy comparator because of its robust effects in treatment-refractory schizophrenia. Clozapine was given open-label because of its safety monitoring requirements; other treatments were double-blind.
As in phase 1, the primary outcome measure was time until discontinuation for any reason. Secondary outcome measures included time to discontinuation because of side effects, patient choice, or lack of efficacy.
Tolerability pathway. Patients who chose the tolerability pathway were randomly assigned to double-blind treatment with ziprasidone, olanzapine, risperidone, or quetiapine.2 Ziprasidone was the major comparator because of clinical data showing a favorable tolerability profile.
The primary outcome measure was time to discontinuation for any reason. Secondary outcomes included reason for discontinuation (as determined by the study clinician), symptomatic ratings, and evaluations of adverse effects.
Trial duration. No patients in either pathway received the same antipsychotics they had taken in phase 1. All patients could continue treatment through the 18 months of the CATIE trial or until they completed 6 months in phase 2.
Efficacy pathway results
Discontinuation. Consistent with literature about its efficacy in treatment-refractory schizophrenia, clozapine showed a robust clinical effect. Overall, more patients receiving clozapine stayed on treatment and for longer periods, compared with patients receiving olanzapine, risperidone, or quetiapine (Table 2).
On secondary measures, discontinuation for lack of efficacy was significantly lower with clozapine (11%) than with:
- olanzapine (35%)
- risperidone or quetiapine (each at 43%).
Discontinuation rates because of adverse effects or by patient choice were the same across all medications (Table 3). Patients on clozapine achieved better ratings in overall psychotic symptoms, positive symptoms, and general function, but not in negative symptoms.
Weight gain. On average, patients gained more weight while taking olanzapine (+1.1 lb/mo) than with:
- risperidone (+0.5 lb/mo)
- clozapine (+0.5 lb/mo)
- quetiapine (+0.5 lb/mo)
Differences in weight gain—or in metabolic parameters or other adverse effects—were not statistically significant, however.
Table 2
Phase 2 efficacy pathway: Discontinuation for any reason
| Measure | Clozapine | Olanzapine | Risperidone | Quetiapine |
|---|---|---|---|---|
| How many patients discontinued | 25 of 49 (56%) | 12 of 19 (71%) | 12 of 16 (86%) | 13 of 15 (93%) |
| Median time to discontinuation | 10.5 months | 2.7 months | 2.8 months | 3.3 months |
Table 3
Reasons patients stopped taking their medications in CATIE phase 2
| Reason | Efficacy pathway | Tolerability pathway |
|---|---|---|
| All cause | 69% | 74% |
| Lack of efficacy | 26% | 29% |
| Lack of tolerability | 10% | 15% |
| Patient choice | 26% | 24% |
Tolerability pathway results
Discontinuation. Patients in the tolerability pathway took olanzapine or risperidone significantly longer—median 6.3 and 7 months, respectively— compared with ziprasidone (4 months) or quetiapine (2.8 months).
- Time to discontinuation during phase 2 was the same across all drugs among patients who entered phase 2 because of intolerable side effects in phase 1.
- Time to discontinuation because of side effects also was similar whether patients discontinued phase 1 for lack of efficacy or intolerable side effects. Patients stopped treatment in the efficacy and tolerability pathways for similar reasons (Table 3).
Weight gain. Patients taking olanzapine gained more weight (average +1.3 lb/mo) than did those taking the other drugs. Patients taking ziprasidone lost weight (average –1.7 lb/mo). Among 61 patients who gained weight during phase 1, 42% of those switched to ziprasidone lost weight in phase 2, as did:
- 20% of those switched to risperidone
- 7% of those switched to quetiapine.
Among those switched to olanzapine in phase 2, no one lost weight and 2% gained weight.
Metabolic effects. Some parameters changed, depending on drug assignment:
- prolactin increased in patients switched to risperidone
- cholesterol and triglycerides increased in patients switched to olanzapine or quetiapine but decreased in those switched to risperidone or ziprasidone
- QTc interval measurements showed no difference across all drugs.
Methodologic caveats
When considering how CATIE’s phase 2 findings might apply to clinical practice, keep in mind four caveats about the study’s design.
Clozapine was given open-label, yet quetiapine, olanzapine, and risperidone were given double-blind in the efficacy pathway. This pathway’s findings are consistent with what we know about clozapine and other SGAs in treatment-refractory schizophrenia, but how the open-label design affected clozapine therapy outcomes is unclear.
Were patients who knew they were taking clozapine more willing to “stay the course” than were patients in the pathway’s double-blind arm?
Discontinuation rates remained high. The 74% “overall discontinuation rate” in phase 1 surprised many psychiatrists because of the perceived high rate at which patients did not adhere to the first medications they received. To some extent, the word “discontinuation” is imprecise, however, because this group includes patients who did not drop out of treatment altogether but chose to move on to phase 2.
It is important to note, however, that nearly one-half of phase 1 patients who were eligible to enter phase 2 (509 of 1,052) did not. This group represents the true drop-out rate, which is substantial. The high rates of discontinuation seen in phase 1 also occurred in both phase 2 pathways (Table 3).
Few patients entered the efficacy pathway. In an approach designed to reflect routine clinical practice, the researchers recommended the efficacy pathway to patients who discontinued phase 1 because of lack of efficacy and the tolerability pathway to those who discontinued phase 1 because of intolerability. Many patients did not follow the recommendations, however, and seemed to choose their pathways based on whether they wanted a chance to receive clozapine or ziprasidone in phase 2.
Thus, among the 543 phase 1 patients who enrolled in phase 2, 99 (18%) entered the efficacy pathway, and 444 (82%) entered the tolerability pathway. The efficacy pathway included 85 patients who discontinued phase 1 for lack of efficacy and 5 for lack of tolerability. The tolerability pathway included 184 patients who discontinued phase 1 for lack of efficacy and 168 for lack of tolerability.
Dosages may not have been equivalent. SGAs’ dosing equivalency is unknown,5,6 which impedes our ability to interpret comparative studies such as CATIE. The study’s designers developed the its dosing ranges by careful consideration, including recommendations from each SGA’s manufacturer. As Nasrallah described,4 the trial’s dosages were not universally consistent with FDA-approved ranges or usual clinical practice (Table 4). In phase 2, for example, ziprasidone dosages were less than psychiatrists usually use, and quetiapine dosages were greater than usual.
Fortunately, studies are underway to determine each SGA’s optimum dosing. This work will help us understand what we can expect when we increase an antipsychotic’s dosage—a key step towards understanding dosing equivalency.
Table 4
Mean modal antipsychotic dosages (mg/d) in CATIE phase 2 pathways*
| Clozapine | Ziprasidone | Olanzapine | Risperidone | Quetiapine | |
|---|---|---|---|---|---|
| Efficacy pathway | 332 | — | 23.4 | 4.8 | 642.9 |
| Tolerability pathway | — | 115.9 | 20.5 | 4.15 | 65.2 |
| * 800 mg/d of quetiapine and 160 mg/d of ziprasidone are generally regarded as therapeutically equivalent to 20 mg/d of olanzapine. | |||||
What clinicians can expect
A recent analysis helps put CATIE’s findings in perspective. Citrome and Stroup7 quantified the results of phase 1 and 2 with respect to:
- number needed to treat (NNT)—how many patients a clinician needs to treat with drug A to see one additional benefit, compared with drug B
- number needed to harm (NNH)—how many patients a clinician needs to treat with drug A to see a given adverse effect, compared with drug B.
In this analysis, the NNT for olanzapine (5.5 to 10) was lowest among the drugs compared in phase 1, and the NNT for clozapine (3) was lowest among those compared in phase 2. A lower number means that, overall, clinicians can expect a more robust treatment response.
On the other hand, the NNH for olanzapine in weight gain and metabolic disturbances (12.4 to 17.7) was the lowest in phase 1, indicating that clinicians can expect more weight gain and metabolic effects with olanzapine than with other SGAs. Ziprasidone had the highest NNH (106 to 208) among the agents in phase 2 for avoiding discontinuation because of weight gain or metabolic disturbances. In other words, ziprasidone appears less likely than other SGAs to cause metabolic problems.
These risk-attribution measures demonstrate the dilemma clinicians face when trying to match schizophrenia patients with antipsychotics. CATIE was “an N of 1,493” subjects, whereas each patient we see in clinical practice is “an N of 1.” One patient may need a more-robust response; another may need improved tolerability.
We strive for balance, seeking to optimize efficacy—often by raising the dosage—while minimizing adverse effects.
What to tell patients
CATIE phases 1 and 2 provide a compelling rationale for individualized treatment, which should be standard clinical practice for schizophrenia:
- All drugs used in phases 1 and 2 worked.
- All showed noteworthy adverse effects that were different for each drug.
- Different patients responded differently to each drug.
Using our clinical judgment and available information, we must match—as best we can—the individual patient’s characteristics with the antipsychotics’ risk: benefit profiles. CATIE phases 1 and 2 provide independent information on the comparative efficacy and tolerability of each medication.
The CATIE investigators and NIMH have done a great service to our field in providing a rich repository of timely information to inform clinical practice. But the CATIE study was not designed to answer all our questions about treating schizophrenia.8,9 Clinicians and patients need to look elsewhere for guidance on the roles of:
- psychosocial treatments
- recovery and the therapeutic alliance in maximizing outcomes
- long-acting SGA formulations
- aripiprazole (addressed in CATIE phase 3)
- SGAs in first-episode schizophrenia
- FGAs when a patient does not adequately respond to an initial SGA.
Related resources
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). www.CATIE.unc.edu.
- Lieberman JA. What the CATIE study means in clinical practice. Psychiatr Serv 2006;57(8):1075.
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Buckley receives research/grant support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, Pfizer, and Solvay Pharmaceuticals, and is a consultant to Abbott Laboratories, Alamo Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, Merck & Co., and Pfizer.
Acknowledgement
The author thanks Del Miller, MD, for comments given on a draft of this paper.
CATIE phase 2 offers insights on efficacy an tolerability
After nearly 3 out of 4 phase 1 patients stopped taking their assigned antipsychotics within 18 months, researchers in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) braced themselves for phase 2.February 2006)
CATIE’s eligibility criteria are broad and include schizophrenia patients with comorbid conditions such as substance abuse and mood disorders. The primary outcome measure is all-cause treatment discontinuation, which incorporates efficacy, safety, tolerability, patient choice, and clinician choice (Table 1).
Phase 1 compared the efficacy and safety of four second-generation antipsychotics (SGA) and one first-generation antipsychotic (FGA).3 Nasrallah concluded that—despite the high discontinuation rate in that phase—there were “no winners or losers” among the five antipsychotics. The results, Nasrallah concluded:
- provide a compelling rationale for clinicians to match medication profiles to individual patients
- support the need for clinicians to have choices among medications when treating patients with schizophrenia.4
Table 1
Drug discontinuation patterns in CATIE phase 1
| Measures | Findings after 18 months | |
|---|---|---|
| % of patients who discontinued medication for any reason | Olanzapine (64%) | Ziprasidone (79%) |
| Risperidone (74%) | Quetiapine (82%) | |
| Perphenazine (75%) | ||
| Time to discontinuation for any reason | Longest (most favorable) with olanzapine, but not statistically longer with olanzapine than with ziprasidone or perphenazine | |
| No statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | ||
| Time to discontinuation for lack of efficacy* | Longer with olanzapine; no statistical difference among risperidone, quetiapine, ziprasidone, and perphenazine | |
| Time to discontinuation for intolerable side effects | No statistical difference among agents | |
| Rate of discontinuation for intolerable side effects | Highest (19%) with olanzapine (primarily because of weight gain or metabolic effects with this medication) | |
| Rate of discontinuation for extrapyramidal effects | Highest (8%) with perphenazine | |
| Rate of discontinuation for intolerability (overall) | Lowest with risperidone (10%) | |
| * Nonequivalent dosing in CATIE phase 1 is an ongoing debate. | ||
What to do next?
When an initial antipsychotic proves inadequate or causes intolerable side effects, how do you choose a more efficacious or tolerable medication? Phase 2 offered CATIE patients and their clinicians two choices—an efficacy and a tolerability pathway (Figure).1,2
CATIE phase 2: Distribution of patients in efficacy and tolerability pathways
Efficacy pathway. Patients who chose the efficacy pathway were randomly assigned to clozapine (50%) or olanzapine, risperidone, or quetiapine.1 Researchers selected clozapine as the major efficacy comparator because of its robust effects in treatment-refractory schizophrenia. Clozapine was given open-label because of its safety monitoring requirements; other treatments were double-blind.
As in phase 1, the primary outcome measure was time until discontinuation for any reason. Secondary outcome measures included time to discontinuation because of side effects, patient choice, or lack of efficacy.
Tolerability pathway. Patients who chose the tolerability pathway were randomly assigned to double-blind treatment with ziprasidone, olanzapine, risperidone, or quetiapine.2 Ziprasidone was the major comparator because of clinical data showing a favorable tolerability profile.
The primary outcome measure was time to discontinuation for any reason. Secondary outcomes included reason for discontinuation (as determined by the study clinician), symptomatic ratings, and evaluations of adverse effects.
Trial duration. No patients in either pathway received the same antipsychotics they had taken in phase 1. All patients could continue treatment through the 18 months of the CATIE trial or until they completed 6 months in phase 2.
Efficacy pathway results
Discontinuation. Consistent with literature about its efficacy in treatment-refractory schizophrenia, clozapine showed a robust clinical effect. Overall, more patients receiving clozapine stayed on treatment and for longer periods, compared with patients receiving olanzapine, risperidone, or quetiapine (Table 2).
On secondary measures, discontinuation for lack of efficacy was significantly lower with clozapine (11%) than with:
- olanzapine (35%)
- risperidone or quetiapine (each at 43%).
Discontinuation rates because of adverse effects or by patient choice were the same across all medications (Table 3). Patients on clozapine achieved better ratings in overall psychotic symptoms, positive symptoms, and general function, but not in negative symptoms.
Weight gain. On average, patients gained more weight while taking olanzapine (+1.1 lb/mo) than with:
- risperidone (+0.5 lb/mo)
- clozapine (+0.5 lb/mo)
- quetiapine (+0.5 lb/mo)
Differences in weight gain—or in metabolic parameters or other adverse effects—were not statistically significant, however.
Table 2
Phase 2 efficacy pathway: Discontinuation for any reason
| Measure | Clozapine | Olanzapine | Risperidone | Quetiapine |
|---|---|---|---|---|
| How many patients discontinued | 25 of 49 (56%) | 12 of 19 (71%) | 12 of 16 (86%) | 13 of 15 (93%) |
| Median time to discontinuation | 10.5 months | 2.7 months | 2.8 months | 3.3 months |
Table 3
Reasons patients stopped taking their medications in CATIE phase 2
| Reason | Efficacy pathway | Tolerability pathway |
|---|---|---|
| All cause | 69% | 74% |
| Lack of efficacy | 26% | 29% |
| Lack of tolerability | 10% | 15% |
| Patient choice | 26% | 24% |
Tolerability pathway results
Discontinuation. Patients in the tolerability pathway took olanzapine or risperidone significantly longer—median 6.3 and 7 months, respectively— compared with ziprasidone (4 months) or quetiapine (2.8 months).
- Time to discontinuation during phase 2 was the same across all drugs among patients who entered phase 2 because of intolerable side effects in phase 1.
- Time to discontinuation because of side effects also was similar whether patients discontinued phase 1 for lack of efficacy or intolerable side effects. Patients stopped treatment in the efficacy and tolerability pathways for similar reasons (Table 3).
Weight gain. Patients taking olanzapine gained more weight (average +1.3 lb/mo) than did those taking the other drugs. Patients taking ziprasidone lost weight (average –1.7 lb/mo). Among 61 patients who gained weight during phase 1, 42% of those switched to ziprasidone lost weight in phase 2, as did:
- 20% of those switched to risperidone
- 7% of those switched to quetiapine.
Among those switched to olanzapine in phase 2, no one lost weight and 2% gained weight.
Metabolic effects. Some parameters changed, depending on drug assignment:
- prolactin increased in patients switched to risperidone
- cholesterol and triglycerides increased in patients switched to olanzapine or quetiapine but decreased in those switched to risperidone or ziprasidone
- QTc interval measurements showed no difference across all drugs.
Methodologic caveats
When considering how CATIE’s phase 2 findings might apply to clinical practice, keep in mind four caveats about the study’s design.
Clozapine was given open-label, yet quetiapine, olanzapine, and risperidone were given double-blind in the efficacy pathway. This pathway’s findings are consistent with what we know about clozapine and other SGAs in treatment-refractory schizophrenia, but how the open-label design affected clozapine therapy outcomes is unclear.
Were patients who knew they were taking clozapine more willing to “stay the course” than were patients in the pathway’s double-blind arm?
Discontinuation rates remained high. The 74% “overall discontinuation rate” in phase 1 surprised many psychiatrists because of the perceived high rate at which patients did not adhere to the first medications they received. To some extent, the word “discontinuation” is imprecise, however, because this group includes patients who did not drop out of treatment altogether but chose to move on to phase 2.
It is important to note, however, that nearly one-half of phase 1 patients who were eligible to enter phase 2 (509 of 1,052) did not. This group represents the true drop-out rate, which is substantial. The high rates of discontinuation seen in phase 1 also occurred in both phase 2 pathways (Table 3).
Few patients entered the efficacy pathway. In an approach designed to reflect routine clinical practice, the researchers recommended the efficacy pathway to patients who discontinued phase 1 because of lack of efficacy and the tolerability pathway to those who discontinued phase 1 because of intolerability. Many patients did not follow the recommendations, however, and seemed to choose their pathways based on whether they wanted a chance to receive clozapine or ziprasidone in phase 2.
Thus, among the 543 phase 1 patients who enrolled in phase 2, 99 (18%) entered the efficacy pathway, and 444 (82%) entered the tolerability pathway. The efficacy pathway included 85 patients who discontinued phase 1 for lack of efficacy and 5 for lack of tolerability. The tolerability pathway included 184 patients who discontinued phase 1 for lack of efficacy and 168 for lack of tolerability.
Dosages may not have been equivalent. SGAs’ dosing equivalency is unknown,5,6 which impedes our ability to interpret comparative studies such as CATIE. The study’s designers developed the its dosing ranges by careful consideration, including recommendations from each SGA’s manufacturer. As Nasrallah described,4 the trial’s dosages were not universally consistent with FDA-approved ranges or usual clinical practice (Table 4). In phase 2, for example, ziprasidone dosages were less than psychiatrists usually use, and quetiapine dosages were greater than usual.
Fortunately, studies are underway to determine each SGA’s optimum dosing. This work will help us understand what we can expect when we increase an antipsychotic’s dosage—a key step towards understanding dosing equivalency.
Table 4
Mean modal antipsychotic dosages (mg/d) in CATIE phase 2 pathways*
| Clozapine | Ziprasidone | Olanzapine | Risperidone | Quetiapine | |
|---|---|---|---|---|---|
| Efficacy pathway | 332 | — | 23.4 | 4.8 | 642.9 |
| Tolerability pathway | — | 115.9 | 20.5 | 4.15 | 65.2 |
| * 800 mg/d of quetiapine and 160 mg/d of ziprasidone are generally regarded as therapeutically equivalent to 20 mg/d of olanzapine. | |||||
What clinicians can expect
A recent analysis helps put CATIE’s findings in perspective. Citrome and Stroup7 quantified the results of phase 1 and 2 with respect to:
- number needed to treat (NNT)—how many patients a clinician needs to treat with drug A to see one additional benefit, compared with drug B
- number needed to harm (NNH)—how many patients a clinician needs to treat with drug A to see a given adverse effect, compared with drug B.
In this analysis, the NNT for olanzapine (5.5 to 10) was lowest among the drugs compared in phase 1, and the NNT for clozapine (3) was lowest among those compared in phase 2. A lower number means that, overall, clinicians can expect a more robust treatment response.
On the other hand, the NNH for olanzapine in weight gain and metabolic disturbances (12.4 to 17.7) was the lowest in phase 1, indicating that clinicians can expect more weight gain and metabolic effects with olanzapine than with other SGAs. Ziprasidone had the highest NNH (106 to 208) among the agents in phase 2 for avoiding discontinuation because of weight gain or metabolic disturbances. In other words, ziprasidone appears less likely than other SGAs to cause metabolic problems.
These risk-attribution measures demonstrate the dilemma clinicians face when trying to match schizophrenia patients with antipsychotics. CATIE was “an N of 1,493” subjects, whereas each patient we see in clinical practice is “an N of 1.” One patient may need a more-robust response; another may need improved tolerability.
We strive for balance, seeking to optimize efficacy—often by raising the dosage—while minimizing adverse effects.
What to tell patients
CATIE phases 1 and 2 provide a compelling rationale for individualized treatment, which should be standard clinical practice for schizophrenia:
- All drugs used in phases 1 and 2 worked.
- All showed noteworthy adverse effects that were different for each drug.
- Different patients responded differently to each drug.
Using our clinical judgment and available information, we must match—as best we can—the individual patient’s characteristics with the antipsychotics’ risk: benefit profiles. CATIE phases 1 and 2 provide independent information on the comparative efficacy and tolerability of each medication.
The CATIE investigators and NIMH have done a great service to our field in providing a rich repository of timely information to inform clinical practice. But the CATIE study was not designed to answer all our questions about treating schizophrenia.8,9 Clinicians and patients need to look elsewhere for guidance on the roles of:
- psychosocial treatments
- recovery and the therapeutic alliance in maximizing outcomes
- long-acting SGA formulations
- aripiprazole (addressed in CATIE phase 3)
- SGAs in first-episode schizophrenia
- FGAs when a patient does not adequately respond to an initial SGA.
Related resources
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). www.CATIE.unc.edu.
- Lieberman JA. What the CATIE study means in clinical practice. Psychiatr Serv 2006;57(8):1075.
Drug brand names
- Aripiprazole • Abilify
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Buckley receives research/grant support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, Pfizer, and Solvay Pharmaceuticals, and is a consultant to Abbott Laboratories, Alamo Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly & Co., Janssen Pharmaceutica, Merck & Co., and Pfizer.
Acknowledgement
The author thanks Del Miller, MD, for comments given on a draft of this paper.
1. Stroup TS, Lieberman JA, McEvoy JP, et al for the CATIE investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
2. McEvoy JP, Lieberman JA, Stroup TS, et al for the CATIE investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. Nasrallah HA. CATIE’s surprises: In antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):52-65.
5. Buckley PF. Dosing equivalency of second-generation antipsychotics. J Clin Psychopharmacol 2005;25(5):501-2.
6. Davis JM. The choice of drugs for schizophrenia. N Engl J Med 2006;354(5):518-20.
7. Citrome L, Stroup TS. Schizophrenia clinical antipsychotic trials intervention effectiveness and number needed to treat: How can CATIE inform clinicians? Int J Clin Pract 2006 (in press).
8. Ragins M. Should the CATIE study be a wake-up call? Psychiatr Serv 2005;56:1489.-
9. Lieberman JA, Hsiao J. Interpreting the results of the CATIE study. Psychiatr Serv 2006;57:139.-
1. Stroup TS, Lieberman JA, McEvoy JP, et al for the CATIE investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
2. McEvoy JP, Lieberman JA, Stroup TS, et al for the CATIE investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. Nasrallah HA. CATIE’s surprises: In antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):52-65.
5. Buckley PF. Dosing equivalency of second-generation antipsychotics. J Clin Psychopharmacol 2005;25(5):501-2.
6. Davis JM. The choice of drugs for schizophrenia. N Engl J Med 2006;354(5):518-20.
7. Citrome L, Stroup TS. Schizophrenia clinical antipsychotic trials intervention effectiveness and number needed to treat: How can CATIE inform clinicians? Int J Clin Pract 2006 (in press).
8. Ragins M. Should the CATIE study be a wake-up call? Psychiatr Serv 2005;56:1489.-
9. Lieberman JA, Hsiao J. Interpreting the results of the CATIE study. Psychiatr Serv 2006;57:139.-