User login
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
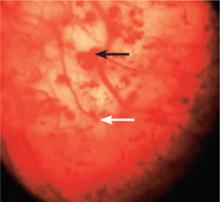
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
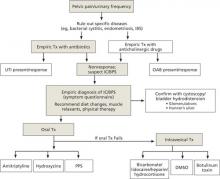
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; theoharis.theoharides@tufts.edu
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; theoharis.theoharides@tufts.edu
• Suspect interstitial cystitis (IC) in a patient who has had suprapubic pain, pressure, or discomfort and frequency of urination for >3 months in the absence of a urinary tract infection or other pelvic condition with similar symptoms. A
• Mild symptoms of IC can be largely contained with dietary changes, off-label oral agents such as amitriptyline or hydroxyzine, and muscle relaxants to reduce pelvic floor muscle spasm. B
• Use pentosan polysulfate with caution; although the drug is approved for the treatment of IC, recent studies indicate it has little benefit. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Jan D, a 27-year-old woman, comes in requesting treatment for pelvic pain and urinary frequency, symptoms she’s had for about 6 months. She describes a feeling of pressure over the suprapubic area that’s relieved by voiding, sensitivity over the vulvar area, and both daytime frequency and nocturia. The patient has a history of allergies and chronic fatigue syndrome (CFS) of 2 years’ duration. When you inquire about her prior medical history, Jan reports that she had frequent urinary tract infections (UTIs) during adolescence.
You order a urinalysis and culture, both of which are negative. If Jan were your patient, what would your next step be?
Interstitial cystitis (IC) is a painful bladder disorder that predominantly affects young and middle-aged women, with an average age of onset of 40 years.1,2 But men can also develop IC, as can women of any age.2 Estimates of prevalence among US women range from less than 1% to more than 6%.2,3 In recent years, however, the number of cases reported has multiplied, the combined result of greater awareness of IC and population surveys based on symptoms rather than on established criteria alone.4
Because the disorder is recognized as a major source of chronic pelvic pain and disability, the term interstitial cystitis/bladder pain syndrome (IC/BPS) is now used by the American Urological Association and many experts to describe it.1,5
Early diagnosis and management of IC/BPS are keys to substantial symptom reduction and improved quality of life. Yet it is often under- or misdiagnosed, both because of the many comorbidities found in patients with the disorder and because its symptoms overlap with those of other common conditions.5
Family physicians are often the first practitioners whom patients with IC/BPS turn to for help. Yet a recent survey of physician practices found significant knowledge gaps with regard to IC/BPS among primary care physicians.6 This evidence-based review is designed to raise awareness of this chronic condition and better prepare you to diagnose and treat it.
IC/BPS: An overview
IC/BPS is characterized by at least 3 to 6 months of pain, pressure, or discomfort over the suprapubic area or the bladder, accompanied by frequency of urination during the day and night in a patient who does not have a UTI.1 There is no known etiology or cure. While evidence suggests that about 90% of those affected are female, some urologists consider chronic bacterial prostatitis to be the male equivalent of IC/BPS, and therefore maintain that the proportion of men with IC/BPS may be considerably higher. 2,3
Chronic pain—the most common symptom—is regional and diffuse over the lower pelvic area, and can be severe. In one study of more than 600 patients with IC/BPS, the most common locations of the pain were the lower abdomen, cited by 80% of those surveyed; the urethral area, cited by 74%; and the low back, by 65%.7 (Dyspareunia is also common, and contributes to the poor quality of life associated with this condition.8)
For about 40% of female patients, the pain and urinary frequency are highest perimenstrually.8 Physical or psychological stress, spicy foods, and smoking can exacerbate symptoms.9,10
Genetics may play a role. Some evidence suggests a genetic predisposition to IC/BPS. In one study, 5 of 8 monozygotic twins of patients with the condition (but 0 of 8 dizygotic twins) were found to have either probable or confirmed IC/BPS. In addition, IC/BPS was 17 times more common in first-degree relatives of patients with the disorder than in the general population.11
There is no established pathogenesis. No infectious organism (bacterial, fungal, or viral) has been identified as a cause for IC/BPS.12 However, an increased number of activated bladder mast cells has been documented in patients with IC/BPS—a possible reason for the pain and some of the histology associated with the condition.9 Inflammation is present to variable degrees and not in all patients.
Some studies suggest that bladder glycosaminoglycans—which form a coating on the luminal surface of the bladder that creates an impermeable, protective barrier—may be compromised in patients with IC/BPS,13 which makes it possible for noxious molecules in the urine to activate sensory nerve endings and lead to chronic pelvic pain.
Overlapping symptoms, comorbidities are common
Symptoms associated with IC/BPS overlap with those of a number of other conditions, including UTIs, sensory urgency, recurrent cystitis, and overactive bladder (OAB), as well as chronic nonbacterial prostatitis in men.4 Comorbidities further complicate the picture.14,15
IC/BPS patients often have a history of allergies,14,15 although they may have negative results on radioallergosorbent (RAST) or skin prick tests, and a number of other comorbidities (TABLE 1). Studies have shown a high correlation between IC/BPS and chronic fatigue syndrome, irritable bowel syndrome, vulvodynia, fibromyalgia, endometriosis, and panic disorder.16-20
TABLE 1
Interstitial cystitis/bladder pain syndrome: Common comorbidities14-20
| Condition | Frequency of comorbidity |
|---|---|
| Allergies | 40%-60% |
| Chronic fatigue syndrome | 35% |
| Endometriosis | 50% |
| Fibromyalgia | 35% |
| Irritable bowel syndrome | 35% |
| Vulvodynia | 20% |
Rule out UTIs and overactive bladder
IC/BPS is largely a diagnosis of exclusion: When a patient presents with suprapubic pain, pressure, or discomfort related to bladder filling and increased urinary frequency lasting for several months, other related conditions—most notably, UTI and OAB—must be ruled out. Often, this can be done with urinalysis and culture, a complete medical history, and symptom assessment. But when doubt remains, a trial of antibiotics (for a UTI) or an anticholinergic agent (for OAB) may be appropriate.
A targeted history and symptom assessment
A history of allergic, gastrointestinal, gynecologic, and/or musculoskeletal disease is often significant.4 In addition, bladder problems in childhood and adolescence are notable, as they are far more common in women with IC/BPS than in the general population.21,22
Identify voiding problems. Question the patient not only about how often she voids, but also about the extent to which the frequency is affecting her life. The severity of the persistent need to void is more significant for an IC/BPS diagnosis than the sudden urge to void for fear of leakage, which is typical of OAB.23
Ask about abuse. Evidence suggests that 50% of women with IC/BPS have been abused, half of them sexually,24 so it is important to include questions about past and present physical, emotional, and sexual abuse in the medical history. Physical or sexual trauma in childhood appears to increase an individual’s lifetime risk for chronic pain syndromes.25
Use these tools to gauge symptoms and severity. Two tools that can aid in diagnosing—or ruling out—IC/BPS are the O’Leary-Sant Symptom and Problem Index, and the Pelvic Pain and Urgency/Frequency (PUF) questionnaire. Both are available at http://www.ichelp.org/Page.aspx?pid=444.
The O’Leary-Sant Index is a measure of urinary and pain symptoms, and of how problematic the symptoms are for the patient.26 The PUF questionnaire also incorporates an assessment of sexual function and the impact of the pain and urinary symptoms,27 but it has not been validated.
The medical work-up
Perform a full gynecologic evaluation of female patients and a rectal examination of men. Include the following laboratory tests in your evaluation: complete blood count (CBC) with differential, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total immunoglobulin E, and liver and thyroid function; test leuteinizing hormone and follicle-stimulating hormone levels for women, as well. Also include urine culture and sensitivity tests in the work-up.
Referral to a urologist is indicated if microscopic hematuria and pyuria are present or the patient’s symptoms are severe. The urologist may conduct a number of tests, for further evaluation or to confirm an IC/BPS diagnosis. These include:
Digital and manometric pelvic floor muscle examination. Manometry is performed using a vaginal or rectal pressure-sensitive probe that measures the strength of contraction and the ability to relax the pelvic floor muscles. In one study, 87% of women with IC/BPS were found to have levator muscle pain described as “consistent with pelvic floor dysfunction.”28
Kaufman Q-tip touch sensitivity test. This involves touching all 4 quadrants of the vulvar and vestibular Skene’s gland ostia to evaluate for vestibulodynia, using a visual analog scale to document the level of pain and sensitivity the patient is experiencing.
Potassium sensitivity test. The physician instills a high concentration of potassium chloride into the bladder to evaluate how much pain it elicits.27 (Although this test is frequently included in the evaluation of patients suspected of having IC/BPS, its use is controversial because it is unnecessarily painful, while its sensitivity and specificity are low.1)
Cystoscopy after hydrodistension, preferably with isotonic saline or isotonic glycine to avoid hypotonic damage to the urothelium, is performed under anesthesia (FIGURE 1). This is the most common procedure performed on patients with IC/BPS29 because it allows visualization of the urothelial glomerulations, or petechiae, and submucosal hemorrhages, found in most patients with this condition. The test would also reveal the presence of the mucosal lesions (Hunner’s ulcers) found in those with “classic” IC/BPS, which represents 5% to 15% of all cases.4
FIGURE 1
IC/BPS: A cystoscopic image
Visualization of the bladder during cystoscopy after hydrodistension reveals submucosal hemorrhages (black arrow) and glomerulations (white arrow), found in most patients with IC/BPS.
Glomerulations can occur in other bladder conditions, however, and may even be found in normal bladders.30 Thus, glomerulations are not diagnostic of IC/BPS in and of themselves, but this finding is often used to confirm the diagnosis.
CASE After Jan’s initial urinalysis, culture, and sensitivity tests, you follow up with a number of other laboratory tests, including CBC with differential, ESR, CRP, and hormonal and immune indexes. All are within the normal range. You also perform a gynecologic examination and use the O’Leary-Sant Symptom and Problem Index to diagnose IC/BPS, and refer the patient to a urologist for further evaluation. The urologist performs cystoscopy with bladder hydrodistension, which reveals multiple submucosal hemorrhages and glomerulations.
Treatment of IC/BPS is multimodal
In January 2011, the American Urological Association (AUA) approved diagnostic and treatment recommendations for IC/BPS (available at http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic-bps). The AUA recommends starting with the most conservative treatments, such as stress management, patient education, and self-care. Interventions may include dietary modification (eliminating bladder irritants such as caffeine and alcohol), self-guided imagery, meditation, yoga, deep breathing, self-hypnosis, and manual physical therapy to the pelvic floor myofascial trigger points, with oral or intravesical medications and other procedures added, as needed (FIGURE 2). Pain management, the AUA notes, should be considered throughout the course of therapy.31
FIGURE 2
Treatment algorithm for interstitial cystitis/bladder pain syndrome (IC/BPS)
DMSO, dimethylsulfoxide; IBS, irritable bowel syndrome; PPS, pentosan polysulfate; UTI, urinary tract infection.
Sources: American Urological Association31; International Incontinence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494).
The other professional society with treatment recommendations for IC/BPS is the International Continence Society (http://www.icsoffice.org/Documents/Documents.aspx?DocumentID=494). Because there is no known cure for IC/BPS, the Society focuses on alleviating symptoms and improving the patient’s quality of life. Treatment is highly individual, the Society states, and may consist of diet modification, oral drugs, bladder instillations or injections, and neuromodulation or surgical interventions, as a last resort. Multiple approaches may be used, often together. 29
Both mild discomfort/pain and urinary frequency in newly diagnosed patients may be treated with a number of oral drugs used off label (TABLE 2), as well as with muscle relaxants, such as diazepam. Pentosan polysulfate—the only oral drug with US Food and Drug Administration approval for treatment of IC/BPS—was initially shown to be “modestly beneficial.”32 However, in 2 recent randomized studies, including one multicenter trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases, it was found to be little (or no) better than placebo.33,34 Thus, we recommend that the drug be tried for no more than 4 to 6 months. If there is no benefit or adverse effects such as GI problems or hair loss develop, the drug should be discontinued.
TABLE 2
Frequently used medications for mild to moderate symptoms
| Agent | Usual dose |
|---|---|
| Amitriptyline | 50-75 mg at bedtime* |
| Diazepam | 2-5 mg up to 4 times per day prn |
| Hydroxyzine | 50-75 mg at bedtime* |
| Pentosan polysulfate | 100 mg tid |
| Pain control | |
| Doxepin cream† | Apply 2-3 times per day |
| Gabapentin | 200 mg tid (starting dose) |
| Tramadol | 100 mg bid |
| *Titrated up over 3-4 weeks. †For vulvodynia. | |
The tricyclic antidepressant amitriptyline is often used to relieve symptoms—both urinary frequency and pain/discomfort—that are mild to moderate. In one small clinical trial, amitriptyline (self-titrated up to 100 mg/d for 4 months) produced a 64% response rate. But nearly a third of the patients in the intervention group (and many more on placebo) dropped out due to nonresponse.35 A recent multicenter, randomized, placebo-controlled trial of amitriptyline showed that only patients who took >50 mg daily had a significantly higher response rate (P=.01) compared with the placebo group.36
Amitriptyline may also be combined with hydroxyzine, especially in patients with allergies, but the combination could result in considerable sedation. The antidepressant doxepin, which is both a histamine-1 (H1) and histamine-2 (H2) receptor antagonist (RA), is an alternative that has been shown to reduce chronic neuropathic pain.37 A doxepin cream may also be used locally for vulvodynia.
Although no comparative studies have been conducted, nontricyclic antidepressants do not appear to have the same benefit for IC symptoms. In an open-label study of 48 women with IC/BPS, the antidepressant duloxetine (titrated to 40 mg bid for 5 weeks) showed no significant improvement of symptoms.38
Hydroxyzine is an H1 RA with mild anxiolytic and antiallergy properties. In an open-label study of IC/BPS patients with allergies (n=37), it was found to reduce symptoms by 55%.39
Is there a role for dietary supplements? In developing its new recommendations for IC/BPS, the AUA did not review studies of dietary supplements.31 The Interstitial Cystitis Association (ICA), however, includes information on its Web site about a number of dietary supplements that may be helpful in controlling symptoms (http://www.ichelp.org/Page.aspx?pid=635).
One such product contains aloe vera, which the ICA describes as having anti-inflammatory actions that have been found to reduce IC/BPS symptoms.40 Another is a dietary supplement that the author (TCT) developed, which contains quercetin, a flavonoid with anti-inflammatory properties, as well as chondroitin and hyaluronate—components of the glycosaminoglycan protective layer in the bladder that may be damaged in patients with ICS. In an open label study of 127 patients with refractory symptoms of IC/BPS, this supplement produced a 51% response rate (P<.0001).41
Pain management is particularly challenging
Intense chronic pain is the most difficult aspect of IC/BPS to treat. Tramadol, an opioid with weaker addiction potential and fewer adverse effects than morphine, is often helpful. Gabapentin, an antiseizure drug, and pregabalin, a similar drug recently approved for fibromyalgia, may also be useful. A fentanyl patch, as well as belladonna and opium suppositories, may be used under the care of a pain management specialist.
If these pain regimens fail, urologists often try intravesical approaches, such as bladder hydrodistension under anesthesia, which has been found to provide short-term (up to 5.3 months) symptom relief in 30% to 50% of patients.4,42,43 Intravesical treatments, in which medication is directly instilled into the bladder, are frequently used, especially in patients with severe symptoms.44
Intravesical dimethylsulfoxide (DMSO) may be given once a week for 6 weeks, but instillation often hurts and DMSO causes the patients to smell of garlic, which severely limits compliance. In one randomized double-blind study involving 11 patients with classic IC/BPS (ie, with Hunner’s ulcers) and 10 IC/BPS patients without Hunner’s ulcers, DMSO reduced urinary frequency and pain only in those with classic cases.45
Intravesical hyaluronate sodium, given in weekly instillations for 4 weeks, resulted in some pain reduction in 2 open-label studies,4 but in a subsequent randomized, double-blind, placebo-controlled, multicenter study, instilling 10 times the amount of hyaluronate failed to show any benefit and was terminated by the sponsor (Seikagaku Corp., written correspondence, March 2004). In one small study, intravesical hyaluronate and chondroitin, given weekly for 20 weeks and then monthly for 3 months, led to significant improvement in frequency, urgency, and pain.46
In a multicenter trial, intravesical instillation of lidocaine, together with sodium bicarbonate, led to 30% improvement, compared with the controls.47 (Such intravesical “cocktails” are often supplemented with other agents, such as heparin or hydrocortisone.)
Other options for refractory pain include intravesical laser ablation, fulguration of bladder lesions, intravesical injections of botulinum toxin, and neuromodulation of the sacral or pudendal nerve via an implanted impulse generator.
CASE After the urologist confirmed Jan’s diagnosis, she returned to your office for treatment. Jan was started on dietary modification, hydroxyzine (50 mg at bedtime), and physical therapy. She had a 50% reduction in symptoms after 3 months of therapy.
CORRESPONDENCE
Theoharis C. Theoharides, MD, PhD, FAAAAI, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111; theoharis.theoharides@tufts.edu
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.
1. Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn. 2010;29:191-198.
2. Association of Reproductive Health Professionals. Screening, tratment, and management of IC/PBS. May 2008. Available at: http://www.arhp.org/Publications-and-Resources/Clinical-Proceedings/Screening-Treatment-and-Management-of-ICPBS/Definition. Accessed May 9, 2011.
3. Interstitial Cystitis Association. 4 to 12 million may have IC. http://www.ichelp.org/Page.aspx?pid=917. Posted January 12, 2010. Accessed May 9, 2011.
4. Hanno PM. Painful bladder syndrome. In: Wein AJ, Kavossi LR, Novick AC, et al, eds. Campbell’s Urology. 9th ed. Philadelphia, Pa: Elsevier; 2007:330-370.
5. American Urological Association. First-ever clinical guidance on interstitial cystitis/bladder pain syndrome released [press release]. March 1, 2011. Available at: http://www.auanet.org/content/press/press_releases/article.cfm?articleNo=224. Accessed May 9, 2011.
6. Clemens JQ, Calhoun EA, Litwin MS, et al. A survey of primary care physician practices in the diagnosis and management of women with interstitial cystitis/painful bladder syndrome. Urology. 2010;76:323-328.
7. FitzGerald MP, Brensinger C, Brubaker L, et al. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:69-72.
8. Powell-Boone T, Ness TJ, Cannon R, et al. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174:1832-1836.
9. Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1-12.
10. Shorter B, Lesser M, Moldwin RM, et al. Effect of comestibles on symptoms of interstitial cystitis. J Urol. 2007;178:145-152.
11. Warren JW, Jackson TL, Langenberg P, et al. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology. 2004;63:17-21.
12. Al-Hadithi HN, Williams H, Hart CA, et al. Absence of bacterial and viral DNA in bladder biopsies from patients with interstitial cystitis/chronic pelvic pain syndrome. J Urol. 2005;174:151-154.
13. Parsons CL, Lilly JD, Stein P. Epithelial dysfunction in nonbacterial cystitis (interstitial cystitis). J Urol. 1991;145:732-735.
14. Erickson DR, Morgan KC, Ordille S, et al. Nonbladder related symptoms in patients with interstitial cystitis. J Urol. 2001;166:557-562.
15. Theoharides TC, Whitmore K, Stanford E, et al. Interstitial cystitis: bladder pain and beyond. Expert Opin Pharmacother. 2008;9:2979-2994.
16. Nickel JC, Tripp DA, Pontari M, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363.
17. Novi JM, Jeronis S, Srinivas S, et al. Risk of irritable bowel syndrome and depression in women with interstitial cystitis: a case-control study. J Urol. 2005;174:937-940.
18. Weissman MM, Gross R, Fyer A, et al. Interstitial cystitis and panic disorder: a potential genetic syndrome. Arch Gen Psychiatr. 2004;61:273-279.
19. Wu EQ, Birnbaum H, Mareva M, et al. Interstitial cystitis: cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55-65.
20. Stanford EJ, Koziol J, Feng A. The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol. 2005;12:43-49.
21. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258-262.
22. Rackow BW, Novi JM, Arya LA, Pfeifer SM. Interstitial cystitis is an etiology of chronic pelvic pain in young women. J Pediatr Adolesc Gynecol. 2009;22:181-185.
23. Diggs C, Meyer WA, Langenberg P, et al. Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology. 2007;69:210-214.
24. Peters KM, Kalinowski SE, Carrico DJ, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891-895.
25. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10:441-447.
26. O’Leary MP, Sant GR, Fowler FJ, Jr, et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49(suppl 5A):S58-S63.
27. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573-578.
28. Peters KM, Carrico DJ, Kalinowski SE, et al. Prevalence of pelvic floor dysfunction in patients with interstitial cystitis. Urology. 2007;70:16-18.
29. Nordling J, Anjum FH, Bade JJ, et al. Primary evaluation of patients suspected of having interstitial cystitis (IC). Eur Urol. 2004;45:662-669.
30. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663-1667.
31. American Urological Association. Guideline on the diagnosis and treatment of interstitial cystitis/bladder pain syndrome (2011). Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=ic.bps. Accessed May 10, 2011.
32. Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922-1929.
33. Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810-815.
34. Buffington CA. Re: cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2006;176:838.-
35. van Ophoven A, Hertle L. Long-term results of amitriptyline treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
36. Foster HE, Jr, Hanno PM, Nickel JC, et al. Effect of amitriptyline on symptoms in treatment naive patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853-1858.
37. Hameroff SR, Weiss JL, Lerman JC, et al. Doxepin’s effects on chronic pain and depression: a controlled study. J Clin Psychiatry. 1984;45(3 Pt 2):PMID 6321454.-
38. van Ophoven A, Hertle L. The dual serotonin and noradrenaline reuptake inhibitor duloxetine for the treatment of interstitial cystitis: results of an observational study. J Urol. 2007;177:552-555.
39. Theoharides TC, Sant GR. Hydroxyzine therapy for interstitial cystitis. Urology. 1997;49(suppl):S108-S110.
40. Interstitial Cystitis Association. IC supplements. Revised April 11, 2011. Available at: http://www.ichelp.org/Page.aspx?pid=635. Accessed May 9, 2011.
41. Theoharides TC, Kempuraj D, Vakali S, et al. Treatment of refractory interstitial cystitis/painful bladder syndrome with CystoProtek—an oral multi-agent natural supplement. Can J Urol. 2008;15:4410-4414.
42. Phatak S, Foster HE, Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45-53.
43. Erickson DR, Kunselman AR, Bentley CM, et al. Changes in urine markers and symptoms after bladder distension for interstitial cystitis. J Urol. 2007;117:556-560.
44. Dawson TE, Jamison J. Intravesical treatments for painful bladder syndrome/interstitial cystitis. Cochrane Database Syst Rev. 2007;(4):CD006113.-
45. Peeker R, Haghsheno MA, Holmang S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis:a prospective, randomized double-blind study. J Urol. 2000;164:1912-1916.
46. Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:943-947.
47. Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103:910-918.