User login
When a woman has advanced prolapse of the anterior vaginal wall, it is highly likely that she has apical prolapse as well. Consider a study by Rooney and associates that determined that clinically significant vault prolapse is present in most women who have anterior vaginal prolapse of stage II or higher.1 For that reason, suspension of the vaginal apex should be considered whenever surgical treatment of anterior wall defects is planned.
Sacrocolpopexy involves suspension of the vaginal vault from the anterior longitudinal ligament of the sacrum, using Y-shaped mesh to augment native tissue (FIGURE).2 It is an effective, durable treatment for vaginal apical prolapse. With a success rate approaching 93%, this procedure has become the gold standard for repair of vault prolapse. Among its advantages are maximization of vaginal depth and preservation of a normal vaginal axis.
Sacrocolpopexy preserves the vaginal axis
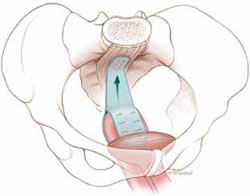
With the vaginal vault suspended from the anterior longitudinal
ligament of the sacrum, the normal vaginal axis is preserved
and vaginal depth is maximized.
Sacrocolpopexy can be performed via the abdominal, laparoscopic, or robotic-assisted approach (TABLE 1). Minimally invasive techniques are attractive because they involve faster recovery than abdominal sacrocolpopexy does. Minimally invasive techniques have also advanced to the point that they are both effective and durable. However, these advantages must be weighed against the effort required to learn the techniques, as well as their higher cost.
TABLE 1
How the 3 approaches to sacrocolpopexy compare
| Approach | Advantages and disadvantages |
|---|---|
| Abdominal | Shortest operative time No significant Trendelenburg position required Highest estimated blood loss Longest length of stay Low rate of complications Longest postoperative recovery Well-established long-term durability |
| Laparoscopic | Longer operative time Moderate Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique least similar to abdominal procedure Low rate of complications Shorter postoperative recovery Long-term durability less firmly established |
| Robotic-assisted | Longest operative time Steep Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique resembles that of abdominal approach Low rate of complications Shorter postoperative recovery Long-term durability appears to be good |
In this article, we highlight:
- a comparison of the laparoscopic and abdominal approaches to sacrocolpopexy
- an investigation of the learning curve associated with robotic-assisted sacrocolpopexy
- a study exploring the durability of robotic-assisted repair
- an estimate of the costs associated with each route of operation.
Laparoscopic vs abdominal sacrocolpopexy—how do they compare?
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192(5):1752–1758.
When surgeons at the Cleveland Clinic performed a retrospective cohort study to compare laparoscopic and abdominal sacrocolpopexy, they found significantly longer operative time with the laparoscopic route, with an average difference of 51 minutes (P < .0001). However, the laparoscopic approach was associated with lower blood loss (although there was no difference between groups in hematocrit on postoperative day 1); shorter hospital stay (average of 1.8 days versus 4 days [P < .001]); and comparable rates of intraoperative and postoperative complications.
Details of the trial
Paraiso and colleagues reviewed the medical charts of 56 consecutive patients who had undergone laparoscopic sacrocolpopexy, comparing them with the charts of 61 consecutive patients who had undergone the procedure using the abdominal approach. The operations had been performed between 1998 and 2003 for treatment of posthysterectomy vaginal prolapse.
The groups underwent similar rates of concurrent procedures. The laparotomy group had a significantly higher number of Burch procedures (P = .007), and the laparoscopic group had a significantly higher rate of adhesiolysis (P = .002).
Among the complications noted— which occurred at comparable rates between groups—were cystotomy, enterotomy, need for transfusion, deep-vein thrombosis, ileus, small bowel obstruction, wound infection, ventral hernia, mesh erosion, and recurrent prolapse. One laparoscopic case was converted to laparotomy because of excessive bleeding during the rectopexy portion of the operation.
Laparoscopy may have taken longer than this trial suggests
This study is one of very few well-designed trials comparing laparoscopic sacrocolpopexy to the historical gold standard of abdominal sacrocolpopexy for vault prolapse.
Twenty-eight percent of laparoscopic procedures in this study used tacking devices in lieu of suturing. Had suturing been performed universally, an even greater difference in surgical time may have been observed.
There may also be differences between groups in the durability of the two types of repair, an outcome not included in this particular study.
The laparoscopic approach offers a shorter hospital stay with no increase in intraoperative or postoperative complications, compared with abdominal sacrocolpopexy. However, it entails a significantly longer operative time than the abdominal approach does.
How steep is the learning curve for robotic-assisted sacrocolpopexy?
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390–2394.
Akl and coworkers reviewed the medical records of all patients who had undergone robotic-assisted sacrocolpopexy at the Mayo Clinics in Arizona and Florida between 2004 and 2007. All operations were performed by the same four urogynecologists, with an average operative time of 197.9 minutes (standard deviation, ± 66.8 minutes). However, after the first 10 cases, the operative time decreased by 64.3 minutes—a decline of 25.4% (P < .01; 95% confidence interval [CI], 16.1–112.4 minutes).
Details of the trial
Researchers collected baseline information on participants’ age, stage of prolapse, and concomitant procedures. They also gathered data on average operative time, estimated blood loss, intraoperative and postoperative complications, conversion to laparotomy, and length of hospitalization.
Of 80 women who had advanced pelvic organ prolapse (stage III/IV) who underwent robotic-assisted sacrocolpopexy, 88% underwent concomitant robotic and vaginal procedures, including robotic supracervical hysterectomy, Burch procedure, paravaginal repair, lysis of adhesions, bilateral salpingooophorectomy, vaginal cystocele or rectocele repair, and placement of a midurethral sling.
Estimated blood loss for the robotic-assisted approach ranged from 25 mL to 300 mL, with a mean loss of 96.8 mL. Average length of hospitalization was 2.6 days. Four cases (5%) were converted to laparotomy because of limited exposure and one intraoperative bladder injury. Other intraoperative complications included small-bowel injury during trocar placement and one ureteral injury. Postoperative complications included one case of ileus and five (6%) vaginal mesh erosions. Three patients developed recurrent prolapse and underwent subsequent correction.
Learning curve could have been measured more precisely
The authors did not specifically measure the learning curve for robotic-assisted sacrocolpopexy, as they took into account the concomitant procedures. For this reason, the decrease in operative time observed after 10 cases may not accurately reflect an improvement in the performance of sacrocolpopexy.
Akl and colleagues consider this detail to be a strength of the study because most women who undergo prolapse surgery have concomitant procedures. However, recording the length of time it took to perform the sacrocolpopexy portion of the procedure would have been more accurate.
The average length of stay approached that of the abdominal route. Length of stay may decline as a surgeon gains experience with the robotic-assisted approach.
Robotic-assisted sacrocolpopexy has a steep learning curve with respect to technique and surgical time.
Does robotic-assisted sacrocolpopexy provide durable support?
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655–659.
Among the few recent series reporting long-term outcomes after robotic-assisted sacrocolpopexy is this observational study from the Mayo Clinic. It involved 30 women who underwent the operation for the treatment of Baden Walker grade 4/4 posthysterectomy vaginal vault prolapse. The authors concluded that advanced prolapse can be treated with robotic-assisted sacrocolpopexy with long-term success and minimal complications.
Details of the trial
Of 30 women in this trial, 52% underwent an anti-incontinence procedure at the time of sacrocolpopexy. Women who had multiple vaginal defects or a history of abdominal surgery were excluded from the study.
Average operative time was 3.1 hours (range, 2.15–4.75 hours) in the early phase of development of operative technique (described in the manuscript) but diminished over time to an average of 2.5 hours.
Twenty-nine patients were discharged from the hospital after an overnight stay. Very few immediate postoperative complications were observed. Two patients experienced mild port-site infections that required outpatient treatment, and one patient had persistent vaginal bleeding from the incision made during the anti-incontinence procedure.
Most patients were followed for at least 1 year
The mean follow-up in this study was 24 months (range, 16–39 months). During this period, 21 women were followed for a full year. Long-term observation revealed that the repair of vault prolapse remained successful in 19 of these women.
One patient experienced recurrent prolapse 7 months after surgery. Another developed a rectocele 9 months after sacrocolpopexy. Vaginal mesh erosions occurred in two patients within 6 months after the procedure; both patients were treated with outpatient resection of the exposed mesh, with no recurrence of the prolapse.
Although a larger sample size and longer follow-up would be ideal, this study demonstrates a low rate of recurrent prolapse 1 year after the procedure.
Robotic sacrocolpopexy appears to provide long-term durability for the treatment of advanced vaginal vault prolapse.
Depending on where you practice, you may have as many as three options: abdominal, laparoscopic, or robotic-assisted. Here are basic questions you should address when choosing one:
- How familiar are you with the technique? if the answer is “not much,” you can anticipate that the cost and time required to perform it will be significantly higher.
- Are the appropriate instruments and surgical team available?
- Does the patient have comorbidities? Consider, for example, the fact that she may not be able to tolerate a steep Trendelenberg position—required for the robotic-assisted approach—if she has severe cardiac or pulmonary disease. However, if she has a risk of poor wound healing, a large abdominal incision may not be advisable and postoperative immobility can be risky. if she is obese, laparoscopic or robotic port placement is challenging, but visualization and retraction will be easier. The need for anticoagulation is another consideration, as it will affect estimated blood loss and the choice of an incision, among other things.
- Let’s not forget the patient. Given the pros and cons, what approach does she prefer?
How much do laparoscopic, abdominal, and robotic-assisted sacrocolpopexy cost?
Judd JP, Siddiqui NY, Barnett JC, et al. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17: 493–499.
This cost-minimization analysis concluded that robotic-assisted sacrocolpopexy incurs the highest hospital charges but is reimbursed by Medicare at a rate similar to reimbursement for the abdominal and laparoscopic routes (TABLE 2).
TABLE 2
Cost of sacrocolpopexy is significant—especially using the robotic approach
| Approach | Cost of a procedure | Operative time, min (range) |
|---|---|---|
| Robotic-assisted | $8,508 | 328 (130–383) |
| Laparoscopic | $7,353 | 269 (97–334) |
| Abdominal | $5,792 | 170 (110–286) |
| Source: Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim invasive Gynecol. 2010;17(4):493–499. | ||
The analysis accounted for realistic practices, such as the inclusion of concurrent hysterectomy and other procedures.
Details of the trial
Surgeons from Duke University developed a decision-analysis model in which a hypothetical group of women with advanced vaginal prolapse could choose between one of the three routes of sacrocolpopexy: abdominal, laparoscopic, or robotic-assisted. Researchers postulated two different scenarios:
- the hospital had ownership of a robotic system
- the hospital invested in the initial purchase and maintenance of such a system.
Researchers reviewed the literature to formulate their estimates of operative time, rate of conversion to laparotomy, rate of transfusion, and length of hospital stay. In addition, the costs of initial anesthesia setup, professional fees, per-minute intraoperative fees, and postanesthesia care were applied to each approach. Operating room costs per minute and the cost of disposable items such as drapes, gowns, gloves, and single-use instruments were added. For the robotic approach, the costs of reusable instruments were distributed across 10 operations. Reusable instruments for laparoscopic and abdominal surgery were assumed to incur no additional investment. Last, postoperative care—including laboratory tests, pharmacy usage, and the need for a hospital room—were individualized for each route of surgery and applied to the cost.
Costs were estimated in 2008 US dollars, based on procedure costs incurred at Duke University Medical Center.
Physician reimbursement data were obtained from Medicare reimbursement rates for anesthesia and from surgeon Current Procedural Terminology (CPT) codes specific to each procedure.
Quality-of-life assessments were not measured. Nor was the cost to society of the postoperative loss of productivity and wages for each surgical route. Had these losses been recognized, the authors observed, the cost of robotic surgery may have been lower.
The cost of robotic surgery was equivalent to the cost of laparoscopy in only two instances:
- when the operative time of robotic surgery was reduced to 149 minutes
- when the cost of robotic disposable items was less than $2,132 (reduced from a baseline cost of $3,293).
Robotic sacrocolpopexy is costly. this is an important consideration when implementing new technology. cost-saving scenarios are useful to maximize patient benefit and minimize financial burden.
We want to hear from you! Tell us what you think.
When a woman has advanced prolapse of the anterior vaginal wall, it is highly likely that she has apical prolapse as well. Consider a study by Rooney and associates that determined that clinically significant vault prolapse is present in most women who have anterior vaginal prolapse of stage II or higher.1 For that reason, suspension of the vaginal apex should be considered whenever surgical treatment of anterior wall defects is planned.
Sacrocolpopexy involves suspension of the vaginal vault from the anterior longitudinal ligament of the sacrum, using Y-shaped mesh to augment native tissue (FIGURE).2 It is an effective, durable treatment for vaginal apical prolapse. With a success rate approaching 93%, this procedure has become the gold standard for repair of vault prolapse. Among its advantages are maximization of vaginal depth and preservation of a normal vaginal axis.
Sacrocolpopexy preserves the vaginal axis

With the vaginal vault suspended from the anterior longitudinal
ligament of the sacrum, the normal vaginal axis is preserved
and vaginal depth is maximized.
Sacrocolpopexy can be performed via the abdominal, laparoscopic, or robotic-assisted approach (TABLE 1). Minimally invasive techniques are attractive because they involve faster recovery than abdominal sacrocolpopexy does. Minimally invasive techniques have also advanced to the point that they are both effective and durable. However, these advantages must be weighed against the effort required to learn the techniques, as well as their higher cost.
TABLE 1
How the 3 approaches to sacrocolpopexy compare
| Approach | Advantages and disadvantages |
|---|---|
| Abdominal | Shortest operative time No significant Trendelenburg position required Highest estimated blood loss Longest length of stay Low rate of complications Longest postoperative recovery Well-established long-term durability |
| Laparoscopic | Longer operative time Moderate Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique least similar to abdominal procedure Low rate of complications Shorter postoperative recovery Long-term durability less firmly established |
| Robotic-assisted | Longest operative time Steep Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique resembles that of abdominal approach Low rate of complications Shorter postoperative recovery Long-term durability appears to be good |
In this article, we highlight:
- a comparison of the laparoscopic and abdominal approaches to sacrocolpopexy
- an investigation of the learning curve associated with robotic-assisted sacrocolpopexy
- a study exploring the durability of robotic-assisted repair
- an estimate of the costs associated with each route of operation.
Laparoscopic vs abdominal sacrocolpopexy—how do they compare?
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192(5):1752–1758.
When surgeons at the Cleveland Clinic performed a retrospective cohort study to compare laparoscopic and abdominal sacrocolpopexy, they found significantly longer operative time with the laparoscopic route, with an average difference of 51 minutes (P < .0001). However, the laparoscopic approach was associated with lower blood loss (although there was no difference between groups in hematocrit on postoperative day 1); shorter hospital stay (average of 1.8 days versus 4 days [P < .001]); and comparable rates of intraoperative and postoperative complications.
Details of the trial
Paraiso and colleagues reviewed the medical charts of 56 consecutive patients who had undergone laparoscopic sacrocolpopexy, comparing them with the charts of 61 consecutive patients who had undergone the procedure using the abdominal approach. The operations had been performed between 1998 and 2003 for treatment of posthysterectomy vaginal prolapse.
The groups underwent similar rates of concurrent procedures. The laparotomy group had a significantly higher number of Burch procedures (P = .007), and the laparoscopic group had a significantly higher rate of adhesiolysis (P = .002).
Among the complications noted— which occurred at comparable rates between groups—were cystotomy, enterotomy, need for transfusion, deep-vein thrombosis, ileus, small bowel obstruction, wound infection, ventral hernia, mesh erosion, and recurrent prolapse. One laparoscopic case was converted to laparotomy because of excessive bleeding during the rectopexy portion of the operation.
Laparoscopy may have taken longer than this trial suggests
This study is one of very few well-designed trials comparing laparoscopic sacrocolpopexy to the historical gold standard of abdominal sacrocolpopexy for vault prolapse.
Twenty-eight percent of laparoscopic procedures in this study used tacking devices in lieu of suturing. Had suturing been performed universally, an even greater difference in surgical time may have been observed.
There may also be differences between groups in the durability of the two types of repair, an outcome not included in this particular study.
The laparoscopic approach offers a shorter hospital stay with no increase in intraoperative or postoperative complications, compared with abdominal sacrocolpopexy. However, it entails a significantly longer operative time than the abdominal approach does.
How steep is the learning curve for robotic-assisted sacrocolpopexy?
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390–2394.
Akl and coworkers reviewed the medical records of all patients who had undergone robotic-assisted sacrocolpopexy at the Mayo Clinics in Arizona and Florida between 2004 and 2007. All operations were performed by the same four urogynecologists, with an average operative time of 197.9 minutes (standard deviation, ± 66.8 minutes). However, after the first 10 cases, the operative time decreased by 64.3 minutes—a decline of 25.4% (P < .01; 95% confidence interval [CI], 16.1–112.4 minutes).
Details of the trial
Researchers collected baseline information on participants’ age, stage of prolapse, and concomitant procedures. They also gathered data on average operative time, estimated blood loss, intraoperative and postoperative complications, conversion to laparotomy, and length of hospitalization.
Of 80 women who had advanced pelvic organ prolapse (stage III/IV) who underwent robotic-assisted sacrocolpopexy, 88% underwent concomitant robotic and vaginal procedures, including robotic supracervical hysterectomy, Burch procedure, paravaginal repair, lysis of adhesions, bilateral salpingooophorectomy, vaginal cystocele or rectocele repair, and placement of a midurethral sling.
Estimated blood loss for the robotic-assisted approach ranged from 25 mL to 300 mL, with a mean loss of 96.8 mL. Average length of hospitalization was 2.6 days. Four cases (5%) were converted to laparotomy because of limited exposure and one intraoperative bladder injury. Other intraoperative complications included small-bowel injury during trocar placement and one ureteral injury. Postoperative complications included one case of ileus and five (6%) vaginal mesh erosions. Three patients developed recurrent prolapse and underwent subsequent correction.
Learning curve could have been measured more precisely
The authors did not specifically measure the learning curve for robotic-assisted sacrocolpopexy, as they took into account the concomitant procedures. For this reason, the decrease in operative time observed after 10 cases may not accurately reflect an improvement in the performance of sacrocolpopexy.
Akl and colleagues consider this detail to be a strength of the study because most women who undergo prolapse surgery have concomitant procedures. However, recording the length of time it took to perform the sacrocolpopexy portion of the procedure would have been more accurate.
The average length of stay approached that of the abdominal route. Length of stay may decline as a surgeon gains experience with the robotic-assisted approach.
Robotic-assisted sacrocolpopexy has a steep learning curve with respect to technique and surgical time.
Does robotic-assisted sacrocolpopexy provide durable support?
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655–659.
Among the few recent series reporting long-term outcomes after robotic-assisted sacrocolpopexy is this observational study from the Mayo Clinic. It involved 30 women who underwent the operation for the treatment of Baden Walker grade 4/4 posthysterectomy vaginal vault prolapse. The authors concluded that advanced prolapse can be treated with robotic-assisted sacrocolpopexy with long-term success and minimal complications.
Details of the trial
Of 30 women in this trial, 52% underwent an anti-incontinence procedure at the time of sacrocolpopexy. Women who had multiple vaginal defects or a history of abdominal surgery were excluded from the study.
Average operative time was 3.1 hours (range, 2.15–4.75 hours) in the early phase of development of operative technique (described in the manuscript) but diminished over time to an average of 2.5 hours.
Twenty-nine patients were discharged from the hospital after an overnight stay. Very few immediate postoperative complications were observed. Two patients experienced mild port-site infections that required outpatient treatment, and one patient had persistent vaginal bleeding from the incision made during the anti-incontinence procedure.
Most patients were followed for at least 1 year
The mean follow-up in this study was 24 months (range, 16–39 months). During this period, 21 women were followed for a full year. Long-term observation revealed that the repair of vault prolapse remained successful in 19 of these women.
One patient experienced recurrent prolapse 7 months after surgery. Another developed a rectocele 9 months after sacrocolpopexy. Vaginal mesh erosions occurred in two patients within 6 months after the procedure; both patients were treated with outpatient resection of the exposed mesh, with no recurrence of the prolapse.
Although a larger sample size and longer follow-up would be ideal, this study demonstrates a low rate of recurrent prolapse 1 year after the procedure.
Robotic sacrocolpopexy appears to provide long-term durability for the treatment of advanced vaginal vault prolapse.
Depending on where you practice, you may have as many as three options: abdominal, laparoscopic, or robotic-assisted. Here are basic questions you should address when choosing one:
- How familiar are you with the technique? if the answer is “not much,” you can anticipate that the cost and time required to perform it will be significantly higher.
- Are the appropriate instruments and surgical team available?
- Does the patient have comorbidities? Consider, for example, the fact that she may not be able to tolerate a steep Trendelenberg position—required for the robotic-assisted approach—if she has severe cardiac or pulmonary disease. However, if she has a risk of poor wound healing, a large abdominal incision may not be advisable and postoperative immobility can be risky. if she is obese, laparoscopic or robotic port placement is challenging, but visualization and retraction will be easier. The need for anticoagulation is another consideration, as it will affect estimated blood loss and the choice of an incision, among other things.
- Let’s not forget the patient. Given the pros and cons, what approach does she prefer?
How much do laparoscopic, abdominal, and robotic-assisted sacrocolpopexy cost?
Judd JP, Siddiqui NY, Barnett JC, et al. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17: 493–499.
This cost-minimization analysis concluded that robotic-assisted sacrocolpopexy incurs the highest hospital charges but is reimbursed by Medicare at a rate similar to reimbursement for the abdominal and laparoscopic routes (TABLE 2).
TABLE 2
Cost of sacrocolpopexy is significant—especially using the robotic approach
| Approach | Cost of a procedure | Operative time, min (range) |
|---|---|---|
| Robotic-assisted | $8,508 | 328 (130–383) |
| Laparoscopic | $7,353 | 269 (97–334) |
| Abdominal | $5,792 | 170 (110–286) |
| Source: Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim invasive Gynecol. 2010;17(4):493–499. | ||
The analysis accounted for realistic practices, such as the inclusion of concurrent hysterectomy and other procedures.
Details of the trial
Surgeons from Duke University developed a decision-analysis model in which a hypothetical group of women with advanced vaginal prolapse could choose between one of the three routes of sacrocolpopexy: abdominal, laparoscopic, or robotic-assisted. Researchers postulated two different scenarios:
- the hospital had ownership of a robotic system
- the hospital invested in the initial purchase and maintenance of such a system.
Researchers reviewed the literature to formulate their estimates of operative time, rate of conversion to laparotomy, rate of transfusion, and length of hospital stay. In addition, the costs of initial anesthesia setup, professional fees, per-minute intraoperative fees, and postanesthesia care were applied to each approach. Operating room costs per minute and the cost of disposable items such as drapes, gowns, gloves, and single-use instruments were added. For the robotic approach, the costs of reusable instruments were distributed across 10 operations. Reusable instruments for laparoscopic and abdominal surgery were assumed to incur no additional investment. Last, postoperative care—including laboratory tests, pharmacy usage, and the need for a hospital room—were individualized for each route of surgery and applied to the cost.
Costs were estimated in 2008 US dollars, based on procedure costs incurred at Duke University Medical Center.
Physician reimbursement data were obtained from Medicare reimbursement rates for anesthesia and from surgeon Current Procedural Terminology (CPT) codes specific to each procedure.
Quality-of-life assessments were not measured. Nor was the cost to society of the postoperative loss of productivity and wages for each surgical route. Had these losses been recognized, the authors observed, the cost of robotic surgery may have been lower.
The cost of robotic surgery was equivalent to the cost of laparoscopy in only two instances:
- when the operative time of robotic surgery was reduced to 149 minutes
- when the cost of robotic disposable items was less than $2,132 (reduced from a baseline cost of $3,293).
Robotic sacrocolpopexy is costly. this is an important consideration when implementing new technology. cost-saving scenarios are useful to maximize patient benefit and minimize financial burden.
We want to hear from you! Tell us what you think.
When a woman has advanced prolapse of the anterior vaginal wall, it is highly likely that she has apical prolapse as well. Consider a study by Rooney and associates that determined that clinically significant vault prolapse is present in most women who have anterior vaginal prolapse of stage II or higher.1 For that reason, suspension of the vaginal apex should be considered whenever surgical treatment of anterior wall defects is planned.
Sacrocolpopexy involves suspension of the vaginal vault from the anterior longitudinal ligament of the sacrum, using Y-shaped mesh to augment native tissue (FIGURE).2 It is an effective, durable treatment for vaginal apical prolapse. With a success rate approaching 93%, this procedure has become the gold standard for repair of vault prolapse. Among its advantages are maximization of vaginal depth and preservation of a normal vaginal axis.
Sacrocolpopexy preserves the vaginal axis

With the vaginal vault suspended from the anterior longitudinal
ligament of the sacrum, the normal vaginal axis is preserved
and vaginal depth is maximized.
Sacrocolpopexy can be performed via the abdominal, laparoscopic, or robotic-assisted approach (TABLE 1). Minimally invasive techniques are attractive because they involve faster recovery than abdominal sacrocolpopexy does. Minimally invasive techniques have also advanced to the point that they are both effective and durable. However, these advantages must be weighed against the effort required to learn the techniques, as well as their higher cost.
TABLE 1
How the 3 approaches to sacrocolpopexy compare
| Approach | Advantages and disadvantages |
|---|---|
| Abdominal | Shortest operative time No significant Trendelenburg position required Highest estimated blood loss Longest length of stay Low rate of complications Longest postoperative recovery Well-established long-term durability |
| Laparoscopic | Longer operative time Moderate Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique least similar to abdominal procedure Low rate of complications Shorter postoperative recovery Long-term durability less firmly established |
| Robotic-assisted | Longest operative time Steep Trendelenburg position required Lower estimated blood loss Shorter length of stay Surgical technique resembles that of abdominal approach Low rate of complications Shorter postoperative recovery Long-term durability appears to be good |
In this article, we highlight:
- a comparison of the laparoscopic and abdominal approaches to sacrocolpopexy
- an investigation of the learning curve associated with robotic-assisted sacrocolpopexy
- a study exploring the durability of robotic-assisted repair
- an estimate of the costs associated with each route of operation.
Laparoscopic vs abdominal sacrocolpopexy—how do they compare?
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol. 2005;192(5):1752–1758.
When surgeons at the Cleveland Clinic performed a retrospective cohort study to compare laparoscopic and abdominal sacrocolpopexy, they found significantly longer operative time with the laparoscopic route, with an average difference of 51 minutes (P < .0001). However, the laparoscopic approach was associated with lower blood loss (although there was no difference between groups in hematocrit on postoperative day 1); shorter hospital stay (average of 1.8 days versus 4 days [P < .001]); and comparable rates of intraoperative and postoperative complications.
Details of the trial
Paraiso and colleagues reviewed the medical charts of 56 consecutive patients who had undergone laparoscopic sacrocolpopexy, comparing them with the charts of 61 consecutive patients who had undergone the procedure using the abdominal approach. The operations had been performed between 1998 and 2003 for treatment of posthysterectomy vaginal prolapse.
The groups underwent similar rates of concurrent procedures. The laparotomy group had a significantly higher number of Burch procedures (P = .007), and the laparoscopic group had a significantly higher rate of adhesiolysis (P = .002).
Among the complications noted— which occurred at comparable rates between groups—were cystotomy, enterotomy, need for transfusion, deep-vein thrombosis, ileus, small bowel obstruction, wound infection, ventral hernia, mesh erosion, and recurrent prolapse. One laparoscopic case was converted to laparotomy because of excessive bleeding during the rectopexy portion of the operation.
Laparoscopy may have taken longer than this trial suggests
This study is one of very few well-designed trials comparing laparoscopic sacrocolpopexy to the historical gold standard of abdominal sacrocolpopexy for vault prolapse.
Twenty-eight percent of laparoscopic procedures in this study used tacking devices in lieu of suturing. Had suturing been performed universally, an even greater difference in surgical time may have been observed.
There may also be differences between groups in the durability of the two types of repair, an outcome not included in this particular study.
The laparoscopic approach offers a shorter hospital stay with no increase in intraoperative or postoperative complications, compared with abdominal sacrocolpopexy. However, it entails a significantly longer operative time than the abdominal approach does.
How steep is the learning curve for robotic-assisted sacrocolpopexy?
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc. 2009;23(10):2390–2394.
Akl and coworkers reviewed the medical records of all patients who had undergone robotic-assisted sacrocolpopexy at the Mayo Clinics in Arizona and Florida between 2004 and 2007. All operations were performed by the same four urogynecologists, with an average operative time of 197.9 minutes (standard deviation, ± 66.8 minutes). However, after the first 10 cases, the operative time decreased by 64.3 minutes—a decline of 25.4% (P < .01; 95% confidence interval [CI], 16.1–112.4 minutes).
Details of the trial
Researchers collected baseline information on participants’ age, stage of prolapse, and concomitant procedures. They also gathered data on average operative time, estimated blood loss, intraoperative and postoperative complications, conversion to laparotomy, and length of hospitalization.
Of 80 women who had advanced pelvic organ prolapse (stage III/IV) who underwent robotic-assisted sacrocolpopexy, 88% underwent concomitant robotic and vaginal procedures, including robotic supracervical hysterectomy, Burch procedure, paravaginal repair, lysis of adhesions, bilateral salpingooophorectomy, vaginal cystocele or rectocele repair, and placement of a midurethral sling.
Estimated blood loss for the robotic-assisted approach ranged from 25 mL to 300 mL, with a mean loss of 96.8 mL. Average length of hospitalization was 2.6 days. Four cases (5%) were converted to laparotomy because of limited exposure and one intraoperative bladder injury. Other intraoperative complications included small-bowel injury during trocar placement and one ureteral injury. Postoperative complications included one case of ileus and five (6%) vaginal mesh erosions. Three patients developed recurrent prolapse and underwent subsequent correction.
Learning curve could have been measured more precisely
The authors did not specifically measure the learning curve for robotic-assisted sacrocolpopexy, as they took into account the concomitant procedures. For this reason, the decrease in operative time observed after 10 cases may not accurately reflect an improvement in the performance of sacrocolpopexy.
Akl and colleagues consider this detail to be a strength of the study because most women who undergo prolapse surgery have concomitant procedures. However, recording the length of time it took to perform the sacrocolpopexy portion of the procedure would have been more accurate.
The average length of stay approached that of the abdominal route. Length of stay may decline as a surgeon gains experience with the robotic-assisted approach.
Robotic-assisted sacrocolpopexy has a steep learning curve with respect to technique and surgical time.
Does robotic-assisted sacrocolpopexy provide durable support?
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. J Urol. 2006;176(2):655–659.
Among the few recent series reporting long-term outcomes after robotic-assisted sacrocolpopexy is this observational study from the Mayo Clinic. It involved 30 women who underwent the operation for the treatment of Baden Walker grade 4/4 posthysterectomy vaginal vault prolapse. The authors concluded that advanced prolapse can be treated with robotic-assisted sacrocolpopexy with long-term success and minimal complications.
Details of the trial
Of 30 women in this trial, 52% underwent an anti-incontinence procedure at the time of sacrocolpopexy. Women who had multiple vaginal defects or a history of abdominal surgery were excluded from the study.
Average operative time was 3.1 hours (range, 2.15–4.75 hours) in the early phase of development of operative technique (described in the manuscript) but diminished over time to an average of 2.5 hours.
Twenty-nine patients were discharged from the hospital after an overnight stay. Very few immediate postoperative complications were observed. Two patients experienced mild port-site infections that required outpatient treatment, and one patient had persistent vaginal bleeding from the incision made during the anti-incontinence procedure.
Most patients were followed for at least 1 year
The mean follow-up in this study was 24 months (range, 16–39 months). During this period, 21 women were followed for a full year. Long-term observation revealed that the repair of vault prolapse remained successful in 19 of these women.
One patient experienced recurrent prolapse 7 months after surgery. Another developed a rectocele 9 months after sacrocolpopexy. Vaginal mesh erosions occurred in two patients within 6 months after the procedure; both patients were treated with outpatient resection of the exposed mesh, with no recurrence of the prolapse.
Although a larger sample size and longer follow-up would be ideal, this study demonstrates a low rate of recurrent prolapse 1 year after the procedure.
Robotic sacrocolpopexy appears to provide long-term durability for the treatment of advanced vaginal vault prolapse.
Depending on where you practice, you may have as many as three options: abdominal, laparoscopic, or robotic-assisted. Here are basic questions you should address when choosing one:
- How familiar are you with the technique? if the answer is “not much,” you can anticipate that the cost and time required to perform it will be significantly higher.
- Are the appropriate instruments and surgical team available?
- Does the patient have comorbidities? Consider, for example, the fact that she may not be able to tolerate a steep Trendelenberg position—required for the robotic-assisted approach—if she has severe cardiac or pulmonary disease. However, if she has a risk of poor wound healing, a large abdominal incision may not be advisable and postoperative immobility can be risky. if she is obese, laparoscopic or robotic port placement is challenging, but visualization and retraction will be easier. The need for anticoagulation is another consideration, as it will affect estimated blood loss and the choice of an incision, among other things.
- Let’s not forget the patient. Given the pros and cons, what approach does she prefer?
How much do laparoscopic, abdominal, and robotic-assisted sacrocolpopexy cost?
Judd JP, Siddiqui NY, Barnett JC, et al. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim Invasive Gynecol. 2010;17: 493–499.
This cost-minimization analysis concluded that robotic-assisted sacrocolpopexy incurs the highest hospital charges but is reimbursed by Medicare at a rate similar to reimbursement for the abdominal and laparoscopic routes (TABLE 2).
TABLE 2
Cost of sacrocolpopexy is significant—especially using the robotic approach
| Approach | Cost of a procedure | Operative time, min (range) |
|---|---|---|
| Robotic-assisted | $8,508 | 328 (130–383) |
| Laparoscopic | $7,353 | 269 (97–334) |
| Abdominal | $5,792 | 170 (110–286) |
| Source: Judd JP, Siddiqui NY, Barnett JC, Visco AG, Havrilesky LJ, Wu JM. Cost-minimization analysis of robotic-assisted, laparoscopic, and abdominal sacrocolpopexy. J Minim invasive Gynecol. 2010;17(4):493–499. | ||
The analysis accounted for realistic practices, such as the inclusion of concurrent hysterectomy and other procedures.
Details of the trial
Surgeons from Duke University developed a decision-analysis model in which a hypothetical group of women with advanced vaginal prolapse could choose between one of the three routes of sacrocolpopexy: abdominal, laparoscopic, or robotic-assisted. Researchers postulated two different scenarios:
- the hospital had ownership of a robotic system
- the hospital invested in the initial purchase and maintenance of such a system.
Researchers reviewed the literature to formulate their estimates of operative time, rate of conversion to laparotomy, rate of transfusion, and length of hospital stay. In addition, the costs of initial anesthesia setup, professional fees, per-minute intraoperative fees, and postanesthesia care were applied to each approach. Operating room costs per minute and the cost of disposable items such as drapes, gowns, gloves, and single-use instruments were added. For the robotic approach, the costs of reusable instruments were distributed across 10 operations. Reusable instruments for laparoscopic and abdominal surgery were assumed to incur no additional investment. Last, postoperative care—including laboratory tests, pharmacy usage, and the need for a hospital room—were individualized for each route of surgery and applied to the cost.
Costs were estimated in 2008 US dollars, based on procedure costs incurred at Duke University Medical Center.
Physician reimbursement data were obtained from Medicare reimbursement rates for anesthesia and from surgeon Current Procedural Terminology (CPT) codes specific to each procedure.
Quality-of-life assessments were not measured. Nor was the cost to society of the postoperative loss of productivity and wages for each surgical route. Had these losses been recognized, the authors observed, the cost of robotic surgery may have been lower.
The cost of robotic surgery was equivalent to the cost of laparoscopy in only two instances:
- when the operative time of robotic surgery was reduced to 149 minutes
- when the cost of robotic disposable items was less than $2,132 (reduced from a baseline cost of $3,293).
Robotic sacrocolpopexy is costly. this is an important consideration when implementing new technology. cost-saving scenarios are useful to maximize patient benefit and minimize financial burden.
We want to hear from you! Tell us what you think.