User login
Whipple's disease
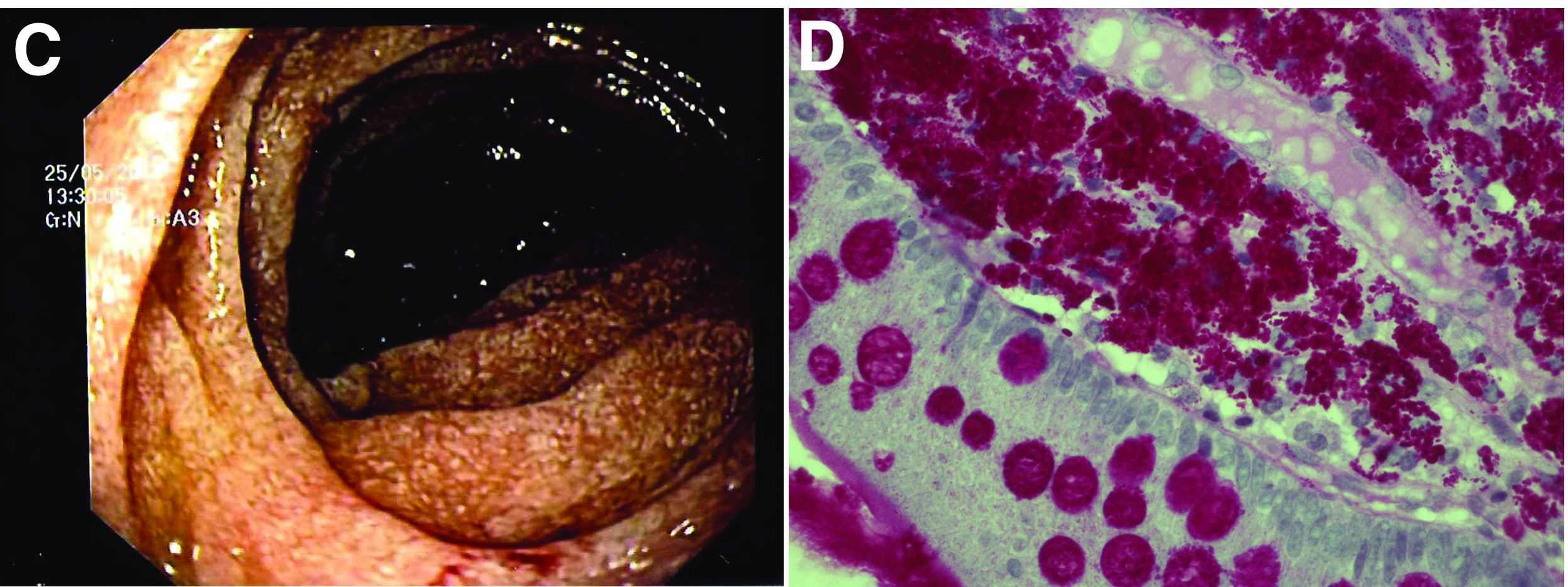
The ultrasound features were highly suggestive of malabsorption, a hypothesis that was supported by the laboratory findings. Celiac disease, one of the most common causes of malabsorption, was excluded by serology tests. Esophagogastroduodenoscopy was therefore repeated: The mucosa of the distal first part and second part of the duodenum appeared completely covered with tiny white spots (Figure C). Histologic examination revealed that the mucosal architecture of the villi was altered by the presence of infiltrates of macrophages with wide cytoplasm filled with round periodic acid-Schiff (PAS)-positive inclusions, associated to aggregates of neutrophils attacking the epithelium (Figure D). These histologic findings are consistent with Whipple's disease.
Whipple's disease is a chronic infectious disease caused by a gram-positive ubiquitous bacterium named Tropheryma whipplei. In predisposed subjects with an insufficient T-helper response, for example, those undergoing treatment with tumor necrosis factor-alpha inhibitors as in our patient, T. whipplei is able to survive and replicate inside the macrophages of the intestinal mucosa and to spread to other organs.1 Whipple's disease can thus manifest as a multisystemic disease or as a single-organ disease with extraintestinal involvement (e.g., central nervous system, eyes, heart, or lung). The classic form is characterized by weight loss, diarrhea, abdominal pain, and signs of malabsorption, typically preceded by a history of arthralgia. The arthralgia is often misdiagnosed as a form of rheumatoid arthritis and therefore treated with immunosuppressant therapy, which favors the onset of the classic intestinal symptoms.
In the literature, few case reports describe the ultrasound findings in patients with Whipple's disease. The most frequent sonographic features include small-bowel dilatation with wall thickening, the presence of peri-intestinal fluid effusion and mesenteric and retroperitoneal lymphadenopathy.2,3
The final diagnosis relies on intestinal biopsy and the histologic finding of foamy macrophages containing large amounts of diastase-resistant PAS-positive particles in the lamina propria of the duodenum, jejunum, ileum, or gastric antral region.
The diagnosis, particularly in cases of extraintestinal involvement, can be confirmed by polymerase chain reaction positivity for T. whipplei in the examined tissue.
Therapy consists of the administration of ceftriaxone (2 g IV once daily) for 2 weeks followed by oral therapy with trimethoprim-sulfamethoxazole for 1 year.
References
1. Schneider T et al. Whipple's disease: New aspects of pathogenesis and treatment. Lancet Infect Dis. 2008;8:179-90.
2. Brindicci D et al. Ultrasonic findings in Whipple's disease. J Clin Ultrasound. 1984;12:286-8.
3. Neye H et al. Der Morbus Whipple's Disease - A rare intestinal disease and its sonographic characteristics. Ultraschall Med. 2012;33(04):314-5.
Whipple's disease
The ultrasound features were highly suggestive of malabsorption, a hypothesis that was supported by the laboratory findings. Celiac disease, one of the most common causes of malabsorption, was excluded by serology tests. Esophagogastroduodenoscopy was therefore repeated: The mucosa of the distal first part and second part of the duodenum appeared completely covered with tiny white spots (Figure C). Histologic examination revealed that the mucosal architecture of the villi was altered by the presence of infiltrates of macrophages with wide cytoplasm filled with round periodic acid-Schiff (PAS)-positive inclusions, associated to aggregates of neutrophils attacking the epithelium (Figure D). These histologic findings are consistent with Whipple's disease.

Whipple's disease is a chronic infectious disease caused by a gram-positive ubiquitous bacterium named Tropheryma whipplei. In predisposed subjects with an insufficient T-helper response, for example, those undergoing treatment with tumor necrosis factor-alpha inhibitors as in our patient, T. whipplei is able to survive and replicate inside the macrophages of the intestinal mucosa and to spread to other organs.1 Whipple's disease can thus manifest as a multisystemic disease or as a single-organ disease with extraintestinal involvement (e.g., central nervous system, eyes, heart, or lung). The classic form is characterized by weight loss, diarrhea, abdominal pain, and signs of malabsorption, typically preceded by a history of arthralgia. The arthralgia is often misdiagnosed as a form of rheumatoid arthritis and therefore treated with immunosuppressant therapy, which favors the onset of the classic intestinal symptoms.
In the literature, few case reports describe the ultrasound findings in patients with Whipple's disease. The most frequent sonographic features include small-bowel dilatation with wall thickening, the presence of peri-intestinal fluid effusion and mesenteric and retroperitoneal lymphadenopathy.2,3
The final diagnosis relies on intestinal biopsy and the histologic finding of foamy macrophages containing large amounts of diastase-resistant PAS-positive particles in the lamina propria of the duodenum, jejunum, ileum, or gastric antral region.
The diagnosis, particularly in cases of extraintestinal involvement, can be confirmed by polymerase chain reaction positivity for T. whipplei in the examined tissue.
Therapy consists of the administration of ceftriaxone (2 g IV once daily) for 2 weeks followed by oral therapy with trimethoprim-sulfamethoxazole for 1 year.
References
1. Schneider T et al. Whipple's disease: New aspects of pathogenesis and treatment. Lancet Infect Dis. 2008;8:179-90.
2. Brindicci D et al. Ultrasonic findings in Whipple's disease. J Clin Ultrasound. 1984;12:286-8.
3. Neye H et al. Der Morbus Whipple's Disease - A rare intestinal disease and its sonographic characteristics. Ultraschall Med. 2012;33(04):314-5.
Whipple's disease
The ultrasound features were highly suggestive of malabsorption, a hypothesis that was supported by the laboratory findings. Celiac disease, one of the most common causes of malabsorption, was excluded by serology tests. Esophagogastroduodenoscopy was therefore repeated: The mucosa of the distal first part and second part of the duodenum appeared completely covered with tiny white spots (Figure C). Histologic examination revealed that the mucosal architecture of the villi was altered by the presence of infiltrates of macrophages with wide cytoplasm filled with round periodic acid-Schiff (PAS)-positive inclusions, associated to aggregates of neutrophils attacking the epithelium (Figure D). These histologic findings are consistent with Whipple's disease.

Whipple's disease is a chronic infectious disease caused by a gram-positive ubiquitous bacterium named Tropheryma whipplei. In predisposed subjects with an insufficient T-helper response, for example, those undergoing treatment with tumor necrosis factor-alpha inhibitors as in our patient, T. whipplei is able to survive and replicate inside the macrophages of the intestinal mucosa and to spread to other organs.1 Whipple's disease can thus manifest as a multisystemic disease or as a single-organ disease with extraintestinal involvement (e.g., central nervous system, eyes, heart, or lung). The classic form is characterized by weight loss, diarrhea, abdominal pain, and signs of malabsorption, typically preceded by a history of arthralgia. The arthralgia is often misdiagnosed as a form of rheumatoid arthritis and therefore treated with immunosuppressant therapy, which favors the onset of the classic intestinal symptoms.
In the literature, few case reports describe the ultrasound findings in patients with Whipple's disease. The most frequent sonographic features include small-bowel dilatation with wall thickening, the presence of peri-intestinal fluid effusion and mesenteric and retroperitoneal lymphadenopathy.2,3
The final diagnosis relies on intestinal biopsy and the histologic finding of foamy macrophages containing large amounts of diastase-resistant PAS-positive particles in the lamina propria of the duodenum, jejunum, ileum, or gastric antral region.
The diagnosis, particularly in cases of extraintestinal involvement, can be confirmed by polymerase chain reaction positivity for T. whipplei in the examined tissue.
Therapy consists of the administration of ceftriaxone (2 g IV once daily) for 2 weeks followed by oral therapy with trimethoprim-sulfamethoxazole for 1 year.
References
1. Schneider T et al. Whipple's disease: New aspects of pathogenesis and treatment. Lancet Infect Dis. 2008;8:179-90.
2. Brindicci D et al. Ultrasonic findings in Whipple's disease. J Clin Ultrasound. 1984;12:286-8.
3. Neye H et al. Der Morbus Whipple's Disease - A rare intestinal disease and its sonographic characteristics. Ultraschall Med. 2012;33(04):314-5.
67-year-old woman presented with a year-long history of general malaise, low-grade fever, diarrhea, and a 20-kg weight loss. She had a history of hypertension and depressive disorder. In the previous 4 years, she had undergone several rheumatologic examinations for polyarthritis and, having been diagnosed with seronegative rheumatoid arthritis, she had been treated with steroids, methotrexate, and etanercept, with little benefit.
Recent laboratory tests showed: hemoglobin, 8.3 g/dL; mean corpuscular volume, 70 fL; erythrocyte sedimentation rate, 78; and C-reactive protein, 6.4 mg/dL. To evaluate the microcytic anemia and the diarrhea, endoscopic investigations had been performed a few months earlier. Esophagogastroduodenoscopy showed villous atrophy at the level of DII; histology was compatible with intramucosal xanthoma. There were no pathologic findings at colonoscopy. The situation had not been further investigated.
At presentation, the physical examination revealed lower-limb edema, skin and mucosal pallor, and a body mass index of 17.4 kg/m2. Laboratory tests showed microcytic anemia (hemoglobin, 10.0 g/dL; mean corpuscular volume, 74 fL), increased acute-phase proteins (erythrocyte sedimentation rate, 59; C-reactive protein, 8.53 mg/dL), and malabsorption (albumin, 2.5 g/dL; multiple electrolytes deficiencies including iron, vitamin A, and vitamin D deficiency).
Abdominal ultrasound examination revealed three small lymph nodes in the periaortic region (maximum diameter, 10 mm), marked mesenteric and ileal wall thickening, mild jejunal wall thickening, an increased number of connivent valves, and a mild amount of peri-intestinal fluid effusion (Figure A, B).

What is the likely diagnosis and the appropriate treatment?