User login
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
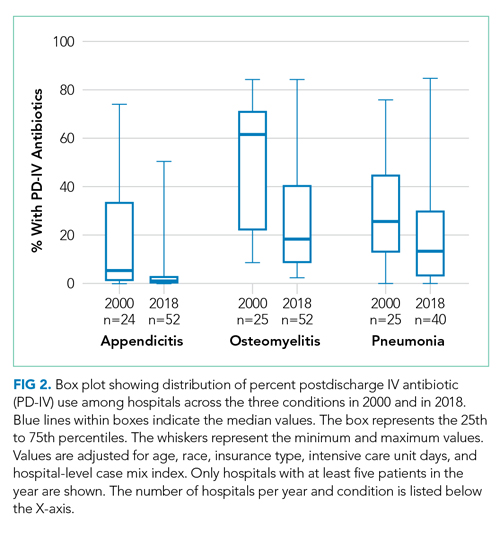
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.
DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.
The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.
DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.

The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
In recent years, mounting evidence has emerged questioning the practice of using prolonged intravenous antibiotic therapy to treat certain serious bacterial infections in children, including complicated appendicitis, osteomyelitis, and complicated pneumonia. Historically, treatment of these conditions was often completed intravenously after hospital discharge using peripherally inserted central catheters (PICCs). Line infections, clots, mechanical problems, and general discomfort complicate PICCs, which led to their removal in more than 20% of children in one study.1 Oral antibiotics avoid these complications and are less burdensome to families.2 Recently, a series of multicenter studies showed no difference in outcomes between oral and postdischarge intravenous antibiotic therapy (PD-IV) for complicated appendicitis, osteomyelitis, and complicated pneumonia.3-5
Despite a growing body of evidence suggesting that oral therapy ought to be the default treatment strategy rather than PD-IV, the extent to which practices have changed is unknown. In this study, we measured national trends in PD-IV use and variation by hospital for complicated appendicitis, osteomyelitis, and complicated pneumonia.
METHODS
We performed a retrospective cohort study of children discharged from hospitals that contributed data to the Pediatric Health Information System (PHIS) database from January 2000 through December 2018. PHIS is an administrative database of children’s hospitals managed by the Children’s Hospital Association (Lenexa, Kansas) and contains deidentified patient-level demographic data, discharge diagnosis and procedure codes, and detailed billing information, including medical supply charges.
The cohorts were defined using International Classification of Diseases, 9th and 10th Revisions (ICD-9 and ICD-10) discharge diagnosis and procedure codes. Patients admitted through September 2015 were identified using ICD-9 codes and patients admitted from October 2015 through December 2018 were identified using ICD-10 codes. The Centers for Medicaid & Medicare Services crosswalk was used to align ICD-9 and ICD-10 codes.6 Inclusion and exclusion criteria identifying cohorts of children hospitalized for complicated appendicitis, osteomyelitis, or complicated pneumonia were based on prior studies using the PHIS database.3-5 These studies augmented the PHIS administrative dataset with local chart review to identify patients from 2009-2012 with the following inclusion and exclusion criteria: Patients with complicated appendicitis were defined by a diagnosis code for acute appendicitis and a procedure code for appendectomy, with postoperative length of stay lasting between 3 and 7 days. Patients with osteomyelitis had a diagnosis code of acute or unspecified osteomyelitis with a hospital length of stay between 2 and 14 days. Patients with complicated pneumonia were defined by a diagnosis code for both pneumonia and pleural effusion with one of these as the primary diagnosis. Patients were excluded if they were older than 18 years or if they were younger than 2 months for osteomyelitis and complicated pneumonia or younger than 3 years for appendicitis. For all three conditions, children with a complex chronic condition7 were excluded. Only the index encounter meeting inclusion and exclusion criteria for each patient was included. PD-IV therapy was defined using procedure codes and hospital charges during the index hospitalization. This definition for PD-IV therapy has been validated among children with complicated pneumonia, demonstrating positive and negative predictive values for PICC exposure of 85% and 99%, respectively.8
Trends in the percentage of patients receiving PD-IV were adjusted for age, race, insurance type, intensive care unit days, and hospital-level case mix index with use of Poisson regression. Calculated risk ratios represent the change in PD-IV across the entire 19-year study period for each condition (as opposed to an annual rate of change). An inflection point for each condition was identified using piecewise linear regression in which the line slope has one value up to a point in time and a second value after that point. The transition point is determined by maximizing model fit.
Some hospitals were added to the database throughout the time period and therefore did not have data for all years of the study. To account for the possibility of a group of high– or low–PD-IV use hospitals entering the cohort and biasing the overall trend, we performed a sensitivity analysis restricted to hospitals continuously contributing data to PHIS every year between 2004 (when a majority of hospitals joined PHIS) and 2018. Significance testing for individual hospital trends was conducted among continuously contributing hospitals, with each hospital tested in the above Poisson model independently.
For the most recent year of 2018, we reported the distribution of adjusted percentages of PD-IV at the individual hospital level. Only hospitals with at least five patients for a given condition are included in the percent PD-IV calculations for 2018. To examine the extent to which an individual hospital might be a low– or high–PD-IV user across conditions, we divided hospitals into quartiles based on PD-IV use for each condition in 2017-2018 and calculated the percent of hospitals in the lowest- and highest-use quartiles for all three conditions. All statistics were performed using Stata 15 (StataCorp).
RESULTS
Among 52 hospitals over a 19-year study period, there were 60,575 hospitalizations for complicated appendicitis, 24,753 hospitalizations for osteomyelitis, and 13,700 hospitalizations for complicated pneumonia. From 2000 to 2018, PD-IV decreased from 13% to 2% (RR, 0.15; 95% CI, 0.14-0.16) for complicated appendicitis, from 61% to 22% (RR, 0.41; 95% CI, 0.39-0.43) for osteomyelitis, and from 29% to 19% (RR, 0.63; 95% CI, 0.58-0.69) for complicated pneumonia (Figure 1). The inflection points occurred in 2009 for complicated appendicitis, 2009 for complicated pneumonia, and 2010 for osteomyelitis. The sensitivity analysis included 31 hospitals that contributed data to PHIS for every year between 2004-2018 and revealed similar findings for all three conditions: Complicated appendicitis had an RR of 0.15 (95% CI, 0.14-0.17), osteomyelitis had an RR of 0.34 (95% CI, 0.32-0.36), and complicated pneumonia had an RR of 0.55 (95% CI, 0.49-0.61). Most individual hospitals decreased PD-IV use (complicated appendicitis: 21 decreased, 8 no change, 2 increased; osteomyelitis: 25 decreased, 6 no change; complicated pneumonia: 14 decreased, 16 no change, 1 increased). While overall decreases in PD-IV were observed for all three conditions, considerable variation remained in 2018 for use of PD-IV (Figure 2), particularly for osteomyelitis (median, 18%; interquartile range [IQR] 9%-40%) and complicated pneumonia (median, 13%; IQR, 3%-30%). In 2017-2018, 1 out of 52 hospitals was in the lowest PD-IV–use quartile for all three conditions, and three hospitals were in the highest-use quartile for all three conditions.
DISCUSSION
Over a 19-year period, we observed a national decline in use of PD-IV for three serious and common bacterial infections. The decline in PD-IV is notable given that it has occurred largely in the absence of nationally coordinated guidelines or improvement efforts. Despite the overall declines, substantial variation in the use of PD-IV for these conditions persists across children’s hospitals.

The observed decrease in PD-IV use is a natural example of deimplementation, the abandonment of medical practices found to be harmful or ineffective.9 What is most compelling about the deimplementation of PD-IV for these infectious conditions is the seemingly organic motivation that propelled it. Studies of physician practice patterns for interventions that have undergone evidence reversals demonstrate that physicians might readily implement new interventions with an early evidence base but be less willing to deimplement them when more definitive evidence later questions their efficacy.10 Therefore, concerted improvement efforts backed by national guidelines are often needed to reduce the use of a widely accepted medical practice. For example, as evidence questioning the efficacy of steroid use in bronchiolitis mounted,11 bronchiolitis guidelines recommended against steroid use12 and a national quality improvement effort led to reductions in exposure to steroids among patients hospitalized with bronchiolitis.13 Complicated intra-abdominal infection guidelines acknowledge oral antibiotic therapy as an option,14 but no such national guidelines or improvement projects exist for osteomyelitis or complicated pneumonia PD-IV.
What is it about PD-IV for complicated appendicitis, osteomyelitis, and complicated pneumonia that fostered the observed organic deimplementation? Our findings that few hospitals were in the top or bottom quartile of PD-IV across all three conditions suggest that the impetus to decrease PD-IV was not likely the product of a broad hospital-wide practice shift. Most deimplementation frameworks suggest that successful deimplementation must be supported by high-quality evidence that the intervention is not only ineffective, but also harmful.15 In this case, the inflection point for osteomyelitis occurred in 2009, the same year that the first large multicenter study suggesting efficacy and decreased complications of early oral therapy for osteomyelitis was published.16 A direct link between a publication and inflection points for complicated pneumonia and appendicitis is less clear. It is possible that growth of the field of pediatric hospital medicine,17 with a stated emphasis on healthcare value,18 played a role. Greater understanding of the drivers and barriers to deimplementation in this and similar contexts will be important.
Our study has some important limitations. While inclusion and exclusion criteria were consistent over the study period, practice patterns (ie, length of stay in uncomplicated patients) change and could alter the case-mix of patients over time. Additionally, the PHIS database largely comprises children’s hospitals, and the trends we observed in PD-IV may not generalize to community settings.
The degree of deimplementation of PD-IV observed across children’s hospitals is impressive, but opportunity for further improvement likely remains. We found that marked hospital-level variation in use of PD-IV still exists, with some hospitals almost never using PD-IV and others using it for most patients. While the ideal amount of PD-IV is probably not zero, a portion of the observed variation likely represents overuse of PD-IV. To reduce costs and complications associated with antibiotic therapy, national guidelines and a targeted national improvement collaborative may be necessary to achieve further reductions in PD-IV.
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
1. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435. https://doi.org/10.1001/jamapediatrics.2013.775
2. Krah NM, Bardsley T, Nelson R, et al. Economic burden of home antimicrobial therapy: OPAT versus oral therapy. Hosp Pediatr. 2019;9(4):234-240. https://doi.org/10.1542/hpeds.2018-0193
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
4. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
5. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
6. Roth J. CMS’ ICD-9-CM to and from ICD-10-CM and ICD-10-PCS Crosswalk or General Equivalence Mappings. National Bureau of Economic Research. May 11, 2016. Accessed June 6, 2018. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
7. Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6):E99. https://doi.org/10.1542/peds.107.6.e99
8. Coon ER, Srivastava R, Stoddard G, Wilkes J, Pavia AT, Shah SS. Shortened IV antibiotic course for uncomplicated, late-onset group B streptococcal bacteremia. Pediatrics. 2018;142(5):e20180345. https://doi.org/10.1542/peds.2018-0345
9. Niven DJ, Mrklas KJ, Holodinsky JK, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13:255. https://doi.org/10.1186/s12916-015-0488-z
10. Niven DJ, Rubenfeld GD, Kramer AA, Stelfox HT. Effect of published scientific evidence on glycemic control in adult intensive care units. JAMA Intern Med. 2015;175(5):801-809. https://doi.org/10.1001/jamainternmed.2015.0157
11. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2013(6):CD004878. https://doi.org/10.1002/14651858.CD004878.pub4
12. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
13. Ralston SL, Garber MD, Rice-Conboy E, et al. A multicenter collaborative to reduce unnecessary care in inpatient bronchiolitis. Pediatrics. 2016;137(1):10. https://doi.org/10.1542/peds.2015-0851
14. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164. https://doi.org/10.1086/649554
15. Norton WE, Chambers DA, Kramer BS. Conceptualizing de-implementation in cancer care delivery. J Clin Oncol. 2019;37(2):93-96. https://doi.org/10.1200/JCO.18.00589
16. Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596
17. Fisher ES. Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107-112. https://doi.org/10.1016/j.cppeds.2012.01.001
18. Landrigan CP, Conway PH, Edwards S, Srivastava R. Pediatric hospitalists: a systematic review of the literature. Pediatrics. 2006;117(5):1736-1744. https://doi.org/10.1542/peds.2005-0609
© 2020 Society of Hospital Medicine
