User login
Although urinary incontinence is one of the most common chronic diseases in women, we still don’t understand its pathophysiology, and treatments have been, of necessity, empiric rather than directed at a specific cause. Fortunately, this bleak scenario may be changing, and I think that is the most exciting news about urinary incontinence in 2005.
Urethral deficiency by any name is still a deficiency
Ironically, the most basic description of urinary incontinence may be the most revealing: Incontinence occurs when the urethra cannot stay closed and fails to hold urine in the bladder, where it should be stored until the “right” time and place for emptying. This description applies equally well to women with stress or urge symptoms, but let’s focus on stress incontinence for now.
By that line of thinking, the urethra is deficient in all women with stress incontinence. I believe this to be true, despite the arbitrary label—intrinsic sphincter deficiency, or ISD—that we apply to women with only the most severe symptoms of stress incontinence.
Surgery does not end the quest
Because surgery focuses on eliminating symptoms, it should come as no surprise that incontinence procedures continue to proliferate while we search for the Holy Grail: the perfect surgery that will effectively and durably “fix” the problem without complications or side effects. However, unless we find and correct the underlying problem that gave rise to the incontinence in the first place, we are doomed to fail in our search.
Doom? Failure? Where is the exciting news I promised?
CARE trial underscores efficacy of Burch procedure
Brubaker L, for the Pelvic Floor Disorders Network. Burch colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. Abstract presented at the American Urogynecologic Society Meeting, September 15–17, 2005, Atlanta.
If you have an exceptional memory, you will recall that, in this Update on Urinary Incontinence last year, results from the CARE trial were promised in 2006. Good news! Early results are available now, at least a year before expected.
Superior results with Burch changed the course of the CARE study
The CARE trial (Colpopexy And Urinary Reduction Efforts) was designed to determine the effect of Burch versus no Burch in women without stress incontinence symptoms but with advanced prolapse who were undergoing abdominal sacrocolpopexy. The trial was sponsored by the National Institute of Child Health and Human Development (NICHD) and performed by the Pelvic Floor Disorders Network of investigators from 7 clinical sites and a central coordinating center.
The original sample size was set at 480 women, to be randomized equally to Burch or no Burch, with the primary stress outcome at 3 months after surgery. However, at the first interim analysis, when about half the sample (232 women) had reached the primary outcome, the results showed such a striking benefit in the Burch group that the Data and Safety Monitoring Board for the Pelvic Floor Disorders Network recommended that enrollment be halted while all women continued to receive scheduled follow-up. Therefore, in February 2005, enrollment in the trial was closed, with 322 women randomized to 1 of the 2 arms.
The following results were presented at the annual meeting of the American Urogynecologic Society in September:
- Stress incontinence symptoms were reduced by about half in women after abdominal sacrocolpopexy (from 44% in the no-Burch group to 24% in the Burch group).
- Stress symptom severity improved with Burch. More women (62%) in the no-Burch stress incontinence group were bothered by their symptoms, compared to 32% of women in the Burch group.
- Urge symptoms were no different with Burch. More surprising, women in both groups had similar levels of symptoms measured as the urge endpoint, which included urge incontinence, urgency, frequency, nocturia, or enuresis; or treatment for any of those 5 symptoms. Almost 33% of women in the Burch group met the urge endpoint, compared with 38% in the no-Burch group (difference not statistically significant).
- Serious adverse events were not significantly different between the 2 groups.
Many more questions will be addressed with further analysis of CARE trial data, such as results of urodynamic testing with and without prolapse reduction, and the potential for predicting which subgroup benefits most when Burch is performed. Long-term follow-up data will address durability of results related to incontinence and prolapse. Follow-up is scheduled for 2 years in the CARE trial, and for up to 10 years in the Extended-CARE trial.
Efficacy of anticholinergics
Trospium chloride (Sanctura): another anticholinergic for overactive bladder. The Medical Letter. 2004;46(1188):63–64.
Solifenacin and darifenacin for overactive bladder. The Medical Letter. 2005;47(1204):23–24.
Three more drugs for overactive bladder won FDA approval in 2004 and 2005:
- trospium chloride (Sanctura)
- solifenacin succinate Vesicare)
- darifenacin hydrobromide (Enablex)
According to The Medical Letter, none appears to offer an advantage over long-acting anticholinergics for overactive bladder. Despite the proliferation of anticholinergic drugs for overactive bladder symptoms—or perhaps because of it—one suspects that these medications are not achieving substantial, long-lasting relief of symptoms. One study reported that two thirds of women discontinued therapy within 4 months.1 A comprehensive review of placebo-controlled trials of anticholinergic drugs for overactive bladder estimated that, as a class, even long-acting agents have a very limited effect on symptoms, with approximately 1 fewer incontinent episode and 1 fewer voiding episode per 48 hours.2
REFERENCES
1. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynaecol Reprod Biol. 2005;119:237-241.
2. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
Do anticholinergics and dementia drugs mix?
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13:324–328.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–498.
Treatment recommendations
As ObGyns become more active in evaluating and treating women with urinary incontinence, we must stay alert for potential adverse drug interactions.
Ideally, behavioral treatment (scheduled voiding, fluid management, bedside commode) and pelvic muscle training should be first-line therapies in elderly women with overactive bladder.
Anticholinergic drugs should be used with caution, if at all, in women taking cholinesterase-inhibiting drugs for dementia.
Elderly patients with overactive bladder are at high risk for drug interactions, especially involving cholinesterase-inhibiting drugs for the treatment of dementia, such as donepezil hydrochloride (Aricept). However, a newer Alzheimer drug, memantine hydrochloride (Namenda), works by a different mechanism and may be less likely to interact directly with anticholinergic drugs for incontinence.
3 studies involving the elderly
Cognitive impairment
Observing a population of older adults with dementia, about half treated with cholinesterase inhibitors for their Alzheimer symptoms, Gill et al found that the patients on cholinesterase inhibitors were more likely to start treatment with an anticholinergic drug for incontinence within a year. They theorized that the cholinesterase-inhibiting drugs possibly contribute to new-onset or worsening urinary incontinence, which in turn leads to treatment with anticholinergic agents.
Jewart and colleagues found better performance in patients with Alzheimer disease who were not taking anticholinergic medication for incontinence.
No or mild cognitive impairment
Lipton et al tested cognitive function with darifenacin for 2 weeks and found no difference between immediate- or controlled-release forms of the drug and placebo. However, the study population consisted of volunteers 65 and older with no or mild cognitive impairment and no use of cholinesterase-inhibiting drugs.
IN THE PIPELINEUrethral injection of muscle-derived cells may restore function
Usiene I, Kim YT, Pruchnic R, et al. Human muscle-derived cells injection increases leak point pressure in a nude rat model of stress urinary incontinence. Abstract presented at the annual meeting of the International Continence Society, August 28–September 2, 2005, Montreal, Quebec. Abstract #2.
Some exciting news: Dr. Michael Chancellor and colleagues at the University of Pittsburgh and at Cook MyoSite in Pittsburgh are working to bring stem cell research to the clinician’s office, with their studies of muscle-derived cells that can be injected into the urethra. (This technique is well-established and currently used for injection of synthetic or biologic material such as bovine collagen.)
What is remarkable about this type of injection is that the muscle cells not only stay put in the urethra, they appear to integrate into the muscle of the urethral sphincter and differentiate into cells that produce new muscle fibers. Newly functioning muscle improves urethral function and, ideally, will be able to restore continence in women with incontinence.
Technique’s success in rats
Could the same be accomplished with muscle-derived stem cells from humans? At the 2005 meeting of the International Continence Society, Chancellor and colleagues described how they injected human muscle-derived stem cells into the urethras of a nude rat model of stress incontinence (via nerve transection). In the injected rats, leak-point pressure measurements were restored to levels similar to those in a control group of rats.
In addition, immunohistochemistry and histology showed persistence of the human muscle-derived stem cells in the injected rats, versus periurethral muscle atrophy in the rats that had nerve transection but no injection.
Clinical testing underway
Clinical trials of this technology in women are now being performed in Toronto. It will be necessary to show safety and efficacy before the stem cell therapy is made available clinically, but there are a couple of factors in its favor:
- In terms of safety, the risks associated with the current crop of injectable materials should not be applicable, at least in theory. Because the injected stem cells are isolated and grown from the patient’s own biopsy, the chance of an adverse immunologic reaction should be zero, absent the rare mistake in labeling or transfer.
- The cell-injection technique is well-established and already used by many clinicians who perform transurethral or periurethral injection of bulking agents for women with incontinence. It will not be necessary to learn a new technique—just injectable material.
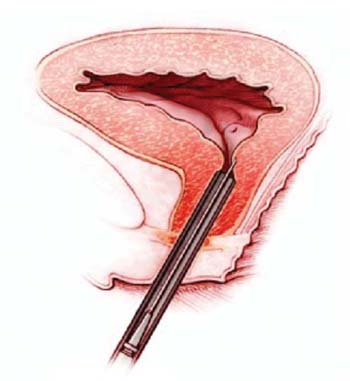
Tissue-engineered sling also in the works
Given that incontinence (and, presumably, the level of urethral damage resulting in some women may have urethral damage so severe as to preclude benefit from the injection of relatively small numbers of researchers is already developing a tissue-engineered sling in the hope that it can be used as a substitute for currently available synthetic or biologic sling materials.
The sling is being developed with the same muscle-derived stem cells, which are seeded onto a scaffold for as little as 2 weeks in a rat model. After the sling was surgically implanted using standard technique, urethral function improved to the level seen among controls.
It may be that the promise of stem cell therapy will come early to the treatment of urinary incontinence. Time will tell.
The author reports no financial relationships relevant to this article.
Although urinary incontinence is one of the most common chronic diseases in women, we still don’t understand its pathophysiology, and treatments have been, of necessity, empiric rather than directed at a specific cause. Fortunately, this bleak scenario may be changing, and I think that is the most exciting news about urinary incontinence in 2005.
Urethral deficiency by any name is still a deficiency
Ironically, the most basic description of urinary incontinence may be the most revealing: Incontinence occurs when the urethra cannot stay closed and fails to hold urine in the bladder, where it should be stored until the “right” time and place for emptying. This description applies equally well to women with stress or urge symptoms, but let’s focus on stress incontinence for now.
By that line of thinking, the urethra is deficient in all women with stress incontinence. I believe this to be true, despite the arbitrary label—intrinsic sphincter deficiency, or ISD—that we apply to women with only the most severe symptoms of stress incontinence.
Surgery does not end the quest
Because surgery focuses on eliminating symptoms, it should come as no surprise that incontinence procedures continue to proliferate while we search for the Holy Grail: the perfect surgery that will effectively and durably “fix” the problem without complications or side effects. However, unless we find and correct the underlying problem that gave rise to the incontinence in the first place, we are doomed to fail in our search.
Doom? Failure? Where is the exciting news I promised?
CARE trial underscores efficacy of Burch procedure
Brubaker L, for the Pelvic Floor Disorders Network. Burch colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. Abstract presented at the American Urogynecologic Society Meeting, September 15–17, 2005, Atlanta.
If you have an exceptional memory, you will recall that, in this Update on Urinary Incontinence last year, results from the CARE trial were promised in 2006. Good news! Early results are available now, at least a year before expected.
Superior results with Burch changed the course of the CARE study
The CARE trial (Colpopexy And Urinary Reduction Efforts) was designed to determine the effect of Burch versus no Burch in women without stress incontinence symptoms but with advanced prolapse who were undergoing abdominal sacrocolpopexy. The trial was sponsored by the National Institute of Child Health and Human Development (NICHD) and performed by the Pelvic Floor Disorders Network of investigators from 7 clinical sites and a central coordinating center.
The original sample size was set at 480 women, to be randomized equally to Burch or no Burch, with the primary stress outcome at 3 months after surgery. However, at the first interim analysis, when about half the sample (232 women) had reached the primary outcome, the results showed such a striking benefit in the Burch group that the Data and Safety Monitoring Board for the Pelvic Floor Disorders Network recommended that enrollment be halted while all women continued to receive scheduled follow-up. Therefore, in February 2005, enrollment in the trial was closed, with 322 women randomized to 1 of the 2 arms.
The following results were presented at the annual meeting of the American Urogynecologic Society in September:
- Stress incontinence symptoms were reduced by about half in women after abdominal sacrocolpopexy (from 44% in the no-Burch group to 24% in the Burch group).
- Stress symptom severity improved with Burch. More women (62%) in the no-Burch stress incontinence group were bothered by their symptoms, compared to 32% of women in the Burch group.
- Urge symptoms were no different with Burch. More surprising, women in both groups had similar levels of symptoms measured as the urge endpoint, which included urge incontinence, urgency, frequency, nocturia, or enuresis; or treatment for any of those 5 symptoms. Almost 33% of women in the Burch group met the urge endpoint, compared with 38% in the no-Burch group (difference not statistically significant).
- Serious adverse events were not significantly different between the 2 groups.
Many more questions will be addressed with further analysis of CARE trial data, such as results of urodynamic testing with and without prolapse reduction, and the potential for predicting which subgroup benefits most when Burch is performed. Long-term follow-up data will address durability of results related to incontinence and prolapse. Follow-up is scheduled for 2 years in the CARE trial, and for up to 10 years in the Extended-CARE trial.
Efficacy of anticholinergics
Trospium chloride (Sanctura): another anticholinergic for overactive bladder. The Medical Letter. 2004;46(1188):63–64.
Solifenacin and darifenacin for overactive bladder. The Medical Letter. 2005;47(1204):23–24.
Three more drugs for overactive bladder won FDA approval in 2004 and 2005:
- trospium chloride (Sanctura)
- solifenacin succinate Vesicare)
- darifenacin hydrobromide (Enablex)
According to The Medical Letter, none appears to offer an advantage over long-acting anticholinergics for overactive bladder. Despite the proliferation of anticholinergic drugs for overactive bladder symptoms—or perhaps because of it—one suspects that these medications are not achieving substantial, long-lasting relief of symptoms. One study reported that two thirds of women discontinued therapy within 4 months.1 A comprehensive review of placebo-controlled trials of anticholinergic drugs for overactive bladder estimated that, as a class, even long-acting agents have a very limited effect on symptoms, with approximately 1 fewer incontinent episode and 1 fewer voiding episode per 48 hours.2
REFERENCES
1. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynaecol Reprod Biol. 2005;119:237-241.
2. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
Do anticholinergics and dementia drugs mix?
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13:324–328.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–498.
Treatment recommendations
As ObGyns become more active in evaluating and treating women with urinary incontinence, we must stay alert for potential adverse drug interactions.
Ideally, behavioral treatment (scheduled voiding, fluid management, bedside commode) and pelvic muscle training should be first-line therapies in elderly women with overactive bladder.
Anticholinergic drugs should be used with caution, if at all, in women taking cholinesterase-inhibiting drugs for dementia.
Elderly patients with overactive bladder are at high risk for drug interactions, especially involving cholinesterase-inhibiting drugs for the treatment of dementia, such as donepezil hydrochloride (Aricept). However, a newer Alzheimer drug, memantine hydrochloride (Namenda), works by a different mechanism and may be less likely to interact directly with anticholinergic drugs for incontinence.
3 studies involving the elderly
Cognitive impairment
Observing a population of older adults with dementia, about half treated with cholinesterase inhibitors for their Alzheimer symptoms, Gill et al found that the patients on cholinesterase inhibitors were more likely to start treatment with an anticholinergic drug for incontinence within a year. They theorized that the cholinesterase-inhibiting drugs possibly contribute to new-onset or worsening urinary incontinence, which in turn leads to treatment with anticholinergic agents.
Jewart and colleagues found better performance in patients with Alzheimer disease who were not taking anticholinergic medication for incontinence.
No or mild cognitive impairment
Lipton et al tested cognitive function with darifenacin for 2 weeks and found no difference between immediate- or controlled-release forms of the drug and placebo. However, the study population consisted of volunteers 65 and older with no or mild cognitive impairment and no use of cholinesterase-inhibiting drugs.
IN THE PIPELINEUrethral injection of muscle-derived cells may restore function
Usiene I, Kim YT, Pruchnic R, et al. Human muscle-derived cells injection increases leak point pressure in a nude rat model of stress urinary incontinence. Abstract presented at the annual meeting of the International Continence Society, August 28–September 2, 2005, Montreal, Quebec. Abstract #2.
Some exciting news: Dr. Michael Chancellor and colleagues at the University of Pittsburgh and at Cook MyoSite in Pittsburgh are working to bring stem cell research to the clinician’s office, with their studies of muscle-derived cells that can be injected into the urethra. (This technique is well-established and currently used for injection of synthetic or biologic material such as bovine collagen.)
What is remarkable about this type of injection is that the muscle cells not only stay put in the urethra, they appear to integrate into the muscle of the urethral sphincter and differentiate into cells that produce new muscle fibers. Newly functioning muscle improves urethral function and, ideally, will be able to restore continence in women with incontinence.
Technique’s success in rats
Could the same be accomplished with muscle-derived stem cells from humans? At the 2005 meeting of the International Continence Society, Chancellor and colleagues described how they injected human muscle-derived stem cells into the urethras of a nude rat model of stress incontinence (via nerve transection). In the injected rats, leak-point pressure measurements were restored to levels similar to those in a control group of rats.
In addition, immunohistochemistry and histology showed persistence of the human muscle-derived stem cells in the injected rats, versus periurethral muscle atrophy in the rats that had nerve transection but no injection.
Clinical testing underway
Clinical trials of this technology in women are now being performed in Toronto. It will be necessary to show safety and efficacy before the stem cell therapy is made available clinically, but there are a couple of factors in its favor:
- In terms of safety, the risks associated with the current crop of injectable materials should not be applicable, at least in theory. Because the injected stem cells are isolated and grown from the patient’s own biopsy, the chance of an adverse immunologic reaction should be zero, absent the rare mistake in labeling or transfer.
- The cell-injection technique is well-established and already used by many clinicians who perform transurethral or periurethral injection of bulking agents for women with incontinence. It will not be necessary to learn a new technique—just injectable material.

Tissue-engineered sling also in the works
Given that incontinence (and, presumably, the level of urethral damage resulting in some women may have urethral damage so severe as to preclude benefit from the injection of relatively small numbers of researchers is already developing a tissue-engineered sling in the hope that it can be used as a substitute for currently available synthetic or biologic sling materials.
The sling is being developed with the same muscle-derived stem cells, which are seeded onto a scaffold for as little as 2 weeks in a rat model. After the sling was surgically implanted using standard technique, urethral function improved to the level seen among controls.
It may be that the promise of stem cell therapy will come early to the treatment of urinary incontinence. Time will tell.
The author reports no financial relationships relevant to this article.
Although urinary incontinence is one of the most common chronic diseases in women, we still don’t understand its pathophysiology, and treatments have been, of necessity, empiric rather than directed at a specific cause. Fortunately, this bleak scenario may be changing, and I think that is the most exciting news about urinary incontinence in 2005.
Urethral deficiency by any name is still a deficiency
Ironically, the most basic description of urinary incontinence may be the most revealing: Incontinence occurs when the urethra cannot stay closed and fails to hold urine in the bladder, where it should be stored until the “right” time and place for emptying. This description applies equally well to women with stress or urge symptoms, but let’s focus on stress incontinence for now.
By that line of thinking, the urethra is deficient in all women with stress incontinence. I believe this to be true, despite the arbitrary label—intrinsic sphincter deficiency, or ISD—that we apply to women with only the most severe symptoms of stress incontinence.
Surgery does not end the quest
Because surgery focuses on eliminating symptoms, it should come as no surprise that incontinence procedures continue to proliferate while we search for the Holy Grail: the perfect surgery that will effectively and durably “fix” the problem without complications or side effects. However, unless we find and correct the underlying problem that gave rise to the incontinence in the first place, we are doomed to fail in our search.
Doom? Failure? Where is the exciting news I promised?
CARE trial underscores efficacy of Burch procedure
Brubaker L, for the Pelvic Floor Disorders Network. Burch colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. Abstract presented at the American Urogynecologic Society Meeting, September 15–17, 2005, Atlanta.
If you have an exceptional memory, you will recall that, in this Update on Urinary Incontinence last year, results from the CARE trial were promised in 2006. Good news! Early results are available now, at least a year before expected.
Superior results with Burch changed the course of the CARE study
The CARE trial (Colpopexy And Urinary Reduction Efforts) was designed to determine the effect of Burch versus no Burch in women without stress incontinence symptoms but with advanced prolapse who were undergoing abdominal sacrocolpopexy. The trial was sponsored by the National Institute of Child Health and Human Development (NICHD) and performed by the Pelvic Floor Disorders Network of investigators from 7 clinical sites and a central coordinating center.
The original sample size was set at 480 women, to be randomized equally to Burch or no Burch, with the primary stress outcome at 3 months after surgery. However, at the first interim analysis, when about half the sample (232 women) had reached the primary outcome, the results showed such a striking benefit in the Burch group that the Data and Safety Monitoring Board for the Pelvic Floor Disorders Network recommended that enrollment be halted while all women continued to receive scheduled follow-up. Therefore, in February 2005, enrollment in the trial was closed, with 322 women randomized to 1 of the 2 arms.
The following results were presented at the annual meeting of the American Urogynecologic Society in September:
- Stress incontinence symptoms were reduced by about half in women after abdominal sacrocolpopexy (from 44% in the no-Burch group to 24% in the Burch group).
- Stress symptom severity improved with Burch. More women (62%) in the no-Burch stress incontinence group were bothered by their symptoms, compared to 32% of women in the Burch group.
- Urge symptoms were no different with Burch. More surprising, women in both groups had similar levels of symptoms measured as the urge endpoint, which included urge incontinence, urgency, frequency, nocturia, or enuresis; or treatment for any of those 5 symptoms. Almost 33% of women in the Burch group met the urge endpoint, compared with 38% in the no-Burch group (difference not statistically significant).
- Serious adverse events were not significantly different between the 2 groups.
Many more questions will be addressed with further analysis of CARE trial data, such as results of urodynamic testing with and without prolapse reduction, and the potential for predicting which subgroup benefits most when Burch is performed. Long-term follow-up data will address durability of results related to incontinence and prolapse. Follow-up is scheduled for 2 years in the CARE trial, and for up to 10 years in the Extended-CARE trial.
Efficacy of anticholinergics
Trospium chloride (Sanctura): another anticholinergic for overactive bladder. The Medical Letter. 2004;46(1188):63–64.
Solifenacin and darifenacin for overactive bladder. The Medical Letter. 2005;47(1204):23–24.
Three more drugs for overactive bladder won FDA approval in 2004 and 2005:
- trospium chloride (Sanctura)
- solifenacin succinate Vesicare)
- darifenacin hydrobromide (Enablex)
According to The Medical Letter, none appears to offer an advantage over long-acting anticholinergics for overactive bladder. Despite the proliferation of anticholinergic drugs for overactive bladder symptoms—or perhaps because of it—one suspects that these medications are not achieving substantial, long-lasting relief of symptoms. One study reported that two thirds of women discontinued therapy within 4 months.1 A comprehensive review of placebo-controlled trials of anticholinergic drugs for overactive bladder estimated that, as a class, even long-acting agents have a very limited effect on symptoms, with approximately 1 fewer incontinent episode and 1 fewer voiding episode per 48 hours.2
REFERENCES
1. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynaecol Reprod Biol. 2005;119:237-241.
2. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
Do anticholinergics and dementia drugs mix?
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13:324–328.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–498.
Treatment recommendations
As ObGyns become more active in evaluating and treating women with urinary incontinence, we must stay alert for potential adverse drug interactions.
Ideally, behavioral treatment (scheduled voiding, fluid management, bedside commode) and pelvic muscle training should be first-line therapies in elderly women with overactive bladder.
Anticholinergic drugs should be used with caution, if at all, in women taking cholinesterase-inhibiting drugs for dementia.
Elderly patients with overactive bladder are at high risk for drug interactions, especially involving cholinesterase-inhibiting drugs for the treatment of dementia, such as donepezil hydrochloride (Aricept). However, a newer Alzheimer drug, memantine hydrochloride (Namenda), works by a different mechanism and may be less likely to interact directly with anticholinergic drugs for incontinence.
3 studies involving the elderly
Cognitive impairment
Observing a population of older adults with dementia, about half treated with cholinesterase inhibitors for their Alzheimer symptoms, Gill et al found that the patients on cholinesterase inhibitors were more likely to start treatment with an anticholinergic drug for incontinence within a year. They theorized that the cholinesterase-inhibiting drugs possibly contribute to new-onset or worsening urinary incontinence, which in turn leads to treatment with anticholinergic agents.
Jewart and colleagues found better performance in patients with Alzheimer disease who were not taking anticholinergic medication for incontinence.
No or mild cognitive impairment
Lipton et al tested cognitive function with darifenacin for 2 weeks and found no difference between immediate- or controlled-release forms of the drug and placebo. However, the study population consisted of volunteers 65 and older with no or mild cognitive impairment and no use of cholinesterase-inhibiting drugs.
IN THE PIPELINEUrethral injection of muscle-derived cells may restore function
Usiene I, Kim YT, Pruchnic R, et al. Human muscle-derived cells injection increases leak point pressure in a nude rat model of stress urinary incontinence. Abstract presented at the annual meeting of the International Continence Society, August 28–September 2, 2005, Montreal, Quebec. Abstract #2.
Some exciting news: Dr. Michael Chancellor and colleagues at the University of Pittsburgh and at Cook MyoSite in Pittsburgh are working to bring stem cell research to the clinician’s office, with their studies of muscle-derived cells that can be injected into the urethra. (This technique is well-established and currently used for injection of synthetic or biologic material such as bovine collagen.)
What is remarkable about this type of injection is that the muscle cells not only stay put in the urethra, they appear to integrate into the muscle of the urethral sphincter and differentiate into cells that produce new muscle fibers. Newly functioning muscle improves urethral function and, ideally, will be able to restore continence in women with incontinence.
Technique’s success in rats
Could the same be accomplished with muscle-derived stem cells from humans? At the 2005 meeting of the International Continence Society, Chancellor and colleagues described how they injected human muscle-derived stem cells into the urethras of a nude rat model of stress incontinence (via nerve transection). In the injected rats, leak-point pressure measurements were restored to levels similar to those in a control group of rats.
In addition, immunohistochemistry and histology showed persistence of the human muscle-derived stem cells in the injected rats, versus periurethral muscle atrophy in the rats that had nerve transection but no injection.
Clinical testing underway
Clinical trials of this technology in women are now being performed in Toronto. It will be necessary to show safety and efficacy before the stem cell therapy is made available clinically, but there are a couple of factors in its favor:
- In terms of safety, the risks associated with the current crop of injectable materials should not be applicable, at least in theory. Because the injected stem cells are isolated and grown from the patient’s own biopsy, the chance of an adverse immunologic reaction should be zero, absent the rare mistake in labeling or transfer.
- The cell-injection technique is well-established and already used by many clinicians who perform transurethral or periurethral injection of bulking agents for women with incontinence. It will not be necessary to learn a new technique—just injectable material.

Tissue-engineered sling also in the works
Given that incontinence (and, presumably, the level of urethral damage resulting in some women may have urethral damage so severe as to preclude benefit from the injection of relatively small numbers of researchers is already developing a tissue-engineered sling in the hope that it can be used as a substitute for currently available synthetic or biologic sling materials.
The sling is being developed with the same muscle-derived stem cells, which are seeded onto a scaffold for as little as 2 weeks in a rat model. After the sling was surgically implanted using standard technique, urethral function improved to the level seen among controls.
It may be that the promise of stem cell therapy will come early to the treatment of urinary incontinence. Time will tell.
The author reports no financial relationships relevant to this article.
